Abstract
Based on network pharmacology methods, we explored the mechanism of the classic Chinese medicine formula Coix seed decoction (CSD) in treating knee osteoarthritis (KOA). We searched each single drug in the CSD in the traditional Chinese medicine systematic pharmacology database in turn to obtain information on the active ingredients and target proteins of the CSD, and obtain the name of the genes corresponding to the target proteins through the UniProt database. We collected KOA-related genes from DisGeNET, GeneCards, comparative toxicogenomics database, and MalaCards database. The Venny online tool identified potential therapeutic targets by intersecting CSD and KOA target genes, while gene ontology and Kyoto encyclopedia of genes and genomes analysis was performed using the Oebiotech Cloud Platform. A protein-protein interaction network was established using the String database; a “CSD-active ingredient-target gene-KOA” network plot was constructed using Cytoscape 3.9.1 software and screened for key targets and hub targets. Finally, molecular docking was performed for hub genes with high Degree values. A total of 227 effective target genes for CSD and 8816 KOA-related target genes were obtained, as well as 191 cross-target genes for CSD and KOA. We screened 37 key gene targets and identified the top 10 hub target genes in descending order of Degree value using protein-protein interaction and Cytoscape 3.9.1 software (TNF, IL-6, MMP-9, IL-1β, AKT-1, VEGFα, STAT-3, PTGS-2, IL-4, TP53). Gene ontology analysis showed that the biological process of CSD treatment of KOA mainly involves cytokine-mediated signaling pathway, negative regulation of apoptotic process, cellular response to hypoxia, cellular response to cadmium ion, response to estradiol, and extrinsic apoptotic signaling pathway in absence of ligand. Kyoto encyclopedia of genes and genomes analysis revealed major signaling pathways including Cellular senescence, TNF signaling pathway, and PI3K-Akt signaling pathway. The molecular docking results show that the core components bind well to the core targets. In conclusion, CSD may exert therapeutic effects on KOA by inhibiting pathological processes such as inflammatory response, apoptosis, cellular senescence, and oxidative stress.
Keywords: Coix seed decoction, mechanism of action, molecular docking, network pharmacology, osteoarthritis of the knee
1. Introduction
Knee osteoarthritis (KOA) is a common degenerative joint disease characterized by histopathological changes such as degeneration of articular cartilage, subchondral bone remodeling, inflammation and fibrosis of the synovial membrane and infrapatellar fat pad, and degeneration of the meniscus,[1–6] and belongs to the category of “Bi Syndrome” in traditional Chinese medicine (TCM). The main signs and symptoms in the early stages include knee pain, edema, and restricted joint movement. Joint pain and mobility difficulties are progressively worse with the development of cartilage loss and the development of synovial and subpatellar fat pad fibrosis,[7,8] and in severe and advanced cases, even the whole loss of knee function may be seen. At this point in time, it is believed that a range of variables contribute to the occurrence of KOA, with age, obesity, heredity, and joint wear and tear all having been linked to the condition risk factors.[9,10] As modern lifestyles change and the world population ages, the proportion of people exposed to high-risk factors is increasing globally each year, driving the rising global incidence of osteoarthritis of the knee.[11] According to Global Burden of Disease data, the worldwide incidence of imaging-confirmed symptomatic KOA reached 3.8% globally as of 2010, and the prevalence of osteoarthritis has climbed by 9% over the course of 28 years, from 1990 to 2017, and is continuing to rise.[12] Oral NSAIDs, joint cavity injections, and surgical procedures make up the majority of the existing KOA therapeutic options.[13,14] However, pharmacological side effects, infections, and financial burden make it difficult to successfully focus KOA long-term therapy and prevention.[15,16] Therefore, to lessen the health burden linked to KOA, the search for a more affordable and effective treatment approach has become essential. Based on the basic theories of Chinese medicine, TCM advocates the use of the corresponding treatment methods for different types of diseases through the method of diagnosis and treatment, and the use of oral Chinese medicine, external application, electro-acupuncture, massage and other characteristic treatment methods to achieve the purpose of treating diseases, and its efficacy and safety have been widely recognized in clinical practice.[17] Currently, studies on the mechanisms related to KOA treatment in Chinese medicine mainly involve oxidative stress, inflammatory response, cell differentiation, proliferation, and apoptosis.[17–21] For instance, Coixenolide can decrease inflammatory response by increasing peripheral serum Foxp3 + CD4 + CD25 + Treg ratio, which in turn controls immunological function in collagen-induced arthritic mice.[18] Artemisinin, the active ingredient in Artemisia annua, was shown to have significant inhibitory effects on the expression of IL-1β, IL-6, and TNF-α in in vitro cellular assays, and could effectively inhibit IL-1β-induced inflammatory responses in rat osteoarthritis (OA) chondrocytes.[22]
Coix seed decoction (CSD) is a classical formula for the treatment of “Bi Syndrome” in TCM recorded in the Chinese medical text “Lei Zheng Zhi Cai.” It is written in “Lei Zheng Zhi Cai” that CSD consists of Yi Yiren (Coix seed/Seed of Job tears), Dang Gui (Angelicae Sinensis Radix), Chuan Xiong (Ligusticum Chuanxiong Rhizoma), Sheng Jiang (Ginger), Gui Zhi (Cinnamomi ramulus), Qiang Huo (Notopterygium incisum), Du Huo (Doubleteeth Pubescent Angelica Root), Fang Feng (Saposhnikovia divaricata), Bai Zhu (Atractylodes macrocephala), Gan Cao (Glycyrrhiza uralensis), Chuan Wu (Aconitum carmichaelii), Ma Huang (Ephedra sinica). In this formula, Coix seed strengthens the spleen and permeates dampness;[23] Chuan Wu,[24–26] Gui Zhi,[27,28] Ma Huang,[29] Sheng Jiang[30,31] warming the meridians and relieving pain. In addition, it has the function of nourishing and energizing the circulation when Chuanxiong is combined with Dang Gui.[32,33] The combination of all the drugs in the formula can dispel wind and dampness, disperse cold and relieve pain, which has good effect on “Bi Syndrome.” However, although clinical observational studies on the treatment of KOA with CSD have been reported in China,[34–37] there is still a lack of higher quality articles describing its clinical effectiveness and mechanism of action. With this in mind, we aimed to investigate potential targets, pathways, and biological processes (BP) of CSD for the treatment of KOA through a network pharmacological approach, and to establish the groundwork for further promotion in the clinic and research into its mechanism of action.
