Abstract
Aims
After an ischaemic stroke, atrial fibrillation (AF) detection allows for improved secondary prevention strategies. This study aimed to compare AF detection and oral anticoagulant (OAC) initiation in patients with an insertable cardiac monitor (ICM) vs. external cardiac monitor (ECM) after ischaemic stroke.
Methods and results
Medicare Fee-for-Service (FFS) insurance claims and Abbott Labs device registration data were used to identify patients hospitalized with an ischaemic stroke in 2017–2019 who received an ICM or ECM within 3 months. Patients with continuous Medicare FFS insurance and prescription drug enrolment in the prior year were included. Patients with prior AF, atrial flutter, cardiac devices, or OAC were excluded. Insertable cardiac monitor and ECM patients were propensity score matched 1:4 on demographics, comorbidities, and stroke hospitalization characteristics. The outcomes of interest were AF detection and OAC initiation evaluated with Kaplan–Meier and Cox proportional hazard regression analyses. A total of 5702 Medicare beneficiaries (ICM, n = 444; ECM, n = 5258) met inclusion criteria. The matched cohort consisted of 2210 Medicare beneficiaries (ICM, n = 442; ECM, n = 1768) with 53% female, mean age 75 years, and mean CHA₂DS₂-VASc score 4.6 (1.6). Insertable cardiac monitor use was associated with a higher probability of AF detection [(hazard ratio (HR) 2.88, 95% confidence interval (CI) (2.31, 3.59)] and OAC initiation [HR 2.91, CI (2.28, 3.72)] compared to patients monitored only with ECM.
Conclusion
Patients with an ischaemic stroke monitored with an ICM were almost three times more likely to be diagnosed with AF and to be prescribed OAC compared to patients who received ECM only.
Keywords: Stroke, Atrial fibrillation, Oral anticoagulation, Insertable cardiac monitors
Graphical Abstract
Graphical Abstract.
Structured Graphical Abstract graphic element shows 2-year atrial fibrillation incident rates by cardiac monitor method, ICM (solid line) and ECM (dashed line). Rates were estimated using the Kaplan–Meier method. Hazard ratios and 95% CIs estimated by Cox proportional hazard models are also shown.
What’s new?
Key question
How do atrial fibrillation (AF) detection and oral anticoagulation (OAC) rates compare between patients with an insertable cardiac monitor (ICM) vs. external cardiac monitor (ECM) after ischaemic stroke in a real-world population?
Key finding
Insertable cardiac monitor use was associated with a higher probability of AF detection [(hazard ratio (HR) 2.88, 95% confidence interval (CI) (2.31, 3.59)] and OAC initiation (HR 2.91, CI (2.28, 3.72)] compared to patients monitored only with ECM.
Take-home message
In a large real-world cohort of patients with stroke of unknown cause, our results highlight that ICMs provide effective AF detection and have increased OAC therapy, with almost three times more AF and OAC rates compared to ECMs.
Introduction
Cerebral thromboembolism related to atrial fibrillation (AF) is responsible for up to a third of ischaemic strokes, a proportion that increases further with age.1 Moreover, in up to a quarter of ischaemic strokes or transient ischaemic attacks (TIAs), the cerebral ischaemia constitutes the first clinical documentation of AF, since the arrhythmia was asymptomatic and previously undetected or unrecognized.2–4 Since AF detection leads to improved secondary prevention strategies, such as prescription of oral anticoagulants (OAC),5–9 one important step of post-stroke care is AF monitoring whenever cardioembolic mechanism is suspected or the stroke remains ‘cryptogenic’.3,10 A common approach to cardiac monitoring after stroke is telemetry during the initial inpatient stay followed by 24–48 h or extended duration (7–30 days) ambulatory monitoring.1,10 However, the American Heart Association/American Stroke Association guidelines for the prevention of recurrent stroke recommend long-term rhythm monitoring to detect AF in patients with cryptogenic stroke (IIa recommendation).11
Insertable cardiac monitors (ICMs) or cardiac electronic devices with atrial sensing capabilities allow continuous monitoring and have extended the capability to detect AF.1,12,13 In CRYSTAL AF, a randomized controlled study, that included 441 patients (mean age 62 years) with cryptogenic stroke, long-term monitoring with an ICM was more effective than conventional follow-up (control) for detecting AF.14 Clinical practice and patient characteristics in the community often differ from controlled clinical trials; therefore, it is important to study the diagnostic role of ICM in a real-world patient population.
The aim of this study was to compare AF detection and OAC initiation in patients followed with ICM vs. intermittent monitoring systems, in US patients who had been hospitalized with an ischaemic stroke.
Methods
Study design and data sources
We performed a retrospective observational study with Medicare Fee-for-Service (FFS) insurance claims linked with Abbott Laboratories device registration data. Medicare claims included inpatient, outpatient, carrier claims, Part D prescription drug fill records, and Master Beneficiary Summary Files (MBSF). The inpatient and outpatient files contained institutional claims for hospital inpatient services and outpatient services, respectively. The carrier files contained non-institutional provider claims for services rendered in any setting. Each of these files included dates of service, diagnosis, and procedure codes. The prescription drug fill records contain information on medications that were paid under Medicare Part D, which is voluntary insurance coverage for outpatient prescription drugs. Master Beneficiary Summary Files contained information on demographics, birth and death dates, Medicare eligibility, and enrolment. Medicare FFS data were available through 31 December 2020, whilst Part D data were available through 31 December 2019. The Abbott device registration database contained patient-level date of birth, sex, device type, implantation dates, implanting facility, and reason for ICM implant.
The study was conducted as a retrospective analysis of de-identified data. We requested and were granted a full waiver of informed consent and a HIPAA waiver from Western IRB for this study.
Study population
The study population included Medicare FFS beneficiaries who received an ICM (Confirm Rx™ Abbott, USA) or an external cardiac monitor (ECM) between 15 November 2017 and 31 December 2019 and had been hospitalized with an ischaemic stroke in the prior 3 months. Supplementary material online, Table S1, in the supplement contains the International Classification of Diseases Tenth Revision (ICD-10) codes used to select patients with ischaemic stroke.
