Abstract
Purpose of review
Early and accurate diagnosis of pancreatic cancer is crucial for improving patient outcomes, and artificial intelligence (AI) algorithms have the potential to play a vital role in computer-aided diagnosis of pancreatic cancer. In this review, we aim to provide the latest and relevant advances in AI, specifically deep learning (DL) and radiomics approaches, for pancreatic cancer diagnosis using cross-sectional imaging examinations such as computed tomography (CT) and magnetic resonance imaging (MRI).
Recent findings
This review highlights the recent developments in DL techniques applied to medical imaging, including convolutional neural networks (CNNs), transformer-based models, and novel deep learning architectures that focus on multitype pancreatic lesions, multiorgan and multitumor segmentation, as well as incorporating auxiliary information. We also discuss advancements in radiomics, such as improved imaging feature extraction, optimized machine learning classifiers and integration with clinical data. Furthermore, we explore implementing AI-based clinical decision support systems for pancreatic cancer diagnosis using medical imaging in practical settings.
Summary
Deep learning and radiomics with medical imaging have demonstrated strong potential to improve diagnostic accuracy of pancreatic cancer, facilitate personalized treatment planning, and identify prognostic and predictive biomarkers. However, challenges remain in translating research findings into clinical practice. More studies are required focusing on refining these methods, addressing significant limitations, and developing integrative approaches for data analysis to further advance the field of pancreatic cancer diagnosis.
Keywords: computer-aided diagnosis, computed tomography, deep learning, medical imaging, magnetic resonance imaging, pancreatic cancer, radiomics
INTRODUCTION
Clinical motivation
Pancreatic cancer is one of the most lethal cancers with a poor prognosis and limited treatment options, with a 5-year relative survival rate of only 12% [1]. Over 90% of pancreatic cancers are exocrine tumors, for which the most common type is pancreatic ductal adenocarcinoma (PDAC), as illustrated in Fig. 1. There is no widely accepted screening for pancreatic cancer yet, and cross-sectional imaging is still the choice of noninvasive method that is widely available [2].
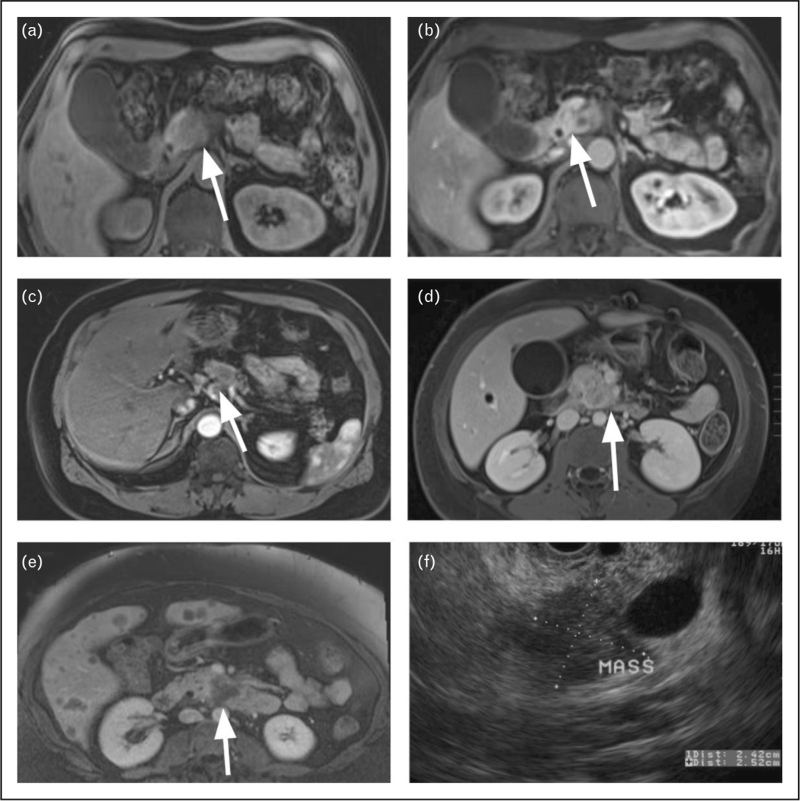
FIGURE 1.
Pancreatic ductal adenocarcinoma (PDAC). (a) T1-weighted (T1W) MRI image with fat suppression (FS); (b) contrast-enhanced (CE) T1W MRI image during the early phase in same patient; (c) PDAC; (d) axial CE-T1W-FS image with PDAC in the uncinate process of the head; (e) axial CE-T1W-FS image with PDAC and liver metastases; (f) EUS image showing PDAC measuring 2.5 cm. The white arrows point to cancer. EUS, endoscopic ultrasound; PDAC, pancreatic ductal adenocarcinoma.
Identifying early precursor changes in the pancreas has the potential to aid in the risk prediction of pancreatic cancer, as these precancerous alterations manifest as morphological and textural changes on abdominal imaging (CT and MRI) [3,4,42,43]. These changes include main pancreatic duct (MPD) stricture and upstream marked MPD dilatation [5]. This observation provides the rationale for our research on computer-aided diagnosis (CAD) methods, specifically deep learning (DL) and radiomics, in identifying pancreatic cancer risk at an early stage. However, detecting these early changes has challenges such as:
-
(1)
variable size, shape and texture of the pancreatic lesions,
-
(2)
limited volume of medical datasets due to cost of examination and manual annotation,
-
(3)
variable imaging techniques collected from different institutions.
Despite these challenges, early detection is crucial, given the high mortality rate associated with pancreatic cancer. Therefore, it is essential to develop robust and reliable CAD systems for medical imaging to improve pancreatic cancer diagnosis.
