Abstract
Here, we report the case of a rare and complex disorder, rapid-onset obesity with hypothalamic dysfunction, hypoventilation, autonomic dysregulation, and neuroendocrine tumor (ROHHADNET) syndrome, in a three-year-old girl with no significant medical history. This is the first such case reported from the UAE. ROHHADNET is a rare disorder of respiratory control and autonomic nervous system regulation with endocrine abnormalities. It typically presents in children older than 18 months with rapid weight gain. This is a challenging diagnosis as there is no clear diagnostic test, and treatment is essentially supportive. This report describes a case of ROHHADNET syndrome in a previously well child who presented with rapid weight gain followed by ophthalmoplegia, dysphagia, electrolyte disturbance, and other comorbidities. The paper outlines in detail the clinical course, investigations, and management of ROHHADNET syndrome. Cerebrospinal fluid analysis revealed oligoclonal bands, which have been reported in only two other cases of ROHHADNET syndrome. Our goal in reporting this case is to increase awareness of this condition among clinicians to facilitate early diagnosis and timely management.
Keywords: neural crest tumor, oligoclonal bands, autonomic dysregulation, hypothalamic dysfunction, rohhadnet, rapid-onset obesity
Introduction
Rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neuroendocrine tumor (ROHHADNET) syndrome is a rare and frequently fatal polymorphic disorder involving multiple systems. It was first reported in 1965, and since then, only 160 cases have been documented [1]. Although the exact cause remains unknown, some evidence suggests a pathological autoimmune response, in some cases, triggered by a neural crest tumor [2-6]. The syndrome may be mistaken for other forms of hypothalamic obesity which result in abnormal hypothalamic-pituitary function, such as Prader-Willi syndrome [7].
Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) may rapidly progress to central hypoventilation and death. This highlights the critical importance of timely diagnosis and management in such patients, particularly the provision of overnight respiratory support to minimize morbidity and mortality [8,9].
Case presentation
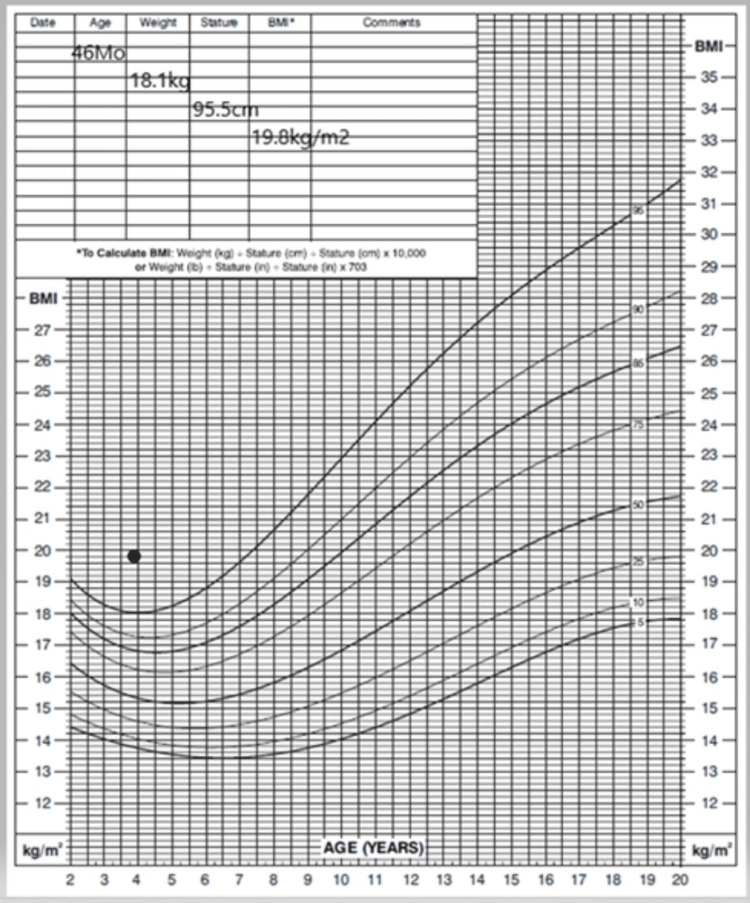
A three-year-old previously healthy girl presented to our hospital with a two-week history of ophthalmoplegia and ataxia. This was associated with mood changes, social withdrawal, markedly increased appetite, and rapid weight gain over three months (Figure 1). Her parents had initially attributed her rapid weight gain and behavioral symptoms to home quarantine, with a consequent lack of physical activity during the COVID-19 pandemic. She had also experienced new-onset dysphagia, polyuria, and nocturnal enuresis. Her weight had increased from 14.0 kg (25th percentile) to 18.1 kg (85.9th percentile) at presentation, corresponding to a body mass index (BMI) of 19.8 kg/m² (98.9th percentile) (Figure 1). She had no prior medical history and was born to healthy, unrelated parents. Her antenatal and birth history was unremarkable. She had normal growth and development until symptom onset. Her family history was negative for any neurological or genetic disorders.