2. Materials and methods
2.1. Compound drug-active ingredient-target gene database establishment
Each individual herbal medicine in CSD was searched sequentially in the traditional Chinese medicine systematic pharmacology database (TCMSP, https://old.tcmsp-e.com/tcmsp.php). Based on the absorption, distribution, metabolism, and excretion criteria, we screened for active compounds with oral bioavailability ≥30 and drug-likeness ≥0.18, and collected their target information (see Supplemental Table 1, http://links.lww.com/MD/J363, Supplemental Content, which illustrates the active ingredient-target protein correspondence of the GSD compound in the database),[38,39] and the active ingredients with no corresponding target proteins in the database were excluded. Using the UniProt database (https://www.uniprot.org/), we searched the obtained target proteins sequentially to obtain the corresponding gene names. After replacing the target protein names with the corresponding gene names, we obtained the CSD compound drug-active ingredient-target gene database (see Supplemental Table 2, http://links.lww.com/MD/J364, Supplemental Content, which illustrates the correspondence of GSD compounded drugs, active ingredients and target genes).
2.2. Disease related target gene database establishment
The keywords “KOA” were searched in GeneCards (https://www.genecards.org/), MalaCards (https://www.malacards.org/). DisGeNetdatabase (https://www.disgenet.org/), CTD (http://ctdbase.org/) databases to obtain KOA-related target gene information for each disease database (see Supplemental Table 3, http://links.lww.com/MD/J365, Supplemental Content, which illustrates the search results of KOA-related target genes in various databases). The search results of each database were aggregated and de-duplicated to create a database of KOA disease-related target genes.
2.3. CSD-drug-active ingredient-potential therapeutic target-disease network construction
The intersection of the CSD target gene set and the KOA target gene set was taken by the Venny online tool (https://bioinfogp.cnb.csic.es/tools/venny/), and the obtained intersection data were the potential therapeutic targets of CSD for KOA. The data was organized to obtain the corresponding relationship data of “CSD-Drug-Active Ingredient-Potential Therapeutic Gene-KOA,” and the data was imported into Cytoscape software (version 3.9.1) in “.xlsx” file format. Finally, the network interaction plot of “YYRD-Drug-Active Ingredient-Potential Therapeutic Gene-KOA” was constructed.
2.4. Protein interaction network graph construction
After importing the set of potential therapeutic genes into the String database (http://string-db.org/) and completing the setup (species selection “Homo sapiens,” confidence value >0.4), protein-protein interaction network analysis was performed. After downloading the potential therapeutic gene protein interaction network data from the String database into Cytoscape software (version 3.9.1) and using the function of CytoHubba plug-in, the data were sorted by MCC, DMNC, maximum neighborhood component, degree, edge percolated component, bottle neck, eceentricity, closeness, radiality, betweenness, stress, clustering coefficient were sorted in descending order and the top ten nodes were filtered out, and then combined and de-weighted to key genes were obtained (see Supplemental Table 4, http://links.lww.com/MD/J366, Supplemental Content, which illustrates the information about each of the above screening conditions for the key genes). The key genes were imported into the String database again according to the above steps to obtain the key gene protein interaction network map. The key genes were arranged in descending order by Degree value using Cytoscape software (version 3.9.1), and the top 10 nodes were the hub genes.
2.5. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis
GO and KEGG enrichment analysis of potential therapeutic gene sets for CSD treatment of KOA using the Cloud Platform of Oebiotech (https://cloud.oebiotech.cn/task/). In the enrichment analysis interface of the Cloud Platform, the species is limited to “H. sapiens,” and the potential therapeutic gene set ID is entered in the common parameters and submitted. After the analysis, we obtained the GO and KEGG database pathway analysis charts of potential therapeutic genes, respectively (see Supplemental Tables 5, http://links.lww.com/MD/J367 and 6, http://links.lww.com/MD/J368 Supplemental Content, which illustrate GO functional class and KEGG enrichment analysis data, respectively).
2.6. Molecular docking validation of predicted targets
We obtained the 3D structure of the proposed docking target in mol2 format from the Pubchem database (https://pubchem.ncbi.nlm.nih.gov/), opened the small ligand molecule with AutodockTools 1.5.6, hydrogenated, charged, detected the ligand root, searched and defined the rotatable bond, and then saved as a pdbqt file. From the RCSB Protein Data Bank (www.rcsb.org), we downloaded the target protein primary 3D structure as a docking protein. Every hydrogen atom was added to AutodockTools 1.5.6 for opening, Gasteiger charge estimation, binding non-polar hydrogen, receptor definition, and saving as pdbqt file. The level of detail parameter was set to 15 and the Vina molecule docking coordinates and box size, while using the default values for the other inputs. The semi-flexible docking procedure was carried out using Autodockvina 1.1.2, and the final docking conformation was chosen based on its affinity.
3. Results
3.1. CSD active ingredient and target information
By searching the TCMSP database, we obtained 9 active ingredients of Coix seed, 2 active ingredients of Dang Gui, 7 active ingredients of Chuan Xiong, 5 active ingredients of Sheng Jiang, 7 active ingredients of Gui Zhi, 15 active ingredients of Qiang Huo, 9 active ingredients of Du Huo, 18 active ingredients of Fang Feng, 7 active ingredients of Bai Zhu, 92 active ingredients of Gan Cao, 3 active ingredients of Chuan Wu, and 23 active ingredients of Ma Huang, of which Fifteen active ingredients were present in multiple drugs at the same time (Table 1). The active ingredients of each drug were combined and de-weighted to obtain 167 active ingredients. The TCMSP database was used again to retrieve 167 active ingredients corresponding to the targets, and after merging and de-duplication and target protein name correction, 227 active targets were finally included. Among the included targets, Coix seed contains 26 species, Dang Gui contains 40 species, Chuanxiong contains 24 species, Sheng Jiang contains 41 species, Gui Zhi contains 32 species, Qiang Huo contains 35 species, Du Huo contains 33 species, Fang Feng contains 66 species, Bai Zhu contains 17 species, Gan Cao contains 206 species, Chuan Wu contains 4 species, and Ma Huang contains 201 species.
Table 1.
Basic information of repeat active ingredient.
| Code | Repeat active ingredient | Related drugs |
|---|---|---|
| A | Quercetin | Gan Cao, Ma Huang |
| B | Beta-sitosterol | Dang Gui, Sheng Jiang, Gui Zhi, Qiang Huo, Du Huo, Fang Feng, Ma Huang |
| C | Sitosterol | Yi Yiren, Chuanxiong, Gui Zhi, Qiang Huo, Fang Feng, Gan Cao |
| D | Kaempferol | Gan Cao, Ma Huang |
| E | STIGMASTEROL | Yi Yiren, Dang Gui, Sheng Jiang, Ma Huang |
| F | (+)-Catechin | Gui Zhi, Ma Huang |
| G | Mandenol | Yi Yiren, Chuanxiong, Fang Feng, Ma Huang |
| H | Poriferast-5-en-3beta-ol | Sheng Jiang, Ma Huang |
| I | Ammidin | Qiang Huo, Du Huo, Fang Feng |
| J | Isoimperatorin | Qiang Huo, Du Huo, Fang Feng |
| K | Phellopterin | Qiang Huo, Fang Feng |
| L | Diosmetin | Qiang Huo, Ma Huang |
| M | Naringenin | Gan Cao, Ma Huang |
| N | Taxifolin | Gui Zhi, Ma Huang |
| O | Nodakenin | Qiang Huo, Du Huo |
3.2. KOA disease target data
Searching the KOA action targets by disease-related databases, we retrieved 80 in GeneCards, 68 in MalaCards, 368 in DisGeNet database, and 7953 in CTD. The search results of each database were combined and de-duplicated to obtain 8116 KOA disease action targets (Fig. 1A).