The ECM patients (control group) were identified in Medicare claims with procedure codes for Holter monitor, outpatient cardiac telemetry, or memory loop event monitor. The ECM index date was the first post-stroke date with an ECM procedure code.
Patients who received an ICM were identified from Abbott Labs device registration data and Medicare data that were linked using probabilistic linking methods.15 Briefly, we analysed Medicare claims data to identify patients who received a cardiac insertable electronic device using Current Procedural Terminology® (CPT) Fourth Edition, Healthcare Common Procedure Coding System (HCPCS), and ICD-10 procedure codes. We then linked those Medicare implant records to the Abbott database using patient date of birth, sex, device type, implantation dates, and implanting facility and selected matches based on best agreement between data sources. The ICM index date was the first post-stroke date with an ICM implant (procedure date from Medicare claims). Insertable cardiac monitor patients who first had an ECM post-stroke were placed in the ICM group and not the ECM group.
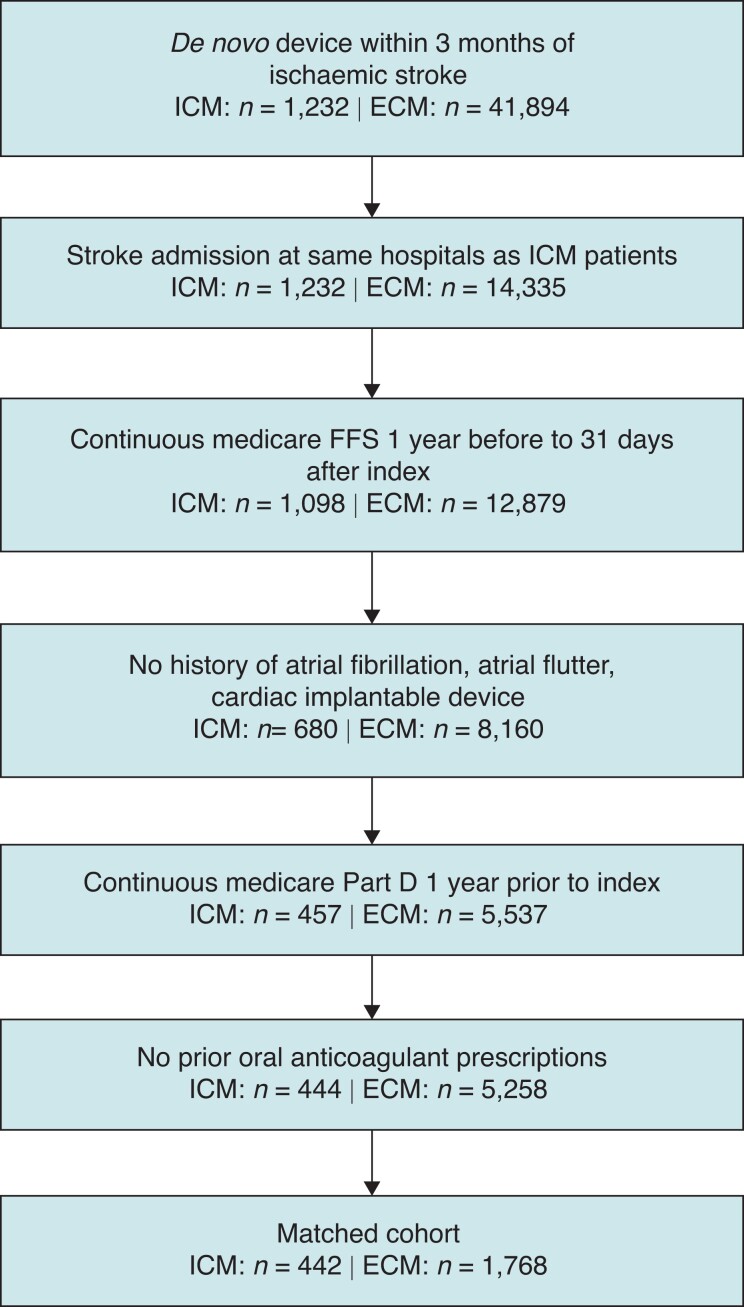
Patients were included in the study if they had continuous Medicare FFS insurance, Part D insurance enrolment, and no Medicare Advantage enrolment between 12-month pre-index and 31-day post-index. Additionally, ECM patients were only included in the study if they were admitted to a hospital where an ICM patient was also admitted for stroke. As ICD-10 diagnosis codes do not specify whether the stroke was cryptogenic, we further excluded patients whose stroke was unlikely cryptogenic by excluding those with a history of atrial tachyarrhythmias, OAC prescriptions, or cardiac implantable electronic devices (including prior ICMs) at index or in the 12 months prior. Data from Abbott ICM device registration database provided information about the reason for ICM implant for a subset of patients in the ICM cohort. See Figure 1 for cohort diagram. See Supplementary material online, Table S1, in supplement for cohort selection diagnosis and procedure codes.
Figure 1.
Cohort diagram.
ECM, external cardiac monitor; ICM, insertable cardiac monitor.
Outcome measures
The outcomes of interest were AF detection and OAC initiation. We identified AF in claims data when at least one inpatient or two hospital outpatient or physician claims with AF diagnosis codes in the first or second positions on the claim were found. The following ICD-10 AF diagnosis codes were included: I48.0, I48.1, I48.11, I48.19, I48.2, I48.20, I48.21, and I48.91. When comparing AF detection between the groups, we considered AF detected after first ICM implant or after first ECM monitoring. Patients in both groups were censored if they had a new cardiac implant or had an ICM explant. Patients were not censored if they received repeat ECM monitoring, so we continued to follow ECM patients for an AF diagnosis whether they were monitored once or more than once. Available Medicare diagnosis data allowed for patients to be followed for 2 years following the index date; therefore, AF was assessed at 2 years following the index date, and patients were censored after the earliest of the following events: (i) 2 years after the index date, (ii) the end of Medicare FFS claims data availability (31 December 2020), (iii) the date when enrolment in Medicare FFS ended, (iv) new cardiac implantable electronic device or ICM explant, or (v) death.
