Background:
Health effects of oxidant gases may be enhanced by components of particulate air pollution that contribute to oxidative stress. Our aim was to examine if within-city spatial variations in the oxidative potential of outdoor fine particulate air pollution (PM2.5) modify relationships between oxidant gases and cardiovascular mortality.
Methods:
We conducted a retrospective cohort study of participants in the Canadian Census Health and Environment Cohort who lived in Toronto or Montreal, Canada, from 2002 to 2015. Cox proportional hazards models were used to estimate associations between outdoor concentrations of oxidant gases (Ox, a redox-weighted average of nitrogen dioxide and ozone) and cardiovascular deaths. Analyses were performed across strata of two measures of PM2.5 oxidative potential and reactive oxygen species concentrations (ROS) adjusting for relevant confounding factors.
Results:
PM2.5 mass concentration showed little within-city variability, but PM2.5 oxidative potential and ROS were more variable. Spatial variations in outdoor Ox were associated with an increased risk of cardiovascular mortality [HR per 5 ppb = 1.028, 95% confidence interval (CI): 1.001, 1.055]. The effect of Ox on cardiovascular mortality was stronger above the median of each measure of PM2.5 oxidative potential and ROS (e.g., above the median of glutathione-based oxidative potential: HR = 1.045, 95% CI: 1.009, 1.081; below median: HR = 1.000, 95% CI: 0.960, 1.043).
Conclusion:
Within-city spatial variations in PM2.5 oxidative potential may modify long-term cardiovascular health impacts of Ox. Regions with elevated Ox and PM2.5 oxidative potential may be priority areas for interventions to decrease the population health impacts of outdoor air pollution.
What this study adds
The effects of gaseous air pollutants may be stronger in areas where particulate matter has greater toxicity, for example, as measured by particle oxidative potential or concentrations of ROS generated in the epithelial lining fluid (ROS). This effect has not been assessed at small spatial scales. We used a population-based cohort in Montreal and Toronto, Canada to assess the association of long-term exposure to oxidant gases with cardiovascular mortality, and whether that relationship varied by particle oxidative potential and ROS. We observed stronger effects of oxidant gas exposures in areas above the median of oxidative potential and ROS.
Introduction
Exposure to fine particulate air pollution (PM2.5) is a known risk factor for cardiovascular morbidity and mortality.1,2 An important mechanism by which PM2.5 induces cardiovascular dysfunction is oxidative stress, which occurs when levels of reactive oxygen species (ROS) exceed normal levels and overcome the body’s antioxidant defenses.1 PM2.5 is only one component in the complex mixture of chemicals that comprise air pollution. Oxidant gases (expressed as Ox, which represents a weighted average of the gases nitrogen dioxide, NO2, and ozone, O3) also contribute to outdoor air pollution. These gases can induce oxidative stress and are associated with cardiovascular mortality.3,4
PM2.5 concentrations depend on regional background levels (i.e., particles transported from distant sources) as well as local sources (e.g., local vehicular traffic or industrial activity)5 but show relatively little spatial variability within cities.6–9 However, Ox concentrations vary considerably within cities (this is driven primarily by local production of NO2).10,11 As well, small-scale spatial variations in PM2.5 components are much greater than variations in total PM2.5 mass concentrations owing to greater differences in composition from local sources.6,7 Moreover, PM components are not equally toxic, and many PM2.5 components (e.g., transition metals) are associated with increased production of free radicals.12 Particle oxidative potential (OP) presents an integrated approach to estimating the ability of particles to induce oxidative stress, and existing evidence suggests that OP is highly variable within cities.6,13 OP can be determined by a number of different acellular assays,14 frequently by measuring depletion of antioxidants [most commonly ascorbate (AA) and glutathione (GSH)] using a cell-free assay based on a synthetic respiratory tract lining fluid exposed to PM2.5 sample extracts.15 Alternatively, the concentration of ROS resulting from the ability of particles to generate ROS and the destruction of ROS by antioxidants can be estimated using a mathematical model based on the content of redox-active components including transition metals in particles.16
Recent evidence suggests that regional variation (i.e., between cities) in PM2.5 OP can modify the acute and chronic health effects of Ox.17,18 Specifically, in a time-stratified case-crossover study across 34 Canadian cities, associations between short-term Ox exposures and respiratory hospitalizations in children were stronger when monthly average GSH-based OP (OPGSH) was higher.18 Similarly, long-term effects were assessed in a cohort study of Canadian adults living within 10 km of one of the 40 monitoring sites across the country, and associations between Ox and mortality (nonaccidental, cardiovascular, and respiratory mortality) were consistently stronger in regions with elevated PM2.5 transition metal/sulfur content and oxidative potential (OPAA, OPGSH, and OP estimated from a dithiothreitol-based assay).17 However, neither of these previous studies examined how PM2.5 OP and ROS concentrations may modify the health impacts of Ox within cities.
In this study, we examined how within-city spatial variations in PM2.5 OP and ROS concentrations influenced associations between long-term exposures to Ox and cardiovascular mortality in Toronto and Montreal, Canada. Our primary focus was on the combined oxidant capacity of oxidant gases O3 and NO2 because these species react together in the atmosphere and are correlated in space (generally inversely). Using the integrated Ox measure allowed us to examine the simultaneous effects of both oxidant gases. However, for the purposes of completeness, we examined the effects of O3 and NO2 separately in addition to the combined Ox measure. Our cohort analysis included more than 1 million members of the Canadian Census Health and Environment Cohort (CanCHEC) and our estimates of PM2.5 OP and ROS were based on dense spatial monitoring campaigns conducted across each city.
