Abstract
Cytotoxic T lymphocytes (CTL) appear to be critical in resolving or reducing the severity of lentivirus infections. Retroviral vectors expressing the Gag/Pr or SU protein of the lentivirus equine infectious anemia virus (EIAV) were constructed and used to evaluate EIAV-specific CTL responses in horses. Three promoters, cytomegalovirus, simian virus SV40, and Moloney murine sarcoma virus (MoMSV) long terminal repeat (LTR), were used, and there was considerable variation in their ability to direct expression of Gag/Pr and SU. Vectors expressing EIAV proteins under the direction of MoMSV LTR and using the gibbon ape leukemia virus (GALV) Env for internalization were efficient at transducing equine kidney (EK) target cells and were effective targets for EIAV-specific CTL lysis. CTL from EIAV-infected horses caused lysis of retroviral vector-transduced EK cells expressing either Gag/Pr or SU in an ELA-A-restricted manner. In contrast, lysis of recombinant vaccinia virus-infected EK cells expressing Gag/Pr and SU/TM was often non-LA-A restricted. Five horses were immunized by direct intramuscular injection with a mixture of retroviral vectors expressing Gag/Pr or SU, and one responded with EIAV-specific CTL. This result indicates that retroviral vector stimulation of CTL in horses needs to be optimized, perhaps by inclusion of appropriate cytokine genes in the constructs. However, the studies demonstrated that retroviral vector-transduced target cells were very effective for in vitro dissection of EIAV-specific CTL responses.
Equine infectious anemia virus (EIAV) is a naturally occurring lentivirus causing disease in horses worldwide (5). Affected animals have episodes of viremia, which are variable in duration and severity, with concomitant anemia, thrombocytopenia, and fever (8, 26). During the first year of infection, these episodes become less frequent and of decreasing severity; more than 90% of affected horses progress to the inapparent carrier state characterized by persistent low viral loads but no apparent clinical disease (22, 33). Initial viremia can be detected as early as 10 days postinfection, with titers reaching as high as 106 50% tissue culture infective doses/ml of plasma (7). These high viral loads allow for horizontal transmission by flies of the Tabanid family that transfer residual, virus-laden blood, on their mouthparts following interrupted feeding (15). Despite high virus titers during these initial episodes, horses control these episodes of EIAV with remarkable regularity. This control, evidenced by progression to the inapparent carrier state, makes EIAV a useful model for the identification of host-virus interactions that can suppress lentivirus replication and the resulting disease.
It has been demonstrated that immune mechanisms are involved in the suppression of EIAV replication by evaluating infection in severe combined immunodeficient (SCID) Arabian foals (40). Foals with this genetic disease lack functional B and T lymphocytes and fail to reduce the initial plasma viremia following inoculation with EIAV, eventually succumbing to disease; in contrast, normal immunocompetent Arabian foals terminate initial plasma viremias. Multiple immune mechanisms have been implicated in the control of EIAV, including the generation of neutralizing antibodies and EIAV-specific cytotoxic T lymphocytes (CTL) (11, 18, 27, 36, 40). Antibody-dependent cellular cytotoxicity (ADCC) is apparently not involved in maintenance of the carrier state, as ADCC-mediating antibodies cannot be detected (48). Neutralizing antibodies which are EIAV variant specific arise following episodes of plasma viremia, contributing to clearance of cell-free virus (18, 38, 51). Normal horses treated by the passive transfer of plasma containing EIAV-specific neutralizing and nonneutralizing antibodies delayed seroconversion following EIAV challenge, but not infection, suggesting a protective role for antibody (44). However, EIAV, like other lentiviruses, undergoes rapid genotypic mutation during RNA-dependent DNA polymerization by an error-prone reverse transcriptase (3). These mutations result in the appearance of antigenic virus variants not recognized by neutralizing antibodies specific for previous variants (4, 18, 33, 36). Proviral integration and subsequent antigenic variation limit the effectiveness of neutralizing antibodies and suggest that other mechanisms, possibly CTL, are also important in lentivirus control.
EIAV-specific, major histocompatibility complex (MHC) class I-restricted, CD8+ CTL are detected as early as 10 days postinfection and recognize cells expressing target antigens without requiring in vitro stimulation (27). These effector CTL (CTLe) persist for as long as 3 months postinfection (27), while relatively high numbers of memory CTL (CTLm) persist in inapparent carriers for years (27, 28). It has been demonstrated that EIAV-specific CTLe and CTLm are directed against multiple proteins (11, 27, 28). Inapparent carrier horses treated with immunosuppressive doses of corticosteroids experience recrudescence of plasma viremia and disease and then suppress virus replication before detectable type-specific neutralizing antibodies develop, further suggesting that CTL have a role in virus control (19).
Further evaluation of the role of EIAV-specific, MHC class I-restricted CTL in the control of EIAV requires the induction of such CTL in horses. Retroviral vectors have been used extensively for gene transfer, and even though these vectors have the potential to present epitopes by the endogenous processing pathway, there are only a few studies of their use for inducing CTL. There are reports demonstrating that retroviral vectors induce CTL responses in mice, baboons, rhesus macaques, and humans (14). These promising results prompted us to make retroviral vectors containing the genes encoding the EIAV Gag/Pr and SU proteins. Retroviral vectors made to express these EIAV proteins by using a Moloney murine sarcoma virus (MoMSV) long terminal repeat (LTR) promoter made Gag and SU proteins detectable by immunobloting. Equine kidney (EK) target cell lines transduced with these retroviral vectors were efficient targets of MHC class I-restricted EIAV-specific CTL. The retroviral vectors expressing Gag and SU proteins were evaluated for their ability to induce EIAV-specific CTL in five naive horses. One of these inoculated horses developed MHC class I-restricted CTL to EIAV-infected target cells. It was clear from these studies that retroviral vectors expressing EIAV proteins could be exploited for in vitro dissection of CTL responses including epitope mapping but that further development was needed to increase the percentage of horses responding to inoculation.
MATERIALS AND METHODS
Experimental horses and EK cell lines.
Three adult horses (mixed-breed ponies) were infected with EIAVWSU5 as previously described (27). All genes were derived from EIAVWSU5, and this strain was used in all in vitro studies described in this report. Prior to infection with EIAV, primary EK cell lines for future CTL assays were established from percutaneous kidney biopsy samples (27). The EIAV-infected horses in this study had at least one episode of fever, viremia, and thrombocytopenia following inoculation. They were defined as inapparent carriers because they had no fever, viremia, or thrombocytopenia during the course of this study. Adult horses (mixed-breed ponies) that were EIAV negative as determined by lack of antibody to the p26 capsid protein (6) were also used. Expression of equine lymphocyte alloantigen-A (ELA-A) alleles encoding for MHC class I molecules were determined for each horse by previously described serologic tests and reagents (23).
Retroviral vector construction.