Oral anticoagulant initiation is defined as a prescription drug fill record with one of the following drugs: warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban. Available Medicare prescription fill data allowed for patients to be followed for 1.5 years following index date; therefore, OAC was assessed at 1.5 years following the index date, and patients were censored after the earliest of the following events: (i) 1.5 years after the index date, (ii) the end of Part D claims data availability (31 December 2019), (ii) the date when enrolment in Part D ended, (iv) new cardiac implantable electronic device or ICM explant, or (v) death.
Statistical analysis
Insertable cardiac monitor and ECM patients were propensity score matched, with a 1:4 ratio, on baseline characteristics obtained from Medicare claims 12 months prior to index, including demographics (age, sex, and race/ethnicity), comorbidities (listed in Table 1 and in Supplementary material online, Table S2, in supplement with diagnosis codes), stroke hospitalization (length of stay, time between stroke, and index), and index year. Individual components of the CHA₂DS₂-VASc score were used for matching rather than the score itself. Matching was done without replacement using the greedy nearest-neighbour matching method with calliper 0.2. After matching, balance between baseline characteristics was evaluated with the standardized mean difference (SMD) test statistic, where less than 0.10 SMD was considered as achieving balance. For baseline characteristics, categorical variables are presented as frequencies with percentages and continuous variables as means with standard deviations or medians with interquartile range (IQR).
Table 1.
Characteristics of Medicare beneficiaries using an ICM or an ECM after an ischaemic stroke
| Unmatched | Unmatched | SMD | Matched | Matched | SMD | |
|---|---|---|---|---|---|---|
| ICM | ECM | ICM | ECM | |||
| n = 444 | n = 5258 | n = 442 | n = 1768 | |||
| n (%) | n (%) | n (%) | n (%) | |||
| Age, mean (SD) | 74.7 (9.0) | 75.0 (9.4) | 0.029 | 74.7 (8.5) | 74.7 (9.4) | 0.010 |
| Female | 238 (53.6) | 2865 (54.5) | 0.018 | 237 (53.6) | 938 (53.1) | 0.011 |
| Race/ethnicity | ||||||
| White | 356 (80.2) | 4304 (81.9) | 0.043 | 356 (80.5) | 1449 (82.0) | 0.036 |
| Black | 52 (11.7) | 491 (9.3) | 0.077 | 51 (11.5) | 188 (10.6) | 0.029 |
| Hispanic | 14 (3.2) | 252 (4.8) | 0.084 | 14 (3.2) | 55 (3.1) | 0.003 |
| Other/unknown | 22 (5.0) | 211 (4.0) | 0.046 | 21 (4.8) | 76 (4.3) | 0.022 |
| Index year | ||||||
| 2017 | 11 (2.5) | 262 (5.0) | 0.040 | 11 (2.5) | 77 (4.4) | 0.020 |
| 2018 | 218 (49.1) | 2437 (46.4) | 217 (49.1) | 822 (46.5) | ||
| 2019 | 215 (48.4) | 2559 (48.7) | 214 (48.4) | 869 (49.2) | ||
| Index to stroke Hospitalization, median (IQR) | −11 (−46, −3) | −14 (−36, −4) | — | −11 (−46, −3) | −17 (−42, −5) | — |
| Index to stroke Hospitalization, mean (SD) | −25.9 (27.6) | −22.9 (23.0) | 0.119 | −26.0 (27.6) | −25.7 (24.4) | 0.012 |
| Stroke hospitalization LOS, mean (SD) | 3.7 (4.1) | 3.1 (2.6) | 0.168 | 3.5 (2.7) | 3.5 (3.1) | 0.006 |
| Comorbidities | ||||||
| Diabetes | 208 (46.9) | 2264 (43.1) | 0.076 | 207 (46.8) | 829 (46.9) | 0.001 |
| Hypertension | 424 (95.5) | 4954 (94.2) | 0.058 | 422 (95.5) | 1701 (96.2) | 0.037 |
| Hyperlipidaemia | 400 (90.1) | 4593 (87.4) | 0.087 | 399 (90.3) | 1601 (90.6) | 0.010 |
| Ischaemic heart disease | 217 (48.9) | 2156 (41.0) | 0.159 | 217 (49.1) | 891 (50.4) | 0.026 |
| Myocardial infarction | 78 (17.6) | 832 (15.8) | 0.047 | 78 (17.7) | 327 (18.5) | 0.022 |
| Congestive heart failure | 83 (18.7) | 960 (18.3) | 0.011 | 83 (18.8) | 333 (18.8) | 0.001 |
| Valvular heart disease | 203 (45.7) | 2331 (44.3) | 0.028 | 201 (45.5) | 835 (47.2) | 0.035 |
| Patent foramen ovale | 44 (9.9) | 338 (6.4) | 0.127 | 43 (9.7) | 174 (9.8) | 0.003 |
| Peripheral vascular disease | 175 (39.4) | 1861 (35.4) | 0.083 | 175 (39.6) | 698 (39.5) | 0.002 |
| Cerebrovascular disease | 132 (29.7) | 1140 (21.7) | 0.185 | 130 (29.4) | 509 (28.8) | 0.014 |
| History of stroke/transient ischaemic attack | 94 (21.2) | 806 (15.3) | 0.152 | 92 (20.8) | 354 (20.0) | 0.020 |
| Chronic obstructive pulmonary disease | 115 (25.9) | 1481 (28.2) | 0.051 | 115 (26.0) | 448 (25.3) | 0.016 |
| Renal disease | 131 (29.5) | 1402 (26.7) | 0.063 | 130 (29.4) | 521 (29.5) | 0.001 |
| Cancer (metastatic or non-metastatic) | 68 (15.3) | 852 (16.2) | 0.024 | 67 (15.2) | 284 (16.1) | 0.025 |
| Dementia | 48 (10.8) | 632 (12.0) | 0.038 | 48 (10.9) | 192 (10.9) | 0.000 |
| CHA2DS2-VASc, mean (SD) | 4.61 (1.6) | 4.44 (1.5) | 0.112 | 4.61 (1.6) | 4.63 (1.6) | 0.013 |
ECM, external cardiac monitor; ICM, insertable cardiac monitor; IQR, interquartile range; LOS, length of stay; SD, standard deviation, SMD, standardized mean difference.