Figure 1.
(A) This figure shows the Veen plots of KOA-related target proteins obtained based on MalaCards, GeneCards, DisGeNetdatabase, CTD databases; (B) This figure shows the Veen plots of drug targets and KOA-related targets based on Coix seed decoction (CSD) complexes. CTD = comparative toxicogenomics database, KOA = knee osteoarthritis.
3.3. CSD-drug-active ingredient-potential therapeutic target-disease network construction
A total of 191 intersecting genes for CSD treatment of KOA were obtained by analysis of the Vine online tool (Fig. 1B). Network tables were constructed based on CSD drug, active ingredient, potential therapeutic target (intersecting gene), and disease information, and then the data attributes are categorized and named in Type tables. The Network file and Type file were imported into Cytoscope software to construct the “CSD-Drug-Active Ingredient-Potential Therapeutic Target-Disease” network diagram (Fig. 2).
Figure 2.
Coix seed decoction (CSD) network diagram of “compound-drug-active-ingredient-potential-target-disease” for KOA. The orange color represents the drug in the compound; the blue hexagon represents the active ingredient; the green hexagon represents the duplicate active ingredient co-existing in multiple drugs; the “V” symbol represents the potential therapeutic target of CSD for KOA, with the dark purple part representing the core target. KOA = knee osteoarthritis.
3.4. Results of gene screening and enrichment analysis
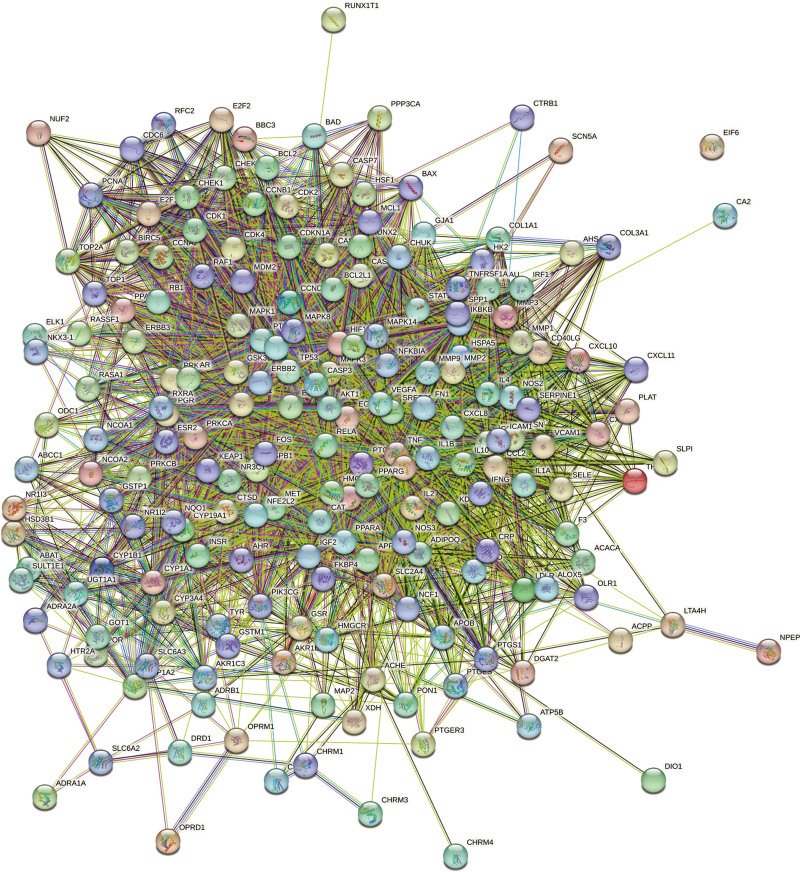
The 191 potential therapeutic targets were analyzed by string online data analysis tool to construct a protein interaction network map (Fig. 3). Using the function of CytoHubba, a plug-in for Cytoscape software, the top ten nodes were filtered by different parameters in descending order, respectively. The first ten nodes of each data were combined and de-weighted to obtain 37 key genes. The key genes were again imported into string online data analysis tool to obtain the key gene protein interaction network map (Fig. 4A). The key genes were imported into Cytoscape software for Degree value descending arrangement, and the top 10 nodes were screened as the hub genes (Table 2), namely TNF, IL-6, MMP-9, IL-1β, AKT-1, VEGFα, STAT-3, PTGS-2, IL-4, TP53 (Fig. 4B). We performed GO and KEGG enrichment analysis on the 37 key targets screened, expecting to further elucidate the pathways of action of CSD for KOA treatment in terms of pathways of action and BP. GO analysis showed 2834 analyzed entries, including 2103 for BP, 444 for molecular function (MF), and 288 for cellular component (CC). The top 10 entries of BP, CC, and MF were filtered by P value calculation ranking (Table 3). The results of the BP screen involve exogenous apoptotic signaling pathway in the absence of ligand (GO:0097192), cellular response to cadmium ion (GO:0071276), response to estradiol (GO:0032355), cellular response to hypoxia (GO:0071456), cytokine-mediated signaling pathway (GO:0019221), gene expression positive regulation of (GO:0010628), negative regulation of apoptotic process (GO:0043066), response to drugs (GO:0042493), positive regulation of transcription by RNA polymerase II (GO:0045944), positive regulation of transcription, DNA-induced (GO:0045893). MF screening results include enzyme binding (GO:0019899), nuclear receptor activity (GO:0004879), homologous protein binding (GO:0042802), protein binding (GO:0005515), protein kinase binding (GO:0019901), DNA binding transcription factor activity (GO:0003700), protein homolog dimerization activity (GO:0042803), transcription factor binding (GO:0008134), protein-containing complex binding (GO:0044877), protease binding (GO:0002020); CC mainly involves extracellular space (GO:0005615), membrane raft (GO:0045121), extracellular region (GO:0005576), cytosol (GO:0005829), chromatin (GO:0000785), cyclin-dependent protein kinase holoenzyme complex (GO:0000307), nucleoplasm (GO:0005654), protein-containing complex (GO:0032991), caveola (GO:0005901), cyptoplasm (GO:0005737) (Fig. 5). KEGG data analysis yielded a total of 267 related pathways, and the top 20 most highly associated pathways were obtained in descending order of enrichment score, namely cellular senescence, TNF signaling pathway, PI3K- Akt signaling pathway, IL-17 signaling pathway, bladder cancer, AGE-RAGE signaling pathway in diabetic complications, pancreatic cancer, prostate cancer, non-small cell lung cancer, small cell lung cancer, endocrine resistance, fluid shear stress and atherosclerosis, hepatitis B, toxoplasmosis, hepatitis C, MicroRNAs in cancer, Kaposi sarcoma-associated herpesvirus infection, human cytomegalovirus infection, proteoglycans in cancer, and pathways in cancer (Fig. 6, Table 4).