Two unique retroviral vectors were constructed by using the parent plasmid pLXSN (provided by A. Dusty Miller, Fred Hutchinson Cancer Research Center, Seattle, Wash.) (31). The first, pLGSN, encoded the gag and 5′ pol region corresponding to nucleotides 462 to 2525 (17). The gag/pr insert was PCR amplified from pEIA5G that contains a 2.2-kb EIAVWSU5 cDNA encoding nucleotides 340 to 2578 (25). The 5′ PCR primer, GCGCGAATTCAAGATGGGAGACCCTTTGAC, encoded an EcoRI restriction enzyme site (underlined), a Kozak consensus sequence (bold) (20), and a start codon (italics). The 3′ PCR primer, CGCCTCGAGGGGTCAAGCAATCCTCTGGA, encoded an XhoI restriction enzyme site (underlined). PCR amplification conditions were as follows: 96°C for 2 min; 30 cycles of 95°C for 1 min, 57°C for 1 min, and 72°C for 1 min, with a 10-s autoextend; and finally 72°C for 7 min. Reactions (100 μl) were carried out with Perkin-Elmer PCR core kit reagents, using 2.5 mM MgCl2, 200 μM nucleotides, 2.5 U of Taq DNA polymerase, and 0.2 μM primers. The amplified 2,045-bp product was double digested with EcoRI and XhoI, separated on 1.5% SeaPlaque GTG low-melting-point agarose, and eluted. This fragment was directionally ligated into pLXSN that was EcoRI-XhoI digested and dephosphorylated. The insert was placed downstream and under the direction of the MoMSV LTR and upstream from the neomycin phosphotransferase gene (neo), which is under the control of the simian virus 40 (SV40) early promoter (31).
The second retroviral vector plasmid, pLGP90SN, encoded the gp90 (SU) of EIAVWSU5 (24) corresponding to nucleotides 5312 to 6644 (17). The gp90 insert was PCR amplified from the parent plasmid pSCEIA5E, which encodes the EIAVWSU5 env gene (27). The 5′ PCR primer, GCGCGAATTCAACATGGTCAGCATGGCATT, encoded an EcoRI restriction enzyme site (underlined), a Kozak consensus sequence (bold), and a start codon (italics). The 3′ PCR primer, GCGCCTCGAGTCATCTCTTATGTCTAATTAG, encoded an XhoI restriction enzyme site (underlined) and a stop codon (bold). PCR amplifications were carried out as described for the gag/pr insert, and the 1,335-bp fragment was directionally ligated into EcoRI-XhoI-digested and dephosphorylated pLXSN.
To compare the effect of different promoters on expression and efficiency of transduced EK cells as CTL targets, we constructed three other retroviral vectors which utilized the cytomegalovirus (CMV) or SV40 promoter to drive expression of the inserted EIAV genes. Similar PCR cloning strategies were used to place the genes encoding SU and Gag/Pr downstream of the SV40 and CMV promoters in the parent plasmids pLNSX and pLNCX, respectively (31). pLNCGP90 and pLNCG encoded SU and Gag/Pr, respectively, under the direction of the CMV promoter. pLNSG encoded Gag/Pr under the direction of the SV40 promoter. Fidelity and orientation of the final constructs were confirmed by DNA sequence analysis of the 5′ and 3′ ends.
Generation of stable retroviral vector-producing cell lines.
To generate vector-producing cell lines, modifications of established protocols were used (31). Six-centimeter-diameter tissue culture dishes were seeded with the amphotropic packaging cell line PA317 (American Type Culture Collection CRL 9078) in the logarithmic phase of growth at a density of 5 × 106 cells/dish. They were cultured overnight in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) (complete DMEM), then transfected with 50 μg of pLXSN, pLGSN, pLGP90SN, pLNSG, pLNCG, or pLNCGP90 DNA admixed with 12 μg of Lipofectamine (Gibco-BRL, Gaithersburg, Md.) in 2 ml of Opti-MEM (Gibco-BRL) serum-free medium, and incubated for 6 h at 37°C in 5% CO2; 2 ml of DMEM containing 20% FBS was then added, and the mixture was incubated overnight. On day 3, the medium was changed to complete DMEM, and another 6-cm-diameter tissue culture dish was seeded with 105 pseudotyped gibbon ape leukemia virus (GALV) packaging cells (American Type Culture Collection CRL 10686) and cultured overnight in complete DMEM. On day 4, PG13 packaging cells were transduced with 1 ml of supernatant harvested from the transfected PA317 cells after passage through 0.45-μm-pore-size filters and addition of Polybrene (4 μg/ml; Sigma Chemical, St. Louis, Mo.). The next day, the transduced PG13 cells were trypsinized and passaged to 75-cm2 tissue culture flasks (Corning) in complete DMEM with G418 sulfate (750 μg/ml; Gibco-BRL). Transduced cells were then expanded into 850-cm2 roller bottles in medium containing 400 μg of G418 sulfate per ml. Supernatants were collected at 16- to 24-h intervals, centrifuged at 3,000 × g for 5 min, filtered (0.45-μm-pore-size filter), and frozen at −80°C. Packaging cells produced retroviral vectors designated vLXSN, vLGSN, vLGP90SN, vLNSG, vLNCG, and vLNCGP90.
Transduced packaging cells were cloned as described above, with the following modifications. On day 3, 6-cm-diameter tissue cultures dishes were plated with 2 × 105 PG13 cells. On day 4, PG13 cells were transduced with 10, 1.0, and 0.1 μl of each PA317 cell supernatant containing the vectors vLXSN, vLGSN, vLGP90SN, vLNSG, vLNCG, and vLNCGP90 described above. On day 5, transduced PG13 cells were passaged into 100-mm-diameter tissue culture dishes in complete DMEM containing 750 μg of G418 sulfate per ml. Individual resistant colonies were isolated with cloning rings and placed into 24-well plates and expanded, and supernatants were harvested, filtered, and tested.
Titration of retroviral vector-containing supernatants.
Titers were determined on EK cells growing in logarithmic phase as previously described (30). Briefly, three 100-mm-diameter tissue culture dishes per supernatant were seeded with 5 × 105 EK cells per dish. On day 2, various amounts of test supernatant were added to each dish. On day 4, these cells were trypsinized, split 1:5, and placed in new 100-mm-diameter dishes in complete DMEM containing 700 μg of G418 sulfate per ml. Fresh medium was added every 3 days for 12 days in total. Surviving colonies were stained with crystal violet and counted. Transducing particles (TP) per milliliter was calculated as follows (30): [no. of colonies/volume of test supernatant (ml)] × (5/3).
Transduction of EK cells.
To establish stable transduced EK cell cultures, 75-cm2 tissue culture flasks were seeded with 7 × 105 EK cells growing in logarithmic phase. The following day, these cells were transduced with retroviral vector-containing supernatants at a multiplicity of infection (MOI) of 2, supplemented with Polybrene (4 μg/ml), and incubated overnight at 37°C. On day 3, the medium was changed to include DMEM plus 5% calf serum and 700 μg of G418 sulfate per ml. Medium was replaced every third day until cells were either used in a CTL assay or frozen for subsequent experiments.