Figure 1. Body mass index.
At presentation, the child was alert and oriented but had impaired horizontal gaze and normal vertical gaze. The pupils were 4 mm and reacted to 3 mm to light, both on direct and consensual testing. The pupils were round and centered in the middle of the iris. She had a complete range of extraocular movements. There was no ptosis. She did not exhibit nystagmus or facial palsy. There was no bulbar weakness or dysfunction, and the uvula was on the midline. The tongue did not deviate and was not atrophic. Hearing and balance were intact. There was no weakness of the Trapezii or sternocleidomastoid muscle. Upper and lower limbs had normal power, tone, and reflexes. Her gait was broad-based and she walked cautiously, partly due to her horizontal gaze difficulty. No dysarthria or cerebellar signs were detected. Apart from obesity, the rest of the physical examination was normal.
Blood tests on admission are summarized in Table 1. The results showed hyponatremia, striking hyperprolactinemia, elevated adrenocorticotropic hormone (ACTH), and slightly elevated morning cortisol in addition to microcytic, hypochromic anemia. Luteinizing hormone (LH), follicle-stimulating hormone (FSH), growth hormone (GH), and insulin-like growth factor-1 (IGF-1) were all within the normal range.
Table 1. Blood, urine, and cerebrospinal fluid test results.
MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; WBC = white blood cell; AST = aspartate transaminase; ALT = alanine transaminase; TSH = thyroid-stimulating hormone; ACTH = adrenocorticotropic hormone; LH = luteinizing hormone; FSH = follicle-stimulating hormone; GH = growth hormone; IGF-1 = insulin-like growth factor 1; CK = creatine kinase; anti-MOG = anti-myelin oligodendrocyte glycoprotein; NMO = neuromyelitis optica; AQP4 = aquaporin‐4
| Investigations | Values |
| Complete blood count | |
| Hemoglobin | 11.3 g/dL |
| MCV | 60.7 fL |
| MCH | 19.9 pg |
| WBC | 15.15 × 103/µL |
| Biochemistry | |
| Osmolality | 283 mOsmol/kg (normal: 275–295) |
| Glucose | 79 mg/dL |
| Sodium | 128 mmol/L (normal: 136–145) |
| Total Bilirubin | 0.22 mg/dL |
| AST | 44 U/L |
| ALT | 29 U/L |
| Endocrinology | |
| TSH | 3.75 mIU/L (normal: 0.7–5.9) |
| T4 | 17.5 pmol/L (normal: 12.3–22.8) |
| Prolactin | 178.4 ng/mL (normal: 4.8–23.3) |
| ACTH | 74 pg/mL (normal: 7.2–63.3) |
| Cortisol AM | 11.5 µg/dL (normal: 3.7–9.4) |
| LH | <0.1 mIU/mL |
| FSH | 0.3 mIU/mL (normal: 0.2–11.1) |
| GH | 1.88 ng/mL (normal: 0–10) |
| IGF-1 | 67 ng/mL (normal: 34.2–155) |
| Specific blood tests | |
| CK | 67 U/L (normal: 0–149) |
| Alpha-fetoprotein | 2.2 ng/mL (normal: 0–8.3) |
| Neuron-specific enolase | 16.3 ng/mL (normal: 0–12.5) |
| Paraneoplastic autoantibodies | Negative |
| Anti-neuronal nuclear antibody (I, II, III) | Negative |
| Anti-glial nuclear antibody (I) | Negative |
