Abstract
Background
In order to prevent overweight and obesity in the general population we need to understand the relationship between the proportion of energy from fat and resulting weight and body fatness in the general population.
Objectives
To assess the effects of proportion of energy intake from fat on measures of weight and body fatness (including obesity, waist circumference and body mass index) in people not aiming to lose weight, using all appropriate randomised controlled trials (RCTs) and cohort studies in adults, children and young people
Search methods
We searched CENTRAL to March 2014 and MEDLINE, EMBASE and CINAHL to November 2014. We did not limit the search by language. We also checked the references of relevant reviews.
Selection criteria
Trials fulfilled the following criteria: 1) randomised intervention trial, 2) included children (aged ≥ 24 months), young people or adults, 3) randomised to a lower fat versus usual or moderate fat diet, without the intention to reduce weight in any participants, 4) not multifactorial and 5) assessed a measure of weight or body fatness after at least six months. We also included cohort studies in children, young people and adults that assessed the proportion of energy from fat at baseline and assessed the relationship with body weight or fatness after at least one year. We duplicated inclusion decisions and resolved disagreement by discussion or referral to a third party.
Data collection and analysis
We extracted data on the population, intervention, control and outcome measures in duplicate. We extracted measures of weight and body fatness independently in duplicate at all available time points. We performed random‐effects meta‐analyses, meta‐regression, subgrouping, sensitivity and funnel plot analyses.
Main results
We included 32 RCTs (approximately 54,000 participants) and data from 25 cohorts. There is consistent evidence from RCTs in adults of a small weight‐reducing effect of eating a smaller proportion of energy from fat; this was seen in almost all included studies and was highly resistant to sensitivity analyses. The effect of eating less fat (compared with usual diet) is a mean weight reduction of 1.5 kg (95% confidence interval (CI) ‐2.0 to ‐1.1 kg), but greater weight loss results from greater fat reductions. The size of the effect on weight does not alter over time and is mirrored by reductions in body mass index (BMI) (‐0.5 kg/m2, 95% CI ‐0.7 to ‐0.3) and waist circumference (‐0.3 cm, 95% CI ‐0.6 to ‐0.02). Included cohort studies in children and adults most often do not suggest any relationship between total fat intake and later measures of weight, body fatness or change in body fatness. However, there was a suggestion that lower fat intake was associated with smaller increases in weight in middle‐aged but not elderly adults, and in change in BMI in the highest validity child cohort.
Authors' conclusions
Trials where participants were randomised to a lower fat intake versus usual or moderate fat intake, but with no intention to reduce weight, showed a consistent, stable but small effect of low fat intake on body fatness: slightly lower weight, BMI and waist circumference compared with controls. Greater fat reduction and lower baseline fat intake were both associated with greater reductions in weight. This effect of reducing total fat was not consistently reflected in cohort studies assessing the relationship between total fat intake and later measures of body fatness or change in body fatness in studies of children, young people or adults.
Plain language summary
Effect of cutting down the fat we eat on body weight
The ideal proportion of energy from fat in our food and its relation to body weight is not clear. This review looked at the effect of cutting down the proportion of energy from fat in our food on body weight and fatness in both adults and children who are not aiming to lose weight. The review found that cutting down on the proportion of fat in our food leads to a small but noticeable decrease in body weight, body mass index and waist circumference. This effect was found both in adults and children. The effect did not change over time.
Summary of findings
Summary of findings for the main comparison. Low dietary fat compared with usual fat for controlling body fatness.
| Low dietary fat compared with usual fat for body fatness | ||||||
|
Patient or population: children, young people and adults from the general population
Settings: general population
Intervention: low dietary fat
Comparison: usual fat Methods: randomised controlled trials | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual fat | Low dietary fat | |||||
| Weight, kg (adults) body weight in kg Follow‐up: 6 to 96 months | Median weight change ‐0.04kg1 | The mean weight, kg (adults) in the low fat groups was 1.54 lower (1.97 to 1.12 lower) | — | 53,647 (30 RCTs) | ⊕⊕⊕⊕ high2,3,4,5,6,7,8 | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The median weight change in the control groups over the course of each study was ‐0.04kg, ranging from ‐1.91kg to 2.13kg.
2While most studies were unblinded for participants and allocation concealment was often unclear (as randomisation was described poorly), RCT results in adults were remarkably consistent in their direction. Sensitivity analyses removing studies without clear allocation concealment did not lose the statistically significant relative weight reduction in the low fat arm, and neither did running fixed‐effect (rather than random‐effects) meta‐analysis or removing studies with attention bias favouring those in the low fat arm, or those with other interventions alongside the fat reduction. The consistent weight loss was despite the fact that none of the studies included intended to alter weight in either arm, so that publication bias on this outcome is unlikely. Together this suggests that the risk of bias was low. 3The direction of effects in these RCTs was remarkably consistent ‐ in almost every study participants eating lower total fat intakes were lower in weight (on average) at the study end than participants eating a higher percentage of total fat. The only inconsistency (where heterogeneity arose) was in the size of this effect. The heterogeneity was partly explained by the degree of reduction of fat intake, and by the level of control group fat intake, which together explained 56% of between‐study variance (in meta‐regression). The reduction in weight in those taking on lower fat diets was seen in very different populations and from six months to several years. It was also consistent when we excluded studies that gave additional support, time or encouragement to the low fat arms, and where we excluded studies that delivered additional dietary interventions (on top of the change in dietary fats). The results were consistent in direction, and much of the heterogeneity in the size of the effect was explained by the selected factors. 4All included RCTs directly compared (and randomised participants to) lower versus usual fat intake; therefore there was no indirectness in intervention. All studies were conducted in industrialised countries so the potential to generalise to other cultural contexts is limited. Nonetheless there is no reason to believe that the effect would be different in different populations. There are changes in diets in many countries around the world, which are resulting in greater similarity in diets in developed and developing countries. Additionally, the industrialised countries represented included a wide variety of baseline (or control group) fat intakes, and the effect was apparent at all of these levels. The studies all addressed weight directly and did not use proxy measures. 5Imprecision was unlikely, as over 40,000 participants were included in RCTs of at least six months duration, and effect sizes were highly statistically significant. There was little imprecision. If the true effect on weight was at either end of the 95% CI we would see the effect in the same way. 6The funnel plot did not suggest publication bias. 7Subgrouping supported the presence of a dose response gradient in that studies that altered the total fat intake between intervention and control by less than 5% of energy had a negligible effect on weight, while greater differences in total fat intake were associated with statistically significant differences in weight. This was supported by the meta‐regression, which suggested a statistically significant relationship between the degree of fat reduction and of weight loss. 8The effects on body weight are supported by similar effects on BMI in adults (‐0.50 kg/m2, 95% CI ‐0.74 to ‐0.26, 10 RCTs, > 45,000 participants), waist circumference in adults (‐0.30 cm, 95% CI ‐0.58 to ‐0.02, one RCT, > 15,000 participants) and BMI reduction in the one RCT in children.
Background
The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) expert consultation on fats and fatty acids in human nutrition debated optimal intakes of total fat in 2008. In light of the rising levels of overweight and obesity, particularly in low‐ and middle‐income countries undergoing rapid nutrition transition, this consultation agreed that any effect of total fat intake on body weight was pivotal in making global recommendations on total fat intake. Overweight and obesity are associated with increased risk of many cancers, coronary heart disease and stroke (Manson 1990; Song 2004; WCRF/AICR 2009).
A previous systematic review found no randomised controlled trials (RCTs) of lower total fat intake that aimed to assess effects on body weight (Kelly 2006), but we were aware of RCTs that had randomised participants to low fat versus usual fat diets, and measured weight or BMI as a process measure (Hooper 2012a). Additionally, meta‐regression within a systematic review assessing RCTs on the effects of step I and II diets (diets designed by the National Heart, Lung and Blood Institute national cholesterol education programme to reduce the risk of cardiovascular disease in the general population and those at increased cardiovascular risk, respectively), found a strong relation between total fat intake and body weight (Yu‐Poth 1999). This review, however, included studies that were as short as three weeks in duration and studies in which weight loss was a goal of the intervention, which may have overstated any relation because the advice was to lower both fat and energy intake. It also excluded many trials of reduction in total fat intake that did not fit the step I or II criteria.
More recent reviews that have explored the long‐term effects of low fat diets either did not explore weight or body fatness as an outcome (Schwingshackl 2013), or looked at low fat intake as part of a wider health promotion intervention (Ni 2010). Other systematic reviews have explored the relationship between fat intake and body fatness but were either limited to the effect low fat dairy versus high fat dairy consumption (Benatar 2013), or investigated it as part of looking at the overall dietary patterns (Ambrosini 2014), or diet quality (Aljadani 2015).
In order to aid the WHO's understanding of the relation between total fat intake and body weight with a view to updating their guidelines on total fat intake, the WHO Nutrition Guidance Expert Advisory Group (NUGAG) subgroup on diet and health (http://www.who.int/nutrition/topics/advisory_group/nugag_dietandhealth_topics/en/) was requested to assess the relationship. The expert advisory group aimed to generate a recommendation on the population impact of total fat intake in the development of obesity. The NUGAG group agreed to exclude studies of populations recruited specifically for weight loss and interventions intended to result in weight loss. These studies were potentially confounded by the implicit objective of reducing calorie intake to produce weight loss and might therefore lead to an overemphasis on studies carried out in highly selected obese populations in North America and Europe, which may have limited transferability to non‐obese populations or those in developing countries or in countries in transition.
To fulfil the requirements for the new guideline, a systematic review was needed of all available evidence of the longer‐term effects of total fat intake on body fatness, in studies not intending to cause weight loss. The WHO therefore commissioned a systematic review and meta‐analysis to assess the relationship between total fat intake and indicators of body fatness (including obesity, waist circumference and body mass index) using all appropriate RCTs and cohort studies in adults and children (Hooper 2012b), which has been updated in 2015.
Objectives
To assess the effects of proportion of energy intake from fat on measures of weight and body fatness (including obesity, waist circumference and body mass index) in people not aiming to lose weight, using all appropriate RCTs and cohort studies in adults, children and young people.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials ( RCTs) of adults and children: trials of reduced fat intake compared with usual diet or modified fat intake with no intention to reduce weight (in any participants in either or both arms), continued for at least six months, unconfounded by non‐nutritional interventions and assessing a measure of body fatness at least six months after the intervention was initiated.
Randomisation of individuals was accepted, or of larger groups where there were at least six of these groups (clusters) randomised. We excluded studies where allocation was not truly randomised (e.g. divisions based on days of the week or first letter of the family name were excluded) or where allocation was not stated as randomised (and no further information was available from the authors). We excluded cross‐over studies (as previous weight gain or weight loss is likely to affect future weight trends) unless the first half of the cross‐over could be used independently.
Cohort studies of adults and children: prospective cohort studies that followed participants for (and assessed final or change in body fatness) at least 12 months after assessment of total fat, and related baseline total fat intake to absolute or change in body fatness at least 12 months later.
Types of participants
We accepted studies of adults (≥ 18 years, no upper age limit) or children and young people (aged ≥ 24 months) at any risk of cardiovascular disease (with or without existing cardiovascular disease). Participants could be of either sex, but we excluded those who were acutely ill, pregnant or lactating. We excluded intervention studies where participants were chosen for raised weight or body mass index (as most appeared to aim to reduce body weight within interventions, even when this was not explicitly stated in the intervention goals).
Types of interventions
Interventions
We considered all randomised controlled trials (RCTs) of interventions stating an intention to reduce dietary fat, when compared with a usual or modified fat intake.
We considered a low fat intake to be one that aimed to reduce fat intake to ≤ 30% energy (≤ 30%E) from fat, and at least partially replace the energy lost with carbohydrates (simple or complex), protein or fruit and vegetables. We considered a modified fat diet to be one that aimed to include > 30% energy from total fats, and included higher levels of mono‐unsaturated or poly‐unsaturated fats than a 'usual' diet.
As we were interested in the effects of fat intake on body weight and fatness in everyday dietary intake (rather than in people aiming to reduce their body weight in weight‐reducing diets) we excluded studies aiming to reduce the weight of some or all participants, as well as those that included only participants who had recently lost weight, or recruited participants according to a raised body weight or BMI. We excluded multifactorial interventions other than diet or supplementation (unless the effects of diet or supplementation could be separated, so the additional intervention was consistent between the intervention and control groups). We excluded Atkins‐type diets aiming to increase protein and fat intake, as well as studies where fat was reduced by means of a fat substitute (like Olestra). We excluded enteral and parenteral feeds, as well as formula weight‐reducing diets.
Examples
We included studies that reduced fats and encouraged physical activity in one arm and compared this with encouraging physical activity in the control. We excluded studies that reduced fats and encouraged physical activity in one arm and compared this with no intervention in the control. We included studies that reduced fats and encouraged fruit and vegetables in one arm and compared this with no intervention in the control.
We included all trials that intended to reduce dietary fat to ≤ 30%E in one arm compared to usual or modified fat intake (> 30%E from fat) in another arm regardless of the degree of difference between fat intake in the two arms (dose). We explored the effects of the difference in %E from fat between control and intervention groups, as well as the effects of fat intake in the control groups and dietary fat goals in the intervention groups, in subgrouping.
Exposures
For cohort studies total fat intake, in grams or as a percentage of dietary energy intake, had to be assessed at baseline and related to a measure of body fatness, or change in body fatness, at least a year later. For cohorts that used multiple dietary assessments to model later body fatness or change in body fatness more than half of the assessments included in the model had to be at least a year before the assessment of body fatness (or the final assessment for a change measure) used in the model.
Types of outcome measures
Primary outcomes
The main outcomes were measures of body fatness, including body weight, body mass index, waist circumference, skinfold thickness or percentage fat. Studies had to report at least one of these measures, or a change in these measures, to be included in the review.
Secondary outcomes
Secondary outcomes included other classic cardiovascular risk factors (systolic or diastolic blood pressure, serum total, low density lipoprotein (LDL) or high density lipoprotein (HDL) cholesterol and triglyceride) and quality of life measures (including informal outcomes such as feelings of health and time off work).
Tertiary outcomes
Tertiary outcomes were process outcomes and included changes in saturated and total fat intakes, as well as other macronutrients, sugars and alcohol.
This is not a systematic review of the effects of reduced fat on these secondary or tertiary outcomes, but we collated the outcomes from included studies in order to understand whether any effects on weight might be compromised by negative effects on secondary or tertiary outcomes.
Search methods for identification of studies
Electronic searches
The search to June 2010 is described in Hooper 2012b. We updated the searches to November 2014 and ran these in MEDLINE (Ovid, see Appendix 1). EMBASE (Ovid) and CINAHL (EBSCO host) searches were based on the MEDLINE search (Appendix 2; Appendix 3). The Cochrane Heart Group ran the update search for adult RCTs on 5 March 2014 in CENTRAL (2014, Issue 1) for a sister review, Hooper 2015 (Appendix 4), and we checked the references for this review.
Searching other resources
We searched the bibliographies of all related identified systematic reviews for further trials and cohort studies for the update, including Aljadani 2015, Ajala 2013, Aljadani 2013, Ambrosini 2014, Benatar 2013, Chaput 2014, Gow 2014, Havranek 2011, Hu 2012, Kratz 2013, Ni 2010, Schwingshackl 2013, Schwingshackl 2013a and Yang 2013.
Data collection and analysis
Selection of studies
We only rejected articles on the initial screen if the review author could determine from the title and abstract that the article was not a relevant RCT or cohort study. We rejected articles if they were not the report of a RCT; the trial did not address a low fat intake; the trial was exclusively in infants (less than 24 months old), pregnant women or the critically ill; participants were chosen for being overweight or obese; there was an intention to reduce weight in some or all participants; the trial was of less than six months duration; or the intervention was multifactorial. We rejected cohort studies where they were not prospective; where participants' total fat intake was not assessed; where they did not follow participants for at least 12 months after assessment of total fat; or where the relationship between total fat at baseline and a measure of absolute or change in body fatness at least 12 months later was not assessed.
When a title/abstract could not be rejected with certainty, we obtained the full text of the article for further evaluation. LH and AA assessed the inclusion of studies independently in duplicate, and we collected studies identified by either review author. LH and AA assessed the full texts collected for inclusion independently in duplicate, and discussed disagreements until agreement was reached.
Data extraction and management
We extracted data concerning participants, interventions or exposures and outcomes, and trial or cohort quality characteristics onto a form designed for the review. We extracted data on potential effect modifiers from RCTs (including duration of intervention, control group fat intake, sex, year of first publication, difference in % energy from fat between the intervention and control groups, type of intervention (food or advice provided), the dietary fat goals set for each arm, baseline BMI and health at baseline). Where provided, we collected data on risk factors for cardiovascular disease (secondary and tertiary outcomes).
All trial outcomes were continuous and where possible we extracted change data (change in the outcome from baseline to outcome assessment) with relevant data on variance for intervention and control arms (along with numbers of participants at that time point). Where change data were not available, we extracted data at study end (or other relevant time point) along with variance and numbers of participants for each arm. LH and AA extracted all data independently in duplicate.
Assessment of risk of bias in included studies
We carried out 'Risk of bias' assessment independently in duplicate. We assessed trial risk of bias using the Cochrane tool for assessment of risk of bias (Higgins 2011b). For included RCTs we also assessed whether trials were free of differences in diet (between intervention and control arms) other than dietary fat intake, and whether there was any systematic difference in attention or care or time given between the intervention and control groups, as we felt that these factors may also cause differences in weight. We used the category 'other bias' to note any further issues of methodological concern. Funding was not formally a part of our assessment of bias in RCTs as it is not a core part of the Cochrane 'Risk of bias' tool.
For cohort studies we assessed the number of participants lost to follow‐up (with reasons), baseline similarity by total fat intake, funding, type of control group (internal or external), method of assessment of total fat intake, number of total fat assessments and factors adjusted for. We also noted factors not adjusted for (age, sex, energy intake, ethnicity, physical activity (and/or TV watching) and socioeconomic (including educational) status for adults and age, sex, energy intake, ethnicity, parental BMI, physical activity (and/or TV watching) and socioeconomic (including educational) status in children).
Measures of treatment effect
The effect measure of choice for continuous outcomes (all review outcomes were continuous outcomes) was the mean difference (MD).
Unit of analysis issues
We did not include any cluster‐randomised or cross‐over trials in this review.
Where there was more than one relevant intervention arm but only one control arm we pooled the relevant intervention arms to create a single pair‐wise comparison (where the intervention arms were equivalently appropriate for this review) as described in Higgins 2011a. We excluded intervention arms that were not appropriate for this review, or less appropriate than another arm. When two arms were appropriate for different subgroups then we used the control group once with each intervention arm, but we did not pool the subgroups overall.
When weight or BMI were assessed at more than one time point we used the data from the latest time point available in general analyses, but we extracted data for all time points for use in subgrouping by study duration.
Dealing with missing data
Where included studies used methods to infer missing data (such as carrying the latest weight data forward) then we used these data in analyses. Where this was not done we used the data as presented.
Assessment of heterogeneity
We examined heterogeneity using the I2 statistic and considered heterogeneity important where the I2 was above 50% (Higgins 2003; Higgins 2011a).
Assessment of reporting biases
We drew funnel plots to examine the possibility of publication bias for measures of body fatness with at least 10 included comparisons (Egger 1997).
Data synthesis
All trial outcomes were continuous and where possible we extracted change data (change in the outcome from baseline to outcome assessment) with relevant data on variance for intervention and control arms (along with numbers of participants at that time point). Where change data were not available, we extracted data at study end (or other relevant time point) along with variance and numbers of participants for each arm. We did not use end data where the difference between the intervention and control groups at baseline was greater than the change in that measure between baseline and endpoint in both arms (instead we used change data in forest plots, but without standard deviations (SDs), so the data did not add to the meta‐analyses but provided comparative information).
We combined data by the inverse variance method in random‐effects meta‐analysis to assess mean differences between lower and higher fat intake arms.
We planned to conduct separate meta‐analyses of data from adult RCTs, data from child RCTs, data from adult cohort studies and data from child cohort studies, where data from separate studies were similar enough to be combined.
We created a 'Summary of findings' table assessing the effects of low dietary fat compared with usual fat for body weight in adults using RCT data.
Subgroup analysis and investigation of heterogeneity
For this update we classified all dietary interventions as low fat versus usual or modified fat. Pre‐specified subgroups for body fat outcomes, to explore the stability of findings in different study subgroups, included:
duration of intervention (6 to < 12 months, 12 to < 24 months, 24 to < 60 months, and 60+ months);
control group total fat intake (> 35%E from fat, > 30%E to 35%E from fat, > 25%E to 30%E from fat);
year of first publication of results (1960s, 1970s, 1980s, 1990s, 2000s, 2010s);
sex (studies of women only, of men only, of men and women mixed);
difference in %E from fat between control and reduced fat groups (up to 5%E from fat, 5%E to < 10%E from fat, 10%E to < 15%E from fat, 15+%E from fat, or unknown difference);
type of intervention (dietary advice, advice plus supplements and diet provided);
by total fat goal in the intervention arm (10%E to < 15%E from fat, 15%E to < 20%E from fat, 20%E to < 25%E from fat, 25%E to < 30%E from fat, 30%E from fat, and no specific goal stated);
achieving fat goals (achieved 30%E from fat or less, did not achieve this);
mean BMI at baseline (< 25, 25 to < 30, 30+);
state of health at baseline (not recruited on the basis of risk factors or disease, recruited on the basis of risk factors such as lipids, hormonal levels etc., recruited on the basis of having or having had diseases such as diabetes, myocardial infarction, cancer, polyps);
assessed energy reduction in the intervention compared with the control group during the intervention period (E intake the same or greater in the low fat group, E intake 1 to 100 kcal/d lower in the low fat group, 101 to 200 kcal/d lower in the low fat group, > 200 Kcal/d lower in the low fat group).
For subgrouping factors that appeared to suggest significant differences in effect size between subgroups we explored the effects using meta‐regression on weight (we also intended to explore the effects on other outcomes, but no other outcome had more than 10 relevant comparisons). We performed random‐effects meta‐regression (Berkley 1995) using the STATA command metareg (Sharp 1998; Sterne 2001; Sterne 2009).
Sensitivity analysis
We carried out sensitivity analyses for primary outcomes, assessing the effect of:
running fixed‐effect meta‐analyses (rather than random‐effects) (Higgins 2011a);
excluding the largest study (WHI with CVD 2006, WHI 2006);
excluding studies that were not free of systematic differences in care (or unclear);
excluding studies that were not free of dietary differences other than fat (or unclear);
excluding studies with unclear or inadequate allocation concealment.
Results
Description of studies
The study flow is shown in Figure 1. The perceived importance of obesity and overweight has increased over the past few years, therefore many trials of reduced fat diets now explicitly or implicitly aim at weight loss. To guard against inclusion of studies that intended weight loss without stating this clearly we decided to exclude RCTs that only included people based according to their BMI or weight classification (i.e. specifically including only people with a BMI > 25). For this reason (and to ensure consistency) we have excluded three RCTs included in the previous version of this review, Hooper 2012b, from this current review (CARMEN 2000; CARMEN MS sub‐study; German Fat Reduced), while we have included an additional adult RCT (Diet and Hormone Study 2003).
1.
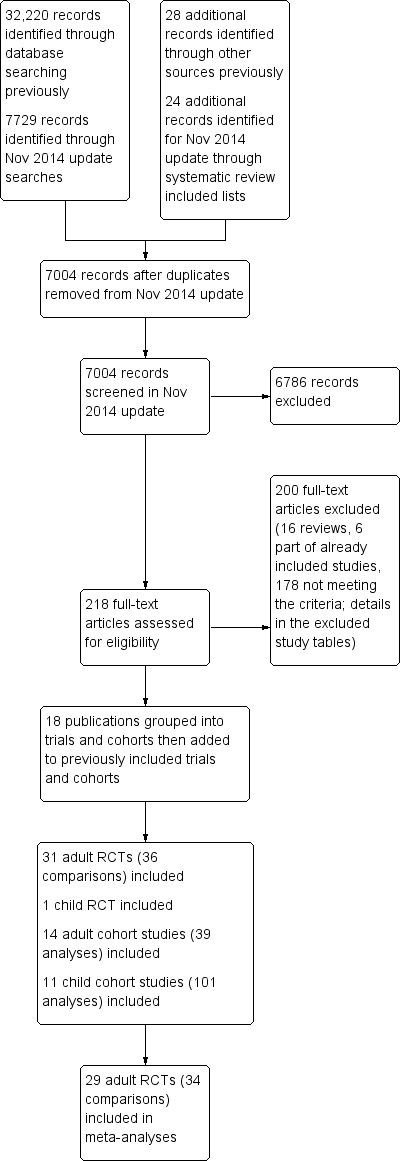
Study flow diagram for this systematic review (update searches run November 2014).
Results of the search
The search for RCTs and cohort studies in the original version of this review identified 32,220 titles and abstracts from the electronic searches plus 28 further potential studies from other sources. For this update the electronic searches identified 7729 possible titles and abstracts, plus we assessed a further 24 potential studies following our check of potentially relevant trials and cohort studies included in other systematic reviews. Of these 7753 potential update titles and abstracts, we assessed 218 full‐text articles for eligibility (additional to the 465 assessed for the original review). We included a total of 32 RCTs (31 in adults, one in children) and 25 prospective cohort studies (17 sets of analyses of 14 cohorts in adults and 13 sets of analyses of 11 cohorts in children) (Figure 1). We included 29 adult RCTs (including 34 comparisons) in meta‐analyses.
Included studies
Of the 31 RCTs in adults (36 comparisons, including roughly 53,626 participants ‐ exact numbers depending on time point in study and endpoint used), 21 were from North America, nine from Europe and one from New Zealand, with none from developing or transitional countries. The duration of the trials varied from six months to more than eight years. In four trials the participants were all men, in 15 all women and in 12 both sexes (one of which reported outcomes by sex). Mean ages and states of health (low, moderate or high risk of cardiovascular disease or breast cancer) varied. The single trial in children analysed 191 Greek 12‐ to 13 ‐year old boys and girls, followed up for 17 months (VYRONAS 2009). See Characteristics of included studies for detailed characteristics of the RCTs in adults and young people.
When discussing the 31 RCTs, the de Bont study (de Bont 1981 non‐obese; de Bont 1981 obese), DEER study (DEER 1998 exercise men; DEER 1998 exercise women; DEER 1998 no exercise men; DEER 1998 no exercise wom), and Kuopio study (Kuopio Reduced & Mod 1993; Kuopio Reduced Fat 1993) are each referred to and counted as a single study, although they appear as individual arms in analyses and in the validity table (suggesting 36 intervention arms).
We included 14 adult cohorts (20 published papers, cohorts presented their results in from one to eight main analyses, 39 analyses in total) which reported on baseline total fat intake and reported on a measure of body fatness at least one year later. Eleven cohorts reported change in weight, BMI and/or waist circumference over the course of the follow‐up, while three cohorts reported absolute weight or BMI at follow‐up. Follow‐up was from one year to over 16 years (median five years). Most cohorts were of mixed sex, though one was men only and two women only. Recruitment included young people (13 years and over in one mixed cohort although most participants recruited were adults, 18 years and over in fully adult cohorts), middle aged and elderly adults (up to 75 years at baseline). Cohorts were recruited in North America (eight cohorts), Europe (five cohorts) and Australia (one).
The 11 included cohorts that recruited children and young people were followed for one to 23 years (median four years). They were reported in 13 published papers, and provided 101 separate analyses. The cohorts recruited children aged from two years to 14 years (although one study, Viva La Familia, may have recruited four‐ to 19‐year olds, so included a few young people older than 14 at baseline), and followed up until later in childhood or early adulthood. Five were based in North America, three in Europe, two in Australia and one in Korea.
The table of characteristics of the adult cohort studies, along with their references, is found in Table 2, and of cohorts of children and young people in Table 3.
1. Characteristics and results of included cohort studies in adults (all or a majority of participants recruited as adults).
| Study | Participants at baseline | + / 0 / ‐ | Results and/or estimate of effect? |
|
CARDIA Ludwig 1999 (1) USA |
2909 healthy black and white young adults Baseline age: 18 to 30 yrs Follow‐up: 10 yrs %E from fat: unclear (lower quintile < 30, upper > 41.7) BMI: unclear |
+ (weight) in black men and women 0 (weight) in white men and women |
Adjusted means of 10‐year body weight according to quintiles of total fat as a percentage of total energy. P for trend 0.32 in white men and women (quintile 1 weight 168.6 lb, quintile 5 weight 169.4 lb), 0.03 for black men and women (quintile 1 weight 182.1 lb, quintile 5 weight 185.7 lb) |
|
Danish Diet Cancer & Health Study Halkjaer 2009 (2‐4) Denmark |
22,570 women and 20,126 men Baseline age: 50 to 64 yrs Follow‐up: 5 yrs %E from fat: unclear (approx 32% in women, 33% in men) BMI: median 24.7 women, 26.1 men |
0 (Δ waist) women 0 (Δ waist) men |
Association between total fat intake at baseline and change in waist circumference over 5 years suggested no statistically significant effects in women (mean change in waist circumference ‐0.03 cm/MJ/d total fat, 95% CI ‐0.20 to 0.14) or men (mean change in waist circumference 0.06 cm/MJ/d total fat, 95% CI ‐0.05 to 0.17) |
| 12,353 women and 10,080 men Baseline age: 50 to 60 yrs Follow‐up: 5 yrs %E from fat: median 33.8% women, 35.2% in men BMI: median 24.4 women, 25.8 men |
0 (Δ waist circumference) 0 (Δ body weight) |
Macronutrient energy substitution where energy from protein was replaced by fat or carbohydrate. Multiple linear regression investigated the association between dietary protein in relation to change in body weight or waist circumference over 5 years. No statistically significant effect of replacing 5%E from fat with protein on change in body weight (8.0 g/year, 95% CI ‐16.6 to 32.5, P value = 0.525) or waist circumference (0.1 mm/year, 95% CI ‐0.3 to 0.4, P value = 0.799) | |
|
Danish MONICA Iqbal 2006 (5) Denmark |
900 women and 862 men Baseline age: 30 to 60 yrs Follow‐up: 5 yrs %E from fat: 43.8% (SD 6.5 women, 42.7 (SD 6.3) men BMI: 23.4 (SD 3.7 women, 25.1 (SD 3.3) men |
0 (Δ weight) women 0 (Δ weight) men |
Regression assessment of total fat as %E and other dietary factors as a function of change in body weight suggested no significant effects of %E from fat on 5‐year change in body weight in women (unadjusted beta 0.47, SE 0.89, P value = 0.60, adjusted beta 0.86, SE 0.92, P value = 0.35) or men (unadjusted beta ‐0.14, SE 0.69, P value = 0.84, adjusted beta 0.11, SE 0.69, P value = 0.87) |
|
Diabetes Control & Complications Trial (DCCT) & EDIC Cundiff 2012 (6) USA |
1055 women and men with diabetes, HbA1c ≤ 9.5 Baseline age: 13 to 39 yrs (mean 27.4) Follow‐up: 14 to 19 yrs (mean 16.4 yrs) %E from fat: 36.2% (90% CI 26.6 to 45.1) BMI: 23.4 (90% CI 19.4 to 27.9) |
0 (Δ BMI/year) | Multiple regression analyses generated the formula linking macronutrient intake and exercise at baseline with change in BMI per year. Univariate analyses suggested no relationship between total fat (as %E) and change in BMI per year (β 0.04 kg/m2/year, P value = 0.22), and only total fat minus polyunsaturated fat (%E, not total fat) was included in the formula predicting BMI change per year |
|
EPIC‐PANACEA Vergnaud 2013 (7) Europe (10 countries) EPIC Beulens 2014 (8) Europe (15 cohorts) |
373,803 men and women from the general European population Baseline age: 25 to 70 yrs Follow‐up: 5 yrs (2 to 11) %E from fat: mean 35.4 (SD unclear) BMI: mean 25.6 women, 26.7 men (SDs unclear) |
0 (Δ weight) when replacing fat with CHO in women or men ‐ (Δ weight) when replacing fat with protein in women or men |
Multivariate substitution models were performed to estimate weight change associated with replacement of 5%E of one macronutrient with another. 5% greater proportion of E from fat at the expense of carbohydrate was not associated with weight change in women or men (P value = 0.36, P value = 0.73). Replacing 5%E from protein with fat was associated with weight reduction in women (β 0.4 kg/5 years, P value < 0.0001) and men (β 0.3 kg/5 years, P value = 0.003) |
| 6192 people with type 2 diabetes Baseline age: unclear Follow‐up: 5 yrs %E from fat: unclear BMI: unclear |
‐ (Δ weight) when replacing CHO with total fat | Linear regression was used to explore the relationship between replacement of CHO with total fat (and also MUFA and PUFA) and 5‐year weight change. This is an abstract so results reported as "5‐year weight change decreased when carbohydrates were substituted with total fat" (no further details) | |
|
Health Professionals Follow‐Up Study (HPFUS) Coakley 1998 (9) USA |
19,478 male health professionals Baseline age: 45 to 75 yrs Follow‐up: 4 yrs %E from fat: unclear, energy adjusted fat intake mean 69.6 g/d (SD 13.8) BMI: unclear |
+ (Δ weight) 45 to 54 yrs men + (Δ weight) 55 to 64 yrs men 0 (Δ weight) 65+ yrs men |
Multivariate regression analyses determined whether total fat intake and other habits were predictive of 4‐year weight change, and found that a change of adjusted fat intake of 10 g/d predicted 0.10 kg of weight change over 4 years (P value < 0.001 for ages 45 to 54 and 55 to 64 years, P value > 0.05 for age 65+) |
|
Melbourne Collaborative Cohort Study (MCCS) MacInnis 2013 (10) Australia |
5879 healthy Australian‐born non‐smokers Baseline age: 40 to 69 yrs Follow‐up: 11.7 yrs %E from fat: 33% (SD 6) women, 33 (SD 5) men BMI: unclear |
+ (weight) overall + (waist circumference) overall + (weight) 40 to 49 yrs 0 (weight) 50 to 59 yrs 0 (weight) 60 to 69 yrs + (waist) 40 to 49 yrs + (waist) 50 to 59 yrs 0 (waist) 60 to 69 yrs |
Multivariable linear regression was used to predict waist circumference and weight at 12‐year follow‐up. Higher percentage of energy from fat at baseline was associated with weight (0.26 kg per 10%E from fat, P value = 0.03) and waist circumference (0.85 cm per 10%E from fat, P value < 0.001) in the whole sample. When assessed in age bands, total fat was associated with weight in those aged 40 to 49 years at baseline (P value = 0.002), but not in those aged 50 to 59 (P value = 0.94) or 60 to 69 years (P value = 0.79), and with waist circumference in those aged 40 to 49 (P value < 0.001) and 50 to 59 (P value = 0.01), but not in those aged 60 to 69 (P value = 0.14) |
|
Memphis Klesges 1992 (11‐13) USA |
152 women and 142 men (Caucasian health professionals) Baseline age: 24 to 52 yrs Follow‐up: 2 yrs %E from fat: mean 36.8 (SD 6.1) women, 36.0 (SD 5.4) men BMI: mean 24.8 (SD 5.0) women, 27.8 (SD 4.3) men |
+ (Δ weight) women 0 (Δ weight) men 0 (Δ waist) women ‐ (Δ waist) men |
Stepwise multivariate regression analyses assessed whether various lifestyle factors were predictive of weight change over 2 years. Percentage of energy as fat was predictive of weight change in women (coefficient 0.53, SE 0.16, P value = 0.0010) but not in men (exact data not provided) Hierarchical linear regression assessed the effects of lifestyle factors on change in waist circumference over 2 years, and found no significant effect in women (coefficient ‐0.04, P value = 0.50) but a statistically significant negative relationship in men (coefficient ‐0.05, P value = 0.04) |
|
NHANES Follow‐up Kant 1995 (14) USA |
4567 women and 2580 men Baseline age: 25 to 74 yrs Follow‐up: mean 10.6 (SD 5) yrs %E from fat: mean 36.4 (SD 5.0) women, 37.0 (SD 10.1) men BMI: mean 25.2 (SD 5.0) women, 25.9 (SD 5.0) men |
+ (Δ weight) < 50 yrs women 0 (Δ weight) 50+ yrs women 0 (Δ weight) < 50 yrs men 0 (Δ weight) 50+ yrs men |
Univariate regression analyses assessed whether fat as %E is predictive of 10‐year weight change and found no significant effects in women (Beta ‐0.011, SE 0.017, P value = 0.51) or men (Beta 0.043, SE 0.022, P value = 0.06). Effects were similar in multivariate regression in women (Beta ‐0.033, SE 0.019, P value = 0.08 for women overall, Beta ‐0.053, SE 0.025, P value = 0.04 for women aged < 50 yrs, Beta ‐0.019, SE 0.030, P value = 0.55 for women aged 50+) or men (Beta 0.021, SE 0.022, P value = 0.33 for men overall, Beta ‐0.004, SE 0.028, P value = 0.88 for men aged < 50 yrs, Beta ‐0.058, SE 0.035, P value = 0.10 for men aged 50+) |
|
Nurses' Health Study Colditz 1990 (15) Field 2007 (16) USA |
31,940 women (nurses) Baseline age: 30 to 55+ Follow‐up: 8 yrs %E from fat: unclear BMI: unclear |
0 (Δ weight) women | Correlation between total fat (g/d) and weight gain over subsequent 4 years (beta ‐0.0007, t ‐0.4), not statistically significant |
| 41,518 women (nurses) Baseline age: 41 to 68 yrs (mean 53.7, SD 7.1 yrs) Follow‐up: 8 yrs %E from fat: 32.8 (SD 5.6) BMI: 25.0 (SD 4.5) |
? unclear (Δ weight) women | Association between a 1% difference in total fat as %E and weight change (in pounds over 8 years) was modelled using linear regression. There was a weak relationship between total fat and weight change (β 0.11 lb/1% total fat difference, P value < 0.0001 stated in text, but no statistical significance indicated in table) | |
|
Pawtucket HHP Parker 1997 (17) USA |
289 women and 176 men Baseline age: 18 to 64 yrs Follow‐up: 4 yrs %E from fat: unclear BMI: mean 26.5 (SD 5.0) |
0 (Δ weight) women and men | Multiple regression assessed association of weight change with different nutrients at baseline. Found no effect of total fat in grams on weight change over 4 years (coefficient 2.30, P value = 0.71) |
|
San Luis Valley Diabetes Study (SLVDS) Mosca 2004 (18) USA |
433 women and 349 men ‐ non‐diabetic, Hispanic and non‐Hispanic white Baseline age: 20 to 74 yrs Follow‐up: 14 yrs %E from fat: mean 38.3 (SD 8.9) white women, 37.2 (8.9) Hispanic women, 38.9 (8.7) white men, 37.8 (9.8) Hispanic men BMI: mean 24.3 (SD 4.4) white women, 25.0 (4.6) Hispanic women, 25.7 (3.3) white men, 24.7 (3.8) Hispanic men |
+ (Δ weight) overall (includes women and men, Hispanic and non‐Hispanic white) | Linear mixed model (random‐effects, PROC MIXED in SAS) was used to assess whether those who generally consume a relatively high fat diet gain more weight over time. They found a significant association between %E from total fat and weight change between participants (β 0.012, P value = 0.0178) after adjusting for potential confounders |
|
SEASONS Ma 2005 (19) USA |
275 healthy women and 297 healthy men Baseline age: 20 to 70 yrs Follow‐up: 1 yr %E from fat: mean 36.7 (SD 9.0) BMI: mean 27.4 (SD 5.5) |
0 (BMI) women and men – with no energy adjustment | Regression analyses to assess effects of total fat %E on BMI. Longitudinal effect was not statistically significant (coefficient 0.005, P value = 0.07) |
|
Women’s Gothenburg Lissner 1997 (20) Sweden |
361 women Baseline age: 38 to 60 yrs Follow‐up: 6 yrs %E from fat: mean 34.1 (SD 4.0) lower fat group, 42.3 (SD 3.0) higher fat group BMI: mean 24.6 (SD 4.1) lower fat group, 24.1 (SD 4.1) higher fat group |
+ (Δ weight) sedentary 0 (Δ weight) moderate 0 (Δ weight) active |
Multivariate regression used to test for interactive effects of dietary fat intake on weight change over 6 years. A significant effect of high vs low %E from fat was found in sedentary women (high fat women gained 2.64 kg while low fat women lost 0.64 kg over 6 years, P value = 0.03) but this was lost with further energy adjustment. No effects were seen in more active women (2 categories), where those with low and high fat intakes all gained 1 to 2 kg on average |
Key:
+ = positive relationship found between fat intake and weight outcome.
0 = no relationship found between fat intake and weight outcome.
‐ = negative (inverse) relationship found between fat intake and weight outcome.
Abbreviations: BMI: body mass index; CHO: carbohydrates; CI: confidence interval; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; SD: standard deviation; SE: standard error.
References for this table:
(1) Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery MI, et al. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA 2006;282:1539‐46.
(2) Halkjaer J, Tjonneland A, Thomsen BL, Overvad K, Sorensen TIA. Intake of macronutrients as predictors of 5‐y changes in waist circumference. American Journal of Clinical Nutrition 2006;84:789‐97.
(3) Halkjaer J, Tjonneland A, Overvad K, Sorensen TIA. Dietary predictors of 5‐year changes in waist circumference. Journal of the American Dietetic Association 2009;109(8):1356‐66.
(4) Ankarfeldt MZA. Interactions of dietary protein and adiposity measures in relation to subsequent changes in body weight and waist circumference. Obesity 2014;22(9):2097‐103.
(5) Iqbal SI, Helge JW, Heitmann BL. Do energy density and dietary fiber influence subsequent 5‐year weight changes in adult men and women? Obesity (Silver Spring) 2006;14:106‐14.
(6) Cundiff DK, Raghuvanshi N. Future body mass index modelling based on macronutrient profiles and physical activity. Theoretical Biology & Medical Modelling 2012;9:43.
(7) Vergnaud A‐CN. Macronutrient composition of the diet and prospective weight change in participants of the EPIC‐PANACEA Study. PLoS One 2013;8(3).
(8) Beulens JWJ. Dietary fat intake in low‐carbohydrate diets and subsequent mortality and weight change in type 2 diabetes. Diabetologia 2014;57(Suppl 1):S311.
(9) Coakley EH, Rimm EB, Colditz GA, Kawachi I, Willett WC. Predictors of weight change in men: results from the health professionals follow‐up study. International Journal of Obesity (Lond) 1998;22:89‐96.
(10) MacInnes RJ, Hodge AM, Dixon HG, Peeters A, Johnson LEA, English DR, et al. Predictors of increased body weight and waist circumference for middle‐aged adults. Public Health Nutrition 2013;17(5):1087‐97.
(11) Eck LH, Pascale RW, Klesges RC, White Ray JA, Klesges LM. Predictors of waist circumference change in healthy young adults. International Journal of Obesity (Lond) 1995;19:765‐9.
(12) Klesges RC, Isbell TR, Klesges LM. Relationship between dietary restraint, energy intake, physical activity, and body weight: a prospective analysis. Journal of Abnormal Psychology 1992;101:668‐74.
(13) Klesges RC, Klesges LM, Haddock CK, Eck LH. A longitudinal analysis of the impact of dietary intake and physical activity on weight change in adults. American Journal of Clinical Nutrition 1992;55:818‐22.
(14) Kant AK, Graubard BI, Schatzkin A, Ballard‐Barbash R. Proportion of energy intake from fat and subsequent weight change in the NHANES I Epidemiologic Followup Study. American Journal of Clinical Nutrition 1995;61:11‐7.
(15) Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. American Journal of Clinical Nutrition 1990;51:1100‐5.
(16) Field AE, Willett WC, Lissner L, Colditz GA. Dietary fat and weight gain among women in the Nurses' Health Study. Obesity (Silver Spring) 2007;15(4):967‐76.
(17) Parker DR, Gonzalez S, Derby CA, Gans KM, Lasater TM, Carleton RA. Dietary factors in relation to weight change among men and women from two southeastern New England communities. International Journal of Obesity (Lond) 1997;21:103‐9.
(18) Mosca CL, Marshall JA, Grunwald GK, Cornier MA, Baxter J. Insulin resistance as a modifier of the relationship between dietary fat intake and weight gain. International Journal of Obesity (Lond) 2004;28:803‐12.
(19) Ma Y, Olendzki BC, Chiriboga D, Hebert JR, Li Y, Li W, et al. Association between dietary carbohydrates and body weight. American Journal of Epidemiology 2005;161:359‐67.
(20) Lissner L, Heitmann BL, Bengtsson C. Low‐fat diets may prevent weight gain in sedentary women. Obesity Research 1997;5(1):43‐8.
2. Characteristics and results of included cohort studies in children and young people (including all cohorts where assessment began in childhood or adolescence).
| Study | Participants at baseline | + / 0 / ‐ | Results and/or estimate of effect |
|
Adelaide Nutrition Study Magarey 2001 (1) Australia |
243 boys and girls Age: diet analysed at 2, 4, 6, 8, 11, 13 and 15 years old Follow‐up: assessed for each gap (e.g. 2 to 4 years, 2 to 6 years, 2 to 8 years, 4 to 6 years etc), 2 to 13 years %E from fat: boys aged 2 yrs 38.4 (SD 5.8), girls aged 2 38.1 (SD 13.4), boys aged 15 33.2 (SD 5.6), girls aged 15 yrs 34.4 (SD 5.6) BMI: boys aged 2 yrs 16.8 (SD 1.7), girls aged 2 16.5 (SD 1.4), boys aged 15 20.2 (SD 2.6), girls aged 15 yrs 21.4 (SD 4.1) |
0 (BMI) for 20 of 21 possible age gaps 0 (triceps skinfold) for 21 of 21 possible age gaps 0 (sub‐scapular skinfold) for 20 of 21 possible age gaps |
Single dietary assessment for each of 21 analyses Analysis: multiple regression analysis was used to predict whether body fatness at a specific age was predicted by macronutrient intake at previous ages. For BMI only one of 21 possible gaps showed a statistically significant relationship between total fat intake as a percentage of energy and later BMI (a significant relationship, P value < 0.01, was only seen between fat at age 6 and BMI at age 8). For triceps skinfold none of 21 possible gaps showed a statistically significant relationship between total fat intake as a percentage of energy and later triceps skinfold. For subscapular skinfold only one of 21 possible gaps showed a statistically significant relationship between total fat intake as a percentage of energy and later sub‐scapular skinfold (a significant relationship, P value < 0.01, was only seen between fat at age 2 and skinfold at age 15) |
|
Amsterdam Growth & Health Long. Study (AGAHLS) Twisk 1998, Koppes 2009 (2;3) Netherlands |
83 boys (then men) and 98 girls (then women) Age: recruited aged 13, diet analysed at ages 13, 14, 15, 16, 21, 27 Follow‐up: 14 yrs (age 27) %E from fat: not reported BMI: boys aged 13 yrs 17.3 (SD 1.6), girls 18.1 (SD 2.1), men aged 27 yrs 22.6 (SD 2.2), women 21.9 (SD 2.5) |
0 (sum of 4 skinfolds) 0 (BMI) Both for absolute fat intake and %E from fat |
Multiple dietary assessments Analysis: first order auto‐regressive model (fatness at each time point related to exposure at the previous time point) estimated by generalised estimating equations. There was no relationship between total fat intake (absolute, g/d) and later fatness as assessed by sum of four skinfolds (P value = 0.41) or BMI (P value = 0.23), or between fat intake as %E and later fatness as assessed by sum of four skinfolds (P value = 0.92) or BMI (P value = 0.69) |
| 168 boys (then men) and 182 girls (then women) Age: recruited aged 13 (SD 0.7), diet analysed at ages 13, 14, 15, 16, 21, 27, 32, 36 Follow‐up: 23 yrs (age 36) %E from fat: not reported BMI: as above |
0 (high %body fat at age 36), 0 of 14 analyses 0 (% body fatness) in men or women |
Multiple dietary assessments Analysis: generalised estimating equation regression analyses found that dietary fat intake (%E) at ages 13, 14, 15, 16, 21, 27 or 32 did not predict high body fatness (> 25% for men, > 35% for women, assessed by DEXA at 36 years) in either men or women (in any of 7 analyses in men or 7 in women). Regression coefficients using all available data gathered between ages 13 and 36 found no relationship between %E from fat and sum of skinfolds in either men (P value = 0.42) or women (P value = 0.89) |
|
|
Bogaert 2003 (4) Australia |
29 boys and 30 girls Age: recruited aged 6 to 9 yrs, mean 8.6 (SE 0.2) yrs Follow‐up: at 6 and 12 mo %E from fat: 33.5 (SD 0.8) in boys aged < 8 yrs, 31.7 (SD 2.7) girls < 8 yrs, 37.5 (SD 1.2) boys aged 8+ yrs, 33.6 (SD 1.7) girls aged 8+ yrs BMI: z scores boys mean 0.3 (SE 0.1), girls mean 0.5 (SE 0.3) |
0 (Δ BMI) |
Single dietary assessment Analysis: correlations were calculated to assess the relation between %E from fat at baseline and BMI z‐score change from baseline to 12 months. No "positive relation" was found |
|
Carruth and Skinner 2001 (5;6) USA |
29 white boys and 24 girls Age: recruited at 24 months, diet assessed at 24 to 32, 28 to 36, 42, 48, 54, 60 months old Follow‐up: body fat assessed at 70 months %E from fat: 31% boys, 32% girls at 27 months, 31% boys, 33% girls at 60 months BMI: 15.7 (SD 1.2) in boys and 15.4 (SD 1.0) in girls at 60 months |
+ (%body fat) + (g body fat) |
Multiple dietary assessments Analysis: regression analyses (general linear models) of total fat intake (averaging over 6 dietary assessments aged 27 to 60 months) predicted body fat at 70 months (assessed as %body fat, P value = 0.02 and grams of body fat, P value = 0.01, both assessed by DEXA) |
| 37 white boys and 33 girls Age: recruited at 24 months (except 2 joined at 1 year, 6 joined at 2 years from similar study), diet assessed at 2.0, 2.3, 2.7, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, 8.0 yrs old Follow‐up: BMI assessed at 8 yrs %E from fat: mean 32% (SD not stated) BMI: 16.5 in boys and 16.2 in girls at 2 yrs, 16.8 in boys and 17.1 in girls at 8 yrs |
+ (BMI) by g/d of fat + (BMI) by %E from fat |
Multiple dietary assessments Analysis: forward stepwise regression was used to assess the relationship between dietary fat (averaged from 9 sets of 3‐day dietary data from ages 2 to 8) and BMI at age 8 years. Whether assessing fat as g/d (P value = 0.004) or %E from fat (P value = 0.010) there was a significant relationship (adjusted for BMI at 2 years and adiposity rebound age) |
|
|
Davison 2001 (7) USA |
197 non‐Hispanic white girls Age: 5.4 (0.4) yrs Follow‐up: 2 yrs (age 7.3 ±0.3) %E from fat: 31 (SD unclear) BMI: 15.8 (1.4) |
+ (Δ BMI) |
Single dietary assessment Analysis: in hierarchical regression models, girls' fat intake (as %E) at 5 yrs had a significant relationship with change in BMI from 5 to 7 years, P value = 0.02 |
|
Etude Longitud. Alimentation Nutrition Croissance des Enfants (ELANCE) Rolland‐Cachera 2013 (8) France |
40 boys and 33 girls whose diets were assessed at 2 yrs Age: 2 yrs Follow‐up: 18 years (age 20) %E from fat: 31.9 (SD 5.7) boys, 32.8 (SD 4.5) girls BMI: unclear |
0 (BMI) 0 (% triceps skinfold) ‐ (% sub‐scapular skinfold) ‐ (fat mass) |
Single dietary assessment (for this analysis) Analysis: association between dietary intake at 2 years and adult body composition was analysed using linear regression models. No statistically significant relationships were found between %E from fat at 2 years and BMI (P value = 0.23), % triceps skinfold (P value = 0.19), or fat‐free mass (P value = 0.98) at age 20. Greater total fat intake predicted lower % subscapular skinfold (P value = 0.03) and fat mass (P value = 0.04). All data presented from the adjusted models |
|
European Youth Heart Study Brixval 2009 (9) Denmark |
171 girls and 137 boys (but total of 384 stated also, numbers vary between tables) Age: boys 9.7 (SD 0.4) yrs, girls 9.6 (SD0.4) yrs Follow‐up: 6 years (age 15 to 16) %E from fat: 32.1 (SD 6.6) boys, 33.3 (SD 6.7) girls BMI: 17.1 (SD 2.0) boys, 17.2 (SD 2.4) girls |
0 (Δ BMI z‐score) boys 0 (Δ BMI z‐score) girls |
Single dietary assessment. Analysis: examined the associations between dietary fat intake at 9 years and subsequent 6‐year weight development using regression analysis. None of the regression models (various levels of adjustment) suggested that fat %E was associated with change in BMI over 6 years (in boys P value = 0.27, girls P value = 0.75 in the most adjusted model) |
|
Klesges 1995 (10) USA |
110 boys and 93 girls Age: 3 to 5 yrs (boys 4.4 (0.5), girls 4.3 (0.5) Follow‐up: 2 yrs %E from fat: boys and girls 33.0 (5.0) BMI: boys 16.1 (1.4), girls 16.1 (1.2) |
0 /+ /0/0 (Δ BMI) |
Multiple dietary assessments Analysis: assessed whether baseline %E from fat, change from baseline to 1 year, 1 yr to 2 yrs, or baseline to 2 yrs (along with other variables) predicted change in BMI over 2 yrs Multiple regression analysis suggested lower baseline %E from fat correlated to lower BMI change (regression coefficient = 0.034, P value = 0.05 – marginal significance) at 2 yrs, 0.17 k/m2per 5% more E from fat Change in %E from fat over the last year was correlated with BMI change (regression numbers not legible, probably P value = 0.01), 0.20 kg/m2 per 5%E from fat change. Change in %E from fat from baseline to 1 yr, and baseline to 2 yrs did not predict change in BMI |
|
Obesity & Metabolic Disorders Cohort in Children (OMDCC) Lee 2012 (11) Korea |
1504 1st and 4th grade children Age: 7.3 (SD 0.3) in 1st graders, 10.0 (SD 0.4) years in 4th graders Follow‐up: 2 years %E from fat: 26.6 (SD 4.9) in 1st graders, 25.2 (SD 5.1) in 4th graders BMI: 16.0 (SD 2.3) in 1st graders, 18.1 (SD 3.0) in 4th graders |
0 (Δ BMI) |
Single dietary assessment Multiple linear regression modelling assessed relationships between baseline environmental factors, parental and lifestyle habits and change in BMI over 2 years. They found no statistically significant relationship between fat intake and change in BMI over 2 years (P value = 0.104) |
|
Trial of Activity for Adolescent Girls (TAAG) Cohen 2014 (12) USA |
265 girls in 8th grade Age: mean 13.9 (SD 0.4) yrs Follow‐up: 2 and 3 yrs %E from fat: unclear BMI: mean 22.1 (SD 5.2) |
0 (BMI percentile) ‐ (% body fat) |
Single dietary assessment Multivariable random coefficients model designed to examine whether habitual physical activity, diet and environmental exposure were predictive of future weight gain or percentage body fat. The multivariate model found no relationship between fat calories at baseline and BMI percentile (P value = 0.16), but suggested a reduction in % body fat associated with increased fat calories (P value = 0.03) |
|
Viva la Familia Study Butte 2007 (13) USA |
1030 Hispanic boys and girls (unclear how many of each) Age: unclear, 4 to 19 yrs? Follow‐up: 1 yr %E from fat: 34.0 (6.0) BMI: not stated |
+ (Δ weight) |
Single dietary assessment Analysis: %E from fat was positively correlated with 1 yr weight gain (kg/y). For 798 participants generalised estimating equations (GEE) suggested coefficient 0.044, SD 0.018, P value = 0.014 |
Key:
+ = positive ss relationship found between fat intake and weight outcome.
0 = no ss relationship found between fat intake and weight outcome.
‐ = negative (inverse) ss relationship found between fat intake and weight outcome.
Abbreviations: BMI: body mass index; DEXA: dual energy X‐ray absorptiometry; SD: standard deviation; SE: standard error; ss: statistically significant
References for this table:
(1) Magarey AM, Daniels LA, Boulton TJC, Cockington RA. Does fat intake predict adiposity in healthy children and adolescents aged 2‐15 y? A longitudinal analysis. European Journal of Clinical Nutrition 2001;55:471‐81.
(2) Twisk JWR, Kempner HCG, van Mechelen W, Post GB, van Lenthe FJ. Body fatness: longitudinal relationship of body mass index and the sum of skinfolds with other risk factors for coronary heart disease. International Journal of Obesity (Lond) 1998;22:915‐22.
(3) Koppes LLJ, Boon N, Nooyens ACJ, van Mechelen W, Saris WHM. Macronutrient distribution over a period of 23 years in relation to energy intake and body fatness. British Journal of Nutrition 2009;101:108‐15.
(4) Bogaert N, Steinbeck KS, Baur LA, Brock K, Bermingham MA. Food, activity and family ‐ environmental vs biochemical predictors of weight gain in children. European Journal of Clinical Nutrition 2003;57:1242‐9.
(5) Carruth BR, Skinner JD. The role of dietary calcium and other nutrients in moderating body fat in preschool children. International Journal of Obesity (Lond) 2001;25:559‐66.
(6) Skinner JD, Bounds W, Carruth BR, Morris M, Ziegler P. Predictors of children's body mass index: a longitudinal study of diet and growth in children aged 2‐8 years. International Journal of Obesity (Lond) 2004;28:476‐82.
(7) Davison KK, Birch LL. Child and parent characteristics as predictors of change in girls' body mass index. International Journal of Obesity (Lond) 2001;25:1834‐42.
(8) Rolland‐Cachera MF, Maillot M, Deheeger M, Souberbielle JC, Peneau S, Hercberg S, et al. Association of nutrition in early life with body fat and serum leptin at adult age. International Journal of Obesity 2013 Aug;37(8):1116‐22.
(9) Brixval CS, Anderson LB, Heitmann BL. Fat intake and weight development from 9 to 16 years of age: the European Youth Heart Study ‐ a Longitudinal Study. Obesity Facts 2009;3:166‐70.
(10) Klesges RC, Klesges LM, Eck LH, Shelton ML. A longitudinal analysis of accelerated weight gain in preschool children. Pediatrics 1995;95:126‐30.
(11) Lee HH, Park HA, Kang JH, Cho YG, Park JK, Lee R, et al. Factors related to body mass index and body mass index change in Korean children: preliminary results from the obesity and metabolic disorders cohort in childhood. Korean Journal of Family Medicine 2012 May;33(3):134‐43.
(12) Cohen DAG. Energy balance in adolescent girls: The trial of activity for adolescent girls cohort. Obesity (Silver Spring) 2014;22(3):772‐80.
(13) Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: The Viva la Familia Study. American Journal of Clinical Nutrition 2007;85:1478‐85.
Excluded studies
Reasons for exclusion of the 345 adult RCTs that we read in full text but excluded from this review are found in Characteristics of excluded studies. Reasons for exclusion of child RCTs are found in Table 4, adult cohort studies in Table 5, and child cohort studies in Table 6, along with their references.