Retroviral vector expression.
Cell lysates of vLGSN- and vLGP90SN-transduced EK cells were examined for EIAV protein expression by immunoblot analysis. Briefly, 2 × 106 transduced EK cells were lysed and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (25). Separated proteins were transferred to nitrocellulose and probed with a monoclonal antibody (MAb) specific for either the EIAV SU (30/8.12) or the capsid protein p26 (EIA6A1) (25, 39). Since vLNSG- and vLNCG-transduced EK cells did not express detectable Gag protein, total RNA was isolated from these cells by using an RNeasy kit (Qiagen) from 5 × 106 stably transduced EK cells and treated with DNase (Gibco-BRL). An RNA template was used in reverse transcriptase PCR (RT-PCR) under the following conditions: reverse transcription for 30 min at 60°C; denaturation for 2 min at 94°C; 40 cycles of 94°C for 45 s and 60° for 45 s; and finally 60°C for 7 min. Reactions (50 μl) were carried out with Perkin-Elmer RT-PCR kit reagents using 300 μM nucleotides, 2.5 mM magnesium acetate, 5.0 U of recombinant Thermus thermophilus DNA polymerase (rTth DNA polymerase; Perkin-Elmer), and 0.45 μM gag-specific primers, which generated a predicted 525-bp fragment.
CTL assays.
To determine if retroviral vector-transduced EK cells were recognized by EIAV-specific CTL, peripheral blood mononuclear cells (PBMC) were isolated from three EIAV-infected carrier horses by using Histopaque 1077. PBMC were placed in 24-well plates at 106 cells per well with 5 × 105 stimulator cells/well in 1 ml of DMEM supplemented with 10% FBS, 20 mM HEPES, and penicillin-streptomycin (100 U-100 μg/ml). EIAV-infected monocytes were used as stimulator cells as previously described (28), with minor modifications; 2 × 108 PBMC were irradiated, and monocytes were isolated on gelatin-coated flasks, and infected with EIAV at an approximate MOI of 5. Cultures were incubated for 7 days and then placed in 24-well plates at a density of 5 × 105 fresh PBMC per well with 5 × 105 stimulators per well and human recombinant interleukin-2 (IL-2; 10 U/ml; Sigma). After 14 days of incubation, dead cells were removed by using Histopaque 1077, and live cells were washed once, counted, and used as effectors cells in CTL assays (28); 3 × 104 EK cell targets, previously transduced with retroviral vectors and under G418 selection for a minimum of 10 days, were plated in 96-well collagen-coated plates; target cells were labeled with 51Cr; effectors were added to experimental wells at effector-to-target cell ratios of 20:1, 10:1, and 5:1 and incubated for 17 h at 37°C in 5% CO2. Percent specific lysis was calculated as [(E − S)/(T − S)] × 100, where E is the mean release from six wells containing EK cells with effectors, S is the mean spontaneous release from six wells containing labeled EK cells but lacking effectors, and T is the mean total release from six wells containing labeled EK cells treated with 2% Triton X-100 in distilled water. Calculation of the standard error (SE) of percent specific lysis accounted for the variability in E, S, and M (45). CTL assays comparing vaccinia virus-infected and retroviral vector-transduced target cells were performed as described above except that vaccinia virus infections were carried out as previously described (27, 28).
Inoculation of horses with retroviral vectors expressing Gag/Pr and SU proteins.
Five normal horses were inoculated with a mixture of the retroviral vectors vLGP90SN and vLGSN. Each of four injection sites, the left and right proximal sternothyroideus and the left and right semitendinosus muscles, 3 cm deep, was injected with 2 ml of 0.5% Bupivicaine. Four days later, a mixture containing 2 × 106 TP of vLGP90SN and 2 × 106 TP of vLGSN was divided and inoculated into the four pretreated injection sites; 14 and 28 days later, each horse was boosted with 2 × 107 TP of each vector, again divided among the four injection sites. Blood was collected 2, 4, and 6 weeks postinoculation, processed, and used in CTL assays.
RESULTS
Construction of retroviral vectors and transduction of EK cells.
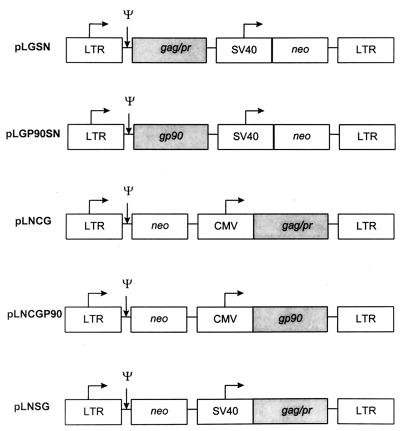
Five unique retroviral vectors encoding either the Gag/Pr or SU protein were constructed (Fig. 1). Two vectors encoding the SU, vLNCGP90 and vLGP90SN, utilized either the CMV or MoMSV LTR promoter. The remaining three vectors, designated vLNCG, vLNSG, and vLGSN, encoded Gag/Pr under the direction of the CMV, SV40, and MoMSV LTR promoters, respectively.
FIG. 1.
Retroviral vectors. The horizontal arrows indicate the direction of transcription. The 5′ and 3′ LTRs are from MoMSV. ψ represents the psi sequence necessary for packaging of full-length RNA and also contains splice donor and splice acceptor sequences. The SV40 early promoter and CMV immediate-early promoter, the selectable marker neo, and inserted EIAV-specific sequences (shaded regions) are indicated.
EK cells were transduced with vLGSN and vLGP90SN and placed under G418 sulfate selection to obtain stable cultures. Transduced cells cultured under selection conditions for 2 weeks and then in medium lacking G418 sulfate retained retroviral sequences for at least 30 days, as evidenced by the lack of visible cell death upon return to selection conditions and their use in subsequent CTL assays. Titers of vLXSN, vLGSN, and vLGP90SN, derived from PG13 packaging cells tested on EK cells were 5 × 106, 6 × 105, and 5 × 106 TP/ml, respectively. Similar techniques resulted in the generation of stable producer cell lines for the vectors vLNCG, vLNSG, and vLNCGP90.
Retroviral vector expression of EIAV Gag/Pr and SU proteins.