| Purkinje cell cytoplasmic antibody (I, II, III) | Negative |
| Normetanephrine | 65.3 pg/mL |
| Metanephrines | 46.5 pg/mL (normal: 0–88.0) |
| Urine investigations | |
| Osmolality | 260 mOsmol/kg (normal: 50–1400) |
| Specific gravity | <1.005 |
| Sodium | 26 mmol/L |
| VMA/Creatinine | 5.7 mg/g creat (normal: 0–11) |
| HVA/Creatinine | 14 mg/g creat (normal: 0–22) |
| Cerebrospinal fluid investigations | |
| Glucose | 59 mg/dL |
| Protein | 16 mg/dL |
| WBC | 4 |
| Neutrophils | 0% |
| Lymphocytes | 100% |
| Viral panel | Negative |
| Culture | Negative |
| Lactate | 1.9 mmol/L (normal: 1.1–2.8) |
| Oligoclonal bands | Positive for oligoclonal bands |
| Anti-MOG antibodies | Negative |
| NMO/AQP4 IgG | Negative |
Upon admission, the patient was evaluated by a multidisciplinary team that included endocrinology, neurology, gastroenterology, pulmonology, cardiology, oncology, pediatric surgery, and speech pathology services.
After admission, polyuria (urine output >4 mL/kg/hour) and nocturnal enuresis with dilute urine (specific gravity <1.010, urine osmolality <300) were noted. Despite hyponatremia, it was felt to be consistent with arginine vasopressin deficiency (previously known as central diabetes insipidus), and she responded well to desmopressin, which normalized her polyuria and hydration [10].
The patient presented initially with oculomotor apraxia along with unsteady gait that progressed over a few days to include motor apraxia involving her upper limbs, and, subsequently, increasing weakness of palmar grasp. Physiotherapy was commenced. Along with her progressive neurological deterioration, she had worsening behavior with marked emotional lability. A lumbar puncture was done, and cerebrospinal fluid (CSF) analysis was positive for oligoclonal bands but otherwise unremarkable.
In view of the known high risk of central apnea, polysomnography was performed and showed moderate sleep apnea with an apnea-hypopnea index of 10 per hour; however, no significant desaturations or hypoventilation occurred during the study. We recommended overnight bilevel-positive airway pressure and oxygen saturation monitoring at home due to her risk of central hypoventilation; however, the parents refused.
The patient had difficulty chewing and swallowing solid food upon presentation and progressed dysphagia for liquids. Her modified barium swallow study showed moderate dysphagia with uncoordinated swallowing. Given her high aspiration risk, she was limited to pureed food and fluids through a nasogastric tube and, later, via gastrostomy.
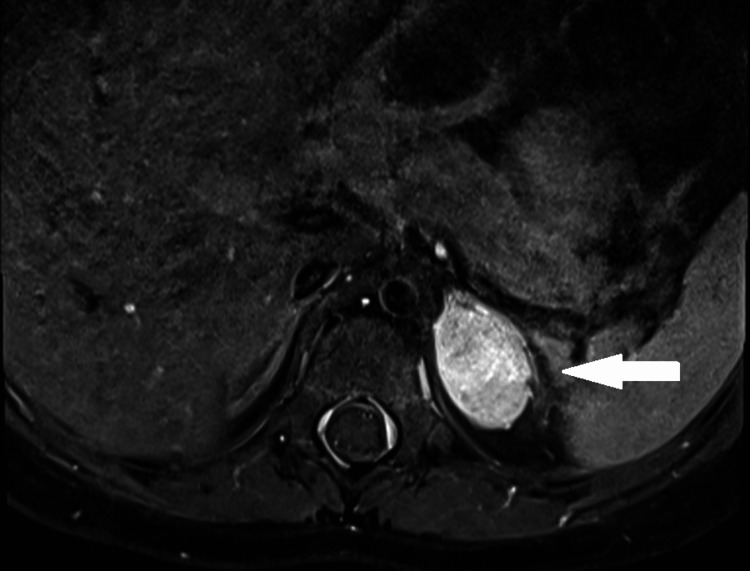
Given her horizontal gaze palsy and progressive dysphagia, she underwent a whole brain and spine MRI which identified a mass in the left suprarenal region (Figure 2). CT of the chest and abdomen confirmed a left adrenal mass measuring approximately 1.6 × 2.6 × 2.4 cm.