Each outcome was analysed using the Kaplan–Meier method, and log-rank tests were conducted to test for differences in outcomes between the two cardiac monitoring groups. Unadjusted and covariate-adjusted Cox proportional hazard regression models were then run, clustered by the hospital where patients were admitted for stroke. Cox models were evaluated for the proportional hazard assumption. Effect size estimates are provided as hazard ratios (HRs) with 95% confidence intervals (CIs). Sex differences were assessed by the addition of an interaction term to the models. The Kaplan–Meier OAC analysis included all patients in the cohort, regardless of whether they were diagnosed with AF. Descriptive analyses were subsequently conducted to provide OAC rates amongst those who were diagnosed with AF within the OAC follow-up time of 1.5 years. All analyses were conducted in SAS Enterprise Guide version 7.15 (SAS Institute Inc.).
Results
Patient characteristics
A total of 5702 Medicare beneficiaries (ICM, n = 444; ECM, n = 5258) met inclusion criteria. Differences in baseline characteristics were observed between ICM and ECM patients, as shown in Table 1. Insertable cardiac monitor patients had a longer average length of stay during their stroke hospitalization (3.7 vs. 3.1 days), as well as time to index cardiac monitoring after stroke hospitalization (25.9 vs. 22.9 days). Insertable cardiac monitor patients were also more likely to have ischaemic heart disease; patent foramen ovale; cerebrovascular disease, including prior stroke/TIA; and a higher CHA₂DS₂-VASc score. After propensity score matching, all baseline characteristics were balanced (SMDs < 0.10) between ICM and ECM patients. The matched cohort consisted of 2210 Medicare beneficiaries (ICM, n = 442; ECM, n = 1768) with 53% female, mean age 75 ± 9, CHA₂DS₂-VASc score 4.6 ± 1.6, and stroke hospitalization length of stay 3.5 ± 3.0 days. Both unmatched and matched baseline characteristics are presented in Table 1. Data from the Abbott ICM device registration database allowed us to characterize the reason for ICM implant for a subset of patients in the ICM cohort (N = 161). Of these patients, 146 (90.7%) had an indication of cryptogenic stroke or suspected atrial fibrillation as the reason for the ICM implant, specifically, 118 (73.3%) were for cryptogenic stroke, and 28 (17.4%) were for suspected atrial fibrillation. The remaining 9.3% of patients had other reasons listed, majority of which were for syncope.
External cardiac monitoring
The types of external cardiac monitoring in the ECM group were as follows: 366 (20.7%) short-term Holter monitors, 473 (26.8%) event monitors, and 929 (52.5%) mobile cardiac telemetry monitors. Amongst ECM patients, there were 176 (10%) who had a repeat ECM within 1 month of index, 77 (4.4%) within 2–3 months of index, and 30 (1.7%) within 4–6 months of index. Over 2 years, 315 (17.8%) of ECM patients had a repeat ECM, with median time to new ECM 30 (4, 109) days. There were 72 (16%) ICM patients who had a post-stroke ECM prior to their ICM implant.
Atrial fibrillation detection
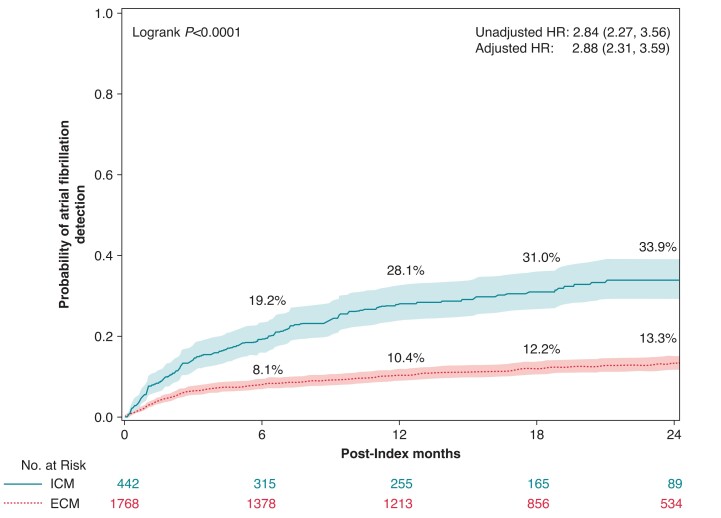
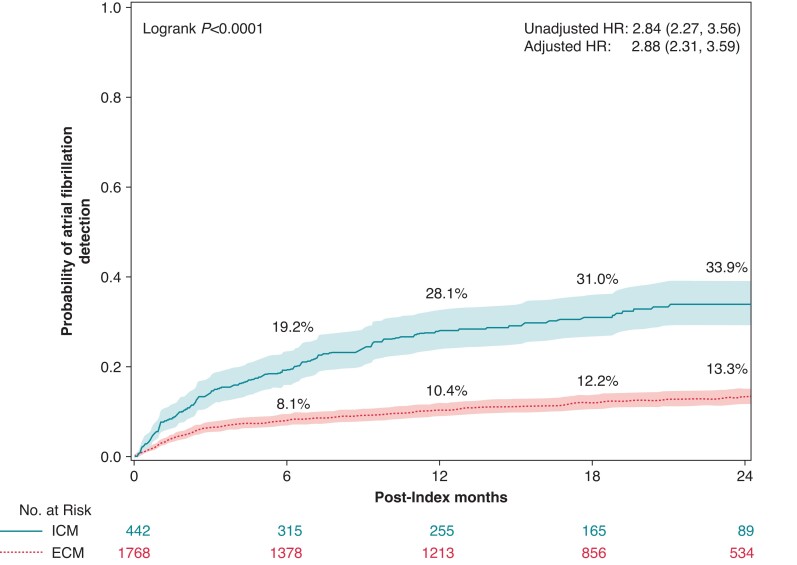
In the AF detection analysis, the median follow-up was 425 (IQR = 141–671) days for ICM and 520 (IQR = 234–730) days for ECM. As detailed in Figure 2, the AF detection rate was 33.9% amongst ICM patients compared with 13.3% amongst ECM patients at 2 years (P < 0.0001), with an unadjusted HR (95% CI) of 2.84 (2.27, 3.56) and adjusted HR (95% CI) of 2.88 (2.31, 3.59) from the Cox proportional hazard models. There were no significant differences between men and women as indicated by the interaction term in the full model (P = 0.946). The proportional hazard assumption was met for the models. The separation of the Kaplan–Meier curves occurred right after index, with AF detection rates of 19.2% amongst ICM patients at 6 months compared to 8.1% amongst ECM patients, 28.1% amongst ICM patients at 12 months compared to 10.4% amongst ECM patients, and 31.0% amongst ICM patients at 18 months compared to 12.2% amongst ECM patients. Log-rank tests indicate all comparisons have P < 0.0001. Whilst Medicare claims data do not contain data on AF duration, the Abbott remote monitoring database had AF duration data for a subset of the ICM cohort (N = 260). The median (IQR) duration of the maximum daily AF burden in these patients was 1.6 h (0.38, 6.1).