Figure 3.
Protein interaction network map of 191 potential therapeutic genes.
Figure 4.
(A) The figure shows the protein interaction network map of 37 key targets; (B) The figure shows the protein interaction network map of the top 10 core targets in terms of Degree value.
Table 2.
The degree value sequence of some key targets of Coix seed decoction (CSD) against knee osteoarthritis (KOA).
| UniProt ID | Gene symbol | Protein names | Degree |
|---|---|---|---|
| P01375 | TNF | Tumor necrosis factor | 32 |
| P05231 | IL6 | Interleukin-6 | 32 |
| P14780 | MMP9 | Matrix metalloproteinase-9 | 31 |
| P01584 | IL1B | Interleukin-1 beta | 31 |
| P31749 | AKT1 | RAC-alpha serine/threonine-protein kinase | 29 |
| P15692 | VEGFA | Vascular endothelial growth factor A | 28 |
| P40763 | STAT3 | Signal transducer and activator of transcription 3 | 28 |
| P35354 | PTGS2 | Prostaglandin G/H synthase 2 | 27 |
| P05112 | IL4 | Interleukin-4 | 27 |
| P04637 | TP53 | Cellular tumor antigen p53 | 26 |
| P01579 | IFNG | Interferon gamma | 26 |
| P03956 | MMP1 | Interstitial collagenase | 25 |
| P42574 | CASP3 | Caspase-3 | 25 |
| P01100 | FOS | Protein c-Fos | 25 |
| P00533 | EGFR | Epidermal growth factor receptor | 25 |
| P01106 | MYC | Myc proto-oncogene protein | 24 |
| P01583 | IL1A | Interleukin-1 alpha | 24 |
| P10451 | SPP1 | Osteopontin | 23 |
| Q14790 | CASP8 | Caspase-8 | 23 |
| P19438 | TNFRSF1A | Tumor necrosis factor receptor superfamily member 1A | 23 |
| P03372 | ESR1 | Estrogen receptor | 22 |
| P16581 | SELE | E-selectin | 22 |
| P24385 | CCND1 | G1/S-specific cyclin-D1 | 22 |
| P02778 | CXCL10 | C-X-C motif chemokine 10 | 21 |
| P04040 | CAT | Catalase | 20 |
| P19875 | CXCL2 | C-X-C motif chemokine 2 | 18 |
| P10914 | IRF1 | Interferon regulatory factor 1 | 18 |
| O14625 | CXCL11 | C-X-C motif chemokine 11 | 14 |
| P06401 | PGR | Progesterone receptor | 14 |
| P07204 | THBD | Thrombomodulin | 8 |
| P02461 | COL3A1 | Collagen alpha-1 (III) chain | 7 |
| P78380 | OLR1 | Oxidized low-density lipoprotein receptor 1 | 6 |
| P03973 | SLPI | Antileukoproteinase | 5 |
| Q14209 | E2F2 | Transcription factor E2F2 | 5 |
Table 3.
The top 10 gene ontology (GO) enrichment items.
| ID | Term | Category |
|---|---|---|
| GO:0097192 | Extrinsic apoptotic signaling pathway in absence of ligand | BP |
| GO:0032355 | Response to estradiol | BP |
| GO:0071276 | Cellular response to cadmium ion | BP |
| GO:0071456 | Cellular response to hypoxia | BP |
| GO:0045893 | Positive regulation of transcription, DNA-templated | BP |
| GO:0045944 | Positive regulation of transcription by RNA polymerase II | BP |
| GO:0043066 | Negative regulation of apoptotic process | BP |
| GO:0042493 | response to drug | BP |
| GO:0010628 | Positive regulation of gene expression | BP |
| GO:0019221 | Cytokine-mediated signaling pathway | BP |
| GO:0005737 | Cytoplasm | CC |
| GO:0005901 | Caveola | CC |
| GO:0032991 | Protein-containing complex | CC |
| GO:0005654 | Nucleoplasm | CC |
| GO:0000307 | Cyclin-dependent protein kinase holoenzyme complex | CC |
| GO:0000785 | Chromatin | CC |
| GO:0005829 | Cytosol | CC |
| GO:0005576 | Extracellular region | CC |
| GO:0045121 | Membrane raft | CC |
| GO:0005615 | Extracellular space | CC |
| GO:0002020 | Protease binding | MF |
| GO:0044877 | Protein-containing complex binding | MF |
| GO:0008134 | Transcription factor binding | MF |
| GO:0042803 | Protein homodimerization activity | MF |
| GO:0003700 | DNA-binding transcription factor activity | MF |
| GO:0019901 | Protein kinase binding | MF |
| GO:0005515 | Protein binding | MF |
| GO:0042802 | Identical protein binding | MF |
| GO:0004879 | Nuclear receptor activity | MF |
| GO:0019899 | Enzyme binding | MF |
BP = biological process, CC = cellular component, MF = molecular function.
Figure 5.
The gene ontology (GO) pathway enrichment analysis.
Figure 6.
The Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis.
Table 4.