3. Excluded child RCTs.
| Study | Reason for exclusion |
| Alexy U, Reinehr T, et al. (2006). Positive changes of dietary habits after an outpatient training program for overweight children. Nutrition Research 26(5): 202‐8 | Weight loss intention |
| Amesz EMS. Optimal growth and lower fat mass in preterm infants fed a protein‐enriched postdischarge formula. Journal of Pediatric Gastroenterology and Nutrition. 2010;50(2):200‐7 | Includes infants |
| Anand SS, Davis AD, et al. (2007). A family‐based intervention to promote healthy lifestyles in an aboriginal community in Canada. Canadian Journal of Public Health Revue Canadienne de Sante Publique. 98(6): 447‐52 | Weight loss intention |
| Angelopoulos PD, Milionis HJ, et al. (2009). Changes in BMI and blood pressure after a school based intervention: the CHILDREN study. European Journal of Public Health 19(3): 319‐25 | Multifactorial intervention |
| Burrows TJ. Long‐term changes in food consumption trends in overweight children in the HIKCUPS intervention. Journal of Pediatric Gastroenterology and Nutrition. 2011;53(5):543‐7 | All obese or overweight at baseline |
| Dal Molin Netto B, Landi Masquio DC, Da Silveira Campos RM, De Lima Sanches P, Campos Corgosinho F, Tock L, et al. The high glycemic index diet was an independent predictor to explain changes in agouti‐related protein in obese adolescents. Nutricion Hospitalaria. 2014;29(2):305‐14 | Obese adolescents |
| Evans RK, Franco RL, et al. (2009). Evaluation of a 6‐month multi‐disciplinary healthy weight management program targeting urban, overweight adolescents: effects on physical fitness, physical activity, and blood lipid profiles. International Journal of Pediatric Obesity 4(3): 130‐3 | Multifactorial intervention, weight loss goal |
| Forneris T, Fries E, et al. (2010). Results of a rural school‐based peer‐led intervention for youth: goals for health. Journal of School Health 80(2): 57‐65 | No relevant outcomes |
| Garnett SPB. Researching Effective Strategies to Improve Insulin Sensitivity in Children and Teenagers ‐ RESIST. A randomised control trial investigating the effects of two different diets on insulin sensitivity in young people with insulin resistance and/or pre‐diabetes. BMC Public Health. 2010;10(pp 575):2010. 2. Garnett SPD. Optimum macronutrient content of the diet for adolescents with pre‐diabetes; RESIST a randomised control trial ACTRN12608000416392. Endocrine Reviews. 2012;Conference(var.pagings) | All obese or overweight at baseline |
| Hernandez TLA. Women with gestational diabetes randomised to a low‐carbohydrate/higher fat diet demonstrate greater insulin resistance and infant adiposity. Diabetes. 2013;Conference(var.pagings):July | Effect on infants |
| Horan MKM. The association of maternal characteristics and macronutrient intake in pregnancy with neonatal body composition. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2014;Conference(var.pagings):June | Infants |
| Jebb SA, Frost G, et al. (2007). The RISCK study: Testing the impact of the amount and type of dietary fat and carbohydrate on metabolic risk. Nutrition Bulletin 32(2): 154‐6 | Design paper |
| Kaitosaari T, Ronnemaa T, et al. (2006). Low‐saturated fat dietary counselling starting in infancy improves insulin sensitivity in 9‐year‐old healthy children: the Special Turku Coronary Risk Factor Intervention Project for Children (STRIP) study. Diabetes Care 29(4): 781‐5 | No relevant outcomes |
| Lagstrom H, Hakanen M, et al. (2008) Growth patterns and obesity development in overweight or normal‐weight 13‐year‐old adolescents: the STRIP study. Pediatrics 122(4): e876‐83 | No relevant exposures |
| Mirza NM, Palmer MG, Sinclair KB, McCarter R, He J, Ebbeling CB, et al. Effects of a low glycemic load or a low‐fat dietary intervention on body weight in obese Hispanic American children and adolescents: a randomised controlled trial. American Journal of Clinical Nutrition. 2013;97(2):276‐85 | All obese at baseline |
| Mobley CCS. Effect of nutrition changes on foods selected by students in a middle school‐based diabetes prevention intervention program: The HEALTHY experience. Journal of School Health. 2012;82(2):82‐90 | No total fat intake assessment |
| Niinikoski H, Lagstrom H, Jokinen E, Siltala M, Ronnemaa T, Viikari J, et al. Impact of repeated dietary counselling between infancy and 14 years of age on dietary intakes and serum lipids and lipoproteins: the STRIP study. Circulation. 2007;116(9):1032‐40 | Aim to reduce saturated fat not total fat |
| Ramon‐Krauel MS. A low‐glycemic‐load versus low‐fat diet in the treatment of fatty liver in obese children. Childhood Obesity. 2013;9(3):252‐60 | All obese at baseline |
| Shalitin S, Ashkenazi‐Hoffnung L, et al. (2010). Effects of a twelve‐week randomised intervention of exercise and/or diet on weight loss and weight maintenance, and other metabolic parameters in obese preadolescent children. Hormone Research 72(5): 287‐301 | Weight loss/unsuitable exposures |
| Sharma SF. One‐year change in energy and macronutrient intakes of overweight and obese inner‐city African American children: Effect of community‐based Taking Action Together type 2 diabetes prevention program. Eating Behaviors. 2012;13(3):271‐4 | All obese or overweight at baseline |
| Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, et al. Nutrition in infancy and long‐term risk of obesity: evidence from 2 randomised controlled trials. American Journal of Clinical Nutrition. 2010;92(5):1133‐44 | Infants |
| Thakwalakwa C, Ashorn P, Phuka J, Cheung YB, Briend A, Puumalainen T, et al. A lipid‐based nutrient supplement but not corn‐soy blend modestly increases weight gain among 6‐ to 18‐month‐old moderately underweight children in rural Malawi. Journal of Nutrition 2010;140(11):2008‐13 | Duration < 26 weeks |
| Williamson DA, Han H, Johnson WD, Martin CK, Newton RL, Jr. Modification of the school cafeteria environment can impact childhood nutrition. Results from the Wise Mind and LA Health studies. Appetite. 2013;61(1):77‐84 | Weight loss aimed |
| Williamson DA, Copeland AL, et al. (2007). Wise Mind project: a school‐based environmental approach for preventing weight gain in children. Obesity 15(4): 906‐17 | Multifactorial intervention |
4. Excluded adult cohort studies.
| Study | Reason for exclusion |
| Adams T, Rini A (2007). Predicting 1‐year change in body mass index among college students. Journal of American College Health 55(6): 361‐5 | No relevant exposures |
| Aerenhouts D, Deriemaeker P, Hebbelinck M, Clarys P, Aerenhouts D, Deriemaeker P, et al. Energy and macronutrient intake in adolescent sprint athletes: a follow‐up study. Journal of Sports Sciences. 2011;29(1):73‐82 | No relationship between total fat and body fatness |
| Ahluwalia N, Ferrieres J, et al. (2009). Association of macronutrient intake patterns with being overweight in a population‐based random sample of men in France. Diabetes & Metabolism 35(2): 129‐36 | Invalid study design |
| Aljadani HM, Patterson A, Sibbritt D, Hutchesson MJ, Jensen ME, Collins CE. Diet quality, measured by fruit and vegetable intake, predicts weight change in young women. Journal of Obesity. 2013;2013:525161 | No relevant outcomes |
| Almoosawi S, Prynne CJ, Hardy R, Stephen AM. Time‐of‐day and nutrient composition of eating occasions: prospective association with the metabolic syndrome in the 1946 British birth cohort. International Journal of Obesity. 2013;37(5):725‐31 | No total fat assessment |
| Al‐Sarraj T, Saadi H, et al. (2010). Metabolic syndrome prevalence, dietary intake, and cardiovascular risk profile among overweight and obese adults 18‐50 years old from the United Arab Emirates. Metabolic Syndrome & Related Disorders 8(1): 39‐46 | Cross‐sectional study |
| Althuizen E, van Poppel MN, de Vries JH, Seidell JC, van MW, Althuizen E, et al. Postpartum behaviour as predictor of weight change from before pregnancy to one year postpartum. BMC Public Health. 2011;11:165 | Total fat assessment is not baseline |
| Bailey BWS. Dietary predictors of visceral adiposity in overweight young adults. British Journal of Nutrition. 2010;103(12):1702‐5 | Cross‐sectional |
| Berg CM, Lappas G, et al. (2008). Food patterns and cardiovascular disease risk factors: the Swedish INTERGENE research program. American Journal of Clinical Nutrition 88(2): 289‐97 | Invalid study design |
| Bes‐Rastrollo M, van Dam RM, et al. (2008) Prospective study of dietary energy density and weight gain in women. American Journal of Clinical Nutrition 88(3): 769‐77 | Not total fat to body fatness |
| Black MHW. High‐fat diet is associated with obesity‐mediated insulin resistance and beta‐cell dysfunction in Mexican Americans. Journal of Nutrition. 2013;143(4):479‐85. 2. Black MHW. Variants in PPARG interact with high‐fat diet to influence longitudinal decline in beta‐cell function in Mexican Americans at risk for type 2 diabetes (T2D). Diabetes. 2014;Conference(var.pagings):June | Not prospective |
| Bujnowski D, Xun P, Daviglus ML, Van HL, He K, Stamler J, et al. Longitudinal association between animal and vegetable protein intake and obesity among men in the United States: the Chicago Western Electric Study. Journal of the American Dietetic Association. 2011;111(8):1150‐5 | No total fat intake assessment |
| Carvalho LKB. Annual variation in body fat is associated with systemic inflammation in chronic kidney disease patients Stages 3 and 4: A longitudinal study. Nephrology Dialysis Transplantation. 2012;27(4):1423‐8 | No total fat assessment and chronic kidney disease |
| Castellanos DC, Connell C, Lee J. Factors affecting weight gain and dietary intake in Latino males residing in Mississippi: a preliminary study. Hispanic Health Care International. 2011;9(2):91‐8 | Cross‐sectional |
| Chang A, Van Horn L, Jacobs Jr DR, Liu K, Muntner P, Newsome B, et al. Lifestyle‐related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) Study. American Journal of Kidney Diseases. 2013;62(2):267‐75 | Assesses dietary patterns |
| Chopra VP. Dietary factors affecting weight gain in midlife women. FASEB Journal. 2013;Conference(var.pagings):April | All overweight or obese at baseline |
| de Groot S, Post MW, Snoek GJ, Schuitemaker M, van der Woude LH. Longitudinal association between lifestyle and coronary heart disease risk factors among individuals with spinal cord injury. Spinal Cord. 2013;51(4):314‐8 | No total fat assessment |
| de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125(14):1735‐41 | No body fatness outcomes |
| Dujmovic M, Kresic G, Mandic ML, Kenjeric D, Cvijanovic O, Dujmovic M, et al. Changes in dietary intake and body weight in lactating and non‐lactating women: prospective study in northern coastal Croatia. Collegium Antropologicum. 2014;38(1):179‐87 | Follow‐up < 1 year |
| Eghtesadi SS‐K. Dietary patterns predicting changes in obesity indices (BMI,WC,WHR) in longitudinal Tehran lipid and glucose study. Annals of Nutrition and Metabolism. 2013;Conference(var.pagings):2013 | No total fat intake assessment |
| Erber E, Hopping BN, Grandinetti A, Park SY, Kolonel LN, Maskarinec G. Dietary patterns and risk for diabetes: the multiethnic cohort. Diabetes Care. 2010;33(3):532‐8 | No total fat intake assessment and no body fatness outcomes |
| Ericson U, Rukh G, Stojkovic I, Sonestedt E, Gullberg B, Wirfalt E, et al. Sex‐specific interactions between the IRS1 polymorphism and intakes of carbohydrates and fat on incident type 2 diabetes. American Journal of Clinical Nutrition. 2013;97(1):208‐16 | Cross‐sectional |
| Hairston KGV. Lifestyle factors and 5‐year abdominal fat accumulation in a minority cohort: The IRAS family study. Obesity. 2012;20(2):421‐7 | No total fat intake assessment |
| Heppe DHMV. Maternal milk consumption, fetal growth, and the risks of neonatal complications: The Generation R Study. American Journal of Clinical Nutrition. 2011;94(2):501‐9 | Fetal growth assessment |
| Holmberg S, Thelin A, Holmberg S, Thelin A. High dairy fat intake related to less central obesity: a male cohort study with 12 years' follow‐up. Scandinavian Journal of Primary Health Care. 2013;31(2):89‐94 | No total fat intake assessment |
| Ibe YT. Food groups and weight gain in Japanese men. Clinical Obesity. 2014;4(3):157‐64 | No relationship between total fat and body fatness assessed |
| Jaacks LMG. Age, period and cohort effects on adult body mass index and overweight from 1991 to 2009 in China: The China Health And Nutrition Survey. International Journal of Epidemiology. 2013;42(3):828‐37 | No total fat intake assessment |
| Jaakkola JH. Eating behavior influences diet, weight, and central obesity in women after pregnancy. Nutrition. 2013;29(10):1209‐13 | No total fat intake assessment |
| Jarvandi S, Gougeon R, Bader A, Dasgupta K, Jarvandi S, Gougeon R, et al. Differences in food intake among obese and non‐obese women and men with type 2 diabetes. Journal of the American College of Nutrition. 2011;30(4):225‐32 | Cross‐sectional |
| Johns DJ, Ambrosini GL, Jebb SA, Sjöström L, Carlsson LMS, Lindroos AK. Tracking of an energy‐dense, high saturated fat, low‐fibre dietary pattern, foods and nutrient composition over 10 years in the severely obese. Journal of Human Nutrition & Dietetics. 2011;24(4):391‐2. 2. Johns DJ, Lindroos AK, Jebb SA, Sjostrom L, Carlsson LM, Ambrosini GL, et al. Tracking of a dietary pattern and its components over 10‐years in the severely obese. PLoS One [Electronic Resource]. 2014;9(5):e97457 | No relevant outcomes |
| Kimokoti RWG. Dietary patterns of women are associated with incident abdominal obesity but not metabolic syndrome. Journal of Nutrition. 2012;142(9):1720‐7. 2. Kimokoti RWN. Diet quality, physical activity, smoking status, and weight fluctuation are associated with weight change in women and men. Journal of Nutrition. 2010;140(7):1287‐93 | No total fat intake assessment |
| Kirk JK, Craven T, Lipkin EW, Katula J, Pedley C, O'Connor PJ, et al. Longitudinal changes in dietary fat intake and associated changes in cardiovascular risk factors in adults with type 2 diabetes: the ACCORD trial. Diabetes Research & Clinical Practice. 2013;100(1):61‐8 | Compares PEP score, not total fat |
| Ko GTC, Chan JCN, et al. (2007). Associations between dietary habits and risk factors for cardiovascular diseases in a Hong Kong Chinese working population‐‐the "Better Health for Better Hong Kong" (BHBHK) health promotion campaign. Asia Pacific Journal of Clinical Nutrition 16(4): 757‐65 | No relevant exposures |
| Laatikainen T, Philpot B, Hankonen N, Sippola R, Dunbar JA, Absetz P, et al. Predicting changes in lifestyle and clinical outcomes in preventing diabetes: The Greater Green Triangle Diabetes Prevention Project. Preventive Medicine. 2012;54(2):157‐61 | No relevant outcomes |
| Manios Y, Kourlaba G, Grammatikaki E, Androutsos O, Ioannou E, Roma‐Giannikou E, et al. Comparison of two methods for identifying dietary patterns associated with obesity in preschool children: the GENESIS study. European Journal of Clinical Nutrition. 2010;64(12):1407‐14 | Cross‐sectional |
| Meidtner KF. Variation in genes related to hepatic lipid metabolism and changes in waist circumference and body weight. Genes and Nutrition. 2014;9(2) | No total fat intake assessment |
| Mejean C, Macouillard P, Castetbon K, Kesse‐Guyot E, Hercberg S, Mejean C, et al. Socio‐economic, demographic, lifestyle and health characteristics associated with consumption of fatty‐sweetened and fatty‐salted foods in middle‐aged French adults. British Journal of Nutrition. 2011;105(5):776‐86 | No total fat intake assessment |
| Mirmiran PB. Association between dietary phytochemical index and 3‐year changes in weight, waist circumference and body adiposity index in adults: Tehran Lipid and Glucose study. Nutrition and Metabolism. 2012(9):108 | No assessment of total fat on body fatness |
| Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, Teede HJ, et al. The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Human Reproduction. 2013;28(8):2276‐83 | Cross‐sectional |
| Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new‐onset diabetes. American Journal of Clinical Nutrition. 2010;92(6):1350‐8 | No body fatness outcomes |
| Naniwadekar AS. Nutritional assessment of patients with chronic pancreatitis and impact of dietary advice. Gastroenterology. 2010;Conference(var.pagings):S393 | Pancreatitis patients |
| Neeland IJT. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA ‐ Journal of the American Medical Association. 2012;308(11):1150‐9 | No total fat intake assessment |
| Niu J, Seo DC, Niu J, Seo DC. Central obesity and hypertension in Chinese adults: a 12‐year longitudinal examination. Preventive Medicine. 2014;62:113‐8 | No relevant outcomes |
| Noori N, Dukkipati R, Kovesdy CP, Sim JJ, Feroze U, Murali SB, et al. Dietary omega‐3 fatty acid, ratio of omega‐6 to omega‐3 intake, inflammation, and survival in long‐term hemodialysis patients. American Journal of Kidney Diseases. 2011;58(2):248‐56 | No total fat assessment and haemodialysis patients |
| Plotnikoff RC, Karunamuni N, et al. (2009) An examination of the relationship between dietary behaviours and physical activity and obesity in adults with type 2 diabetes. Canadian Journal of Diabetes 33(1): 27‐34 | No relevant exposures |
| Qi QR. Consumption of branched chain amino acids and risk of coronary heart disease in us men and women. Circulation. 2013;Conference(var.pagings) | No total fat intake on weight assessment |
| Quatromoni PA, Pencina M, Cobain MR, Jacques PF, D'Agostino RB. Dietary quality predicts adult weight gain: findings from the Framingham Offspring Study. Obesity (Silver Spring, Md). 2006;14(8):1383‐91 | No relevant outcomes |
| Rautiainen SW. Dairy consumption and risk of becoming overweight or obese in middle‐aged and older women. Circulation. 2014;Conference(var.pagings):25 | No total fat intake assessment |
| Rukh G, Sonestedt E, Melander O, Hedblad B, Wirfalt E, Ericson U, et al. Genetic susceptibility to obesity and diet intakes: association and interaction analyses in the Malmo Diet and Cancer Study. Genes & Nutrition. 2013;8(6):535‐47 2. Rukh GS. Genetic susceptibility for obesity increases the risk of type 2 diabetes and is modified by macronutrient intakes. Diabetologia. 2010;Conference(var.pagings):September 3. Rukh GS. Genetic susceptibility to obesity associates with type 2 diabetes and interacts with dietary intake to predispose for obesity. Obesity Reviews. 2010;Conference(var.pagings):July | Not prospective |
| Sammel MD, Grisson JA, Freeman EW, Hollander L, Liu L, Liu S, et al. Weight gain among women in the late reproductive years. Family Practice 2003; 20: 401–9 | No total fat assessment |
| Sanchez‐Villegas A, Bes‐Rastrollo M, Martinez‐Gonzalez MA, Serra‐Majem L. Adherence to a Mediterranean dietary pattern and weight gain in a follow‐up study: the SUN cohort. International Journal of Obesity 2006; 30: 350–8 | No relevant outcomes |
| Sayon‐Orea CB‐R. Longitudinal association between yogurt consumption and weight gain, and the risk of overweight/obesity: The SUN cohort study. Obesity Facts. 2014;Conference(var.pagings):May | No total fat intake assessment |
| Scholz U, Ochsner S, Hornung R, Knoll N, Scholz U, Ochsner S, et al. Does social support really help to eat a low‐fat diet? Main effects and sex differences of received social support within the Health Action Process Approach. Applied Psychology. 2013;Health and Well‐being. 5(2):270‐90 | All obese or overweight at baseline |
| Schulz M, Kroke A, Liese AD, Hoffmann K, Bergmann MM, Boeing H. Food groups as predictors for short‐term weight changes in men and women of the EPIC Potsdam cohort. Journal of Nutrition 2002; 132: 1335–40 | No total fat assessment |
| Sherafat‐Kazemzadeh R, Egtesadi S, Mirmiran P, Gohari M, Farahani SJ, Esfahani FH, et al. Dietary patterns by reduced rank regression predicting changes in obesity indices in a cohort study: Tehran Lipid and Glucose Study. Asia Pacific Journal of Clinical Nutrition. 2010;19(1):22‐32.2. Sherafat‐Kazemzadeh R, Egtesadi S, Mirmiran P, Hedayati M, Gohari M, Vafa M, et al. Predicting of changes in obesity indices regarding to dietary patterns in longitudinal Tehran lipid and glucose study. Iranian Journal of Endocrinology & Metabolism. 2010;12(2):197 | No assessment of total fat on body fatness |
| Simpson A, Maynard V, Simpson A, Maynard V. A longitudinal study of the effect of Antarctic residence on energy dynamics and aerobic fitness. International Journal of Circumpolar Health. 2012;71:17227 | No total fat intake assessment |
| Tanisawa KI. Strong influence of dietary intake and physical activity on body fatness in elderly Japanese men: age‐associated loss of polygenic resistance against obesity. Genes and Nutrition. 2014;9(5) | Cross‐sectional |
| Threapleton DE, Greenwood DC, Burley VJ, Aldwairji M, Cade JE, Threapleton DE, et al. Dietary fibre and cardiovascular disease mortality in the UK Women's Cohort Study. European Journal of Epidemiology. 2013;28(4):335‐46 | No total fat intake assessment |
| Vadiveloo M, Scott M, Quatromoni P, Jacques P, Parekh N, Vadiveloo M, et al. Trends in dietary fat and high‐fat food intakes from 1991 to 2008 in the Framingham Heart Study participants. British Journal of Nutrition. 2014;111(4):724‐34. 2. Vadiveloo MS. Increases in dietary fat intake among the Framingham heart study participants: Trends from 1991‐2008. Circulation. 2012;Conference(var.pagings) | No assessment of total fat on body fatness |
| Verheijden MW, van der Veen JE, van Zadelhoff WM, Bakx C, Koelen MA, van den Hoogen HJ, et al. Nutrition guidance in Dutch family practice: behavioral determinants of reduction of fat consumption. American Journal of Clinical Nutrition. 2003;77(4 Suppl):1058s‐64s | No relevant outcomes |
| Wang HT. Longitudinal association between dairy consumption and changes of body weight and waist circumference: The Framingham Heart Study.International Journal of Obesity. 2014;38(2):299‐305 | No total fat intake assessment |
| Wolongevicz DM, Zhu L, Pencina MJ, Kimokoti RW, Newby PK, D'Agostino RB, et al. Diet quality and obesity in women: the Framingham Nutrition Studies. British Journal of Nutrition. 2010;103(8):1223‐9 | No relevant outcomes |
| Yadav VM. Effects of a low fat plant based diet in multiple sclerosis (MS): results of a 1‐year long randomised controlled (RC) study. Neurology. 2014;Conference(var.pagings) | Multiple sclerosis patients |
| Yin JQ. Maternal diet, breastfeeding and adolescent body composition: A 16‐year prospective study. European Journal of Clinical Nutrition. 2012;66(12):1329‐34 | No total fat intake assessment |
| Yoshimura YK. Relations of nutritional intake to age, sex and body mass index in Japanese elderly patients with type2 diabetes: The Japanese Elderly Diabetes Intervention Trial. Geriatrics and Gerontology International. 2012;12(SUPPL.1):29‐40 | Cross‐sectional |
| Younossi ZMS. Prevalence and independent predictors of non‐alcoholic fatty liver disease (NAFLD) in lean U.S population. Hepatology. 2011;Conference(var.pagings):October | NAFLD |
| Yuan BD. Study on transition of dietary patterns in Jiangsu province, 1989‐2009, China. FASEB Journal. 2011;Conference(var.pagings):April. 2. Yuan BD. Nutrition transition in Jiangsu, China, 1989‐2009. Annals of Nutrition and Metabolism. 2013;Conference(var.pagings):2013 | No total fat intake assessment |
| Zamora D, Gordon‐Larsen P, Jacobs DR, Jr., Popkin BM, Zamora D, Gordon‐Larsen P, et al. Diet quality and weight gain among black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study (1985‐2005). American Journal of Clinical Nutrition. 2010;92(4):784‐93 | No assessment of total fat on body fatness |
| Zelber‐Sagi SL. Non‐alcoholic fatty liver disease (NAFLD) independently predicts type‐2 diabetes and pre‐diabetes during a seven‐year prospective follow‐up. Journal of Hepatology. 2012;Conference(var.pagings):April | No relevant outcomes |
5. Excluded child cohort studies.
| Study | Reason for exclusion |
| Alexy U, Libuda L, Mersmann S, Kersting M, Alexy U, Libuda L, et al. Convenience foods in children's diet and association with dietary quality and body weight status. European Journal of Clinical Nutrition. 2011;65(2):160‐6 | Not longitudinal |
| Ambrosini GLE. Identification of a dietary pattern prospectively associated with increased adiposity during childhood and adolescence. International Journal of Obesity (2005). 2012;36(10):1299‐305. 2.Ambrosini GLE. Tracking a dietary pattern associated with increased adiposity in childhood and adolescence. Obesity. 2014;22(2):458‐65. 3. Ambrosini GLL. An energy‐dense, high fat, low fibre dietary pattern is prospectively associated with greater adiposity in adolescent girls in the Avon longitudinal study of parents and children. Obesity Reviews. 2010;Conference(var.pagings):July | No total fat intake assessment |
| Barton AJ, Gilbert L, et al. (2006). Cardiovascular risk in Hispanic and non‐Hispanic preschoolers. Nursing Research 55(3): 172‐9 | Cross‐sectional study |
| Berz JP, Singer MR, Guo X, Daniels SR, Moore LL, Berz JPB, et al. Use of a DASH food group score to predict excess weight gain in adolescent girls in the National Growth and Health Study. Archives of Pediatrics & Adolescent Medicine. 2011;165(6):540‐6 | No total fat assessment |
| Bigornia SJL. Dairy intakes at age 10 years do not adversely affect risk of excess adiposity at 13 years. Journal of Nutrition. 2014;144(7):1081‐90 | No total fat assessment |
| Boreham C, Twisk J, van Mechelen W, Savage M, Strain J, Cran G. Relationships between the development of biological risk factors for coronary heart disease and lifestyle parameters during adolescence: The Northern Ireland Young Hearts Project. Public Health. 1999;113(1):7‐12 | No relevant outcomes |
| Burke V, Beilin LJ, Simmer K, Oddy WH, Blake KV, Doherty D, et al. Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study.International Journal of Obesity (Lond). 2005;29(1):15‐23 | No baseline fat intake |
| Burke V, Beilin LJ, et al. (2006). Television, computer use, physical activity, diet and fatness in Australian adolescents. International Journal of Pediatric Obesity 1(4): 248‐55 | Cross‐sectional study |
| Chaput J‐P, Tremblay A, et al. (2008). A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. American Journal of Clinical Nutrition 87(2): 303‐9 | No relevant exposures |
| Davis JN, Alexander KE, et al. Inverse relation between dietary fiber intake and visceral adiposity in overweight Latino youth. American Journal of Clinical Nutrition 2009; 90(5): 1160‐6 | Unsuitable analyses |
| Deshmukh UJ. Growth and body composition changes in Indian undernourished children. Annals of Nutrition and Metabolism. 2013;Conference(var.pagings):2013 | No relevant outcomes |
| Dubois L, Farmer A, et al. (2007). Regular sugar‐sweetened beverage consumption between meals increases risk of overweight among preschool‐aged children. Journal of the American Dietetic Association 107(6): 924‐34 | Invalid study design |
| Elliott SAT. Associations of body mass index and waist circumference with: energy intake and percentage energy from macronutrients, in a cohort of Australian children. Nutrition Journal. 2011;10(1) | Cross‐sectional |
| Enes CC, Slater B, Enes CC, Slater B. Variation in dietary intake and physical activity pattern as predictors of change in body mass index (BMI) Z‐score among Brazilian adolescents. Revista Brasileira de Epidemiologia. 2013;16(2):493‐501 | Not prospective |
| Faith MS, Dennison BA, et al. (2006). Fruit juice intake predicts increased adiposity gain in children from low‐income families: weight status‐by‐environment interaction. Pediatrics 118(5): 2066‐75 | No relevant exposures |
| Frohnert BIJ. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes. 2013;62(9):3163‐9 | No total fat assessment |
| Heppe DH, Kiefte‐de Jong JC, Durmus B, Moll HA, Raat H, Hofman A, et al. Parental, fetal, and infant risk factors for preschool overweight: the Generation R Study. Pediatric Research. 2013;73(1):120‐7 | No total fat intake assessment |
| Hooley M, Skouteris H, Millar L, Hooley M, Skouteris H, Millar L. The relationship between childhood weight, dental caries and eating practices in children aged 4‐8 years in Australia, 2004‐2008. Pediatric Obesity. 2012;7(6):461‐70 | No total fat intake assessment |
| Hopkins DS. The effect on growth of using cows milk as the main drink for infants. Annals of Nutrition and Metabolism. 2011;Conference(var.pagings):October | Infants |
| Huh SYR. Prospective association between milk intake and adiposity in preschool‐aged children. Journal of the American Dietetic Association. 2010;110(4):563‐70 | No total fat intake assessment |
| Humenikova L, Gates GE (2007). Dietary intakes, physical activity, and predictors of child obesity among 4‐6th graders in the Czech Republic. Central European Journal of Public Health 15(1): 23‐8 | Cross‐sectional |
| Isharwal S, Arya S, et al. (2008). Dietary nutrients and insulin resistance in urban Asian Indian adolescents and young adults. Annals of Nutrition & Metabolism 52(2): 145‐51 | Invalid study design |
| Kagura J, Feeley AB, Micklesfield LK, Pettifor JM, Norris SA, Kagura J, et al. Association between infant nutrition and anthropometry, and pre‐pubertal body composition in urban South African children. Journal of Developmental Origins of Health and Disease. 2012;3(6):415‐23 | No total fat intake assessment |
| Khalil HM. Developmental trajectories of body mass index (BMI) from birth to late childhood and their relation with paternal and child nutrients intake. Obesity Facts. 2014;Conference(var.pagings):May | No relevant outcomes |
| Labayen I, Ruiz JR, Ortega FB, Huybrechts I, Rodríguez G, Jiménez‐Pavón D, et al. High fat diets are associated with higher abdominal adiposity regardless of physical activity in adolescents; the HELENA study. Clinical Nutrition. 2014;33(5):859‐66 | Cross‐sectional |
| Li SF. Dairy consumption with onset of overweight and obesity among U.S. adolescents.FASEB Journal. 2014;Conference(var.pagings) | No total fat intake assessment |
| Magnussen CG, Thomson R, Cleland VJ, Ukoumunne OC, Dwyer T, Venn A, et al. Factors affecting the stability of blood lipid and lipoprotein levels from youth to adulthood: evidence from the Childhood Determinants of Adult Health Study. Archives of Pediatrics & Adolescent Medicine. 2011;165(1):68‐76 | No relevant outcomes |
| Manios Y. (2006). Design and descriptive results of the "Growth, Exercise and Nutrition Epidemiological Study in preSchoolers": The GENESIS Study. BMC Public Health 6(32) | No fat to weight relationship |
| Mete MS. Dietary patterns and depression in a population with high prevalence of obesity: The strong heart family study. Circulation. 2012;Conference(var.pagings) | No total fat intake assessment |
| Millar L, Rowland B, Nichols M, Swinburn B, Bennett C, Skouteris H, et al. Relationship between raised BMI and sugar sweetened beverage and high fat food consumption among children. Obesity. 2014;22(5):E96‐103. 2. Millar LMR. Sugar sweetened beverage and high fat food consumption are related to raised BMI z‐scores among a cohort of Australian children from 4 to 10 years of age. Obesity Facts. 2013;Conference(var.pagings):May. | No total fat assessment |
| Oldewage‐Theron W, Napier C, Egal A. Dietary fat intake and nutritional status indicators of primary school children in a low‐income informal settlement in the Vaal region... [corrected] [published erratum appears in S AFR J CLIN NUTR 2011; 24(3):164]. South African Journal of Clinical Nutrition. 2011;24(2):99‐104 | Cross‐sectional |
| Pala VL. Dietary patterns and longitudinal change in body mass in European children: a follow‐up study on the IDEFICS multicenter cohort. European Journal of Clinical Nutrition. 2013;67(10):1042‐9 | No total fat intake assessment |
| Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB, et al. Changes in water and beverage intake and long‐term weight changes: results from three prospective cohort studies. International Journal of Obesity. 2013;37(10):1378‐85 | No total fat intake assessment |
| Puengputtho WL. Salt intake and salt reduction in secondary school‐age students of Princess Chulabhorn's College Chiangrai (Regional science school). Annals of Nutrition and Metabolism. 2013;Conference(var.pagings):2013 | No total fat intake on weight assessment |
| Riedel CV. Interactions of genetic and environmental risk factors with respect to body fat mass in children: Results from the ALSPAC study. Obesity. 2013;21(6):1238‐42 | No total fat intake assessment |
| Scharf RJ, Demmer RT, Deboer MD. Longitudinal evaluation of milk type consumed and weight status in preschoolers. Archives of Disease in Childhood. 2013;98(5):335‐40 | No total fat intake assessment |
| Serra‐Majem L, Aranceta‐Bartrina J, et al. Prevalence and determinants of obesity in Spanish children and young people. British Journal of Nutrition. 2006;96 Suppl 1: S67‐72 | Cross‐sectional |
| Vazaiou AP. Protein intake of toddlers in Greece and its nutritional consequences. Hormone Research in Paediatrics. 2011;Conference(var.pagings):October | No assessment of total fat on body fatness |
| Weijs PJM. High beverage sugar as well as high animal protein intake at infancy may increase overweight risk at 8 years: a prospective longitudinal pilot study. Nutrition Journal. 2011;10(1) | Infants |
| Williams CL, Strobino BA. Childhood diet, overweight, and CVD risk factors: the Healthy Start project. Preventive Cardiology. 2008;11(1):11‐20 | No relevant outcomes |
| Wosje KS, Khoury PR, Claytor RP, Copeland KA, Hornung RW, Daniels SR, et al. Dietary patterns associated with fat and bone mass in young children. American Journal of Clinical Nutrition. 2010;92(2):294‐303 | No total fat intake assessment |
| Yin JQ. Maternal diet, breastfeeding and adolescent body composition: A 16‐year prospective study. European Journal of Clinical Nutrition. 2012;66(12):1329‐34 | No total fat intake assessment |
| Zaki MH. Identifying obesogenic dietary factors among Egyptian obese adolescents. Annals of Nutrition and Metabolism. 2013;Conference(var.pagings):2013 | No relevant outcomes |
| Zhang ZG. Added sugar intake and lipids profile among us adolescents: Nhanes 2005‐2010. Circulation. 2014;Conference(var.pagings):25 | Cross‐sectional |
Risk of bias in included studies
To understand the risk of bias in the individual included RCTs in a visual way, see Figure 2. 'Risk of bias' assessments of included adult cohort analyses are found in Table 7, and of child and young people's cohort analyses in Table 8.
2.
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included adult and child RCT comparison.
6. Risk of bias of included adult cohort studies.
| Study | Number lost to follow‐up | Baseline similarity by total fat intake, funding, control groups | Adjustments (where stratified not counted as not being adjusted)* | Method of assessment | Risk of bias** |
|
CARDIA Ludwig 1999 (1) USA |
5111 attended original screening, 3609 attended at years 1, 7 and 10, 2909 included in analysis 43% lost or not analysed Reasons: exclusion of those who were pregnant or lactating, with diabetes, on lipid or BP medication or with extreme dietary factors |
Different. Those with lower total fat intake were more likely to be women, non‐smokers, more physically active, with higher alcohol and vitamin supplement intake Funded by: NHLBI, NIDDKD Control group: internal |
Weight was adjusted for baseline weight. Analysis adjusted for energy, sex, age, field centre, education, energy intake, physical activity, cigarette smoking, alcohol intake, vitamin supplement use. All adjusted for |
Interviewer‐ administered FFQ (700 foods) Single (multiple dietary assessments – but appear to use baseline data only in analysis) |
High |
|
Danish Diet Cancer & Health Study Halkjaer 2009 (2‐4) Denmark |
57,043 at baseline, 44,897 re‐assessed 5 years later 21% lost or not analysed Reasons: 1781 had died, 435 emigrated, remainder did not want to participate or did not reply |
Data not reported Unclear Funded by: National Danish Research Foundation, DiOGenes (EU funding) Control group: internal |
BMI, energy, age, smoking, alcohol, wine, beer, spirits, sporting activity Not adjusted for ethnicity, or socioeconomic status |
192‐item semi‐quantitative FFQ checked by dietitian Single dietary assessment used |
High |
| 57,053 at baseline, 22,433 included in 5‐year analysis. 61% lost or not analysed Reasons: excluded aged ≥ 60 years (baseline) or ≥ 65 years (follow‐up), did not attend follow‐up, illness at baseline or during follow‐up, average weight gain or loss > 5 kg/year or waist circumference > 7 cm/year, lack of blood sample or other baseline data |
Data not reported. Unclear Funded by: National Danish Research Foundation, DiOGenes (EU funding) Control group: internal |
Age, sex, physical activity, smoking, education, follow‐up time, fibre intake, glycaemic index, hormone treatment and baseline body weight or waist circumference (analysed as %E from fat, so adjusted for E) Not adjusted for ethnicity |
192‐item semi‐quantitative FFQ checked by dietitian Single dietary assessment used |
High | |
|
Danish MONICA Iqbal 2006 (5) Denmark |
2025 at baseline, 1762 re‐assessed 5 years later 13% lost or not analysed Reasons: missing or very high energy or unknown history of family obesity |
Data not reported Unclear Funded by: Apotekerfonden & Danish Ministry for Health Control group: internal |
Baseline BMI, age, physical activity, smoking, education level, cohort, volume, energy intake Not adjusted for ethnicity |
Weighed 7‐day food record Single dietary assessment used |
Moderate |
|
Diabetes Control & Complications Trial (DCCT) & EDIC Cundiff 2012 (6) |
1441 at baseline, 1055 analysed at 14 to 19 years 27% lost or not analysed Reasons: omitted 137 with HbA1c > 9.5, otherwise losses not described in this publication Note: also analysed FAO/WHO data from 167 countries, but these appear cross‐sectional |
Data not reported Unclear Funded by: Data collection by NIH, General Clinical Research Center Program (NCRR), analysis not funded Control group: internal |
Energy, fibre, saturated, mono‐ and poly‐unsaturated fat, alcohol, exercise (probably) Not adjusted for age, sex, ethnicity or SES |
1 week food record (unclear whether recall or diary based) Multiple dietary assessments (baseline, 2, 5 yrs and completion averaged) |
High |
|
EPIC‐PANACEA Vergnaud 2013 (7) EPIC Beulens 2014 (8) |
521,448 recruited, 373,803 included in analysis 28% lost or not analysed Reasons: omitted 23,713 with missing or implausible baseline data, 121,866 with missing follow‐up weight, 2066 with implausible weight changes |
Those with lower fat intake tended to be older, more physically active and less likely to smoke Dissimilar Funded by: EU and a wide range of charities and government funders Control group: internal |
Adjusted for age, baseline BMI, study centre, weekday, season, total E (from non‐alcohol sources, and from alcohol sources), smoking, education, physical activity Not adjusted for ethnicity |
Quant. dietary questionnaire of 88‐266 items (country‐specific) Single dietary assessment used |
High |
| Unclear how many were included compared with recruited unclear% lost or not analysed Reasons: unclear |
Data not reported Unclear Funded by: unclear Control group: internal |
Adjustments unclear Not adjusted for … unclear |
Country‐specific FFQs | High | |
|
Health Professionals Follow‐Up Study (HPFUS) Coakley 1998 (9) USA |
36,353 returned 1992 questionnaires, of whom 19,478 were included in this analysis 46% lost or not analysed Reasons: 9345 had cancer, heart disease, diabetes or stroke, 7530 were missing key information |
Data not reported Unclear Funded by: NIH and Centres for Disease Control Control group: internal |
Baseline weight, energy, height, activity, TV viewing, high BP, high cholesterol Not adjusted for ethnicity, socioeconomic status |
FFQ Single dietary assessment used |
High |
|
Melbourne Collaborative Cohort Study (MCCS) MacInnis 2013 (10) Australia |
Of 9066 at baseline, 5879 included in analyses. 35% lost or not analysed Reasons: 656 died, 1894 declined, 21 did not have waist circumference or weight at follow‐up, and 616 lost ≥ 5 kg weight so excluded |
Data not reported Unclear Funded by: Cancer Council Victoria, VicHealth, National Health and Medical Research Council Control group: internal |
Weight adjusted for baseline weight, waist for baseline waist circumference. All adjusted for sex, age, physical activity, alcohol, education, smoking, marital status, SES, total energy intake. Not adjusted for ethnicity (all described as "Australian‐born" but > 20% born in Europe) |
Self administered 121‐item FFQ developed for study Single dietary assessment used |
High |
|
Memphis Klesges 1992 (11‐13) USA |
417 were enrolled, 294 were included in weight change analysis, and 230 in the waist circumference change analysis 29% lost or not analysed (weight), 45% (waist) Reasons: "attrition" for weight change, no explanation of further losses for waist circumference data |
Data not reported Unclear Funded by: NHLBI and Tennessee Centres of Excellence Control group: internal |
Sex, age, pregnancy status, smoking, alcohol, family risk of obesity, energy intake, sports activity, work activity, leisure activity, change from baseline of energy, fat intake, activity, cigarettes Not adjusted for socioeconomic status |
Willett's FFQ Single (multiple dietary assessments – but appear to be using baseline data in analysis) |
High |
|
NHANES Follow‐up Kant 1995 (14) USA |
14,407 were enrolled and eligible, 7147 were included in analysis. 50% lost or not analysed Reasons: no dietary info, unsatisfactory 24‐hour recalls, atypical intake, proxies, mistakes, pregnant or lactating participants, lack of weight data, death |
Higher fat as %E associated with younger age, more smoking, higher levels of morbidity Funded by: unclear Control group: internal |
Baseline age, race, education, BMI, energy intake, smoking, physical activity, duration of follow‐up, alcohol, morbidity, special diet, parity All adjusted for |
24‐hour dietary recall Single dietary assessment used |
High |
|
Nurses' Health Study Colditz 1990 (15) Field 2007 (16) USA |
Of 121,700 women enrolled, 38,724 were eligible for this study, 31,940 women included in analyses 17% lost or not analysed Reasons: non‐respondent or invalid FFQ |
Data not reported Unclear Funded by: NIH Control group: internal |
Age, BMI, energy intake Not adjusted for ethnicity, physical activity, socioeconomic status |
61‐item FFQ Single dietary assessment used |
High |
| Of 121,700 women enrolled, 41,518 included in analyses 66% lost or not analysed Reasons: of 121,700, 41,518 assessed in 1986 and at 8 years, were free of cancer, hypertension and diabetes, and eligible for this study |
Greater fat intake associated with greater baseline weight Unclear Funded by: Boston Obesity Nutrition Research Center and National Cancer Institute Control group: internal |
Age, baseline BMI, activity, menopausal status, smoking, protein intake, change in protein intake Not adjusted for ethnicity or SES |
136‐item FFQ in 1986 Single dietary assessment used |
High | |
|
Pawtucket HHP Parker 1997 (17) USA |
Of 1081 enrolled, FFQ administered to random sub‐sample of 556, 465 included in analysis 16% lost or not analysed Reasons: those excluded were those who did not attend both relevant appointments, and were more male, less educated, less active, greater BMI |
Data not reported Unclear Funded by: NHLBI Control group: internal |
Age, BMI, energy, smoking, activity Not adjusted for sex, ethnicity or socioeconomic status |
Willett's FFQ with categories added for fats, oils, sweets, snacks and dairy products Single dietary assessment used |
High |
|
San Luis Valley Diabetes Study (SLVDS) Mosca 2004 (18) USA |
Of 1351 enrolled, 782 "included in analysis", unclear how many in prospective analysis unclear% lost or not analysed Reasons: unclear how many lost and how many excluded. Of 1351, 1027 had and 782 continued to have normal glucose tolerance tests, 140 altered smoking status or became pregnant and were excluded. 782 completed visit 1, 536 visit 2 and 375 visit 3 |
Data not reported Unclear Funded by: not stated Control group: internal |
Sex, ethnicity, physical activity, baseline BMI, age, smoking status, energy intake Not adjusted for SES |
24‐hour diet recall (bilingual interviewers) with visual aids for food portions | High |
|
SEASONS Ma 2005 (19) USA |
Of 1257 in original cohort, 641 completed baseline questionnaire and one blood draw, 572 included in analyses 11% lost or not analysed Reasons: unclear, did not attend further appointments |
Data not reported Unclear Funded by: NHLBI Control group: internal |
None (but analysed as %E from fat, so energy adjusted for indirectly) Not adjusted for age, sex, ethnicity, physical activity or socioeconomic status |
7‐day dietary recall Single (Multiple dietary assessments – but appear to be using baseline data in analysis) |
High |
|
Women's Gothenburg Lissner 1997 (20) Sweden |
Of 1462 in main cohort, 437 randomly selected and asked for dietary information, 361 included in analysis. 17% lost or not analysed Reasons: 64 did not return for weight assessment, 12 had chronic illness so excluded |
Higher fat as %E associated with younger age, higher energy intake, more walking and lifting at work, greater likelihood of being a smoker Funded by: Swedish Medical Research Council Control group: internal |
Baseline body weight, activity, smoking, age, energy Not adjusted for ethnicity or socioeconomic status |
Dietary interview including frequency of 69 food items Single dietary assessment used |
High |
*Of age, sex, energy intake, ethnicity, physical activity (and/or TV watching) and socioeconomic (which includes educational) status.
**Moderate risk of bias was suggested where < 20% were lost to follow‐up, up to two factors were unadjusted for in the design or analysis, and diet was assessed using a 24‐hour recall or diet diary. All other studies were at high risk of bias.
Reference numbers relate to references below Table 2.
Abbreviations: BMI: body mass index; BP: blood pressure; FAO: Food and Agriculture Organization; FFQ: food frequency questionnaire; NIH: National Institutes of Health; NHLBI: National Heart, Lung and Blood Institute; NIDDKD: National Institute of Diabetes and Digestive and Kidney Diseases; SES: socioeconomic status; WHO: World Health Organization
7. Risk of bias of included cohort studies in children and young people.
| Study | Number lost to follow‐up | Baseline similarity, funding, control group | Adjustments* | Method of dietary assessment | Risk of bias** |
|
Adelaide Nutrition Study Magarey 2001 (1) Australia |
Of 500 recruited to ANS at birth only 130 were seen at age 11, so a further 113 from a separate cohort were added at age 11 ˜74% lost (varied for different follow‐ups) Reason: did not attend Lost characteristics: not stated |
Data not reported Unclear Funded by: National Heart Foundation of Australia, Adelaide Children's Hospital Research Foundation, National Health and Medical Research Council of Australia Control group: internal |
Adjusted for energy intake, previous adiposity, adiposity of parent at a specific age Not adjusted for sex, ethnicity, physical activity or SES (4) |
3‐day weighed food record | High |
|
Amsterdam Growth & Health Long. Study (AGAHLS) Twisk 1998, Koppes 2009 (2;3) Netherlands |
Of 307 13‐year olds recruited 181 were reassessed at age 27 41% lost Reason: unclear Lost characteristics: "for the variables of interest no drop‐out effects were observed" |
Data not reported Unclear Funded by: Dutch Heart Foundation, Dutch Prevention Fund, Dutch Ministry of Wellbeing and Public Health, Dairy Foundation on Nutrition and Health, Netherlands Olympic Committee, Netherlands Sports Fed., no additional funding was stated for the 36‐year old analysis Control group: internal |
Adjusted for physical activity, smoking, alcohol, dietary energy and macronutrient intake. Did not adjust for sex, would have if appropriate. Not adjusted for ethnicity, parental BMI, or SES (3) |
Modified cross‐check dietary history interview relating to previous month | High |
| Of 698 13‐year olds recruited (those above plus another school with fewer assessments) 350 had complete data at age 36 50% lost Reason: unclear Lost characteristics: girls who completed follow‐up had slightly lower body fat %age, and boys who completed had lower tobacco and alcohol use at baseline |
Carried out for boys and girls separately, at each age. Skinfold data (not % body fat) additionally adjusted for physical activity Not adjusted for ethnicity, parental BMI, physical activity or SES (4) |
As above | High | ||
|
Bogaert 2003 (4) Australia |
Of 59 recruited, 41 were re‐assessed at 12 months 31% lost Reason: unclear Lost characteristics: unclear |
Data not reported Unclear Funded by: Australian Rotary Health Found., Financial Markets Found. for Children, National Health & Medical Research Council Control group: internal |
Adjustment not described (or not done) – unclear Assume not adjusted for age, sex, ethnicity, parental BMI, physical activity or SES (6) |
2 food records and 1 24‐hour recall from | High |
|
Carruth & Skinner 2001 (5;6) USA |
Of 72 recruited 53 took part at 70 months 26% lost Reason: 7 parents declined, 7 not in area, 5 could not be scheduled in timeframe Lost characteristics: unclear |
Data not reported Unclear Funded by: Gerber products, Tennessee Agricultural Experiment Station Control group: internal |
Adjusted for BMI (all children white and of same age) Not adjusted for sex, energy intake, parental BMI, physical activity or SES (5) |
3‐day dietary intake interviews by dietitian | High |
| 62 of 72 recruited (98 recruited at 2 mo of age), plus 2 added at 1 year and 6 added at 2 years took part unclear % lost Reason: as above? Lost characteristics: unclear |
Adjusted for BMI at 2 years and adiposity rebound age, assessed across ages 2 to 8, all children white and "predominantly middle or upper socioeconomic status" Factors assessed but found non‐significant so not adjusted for included sex, TV‐watching, parental BMI All adjusted for (0) |
3‐day dietary intake interviews | High | ||
| Davison 2001 (7) | 197 participants at study entry, 192 re‐assessed 2 years later 3% lost Reason: unclear Lost characteristics: none stated |
Data not reported Unclear Funded by: NIH Control group: internal |
BMI, levels of activity, familial risk of overweight, change in BMI (mother), enjoyment of activity (father), total energy intake (father), and girls' percentage fat intake (girls). Not adjusted for SES (1) |
24‐hour dietary recall | Moderate |
|
ELANCE Rolland‐Cachera 2013 (8) France |
Unclear how many 10‐month olds, but 222 attended at 10 months and either 2 or 4 years, 73 attended at 20 years, 68 included in analyses. > 67% lost Reason: unclear Lost characteristics: "similar" between those lost to follow‐up and those included |
Data not reported Unclear Funded by: Institut Benjamin Delessert Control group: internal |
Total energy intake, sex, breast feeding, mother's BMI, father’s occupation Not adjusted for ethnicity or physical activity (2) |
Dietary history (dietitian discussion of diet with parent over past month) | High |
|
European Youth Heart Study Brixval 2009 (9) Denmark |
384 of 589 baseline children attended follow‐up, 308 in regression model 48% lost Reason: "due to ethical consideration it was not permitted to contact subjects who decided not to participate at follow‐up" Lost characteristics: not stated |
Data not reported Unclear Funded by: not stated Control group: internal |
Age, puberty status, total energy intake, parental income, activity, overweight parents, protein intake, birth weight. Presented by sex Not adjusted for ethnicity (1) |
Interview and questionnaire of children and parents relating to past 24 hours | High |
|
Klesges 1995 (10) USA |
203 children at baseline, 146 at follow‐up 28% lost Reason: unclear Lost characteristics: "no significant differences" (P value > 0.15) in BMI, energy intake, fat as %E, physical activity, sex or familial obesity risk between those attending at 2 years and those not attending |
Data not reported Unclear Funded by: National Heart Lung and Blood Institute Control group: internal |
Age, sex, BMI, physical activity Not adjusted for ethnicity, SES (2) |
Dietary FFQ | High |
|
OMDCC Lee 2012 (11) Korea |
2740+ baseline children (unclear), 1504 followed up 45% lost Reasons: "analytic sample" – no reasons given Lost characteristics: unclear |
Data not reported Unclear Funded by: unclear Control group: internal |
Age, sex, sexual maturation, baseline BMI, exercise, TV time, sleep, parental BMI and education, energy intake, food habits and household income Not adjusted for ethnicity (1) |
24‐hour recall for 2 weekdays and 1 weekend day | High |
|
TAAG Cohen 2014 (12) |
Of 303 randomly selected at baseline, 265 analysed 13% lost Reasons: 38 did not have complete data Lost characteristics: no difference in race, age, mother's education |
Data not reported Unclear Funded by: National Heart Lung and Blood Institute Control group: internal |
Age, ethnicity, physical activity Not adjusted for energy intake, parental BMI or SES (3) |
FFQ | High |
|
Viva la Familia Study Butte 2007 (13) USA |
1030 at baseline, with 879 returning after 1 year 15% lost Reasons: unclear Lost characteristics: none stated |
Data not reported Unclear Funded by: NIH, USDA/ARS Control group: internal |
Adjusted for sex, age, age squared, and Tanner stage and BMI status in Generalised Estimating Equations Not adjusted for parental BMI, physical activity and SES (3) |
24‐hour recall, measured by a registered dietitian | High |
* Of age, sex, energy intake, ethnicity, parental BMI, physical activity (and/or TV watching) and socioeconomic (which includes educational) status
** Moderate risk of bias was suggested where < 20% were lost to follow‐up, up to three factors were unadjusted for in the design or analysis, and diet was assessed using a 24‐hour recall or diet diary. All other studies were at high risk of bias.
References are the same as those following Table 3.
Abbreviations: ANS: Adelaide Nutrition Study; BMI: body mass index; FFQ: food frequency questionnaire; NIH: National Institutes of Health; SES: socioeconomic status; USDA/ARS: US Department of Agriculture/ Agricultural Research Service.
Validity of RCTs
Allocation
Twenty‐two RCTs and the single child RCT, VYRONAS 2009, had low risk of bias from random sequence generation; the remainder were at unclear risk. Eleven adult RCTs and the single child RCT were at low risk of selection bias arising from poor or unclear allocation concealment or randomisation, one was at high risk (Sondergaard 2003), and the remaining RCTs were at unclear risk.
Blinding
There was a high risk of performance and detection bias due to lack of blinding (which is usual in dietary trials) in all included RCTs except the National Diet and Heart Studies (NDHS Open 1st L&M 1968; NDHS Open 2nd L&M 1968), which provided trial shops that blinded purchases of usual or low fat products.
Incomplete outcome data
For RCTs we assessed those studies that lost more than 5% of participants per year as at high risk of attrition bias; others were at low risk of attrition bias. Eight RCTs were at low risk of attrition bias, two were unclear and the remainder (including the one child RCT) at high risk.
Selective reporting
Most RCTs were at unclear risk of reporting bias (due to the paucity of accessible protocols, so that we could not assess reporting bias), but three adult RCTs were at low risk and one at high risk of bias. We examined the possible presence of reporting bias by using the list of included studies from a recent review of RCTs of the effects of reduced and modified fat on cardiovascular events (Hooper 2012b). Of 48 included RCTs in the other review, we included 21 in the current review. Of the remaining 27 RCTs, 10 did not compare reduced fat intake with usual fat intake (they were included as they modified fat compared with usual fat intake), 13 aimed to reduce weight in some or all participants and three included only participants with a high BMI. Only one trial was eligible for this review but was not included as no data were provided on any measure of body fatness (Toronto Polyp Prev 1994). The risk of reporting bias, related to the proportion of studies not included in a meta‐analysis, seems minimal here (Furukawa 2007).
Other potential sources of bias
We considered all the adult RCTs to be at low risk of other types of bias, but the child RCT, VYRONAS 2009, was felt to be at high risk due to individual randomisation in a school setting, which raised the issue of contamination of the intervention between intervention and control children. Eight adult RCTs had low risk of systematic differences in level of care between the intervention and control groups, while 24 had high risk of such differences in care, as did the child RCT. Differences in attention, training, time from health professionals, number of health checks and/or group support could potentially alter feelings of self efficacy and increase contact with healthcare professionals offering various types of support, and alter participants' ability to look after themselves and maintain a healthy weight. Some dietary interventions to reduce fat also had specific goals around fruit, vegetables, fibre, alcohol etc., which raises the possibility that any changes in weight may result from these alterations, not from change in fat intake. Ten adult RCTs and the child RCT were at high risk of effects from dietary differences other than fat; the remaining 22 RCTs were at low risk of effects from other dietary advice.
Validity of cohort studies
We considered the cohort studies to be at either moderate or high risk of bias. Moderate risk of bias was suggested where less than 20% were lost to follow‐up, two factors or fewer were unadjusted for in the design or analysis (of age, sex, energy intake, ethnicity, physical activity and/or TV watching and socioeconomic status (which includes educational status for adult cohorts), and diet was assessed using a 24‐hour recall or diet diary. For child cohorts factors assessed for adjustment included age, sex, energy intake, ethnicity, parental BMI, physical activity and/or TV watching) and socioeconomic factors, including educational status. We considered all other studies to be at high risk of bias.
We considered all adult cohort analyses to be at high risk of bias, apart from the MONICA study analysis. We likewise considered all cohort studies of children and young people to be at high risk of bias, except for Davison 2001, which was at moderate risk of bias. Cohort studies overall suffered from high dropout rates, lack of complete adjustment for relevant potential confounders and poor assessment of total fat intake.
Effects of interventions
See: Table 1
A 'Summary of findings' table assessing the effects of low dietary fat compared with usual fat for body weight in adults using randomised controlled trial (RCT) data is presented (Table 1).
Effects of reducing dietary fat on weight and body fatness in adults (as seen in RCTs)
Weight
Eating a lower proportion of energy as fat results in lower weight (or lower weight gain, or greater weight reductions) than eating the usual proportion of fat (‐1.5 kg, 95% confidence interval (CI) ‐2.0 to ‐1.1, 53,647 participants, 24 estimable comparisons, I2 = 77%, Analysis 1.1; Figure 3). The effect was small but statistically significant, and the best estimate of effect being a reduction in weight was consistent across 21 of the 24 comparisons with numerical data. Additionally, all of the six comparisons that did not have an estimable effect size, due to lack of variance data or large baseline differences, were consistent with greater weight reduction in the reduced fat arms (Figure 3). The same effect was reported in two of the three comparisons that were not included in the forest plot (as they provided insufficient information). The exception was Sondergaard 2003, which reported "in both groups, body weight remained unchanged after 12 months".
1.1. Analysis.
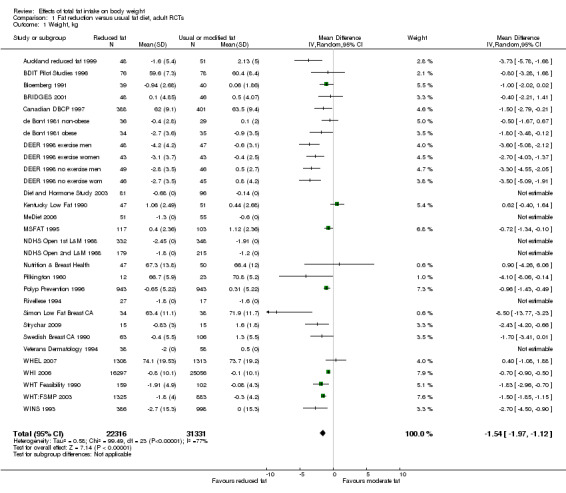
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 1 Weight, kg.
3.
Forest plot of comparison: 1 Fat reduction versus usual fat diet, adult RCTs, outcome: 1.1 Weight, kg.
The statistical significance of this relative weight reduction was not lost when we removed studies providing greater time or resources to the reduced fat group (‐1.3 kg, 95% CI ‐2.1 to ‐0.4), when we removed studies with additional dietary interventions (‐1.9 kg, 95% CI ‐2.6 to ‐1.3), when we used fixed‐effect meta‐analysis (rather than random‐effects analysis) (‐1.0 kg, 95% CI ‐1.2 to ‐0.9), when we removed the largest RCT (WHI 2006) (‐1.6 kg, 95% CI ‐2.1 to ‐1.2), or when we removed studies with high or unclear risk of selection bias (‐1.0 kg, 95% CI ‐1.4 to ‐0.5).
We examined the influence of potential effect modifiers through subgrouping (Table 9). There was a suggestion of a dose effect, with studies that reduced total fat in the intervention group by a greater amount compared with the control group showing greater reductions in weight (test for subgroup differences: P value = 0.003). Where the reduction in total fat was less than 5%E compared with control, weight loss was not statistically significant (mean difference (MD) ‐0.2 kg, 95% CI ‐0.9 to 0.6), but as the difference in total fat increased, weight reductions were seen (5%E to < 10%E from fat difference between intervention and control groups, MD ‐2.1 kg, 95% CI ‐2.9 to ‐1.4, and 10%E to < 15%E from fat difference, MD ‐1.3 kg, 95% CI ‐1.7 to ‐1.0). As few studies altered the %E from fat by 15% or more, power was limited so the suggested effect size was large but non‐significant (MD ‐3.9 kg, 95% CI ‐8.8 to 1.0). Similarly there was a suggestion that in low fat arms with greater reductions in energy intake there were greater relative falls in weight (test for subgroup differences: P value = 0.04).