Figure 2.
Two-year atrial fibrillation incident rates by cardiac monitor method, ICM (solid line) and ECM (dashed line). Rates were estimated using the Kaplan–Meier method. Hazard ratios and 95% CIs estimated by Cox proportional hazard models are also shown. ECM, external cardiac monitor; ICM, insertable cardiac monitor.
Oral anticoagulant prescriptions
In the OAC prescription analysis, the median follow-up was 241 (IQR = 97–414) days for ICM and 250 (IQR = 84–463) days for ECM. The results of the Kaplan–Meier analysis of OAC prescription fill rate are described in Table 2. At 18-month follow-up, the OAC prescription rate, as estimated via Kaplan–Meier analysis, was 35.9% amongst ICM patients and 16.8% amongst ECM patients (log-rank tests P < 0.0001), with an unadjusted HR (95% CI) of 2.82 (2.20, 3.62), and adjusted HR (95% CI) of 2.91 (2.28, 3.72) from the Cox proportional hazard models. There were no significant differences between men and women as indicated by the interaction term in the full model (P = 0.199). Due to non-proportional hazards, an interaction term between the treatment variable and time was included in the models. Amongst patients who had an AF diagnosis within 1.5 years of index, 59% initiated OACs in both groups; specifically, amongst ECM patients, 35% initiated OAC after AF detection, whilst 24% initiated OAC prior to AF detection, and amongst ICM patients, 47% initiated OAC after AF detection, whilst 12% initiated OAC prior to AF detection.
Table 2.
Oral anticoagulant prescription rate by cardiac monitoring method amongst Medicare patients hospitalized with ischaemic stroke, 2017–2019
| Follow-up (months) | ICM % (95% CI) | ECM % (95% CI) |
|---|---|---|
| 6 | 13.6 (10.5–17.5) | 11.2 (9.7–12.9) |
| 12 | 30.9 (25.7–36.8) | 14.7 (12.9–16.8) |
| 18 | 35.9 (30.1–42.4) | 16.8 (14.7–19.2) |
The table shows 1.5-year oral anticoagulant prescription rates and 95% CIs by cardiac monitoring method, ICM and ECM. Rates were estimated using the Kaplan–Meier method.
ECM, external cardiac monitor; ICM, insertable cardiac monitor.
Death
There were 197 (11.1%) ECM patients and 40 (9.1%) ICM patients who died during the study period. The median follow-up was 583 (IQR = 393–730) days for ICM and 593 (IQR = 374–730) days for ECM.
Stroke/transient ischaemic attack
There were 140 (7.9%) ECM patients and 37 (8.4%) ICM patients who had another stroke or TIA during the study period. The median follow-up was 591 (IQR = 376–730) for ECM patients and 577 (IQR = 382–730) for ICM patients.
Discussion
Main findings
In this nationwide study with more than 2000 matched subjects with linked ICM and outcomes data, we found that an ICM monitoring strategy was associated with a much higher rate of AF detection in persons with ischaemic or cardioembolic stroke when compared with external cardiac monitoring. Moreover, we found that ICM monitoring was associated with a much higher rate of initiation of oral anticoagulation. These findings have important implications for the care of patients with stroke because AF may remain undetected even after a first stroke, and this may prevent or delay effective treatment strategies and increase the risk of recurrent stroke.1
Comparison with previous findings
In our clinical practice study of older US adults who were hospitalized with ischaemic stroke, which was cryptogenic in about 90% of patients, ICM patients were almost three times more likely to be diagnosed with AF and to be prescribed OAC compared to ECM patients. In particular, the AF detection rate was 33.9% amongst ICM patients compared with 13.3% amongst ECM patients at 2 years.
Only a small number of studies have evaluated the clinical impact of ICM usage in cryptogenic stroke patients treated in clinical practice.16–18 Ziegler et al.16 evaluated a cohort of 1247 US patients with cryptogenic stroke and reported an AF detection rate of 12.2% at 6 months and 21.5% at 2 years. Ungar et al.17 followed 334 cryptogenic stroke Italian patients with a mean age of 67 ± 12 years. In 62% of these patients, short-term Holter monitoring was performed before ICM implant. During a median follow-up of 23.6 months, subclinical AF was detected in 22.0%, 24.1%, and 31.5% at 6, 12, and 24 months, respectively, after ICM implantation, similar to the findings of our study. Furthermore, AF was asymptomatic in 88.1% of their patients.
In our clinical practice study, ICM patients were almost three times more likely to be prescribed OAC compared to ECM patients. In particular, at 18 months, the OAC prescription rate was 35.9% amongst ICM patients and 16.8% amongst ECM patients. Yaghi et al.18 performed an analysis on 12 994 US patients with incident hospitalization for cryptogenic stroke, identified in the Optum® claims database with 1949 ICM patients and 11 045 ECM patients. This study reported that ICM provided faster AF diagnosis compared with ECM and OAC drugs were prescribed in 30% of ICM patients vs. 19% of ECM patients at 18 months.