Methods
Cohort description and mortality outcomes
The Canadian Census Health and Environment Cohort (CanCHEC) has been described previously.19,20 Briefly, this is a population-based cohort established in 2008, when the 1991 Canadian Census was linked to 10 years of death records, and includes non-institutionalized Canadians (aged 25 and older) who were among the approximately 20% of households selected for enumeration by the long-form Census questionnaire. The cohort now includes multiple cycles of follow-up.21 These datasets were linked to postal code histories for annual place of residence using Historical Tax Summary Files. CanCHEC includes information from Census questionnaires on socioeconomic indicators, ethnicity, and place of residence, as well as neighborhood-level characteristics including environmental conditions.19 Cause-specific mortality data were linked from the Canadian Vital Statistics Death Database using deterministic and probabilistic methods. The CanCHEC dataset was created under the authority of the Statistics Act and approved by the Executive Management Board at Statistics Canada (reference: 045-2015). This is equivalent to standard research ethics board approval. Informed consent was waived because the database used in this study contains only deidentified individual records.
The present study population was limited to individuals in the 1991, 1996, 2001, or 2006 CanCHEC cycles who were between the ages of 25 and 90 years and who lived in Toronto or Montreal for at least 2 years during follow-up. Individuals who were enumerated in more than one long-form census cycle were assigned to the earliest cohort in which they appeared. All participants were followed for cardiovascular mortality (ICD-10 codes I10-I69) from the date they entered the study area (on or after census day in 2001 for the 1991, 1996, and 2001 cohorts, or on or after census day in 2006 for the 2006 cohort) to 31 December 2015, which was the end of data availability for O3 concentrations. This restricted follow-up period was implemented to reduce potential error caused by extrapolation of OP and ROS exposures far into the past (i.e., for the 1991 cohort).
Exposure assignment
Outdoor concentrations of Ox, PM2.5 mass concentrations, and PM2.5 OP and ROS were assigned to the residential postal codes across each city (6-digit postal codes, about the size of one city block face). Residential postal code histories from annual income tax filings were used to estimate time-varying exposures for Ox (and PM2.5) over the duration of the follow-up period to account for residential mobility within and between cities (i.e., between Montreal and Toronto). Specifically, exposures were assigned as 3-year moving averages with a 1-year lag (e.g., an individual’s exposure for 2008 was equal to the mean of their exposures for 2005, 2006, and 2007), as in Pinault et al.22 This exposure assignment procedure ensured that the exposure always preceded the event. Although PM2.5 OP and ROS exposures were measured or estimated based on 2018 data, they were updated annually to account for residential mobility. Person-time was considered at risk of exposure effects if the individual resided in the study area during at least two of the preceding 3 years.
Outdoor oxidant gas and PM2.5 concentrations
Outdoor Ox concentrations were calculated as a redox-weighted average of ozone (O3) and nitrogen dioxide (NO2) based on the following equation: Ox = ((1.07 × NO2) + (2.075 × O3))/3.14.3,23 O3 data were estimated using chemical transport models of surface observations incorporating ground monitor data.24,25 O3 concentrations reflected the daily maximum of 8-hour average concentrations26 and were assigned as annual averages to postal codes. The O3 models had a spatial resolution of 21 km2 before 2009 and 10 km2 from 2009 to 2015. Annual average outdoor NO2 concentrations were estimated from a land-use regression model27 developed from 2006 data, combining NO2 estimates derived from remote sensing and National Air Pollution Surveillance monitoring data. The NO2 model had a spatial resolution of 100 m2. NO2 and O3 data indexed to DMTI Spatial Inc. postal codes were provided by CANUE (Canadian Urban Environmental Health Research Consortium). The resulting calculated Ox exposures combined NO2 exposures estimated at a 100 m2 spatial scale with lower-resolution O3 exposures estimated at a larger spatial scale of 10 km2 (21 km2). Finally, annual average outdoor PM2.5 mass concentrations were estimated using previously developed models.28 Briefly, PM2.5 concentrations were estimated at a resolution of 1 × 1 km using a combination of aerosol optical depth, a chemical transport model, and land-use data.28,29 Spatial and temporal resolutions of air pollutant concentrations are summarized in Table S1; http://links.lww.com/EE/A225.
Spatial monitoring studies and laboratory analyses for PM2.5 oxidative potential and modeling reactive oxygen species concentrations
Outdoor PM2.5 monitoring campaigns were conducted in 2018 in Toronto and Montreal, Canada. Monitoring sites were identified to capture the variability of ambient PM2.5 in each city with maximal spatial coverage.22 In total, 110 sites were monitored in Toronto (a geographic area of 630.2 km2) and 124 sites in Montreal (472.6 km2) in the summer season; daily mean temperatures ranged from 14.4 °C to 23.7 °C in Montreal and 19.8 °C to 26.6 °C in Toronto. Winter season sampling was planned but could not be carried out in both cities due to technical issues. A smaller subset of sites was monitored in Montreal during the winter season, and spatial patterns of OP were similar between summer and winter.6
Integrated 2-week PM2.5 samples were collected at each site using Teflon filters with a mix of Ultrasonic Personal Air Sample monitors (Access Sensor Technologies, Fort Collins, CO) at a flow rate of 1 L/min and cascade impactors at a flow rate of 5 L/min. Samples were collected simultaneously within each city using preset timers to eliminate the need for temporal adjustment of estimates; however, the Toronto monitoring period was approximately one month after the Montreal monitoring period, due to the practical limitations of simultaneous monitoring at a large number of sites. As our focus was on within-city exposure effects, we did not perform temporal adjustment of the OP measurements between the two cities. The oxidative potential of PM2.5 samples was analyzed using two acellular in vitro assays, namely the ascorbate (AA) assay and the GSH assay, according to procedures described previously.15,30 Briefly, PM2.5 samples were extracted, re-suspended and then incubated with a synthetic human respiratory tract lining fluid for 4 hours at 37 °C. This fluid was a composite solution of physiologically relevant antioxidants including equimolar concentrations (200 µM) of ascorbate (AA), GSH, and urate. PM2.5 oxidative potential was measured by depletion of AA (% change in absorbance at 260 nm wavelength) and GSH (% change in absorbance at 405 nm wavelength). Measures of AA-related and GSH-related PM2.5 oxidative potential were expressed per unit mass (% depletion/µg).