8. Subgrouping: effects on weight of reducing fat.
| Factor assessed | Subgroup | Effect on weight, kg (95% CI) | Number of comparisons | Number of participants | I2 for subgroup | Chi2 test for subgroup differences |
| Duration of dietary advice | 6 to < 12 months | ‐1.7 (‐2.3 to ‐1.1) | 10 | 5305 | 71% | P value = 0.04 |
| 12 to < 24 months | ‐2.0 (‐2.5 to ‐1.5) | 17 | 51367 | 71% | ||
| 24 to < 60 months | ‐1.2 (‐1.7 to ‐0.7) | 9 | 49,286 | 56% | ||
| 60+ months | ‐0.7 (‐1.7 to 0.3) | 4 | 40,838 | 58% | ||
| Fat intake in the control group assessed during trial (equivalent to baseline fat intake) | > 35%E from fat | ‐0.9 (‐1.1 to ‐0.8) | 9 | 45,103 | 64% | P value < 0.00001 |
| > 30% to 35%E from fat | ‐0.8 (‐1.2 to ‐0.5) | 9 | 7123 | 73% | ||
| > 25% to 30%E from fat | ‐3.0 (‐3.6 to ‐2.3) | 5 | 2109 | 1% | ||
| Sex | Women only | ‐1.4 (‐1.9 to ‐0.9) | 15 | 50,154 | 72% | P value = 0.20 |
| Men only | ‐2.7 (‐4.3 to ‐1.2) | 4 | 1719 | 76% | ||
| Mixed men and women | ‐1.1 (‐2.0 to ‐0.2) | 5 | 2492 | 79% | ||
| Year of first publication of the trial | 1960s | ‐4.1 (‐8.1 to ‐0.1) | 1 | 1450 | ‐ | P value = 0.07 |
| 1970s | ‐ | 0 | 0 | ‐ | ||
| 1980s | ‐0.9 (‐1.8 to ‐0.01) | 3 | 288 | 0% | ||
| 1990s | ‐1.9 (‐2.6 to ‐1.3) | 14 | 5941 | 80% | ||
| 2000s | ‐0.9 (‐1.6 to ‐0.3) | 6 | 46,686 | 77% | ||
| 2010s | ‐ | 0 | 0 | ‐ | ||
| Difference in %E from fat between intervention and control groups | Up to 5%E from fat | ‐0.2 (‐0.9 to 0.6) | 5 | 4567 | 30% | P value = 0.003 |
| 5 to < 10%E from fat | ‐2.1 (‐2.9 to ‐1.4) | 11 | 44,356 | 84% | ||
| 10 to < 15%E from fat | ‐1.3 (‐1.7 to ‐1.0) | 4 | 8311 | 26% | ||
| 15+%E from fat | ‐3.9 (‐8.8 to 1.0) | 3 | 319 | 68% | ||
| Dietary advice or diet provided | Dietary advice | ‐1.6 (‐2.0 to ‐1.1) | 22 | 52,594 | 78% | P value = 0.04 |
| Diet provided | ‐0.7 (‐1.3 to ‐0.1) | 1 | 1741 | ‐ | ||
| Dietary fat goals for intervention (these were not necessarily achieved) | 30%E from fat | ‐1.0 (‐1.7 to ‐0.3) | 3 | 1628 | 0% | P value = 0.34 |
| 25 to < 30%E from fat | ‐2.5 (‐4.3 to ‐0.6) | 5 | 509 | 90% | ||
| 20 to < 25%E from fat | ‐0.9 (‐1.2 to ‐0.6) | 5 | 43,878 | 31% | ||
| 15 to < 20%E from fat | ‐1.3 (‐2.2 to ‐0.4) | 7 | 7860 | 58% | ||
| Total fat achieved in intervention group | > 30%E from fat | ‐0.8 (‐1.3 to ‐0.4) | 5 | 1767 | 0% | P value = 0.42 |
| ≤ 30%E from fat | ‐1.1 (‐1.6 to ‐0.6) | 13 | 50,099 | 76% | ||
| BMI at baseline (body mass index, kg/m2) | < 25 | ‐1.0 (‐1.7 to ‐0.2) | 8 | 1781 | 56% | P value = 0.17 |
| 25 to < 30 | ‐1.8 (‐2.4 to ‐1.3) | 15 | 51,297 | 83% | ||
| 30+ | ‐1.8 (‐3.5 to ‐0.1) | 1 | 69 | ‐ | ||
| Baseline health of participants | Healthy | ‐1.0 (‐1.6 to ‐0.4) | 3 | 45,032 | 87% | P value = 0.12 |
| With risk factors | ‐2.2 (‐3.2 to ‐1.2) | 12 | 2166 | 79% | ||
| With disease | ‐1.2 (‐1.9 to ‐0.6) | 9 | 6449 | 44% | ||
| Amount of energy reduction in the low fat arm | E intake the same or greater in low fat group | ‐0.5 (‐1.5 to 0.5) | 4 | 3352 | 25% | P value = 0.04 |
| 1 to 100 kcal/d less in low fat arm | ‐1.5 (‐2.9 to ‐0.1) | 4 | 2398 | 66% | ||
| 101 to 200 kcal/d less in low fat arm | ‐1.1 (‐2.2 to ‐0.04) | 5 | 43,755 | 80% | ||
| 201+ kcal/d less in low fat arm | ‐2.2 (‐3.0 to ‐1.5) | 8 | 3954 | 78% |
Note: studies that provide data at different time points or that fit into different categories have all been included, so studies may appear more than once in any series of subgroups.
The time point at which weight is assessed following the onset of a reduced compared with a moderate fat diet may be important. The effect in studies that assessed weight from six to up to 12 months, 12 to up to 24 months and 24 to up to 60 months was statistically significant, but at 60+ months (MD ‐0.7 kg, 95% CI ‐1.7 to 0.3) statistical significance was lost (test for subgroup differences: P value = 0.04).
The level of fat in the control group may also be important. Weight loss was statistically significant where the control group intake was over 35% of energy from fat, over 30% to 35% of energy or over 25% to 30% of energy, with a suggestion of greater weight loss in groups with lower baseline fat intake (test for subgroup differences: P value < 0.00001) (see Table 9).
There was a suggestion that dietary advice was more effective in weight reduction with low fat eating than provision of low fat foods, however the power of the analysis was limited (only one study that provided foods also supplied numerical data for meta‐analysis (test for subgroup differences: P value = 0.04).
There were no clear effects of: sex on weight (studies in men, in women and in mixed sexes all showed significant weight loss; test for subgroup differences: P value = 0.20), year of first publication (studies published in the 1960s, 1980s, 1990s and 2000s were all statistically significant; test for subgroup differences: P value = 0.07), the total fat intake goal in the intervention group (test for subgroup differences: P value = 0.34), whether the low fat arm achieved a fat intake of ≤ 30%E or not (test for subgroup differences: P value = 0.42), body mass index at baseline (test for subgroup differences: P value = 0.17), or whether participants were recruited as healthy, with risk factors (such as lipids, hormone levels or breast cancer risk factors), or with existing disease (such as diabetes, previous myocardial infarction or polyps) (test for subgroup differences: P value = 0.12). For all of these subgroupings all of the subgroups examined showed statistically significant weight loss in the low fat arms compared with the control arms.
Meta‐regression (multiple regression model on dose, duration and control group fat intake, all at once) suggested that the degree of fat reduction was significantly associated with the degree of weight loss in the intervention arm compared with the control arm (coefficient ‐0.20 kg/1% energy from total fat reduction, 95% CI ‐0.34 to ‐0.05, P value = 0.010), suggesting that greater reduction in fat intake was associated with greater weight loss. Fat intake in the control group (equivalent to baseline fat intake) was also significantly associated with the degree of weight loss in the intervention group (coefficient 0.17 kg/1% energy from fat in the control group, 95% CI 0.04 to 0.29, P value = 0.010), suggesting that a reduction in fat intake was more effective at reducing weight in those with a lower baseline fat intake. There was no clear association between trial duration and degree of weight loss (coefficient 0.01 kg/month, 95% CI ‐0.006 to 0.030, P value = 0.19). Together these factors explained 56% of variance between studies, using the equation: weight change (kg) = ‐5.97 kg + 0.17 kg/1% energy from total fat in control group ‐0.20 kg/1% decrease in energy from total fat in intervention group + 0.01 kg/months' duration.
Body mass index (BMI), waist circumference and other measures of body fatness
Fewer studies reported BMI than weight, but the effect of a lower proportion of energy from fat on BMI appeared similar to that on weight (‐0.5 kg, 95% CI ‐0.7 to ‐0.3, 45,703 participants, 10 comparisons, I2 = 74%) (Analysis 1.2; Figure 4). As there were fewer studies than for weight, we did not attempt sensitivity analyses and subgrouping for BMI.
1.2. Analysis.
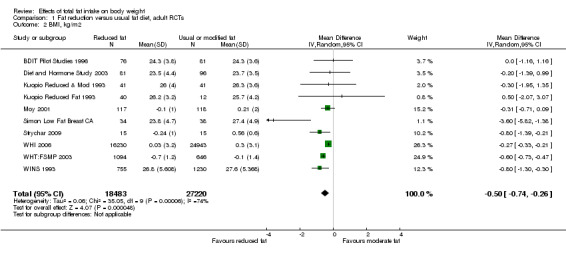
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 2 BMI, kg/m2.
4.
Forest plot of comparison: 1 Fat reduction versus usual fat diet, adult RCTs, outcome: 1.2 BMI, kg/m2.
Only one RCT reported waist circumference, finding that waist circumference in those on low fat diets was significantly lower than in those on usual fat diets at five and seven years (by 0.3 cm, 95% CI ‐0.6 to ‐0.02, 15,671 women) (WHI 2006). No adult RCTs reported other measures of body fatness.
Secondary outcomes ‐ lipids and blood pressure
There was no suggestion of harms associated with low fat diets that might mitigate any benefits on weight.
Effects of reduced fat compared with usual or modified fat diets suggested that the lower fat diets were associated with lower total and low‐density lipoprotein (LDL) cholesterol, without important effects on high‐density lipoprotein (HDL) or triglycerides. Effects on LDL (‐0.1 mmol/L, 95% CI ‐0.2 to ‐0.03, 7285 participants, 18 comparisons, I2 = 65%) were similar to those on total cholesterol (‐0.2 mmol/L, 95% CI ‐0.3 to ‐0.1, 7715 participants, 20 comparisons, I2 = 54%). The effect on HDL suggested slight harm from lower fat diets (‐0.01 mmol/L, 95% CI ‐0.03 to 0.00, P value = 0.11, 7166 participants, 19 comparisons, I2 = 0%). Given the weight loss, there was little evidence of a benefit on triglycerides (‐0.02 mmol/L, 95% CI ‐0.12 to 0.08, 6976 participants, 17 comparisons, I2 = 56%). There was a reduction in total cholesterol/HDL ratio over the seven comparisons that reported it (‐0.10, 95% CI ‐0.16 to ‐0.04, 3332 participants, I2 = 0%).
There were small and statistically significant beneficial effects of a lower fat diet on systolic and diastolic blood pressure (although these were reported in relatively few studies). The effect on systolic blood pressure (‐1.2 mmHg, 95% CI ‐2.0 to ‐0.4, 5159 participants, nine comparisons, I2 = 0%) was greater than that on diastolic blood pressure (‐0.7 mmHg, 95% CI ‐1.4 to ‐0.1, 5159 participants, nine comparisons, I2 = 23%).
Secondary outcomes ‐ effects of reducing fat intake on intakes of energy, protein, carbohydrate, sugars and alcohol
Indications were that during the studies energy intake was usually lower in the low fat group than in the control or usual fat groups. Sugar intake was not measured often but where reported sugar intake appeared higher in low fat arms (except in MeDiet 2006, see Table 10). Carbohydrate intakes appeared almost universally higher in low fat arms than in usual fat arms, and protein intakes were sometimes higher and sometimes similar. There was no consistent pattern in alcohol intake.
9. Data on dietary intake of energy, sugars, carbohydrate, protein and alcohol during the diet period of RCTs comparing low fat with usual fat intake.
| Trial | Energy intake (SD), kcal | Sugars intake, %E | CHO intake, %E | Protein intake, %E | Alcohol intake, %E | No. of participants | ||||||
| Int. | Cont | Int. | Cont | Int. | Cont | Int. | Cont | Int. | Cont | Int. | Cont | |
| Auckland reduced fat, 1 yr | 1887 (672) | 2269 (750) | — | — | 54.2 (10.5) | 45.8 (10.9) | 18.4 (3.5) | 16.6 (3.9) | 3.6 (7.0) | 5.7 (7.0) | 49 | 61 |
| BDIT pilot studies, 9 yrs | 1460 (376) | 1578 (365) | — | — | 49.6 (7.5) | 46.9 (6.2) | 15.5 (2.4) | 15.3 (2.6) | 2.3 (3.3) | 1.7 (2.4) | 76 | 81 |
| BeFIT | (data not reported in control groups) | |||||||||||
| Bloemberg, Δ to 6 mo | — | — | — | — | 4.4 (6.5) | 1.2 (6.1) | 0.33 (2.9) | 0.57 (1.7) | — | — | 39 | 41 |
| BRIDGES, 6 mo | ‐34 (79) | + 22 (79) | — | — | — | — | — | — | — | — | 48 | 46 |
| Canadian DBCP, 2 yrs | 1540 (317) | 1759 (437) | — | — | 60.3 (8.3) | 48.8 (8.1) | 18.0 (3.2) | 16.9 (2.8) | — | — | 104 | 100 |
| De Bont, Δ to 6 mo | ‐98 (369) | ‐120 (485) | — | — | 7.9 (9.5) | ‐0.1 (10.9) | 2.4 (7.0) | 1.7 (5.9) | ‐0.2 (1.6) | ‐0.4 (2.6) | 71 | 65 |
| DEER (diet alone), Δ to 1 yr | Women: ‐220 (356) Men: ‐285 (541) |
Women: ‐19 (367) Men: ‐25 (482) |
— | — | Women: +5.5 (8.0) Men: +8.0 (9.3) |
Women: ‐0.2 (7.3) Men: +1.1 (6.6) |
— | — | — | — | 46, 49 | 45, 46 |
| DEER (diet and ex), Δ to 1 yr | Women: ‐191 (343) Men: ‐167 (516) |
Women: ‐54 (410) Men: +141 (437) |
— | — | Women: +7.8 (6.2) Men: +9.3 (8.3) |
Women: ‐0.3 (7.9) Men: +1.4 (6.3) |
— | — | — | — | 43, 48 | 43, 47 |
| Diet and hormone study, 1 yr | 1921 (386) | 2063 (610) | — | — | 64.3 (9.0) | 54.6 (9.2) | 14.5 (2.9) | 14.1 (3.8) | est: 1 (2) | est: 1 (2) | 81 | 96 |
| Kentucky low fat, 1 yr | 1882 (521) | 2010 (528) | — | — | 53 (8.9) | 50 (7.9) | 17 (3.4) | 18 (4.3) | — | — | 47 | 51 |
| Kuopio, wks 14 to 28 | AHA 1791 (382) Mono 1887 (478) Low fat 1648 (430) |
1982 (406) | — | — | AHA 48 (5) Mono 47 (6) Low fat 51 (5) |
46 (6) | AHA 17 (2) Mono 17 (20) Low fat 19 (3) |
16 (2) | — | — | AHA 41 Mono 41 Low fat 40 |
37 |
| Mastopathy diet, 6 mo | 1491 (NR) | 1676 (NR) | — | — | 56.3 (NR) | 48.1 (NR) | 17.9 (NR) | 15.8 (NR) | 4.8 (NR) | 4.2 (NR) | 10 | 9 |
| MeDiet, 6 mo | 1676 (639) | 1654 (498) | 18.7 (6.9) | 21.9 (9.2) | 27.2 (17.0) | 25.8 (11.0) | 14.9 (4.7) | 16.2 (5.1) | 5.6 (11.1) | 1.6 (2.2) | 51? | 55? |
| Moy, 2 yrs | 1825 (NR) | 2092 (NR) | — | — | — | — | — | — | — | — | 117 | 118 |
| MSFAT, 6 mo | 2460 (NR) | 2699 (NR) | — | — | 47 (NR) | 41 (NR) | 16 (NR) | 14 (NR) | 3 (NR) | 3 (NR) | 117 | 103 |
| NDHS open 1st 6 mo (for definitions of groups B, C and D see Characteristics of Included Studies) |
B: 2154 (432) | C: 2262 (435) D: 2228 (456) |
— | — | B: 48.7 (12.3) | C: 45.3 (12.1) D: 44.7 (11.7) |
B: 18.6 (3.4) | C: 17.6 (3.1) D: 17.4 (3.1) |
B: 3.7 (3.7) | C: 3.6 (4.0) D: 3.8 (4.0) |
B: 339 | C: 355 D: 346 |
| NDHS open 2nd 6 mo (for definitions of groups BC, F and G see Characteristics of Included Studies) |
BC: 2249 (492) | F: 2196 (427) G: 2169 (420) |
— | — | BC: 45.7 (12.7) | F: 44.1 (11.1) G: 43.3 (11.4) |
BC: 17.3 (3.5) | F: 7.3 (3.0) G: 17.7 (2.9) |
BC: 3.5 (4.2) | F: 4.2 (4.0) G: 4.0 (4.5) |
BC: 491 | F: 214 G: 194 |
| Nutrition and breast health, 1 yr | 1780 and 1960 | 1571 and 1687 | — | — | — | — | — | — | — | — | 23 and 25 | 24 and 23 |
| Nutrition education study, 6 to 9 mo | 1534 (448) | 1721 (620) | — | — | 43.4 (9.5) | 41.5 (8.9) | 19.9 (3.7) | 18.7 (4.4) | 4.5 (7.2) | 4.8 (9.3) | 224 | 69 |
| Pilkington, 1 yr | NR | NR | — | — | — | — | — | — | — | — | 12 | 23 |
| Polyp prevention trial, yr 4 | 1978 (471) | 2030 (518) | — | — | 58.3 (7.4) | 47.1 (7.2) | 17.3 (2.5) | 16.5 (2.4) | — | — | 605 | 581 |
| Rivellese, 6 mo | NR | NR | 14 | 10 | 55 | 48 | 18 | 16 | — | — | 27 | 17 |
| Simon low fat, 1 yr | 1570 (NR) | 1594 (NR) | — | — | — | — | — | — | — | — | 65 | 68 |
| Sondergaard, 12 mo | — | — | — | — | 52.3 (6.4) | 48.5 (8.7) | 17.0 (2.9) | 16.6 (3.1) | 4.5 (5.3) | 6.4 (7.4) | 62 | 51 |
| Strychar, 6 mo | NR | NR | — | — | — | — | — | — | — | — | 15 | 15 |
| Swedish breast CA, Δ to 2 yrs | ‐215 (P value < 0.01) | ‐143 (P value < 0.01) | +4.8 (P value < 0.01) | +1.4 (P value < 0.01) | +11.0 (P value < 0.01) | +2.7 (P value < 0.01) | +1.7 (P value < 0.01) | +0.3 (P value > 0.05) | +0.2 (P value > 0.05) | +0.4 (P value > 0.05) | 63 | 106 |
| Veteran's dermatology, during trial | 1995 (564) | 2196 (615) | — | — | 60.3 (6.3) | 44.6 (6.9) | 17.7 (2.2) | 15.7 (2.4) | 3.2 (3.4) | 3.2 (3.9) | 57? | 58? |
| WHEL, 1 yr | 1664 (345) | 1635 (384) | — | — | 65.3 (8.5) | 57.1 (9.3) | — | — | — | — | 197 | 196 |
| WHI, 7.5 yrs | 1446 (510) | 1564 (595) | — | — | 52.7 (9.8) | 44.7 (8.5) | — | — | — | — | 14246 | 22083 |
| WHT: feasibility, 2 yrs | 1356 (358) | 1617 (391) | — | — | 59.0 (8.8) | 46.9 (8.9) | 19.2 (3.9) | 16.8 (3.8) | — | — | 163 | 101 |
| WHT: FSMP, Δ to 18 mo | ‐488 (NR) | ‐255 (NR) | — | — | — | — | — | — | — | — | 285 | 194 |
| WINS, 5 yrs | ‐167 (p value < 0.0001 vs cont) | 0 | — | — | — | — | — | — | — | — | 380 | 648 |
est: estimated by review authors from data on g/d and mean energy intakes
Abbreviations: AHA: American Heart Association; CHO: carbohydrates; DBCP: Diet and Breast Cancer Prevention; SD: standard deviation
Secondary outcomes ‐ effects of reducing fat intake on quality of life measures
Quality of life outcomes were rarely measured or reported. It appears that quality of life was assessed in WHI 2006 but we were unable to find any reference to this outcome by dietary intervention group. No other relevant data were located.
Publication bias
The funnel plot of studies assessing effects on weight did not suggest any serious publication bias (Figure 5), and neither did the funnel plot of effects on BMI (not shown). The studies that assessed weight, but where we could not include the data provided in meta‐analysis, did not appear to differ importantly in their results from the studies that provided variance data and were included in the analyses.
5.
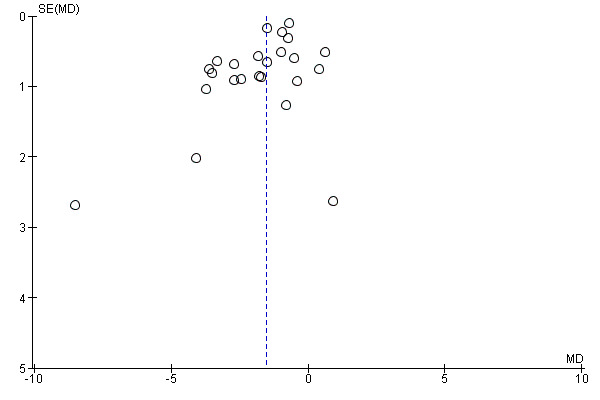
Funnel plot of comparison: 1 Fat reduction versus usual fat diet, outcome: 1.1 Weight, kg.
Effects of reducing dietary fat on weight and body fatness in children (as seen in RCTs)
As part of the single RCT in children, VYRONAS 2009 randomised 213 students aged 12 to 13 years at baseline to intervention or usual diet, of whom 191 were analysed at 17 months. The validity of this RCT was discussed with the adult RCTs and is shown in Figure 2). The intervention group (n = 98) had a 12‐week school‐based health and nutrition interventional programme with a 17‐month follow‐up period. After 17 months, total fat intake (as %E) showed a significant reduction 31.3% (standard deviation (SD) 4.4) compared with baseline intake of 35.4% (SD 4.7) in the intervention group (P value < 0.001). In the control group fat intake at 17 months was 36.2% (SD 5.2) compared with 36.9% (SD 4.8) at baseline (P value = 0.343). Mean BMI (kg/m2) also decreased significantly (adjusting for age and sex) to 23.3 kg/m2 (SD 2.8) compared with 24.0 kg/m2 (SD 3.1) at baseline in the intervention group (P value < 0.001), but was more similar in the control group (24.8 (SD 3.8) versus 24.3 (SD 3.3), P value = 0.355). The difference in weight between intervention and control arms was not reported, and as the difference between intervention and control groups for baseline BMI was greater than the changes in BMI in either arm a direct comparison of BMI is probably inappropriate statistically. Mean change in BMI was a fall of 0.7 kg/m2 in the intervention group and an increase of 0.5 kg/m2 in the control group, a difference of 1.2 kg/m2 (but we do not have variance data for these changes, so cannot comment on statistical significance). Analysis of 17‐month BMI data by the review authors in RevMan (RevMan 2014) suggested that the effect of a low fat diet compared with a usual fat diet in children was ‐1.50 kg/m2 (95% CI ‐2.45 to ‐0.55), however this was assessed on adjusted data, with a large baseline difference in BMI between groups. Without analysis of the original data set this should therefore be considered with caution.
Associations between total dietary fat and measures of body fatness in adults (as seen in cohorts)
We included 14 adult cohorts (20 published papers, cohorts presented their results in from one to eight main analyses, 39 analyses in total) which reported on baseline total fat intake and reported on a measure of body fatness at least one year later. Eleven cohorts reported change in weight, BMI and/or waist circumference over the course of the follow‐up, while three cohorts reported absolute weight or BMI at follow‐up (for characteristics of these studies see Table 2). We considered meta‐analysis of beta values, but the different methodologies, methods of modelling, numbers of baseline dietary assessments, numbers of relevant statistical analyses per single cohort, time periods between dietary assessment and body fatness assessment, ages at baseline and outcome measures (weight, change in weight, BMI, change in BMI, change in waist circumference) were so varied that we felt combining studies in meta‐analysis was inappropriate.
The single study at moderate risk of bias (Danish MONICA, Iqbal 2006, Table 2) found no relationship between fat intake and change in weight five years later. Four further cohorts reported no relationship between fat intake and measures of body fatness in the whole cohort or in any reported subgroup (Cundiff 2012; Ma 2005; Parker 1997; Halkjaer 2009). Eight cohorts reported relationships in some subgroups but not others (CARDIA found a relationship for black men and women, but not white men and women; EPIC negative relationships when replacing fat with protein, and when replacing carbohydrates with total fat, but not when replacing fat with carbohydrates; Coakley 1998 a relationship between total fat and change in weight in 45 to 54 year old men and 55 to 64 year old men, but not in men aged 65 or more; MacInnis 2013 found associations between baseline fat intake with final weight and waist circumference overall, but this was only significant in some age subgroupings; Klesges 1992 found a positive relationship with change in weight in women, but not in men, and a negative relationship with change in waist circumference in men, but not in women; Kant 1995 found a relationship with change in weight in younger women, but not in older women or men of either age group; Nurses Health Study found no relationship with change in weight in one paper, and the relationship was unclear in another paper; Lissner 1997 found a relationship between fat intake and change in weight in sedentary participants, but not in moderate or active participants). One cohort reported a positive association between total fat intake and change in weight in a mixed group of Hispanic and non‐Hispanic men and women (Mosca 2004).
Overall, of the 39 reported analyses of the relationship between total fat intake and measures of body fatness in adults, 12 suggested a positive relationship, three a negative relationship and one was unclear. The remainder (23 analyses) were neutral (no statistically significant relationship).
Associations between total dietary fat in youth and measures of body fatness in children, young people and adults (as seen in cohorts)
The 11 included cohorts that recruited children and young people were reported in 13 published papers, and provided 101 separate analyses. Two cohorts assessed outcomes in adulthood, the remainder later in childhood.
Of the nine child or young person cohorts that assessed effects on body fatness in childhood or adolescence, three cohorts, including the study at moderate risk of bias, Davison 2001) suggested that higher dietary fat intakes predicted greater body fatness (assessed as % body fat and BMI in Carruth & Skinner 2001, change in BMI in Davison 2001, and change in weight in Viva la Familia). Four cohorts suggested no clear relationship between fat intake and fatness (assessed as BMI, triceps skinfold and subscapular skinfold in the Adelaide Nutrition Study, change in BMI in Bogaert 2003 and Obesity and Metabolic Disorders Cohort in Children, and change in BMI z‐score in the European Youth Heart Study). Two cohorts reported effects in some measures of body fatness or some analysed age groups but not others (Trial of Activity for Adolescent Girls found no relationship of fat with BMI percentile, but a negative relationship with % body fat, while Klesges 1995 found no relationship in 3 of four assessments of change in BMI). For details of these cohort studies see Table 3.
We considered meta‐analysis, but the different methodologies, methods of modelling, numbers of baseline dietary assessments, numbers of relevant statistical analyses per single cohort (from 1 to 63), time periods between dietary assessment and body fatness assessment, ages at baseline and outcome measures (weight, change in weight, BMI, change in BMI z‐score, change in BMI, body fat percentage, various skinfold measures) were so varied that we felt combining studies in meta‐analysis was inappropriate.
The two cohorts (two analyses of the Amsterdam Growth and Health Longitudinal Study, and one of ELANCE, Table 3), which assessed the relationship between fat intake in childhood and body fatness in early adulthood (ages 20, 27 and 36), found no clear relationships between baseline fat intake and BMI, percentage body fat, sum of skinfolds or % triceps skinfold. The exception was ELANCE, which found that greater total fat intake in youth was related to lower percentage sub‐scapular skinfold and fat mass (though not to BMI or % triceps skinfold).
Overall, the included cohorts reported a total of 101 analyses of the relationship between total fat intake and body fatness in cohorts recruiting children and young people. Nine suggested positive relationships and three suggested negative relationships. The vast majority were neutral.
Discussion
Summary of main results
Randomised controlled trials (RCTs) of the effects on body fatness of reducing total fat intake (without any intention to reduce body weight) show a small but consistent reduction in weight in the low fat arm compared with the usual fat arm. There is some heterogeneity between studies in the size of this effect, but not in its presence, and the effect was highly resistant to sensitivity analyses. The heterogeneity was explained by the degree of total fat reduction and baseline total fat intake (in meta‐regression and in subgrouping). The small reduction in weight (1.5 kg, 95% confidence interval (CI) ‐2.0 to ‐1.1 kg) was also reflected in a reduction in body mass index (BMI) (‐0.50 kg/m2, 95% CI ‐0.74 to ‐0.26) and waist circumference (0.3 cm, 95% CI ‐0.6 to ‐0.02) in the adult studies that reported these data, and in a suggested reduction in BMI in the one child study (VYRONAS 2009): a fall of 0.7 kg/m2 in the intervention arm and a rise of 0.5 kg/m2 in the control arm). Additionally, there was no suggestion of harms that might mitigate any benefits on weight, and some suggestion of benefit to serum lipids and blood pressure resulting from low fat diets.
Cohort studies in adults and children generally found no clear relationship between total fat intake and measures of body fatness later in life, but a few did see positive relationships (higher total fat intake was associated with higher later body fatness), and fewer suggested negative relationships.
Overall completeness and applicability of evidence
We have searched very carefully and used a set of comprehensive search strategies to find the full set of RCTs and cohort studies assessing the relationship between total fat intake and measures of body fatness. We did this by searching for trials that reduced total fat in one arm and not in the other, regardless of the primary aims or outcomes mentioned in the title or abstracts. Indeed, the included RCTs rarely had weight as a key outcome. Reflecting this, there was little suggestion (from the funnel plot of adult RCTs assessing effects on weight and BMI) that we have missed a sample of RCTs. However, we are limited in how well we are able to assess this for cohort studies, where the risk of missing studies is keener (where sometimes the relevant analysis is added into the text as an afterthought (e.g. Working Well 1996) and does not appear in the title or abstract).
The studies are highly applicable to the question, allowing us to draw conclusions on the effect of altering the percentage of energy from total fat on body fatness.
Quality of the evidence
The included RCTs were often at unclear risk of selection bias due to unclear allocation concealment, but this did not appear to affect the results of the review as omitting all RCTs with unclear or poor allocation concealment still resulted in a statistically significant weight reduction in the intervention arms. Lack of blinding was a validity issue in most included RCTs, reflecting the difficulties of blinding dietary intervention studies. We assessed the effects of attention bias in sensitivity analyses, removing studies that provided more time or review or education to the intervention group compared with the control group, and also the effect of removing studies that provided dietary advice other than on dietary fat (in case effects were being driven by other dietary interventions) and in neither case did we lose the significant weight reduction seen in the low fat arms. In each case the higher validity trials reflect the main message, that eating a lower proportion of energy from fat results in slightly lower body fatness.
The included cohort studies were generally at high risk of bias due to the high proportion of participants lost to follow‐up or lack of adjustment for potential confounders. Although the included cohorts reported on a large number of participants, they did not add significantly to the conclusions of the review as their findings were not conclusive.
Potential biases in the review process
When compiling the included studies we tried to locate RCTs that investigated the effects of reducing total dietary fat for at least six months. There was a high degree of heterogeneity among trials from different sources, including the type and number of participants, the duration and nature of interventions, control methods and follow‐up. However, our sensitivity analyses and subgrouping to examine the effect of the potential effect modifiers mentioned above did not affect the statistical significance of the suggested effect, finding it remarkably robust to subgroup and sensitivity analyses.
Our review included only published studies (we did not seek unpublished data), which could bias the results due to the lack of publication of negative or inconclusive studies. However, our funnel plots did not suggest serious publication bias (Figure 5).
Our decision to exclude trials that explicitly or implicitly aimed to reduce weight may have led to missing some trials or restricting the number of included studies, especially excluding studies where there was no energy restriction, no explicit aim of weight loss, or encouraging of weight loss for some and not all participants. However, this decision makes the effect we found on weight and other measures of body fatness more reliable and avoids the potential confounding effects of dieting and unconscious energy restriction or other diet changes.
The restriction of inclusion to studies with a minimum of six months duration for RCTs or one year for cohorts led to missing some potentially relevant studies (for example, studies of 24 weeks duration, which just missed the 26‐week limit). However, it is essential to draw the line at some point, and longer trials and follow‐up ensure that the data are relevant to long‐term fatness, which affects long‐term health.
A limitation of the review was that we did not assess the causal pathway between restriction of energy from fat and weight and so the mechanism of the effect is not clear. It is likely that restricting energy from fat also reduces energy intake (see Table 10), which leads to lower body weight. Further evidence that energy intake is important in mediating the effect of lowering fat intake on body weight is suggested by a higher relative weight loss in the low fat arms with greater energy reduction.
Most (22 of 32) included RCTs were published before the year 2000 ‐ this is primarily because most recent studies have focused on weight reduction so were ineligible for this review. However, there was no suggestion when subgrouping by decade of publication that effects have altered over time.
Agreements and disagreements with other studies or reviews
The conclusions of this updated review have not altered in overall import from the original review (Hooper 2012b). Yu‐Poth 1999 found that dietary trials (excluding trials that also assessed exercise interventions) of the National Cholesterol Education Program's Step I and Step II dietary intervention programmes resulted in weight reductions (compared with control groups) of just under 3 kg, and that this was related to the degree of total fat reduction. Their regression suggested that for every 1% decrease in energy as total fat, there was a 0.28 kg decrease in body weight, while our meta‐regression found that for every 1% decrease in energy as total fat there was a slightly smaller 0.20 kg decrease in weight (95% CI ‐0.34 to ‐0.05, P value = 0.010). The slightly smaller effect size in this review may be due to our excluding shorter duration studies and studies that aimed to reduce weight in the intervention arm.
However, some recent cardiovascular disease prevention guidelines have not mentioned total fat intake as regards to either weight control or prevention of cardiovascular disease (Joint ESC guidelines 2012).
Authors' conclusions
Implications for practice.
Attempts should be made to reduce total fat intake in populations where mean total fat intake is 30% or more of energy, in order to support maintenance of healthy weights. For populations where the mean total fat intake is below 30% of energy, then interventions to restrict increases in total fat intake to over 30% of energy may help to avoid obesity.
Implications for research.
High quality trials are needed to investigate the effect on body weight of reducing fat intake in developing or transitional countries with total fat intakes greater than 30% of energy, and of preventing total fat intake rising above 30% of energy in countries with total fat intakes of 25% to 30% of energy. High quality trials are also required in children.
Feedback
Tobias 2016, 7 July 2016
Summary
In their systematic review and meta‐analysis of 32 randomized controlled trials, representing 54,000 participants, Hooper et al. reported that a lower proportion of energy intake from total fat was associated with a small reduction in body weight (difference = 1.5 kg).1 The authors’ conclusion, however, was contradicted by findings from their parallel meta‐analysis of 25 observational cohort studies. The erroneous conclusion from the review of trials is a consequence of biased study selection criteria, inclusion of short‐term follow‐up (<12 months), and other methodologic flaws.
First, their criteria explicitly included only trials in which weight loss was not an objective of the intervention. This led to the exclusion of several long‐term, rigorously conducted RCTs designed specifically to test the hypothesis that the fat composition of the diet affects weight change. The criteria used by Hooper et al. resulted in a heterogeneous subset of the of low‐fat dietary intervention RCTs, which included trials conducted to test the effects of low‐fat diets on endpoints such as cancer incidence or lipids in higher risk study populations. In fact, only three trials in their meta‐analysis were among healthy participants, not recruited on the basis of risk factors or disease. The authors’ contend that including only studies not intending to alter weight would reduce potential publication bias. On the contrary, we believe this would increase the likelihood of publication bias, since investigators of diet trials not explicitly conducted for weight loss would not be motivated to publish null or contrary results. Since the point of this work is to advise generally healthy individuals as to how to maintain or lose weight, it is bizarre to specifically exclude trials designed to answer that question.
Second, the authors’ included short‐term trials (of as little as 6 months duration). Six months is typically when the effect of dietary interventions on body weight wane and weight regain commences; thus short‐term results do not reflect sustained effects at 1 year or longer, which is of primary interest.2
Third, most of the studies included by Hooper et al. were seriously confounded by factors other than the fat content of the diet. Some of the trials coupled a low‐fat intervention with other advice, such as eating more fruits and vegetables, which obscures the interpretation of the findings. The other key characteristic is the differences in intensity or attention between intervention groups (e.g., fewer or no in‐person visits, dietary counseling meetings, etc), because the control group was often simply assigned to maintain their usual diet. Aspects related to the intensity of a dietary intervention, such as behavioral support, are modest predictors of weight loss success;3 thus, most RCT’s designed to assess the effects of diet composition on weight intentionally balanced the intensity of interventions, but these were the studies explicitly excluded by Hooper it al. In our previous meta‐analysis of RCTs comparing low‐fat vs. higher fat dietary interventions, we conducted stratified analyses by these key trial characteristics.4 We observed that significant long‐term weight loss favoring low‐fat interventions was observed only for trials in which the comparator group was “usual diet” or received less attention during the intervention from study investigators. This was true regardless of whether the RCTs had a weight loss focus or not. Comparisons between low‐fat and higher fat interventions of similar intensity demonstrated no benefit of low‐fat over higher fat diets, regardless of weight loss goal. Indeed, the overall results of these trials favored a small but statistically significant greater weight loss with higher fat diets. Our findings clearly demonstrated the biased impact of differential attention across treatment groups.
Only 4 RCTs in Hooper’s meta‐analysis (419 total participants) remained after exclusion of trials in which control groups were asked simply to maintain usual diet or received differentially less attention than the low‐fat intervention arms. Three were 6 month trials, and the fourth was published in 1960 among men with recent myocardial infarction to examine lipid changes after a 1 year intervention with either a low‐fat or a “unsaturated‐fat” diet.5 These 4 RCTs also were judged by Hooper et al. to have relatively high “risk of bias” according to authors’ methodological quality criteria.
In summary, the results from the most recent Hooper et al. meta‐analysis provide no convincing evidence for recommending a low‐fat diet for the prevention of weight gain and obesity in the general population. In fact, their strict exclusion criteria restricting the analysis only to trials in which weight‐loss was not intended led to biased results. Although the authors’ felt that limiting their analysis to non‐weight loss trials would enhance validity, this selectively excluded trials designed to avoid confounding by intensity of intervention and other factors. Analysis of trials that include those specifically testing interventions for weight control, that exclude short‐term trials, and account for key trial characteristics yield consistent results that are consonant with observational studies. Would we derive recommendations for statin use in the primary prevention of coronary heart disease solely from trials with a completely different disease endpoint? Promoting low fat diets for weight control can lead to increased consumption of refined carbohydrates, causing increased weight gain,4 an array of adverse metabolic effects,6 and premature death.7 The overall body of scientific evidence clearly demonstrates that dietary recommendations should focus not on lowering the total fat content of the diet but rather on specific types of fats and carbohydrates and, more importantly, on specific foods and overall dietary patterns.8
References
Hooper L, Abdelhamid A, Bunn D, Brown T, Summerbell CD, Skeaff CM. Effects of total fat intake on body weight. The Cochrane database of systematic reviews. 2015(8):CD011834.
Willett WC. Dietary fat plays a major role in obesity: no. Obesity reviews : an official journal of the International Association for the Study of Obesity. May 2002;3(2):59‐68.
Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta‐analysis. Jama. Sep 3 2014;312(9):923‐933.
Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low‐fat diet interventions versus other diet interventions on long‐term weight change in adults: a systematic review and meta‐analysis. The lancet. Diabetes & endocrinology. Dec 2015;3(12):968‐979.
Pilkington TR, Stafford JL, Hankin VS, Simmonds FM, Koerselman HB. Practical Diets for Lowering Serum Lipids. British medical journal. Jan 2 1960;1(5165):23‐25.
Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. Jama. Nov 16 2005;294(19):2455‐2464.
Wang DD, Li Y, Chiuve SE, et al. Association of Specific Dietary Fats With Total and Cause‐Specific Mortality. JAMA internal medicine. Jul 5 2016.
U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Reply
Thank you for your interest in our systematic review (1). You are incorrect, we did not state anywhere in the review that “a lower proportion of energy intake from total fat was associated with a small reduction in body weight (difference = 1.5 kg)”. We were not interested in associations, we were interested in causality, so we included RCTs that reduced total fat in one randomised arm and not in the other. In the abstract we stated “There is consistent evidence from RCTs in adults of a small weight‐reducing effect of eating a smaller proportion of energy from fat; this was seen in almost all included studies and was highly resistant to sensitivity analyses. The effect of eating less fat (compared with usual diet) is a mean weight reduction of 1.5 kg (95% confidence interval (CI) ‐2.0 to ‐1.1 kg), but greater weight loss results from greater fat reductions.”
Yes, we only included studies where weight loss was NOT a goal (where fat reduction was assessed for its effect on cardiovascular disease, cancer risk or other health issues). The reason for this was that we were interested not in weight reducing diets for overweight people, but in usual diets eaten day to day by generally healthy people all over the world. This issue was discussed in great detail by the World Health Organization NUGAG committee before the review was commissioned and the committee was very clear that their instructions were in setting goals for generally healthy populations and not therapeutic diets for those who were already overweight or obese. Therapeutic weight reducing diets are very different and, whatever their macronutrient or food composition, cannot be disentangled from the overriding and conscious requirement to eat less food (i.e. reduce energy intake). Indeed, and importantly, the participants in the studies we reviewed were not recruited to studies that aimed to promote weight loss in participants, or where participants were aware that one of the aims of the study was to promote a loss in their weight to achieve a healthy weight. This also meant that we did not include studies where low fat diets were compared to other therapeutic diets (such as very low carbohydrate diets).
Our review assesses the effects on weight of encouraging normal populations to reduce their total fat intake over the long term. The studies included durations of 6 months up to over 8 years. The effect in studies of between 6 and 12 months duration was a reduction of 1.74kg in the low fat group compared to control (95% CI ‐2.34 to ‐1.13), similar to that at 12 to 24 months (‐2.00kg, 95% CI ‐2.51 to ‐1.48) and at 24 to 60 months (‐1.18kg, 95% CI ‐1.65 to ‐0.70). The effect over more than 5 years was smaller (‐0.68kg, 95% CI ‐1.66 to 0.29) but two of the four large RCTs still showed statistically significantly lower weight in the intervention groups (perhaps reflecting differences in the intensity of the intervention delivery and support this far into the trials), and meta‐regression did not suggest a significant effect of duration on the extent of weight reduction in the low fat group compared to control. Dr Tobias’ own systematic review also clearly shows that in studies where there was no intention to reduce weight “that low‐fat interventions led to greater weight loss” compared to usual diets (abstract of (2)).
Strategies to help obese adults and children to lose weight are also clearly very important – but how to lose weight is a different question from how populations should eat day to day, year to year (there are a set of specific systematic reviews about weight reduction strategies in different populations on the Cochrane Library).
We used sensitivity analysis to assess the effect of “attention bias” (see Analysis 3.1). We removed studies where there appeared to have been more attention and/or time spent on the intervention group than the control group. Five studies provided data for this meta‐analysis, finding that there was still a statistically significantly reduced weight in the low fat group (‐1.25kg, 95% CI ‐2.09 to ‐0.41). Three further trials did not provide variance data so could not be included in the meta‐analysis, but they all clearly showed greater weight reduction in the low fat compared to usual fat arms, on average (though their statistical significance cannot be assessed). This is a very consistent effect, is not dependent on short duration, and does not rely on increased attention or behavioural strategies in the low fat arms.
We reiterate, “Trials where participants were randomised to a lower fat intake versus usual or moderate fat intake, but with no intention to reduce weight, showed a consistent, stable but small effect of low fat intake on body fatness: slightly lower weight, BMI and waist circumference compared with controls. Greater fat reduction and lower baseline fat intake were both associated with greater reductions in weight.”
References
Hooper L, Abdelhamid A, Bunn DK, Brown T, Summerbell CD, Skeaff CM. Effects of total fat intake on body weight. Cochrane Database Syst Rev 2015;8:Art. No.: CD011834.doi: 10.1002/14651858.CD011834
Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low‐fat diet interventions versus other diet interventions on long‐term weight change in adults: a systematic review and meta‐analysis. The Lancet Diabetes & Endocrinology 2015;3:968‐79.
Contributors
Julia Lowe, feedback editor for Cochrane Heart
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2016 | Feedback has been incorporated | Comment and authors' response added. |
| 2 March 2016 | Amended | The description of data included in the main analysis for the WHI study was incorrect, so the entry for the "Characteristics of Included Studies" table now reflects that the weight, BMI and waist circumference data used in the main analyses were 7.5 year follow up data (as is appropriate). The data in the forest plots were already correct. Additionally the main reference for WHI is now indicated as the paper that provides this 7.5 year follow up data. The first paragraph of the text on "Associations between total dietary fat in youth and measures of body fatness in children, young people and adults (as seen in cohorts)" was unclear, so we have tried to clarify these results. Table 2 is helpful to read in understanding this section. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 8, 2015
| Date | Event | Description |
|---|---|---|
| 21 July 2015 | New search has been performed | The searches were run on 12 November 2014. |
| 11 July 2015 | New citation required and conclusions have changed | We split a previously published review (Reduced and modified dietary fat for preventing cardiovascular disease, DOI: 10.1002/14651858.CD002137.pub3) into six smaller review updates. The conclusions are therefore now focused on the effects of total fat intake on body weight instead of the effects of reducing or modifying fat intake overall on cardiovascular disease risk. At the request of the World Health Organization (WHO) Nutrition Guidance Expert Advisory Group (NUGAG) group we extended this review to include cohort studies, and studies in children and young people. This split review update includes 32 randomised controlled trials and also 30 sets of analyses of 25 cohorts. |
| 11 June 2010 | New citation required and conclusions have changed | — |
| 9 September 2008 | Amended | — |
| 1 February 2000 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We thank the members of the WHO NUGAG subgroup on diet and health for their work in setting up the question and the protocol for this review (agreed in outline at its first meeting in February 2010, but not published), offering further studies for examination and assessment for inclusion during the initial version of this review, and in ensuring robust analysis. We thank the WHO for funding the update of this review and agreeing with the publication of this systematic review as a scientific paper.
Appendices
Appendix 1. MEDLINE search run to collect adult and child RCTs and cohort studies 15 November 2014
Search adapted from that run in 2010, to search for both adult and child RCTs and cohort studies, but omitting dietary exposures other than dietary fat.
Run 15 November 2014.
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Present> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Weight Gain/ (24259) 2 exp Weight Loss/ (30933) 3 obesity.ab,ti. (152189) 4 obese.ab,ti. (86464) 5 adipos$.ab,ti. (71315) 6 weight gain.ab,ti. (44371) 7 weight loss.ab,ti. (59414) 8 overweight.ab,ti. (42626) 9 over weight.ab,ti. (349) 10 overeat$.ab,ti. (1934) 11 over eat$.ab,ti. (275) 12 weight change$.ab,ti. (8042) 13 ((bmi or body mass index) adj2 (gain or loss or change)).ab,ti. (2786) 14 body fat$.ab,ti. (24784) 15 body composition.ab,ti. (23804) 16 body constitution.ab,ti. (257) 17 exp Dietary Fats/ (73523) 18 exp Diet, Fat‐Restricted/ (3040) 19 (fat$ adj2 (total or intake or consum$ or ate or eat or reduce$ or restrict$ or low$ or diet$)).ab,ti. (63037) 20 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 (366287) 21 17 or 18 or 19 (114331) 22 20 and 21 (28779) 23 randomized controlled trial.pt. (399992) 24 controlled clinical trial.pt. (90666) 25 Randomized controlled trials/ (99585) 26 random allocation.sh. (84070) 27 double blind method.sh. (132423) 28 single‐blind method.sh. (20589) 29 23 or 24 or 25 or 26 or 27 or 28 (658672) 30 (animals not (human and animals)).sh. (5551801) 31 29 not 30 (590901) 32 clinical trial.pt. (501242) 33 exp Clinical trial/ (816129) 34 (clin$ adj25 trial$).ti,ab. (291641) 35 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. (137043) 36 placebos.sh. (34004) 37 placebo$.ti,ab. (169148) 38 random$.ti,ab. (764596) 39 research design.sh. (82260) 40 comparative study.sh. (1730651) 41 exp Evaluation studies/ (206135) 42 follow up studies.sh. (520109) 43 prospective studies.sh. (390949) 44 (control$ or prospectiv$ or volunteer$).ti,ab. (3243146) 45 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 (5767873) 46 45 not 30 (4293785) 47 31 or 46 (4323589) 48 exp Cohort Studies/ (1438154) 49 (cohort$ or quintile$ or quartile$ or quantile$ or tertile$).mp. (411555) 50 (follow‐up$ or followup$).mp,tw. (970994) 51 longitud$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] (208935) 52 ((prospectiv$ or observation$) adj5 (research$ or data$ or stud$)).mp. (587538) 53 48 or 49 or 50 or 51 or 52 (2092058) 54 53 not 30 (1996509) 55 47 or 54 (4973664) 56 22 and 55 (9237) 57 limit 56 to (english language and yr="2010 ‐ 2015") (3294) 58 exp Case‐Control Studies/ (710182) 59 (case adj3 control$).tw. (93452) 60 (case adj3 series).tw. (42174) 61 case study/ (1736496) 62 letter.pt. (885169) 63 exp Drug Therapy/ (1125358) 64 exp Surgery/ (35422) 65 exp Biochemical Phenomena/ (3179065) 66 exp OBESITY/dt, ec, ra, ri, rt, su, ve [Drug Therapy, Economics, Radiography, Radionuclide Imaging, Radiotherapy, Surgery, Veterinary] (21417) 67 exp HIV/ (89024) 68 exp HIV infections/ (246055) 69 cancer.ti. (653428) 70 (tumour or tumor).ti. (242371) 71 lung.ti. (197074) 72 asthma.ti. (66394) 73 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 (8021499) 74 57 not 73 (1961)
Appendix 2. EMBASE search run to collect adult and child RCTs and cohort studies 14 November 2014
Search adapted from that run in 2010, to search for both adult and child RCTs and cohort studies, but omitting dietary exposures other than dietary fat.
Run 14 November 2014.
Database: EMBASE <1974 to 2014 November 14> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Weight Gain/ (67847) 2 exp weight reduction/ (104267) 3 obesity.ab,ti. (197751) 4 obese.ab,ti. (114407) 5 overweight.ab,ti. (55916) 6 over weight.ab,ti. (671) 7 ((weight or bmi or body mass index) adj2 (gain or loss or change or reduc$)).ab,ti. (154396) 8 exp fat intake/ (42075) 9 exp low fat diet/ (6962) 10 (fat$ adj2 (total or intake or consum$ or ate or eat or reduce$ or restrict$ or low$ or diet$)).ab,ti. (76246) 11 1 or 2 or 3 or 4 or 5 or 6 or 7 (440097) 12 8 or 9 or 10 (102724) 13 11 and 12 (27385) 14 controlled study/ (4458191) 15 randomized controlled trial/ (355956) 16 clinical trial/ (839688) 17 major clinical study/ (2275896) 18 (trial$ or control$).tw. (3805000) 19 (blind$ or placebo).tw. (383515) 20 placebo/ (260940) 21 14 or 15 or 16 or 17 or 18 or 19 or 20 (8434269) 22 exp human/ (15270878) 23 nonhuman/ (4404779) 24 23 not 22 (3499956) 25 21 not 24 (6542287) 26 exp Longitudinal Study/ (70712) 27 exp Prospective Study/ (266457) 28 (cohort$ or quintile$ or quartile$ or tertile$ or quantile$).mp. (498531) 29 (follow‐up$ or followup$).mp,tw. (1184342) 30 longitud$.mp. (214152) 31 ((prospectiv$ or observation$) adj5 (research$ or data$ or stud$)).mp. (615851) 32 26 or 27 or 28 or 29 or 30 or 31 (2100044) 33 32 not 24 (2060027) 34 33 or 25 (7492226) 35 13 and 34 (12448) 36 limit 35 to (english language and yr="2010 ‐ 2015") (6329) 37 exp Case‐Control Studies/ (90210) 38 (case adj3 control$).tw. (107292) 39 (case adj3 series).tw. (51300) 40 case study/ (28823) 41 letter.pt. (860483) 42 exp Drug Therapy/ (1859698) 43 exp Surgery/ (3481521) 44 exp Biochemical Phenomena/ (81777) 45 exp obesity/cn, di, dr, dt, rt, su [Congenital Disorder, Diagnosis, Drug Resistance, Drug Therapy, Radiotherapy, Surgery] (33545) 46 exp HIV/ (138030) 47 exp HIV infections/ (303673) 48 cancer.ti. (812504) 49 (tumour or tumor).ti. (277200) 50 lung.ti. (240253) 51 asthma.ti. (82529) 52 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (6915750) 53 36 not 52 (5003)
Appendix 3. CINAHL search run to collect adult and child RCTs and cohort studies 1 December 2014 (Interface EBSCO host Research Databases, Advanced Search, CINAHL Complete)
| # | Query | Limiters/Expanders | Results |
| S1 | (MH "weight gain+") | Search modes ‐ Boolean/Phrase | 62,681 |
| S2 | (MH "weight loss+") | Search modes ‐ Boolean/Phrase | 14,411 |
| S3 | TI obesity OR AB obesity | Search modes ‐ Boolean/Phrase | 32,659 |
| S4 | TI obese OR AB obese | Search modes ‐ Boolean/Phrase | 15,905 |
| S5 | TI adipos* OR AB adipos* | Search modes ‐ Boolean/Phrase | 6,462 |
| S6 | TI weight gain OR AB weight gain | Search modes ‐ Boolean/Phrase | 6,645 |
| S7 | TI weight loss OR AB weight loss | Search modes ‐ Boolean/Phrase | 11,452 |
| S8 | TI overweight OR AB overweight | Search modes ‐ Boolean/Phrase | 12,405 |
| S9 | TI over weight OR AB over weight | Search modes ‐ Boolean/Phrase | 1,157 |
| S10 | TI overeat* OR AB overeat* | Search modes ‐ Boolean/Phrase | 418 |
| S11 | TI over eat* OR AB over eat* | Search modes ‐ Boolean/Phrase | 321 |
| S12 | TI weight change* OR AB weight change* | Search modes ‐ Boolean/Phrase | 3,689 |
| S13 | (TI ((bmi or body mass index) N2 (gain or loss or change))) OR (AB ((bmi or body mass index) N2 (gain or loss or change))) | Search modes ‐ Boolean/Phrase | 862 |
| S14 | TI body fat* OR AB body fat* | Search modes ‐ Boolean/Phrase | 5,932 |
| S15 | TI body composition OR AB body composition | Search modes ‐ Boolean/Phrase | 5,353 |
| S16 | TI body constitution OR AB body constitution | Search modes ‐ Boolean/Phrase | 26 |
| S17 | (MH "Dietary Fats+") | Search modes ‐ Boolean/Phrase | 17,455 |
| S18 | (MM "Diet, Fat‐Restricted") | Search modes ‐ Boolean/Phrase | 901 |
| S19 | (TI (fat* N2 (total or intake or consum* or ate or eat or reduc* or restrict* or low* or diet*))) OR (AB (fat* N2 (total or intake or consum* or ate or eat or reduc* or restrict* or low* or diet*))) | Search modes ‐ Boolean/Phrase | 11,074 |
| S20 | (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16) | Search modes ‐ Boolean/Phrase | 99,408 |
| S21 | (S17 OR S18 OR S19) | Search modes ‐ Boolean/Phrase | 25,122 |
| S22 | (S20 AND S21) | Search modes ‐ Boolean/Phrase | 6,404 |
| S23 | PT randomized controlled trial | Search modes ‐ Boolean/Phrase | 45,326 |
| S24 | TX "controlled clinical trial" | Search modes ‐ Boolean/Phrase | 7,628 |
| S25 | MM "Randomized Controlled Trials" | Search modes ‐ Boolean/Phrase | 668 |
| S26 | MM "Random Assignment" | Search modes ‐ Boolean/Phrase | 147 |
| S27 | MM "Double‐Blind Studies" | Search modes ‐ Boolean/Phrase | 76 |
| S28 | MM "Single‐Blind Studies" | Search modes ‐ Boolean/Phrase | 26 |
| S29 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 | Search modes ‐ Boolean/Phrase | 52,650 |
| S30 | SU (animals not (human and animals)) | Search modes ‐ Boolean/Phrase | 53,619 |
| S31 | S29 NOT S30 | Search modes ‐ Boolean/Phrase | 52,575 |
| S32 | PT clinical trial | Search modes ‐ Boolean/Phrase | 77,533 |
| S33 | MH "Clinical Trials+" | Search modes ‐ Boolean/Phrase | 184,793 |
| S34 | TI (clin* N25 trial*) OR AB (clin* N25 trial*) | Search modes ‐ Boolean/Phrase | 53,327 |
| S35 | TI ((singl* or doubl* or trebl* or tripl* or quad*) N (blind* or mask*)) OR AB ((singl* or doubl* or trebl* or tripl* or quad*) N (blind* or mask*)) | Search modes ‐ Boolean/Phrase | 300 |
| S36 | MM "Placebos" | Search modes ‐ Boolean/Phrase | 828 |
| S37 | TI placebo* OR AB placebo* | Search modes ‐ Boolean/Phrase | 27,852 |
| S38 | TI random* OR AB random* | Search modes ‐ Boolean/Phrase | 144,733 |
| S39 | MM "study design" | Search modes ‐ Boolean/Phrase | 5,275 |
| S40 | MM "comparative studies" | Search modes ‐ Boolean/Phrase | 283 |
| S41 | MH "Evaluation Research+" | Search modes ‐ Boolean/Phrase | 20,984 |
| S42 | MM "prospective studies" | Search modes ‐ Boolean/Phrase | 800 |
| S43 | TI (control* or prospectiv* or volunteer*) OR AB (control* or prospectiv* or volunteer*) | Search modes ‐ Boolean/Phrase | 357,450 |
| S44 | S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 | Search modes ‐ Boolean/Phrase | 542,974 |
| S45 | S44 NOT S30 | Search modes ‐ Boolean/Phrase | 535,502 |
| S46 | S31 OR S45 | Search modes ‐ Boolean/Phrase | 541,731 |
| S47 | MH "prospective studies+" | Search modes ‐ Boolean/Phrase | 254,176 |
| S48 | TX cohort* or quintile* or quartile* or quantile* or tertile* | Search modes ‐ Boolean/Phrase | 152,914 |
| S49 | TX follow‐up* or followup* | Search modes ‐ Boolean/Phrase | 249,854 |
| S50 | TX longitud* | Search modes ‐ Boolean/Phrase | 103,954 |
| S51 | TX ((prospectiv* or observation*) N5 (research* or data* or stud*)) | Search modes ‐ Boolean/Phrase | 382,309 |
| S52 | S47 OR S48 OR S49 OR S50 OR S51 | Search modes ‐ Boolean/Phrase | 613,040 |
| S53 | S52 NOT S30 | Search modes ‐ Boolean/Phrase | 610,840 |
| S54 | S46 OR S53 | Search modes ‐ Boolean/Phrase | 963,714 |
| S55 | S22 AND S54 | Search modes ‐ Boolean/Phrase | 3,017 |
| S56 | S22 AND S54 | Limiters ‐ Published Date: 20100101‐20151231; English Language Search modes ‐ Boolean/Phrase | 1,236 |
| S57 | MH "Case Control Studies+" | Limiters ‐ Published Date: 20100101‐20151231; English Language Search modes ‐ Boolean/Phrase | 23,820 |
| S58 | TX case N3 control* | Limiters ‐ Published Date: 20100101‐20151231; English Language Search modes ‐ Boolean/Phrase | 35,592 |
| S59 | TX case N3 series | Limiters ‐ Published Date: 20100101‐20151231; English Language Search modes ‐ Boolean/Phrase | 10,407 |
| S60 | MM "Case Studies" | Search modes ‐ Boolean/Phrase | 623 |
| S61 | PT letter | Search modes ‐ Boolean/Phrase | 198,888 |
| S62 | MH "Drug Therapy+" | Search modes ‐ Boolean/Phrase | 109,541 |
| S63 | MH "Surgery, Operative+" | Search modes ‐ Boolean/Phrase | 385,583 |
| S64 | MH "Biochemical Phenomena+" | Search modes ‐ Boolean/Phrase | 29,949 |
| S65 | MH "Obesity+/DT/EC/RA/RT/SU" | Search modes ‐ Boolean/Phrase | 5,470 |
| S66 | MH "Human Immunodeficiency Virus+" | Search modes ‐ Boolean/Phrase | 5,947 |
| S67 | MH "HIV Infections+" | Search modes ‐ Boolean/Phrase | 62,282 |
| S68 | TI cancer | Search modes ‐ Boolean/Phrase | 137,532 |
| S69 | TI tumor OR tumour | Search modes ‐ Boolean/Phrase | 21,392 |
| S70 | TI lung | Search modes ‐ Boolean/Phrase | 24,925 |
| S71 | TI asthma | Search modes ‐ Boolean/Phrase | 15,732 |
| S72 | S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 | Search modes ‐ Boolean/Phrase | 913,702 |
| S73 | S56 NOT S72 | Search modes ‐ Boolean/Phrase | 765 |
Appendix 4. CENTRAL search run as part of the update in March 2014
#1 lipid near (low* or reduc* or modifi*)
#2 cholesterol* near (low* or modifi* or reduc*)
#3 (#1 or #2)
#4 MeSH descriptor: [Nutrition Therapy] explode all trees
#5 diet* or food* or nutrition*
#6 (#4 or #5)
#7 (#3 and #6)
#8 fat* near (low* or reduc* or modifi* or animal* or saturat* or unsaturat*)
#9 MeSH descriptor: [Diet, Atherogenic] explode all trees
#10 MeSH descriptor: [Diet Therapy] explode all trees
#11 (#7 or #8 or #9 or #10)
#12 MeSH descriptor: [Cardiovascular Diseases] this term only
#13 MeSH descriptor: [Heart Diseases] explode all trees
#14 MeSH descriptor: [Vascular Diseases] explode all trees
#15 MeSH descriptor: [Cerebrovascular Disorders] this term only
#16 MeSH descriptor: [Brain Ischemia] explode all trees
#17 MeSH descriptor: [Carotid Artery Diseases] explode all trees
#18 MeSH descriptor: [Dementia, Vascular] explode all trees
#19 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees
#20 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees
#21 MeSH descriptor: [Intracranial Hemorrhages] explode all trees
#22 MeSH descriptor: [Stroke] explode all trees
#23 coronar* near (bypas* or graft* or disease* or event*)
#24 cerebrovasc* or cardiovasc* or mortal* or angina* or stroke or strokes or tia or ischaem* or ischem*
#25 myocardi* near (infarct* or revascular* or ischaem* or ischem*)
#26 morbid* near (heart* or coronar* or ischaem* or ischem* or myocard*)
#27 vascular* near (peripheral* or disease* or complication*)
#28 heart* near (disease* or attack* or bypas*)
#29 (#12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28)
#30 (#11 and #29)
Data and analyses
Comparison 1. Fat reduction versus usual fat diet, adult RCTs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight, kg | 30 | 53647 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐1.97, ‐1.12] |
| 2 BMI, kg/m2 | 10 | 45703 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.74, ‐0.26] |
| 3 Waist circumference, cm | 1 | 15671 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.58, ‐0.02] |
| 4 LDL cholesterol, mmol/L | 18 | 7285 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.23, ‐0.03] |
| 5 HDL cholesterol, mmol/L | 19 | 7166 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.00] |
| 6 Total cholesterol, mmol/L | 20 | 7715 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.29, ‐0.11] |
| 7 Triglycerides, mmol/L | 17 | 6976 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 8 Total cholesterol/HDL | 7 | 3332 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.04] |
| 9 Systolic blood pressure, mmHg | 9 | 5159 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.95, ‐0.37] |
| 10 Diastolic blood pressure, mmHg | 9 | 5159 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.40, ‐0.08] |
1.3. Analysis.
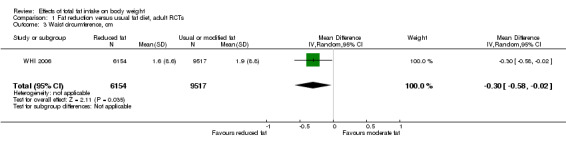
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 3 Waist circumference, cm.