The top 20 Kyoto encyclopedia of genes and genomes (KEGG) enrichment items.
| ID | Term | P value |
|---|---|---|
| hsa05200 | Pathways in cancer | 1.23E-38 |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 1.10E-31 |
| hsa05215 | Prostate cancer | 5.95E-28 |
| hsa05161 | Hepatitis B | 2.46E-26 |
| hsa05219 | Bladder cancer | 1.97E-24 |
| hsa05160 | Hepatitis C | 3.08E-23 |
| hsa05212 | Pancreatic cancer | 5.16E-23 |
| hsa05418 | Fluid shear stress and atherosclerosis | 6.95E-23 |
| hsa05167 | Kaposi sarcoma-associated herpesvirus infection | 2.35E-22 |
| hsa05163 | Human cytomegalovirus infection | 4.54E-22 |
| hsa05223 | Non-small cell lung cancer | 6.64E-21 |
| hsa04657 | IL-17 signaling pathway | 1.91E-20 |
| hsa04668 | TNF signaling pathway | 1.35E-19 |
| hsa05206 | MicroRNAs in cancer | 1.42E-19 |
| hsa05222 | Small cell lung cancer | 1.92E-19 |
| hsa04151 | PI3K-Akt signaling pathway | 1.98E-19 |
| hsa01522 | Endocrine resistance | 7.46E-19 |
| hsa05145 | Toxoplasmosis | 1.98E-18 |
| hsa04218 | Cellular senescence | 7.40E-18 |
| hsa05205 | Proteoglycans in cancer | 1.20E-17 |
3.5. Molecular docking results
From the CSD compound drug-active ingredient-target gene database, we screened 4 active ingredients (quercetin, kaempferol, luteolin, and wogonin) as key active compounds for molecular docking with the first 4 hub genes (TNF, IL-6, MMP-9, and IL-1). These key active compounds not only act on the proteins expressed by the 4 hub genes mentioned above, but also have as many proteins expressed by other genes as possible as targets. Small molecule ligands can spontaneously bind to protein receptors when the binding energy is <0 kJ mol. If the binding energy is <−5.0 kJ mol or lower, it indicates a better binding ability of both. 16 docking results were generated by the docking simulation (Table 5). They both have binding energies <−5 kJ mol, which means that they both bind well. This chemical docking result raises the prospect that substances like quercetin may be essential in the way CSD treats KOA. Finally, Pymol software was employed to visualize the complexes of the 4 groups of docked proteins and chemicals (Fig. 7).
Table 5.
The binding energy of Molecular docking (kJ mol).
| Hub gens Active chemicals |
TNF | IL6 | MMP-9 | IL-1β |
|---|---|---|---|---|
| Quercetin | −6.3 | −6.3 | −9.8 | −6.2 |
| Kaempferol | −6.0 | −6.1 | −9.4 | −6.1 |
| Luteolin | −6.4 | −6.3 | −10.1 | −6.5 |
| Wogonin | −6.0 | −5.8 | −9.3 | −6.1 |
Figure 7.
Molecular docking diagram and local enlargement. (A) TNF-luteolin; (B) MMP9-luteolin; (C) IL1β-quercetin; (D) IL6-quercetin.
4. Discussion
KOA is a common clinical condition that is now a major health threat affecting the global middle-aged and elderly population, driven by an aging population and changes in modern lifestyles.[40] However, due to its complex etiology and wide range of lesion sites, the specific pathogenesis of KOA is not yet fully understood.[41] Therefore, it is important to explore the pathogenesis of KOA and seek convenient, effective and economical treatment methods to alleviate the health crisis caused by KOA. Due to its convenience, effectiveness, affordability, and low side effects, TCM is emerging as a new approach for treating KOA. However, the fact that TCM contains a sizable number of active ingredients and is associated with multiple pathways of combined action has made it challenging to thoroughly research the therapeutic mechanisms of TCM compounding. In contrast, network pharmacology has shed light on the synergistic effects and potential mechanisms of multi-compound and multi-target drugs by examining the various intricate interaction networks in Chinese medicine, which are now frequently used in the investigation of the workings of Chinese medicine monomeric drugs and compounding.[42,43] Therefore, this study was conducted to capture the relevant target information of CSD for the treatment of KOA by searching relevant literature and databases, aiming to analyze the BPs and related pathways involved by means of network pharmacology, to analyze the mechanism of CSD for the treatment of KOA at the molecular level, and to reveal its multi-component and multi-target mechanism of action.
We captured the active ingredients of CSD drugs and KOA disease-related targets respectively, and obtained 191 potential therapeutic targets by taking the intersection, from which 37 key genes with high influence were screened and arranged in descending order by Degree value, and the top ten core targets were: TNF, IL-6, MMP-9, IL-1β, AKT-1, VEGFα, STAT-3, PTGS-2, IL-4, TP53. Meanwhile, we performed GO and KEGG enrichment analysis with 37 key genes. GO analysis showed that the BPs associated with CSD treatment of KOA mainly involved the exogenous apoptotic signaling pathway of ligand deletion, cellular response to cadmium ion, response to estradiol, cellular response to hypoxia, negative regulation of apoptotic process, and cytokine-mediated signaling pathway. The results of KEGG enrichment analysis showed that cellular senescence, TNF signaling pathway, and PI3K-Akt signaling pathway were the main relevant enrichment pathways. The molecular docking results between 4 important active ingredients and the related proteins of the first 4 hub genes showed that all 16 obtained results were <−5.0 kJ/mol, indicating that these active ingredients can bind well with the hub genes and may be an important potent substance basis for the therapeutic effects of CSD. For instance,[44] Fei et al found that luteolin could effectively reduce the expression of MMP-9 and TNF-α in mouse chondrocytes induced by LI-1β, and further demonstrated its inhibitory effects on the degradation of type II collagen through in vivo experiments. Meanwhile, Wu et al[45] discovered that the inhibitory effect of luteolin on MMP-9 expression may be associated with the Akt-mTOR signaling pathway. This finding is intriguing to us, as it is consistent with our network pharmacology analysis despite not having been confirmed in studies related to OA. Furthermore, studies have demonstrated varying degrees of inhibition in extracellular matrix degradation and inflammatory responses during OA progression for quercetin, wogonin, and kaempferol.[46–50]
IL-6 itself is a cytokine that activates the systemic immune system and enhances the inflammatory response, and in KOA acts on articular cartilage to inhibit type II collagen production and promote the expression of MMPs group.[51] In KOA-related studies, IL-6 has often been noted as an indicator of inflammation because of its positive correlation between synovial fluid concentration and the severity of KOA imaging.[52,53] And the increase in IL-6 concentration in synovial fluid is thought to be closely related to TNF-α and IL-1β. It was shown that TNF-α and IL-1β induced IL-6 production by chondrocytes, synovial fibroblasts, macrophages, osteoblasts, and adipocytes, and also stimulated the upregulation and expression of chemokines such as IL-8, MCP-1, Rantes, and some inflammatory factors in joints, thus participating in regulating the inflammatory pathological process of KOA.[54] In addition, these 2 cytokines can lead to the production of reactive oxygen species and the down-regulation of reactive oxygen antioxidant enzymes, thereby promoting oxidative stress and apoptosis, thereby accelerating cartilage destruction.[55] Moreover, cyclooxygenase 2 (COX-2) is an important element in the inflammatory response. On the one hand, COX-2 is compiled by the gene PTGS-2, which is difficult to detect in normal tissues and can be induced to be expressed by inflammatory factors (e.g., IL-1β, TNFα) and is often used as a test for inflammatory responses in OA.[44,56–58] On the other hand, COX-2 is one of the important catalysts in the conversion of arachidonic acid to prostaglandin E2 (PGE2), and its metabolite PGE2 can increase vascular permeability and exacerbate inflammatory tissue edema, therefore it is also an important target of current anti-inflammatory and analgesic drugs for OA (e.g., non-steroidal anti-inflammatory drugs, COX-2 specific inhibitors).[58,59]
The combined action of multiple factors leads to the overexpression and aggregation of pro-inflammatory factors represented by these inflammatory factors and mediators in KOA, inducing adverse events such as chondrocyte apoptosis and extracellular matrix degradation, and accelerating the progression of KOA. In response to the threat posed by these pro-inflammatory factors, the body will also initiate corresponding anti-inflammatory mechanisms for intervention. IL-4 is an important representative of anti-inflammatory factors involved in the inflammatory response of OA. It can not only effectively inhibit the secretion of the above inflammatory factors such as TNFα, IL-6, and inflammatory mediators COX-2, PGE2, but also reduce the secretion of MMPs in articular cartilage and the degradation of proteoglycans, inhibit the apoptosis of chondrocytes and synovial fibroblasts, and delay the progression of KOA.[54] On the other hand, the overexpression of inflammatory factors can also activate corresponding pathways in the human body to inhibit inflammatory responses. For example,[60–62] IL-6 can activate JAK-STAT signaling pathway, which is one of the few multi-effect cascades. IL-6 first binds to the receptor on the cell membrane to activate the receptor, and the activated receptor induces JAK phosphorylation to further phosphorylate STAT-3, and the activated STAT-3 dimerizes into the nucleus to regulate the expression of anti-apoptotic genes such as Bcl-2, MCL1, and cell cycle-related genes such as c-Myc and p21.