In addition to the OP assays, the concentration of ROS in the epithelial lining fluid due to generation from redox reactions of transition metals and destruction by reactions with antioxidants was estimated using the KM-SUB-ELF model described by Lakey et al.16 This mathematical model simulates chemical reactions that occur in the respiratory tract’s epithelial lining fluid following inhalation of particles as determined by the particles’ Fe and Cu content. Concentrations of Cu and Fe in PM2.5 samples were determined by x-ray fluorescence according to EPA Method IO-3.3 in Compendium of Methods for the Determination of Metals in Ambient Particulate Matter (EPA 625/R-96/010a).
Spatial variations in outdoor PM2.5 OP and ROS were assigned based on the measurements collected across each city (i.e., the closest monitoring site to a given residential postal code centroid). We used measured values rather than modeled estimates since the measured sites were densely concentrated across the study areas. We were not able to extrapolate OP and ROS estimates into the past due to the absence of historical data. However, we assume relative stability of the spatial distributions of OP and ROS over time since these measures are driven largely by vehicular traffic and the locations of major roadways/highways have not changed significantly over the time period of follow-up.
Statistical analyses
We used stratified Cox proportional hazards models to estimate hazard ratios describing relationships between within-city spatial variations in Ox concentrations and cardiovascular mortality, overall and within strata of OP/ROS of PM2.5. Models were stratified by age (10-year age groups), sex (male/female), Census cohort year (1991, 1996, 2001, and 2006), city of residence (Toronto/Montreal), and immigrant status (Canadian-born/immigrant). Covariates were chosen with the aid of a Directed Acyclic Graph (see Figure S1; http://links.lww.com/EE/A225). Additionally, models were adjusted for several indicators of socioeconomic status including visible minority status, occupational level, educational attainment, labor force status, marital status, and income quintile, as well as an additional variable indicating relative age within the 10-year age group (to address possible residual confounding by age), and PM2.5 mass concentration. In addition, we included neighborhood-level variables for four dimensions of the Canadian Marginalization Index (CAN-Marg) which describes inequalities in terms of material deprivation, residential instability, dependency, and ethnic concentration.31
Follow-up time started with census day 2001 for the 1991, 1996 and 2001 cohorts, and census day 2006 for the 2006 cohort. Subjects were censored if they moved outside the cities of Montreal or Toronto, if they were lost to follow up, at the end of the study period, or at time of death from a non-cardiovascular cause. Data were accessed and analyzed in the secure facilities of the McGill-Concordia Research Data Centre located at McGill University. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Hazard ratios were expressed per 5 ppb increase in Ox.
The CanCHEC datasets lack information on potential individual-level confounders such as smoking and body mass index (BMI). Although these are not likely to be strong confounders of the relationship between outdoor concentrations of Ox and cardiovascular mortality (i.e., because individual-level smoking is not a cause of long-term average outdoor Ox concentrations), we evaluated correlations between Ox and smoking and BMI in order to assess the potential for confounding by chance correlations with Ox. To do this, we used the Canadian Community Health Survey (CCHS) cohort population (multiple cycles: 2001–2008), an ancillary population-based cohort which has individual-level data on these lifestyle variables. From the CCHS cohort, we selected people who lived in Toronto or Montreal; and who were aged between 25 and 89 years at baseline. We assigned pollutant exposure values to these people in CCHS based on postal code and year of survey and calculated correlations between Ox and smoking and BMI.
Finally, we assessed the relationship between co-exposure to both high (i.e., above the median) levels of OPAA, OPGSH, and ROS and high (above the median) Ox and each dimension of the Canadian Marginalization Index. For each measure of OP/ROS, we created a three-level categorical variable representing high, low, or mixed exposure to both pollutants (i.e., exposure to below-median values of Ox and below-median values of OP; exposure to above-median values of Ox and above-median values of OP; and exposure to above-median OP but below-median Ox, or vice versa). Finally, we calculated correlations between co-exposure to each of the 3-level OP/Ox variables and each of the 5-level quintiles of the four CanMARG dimensions using Spearman’s rank order correlation.32
Results
Cohort characteristics
In total, approximately 36,800 deaths from cardiovascular causes were included in the analyses, occurring over 10,987,500 person-years (rounded to the nearest 100 to comply with Statistics Canada confidentiality requirements) in approximately 1.1 million individuals (Table 1).
Table 1.
Descriptive statistics at baseline for the study cohort of people living in Toronto or Montreal (1991, 1996, 2001, and 2006 CanCHEC cohorts).