Multiple imaging modalities are available for diagnosis of pancreas cancer: CT, MRI, endoscopic ultrasound (EUS), and transabdominal ultrasound. In this review, we primarily focus on CT and MRI as they are the most common noninvasive diagnostic imaging methods with certain superiorities available. Among CT and MRI, CT is the most utilized modality in cancer diagnosis due to its availability, high spatial resolution, and fast acquisition times. However, MRI offers superior soft tissue contrast resolution and provide more insight into tissue characteristics such as signal intensity on T1- or T2-weighted images, better visualization of ductal strictures, as well as advanced imaging, such as diffusion-weighted imaging (DWI) and perfusion imaging. Figure 1 shows both MRI and corresponding EUS images of pancreatic cancer.
Cross-sectional imaging is the most commonly used modality for the diagnosis of pancreatic cancer. However, this practice has challenges, as tumors might be missed or can be confused with other benign pancreatic lesions or abnormalities. CAD systems have been developed over years to alleviate some of the difficulties in the current standard. For example, a CAD system can help identify small tumors in the pancreas region that may be missed or help predict patient outcomes after diagnosis of pancreatic cancer, surgical planning, or therapy response assessment in radiation oncology. Related to the role of artificial intelligence (AI) in pancreatic cancer diagnosis, we explore two prominent families of methods of medical image classification for pancreatic cancer that have garnered significant attention and demonstrated success: deep learning and radiomics. Gaining a comprehensive understanding of the current state of research in these areas is essential for enhancing diagnostic accuracy and improving patient outcomes. We discuss recent advances, challenges, and future perspectives too.
Box 1.
no caption available
Development of radiomics and artificial intelligence
A typical workflow of a CAD system for pancreatic cancer diagnosis involves image preprocessing, region of interest (ROI) segmentation, and tumor classification, after which the results are presented to a clinician for further evaluation and treatment/surgical planning or response assessment (Fig. 2). Radiomics and DL are essential in tumor segmentation, classification, and early diagnosis. Although radiomics is often considered a pre-DL era method of extracting hand-crafted features for auto-diagnosis, DL is regarded as a feature exploration method for the same tasks. DL can be used for tumor segmentation, crucial for diagnosis and prognostic purposes because the size, shape, and location of the tumor are critical in such tasks.
FIGURE 2.
A typical pancreatic cancer CAD system workflow, including two strategies, deep learning and radiomics. CAD, computer-aided diagnosis.
These advanced techniques (DL and radiomics) are becoming increasingly significant as they can detect subtle changes and aid in the early detection and characterization of pancreatic tumors. One may improve diagnostic accuracy in differentiating benign and malignant lesions by leveraging quantitative imaging biomarkers extracted from radiomics and high-level features from deep learning algorithms. However, challenges remain regarding image registration (alignment), data availability, clinical interpretability, robustness and generalization ability.
Evaluation criteria
Evaluation metrics are essential for providing clear, objective, and quantifiable ways to assess and compare research findings; therefore, herein, we enlist commonly used evaluation strategies in CAD systems for identifying weaknesses and strengths of available methods. Several widely used metrics from the confusion matrix and the receiver operating characteristic (ROC) curve are employed for determining the effectiveness and reliability (Fig. 3): sensitivity, specificity, accuracy, precision, recall, F-score and area under the receiver operating characteristic curve (AUC). The key metrics are explained here:
FIGURE 3.
Common evaluation metrics. Left: confusion matrix; right: ROC curve. ROC, receiver operating characteristic.
-
(1)
Sensitivity: Sensitivity, also known as the true positive rate, measures the proportion of confirmed cases that the CAD system correctly identifies as positive. It is a measure of the system's ability to detect cancerous lesions in medical images. High sensitivity implies proficiency at identifying pancreatic cancer cases, minimizing the chances of false-negative results.
-
(2)
Specificity: Specificity, or the true negative rate, reflects the system's ability to distinguish noncancerous tissue from cancers. High specificity indicates that the CAD system effectively avoids false-positive results, reducing the likelihood of unnecessary additional tests or treatments.
-
(3)
Accuracy: Sometimes, balanced accuracy is used to evaluate performance on an unbalanced dataset. Accuracy represents the proportion of all cases (both positive and negative) that the CAD system correctly classifies. In pancreatic cancer diagnosis, accuracy is an overall measure of the CAD system's performance in correctly distinguishing cancerous and noncancerous lesions.
-
(4)
AUC: ROC curve is a graphical representation of a classifier's performance across various threshold settings. The curve plots the true positive rate (TPR = sensitivity) against the false positive rate (FPR = 1 – specificity) at different thresholds. The AUC is a summary metric that quantifies the overall performance of the CAD system across all possible thresholds. As shown in Fig. 3, an AUC of 1 (line C-A-D) indicates perfect classification, while an AUC of 0.5 (dotted line C-D) suggests that the CAD system's performance is no better than random chance. A high AUC value implies that the CAD system effectively distinguishes between pancreatic cancer and noncancer cases across a range of threshold settings.
In conclusion, these evaluation metrics provide a comprehensive performance assessment, as each metric contributes unique insights into the classifier's ability to detect cancerous lesions and avoid false diagnoses.
Search criteria
The literature search keywords used to identify relevant articles include pancreatic cancer, computed tomography (CT), magnetic resonance imaging (MRI), pancreatic ductal adenocarcinoma (PDAC), intraductal papillary mucinous neoplasms (IPMN), pancreatic cysts, auto-diagnosis, CAD systems for pancreatic cancer, and a mixture of them. We used Google Scholar, PubMed, and Scopus to conduct a literature search and manually reviewed the reference lists of selected articles to identify additional relevant studies. Articles were included if they met the following criteria:
-
(1)
Published within the specified time window (November 2021–April 2023).