The results from our analysis extend these observations16–18 and highlight the clinical implications of enhanced AF detection capabilities that ICM can obtain in an older, sicker patient population with ischaemic stroke. Other studies, both real-world and clinical trial, were mostly focused on younger and healthier patient populations; for example, the patients in our study had higher CHA₂DS₂-VASc scores and were ∼13 years older than those enrolled in the CRYSTAL AF clinical trial (75 vs. 62 years).14 Age is an important factor since AF, including long-duration AF and asymptomatic AF, is more frequent with older age.19,20 Older patients who experienced a stroke could substantially benefit from reduction of stroke recurrences that anticoagulation may allow if an associated AF is detected. Patient age also emerged as independently associated with increased AF detection through an ICM in a secondary analysis of the CRYSTAL AF trial.21
There is substantial uncertainty and variability in the interpretation of so-called ‘subclinical AF’, but usually after stroke, the threshold for prescribing OAC is low, even if an AF episode lasting only a few minutes is detected.22,23 In the CRYSTAL AF trial,24 92% of patients with detected AF were prescribed OAC, and AF duration influenced OAC prescription; all patients with at least one long AF episode (>1 h) were prescribed OAC therapy compared with 70% of patients with only brief episodes (<1 h). The median duration of the maximum daily AF burden in a subset of our ICM cohort was 1.6 h. Our study was based on AF diagnosis codes and therefore relates to clinical AF detected by ICM or ECM. In both groups, 59% of patients with an AF diagnosis had an OAC prescription. This is consistent with other US-based studies on OAC underutilization in patients with AF, which may be related to various reasons25–27, whilst rates of OAC initiation in European patient populations tend to be higher.27–29 It is noteworthy that in some cases, prescription of OAC was done even before AF diagnosis, which could be due to clinical judgement or an artefact of using administrative data. In fact, other real-world studies30,31 were designed to look for OAC before and after AF diagnosis as well. Using Medicare claims data, Norby et al. defined first OAC as OAC found in claims 30 days prior to any time after first AF diagnosis. Using commercial claims data, O’Neal et al. defined OAC as OAC found in claims 3 months prior to 6 months after first AF diagnosis.
Clinical implications
In a large real-world cohort of patients with a stroke of unknown cause, our results highlight that ICMs lead to increased rates of AF detection and increased use of OAC therapy by approximately three times compared to ECMs. Improved detection of AF may translate into improved treatment and consequently reduced risk of stroke and death. Indeed in the study recently published by Yaghi et al.,18 ICM use was associated with a significantly reduced risk of death with HR = 0.70, CI 0.55–0.89. Also in the meta-analysis performed by Tsivgoulis et al.32 ICM use, compared with conventional monitoring in cryptogenic stroke patients, was associated with increased AF detection yield, higher OAC initiation, and decreased risk of recurrent stroke with ICM. Our data do not show a significant reduction of death in ICM patients vs. ECM patients, likely due to the sample size and limited follow-up length for studying rare events such as death.
The benefit of continuous vs. intermittent AF monitoring is of particular clinical relevance when AF is infrequent, paroxysmal, and asymptomatic.33–38 Detection of AF in patients with cryptogenic stroke and subsequent treatment with OAC is also important because silent brain infarcts have an impact on cognitive function in AF patients.39 Moreover, continuous rhythm monitoring enables an improved characterization of diverse AF patterns and their longitudinal changes, which may bring attention to progressive remodelling of the atrial substrate or worsening underlying diseases.40–44
Strengths and limitations
We performed a retrospective analysis of a large real-world database of patients with a stroke of unknown cause. The impact of monitoring strategies for AF detection in cryptogenic stroke has mostly been evaluated in randomized controlled trials (RCTs),14,21,24,45 which are performed in selected patient populations, with variable risk of AF detection,33 and often with important differences compared with clinical practice.46 Compared with prior studies, our results may be more generalizable to an older, sicker real-world patient population that more closely reflects the population affected by AF.
Whilst the use of observational data has many advantages, there are also important limitations that must be kept in mind. Initially there were important differences in patient characteristics between the two groups; we therefore used propensity score matching and further covariate adjustment to balance patient characteristics. Specifically, ICM patients had higher rates of cardiovascular diseases and higher CHA₂DS₂-VASc scores prior to matching. We attempted to minimize confounding with propensity score matching and further adjusting models for covariates and by limiting ECM patients to those who were admitted to the same hospitals as ICM patients for stroke. However, we cannot exclude the possibility that residual confounding impacted our results. Additionally, there are currently no specific diagnosis codes for cryptogenic stroke. We have minimized this limitation by excluding patients who likely did not have cryptogenic stroke, such as patients with a history of atrial tachyarrhythmias, implantable cardiac electronic devices, and OAC prescriptions, and our ICM registration data indicate that in a subset of the ICM cohort with data on reason for ICM implant, majority had the reason of cryptogenic stroke. We also could not adjust for stroke severity since validated data on this measure were not available in insurance claims; however, we adjusted for hospital length of stay, which is related to stroke severity. The follow-up time in our study differed according to the study endpoint due to differences in censoring, e.g. the follow-up for OAC was shorter due to a lag in data availability for Medicare Part D compared to Medicare FFS. We also could not evaluate recurrent stroke and other clinical outcomes due to low statistical power. The study population was limited to US patients with Medicare FFS insurance and may not be generalizable to younger patients. With this regard, it is noteworthy that there is an important heterogeneity of reimbursement practices across Europe, and some revision and update of related policies would be desirable, also taking into account innovative approaches.47–49
Conclusions
In a nationwide cohort of older patients with ischaemic stroke, we compared different cardiac monitoring strategies to detect AF as a potential cause of stroke. Long-term monitoring through an ICM yielded more frequent and timely AF detection rates and OAC prescription fills compared to short-term ECMs. Patients monitored with an insertable monitor were almost three times more likely to be diagnosed with AF and to be prescribed OAC compared to patients monitored with an external monitor.