| Characteristic | Person-years | Participants | Cardiovascular deaths |
|---|---|---|---|
| Total | 10,987,500 | 1,121,000 | 36,800 |
| Sex | |||
| Male | 4,994,700 | 520,400 | 19,400 |
| Female | 5,992,800 | 600,700 | 17,400 |
| Immigrant status | |||
| Nonimmigrant | 5,811,100 | 606,400 | 20,500 |
| Immigrant | 5,176,400 | 514,600 | 16,300 |
| City of residence | |||
| Toronto | 6,012,200 | 616,400 | 19,500 |
| Montreal | 4,975,300 | 504,600 | 17,400 |
| Age group | |||
| 25–34 | 917,100 | 108,300 | NA |
| 35–44 | 2,662,100 | 259,800 | 700 |
| 45–54 | 2,855,300 | 271,800 | 2,000 |
| 55–64 | 2,050,600 | 197,400 | 4,100 |
| 65–74 | 1,495,400 | 151,100 | 9,600 |
| 75–84 | 781,800 | 99,500 | 13,800 |
| 85–89 | 225,300 | 33,200 | 6,500 |
| Occupational class | |||
| Management | 927,100 | 92,400 | 1,200 |
| Professional | 1,748,300 | 169,800 | 1,500 |
| Skilled, technical, and supervisory | 2,035,100 | 202,300 | 2,700 |
| Semiskilled | 2,441,900 | 242,900 | 3,300 |
| Unskilled | 800,800 | 79,200 | 1,500 |
| No occupation | 3,034,500 | 334,300 | 26,700 |
| Labor force status | |||
| Employed | 7,078,100 | 697,500 | 8,100 |
| Unemployed | 618,500 | 62,700 | 1,000 |
| Not in labor force | 3,290,900 | 360,800 | 27,700 |
| Income quintile | |||
| Lowest | 2,194,000 | 224,500 | 6,100 |
| Second lowest | 2,193,700 | 236,200 | 13,900 |
| Middle | 2,202,800 | 226,400 | 7,400 |
| Second highest | 2,195,700 | 220,700 | 5,000 |
| Highest | 2,201,300 | 213,200 | 4,400 |
| Educational attainment | |||
| Less than high school graduation | 2,936,300 | 305,000 | 8,900 |
| High school graduation with/without trades certificate | 3,242,700 | 330,300 | 10,300 |
| Some postsecondary or college diploma | 1,986,800 | 206,100 | 3,800 |
| University degree | 2,821,700 | 279,600 | 3,900 |
| Marital status | |||
| Divorced/separated/widowed | 1,561,200 | 173,500 | 10,700 |
| Married (including common law) | 7,265,400 | 720,900 | 21,100 |
| Single | 2,160,810 | 226,600 | 5,000 |
| Visible minority status | |||
| Not defined as visible minority | 8,672,300 | 881,100 | 33,100 |
| Visible minority | 2,315,100 | 239,900 | 3,800 |
| Marginalization index | |||
| Can-Marg: residential instability | |||
| Lowest | 2,212,300 | 206,700 | 5,700 |
| Second lowest | 2,185,600 | 214,300 | 6,700 |
| Middle | 2,189,000 | 226,100 | 6,900 |
| Second highest | 2,202,300 | 234,600 | 7,600 |
| Highest | 2,198,400 | 239,500 | 10,000 |
| Can-Marg: material deprivation | |||
| Lowest | 2,214,100 | 214,000 | 7,400 |
| Second lowest | 2,178,200 | 213,200 | 7,100 |
| Middle | 2,192,600 | 223,800 | 7,200 |
| Second highest | 2,207,700 | 231,700 | 7,300 |
| Highest | 2,194,700 | 238,200 | 7,800 |
| Can-Marg: dependency | |||
| Lowest | 2,186,700 | 241,200 | 5,400 |
| Second lowest | 2,233,900 | 232,900 | 6,000 |
| Middle | 2,171,600 | 219,100 | 6,300 |
| Second highest | 2,204,100 | 218,100 | 7,500 |
| Highest | 2,191,200 | 209,800 | 11,800 |
| Can-Marg: ethnic concentration | |||
| Lowest | 2,181,000 | 209,200 | 8,000 |
| Second lowest | 2,214,700 | 220,500 | 7,900 |
| Middle | 2,202,700 | 221,100 | 7,100 |
| Second highest | 2,185,400 | 224,700 | 7,300 |
| Highest | 2,203,700 | 245,500 | 6,600 |
All numbers are rounded to the nearest 100 for confidentiality and may not add up to the total; NA denotes counts below 100 and are suppressed for confidentiality.
Characteristics of pollutant exposures
Ox and PM2.5 exposures across all person-years, as well as PM2.5 OP and ROS, are summarized in Table 2. Ox and PM2.5 distributions were similar within strata of PM2.5 OP and ROS (see Tables S2 and S3; http://links.lww.com/EE/A225). In Montreal, the median distance from postal code centroids to the nearest monitored site was 916.5 m, with a maximum of 5,550.2 m. In Toronto, the median distance was 1,338.3 m and maximum 4,863.1 m. Ox exposures were weakly/moderately correlated with measures of PM2.5 oxidative potential (Pearson correlation coefficients as follows: OPAA, 0.33; OPGSH: 0.51; ROS, 0.29). The three OP and ROS measures were positively correlated, with the highest correlation observed between OPAA and OPGSH (Pearson correlation = 0.54), and weaker correlations between ROS and OPGSH (Pearson correlation = 0.49) and ROS and OPAA (Pearson correlation = 0.17) (for a full table of correlations between pollutants and OP measures, see Table S4; http://links.lww.com/EE/A225).
Table 2.
Descriptive statistics for ambient pollutant concentrations across all person-years.
| Pollutant | Mean (SD) | Median | IQR | Percentile | |
|---|---|---|---|---|---|
| 1st | 99th | ||||
| PM2.5 (µg/m3) | 9.5 (1.3) | 9.5 | 1.6 | 7.0 | 13.0 |
| OPAA (% depletion/µg) | 0.092 (0.036) | 0.085 | 0.034 | 0.016 | 1.036 |
| OPGSH (% depletion/µg) | 0.337 (0.070) | 0.332 | 0.068 | 0.182 | 1.442 |
| ROS (nmol/L) | 71.474 (10.807) | 73.399 | 14.311 | 44.525 | 95.110 |
| NO2 (ppb) | 20.027 (5.475) | 19.80 | 7.60 | 8.64 | 35.00 |
| O3 (ppb) | 38.697 (5.081) | 37.75 | 6.73 | 29.41 | 51.88 |
| Ox (ppb) | 32.345 (3.804) | 31.88 | 5.01 | 25.56 | 42.66 |
IQR indicates interquartile range; SD, standard deviation.