1.4. Analysis.
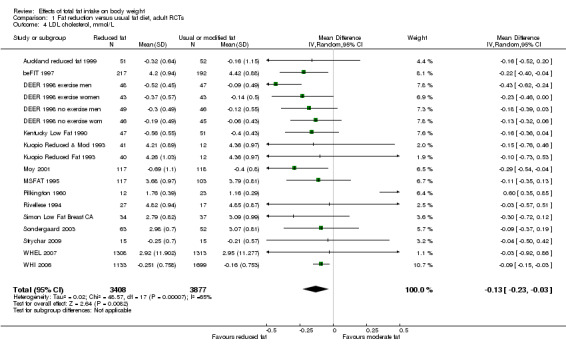
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 4 LDL cholesterol, mmol/L.
1.5. Analysis.
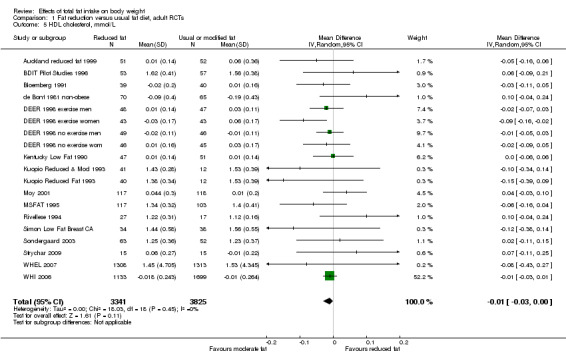
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 5 HDL cholesterol, mmol/L.
1.6. Analysis.
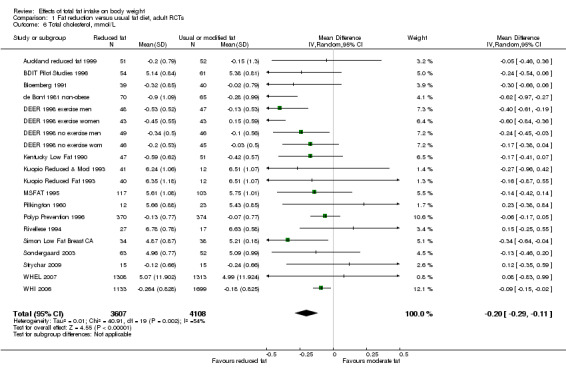
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 6 Total cholesterol, mmol/L.
1.7. Analysis.
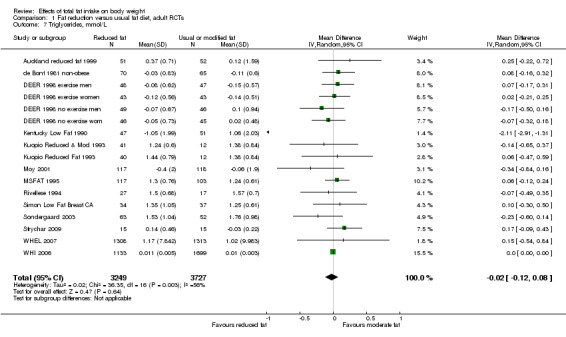
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 7 Triglycerides, mmol/L.
1.8. Analysis.
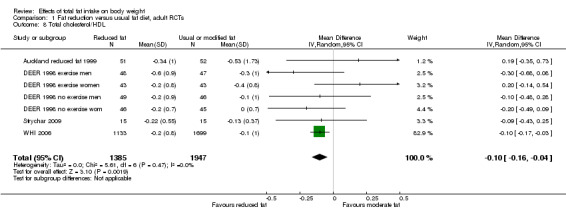
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 8 Total cholesterol/HDL.
1.9. Analysis.
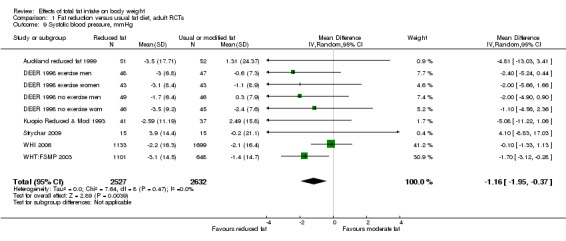
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 9 Systolic blood pressure, mmHg.
1.10. Analysis.
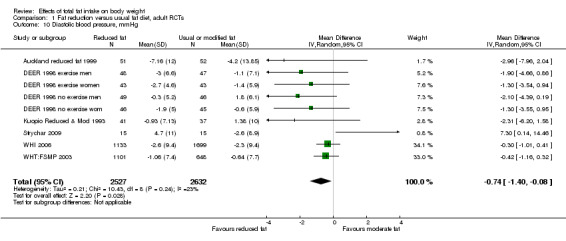
Comparison 1 Fat reduction versus usual fat diet, adult RCTs, Outcome 10 Diastolic blood pressure, mmHg.
Comparison 2. Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight ‐ subgrouped by duration of advice | 30 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 to < 12 months | 16 | 5305 | Mean Difference (IV, Random, 95% CI) | ‐1.74 [‐2.34, ‐1.13] |
| 1.2 12 to < 24 months | 18 | 51367 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐2.51, ‐1.48] |
| 1.3 24 to < 60 months | 10 | 49286 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.65, ‐0.70] |
| 1.4 60+ months | 4 | 40838 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.66, 0.29] |
| 2 Weight, subgrouped by control group fat intake | 29 | 54335 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐1.15, ‐0.86] |
| 2.1 > 35%E from fat | 13 | 45103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.07, ‐0.75] |
| 2.2 > 30% to 35%E from fat | 11 | 7123 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.21, ‐0.48] |
| 2.3 > 25% to 30%E from fat | 5 | 2109 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐3.60, ‐2.34] |
| 3 Weight, subgrouped by sex | 30 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Studies of women only | 17 | 50154 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.93, ‐0.91] |
| 3.2 Studies of men only | 6 | 1719 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐4.32, ‐1.17] |
| 3.3 Studies of men and women | 7 | 2492 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [0.00, ‐0.18] |
| 4 Weight, subgrouped by year of first publication of results | 30 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 1960s | 3 | 1450 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐8.06, ‐0.14] |
| 4.2 1970s | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 1980s | 3 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.80, ‐0.01] |
| 4.4 1990s | 16 | 5941 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐2.62, ‐1.25] |
| 4.5 2000s | 8 | 46686 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.59, ‐0.29] |
| 4.6 2010s | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Weight, subgrouped by difference in %E from fat between control and reduced fat groups | 32 | 57583 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐1.97, ‐1.12] |
| 5.1 Up to 5%E from fat | 8 | 4567 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.91, 0.59] |
| 5.2 5% to < 10%E from fat | 14 | 44356 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐2.87, ‐1.35] |
| 5.3 10% to < 15%E from fat | 5 | 8311 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐1.70, ‐0.98] |
| 5.4 15+%E from fat | 4 | 319 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐8.76, 0.99] |
| 5.5 Unknown difference in %E from fat | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐4.20, ‐0.66] |
| 6 Weight ‐ subgrouped by advice vs provided | 29 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Dietary advice | 25 | 52594 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐2.00, ‐1.10] |
| 6.2 Advice plus supplements | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Diet provided | 4 | 1741 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.34, ‐0.10] |
| 7 Weight subgrouped by fat goals | 29 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 30%E from fat goal | 5 | 1628 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.66, ‐0.26] |
| 7.2 25% to < 30%E from fat goal | 6 | 509 | Mean Difference (IV, Random, 95% CI) | ‐2.45 [‐4.27, ‐0.64] |
| 7.3 20% to < 25%E from fat goal | 6 | 43878 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.24, ‐0.55] |
| 7.4 15% to < 20%E from fat goal | 8 | 7860 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐2.19, ‐0.37] |
| 7.5 10% to < 15%E from fat goal | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 No specific goal stated | 4 | 460 | Mean Difference (IV, Random, 95% CI) | ‐2.49 [‐5.03, 0.05] |
| 8 Weight, kg subgrouped of above below 30%E from fat | 24 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Int achieved > 30%E from fat | 8 | 1767 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.28, ‐0.37] |
| 8.2 Int achieved 30%E from fat or less | 16 | 50099 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.62, ‐0.60] |
| 9 Weight, kg subgrouped by BMI baseline | 28 | 53147 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐1.97, ‐1.12] |
| 9.1 BMI at baseline < 25 | 10 | 1781 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.69, ‐0.22] |
| 9.2 BMI at baseline ≥ 25 to 29.9 | 17 | 51297 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐2.38, ‐1.28] |
| 9.3 BMI at baseline ≥ 30 | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.48, ‐0.12] |
| 10 Weight, kg subgrouped by healthy vs patient | 30 | 53647 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐1.97, ‐1.12] |
| 10.1 Healthy ‐ not recruited on the basis of risk factors or disease | 6 | 45032 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.56, ‐0.41] |
| 10.2 Recruited on basis of risk factors, e.g. lipids, BMI, hormonal levels, breast CA risk | 14 | 2166 | Mean Difference (IV, Random, 95% CI) | ‐2.18 [‐3.17, ‐1.20] |
| 10.3 People with disease such as DM, MI, cancer, polyps | 10 | 6449 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.85, ‐0.56] |
| 11 Weight, kg subgrouped by energy reduction in int group | 26 | 53459 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐1.97, ‐1.07] |
| 11.1 E intake same or greater in low fat group | 6 | 3352 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.49, 0.47] |
| 11.2 E intake 1 to 100 kcal/d less in low fat group | 5 | 2398 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐2.92, ‐0.06] |
| 11.3 E intake 101 to 200 kcal/d less in low fat group | 6 | 43755 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.24, ‐0.04] |
| 11.4 E intake > 201 kcal/d less in low fat group | 9 | 3954 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐2.97, ‐1.49] |
2.1. Analysis.
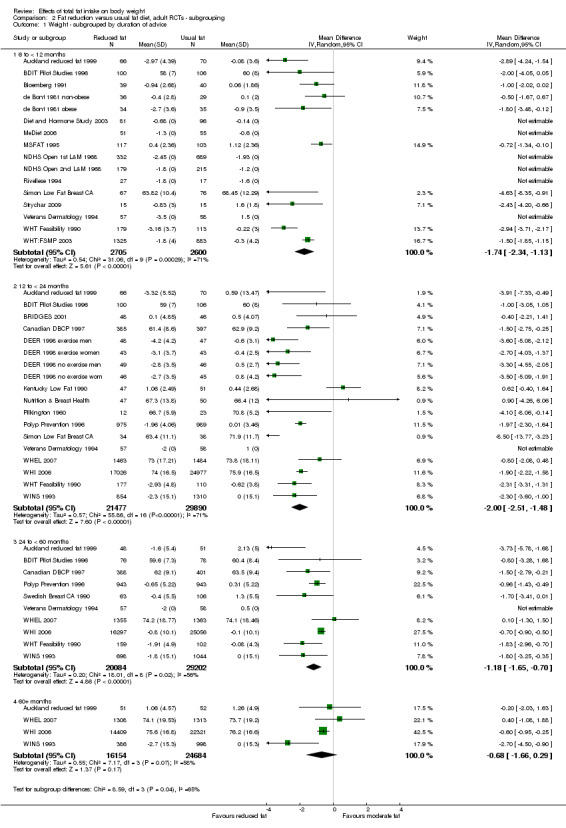
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 1 Weight ‐ subgrouped by duration of advice.
2.2. Analysis.
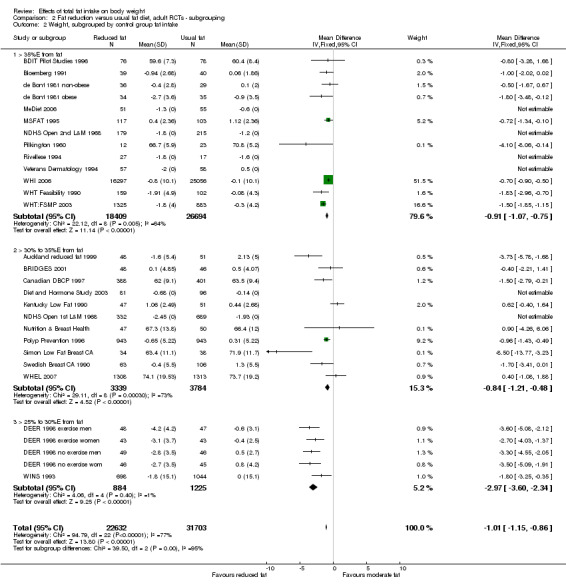
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 2 Weight, subgrouped by control group fat intake.
2.3. Analysis.
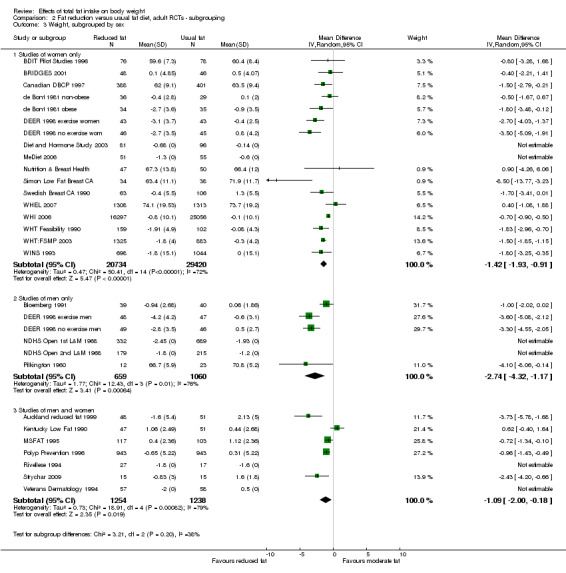
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 3 Weight, subgrouped by sex.
2.4. Analysis.
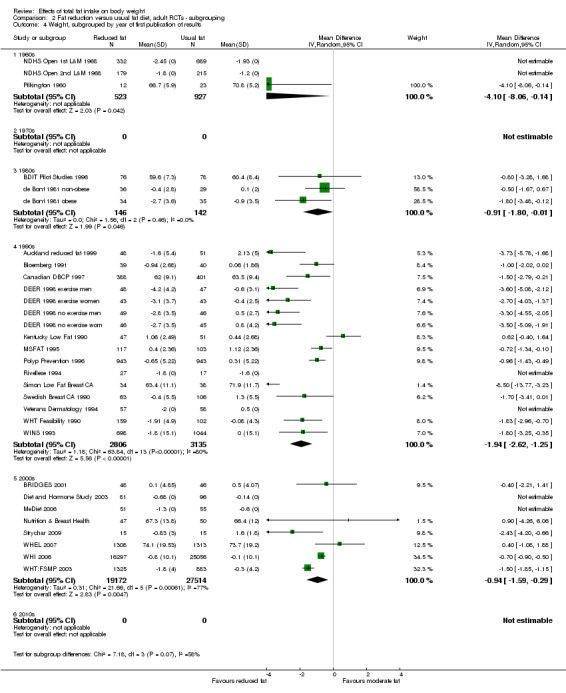
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 4 Weight, subgrouped by year of first publication of results.
2.5. Analysis.
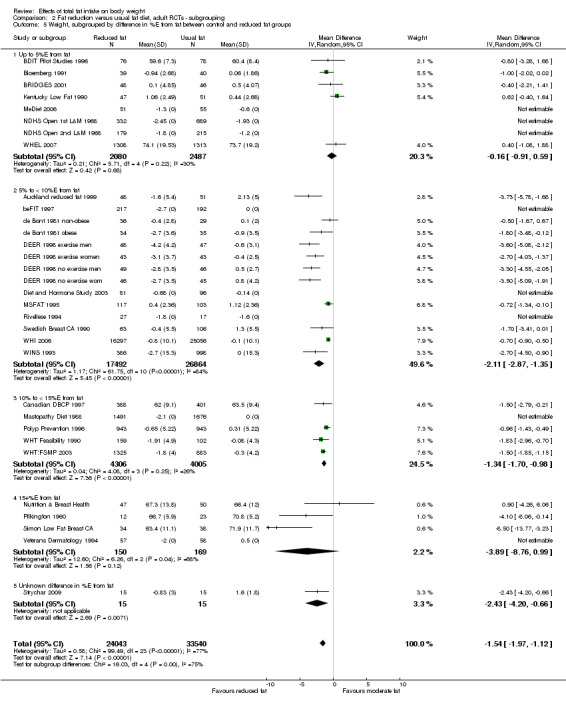
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 5 Weight, subgrouped by difference in %E from fat between control and reduced fat groups.
2.6. Analysis.
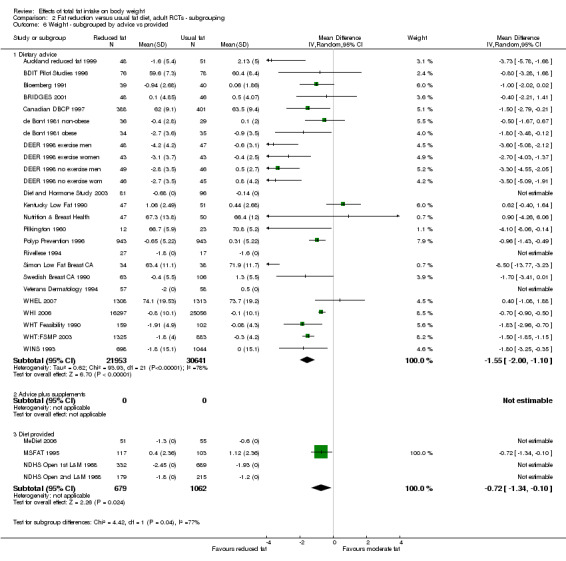
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 6 Weight ‐ subgrouped by advice vs provided.
2.7. Analysis.
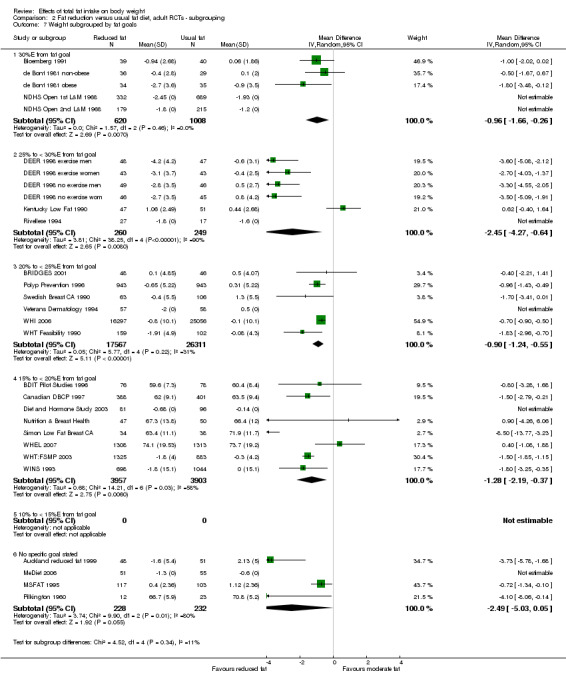
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 7 Weight subgrouped by fat goals.
2.8. Analysis.
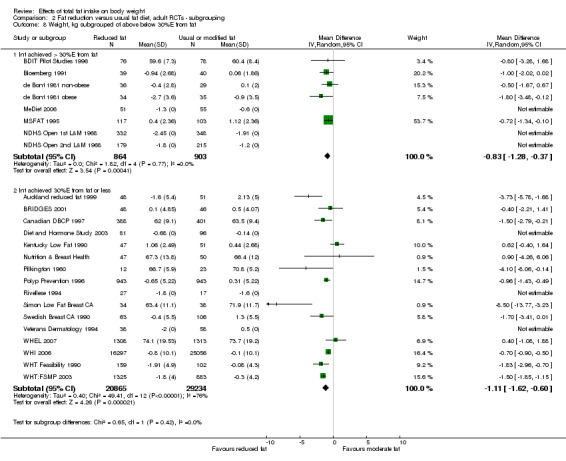
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 8 Weight, kg subgrouped of above below 30%E from fat.
2.9. Analysis.
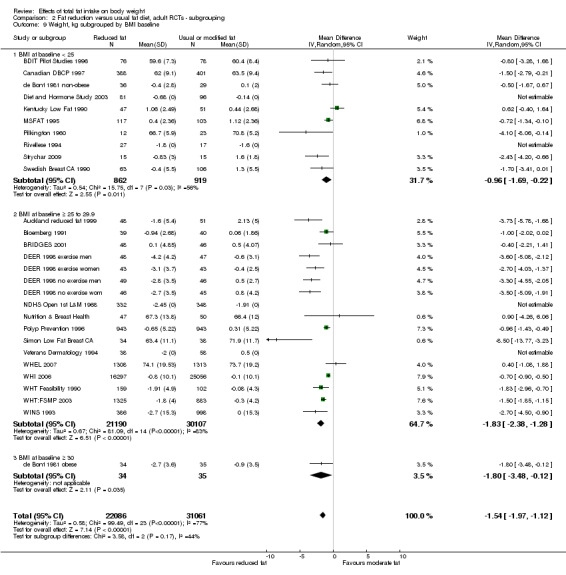
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 9 Weight, kg subgrouped by BMI baseline.
2.10. Analysis.
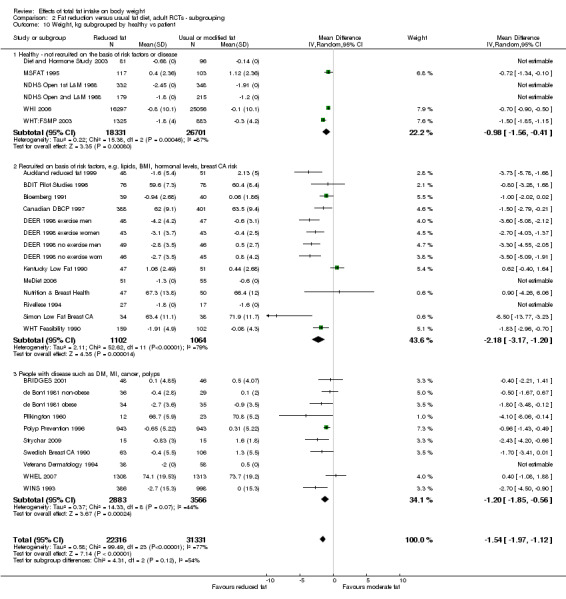
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 10 Weight, kg subgrouped by healthy vs patient.
2.11. Analysis.
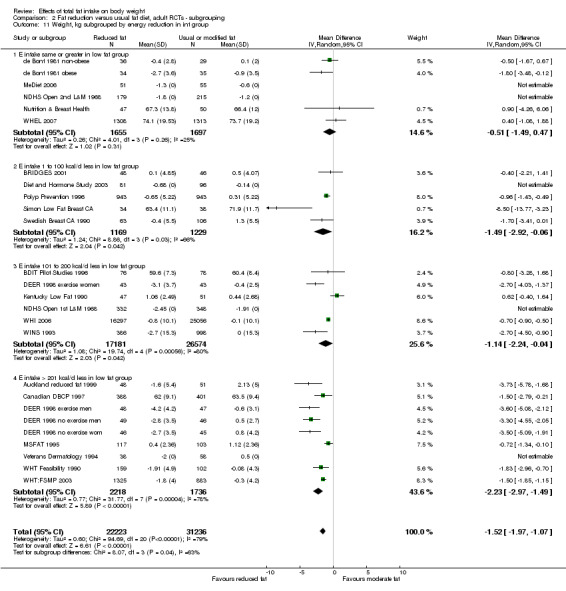
Comparison 2 Fat reduction versus usual fat diet, adult RCTs ‐ subgrouping, Outcome 11 Weight, kg subgrouped by energy reduction in int group.
Comparison 3. Fat reduction versus usual fat diet, adult RCTs ‐ sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight, kg ‐ removing studies with more attention to low fat arms | 8 | 1537 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐2.09, ‐0.41] |
| 2 Weight, kg ‐ removing studies with dietary interventions other than fat | 22 | 5516 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐2.57, ‐1.26] |
| 3 Weight, kg ‐ fixed‐effect analysis | 30 | 54005 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.16, ‐0.87] |
| 4 Weight, kg ‐ removing WHI | 29 | 12294 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐2.12, ‐1.16] |
| 5 Weight, kg ‐ removing studies without good allocation concealment | 11 | 49617 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.40, ‐0.51] |
3.1. Analysis.
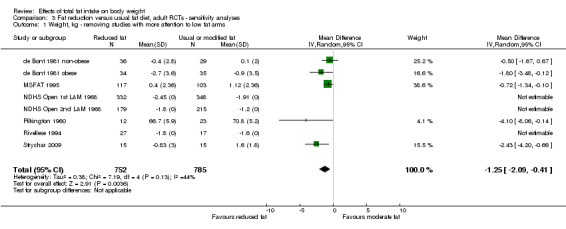
Comparison 3 Fat reduction versus usual fat diet, adult RCTs ‐ sensitivity analyses, Outcome 1 Weight, kg ‐ removing studies with more attention to low fat arms.
3.2. Analysis.
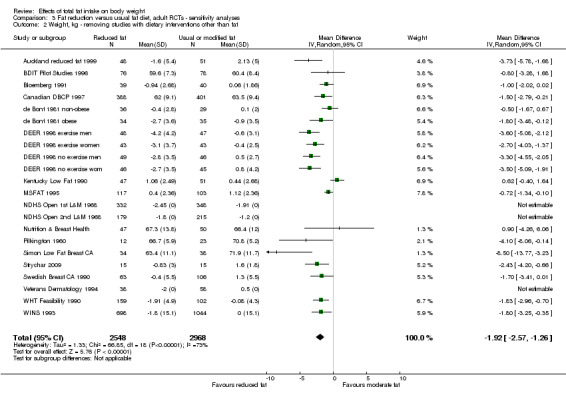
Comparison 3 Fat reduction versus usual fat diet, adult RCTs ‐ sensitivity analyses, Outcome 2 Weight, kg ‐ removing studies with dietary interventions other than fat.
3.3. Analysis.
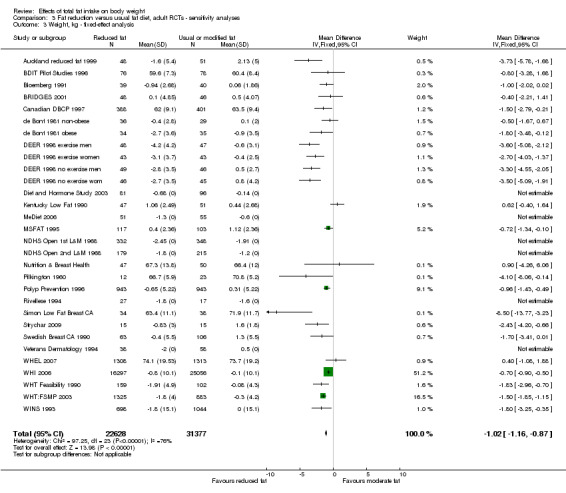
Comparison 3 Fat reduction versus usual fat diet, adult RCTs ‐ sensitivity analyses, Outcome 3 Weight, kg ‐ fixed‐effect analysis.
3.4. Analysis.
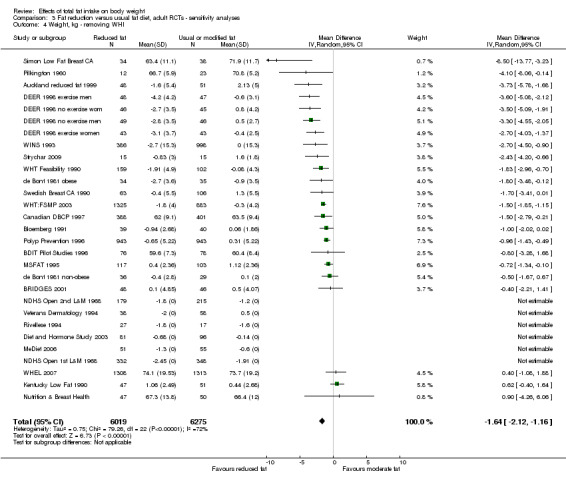
Comparison 3 Fat reduction versus usual fat diet, adult RCTs ‐ sensitivity analyses, Outcome 4 Weight, kg ‐ removing WHI.
3.5. Analysis.
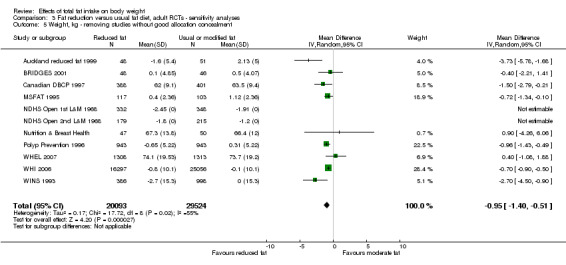
Comparison 3 Fat reduction versus usual fat diet, adult RCTs ‐ sensitivity analyses, Outcome 5 Weight, kg ‐ removing studies without good allocation concealment.
Comparison 4. Fat reduction versus usual fat, child RCTs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BMI, kg/m2 ‐ in child RCTs | 1 | 191 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.45, ‐0.55] |
4.1. Analysis.
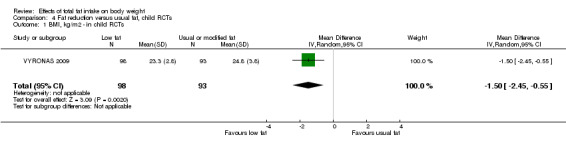
Comparison 4 Fat reduction versus usual fat, child RCTs, Outcome 1 BMI, kg/m2 ‐ in child RCTs.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Auckland reduced fat 1999.
| Methods | RCT | |
| Participants | People with impaired glucose intolerance or high normal blood glucose (New Zealand)
CVD risk: moderate
Control: unclear how many randomised (176 between both groups), 51 analysed
Intervention: unclear how many randomised (176 between both groups), 48 analysed
Mean years in trial: 4.1 over whole trial
% male: control 80%, intervention 68%
Age: mean control 52.0 (SE 0.8), intervention 52.5 (SE 0.8) Baseline BMI: mean control 29.1 (SE 0.6), intervention 29.3 (SE 0.6) |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet Intervention aims: reduced fat diet (no specific goal stated) Control methods: usual intake Intervention methods: monthly meetings to follow a 1‐year structured programme aimed at reducing fat in the diet; includes education, personal goal setting, self monitoring Weight goals: weight and calories not mentioned, diet was "aimed solely at reducing the total amount of fat in their diet" Total fat intake (at 1 year): low fat 26.1 (SD 7.7), cont 33.6 (SD 7.8) %E Saturated fat intake (at 1 year): low fat 10.0 (SD 4.2), cont 13.4 (SD 4.7) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: lipids, glucose, blood pressure Available outcomes: weight, total, LDL and HDL cholesterol, TG, BP |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unmarked opaque envelopes were opened by the person recruiting, unable to alter allocation later |
| Allocation concealment (selection bias) | Low risk | Unmarked opaque envelopes were opened by the person recruiting, unable to alter allocation later |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, outcome assessors were |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 77 of 176 recruited lost to follow‐up, 44% over 5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
BDIT Pilot Studies 1996.
| Methods | RCT | |
| Participants | Women with mammographic dysplasia (Canada)
CVD risk: low
Control: 147 randomised, 78 analysed
Intervention: 148 randomised, 76 analysed
Mean years in trial: control 7.5, intervention 6.8
% male: 0
Age: mean control 45, intervention 44 (all > 30) Baseline BMI: mean intervention 24.3 (SD 3.8), control 24.3 (SD 3.6) |
|
| Interventions | Reduced fat intake vs usual diet Control aims: healthy diet advice, no alteration in dietary fat advised, aim to maintain weight Intervention aims: total fat 15%E, replace fat by complex CHO, aim to maintain weight Control methods: seen for advice once every 4 months for 12 months Intervention methods: seen for advice once a month for 12 months Weight goal: low fat group ‐ "isocaloric exchange of complex carbohydrate for fat. We tried to maintain an isocaloric diet to avoid weight loss...". Not discussed for control group Total fat intake (at 9.2 years): low fat 31.7 (SD 7.3) %E, control 35.3 (SD 5.6) %E Saturated fat intake (at 9.2 years): low fat 10.6 (SD 4.6) %E, control 12.3 (SD 4.6) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary fat, serum cholesterol Available outcomes: weight, BMI, total and HDL cholesterol |
|
| Notes | Weight data available for 1 year, 2 years and 9 years. Unclear whether participants were still in the trial by 9 years, so 2‐year data used in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants not blinded, but outcome assessors blinded to intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 141 of 295 (48%) lost over 8 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Minor: women in intervention group seen more frequently. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
beFIT 1997.
| Methods | RCT | |
| Participants | Women and men with mild hypercholesterolaemia (USA)
CVD risk: moderate
Control: unclear how many randomised, 192 analysed
Intervention: unclear how many randomised, 217 analysed
Mean years in trial: unclear (max duration 0.5 years)
% male: 52 (not divided by intervention group)
Age: mean 43.2 (not divided by intervention group) (all > 30) Baseline BMI (not reported by intervention): women with hypercholesterolaemia (n = 84) mean 25.9 (SD 4.9), women with combined hyperlipidaemia (n = 94) mean 29.2 (SD 6.1), men with hypercholesterolaemia (n = 123) mean 26.6 (SD 3.3), men with combined hyperlipidaemia (n = 108) mean 27.5 (SD 3.2) |
|
| Interventions | Reduced and modified fat vs usual diet Control aims: asked to delay dietary changes (provided intervention after the randomised trial) Intervention aims: total fat < 30%E, SFA < 7%E, dietary cholesterol < 200 mg/d Control methods: usual intake Intervention methods: 8 weekly classes with nutrition info and behaviour modification with spouses, plus individual appointments at 3 and 6 months Weight goals: intervention group "assigned food group pattern for their calorie needs", no information for control group. Total fat intake (at 6 months): intervention 25.2 (SD unclear) %E, control unclear ‐ no significant difference from baseline 34 (SD unclear) %E Saturated fat intake (at 6 months): intervention 7.6% (SD unclear) %E, control unclear ‐ no significant difference from baseline 12 (SD unclear)%E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: lipids Available outcomes: weight, total, LDL and HDL cholesterol, TG (but variance data only provided for the randomised comparison for LDL cholesterol) |
|
| Notes | Weight: control 'no change', intervention ‐2.7 kg at 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random sampling scheme |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants knew their allocation, unclear for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear what proportion lost over trial as unclear how many recruited |
| Selective reporting (reporting bias) | High risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Intensive intervention for intervention group, but no intervention during the 6 months of the randomised part of the study for the control group. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Bloemberg 1991.
| Methods | RCT | |
| Participants | Men with untreated raised total cholesterol (the Netherlands)
CVD risk: moderate
Control: randomised 41, analysed 40
Intervention: randomised 39, analysed 39
Mean years in trial: control 0.5, randomised 0.5
% male: 100%
Age: mean control 47.5 (SD 8.0), intervention 47.2 (SD 8.3) Baseline BMI: mean control 26.3 (SD 2.3), intervention 26.0 (SD 2.6) |
|
| Interventions | Reduced and modified fat vs usual diet Control aims: usual diet Intervention aims: 30%E from fat, PUFA/SFA 1.0, dietary cholesterol 20 mg Control methods: no advice provided Intervention methods: individual advice provided face to face, followed by 2 phone calls and 5 mailings of information on healthy foods Weight goals: weight and calories not mentioned Total fat intake (change to 6 months): intervention ‐5.0 (SD 6.5) (33.5 overall), control ‐1.5 (SD 5.9) (36.8 overall) %E Saturated fat intake (change to 6 months): intervention‐4.3 (SD 3.9), control ‐0.7 (SD 2.9) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: lipids Available outcomes: weight, total and HDL cholesterol |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised" and stratified by age and BMI (each dichotomised) |
| Allocation concealment (selection bias) | Unclear risk | No method stated (as above) |
| Blinding (performance bias and detection bias) All outcomes | High risk | No for participants, yes for laboratory staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 of 80 (< 1%) lost over 0.5 years (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Much more time spent on those in the intervention group |
| Free of dietary differences other than fat? | Low risk | Dietary focus on fats alone |
BRIDGES 2001.
| Methods | RCT | |
| Participants | Women diagnosed with stage I or II breast cancer over the past 2 years (USA)
CVD risk: low
Control: randomised unclear (at least 56), analysed 46
Intervention: randomised unclear (at least 50), analysed 48
Mean years in trial: unclear (1 year max follow‐up)
% male: 0
Age: mean control unclear (71% postmenopausal), intervention unclear (56% postmenopausal) (all 20 to 65) Baseline BMI: not reported |
|
| Interventions | Reduced fat vs usual diet Control aims: no formal intervention Intervention diet aims: total fat 20%E, high fibre, plant‐based micronutrients Intervention stress: separate parallel arm, stress reduction programme (data not used here) Control methods: no formal intervention Intervention methods: nutrition intervention programme, 15 sessions (42 hours) over 15 weeks, group‐based, dietitian led, 2 individual sessions using social cognitive theory and patient centred counselling to increase self efficacy and confidence Weight goals: "reduction in body mass was not a primary goal of NEP. (NEP was neither designed nor presented to participants as a weight loss or weight control program)." The control group was presented as "individual choice". Total fat intake (at 12 months): low fat 29.9 (SD unclear), control 33.6 (SD unclear) %E Saturated fat intake: unclear Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: diet and BMI Available outcomes: weight |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised", stratified by medical centre, cancer stage and age, randomised number/envelope method by project co‐ordinator |
| Allocation concealment (selection bias) | Low risk | The project co‐ordinator had contact with those from the University of Massachusetts, but not those from the other 3 centres, and allocation could not be altered later |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants not blinded, unclear about researchers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear how many recruited, so unclear how many were lost to follow‐up (at least 12 of 106 (11%) over 1 year, so > 5%/year |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | High‐intensity programme for intervention group, nothing for control group. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | High risk | Intervention also focused on fibre and plant based micronutrients. See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Canadian DBCP 1997.
| Methods | RCT | |
| Participants | Women with mammographic densities > 50% breast area (Canada)
CVD risk: low
Control: randomised 448+, analysed 401
Intervention: randomised 448+, analysed 388
Mean years in trial: control 2.0, randomised 2.0 (note, papers suggest a 10‐year follow‐up overall)
% male: 0%
Age: mean control 45.9 (SD unclear), intervention 46.5 (SD unclear) Baseline BMI: mean control 23.6, intervention 23.4, no variance reported |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet Intervention aims: total fat 15%E, protein 20%E, CHO 65%E, isocaloric diet Control methods: encouraged to continue usual diet, interviewed by dietitian every 4 months during first year, then every 3 months in the second year Intervention methods: dietary prescription using food exchange (fat calories replaced by CHO), met with dietitian monthly during first year, then every 3 months. Scales, recipes, shopping guide provided Weight goals: "calories derived from fat were replaced by isocaloric exchange with carbohydrate" Total fat intake (at 2 years): intervention 21.3 (SD 6.2), control 31.8 (SD 6.7) %E Saturated fat intake (at 2 years): intervention 7.1 (SD 2.5), control 11.5 (SD 3.3) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: incidence of breast cancer Available outcomes: weight |
|
| Notes | Weight data available for 1 and 2 years, 2‐year data used in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated by telephone to Dept. of Biostatistics at Ontario Cancer Institute, stratified by centre |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants knew what arm they were in |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At least 107 of at least 896 (12%) lost over 2 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Minor difference in attention for participants in intervention and control in first year |
| Free of dietary differences other than fat? | Low risk | Focus on dietary fat |
de Bont 1981 non‐obese.
| Methods | RCT | |
| Participants | Women with type 2 diabetes (UK)
CVD risk: moderate
Control: randomised unclear (total in control and intervention 148), analysed 65 (for obese and non‐obese)
Intervention: randomised unclear, analysed 71 (for obese and non‐obese)
Mean years in trial: control 0.5, randomised 0.5
% male: 0%
Age: mean control 54 (SD 8), intervention 56 (SD 7), (all 35 to 64) (for obese and non‐obese) Baseline BMI: chosen for BMI < 28, mean not reported |
|
| Interventions | Reduced and modified fat vs usual diet Control aims: usual diet but with CHO ≤ 40%E Intervention aims: 30%E from fat, focus on reducing meat fat, dairy foods and substituting margarines to improve the SFA/PUFA ratio, CHO increased to maintain energy intake Control methods: 3 home visits from a nutritionist over the 6 months of the trial Intervention methods: 3 home visits from a nutritionist over the 6 months of the trial Weight goals: to maintain the required total energy intake the proportion of carbohydrates in these diets was increased. Total fat intake (change to 6 months): intervention‐10.1 (SD 10.8) (overall 31.1), control ‐1.0 (SD 10.5) (overall 41.8) %E (for obese and non‐obese) Saturated fat intake (change to 6 months): intervention‐8.1 (SD 5.8), control ‐1.1 (SD 5.7) %E (for obese and non‐obese) Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: diet, weight, lipids Available outcomes: weight, total and HDL cholesterol, triglycerides |
|
| Notes | Outcome data separated by those obese (BMI ≥ 28) or not obese at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | No for participants, unclear for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 of 148 (8%) lost over 0.5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Follow‐up similar |
| Free of dietary differences other than fat? | Low risk | Diet focusses on fat |
de Bont 1981 obese.
| Methods | RCT | |
| Participants | Women with type 2 diabetes (UK)
CVD risk: moderate
Control: randomised unclear (total in control and intervention 148), analysed 71 (for obese and non‐obese)
Intervention: randomised unclear, analysed 65 (for obese and non‐obese)
Mean years in trial: control 0.5, randomised 0.5
% male: 0%
Age: mean control 54 (SD 8), intervention 56 (SD 7), (all 35 to 64) (for obese and non‐obese) Baseline BMI: chosen for BMI ≥ 28, mean not reported |
|
| Interventions | Reduced and modified fat vs usual diet Control aims: usual diet but with CHO ≤ 40%E Intervention aims: 30%E from fat, focus on reducing meat fat, dairy foods and substituting margarines to improve the SFA/PUFA ratio, CHO increased to maintain energy intake Control methods: 3 home visits from a nutritionist over the 6 months of the trial Intervention methods: 3 home visits from a nutritionist over the 6 months of the trial Weight goals: to maintain the required total energy intake the proportion of carbohydrates in these diets was increased Total fat intake (change to 6 months): intervention‐10.1 (SD 10.8) (overall 31.1), control ‐1.0 (SD 10.5) (overall 41.8) %E (for obese and non‐obese) Saturated fat intake (change to 6 months): intervention‐8.1 (SD 5.8), control ‐1.1 (SD 5.7) %E (for obese and non‐obese) Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: diet, weight, lipids Available outcomes: weight, total and HDL cholesterol, triglycerides |
|
| Notes | Outcome data separated by those obese (BMI ≥ 28) or not obese at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | No for participants, unclear for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 of 148 (8%) lost over 0.5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Similar follow‐up |
| Free of dietary differences other than fat? | Low risk | Focus on fat |
DEER 1998 exercise men.
| Methods | RCT | |
| Participants | Men with raised LDL and low HDL cholesterol (USA)
CVD risk: moderate
Control: randomised 50, analysed 47
Intervention: randomised 51, analysed 48
Mean years in trial: control 1.0, intervention 1.0
% male: 100%
Age: mean 47.8 (SD 8.9) for all men (including the non‐exercise part of this trial) Baseline BMI: intervention 26.6 (SD 2.6), control 26.9 (SD 2.6) |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet (and exercise intervention) Intervention aims: NCEP step 2 diet: < 30%E from fat, < 7%E from SFA, < 200 mg/d cholesterol (and exercise intervention) Control methods: no advice provided Intervention methods: individual advice provided face to face, followed by 8 1‐hour group sessions during first 12 weeks, then monthly contact with dietitians by mail, phone, individual or group appointment Weight goals: "weight loss was not emphasised" Total fat intake (change to 12 months): intervention‐8.2 (SD 5.9) (22.2 overall), control ‐0.5 (SD 5.7) (29.9 overall) %E Saturated fat intake (change to 12 months): intervention‐3.9 (SD 2.6), control ‐0.1 (SD 2.6) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake and lipids Available outcomes: weight, total, LDL and HDL cholesterol, triglycerides, systolic and diastolic BP |
|
| Notes | Factorial trial re. exercise and reported by sex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignments by computer, modified Efron procedure, balanced by HDL and LDL |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of randomisation group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 of 101 (6%) lost over 1 year (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Very different levels of attention and review |
| Free of dietary differences other than fat? | Low risk | Dietary focus on fat |
DEER 1998 exercise women.
| Methods | RCT | |
| Participants | Postmenopausal women with raised LDL and low HDL cholesterol (USA)
CVD risk: moderate
Control: randomised 44, analysed 43
Intervention: randomised 43, analysed 43
Mean years in trial: control 1.0, intervention 1.0
% male: 0%
Age: mean 56.9 (SD 5.1) for all women (including the non‐exercise part of this trial) Baseline BMI: intervention 26.4 (SD 3.5), control 25.9 (SD 2.4) |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet (and exercise intervention) Intervention aims: NCEP step 2 diet: < 30%E from fat, < 7%E from SFA, < 200 mg/d cholesterol (and exercise intervention) Control methods: no advice provided Intervention methods: individual advice provided face to face, followed by 8 1‐hour group sessions during first 12 weeks, then monthly contact with dietitians by mail, phone, individual or group appointment Weight goals: "weight loss was not emphasised" Total fat intake (change to 12 months): intervention‐8.0 (SD 5.8) (20.4 overall), control 0.3 (SD 6.9) (28.7 overall) %E Saturated fat intake (change to 12 months): intervention‐3.0 (SD 2.3), control 0.2 (SD 3.1) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake and lipids Available outcomes: weight, total, LDL and HDL cholesterol, triglycerides, systolic and diastolic BP |
|
| Notes | Factorial trial re. exercise and reported by sex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignments by computer, modified Efron procedure, balanced by HDL and LDL |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of randomisation group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 of 87 (1%) lost over 1 year (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Very different levels of attention and review |
| Free of dietary differences other than fat? | Low risk | Focus on dietary fat |
DEER 1998 no exercise men.
| Methods | RCT | |
| Participants | Men with raised LDL and low HDL cholesterol (USA)
CVD risk: moderate
Control: randomised 47, analysed 46
Intervention: randomised 49, analysed 49
Mean years in trial: control 1.0, intervention 1.0
% male: 100%
Age: mean 47.8 (SD 8.9) for all men (including the exercise part of this trial) Baseline BMI: intervention 26.9 (SD 3.1), control 26.7 (SD 3.2) |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet (and usual exercise) Intervention aims: NCEP step 2 diet: < 30%E from fat, < 7%E from SFA, < 200 mg/d cholesterol (and usual exercise) Control methods: no advice provided Intervention methods: individual advice provided face to face, followed by 8 1‐hour group sessions during first 12 weeks, then monthly contact with dietitians by mail, phone, individual or group appointment Weight goals: "weight loss was not emphasised" Total fat intake (change to 12 months): intervention‐8.0 (SD 8.1) (22.4 overall), control ‐0.7 (SD 5.9) (29.7 overall) %E Saturated fat intake (change to 12 months): intervention‐3.4 (SD 3.2), control 0.0 (SD 2.4) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake and lipids Available outcomes: weight, total, LDL and HDL cholesterol, triglycerides, systolic and diastolic BP |
|
| Notes | Factorial trial re. exercise and reported by sex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignments by computer, modified Efron procedure, balanced by HDL and LDL |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of randomisation group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 of 96 (1%) lost over 1 year (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Very different levels of attention and review |
| Free of dietary differences other than fat? | Low risk | Focus on dietary fat |
DEER 1998 no exercise wom.
| Methods | RCT | |
| Participants | Postmenopausal women with raised LDL and low HDL cholesterol (USA)
CVD risk: moderate
Control: randomised 47, analysed 46
Intervention: randomised 46, analysed 45
Mean years in trial: control 1.0, intervention 1.0
% male: 0%
Age: mean 56.9 (SD 5.1) for all women (including the exercise part of this trial) Baseline BMI: intervention 26.6 (SD 2.8), control 26.0 (SD 3.9) |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet (and usual exercise) Intervention aims: NCEP step 2 diet: < 30%E from fat, < 7%E from SFA, < 200 mg/d cholesterol (and usual exercise) Control methods: no advice provided Intervention methods: individual advice provided face to face, followed by 8 1‐hour group sessions during first 12 weeks, then monthly contact with dietitians by mail, phone, individual or group appointment Weight goals: "weight loss was not emphasised" Total fat intake (change to 12 months): intervention‐5.7 (SD 7.4) (overall 22.7), control ‐0.2 (SD 6.7) (overall 28.2) %E Saturated fat intake (change to 12 months): intervention‐2.4 (SD 2.8), control 0.2 (SD 2.8) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake and lipids Available outcomes: weight, total, LDL and HDL cholesterol, triglycerides, systolic and diastolic BP |
|
| Notes | Factorial trial re. exercise and reported by sex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignments by computer, modified Efron procedure, balanced by HDL and LDL |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of randomisation group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 93 (2%) lost over 1 year (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Very different levels of attention and review |
| Free of dietary differences other than fat? | Low risk | Focus on dietary fat |
Diet and Hormone Study 2003.
| Methods | RCT | |
| Participants | Healthy premenopausal women aged 20 to 40 years (USA)
CVD risk: low Control: randomised 107, analysed 96 Intervention: randomised 106, analysed 81 Mean years in trial: control 0.95, intervention 0.88 % male: 0% Age: control mean 33.3, intervention 33.5 (SDs not given) Baseline BMI: mean control 23.8 (SD 3.5), intervention 23.7 (SD 4.2) |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet Intervention aims: < 20%E from fat, 25 to 30 g/d fibre, > 8 servings/d fruit and vegetables, CHO 60% to 65%E, protein 15% to 20%E Control methods: received a pamphlet on healthy eating (minimal intervention) Intervention methods: classroom nutrition education (18 group classes) plus 2 individual counselling sessions over 12 months covering knowledge and behavioural skills, appropriate foods served at intervention sessions Weight goals: "not encouraged to reduce total caloric intake and weight was monitored to maintain within 2 kg of baseline weight" Total fat intake (at 12 cycles/months): intervention 22.2 (SD 7.2), control 30.7 (SD 7.5) %E Saturated fat intake (at 12 cycles/months): intervention 14.9 (SD 6.7), control 23.9 (SD 13.2) g/d Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: hormonal responses Available outcomes: weight, BMI, dietary intake, hormones, menstrual cycle length |
|
| Notes | No answer to requests for data on deaths or health events. Weight and BMI data provided at 4 and 12 cycles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned by reference to a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of randomisation group, unclear for assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36 of 213 (17%) lost over 1 year (> 5% per year). Reasons not stated, greater losses in intervention group |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Very different levels of attention and review |
| Free of dietary differences other than fat? | High risk | Intervention group also asked to increase fibre, fruit and vegetables substantially |
Kentucky Low Fat 1990.
| Methods | RCT | |
| Participants | Moderately hypercholesterolaemic, non‐obese Caucasian men and women aged 30 to 50 (USA)
CVD risk: moderate
Control: randomised 62, analysed 51
Intervention: randomised 56, analysed 47
Mean years in trial: control 0.91, intervention 0.92
% male: control 61, intervention 66
Age: mean control 40.3 (SD 5.4), intervention 40.7 (SD 5.2) (all 30 to 50) Baseline BMI: not reported |
|
| Interventions | Reduced fat diet vs usual diet Control aims: no diet intervention Intervention aims: 25%E from fats, 20%E from protein, 55%E from CHO, < 200 mg cholesterol/day (Also an intervention arm with similar aims plus increased fibre intake) Control methods: no intervention Intervention methods: seminars and individual eating patterns taught, 10 weeks teaching and 40 weeks maintenance Weight goals: participants were directed to maintain initial body weight throughout the study Total fat intake (at 1 year): low fat 30 (SD 7.5), control 31 (SD 5.7) %E Saturated fat intake (at 1 year): low fat 9 (SD 2.7), control 10 (SD 2.9) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: diet composition, lipids Available outcomes: weight, total, LDL and HDL cholesterol |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "matched on age, gender & cholesterol level, randomly assigned to intervention group using systematic random procedure" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were aware of their dietary advice, researchers were not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20 of 118 (17%) lost over 1 year (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | (As the high fibre arm has not been used in the data set). See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Kuopio Reduced & Mod 1993.
| Methods | RCT (4 arms have been used here as 2 RCTs) | |
| Participants | Free‐living people aged 30 to 60 with serum total cholesterol levels 6.5 to 8.0 mmol/L (Finland)
CVD risk: moderate
Control (monoene enriched): randomised 41, analysed 41
Intervention AHA: randomised 41, analysed 41 Mean years in trial: for all 4 groups 0.5 % male: control 46, AHA 46 Age: mean control 46.4, AHA 47.3 (all 30 to 60) Baseline BMI: mean control 26.6 (SD 3.8), intervention 26.2 (SD 4.0) |
|
| Interventions | Reduced and modified fat vs modified fat diet
Control aims mono: total fat 38%E, SFA < 14%E, MUFA 18%E, PUFA < 6%E, rapeseed oil, rapeseed spread and skimmed milk provided
Intervention aims AHA: total fat 30%E, SFA < 10%E, MUFA 10%E, PUFA 10%E, sunflower oil, sunflower spread and skimmed milk provided Control and intervention methods: given written dietary instructions and a diet plan with checking and reinforcement for 3 visits, then at 2, 6, 12, 18 and 26 weeks Weight goals: dietary written instructions were designed for 5 energy levels (1800, 2000, 2400, 2800 and 3200) based on individual diet and activity assessment Total fat intake (weeks 14 to 28): low and mod fat 34 (SD 4), control 35 (SD 5) %E Saturated fat intake (weeks 14 to 28): low and mod fat 11 (SD 2), control 11 (SD 2) %E Style: dietary advice and supplement (food) Setting: community |
|
| Outcomes | Stated trial outcomes: lipids and blood pressure Available outcomes: BMI, total, LDL and HDL cholesterol, TG, BP |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation stratified for men and women, singles and couples, random number tables" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and researchers knew allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0 of 82 (0%) lost over 0.5 years (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Similar intensity and duration in both groups. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Kuopio Reduced Fat 1993.
| Methods | RCT (4 arms have been used here as 2 RCTs) | |
| Participants | Free‐living people aged 30 to 60 with serum total cholesterol levels 6.5 to 8.0 mmol/L (Finland)
CVD risk: moderate
Control (high saturated fat): randomised 37, analysed 12
Intervention low fat: randomised 40, analysed 40
Mean years in trial: for both groups 0.5
% male: control 46, low fat 48
Age: mean control 43.2, low fat 45.8 (all 30 to 60) Baseline BMI: mean control 25.6 (SD 4.2), intervention 26.5 (SD 3.4) |
|
| Interventions | Reduced fat vs usual diet (low fat vs control)
Control aims: advised total fat 38%E, SFA < 18%E, MUFA 15%E, PUFA < 5%E, rapeseed oil, butter and semi‐skimmed milk provided
Intervention aims low fat: total fat 28%E to 30%E, SFA < 14%E, MUFA 10%E, PUFA 4%E, butter and rapeseed spread and skimmed milk provided Control and intervention methods: given written dietary instructions and a diet plan with checking and reinforcement for 3 visits, then at 2, 6, 12, 18 and 26 weeks Weight goals: dietary written instructions were designed for 5 energy levels (1800, 2000, 2400, 2800 and 3200) based on individual diet and activity assessment Total fat intake (weeks 14 to 28): low fat 31 (SD 5), control 36 (SD 5) %E Saturated fat intake (weeks 14 to 28): low fat 12 (SD 2), control 15 (SD 2) %E Style: dietary advice and supplement (food) Setting: community |
|
| Outcomes | Stated trial outcomes: lipids and blood pressure Available outcomes: BMI, total, LDL and HDL cholesterol, TG, BP |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation stratified for men and women, singles and couples, random number tables" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and researchers knew allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25 of 77 (32%) lost over 0.5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Similar intensity and duration in both groups. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Mastopathy Diet 1988.
| Methods | RCT | |
| Participants | Women with severe cyclical mastopathy for at least 5 years (Canada)
CVD risk: low
Control: randomised 10, analysed 9
Intervention: randomised 11, analysed 10
Mean years in trial: control 0.45, intervention 0.45
% male: 0%
Age: mean control 36, intervention 38 (variances unclear) Baseline BMI: no data provided |
|
| Interventions | Reduced fat vs usual diet Control aims: given principles of healthy diet, not counselled to alter fat content Intervention aims: total fat 15%E, CHO 65%E Control methods: seen every 2 months to monitor symptoms, nutrition and biochemistry Intervention methods: seen monthly to monitor symptoms, nutrition and biochemistry, teaching materials included food guide, recipes, product information and advice on eating out Weight goals: the intervention goals included the isocaloric replacement of complex carbohydrate for fat (no mention for control group) Total fat intake (at 6 months): low fat 22.8 (SD unclear), control 33.4 (SD unclear) %E Saturated fat intake (at 6 months): low fat 8.8 (SD unclear), control 12.3 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: mastopathy symptoms, plasma hormone and lipids Available outcomes: weight, total cholesterol (but variance data not provided) |
|
| Notes | Total cholesterol rose by 0.09 mmol/L in control group (from 4.5 to 4.59) and fell by 0.15 mmol/L in intervention group (4.84 to 4.69). Weight changed in the intervention group (mean fall of 2.1 kg over 6 months, no variance provided), but change, or otherwise, in control group not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, those assessing physical outcomes were blinded, those assessing symptoms were not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 of 21 (10%) lost over 0.5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Minor differences in follow‐up frequency. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
MeDiet 2006.
| Methods | RCT | |
| Participants | Healthy postmenopausal women with above median serum testosterone (Italy)
CVD risk: low
Control: randomised 57, analysed at 6 months 55
Intervention: randomised 58, analysed at 6 months 51
Mean years in trial: control 4.38, intervention 4.28
% male: 0
Age: mean unclear (age range 48 to 69) Baseline BMI: not reported |
|
| Interventions | Reduced and modified fat vs usual diet Control aims: advised to increase fruit and vegetable intake Intervention aims: taught Sicilian diet including reduced total, saturated and omega‐6 fats, increased blue fish (high in omega 3), increased whole cereals, legumes, seeds, fruit and vegetables Control methods: advice Intervention methods: taught Sicilian diet and cooking by professional chefs, with a weekly cooking course including social dinners Weight goals: not mentioned Total fat intake (at 6 months): low and mod fat 30.9 (SD 11.4), control 34.0 (SD 11.8) %E Saturated fat intake (at 6 months): low and mod fat 8.4 (SD 3.0), control 11.2 (SD 5.0) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: breast cancer, weight, lipids, well being Available outcomes: weight |
|
| Notes | Weight data provided at 6 months (fall of 0.6 kg in control group, fall of 1.3 kg in intervention group), but without variance information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "individually randomised" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were aware of assignment, researchers unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 of 115 (8%) lost over 4 years (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Intensive cookery course with social element compared with brief advice. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | High risk | Both groups encouraged to increase fruit and vegetables, but intervention group also encouraged to increase fish, pulses, seeds and whole grains |
Moy 2001.