-
(2)
Focused on pancreatic cancer diagnosis using CT or MRI.
-
(3)
Employed radiomics, deep learning, or a combination of both methods for image analysis.
-
(4)
Investigated pancreatic cancer types or predisposing conditions mentioned above.
RADIOMICS
Basics
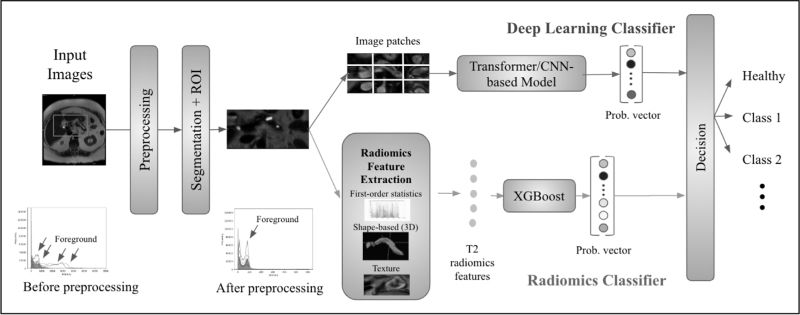
Radiomics has been applied to pancreatic cancer imaging data to provide prognostic and predictive information that can guide clinical decision making. Radiomics is an advanced image analysis technique that systematically extracts quantitative features from images, known as manually crafted features [5]. These features capture detailed information about the morphology, texture, and intensity distribution within the pancreas. The radiomics workflow typically involves several steps, including image preprocessing, region of interest (ROI) segmentation, feature extraction, feature selection, and classification or prediction using machine learning algorithms (Fig. 4).
FIGURE 4.
A detailed radiomics-based diagnosis workflow.
The primary advantage of radiomics lies in its ability to provide an extensive and objective assessment of pancreas heterogeneity, which can significantly enhance clinical decision-making. By leveraging high-dimensional feature spaces, radiomics enables the discovery of complex patterns and correlations that may not be readily apparent through traditional visual inspection by physicians (or radiologists). Consequently, radiomics has shown promise [6–8,44] in improving diagnostic accuracy with an AUC of 0.7–0.8, depending on the specific dataset and implementation [9▪,10▪–12▪,13▪▪,14▪].
Recent advances
Recent advances in radiomics methods for pancreatic cancer diagnosis tasks have demonstrated significant progress in medical imaging analysis, benefiting from a mature methodology pipeline. These studies often integrate clinical features, employ feature selection techniques, and compare the performance of various machine learning classifiers, as illustrated in detail in Table 1.
Table 1.
Recent advances in deep learning for pancreatic cancer diagnosis based on imaging. Ground truth diagnoses in the dataset are usually based on pathology data
| Title | Methodology | Performance | Dataset |
| Retrospective analysis of the value of enhanced CT radiomics analysis in the differential diagnosis between pancreatic cancer and chronic pancreatitis [9▪] | Ma et al. built a multivariable logistic regression model based on selected radiomics features, combined with clinical features and established a nomogram | An AUC of 0.980, with a sensitivity of 94.7% and specificity of 91.7%. | A retrospective dataset of 151 pancreatic cancer cases and 24 chronic pancreatitis cases, CT images |
| Predicting PDAC using artificial intelligence analysis of prediagnostic computed tomography images [10▪] | Ahmad et al. developed a naïve Bayes classifier for the automatic classification of CT scans after selecting features potentially predictive of PDAC | Average accuracy of 86% | 108 retrospective CT scans from 72 subjects, with 36 scans each from healthy control, prediagnostic, and diagnostic groups |
| Radiomics-based machine-learning models can detect pancreatic cancer on prediagnostic computed tomography scans at a substantial lead time before clinical diagnosis [11▪] | A total of 34 features were selected through the least absolute shrinkage and selection operator-based feature selection method. Four ML classifiers were evaluated: KNN, SVM, RF and XGBoost | SVM achieved the highest sensitivity (95.5%), specificity (90.3%), F1-score (89.5%), AUC (0.98), and accuracy (92.2%) | 155 prediagnostic CT scans of PDAC patients and 265 age-matched CT scans of subjects with normal pancreas |
| Applying a radiomics-based CAD scheme to classify between malignant and benign pancreatic tumors using CT images [12▪] | Gai et al. developed a radiomics-based CAD scheme for CT images, including preprocessing, segmentation, feature extraction and classification (SVM) | AUC of 0.75 | A retrospective dataset of 77 patients with suspicious pancreatic tumors detected on CT images, including 33 malignant tumors |
| Branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs): an MRI-based radiomic model to determine the malignant degeneration potential [13▪▪] | Flammia et al. aimed to create an MRI-based radiomic model to identify features linked to a higher risk of malignant degeneration in BD-IPMNs. Features that showed significant differences were included in a LASSO regression method to build a radiomics-based predictive model | The radiomic-based predictive model identified 16 significant features, including 5 for T1-W, 6 for postcontrast T1-W, 3 for T2-W, and 2 for the apparent diffusion coefficient | 50 patients with BD-IPMN, MRI images |
| Radiomics analysis for predicting malignant potential of intraductal papillary mucinous neoplasms of the pancreas: comparison of CT and MRI [14▪] | Intraclass correlation coefficients were calculated to assess interobserver reproducibility. The least absolute shrinkage and selection operator algorithm was used for feature selection. Radiomics models were constructed based on selected features with logistic regression (LR) and SVM | MRI radiomics models achieved better AUCs (0.879 with LR and 0.940 with SVM) than CT radiomics models (0.811 with LR and 0.864 with SVM) | 60 patients with surgically confirmed IPMNs (37 malignant and 23 benign), both CT and MRI |
AUC, area under the receiver operating characteristic curve; CAD, computer-aided diagnosis; CNN, convolutional neural network.