Relationship between Ox and cardiovascular mortality within strata of particle OP/ROS
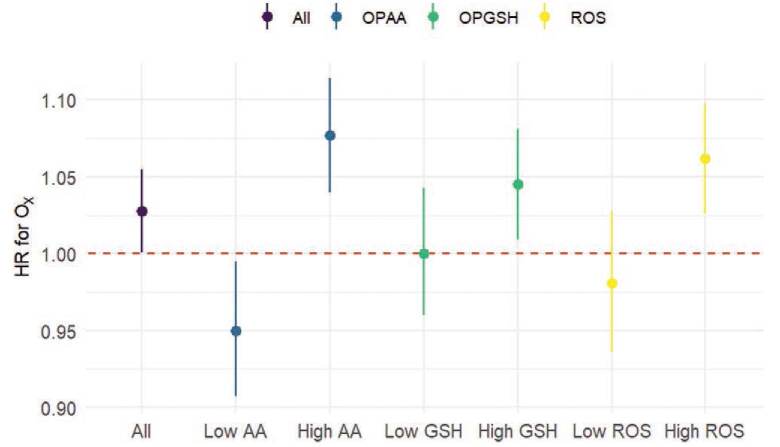
Figure 1 shows the hazard ratios for associations between Ox and cardiovascular mortality. Overall, each 5 ppb increase in Ox (IQR = 5.01) was associated with an increased risk of cardiovascular mortality (HR: 1.028, 95% CI: 1.001, 1.055). The relationship was stronger above the median of OPAA, OPGSH, and ROS concentrations (for numeric values see Table S5; http://links.lww.com/EE/A225). The overall effect of Ox was stronger in women than in men (HR for women: 1.039, 95% CI: 1.000, 1.080; HR for men: 1.019, 95% CI: 0.983, 1.055), but differences in HRs for Ox across strata of OP were larger in men (see Table S6; http://links.lww.com/EE/A225). Little variation in outdoor PM2.5 mass concentration exposures was observed across the study area (IQR = 1.60 μg/m3) and was not associated with the risk of cardiovascular mortality (HR per 1 μg/m3: 0.988, 95% CI: 0.960, 1.017). Although our primary focus was on the combined weighted redox capacity of NO2 and O3, we also observed higher effects above the median of OPAA, OPGSH, and ROS for NO2 (see Table S7; http://links.lww.com/EE/A225), and above the median of OPAA and OPGSH for O3 (see Table S8; http://links.lww.com/EE/A225). For example, overall O3 exposures were associated with an increased risk of cardiovascular mortality [HR per IQR (6.73 ppb): 1.035, 95% CI: 1.010, 1.062], and a higher risk above the median OPGSH (HR: 1.021, 95% CI: 1.008, 1.079) than below the median OPGSH (HR: 1.014, 95% CI: 0.973, 1.022). However, opposite to the general trend, the effects of O3 were higher below the median of ROS relative to above the median (see Table S8; http://links.lww.com/EE/A225). In analyses stratified above/below the median, estimates under the median of OPAA were significantly below the null for Ox (Table S6; http://links.lww.com/EE/A225). Similarly, estimates below the median of OPAA or ROS were significantly below the null for NO2 (Table S7; http://links.lww.com/EE/A225).
Figure 1.
Hazard ratios (95% CI) of Ox exposures (per 5 ppb) on cardiovascular mortality overall and across strata (low = below median, high = above median) of PM2.5 oxidative potential (AA, GSH) and ROS concentrations.
Sensitivity analysis for unmeasured confounding by smoking and body mass index
We used 24,200 individuals at baseline in the CCHS cohorts to check correlations between Ox exposures and unmeasured lifestyle confounders (smoking and BMI) (see Tables S9 and S10; http://links.lww.com/EE/A225, for characteristics of this population). Within each city, we observed weak and generally inverse correlations between outdoor Ox concentrations and smoking [r = −0.032 (Toronto); r = −0.056 (Montreal)] and BMI [r = −0.013 (Toronto); r = −0.0055 (Montreal)] (for all correlations, see Table S11; http://links.lww.com/EE/A225).
Spatial distributions of co-occurring high levels of Ox and PM2.5 OP
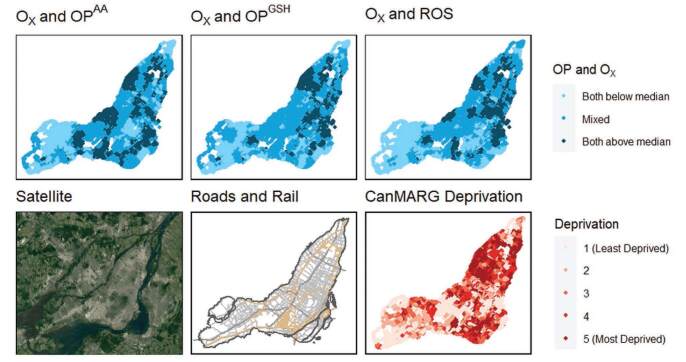
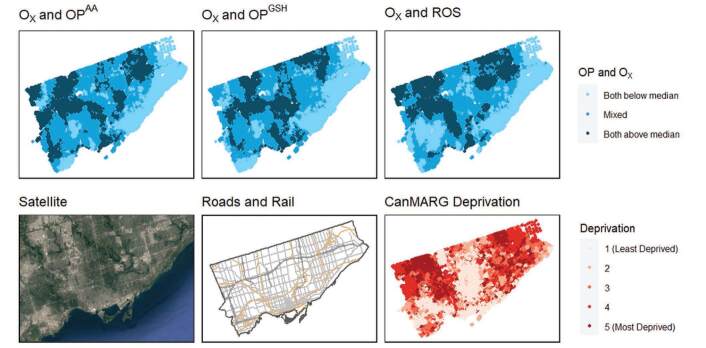
In Montreal, areas with co-occurring high levels of Ox and OP and ROS (i.e., above the median) tended to be near major highways, in the downtown core, and in the east end of the city where industrial activity is prevalent (Figure 2). Similarly, in Toronto, these areas occurred in the north-west quadrant of the city where two major highways intersect near an international airport, as well as in the eastern area of the city where there is a major north-south highway (Figure 3).
Figure 2.