| Methods | RCT | |
| Participants | Middle‐aged siblings of people with early CHD, with at least one CVD risk factor (USA)
CVD risk: moderate
Control: randomised 132, analysed 118
Intervention: randomised 135, analysed 117
Mean years in trial: 1.9
% male: control 49%, intervention 55%
Age: control mean 45.7 (SD 7), intervention 46.2 (SD 7) Baseline BMI: control mean 29.5 (SD 7), intervention 28.5 (SD 5) |
|
| Interventions | Reduced fat intake vs usual diet Control: physician management (physicians informed on risk factor management) Intervention: nurse management, aim total fat 40 g/d or less Control methods: physician management with risk factor management at 0, 1 and 2 years Intervention methods: nurse management, appointments 6‐ to 8‐weekly for 2 years Weight goals: not mentioned Total fat intake (at 2 years): low fat 34.1 (SD unclear), control 38.0 (SD unclear) %E Saturated fat intake (at 2 years): low fat 11.5 (SD unclear), control 14.4 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake Available outcomes: BMI, HDL and LDL cholesterol, TG |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned via computerised schema after all eligible siblings from a family had been screened |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and trialists clear about their allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32 of 267 (12%) lost over 2 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Differences in frequency of follow‐up, but unclear what differences in care occurred between the physician and nurse‐led care. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Unclear risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
MSFAT 1995.
| Methods | RCT | |
| Participants | Healthy people aged 20 to 55 (Netherlands)
CVD risk: low
Control: randomised unclear (120?), analysed 103
Intervention: randomised unclear (120?), analysed 117
Mean years in trial: control 0.46, intervention 0.49
% male: control 50%, intervention 50%
Age: mean control men 35.6 (SD 10), control women 36.0 (SD 11), intervention men 35.5 (SD 11), intervention women 36.0 (SD 12) (all 19 to 55) Baseline BMI: mean control men 24.9 (SD 2.2), control women 25 (SD 2), intervention men 24.9 (SD 2.3), intervention women 24.7 (SD 2) |
|
| Interventions | Reduced fat vs usual diet Control aims: advised to use products from trial shop ad lib. (usual fat products provided) Intervention aims: advised to use products from trial shop ad lib. (low fat products provided) Control methods: participants obtained foods in a study shop at least once a week Intervention methods: participants obtained foods in a study shop at least once a week Weight goals: ad libitum diet Total fat intake (at 6 months): low fat 34.7 (SD unclear), control 42.7 (SD unclear) %E Saturated fat intake (at 6 months): low fat 14.2 (SD unclear), control 18.2 (SD unclear) %E Style: food provided Setting: community |
|
| Outcomes | Stated trial outcomes: weight, vitamin and fatty acid intake, anti‐oxidative capacity Available outcomes: weight (for subgroup), weight and lipids provided for larger group, but without variance data |
|
| Notes | Change from baseline to 6 months for whole group (control 103, intervention 117): Weight, kg: 1.1, 0.4 Total cholesterol, mmol/L: 0.07, ‐0.09 HDL cholesterol, mmol/L: ‐0.03, ‐0.06 LDL cholesterol, mmol/L: 0.15, 0.16 TG, mmol/L: 0.04, ‐0.04 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "stratified randomisation (according to sex, age, QI index and eating behaviour) by co‐ordinating centre", a statistician at Unilever Research, SAS software, and allocation could not be altered later |
| Allocation concealment (selection bias) | Low risk | "stratified randomisation (according to sex, age, QI index and eating behaviour) by co‐ordinating centre", a statistician at Unilever Research, SAS software, and allocation could not be altered later |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of allocation, those analysing biochemistry were not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20 of 240 (8%) lost over 0.5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Both groups used study shop. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
NDHS Open 1st L&M 1968.
| Methods | RCT | |
| Participants | Free‐living men (USA)
CVD risk: low
Control: randomised 382, analysed 348
Intervention B: randomised 385, analysed 332 Intervention X: randomised 54, analysed 46 Mean years in trial: control 1.0, B 0.9, C 0.9, X 0.9 % male: 100 Age: unclear (all 45 to 54) Baseline BMI: not reported |
|
| Interventions | Reduced and modified fat diet vs usual diet Control aims: total fat 40%E, SFA 16%E to 18%E, dietary cholesterol 650 to 750 mg/d, P/S 0.4 Intervention B: total fat 30%E, SFA < 9%E, dietary cholesterol 350 to 450 mg/d, PUFA 15%E, P/S 1.5 Intervention X: total fat 30%E, SFA < 9%E, dietary cholesterol 350 to 450 mg/d, PUFA 15%E, P/S 1.5 Control methods: dietary advice to reduce saturated fat and cholesterol (plus 10 follow‐up visits with nutritionist), purchase of 'usual fat' items from a trial shop Intervention B methods: dietary advice to reduce saturated fat and cholesterol (plus 10 follow‐up visits with nutritionist), plus purchase of appropriately reduced and modified fat items from a trial shop Intervention X methods: dietary advice but no trial shop Weight goals: weight and calories not mentioned Total fat intake (through study): B 29.7 (SD unclear) %E, X 31.7 (SD unclear), control 34.9 (SD unclear) %E Saturated fat intake (through study): B 7.1 (SD unclear) %E, X 8.9 (SD unclear), control 11.6 (SD unclear) %E Style: B diet provided, X ‐ diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: lipid levels and dietary assessment Available outcomes: total cholesterol (some weight and BP data presented but no variance info) |
|
| Notes | At 52 weeks weight change in the control was not presented, weight change in B was ‐2.4 kg. Average weight change over the first year (mean of weights at weeks 6, 12, 20, 28, 36 and 44 weeks) was ‐2.45 kg (‐5.4lb) for the low fat group (B) and ‐1.91 kg (‐4.2lb) for the modified fat group (C) and ‐1.95 kg (‐4.3lb) for the control group (D) At 52 weeks diastolic BP change from baseline was ‐2.2 kg in control, ‐1.9 in B and ‐5.8 in X |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation by the statistical centre |
| Allocation concealment (selection bias) | Low risk | Stratified randomisation by the statistical centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Intervention B: all reduced saturated fat and purchased blinded foods from a trial shop, double‐blind Intervention X: no trial shop, so participants not blinded, though those analysing blood samples etc. were |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 87 of 821 (11%) lost over 1 year (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Yes for intervention B (as both intervention and control received dietary advice and purchased food from trial shop). No for intervention X (as it did not include a trial shop as in the control group). See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
NDHS Open 2nd L&M 1968.
| Methods | RCT | |
| Participants | Free‐living men who had participated in NDHS 1st studies (USA)
CVD risk: low
Control: randomised 304, analysed 215
Intervention BC (this study had a range of interventions, we were interested in BC for the systematic review): randomised 194, analysed 179
Mean years in trial: control 0.6, intervention BC 0.6
% male: 100
Age: unclear (all 45 to 54) Baseline BMI: not reported |
|
| Interventions | Reduced and modified fat vs usual diet Control aims: total fat 40%E, SFA 16%E to 18%E, dietary cholesterol 650 to 750 mg/d, P/S 0.4, X ‐ advice to continue usual diet Intervention aims: BC total fat 30%E to 40%E, SFA reduced, dietary cholesterol 350 to 450 mg/d, increased PUFA, P/S 1.5 to 2.0 Control methods: dietary advice to reduce saturated fat and cholesterol (plus 10 follow‐up visits with nutritionist), purchase of 'usual fat' items from a trial shop Intervention BC methods: dietary advice to reduce saturated fat and cholesterol (plus 10 follow‐up visits with nutritionist), plus purchase of appropriately reduced and modified fat items from a trial shop Weight goals: weight and calories not mentioned Total fat intake (through study): BC 32.5 (SD unclear) %E, control 35.5 (SD unclear) %E Saturated fat intake (through study): BC 7.4 (SD unclear) %E, control 12.0 (SD unclear) %E Style: food provided Setting: community |
|
| Outcomes | Stated trial outcomes: lipid levels and dietary assessment Available outcomes: weight |
|
| Notes | Weight data provided for the BC intervention group ‐1.8 kg (‐4 lb over 6 months), and ‐0.9 kg (‐2 lb) for modified fat diet G, ‐1.4 kg (‐3 lb) for modified fat diet F. No info provided for the control group (D) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation by the statistical centre |
| Allocation concealment (selection bias) | Low risk | Stratified randomisation by the statistical centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Some participants continued with advice to reduce saturated fat and purchased blinded foods from a trial shop, but half of the participants were instructed in their own purchase of appropriate foods from normal shops to compile their own dietary regimen |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 104 of 498 (21%) lost over 0.6 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Trial shop used by both groups, plus dietary advice. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Nutrition & Breast Health.
| Methods | RCT | |
| Participants | Pre‐menopausal women at increased risk of breast cancer (USA)
CVD risk: low
Control: randomised 53, analysed 50
Intervention: randomised 69, analysed 47
Mean years in trial: control 1.0, intervention 0.8
% male: control 0%, intervention 0%
Age: mean 38 (SD 7) ‐ not provided by study arm (all 21 to 50) Baseline BMI: not reported |
|
| Interventions | Reduced fat vs usual diet Control aims: followed usual diet, given daily food guide pyramid (half of this group randomised to 9 portions/d of fruit and vegetables advice) Intervention aims: total fat 15%E (half of this group randomised to 9 portions/d of fruit and vegetables advice) Control methods: no dietary counselling (offered this at the end of study), but those given fruit and vegetables advice had support as below Intervention methods: met dietitian every 2 weeks until compliant, monthly group meetings, counselling on home diets, restaurants, parties, social support, eating at work, exchange booklets, cookbook Weight goals: "goals were derived such that baseline energy intake would be maintained while meeting study goals" Total fat intake (at 12 months): low fat 15.7 (SD 5.1) %E, control 32.7 (SD 6.1) %E Saturated fat intake (at 12 months): low fat 7.2 (SD unclear) %E, control 11.6 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: body weight, dietary compliance Available outcomes: weight, total, LDL and HDL cholesterol, TG, BMI (but variance data not provided for any but weight) |
|
| Notes | Change from baseline to 12 months for the control (n = 23), control plus fruit and vegetables (n = 25), low fat (n = 24), low fat plus fruit and vegetables (n = 23): Total cholesterol mg/dl: 9, 2, ‐8, 0 TG mg/dl: ‐7, 1, 5, 8 HDL cholesterol mg/dl: 0, 0, ‐4, 0 LDL cholesterol mg/dl: 11, 2, ‐6, ‐2 BMI kg/m2: 0, 4, ‐13, 0 For weight end data only are provided (no change data) although the intervention group were considerably heavier at baseline (149 lb and 154 lb) than control groups (both 143 lb) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The statistician made envelopes ahead of time, dietitians handed out envelopes at first visit |
| Allocation concealment (selection bias) | Low risk | Allocation could not be altered once made |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were aware of allocation, researchers and those assessing lipids were not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 of 122 (12%) lost over 1 year (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | High levels of intervention for those on low fat or high fruit and vegetable diets. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | Randomisation to fruit and vegetable intervention was independent of low fat allocation |
Pilkington 1960.
| Methods | RCT | |
| Participants | Men with angina or who have had a MI (UK)
CVD risk: high
Reduced fat: randomised unclear, analysed 12
Modified fat: randomised unclear, analysed 23
Mean years in trial:reduced fat 1.1, modified fat 1.1
% male: reduced fat 100%, modified fat 100%
Age: not stated Baseline BMI: not reported |
|
| Interventions | Reduced fat vs modified fat diet Reduced fat aims: total fat 20 g/d, advice to avoid dairy fats except skimmed milk plus 1 egg or 21 g cheese/d. Lean meat and fish each allowed once/d, other non‐fatty foods allowed in unlimited quantities Modified fat aims: fat aims not stated, dairy produce avoided except skimmed milk, 90 ml/d soya oil provided, lean meat originally prohibited but allowed after 6 months along with 113 g/wk of 'relatively unsaturated margarine'. Fish and vegetables allowed freely Reduced fat methods: unclear, "dietary histories taken before and during treatment" Modified fat methods: unclear, "dietary histories taken before and during treatment" Weight goals: non‐fatty foods not restricted, no weight goals mentioned Total fat intake (during treatment): low fat 15.8 (SD unclear) %E, mod fat 36 (SD unclear) %E Saturated fat intake: unclear Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: lipids Available outcomes: weight, total and LDL cholesterol |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | No for participants, unclear for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear exactly how many were randomised, but paper suggests that all randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Appear to be similar levels of assessment and support in both arms |
| Free of dietary differences other than fat? | Low risk | Dietary focus entirely on fat |
Polyp Prevention 1996.
| Methods | RCT | |
| Participants | People with at least one adenomatous polyp of the large bowel removed (USA)
CVD risk: low Control: 1042 randomised, 943 analysed Intervention: 1037 randomised, 943 analysed Mean years in trial: control 3.05, intervention 3.05 % male: control 64%, intervention 66% Age: mean control 61.5, intervention 61.4 (all at least 35) Baseline BMI: mean control 27.5 (SE 0.12), intervention 27.6 (SE 0.13) |
|
| Interventions | Low fat vs usual diet Control: general dietary guidelines Intervention: total fat 20%E, 18 g fibre/1000 kcal, 5 to 8 servings fruit and vegetables daily Control methods: leaflet, no additional information or behaviour modification Intervention methods: > 50 hours of counselling over 4 years, included skill building, behaviour modification, self monitoring and nutritional materials Weight goals: "weight loss is permitted but not encouraged....counselled to replace fat intake with increased intake of fruit, vegetable and grain products rather than reduce total calorie intake." Total fat intake (at 4 years): low fat 23.8 (SD 6.0), control 33.9 (SD 5.9) %E Saturated fat intake: unclear Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: recurrence of polyps, prostate cancer Available outcomes: weight, total cholesterol |
|
| Notes | Weight data reported at 1, 2, 3 and 4 years. 3‐year data used in main analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned" by computer randomisation centre, stratified according to centre |
| Allocation concealment (selection bias) | Low risk | Phone call to computer randomisation centre, stratified according to centre |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcome assessors blinded, participants not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 193 of 2079 (9%) lost over 3 years (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | 50 hours behaviour modification in intervention group, not in control. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | High risk | Fibre, fruit and vegetable goals in intervention group |
Rivellese 1994.
| Methods | RCT | |
| Participants | Adults with primary hyperlipoproteinaemia (Italy)
CVD risk: moderate
Intervention reduced fat: 33 randomised, 27 analysed
Intervention modified fat: 30 randomised, 17 analysed
Mean years in trial: reduced fat 0.4, modified fat 0.4
% male: reduced fat 82%, modified fat 63%
Age, years: reduced fat 47.4 mean (SD 10.3), modified fat 48.6 (SD 8.1) Baseline BMI: reduced fat 24.4 mean (SD 2.9), modified fat 25.2 (SD 2.7) |
|
| Interventions | Reduced fat vs modified fat diet Reduced fat aims: total fat 25%E, SFA 8%E, MUFA 15%, PUFA 2%, dietary cholesterol < 300 mg/d, CHO 58%, protein 17%E, soluble fibre 41 g/d Modified fat aims: total fat 38%E, SFA < 10%E, MUFA 20%E, PUFA 10%E, dietary cholesterol < 300 mg/d, CHO 47%E, protein 15%E, soluble fibre 19 g/d Reduced fat methods: seen monthly by dietitian and doctor, feedback based on 7‐day food diary each time Modified fat methods: seen monthly by dietitian and doctor, feedback based on 7‐day food diary each time Weight goals: neither weight or energy intake goals mentioned for either group Total fat intake (at 5 to 6 months): low fat 27 (SD unclear) %E, mod fat 36 (SD unclear) %E Saturated fat intake (at 5 to 6 months): low fat 6 (SD unclear) %E, mod fat 7 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: metabolic effects Available outcomes: weight, total, LDL and HDL cholesterol, TG |
|
| Notes | Weight data were presented without variance info. Participants in the low fat arm lost 1.8 kg over the 6 months, the modified fat diet arm lost 1.6 kg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following 3 or 6 weeks compliance with control diet run‐in, stratified block randomisation with tables of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | None |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19 of 63 (30%) lost over 0.4 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Identical follow‐up. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | High risk | Some differences in soluble fibre intake |
Simon Low Fat Breast CA.
| Methods | RCT | |
| Participants | Women with a high risk of breast cancer (USA)
CVD risk: low
Control: randomised 96, analysed 38
Intervention: randomised 98, analysed 34
Mean years in trial: control 1.8, intervention 1.7
% male: 0
Age: mean control 46, intervention 46 Baseline BMI: mean intervention 25.2 (SE 0.8), control 28.1 (SE 0.8) |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet Intervention aims: total fat 15%E Control methods: continued usual diet Intervention methods: biweekly individual dietetic appointments over 3 months followed by monthly individual or group appointments, including education, goal setting, evaluation, feedback and self monitoring Weight goals: weight and calorie goals not discussed Total fat intake (at 12 months): low fat 18.0 (SD 5.6), control 33.8 (SD 7.4) %E Saturated fat intake (at 12 months): low fat 6.0 (SD unclear), control 11.3 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: intervention feasibility Available outcomes: weight, total, LDL and HDL cholesterol, TG |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by age and randomised (block size 2) |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants knew their allocation, unclear whether physicians did |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 122 of 194 (63%) lost over 2 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Very different contact time with dietitian, but medical appointments same in both groups. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Sondergaard 2003.
| Methods | RCT | |
| Participants | People with IHD plus total cholesterol at least 5 mmol/L (Denmark)
CVD risk: high
Control: 63 randomised, 52 analysed
Intervention: 68 randomised, 63 analysed
Mean years in trial: 1.0
% male: control 79%, intervention 62%
Age: control mean 62.8 (SD 10.5), intervention mean 62.1 (SD 9.3) Baseline BMI: intervention 26.6 (SD 3.9), control 26.7 (SD 4.2) |
|
| Interventions | Reduced and modified fat intake vs usual diet Control: aims unclear Intervention: aims reductions in total and saturated fat, replace fats with oils, 600 g fruit and vegetables/d, fatty fish at least once a week, eat plenty of bread and cereals Control methods: booklets plus one dietetic interview, and 3 monthly clinical review Intervention methods: 1‐hour nutrition interview every 3 months, plus 3 monthly clinical review Weight goals: weight not mentioned Total fat intake (at 12 months): low and mod fat 26.2 (SD 5.1), control 28.9 (SD 7.9) %E Saturated fat intake (at 12 months): unclear Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: endothelial function Available outcomes: weight, total, LDL and HDL cholesterol, TG |
|
| Notes | No outcome data provided on weight, except the statement "in both groups, body weight remained unchanged after 12 months". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised in unblinded 1:1 fashion" |
| Allocation concealment (selection bias) | High risk | "randomised in unblinded 1:1 fashion" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of allocation, unclear about others |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 of 131 (12%) lost over 1 year (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Additional dietetic time for intervention group. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | High risk | Additional dietary advice for intervention group (fruit, vegetables, fish, cereals) |
Strychar 2009.
| Methods | RCT | |
| Participants | People with well controlled type I diabetes mellitus (Canada)
CVD risk: moderate
Intervention reduced fat: 18 randomised, 15 analysed
Intervention modified fat: 17 randomised, 15 analysed
Mean years in trial: reduced fat 0.46, modified fat 0.47
% male: reduced fat unclear, modified fat unclear
Age, years: 37.9 (8.1 SD) (not specified by study arm) Baseline BMI: mean reduced fat 24.3 (SD 2.6), modified fat 24.3 (SD 2.7) |
|
| Interventions | Reduced fat vs modified fat diet Reduced fat aims: total fat 27%E to 30%E, SFA ≤ 10%E, MUFA 10%, CHO 54% to 57% Modified fat aims: total fat 37%E to40%E, SFA ≤ 10%E, MUFA 20%E, CHO 43%E to 46%E Reduced fat methods: after initial dietary advice monitored weekly by phone by a dietitian (24‐hour food recall). Glycaemia, insulin doses, CHO at meals, hypoglycaemic attacks all self monitored daily and reported weekly Modified fat methods: after initial dietary advice monitored weekly by phone by a dietitian (24‐hour food recall). Glycaemia, insulin doses, CHO at meals, hypoglycaemic attacks all self monitored daily and reported weekly Total fat intake (at 6 months): not stated Saturated fat intake (at 6 months): not stated Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: triglycerides and other CVD risk factors Available outcomes: weight; BMI; total, LDL and HDL cholesterol; TG; systolic and diastolic blood pressure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details provided, but participants had to make decisions about what they ate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 of 35 (14%) lost over 0.5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | Low risk | Similar intervention in both groups |
| Free of dietary differences other than fat? | Low risk | Focus on fat and CHO intake |
Swedish Breast CA 1990.
| Methods | RCT | |
| Participants | Women who had had surgery for breast cancer (Sweden)
CVD risk: low
Control: randomised 121, analysed 63
Intervention: randomised 119, analysed 106
Mean years in trial: control 1.9, randomised 1.5
% male: 0%
Age: mean 58 (not described by randomisation group) Baseline BMI: intervention 6 BMI < 20, 81 BMI 20 to 24.9, 34 BMI ≥ 25; control 9 BMI < 20, 74 BMI 20 to 24.9, 36 BMI ≥ 25 |
|
| Interventions | Reduced fat vs usual diet Control aims: usual diet Intervention aims: 20%E to 25%E from fat, increase energy from CHO to replace lost energy Control methods: no advice provided, only seen at baseline and 2 years Intervention methods: 4 to 6 sessions during the first 2 months, group meetings every 6 to 8 weeks, evening classes in low fat cooking, 3 monthly counselling during the first year, then at 18 months Weight goals: "The total energy and/or protein intake was to be held constant" Total fat intake (at 2 years): intervention ‐12.9 (SD unclear) (24 overall), control ‐3.1 (SD unclear) (34.1 overall) %E Saturated fat intake (change to 2 years): intervention ‐6.8 (SD unclear), control ‐1.9 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake Available outcomes: weight, BMI |
|
| Notes | No exact variance or P values reported for weight and BMI outcomes, so have estimated variance from P value < 0.05 for the difference between the 2 arms for weight. As P value > 0.05 for BMI no variance could be estimated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | No for participants, unclear for those assessing outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data ignored for those who dropped out (48% of the intervention group), > 5%/year |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Different levels of time and follow‐up in the 2 groups |
| Free of dietary differences other than fat? | Low risk | Focus on fat |
Veterans Dermatology 1994.
| Methods | RCT | |
| Participants | People with non‐melanoma skin cancer (USA)
CVD risk: low
Control: randomised 67, analysed 58
Intervention: randomised 66, analysed 38
Mean years in trial: 1.9
% male: control 67%, intervention 54%
Age: mean control 52.3 (SD 13.2), intervention 50.6 (SD 9.7) Baseline BMI: data not provided |
|
| Interventions | Reduced fat vs usual diet Control aims: no dietary advice Intervention aims: total fat 20%E, protein 15%E, CHO 65%E Control methods: no dietary change, 4 monthly clinic visits Intervention methods: 8 weekly classes, with behavioural techniques, plus 4 monthly clinic visits Weight goals: "to maintain body weight .... patients were instructed to increase their intake of carbohydrate, particularly complex carbohydrate" Total fat intake ("during study" months 4 to 24): low fat 20.7 (SD 5.5), control 37.8 (SD 4.1) %E Saturated fat intake ("during study, months 4 to 24): low fat 6.6 (SD 1.8), control 12.8 (SD 2.0) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: incidence of actinic keratosis and non‐melanoma skin cancer Available outcomes: none (weight data provided, but no variance info) |
|
| Notes | At 2 years control ‐1.5 kg n = 50?, intervention ‐1 kg n = 51? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "list of randomly generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Physician blinding: adequate Participant blinding: inadequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37 of 133 (28%) lost over 2 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Minor: all have 4 monthly clinic visits, the intervention group had 8 behavioural technique classes that the control group did not have |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
VYRONAS 2009.
| Methods | RCT | |
| Participants | 12 to 13‐year olds attending schools in Vyronas, Athens (Greece)
CVD risk: low
Control: randomised n = 105, analysed at 17 months n = 93
Intervention: randomised n = 108, analysed at 17 months n = 98
Mean years in trial: control 1.3, intervention 1.4
% male: control 49.5%, intervention 49.0%
Age: control mean 13.3 (SD 0.9), intervention 13.1 (SD 0.8) Baseline BMI: control mean 24.3 (SD 3.3), intervention 24 (SD 3.1) |
|
| Interventions | Reduced fat vs usual diet Control aims: not stated, usual intake assumed Intervention aims: unclear, but appears to have been low fat and dental hygiene Control methods: screening results were posted to parents, no other information Intervention methods: 12 hours of classroom materials over 12 weeks, taught by home economics teacher supervised by health visitor or family doctor, including multi‐component workbooks, "interactions among environmental, cognitive and behavioural factors", "classroom modules developed behavioural capability, expectations and self‐efficacy for healthful eating and healthy foods selection", 2 meetings including presentations were held with parents Weight goals: not mentioned except that note was made of obese children (unclear in what respect) Total fat intake (at 17 months): low fat 31.3 (SD 4.4), control 36.9 (SD 4.8) %E Saturated fat intake (at 17 months): low fat 10.3 (SD 1.9), control 13.4 (SD 2.8) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: diet, nutrition intake and BMI Available outcomes: nutritional intake, BMI |
|
| Notes | BMI reported compared with baseline in each group, but change in BMI not directly compared between intervention and control groups (calculated by review authors) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerised random number generator" |
| Allocation concealment (selection bias) | Low risk | Recruitment appeared to have been completed before allocation occurred |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Because of the nature of the intervention, blinding was not feasible" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Similar in both arms, paper mentions loss of 5 participants during trial (due to health problems, lack of interest and move to other schools). Of 109 allocated in each arm 10 were not included in analysis of the intervention group and 12 in the control (reasons unclear). 22 of 213 (10%) lost over 17 months (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | High risk | Unclear how intervention was delivered to some children but not others as randomisation appeared to be individual, not by class. Intervention methods imply an individualised intervention, but unclear what elements were individualised |
| Free of systematic difference in care? | High risk | No, intervention group appear to have received modules designed to develop behavioural capability, expectations and self efficacy, and included motivational methods and strategies as well as social influence |
| Free of dietary differences other than fat? | High risk | Exact goals of intervention unclear, but appears to have focused on "mainly dietary issues, but also dental health hygiene and consumption attitudes" |
WHEL 2007.
| Methods | RCT | |
| Participants | Women with previously treated early breast cancer (USA)
CVD risk: low
Control: randomised 1561, analysed 1313
Intervention: randomised 1546, analysed 1308
Mean years in trial: unclear, 11 years max, around 11 years mean?
% male: 0
Age: control mean 53.0 (SD 9.0), intervention mean 53.3 (SD 8.9) Baseline BMI: control mean 27.2 (SD 6.1), intervention mean 27.2 (SD 6.1) |
|
| Interventions | Reduced fat intake vs usual diet Control: aim 30%E from fat Intervention: aim 15%E to 20%E from fat, 5 vegetables/d, 3 fruit/d, 16 oz vegetable juice and 30 g/d fibre Control methods: given print materials only Intervention methods: telephone counselling programme (31 calls by study end), cooking classes (12 offered in first year, 4 attended on average) and monthly newsletters (48 by study end), all focused on self efficacy, self monitoring and barriers, retaining motivation Weight goal: intervention goal was to achieve the change in dietary pattern without weight reduction, weight and calories not mentioned in the control group Total fat intake (at 72 months): low fat 28.9 (SD 9.0), control 32.4 (SD 8.0) %E Saturated fat intake (at 72 months): low fat 7.2 (SD unclear), control 8.9 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: mortality, invasive breast cancer Available outcomes: weight, total, LDL and HDL cholesterol, TG |
|
| Notes | Weight reported at 1, 2, 3, 4 and 6 years, and 3‐year data used in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via computer program |
| Allocation concealment (selection bias) | Low risk | Randomisation via computer program |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 486 of 3107 (16%) lost over 11 years (< 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | High‐intensity intervention compared with leaflets. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | High risk | Fruit and vegetable intervention in low fat arm, not in control |
WHI 2006.
| Methods | RCT | |
| Participants | Postmenopausal women aged 50 to 79 (USA)
CVD risk: mixed, mostly low but some participants had CVD at baseline
Control: randomised 29,294, analysed 25,056
Intervention: randomised 19,541, analysed 16,297
Mean years in trial: control 8.1, intervention 8.1
% male: 0
Age: mean intervention 62.3 (SD 6.9), control 62.3 (SD 6.9) Baseline BMI: mean intervention 29.1 (SD 5.9), control 29.1 (SD 5.9) |
|
| Interventions | Reduced fat vs usual diet Control: diet‐related education materials Intervention: low fat diet (20%E from fat) with increased fruit and vegetables Control methods: given copy of 'Dietary Guidelines for Americans' Intervention methods: 18 group sessions with trained and certified nutritionists in the first year, quarterly maintenance sessions thereafter, focusing on diet and behaviour modification Weight goals: "the intervention did not include total energy reduction or weight‐loss goals" Total fat intake (at 6 years): intervention 28.8 (SD 8.4) %E, control 37.0 (SD 7.3) %E Saturated fat intake (at 6 years): intervention 9.5 (SD 3.2) %E, control 12.4 (SD 3.1) %E Style: dietary advice Setting: community |
|
| Outcomes | Stated trial outcomes: breast cancer, mortality, other cancers, cardiovascular events, diabetes Available outcomes: weight, BMI, total, LDL and HDL cholesterol, TG, systolic and diastolic BP |
|
| Notes | Weight data available at 1 year, 3 years, 6 years and 7.5 years. Latest (7.5 year) data used for main analysis for weight, BMI and waist circumference. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer algorithm |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7482 of 48,835 (15%) lost over 8 years (< 5% per year) |
| Selective reporting (reporting bias) | Low risk | Weight and secondary outcomes reported as in protocol |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Intervention participants received 18 group sessions with behavioural modification plus quarterly maintenance sessions thereafter. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | High risk | Also fruit and vegetable intervention. See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
WHT Feasibility 1990.
| Methods | RCT | |
| Participants | Women at increased risk of breast cancer (USA)
CVD risk: low
Control: randomised 184, analysed 159
Intervention: randomised 119, analysed 102
Mean years in trial: control 1.9, randomised 1.9
% male: 0%
Age: mean control 55.6 (SD 6.3), intervention 55.6 (SD 6.2) Baseline BMI: mean intervention 26 (SD 4), control 25 (SD 4) |
|
| Interventions | Reduced fat vs usual diet Control aims: maintain usual diet Intervention aims: 20%E from fat Control methods: no advice provided, only seen at baseline, then 6, 12 and 24 months for assessment Intervention methods: women were given flexible diet plans and responsible for their own monitoring, they had individual appointments with a nutritionist at 2 and 12 weeks, plus small group meetings (weekly for 8 weeks, then biweekly for 8 weeks, then monthly to 2 years) Weight goals: weight and calories not mentioned Total fat intake (at 2 years): intervention 22.6 (SD 7.1), control 36.8 (SD 8.0) %E Saturated fat intake (at 2 years): intervention 7.2 (SD 2.7), control 12.3 (SD 3.6) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake/feasibility Available outcomes: weight, total cholesterol |
|
| Notes | Weight data provided at 6, 12 and 24 months. 2‐year data used in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42 of 303 (14%) lost over 2 years (> 5% per year) |
| Selective reporting (reporting bias) | Low risk | Design paper published, weight and serum total cholesterol reported |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Different levels of attention and time |
| Free of dietary differences other than fat? | Low risk | Focus on fat only |
WHT:FSMP 2003.
| Methods | RCT | |
| Participants | Postmenopausal women from diverse ethnic and socioeconomic backgrounds (USA)
CVD risk: low
Control: randomised 883, analysed 649 at 6 mo, 443 at 12 mo, 194 at 18 mo
Intervention: randomised 1325, analysed 1071 at 6 mo, 698 at 12 mo, 285 at 18 mo
Mean years in trial: unclear, follow‐up from 6 to 18 months
% male: 0%
Age: mean control 59.8 (SD 6.6), intervention 60.1 (SD 6.6) Baseline BMI: 28.8 (SD 4.7) for all |
|
| Interventions | Reduced fat vs usual diet Control aims: maintain usual diet Intervention aims: up to 20%E from fat, reduced saturated fat and dietary cholesterol, increased fruit, vegetables and whole grains Control methods: pamphlet on general dietary guidelines provided, no other follow‐up, seen at baseline, then 6, 12 and 18 months for assessment Intervention methods: women allocated to groups of 8 to 15 women with a nutritionist leader, meeting weekly for 6 weeks, bi‐weekly for 9 months then quarterly. Women provided with personal fat gram goals Weight goals: weight and calories not mentioned Total fat intake (at 1 year): intervention 25.4 (SD unclear), control 36.0 (SD unclear) %E Saturated fat intake (at 1 year): intervention 8.7 (SD unclear), control 12.1 (SD unclear) %E Style: diet advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary intake/feasibility Available outcomes: weight, BMI, blood pressure |
|
| Notes | Weight and BMI data only found for 6 months of intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding (performance bias and detection bias) All outcomes | High risk | No for participants, though outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those randomised were analysed for weight |
| Selective reporting (reporting bias) | Low risk | For weight |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Greater time and support provided to intervention group |
| Free of dietary differences other than fat? | High risk | Suggestion to intervention group to increase fruit, vegetable and whole grain intakes |
WINS 1993.
| Methods | RCT | |
| Participants | Women with localised resected breast cancer (USA)
CVD risk: low Control: 1462 randomised, 998 analysed Intervention: 975 randomised, 386 analysed Mean years in trial: overall 5.0 % men: 0 Age: control mean 58.5 (95% CI 43.6 to 73.4), intervention mean 58.6 (95% CI 44.4 to 72.8) (all postmenopausal) Baseline BMI: mean intervention 27.6 (95% CI 27.2 to 28.0), control 27.5 (95% CI 27.2 to 27.8) |
|
| Interventions | Reduced fat intake vs usual diet Control aims: minimal nutritional counselling focused on nutritional adequacy Intervention aims: total fat 15%E to 20%E Control methods: 1 baseline dietetic session plus 3‐monthly sessions Intervention methods: 8 bi‐weekly individual dietetic sessions, then optional monthly group sessions, incorporating individual fat gram goals, social cognitive theory, self monitoring, goal setting, modelling, social support and relapse prevention and management Weight goals: "fat gram goals were based on energy needed to maintain weight, and no counselling on weight reduction was provided", not mentioned for control Total fat intake (at 1 year): low fat 20.3 (SD 8.1), control 29.2 (SD 7.4) %E Saturated fat intake (at 1 year): low fat 10.4 (SD 6.7), control 16.6 (SD 9.3) %E Style: dietary advice Setting: community |
|
| Outcomes | Stated trial outcomes: dietary fat intake, total cholesterol, weight and waist Available outcomes: weight, BMI |
|
| Notes | Weight data reported at 1, 3 and 5. 3‐year data used in main analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random stratified permuted block design, carried out at the statistical co‐ordinating centre of WINS |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants not blinded, not relevant for assessment of mortality by researchers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1053 of 2437 (43%) lost over 5 years (> 5% per year) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Low risk | |
| Free of systematic difference in care? | High risk | Differences in attention ‐ more time for those in intervention group. See 'Control methods' and 'Intervention methods' in the 'Interventions' section above |
| Free of dietary differences other than fat? | Low risk | See 'Control aims' and 'Intervention aims' in the 'Interventions' section above |
Abbreviations: %E: percentage of total energy intake
AHA: American Heart Association
BC: BMI: body mass index BP: blood pressure CHD: coronary heart disease CHO: carbohydrates CI: confidence interval CVD: cardiovascular disease HDL: high‐density lipoprotein
IHD: ischaemic heart disease LDL: low‐density lipoprotein MI: myocardial infarction
MUFA: monounsaturated fatty acid
NCEP: National Cholesterol Education Program
NEP: Nutrition Education Program NDHS: National Diet‐Heart Study P/S: polyunsaturated/saturated fat ratio
PUFA: polyunsaturated fatty acid RCT: randomised controlled trial SD: standard deviation SE: standard error
SFA: saturated fatty acid TG: triglycerides
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agewall 2001 | Multifactorial intervention |
| Ammerman 2003 | No appropriate control group (and not low fat vs modified fat) |
| Anti‐Coronary C 1966 | Not randomised |
| Aquilani 2000 | No appropriate control group (and not low fat vs modified fat) |
| Arne 2014 | Intervention aimed at weight management |
| Arntzenius 1985 | No appropriate control group (and not low fat vs modified fat) |
| Aro 1990 | Intervention and randomised follow‐up less than 6 months |
| ASSIST 2001 | Intervention is not dietary fat modification or low fat diet |
| Australian Polyp Prev | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Baer 1993 | Not randomised |
| Bakx 1997 | Multifactorial intervention |
| Barnard 2009 | Weight reduction encouraged in the conventional diet, but not in the vegan diet arm |
| Barndt 1977 | No appropriate control group (and not low fat vs modified fat) |
| Baron 1990 | Multifactorial intervention |
| Barr 1990 | Intervention and randomised follow‐up less than 6 months |
| Baumann 1982 | Intervention and randomised follow‐up less than 6 months |
| Bazzano 2012 | Participants selected on basis of BMI (30 to 45) |
| Beckmann 1988 | Not randomised |
| Beckmann 1995 | Intervention is not dietary fat modification or low fat diet |
| Beresford 1992 | Intervention and randomised follow‐up less than 6 months |
| Bergstrom 1967 | Intervention and randomised follow‐up less than 6 months |
| Bierenbaum 1963 | No appropriate control group (and not low fat vs modified fat) |
| Bloomgarden 1987 | Multifactorial intervention |
| Bonnema 1995 | No appropriate control group (and not low fat vs modified fat) |
| Bosaeus 1992 | Intervention and randomised follow‐up less than 6 months |
| Boyar 1988 | Not randomised |
| Brehm 2009 | Participants recruited on basis of being overweight or obese |
| Brensike 1982 | No appropriate control group (and not low fat vs modified fat) |
| Broekmans 2003 | Intervention is not dietary fat modification or low fat diet |
| Brown 1984 | No appropriate control group (and not low fat vs modified fat) |
| Bruce 1994 | No appropriate control group (and not low fat vs modified fat) |
| Bruno 1983 | Multifactorial intervention |
| Butcher 1990 | Intervention and randomised follow‐up less than 6 months |
| Butowski 1998 | Not randomised |
| Byers 1995 | No appropriate control group (and not low fat vs modified fat) |
| Caggiula 1996 | No appropriate control group (and not low fat vs modified fat) |
| CARMEN 2000 | Participants recruited on basis of BMI (26 to 34) |
| CARMEN MS sub‐study | Substudy of CARMEN 2000, participants recruited on basis of BMI |
| Cerin 1993 | Intervention and randomised follow‐up less than 6 months |
| Chan 1993 | Intervention and randomised follow‐up less than 6 months |
| Chapman 1950 | Intervention and randomised follow‐up less than 6 months |
| Charbonnier 1975 | Intervention and randomised follow‐up less than 6 months |
| Cheng 2004 | Intervention and randomised follow‐up less than 6 months |
| Chicago CPEP 1977 | Not randomised |
| Chiostri 1988 | Intervention and randomised follow‐up less than 6 months |
| Choudhury 1984 | Intervention and randomised follow‐up less than 6 months |
| Clark 1997 | Multifactorial intervention |
| Clifton 1992 | Intervention and randomised follow‐up less than 6 months |
| Cobb 1991 | Intervention and randomised follow‐up less than 6 months |
| Cohen 1991 | Intervention is not dietary fat modification or low fat diet |
| Cole 1988 | Intervention and randomised follow‐up less than 6 months |
| Colquhoun 1990 | Intervention and randomised follow‐up less than 6 months |
| Consolazio 1946 | Intervention and randomised follow‐up less than 6 months |
| Coppell 2010 | Weight loss recommended |
| Cox 1996 | Multifactorial intervention |
| Croft 1986 | Intervention is not dietary fat modification or low fat diet |
| Crouch 1986 | Not randomised |
| Da Qing IGT 1997 | Intervention is not dietary fat modification or low fat diet |
| Dalgard 2001 | No appropriate control group (and not low fat vs modified fat) |
| DAS 1989 | No appropriate control group (and not low fat vs modified fat) |
| DASH 1997 | Intervention and randomised follow‐up less than 6 months |
| Davey Smith 2005 | Multifactorial intervention |
| de Boer 1983 | Intervention and randomised follow‐up less than 6 months |
| DeBusk 1994 | Multifactorial intervention |
| Delahanty 2001 | No appropriate control group (and not low fat vs modified fat) |
| Delius 1969 | Intervention is not dietary fat modification or low fat diet |
| Demark 1990 | Intervention and randomised follow‐up less than 6 months |
| Dengel 1995 | No appropriate control group (and not low fat vs modified fat) |
| Denke 1994 | Intervention and randomised follow‐up less than 6 months |
| Diabetes CCT 1995 | Intervention is not dietary fat modification or low fat diet |
| DIET 1998 | Multifactorial intervention |
| Ding 1992 | Intervention and randomised follow‐up less than 6 months |
| DIRECT 2009 | Weight reduction aim |
| DO IT 2004 | "Overweight subjects were encouraged to adopt a calorie‐restricted diet" |
| Dobs 1991 | No appropriate control group (and not low fat vs modified fat) |
| Duffield 1982 | Multifactorial intervention |
| Dullaart 1997 | Not randomised |
| Dutch Nutrition Guide | No data on weight or body fatness, or any cardiovascular outcomes |
| Eating Patterns 1997 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Eckard 2013 | Energy restricted diet |
| Ehnholm 1982 | Intervention and randomised follow‐up less than 6 months |
| Ehnholm 1984 | Intervention and randomised follow‐up less than 6 months |
| Eisenberg 1990 | Intervention and randomised follow‐up less than 6 months |
| Elder 2000 | No appropriate control group (and not low fat vs modified fat) |
| Ellegard 1991 | Intervention and randomised follow‐up less than 6 months |
| Esposito 2003 | No appropriate control group (and not low fat vs modified fat) |
| Esposito 2004 | No appropriate control group (both groups aimed at < 30%E from fat) |
| Esposito 2014 | Energy restricted diet |
| EUROACTION 2008 | Multifactorial intervention |
| FARIS 1997 | Multifactorial intervention |
| Fasting HGS 1997 | No appropriate control group (and not low fat vs modified fat) |
| Ferrara 2000 | No appropriate control group (and not low fat vs modified fat) |
| Fielding 1995 | Intervention and randomised follow‐up less than 6 months |
| Finckenor 2000 | Not randomised |
| Finnish Diabetes 2000 | Multifactorial intervention |
| Finnish Mental 1972 | Not randomised (cluster‐randomised, but < 6 clusters) |
| Fisher 1981 | Intervention and randomised follow‐up less than 6 months |
| Fleming 2002 | No appropriate control group (and not low fat vs modified fat) |
| Fortmann 1988 | Intervention is not dietary fat modification or low fat diet |
| Foster 2003 | Weight reduction in one arm but not the other |
| FRESH START 2007 | Participants were newly diagnosed with cancer |
| Friedman 2012 | Weight loss diets |
| Gambera 1995 | Intervention and randomised follow‐up less than 6 months |
| Gaullier 2007 | No appropriate control group (and not low fat vs modified fat) |
| German Fat Reduced | Participants recruited on basis of their BMI (24 to 29) |
| Ginsberg 1988 | Intervention and randomised follow‐up less than 6 months |
| Gjone 1972 | Intervention and randomised follow‐up less than 6 months |
| Glatzel 1966 | No appropriate control group (and not low fat vs modified fat) |
| Goodpaster 1999 | No appropriate control group (and not low fat vs modified fat) |
| Gower 2012 | Participants recruited on basis of high BMI |
| Gregg 2013 | Participants recruited on basis of high BMI |
| Grundy 1986 | Intervention and randomised follow‐up less than 6 months |
| Gudlaugsson 2013 | Multifactorial intervention |
| Guelinckx 2010 | Participants recruited on basis of high BMI |
| Guldbrand 2012 | Weight loss intended |
| Hardcastle 2008 | Multifactorial intervention |
| Harris 1990 | Intervention and randomised follow‐up less than 6 months |
| Hartman 1993 | No appropriate control group (and not low fat vs modified fat) |
| Hartwell 1986 | No appropriate control group (and not low fat vs modified fat) |
| Hashim 1960 | Intervention and randomised follow‐up less than 6 months |
| Haynes 1984 | Intervention is not dietary fat modification or low fat diet |
| Heber 1991 | Intervention and randomised follow‐up less than 6 months |
| Heine 1989 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Heller 1993 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Hildreth 1951 | No appropriate control group (and not low fat vs modified fat) |
| Hood 1965 | Not randomised |
| Horlick 1957 | Intervention and randomised follow‐up less than 6 months |
| Horlick 1960 | Intervention and randomised follow‐up less than 6 months |
| Howard 1977 | Intervention and randomised follow‐up less than 6 months |
| Hunninghake 1990 | Intervention and randomised follow‐up less than 6 months |
| Hutchison 1983 | No appropriate control group (and not low fat vs modified fat) |
| Hyman 1998 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Iacono 1981 | Not randomised; intervention and randomised follow‐up less than 6 months |
| IMPACT 1995A | Multifactorial intervention |
| Ishikawa 1995 | Not randomised |
| Iso 1991 | No appropriate control group (and not low fat vs modified fat) |
| Ives 1993 | Multifactorial intervention |
| Jalkanen 1991 | Multifactorial intervention |
| Janus 2012 | Weight loss intended |
| Jepson 1969 | Not randomised |
| Jerusalem Nut 1992 | Intervention and randomised follow‐up less than 6 months |
| Jonasson 2014 | Energy restricted diet |
| Juanola‐Falgarona 2014 | Energy restricted diet |
| Jula 1990 | Multifactorial intervention |
| Junker 2001 | Intervention and randomised follow‐up less than 6 months |
| Karmally 1990 | Intervention and randomised follow‐up less than 6 months |
| Karvetti 1992 | Multifactorial intervention |
| Kastarinen 2002 | Multifactorial intervention |
| Kather 1985 | Intervention and randomised follow‐up less than 6 months |
| Kattelmann 2010 | Weight loss intended |
| Katzel 1995 | Not randomised |
| Katzel 1995A | Intervention is not dietary fat modification or low fat diet |
| Kawamura 1993 | Intervention and randomised follow‐up less than 6 months |
| Keidar 1988 | Intervention and randomised follow‐up less than 6 months |
| Kempner 1948 | No appropriate control group (and not low fat vs modified fat) |
| Keys 1952 | Not randomised |
| Keys 1957 | Intervention and randomised follow‐up less than 6 months |
| Keys 1957A | Intervention and randomised follow‐up less than 6 months |
| Keys 1957B | Intervention and randomised follow‐up less than 6 months |
| Khan 2003 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| King 2000 | Intervention and randomised follow‐up less than 6 months |
| Kingsbury 1961 | Intervention and randomised follow‐up less than 6 months |
| Klemsdal 2010 | Participants recruited on basis of high BMI |
| Kohler 1986 | Not randomised |
| Kontogianni 2012 | Not randomised |
| Koopman 1990 | Intervention and randomised follow‐up less than 6 months |
| Koranyi 1963 | Unclear whether randomised |
| Korhonen 2003 | Multifactorial intervention |
| Kriketos 2001 | Intervention and randomised follow‐up less than 6 months |
| Kris 1994 | Intervention and randomised follow‐up less than 6 months |
| Kristal 1997 | Multifactorial intervention |
| Kromhout 1987 | No appropriate control group (and not low fat vs modified fat) |
| Kummel 2008 | Intervention is not dietary fat modification or low fat diet |
| Laitinen 1993 | Multifactorial intervention |
| Laitinen 1994 | Multifactorial intervention |
| Larsen 2011 | Energy restricted diet |
| Leduc 1994 | Multifactorial intervention |
| Leibbrandt 2010 | Participants recruited on basis of high BMI |
| Lewis 1958 | Intervention and randomised follow‐up less than 6 months |
| Lewis 1981 | Intervention and randomised follow‐up less than 6 months |
| Lewis 1985 | Multifactorial intervention |
| Lichtenstein 2002 | Intervention and randomised follow‐up less than 6 months |
| Linko 1957 | Intervention and randomised follow‐up less than 6 months |
| Lipid Res Clinic 1984 | No appropriate control group (and not low fat vs modified fat) |
| Little 1990 | Intervention and randomised follow‐up less than 6 months |
| Little 1991 | Not randomised |
| Little 2004 | Intervention is not dietary fat modification or low fat diet |
| Lottenberg 1996 | Intervention and randomised follow‐up less than 6 months |
| Luoto 2012 | No assessment of total fat intake |
| Luszczynska 2007 | No appropriate control group (and not low fat vs modified fat) |
| Lyon Diet Heart 1994 | Intervention is not dietary fat modification or low fat diet |
| Lysikova 2003 | Intervention and randomised follow‐up less than 6 months |
| Macdonald 1972 | Intervention and randomised follow‐up less than 6 months |
| Mansel 1990 | Intervention is not dietary fat modification or low fat diet |
| Marckmann 1993 | Not randomised |
| MARGARIN | No appropriate control group (and not low fat vs modified fat) |
| Martin 2011 | Participants recruited on basis of high BMI |
| Maruthur 2014 | No relevant outcomes available |
| Mattson 1985 | Intervention and randomised follow‐up less than 6 months |
| Mayneris‐Perxachs 2014 | No assessment of total fat intake |
| McCarron 1997 | Intervention and randomised follow‐up less than 6 months |
| McCarron 2001 | Intervention is not dietary fat modification or low fat diet |
| McManus 2001 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| McNamara 1981 | Intervention and randomised follow‐up less than 6 months |
| Medi‐RIVAGE 2004 | Weight reduction for some low fat diet participants (those with BMI > 25) but not in Mediterranean group |
| Mensink 1987 | Intervention and randomised follow‐up less than 6 months |
| Mensink 1989 | Intervention and randomised follow‐up less than 6 months |
| Mensink 1990 | Intervention and randomised follow‐up less than 6 months |
| Mensink 1990A | Intervention and randomised follow‐up less than 6 months |
| Merrill 2011 | Multifactorial intervention |
| Metroville Health 2003 | No assessment of outcomes further than reduction in fat |
| Michalsen 2006 | Diet plus stress management vs no intervention |
| Miettinen 1994 | Intervention and randomised follow‐up less than 6 months |
| Millar 1973 | No appropriate control group (and not low fat vs modified fat) |
| Miller 1998 | Intervention and randomised follow‐up less than 6 months |
| Miller 2001 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Milne 1994 | No appropriate control group (and not low fat vs modified fat) ‐ the high CHO diet is neither 'usual' or 'low fat' to compare with the modified fat diet |
| Minnesota HHP 1990 | No appropriate control group (and not low fat vs modified fat) |
| Mishra 2013 | Intervention and randomised follow‐up less than 6 months |
| Mitchell 2011 | No relevant outcomes available |
| Mokuno 1988 | Intervention and randomised follow‐up less than 6 months |
| Moreno 1994 | Not randomised |
| Morrison 1950 | Not randomised |
| Morrison 1951 | Not randomised |
| Morrison 1960 | Not randomised |
| Mortensen 1983 | Intervention and randomised follow‐up less than 6 months |
| Moses 2014 | Intervention and randomised follow‐up less than 6 months |
| MRFIT substudy 1986 | Intervention and randomised follow‐up less than 6 months |
| MSDELTA 1995 | Intervention and randomised follow‐up less than 6 months |
| MUFObes low fat 2007 | Trial aims to assess weight maintenance following major weight loss |
| MUFObes low vs mod 2007 | Trial aims to assess weight maintenance following major weight loss |
| Mujeres Felices 2003 | Diet and breast self examination vs no intervention |
| Munsters 2010 | Weight loss intended |
| Mutanen 1997 | Intervention and randomised follow‐up less than 6 months |
| Muzio 2007 | Intervention and randomised follow‐up less than 6 months |
| Naglak 2000 | Dietary fat intervention unclear |
| NAS 1987 | Intervention and randomised follow‐up less than 6 months |
| NCEP weight | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Neil 1995 | No appropriate control group (and not low fat vs modified fat) |
| Neverov 1997 | Multifactorial intervention |
| Next Step 1995 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Nordoy 1971 | Intervention and randomised follow‐up less than 6 months |
| Norway Veg Oil 1968 | No appropriate control group (and not low fat vs modified fat) |
| Novotny 2012 | Weight loss intended |
| Nutrition Ed Study 1980 | Those who were overweight were provided with a weight reduction booklet |
| O'Brien 1976 | Intervention and randomised follow‐up less than 6 months |
| ODES 2001 | The study aimed for weight loss in some participants |
| Oldroyd 2001 | Multifactorial intervention |
| Orazio 2011 | Weight loss intended |
| ORIGIN 2008 | Intervention is not dietary fat modification or low fat diet |
| Ornish 1990 | Multifactorial intervention (diet, smoking, stress and exercise) compared to no intervention |
| Oslo Study 1980 | Multifactorial intervention |
| Otago Weight Loss 2005 | Although intake was ad libitum the aim was for weight loss to occur ‐ participants presumably joined the study on the basis that it was assessing effects on weight loss, so were keen to lose weight |
| Pandey 2013 | Not randomised |
| Pascale 1995 | Multifactorial intervention |
| Paz‐Tal 2013 | No relevant outcomes available |
| PEP 2001 | Multifactorial intervention |
| PHYLLIS 1993 | No appropriate control group (and not low fat vs modified fat) |
| PREDIMED 2007 | Modified fat group is clearly defined, but no fat goals were set for the low fat group. We were unable to verify whether the fat aim was ≤ 30%E |
| PREMIER 2003 | Overweight participants were encouraged to lose weight |
| Pritchard 2002 | The study aimed for weight loss in one arm and not in the comparison arm |
| Puget Sound EP | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Rabast 1979 | Intervention and randomised follow‐up less than 6 months |
| Rabkin 1981 | Intervention and randomised follow‐up less than 6 months |
| Radack 1990 | Intervention and randomised follow‐up less than 6 months |
| Rasmussen 1995 | Intervention and randomised follow‐up less than 6 months |
| Reaven 2001 | Intervention and randomised follow‐up less than 6 months |
| Reid 2002 | No appropriate control group (and not low fat vs modified fat) |
| Renaud 1986 | Not randomised |
| Rivellese 2003 | Intervention and randomised follow‐up less than 6 months |
| Roderick 1997 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Roman CHD prev 1986 | Multifactorial intervention |
| Rose 1987 | No appropriate control group (and not low fat vs modified fat) |
| Rusu 2013 | Energy restricted diet |
| Sacks 2009 | All arms aimed at a 750 kcal/day deficit to ensure weight loss |
| Salas‐Salvado 2014 | No assessment of total fat intake |
| Sandstrom 1992 | Not randomised |
| Sasaki 2000 | Not randomised |
| Schaefer 1995 | Intervention and randomised follow‐up less than 6 months |
| Schaefer 1995A | Intervention and randomised follow‐up less than 6 months |
| Schectman 1996 | Multifactorial intervention |
| Schlierf 1995 | Multifactorial intervention |
| Seppanen‐Laakso | Intervention and randomised follow‐up less than 6 months |
| Shai 2012 | Energy restricted diet |
| Singh 1990 | Not randomised |
| Singh 1991 | Multifactorial intervention |
| Singh 1992 | No appropriate control group (and not low fat vs modified fat) |
| Siqueira‐Catania 2010 | Weight loss intended |
| Sirtori 1992 | Intervention and randomised follow‐up less than 6 months |
| SLIM 2008 | Multifactorial intervention |
| Sollentuna Diet | The study aimed for weight loss in one arm and not in the comparison arm |
| Sollentuna Diet & Ex | The study aimed for weight loss in one arm and not in the comparison arm |
| Sopotsinskaia 1992 | The study aimed for weight loss in one arm and not in the comparison arm |
| Staff HHP 1994 | Not randomised |
| Stanford NAP 1997 | Intervention and randomised follow‐up less than 6 months |
| Stanford Weight | The study aimed for weight loss in one arm and not in the comparison arm |
| Starmans 1995 | Intervention and randomised follow‐up less than 6 months |
| Steinbach 1996 | Multifactorial intervention |
| Steptoe 2001 | No appropriate control group (and not low fat vs modified fat) |
| Stevens 2002 | Diet plus breast self examination vs no intervention |
| Stevenson 1988 | No appropriate control group (and not low fat vs modified fat) |
| Sweeney 2004 | Intervention is not dietary fat modification or low fat diet |
| TAIM 1989 | Intervention is not dietary fat modification or low fat diet |
| Take Heart II 1997 | Not randomised |
| Tapsell 2004 | No weight data or cardiovascular outcomes reported |
| Taylor 1991 | Not randomised |
| THIS DIET 2008 | Study states "although this was not a weight loss intervention, participants who were overweight or obese were encouraged to reduce calories to facilitate weight loss". |
| TOHP I 1992 | Multifactorial intervention |
| TONE 1997 | Intervention is not dietary fat modification or low fat diet |
| Toobert 2003 | Multifactorial intervention |
| Toronto Polyp Prev 1994 | No weight or BMI data presented |
| Towle 1994 | Intervention and randomised follow‐up less than 6 months |
| TRANSFACT 2006 | Intervention and randomised follow‐up less than 6 months |
| Treatwell 1992 | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Tromso Heart 1989 | Multifactorial intervention |
| Turku Weight | Both intervention groups aimed to lose weight, while the control group did not |
| Turpeinen 1960 | Not randomised |
| UK PDS 1996 | No appropriate control group (and not low fat vs modified fat) |
| Urbach 1952 | No appropriate control group (and not low fat vs modified fat) |
| Uusitupa 1993 | Multifactorial intervention |
| Uusitupa 2013 | Intervention and randomised follow‐up less than 6 months |
| Vavrikova 1958 | Intervention and randomised follow‐up less than 6 months |
| Wan 2013 | Not a RCT |
| Wass 1981 | Intervention and randomised follow‐up less than 6 months |
| Wassertheil 1985 | Intervention is not dietary fat modification or low fat diet |
| WATCH | Neither mortality nor cardiovascular morbidity data available (only decided after contact with at least one author) |
| Watts 1988 | Intervention and randomised follow‐up less than 6 months |
| Weintraub 1992 | No appropriate control group (and not low fat vs modified fat) |
| Westman 2006 | Intervention is not dietary fat modification or low fat diet |
| Weststrate 1998 | Intervention and randomised follow‐up less than 6 months |
| WHO primary prev 1979 | Multifactorial intervention |
| WHT | Neither mortality nor cardiovascular morbidity data available as such data were not collected in the study |
| Wilke 1974 | Intervention and randomised follow‐up less than 6 months |
| Williams 1990 | Intervention is not dietary fat modification or low fat diet |
| Williams 1992 | Intervention is not dietary fat modification or low fat diet |
| Williams 1994 | Intervention is not dietary fat modification or low fat diet |
| Wilmot 1952 | No appropriate control group (and not low fat vs modified fat) |
| Wing 1998 | No appropriate control group (and not low fat vs modified fat) |
| Wolever 2008 | Weight loss intended in some participants |
| WOMAN 2007 | Lifestyle intervention includes exercise and weight as well as diet |
| Wood 1988 | Intervention is not dietary fat modification or low fat diet |
| Woollard 2003 | Multifactorial intervention including smoking, weight, exercise and alcohol components |
| Working Well 1996 | Multifactorial intervention |
| Young 2010 | Weight loss intended |
| Zock 1995 | Intervention and randomised follow‐up less than 6 months |
BMI: body mass index RCT: randomised controlled trial
Contributions of authors
The WHO NUGAG subgroup on diet and health (which included LH, MS and CDS) discussed and developed the question for this review. The protocol was drafted by LH and approved by the NUGAG subgroup on diet and health. LH, WD, and HJM carried out the searches for the first version of the review, AA and LH carried out searches for the update. LH, AA, WD, HJM and CSE assessed the eligibility of the studies for inclusion of the first review, extracted data and assessed trial validity, while AA, DKB, TB and LH carried this out for the update. LH carried out the first GRADE assessment, which was refined by the NUGAG subgroup on diet and health, LH carried out the GRADE assessment for this update. LH wrote the first drafts of the original paper and this update. All authors contributed to the analysis, as did the NUGAG subgroup on diet and health in response to the first draft of the review. All authors agreed on the final draft of this review. LH is the guarantor.
Sources of support
Internal sources
-
University of East Anglia, UK.
For the original version of this systematic review: help with acquiring papers for the review, time for Lee Hooper to work on the review.