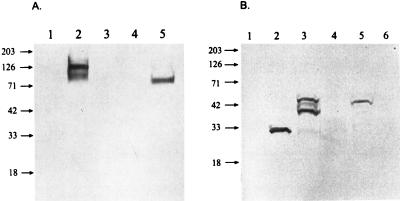
For immunoblots, transduced EK cells were placed under G418 sulfate selection for 2 weeks, cell lysates were separated by SDS-PAGE, and proteins were transferred to nitrocellulose and probed for expression. Positive control cell lysates infected with EIAVWSU5 had 125- and 105-kDa proteins reactive with anti-SU MAb 30/8.12, assumed to represent Env precursor and SU (Fig. 2A, lane 2). vLGP90SN-transduced EK cell lysates had a single reactive protein at 90 kDa corresponding to the SU but migrating at slightly lower molecular weight than SU from EIAV-infected cell lysates (Fig. 2A, lane 5). Positive control lysates of EK cells infected with EIAVWSU5 had a single protein reactive with anti-p26 MAb EIA6A1 (Fig. 2B, lane 2). Immunoblots of lysates from EK cells infected with recombinant vaccinia virus vGag/Pr, which contains the same EIAV genes as vLGSN, had anti-p26 MAb-reactive proteins of 55, 40, and 35 kDa (Fig. 2B, lane 3). Lysates of cells transduced with vLGSN had similar anti-p26 MAb-reactive proteins except that the 55-kDa protein was predominant (Fig. 2B, lane 5). Proteolytic cleavage of the vLGSN-expressed 55-kDa precursor protein to p26 was not evident. Lysates from negative control EK cells were unreactive with the MAb (Fig. 2).
FIG. 2.
Immunoblot of Env proteins expressed by EIAV-infected EK cells and retroviral vector-transduced EK cells reacted with MAb 30/8.12 (A) and MAb EIA6A1 (B). (A) Lysates from normal (lane 1), EIAV-infected (lane 2), vLXSN-transduced (lane 3), vLGSN-transduced (lane 4), and vLGP90SN-transduced (lane 5) EK cells. (B) Lysates from normal (lane 1), EIAV-infected (lane 2), recombinant vaccinia virus (VGag/Pr)-infected (lane 3), vLXSN-transduced (lane 4), vLGSN-transduced (lane 5), and vLGP90SN-transduced (lane 6) EK cells. Sizes are indicated in kilodaltons.
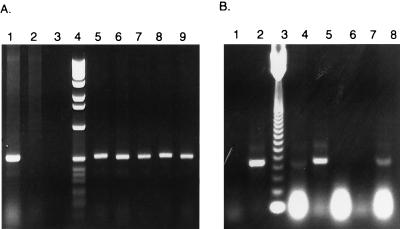
Detectable Gag protein expression was not evident from bulk cultures or clones of EK cells transduced with vectors utilizing the SV40 (vLNSG) and CMV (vLNCG) promoters. However, all clones of vLNCG- and vLNSG-transduced EK cells tested contained proviral DNA, as evidenced by amplification of the predicted 525-bp gag fragment (Fig. 3A shows results for vLNCG). Total RNA was isolated from the five clones used for Fig. 3A and amplified by RT-PCR to determine if gag-encoded mRNA was transcribed. Only two produced a transcript of the correct size; another appeared to produce a slightly truncated message (Fig. 3B). Clones producing detectable transcript were further evaluated for protein expression by direct fluorescent antibody assay (36). No detectable Gag expression was evident, consistent with observations by other investigators (46). However, immediately following transfection with pLNCG and pLNSG plasmid DNAs, Gag protein expression was easily detected in EK cells by direct fluorescent antibody. This suggested that the CMV and SV40 promoters were initially functional in EK cells, but expression was blocked at the transcriptional or translational level over time, especially in transduced cells.
FIG. 3.
PCR and RT-PCR analysis of five vLNCG-transduced EK cell clones. (A) Detection of EIAV gag-specific DNA from EIAV-infected EK and vLNCG-transduced EK cell genomic DNA. Lanes contain PCR mixtures with DNA from EIAV-infected EK cells (lane 1), normal EK cells (lane 2), no-DNA control (lane 3), 1-kb ladder (lane 4), and vLNCG-transduced EK cell clones 1 to 5 (lanes 5 to 9, respectively). (B) Detection of EIAV gag-specific RNA from EIAV-infected and vLNCG-transduced EK cells. Lanes contain RT-PCR reaction mixtures from no-RNA control (lane 1), EIAV-infected EK cell total RNA (lane 2), 123-bp ladder (lane 3), and total RNA from vLNCG-transduced EK cell clones 1 to 5 (lanes 4 to 8, respectively).
CTL activity against retroviral vector-transduced EK cells.
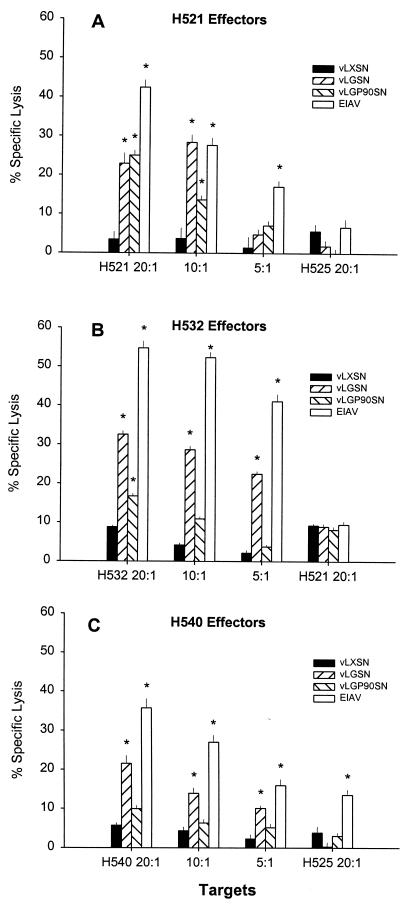
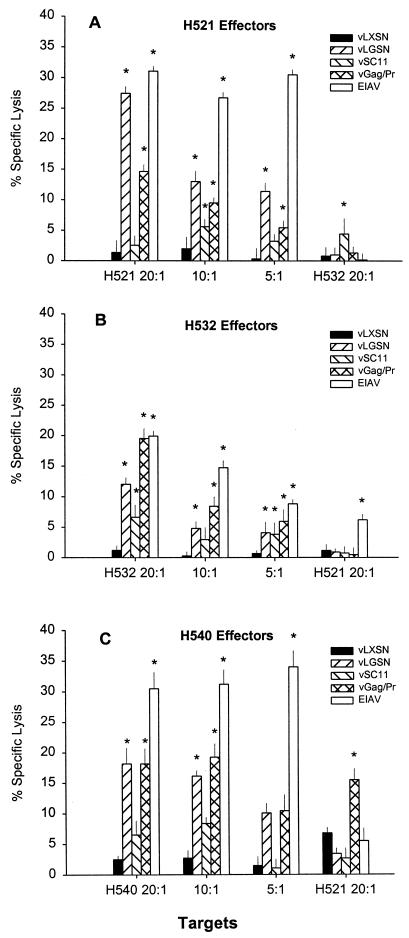
Assays were performed on EK target cells transduced with vLXSN, vLGSN, or vLGP90SN to determine if CTLm derived from EIAV-infected carrier horses H521, H532, and H540 recognized epitopes encoded by the retroviral vectors. CTLm from all three carrier horses caused significant killing of autologous EIAVWSU5-infected EK cell targets and of targets transduced with retroviral vectors expressing SU and Gag/Pr (Fig. 4). Specific lysis ranged from 22 to 33% for vLGSN-transduced targets and from 10 to 25% for vLGP90SN-transduced targets. Significant killing was defined as percent specific lysis that was 3 SE greater than that occurring with either autologous vLXSN-transduced EK target cells or ELA-A-mismatched vLGSN- and vLP90SN-transduced EK target cells. CTLm from H540 caused significant killing (14% specific lysis) of EIAV-infected ELA-A-mismatched EK target cells (Fig. 4C).