A common practice in these studies is to use feature selection methods to identify the most relevant radiomics features for tumor classification. Ma et al.[9▪] and Flammia et al.[13▪▪] both utilized the least absolute shrinkage and selection operator (LASSO) method to select features that were then integrated into their respective models. LASSO is a linear regression analysis method often used in statistics and machine learning as a regularization strategy to prevent overfitting. Moreover, LASSO offers greater interpretability than other complex machine learning and statistical methods, as it shrinks less important parameters to zero while explaining the regression results using the remaining coefficients. This feature makes LASSO particularly useful for identifying the most relevant radiomics features and building more transparent models. Another study by Ahmad et al.[10▪] employed a naïve Bayes classifier after selecting features potentially predictive of PDAC. Naive Bayes classification is often used when features are considered conditionally independent given the output label. Incorporating clinical features is beneficial [9▪], and researchers continue investigating more informative hand-crafted features [13▪▪].
In model development, various machine learning classifiers have been used in these studies [9▪,10▪–12▪,13▪▪,14▪]. Support Vector Machines (SVM) and XGBoost have emerged as robust classifiers for such tasks [11▪,12▪,14▪], with most advances focusing on application aspects, such as the comparison between CT and MRI [14▪]. Experiments on diverse datasets from different regions have further validated the value of radiomics in pancreatic cancer diagnosis tasks [15].
DEEP LEARNING
Basics
Deep learning has demonstrated remarkable potential in pancreatic cancer diagnosis, with numerous studies reporting promising results [16–20]. Widely utilized models include CNN-based architectures such as UNet and its variations [21]. Transfer learning is often employed to adapt pretrained CNN models, including VGG [22], Inception [23], and ResNet [24], to discern specific features in pancreatic cancer imaging. Despite its promise, deep learning faces challenges such as data scarcity, model interpretability, and generalizability. Several data augmentation techniques are frequently employed to address these issues. [16,25,26], increasing the size and diversity of training datasets, thereby enhancing model performance on previously unseen images or domains.
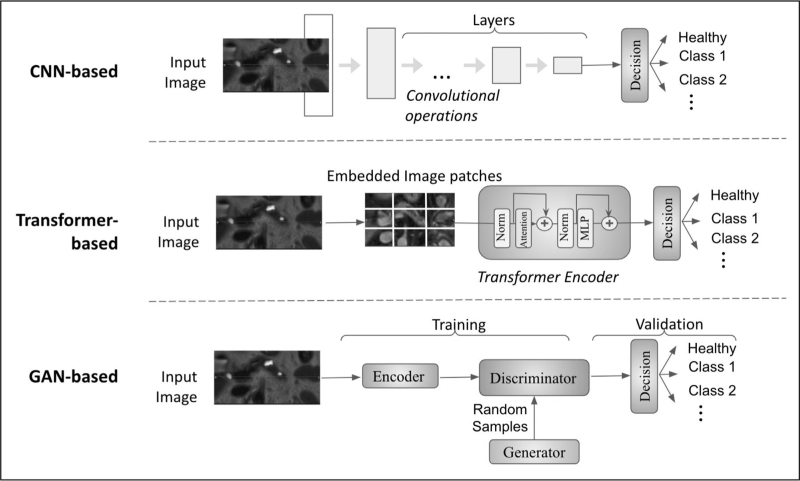
Figure 5 illustrates a simplified diagram of commonly-employed deep learning approaches. To explain the differences among deep learning strategies: CNN is the most typical deep learning architecture, consisting of multiple convolutional layers and other network architecture elements, and is being successfully used for many image classifications, segmentation and detection tasks. Transformers, on the contrary, have recently gained momentum and have been shown to be state-of-the-art, replacing CNN-based methods. The main premise behind the Transformers is to have a procedure called self-attention mechanism, allowing them one to weigh and consider different parts of the images when making predictions. Lastly, generative adversarial network (GAN) based methods encompass encoder, generator, and discriminator components, functioning in two stages: training and validation. These are substantially different from CNNs and transformers in those GANs are often used to generate new data samples, while CNNs and Transformers are used for classification and detection tasks. One should note that GANs are generative models, and CNNs and Transformers are discriminative models. All these families of deep learning methods have been used in pancreatic cancer detection and analysis studies; therefore, it is essential to enlist their basic architecture and differences, as in Fig. 5.
FIGURE 5.
Deep learning architectures used for pancreatic cancer diagnosis. The diagram is organized into three main categories: CNN-based models, transformer-based models, and cycleGAN. GAN, generative adversarial network; CNN, convolutional neural network.
Recent advances
Most research until today were conducted on CT images, with a few investigations involving MRI [33▪▪] and EUS [27,28]. Several studies have developed end-to-end DL solutions for pancreatic tumor detection and classification. For instance, Park et al.[29▪], Althobaiti et al.[30▪], and Chen et al.[31▪▪] employed different deep learning architectures, such as nnU-Net, Capsule Network, and CNNs, respectively, for detecting and segmenting pancreatic tumors. These studies further show the potential of deep learning in automating the diagnosis process and reducing the workload for radiologists.