External sources
-
The World Health Organization (WHO) provided funding to Durham University towards the cost of carrying out the original version of this systematic review, Not specified.
No funding was received for the searching, analysis, or writing up of the data from randomised controlled trials in adults for the first version of the review. The funders did not have any vested interests in the findings of this research
WHO provided funding to the University of East Anglia (PI Lee Hooper) for the update of this systematic review and translation into a Cochrane review, Not specified.
Declarations of interest
AA: none known.
TB: none known.
DB: none known.
LH: the World Health Organization (WHO) provided funding to the University of East Anglia towards the cost of carrying out the update of this systematic review. LH is a member of the WHO NUGAG subgroup on diet and health and received funding from WHO to cover expenses associated with attendance at meetings of the NUGAG subgroup on diet and health.
CMS: none known
CDS: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Auckland reduced fat 1999 {published and unpublished data}
- Ley SJ, Metcalf PA, Scragg RKR, Swinburn BA. Long‐term effects of a reduced fat diet intervention on cardiovascular disease risk factors in individuals with glucose intolerance. Diabetes Research and Clinical Practice 2004;63:103‐12. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Metcalf PA, Ley SJ. Long‐term (5‐year) effects of a reduced‐fat diet intervention in individuals with glucose intolerance. Diabetes Care 2001;24(4):619‐24. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Woollard GA, Chang EC, Wilson MR. Effects of reduced‐fat diets consumed ad libitum on intake of nutrients particularly antioxidant vitamins. Journal of the American Dietetic Association 1999;99(11):1400‐5. [DOI] [PubMed] [Google Scholar]
BDIT Pilot Studies 1996 {published and unpublished data}
- Boyd NF, Cousins M, Beaton M, Fishell E, Wright B, Fish E, et al. Clinical trial of low‐fat, high‐carbohydrate diet in subjects with mammographic dysplasia: report of early outcomes. Journal of the National Cancer Institute 1988;80:1244‐8. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Cousins M, Beaton M, Han L, McGuire V. Methodological issues in clinical trials of dietary fat reduction in patients with breast dysplasia. Progress in Clinical and Biological Research 1986;222:117‐24. [PubMed] [Google Scholar]
- Boyd NF, Cousins M, Beaton M, Kriukov V, Lockwood G, Tritchler D. Quantitative changes in dietary fat intake and serum cholesterol in women: results from a randomized, controlled trial. American Journal of Clinical Nutrition 1990;52(3):470‐6. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Cousins M, Kriukov V. A randomised controlled trial of dietary fat reduction: the retention of subjects and characteristics of drop outs. Journal of Clinical Epidemiology 1992;45(1):31‐8. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Cousins M, Lockwood G, Tritchler D. Dietary fat and breast cancer risk: the feasibility of a clinical trial of breast cancer prevention. Lipids 1992;27(10):821‐6. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Cousins M, Lockwood G, Tritchler D. The feasibility of testing experimentally the dietary fat‐breast cancer hypothesis. Progress in Clinical and Biological Research 1990;346:231‐41. [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Beaton M, Cousins M, Kriukov V. Long‐term effects of participation in a randomized trial of a low‐fat, high‐carbohydrate diet. Cancer Epidemiology, Biomarkers and Prevention 1996;5(3):217‐22. [PubMed] [Google Scholar]
- Lee‐Han H, Cousins M, Beaton M, McGuire V, Kriukov V, Chipman M, et al. Compliance in a randomized clinical trial of dietary fat reduction in patients with breast dysplasia. American Journal of Clinical Nutrition 1988;48(3):575‐86. [DOI] [PubMed] [Google Scholar]
beFIT 1997 {published and unpublished data}
- Retzlaff BM, Walden CE, McNeney WB, Buck BL, McCann BS, Knopp RH. Nutritional intake of women and men on the NCEP Step I and Step II diets. Journal of the American College of Nutrition 1997;16(1):52‐61. [DOI] [PubMed] [Google Scholar]
- Walden CE, Retzlaff BM, Buck BL, McCann BS, Knopp RH. Lipoprotein lipid response to the National Cholesterol Education Program Step II diet by hypercholesterolemic and combined hyperlipidemic women and men. Arteriosclerosis, Thrombosis and Vascular Biology 1997;17:375‐82. [DOI] [PubMed] [Google Scholar]
- Walden CE, Retzlaff BM, Buck BL, Wallick S, McCann BS, Knopp RH. Differential effect of National Cholesterol Education Program (NCEP) Step II Diet on HDL cholesterol, its subfractions, and apoprotein A‐1 levels in hypercholesterolemic women and men after 1 year: the beFIT study. Arteriosclerosis, Thrombosis and Vascular Biology 2000;20(6):1580‐7. [DOI] [PubMed] [Google Scholar]
Bloemberg 1991 {published and unpublished data}
- Bloemberg BPM, Kromhout D, Goddijn HE, Jansen A, Obermann de Boer GL. The impact for the guidelines for a healthy diet of the Netherlands Nutrition Council on total and high density lipoprotein cholesterol in hypercholesterolemic free living men. American Journal of Epidemiology 1991;134:39‐48. [DOI] [PubMed] [Google Scholar]
BRIDGES 2001 {published and unpublished data}
- Hebert JR, Ebbeling CB, Olendzki BC, Hurley TG, Ma Y, Saal N, et al. Change in women's diet and body mass following intensive intervention for early‐stage breast cancer. Journal of the American Dietetic Association 2001;101(4):421‐31. [DOI] [PubMed] [Google Scholar]
Canadian DBCP 1997 {published data only (unpublished sought but not used)}
- Boyd NF, Greenberg C, Lockwood G, Little L, Martin L, Byng J, et al. Effects at two years of a low‐fat, high‐carbohydrate diet on radiologic features of the breast: results from a randomized trial. Canadian Diet and Breast Cancer Prevention Study Group. Journal of the National Cancer Institute 1997;89(7):488‐96. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Greenberg C, Martin L, Stone J, Hammond G, Minkin S, et al. Lack of effect of a low‐fat high‐carbohydrate diet on ovarian hormones in premenopausal women: results from a randomized trial. IARC Scientific Publications 2002;156:445‐50. [PubMed] [Google Scholar]
- Boyd NF, Lockwood GA, Greenberg CV, Martin LJ, Tritchler DL, Boyd NF, et al. Effects of a low‐fat high‐carbohydrate diet on plasma sex hormones in premenopausal women: results from a randomized controlled trial. Canadian Diet and Breast Cancer Prevention Study Group. British Journal of Cancer 1997;76(1):127‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JA, Martin LJ, Greenberg CV, Lockwood GA, Byng JW, Yaffe MJ, et al. Macronutrient intake and change in mammographic density at menopause: results from a randomized trial. Cancer Epidemiology, Biomarkers & Prevention 1999;8(2):123‐8. [PubMed] [Google Scholar]
- Leyenaar J, Sutherland HJ, Lockwood GA, Martin LJ, Kriukov V, Greenberg CV, et al. Self‐reported physical and emotional health of women in a low‐fat, high‐carbohydrate dietary trial (Canada). Cancer Causes & Control 1998;9(6):601‐10. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Greenberg CV, Kriukov V, Minkin S, Jenkins DJ, Boyd NF, et al. Intervention with a low‐fat, high‐carbohydrate diet does not influence the timing of menopause. American Journal of Clinical Nutrition 2006;84(4):920‐8. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Greenberg CV, Kriukov V, Minkin S, Jenkins DJ, Yaffe M, et al. Effect of a low‐fat, high‐carbohydrate dietary intervention on change in mammographic density over menopause. Breast Cancer Research & Treatment 2009;113(1):163‐72. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Lockwood GA, Kristal AR, Kriukov V, Greenberg C, Shatuck AL, et al. Assessment of a food frequency questionnaire as a screening tool for low fat intakes. Controlled Clinical Trials 1997;18(3):241‐50. [DOI] [PubMed] [Google Scholar]
- Sutherland HJ, Carlin K, Harper W, Martin LJ, Greenberg CV, Till JE, et al. A study of diet and breast cancer prevention in Canada: why healthy women participate in controlled trials. Cancer Causes & Control 1993;4(6):521‐8. [DOI] [PubMed] [Google Scholar]
de Bont 1981 non‐obese {published and unpublished data}
- Bont AJ, Baker IA, Leger AS, Sweetnam PM, Wragg KG, Stephens SM, et al. A randomised controlled trial of the effect of low fat diet advice on dietary response in insulin independent diabetic women. Diabetologia 1981;21(6):529‐33. [DOI] [PubMed] [Google Scholar]
de Bont 1981 obese {published and unpublished data}
- Bont AJ, Baker IA, Leger AS, Sweetnam PM, Wragg KG, Stephens SM, et al. A randomised controlled trial of the effect of low fat diet advice on dietary response in insulin independent diabetic women. Diabetologia 1981;21(6):529‐33. [DOI] [PubMed] [Google Scholar]
DEER 1998 exercise men {published data only}
- Camhi SM, Stefanick ML, Katzmarzyk PT, Young DR. Metabolic syndrome and changes in body fat from a low‐fat diet and/or exercise randomized controlled trial. Obesity 2010;18(3):548‐54. [DOI: 10.1038/oby.2009.304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi SM, Stefanick ML, Ridker PM, Young DR. Changes in C‐reactive protein from low‐fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism 2010;59(1):54‐61. [DOI: 10.1016/j.metabol.2009.07.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanick ML, Mackey S, Sheehan RD, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. New England Journal of Medicine 1998;339(1):12‐20. [DOI] [PubMed] [Google Scholar]
DEER 1998 exercise women {published data only}
- Camhi SM, Stefanick ML, Katzmarzyk PT, Young DR. Metabolic syndrome and changes in body fat from a low‐fat diet and/or exercise randomized controlled trial. Obesity 2010;18(3):548‐54. [DOI: 10.1038/oby.2009.304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi SM, Stefanick ML, Ridker PM, Young DR. Changes in C‐reactive protein from low‐fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism 2010;59(1):54‐61. [DOI: 10.1016/j.metabol.2009.07.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanick ML, Mackey S, Sheehan RD, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. New England Journal of Medicine 1998;339(1):12‐20. [DOI] [PubMed] [Google Scholar]
DEER 1998 no exercise men {published data only (unpublished sought but not used)}
- Camhi SM, Stefanick ML, Katzmarzyk PT, Young DR. Metabolic syndrome and changes in body fat from a low‐fat diet and/or exercise randomized controlled trial. Obesity 2010;18(3):548‐54. [DOI: 10.1038/oby.2009.304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi SM, Stefanick ML, Ridker PM, Young DR. Changes in C‐reactive protein from low‐fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism 2010;59(1):54‐61. [DOI: 10.1016/j.metabol.2009.07.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanick ML, Mackey S, Sheehan RD, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. New England Journal of Medicine 1998;339(1):12‐20. [DOI] [PubMed] [Google Scholar]
DEER 1998 no exercise wom {published data only}
- Camhi SM, Stefanick ML, Katzmarzyk PT, Young DR. Metabolic syndrome and changes in body fat from a low‐fat diet and/or exercise randomized controlled trial. Obesity 2010;18(3):548‐54. [DOI: 10.1038/oby.2009.304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi SM, Stefanick ML, Ridker PM, Young DR. Changes in C‐reactive protein from low‐fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism 2010;59(1):54‐61. [DOI: 10.1016/j.metabol.2009.07.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanick ML, Mackey S, Sheehan RD, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. New England Journal of Medicine 1998;339(1):12‐20. [DOI] [PubMed] [Google Scholar]
Diet and Hormone Study 2003 {published data only (unpublished sought but not used)}
- Gann PH, Chatterton RT, Gapstur SM, Liu K, Garside D, Giovannazzi S, et al. The effects of a low‐fat/high‐fiber diet on sex hormone levels and menstrual cycling in premenopausal women: a 12‐month randomized trial (the Diet and Hormone Study). Cancer 2003;98:1870‐9. [DOI] [PubMed] [Google Scholar]
Kentucky Low Fat 1990 {published and unpublished data}
- Anderson JW, Garrity TF, Smith BM, Whitis SE. Follow‐up on a clinical trial comparing the effects of two lipid lowering diets. Arteriosclerosis 1990;10(5):882a. [Google Scholar]
- Anderson JW, Garrity TF, Wood CL, Whitis SE, Smith BM, Oeltgen PR. Prospective, randomized, controlled comparison of the effects of low‐fat and low‐fat plus high‐fiber diets on serum lipid concentrations. American Journal of Clinical Nutrition 1992;56(5):887‐94. [DOI] [PubMed] [Google Scholar]
Kuopio Reduced & Mod 1993 {published and unpublished data}
- Makinen E, Uusitupa MI, Pietinen P, Aro A, Penttila I. Long term effects of three fat modified diets on serum lipids in free living hypercholesterolaemic subjects (abstract). European Heart Journal 1991;12:162. [Google Scholar]
- Sarkkinen E. Long‐term feasibility and effects of three different fat‐modified diets in free‐living hypercholesterolemic subjects [PhD Thesis]. Department of Clinical Nutrition, Faculty of Medicine, University of Kuopio, 1995. [Google Scholar]
- Sarkkinen ES, Agren JJ, Ahola I, Ovaskainen ML, Uusitupa MI. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long‐term adherence to fat‐modified diets. American Journal of Clinical Nutrition 1994;59(2):364‐70. [DOI] [PubMed] [Google Scholar]
- Sarkkinen ES, Uusitupa MI, Nyyssonen K, Parviainen M, Penttila I, Salonen JT. Effects of two low‐fat diets, high and low in polyunsaturated fatty acids, on plasma lipid peroxides and serum vitamin E levels in free‐living hypercholesterolaemic men. European Journal of Clinical Nutrition 1993;47(9):623‐30. [PubMed] [Google Scholar]
- Sarkkinen ES, Uusitupa MI, Pietinen P, Aro A, Ahola I, Penttila I, et al. Long‐term effects of three fat‐modified diets in hypercholesterolemic subjects. Atherosclerosis 1994;105(1):9‐23. [DOI] [PubMed] [Google Scholar]
- Uusitupa MI, Sarkkinen ES, Torpstrom J, Pietinen P, Aro A. Long‐term effects of four fat‐modified diets on blood pressure. Journal of Human Hypertension 1994;8(3):209‐18. [PubMed] [Google Scholar]
Kuopio Reduced Fat 1993 {published and unpublished data}
- Makinen E, Uusitupa MI, Pietinen P, Aro A, Penttila I. Long term effects of three fat modified diets on serum lipids in free living hypercholesterolaemic subjects (abstract). European Heart Journal 1991;12:162. [Google Scholar]
- Sarkkinen E. Long‐term feasibility and effects of three different fat‐modified diets in free‐living hypercholesterolemic subjects [PhD Thesis]. Department of Clinical Nutrition, Faculty of Medicine, University of Kuopio, 1995. [Google Scholar]
- Sarkkinen ES, Agren JJ, Ahola I, Ovaskainen ML, Uusitupa MI. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long‐term adherence to fat‐modified diets. American Journal of Clinical Nutrition 1994;59(2):364‐70. [DOI] [PubMed] [Google Scholar]
- Sarkkinen ES, Uusitupa MI, Nyyssonen K, Parviainen M, Penttila I, Salonen JT. Effects of two low‐fat diets, high and low in polyunsaturated fatty acids, on plasma lipid peroxides and serum vitamin E levels in free‐living hypercholesterolaemic men. European Journal of Clinical Nutrition 1993;47(9):623‐30. [PubMed] [Google Scholar]
- Sarkkinen ES, Uusitupa MI, Pietinen P, Aro A, Ahola I, Penttila I, et al. Long‐term effects of three fat‐modified diets in hypercholesterolemic subjects. Atherosclerosis 1994;105(1):9‐23. [DOI] [PubMed] [Google Scholar]
- Uusitupa MI, Sarkkinen ES, Torpstrom J, Pietinen P, Aro A. Long‐term effects of four fat‐modified diets on blood pressure. Journal of Human Hypertension 1994;8(3):209‐18. [PubMed] [Google Scholar]
Mastopathy Diet 1988 {published and unpublished data}
- Boyd NF, McGuire V, Shannon P, Cousins M, Kriukov V, Mahoney L, et al. Effect of a low‐fat high‐carbohydrate diet on symptoms of cyclical mastopathy. Lancet 1988;2(8603):128‐32. [DOI] [PubMed] [Google Scholar]
MeDiet 2006 {published and unpublished data}
- Carruba G, Granata OM, Pala V, Campisi I, Agostara B, Cusimano R, et al. A traditional Mediterranean diet decreases endogenous estrogens in healthy postmenopausal women. Nutrition and Cancer 2006;56(2):253‐9. [DOI] [PubMed] [Google Scholar]
- Castagnetta L, Granata OM, Cusimano R, Ravazzolo B, Liquori M, Polito L, et al. The Mediet Project. Annals of the New York Academy of Science 2002;963:282‐9. [DOI] [PubMed] [Google Scholar]
- Granata OM, Traina A, Ramirez S, Campisi I, Zarcone M, Amodio R, et al. Dietary enterolactone affects androgen and estrogen levels in healthy postmenopausal women. Annals of the New York Academy of Science 2009;1155:232‐6. [DOI] [PubMed] [Google Scholar]
Moy 2001 {published and unpublished data}
- Moy TF, Yanek LR, Raqueno JV, Bezirdjian PJ, Blumenthal RS, Wilder LB, et al. Dietary counseling for high blood cholesterol in families at risk of coronary disease. Preventive Cardiology 2001;4(4):158‐64. [DOI] [PubMed] [Google Scholar]
MSFAT 1995 {published and unpublished data}
- Velthuis‐te WE, Leeuwen REW, Hendriks HF, Verhagen H, Loft S, Poulsen HE, et al. Short‐term moderate energy restriction does not affect indicators of oxidative stress and genotoxicity in humans. Journal of Nutrition 1995;125:2631‐9. [DOI] [PubMed] [Google Scholar]
- Velthuis‐te Wierik EJ, Berg H, Weststrate JA, het Hof KH, Graaf C. Consumption of reduced‐fat products: effects on parameters of anti‐oxidative capacity. European Journal of Clinical Nutrition 1996;50(4):214‐9. [PubMed] [Google Scholar]
- Weststrate JA, het Hof KH, Berg H, Velthuis‐te WE, Graaf C, Zimmermanns NJ, et al. A comparison of the effect of free access to reduced fat products or their full fat equivalents on food intake, body weight, blood lipids and fat‐soluble antioxidants levels and haemostasis variables. European Journal of Clinical Nutrition 1998;52:389‐95. [DOI] [PubMed] [Google Scholar]
- het Hof KH, Weststrate JA, Berg H, Velthuis‐te Wierik EJ, Graaf C, Zimmermanns NJ, at al. A long‐term study on the effect of spontaneous consumption of reduced fat products as part of a normal diet on indicators of health. International Journal of Food Sciences and Nutrition 1997;48(1):19‐29. [DOI] [PubMed] [Google Scholar]
NDHS Open 1st L&M 1968 {published data only}
- Anon. The National Diet‐Heart Study. Nutrition Reviews 1968;26(5):133‐6. [DOI] [PubMed] [Google Scholar]
- Baker BM, Frantz ID Jr, Keys A, Kinsell LW, Page IH, Stamler J, et al. The National Diet‐Heart Study: an initial report. JAMA 1963;185:105‐6. [DOI] [PubMed] [Google Scholar]
- Brown HB. The National Diet Heart Study ‐ implications for dietitians and nutritionists. Journal of the American Dietetic Association 1968;52:279‐87. [PubMed] [Google Scholar]
- NDHS. The national diet‐heart study final report. Circulation 1968;37(II):1‐428. [PubMed] [Google Scholar]
- Page IH, Brown HB. Some observations on the National Diet‐Heart Study. Circulation 1968;37:313‐5. [DOI] [PubMed] [Google Scholar]
NDHS Open 2nd L&M 1968 {published data only}
- Anon. The National Diet‐Heart Study. Nutrition Reviews 1968;26(5):133‐6. [DOI] [PubMed] [Google Scholar]
- Baker BM, Frantz ID Jr, Keys A, Kinsell LW, Page IH, Stamler J, et al. The National Diet‐Heart Study: an initial report. JAMA 1963;185:105‐6. [DOI] [PubMed] [Google Scholar]
- Brown HB. The National Diet Heart Study ‐ implications for dietitians and nutritionists. Journal of the American Dietetic Association 1968;52:279‐87. [PubMed] [Google Scholar]
- NDHS. The national diet‐heart study final report. Circulation 1968;37(II):1‐428. [PubMed] [Google Scholar]
- Page IH, Brown HB. Some observations on the National Diet‐Heart Study. Circulation 1968;37:313‐5. [DOI] [PubMed] [Google Scholar]
Nutrition & Breast Health {published and unpublished data}
- Djuric Z, Poore KM, Depper JB, Uhley VE, Lababidi S, Covington C, et al. Methods to increase fruit and vegetable intake with and without a decrease in fat intake: compliance and effects on body weight in the Nutrition and Breast Health Study. Nutrition and Cancer 2002;43(2):141‐51. [DOI] [PubMed] [Google Scholar]
Pilkington 1960 {published and unpublished data}
- Pilkington TRE, Stafford JL, Hankin VS, Simmonds FM, Koerselman HB. Practical diets for lowering serum lipids. British Medical Journal 1960;2 Jan:23‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polyp Prevention 1996 {published and unpublished data}
- Lanza E, Schatzkin A, Ballard BR, Clifford DC, Paskett E, Hayes D, et al. The polyp prevention trial II: dietary intervention program and participant baseline dietary characteristics. Cancer Epidemiology, Biomarkers and Prevention 1996;5(5):385‐92. [PubMed] [Google Scholar]
- Schatzkin A, Lanza E, Freedman LS, Tangrea J, Cooper MR, Marshall JR, et al. The polyp prevention trial I: rationale, design, recruitment, and baseline participant characteristics. Cancer Epidemiology, Biomarkers and Prevention 1996;5(5):375‐83. [PubMed] [Google Scholar]
Rivellese 1994 {published and unpublished data}
- Rivellese AA, Auletta P, Marotta G, Saldalamacchia G, Giacoo A, Mastrilli V, et al. Long term metabolic effects of two dietary methods of treating hyperlipidaemia. BMJ 1994;308:227‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon Low Fat Breast CA {published and unpublished data}
- Djuric Z, Heilbrun LK, Reading BA, Boomer A, Valeriote FA, Martino S. Effects of a low fat diet on levels of oxidative damage to DNA in human peripheral nucleated blood cells. Journal of the National Cancer Institute 1991;83(11):766‐9. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Martino S, Heilbrun LK, Hart RW. Dietary modulation of oxidative DNA damage. Advances In Experimental Medicine and Biology 1994;354:71‐83. [DOI] [PubMed] [Google Scholar]
- Kasim SE, Martino S, Kim P‐N, Khilnani S, Boomer A, Depper J, et al. Dietary and anthropometric determinants of plasma lipoproteins during a long‐term low‐fat diet in healthy women. American Journal of Clinical Nutrition 1993;57:146‐53. [DOI] [PubMed] [Google Scholar]
- Simon MS, Heilbrun LK, Boomer A, Kresge C, Depper J, Kim PN, et al. A randomised trial of a low‐fat dietary intervention in women at high risk for breast cancer. Nutrition and Cancer 1997;27(2):136‐42. [DOI] [PubMed] [Google Scholar]
Sondergaard 2003 {published and unpublished data}
- Sondergaard E, Moller JE, Egstrup K. Effect of dietary intervention and lipid‐lowering treatment on brachial vasoreactivity in patients with ischemic heart disease and hypercholesterolemia. American Heart Journal 2003;145(5):E19. [DOI] [PubMed] [Google Scholar]
Strychar 2009 {published and unpublished data}
- Strychar I, Cohn JS, Renier G, Rivard M, Aris‐Jilwan N, Beauregard H, et al. Effects of a diet higher in carbohydrate/lower in fat versus lower in carbohydrate/higher in monounsaturated fat on postmeal triglyceride concentrations and other cardiovascular risk factors in type 1 diabetes. Diabetes Care 2009;32(9):1597‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swedish Breast CA 1990 {published data only (unpublished sought but not used)}
- Holm LE, Nordevang E, Ikkala E, Hallstrom L, Callmer E. Dietary intervention as adjuvant therapy in breast cancer patients‐‐a feasibility study. Breast Cancer Research and Treatment 1990;16(2):103‐9. [DOI] [PubMed] [Google Scholar]
- Nordevang E, Callmer E, Marmur A, Holm LE. Dietary intervention in breast cancer patients: effects on food choice. European Journal of Clinical Nutrition 1992;46(6):387‐96. [PubMed] [Google Scholar]
- Nordevang E, Ikkala E, Callmer E, Hallstrom L, Holm LE. Dietary intervention in breast cancer patients: effects on dietary habits and nutrient intake. European Journal of Clinical Nutrition 1990;44(9):681‐7. [PubMed] [Google Scholar]
Veterans Dermatology 1994 {published and unpublished data}
- Black HS, Herd JA, Goldberg LH, Wolf‐JE J, Thornby JI, Rosen T, et al. Effect of a low‐fat diet on the incidence of actinic keratosis. New England Journal of Medicine 1994;330(18):1272‐5. [DOI] [PubMed] [Google Scholar]
- Black HS, Thornby JI, Wolf‐JE J, Goldberg LH, Herd JA, Rosen T, et al. Evidence that a low‐fat diet reduces the occurrence of non‐melanoma skin cancer. International Journal of Cancer 1995;62(2):165‐9. [DOI] [PubMed] [Google Scholar]
- Jaax S, Scott LW, Wolf‐JE J, Thornby JI, Black HS. General guidelines for a low‐fat diet effective in the management and prevention of nonmelanoma skin cancer. Nutrition and Cancer 1997;27(2):150‐6. [DOI] [PubMed] [Google Scholar]
VYRONAS 2009 {published data only}
- Mihas C, Mariolis A, Manios Y, Naska A, Arapaki A, Mariolis‐Sapsakos T, et al. Evaluation of a nutrition intervention in adolescents of an urban area in Greece: short‐ and long‐term effects of the VYRONAS study. Public Health Nutrition 2010;13(5):712‐9. [DOI: 10.1017/S1368980009991625] [DOI] [PubMed] [Google Scholar]
WHEL 2007 {published data only}
- Bardwell WA, Profant J, Casden DR, Dimsdale JE, Ancoli‐Israel S, Natarajan L, et al. The relative importance of specific risk factors for insomnia in women treated for early‐stage breast cancer. Psycho‐Oncology 2008;17(1):9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caan BJ, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP, et al. Low‐energy reporting in women at risk for breast cancer recurrence. Women's Healthy Eating and Living Group. Cancer Epidemiology, Biomarkers & Prevention 2000;9(10):1091‐7. [PubMed] [Google Scholar]
- Gold EB, Flatt SW, Pierce JP, Bardwell WA, Hajek RA, Newman VA, et al. Dietary factors and vasomotor symptoms in breast cancer survivors: the WHEL Study. Menopause 2006;13(3):423‐33. [DOI] [PubMed] [Google Scholar]
- Gold EB, Pierce JP, Natarajan L, Stefanick ML, Laughlin GA, Caan BJ, et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: the women's healthy eating and living trial. Journal of Clinical Oncology 2009;27(3):352‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Valero MA, Thomson CA, Hernandez M, Tran T, Detry MA, Theriault RL, et al. Comparison of baseline dietary intake of Hispanic and matched non‐Hispanic white breast cancer survivors enrolled in the Women's Healthy Eating and Living study. Journal of the American Dietetic Association 2008;108(8):1323‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Bardwell WA, Natarajan L, Flatt SW, Rock CL, Newman VA, et al. Correlates of physical activity level in breast cancer survivors participating in the Women's Healthy Eating and Living (WHEL) Study. Breast Cancer Research & Treatment 2007;101(2):225‐32. [DOI] [PubMed] [Google Scholar]
- Hyder JA, Thomson CA, Natarajan L, Madlensky L, Pu M, Emond J, et al. Adopting a plant‐based diet minimally increased food costs in WHEL Study. American Journal of Health Behavior 2009;33(5):530‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlensky L, Natarajan L, Flatt SW, Faerber S, Newman VA, Pierce JP, et al. Timing of dietary change in response to a telephone counseling intervention: evidence from the WHEL study. Health Psychology 2008;27(5):539‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JE, Flatt SW, Parker BA, Gold EB, Wasserman L, Natarajan L, et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Research & Treatment 2008;108(3):421‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman VA, Thomson CA, Rock CL, Flatt SW, Kealey S, Bardwell WA, et al. Achieving substantial changes in eating behavior among women previously treated for breast cancer‐‐an overview of the intervention. Journal of the American Dietetic Association 2005;105(3):382‐91. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, et al. A randomized trial of the effect of a plant‐based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Controlled Clinical Trials 2002;23(6):728‐56. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Caan BJ, Flatt SW, Kealey S, Gold EB, et al. Dietary change and reduced breast cancer events among women without hot flashes after treatment of early‐stage breast cancer: subgroup analysis of the Women's Healthy Eating and Living Study. American Journal of Clinical Nutrition 2009;89(5):1565S‐71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA 2007;298(3):289‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. [see comment]. JAMA 2007;298(3):289‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Sun S, Al‐Delaimy W, Flatt SW, Kealey S, et al. Increases in plasma carotenoid concentrations in response to a major dietary change in the women's healthy eating and living study. Cancer Epidemiology, Biomarkers & Prevention 2006;15(10):1886‐92. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Newman VA, Flatt SW, Faerber S, Rock CL, Natarajan L, et al. Telephone counseling intervention increases intakes of micronutrient‐ and phytochemical‐rich vegetables, fruit and fiber in breast cancer survivors. Journal of Nutrition 2004;134(2):452‐8. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Pierce John P. Diet and breast cancer prognosis: making sense of the Women's Healthy Eating and Living and Women's Intervention Nutrition Study trials. [Review] [33 refs]. Current Opinion in Obstetrics & Gynecology 2009;21(1):86‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JPF. A randomized trial of the effect of a plant‐based dietary pattern on additional breast cancer events and survival: The Women's Healthy Eating and Living (WHEL) Study. Controlled Clinical Trials 2002;23(6):728‐56. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Laughlin GA, Gold EB, Thomson CA, Natarajan L, et al. Reproductive steroid hormones and recurrence‐free survival in women with a history of breast cancer. Cancer Epidemiology, Biomarkers & Prevention 2008;17(3):614‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, et al. Factors associated with weight gain in women after diagnosis of breast cancer. Women's Healthy Eating and Living Study Group. Journal of the American Dietetic Association 1999;99(10):1212‐21. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Thomson CA, Stefanick ML, Newman VA, Jones L, et al. Plasma triacylglycerol and HDL cholesterol concentrations confirm self‐reported changes in carbohydrate and fat intakes in women in a diet intervention trial. Journal of Nutrition 2004;134(2):342‐7. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Thomson CA, Stefanick ML, Newman VA, Jones L, et al. Plasma triacylglycerol and HDL cholesterol concentrations confirm self‐reported changes in carbohydrate and fat intakes in women in a diet intervention trial. Journal of Nutrition 2004;134(2):342‐7. [DOI] [PubMed] [Google Scholar]
- Rock CL, Natarajan L, Pu M, Thomson CA, Flatt SW, Caan BJ, et al. Longitudinal biological exposure to carotenoids is associated with breast cancer‐free survival in the Women's Healthy Eating and Living Study. Cancer Epidemiology, Biomarkers & Prevention 2009;18(2):486‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saquib N, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Caan B, et al. Weight gain and recovery of pre‐cancer weight after breast cancer treatments: evidence from the women's healthy eating and living (WHEL) study. Breast Cancer Research & Treatment 2007;105(2):177‐86. [DOI] [PubMed] [Google Scholar]
- Saxe GA, Madlensky L, Kealey S, Wu DP, Freeman KL, Pierce JP, et al. Disclosure to physicians of CAM use by breast cancer patients: findings from the Women's Healthy Eating and Living Study. Integrative Cancer Therapies 2008;7(3):122‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHI 2006 {published data only}
- Anderson G, Cummings S, Freedman LS, Furberg C, Henderson M, Johnson SR, et al. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women's Health Initiative study design. Annals of Epidemiology 2003;13(9 Suppl):S5‐17. [DOI] [PubMed] [Google Scholar]
- Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low‐fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):643‐54. [DOI] [PubMed] [Google Scholar]
- Bowen D, Ehret C, Pedersen M, Snetselaar L, Johnson M, Tinker L, et al. Results of an adjunct dietary intervention program in the Women's Health Initiative. Journal of the American Dietetic Association 2002;102(11):1631‐7. [DOI] [PubMed] [Google Scholar]
- Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Annals of Epidemiology 2003;13(9 Suppl):S122‐8. [DOI] [PubMed] [Google Scholar]
- Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Annals of Epidemiology 2003;13(9 Suppl):S18‐77. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Patterson RE, Gorfine M, Ebbeling CB, Jeor ST, Chlebowski RT, et al. Differences between estimated caloric requirements and self‐reported caloric intake in the women's health initiative. Annals of Epidemiology 2003;13(9):629‐37. [DOI] [PubMed] [Google Scholar]
- Howard BV. Dietary fat and cardiovascular disease: putting the Women's Health Initiative in perspective. Nutrition Metabolism & Cardiovascular Diseases 2007;17(3):171‐4. [DOI] [PubMed] [Google Scholar]
- Howard BV, Curb JD, Eaton CB, Kooperberg C, Ockene J, Kostis JB, et al. Low‐fat dietary pattern and lipoprotein risk factors: the Women's Health Initiative Dietary Modification Trial. American Journal of Clinical Nutrition 2010;91:860‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, et al. Low‐fat dietary pattern and weight change over 7 years: the Women's Health Initiative Dietary Modification Trial. JAMA 2006;295(1):39‐49. [DOI] [PubMed] [Google Scholar]
- Howard BV, Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil‐Smoller S, et al. Low‐fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):655‐66. [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, et al. Use of recovery biomarkers to calibrate nutrient consumption self‐reports in the Women's Health Initiative. American Journal of Epidemiology 2008;167(10):1247‐59. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Kristal A, Rodabough R, Caan B, Lillington L, Mossavar‐Rahmani Y, et al. Changes in food sources of dietary fat in response to an intensive low‐fat dietary intervention: early results from the Women's Health Initiative. Journal of the American Dietetic Association 2003;103(4):454‐60. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs‐Collins T, et al. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Annals of Epidemiology 1999;9(3):178‐87. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low‐fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):629‐42. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, et al. Low‐fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. Journal of the National Cancer Institute 2007;99(20):1534‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels‐Tinker L, Howard B, et al. The Women's Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Annals of Epidemiology 2003;13(9 Suppl):S87‐97. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Finnegan LP, Harlan WR, Pinn VW, Clifford C, McGowan JA. The evolution of the Women's Health Initiative: perspectives from the NIH. Journal of the American Medical Women's Association 1995;50(2):50‐5. [PubMed] [Google Scholar]
- The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19(1):61‐109. [DOI] [PubMed] [Google Scholar]
- Tinker LF, Bonds DE, Margolis KL, Manson JE, Howard BV, Larson J, et al. Low‐fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women's Health Initiative randomized controlled dietary modification trial. Archives of Internal Medicine 2008;168(14):1500‐11. [DOI] [PubMed] [Google Scholar]
- Tinker LF, Perri MG, Patterson RE, Bowen DJ, McIntosh M, Parker LM, et al. The effects of physical and emotional status on adherence to a low‐fat dietary pattern in the Women's Health Initiative. Journal of the American Dietetic Association 2002;102(6):789‐800. [DOI] [PubMed] [Google Scholar]
- Tinker LF, Rosal MC, Young AF, Perri MG, Patterson RE, Horn L, et al. Predictors of dietary change and maintenance in the Women's Health Initiative Dietary Modification Trial. Journal of the American Dietetic Association 2007;107(7):1155‐66. [DOI] [PubMed] [Google Scholar]
- Women's Health Initiative Study Group. Dietary adherence in the Women's Health Initiative Dietary Modification Trial. Journal of the American Dietetic Association 2004;104(4):654‐8. [DOI] [PubMed] [Google Scholar]
WHT:FSMP 2003 {published and unpublished data}
- Hall WD, Feng Z, George VA, Lewis CE, Oberman A, Huber M, et al for the WHT:FSMP. Low‐fat diet: effect on anthropometrics, blood pressure, glucose and insulin in older women. Ethnicity and Disease 2003;13:337‐43. [PubMed] [Google Scholar]
WHT Feasibility 1990 {published and unpublished data}
- Bowen D, Clifford CK, Coates R, Evans M, Feng Z, Fouad M, et al. The Women's Health Trial Feasibility Study in Minority Populations: design and baseline descriptions. Annals of Epidemiology 1996;6(6):507‐19. [DOI] [PubMed] [Google Scholar]
- Hall WD, Feng Z, George VA, Lewis CE, Oberman A, Huber M, et al. Low‐fat diet: effect on anthropometrics, blood pressure, glucose, and insulin in older women. Ethnicity and Disease 2003;13:337‐43. [PubMed] [Google Scholar]
WINS 1993 {published and unpublished data}
- Chlebowski RT, Blackburn GL, Buzzard IM, Rose DP, Martino S, Khandekar JD, et al. Adherence to a dietary fat intake reduction program in postmenopausal women receiving therapy for early breast cancer. The Women's Intervention Nutrition Study. Journal of Clinical Oncology 1993;11(11):2072‐80. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. JNCI Journal of the National Cancer Institute 2006;98(24):1767‐76. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Rose DP, Buzzard IM, Blackburn GL, York M, Insull W, et al. Dietary fat reduction in adjuvant breast cancer therapy: current rationale and feasibility issues. Adjuvant Ther Cancer 1990;6:357‐63. [Google Scholar]
- Hoy MK, Winters BL, Chlebowski RT, Papoutsakis C, Shapiro A, Lubin MP, et al. Implementing a low‐fat eating plan in the Women's Intervention Nutrition Study. Journal of the American Dietetic Association 2009;109(4):688‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DP, Chlebowski RT, Connolly JM, Jones LA, Wynder EL. Effects of tamoxifen adjuvant therapy and a low‐fat diet on serum binding proteins and estradiol bioavailability in postmenopausal breast cancer patients. Cancer Research 1992;52:5386‐90. [PubMed] [Google Scholar]
- Rose DP, Connolly JM, Chlebowski RT, Buzzard IM, Wynder EL. The effects of a low‐fat dietary intervention and tamoxifen adjuvant therapy on the serum estrogen and sex hormone‐binding globulin concentrations of postmenopausal breast cancer patients. Breast Cancer Research & Treatment 1993;27(3):253‐62. [DOI] [PubMed] [Google Scholar]
- Wynder EL, Cohen LA, Winters BL. The challenges of assessing fat intake in cancer research investigations. Journal of the American Dietetic Association 1997;97(7 Suppl):S5‐S8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agewall 2001 {published data only}
- Agewall S. Multiple risk intervention trial in high risk hypertensive men: comparison of ultrasound intima‐media thickness and clinical outcome during 6 years of follow‐up. Journal of Internal Medicine 2001;249(4):305‐14. [DOI] [PubMed] [Google Scholar]
Ammerman 2003 {published data only}
- Ammerman AS, Keyserling TC, Atwood JR, Hosking JD, Zayed H, Krasny C. A randomized controlled trial of a public health nurse directed treatment program for rural patients with high blood cholesterol. Preventive Medicine 2003;36(3):340‐51. [DOI] [PubMed] [Google Scholar]
Anti‐Coronary C 1966 {published data only}
- Christakis G, Rinzler SH, Archer M, Kraus A. Effect of the Anti‐Coronary Club Program on coronary heart disease risk factor status. JAMA 1969;198:129‐36. [PubMed] [Google Scholar]
- Christakis G, Rinzler SH, Archer M, Maslansky E. Summary of the research activities of the Anti‐Coronary Club. Public Health Reports (Washington) 1966;81:64‐70. [PMC free article] [PubMed] [Google Scholar]
- Jolliffe N, Baumgarter L, Rinzler SH, Archer M, Stephenson JH, Christakis GJ. The Anti‐Coronary Club: the first four years. New York State Journal of Medicine 1963;63:69‐79. [PubMed] [Google Scholar]
- Singman HS, Berman SN, Cowell C, Maslansky E, Archer M. The Anti‐Coronary Club: 1957‐1972. American Journal of Clinical Nutrition 1980;33(6):1183‐91. [DOI] [PubMed] [Google Scholar]
Aquilani 2000 {published data only}
- Aquilani R, Tramarin R, Pedretti RFE, Bertolotti G, Sommaruga M, Mariani P, et al. Can a very‐low‐fat diet achieve cholesterol goals in CAD?. Cardiology Review 2000;17(10):36‐40. [Google Scholar]
Arne 2014 {published data only}
- Arne A. Diet in the role of prevention and management of obesity: from caloric restriction to optimized diet composition. Obesity Reviews 2014;15(Suppl S2):PL01. [Google Scholar]
Arntzenius 1985 {published data only}
- Arntzenius AC, Kromhout D, Bartn JE, Reiber JHC, Bruschke AVG, Buis Van Gent CM. Diet, lipoprotiens and progression of coronary atherosclerosis: the Leiden intervention trial. New England Journal of Medicine 1985;312:805‐8. [DOI] [PubMed] [Google Scholar]
Aro 1990 {published data only}
- Aro A, Ahola I, Jauhiainen M, et al. Effects of plasma phospholipid fatty acids of rapeseed oil and sunflower oil diets [Abstract]. Arteriosclerosis 1990;10:877a. [Google Scholar]
ASSIST 2001 {published data only}
- Moher M, Yudkin P, Wright L, Turner R, Fuller A, Schofield T, et al. Cluster randomised controlled trial to compare three methods of promoting secondary prevention of coronary heart disease in primary care. BMJ 2001;322(7298):1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Polyp Prev {published and unpublished data}
- MacLennan R, Macrae F, Bain C, Battistutta D, Chapuis P, Gratten H, et al. Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas. The Australian Polyp Prevention Project. Journal of the National Cancer Institute 1995;87(23):1760‐6. [DOI] [PubMed] [Google Scholar]
- MacLennan R, et al. Effect of fat, fibre and beta‐carotene on colorectal adenomas after 24 months. Gastroenterology 1991;100:A382. [Google Scholar]
- Macrae FA, Hughes NR, Bhathal PS, Tay D, Selbie L, MacLennan R. Dietary suppression of rectal epthelial cell proliferation. Gastroenterology 1991;100:A383. [Google Scholar]
Baer 1993 {published data only}
- Baer JT. Improved plasma cholesterol levels in men after a nutrition education program at the worksite. Journal of the American Dietetic Association 1993;93(6):658‐63. [DOI] [PubMed] [Google Scholar]
Bakx 1997 {published data only}
- Bakx JC, Stafleu A, van SW, van‐den HH, van WC. Long‐term effect of nutritional counseling: a study in family medicine. American Journal of Clinical Nutrition 1997;65(6 Suppl):1946S‐50S. [DOI] [PubMed] [Google Scholar]
Barnard 2009 {published data only}
- Barnard ND, Cohen J, Jenkins DJ, Turner‐McGrievy G, Gloede L, Green A, et al. A low‐fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74‐wk clinical trial. American Journal of Clinical Nutrition 2009;89(5):1588S‐96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barndt 1977 {published data only}
- Barndt R, Blankenhorn CH, Crawford DW, et al. Regression and progression of early femoral atherosclerosis in treated hyperlipidaemic patients. Annals of Internal Medicine 1977;86:139‐46. [DOI] [PubMed] [Google Scholar]
Baron 1990 {published data only}
- Baron JA, Gleason R, Crowe B, Mann JI. Preliminary trial of the effect of general practice based nutritional advice. British Journal of General Practice 1990;40(333):137‐41. [PMC free article] [PubMed] [Google Scholar]
Barr 1990 {published data only}
- Barr SL, Ramakrishnan R, Holleran S, et al. A 30% fat diet high in polyunsaturates and a 30% fat diet high in monounsaturates both lower total and low density lipoprotein cholesterol levels in normal males [Abstract]. Arteriosclerosis 1990;10:872a. [Google Scholar]
Baumann 1982 {published data only}
- Baumann J, Martschick R. Therapy of hyperlipidemia with xanthinol nicotinate as opposed to low fat diet [Therapie der Hyperlipidamie mit Xantinolnicotinat gegenuber fettarmer Diat]. Die Medizinische Welt 1982;33(4):139‐41. [PubMed] [Google Scholar]
Bazzano 2012 {published data only}
- Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low‐carbohydrate and low‐fat diets: a randomized trial. Annals of Internal Medicine 2014;161(5):309‐18. [10.7326/P14‐9029; PMID: 25178581]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano LAR. Effect of a low‐carbohydrate diet on weight and cardiovascular risk factors: a randomized controlled trial. Circulation 2012;125:AP306. [Google Scholar]
Beckmann 1988 {published data only}
- Beckmann SL, Os I, Kjeldsen SE, Mogensen B, Norum KR, Hjermann I. Non‐pharmacological treatment of mild to moderate hypertension. A randomized, controlled study‐‐results 1 1/2 years later. Tidsskrift For Den Norske Laegeforening 1988;108:1593‐7. [PubMed] [Google Scholar]
Beckmann 1995 {published data only}
- Beckmann SL, Os I, Kjeldsen SE, Eide IK, Westheim AS, Hjermann I. Effect of dietary counselling on blood pressure and arterial plasma catecholamines in primary hypertension. American Journal of Hypertension 1995;8(7):704‐11. [DOI] [PubMed] [Google Scholar]
Beresford 1992 {published data only}
- Beresford SAA, Farmer EMZ, Feingold L, Graves KL, Sumner SK, Baker RM. Evaluation of a self‐help dietary intervention in a primary care setting. American Journal of Public Health 1992;82:79‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergstrom 1967 {published data only}
- Bergstrom G, Svanborg A. Dietary treatment of acute myocardial infarction. Acta Medica Scandinavica 1967;181(6):717‐21. [DOI] [PubMed] [Google Scholar]
Bierenbaum 1963 {published data only}
- Bierenbaum ML, Fleischman AI, Raichelson RI, Hayton T, Watson P. Ten year experience of modified fat diets on younger men with coronary heart disease. Lancet 1973;i:1404‐7. [DOI] [PubMed] [Google Scholar]
- Bierenbaum ML, Green DP, Florin A, Fleischman AI, Caldwell AB. Modified‐fat dietary management of the young male with coronary disease. A five‐year report. JAMA 1967;202(13):1119‐23. [PubMed] [Google Scholar]
- Bierenbaum ML, Green DP, Gherman C, Florin A, Caldwell AB. The effects of two low fat dietary patterns on the blood cholesterol levels of young male coronary patients. Journal of Chronic Diseases 1963;16:1073‐83. [DOI] [PubMed] [Google Scholar]
Bloomgarden 1987 {published data only}
- Bloomgarden ZT, Karmally W, Metzger MJ, Brothers M, Nechemias C, Bookman J, et al. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care 1987;10:263‐72. [DOI] [PubMed] [Google Scholar]
Bonnema 1995 {published data only}
- Bonnema SJ, Jespersen LT, Marving J, Gregersen G. Supplementation with olive oil rather than fish oil increases small arterial compliance in diabetic patients. Diabetes, Nutrition and Metabolism Clinical and Experimental 1995;8:81‐7. [Google Scholar]
Bosaeus 1992 {published data only}
- Bosaeus I, Belfrage L, Lindgren C, Andersson H. Olive oil instead of butter increases net cholesterol excretion from the small bowel. European Journal of Clinical Nutrition 1992;46(2):111‐5. [PubMed] [Google Scholar]
Boyar 1988 {published data only}
- Boyar AP, Rose DP, Loughridge JR, Engle A, Palge A, Laakso K, et al. Response to a diet low in total fat in women with postmenopausal breast cancer: a pilot study. Nutrition and Cancer 1988;11:93‐9. [DOI] [PubMed] [Google Scholar]
Brehm 2009 {published data only (unpublished sought but not used)}
- Brehm BJ, Lattin BL, Summer SS, Boback JA, Gilchrist GM, Jandacek RJ, et al. One‐year comparison of a high‐monounsaturated fat diet with a high‐carbohydrate diet in type 2 diabetes. Diabetes Care 2009;32(2):215‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brensike 1982 {published data only}
- Brensike JF, Kelsey SF, Passamani ER, Fisher MR, Richardson JM, Loh IK, et al. National Heart, Lung, and Blood Institute type II Coronary Intervention Study: design, methods, and baseline characteristics. Controlled Clinical Trials 1982;3(2):91‐111. [DOI] [PubMed] [Google Scholar]
Broekmans 2003 {published and unpublished data}
- Broekmans WMR, Klopping‐Ketelaars IAA, Weststrate JA, Tijburg LBM, Poppel G, Vink AA, et al. Decreased carotenoid concentrations due to dietary sucrose polyesters do not affect possible markers of disease risk in humans. Journal of Nutrition 2003;133:720‐6. [DOI] [PubMed] [Google Scholar]
Brown 1984 {published data only}
- Brown GD, Whyte L, Gee MI, Crockford PM, Grace M, Oberle K, et al. Effects of two "lipid‐lowering" diets on plasma lipid levels of patients with peripheral vascular disease. Journal of the American Dietetic Association 1984;84(5):546‐50. [PubMed] [Google Scholar]
Bruce 1994 {published data only}
- Bruce SL, Grove SK. The effect of a coronary artery risk evaluation program on serum lipid values and cardiovascular risk levels. Applied Nursing Research 1994;7(2):67‐74. [DOI] [PubMed] [Google Scholar]
Bruno 1983 {published data only}
- Bruno R, Arnold C, Jacobson L, Winick M, Wynder E. Randomized controlled trial of a nonpharmacologic cholesterol reduction program at the worksite. Preventive Medicine 1983;12(4):523‐32. [DOI] [PubMed] [Google Scholar]
Butcher 1990 {published data only}
- Butcher LA, O'Dea K, Sinclair AJ, Parkin JD, Smith IL, Blombery P. The effects of very low fat diets enriched with fish or kangaroo meat on cold‐induced vasoconstriction and platelet function. Prostaglandins Leukot Essent. Fatty Acids 1990;39(3):221‐6. [DOI] [PubMed] [Google Scholar]
Butowski 1998 {published data only}
- Butowski PF, Winder AF. Usual care dietary practice, achievement and implications for medication in the management of hypercholesterolaemia. European Heart Journal 1998;19:1328‐33. [DOI] [PubMed] [Google Scholar]
Byers 1995 {published data only}
- Byers T, Mullis R, Anderson J, Dusenbury L, Gorsky R, Kimber C, et al. The costs and effects of a nutritional education program following work‐site cholesterol screening. American Journal of Public Health 1995;85(5):650‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caggiula 1996 {published data only}
- Caggiula AW, Watson JE, Kuller LH, Olson MB, Milas NC, Berry M, et al. Cholesterol‐lowering intervention program. Effect of the step I diet in community office practices. Archives of Internal Medicine 1996;156(11):1205‐13. [DOI] [PubMed] [Google Scholar]
CARMEN 2000 {published and unpublished data}
- Poppitt SD, Keogh GF, Prentice AM, Williams DEM, Sonnemans HMW, Valk EEJ, et al. Long‐term effects of ad libitum low‐fat, high‐carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. American Journal of Clinical Nutrition 2002;75:11‐20. [DOI] [PubMed] [Google Scholar]
- Raben A, Astrup A, Vasilaras TH, Prentice AM, Zunft H‐JF, Formiguera X, et al. The CARMEN study [CARMEN‐studiet]. Ugeskrift fur Laeger 2002;164(5):627‐31. [PubMed] [Google Scholar]
- Saris WHM, Astrup A, Prentice AM, Zunft FJF, Formiguera X. CARMEN Project: European multicentre study on the impact of dietary fat/CHO ratio and simple/complex CHO changes on long term weight control in overweight subjects. International Journal of Obesity 1997;21(Suppl 2):S71. [Google Scholar]
- Saris WHM, Astrup A, Prentice AM, Zunft HJF, Formiguera X, Verboeket‐van de Venne WPHG, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. International Journal of Obesity 2000;24:1310‐8. [DOI] [PubMed] [Google Scholar]
- Vasilaras TH, Astrup A, Raben A. Micronutrient intake in overweight subjects is not deficient on and ad libitum fat‐reduced, high‐simple carbohydrate diet. European Journal of Clinical Nutrition 2004;58:326‐36. [DOI] [PubMed] [Google Scholar]
CARMEN MS sub‐study {published and unpublished data}
- Poppitt SD, Keogh GF, Prentice AM, Williams DEM, Sonnemans HMW, Valk EEJ, et al. Long‐term effects of ad libitum low‐fat, high‐carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. American Journal of Clinical Nutrition 2002;75:11‐20. [DOI] [PubMed] [Google Scholar]
Cerin 1993 {published data only}
- Cerin A, Collins A, Landgren BM, Eneroth P. Hormonal and biochemical profiles of premenstrual syndrome. Treatment with essential fatty acids. Acta Obstetricia et Gynecologica Scandinavica 1993;72(5):337‐43. [DOI] [PubMed] [Google Scholar]
Chan 1993 {published data only}
- Chan JK, McDonald BE, Gerrard JM, Bruce VM, Weaver BJ, Holub BJ. Effect of dietary alpha‐linolenic acid and its ratio to linolenic acid on platelet and plasma fatty acids and thrombogenesis. Lipids 1993;28:811‐7. [DOI] [PubMed] [Google Scholar]
Chapman 1950 {published data only}
- Chapman CB, Gibbons T, Henschel A. The effect of the rice‐fruit diet on the composition of the body. New England Journal of Medicine 1950;243:899‐905. [DOI] [PubMed] [Google Scholar]
Charbonnier 1975 {published data only}
- Charbonnier A, Nepveux P, Fluteau G, Fluteau D. Immediate effects of ingestion of olive oil on the principal lipid constituents of the plasma. Comparison with other edible fats. Médecine & Chirurgie Digestives 1975;4 Suppl 2:73‐9. [PubMed] [Google Scholar]
Cheng 2004 {published data only}
- Cheng C, Graziani C, Diamond JJ, Cheng C, Graziani C, Diamond JJ. Cholesterol‐lowering effect of the Food for Heart Nutrition Education Program. Journal of the American Dietetic Association 2004;104(12):1868‐72. [DOI] [PubMed] [Google Scholar]
Chicago CPEP 1977 {published data only}
- Farinaro E, Stamler J, Upton M, Mojonnier L, Hall Y, Moss D, et al. Plasma glucose levels: long term effect of diet in the Chicago Coronary Prevention Evaluation Program. Annals of Internal Medicine 1977;86:147‐54. [DOI] [PubMed] [Google Scholar]
Chiostri 1988 {published data only}