As mentioned above, TNF-α can mediate inflammation, immunity, apoptosis, oxidative stress and other processes, and is one of the important signaling molecules for KOA, and one of the important targets we screened for the treatment of KOA by CSD. And through enrichment analysis, we speculate that CSD may mediate the activation of its downstream NF-κB signaling pathway via the TNF signaling pathway to promote cell proliferation, inhibit apoptosis, and reduce the occurrence of inflammatory reactions in order to treat KOA. There are 2 types of receptors in the TNF signaling pathway, TNFR1 and TNFR2, the former has a death structure domain in the inner cell membrane and plays a dominant role in the TNF signaling pathway.[63] The TNF signaling pathway activates the downstream pathway NF-κ B signaling pathway mainly through the TNFR1 signaling complex (TNFR1-SC) composed of TRAF-2/5 combined with the N-terminal non-dead structural domain part of TRADD, which activates the IκB kinase complex to promote the detachment of IκB from the NF-κB trimer, thus allowing the NF-κB dimer to enter the nucleus and bind to DNA-specific sequences, inducing the expression of pro-inflammatory factor-related genes such as TNF-α, IL-6, and cell proliferation-related genes such as VEGF, MMP-9.[64] VEGF-α mainly acts on endothelial cells, promoting angiogenesis by activating receptors on them. Multiple studies have shown that angiogenesis can induce osteophyte formation, deep bone calcification in articular cartilage, and is one of the important mechanisms for the pathogenesis and progression of OA.[65,66] In addition, studies have shown that VEGF-α can also promote capillary permeability and promote inflammatory response in OA.[67] MMP-9 belongs to the family of matrix metalloproteinases, which are induced and activated in inflammatory environments. They promote leukocyte infiltration by cutting and degrading extracellular matrix, which is beneficial for clearing infections. However, in the pathological process of OA, the degradation of extracellular matrix is an important cause of cartilage degeneration.
Unlike the pro-inflammatory effect mediated by TNFR-1, activation of TNFR-2 pathway promotes cell proliferation and has anti-inflammatory effects.[68,69] TNFR-2 can still activate the downstream NF-κB pathway despite the absence of the death structural domain, which is closely linked to its involvement in the regulation of PI3K-Akt-related pathways. And by searching the KEGG database and reviewing the literature, we also found that TNFR-2 can inactivate the TSC1/2 complex by promoting Akt phosphorylation of TSC2, which in turn activates mTORC1 to promote cell growth, protein synthesis and other processes.[69–71]
In addition, among the 3 subtypes of AKT that have been discovered so far, AKT1, AKT2, and AKT3. AKT1 is widely distributed and mainly expressed in tissues, with an anti-apoptotic effect, which may be the result of the inhibition of p53 after activation of the PI3K-Akt signaling pathway.[72] P53, a protein transcription factor encoded by the TP53 gene, has a role in inducing cell cycle termination and apoptosis, and is one of the major regulators of cell growth and apoptosis.[73] In the PI3K-Akt-p53 signaling pathway, the activation of the PI3K-Akt pathway leads to the phosphorylation of Mdm2 oncoprotein at serine 166 and serine 186 sites. Consequently, Mdm2 trans-locates from the cytoplasm to the nucleus where it inhibits the expression and transcription of p53. This ultimately results in a negative regulation of apoptosis and cell cycle termination.[74]
While we were able to obtain results on the therapeutic mechanism of CSD for KOA, our network pharmacology analysis was limited by the data available in our selected database. Specifically, the analysis did not take into account factors such as drug concentration, and there may be drug targets and disease-associated genes that have not yet been identified or entered into the database. As such, the results we obtained should be interpreted with caution as they may only reflect a portion of the therapeutic mechanism of CSD. Moreover, the interactions between various cytokines and signaling pathways in the body form a complex network structure, and we have only been able to analyze and reason about a small part of this complex network. Nonetheless, this also provides a preliminary direction for subsequent experimental validation of the relevant pathways, which is crucial for exploring the mechanism underlying CSD efficacy in treating KOA.
5. Conclusion
In summary, we believe that CSD reduces apoptosis, inflammatory cytokine secretion, and neoangiogenesis in the development of KOA by inhibiting the expression of caspase-8 mediated by the TNFR1 pathway in the TNF signaling pathway, as well as the activation of downstream NF-κB signaling pathway. On the other hand, CSD enhances the expression of anti-inflammatory cytokines such as IL-4, which in turn reduces the secretion and aggregation of inflammatory factors such as TNF-α, IL-6, COX-2, PGE2, and their related oxidative stress reactions. Additionally, CSD activates TNFR2-mediated cell proliferation and PI3K-Akt pathway-related anti-apoptotic and anti-inflammatory effects, thereby achieving therapeutic effects for KOA.
Author contributions
Data curation: Rui Chen.
Formal analysis: Chao Song.
Methodology: Xiaoqiang Wang, Wei Xiang.
Resources: Sanjun Huang, Qifan Su, Guanghui Deng.
Software: Jiaqi Wu.
Writing – original draft: Junjie Qiu.
Writing – review & editing: Xiaojun Chen.