Despite further investigation into CNN-based models, recent advances in deep learning methods for pancreatic cancer diagnosis have led to the development of transformer-based models, which have demonstrated significant potential in various aspects of medical imaging. Transformer models are known for making robust encoders [37], and vision transformers have been specifically applied for the classification of intraductal papillary mucosal neoplasms (IPMN) in MRI images [33▪▪].
In addition to using established deep learning architectures, some studies have proposed novel deep learning models. For instance, Vaiyapuri et al.[32▪] introduced the IDLDMS-PTC, a system that combines MobileNet for feature extraction with an optimal autoencoder and multileader optimization for classification. Another study introduced a meta-information-aware dual-path transformer for pancreatic lesion classification and segmentation, highlighting the potential of incorporating auxiliary information to improve model performance [36▪].
Performance details are summarized in the following Table 2. It is worth noting that dataset bias could potentially impact performance. Nevertheless, these recent advancements highlight the growing potential of transformer-based deep learning techniques in the diagnosis and management of pancreatic cancer.
Table 2.
Recent advances in deep learning for pancreatic cancer diagnosis based on imaging. Ground truth diagnoses in the dataset are usually based on clinical manifestations and pathology data
| Title | Methodology | Performance | Dataset |
| Deep learning–based detection of solid and cystic pancreatic neoplasms at contrast-enhanced CT [29▪] | Park et al. developed a three-dimensional nnU-Net-based deep learning model for automatically identifying patients with various solid and cystic pancreatic neoplasms in abdominal CT scan | AUC of 0.91 in test set 1 and 0.87 in test set 2; comparable sensitivity to radiologists for solid and cystic lesions | 852 patients in the training set, 603 in test set 1, and 589 in test set 2, CT images |
| Design of optimal deep learning-based pancreatic tumor and nontumor classification model using computed tomography scans [30▪] | Althobaiti et al. developed an optimal deep learning-based pancreatic tumor and nontumor classification (ODL-PTNTC) model using CT images, which includes adaptive window filtering for noise removal, sailfish optimizer-based Kapur's thresholding for segmentation, Capsule Network for feature extraction, and Political Optimizer with Cascade Forward Neural Network | Achieved sensitivity, specificity, accuracy, and F-score of 98.73%, 97.75%, 98.40%, and 98.82%, respectively | the benchmark BioGPS dataset [41], CT images |
| Pancreatic cancer detection on CT scans with deep learning: a nationwide population-based study [31▪▪] | Chen et al. developed an end-to-end deep learning tool for pancreatic cancer detection on CT scans, comprising a segmentation CNN for pancreas localization and a classifier ensemble of five CNNs for cancer identification | 89.9% sensitivity, 95.9% specificity (internal test set); 89.7% sensitivity, 92.8% specificity (real-world test set); 74.7% sensitivity for malignancies <2 cm | Retrospectively collected contrast-enhanced CT studies from 546 pancreatic cancer patients and 733 control subjects between January 2004 and December 2019, CT images |
| Intelligent deep-learning-enabled decision-making medical system for pancreatic tumor classification on CT images [32▪] | Vaiyapuri et al. proposed an intelligent deep-learning-enabled decision-making medical system for pancreatic tumor classification (IDLDMS-PTC) using CT images, which includes an emperor penguin optimizer with multilevel thresholding (EPO-MLT) for segmentation, MobileNet for feature extraction, and optimal autoencoder (AE) with multileader optimization (MLO) for classification | The IDLDMS-PTC model achieved an average sensitivity of 0.9935, specificity of 0.9884, accuracy of 0.9935, and F-score of 0.9948 | Various sources, a total of 500 images, with 250 images under pancreatic tumor and 250 images under nonpancreatic tumor, CT images |
| Neural transformers for classification of intraductal papillary mucinous neoplasm (IPMN) using MRI [33▪▪] | The authors proposed an AI-based IPMN classifier using a transformer-based architecture, specifically ViT, which employs the encoder of the original transformer model on a sequence of image patches | Achieved an accuracy of 0.7 ± 0.11, precision of 0.67 ± 0.19, and recall of 0.64 ± 0.12 | 139 MRI scans from distinct patients |
| The FELIX project: deep networks to detect pancreatic neoplasms [34▪▪] | The FELIX Project presents a suite of deep learning algorithms designed to recognize pancreatic lesions from CT images without human input. The deep networks were developed to detect pancreatic neoplasms, particularly PDACs. | >95% specificity and >95% sensitivity. The models also showcase the ability to generalize to other institutions and other pancreatic tumor types | Collected ∼2000 CT abdominal images, including healthy, PDAC, PanNET and cyst |
| Towards a single unified model for effective detection, segmentation, and diagnosis of eight major cancers using a large collection of CT scans [35▪] | Chen et al. Developed a Unified Tumor Transformer (UniT) model for detecting and diagnosing eight major cancers in CT scans using a query-based Mask Transformer with multiorgan and multitumor semantic segmentation | 95% sensitivity for pancreatic cancer | 10 042 patients with CT images of eight cancer types and noncancer tumors; 631 patients in the test set |
| Meta-information-aware dual-path transformer for differential diagnosis of multitype pancreatic lesions in multiphase CT [36▪] | The study introduces a meta-information-aware dual-path transformer for pancreatic lesion classification and segmentation | The method outperforms previous baselines and approaches to the accuracy of radiology reports. | 3096 patients with multiphase CT scans |
CNN, convolutional neural network; PDAC, pancreatic ductal adenocarcinoma.