FIG. 4.
EIAV-specific CTL activity against retroviral vector-transduced and EIAV-infected EK cell targets at effector-to-target cell ratios of 20:1, 10:1, and 5:1. ELA-A-mismatched EK cell target controls (far right in each panel) were evaluated at a ratio of 20:1. Vertical lines represent SEs, and an asterisk denotes significant specific lysis that was 3 SE above that for vLXSN-transduced ELA-A-mismatched target EK cells.
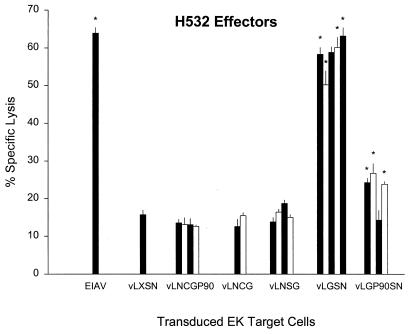
CTL assays were also performed to evaluate clones of EK cells transduced with vectors utilizing different internal promoters (Fig. 5). EIAV-specific CTL did not lyse those vectors utilizing the CMV and SV40 promoters for expression of EIAV proteins, including two clones of vLNCG-transduced EK cells that were positive for gag-specific message in Fig. 3B. The lack of CTL reactivity with vLNCGP90-, vLNCG-, and vLNSG-transduced targets was in marked contrast to clones of EK cells transduced with vLGSN and vLGP90SN, which utilized the MoMSV LTR for expression of Gag and SU proteins. Specific lysis of vLGSN-transduced EK cell clones was highest among the transduced targets and ranged 51 to 63% (Fig. 5). Three of four vLGP90SN-transduced EK target cell clones had statistically significant lysis ranging from 24 to 27%. EIAV-infected EK cells had 64% specific lysis, while negative-control vLXSN-transduced EK cells had 16% specific lysis.
FIG. 5.
EIAV-specific CTL activity against autologous retroviral vector-transduced EK cell clones and EIAV-infected EK cell targets. vLXSN is the negative control vector. Each column represents percent specific lysis on a vector-transduced target EK clone. Vertical lines represent SEs, and an asterisk denotes significant specific lysis that was 3 SE above that for vLXSN-transduced autologous target EK cells.
Vaccinia virus-infected and retroviral vector-transduced EK cells as CTL targets.
To compare retroviral vector-transduced with vaccinia virus-infected CTL targets, EK cells were infected with an MOI of 5 with a vaccinia virus construct (vGag/Pr) encoding for the same insert as vLGSN (Fig. 6). H521 effectors lysed vLGSN-transduced targets more efficiently (27%) than vGag/Pr-infected cells (15%) (Fig. 6A). However, H532 effectors caused significantly higher specific lysis (19.5%) of vGag/Pr-infected than of vLGSN-transduced targets (12%) (Fig. 6B). In another assay (Fig. 6C), utilizing H540 effectors, both targets had approximately 18% specific lysis. Of note, H540 effectors lysed ELA-A-mismatched vGag/Pr-infected target cells at the 15.5% level, in comparison to 3.4% with mismatched vLGSN-transduced EK cells (Fig. 6C).
FIG. 6.
EIAV-specific CTL activity against autologous retroviral vector-transduced (vLXSN and vLGSN), vaccinia virus-infected (vSC11 and vGag/Pr), and EIAV-infected EK cell targets at effector-to-target cell ratios of 20:1, 10:1, and 5:1. ELA-A-mismatched EK cell target controls (far right in each panel) and were evaluated at a ratio of 20:1. Vertical lines represent SEs, and an asterisk denotes significant specific lysis that was 3 SE above that for vLXSN-transduced, ELA-A-mismatched, target EK cells.
Induction of CTL in vivo with retroviral vectors.
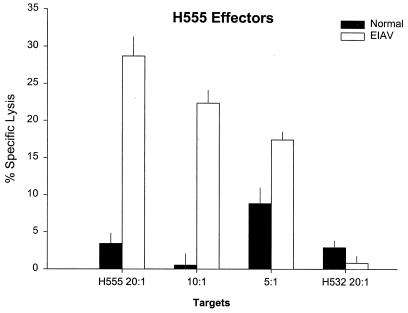
Five horses were inoculated with a mixture of the retroviral vectors vLGSN and vLGP90SN to determine if EIAV-specific CTL responses could be induced. None of the horses had CTLm before inoculation. Only one of the inoculated horses (H555) developed significant EIAV-specific CTLm activity (29% specific lysis) 2 weeks after the second inoculation (Fig. 7). CTL killing by H555 effectors was significant at all effector-to-target cell ratios tested at the 4- and 6-week time points, as defined by percent specific lysis that was 3 SE greater than that with either autologous EIAV-infected EK target cells or ELA-A-mismatched EIAV-infected EK target cells. Results of immunoblot analysis of sera 6 weeks after retroviral vector inoculation were negative at a 1:100 dilution against whole EIAV.
FIG. 7.
EIAV-specific CTL activity in H555 4 weeks after inoculation with vLGSN and vLGP90SN retroviral vectors. Autologous EIAV-infected EK cell targets were used at effector-to-target cell ratios of 20:1, 10:1, and 5:1; ELA-A-mismatched EIAV-infected EK cell targets were used at a ratio of 20:1 (far right). Vertical lines represent SEs.
DISCUSSION
This study describes the development and use of retroviral vectors to evaluate EIAV-specific CTL responses. Vector constructs expressing EIAV Gag/Pr and SU proteins which were efficient transducers of EK cells and expressed easily detectable levels of protein were identified. These vectors were also shown to be efficient targets for EIAV-specific lysis, in contrast to previous studies using a similar retroviral vector system for evaluation of feline immunodeficiency virus capsid-specific responses (46). SU-specific CTL responses were demonstrated in EIAV-infected horses, extending previous studies demonstrating Env-specific (SU and/or TM) CTL in both acutely and chronically infected horses on vaccinia virus-infected targets (27, 28). Furthermore, preliminary studies suggested that these vectors could also induce EIAV-specific CTL in vivo.