Figure 2. MRI: Left suprarenal mass showing contrast enhancement of her left adrenal mass, which was confirmed on histology to be a ganglioneuroma.
At laparoscopy, a 4.5 × 3.0 × 2.0 cm smooth, encapsulated adrenal mass was found and completely resected. Histology of the tumor showed Schwannian stroma and ganglion cells, typical of a ganglioneuroma. There were multiple foci of calcification along with clusters of lymphocytic infiltration, without any noted neuroblast or neuropil tissue.
Molecular analysis for PHOX2B was performed to rule out congenital central hypoventilation syndrome (CCHS). With the rapid development of obesity, disordered water balance (hyponatremia), hyperprolactinemia, abnormal polysomnography, and the absence of PHOX2B mutations, our patient’s diagnosis was confirmed to be ROHHADNET.
She received intravenous immunoglobulin (IVIG) 2 g/kg over two days, and we planned to proceed to rituximab and cyclophosphamide if she proved refractory to IVIG therapy. At follow-up, she had greatly improved behavior and swallowing. Unfortunately, the parents subsequently defaulted from follow-up, and she died in her sleep six months later.
Discussion
We describe the case of a three-year-old girl who presented with classical signs and symptoms of ROHHAD. The investigation confirmed the presence of a left adrenal neuroendocrine tumor and the absence of PHOX2B mutation, confirming the full ROHHADNET syndrome. Although the diagnosis of this syndrome is often delayed, in our case, it was suspected at the initial presentation. Management is very challenging and requires a multidisciplinary approach involving pulmonology, endocrine, autonomic medicine, oncology, psychiatry, surgery, ENT, cardiology, psychology, and dietetics [9,11].
Our differential diagnosis included endocrinological etiologies such as hypercortisolism and hypothyroidism, which were excluded on initial tests. The finding of hyponatremia coupled with polyuria was contradictory, but the patient responded extremely well to DDAVP therapy, with the restoration of hydration and consequent improved well-being. The co-occurrence of arginine vasopressin deficiency and cerebral salt wasting is a potential explanation for this anomalous presentation. Striking hyperprolactinemia was noted at presentation and is characteristic of ROHHAD, indicating a more generalized hypothalamic dysfunction. Dysnatremia and hyperprolactinemia were seen in 97% and 96% of ROHHAD cases, respectively [9]. Her 9 am ACTH and cortisol, although trivially elevated, were not consistent with Cushing’s disease, and were most likely due to stress.
One of the other differential diagnoses was CCHS which was suspected based on the clinical presentation of ventilatory dysfunction, especially during sleep, without evidence of primary respiratory, cardiac, or neurologic disorders [12]. The diagnosis of CCHS can be confirmed by polysomnography findings and genetic testing revealing a PHOX2B mutation [12].
Although an underlying genetic component has been proposed for the pathogenesis of ROHHAD due to its similarity with CCHS, extensive screening and chromosomal analysis failed to identify any genetic mutation [3,4,13]. Nevertheless, genetic testing is recommended for all suspected cases to rule out a PHOX2B mutation and genetic obesity syndromes [14].
Prader-Willi syndrome is another differential, a rare, complex, multisystem genetic disorder recognized as the most commonly known genetic cause of life-threatening obesity in humans. The cardinal clinical features include severe infantile hypotonia, hyperphagia with the onset of obesity during early childhood if not controlled, developmental delay with learning and behavioral problems, short stature with small hands/feet, and hypogonadism/hypogenitalism due to growth hormone and other endocrine deficiencies [15].
One of the early factors in suspecting ROHHAD syndrome in a patient is rapid weight gain with increased BMI. The standard measure of obesity for children above than age of two years is a BMI ≥95th percentile for age and sex, as in our case [16,17]. The criteria for diagnosis are summarized in Table 2.
Table 2. Diagnostic criteria for ROHHADNET syndrome.