Supplementary Material
Abbreviations:
- BP
- biological process
- CC
- cellular component
- COX-2
- cyclooxygenase 2
- CSD
- Coix seed decoction
- CTD
- comparative toxicogenomics database
- GO
- gene ontology
- KEGG
- Kyoto encyclopedia of genes and genomes
- KOA
- knee osteoarthritis
- MF
- molecular function
- OA
- osteoarthritis
- PGE2
- prostaglandin E2
- TCM
- traditional Chinese medicine
- TCMSP
- traditional Chinese medicine systematic pharmacology database
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
Not necessary, this article does not involve clinical or animal testing.
This study was supported by the Sichuan Provincial Administration of Traditional Chinese Medicine Science and Technology Research Special Project (2023MS395).
How to cite this article: Qiu J, Chen R, Song C, Wang X, Xiang W, Huang S, Su Q, Deng G, Wu J, Chen X. Network pharmacological analysis on the mechanism of Coix seed decoction for osteoarthritis of the knee. Medicine 2023;102:31(e34464).
Contributor Information
Rui Chen, Email: chenxj2012@163.com.
Chao Song, Email: 591065330@qq.com.
Xiaoqiang Wang, Email: 476536228@qq.com.
Wei Xiang, Email: xw1064379543@163.com.
Sanjun Huang, Email: Alexhsjunjun97@163.com.
Qifan Su, Email: 949873857@qq.com.
Guanghui Deng, Email: 1295667386@qq.com.
Jiaqi Wu, Email: wujiaqi15@swmu.edu.cn.
Xiaojun Chen, Email: chenxj2012@163.com.
References
- [1].Poole AR. Osteoarthritis as a whole joint disease. HSS J. 2012;8:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sanchez-Lopez E, Coras R, Torres A, et al. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019;4:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ozeki N, Koga H, Sekiya I. Degenerative meniscus in knee osteoarthritis: from pathology to treatment. Life (Basel). 2022;12:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Battistelli M, Favero M, Burini D, et al. Morphological and ultrastructural analysis of normal, injured and osteoarthritic human knee menisci. Eur J Histochem. 2019;63:2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Belluzzi E, Macchi V, Fontanella CG, et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int J Mol Sci. 2020;21:6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schroeppel JP, Crist JD, Anderson HC, et al. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol Histopathol. 2011;26:377–94. [DOI] [PubMed] [Google Scholar]
- [8].Zhang L, Xing R, Huang Z, et al. Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis. Mediators Inflamm. 2019;2019:2165918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Long H, Cao R, Yin H, et al. Associations between obesity, diabetes mellitus, and cardiovascular disease with progression states of knee osteoarthritis (KOA). Aging Clin Exp Res. 2023;35:333–40. [DOI] [PubMed] [Google Scholar]
- [10].Berenbaum F, Walker C. Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgrad Med. 2020;132:377–84. [DOI] [PubMed] [Google Scholar]
- [11].Quicke JG, Conaghan PG, Corp N, et al. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthritis Cartilage. 2022;30:196–206. [DOI] [PubMed] [Google Scholar]
- [12].Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. [DOI] [PubMed] [Google Scholar]
- [13].Serrano DV, Saseendar S, Shanmugasundaram S, et al. Spontaneous osteonecrosis of the knee: state of the art. J Clin Med. 2022;11:6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Biazzo A, D'Ambrosi R, Masia F, et al. Autologous adipose stem cell therapy for knee osteoarthritis: where are we now? Phys Sportsmed. 2020;48:392–9. [DOI] [PubMed] [Google Scholar]
- [15].Kiadaliri A, Englund M. Trajectory of excess healthcare consultations, medication use, and work disability in newly diagnosed knee osteoarthritis: a matched longitudinal register-based study. Osteoarthritis Cartilage. 2021;29:357–64. [DOI] [PubMed] [Google Scholar]
- [16].Shanmugasundaram S, Solanki K, Saseendar S, et al. Role of doxycycline as an osteoarthritis disease-modifying drug. J Clin Med. 2023;12:2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang M, Liu L, Zhang CS, et al. Mechanism of traditional Chinese medicine in treating knee osteoarthritis. J Pain Res. 2020;13:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zheng H, Zhang W, Zhou H, et al. Effect of coixenolide on Foxp3+ CD4+ CD25+ regulatory T cells in collagen-induced arthritis mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36:348–50. [PubMed] [Google Scholar]
- [19].Han X, Lin D, Huang W, et al. Mechanism of NLRP3 inflammasome intervention for synovitis in knee osteoarthritis: a review of TCM intervention. Front Genet. 2023;14:1159167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zeng X, Lin S, Li Y. Effects of modified Duhuo Jisheng decoction combined with arthroscopic surgery on bone metabolism, oxidative stress, and serum TLR4 and TGF-β1 in patients with knee osteoarthritis. J Environ Public Health. 2022;2022:1933504. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [21].Zhang P. Ginsenoside-Rg5 treatment inhibits apoptosis of chondrocytes and degradation of cartilage matrix in a rat model of osteoarthritis. Oncol Rep. 2017;37:1497–502. [DOI] [PubMed] [Google Scholar]
- [22].Zhong G, Liang R, Yao J, et al. Artemisinin ameliorates osteoarthritis by inhibiting the Wnt/β-catenin signaling pathway. Cell Physiol Biochem. 2018;51:2575–90. [DOI] [PubMed] [Google Scholar]
- [23].Han X, Ji X, Zhao H, et al. Mechanisms of Coix seed compositions in the treatment of spleen deficiency and wet dampness Zheng. Afr J Tradit Complement Altern Med. 2017;14:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gao Y, Fan H, Nie A, et al. Aconitine: a review of its pharmacokinetics, pharmacology, toxicology and detoxification. J Ethnopharmacol. 2022;293:115270. [DOI] [PubMed] [Google Scholar]
- [25].Fu YP, Zou YF, Lei FY, et al. Aconitum carmichaelii Debeaux: a systematic review on traditional use, and the chemical structures and pharmacological properties of polysaccharides and phenolic compounds in the roots. J Ethnopharmacol. 2022;291:115148. [DOI] [PubMed] [Google Scholar]
- [26].Xia X, Wang F, Zheng X, et al. Effects of extracts from Chuanwu (Aconitum Carmichaelii) and Banxia (Rhizoma Pinelliae) on excisional wound healing in a rat’s model. J Tradit Chin Med. 2019;39:65–73. [PubMed] [Google Scholar]
- [27].Liu J, Zhang Q, Li RL, et al. Anti-proliferation and anti-migration effects of an aqueous extract of Cinnamomi ramulus on MH7A rheumatoid arthritis-derived fibroblast-like synoviocytes through induction of apoptosis, cell arrest and suppression of matrix metalloproteinase. Pharm Biol. 2020;58:863–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu J, Zhang Q, Li RL, et al. The traditional uses, phytochemistry, pharmacology and toxicology of Cinnamomi ramulus: a review. J Pharm Pharmacol. 2020;72:319–42. [DOI] [PubMed] [Google Scholar]