To determine which proteins are recognized by CTL and to express truncated genes for preliminary epitope mapping, investigators have used viral vectors, primarily vaccinia virus (12, 35, 47). Retroviral vectors have been used to transduce CTL target cells in a limited number of reports (14, 16, 49). We found retroviral vectors easier to develop than recombinant vaccinia viruses, as recombination, selection, and cloning were not necessary for establishing high-titer supernatants for CTL target cell transduction. Retroviral vector-transduced target cells have several other advantages, which include failure to down regulate MHC class I molecules (52), expression of only the protein of interest and the selectable marker neo, lack of cytotoxicity of transduced target cells, and failure to co-opt synthesis of host cell proteins (2). The disadvantages to these vectors include the necessity for in vitro cell line selection, though once expanded target cells can be frozen for future assays; the variability of expression, though again this disadvantage can be overcome by bulk target cell culture of target cells with demonstrated expression; and relative instability when utilizing some promoter-protein combinations as described below.
We constructed a total of six retroviral vectors expressing EIAV proteins which varied in the promoter and the order in which the gene of interest and the selectable marker were expressed. Significant differences among the vectors were found in levels of mRNA expression, protein expression, and cytolysis by EIAV-specific CTLm. EK cells transduced by retroviral vectors using the MoMSV LTR to promote expression of inserted genes upstream of the neo gene had consistently good EIAV protein expression and were consistently good CTL targets. Those vectors utilizing the CMV immediate-early promoter or SV40 early promoter and expressing the gene of interest downstream of the selectable marker neo did not express detectable levels of EIAV protein in either cloned EK cells or bulk EK cell cultures following selection with G418 sulfate. However, when these preparations were examined for insert-specific mRNA transcript, variable but occasionally detectable levels of mRNA were evident. Variability of expression in retroviral vector-transduced cells has been recognized in previous studies (46) and attributed to cell-specific promoter activity (41–43), DNA integration into regions of the genome not suitable for efficient transcription (9), or promoter selection (29). It is not evident which of these effects was responsible for the lack of protein expression by vectors using CMV and SV40 promoters; however, only vectors vLGSN and vLGP90SN, which used the MoMSV LTR to express EIAV proteins, were evaluated further.
vLGSN and vLGP90SN were both produced in the packaging cell line PG13, demonstrating that the GALV Env could be used to internalize retroviral vector virions into equine cell lines. vLGSN encoded the entire gag and 5′ pol gene of EIAV. The gag gene is expressed as a 55-kDa polyprotein and subsequently cleaved into four major internal proteins designated p15, p26, p11, and p9 by the protease encoded within the 5′ pol (13). Gag proteins account for approximately 80% of total EIAV virion structural proteins by weight, and significant sequence homology has been identified between the p26 of EIAV isolates and p24 of human immunodeficiency virus type 1 (13). This sequence homology is reflected in antigenic cross-reactivity and suggests interspecies conservation of lentiviral core proteins (10, 34). For these reasons, the gag gene was included in the vLGSN construct, whereas the protease gene was included to more closely mimic antigen presentation in wild-type EIAV-infected cells. In expression analysis, however, easily detectable levels of the Pr55 were noted, but detectable p26 cleavage was not evident in transduced EK cell lysates. This result does not preclude the possibility that undetectable levels of processing occurred, since in previous studies, processed p26 protein was not evident in vaccinia virus (vGag/Pr)-infected EK lysates without concentrating subviral particles (25). The EIAV env gene encodes two glycosylated proteins, SU (gp90) and TM (gp45) (1). The EIAV env sequence has insignificant identity with related lentiviruses, but significant structural, and therefore putative functional, similarities do exist (3, 17, 24, 38, 50). As in other lentivirus systems, significant CTL activity and antibody reactivity against the EIAV env gene products have been demonstrated, but further characterization is necessary (51). For these reasons, we constructed the retroviral vector vLGP90SN encoding EIAV SU, which expressed a single reactive 90-kDa protein on immunoblot analysis.
All EIAV-infected horses examined had significant CTL activity to Gag proteins, reflecting the possible immunodominance of these proteins. In contrast, the PBMC isolated from these same horses often had significant, but usually lower, SU-specific CTL activity. This may be due to the relative stability of Gag epitopes, allowing for the continued antigenic stimulation of CTLm in comparison to the more variable SU. Results of assays designed to compare the relative efficiencies of EIAV-specific CTL lysis of recombinant vaccinia virus-infected and retroviral vector-transduced EK cell targets were equivocal in that experimental results varied depending on donor horse (Fig. 6). Experimental variation in bulk CTL assays or differences in retroviral vector expression in EK cells from MHC class I-dissimilar donor horses may also explain the experimental results. In some assays, EK cell targets infected with EIAV or vGag/Pr were significantly killed by ELA-A-mismatched effectors, while the same EK cell targets transduced with the retroviral vectors were not killed (Fig. 4C and 6C). This may be because retroviral vectors do not perturb host cell machinery and do not cause decreased MHC class I molecule expression, thereby avoiding recognition by natural killer cells activated during the in vitro stimulation of PBMC (21). MHC class I-unrestricted CTL killing has been observed by other investigators and can be quenched by the addition of cold CTL targets (37).
The ability of retroviral vectors to stimulate EIAV-specific CTL in vivo was also examined. There is a paucity of information regarding the use of retroviral vectors in vivo for induction of antiviral immune responses, as only four studies have been reported (14, 16, 49, 52). A preliminary study utilizing five EIAV-negative horses was undertaken to determine if intramuscular inoculation with a mixture of vLGSN and vLGP90SN could induce CTL. Of five horses inoculated, one, H555, developed EIAV-specific CTL. These results are in contrast to results obtained in murine and nonhuman primate models (14, 16, 49) but similar to results of a study in humans (52). Though retroviral vectors have multiple advantages with regard to antigen presentation, hurdles exist for efficient in vivo transduction and immune stimulation. Stable transduction is most efficient when cells enter S phase immediately following vector uptake, allowing for integration into the host cell chromosome (32). Induction and timing of S phase are difficult in myocytes that normally reside in G0 but were attempted in this study through the use an acidic (pH 4.2) local anesthetic, Bupivicaine. It is possible that lack of detectable CTL induction in four of five animals was due to improper timing of pretreatment of injection sites, resulting in poor transduction efficiency. Another explanation may be that since retroviral vectors do not perturb host cell machinery, transduced cells may not be recognized as infected, evidenced by the lack of NK-like activity in CTL assays. This may result in only the protein of interest being expressed, without stimulation of the cytokines IL-2, IL-12, and gamma interferon, which have been demonstrated as being important for CTL activation. Polycistronic expression vectors encoding for these important costimulatory molecules may help to overcome this possible shortcoming.
In conclusion, these studies have further demonstrated the utility of retroviral vectors in dissection of CTL responses in vitro. Retroviral vectors expressing EIAV Gag/Pr and SU and using the GALV Env for internalization transduced EK cells which were efficiently lysed by EIAV-specific CTL. These results confirmed CTL activity to EIAV Gag/Pr proteins and provided information regarding CTL epitope localization by demonstrating CTL reactivity to the SU protein of EIAV. Finally, preliminary studies confirmed that even though methods to enhance responses to their expressed proteins may be needed, retroviral vectors have the potential of eliciting virus-specific CTL.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Emma Karel, Eldon Libstaff, and David Auyong.