Notably, there has been a growing interest in developing deep learning algorithms that can recognize multiple tumor types or provide multiorgan and multitumor semantic segmentation. Chen et al.'s [35▪] Unified Tumor Transformer (UniT) model exemplify this approach, detecting and diagnosing eight major cancers from CT scans. Furthermore, the FELIX Project presents a suite of deep learning algorithms designed to recognize pancreatic lesions, specifically PDAC [34▪▪].
DISCUSSION
In summary, there are exciting recent advances in AI, specifically DL and radiomics approaches, for the diagnosis of pancreatic cancer using medical imaging modalities such as CT and MRI. Looking forward, there are multiple potential avenues for future research as well as a need to address the challenges in translating these findings into clinical practice.
Fusion models
Integrating DL and radiomics approaches could potentially lead to more powerful diagnostic tools for pancreatic cancer. Combining these methods may result in enhanced model performance, benefiting from the strengths of both techniques, as suggested by several studies [38–40]. Future research should investigate the development and application of fusion models in pancreatic cancer diagnosis using medical imaging.
Public large datasets and benchmarks
Most studies discussed in this review utilized internal datasets, which could introduce bias when comparing model performance. There is an urgent need to develop large volume public datasets and standardized benchmarks to address this issue. These resources would facilitate more accurate model comparisons and improve the generalizability of findings across different patient populations and institutions.
Generalizability across tumor types, domains, and organs
Future research should focus on developing models that can be used across different types of pancreatic neoplasia, such as PDAC and IPMN. Additionally, there is potential for creating models capable of generalizing across organs, which could improve healthcare practice by enabling the diagnosis of various tumor types using a single model. However, this presents challenges, as different organs have varying difficulty levels regarding segmentation and classification. For instance, abdominal segmentation, particularly for the pancreas, is more challenging than brain segmentation due to the complex shapes and structures involved. Developing automatic or self-adaptive models that can effectively address these challenges remains an open question.
Integration of diagnostic functions into computer-aided diagnosis systems
Recent research has begun to explore the integration of various diagnostic procedures, such as preprocessing, segmentation, and classification, into CAD systems for pancreatic cancer. This holistic approach could streamline the diagnostic process and improve the efficiency of medical imaging-based pancreatic cancer diagnosis. Future studies should continue investigating the development and implementation of such integrated CAD systems.
DL and radiomics with medical imaging have shown great promise for improving the diagnostic accuracy of pancreatic cancer, facilitating personalized treatment planning, and identifying prognostic and predictive biomarkers. However, challenges remain in translating these research findings into clinical practice. By addressing these challenges and focusing on the areas discussed above, future research can continue to advance the field of pancreatic cancer diagnosis using medical imaging.
CONCLUSION
In conclusion, the application of artificial intelligence techniques, specifically deep learning and radiomics, for the diagnosis of pancreatic cancer (especially at the early stage) using cross-sectional imaging modalities such as CT and MRI has shown significant potential for improving diagnostic accuracy and patient outcomes. This review has highlighted recent advances:
-
(1)
Radiomics methods have made significant progress in pancreatic cancer diagnosis, integrating clinical features, employing feature selection techniques, and utilizing various machine learning classifiers such as SVM and XGBoost, with recent advances focusing on application aspects and comparisons between CT and MRI imaging modalities.
-
(2)
Deep learning approaches have demonstrated potential in automating pancreatic tumor detection and classification by employing CNNs, transformer-based models, and novel deep learning architectures focusing on pancreatic lesions, organ, and tumor segmentation, as well as incorporating auxiliary information to further improve model performance.
Despite the progress made thus far, several challenges remain to be addressed before these methods can be fully integrated into clinical practice. These challenges include the development of fusion models, the establishment of large volume public datasets and standardized benchmarks, the improvement of generalizability across tumor types, domains, and organs, and integrating these functions into CAD systems.
To move the field forward, researchers must address these challenges and refine the current methods to enhance their utility in the clinical setting. Future research should focus on developing more robust, interpretable, and clinically relevant models that can leverage the complementary information from both methods and improve the personalized management of pancreatic cancer patients. The collaboration between experts in AI, medical imaging, pancreatology and oncology will be vital in advancing the field and ultimately improving the early and accurate diagnosis of pancreatic cancer. These efforts, if successful, will contribute to better treatment planning and personalized care, ultimately improving patient outcomes.
Acknowledgements
We thank our close collaborators for their significance guidance and help in our projects: Rajesh N. Keswani, Amir A. Bourhani, Frank H. Miller, Michael Wallace.
Financial support and sponsorship
This project is supported by NIH funding: R01-CA246704 and R01-CA240639.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Bakasa W, Viriri S. Pancreatic cancer survival prediction: a survey of the state-of-the-art. Comput Math Methods Med 2021; 2021:1188414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg 2013; 257:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn SS, Kim MJ, Choi JY, et al. Indicative findings of pancreatic cancer in prediagnostic CT. Eur Radiol 2009; 19:2448–2455. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka S, Nakaizumi A, Ioka T, et al. Main pancreatic duct dilatation: a sign of high risk for pancreatic cancer. Jpn J Clin Oncol 2002; 32:407–411. [DOI] [PubMed] [Google Scholar]
- 5.Bera K, Braman N, Gupta A, et al. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol 2022; 19:132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preuss K, Thach N, Liang X, et al. Using quantitative imaging for personalized medicine in pancreatic cancer: a review of radiomics and deep learning applications. Cancers 2022; 14:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granata V, Grassi R, Fusco R, et al. Pancreatic cancer detection and characterization: state of the art and radiomics. Eur Rev Med Pharmacol Sci 2021; 25:3684–3699. [DOI] [PubMed] [Google Scholar]
- 8.Marti-Bonmati L, Cerdá-Alberich L, Pérez-Girbés A, et al. Pancreatic cancer, radiomics and artificial intelligence. Br J Radiol 2022; 95:20220072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Ma X, Wang YR, Zhuo LY, et al. Retrospective analysis of the value of enhanced CT radiomics analysis in the differential diagnosis between pancreatic cancer and chronic pancreatitis. Int J Gen Med 2022; 15:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study combines radiomics with clinical feature that requires invasive procedure. It establishes the feasibility of enhanced CT radiomics analysis to differentiate between pancreatic cancer and chronic pancreatitis.