- [29].Zheng Q, Mu X, Pan S, et al. Ephedrae herba: a comprehensive review of its traditional uses, phytochemistry, pharmacology, and toxicology. J Ethnopharmacol. 2023;307:116153. [DOI] [PubMed] [Google Scholar]
- [30].Rondanelli M, Fossari F, Vecchio V, et al. Clinical trials on pain lowering effect of ginger: a narrative review. Phytother Res. 2020;34:2843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ballester P, Cerdá B, Arcusa R, et al. Effect of ginger on inflammatory diseases. Molecules. 2022;27:7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li W, Tang Y, Guo J, et al. Comparative metabolomics analysis on hematopoietic functions of herb pair Gui-Xiong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach. J Chromatogr A. 2014;1346:49–56. [DOI] [PubMed] [Google Scholar]
- [33].Jin Y, Qu C, Tang Y, et al. Herb pairs containing Angelicae Sinensis Radix (Danggui): a review of bio-active constituents and compatibility effects. J Ethnopharmacol. 2016;181:158–71. [DOI] [PubMed] [Google Scholar]
- [34].汪 国强. 薏苡仁汤治疗风寒湿痹型膝骨关节炎的临床研究. 成都中医药大学. 2020. [Google Scholar]
- [35].杨 雪, 李 素华, 丛 珊, et al. 薏苡仁汤治疗寒湿痹阻型类风湿关节炎有效性和安全性的Meta分析. 风湿病与关节炎. 2022;11:26–31 + 66. [Google Scholar]
- [36].叶 景林. 薏苡仁汤、独活寄生汤分期治疗风寒湿痹型膝关节骨性关节炎. 新中医. 2013;45:86–7. [Google Scholar]
- [37].王 健, 袁 海洲. 温针灸联合薏苡仁汤治疗风寒湿痹型膝骨关节炎的临床观察. 中国民间疗法. 2021;29:47–50. [Google Scholar]
- [38].Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhu N, Hou J, Ma G, et al. Network pharmacology identifies the mechanisms of action of Shaoyao Gancao Decoction in the treatment of osteoarthritis. Med Sci Monit. 2019;25:6051–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–8. [DOI] [PubMed] [Google Scholar]
- [41].Zheng L, Zhang Z, Sheng P, et al. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev. 2021;66:101249. [DOI] [PubMed] [Google Scholar]
- [42].Li X, Wei S, Niu S, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput Biol Med. 2022;144:105389. [DOI] [PubMed] [Google Scholar]
- [43].Xu H-H, Li S-M, Xu R, et al. Predication of the underlying mechanism of Bushenhuoxue formula acting on knee osteoarthritis via network pharmacology-based analyses combined with experimental validation. J Ethnopharmacol. 2020;263:113217. [DOI] [PubMed] [Google Scholar]
- [44].Fei J, Liang B, Jiang C, et al. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586–92. [DOI] [PubMed] [Google Scholar]
- [45].Wu HT, Lin J, Liu YE, et al. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine. 2021;81:153437. [DOI] [PubMed] [Google Scholar]
- [46].Hu Y, Gui Z, Zhou Y, et al. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med. 2019;145:146–60. [DOI] [PubMed] [Google Scholar]
- [47].Zhuang Z, Ye G, Huang B. Kaempferol alleviates the interleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB. Med Sci Monit. 2017;23:3925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Khan NM, Haseeb A, Ansari MY, et al. Dataset of effect of Wogonin, a natural flavonoid, on the viability and activation of NF-κB and MAPKs in IL-1β-stimulated human OA chondrocytes. Data Brief. 2017;12:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Khan NM, Haseeb A, Ansari MY, et al. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic Biol Med. 2017;106:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Estakhri F, Panjehshahin MR, Tanideh N, et al. The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. Knee. 2020;27:817–32. [DOI] [PubMed] [Google Scholar]
- [51].Latourte A, Cherifi C, Maillet J, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76:748–55. [DOI] [PubMed] [Google Scholar]
- [52].Kaneko S, Satoh T, Chiba J, et al. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6:71–9. [DOI] [PubMed] [Google Scholar]
- [53].Lin Z, Liu T, Hu Z, et al. Effects of different running intensity on serum levels of IL-6 and TNF-α in patients with early knee osteoarthritis. J Coll Physicians Surg Pak. 2022;32:899–903. [DOI] [PubMed] [Google Scholar]
- [54].Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- [56].Xu Z, Ke T, Zhang Y, et al. Danshensu inhibits the IL-1β-induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway. Mol Med. 2021;27:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang G, Wang K, Song H, et al. Celastrol ameliorates osteoarthritis via regulating TLR2/NF-κB signaling pathway. Front Pharmacol. 2022;13:963506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Colletti A, Cicero AFG. Nutraceutical approach to chronic osteoarthritis: from molecular research to clinical evidence. Int J Mol Sci. 2021;22:12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11:81s–110s. [DOI] [PubMed] [Google Scholar]
- [60].Hu X, Li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Roskoski R. Janus kinase (JAK) inhibitors in the treatment of neoplastic and inflammatory disorders. Pharmacol Res. 2022;183:106362. [DOI] [PubMed] [Google Scholar]
- [62].Xue C, Yao Q, Gu X, et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. 2023;8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chu W-M. Tumor necrosis factor. Cancer Lett. 2013;328:222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Naudé PJW, Den Boer JA, Luiten PGM, et al. Tumor necrosis factor receptor cross-talk. FEBS J. 2011;278:888–98. [DOI] [PubMed] [Google Scholar]
- [65].MacDonald IJ, Liu SC, Su CM, et al. Implications of angiogenesis involvement in arthritis. Int J Mol Sci. 2018;19:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–8. [DOI] [PubMed] [Google Scholar]
- [67].Yoo SA, Bae DG, Ryoo JW, et al. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. J Immunol. 2005;174:5846–55. [DOI] [PubMed] [Google Scholar]
- [68].Fu W, Hettinghouse A, Chen Y, et al. 14-3-3 epsilon is an intracellular component of TNFR2 receptor complex and its activation protects against osteoarthritis. Ann Rheum Dis. 2021;80:1615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chan FK-M, Shisler J, Bixby JG, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–21. [DOI] [PubMed] [Google Scholar]
- [70].Pomerantz JL, Baltimore D. NF-κB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–93. [DOI] [PubMed] [Google Scholar]
- [72].Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Simabuco FM, Morale MG, Pavan ICB, et al. p53 and metabolism: from mechanism to therapeutics. Oncotarget. 2018;9:34030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.