- Chiostri JE, Kwiterovich PO. Effect of American Heart Association Phase 2 diet versus eater's choice based diet on hypercholesterolaemia. Circulation 1988;78(4):II‐385. [Google Scholar]
Choudhury 1984 {published data only}
- Choudhury S, Jackson P, Katan MB, Marenah CB, Cortese C, Miller NE, et al. A multifactorial diet in the management of hyperlipidaemia. Atherosclerosis 1984;50:93‐103. [DOI] [PubMed] [Google Scholar]
Clark 1997 {published data only}
- Clark M, Ghandour G, Miller NH, Taylor CB, Bandura A, DeBusk RF. Development and evaluation of a computer‐based system for dietary management of hyperlipidemia. Journal of the American Dietetic Association 1997;97(2):146‐50. [DOI] [PubMed] [Google Scholar]
Clifton 1992 {published data only}
- Clifton PM, Wight MB, Nestel PJ. Is fat restriction needed with HMGCoA reductase inhibitor treatment?. Atherosclerosis 1992;93(1‐2):59‐70. [DOI] [PubMed] [Google Scholar]
Cobb 1991 {published data only}
- Cobb MM, Teitelbaum HS, Breslow JL. Lovastatin efficacy in reducing low‐density lipoprotein cholesterol levels on high‐ vs low‐fat diets. JAMA 1991;265(8):997‐1001. [PubMed] [Google Scholar]
Cohen 1991 {published data only}
- Cohen MD, D'Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Family Medicine 1991;23(1):25‐8. [PubMed] [Google Scholar]
Cole 1988 {published data only}
- Cole TG, Schmeisser D, Prewitt TE, et al. AHA phase 3 diet reduces cholesterol in moderately hypercholesterolemic premenopausal women [Abstract]. Circulation 1988;78(4):II‐73. [Google Scholar]
Colquhoun 1990 {published data only}
- Colquhoun DM, Moores D, Somerset SM. Comparison of the effects of an avocado enriched and American Heart Association diets on lipid levels [Abstract]. Arteriosclerosis 1990;10:875a. [Google Scholar]
Consolazio 1946 {published data only}
- Consolazio FC, Forbes WH. The effects of high fat diet in a temperate environment. Journal of Nutrition 1946;32:195‐204. [DOI] [PubMed] [Google Scholar]
Coppell 2010 {published data only}
- Coppell KJK. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment ‐ Lifestyle over and above drugs in diabetes (LOADD) study: randomised controlled trial. BMJ 2010;341:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 1996 {published data only}
- Cox RH, Gonzales‐Vigilar MCRV, Novascone MA, Silva‐Barbeau I. Impact of a cancer intervention on diet‐related cardiovascular disease risks of white and African‐American EFNEP clients. Journal of Nutrition Education 1996;28:209‐18. [Google Scholar]
Croft 1986 {published data only}
- Croft PR, Brigg D, Smith S, Harrison CB, Branthwaite A, Collins MF. How useful is weight reduction in the management of hypertension?. Journal of the Royal College of General Practitioners 1986;36(291):445‐8. [PMC free article] [PubMed] [Google Scholar]
Crouch 1986 {published data only}
- Crouch M, Sallis JF, Farquar JW, Haskell WL, Ellsworth NM, King AB, et al. Personal and mediated health counselling for sustained dietary reduction of hypercholesterolaemia. Preventive Medicine 1986;15:282‐91. [DOI] [PubMed] [Google Scholar]
Dalgard 2001 {published data only}
- Dalgard C, Thuroe A, Haastrup B, Haghfelt T, Stender S. Saturated fat intake is reduced in patients with ischemic heart disease 1 year after comprehensive counseling but not after brief counseling. Journal of the American Dietetic Association 2001;101(12):1420‐9. [DOI] [PubMed] [Google Scholar]
Da Qing IGT 1997 {published data only}
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20(4):537‐44. [DOI] [PubMed] [Google Scholar]
DAS 1989 {published data only}
- Bovbjerg VE, McCann BS, Brief DJ, Follette WC, Retzlaff BM, Dowdy AA, et al. Spouse support and long‐term adherence to lipid‐lowering diets. American Journal of Epidemiology 1995;141(5):451‐60. [DOI] [PubMed] [Google Scholar]
- Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One‐year effects of increasingly fat‐restricted, carbohydrate‐enriched diets on lipoprotein levels in free‐living subjects. Proceedings of the Society for Experimental Biology & Medicine 2000;225(3):191‐9. [DOI] [PubMed] [Google Scholar]
- Knopp RH, Walden CE, McCann BS, Retzlaff B, Dowdy A, Gey G, et al. Serial changes in lipoprotein cholesterol in hypercholesterolemic men treated with alternative diets [abstract]. Arteriosclerosis 1989;9:745A. [Google Scholar]
- Knopp RH, Walden CE, Retzlaff BM, McCann BS, Dowdy AA, Albers JJ, et al. Long‐term cholesterol‐lowering effects of 4 fat‐restricted diets in hypercholesterolaemic and combined hyperlipidaemic men: The Dietary Alternatives Study. JAMA 1997;278:1509‐15. [PubMed] [Google Scholar]
- Walden CE, McCann BS, Retzlaff B, Dowdy A, Hanson M, Fish B, et al. Alternative fat‐restricted diets for hypercholesterolemia and combined hyperlipidemia: feasibility, design, subject recruitment, and baseline characteristics of the. Journal of the American College of Nutrition 1991;10(5):429‐42. [DOI] [PubMed] [Google Scholar]
DASH 1997 {published data only}
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. New England Journal of Medicine 1997;336(16):1117‐24. [DOI] [PubMed] [Google Scholar]
- Blackburn GL. Functional foods in the prevention and treatment of disease: significance of the Dietary Approaches to Stop Hypertension Study. American Journal of Clinical Nutrition 1997;66(5):1067‐71. [DOI] [PubMed] [Google Scholar]
Davey Smith 2005 {published data only}
- Davey Smith G, Bracha Y, Svendsen KH, Neaton JD, Haffner SM, Kuller LH, et al. Incidence of type 2 diabetes in the randomized multiple risk factor intervention trial. Annals of Internal Medicine 2005;142(5):313‐22. [DOI] [PubMed] [Google Scholar]
de Boer 1983 {published data only}
- Boer AC, Turek JV, Pannebakker MA, den OG. The effect of diets high in polyunsaturated and high in saturated fatty acids on blood lipids and platelet tests in patients with coronary artery disease (CAD) [abstract]. Thrombosis And Haemostasis 1983;50:96. [Google Scholar]
DeBusk 1994 {published data only}
- DeBusk RF, Miller NH, Superko HR, Dennis CA, Thomas RJ, Lew HT, et al. A case‐management system for coronary risk factor modification after acute myocardial infarction [see comments]. Annals of Internal Medicine 1994;120(9):721‐9. [DOI] [PubMed] [Google Scholar]
Delahanty 2001 {published data only}
- Delahanty LM, Hayden D, Ammerman A, Nathan DM. Medical nutrition therapy for hypercholesterolemia positively affects patient satisfaction and quality of life outcomes. Annals of Behavioral Medicine 2002;24(4):269‐78. [DOI] [PubMed] [Google Scholar]
- Delahanty LM, Sonnenberg LM, Hayden D, Nathan DM. Clinical and cost outcomes of medical nutrition therapy for hypercholesterolemia: a controlled trial. Journal of the American Dietetic Association 2001;101(9):1012‐23. [DOI] [PubMed] [Google Scholar]
Delius 1969 {published data only}
- Delius L. Treatment of hypotensive circulatory disorder [Die Behandlung der hypotonen Kreislaufregulationsstorung]. Deutsche Medizinische Wochenschrift 1969;94(42):2172‐3. [DOI] [PubMed] [Google Scholar]
Demark 1990 {published data only}
- Demark WW, Bowering J, Cohen PS. Reduced serum cholesterol with dietary change using fat‐modified and oat bran supplemented diets. Journal of the American Dietetic Association 1990;90(2):223‐9. [PubMed] [Google Scholar]
Dengel 1995 {published data only}
- Dengel JL, Katzel LI, Goldberg AP. Effect of an American Heart Association diet, with or without weight loss, on lipids in obese middle‐aged and older men. American Journal of Clinical Nutrition 1995;62(4):715‐21. [DOI] [PubMed] [Google Scholar]
Denke 1994 {published data only}
- Denke MA, Grundy SM. Individual responses to a cholesterol lowering diet in 50 men with moderate hypercholesterolaemia. Archives of Internal Medicine 1994;154:17‐25. [PubMed] [Google Scholar]
Diabetes CCT 1995 {published data only}
- Anon. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. American Journal of Cardiology 1995;75:894‐903. [DOI] [PubMed] [Google Scholar]
DIET 1998 {published data only}
- Dornelas EA, Wylie‐Rosett J, Swencionis C. The DIET study: long term outcomes of a cognitive‐behavioural weight control intervention in independent‐living elders. Journal of the American Dietetic Association 1998;98(11):1276‐81. [DOI] [PubMed] [Google Scholar]
Ding 1992 {published data only}
- Ding Q. Clinical study of qianxining in the treatment of 60 cases of yang hyperactivity due to yin deficiency type of hypertension. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih 1992;12:409‐11, 388. [PubMed] [Google Scholar]
DIRECT 2009 {published data only (unpublished sought but not used)}
- Ben‐Avraham S, Harman‐Boehm I, Schwarzfuchs D, Shai I. Dietary strategies for patients with type 2 diabetes in the era of multi‐approaches; review and results from the Dietary Intervention Randomized Controlled Trial (DIRECT). Diabetes Research and Clinical Practice 2009;86(Suppl 1):S41‐8. [DOI] [PubMed] [Google Scholar]
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al for the Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low‐carbohydrate, Mediterranean, or low‐fat diet. New England Journal of Medicine 2008;359:229‐41. [DOI] [PubMed] [Google Scholar]
Dobs 1991 {published data only}
- Dobs AS, Sarma PS, Wilder L. Lipid‐lowering diets in patients taking pravastatin, a new HMG‐CoA reductase inhibitor: compliance and adequacy. American Journal of Clinical Nutrition 1991;54(4):696‐700. [DOI] [PubMed] [Google Scholar]
DO IT 2004 {published and unpublished data}
- Berstad P, Seljeflot I, Veierod MB, Hjerkinn EM, Arnesen H, Pedersen JI, et al. Supplementation with fish oil affects the association between very long‐chain n‐3 polyunsaturated fatty acids in serum non‐esterified fatty acids and soluble vascular cell adhesion molecule‐1. Clinical Science 2003;105(1):13‐20. [DOI] [PubMed] [Google Scholar]
- Ellingsen I, Hjerkinn EM, Seljeflot I, Arnesen H, Tonstad S, Ellingsen I, et al. Consumption of fruit and berries is inversely associated with carotid atherosclerosis in elderly men.[Erratum appears in Br J Nutr. 2008 Mar;99(3):697]. British Journal of Nutrition 2008;99(3):674‐81. [DOI] [PubMed] [Google Scholar]
- Ellingsen I, Seljeflot I, Arnesen H, Tonstad S. Vitamin C consumption is associated with less progression in carotid intima media thickness in elderly men: a 3‐year intervention study. Nutrition Metabolism & Cardiovascular Diseases 2009;19(1):8‐14. [DOI] [PubMed] [Google Scholar]
- Furenes EB, Seljeflot I, Solheim S, Hjerkinn EM, Arnesen H, Furenes EB, et al. Long‐term influence of diet and/or omega‐3 fatty acids on matrix metalloproteinase‐9 and pregnancy‐associated plasma protein‐A in men at high risk of coronary heart disease. [Review] [39 refs]. Scandinavian Journal of Clinical & Laboratory Investigation 2008;68(3):177‐84. [DOI] [PubMed] [Google Scholar]
- Hjerkinn EM, Abdelnoor M, Breivik L, Bergengen L, Ellingsen I, Seljeflot I, et al. Effect of diet or very long chain omega‐3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima‐media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. European Journal of Cardiovascular Prevention & Rehabilitation 2006;13(3):325‐33. [DOI] [PubMed] [Google Scholar]
- Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, et al. Influence of long‐term intervention with dietary counselling, long‐chain n‐3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long‐standing hyperlipidemia. American Journal of Clinical Nutrition 2005;81(3):583‐9. [DOI] [PubMed] [Google Scholar]
- Lindman AS, Pedersen JI, Hjerkinn EM, Arnesen H, Veierod MB, Ellingsen I, et al. The effects of long‐term diet and omega‐3 fatty acid supplementation on coagulation factor VII and serum phospholipids with special emphasis on the R353Q polymorphism of the FVII gene. Thrombosis & Haemostasis 2004;91(6):1097‐104. [DOI] [PubMed] [Google Scholar]
- Troseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin‐18 are reduced by diet and n‐3 fatty acid intervention in elderly high‐risk men. Metabolism: Clinical & Experimental 2009;58(11):1543‐9. [DOI] [PubMed] [Google Scholar]
- Troseid M, Seljeflot I, Hjerkinn EM, Arnesen H. Interleukin‐18 is a strong predictor of cardiovascular events in elderly men with the metabolic syndrome: synergistic effect of inflammation and hyperglycemia. Diabetes Care 2009;32(3):486‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffield 1982 {published data only}
- Duffield RG, Lewis B, Miller NE, Jamieson CW, Brunt JN, Colchester AC. Treatment of hyperlipidaemia retards progression of symptomatic femoral atherosclerosis. A randomised controlled trial. Lancet 1983;2(8351):639‐42. [DOI] [PubMed] [Google Scholar]
- Duffield RG, Miller NE, Jamieson CW, Lewis B. A controlled trial of plasma lipid reduction in peripheral atherosclerosis‐‐an interim report. British Journal of Surgery 1982;69 Suppl:S3‐S5. [DOI] [PubMed] [Google Scholar]
Dullaart 1997 {published and unpublished data}
- Dullaart RP, Hoogenberg K, Riemens SC, Groener JE, Tol A, Sluiter WJ, Stulp BK. Cholesteryl ester transfer protein gene polymorphism is a determinant of HDL cholesterol and of the lipoprotein response to a lipid‐lowering diet in type 1 diabetes. Diabetes 1997;46(12):2082‐7. [DOI] [PubMed] [Google Scholar]
Dutch Nutrition Guide {published data only (unpublished sought but not used)}
- Verheiden MW, Veen JE, Zadelhoff WM, Bakx C, Koelen MA, Hoogen HJM, et al. Nutrition guidance in Dutch family practice: behavioural determinants of reduction in fat consumption. American Journal of Clinical Nutrition 2003;77(Suppl):1058S‐64S. [DOI] [PubMed] [Google Scholar]
Eating Patterns 1997 {published and unpublished data}
- Beresford SA, Curry SJ, Kristal AR, Lazovich D, Feng Z, Wagner EH. A dietary intervention in primary care practice: the Eating Patterns Study. American Journal of Public Health 1997;87(4):610‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eckard 2013 {published data only}
- Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therapeutic Advances in Gastroenterology 2013;6:249‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehnholm 1982 {published data only}
- Ehnholm C, Huttunen JK, Pietinen P, Leino U, Mutanen M, Kostiainen E, et al. Effect of diet on serum lipoproteins in a population with a high risk of coronary heart disease. New England Journal of Medicine 1982;307:850‐5. [DOI] [PubMed] [Google Scholar]
Ehnholm 1984 {published data only}
- Ehnholm C, Huttunen JK, Pietinen P, Leino U, Mutanen M, Kostiainen E, et al. Effect of a diet low in saturated fatty acids on plasma lipids, lipoproteins, and HDL subfractions. Arteriosclerosis 1984;4(3):265‐9. [DOI] [PubMed] [Google Scholar]
Eisenberg 1990 {published data only}
- Eisenberg S. The effect of dietary substitution of monounsaturated fatty acids with carbohydrates on lipoprotein levels, structure, and function in a free‐living population [abstract]. Arteriosclerosis 1990;10:872A. [Google Scholar]
Elder 2000 {published data only}
- Elder JP, Candelaria JI, Woodruff SI, Criqui MH, Talavera GA, Rupp JW. Results of language for health: cardiovascular disease nutrition education for Latino English‐as‐a‐second‐language students. Health Education & Behavior 2000;27(1):50‐63. [DOI] [PubMed] [Google Scholar]
Ellegard 1991 {published data only}
- Ellegard L, Bosaeus I. Sterol and nutrient excretion in ileostomists on prudent diets. European Journal of Clinical Nutrition 1991;45(9):451‐7. [PubMed] [Google Scholar]
Esposito 2003 {published data only}
- Esposito K, Pontillo A, Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289(14):1799‐804. [DOI] [PubMed] [Google Scholar]
Esposito 2004 {published data only}
- Esposito K, Marfella R, Ciotola M, Palo C, Giugliano F, Giugliano G, et al. Effect of a Mediterranean‐style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004;292(12):1440‐6. [DOI] [PubMed] [Google Scholar]
Esposito 2014 {published data only}
- Esposito K, Maiorino MI, Petrizzo M, Bellastella G, Giugliano D. The effects of a Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: follow‐up of a randomized trial. Diabetes Care 2014;37:1824‐30. [DOI] [PubMed] [Google Scholar]
EUROACTION 2008 {published data only}
- Wood DA, Kotseva K, Connolly S, Jennings C, Mead A, Jones J, et al. Nurse‐coordinated multidisciplinary, family‐based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster‐randomised controlled trial. Lancet 2008;371(9629):1999‐2012. [DOI] [PubMed] [Google Scholar]
FARIS 1997 {published data only}
- Goble A, Jackson B, Phillips P, Race E, Oliver RG, Worcester MC. The Family Atherosclerosis Risk Intervention Study (FARIS): risk factor profiles of patients and their relatives following an acute cardiac event. Australian and New Zealand Journal of Medicine 1997;27:568‐77. [DOI] [PubMed] [Google Scholar]
Fasting HGS 1997 {published data only}
- Dyson PA, Hammersley MS, Morris RJ, Holman RR, Turner RC. The Fasting Hyperglycaemia Study: II. Randomized controlled trial of reinforced healthy‐living advice in subjects with increased but not diabetic fasting plasma glucose. Metabolism 1997;46(12 Suppl 1):50‐5. [DOI] [PubMed] [Google Scholar]
Ferrara 2000 {published data only}
- Ferrara LA, Raimondi AS, d'Episcopo L, Guida L, Dello Russo A, Marotta T. Olive oil and reduced need for antihypertensive medications. Archives of Internal Medicine 2000;160:837‐42. [DOI] [PubMed] [Google Scholar]
Fielding 1995 {published data only}
- Fielding CJ, Havel RJ, Todd KM, Yeo KE, Schloetter MC, Weinberg V, et al. Effects of dietary cholesterol and fat saturation on plasma lipoproteins in an ethnically diverse population of healthy young men. Journal of Clinical Investigation 1995;95(2):611‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finckenor 2000 {published data only}
- Finckenor M. Nutrition intervention group program based on preaction‐stage‐oriented change processes of the transtheoretical model promotes long‐term reduction in dietary fat intake. Journal of the American Dietetic Association 2000;100(3):335‐42. [DOI] [PubMed] [Google Scholar]
Finnish Diabetes 2000 {published data only}
- Uusitupa M, Louheranta A, Lindstrom J, Valle T, Sundvall J, Eriksson J, et al. The Finnish Diabetes Prevention Study. British Journal of Nutrition 2000;83 Suppl 1:S137‐42. [DOI] [PubMed] [Google Scholar]
Finnish Mental 1972 {published data only}
- Miettinen M, Turpeinen O, Karvonen MJ, Elosuo R, Paavilainen E. Effect of cholesterol‐lowering diet on mortality from coronary heart‐disease and other causes. A twelve‐year clinical trial in men and women. Lancet 1972;2(782):835‐8. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Turpeinen O, Karvonen MJ, Pekkarinen M, Paavilainen E, Elosuo R. Dietary prevention of coronary heart disease in women: the Finnish mental hospital study. International Journal of Epidemiology 1983;12(1):17‐25. [DOI] [PubMed] [Google Scholar]
- Turpeinen O, Miettinen M, Karvonen M, Roine P, Pekkarinen M, Lehtosuo EJ, et al. Dietary prevention of coronary heart disease: long‐term experiment. I. Observations on male. American Journal of Clinical Nutrition 1968;21(4):255‐76. [DOI] [PubMed] [Google Scholar]
Fisher 1981 {published data only}
- Fisher EA, Breslow JL, Zannis VI, Shen G, Blum CB. Dietary saturated fat, not cholesterol, affects plasma lipids and Apo E. Arteriosclerosis 1981;1(5):364a. [Google Scholar]
Fleming 2002 {published data only}
- Fleming RM. The effect of high‐, moderate‐, and low‐fat diets on weight loss and cardiovascular disease risk factors. Preventive Cardiology 2002;5:110‐5. [DOI] [PubMed] [Google Scholar]
Fortmann 1988 {published data only}
- Fortmann SP, Haskell WL, Wood PD. Effects of weight loss on clinic and ambulatory blood pressure in normotensive men. American Journal of Cardiology 1988;62(1):89‐93. [DOI] [PubMed] [Google Scholar]
Foster 2003 {published data only}
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low‐carbohydrate diet for obesity. New England Journal of Medicine 2003;348(21):2082‐90. [DOI] [PubMed] [Google Scholar]
FRESH START 2007 {published data only}
- Denmark‐Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. Journal of Clinical Oncology 2007;25(19):2709‐18. [DOI] [PubMed] [Google Scholar]
Friedman 2012 {published data only}
- Friedman AN, Ogden LG, Foster GD, Klein S, Stein R, Miller B, et al. Comparative effects of low‐carbohydrate high‐protein versus low‐fat diets on the kidney. Clinical Journal of the American Society of Nephrology 2012;7:1103‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gambera 1995 {published data only}
- Gambera PJ, Schneeman BO, Davis PA. Use of the Food Guide Pyramid and US Dietary Guidelines to improve dietary intake and reduce cardiovascular risk in active‐duty Air Force members. Journal of the American Dietetic Association 1995;95(11):1268‐73. [DOI] [PubMed] [Google Scholar]
Gaullier 2007 {published data only}
- Gaullier J‐M, Halse J, Hoivik HO, Hoye K, Syvertsen C, Nurminiemi M, et al. Six months supplementation with conjugated linoleic acid induces regional‐specific fat mass decreases in overweight and obese. British Journal of Nutrition 2007;97:550‐60. [DOI] [PubMed] [Google Scholar]
German Fat Reduced {published and unpublished data}
- Seppelt B, Weststrate JA, Reinert A, Johnson D, Luder W, Zunft HJ. Long‐term effects of nutrition with fat‐reduced foods on energy consumption and body weight [Langzeiteffekte einer Ernahrung mit fettreduzierten Lebensmitteln auf die Energieaufnahme und das Korpergewicht]. Zeitschrift fur Ernahrungswissenschaft 1996;35(4):369‐77. [DOI] [PubMed] [Google Scholar]
Ginsberg 1988 {published data only}
- Ginsberg H. Both a high monounsaturated fat diet and the step 1 AHA diet significantly reduce plasma cholesterol levels in healthy males [abstract]. Circulation 1988;78:II73. [Google Scholar]
Gjone 1972 {published data only}
- Gjone E, Nordoy A, Blomhoff JP, Wiencke I. The effects of unsaturated and saturated dietary fats on plasma cholesterol, phospholipids and lecithin: cholesterol acyltransferase activity. Acta Medica Scandinavica 1972;191(6):481‐4. [PubMed] [Google Scholar]
Glatzel 1966 {published data only}
- Glatzel H. The relationship between postprandial triglyceridemia and the fat content of the basic diet [Die Abhangigkeit der postcenalen Triglyceridamie von Fettgehalt der Grundkost]. Klinische Wochenschrift 1966;44(5):283‐4. [DOI] [PubMed] [Google Scholar]
Goodpaster 1999 {published data only}
- Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 1999;48:839‐47. [DOI] [PubMed] [Google Scholar]
Gower 2012 {published data only}
- Gower B A, Goree L L, Chandler‐Laney P C, Ellis A C, Casazza K, Granger W M. A higher‐carbohydrate, lower‐fat diet reduces fasting glucose concentration and improves beta‐cell function in individuals with impaired fasting glucose. Metabolism 2012;61:358‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BAG. Impact of dietary macronutrient composition on insulin sensitivity, fasting glucose, and beta‐cell response in healthy, overweight, men and women. Endocrine Reviews 2011;Conference:SAT‐110. [Google Scholar]
Gregg 2013 {published data only}
- Gregg EWK. An intensive lifestyle intervention increased remission from type 2 diabetes in overweight adults. Annals of Internal Medicine 2013;158:4. [DOI] [PubMed] [Google Scholar]
Grundy 1986 {published data only}
- Grundy SM, Nix D, Whelan MF, Franklin L. Comparison of three cholesterol‐lowering diets in normolipidaemic men. JAMA 1986;256:2351‐5. [PubMed] [Google Scholar]
Gudlaugsson 2013 {published data only}
- Gudlaugsson J, Gudnason V. Effects of exercise training and nutrition counseling on body composition and cardiometabolic factors in old individuals. European Geriatric Medicine 2013;4:431‐7. [Google Scholar]
Guelinckx 2010 {published data only}
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. American Journal of Clinical Nutrition 2010;91:373‐80. [DOI] [PubMed] [Google Scholar]
Guldbrand 2012 {published data only}
- Guldbrand H, Dizdar B, Bunjaku B, Lindstrom T, Bachrach‐Lindstrom M, Fredrikson M, et al. In type 2 diabetes, randomisation to advice to follow a low‐carbohydrate diet transiently improves glycaemic control compared with advice to follow a low‐fat diet producing a similar weight loss. Diabetologia 2012;55:2118‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardcastle 2008 {published data only}
- Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Education & Counseling 2008;70(1):31‐9. [DOI] [PubMed] [Google Scholar]
Harris 1990 {published data only}
- Harris WS, Feldman EB. Intensive dietary intervention in hypercholesterolemic patients. Observed versus predicted changes in cholesterol levels [abstract]. Arteriosclerosis 1990;10:853A. [Google Scholar]
Hartman 1993 {published data only}
- Hartman T, McCarthy P, Himes J. Use of eating pattern messages to evaluate changes in eating behaviors in a worksite cholesterol education program. Journal of the American Dietetic Association 1993;93:1119‐23. [DOI] [PubMed] [Google Scholar]
Hartwell 1986 {published data only}
- Hartwell SL, Kaplan RM, Wallace JP. Comparison of behavioral interventions for control of type II diabetes mellitus. Behavior Therapy 1986;17:447‐61. [Google Scholar]
Hashim 1960 {published data only}
- Hashim SA, Arteaga A, Itallie TB. Effect of saturated medium‐chain triglyceride on serum‐lipids in man. Lancet 1960;1:1105‐7. [DOI] [PubMed] [Google Scholar]
Haynes 1984 {published data only}
- Haynes RB, Harper AC, Costley SR, Johnston M, Logan AG, Flanagan PT, et al. Failure of weight reduction to reduce mildly elevated blood pressure: a randomized trial. Journal of Hypertension 1984;2(5):535‐9. [DOI] [PubMed] [Google Scholar]
Heber 1991 {published data only}
- Heber D, Ashley JM, Leaf DA, Barnard JA. Reduction of serum estradiol in postmenopausal women given free access to low‐fat high carbohydrate diet. Nutrition 1991;7:137‐41. [PubMed] [Google Scholar]
Heine 1989 {published and unpublished data}
- Heine RJ, Mulder C, Popp‐Snijders C, Meer J, Veen EA. Linoleic‐acid‐enriched diet: long‐term effects on serum lipoprotein and apolipoprotein concentration and insulin sensitivity in noninsulin‐dependent diabetic patients. American Journal of Clinical Nutrition 1989;49:448‐56. [DOI] [PubMed] [Google Scholar]
Heller 1993 {published and unpublished data}
- Heller RF, Knapp JC, Valenti LA, Dobson AJ. Secondary prevention after acute myocardial infarction. American Journal of Cardiology 1993;72(11):759‐62. [DOI] [PubMed] [Google Scholar]
- Heller RF, Walker RJ, Boyle CA, O'Connell DL, Rusakaniko S, Dobson AJ. A randomised controlled trial of a dietary advice program for relatives of heart attack victims. Medical Journal of Australia 1994;161(9):529‐31. [DOI] [PubMed] [Google Scholar]
Hildreth 1951 {published data only}
- Hildreth EA, Mellinkoff SM, Blair GW, Hildreth DM. The effect of vegetable fat ingestion on human serum cholesterol concentration. Circulation 1951;3:641‐?. [DOI] [PubMed] [Google Scholar]
Hood 1965 {published data only}
- Hood B, Sanne H, Orndahl G, Ahlstrom M, Welin G. Long term prognosis in essential hypercholesterolaemia: the effect of a strict diet. Acta Medica Scandinavica 1965;178:161‐73. [DOI] [PubMed] [Google Scholar]
Horlick 1957 {published data only}
- Horlick L, Craig BM. Effect of long‐chain polyunsaturated and saturated fatty acids on the serum‐lipids of man. Lancet 1957;2:566‐9. [DOI] [PubMed] [Google Scholar]
Horlick 1960 {published data only}
- Horlick L, O'Neil JB. Effect of modified egg‐yolk fats on blood‐cholesterol levels [letter]. Lancet 1960;1:438. [DOI] [PubMed] [Google Scholar]
Howard 1977 {published data only}
- Howard AN, Marks J. Hypocholesterolaemic effect of milk [letter]. Lancet 1977;2(8031):255‐6. [DOI] [PubMed] [Google Scholar]
Hunninghake 1990 {published data only}
- Hunninghake DB, Laskarzewski PM. Gender difference in the response to lovastatin administration with and without a cholesterol lowering diet [abstract]. Arteriosclerosis 1990;10:786A. [Google Scholar]
Hutchison 1983 {published data only}
- Hutchison K, Oberle K, Crockford P, Grace M, Whyte L, Gee M, et al. Effects of dietary manipulation on vascular status of patients with peripheral vascular disease. JAMA 1983;249(24):3330. [DOI] [PubMed] [Google Scholar]
Hyman 1998 {published and unpublished data}
- Hyman DJ, Ho KSI, Dunn K, Simons‐Morton D. Dietary intervention for cholesterol reduction in public clinic patients. American Journal of Preventive Medicine 1998;15:139‐45. [DOI] [PubMed] [Google Scholar]
Iacono 1981 {published data only}
- Iacono JM, Judd JT, Marshall MW, Canary JJ, Dougherty RM, Mackin JF, et al. The role of dietary essential fatty acids and prostaglandins in reducing blood pressure. Progress in Lipid Research 1981;20:349‐64. [DOI] [PubMed] [Google Scholar]
IMPACT 1995A {published data only}
- Fielding JE, Mason T, Knight K, Klesges R, Pelletier KR. A randomized trial of the IMPACT worksite cholesterol reduction program. American Journal Of Preventive Medicine 1995;11:120‐3. [PubMed] [Google Scholar]
Ishikawa 1995 {published data only}
- Ishikawa H, Akedo I, Suzuki T, Otani T, Sobue T. Interventional trial for colorectal cancer prevention in Osaka: an introduction to the protocol. Japanese Journal of Cancer Research 1995;86(8):707‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iso 1991 {published data only}
- Iso H, Konishi M, Terao A, Kiyama M, Tanigaki M, Baba M, et al. A community‐based education program for serum cholesterol reduction in urban hypercholesterolemic persons‐‐comparison of intensive and usual education groups. Nippon Koshu Eisei Zasshi 1991;38(9):751‐61. [PubMed] [Google Scholar]
Ives 1993 {published data only}
- Ives DG, Kuller LH, Traven ND. Use and outcomes of a cholesterol‐lowering intervention for rural elderly subjects. American Journal of Preventive Medicine 1993;9(5):274‐81. [PubMed] [Google Scholar]
Jalkanen 1991 {published data only}
- Jalkanen L. The effect of a weight reduction program on cardiovascular risk factors among overweight hypertensives in primary health care. Scandinavian Journal of Social Medicine 1991;19(1):66‐71. [DOI] [PubMed] [Google Scholar]
Janus 2012 {published data only}
- Janus ED, Best JD, Davis‐Lameloise N, Philpot B, Hernan A, Bennett CM, et al. Scaling‐up from an implementation trial to state‐wide coverage: results from the preliminary Melbourne Diabetes Prevention Study. Trials [Electronic Resource] 2012;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jepson 1969 {published data only}
- Jepson EM, Fahmy MF, Torrens PE, Billimoria JD, Maclagan NF. Treatment of essential hyperlipidaemia. Lancet 1969;2(7634):1315‐9. [DOI] [PubMed] [Google Scholar]
Jerusalem Nut 1992 {published data only}
- Berry EM, Eisenberg S, Friedlander Y, Harats D, Kaufmann NA, Norman Y, et al. Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins‐‐the Jerusalem Nutrition Study. II. Monounsaturated fatty acids vs carbohydrates. American Journal of Clinical Nutrition 1992;56(2):394‐403. [DOI] [PubMed] [Google Scholar]
Jonasson 2014 {published data only}
- Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH. Advice to follow a low‐carbohydrate diet has a favourable impact on low‐grade inflammation in type 2 diabetes compared with advice to follow a low‐fat diet. Annals of Medicine 2014;46:182‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juanola‐Falgarona 2014 {published data only}
- Juanola‐Falgarona M, Salas‐Salvado J, Ibarrola‐Jurado N, Rabassa‐Soler A, Bullo M. Effect of dietary glycemic index and glycemic load on body weight and cardiovascular risk factors: The GLYNDIET Study. Obesity Facts. 20th European Congress on Obesity, ECO 2013 Liverpool United Kingdom. 2013; Vol. 6:111.
- Juanola‐Falgarona Martí, Salas‐Salvado Jordi, Ibarrola‐Jurado Núria, Rabassa‐Soler Antoni, Diaz‐Lopez Andres, Guasch‐Ferré Marta, et al. Effect of the glycemic index of the diet on weight loss, modulation of satiety, inflammation, and other metabolic risk factors: a randomized controlled trial. American Journal of Clinical Nutrition 2014;100:27‐35. [DOI] [PubMed] [Google Scholar]
- Juanola‐Falgarona Martí, Salas‐Salvado Jordi, Ibarrola‐Jurado Núria, Rabassa‐Soler Antoni, Diaz‐Lopez Andres, Guasch‐Ferré Marta, et al. Effect of the glycemic index of the diet on weight loss, modulation of satiety, inflammation, and other metabolic risk factors: a randomized controlled trial. American Journal of Clinical Nutrition 2014;100:27‐35. [DOI] [PubMed] [Google Scholar]
Jula 1990 {published data only}
- Jula A, Ronnemaa T, Rastas M, Karvetti RL, Maki J. Long‐term nopharmacological treatment for mild to moderate hypertension. Journal of Internal Medicine 1990;227(6):413‐21. [DOI] [PubMed] [Google Scholar]
Junker 2001 {published data only}
- Junker R, Pieke B, Schulte H, Nofer R, Neufeld M, Assmann G, et al. Changes in hemostasis during treatment of hypertriglyceridemia with a diet rich in monounsaturated and n‐3 polyunsaturated fatty acids in comparison with a low‐fat diet. Thrombosis Research 2001;101(5):355‐66. [DOI] [PubMed] [Google Scholar]
Karmally 1990 {published data only}
- Karmally W, Carpentiri C, Viscardi T, Cheverez V, Holleran S, Ramakrishnan R, et al. Replacing monounsaturated by polyunsaturated fatty acids within an AHA step I diet does not affect the plasma levels or metabolism of low density and high density lipoproteins in normal men [abstract]. Arteriosclerosis 1990;10:877A. [Google Scholar]
Karvetti 1992 {published data only}
- Karvetti RL, Hakala P. A seven‐year follow‐up of a weight reduction programme in Finnish primary health care. European Journal of Clinical Nutrition 1992;46:743‐52. [PubMed] [Google Scholar]
Kastarinen 2002 {published data only}
- Kastarinen MJ, Puska PM, Korhonen MH, Mustonen JN, Salomaa VV, Sundvall JE, et al. Non‐pharmacological treatment of hypertension in primary health care: a 2‐year open randomized controlled trial of lifestyle intervention against hypertension in eastern Finland. Journal of Hypertension 2002;20(12):2505‐12. [DOI] [PubMed] [Google Scholar]
Kather 1985 {published data only}
- Kather H, Wildenberg U, Wieland E. Influence of different dietary conditions in ideal‐weight subjects on serum levels of free fatty acids and of glycerol in vivo and on lipid mobilization in vitro [abstract]. European Journal of Clinical Investigation 1985;15:A. [Google Scholar]
Kattelmann 2010 {published data only}
- Kattelmann KK, Conti K, Ren C, Kattelmann Kendra K, Conti Kibbe, Ren Cuirong. The Medicine Wheel nutrition intervention: a diabetes education study with the Cheyenne River Sioux Tribe.[Reprint of J Am Diet Assoc. 2009 Sep;109(9):1532‐9; PMID: 19699832]. Journal of the American Dietetic Association 2010;110:S44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katzel 1995 {published data only}
- Katzel LI, Coon PJ, Dengel J, Goldberg AP. Effect of an American Heart Association Step I diet and weight loss on lipoprotein lipid levels in obese men with silent myocardial ischaemia and reduced high density lipoprotein cholesterol. Metabolism 1995;44:307‐14. [DOI] [PubMed] [Google Scholar]
Katzel 1995A {published data only}
- Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle‐aged and older men. A randomized controlled trial [see comments]. JAMA 1995;274(24):1915‐21. [DOI] [PubMed] [Google Scholar]
Kawamura 1993 {published data only}
- Kawamura M, Akasaka T, Kasatsuki T, Nakajima J, Onodera S, Fujiwara T, et al. Blood pressure is reduced by short‐time calorie restriction in overweight hypertensive women with a constant intake of sodium and potassium. Journal of Hypertension. Supplement 1993;11 Suppl 5:S320‐1. [PubMed] [Google Scholar]
Keidar 1988 {published data only}
- Keidar S, Krul ES, Goldberg AC, Bateman J, Schonfield G. Fat‐free diet modulates epitope expression of LDL‐apo_ [abstract]. Arteriosclerosis 1988;8:565A. [PubMed] [Google Scholar]
Kempner 1948 {published data only}
- Kempner W. Treatment of hypertensive vascular disease with rice diet. American Journal of Medicine 1948;4:545‐77. [DOI] [PubMed] [Google Scholar]
Keys 1952 {published data only}
- Keys A. Human atherosclerosis and the diet. Circulation 1952;5:115‐8. [DOI] [PubMed] [Google Scholar]
Keys 1957 {published data only}
- Keys A, Anderson JT, Grande F. Serum‐cholesterol response to dietary fat [letter]. Lancet 1957;1:787. [Google Scholar]
Keys 1957A {published data only}
- Keys A, Anderson JT, Grande F. Essential fatty acids, degree of unsaturation, and effect of corn (maize) oil on the serum‐cholesterol level in man. Lancet 1957;1:66‐8. [DOI] [PubMed] [Google Scholar]
Keys 1957B {published data only}
- Keys A. Prediction of serum‐cholesterol responses of man to changes in fats in the diet. Lancet 1957;2:959‐66. [DOI] [PubMed] [Google Scholar]
Khan 2003 {published and unpublished data}
- Khan F, Elherik K, Bolton‐Smith C, Barr R, Hill A, Murrie I, et al. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovascular Research 2003;59:955‐62. [DOI] [PubMed] [Google Scholar]
King 2000 {published data only}
- King S, David S, Newton H, Hevey D, Rafferty F, Horgan JH. The effect of dietary modification on the training outcome and body composition in patients undergoing a cardiac rehabilitation programme. Coronary Health Care 2000;4(2):76‐81. [Google Scholar]
Kingsbury 1961 {published data only}
- Kingsbury KJ, Morgan DM, Aylott C, Emmerson R. Effects of ethyl arachidonate, cod‐liver oil, and corn oil on the plasma‐cholesterol level: a comparison in normal volunteers. Lancet 1961;1:739‐41. [DOI] [PubMed] [Google Scholar]
Klemsdal 2010 {published data only}
- Klemsdal TO, Holme I, Nerland H, Pedersen TR, Tonstad S, Klemsdal TO, et al. Effects of a low glycemic load diet versus a low‐fat diet in subjects with and without the metabolic syndrome. Nutrition Metabolism & Cardiovascular Diseases 2010;20:195‐201. [DOI] [PubMed] [Google Scholar]
Kohler 1986 {published data only}
- Kohler VH, Voigt H, Reuter W, Peters H‐J, Kuklinski B, Scheel H, et al. Results of a long‐term study of arteriosclerotic circulatory disorders with polyene fatty acid therapy [German]. Zeitschrift für die Gesamte Innere Medizin und ihre Grenzgebiete 1986;41:91‐3. [PubMed] [Google Scholar]
Kontogianni 2012 {published data only}
- Kontogianni MDL. Changes in dietary habits and their association with metabolic markers after a non‐intensive, community‐based lifestyle intervention to prevent type 2 diabetes, in Greece. The DEPLAN study. Diabetes Research and Clinical Practice 2012;95:207‐14. [DOI] [PubMed] [Google Scholar]
Koopman 1990 {published data only}
- Koopman H, Spreeuwenberg C, Westerman RF, Donker AJ. Dietary treatment of patients with mild to moderate hypertension in a general practice: a pilot intervention study (2). Beyond three months. Journal of Human Hypertension 1990;4(4):372‐4. [PubMed] [Google Scholar]
Koranyi 1963 {published data only}
- Koranyi A. Prophylaxis and treatment of the coronary syndrome. Therapia Hungarica 1963;11:17‐20. [PubMed] [Google Scholar]
Korhonen 2003 {published data only}
- Korhonen M, Kastarinen M, Uusitupa M, Puska P, Nissinen A. The effect of intensified diet counseling on the diet of hypertensive subjects in primary health care: a 2‐year open randomized controlled trial of lifestyle intervention against hypertension in eastern Finland. Preventive Medicine 2003;36(1):8‐16. [DOI] [PubMed] [Google Scholar]
Kriketos 2001 {published data only}
- Kriketos AD, Robertson RM, Sharp TA, Drougas H, Reed GW, Storlien LH, et al. Role of weight loss and polyunsaturated fatty acids in improving metabolic fitness in moderately obese, moderately hypertensive subjects. Journal of Hypertension 2001;19(10):1745‐54. [DOI] [PubMed] [Google Scholar]
Kris 1994 {published data only}
- Kris EP, Mustad VA. Chocolate feeding studies: a novel approach for evaluating the plasma lipid effects of stearic acid. American Journal of Clinical Nutrition 1994;60(6 Suppl):1029S‐36S. [DOI] [PubMed] [Google Scholar]
Kristal 1997 {published data only}
- Kristal AR, Shattuck AL, Bowen DJ, Sponzo RW, Nixon DW. Feasibility of using volunteer research staff to deliver and evaluate a low‐fat dietary intervention: the American Cancer Society Breast Cancer Dietary Intervention Project. Cancer Epidemiology, Biomarkers and Prevention 1997;6(6):459‐67. [PubMed] [Google Scholar]
Kromhout 1987 {published data only}
- Kromhout D, Arntzenius AC, Kempen‐Voogd N, Kempen HJ, Barth JD, Voort HA, et al. Long‐term effects of linoleic‐acid enriched diet, changes in body weight and alcohol consumption on serum total and HDL cholesterol. Atherosclerosis 1987;66:99‐105. [DOI] [PubMed] [Google Scholar]
Kummel 2008 {published data only}
- Kummel MV. Effects of an intervention on health behaviors of older coronary artery bypass (CAB) patients. Archives of Gerontology and Geriatrics 2008;2(2):227‐44. [DOI] [PubMed] [Google Scholar]
Laitinen 1993 {published data only}
- Laitinen JH, Ahola IE, Sarkkinen ES, Winberg RL, Harmaakorpi IP, Uusitupa MI. Impact of intensified dietary therapy on energy and nutrient intakes and fatty acid composition of serum lipids in patients with recently diagnosed non‐insulin‐dependent diabetes mellitus. Journal of the American Dietetic Association 1993;93(3):276‐83. [DOI] [PubMed] [Google Scholar]
Laitinen 1994 {published data only}
- Laitinen J, Uusitupa M, Ahola I, Siitonen O. Metabolic and dietary determinants of serum lipids in obese patients with recently diagnosed non‐insulin‐dependent diabetes. Annals of Medicine 1994;26(2):119‐24. [DOI] [PubMed] [Google Scholar]
Larsen 2011 {published data only}
- Larsen RN, Mann NJ, Maclean E, Shaw JE, Larsen RN, Mann NJ, et al. The effect of high‐protein, low‐carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial. Diabetologia 2011;54:731‐40. [DOI] [PubMed] [Google Scholar]
Leduc 1994 {published data only}
- Leduc CP, Cherniak D, Faucher J. Effectiveness of a group dietary intervention on hypercholesterolaemia: a randomised controlled clinical trial (poster abstract). Atherosclerosis 1994;?:149. [Google Scholar]
Leibbrandt 2010 {published data only}
- Leibbrandt AJ, Kiefte‐de Jong JC, Hogenelst MHE, Snoek FJ, Weijs PJM. Effects of the PRo‐active interdisciplinary Self‐MAnagement (PRISMA, Dutch DESMOND) program on dietary intake in type 2 diabetes outpatients: a pilot study. Clinical Nutrition 2010;29:199‐205. [DOI] [PubMed] [Google Scholar]
Lewis 1958 {published data only}
- Lewis B. Effect of certain dietary oils on bile‐acid secretion and serum‐cholesterol. Lancet 1958;1:1090‐2. [DOI] [PubMed] [Google Scholar]
Lewis 1981 {published data only}
- Lewis B, Hammett F, Katan M, Kay RM, Merkx I, Nobels A, et al. Towards an improved lipid‐lowering diet: additive effects of changes in nutrient intake. Lancet 1981;2(8259):1310‐3. [DOI] [PubMed] [Google Scholar]
Lewis 1985 {published data only}
- Lewis B. Randomised controlled trial of the treatment of hyperlipidaemia on progression of atherosclerosis. Acta Medica Scandinavica. Supplementum 1985;701:53‐7. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2002 {published data only}
- Lichtenstein AH, Ausman LM, Jalbert SM, Vilella‐Bach M, Jauhiainen M, McGladdery S, et al. Efficacy of a Therapeutic Lifestyle Change/Step 2 diet in moderately hypercholesterolemic middle‐aged and elderly female and male subjects. Journal of Lipid Research 2002;43(2):264‐73. [PubMed] [Google Scholar]
Linko 1957 {published data only}
- Linko E. Vegetable oils and serum cholesterol: short‐term experiments with rapeseed and sunflower oils. Acta Medica Scandinavica. Supplementum 1957;159:475‐88. [PubMed] [Google Scholar]
Lipid Res Clinic 1984 {published data only}
- Anon. The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251(3):351‐64. [DOI] [PubMed] [Google Scholar]
- Anon. The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251(3):365‐74. [PubMed] [Google Scholar]
- Gordon DJ, Salz KM, Roggenkamp KJ. Dietary determinants of plasma cholesterol change in the recruitment phase of the Lipid Research Clinics Coronary Primary Prevention Trial. Arteriosclerosis 1982;2(6):537‐48. [DOI] [PubMed] [Google Scholar]
Little 1990 {published data only}
- Little P, Girling G, Hasler A, Craven A, Trafford A. The effect of a combination low sodium, low fat, high fibre diet on serum lipids in treated hypertensive patients. European Journal of Clinical Nutrition 1990;44(4):293‐300. [PubMed] [Google Scholar]
Little 1991 {published data only}
- Little P, Girling G, Hasler A, Trafford A. A controlled trial of a low sodium, low fat, high fibre diet in treated hypertensive patients: effect on antihypertensive drug requirement in clinical practice. Journal of Human Hypertension 1991;5(3):175‐81. [PubMed] [Google Scholar]
Little 2004 {published data only}
- Little P, Kelly J, Barnett J, Dorward M, Margetts B, Warm D, et al. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. BMJ 2004;328(7447):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lottenberg 1996 {published data only}
- Lottenberg AM, Nunes VS, Lottenberg SA, Shimabukuro AF, Carrilho AJ, Malagutti S, et al. Plasma cholesteryl ester synthesis, cholesteryl ester transfer protein concentration and activity in hypercholesterolemic women: effects of the degree of saturation of dietary fatty acids in the fasting and postprandial states. Atherosclerosis 1996;126(2):265‐75. [DOI] [PubMed] [Google Scholar]
Luoto 2012 {published data only}
- Luoto R, Laitinen K, Nermes M, Isolauri E, Luoto Raakel, Laitinen Kirsi, et al. Impact of maternal probiotic‐supplemented dietary counseling during pregnancy on colostrum adiponectin concentration: a prospective, randomized, placebo‐controlled study. Early Human Development 2012;88:339‐44. [DOI] [PubMed] [Google Scholar]
Luszczynska 2007 {published data only}
- Luszczynska A, Scholz U, Sutton S. Planning to change diet: a controlled trial of an implementation intentions training intervention to reduce saturated fat intake among patients after myocardial infarction. Journal of Psychosomatic Research 2007;63(5):491‐7. [DOI] [PubMed] [Google Scholar]
Lyon Diet Heart 1994 {published data only}
- Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha‐linolenic acid‐rich diet in secondary prevention of coronary heart disease. Lancet 1994;343(8911):1454‐9. [DOI] [PubMed] [Google Scholar]
- Lorgeril M, Salen P. Mediterranean diet in secondary prevention of coronary heart disease. Australian Journal of Nutrition and Dietetics 1998;55(Suppl):s16‐s20. [Google Scholar]
- Lorgeril M, Salen P, Caillat‐Vallet E, Hanauer M‐T, Barthelemy JC, Mamelle N. Control of bias in dietary trial to prevent coronary recurrences: the Lyon diet heart study. European Journal of Clinical Nutrition 1997;51(2):116‐22. [DOI] [PubMed] [Google Scholar]
- Lorgeril M, Salen P, Martin J‐L, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon diet heart study. Circulation 1999;99:779‐85. [DOI] [PubMed] [Google Scholar]
- Lorgeril M, Salen P, Martin JL, Mamelle N, Monjaud I, Touboul P, et al. Effect of a Mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the cardioprotective effect of certain nutriments. Journal of the American College of Cardiology 1996;28:1103‐8. [DOI] [PubMed] [Google Scholar]
- Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N. Mediterranean dietary pattern in a randomised trial. Archives of Internal Medicine 1998;158:1181‐7. [DOI] [PubMed] [Google Scholar]
- Renaud S, Lorgeril M, Delaye J, Guidollet J, Jacquard F, Mamelle N, et al. Cretan Mediterranean diet for prevention of coronary heart disease. American Journal of Clinical Nutrition 1995;61(6 Suppl):1360S‐7S. [DOI] [PubMed] [Google Scholar]
Lysikova 2003 {published data only}
- Lysikova SL, Pogozheva AV, Akol'zina SE, Vasil'ev AV, Vorob'eva LS. The study of the clinical potency of antiatherogenic diet containing flavonoids in cardiovascular patients [Russian]. Voprosy Pitaniia 2003;72(3):8‐11. [PubMed] [Google Scholar]
Macdonald 1972 {published data only}
- Macdonald I. Relationship between dietary carbohydrates and fats in their influence on serum lipid concentrations. Clinical Science 1972;43(2):265‐74. [DOI] [PubMed] [Google Scholar]
Mansel 1990 {published data only}
- Mansel RE, Harrison BJ, Melhuish J, Sheridan W, Pye JK, Pritchard G, et al. A randomized trial of dietary intervention with essential fatty acids in patients with categorized cysts. Annals of the New York Academy of Sciences 1990;?:288‐94. [DOI] [PubMed] [Google Scholar]
Marckmann 1993 {published data only}
- Marckmann P, Sandstrom B, Jespersen J. Favorable long‐term effect of a low‐fat/high fiber diet on human blood coagulation and fibrinolysis. Arteriosclerosis and Thrombosis 1993;13:505‐11. [DOI] [PubMed] [Google Scholar]
MARGARIN {published data only}
- Bemelmans WJE, Broer J, Feskens EJM, Smit AJ, Muskiet FAJ, Lefrandt JD, et al. Effect of an increased intake of alpha‐linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean Alpha‐linolenic Enriched Groningen Dietary Intervention (MARGARIN) study. American Journal of Clinical Nutrition 2002;75:221‐7. [DOI] [PubMed] [Google Scholar]
Martin 2011 {published data only}
- Martin CK, Rosenbaum D, Han H, Geiselman PJ, Wyatt HR, Hill JO, et al. Change in food cravings, food preferences, and appetite during a low‐carbohydrate and low‐fat diet. Obesity 2011;19:1963‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maruthur 2014 {published data only}
- Maruthur N, Yau MS, Jablonski KA, Delahanty L, Franks PW, Knowler WC, et al. Genetic variation and response to weight, physical activity, and diet change to prevent diabetes in the diabetes prevention program. Diabetes. 2014; Vol. 63:A415.
Mattson 1985 {published data only}
- Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. Journal of Lipid Research 1985;26:194‐202. [PubMed] [Google Scholar]
Mayneris‐Perxachs 2014 {published data only}
- Mayneris‐Perxachs J, Sala‐Vila A, Chisaguano M, Castellote AI, Estruch R, Covas MI, et al. Effects of 1‐year intervention with a Mediterranean diet on plasma fatty acid composition and metabolic syndrome in a population at high cardiovascular risk. PloS One 2014;9:e85202. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarron 1997 {published data only}
- McCarron DA, Oparil S, Chait A, Haynes RB, Kris EP, Stern JS, et al. Nutritional management of cardiovascular risk factors. A randomized clinical trial. Archives of Internal Medicine 1997;157(2):169‐77. [PubMed] [Google Scholar]
McCarron 2001 {published data only}
- McCarron DA, Reusser ME. Reducing cardiovascular disease risk with diet. Obesity Research 2001;9 Suppl 4:335S‐40S. [DOI] [PubMed] [Google Scholar]
McManus 2001 {published and unpublished data}
- McManus K, Antinoro L, Sacks F. Randomized controlled trial of a moderate‐fat low‐energy diet compared with a low fat, low‐energy diet for weight loss in overweight adults. International Journal of Obesity 2001;25:1503‐11. [DOI] [PubMed] [Google Scholar]
McNamara 1981 {published data only}
- McNamara DJ, Kolb R, Parker T, Batwin H, Brown C, Samuel P, et al. Diet and cholesterol homeostasis in men [abstract]. Arteriosclerosis 1981;1:369A. [Google Scholar]
Medi‐RIVAGE 2004 {published and unpublished data}
- Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent‐Baudry S, et al. Human fasting plasma concentrations of vitamin E and carotenoids, and their association with genetic variants in apo C‐III, cholesteryl ester transfer protein, hepatic lipase, intestinal fatty acid binding protein and microsomal triacylglycerol transfer protein. British Journal of Nutrition 2009;101(5):680‐7. [DOI] [PubMed] [Google Scholar]
- Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent‐Baudry S, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. Journal of Nutrition 2007;137(12):2653‐9. [DOI] [PubMed] [Google Scholar]
- Gastaldi M, Diziere S, Defoort C, Portugal H, Lairon D, Darmon M, et al. Sex‐specific association of fatty acid binding protein 2 and microsomal triacylglycerol transfer protein variants with response to dietary lipid changes in the 3‐mo Medi‐RIVAGE primary intervention study. American Journal of Clinical Nutrition 2007;86(6):1633‐41. [DOI] [PubMed] [Google Scholar]
- Vincent S, Gerber M, Bernard MC, Defoort C, Loundou A, Portugal H, et al. The Medi‐RIVAGE study (Mediterranean Diet, Cardiovascular Risks and Gene Polymorphisms): rationale, recruitment, design, dietary intervention and baseline characteristics of participants. Public Health Nutrition 2004;7(4):531‐42. [DOI] [PubMed] [Google Scholar]
- Vincent‐Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, et al. The Medi‐RIVAGE study: reduction of cardiovascular disease risk factors after a 3‐mo intervention with a Mediterranean‐type diet or a low‐fat diet. American Journal of Clinical Nutrition 2005;82(5):964‐71. [DOI] [PubMed] [Google Scholar]
Mensink 1987 {published data only}
- Mensink RP, Katan MB. Effect of monounsaturated fatty acids versus complex carbohydrates on high‐density lipoproteins in healthy men and women. Lancet 1987;1(8525):122‐5. [DOI] [PubMed] [Google Scholar]
Mensink 1989 {published data only}
- Mensink RP, Katan MB. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low density and high density lipoprotein cholesterol in healthy women and men. New England Journal of Medicine 1989;321:436‐41. [DOI] [PubMed] [Google Scholar]
Mensink 1990 {published data only}
- Mensink RP, Katan MB. Effect of dietary trans fatty acids on high density and low density lipoprotein cholesterol levels in healthy subjects. New England Journal of Medicine 1990;323:439‐45. [DOI] [PubMed] [Google Scholar]
Mensink 1990A {published and unpublished data}
- Mensink RP. Effect of monounsaturated fatty acids on high‐density and low‐density lipoprotein cholesterol levels and blood pressure in healthy men and women. PhD Thesis 1990.