This research was supported in part by National Institute of Health grants AI01260 and AI24291 and by AVMA Research Foundation grant 95-11.
REFERENCES
- 1.Ball J M, Payne S L, Issel C J, Montelaro R C. EIAV genomic organization: further characterization by sequencing of purified glycoproteins and cDNA. Virology. 1988;165:601–605. doi: 10.1016/0042-6822(88)90605-8. [DOI] [PubMed] [Google Scholar]
- 2.Bennink J R, Yewdell J W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- 3.Burns D P, Desrosiers R C. Envelope sequence variation, neutralizing antibodies, and primate lentivirus persistence. Curr Top Microbiol Immunol. 1994;188:185–219. doi: 10.1007/978-3-642-78536-8_11. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter S, Evans L H, Sevoian M, Chesebro B. Role of the host immune response in selection of equine infectious anemia virus variants. J Virol. 1987;61:3783–3789. doi: 10.1128/jvi.61.12.3783-3789.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffin J M. Retroviridae and their replication. In: Fields B N, Knipe D M, et al., editors. Virology. New York, N.Y: Raven Press, Ltd.; 1990. pp. 1437–1500. [Google Scholar]
- 6.Coggins L, Norcross N L, Nusbaum S R. Diagnosis of equine infectious anemia by immunodiffusion test. Am J Vet Res. 1972;33:11–18. [PubMed] [Google Scholar]
- 7.Crawford T B, Cheevers W P, Klevjer-Anderson P, McGuire T C. Equine infectious anemia: virion characteristics, virus-cell interaction and host responses. ICN/UCLA Symp Mol Cell Biol. 1978;11:727–749. [Google Scholar]
- 8.Crawford T B, Wardrop K J, Tornquist S J, Reilich E, Meyers K M, McGuire T C. A primary production deficit in the thrombocytopenia of equine infectious anemia. J Virol. 1996;70:7842–7850. doi: 10.1128/jvi.70.11.7842-7850.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita K, Maldarelli F, Purcell D F, Silver J. Murine retroviral vector that induces long-term expression of HIV-1 envelope protein. J Virol Methods. 1994;50:293–311. doi: 10.1016/0166-0934(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 10.Gerencer M, Valpotic I, Jukic B, Tomaskovic M, Basic I. Qualitative analyses of cellular immune functions in equine infectious anemia show homology with AIDS. Arch Virol. 1989;104:249–257. doi: 10.1007/BF01315547. [DOI] [PubMed] [Google Scholar]
- 11.Hammond S A, Cook S J, Lichtenstein D L, Issel C J, Montelaro R C. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 13.Henderson L E, Sowder R C, Smythers G W, Oroszlan S. Chemical and immunological characterizations of equine infectious anemia virus gag-encoded proteins. J Virol. 1987;61:1116–1124. doi: 10.1128/jvi.61.4.1116-1124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin M J, Laube L S, Lee V, Austin M, Chada S, Anderson C G, Townsend K, Jolly D J, Warner J F. Direct injection of a recombinant retroviral vector induces human immunodeficiency virus-specific immune responses in mice and nonhuman primates. J Virol. 1994;68:5036–5044. doi: 10.1128/jvi.68.8.5036-5044.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issel C J, Foil L D. Studies on equine infectious anemia virus transmission by insects. J Am Vet Med Assoc. 1984;184:293–297. [PubMed] [Google Scholar]
- 16.Jolly D J, Warner J F. Induction of anti-HIV-1 immune responses by retroviral vectors. Biotechnol Ther. 1991;2:179–193. [PubMed] [Google Scholar]
- 17.Kawakami T, Sherman L, Dahlberg J, Gazit A, Yaniv A, Tronick S R, Aaronson S A. Nucleotide sequence analysis of equine infectious anemia virus proviral DNA. Virology. 1987;158:300–312. doi: 10.1016/0042-6822(87)90202-9. [DOI] [PubMed] [Google Scholar]
- 18.Kono Y. Antigenic variation of equine infectious anemia virus as detected by virus neutralization. Brief report. Arch Virol. 1988;98:91–97. doi: 10.1007/BF01321009. [DOI] [PubMed] [Google Scholar]
- 19.Kono Y, Hirasawa K, Fukunaga Y, Taniguchi T. Recrudescence of equine infectious anemia by treatment with immunosuppressive drugs. Natl Inst Anim Health Q. 1976;16:8–15. [PubMed] [Google Scholar]
- 20.Kozak M. Adherence to the first-AUG rule when a second AUG codon follows closely upon the first. Proc Natl Acad Sci USA. 1995;92:2662–2666. doi: 10.1073/pnas.92.7.2662. . (Erratum, 92:7134, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurago Z B, Smith K D, Lutz C T. NK cell recognition of MHC class I. NK cells are sensitive to peptide-binding groove and surface alpha-helical mutations that affect T cells. J Immunol. 1995;154:2631–2641. [PubMed] [Google Scholar]
- 22.Langemeier J L, Cook S J, Cook R F, Rushlow K E, Montelaro R C, Issel C J. Detection of equine infectious anemia viral RNA in plasma samples from recently infected and long-term inapparent carrier animals by PCR. J Clin Microbiol. 1996;34:1481–1487. doi: 10.1128/jcm.34.6.1481-1487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazary S, Antczak D F, Bailey E, Bell T K, Bernoco D, Byrns G, McClure J J. Joint Report of the Fifth International Workshop on Lymphocyte Alloantigens of the Horse, Baton Rouge, Louisiana, 31 October-1 November 1987. Anim Genet. 1988;19:447–456. doi: 10.1111/j.1365-2052.1988.tb00836.x. [DOI] [PubMed] [Google Scholar]
- 24.McGuire T C, Lacy P A, O’Rourke K I. cDNA sequence of the env gene of a pathogenic equine infectious anemia lentivirus variant. Nucleic Acids Res. 1990;18:196. doi: 10.1093/nar/18.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire T C, O’Rourke K I, Baszler T V, Leib S R, Brassfield A L, Davis W C. Expression of functional protease and subviral particles by vaccinia virus containing equine infectious anaemia virus gag and 5′ pol genes. J Gen Virol. 1994;75:895–900. doi: 10.1099/0022-1317-75-4-895. [DOI] [PubMed] [Google Scholar]
- 26.McGuire T C, O’Rourke K I, Perryman L E. Immunopathogenesis of equine infectious anemia lentivirus disease. Dev Biol Stand. 1990;72:31–37. [PubMed] [Google Scholar]
- 27.McGuire T C, Tumas D B, Byrne K M, Hines M T, Leib S R, Brassfield A L, O’Rourke K I, Perryman L E. Major histocompatibility complex-restricted CD8+ cytotoxic T lymphocytes from horses with equine infectious anemia virus recognize Env and Gag/PR proteins. J Virol. 1994;68:1459–1467. doi: 10.1128/jvi.68.3.1459-1467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuire T C, Zhang W, Hines M T, Henney P J, Byrne K M. Frequency of memory cytotoxic T-lymphocytes to equine infectious anemia virus proteins in blood from carrier horses. Virology. 1997;238:85–93. doi: 10.1006/viro.1997.8795. [DOI] [PubMed] [Google Scholar]
