Abstract
The Biobehavioral Working Group of BACPAC was charged to evaluate a range of psychosocial, psychophysical, and behavioral domains relevant to chronic low back pain, and recommend specific assessment tools and procedures to harmonize biobehavioral data collection across the consortium. Primary references and sources for measure selection were the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials, the Minimum Data Set from the National Institutes of Health (NIH) Research Task Force on Standards for Chronic Low Back Pain, the Patient-Reported Outcomes Measurement Information System, and NeuroQOL. The questionnaire’s recommendations supplemented the NIH HEAL Common Data Elements and BACPAC Minimum Data Set. Five domains were identified for inclusion: Pain Characteristics and Qualities; Pain-Related Psychosocial/Behavioral Factors; General Psychosocial Factors; Lifestyle Choices; and Social Determinants of Health/Social Factors. The Working Group identified best practices for required and optional Quantitative Sensory Testing of psychophysical pain processing for use in BACPAC projects.
Keywords: Behavioral Assessments, Psychosocial Assessments, Patient-Reported Outcomes, Quantitative Sensory Testing, Chronic Low Back Pain
Introduction
Chronic low back pain (cLBP) currently ranks as the most disabling and costly condition affecting the US population [1]. Efforts to understand and treat cLBP have historically focused on biological mechanisms along with biomedical and biomechanical treatments. In recent decades, the biopsychosocial model of pain has broadened our understanding of the many factors that are associated with pain processing in the context of cLBP [2]. Greater understanding of the interplay between biological, psychological and social factors in the production of pain facilitates better precision in matching treatments with the needs of patients [3]. Examples of factors with known influence on pain processing include emotional state, cognitive or thinking styles, habitual coping methods, behavioral lifestyle choices, socioeconomic factors, history of trauma and memories of past pain experiences, and availability of social supports.
This article describes the methods and recommendations of the BACPAC Biobehavioral Research Working Group (BWG). The charge and responsibilities of the BWG were to examine a range of psychosocial, psychophysical, and behavioral domains relevant to the various BACPAC intervention and phenotyping projects, to make recommendations for harmonizing the domains to be assessed, and to recommend specific assessment tools and procedures.
Deliverables of the BWG included developing a set of recommendations regarding patient-reported outcomes (PROs)/psychosocial domains and specific questionnaires to 1) assess meaningful outcomes following interventions (outcomes assessment) and 2) contribute to prediction of who would respond to which specific interventions (phenotyping for precision medicine). The recommendations of a broader set of questionnaires to be included in BACPAC projects were meant to supplement the National Institutes of Health (NIH) HEAL Common Data Elements (CDE) and the BACPAC Minimum Dataset (BMD) of PROs and demographics. The BWG also provided best practice guidelines for quantitative sensory testing (QST) in BACPAC projects [4]. Table 1 provides a list of abbreviations. Although harmonization across sites was an important goal, we recognized that testing sites would choose the measures and procedures most pertinent to their projects and would strongly consider participant burden. The overall goal was to find a balance between maximizing harmonization for the assessment tools between the various BACPAC sites and allowing flexibility for individual teams’ objectives.
Table 1.
Abbreviations
| BACPAC | Back Pain Consortium (BACPAC) Research Program |
|---|---|
| BMD | BACPAC minimum dataset |
| BWG | Biobehavioral WG |
| CDE | Common data elements |
| cLBP | Chronic low back pain |
| CPM | Conditioned Pain Modulation |
| HEAL | The Helping to End Addiction Long-termSM Initiative |
| IMMPACT | Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials |
| MDS | Minimum dataset |
| MRC | Interdisciplinary Mechanistic Research Center |
| NIH | National Institutes of Health |
| NIMHD | National Institute on Minority Health and Health Disparities |
| PPT | Pressure Pain Threshold |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| PROs | Patient-Reported Outcomes |
| QOL | Quality of life |
| QST | Quantitative Sensory Testing |
| SDoH | Social determinants of health |
| WG | Working Group |
Abbreviations for questionnaires are included in Table 2.
Methods
BWG Membership
The BWG was composed of self-nominated investigators from the BACPAC sites, including the directors of the Behavioral Cores at each BACPAC Mechanistic Research Center (MRC). The BWG members were behavioral scientists and physicians with expertise in PROs, psychosocial assessment, clinical pain assessment and treatment, and psychophysical assessment using quantitative sensory testing. Of note, several members of the BWG served on additional BACPAC WGs, including the Minimum Data Set WG, Theoretical Model WG, and Biomechanical and Physical Function WG. This overlap allowed for greater integration and harmonization across projects and fostered ongoing connections for potential new collaborative research endeavors.
BWG Procedures
The BWG established a schedule of online meetings. In initial discussions about its charge and deliverables, members of the BWG self-selected into two subgroups: 1) PROs/Psychosocial Questionnaires and 2) Psychophysical Assessment/QST.