ROHHADNET = rapid-onset obesity with hypothalamic dysfunction, hypoventilation, autonomic dysregulation, and neuroendocrine tumor
| Diagnostic Criteria for ROHHADNET | Our patient | |
| 1 | Rapid-onset obesity starting after the age of 18 months | Yes |
| 2 | Evidence of hypothalamic dysfunction, as defined by at least one of the following findings: | 2/6 |
| 2A | Hyperprolactinemia | Yes |
| 2B | Central hypothyroidism | No |
| 2C | Disordered water balance | Yes |
| 2D | Growth hormone deficiency | No |
| 2E | Corticotrophin deficiency | No |
| 2F | Delayed or precocious puberty | No |
| 3 | Alveolar hypoventilation during sleep starting after the age of 18 months | Yes |
| 4 | Features of autonomic dysregulation | Yes |
| Rapid development of obesity was noticed at 3½ years of age, with BMI rising from the 25th to the 95th centile in just three months. Disordered water balance (hyponatremia) and hyperprolactinemia were identified at the initial presentation, Cyanotic episodes attributed to central hypoventilation were noticed four months later | ||
In a systematic review of ROHHADNET in 2018 by Lee et al., the most common presentation of patients with ROHHAD/NET was rapid obesity and hypothalamic dysfunction (83%), followed by hypoventilation (75%). Ocular symptoms were reported in 25% of cases [9].
At the time of diagnosis, a significant proportion of patients (42%) require respiratory support and mechanical ventilation [9]. Given our patient’s abnormal polysomnography findings along with episodes of cyanosis, central hypoventilation syndrome was diagnosed.
Our patient presented with oculomotor apraxia and a left adrenal mass. Opsoclonus-myoclonus syndrome (OMS) was excluded by the absence of clinical findings. Furthermore, tumor histology showed ganglioneuroma. OMS is typically seen with less differentiated neuroblastomas or ganglioneuroblastomas. Plasma metanephrines and normetanephrines and urine catecholamines were normal.
Lee et al. reported that the most common neuroendocrine tumors in ROHHADNET were ganglioneuromas (60%), as in our case. Although the lesions usually presented as intra-abdominal masses, two cases with mediastinal masses were reported. The characteristic findings on histology are Schwannian stroma and ganglion cells [2-6,9]. They are benign, differentiated tumors and complete resection is the preferred modality of treatment [18].
Behavioral change is a common manifestation of cognitive dysfunction (60% of cases), with symptoms such as mood changes, fatigue, social withdrawal, poor school performance, and intellectual disability, which were also noted in our patient. Other manifestations include seizures, altered consciousness, sleep disturbance, and developmental delay [9].
Although, the pathogenesis of ROHHAD has not been yet identified, an autoimmune origin is suspected [14]. The finding of oligoclonal bands in our patient and two previously reported cases support an immune origin for ROHHAD [14,19,20]. This is supported by the patient’s partial, albeit significant, clinical improvement with IVIG treatment [21].
Immunosuppressive treatment with high-dose cyclophosphamide has been reported to have positive effects on BMI stability and neuropsychological function in two cases [13,19]. Immune treatments were previously employed in six reported patients, which included glucocorticoids, IVIG, cyclophosphamide, and rituximab, with variable but generally positive responses [14].
The management of ROHHADNET syndrome requires a multidisciplinary approach (Table 3). The aim is to manage each aspect of the disease to improve quality of life and life expectancy. This necessitates the early involvement of a multidisciplinary team to manage all aspects of this polymorphic disorder. Due to the high risk of central hypoventilation, overnight polysomnography is essential for the detection of obstructive and/or central sleep apnea. Cardiac evaluation for autonomic neuropathy is needed. Hypertension requiring antihypertensive medication is common, and a pacemaker may be required to manage dysrhythmias. Neurological and ophthalmological manifestations, such as ophthalmoplegia and seizures, necessitate early involvement of these specialties [14].
Table 3. Multidisciplinary team in treating ROHHADNET.