Spatial distributions of co-occurring oxidative potential measures and Ox concentrations at postal codes in comparison to land-use patterns, traffic infrastructure and quintiles of socioeconomic deprivation in Montreal, Canada.
Figure 3.
Spatial distributions of co-occurring oxidative potential measures and Ox concentrations at postal codes in comparison to land-use patterns, traffic infrastructure and quintiles of socioeconomic deprivation in Toronto, Canada.
In Montreal, areas with higher marginalization appeared to have a more harmful mixture of pollutants in terms of combined levels of Ox and OP/ROS (Figure 3). Specifically, co-exposure to both Ox above the median and OP above the median (for all three OP measures) was weakly to moderately correlated with material deprivation (a measure of access to and attainment of basic material needs, which includes factors such as percent unemployment and percent without a high school degree) in Montreal (Spearman’s rank correlations of 0.377–0.421) (see Table S12; http://links.lww.com/EE/A225). Similarly, the CanMARG dimension of residential instability (which includes indicators that measure types and density of residential accommodations as well as family structure characteristics) was correlated with co-exposure to both Ox above the median and OP above the median (Spearman’s rank correlations of 0.233–0.436) in Montreal (see Table S11; http://links.lww.com/EE/A225). However, the CanMARG dimensions of ethnic concentration and dependency were not consistently correlated with combined OP/Ox exposures. Significant correlations between marginalization variables and combined OP/Ox were not observed in Toronto.
Discussion
In this study we investigated how within-city spatial variations in outdoor PM2.5 OP and ROS concentrations may modify associations between long-term exposure to Ox and cardiovascular mortality. Estimates of spatial variations in OP/ROS were based on monitoring campaigns of 110 sites in Toronto and 124 sites in Montreal to estimate the intraurban spatial variability of OP/ROS exposures.
Our results suggest that exposures to Ox are associated with higher risks of cardiovascular mortality in areas where the ability of PM2.5 to induce oxidative stress is elevated. Recent work has demonstrated effect modification of the effects of Ox by OP at the regional scale. For example, between cities, associations between Ox and mortality (nonaccidental, cardiovascular, and respiratory mortality) were consistently stronger in regions where PM2.5 oxidative potential was higher.17 Similarly, another study found that the association of Ox with respiratory hospitalizations in children was higher when monthly average GSH-based OP (OPGSH) was higher.18 However, each of these studies was conducted across a wide spatial area using a single site monitor or a small number of monitors in each city/region, so complex small-scale spatial variations in OP were not captured. Our study is the first to present evidence that PM2.5 OP can influence the effect of Ox within cities.
Although the number of studies showing the influence of OP on Ox exposures is small, a substantial body of evidence demonstrates that OP modifies the effect of PM2.5 mass concentration exposures on health outcomes. For example, in a cohort study in Ontario, OPGSH-related oxidative burden (i.e., PM2.5 mass weighted by OPGSH) was more strongly associated with elevated risks of mortality than PM2.5 mass concentration alone, but this was not observed for OPAA-related oxidative burden.33 Further, in a case-crossover study conducted in 16 studies across Ontario, the strongest associations between PM2.5 and emergency room visits for myocardial infarction occurred in areas where both Ox and OPGSH were high.34 Given that both Ox and PM2.5 mass concentration exposures induce oxidative stress, which is thought to be an important mechanism contributing to the observed adverse health effects, it is plausible that areas where PM2.5 exposures have a greater ability to induce oxidative stress will see greater health effects from both PM2.5 mass concentrations and Ox. In our study, spatial variations in PM2.5 exposures were minimal at the within-city scale and were not associated with an increased risk of cardiovascular mortality; however, since Ox varies substantially within cities, it was possible to identify an effect of spatial variations in Ox exposures on cardiovascular mortality and to examine how OP enhances that effect.
Areas in Montreal and Toronto with high co-occurring levels of Ox and OP or ROS concentrations appeared to be spatially distributed near sources of traffic emissions. This is consistent with previous work that showed traffic-related variables to be the strongest predictors of OPAA, OPGSH, and ROS in this study area.6 Transition metals including Cu and Fe are drivers of OP derived from vehicular non-tailpipe emissions; however, OP also responds to other, nonmetal components of PM2.5 (e.g., organic compounds).14 Importantly, our findings suggest that neighborhoods in Montreal with higher material deprivation and residential instability (i.e., lower socioeconomic status) may also face a more dangerous mix of air pollution in terms of combined exposure Ox and OP/ROS. We previously described a relationship between material deprivation and measured OP which was evident in Montreal but not Toronto6; given our present findings that combined OP/Ox exposures tend to be elevated in areas of Montreal with greater deprivation, it is likely that more-deprived neighborhoods are exposed to a more toxic air pollution mixture relative to less-deprived neighborhoods. Existing evidence suggests that a disproportionate burden of traffic-related air pollution falls upon marginalized populations in Canada’s largest cities.11,35–38 Moreover, recent evidence suggests that targeted, location-specific reductions in emissions can efficiently reduce national inequalities in PM2.5 exposures39 and exposures to metals in PM2.5.40 In the same manner, identifying areas in cities where both Ox and PM2.5 OP are elevated may be an efficient approach to targeting local interventions aimed at reducing the population health impacts of air pollution.
The study has several notable strengths including the availability of annual updated exposure information (for NO2, O3, and PM2.5), a large study population, and detailed individual-level information on socioeconomic factors. Additionally, we have exposure data from a dense network of monitors allowing measurement of PM2.5 oxidative potential/ROS at a high spatial resolution. Nonetheless, we acknowledge some limitations of the study. First, we assume that the use of 2-week monitoring periods represents a sufficient approximation to long-term average spatial variations in PM2.5 OP. This assumption is supported by previous studies that have suggested that the spatial pattern of pollutant concentrations derived from short-term monitoring campaigns remains relatively stable over time.41,42 Although the OP measurements were collected after the end of follow-up, spatial contrasts are assumed to be representative of earlier spatial contrasts within each city during the follow-up period. Since spatial contrasts were classified as above/below the median of OP or ROS, this error would only change the observed results if it resulted in a location moving across categories (i.e., above/below the median). While we cannot rule out this possibility entirely, the locations of major roadways/highways and industrial areas have not changed over the duration of follow-up and thus we do not expect considerable differences in the spatial distribution of OP/ROS over the follow-up period.