The BWG PROs/Psychosocial Questionnaires subgroup used a literature review, discussion, and consensus decision-making approach to identify important assessment domains and concepts, and to recommend specific questionnaires for inclusion in BACPAC projects. The BWG PROs/psychosocial questionnaires members reviewed existing publications regarding psychosocial, behavioral, and pain-related domains. An iterative process identified domains based on their contribution to understanding the cLBP patients’ experience and on their prognostic value for treatment outcomes. Primary references were the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations [5–11] and the NIH Research Task Force Minimum Data Set (MDS) [12]. We also evaluated NeuroQOL and Patient-Reported Outcomes Measurement Information System® (PROMIS) measures [13]. Advantages of PROMIS questionnaires include their rigorous instrument development methodology [14], simple score interpretation with mean T-score of 50 and Standard deviation of 10, and the availability of comparison tables so that PROMIS scores can be “linked” or translated into scores on other frequently used or “legacy” questionnaires [15–17]. The BWG also considered the domains and conceptual areas identified during the comprehensive literature reviews conducted by the Theoretical Models WG. Once conceptually important domains were identified, we evaluated specific questionnaires for inclusion in the list of recommended measures. We evaluated the evidence for their validity as prognostic indicators or responsiveness to treatment in persons with cLBP. We further evaluated practicality and feasibility, considering factors such as length of questionnaires, conceptual clarity, and simplicity of items and scoring. The BWG aimed to include both resilience factors as well as risk factors and aimed to balance breadth of conceptual domain coverage with brevity of validated questionnaires in order to minimize participant burden. Final decisions regarding recommendations of specific questionnaires were made by consensus.
Recognizing that BACPAC projects differ regarding their aims and interests, the BWG also reviewed discretionary or optional domains and questionnaires. While BWG recommended questionnaires are meant to supplement the HEAL CDE and BMD for all BACPAC projects, the discretionary questionnaires are options that can be administered in studies where the additional participant burden is not a barrier, and where the concept is particularly relevant to the study aims.
The BWG QST subgroup developed best practice guidelines for harmonizing psychophysical testing across BACPAC sites conducting QST. The recommendations of the BWG were documented and provided to the BACPAC Executive and Steering Committees, reviewed, and revised as needed until approval was received from the Steering Committee. The members of this subgroup searched the literature for studies that used QST procedures in cLBP, compiled a list of the most commonly used QST procedures in this patient population, and developed a minimal set of QST procedures through a consensus process.
The primary work of the BWG was accomplished during January to May 2020. However, members of the BWG have reconvened as needed to address additional issues as they would come up, including modifications to procedures and additional questions needed as the BACPAC projects readied for recruitment of participants.
Results and Recommendations
Patient-reported Outcomes/Psychosocial Domains and Questionnaires
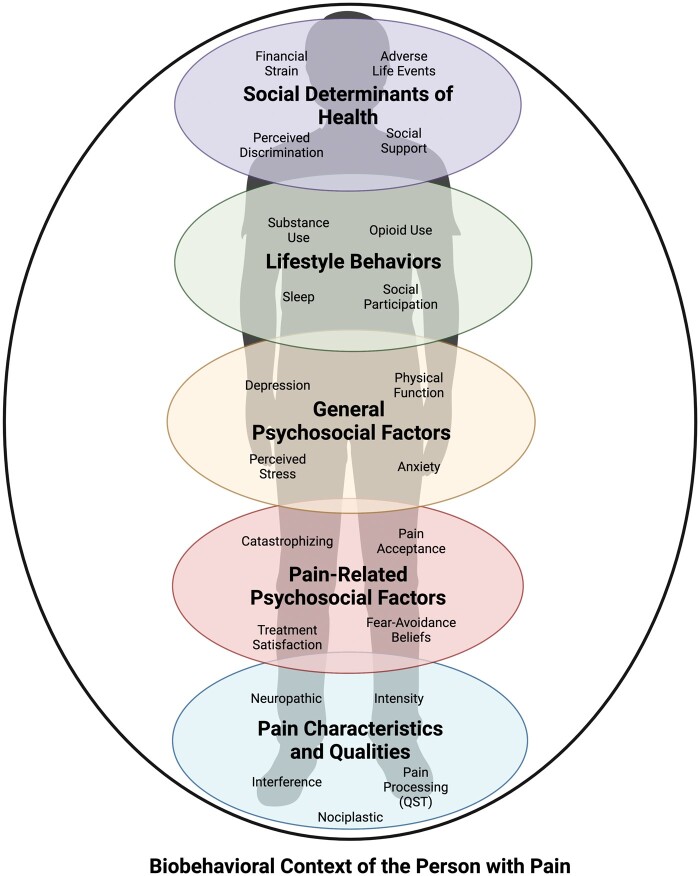
BWG-recommended PRO/psychosocial domains for inclusion in BACPAC are pain characteristics, pain-related psychosocial and behavioral factors, general psychosocial factors, lifestyle choice factors, and social determinants of health (SDoH)/social factors (Figure 1). Each of these domains include several categories or constructs. In the following paragraphs we summarize the domains, domain constructs, and list specific recommended and discretionary/optional questionnaires. Table 2 lists the domains, constructs, and questionnaires, and identifies the BWG-recommended questionnaires that are also included in the NIH HEAL CDE (required in HEAL studies in humans) and the BMD (required in BACPAC studies). As indicated in Table 2, the BWG added several recommendations and options for broad inclusion beyond the BMD and HEAL CDE. BWG-recommended questionnaires are intended for use in all BACPAC projects. The discretionary questionnaires listed on Table 2 are validated but non-required options that may be chosen based on the aims and interests of particular BACPAC projects.