Supplementary Material
Contributor Information
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Via del Pozzo, 71, Modena 41124, Italy.
Angelo Auricchio, Division of Cardiology, Cardiocentro Ticino, Lugano, Switzerland.
Giovanni Luca Botto, Department of Cardiology—Electrophysiology, ASST Rhodense, Civile Hospital Rho and Salvini Hospital Garbagnate Milanese Hospital, Milan, Italy.
Jennifer M Joseph, Medical Devices, Abbott, Santa Clara, CA, USA.
Gregory J Roberts, Medical Devices, Abbott, Sylmar, CA, USA.
Andrea Grammatico, Medical Devices, Abbott, Rome, Italy.
Yelena Nabutovsky, Medical Devices, Abbott, Santa Clara, CA, USA.
Jonathan P Piccini, Duke Clinical Research Institute, Duke University, Durham, NC, USA.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was funded by Abbott.
Data availability
Centers for Medicare & Medicaid Services data can be requested under an approved research protocol via ResDAC (www.resdac.org). Restrictions apply to the availability of data generated and analysed for this study to preserve patient confidentiality. Data were used under a data use agreement. Requests to access these data sets should be directed to ResDAC, resdac@umn.edu.
References
- 1. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann Jet al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN international collaboration. Circulation 2020;141:e99. [DOI] [PubMed] [Google Scholar]
- 2. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LHet al. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015;128:509–18.e2. [DOI] [PubMed] [Google Scholar]
- 3. Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015;14:377–87. [DOI] [PubMed] [Google Scholar]
- 4. Sgreccia D, Manicardi M, Malavasi VL, Vitolo M, Valenti AC, Proietti Met al. Comparing outcomes in asymptomatic and symptomatic atrial fibrillation: a systematic review and meta-analysis of 81,462 patients. J Clin Med 2021;10:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman Bet al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121–201. [DOI] [PubMed] [Google Scholar]
- 6. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. Corrigendum to: 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:4194. [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jret al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019;16:e66–93. [DOI] [PubMed] [Google Scholar]
- 8. Boriani G, Vitolo M, Lane DA, Potpara TS, Lip GY. Beyond the 2020 guidelines on atrial fibrillation of the European society of cardiology. Eur J Intern Med 2021;86:1–11. [DOI] [PubMed] [Google Scholar]
- 9. Imberti JF, Mei DA, Vitolo M, Bonini N, Proietti M, Potpara Tet al. Comparing atrial fibrillation guidelines: focus on stroke prevention, bleeding risk assessment and oral anticoagulant recommendations. Eur J Intern Med 2022;101:1–7. [DOI] [PubMed] [Google Scholar]
- 10. Lip GYH, Lane DA, Lenarczyk R, Boriani G, Doehner W, Benjamin LAet al. Integrated care for optimizing the management of stroke and associated heart disease: a position paper of the European Society of Cardiology Council on Stroke. Eur Heart J 2022;43:2442–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill Det al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021;52:e364–467. [DOI] [PubMed] [Google Scholar]
- 12. Boriani G, Pettorelli D. Atrial fibrillation burden and atrial fibrillation type: clinical significance and impact on the risk of stroke and decision making for long-term anticoagulation. Vascul Pharmacol 2016;83:26–35. [DOI] [PubMed] [Google Scholar]
- 13. Freedman B, Boriani G, Glotzer TV, Healey JS, Kirchhof P, Potpara TS. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol 2017;14:701–14. [DOI] [PubMed] [Google Scholar]
- 14. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CAet al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- 15. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziegler PD, Rogers JD, Ferreira SW, Nichols AJ, Richards M, Koehler JLet al. Long-term detection of atrial fibrillation with insertable cardiac monitors in a real-world cryptogenic stroke population. Int J Cardiol 2017;244:175–9. [DOI] [PubMed] [Google Scholar]
- 17. Ungar A, Pescini F, Rafanelli M, De Angelis MV, Faustino M, Tomaselli Cet al. Detection of subclinical atrial fibrillation after cryptogenic stroke using implantable cardiac monitors. Eur J Intern Med 2021;92:86–93. [DOI] [PubMed] [Google Scholar]
- 18. Yaghi S, Ryan MP, Gunnarsson CL, Irish W, Rosemas SC, Neisen Ket al. Longitudinal outcomes in cryptogenic stroke patients with and without long-term cardiac monitoring for atrial fibrillation. Heart Rhythm O2 2022;3:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boriani G, Vitolo M, Diemberger I, Proietti M, Valenti AC, Malavasi VLet al. Optimizing indices of atrial fibrillation susceptibility and burden to evaluate atrial fibrillation severity, risk and outcomes. Cardiovasc Res 2021;117:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benjamin EJ, Go AS, Desvigne-Nickens P, Anderson CD, Casadei B, Chen LYet al. Research priorities in atrial fibrillation screening: A report from a national heart, lung, and blood institute virtual workshop. Circulation 2021;143:372–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RAet al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol 2016;9:e003333. [DOI] [PubMed] [Google Scholar]
- 22. Boriani G, Healey JS, Schnabel RB, Lopes RD, Calkins H, Camm JAet al. Oral anticoagulation for subclinical atrial tachyarrhythmias detected by implantable cardiac devices: an international survey of the AF-SCREEN Group. Int J Cardiol 2019;296:65–70. [DOI] [PubMed] [Google Scholar]
- 23. Proietti M, Romiti GF, Vitolo M, Borgi M, Rocco AD, Farcomeni Aet al. Epidemiology of subclinical atrial fibrillation in patients with cardiac implantable electronic devices: a systematic review and meta-regression. Eur J Intern Med 2022;103:84–94. [DOI] [PubMed] [Google Scholar]
- 24. Thijs VN, Brachmann J, Morillo CA, Passman RS, Sanna T, Bernstein RAet al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology 2016;86:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–45.e4. [DOI] [PubMed] [Google Scholar]