Merrill 2011 {published data only}
- Merrill RM, Aldana SG, Garrett J, Ross C, et al. Effectiveness of a workplace wellness program for maintaining health and promoting healthy behaviors. Journal of Occupational & Environmental Medicine 2011;53:782‐7. [DOI] [PubMed] [Google Scholar]
Metroville Health 2003 {published data only (unpublished sought but not used)}
- Aziz KU, Dennis B, Davis CE, Sun K, Burke G, Manolio T, et al. Efficacy of CVD risk factor modification in a lower‐middle class community in Pakistan: the Metroville Health Study. Asia Pacific Journal of Public Health 2003;15(1):30‐6. [DOI] [PubMed] [Google Scholar]
Michalsen 2006 {published and unpublished data}
- Michalsen A, Lehmann N, Pithan C, Knoblauch NT, Moebus S, Kannenberg F, et al. Mediterranean diet has no effect on markers of inflammation and metabolic risk factors in patients with coronary artery disease. European Journal of Clinical Nutrition 2006;60(4):478‐85. [DOI] [PubMed] [Google Scholar]
Miettinen 1994 {published data only}
- Miettinen TA, Vanhanen H. Dietary sitostanol related to absorption, synthesis and serum level of cholesterol in different apolipoprotein E phenotypes. Atherosclerosis 1994;105(2):217‐26. [DOI] [PubMed] [Google Scholar]
Millar 1973 {published data only}
- Millar JH, Zilkha KJ, Langman MJS, Payling‐Wright H, Smith AD, Belin J, et al. Double‐blind trial of linoleate supplementation of the diet in multiple sclerosis. BMJ 1973;i:765‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1998 {published data only}
- Miller ER, Appel LJ, Risby TH. Effect of dietary patterns on measures of lipid peroxidation: results from a randomised clinical trial. Circulation 1998;98:2390‐5. [DOI] [PubMed] [Google Scholar]
Miller 2001 {published and unpublished data}
- Miller SL, Reber RJ, Chapman‐Novakofski K. Prevalence of CVD risk factors and impact of a two‐year education program for premenopausal women. Women's Health Issues 2001;11(6):486‐93. [DOI] [PubMed] [Google Scholar]
Milne 1994 {published data only}
- Milne RM, Mann JI, Chisholm AW, Williams SM. Long‐term comparison of three dietary prescriptions in the treatment of NIDDM. Diabetes Care 1994;17(1):74‐80. [DOI] [PubMed] [Google Scholar]
Minnesota HHP 1990 {published data only}
- Murray DM, Kurth C, Mullis R, Jeffery RW. Cholesterol reduction through low‐intensity interventions: results from the Minnesota Heart Health Program. Preventive Medicine 1990;19(2):181‐9. [DOI] [PubMed] [Google Scholar]
Mishra 2013 {published data only}
- Mishra S, Barnard ND, Gonzales J, Xu J, Agarwal U, Levin S, et al. Nutrient intake in the GEICO multicenter trial: the effects of a multicomponent worksite intervention. European Journal of Clinical Nutrition 2013;67:1066‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2011 {published data only}
- Mitchell D, Alaniz G, Castaneda X, Schenker M. Application of a diabetes prevention programme in immigrant Latino farm workers. Occupational and Environmental Medicine 2011;68:A50. [Google Scholar]
Mokuno 1988 {published data only}
- Mokuno H, Yamada N, Sugimoto T, et al. Cholesterol free diet in heterozygous familial hypercholesterolaemia: significant lowering effect on plasma cholesterol (abstract). Arteriosclerosis 1988;8(5):590a. [DOI] [PubMed] [Google Scholar]
Moreno 1994 {published data only}
- Moreno VJ, Garcia AJ, Campillo AJ. Influence of diet and physical exercise on plasma lipid concentrations in an homogeneous sample of young Spanish Air Force pilots. European Journal of Applied Physiology 1994;69(1):75‐80. [DOI] [PubMed] [Google Scholar]
Morrison 1950 {published data only}
- Morrison LM, Awierlein M, Wolfson E. The effects of low fat low cholesterol diets on the serum lipids. Circulation 1950;2:475‐6. [Google Scholar]
Morrison 1951 {published data only}
- Morrison LM. Reduction of mortality rate in coronary atherosclerosis by a low cholesterol low fat diet. American Heart Journal 1951;42:538‐45. [DOI] [PubMed] [Google Scholar]
Morrison 1960 {published data only}
- Morrison LM. Diet in coronary atherosclerosis. JAMA 1960;173:884‐8. [DOI] [PubMed] [Google Scholar]
Mortensen 1983 {published data only}
- Mortensen JZ, Schmidt EB, Nielsen AH, Dyerberg J. The effect of N‐6 and N‐3 polyunsaturated fatty acids on hemostasis, blood lipids and blood pressure. Thrombosis and Haemostasis 1983;50(2):543‐6. [PubMed] [Google Scholar]
Moses 2014 {published data only}
- Moses RG, Casey SA, Quinn EG, Cleary JM, Tapsell LC, Milosavljevic M, et al. Pregnancy and Glycemic Index Outcomes study: effects of low glycemic index compared with conventional dietary advice on selected pregnancy outcomes. American Journal of Clinical Nutrition 2014;99:517‐23. [DOI] [PubMed] [Google Scholar]
MRFIT substudy 1986 {published data only}
- Daniel GJ, Dolecek TA, Caggiula AW, Van HL, Epley L, Randall BL. Increasing the use of meatless meals: a nutrition intervention substudy in the Multiple Risk Factor Intervention Trial (MRFIT). Journal of the American Dietetic Association 1986;86(6):778‐81. [PubMed] [Google Scholar]
MSDELTA 1995 {published data only}
- Ginsberg HN. New directions in dietary studies and heart disease: the National Heart, Lung and Blood Institute sponsored Multicenter Study of Diet Effects on Lipoproteins and Thrombogenic Activity. Advances In Experimental Medicine and Biology 1995;369:241‐7. [DOI] [PubMed] [Google Scholar]
MUFObes low fat 2007 {published and unpublished data}
- Due A, Larsen TM, Hermansen K, Stender S, Holst JJ, Toubro S, et al. Comparison of the effects on insulin resistance and glucose tolerance of 6‐mo high‐monounsaturated‐fat, low‐fat, and control diets. American Journal of Clinical Nutrition 2008;87(4):855‐62. [DOI] [PubMed] [Google Scholar]
- Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight‐loss maintenance, risk of cardiovascular disease, and diabetes: a 6‐mo randomized, controlled trial. American Journal of Clinical Nutrition 2008;88(5):1232‐41. [DOI] [PubMed] [Google Scholar]
- Rasmussen LG, Larsen TM, Mortensen PK, Due A, Astrup A, Rasmussen Lone G, et al. Effect on 24‐h energy expenditure of a moderate‐fat diet high in monounsaturated fatty acids compared with that of a low‐fat, carbohydrate‐rich diet: a 6‐mo controlled dietary intervention trial. American Journal of Clinical Nutrition 2007;85(4):1014‐22. [DOI] [PubMed] [Google Scholar]
- Sloth B, Due A, Larsen TM, Holst JJ, Heding A, Astrup A, et al. The effect of a high‐MUFA, low‐glycaemic index diet and a low‐fat diet on appetite and glucose metabolism during a 6‐month weight maintenance period. British Journal of Nutrition 2009;101(12):1846‐58. [DOI] [PubMed] [Google Scholar]
MUFObes low vs mod 2007 {published and unpublished data}
- Due A, Larsen TM, Hermansen K, Stender S, Holst JJ, Toubro S, et al. Comparison of the effects on insulin resistance and glucose tolerance of 6‐mo high‐monounsaturated‐fat, low‐fat, and control diets. American Journal of Clinical Nutrition 2008;87(4):855‐62. [DOI] [PubMed] [Google Scholar]
- Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A, et al. Comparison of 3 ad libitum diets for weight‐loss maintenance, risk of cardiovascular disease, and diabetes: a 6‐mo randomized, controlled trial. American Journal of Clinical Nutrition 2008;88(5):1232‐41. [DOI] [PubMed] [Google Scholar]
- Rasmussen LG, Larsen TM, Mortensen PK, Due A, Astrup A, Rasmussen Lone G, et al. Effect on 24‐h energy expenditure of a moderate‐fat diet high in monounsaturated fatty acids compared with that of a low‐fat, carbohydrate‐rich diet: a 6‐mo controlled dietary intervention trial. American Journal of Clinical Nutrition 2007;85(4):1014‐22. [DOI] [PubMed] [Google Scholar]
- Sloth B, Due A, Larsen TM, Holst JJ, Heding A, Astrup A, et al. The effect of a high‐MUFA, low‐glycaemic index diet and a low‐fat diet on appetite and glucose metabolism during a 6‐month weight maintenance period. British Journal of Nutrition 2009;101(12):1846‐58. [DOI] [PubMed] [Google Scholar]
Mujeres Felices 2003 {published data only}
- Fitzgibbon ML, Gapstur SM, Knight SJ. Mujeres felices por ser saludables: a breast cancer risk reduction program for Latino women. Preventive Medicine 2003;36(5):536‐46. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon ML, Gapstur SM, Knight SJ. Results of Mujeres Felices por ser Saludables: a dietary/breast health randomized clinical trial for Latino women. Annals of Behavioral Medicine 2004;28(2):95‐104. [DOI] [PubMed] [Google Scholar]
Munsters 2010 {published data only}
- Munsters MJ, Saris WH. The effect of sugar‐sweetened beverage intake on energy intake in an ad libitum 6‐month low‐fat high‐carbohydrate diet. Annals of Nutrition & Metabolism 2010;57:116‐23. [DOI] [PubMed] [Google Scholar]
Mutanen 1997 {published data only}
- Mutanen M. Comparison between dietary monounsaturated and polyunsaturated fatty acids as regards diet‐related diseases. Biomedicine and Pharmacotherapy 1997;51(8):314‐7. [DOI] [PubMed] [Google Scholar]
Muzio 2007 {published data only}
- Muzio F, Mondazzi L, Harris WS, Sommariva D, Branchi A, Muzio Fulvio, et al. Effects of moderate variations in the macronutrient content of the diet on cardiovascular disease risk factors in obese patients with the metabolic syndrome. American Journal of Clinical Nutrition 2007;86(4):946‐51. [DOI] [PubMed] [Google Scholar]
Naglak 2000 {published data only (unpublished sought but not used)}
- Naglak MC, Mitchell DC, Shannon BM, Pearson TA, Harkness WL, Kris‐Etherton PM. Nutrient adequacy of diets of adults with hypercholesterolemia after a cholesterol‐lowering intervention: long term assessment. Journal of the American Dietetic Association 2000;100(11):1385‐91. [DOI] [PubMed] [Google Scholar]
NAS 1987 {published data only}
- Chlebowski RT, Nixon DW, Blackburn GL, Jochimsen P, Scanlon EF, Insull W, et al. A breast cancer Nutrition Adjuvant Study (NAS): protocol design and initial patient adherence. Breast Cancer Research and Treatment 1987;10(1):21‐9. [DOI] [PubMed] [Google Scholar]
NCEP weight {published and unpublished data}
- Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight‐reducing diet, with or without exercise, in overweight men and women. New England Journal of Medicine 1991;325(7):461‐6. [DOI] [PubMed] [Google Scholar]
Neil 1995 {published data only}
- Neil HA, Roe L, Godlee RJ, Moore JW, Clark GM, Brown J, et al. Randomised trial of lipid lowering dietary advice in general practice: the effects on serum lipids, lipoproteins, and antioxidants [see comments]. BMJ 1995;310(6979):569‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neverov 1997 {published data only}
- Neverov NI, Kaysen GA, Tareyeva IE. Effect of lipid‐lowering therapy on the progression of renal disease in nondiabetic nephrotic patients. Contributions to Nephrology 1997;120:68‐78. [DOI] [PubMed] [Google Scholar]
Next Step 1995 {published and unpublished data}
- Tilley BC, Vernon SW, Glanz K, Myers R, Sanders K, Lu M, et al. Worksite cancer screening and nutrition intervention for high‐risk auto workers: design and baseline findings of the Next Step Trial. Preventive Medicine 1997;26(2):227‐35. [DOI] [PubMed] [Google Scholar]
- Tilley BC, Vernon SW, Myers R, Glanz K, Lu M, Sanders K, et al. Planning the next step. A screening promotion and nutrition intervention trial in the work site. Annals of the New York Academy of Sciences 1995;?:296‐9. [DOI] [PubMed] [Google Scholar]
Nordoy 1971 {published data only}
- Nordoy A, Rodset JM. The influence of dietary fats on platelets in man. Acta Medica Scandinavica 1971;190(1‐2):27‐34. [PubMed] [Google Scholar]
Norway Veg Oil 1968 {published data only}
- Natvig H, Borchgrevink CF, Dedichen J, Owren PA, Schiotz EH, Westlund K. A controlled trial of the effect of linolenic acid on incidence of coronary heart disease: the Norwegian Vegetable Oil Experiment of 1965‐66. Scandinavian Journal of Clinical and Laboratory Investigation. Supplement 1968;105:1‐20. [PubMed] [Google Scholar]
Novotny 2012 {published data only}
- Novotny R, Chen C, Williams AE, Albright CL, Nigg CR, Oshiro CE, et al. US acculturation is associated with health behaviors and obesity, but not their change, with a hotel‐based intervention among Asian‐Pacific Islanders. Journal of the Academy of Nutrition & Dietetics 2012;112:649‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nutrition Ed Study 1980 {published data only (unpublished sought but not used)}
- Mojonnier ML, Hall Y, Berkson DM, Robinson E, Wethers B, Pannbacker B, et al. Experience in changing food habits of hyperlipidaemic men and women. Journal of the American Dietetic Association 1980;77:140‐8. [PubMed] [Google Scholar]
O'Brien 1976 {published data only}
- O'Brien JR, Etherington MD, Jamieson S. Effect of a diet of polyunsaturated fats on some platelet‐function tests. Lancet 1976;2(7993):995‐6. [DOI] [PubMed] [Google Scholar]
ODES 2001 {published data only}
- Anderssen S, Holme I, Urdal P, Hjermann I. Diet and exercise intervention have favourable effects on blood pressure in mild hypertensives: the Oslo Diet and Exercise Study (ODES). Blood Pressure 1995;4(6):343‐9. [DOI] [PubMed] [Google Scholar]
- Anderssen SA, Hjermann I, Urdal P, Torjesen PA, Holme I. Improved carbohydrate metabolism after physical training and dietary intervention in individuals with the "atherothrombogenic syndrome'. Oslo Diet and Exercise Study (ODES). A randomized trial. Journal of Internal Medicine 1996;240(4):203‐9. [DOI] [PubMed] [Google Scholar]
- Holme I, Haaheim LL, Tonstad S, Hjermann I, Holme I, Haaheim LL, et al. Effect of dietary and antismoking advice on the incidence of myocardial infarction: a 16‐year follow‐up of the Oslo Diet and Antismoking Study after its close. Nutrition Metabolism & Cardiovascular Diseases 2006;16(5):330‐8. [DOI] [PubMed] [Google Scholar]
- Rokling‐Andersen MH, Reseland JE, Veierod MB, Anderssen SA, Jacobs DR Jr, Urdal P, et al. Effects of long‐term exercise and diet intervention on plasma adipokine concentrations. American Journal of Clinical Nutrition 2007;86(5):1293‐301. [DOI] [PubMed] [Google Scholar]
- The ODES Investigators. The Oslo Diet and Exercise Study (ODES): design and objectives. Controlled Clinical Trials 1993;14(3):229‐43. [DOI] [PubMed] [Google Scholar]
- Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care 1997;20(1):26‐31. [DOI] [PubMed] [Google Scholar]
Oldroyd 2001 {published data only}
- Oldroyd JC, Unwin NC, White M, Mathers JC, Alberti KG, et al. Randomised controlled trial evaluating lifestyle interventions in people with impaired glucose tolerance. Diabetes Research & Clinical Practice 2006;72(2):117‐27. [DOI] [PubMed] [Google Scholar]
- Oldroyd JCU. Randomised controlled trial evaluating the effectiveness of behavioural interventions to modify cardiovascular risk factors in men and women with impaired glucose tolerance: outcomes at 6 months. Diabetes Research and Clinical Practice 2001;?(1):29‐43. [DOI] [PubMed] [Google Scholar]
Orazio 2011 {published data only}
- Orazio LK, Isbel NM, Armstrong KA, Tarnarskyj J, Johnson DW, Hale RE, et al. Evaluation of dietetic advice for modification of cardiovascular disease risk factors in renal transplant recipients. Journal of Renal Nutrition 2011;21:462‐71. [DOI] [PubMed] [Google Scholar]
ORIGIN 2008 {published data only}
- Origin Trial I, Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). American Heart Journal 2008;155(1):26‐32, 32. [DOI] [PubMed] [Google Scholar]
Ornish 1990 {published data only}
- Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 1990;336:129‐33. [DOI] [PubMed] [Google Scholar]
Oslo Study 1980 {published data only}
- Hjerkinn EM, Sandvik L, Hjermann I, Arnesen H. Effect of diet intervention on long‐term mortality in healthy middle‐aged men with combined hyperlipidaemia. Journal of Internal Medicine 2004;255(1):68‐73. [DOI] [PubMed] [Google Scholar]
- Hjermann I. Intervention of smoking and eating habits in healthy men carrying high risk for coronary heart disease. The Oslo Study. Acta Medica Scandinavica. Supplementum 1981;651:281‐4. [DOI] [PubMed] [Google Scholar]
- Hjermann I. Smoking and diet intervention in healthy coronary high risk men. Methods and 5‐year follow‐up of risk factors in a randomized trial. The Oslo study. Journal of the Oslo City Hospitals 1980;30(1):3‐17. [PubMed] [Google Scholar]
- Hjermann I, Leren P, Norman N, Helgeland A, Holme I. Serum insulin response to oral glucose load during a dietary intervention trial in healthy coronary high risk men: the Oslo study. Scandinavian Journal of Clinical and Laboratory Investigation 1980;40(1):89‐94. [DOI] [PubMed] [Google Scholar]
- Hjermann I, Velve BK, Holme I, Leren P. Effect of diet and smoking intervention on the incidence of coronary heart disease. Report from the Oslo Study Group of a randomised trial in healthy men. Lancet 1981;2(8259):1303‐10. [DOI] [PubMed] [Google Scholar]
Otago Weight Loss 2005 {published and unpublished data}
- McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW, et al. Comparison of a high‐fat and high‐protein diets with a high‐carbohydrate diet in insulin‐resistant obese women. Diabetologia 2005;48:8‐16. [DOI] [PubMed] [Google Scholar]
- McAuley KA, Smith KJ, Taylor RW, McLay RT, Williams SM, Mann JI. Long‐term effects of popular dietary approaches on weight loss and features of insulin resistance. International Journal of Obesity 2006;30:342‐9. [DOI] [PubMed] [Google Scholar]
Pandey 2013 {published data only}
- Pandey RM, Agrawal A, Misra A, Vikram NK, Misra P, Dey S, et al. Population‐based intervention for cardiovascular diseases related knowledge and behaviours in Asian Indian women. Indian Heart Journal 2013;65:40‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pascale 1995 {published data only}
- Pascale RW, Wing RR, Butler BA, Mullen M, Bononi P. Effects of a behavioral weight loss program stressing calorie restriction versus calorie plus fat restriction in obese individuals with NIDDM or a family history of diabetes. Diabetes Care 1995;18(9):1241‐8. [DOI] [PubMed] [Google Scholar]
Paz‐Tal 2013 {published data only}
- Paz‐Tal O, Canfi A, Marko R, Katorza E, Karpas Z, Schwarzfuchs D, et al. Dynamics of magnesium, copper, selenium and zinc serum concentrations for 2‐year dietary intervention. e‐SPEN Journal 2013;8:e100‐7. [DOI] [PubMed] [Google Scholar]
PEP 2001 {published data only}
- Ohrig E, Geib HC, Haas G‐M, Schwandt P. The prevention education program (PEP) Nuremberg: design and baseline data of a family oriented intervention study. International Journal of Obesity 2001;25(Suppl 1):S89‐92. [DOI] [PubMed] [Google Scholar]
PHYLLIS 1993 {published data only}
- Anon. Plaque Hypertension Lipid‐Lowering Italian Study (PHYLLIS): a protocol for non‐invasive evaluation of carotid atherosclerosis in hypercholesterolaemic hypertensive subjects. Journal of Hypertension. Supplement 1993;11(Suppl 5):S314‐5. [PubMed] [Google Scholar]
- Bond GM, Crepaldi G, Zanchetti A, Avogaro P, Marubini E, Maseri A, et al. Plaque hypertension lipid‐lowering Italian study (PHYLLIS): a protocol for non‐invasive evaluation of carotid atherosclerosis in hypercholesterolaemic hypertensive subjects. Journal of Hypertension 1993;11(Suppl 5):S314‐5. [PubMed] [Google Scholar]
PREDIMED 2007 {published data only (unpublished sought but not used)}
- Buil‐Cosiales P, Irimia P, Ros E, Riverol M, Gilabert R, Martinez‐Vila E, et al. Dietary fibre intake is inversely associated with carotid intima‐media thickness: a cross‐sectional assessment in the PREDIMED study. European Journal of Clinical Nutrition 2009;63(10):1213‐9. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martinez‐Gonzalez MA, Corella D, Salas‐Salvado J, Ruiz‐Gutierrez V, Covas MI, et al. Effects of a Mediterranean‐style diet on cardiovascular risk factors: a randomized trial.[Summary for patients in Ann Intern Med. 2006 Jul 4;145(1):I11; PMID: 16818920]. Annals of Internal Medicine 2006;145(1):1‐11. [DOI] [PubMed] [Google Scholar]
- Razquin C, Martinez JA, Martinez‐Gonzalez MA, Mitjavila MT, Estruch R, Marti A, et al. A 3 years follow‐up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. European Journal of Clinical Nutrition 2009;63(12):1387‐93. [DOI] [PubMed] [Google Scholar]
- Salas‐Salvado J, Fernandez‐Ballart J, Ros E, Martinez‐Gonzalez MA, Fito M, Estruch R, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one‐year results of the PREDIMED randomized trial. Archives of Internal Medicine 2008;168(22):2449‐58. [DOI] [PubMed] [Google Scholar]
- Salas‐Salvado J, Garcia‐Arellano A, Estruch R, Marquez‐Sandoval F, Corella D, Fiol M, et al. Components of the Mediterranean‐type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. European Journal of Clinical Nutrition 2008;62(5):651‐9. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Tainta A, Estruch R, Bullo M, Corella D, Gomez‐Gracia E, Fiol M, et al. Adherence to a Mediterranean‐type diet and reduced prevalence of clustered cardiovascular risk factors in a cohort of 3,204 high‐risk patients. European Journal of Cardiovascular Prevention & Rehabilitation 2008;15(5):589‐93. [DOI] [PubMed] [Google Scholar]
- Schroder H, Torre R, Estruch R, Corella D, Martinez‐Gonzalez MA, Salas‐Salvado J, et al. Alcohol consumption is associated with high concentrations of urinary hydroxytyrosol. American Journal of Clinical Nutrition 2009;90(5):1329‐35. [DOI] [PubMed] [Google Scholar]
- Toledo E, Delgado‐Rodriguez M, Estruch R, Salas‐Salvado J, Corella D, Gomez‐Gracia E, et al. Low‐fat dairy products and blood pressure: follow‐up of 2290 older persons at high cardiovascular risk participating in the PREDIMED study. British Journal of Nutrition 2009;101(1):59‐67. [DOI] [PubMed] [Google Scholar]
- Waterhouse AL. "Resveratrol metabolites in urine as biomarker of wine intake in free‐living subjects: The PREDIMED Study". Free Radical Biology & Medicine 2009;46(12):1561. [DOI] [PubMed] [Google Scholar]
- Zamora‐Ros R, Urpi‐Sarda M, Lamuela‐Raventos RM, Estruch R, Martinez‐Gonzalez MA, Bullo M, et al. Resveratrol metabolites in urine as a biomarker of wine intake in free‐living subjects: The PREDIMED Study. Free Radical Biology & Medicine 2009;46(12):1562‐6. [DOI] [PubMed] [Google Scholar]
- Zazpe I, Estruch R, Toledo E, Sanchez‐Tainta A, Corella D, Bullo M, et al. Predictors of adherence to a Mediterranean‐type diet in the PREDIMED trial. European Journal of Nutrition 2010;49(2):91‐9. [DOI] [PubMed] [Google Scholar]
- Zazpe I, Sanchez‐Tainta A, Estruch R, Lamuela‐Raventos RM, Schroder H, Salas‐Salvado J, et al. A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean‐type diets: the PREDIMED study. Journal of the American Dietetic Association 2008;108(7):1134‐44. [DOI] [PubMed] [Google Scholar]
PREMIER 2003 {published and unpublished data}
- Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003;289(16):2083‐93. [DOI] [PubMed] [Google Scholar]
- Elmer PJ, Obarzanek E, Vollmer WM, Simons‐Morton D, Stevens VJ, Young DR, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18‐month results of a randomized trial. Annals of Internal Medicine 2006;144(7):485‐95. [DOI] [PubMed] [Google Scholar]
- Ledikwe JH, Rolls BJ, Smiciklas‐Wright H, Mitchell DC, Ard JD, Champagne C, et al. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. American Journal of Clinical Nutrition 2007;85(5):1212‐21. [DOI] [PubMed] [Google Scholar]
- Lien LF, Brown AJ, Ard JD, Loria C, Erlinger TP, Feldstein AC, et al. Effects of PREMIER lifestyle modifications on participants with and without the metabolic syndrome. Hypertension 2007;50(4):609‐16. [DOI] [PubMed] [Google Scholar]
- Lin PH, Appel LJ, Funk K, Craddick S, Chen C, Elmer P, et al. The PREMIER intervention helps participants follow the Dietary Approaches to Stop Hypertension dietary pattern and the current Dietary Reference Intakes recommendations. Journal of the American Dietetic Association 2007;107(9):1541‐51. [DOI] [PubMed] [Google Scholar]
- Lin PH, Wang Y, Grambow SC, Goggins W, Almirall D. Dietary saturated fat intake is negatively associated with weight maintenance among the PREMIER participants. Obesity 2012;20:571‐5. [DOI] [PubMed] [Google Scholar]
- Lin PH, Yancy WS Jr, Pollak KI, Dolor RJ, Marcello J, Samsa GP, et al. The influence of a physician and patient intervention program on dietary intake. Journal of the Academy of Nutrition & Dietetics 2013;113:1465‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation 2009;119(15):2026‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire HL, Svetkey LP, Harsha DW, Elmer PJ, Elmer PJ, Appel LJ, et al. Comprehensive lifestyle modification and blood pressure control: a review of the PREMIER trial. Journal of Clinical Hypertension (Greenwich., Conn.) 2004;6(7):383‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obarzanek E, Vollmer WM, Lin PH, Cooper LS, Young DR, Ard JD, et al. Effects of individual components of multiple behavior changes: the PREMIER trial. American Journal of Health Behavior 2007;31(5):545‐60. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Erlinger TP, Vollmer WM, Feldstein A, Cooper LS, Appel LJ, et al. Effect of lifestyle modifications on blood pressure by race, sex, hypertension status, and age. Journal of Human Hypertension 2005;19(1):21‐31. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Harsha DW, Vollmer WM, Stevens VJ, Obarzanek E, Elmer PJ, et al. Premier: a clinical trial of comprehensive lifestyle modification for blood pressure control: rationale, design and baseline characteristics. Annals of Epidemiology 2003;13(6):462‐71. [DOI] [PubMed] [Google Scholar]
Pritchard 2002 {published data only}
- Pritchard JE, Nowson CA, Billington T, Wark JD. Benefits of a year‐long workplace weight loss program on cardiovascular risk factors. Nutrition and Dietetics 2002;59(2):87‐96. [Google Scholar]
Puget Sound EP {published and unpublished data}
- Kristal AR, Curry SJ, Shattuck AL, Feng Z, Li S. A randomized trial of a tailored, self‐help dietary intervention: the Puget Sound Eating Patterns Study. Preventive Medicine 2000;31:380‐9. [DOI] [PubMed] [Google Scholar]
Rabast 1979 {published data only}
- Rabast U, Schonborn J, Kasper H. Dietetic treatment of obesity with low and high‐carbohydrate diets: comparative studies and clinical results. International Journal of Obesity 1979;3(3):201‐11. [PubMed] [Google Scholar]
Rabkin 1981 {published data only}
- Rabkin SW, Boyko E, Streja DA. Relationship of weight loss and cigarette smoking to changes in high‐density lipoprotein cholesterol. American Journal of Clinical Nutrition 1981;34:1764‐8. [DOI] [PubMed] [Google Scholar]
Radack 1990 {published data only}
- Radack K, Deck C, Huster G. The comparative effects of n‐3 and n‐6 polyunsaturated fatty acids on plasma fibrinogen levels: a controlled clinical trial in hypertriglyceridemic subjects. Journal of the American College of Nutrition 1990;9(4):352‐7. [DOI] [PubMed] [Google Scholar]
Rasmussen 1995 {published data only}
- Rasmussen OW, Thomsen CH, Hansen KW, Vesterlund M, Winther E, Hermansen K. Favourable effect of olive oil in patients with non‐insulin‐dependent diabetes. The effect on blood pressure, blood glucose and lipid levels of a high‐fat diet rich in monounsaturated fat compared with a carbohydrate‐rich diet [Gunstig virkning af olivenolie hos ikkeinsulinkraevende diabetikere. Virkningen pa blodtryk, blodglukose og lipidniveauer af en dioet med et hojt indhold af monoumoettet fedt sammenlignet med en kulhydratrig dioet]. Ugeskrift for Laeger 1995;157(8):1028‐32. [PubMed] [Google Scholar]
Reaven 2001 {published data only}
- Reaven GM, Abbasi F, Bernhart S, Coulston A, Darnell B, Dashti N, et al. Insulin resistance, dietary cholesterol, and cholesterol concentration in postmenopausal women. Metabolism: Clinical & Experimental 2001;50(5):594‐7. [DOI] [PubMed] [Google Scholar]
Reid 2002 {published data only}
- Reid R, Fodor G, Lydon‐Hassen K, D'Angelo MS, McCrea J, Bowlby M, et al. Dietary counselling for dyslipidemia in primary care: results of a randomized trial. Canadian Journal of Dietetic Practice & Research 2002;63(4):169‐75. [DOI] [PubMed] [Google Scholar]
Renaud 1986 {published data only}
- Renaud S, Godsey F, Dumont E, Thevenon C, Ortchanian E, Martin JL. Influence of long‐term diet modification on platelet function and composition in Moselle farmers. American Journal of Clinical Nutrition 1986;43:136‐50. [DOI] [PubMed] [Google Scholar]
Rivellese 2003 {published data only}
- Rivellese AA, Maffettone A, Vessby B, Uusitupa M, Hermansen K, Berglund L, et al. Effects of dietary saturated, monounsaturated and n‐3 fatty acids on fasting lipoproteins, LDL size and post‐prandial lipid metabolism in healthy subjects. Atherosclerosis 2003;167(1):149‐58. [DOI] [PubMed] [Google Scholar]
Roderick 1997 {published and unpublished data}
- Roderick P, Ruddock V, Hunt P, Miller G. A randomized trial to evaluate the effectiveness of dietary advice by practice nurses in lowering diet‐related coronary heart disease risk. British Journal of General Practice 1997;47(414):7‐12. [PMC free article] [PubMed] [Google Scholar]
Roman CHD prev 1986 {published data only}
- Anon. The Roman Coronary Disease Prevention Project: effectiveness of intervention and reduction of mortality over a 10‐year period [II Progetto Romano di Prevenzione della Cardiopatia Coronarica: efficacia dell'intervento e riduzione della mortalita in 10 anni]. Giornale Italiano di Cardiologia 1986;16(3):196‐202. [PubMed] [Google Scholar]
- Research Group of the Rome Project of Coronary Heart Disease Prevention. Eight‐year follow‐up results from the Rome Project of Coronary Heart Disease Prevention. Research Group of the Rome Project of Coronary Heart Disease Prevention. Preventive Medicine 1986;15(2):176‐91. [DOI] [PubMed] [Google Scholar]
Rose 1987 {published data only}
- Rose DP, Boyar AP, Cohen C, Strong LE. Effect of a low fat diet on hormone levels in women with cystic breast disease I Serum steroids and gonadotropins. Journal of the National Cancer Institute 1987;78:623‐6. [PubMed] [Google Scholar]
Rusu 2013 {published data only}
- Rusu E, Jinga M, Enache G, Rusu F, Dragomir AD, Ancuta I, et al. Effects of lifestyle changes including specific dietary intervention and physical activity in the management of patients with chronic hepatitis C‐‐a randomized trial. Nutrition Journal 2013;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu ED, Jinga M, Enache G, Rusu F, Dragomir A, Ancuta I, et al. Effects of the prudent diet versus low fat diet in cytokines profile in patients with diabetes and chronic hepatitis C. Diabetologia 2012;55:S361‐2. [Google Scholar]
Sacks 2009 {published and unpublished data}
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight‐loss diets with different compositions of fat, protein, and carbohydrates. New England Journal of Medicine 2009;360(9):859‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salas‐Salvado 2014 {published data only}
- Salas‐Salvado J, Bullo M, Estruch R, Ros E, Covas M I, Ibarrola‐Jurado N, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Annals of Internal Medicine 2014;160:1‐10. [DOI] [PubMed] [Google Scholar]
Sandstrom 1992 {published data only}
- Sandstrom B, Marckmann P, Bindslev N. An eight‐month controlled study of a low‐fat high‐fibre diet: effects on blood lipids and blood pressure in healthy young subjects. European Journal of Clinical Nutrition 1992;46(2):95‐109. [PubMed] [Google Scholar]
Sasaki 2000 {published data only}
- Sasaki S. Change and 1‐year maintenance of nutrient and food group intakes at a 12‐week worksite dietary intervention trial for men at high risk of coronary heart disease. Journal of Nutritional Science & Vitaminology 2000;46(1):15‐22. [DOI] [PubMed] [Google Scholar]
Schaefer 1995 {published data only}
- Schaefer EJ, Lichtenstein AH, Lamon‐Fava S, McNamara JR, Schaefer MM, Rasmussen H, et al. Body weight and low density lipoprotein cholesterol changes after consumption of a low fat ad libitum diet. JAMA 1995;274:1450‐5. [DOI] [PubMed] [Google Scholar]
Schaefer 1995A {published data only}
- Schaefer EJ, Lichtenstein AH, Lamon‐Fava S, Contois JH, Li Z, Rasmussen H, et al. Efficacy of a National Cholesterol Education Program Step 2 diet in normolipidaemic and hypercholesterolaemic middle‐aged and elderly men and women. Arteriosclerosis, Thrombosis, and Vascular Biology 1995;15:1079‐85. [DOI] [PubMed] [Google Scholar]
Schectman 1996 {published data only}
- Schectman G, Wolff N, Byrd JC, Hiatt JG, Hartz A. Physician extenders for cost‐effective management of hypercholesterolemia. Journal of General Internal Medicine 1996;11(5):277‐86. [DOI] [PubMed] [Google Scholar]
Schlierf 1995 {published data only}
- Schlierf G, Schuler G, Hambrecht R, Niebauer J, Hauer K, Vogel G, et al. Treatment of coronary heart disease by diet and exercise. Journal of Cardiovascular Pharmacology 1995;25 Suppl 4:S32‐4. [PubMed] [Google Scholar]
Seppanen‐Laakso {published data only}
- Seppanen‐Laakso T, Vanhanen H, Laakso I, Kohtamaki H, Viikari J. Replacement of butter on bread by rapeseed oil and rapeseed oil‐containing margarine: effects on plasma fatty acid composition and serum cholesterol. British Journal of Nutrition 1992;68:639‐54. [DOI] [PubMed] [Google Scholar]
Shai 2012 {published data only}
- Shai I. The effect of low‐carb, Mediterranean and low‐fat diets on renal function; a 2‐year dietary intervention randomized controlled trial (direct). Obesity Facts 2012;5:19. [Google Scholar]
- Shai I, Spence JD, Schwarzfuchs D, Henkin Y, Parraga G, Rudich A, et al. Dietary intervention to reverse carotid atherosclerosis. Circulation 2010;121:1200‐8. [DOI] [PubMed] [Google Scholar]
Singh 1990 {published data only}
- Singh RB, Sircar AR, Rastogi SS, Singh R. Dietary modulators of blood pressure in hypertension. European Journal of Clinical Nutrition 1990;44(4):319‐27. [PubMed] [Google Scholar]
Singh 1991 {published data only}
- Singh RB, Rastogi SS, Sircar AR. Dietary strategies for risk‐factor modification to prevent cardiovascular diseases. Nutrition 1991;7(3):210‐4. [PubMed] [Google Scholar]
Singh 1992 {published data only}
- Singh RB, Niaz MA, Agarwal P, Begom R, Rastogi SS. Effect of antioxidant‐rich foods on plasma ascorbic acid, cardiac enzyme, and lipid peroxide levels in patients hospitalized with acute myocardial infarction. Journal of the American Dietetic Association 1995;95(7):775‐80. [DOI] [PubMed] [Google Scholar]
- Singh RB, Niaz MA, Ghosh S. Effect on central obesity and associated disturbances of low‐energy, fruit‐ and vegetable‐enriched prudent diet in north Indians. Postgraduate Medical Journal 1994;70(830):895‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RB, Rastogi SS, Verma R, Bolaki L, Singh R. An Indian experiment with nutritional modulation in acute myocardial infarction. American Journal of Cardiology 1992;69(9):879‐85. [DOI] [PubMed] [Google Scholar]
- Singh RB, Rastogi SS, Verma R, Laxmi B, Singh R, Ghosh S, et al. Randomised controlled trial of cardioprotective diet in patients with recent acute myocardial infarction: results of one year follow up. BMJ 1992;304(6833):1015‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siqueira‐Catania 2010 {published data only}
- Siqueira‐Catania A Barros. Cardiometabolic benefits induced by lifestyle changes are mediated by inflammation in a Brazilian prevention program. Diabetes 2010;Conference:2010. [Google Scholar]
Sirtori 1992 {published data only}
- Sirtori CR, Gatti E, Tremoli E, Galli C, Gianfranceschi G, Franceschini G, et al. Olive oil, corn oil, and n‐3 fatty acids differently affect lipids, lipoproteins, platelets, and superoxide formation in type II hypercholesterolemia. American Journal of Clinical Nutrition 1992;56(1):113‐22. [DOI] [PubMed] [Google Scholar]
SLIM 2008 {published data only}
- Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE, et al. Impact of 3‐year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabetic Medicine 2008;25(5):597‐605. [DOI] [PubMed] [Google Scholar]
Sollentuna Diet {published and unpublished data}
- Hellenius M‐L. Prevention of cardiovascular disease: studies on the role of diet and exercise in the prevention of cardiovascular disease among middle‐aged men [PhD Thesis]. Huddinge, Sweden: Karolinska Institute, 1995. [Google Scholar]
- Hellenius M‐L, Krakau I, Faire U. Favourable long‐term effects from advice on diet and exercise given to healthy men with raised cardiovascular risks. Nutrition, Metabolism & Cardiovascular Diseases 1997;7:293‐300. [Google Scholar]
- Hellenius ML, Brismar KE, Berglund BH, de FU. Effects on glucose tolerance, insulin secretion, insulin‐like growth factor 1 and its binding protein, IGFBP‐1, in a randomized controlled diet and exercise study in healthy, middle‐aged men. Journal of Internal Medicine 1995;238(2):121‐30. [DOI] [PubMed] [Google Scholar]
- Hellenius ML, Dahlof C, Aberg H, Krakau I, de FU. Quality of life is not negatively affected by diet and exercise intervention in healthy men with cardiovascular risk factors. Quality of Life Research 1995;4(1):13‐20. [DOI] [PubMed] [Google Scholar]
- Hellenius ML, de FU, Berglund B, Hamsten A, Krakau I. Diet and exercise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis 1993;103(1):81‐91. [DOI] [PubMed] [Google Scholar]
- Naslund GK, Fredrikson M, Hellenius ML, de FU. Effect of diet and physical exercise intervention programmes on coronary heart disease risk in smoking and non‐smoking men in Sweden. Journal of Epidemiology and Community Health 1996;50(2):131‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sollentuna Diet & Ex {published and unpublished data}
- Hellenius M‐L. Prevention of cardiovascular disease: studies on the role of diet and exercise in the prevention of cardiovascular disease among middle‐aged men [PhD Thesis]. Huddinge, Sweden: Karolinska Institute, 1995. [Google Scholar]
- Hellenius M‐L, Krakau I, Faire U. Favourable long‐term effects from advice on diet and exercise given to healthy men with raised cardiovascular risks. Nutrition, Metabolism, and Cardiovascular Diseases 1997;7:293‐300. [Google Scholar]
- Hellenius ML, Brismar KE, Berglund BH, de FU. Effects on glucose tolerance, insulin secretion, insulin‐like growth factor 1 and its binding protein, IGFBP‐1, in a randomized controlled diet and exercise study in healthy, middle‐aged men. Journal of Internal Medicine 1995;238(2):121‐30. [DOI] [PubMed] [Google Scholar]
- Hellenius ML, Dahlof C, Aberg H, Krakau I, de FU. Quality of life is not negatively affected by diet and exercise intervention in healthy men with cardiovascular risk factors. Quality of Life Research 1995;4(1):13‐20. [DOI] [PubMed] [Google Scholar]
- Hellenius ML, de FU, Berglund B, Hamsten A, Krakau I. Diet and exercise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis 1993;103(1):81‐91. [DOI] [PubMed] [Google Scholar]
- Naslund GK, Fredrikson M, Hellenius ML, de FU. Effect of diet and physical exercise intervention programmes on coronary heart disease risk in smoking and non‐smoking men in Sweden. Journal of Epidemiology and Community Health 1996;50(2):131‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sopotsinskaia 1992 {published data only}
- Sopotsinskaia EB, Balitskii KP, Tarutinov VI, Zhukova VM, Semenchuk DD, Kozlovskaia SG, et al. Experience with the use of a low‐calorie diet in breast cancer patients to prevent metastasis [Opyt primeneniia nizkokaloriinoi diety u bol'nykh rakom molochnoi zhelezy s tsel'iu profilaktiki metastazi]. Voprosy Onkologii 1992;38(5):592‐9. [PubMed] [Google Scholar]
Staff HHP 1994 {published data only}
- Barratt A, Reznik R, Irwig L, Cuff A, Simpson JM, Oldenburg B, et al. Work‐site cholesterol screening and dietary intervention: the Staff Healthy Heart Project. Steering Committee. American Journal of Public Health 1994;84(5):779‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanford NAP 1997 {published data only}
- Howard PB, Winkleby MA, Albright CL, Bruce B, Fortmann SP. The Stanford Nutrition Action Program: a dietary fat intervention for low‐literacy adults. American Journal of Public Health 1997;87(12):1971‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanford Weight {published and unpublished data}
- Williams PT, Krauss RM, Stefanick ML, Vranizan KM, Wood PD. Effects of low‐fat diet, calorie restriction, and running on lipoprotein subfraction concentrations in moderately overweight men. Metabolism 1994;43(5):655‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Starmans 1995 {published data only}
- Starmans KM, Lustermans FT, Kragten HA, Struijker BH, Rilla H. Lowering cholesterol in patients with mild hypercholesterolaemia does not improve functional properties of large arteries [Abstract]. Netherlands Journal Of Medicine 1995;46:A70. [Google Scholar]
Steinbach 1996 {published data only}
- Steinbach M. A Romanian contribution to the epidemiology and prevention of cardiovascular diseases. Romanian Journal of Internal Medicine 1996;34(1‐2):137‐48. [PubMed] [Google Scholar]
Steptoe 2001 {published data only}
- Steptoe A, Kerry S, Rink E, Hilton S. The impact of behavioral counseling on stage of change in fat intake, physical activity, and cigarette smoking in adults at increased risk of coronary heart disease. American Journal of Public Health 2001;91(2):265‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2002 {published and unpublished data}
- Stevens VJ, Glasgow RE, Toobert DJ, Karanja N, Smith KS. One‐year results from a brief, computer‐assisted intervention to decrease consumption of fat and increase consumption of fruits and vegetables. Preventive Medicine 2003;36:594‐600. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Glasgow RE, Toobert DJ, Karanja N, Smith KS. Randomized trial of a brief dietary intervention to decrease consumption of fat and increase consumption of fruits and vegetables. American Journal of Health Promotion 2002;16(3):129‐34. [DOI] [PubMed] [Google Scholar]
Stevenson 1988 {published data only}
- Stevenson DW, Darga LL, Spafford TR, Ahmad N, Lucas CP. Variable effects of weight loss on serum lipids and lipoproteins in obese patients. International Journal of Obesity 1988;12:495‐502. [PubMed] [Google Scholar]
Sweeney 2004 {published data only}
- Sweeney M. Effects of very low‐fat diets on anginal symptoms. Medical Hypotheses 2004;63(3):553. [DOI] [PubMed] [Google Scholar]
TAIM 1989 {published data only}
- Davis BR, Blaufox MD, Hawkins CM, Langford HG, Oberman A, Swencionis C, et al. Trial of antihypertensive interventions and management. Design, methods, and selected baseline results. Controlled Clinical Trials 1989;10(1):11‐30. [DOI] [PubMed] [Google Scholar]
- Davis BR, Blaufox MD, Oberman A, Wassertheil SS, Zimbaldi N, Cutler JA, et al. Reduction in long‐term antihypertensive medication requirements. Effects of weight reduction by dietary intervention in overweight persons with mild hypertension. Archives of Internal Medicine 1993;153(15):1773‐82. [DOI] [PubMed] [Google Scholar]
- Davis BR, Oberman A, Blaufox MD, Wassertheil SS, Hawkins CM, Cutler JA, et al. Effect of antihypertensive therapy on weight loss. The Trial of Antihypertensive Interventions and Management Research Group. Hypertension 1992;19(4):393‐9. [DOI] [PubMed] [Google Scholar]
- Langford HG, Davis BR, Blaufox D, Oberman A, Wassertheil Smoller S, Hawkins M. Effect of drug and diet treatment of mild hypertension on diastolic blood pressure. The TAIM Research. Hypertension 1991;17(2):210‐7. [DOI] [PubMed] [Google Scholar]
- Oberman A, Wassertheil Smoller S, Langford HG, Blaufox MD, Davis BR, Blaszkowski T, et al. Pharmacologic and nutritional treatment of mild hypertension: changes in cardiovascular risk status. Annals of Internal Medicine 1990;112(2):89‐95. [DOI] [PubMed] [Google Scholar]
- Wassertheil Smoller S, Davis BR, Breuer B, Chee JC, Oberman A, Blaufox MD. Differences in precision of dietary estimates among different population subgroups. Annals of Epidemiology 1993;3:619‐28. [DOI] [PubMed] [Google Scholar]
- Wassertheil Smoller S, Oberman A, Blaufox MD, Davis B, Langford H. The Trial of Antihypertensive Interventions and Management (TAIM) Study. Final results with regard to blood pressure, cardiovascular risk, and quality of life. American Journal of Hypertension 1992;5(1):37‐44. [DOI] [PubMed] [Google Scholar]
- Wylie Rosett J, Wassertheil Smoller S, Blaufox MD, Davis BR, Langford HG, Oberman A, et al. Trial of antihypertensive intervention and management: greater efficacy with weight reduction than with a sodium‐potassium intervention. Journal of the American Dietetic Association 1993;93(4):408‐15. [DOI] [PubMed] [Google Scholar]
Take Heart II 1997 {published data only}
- Glasgow RE, Terborg JR, Strycker LK, Boles SM, Hollis JF. Take Heart II: Replication of a worksite health promotion trial. Journal of Behavioral Medicine 1997;20:143‐61. [DOI] [PubMed] [Google Scholar]
Tapsell 2004 {published data only (unpublished sought but not used)}
- Tapsell LC, Hokman A, Sebastiao A, Denmeade S, Martin G, Calvert GD, et al. The impact of usual dietary patterns, selection of significant foods and cuisine choices on changing dietary fat under 'free living' conditions. Asia Pacific Journal of Clinical Nutrition 2004;13(1):86‐91. [PubMed] [Google Scholar]
Taylor 1991 {published data only}
- Taylor CB, Fortmann SP, Flora J, Kayman S, Barrett DC, Jatulis D, et al. Effect of long‐term community health education on body mass index. The Stanford Five‐City Project. American Journal of Epidemiology 1991;134:235‐49. [DOI] [PubMed] [Google Scholar]
THIS DIET 2008 {published data only}
- Tuttle KR, Shuler LA, Packard DP, Milton JE, Daratha KB, Bibus DM, et al. Comparison of low‐fat versus Mediterranean‐style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). American Journal of Cardiology 2008;101(11):1523‐30. [DOI] [PubMed] [Google Scholar]
TOHP I 1992 {published data only}
- Anon. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA 1992;267(9):1213‐20. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Hebert PR, Cutler JA, Lasser VI, Sugars CP, Steffen Batey L, et al. Feasibility and efficacy of sodium reduction in the Trials of Hypertension Prevention, phase I. Trials of Hypertension Prevention Collaborative Research Group. Hypertension 1993;22(4):502‐12. [DOI] [PubMed] [Google Scholar]
- Satterfield S, Cutler JA, Langford HG, Applegate WB, Borhani NO, Brittain E, et al. Trials of hypertension prevention. Phase I design. Annals of Epidemiology 1991;1(5):455‐71. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, et al. Weight loss intervention in phase I of the trials of hypertension prevention. The TOHP Collaborative Research Group. Archives of Internal Medicine 1993;153(7):849‐58. [PubMed] [Google Scholar]
- Whelton PK, Hebert PR, Cutler J, Applegate WB, Eberlein KA, Klag MJ, et al. Baseline characteristics of participants in phase I of the Trials of Hypertension Prevention. Annals of Epidemiology 1992;2(3):295‐310. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Kumanyika SK, Cook NR, Cutler JA, Borhani NO, Hennekens CH, et al. Efficacy of nonpharmacologic interventions in adults with high‐normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. American Journal of Clinical Nutrition 1997;65(2 Suppl):652S‐60S. [DOI] [PubMed] [Google Scholar]
TONE 1997 {published data only}
- Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger‐WH J, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998;279(11):839‐46. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Babnson J, Appel LJ, Charleston J, Cosgrove N, Espeland MA, et al. Recruitment in the Trial of Nonpharmacologic Intervention in the Elderly (TONE). Journal of the American Geriatrics Society 1997;45(2):185‐93. [DOI] [PubMed] [Google Scholar]
Toobert 2003 {published data only}
- Toobert DJ, Glasgow RE, Strycker LA, Barrera M Jr, Radcliffe JL, Wander RC, et al. Biologic and quality‐of‐life outcomes from the Mediterranean Lifestyle Program: a randomized clinical trial. Diabetes Care 2003;26(8):2288‐93. [DOI] [PubMed] [Google Scholar]
Toronto Polyp Prev 1994 {published and unpublished data}
- McKeown‐Eyssen GE, Bright SE, Bruce WR, Jazmaji V. A randomized trial of a low fat high fibre diet in the recurrence of colorectal polyps. Toronto Polyp Prevention Group. Journal of Clinical Epidemiology 1994;47(5):525‐36. [DOI] [PubMed] [Google Scholar]
Towle 1994 {published data only}
- Towle LA, Bergman EA, Joseph E. Low‐fat bison‐hybrid ground meat has no effects on serum lipid levels in a study of 12 men. Journal of the American Dietetic Association 1994;94(5):546‐8. [DOI] [PubMed] [Google Scholar]
TRANSFACT 2006 {published data only}
- Chardigny JM, Malpuech‐Brugere C, Dionisi F, Bauman DE, German B, Mensink RP, et al. Rationale and design of the TRANSFACT project phase I: a study to assess the effect of the two different dietary sources of trans fatty acids on cardiovascular risk factors in humans. Contemporary Clinical Trials 2006;27(4):364‐73. [DOI] [PubMed] [Google Scholar]
- Chardigny JMD. Do trans fatty acids from industrially produced sources and from natural sources have the same effect on cardiovascular disease risk factors in healthy subjects? Results of the trans Fatty Acids Collaboration (TRANSFACT) study. American Journal of Clinical Nutrition 2008;108(3):558‐66. [DOI] [PubMed] [Google Scholar]
Treatwell 1992 {published and unpublished data}
- Sorensen G, Morris DM, Hunt MK, Hebert JR, Harris DR, Stoddard A, et al. Work‐site nutrition intervention and employees' dietary habits: the Treatwell program. American Journal of Public Health 1992;82(6):877‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tromso Heart 1989 {published data only}
- Knutsen SF, Knutsen R. The Tromso Heart Study: family approach to intervention on CHD. Feasibility of risk factor reduction in high‐risk persons‐‐project description. Scandinavian Journal of Social Medicine 1989;17:109‐19. [DOI] [PubMed] [Google Scholar]
Turku Weight {published and unpublished data}
- Hakala P, Karvetti RL. Weight reduction on lactovegetarian and mixed diets. European Journal of Clinical Nutrition 1989;43:421‐30. [PubMed] [Google Scholar]
- Marniemi J, Seppanen A, Hakala P. Long‐term effects on lipid metabolism of weight reduction on lactovegetarian and mixed diet. International Journal of Obesity 1990;14:113‐25. [PubMed] [Google Scholar]
Turpeinen 1960 {published data only}
- Turpeinen O, Roine P, Pekkarinen M, Karvonen MJ, Rautanen Y, Runeberg J, et al. Effect on serum‐cholesterol level of replacement of dietary milk fat by soybean oil. Lancet 1960;1:196‐8. [DOI] [PubMed] [Google Scholar]
UK PDS 1996 {published data only}
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9‐year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non‐insulin‐dependent diabetes mellitus. Annals of Internal Medicine 1996;124(1 Pt 2):136‐45. [DOI] [PubMed] [Google Scholar]
- Turner RC, Holman RR. Lessons from UK prospective diabetes study. Diabetes Research and Clinical Practice 1995;28 Suppl:S151‐7. [DOI] [PubMed] [Google Scholar]
Urbach 1952 {published data only}
- Urbach R, Hildreth EA, Wackerman MT. The therapeutic uses of low fat, low cholesterol diets: I. Treatment of essential familial xanthomatosis. Journal of Clinical Nutrition 1952;1:52‐6. [DOI] [PubMed] [Google Scholar]
Uusitupa 1993 {published data only}
- Uusitupa M, Laitinen J, Siitonen O, Vanninen E, Pyorala K. The maintenance of improved metabolic control after intensified diet therapy in recent type 2 diabetes. Diabetes Research and Clinical Practice 1993;19(3):227‐38. [DOI] [PubMed] [Google Scholar]
Uusitupa 2013 {published data only}
- Uusitupa M, Hermansen K, Savolainen M J, Schwab U, Kolehmainen M, Brader L, et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome ‐ a randomized study (SYSDIET). Journal of Internal Medicine 2013;274:52‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vavrikova 1958 {published data only}
- Vavrikova J. Essential fatty acids, lipid metabolism, and atherosclerosis [letter]. Lancet 1958;1:1337. [DOI] [PubMed] [Google Scholar]
Wan 2013 {published data only}
- Wan Abdul Manan WMW. The effects of physical activity and dietary management in adults with metabolic syndrome in a rural district in Malaysia: An intervention study. Annals of Nutrition and Metabolism 2013;Conference:2013. [Google Scholar]
Wass 1981 {published data only}
- Wass VJ, Jarrett RJ, Meilton V, Start MK, Mattock M, Ogg CS, et al. Effect of a long‐term fat‐modified diet on serum lipoprotein levels of cholesterol and triglyceride in patients on home haemodialysis. Clinical Science 1981;60(1):81‐6. [DOI] [PubMed] [Google Scholar]
Wassertheil 1985 {published data only}
- Wassertheil SS, Blaufox MD, Langford HG, Oberman A, Cutter G, Pressel S. Prediction of response to sodium intervention for blood pressure control. Journal of Hypertension. Supplement 1986;4(5):S343‐6. [PubMed] [Google Scholar]
- Wassertheil SS, Langford HG, Blaufox MD, Oberman A, Hawkins M, Levine B, et al. Effective dietary intervention in hypertensives: sodium restriction and weight reduction. Journal of the American Dietetic Association 1985;85(4):423‐30. [PubMed] [Google Scholar]
WATCH {published and unpublished data}
- Ockene IS, Hebert JR, Ockene JK, Saperia GM, Stanek E, Nicolosi R, et al. Effect of a physician‐delivered nutrition counselling training and an office‐support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia. Archives of Internal Medicine 1999;159:725‐31. [DOI] [PubMed] [Google Scholar]
Watts 1988 {published data only}
- Watts GF, Ahmed W, Quiney J, Houlston R, Jackson P, Iles C, et al. Effective lipid lowering diets including lean meat. British Medical Journal (Clinical Research Ed.) 1988;296(6617):235‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weintraub 1992 {published data only}
- Weintraub M, Sundaresan PR, Schuster B. Long‐term weight control study. VII (weeks 0 to 210). Serum lipid changes. Clinical Pharmacology and Therapeutics 1992;51(5):634‐41. [DOI] [PubMed] [Google Scholar]
Westman 2006 {published data only}
- Westman EC, Yancy WS Jr, Olsen MK, Dudley T, Guyton JR, Westman Eric C, et al. Effect of a low‐carbohydrate, ketogenic diet program compared to a low‐fat diet on fasting lipoprotein subclasses. International Journal of Cardiology 2006;110(2):212‐6. [DOI] [PubMed] [Google Scholar]
Weststrate 1998 {published data only}
- Weststrate JA, Meijer GW. Plant sterol enriched margarines and reduction of plasma total‐and LDL‐cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. European Journal of Clinical Nutrition 1998;52:334‐43. [DOI] [PubMed] [Google Scholar]
WHO primary prev 1979 {published data only}
- Anon. Primary prevention of ischaemic heart disease: WHO coordinated cooperative trial. A summary report. Bulletin Of The World Health Organization 1979;57:801‐5. [PMC free article] [PubMed] [Google Scholar]
WHT {published and unpublished data}
- Bowen D. The role of participation in the women's health trial: feasibility study in minority populations. Preventive Medicine 2000;31(5):474‐80. [DOI] [PubMed] [Google Scholar]
- Bowen D, Clifford CK, Coates R, Evans M, Feng Z, Fouad M, et al. The Women's Health Trial Feasibility Study in Minority Populations: design and baseline descriptions. Annals of Epidemiology 1996;6(6):507‐19. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Kestin M, McTiernan A, Carrell D, Green P. Effects of dietary fat intervention on mental health in women. Cancer Epidemiology, Biomarkers and Prevention 1995;4(5):555‐9. [PubMed] [Google Scholar]
- Gorbach SL, Morrill LA, Woods MN, Dwyer JT, Selles WD, Henderson M, et al. Changes in food patterns during a low‐fat dietary intervention in women. Journal of the American Dietetic Association 1990;90(6):802‐9. [PubMed] [Google Scholar]
- Henderson MM, Kushi LH, Thompson DJ, Gorbach SL, Clifford CK, Insull W, et al. Feasibility of a randomized trial of a low‐fat diet for the prevention of breast cancer: dietary compliance in the Women's Health Trial Vanguard Study. Preventive Medicine 1990;19(2):115‐33. [DOI] [PubMed] [Google Scholar]
- Insull W, Henderson MM, Prentice RL, Thompson DJ, Clifford C, Goldman S, et al. Results of a randomized feasibility study of a low‐fat diet. Archives of Internal Medicine 1990;150(2):421‐7. [PubMed] [Google Scholar]
- Kristal AR, White E, Shattuck AL, Curry S, Anderson GL, Fowler A, et al. Long‐term maintenance of a low‐fat diet: durability of fat‐related dietary habits in the Women's Health Trial. Journal of the American Dietetic Association 1992;92(5):553‐9. [PubMed] [Google Scholar]
- Prentice RL, Kakar F, Hursting S, Sheppard L, Klein R, Kushi LH. Aspects of the rationale for the Women's Health Trial. Journal of the National Cancer Institute 1988;80(11):802‐14. [DOI] [PubMed] [Google Scholar]
- Self S, Prentice R, Iverson D, Henderson M, Thompson D, Byar D, et al. Statistical design of the Women's Health Trial. Controlled Clinical Trials 1988;9(2):119‐36. [DOI] [PubMed] [Google Scholar]
- Sheppard L, Kristal AR, Kushi LH. Weight loss in women participating in a randomised trial of low‐fat diets. American Journal of Clinical Nutrition 1991;54:821‐8. [DOI] [PubMed] [Google Scholar]
- Urban N, Baker M. The Women's Health Trial as an investment. Medical Decision Making 1989;9(1):59‐64. [DOI] [PubMed] [Google Scholar]
- White E, Shattuck AL, Kristal AR, Urban N, Prentice RL, Henderson MM, et al. Maintenance of a low‐fat diet: follow‐up of the Women's Health Trial. Cancer Epidemiology, Biomarkers and Prevention 1992;1(4):315‐23. [PubMed] [Google Scholar]
Wilke 1974 {published data only}
- Wilke H, Frahm H. Influence of low‐caloric‐diet and d‐triiodothyronine on serum lipids and body weight (author's trans) [Verhalten der Serumlipide und des Korpergewichts unter Reduktionsdiat und medikamentoser Behandlung mit D‐Trijodthyronin]. Medizinische Klinik 1974;69(48):1986‐9. [PubMed] [Google Scholar]
Williams 1990 {published data only}
- Williams PT, Krauss RM, Vranizan KM, Wood PS. Changes in lipoprotein subfractions during diet‐induced and exercise‐induced weight loss in moderately overweight men. Circulation 1990;81:1293‐304. [DOI] [PubMed] [Google Scholar]
Williams 1992 {published data only}
- Williams PT, Krauss RM, Vranizan KM, Albers JJ, Wood PD. Effects of weight‐loss by exercise and by diet on apolipoproteins A‐I and A‐II and the particle‐size distribution of high‐density lipoproteins in men. Metabolism: Clinical and Experimental 1992;41:441‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1994 {published data only}
- Williams PT, Stefanick ML, Vranizan KM, Wood PD. The effects of weight loss by exercise or by dieting on plasma high‐density lipoprotein (HDL) levels in men with low, intermediate, and normal‐to‐high HDL at baseline. Metabolism 1994;43(7):917‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilmot 1952 {published data only}
- Wilmot VA, Swank RL. The influence of low fat diet on blood lipid levels in health and in multiple sclerosis. American Journal of the Medical Sciences 1952;223:25‐34. [DOI] [PubMed] [Google Scholar]
Wing 1998 {published data only}
- Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21(3):350‐9. [DOI] [PubMed] [Google Scholar]
Wolever 2008 {published data only}
- Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1‐y controlled trial of low‐glycemic‐index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C‐reactive protein. American Journal of Clinical Nutrition 2008;87(1):114‐25. [DOI] [PubMed] [Google Scholar]
WOMAN 2007 {published data only}
- Kuller LH, Kriska AM, Kinzel LS, Simkin‐Silverman LR, Sutton‐Tyrrell K, Johnson BD, et al. The clinical trial of Women On the Move through Activity and Nutrition (WOMAN) study. Contemporary Clinical Trials 2007;28(4):370‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 1988 {published data only}
- Wood PD, Stefanick ML, Dreon DM, Frey HB, Garay SC, Williams PT, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. New England Journal of Medicine 1988;319(18):1173‐9. [DOI] [PubMed] [Google Scholar]
Woollard 2003 {published data only}
- Woollard J, Burke V, Beilin LJ, Verheijden M, Bulsara MK. Effects of a general practice‐based intervention on diet, body mass index and blood lipids in patients at cardiovascular risk. Journal of Cardiovascular Risk 2003;10(1):31‐40. [DOI] [PubMed] [Google Scholar]
Working Well 1996 {published data only}
- Sorensen G, Thompson B, Glanz K, Feng Z, Kinne S, DiClemente C, et al. Work site‐based cancer prevention: primary results from the Working Well Trial. American Journal of Public Health 1996;86(7):939‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2010 {published data only}
- Young DR, Coughlin J, Jerome GJ, Myers V, Chae SE, Brantley PJ, et al. Effects of the PREMIER interventions on health‐related quality of life. Annals of Behavioral Medicine 2010;40:302‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zock 1995 {published and unpublished data}
- Zock PL. Dietary fatty acids and risk factors for coronary heart disease: controlled studies in healthy volunteers. PhD Thesis 1995.
- Zock PL, Mensink RP, Harryvan J, de VJ, Katan MB. Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. American Journal of Epidemiology 1997;145(12):1114‐22. [DOI] [PubMed] [Google Scholar]
Additional references
Ajala 2013
- Ajala O, English P, Pinkney J. Systematic review and meta‐analysis of different dietary approaches to the management of type 2 diabetes. American Journal of Clinical Nutrition 2013;97:505‐16. [DOI] [PubMed] [Google Scholar]
Aljadani 2013
- Aljadani H, Patterson A, Sibbritt D, Collins C. The association between diet quality and weight change in adults over time: a systematic review of prospective cohort studies. Diet Quality: An Evidence Based Approach. 2. New York: Springer, 2013:3‐27. [DOI: 10.1007/978-1-4614-7315-2_1] [DOI] [Google Scholar]
Aljadani 2015
- Aljadani H, Patterson A, Sibbritt D, Collins CE. Diet quality and weight change in adults over time: a systematic review of cohort studies. Current Nutrition Reports 2015;4:88‐101. [Google Scholar]
Ambrosini 2014
- Ambrosini GL. Childhood dietary patterns and later obesity: a review of the evidence. Proceedings of the Nutrition Society 2014;73:137‐46. [DOI] [PubMed] [Google Scholar]
Benatar 2013
- Benatar JR, Sidhu K, Stewart RA, Benatar JR, Sidhu K, Stewart RAH. Effects of high and low fat dairy food on cardio‐metabolic risk factors: a meta‐analysis of randomized studies. PloS One 2013;8:e76480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkley 1995
- Berkley CS, Hoaglin DC, Mosteller F, Colditz GA. A random‐effects regression model for meta‐analysis. Statistics in Medicine 1995;14:395‐411. [DOI] [PubMed] [Google Scholar]
Chaput 2014
- Chaput JP. Findings from the Quebec Family Study on the Etiology of Obesity: Genetics and Environmental Highlights. Current Obesity Reports 2014;3:54‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Watanabe N, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta‐analyses. JAMA 2007;297:468‐70. [DOI] [PubMed] [Google Scholar]
Gow 2014
- Gow ML, Ho M, Burrows TL, Baur LA, Stewart L, Hutchesson MJ, et al. Impact of dietary macronutrient distribution on BMI and cardiometabolic outcomes in overweight and obese children and adolescents: a systematic review. Nutrition Reviews 2014;72:453‐70. [DOI] [PubMed] [Google Scholar]
Havranek 2011
- Havranek EP. A Mediterranean diet reduces cardiovascular risk factors in overweight patients compared with a low‐fat diet. ACP Journal Club 2011;155(12):JC6‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Available from www.cochrane‐handbook.org: The Cochrane Collaboration.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hooper 2012a
- Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore HJ, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooper 2015
- Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011737] [DOI] [PubMed] [Google Scholar]
Hu 2012
- Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS Jr, et al. Effects of low‐carbohydrate diets versus low‐fat diets on metabolic risk factors: a meta‐analysis of randomized controlled clinical trials. American Journal of Epidemiology 2012;176 Suppl 7:S44‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joint ESC guidelines 2012
- The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). European Heart Journal 2012;33:1635‐701. [DOI: 10.1093/eurheartj/ehs092] [DOI] [PubMed] [Google Scholar]
Kelly 2006
- Kelly S, Hillier F, Whittaker V, Ells LJ, Edmunds LD, Smith S, et al. The associations between food, nutrition, physical activity and the risk of weight gain, overweight and obesity and underlying mechanisms: systematic literature review. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective (www.dietandcancerreport.org/cancer_resource_center/downloads/SLR/Obesity_SLR.pdf.5). World Cancer Research Fund/American Institute for Cancer Research, 2006. [Google Scholar]
Kratz 2013
- Kratz MB. The relationship between high‐fat dairy consumption and obesity, cardiovascular, and metabolic disease. European Journal of Nutrition 2013;52:1‐24. [DOI] [PubMed] [Google Scholar]
Manson 1990
- Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, et al. A prospective study of obesity and risk of coronary heart disease in women. New England Journal of Medicine 1990;322:882‐9. [DOI: 10.1056/NEJM199003293221303] [DOI] [PubMed] [Google Scholar]
Ni 2010
- Ni MC, Aston LM, Jebb SA. Effects of worksite health promotion interventions on employee diets: a systematic review. BMC Public Health 2010;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schwingshackl 2013
- Schwingshackl L, Hoffmann G. Comparison of effects of long‐term low‐fat vs high‐fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta‐analysis. Journal of the Academy of Nutrition & Dietetics 2013;113:1640‐61. [DOI] [PubMed] [Google Scholar]
Schwingshackl 2013a
- Schwingshackl L, Hoffmann G. Long‐term effects of low‐fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta‐analysis. Nutrition Journal 2013;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 1998
- Sharp S. Meta‐analysis regression. Stats Technical Bulletin 1998;42:16‐22. [Google Scholar]
Song 2004
- Song Y‐M, Sung J, Davey Smith G, Ebrahim S. Body mass index and ischemic and hemorrhagic stroke: a prospective study in Korean men. Stroke 2004;35:831‐6. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Bradburn MJ, Egger M. Meta‐analysis in STATA. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. London: BMJ Books, 2001. [Google Scholar]
Sterne 2009
- Sterne JAC. Meta‐analysis in Stata: an Updated Collection from the Stata Journal. Texas, USA: STATA Press, 2009. [Google Scholar]
WCRF/AICR 2009
- World Cancer Research Fund/American Institute for Cancer Research. Preventability of cancer by food, nutrition, and physical activity: Appendix A. Policy and Action for Cancer Prevention. Food, Nutrition, and Physical Activity: a Global Perspective. Washington DC: AICR, 2009. [Google Scholar]
Yang 2013
- Yang Z, Huffman SL. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Maternal & Child Nutrition 2013;9(Suppl 1):105‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu‐Poth 1999
- Yu‐Poth S, Zhao G, Etherton T, Naglak M, Jonnalagadda S, Kris‐Etherton PM. Effects of the National Cholesterol Education Program's Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta‐analysis. American Journal of Clinical Nutrition 1999;69:632‐46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hooper 2000
- Hooper L, Summerbell CD, Higgins JPT, Thompson RL, Clements G, Capps N, et al. Reduced or modified dietary fat for prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002137] [DOI] [PubMed] [Google Scholar]
Hooper 2001
- Hooper L, Summerbell CD, Higgins JPT, Thompson RL, Capps N, Davey Smith G, et al. Dietary fat intake and prevention of cardiovascular disease: systematic review. BMJ 2001;322:757‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooper 2012b
- Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta‐analysis of randomised controlled trials and cohort studies. BMJ 2012;345:e7666. [DOI: 10.1136/bmj.e7666] [DOI] [PMC free article] [PubMed] [Google Scholar]