- 29.McLachlin J R, Mittereder N, Daucher M B, Kadan M, Eglitis M A. Factors affecting retroviral vector function and structural integrity. Virology. 1993;195:1–5. doi: 10.1006/viro.1993.1340. [DOI] [PubMed] [Google Scholar]
- 30.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–982. [PMC free article] [PubMed] [Google Scholar]
- 32.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montelaro R C, Ball J M, Rushlow K E. Equine retroviruses. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1993. pp. 257–360. [Google Scholar]
- 34.Montelaro R C, Robey W G, West M D, Issel C J, Fischinger P J. Characterization of the serological cross-reactivity between glycoproteins of the human immunodeficiency virus and equine infectious anaemia virus. J Gen Virol. 1988;69:1711–1717. doi: 10.1099/0022-1317-69-7-1711. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y, Kameoka M, Tobiume M, Kaya M, Ohki K, Yamada T, Ikuta K. A chain section containing epitopes for cytotoxic T, B and helper T cells within a highly conserved region found in the human immunodeficiency virus type 1 Gag protein. Vaccine. 1997;15:489–496. doi: 10.1016/s0264-410x(96)00224-1. [DOI] [PubMed] [Google Scholar]
- 36.O’Rourke K, Perryman L E, McGuire T C. Antiviral, anti-glycoprotein and neutralizing antibodies in foals with equine infectious anemia virus. J Gen Virol. 1988;69:667–674. doi: 10.1099/0022-1317-69-3-667. [DOI] [PubMed] [Google Scholar]
- 37.Ozaki S, York-Jolley J, Kawamura H, Berzofsky J A. Cloned protein antigen-specific, Ia-restricted T cells with both helper and cytolytic activities: mechanisms of activation and killing. Cell Immunol. 1987;105:301–316. doi: 10.1016/0008-8749(87)90079-7. [DOI] [PubMed] [Google Scholar]
- 38.Payne S L, Fang F D, Liu C P, Dhruva B R, Rwambo P, Issel C J, Montelaro R C. Antigenic variation and lentivirus persistence: variations in envelope gene sequences during EIAV infection resemble changes reported for sequential isolates of HIV. Virology. 1987;161:321–331. doi: 10.1016/0042-6822(87)90124-3. [DOI] [PubMed] [Google Scholar]
- 39.Perryman L E, O’Rourke K I, Mason P H, McGuire T C. Equine monoclonal antibodies recognize common epitopes on variants of equine infectious anaemia virus. Immunology. 1990;71:592–594. [PMC free article] [PubMed] [Google Scholar]
- 40.Perryman L E, O’Rourke K I, McGuire T C. Immune responses are required to terminate viremia in equine infectious anemia lentivirus infection. J Virol. 1988;62:3073–3076. doi: 10.1128/jvi.62.8.3073-3076.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petropoulos C J, Hughes S H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991;65:3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray J, Gage F H. Gene transfer into established and primary fibroblast cell lines: comparison of transfection methods and promoters. BioTechniques. 1992;13:598–603. [PubMed] [Google Scholar]
- 43.Rettinger S D, Kennedy S C, Wu X, Saylors R L, Hafenrichter D G, Flye M W, Ponder K P. Liver-directed gene therapy: quantitative evaluation of promoter elements by using in vivo retroviral transduction. Proc Natl Acad Sci USA. 1994;91:1460–1464. doi: 10.1073/pnas.91.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rushlow K E. Evaluation of protective host immune responses during persistent infection with equine infectious anemia. In: Girard M, Valette L, editors. Proceeding of the 5th Colloque des Cent Garde: Retroviruses of human AIDS and related animal diseases. Lyon, France: Foundation Merieux; 1990. pp. 133–138. [Google Scholar]
- 45.Siliciano R F, Keegan A D, Dintzis R Z, Dintzis H M, Shin H S. The interaction of nominal antigen with T cell antigen receptors. I. Specific binding of multivalent nominal antigen to cytolytic T cell clones. J Immunol. 1985;135:906–914. [PubMed] [Google Scholar]
- 46.Song W, Collisson E W, Li J, Wolf A M, Elder J H, Grant C K, Brown W C. Feline immunodeficiency virus (FIV)-specific cytotoxic T lymphocytes from chronically infected cats are induced in vitro by retroviral vector-transduced feline T cells expressing the FIV capsid protein. Virology. 1995;209:390–399. doi: 10.1006/viro.1995.1271. [DOI] [PubMed] [Google Scholar]
- 47.Sparer T E, Wynn S G, Clark D J, Kaplan J M, Cardoza L M, Wadsworth S C, Smith A E, Gooding L R. Generation of cytotoxic T lymphocytes against immunorecessive epitopes after multiple immunizations with adenovirus vectors is dependent on haplotype. J Virol. 1997;71:2277–2284. doi: 10.1128/jvi.71.3.2277-2284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tschetter J R, Byrne K M, Perryman L E, McGuire T C. Control of equine infectious anemia virus is not dependent on ADCC mediating antibodies. Virology. 1997;230:275–280. doi: 10.1006/viro.1997.8502. [DOI] [PubMed] [Google Scholar]
- 49.Warner J F, Anderson C G, Laube L, Jolly D J, Townsend K, Chada S, St. Louis D. Induction of HIV-specific CTL and antibody responses in mice using retroviral vector-transduced cells. AIDS Res Hum Retroviruses. 1991;7:645–655. doi: 10.1089/aid.1991.7.645. [DOI] [PubMed] [Google Scholar]
- 50.Willbold D, Rosin Arbesfeld R, Sticht H, Frank R, Rosch P. Structure of the equine infectious anemia virus Tat protein. Science. 1994;264:1584–1587. doi: 10.1126/science.7515512. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y H, Sentsui H, Nakaya T, Kono Y, Ikuta K. In vivo dynamics of equine infectious anemia viruses emerging during febrile episodes: insertions/duplications at the principal neutralizing domain. J Virol. 1997;71:5031–5039. doi: 10.1128/jvi.71.7.5031-5039.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziegner U H, Peters G, Jolly D J, Mento S J, Galpin J, Prussak C E, Barber J R, Hartnett D E, Bohart C, Klump W, et al. Cytotoxic T-lymphocyte induction in asymptomatic HIV-1-infected patients immunized with retrovector-transduced autologous fibroblasts expressing HIV-1IIIB Env/Rev proteins. AIDS. 1995;9:43–50. doi: 10.1097/00002030-199501000-00006. [DOI] [PubMed] [Google Scholar]