- 26. Piccini JP, Hernandez AF, Zhao X, Patel MR, Lewis WR, Peterson EDet al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. JACC 2009;54:1280–9. [DOI] [PubMed] [Google Scholar]
- 27. Grymonprez M, Simoens C, Steurbaut S, De Backer TL, Lahousse L. Worldwide trends in oral anticoagulant use in patients with atrial fibrillation from 2010 to 2018: a systematic review and meta-analysis. Europace 2022;24:887–98. [DOI] [PubMed] [Google Scholar]
- 28. Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer Met al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. Europace 2018;20:747–57. PMID: 29016832. [DOI] [PubMed] [Google Scholar]
- 29. Ritchie LA, Lane DA, Lip GYH. Worldwide trends in antithrombotic therapy prescribing for atrial fibrillation: observations on the ‘transition era’ to non-vitamin K antagonist oral anticoagulants. Europace 2022;24:871–3. Erratum in: Europace. 2023 Feb16; 25(2):505. PMID: 34964471. [DOI] [PubMed] [Google Scholar]
- 30. Norby FL, Lutsey PL, Shippee ND, Chen LY, Henning-Smith C, Alonso Aet al. Direct oral anticoagulants and warfarin for atrial fibrillation treatment: rural and urban trends in Medicare beneficiaries. Am J Cardiovasc Drugs 2022;22:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Neal WT, Sandesara PB, Claxton JS, MacLehose RF, Chen LY, Bengtson LGSet al. Provider specialty, anticoagulation prescription patterns, and stroke risk in atrial fibrillation. J Am Heart Assoc 2018;7:e007943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsivgoulis G, Katsanos AH, Grory BM, Köhrmann M, Ricci BA, Tsioufis Ket al. Prolonged cardiac rhythm monitoring ad secondary stroke prevention in patients with cryptogenic cerebral ischemia. Stroke 2019;50:2175–80. [DOI] [PubMed] [Google Scholar]
- 33. Skrebelyte-Strøm L, Rønning OM, Dahl FA, Steine K, Kjekshus H. Prediction of occult atrial fibrillation in patients after cryptogenic stroke and transient ischaemic attack: PROACTIA. Europace 2022;24:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koh KT, Law WC, Zaw WM, Foo DHP, Tan CT, Steven Aet al. Smartphone electrocardiogram for detecting atrial fibrillation after a cerebral ischaemic event: a multicentre randomized controlled trial. Europace 2021;23:1016–23. [DOI] [PubMed] [Google Scholar]
- 35. McIntyre WF, Wang J, Benz AP, Johnson L, Connolly SJ, Van Gelder ICet al. Estimated incidence of previously undetected atrial fibrillation on a 14-day continuous electrocardiographic monitor and associated risk of stroke. Europace 2022;24:1058–64. [DOI] [PubMed] [Google Scholar]
- 36. Vitolo M, Imberti JF, Maisano A, Albini A, Bonini N, Valenti ACet al. Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: a systematic review and meta-analysis. Eur J Intern Med 2021;92:100–6. [DOI] [PubMed] [Google Scholar]
- 37. Lopez Perales CR, Van Spall HGC, Maeda S, Jimenez A, Laţcu DG, Milman Aet al. Mobile health applications for the detection of atrial fibrillation: a systematic review. Europace 2021;23:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyazawa K, Pastori D, Martin DT, Choucair WK, Halperin JL, Lip GYH, et al. Characteristics of patients with atrial high rate episodes detected by implanted defibrillator and resynchronization devices. Europace 2022;24:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kühne M, Krisai P, Coslovsky M, Rodondi N, Müller A, Beer JHet al. Silent brain infarcts impact on cognitive function in atrial fibrillation. Eur Heart J 2022;43:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De With RR, Erküner Ö, Rienstra M, Nguyen BO, Körver FWJ, Linz Det al. Temporal patterns and short-term progression of paroxysmal atrial fibrillation: data from RACE V. Europace 2020;22:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malavasi VL, Fantecchi E, Tordoni V, Melara L, Barbieri A, Vitolo Met al. Atrial fibrillation pattern and factors affecting the progression to permanent atrial fibrillation. Intern Emerg Med 2021;16:1131–40. [DOI] [PubMed] [Google Scholar]
- 42. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CMet al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Linz D, Hermans A, Tieleman RG. Early atrial fibrillation detection and the transition to comprehensive management. Europace 2021;23:ii46–51. [DOI] [PubMed] [Google Scholar]
- 44. Hartley A, Shalhoub J, Ng FS, Krahn AD, Laksman Z, Andrade JGet al. Size matters in atrial fibrillation: the underestimated importance of reduction of contiguous electrical mass underlying the effectiveness of catheter ablation. Europace 2021;23:1698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall Jet al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- 46. Boriani G, Maniadakis N, Auricchio A, Müller-Riemenschneider F, Fattore G, Leyva Fet al. Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European Heart Rhythm Association. Eur Heart J 2013;34:1869–74. [DOI] [PubMed] [Google Scholar]
- 47. Boriani G, Burri H, Svennberg E, Imberti JF, Merino JL, Leclercq C. Current status of reimbursement practices for remote monitoring of cardiac implantable electrical devices across Europe. Europace 2022;24:1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boriani G, Vitolo M, Svennberg E, Casado-Arroyo R, Merino JL, Leclercq C. Performance-based risk-sharing arrangements for devices and procedures in cardiac electrophysiology: an innovative perspective. Europace 2022;24:1541–7. [DOI] [PubMed] [Google Scholar]
- 49. Boriani G, Svennberg E, Guerra F, Linz D, Casado-Arroyo R, Malaczynska-Rajpold Ket al. Reimbursement practices for use of digital devices in atrial fibrillation and other arrhythmias: a European Heart Rhythm Association survey. Europace 2022;24:1834–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Centers for Medicare & Medicaid Services data can be requested under an approved research protocol via ResDAC (www.resdac.org). Restrictions apply to the availability of data generated and analysed for this study to preserve patient confidentiality. Data were used under a data use agreement. Requests to access these data sets should be directed to ResDAC, resdac@umn.edu.