- 10▪.Qureshi TA, Gaddam S, Wachsman AM, et al. Predicting pancreatic ductal adenocarcinoma using artificial intelligence analysis of prediagnostic computed tomography images. Cancer Biomarkers 2022; 33:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows a standard radiomics study pipeline with 108 retrospective CT scans with an ability of generalization. It indicates CT radiomics can unveilt micro-level changes in the pancreas.
- 11▪.Mukherjee S, Patra A, Khasawneh H, et al. Radiomics-based machine-learning models can detect pancreatic cancer on prediagnostic computed tomography scans at a substantial lead time before clinical diagnosis. Gastroenterology 2022; 163:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study evaluates 4 ML classifiers for the task of detecting pancreatic cancer with CT radiomics. The authors suggest that ML models have the potential to detect PDAC from normal pancreas better than human experts.
- 12▪.Gai T, Thai T, Jones M, et al. Applying a radiomics-based CAD scheme to classify between malignant and benign pancreatic tumors using CT images. J Xray Sci Technol 2022; 30:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study develops a CAD system for radiomics-based pancreatic cancer detection, including image-preprocessing, pancreas segmentation, tumor detection and radiomics-based classification. It supports further efforts on CAD schemes.
- 13▪▪.Flammia F, Innocenti T, Galluzzo A, et al. Branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs): an MRI-based radiomic model to determine the malignant degeneration potential. Radiol Med 2023; 128:383–392. [DOI] [PubMed] [Google Scholar]; This study identifies MRI radiomics features linked to a higher risk of malignant IPMN, which can serve as a reference for future radiomics study.
- 14▪.Cheng S, Shi H, Lu M, et al. Radiomics analysis for predicting malignant potential of intraductal papillary mucinous neoplasms of the pancreas: comparison of CT and MRI. Acad Radiol 2022; 29:367–375. [DOI] [PubMed] [Google Scholar]; This article compares radiomics from CT and MRI to determine which is a better modality for predicting IPMN risk. This article shows direct comparison of different imaging techniques.
- 15.Casà C, Piras A, D’Aviero A, et al. The impact of radiomics in diagnosis and staging of pancreatic cancer. Ther Adv Gastrointest Endosc 2022; 15:26317745221081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Si K, Xue Y, Yu X, et al. Fully end-to-end deep-learning-based diagnosis of pancreatic tumors. Theranostics 2021; 11:1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu KL, Wu T, Chen PT, et al. Deep learning to distinguish pancreatic cancer tissue from noncancerous pancreatic tissue: a retrospective study with cross-racial external validation. Lancet Digital Health 2020; 2:e303–e313. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Z, Xia Y, Xie L, et al. Multiscale coarse-to-fine segmentation for screening pancreatic ductal adenocarcinoma. In: Medical Image Computing and Computer Assisted Intervention–MICCAI 2019: 22nd International Conference, Shenzhen, China, October 13–17, 2019, Proceedings, Part VI 22. Springer International Publishing; 2019:3–12. [Google Scholar]
- 19.Chu LC, Park S, Kawamoto S, et al. Application of deep learning to pancreatic cancer detection: lessons learned from our initial experience. J Am Coll Radiol 2019; 16:1338–1342. [DOI] [PubMed] [Google Scholar]
- 20.Hussein S, Kandel P, Bolan CW, et al. Lung and pancreatic tumor characterization in the deep learning era: novel supervised and unsupervised learning approaches. IEEE Trans Med Imaging 2019; 38:1777–1787. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Li Y, Zhang Z, et al. Hyper-pairing network for multiphase pancreatic ductal adenocarcinoma segmentation. In: Medical Image Computing and Computer Assisted Intervention–MICCAI 2019: 22nd International Conference, Shenzhen, China, October 13–17, 2019, Proceedings, Part II 22. Springer International Publishing; 2019:155–163. [Google Scholar]
- 22.Alves N, Schuurmans M, Litjens G, et al. Fully automatic deep learning framework for pancreatic ductal adenocarcinoma detection on computed tomography. Cancers 2022; 14:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hameed BS, Krishnan UM. Artificial intelligence-driven diagnosis of pancreatic cancer. Cancers 2022; 14:5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoudi T, Kouzahkanan ZM, Radmard AR, et al. Segmentation of pancreatic ductal adenocarcinoma (PDAC) and surrounding vessels in CT images using deep convolutional neural networks and texture descriptors. Sci Rep 2022; 12:3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonozuka R, Itoi T, Nagata N, et al. Deep learning analysis for the detection of pancreatic cancer on endosonographic images: a pilot study. J Hepatobiliary Pancreat Sci 2021; 28:95–104. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Li S, Wang Z, Lu Y. A novel and efficient tumor detection framework for pancreatic cancer via CT images. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE; 2020:1160–1164. [DOI] [PubMed] [Google Scholar]
- 27.Schulz D, Schmid R, Abdelhafez M. Deep learning can accurately distinguish between low grade dysplasia and high grade dysplasia/invasive carcinoma in IPMN by unilizing endosonographic images. Endoscopy 2022; 54 (S 01):e421.34496434 [Google Scholar]
- 28.Schulz D, Heilmaier M, Phillip V, et al. Accurate prediction of histological grading of intraductal papillary mucinous neoplasia using deep learning. Endoscopy 2023; 55:415–422. [DOI] [PubMed] [Google Scholar]
- 29▪.Park HJ, Shin K, You MW, et al. Deep learning–based detection of solid and cystic pancreatic neoplasms at contrast-enhanced CT. Radiology 2023; 306:140–149. [DOI] [PubMed] [Google Scholar]; This study shows proof that deep learning-based approach demonstrates comparably high performance with radiologists in identifying patients with various solid and cystic pancreatic lesions at CT.