The potential for residual confounding due to lack of individual-level data on potential confounders (such as smoking and BMI) is an important limitation of studies using the CanCHEC data. We used an ancillary dataset to examine correlations between Ox concentrations and smoking and BMI and found that Ox was generally negatively correlated with both smoking and BMI, and thus residual confounding by these factors would tend to lead to negative confounding43 and an underestimate of the magnitude of association between Ox exposures and cardiovascular mortality. Similar results were reported in a previous study of regional-scale spatial variations in Ox by Olaniyan et al.17
Some air pollutant concentrations were estimated with greater spatial precision than others. To some extent this reflects the spatial scale of variation of different species (i.e., ozone tends to vary regionally, whereas NO2 concentrations decay quickly with distance from source). However, the larger spatial resolutions for older ozone exposures in particular are likely a source of exposure measurement error, which would likely tend to bias the observed results toward the null, as observed in a previous study by Garriazzo et al: HRs for health outcomes were closer to the null as exposure resolution increased.44 Finally, there is potential for exposure measurement error in the assignment of OP values from sites directly to monitors, as some people live closer to a monitor than others. However, in comparison to previous studies which assigned exposures to individuals residing within a 10-km radius of a measured site,17 our median distance-to-monitor of approximately 1 km yields a more accurate exposure assignment.
In conclusion, our findings suggest that within-city variations in PM2.5 oxidative potential may modify associations between long-term exposure to Ox on mortality from cardiovascular diseases. Additional studies are needed to confirm these results since these patterns may differ in other cities. Nonetheless, our findings suggest that the effect modification previously observed at a regional scale is relevant even at a much smaller spatial scale. Moreover, our results suggest that areas with greater material deprivation and residential instability in Montreal are more likely to be exposed to both Ox and OP above the median, which is a potential environmental justice issue given the increased risk of cardiovascular mortality in these areas.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Supplementary Material
Footnotes
This research was funded by a Canadian Institutes of Health Research (CIHR) Doctoral Award (S.R.), as well as a CIHR Foundation Grant (S.W.). M.S. acknowledges funding from the Health Effects Institute (Walter A. Rosenblith New Investigator Award, No. 4964-RFA17-3/18–6), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. CR-83590201) and certain motor vehicle and engine manufacturers.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
CanCHEC cohort data are held in secure Research Data Centers facilities managed by Statistics Canada. These can be accessed through the microdata access portal application process. The application process and procedures are available online here: www.statcan.gc.ca/en/microdata/data-centres/access.
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. ; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 2.Kelly FJ, Fussell JC. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic Biol Med. 2017;110:345–367. [DOI] [PubMed] [Google Scholar]
- 3.Weichenthal S, Pinault LL, Burnett RT. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci Rep. 2017;7:16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouse DL, Peters PA, Hystad P, et al. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Health Perspect. 2015;123:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook JR, Poirot RL, Dann TF, Lee PKH, Lillyman CD, Ip T. Assessing sources of PM2.5 in cities influenced by regional transport. J Toxicol Environ Health A. 2007;70:191–199. [DOI] [PubMed] [Google Scholar]
- 6.Ripley S, Minet L, Zalzal J, et al. Predicting spatial variations in multiple measures of PM2.5 oxidative potential and magnetite nanoparticles in Toronto and Montreal, Canada. Environ Sci Technol. 2022;56:7256–7265. [DOI] [PubMed] [Google Scholar]
- 7.Weichenthal S, Shekarrizfard M, Kulka R, et al. Spatial variations in the estimated production of reactive oxygen species in the epithelial lung lining fluid by iron and copper in fine particulate air pollution. Environ Epidemiol. 2018;2:e020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smargiassi A, Baldwin M, Pilger C, Dugandzic R, Brauer M. Small-scale spatial variability of particle concentrations and traffic levels in Montreal: a pilot study. Sci Total Environ. 2005;338:243–251. [DOI] [PubMed] [Google Scholar]
- 9.van Donkelaar A, Martin RV, Spurr RJ, Burnett RT. High-resolution satellite-derived PM2.5 from optimal estimation and geographically weighted regression over North America. Environ Sci Technol. 2015;49:10482–10491. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt CN. Spatial variations in nitrogen dioxide concentrations in an urban area. Atmos Environ. 1991;25B:429–434. [Google Scholar]
- 11.Buzzelli M, Jerrett M. Geographies of susceptibility and exposure in the city: environmental inequity of traffic-related air pollution in Toronto. Can J Reg Sci. 2007;30:195–210. [Google Scholar]
- 12.See SW, Wang YH, Balasubramanian R. Contrasting reactive oxygen species and transition metal concentrations in combustion aerosols. Environ Res. 2007;103:317–324. [DOI] [PubMed] [Google Scholar]
- 13.Weichenthal S, Shekarrizfard M, Traub A, et al. Within-city spatial variations in multiple measures of PM2.5 oxidative potential in Toronto, Canada. Environ Sci Technol. 2019;53:2799–2810. [DOI] [PubMed] [Google Scholar]
- 14.Gao D, Ripley S, Weichenthal S, Godri Pollitt KJ. Ambient particulate matter oxidative potential: Chemical determinants, associated health effects, and strategies for risk management. Free Radic Biol Med. 2020;151:7–25. [DOI] [PubMed] [Google Scholar]