ROHHADNET = rapid-onset obesity with hypothalamic dysfunction, hypoventilation, autonomic dysregulation, and neuroendocrine tumor
| Investigation/Screening | Therapeutic options |
| Rapid obesity | |
| Initial clinical evaluation, brain MRI, complete endocrine workup to exclude other differential diagnosis of precautious puberty, and evaluation of metabolic disturbance/year | Body mass index stabilization using strict calorie intake and regular exercise. Antidiabetic drugs and antilipid drugs |
| Hypothalamic dysfunction | |
| Hormonal investigation/Year for hypothyroidism, hyperprolactinemia, growth hormone deficiency, puberty delay, and adrenalin insufficiency | Specific hormonal substitution |
| Hypoventilation | |
| Polysomnography with nocturnal gas exchange/year if negative in the first five years, and prevention of respiratory infection | Artificial ventilation and influenza vaccine/year |
| Autonomic dysregulation | |
| ECG, echocardiography, 72-hour Holter/year, blood pressure/three months, gastroenterology screening/year for celiac disease, food intolerance and transient dysregulation, and ophthalmologic evaluation/year | Cardiac pacemaker, antihypertensive drugs, gluten-free diet, lactose-free diet, and drugs for transient control |
| Neural Tumor | |
| Screening to detect neuroendocrine tumors, and chest and abdominal MRI/year | In case of neuroendocrine tumor: staging and treatment |
| Neurologic Impact | |
| Electroencephalography in case of seizure, and evaluation for behavioral disturbances. | Antiepileptic drugs and antipsychotic drugs |
| Genetic considerations | |
| Exclude a PHOX2B mutation and genetic obesity | |
ROHHAD(NET) syndrome is a life-threatening condition with high mortality and few long-term survivors [22]. A systematic review by Julie et al. found the median age of death to be 4.6 years and that two of six patients died due to sudden death [14]. Death at a young age may be due to delay in diagnosis, severe initial presentation, or, as in our case, parents not adhering to medical advice or follow-up.
Conclusions
ROHHAD syndrome is a rare and potentially fatal disease that requires timely diagnosis and management by a multidisciplinary team. The finding of CSF oligoclonal bands in ROHHAD syndrome, as previously noted in two other cases, is further evidence of an immune etiology for this condition and may point the way to the use of immune therapies as a strategy for improving the prognosis of this very severe condition.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.ROHHAD syndrome - a still unrecognized cause of childhood obesity: report of three cases. Filippidou M, Petropoulou T, Botsa E, et al. J Pediatr Endocrinol Metab. 2020;33:1341–1348. doi: 10.1515/jpem-2020-0111. [DOI] [PubMed] [Google Scholar]
- 2.Late-onset central hypoventilation with hypothalamic dysfunction: a distinct clinical syndrome. Katz ES, McGrath S, Marcus CL. Pediatr Pulmonol. 2000;29:62–68. doi: 10.1002/(sici)1099-0496(200001)29:1<62::aid-ppul10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Ize-Ludlow D, Gray JA, Sperling MA, et al. Pediatrics. 2007;120:0–88. doi: 10.1542/peds.2006-3324. [DOI] [PubMed] [Google Scholar]
- 4.Delineation of late onset hypoventilation associated with hypothalamic dysfunction syndrome. De Pontual L, Trochet D, Caillat-Zucman S, et al. Pediatr Res. 2008;64:689–694. doi: 10.1203/PDR.0b013e318187dd0e. [DOI] [PubMed] [Google Scholar]
- 5.Endocrine manifestations of the rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neural tumor syndrome in childhood. Bougnères P, Pantalone L, Linglart A, Rothenbühler A, Le Stunff C. J Clin Endocrinol Metab. 2008;93:3971–3980. doi: 10.1210/jc.2008-0238. [DOI] [PubMed] [Google Scholar]
- 6.Hypothalamic dysfunction associated with neuroblastoma: evidence for a new paraneoplastic syndrome? Sirvent N, Bérard E, Chastagner P, Feillet F, Wagner K, Sommelet D. Med Pediatr Oncol. 2003;40:326–328. doi: 10.1002/mpo.10157. [DOI] [PubMed] [Google Scholar]
- 7.A case of rapid-onset obesity with hypothalamic dysfunction, hypoventilation, autonomic dysregulation, and neural crest tumor: ROHHADNET syndrome. Abaci A, Catli G, Bayram E, et al. Endocr Pract. 2013;19:0–6. doi: 10.4158/EP12140.CR. [DOI] [PubMed] [Google Scholar]
- 8.Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) syndrome: a case report. Bagheri B, Pourbakhtyaran E, Talebi Kiasari F, Taherkhanchi B, Salarian S, Sadeghi AA. Arch Pediatr Infect Dis. 2017;5:0. [Google Scholar]
- 9.Rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neuroendocrine tumors (ROHHADNET) syndrome: a systematic review. Lee JM, Shin J, Kim S, et al. Biomed Res Int. 2018;2018:1250721. doi: 10.1155/2018/1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Changing the name of diabetes insipidus: a position statement of The Working Group for Renaming Diabetes Insipidus. Arima H, Cheetham T, Christ-Crain M, et al. Endocr J. 2022;69:1281–1284. doi: 10.1507/endocrj.EJ20220831. [DOI] [PubMed] [Google Scholar]
- 11.Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation. National Organization for Rare Disorders. [ May; 2023 ]. 2023. https://rarediseases.org/rare-diseases/rapid-onset-obesity-with-hypothalamic-dysfunction-hypoventilation-and-autonomic-dysregulation/. https://rarediseases.org/rare-diseases/rapid-onset-obesity-with-hypothalamic-dysfunction-hypoventilation-and-autonomic-dysregulation/.