- 30▪.Althobaiti MM, Almulihi A, Ashour AA, et al. Design of optimal deep learning-based pancreatic tumor and nontumor classification model using computed tomography scans. J Healthc Eng 2022; 2872461. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; This study combines efficient operations to develop a deep learning algorithm (ODL-PTNTC) to detect pancreatic cancer from CT scans. The model demonstrates promising ability to classify pancreatic cancer.
- 31▪▪.Chen PT, Wu T, Wang P, et al. Pancreatic cancer detection on CT scans with deep learning: a nationwide population-based study. Radiology 2023; 306:172–182. [DOI] [PubMed] [Google Scholar]; This study develops an CNN-based end-to-end CAD system for pancreatic cancer detection from CT scans. It involves a large real world dataset of 1279 patients.
- 32▪.Vaiyapuri T, Dutta AK, Punithavathi IH, et al. Intelligent deep-learning-enabled decision-making medical system for pancreatic tumor classification on CT images. Healthcare 2022; 10:677.MDPI. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study develops a novel end-to-end approach (IDLDMS-PTC) for pancreatic cancer detection from CT scans, encompassing efficient operations at each processing stage. The model shows promising performance over the existing methods.
- 33▪▪.Proietto Salanitri F, Bellitto G, Palazzo S, et al. Neural transformers for intraductal papillary mucosal neoplasms (IPMN) classification in MRI images. Annu Int Conf IEEE Eng Med Biol Soc 2022; 2022:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study first applies transformer-based deep learning model on the task of IPMN classification with MRI scans. This study indicates that transformers have more flexibility and interpretability compared to CNN counterparts.
- 34▪▪.Xia Y, Yu Q, Chu L, et al. The felix project: deep networks to detect pancreatic neoplasms. medRxiv 2022; 2022–2029. [Google Scholar]; The FELIX Project presents a suite of deep learning algorithms designed to recognize pancreatic lesions from CT images without human input, reaching a specificity of >95%. It involves around 2000 CT abdominal images.
- 35▪.Chen J, Xia Y, Yao J, et al. Towards a single unified model for effective detection, segmentation, and diagnosis of eight major cancers using a large collection of CT scans. arXiv preprint arXiv:2301.12291. 2023. [Google Scholar]; UniT is a query-based Transformer for tumor segmentation and detection. Pancreas is among 8 organs that UniT is trained for. This study moves one step closer to a universal high-performance cancer screening tool.
- 36▪.Springer, Cham, Zhou B, Xia Y, Yao J. Frangi A, de Bruijne M, Wassermann D, Navab N, et al. Meta-information-aware dual-path transformer for differential diagnosis of multitype pancreatic lesions in multiphase CT. Information processing in Medical Imaging. IPMI 2023. Lecture Notes in Computer Science, vol 13939 2023. [Google Scholar]; This study is another exploration on transformer-based model on pancreatic lesions classification. It incorporates patient meta-information for better diagnosis.
- 37.Chen J, Lu Y, Yu Q, et al. Transunet: transformers make strong encoders for medical image segmentation. arXiv preprint arXiv:2102.04306. 2021. [Google Scholar]
- 38.Dmitriev K, Kaufman AE, Javed AA, et al. Classification of pancreatic cysts in computed tomography images using a random forest and convolutional neural network ensemble. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2017: 20th International Conference, Quebec City, QC, Canada, September 11-13, 2017, Proceedings, Part III 20 (pp. 150–158). Springer International Publishing. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee S, Patra A, Khasawneh H, et al. Radiomics-based machine learning models can detect pancreatic cancer on prediagnostic CTs at a substantial lead time prior to clinical diagnosis. Gastroenterology 2022; 163:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei W, Jia G, Wu Z, et al. A multidomain fusion model of radiomics and deep learning to discriminate between PDAC and AIP based on 18F-FDG PET/CT images. Jpn J Radiol 2022; 41:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xuan W, You G. Detection and diagnosis of pancreatic tumor using deep learning-based hierarchical convolutional neural network on the Internet of medical things platform. Future Gener Comput Syst 2020; 111:132–142. [Google Scholar]
- 42.Qureshi TA, Javed S, Sarmadi T, et al. Artificial intelligence and imaging for risk prediction of pancreatic cancer. Chinese Clin Oncol 2022; 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado NO, Al Qadhi H, Al Wahibi K. Intraductal papillary mucinous neoplasm of pancreas. N Am J Med sci 2015; 7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ha J, Choi SH, Kim KW, et al. MRI features for differentiation of autoimmune pancreatitis from pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Digest Liver Dis 2022; 54:849–856. [DOI] [PubMed] [Google Scholar]