- 15.Godri KJ, Duggan ST, Fuller GW, et al. Particulate matter oxidative potential from waste transfer station activity. Environ Health Perspect. 2010;118:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakey PS, Berkemeier T, Tong H, et al. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci Rep. 2016;6:32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olaniyan T, Lavigne E, Traub A, et al. Long-term exposure to oxidant gases and mortality: Effect modification by PM2.5 transition metals and oxidative potential. Epidemiology. 2022;33:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korsiak J, Lavigne E, You H, et al. Air pollution and pediatric respiratory hospitalizations: effect modification by particle constituents and oxidative potential. Am J Respir Crit Care Med. 2022;206:1370–1378. [DOI] [PubMed] [Google Scholar]
- 19.Peters PA, Tjepkema M, Wilkins R, et al. Data resource profile: 1991 Canadian census cohort. Int J Epidemiol. 2013;42:1319–1326. [DOI] [PubMed] [Google Scholar]
- 20.Crouse DL, Peters PA, van Donkelaar A, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. 2012;120:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christidis T, Labrecque-Synnott F, Pinault L, Saidi A, Tjepkema M. The 1996 CanCHEC: Canadian Census Health and Environment Cohort Profile. In: Division HADaHSM, ed. Analytical Studies: Methods and References. Ottawa: Statistics Canada, 2018. [Google Scholar]
- 22.Pinault LL, Weichenthal S, Crouse DL, et al. Associations between fine particulate matter and mortality in the 2001 Canadian census health and environment cohort. Environ Res. 2017;159:406–415. [DOI] [PubMed] [Google Scholar]
- 23.Bratsch SG. Standard electrode potentials and temperature coefficients in water at 298.15 K. J Chem Phys. 1989;18:154104. [Google Scholar]
- 24.Robichaud A, Ménard R. Multi-year objective analyses of warm season ground-level ozone and PM2.5 over North America using real-time observations and Canadian operational air quality models. Atmos Chem Phys. 2014;14:1769–1800. [Google Scholar]
- 25.Robichaud A, Ménard R, Zaïtseva Y, Anselmo D. Multi-pollutant surface objective analyses and mapping of air quality health index over North America. Air Qual Atmos Health. 2016;9:743–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canada E. National Air Pollution Surveillance (NAPS) Program. December 2019. Available at: https://www.canada.ca/en/environment-climate-change/services/airpollution/monitoring-networks-data/national-air-pollution-program.html. Accessed 12 September 2022. [Google Scholar]
- 27.Hystad P, Setton E, Cervantes A, et al. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect. 2011;119:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammer MS, van Donkelaar A, Li C, et al. Global estimates and long-term trends of fine particulate matter concentrations (1998-2018). Environ Sci Technol. 2020;54:7879–7890. [DOI] [PubMed] [Google Scholar]
- 29.CanMap Postal Code Suite. 3 ed. DMTI Spatial Inc. 2015. [Google Scholar]
- 30.Maikawa CL, Weichenthal S, Wheeler AJ, et al. Particulate oxidative burden as a predictor of exhaled nitric oxide in children with asthma. Environ Health Perspect. 2016;124:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matheson FI, Dunn JR, Smith KLW, Moineddin R, Glazier RH. Development of the canadian marginalization index: a new tool for the study of inequality. Can J Public Health. 2012;103:S12–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fieller EC, Hartley HO, Pearson ES. Tests for rank correlation coefficients I. Biometrika. 1957;44:470–481. [Google Scholar]
- 33.Weichenthal S, Crouse DL, Pinault L, et al. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Res. 2016;146:92–99. [DOI] [PubMed] [Google Scholar]
- 34.Weichenthal S, Lavigne E, Evans G, Pollitt K, Burnett RT. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: Impact of regional PM2.5 oxidative potential: a case-crossover study. Environ Health. 2016;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinault L, Crouse D, Jerrett M, Brauer M, Tjepkema M. Spatial associations between socioeconomic groups and NO2 air pollution exposure within three large Canadian cities. Environ Res. 2016;147:373–382. [DOI] [PubMed] [Google Scholar]
- 36.Buzzelli M, Jerrett M, Burnett R, Finklestein N. Spatiotemporal perspectives on air pollution and environmental justice in Hamilton, Canada, 1985–1996. Ann Am Assoc Geogr. 2003;93:557–573. [Google Scholar]
- 37.Carrier M, Apparicio P, Séguin A-M, Crouse D. The application of three methods to measure the statistical association between different social groups and the concentration of air pollutants in Montreal: a case of environmental equity. Transp Res D Transp Environ. 2014;30:38–52. [Google Scholar]
- 38.Crouse DL, Ross NA, Goldberg MS. Double burden of deprivation and high concentrations of ambient air pollution at the neighbourhood scale in Montreal, Canada. Soc Sci Med. 2009;69:971–981. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Apte JS, Hill JD, et al. Location-specific strategies for eliminating US national racial-ethnic PM2.5 exposure inequality. Proc Natl Acad Sci USA. 2022;119:e2205548119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kodros JK, Bell ML, Dominici F, et al. Unequal airborne exposure to toxic metals associated with race, ethnicity, and segregation in the USA. Nat Commun. 2022;13:6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebret E, Briggs D, van Reeuwijk H, et al. Small area variations in ambient NO2 concentrations in four European areas. Atmos Environ. 2000;34:177–185. [Google Scholar]
- 42.Sahsuvaroglu T, Arain A, Kanaroglou P, et al. A land use regression model for predicting ambient concentrations of nitrogen dioxide in Hamilton, Ontario, Canada. J Air Waste Manag Assoc. 2006;56:1059–1069. [DOI] [PubMed] [Google Scholar]
- 43.Skzlo M, Javier-Nieto F. Epidemiology: Beyond the basics. Aspen Publishers, Inc. 2000. [Google Scholar]
- 44.Gariazzo C, Carlino G, Silibello C, et al. Impact of different exposure models and spatial resolution on the long-term effects of air pollution. Environ Res. 2021;192:110351. [DOI] [PubMed] [Google Scholar]