- 12.Failure of automatic control of ventilation (Ondine's curse). Report of an infant born with this syndrome and review of the literature. Mellins RB, Balfour HH Jr, Turino GM, Winters RW. https://journals.lww.com/md-journal/Citation/1970/11000/FAILURE_OF_AUTOMATIC_CONTROL_OF_VENTILATION.3.aspx. Medicine (Baltimore) 1970;49:487–504. [PubMed] [Google Scholar]
- 13.Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: analysis of hypothalamic and autonomic candidate genes. Rand CM, Patwari PP, Rodikova EA, et al. Pediatr Res. 2011;70:375–378. doi: 10.1203/PDR.0b013e318229474d. [DOI] [PubMed] [Google Scholar]
- 14.ROHHAD(NET) syndrome: systematic review of the clinical timeline and recommendations for diagnosis and prognosis. Harvengt J, Gernay C, Mastouri M, Farhat N, Lebrethon MC, Seghaye MC, Bours V. J Clin Endocrinol Metab. 2020;105:0. doi: 10.1210/clinem/dgaa247. [DOI] [PubMed] [Google Scholar]
- 15.Prader-Willi syndrome - clinical genetics, diagnosis and treatment approaches: an update. Butler MG, Miller JL, Forster JL. Curr Pediatr Rev. 2019;15:207–244. doi: 10.2174/1573396315666190716120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Deurenberg P, Weststrate JA, Seidell JC. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 17.Overweight children and adolescents: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Baker S, Barlow S, Cochran W, et al. J Pediatr Gastroenterol Nutr. 2005;40:533–543. doi: 10.1097/01.mpg.0000161147.16590.12. [DOI] [PubMed] [Google Scholar]
- 18.Treatment and outcome of ganglioneuroma and ganglioneuroblastoma intermixed. Decarolis B, Simon T, Krug B, et al. BMC Cancer. 2016;16:542. doi: 10.1186/s12885-016-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Improved behavior and neuropsychological function in children with ROHHAD after high-dose cyclophosphamide. Jacobson LA, Rane S, McReynolds LJ, Steppan DA, Chen AR, Paz-Priel I. Pediatrics. 2016;138:0. doi: 10.1542/peds.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cyclophosphamide for rapid-onset obesity, hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome. Paz-Priel I, Cooke DW, Chen AR. J Pediatr. 2011;158:337–339. doi: 10.1016/j.jpeds.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Intrathecal synthesis of oligoclonal bands in rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome: new evidence supporting immunological pathogenesis. Sartori S, Priante E, Pettenazzo A, et al. J Child Neurol. 2014;29:421–425. doi: 10.1177/0883073812469050. [DOI] [PubMed] [Google Scholar]
- 22.Rapid-onset obesity, hypoventilation, hypothalamic dysfunction, autonomic dysregulation and neuroendocrine tumor syndrome with a homogenous enlargement of the pituitary gland: a case report. Aljabban L, Kassab L, Bakoura NA, Alsalka MF, Maksoud I. J Med Case Rep. 2016;10:328. doi: 10.1186/s13256-016-1116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]