Figure 1.
Biobehavioral, psychosocial and social context domains relevant to persons with chronic pain, with key categories and constructs.
Table 2.
PRO/Psychosocial domains and patient-reported questionnaires
DOMAIN
|
BWG RECOMMENDED (REC) and DISCRETIONARY (DISC) Patient-Reported Questionnaires (Number of Items) | REC, DISC | BACPAC Minimum Dataset (BMD) | NIH HEAL Common Data Element (CDE) |
|---|---|---|---|---|
| Pain Characteristics and Qualities | ||||
|
PEG (3) | REC | X | X |
| Pain Intensity NRS for low back pain (1) | X | |||
|
PEG (has both pain intensity and interference) (3) | REC | X | X |
| PROMIS-29 + 2 Pain Interference (4) | X | |||
|
Low-back pain duration and frequency (items from NIH RTF MDS) (2) | REC | X | |
|
Radicular pain questions adapted from NIH RTF MDS (2) | REC | X | |
|
Bothersome stomach pain, headaches (2) | REC | X | X |
|
Widespread Pain Inventory (7) | REC | X | |
| Michigan Body Map (1) | REC | |||
|
PainDETECT (9) | REC | ||
|
McGill SF (16) | DISC | ||
|
Oswestry Disability Index (10) | DISC | ||
| Pain-related Psychosocial | ||||
|
PCS SF (6) | REC | X | X |
|
Patient Global Impression of Change (since start of a treatment) (1) | REC | X | X |
|
FABQ-Physical Activity scale (5) | REC | ||
| Tampa Scale of Kinesiophobia (17) | DISC | |||
|
PSEQ (4) | REC | ||
|
CPAQ (8) | REC | ||
|
Expectation of pain relief (with or without treatment) (1) | DISC | ||
| HEAL Treatment Expectancy item (1) | DISC | |||
|
Pain Anxiety Symptoms Scale SF (20) Avoidance and Physiological Pain Anxiety subscales | DISC | ||
|
Coping Strategies Questionnaire-brief version (24) | DISC | ||
| Psychosocial factors—General | ||||
|
PROMIS Physical Function—6b (6) | REC | X | X |
|
PHQ2 Depression (2) | REC | X | X |
| PROMIS-29 + 2 Depression (4) | REC | X | ||
|
GAD2 Anxiety (2) | REC | X | X |
| PROMIS-29 + 2 Anxiety (4) | REC | X | ||
|
PSS (4, or 10) | REC | ||
|
PANAS SF (10) or Positive affect alone (5) | DISC | ||
|
HEAL Positive outlook scale (6) | DISC | ||
|
PROMIS-29 + 2 memory and concentration (2) | DISC | ||
|
MAIA-2 or selected subscales (37) | DISC | ||
|
PROMIS Self-efficacy for Managing symptoms (4), or PROMIS General Self-efficacy (4) | DISC, DISC | ||
| Lifestyle behaviors | ||||
|
PROMIS Sleep Disturbance 6a (6) | REC | X | X |
| Sleep duration (hours, minutes per night) (1) | REC | X | X | |
| PROMIS Sleep-Related Impairment 4a or 8a (4, 8) | DISC | |||
|
TAPS Screener—part 1 (5) | REC | X | X |
|
Current daily opioid use (1) | REC | X | X |
|
PROMIS-29 + 2 ability to participate in social roles and activities (4) | REC | ||
| Social Determinants of Health (SDOH) | ||||
|
PC-PTSD-5 Exposure to traumatic event(s) (1–7) adapted to include childhood or adulthood exposure question (1), or | DISC | ||
| Life Events Checklist (17) | DISC | |||
|
Difficulty paying for basic needs (1) | DISC | ||
| Thrive SDOH tool (11) | DISC | |||
|
Perceptions of being treated unfairly due to 1) race, ethnicity, and 2) sexual orientation or gender identity (1 or 2 questions) | DISC | ||
|
PROMIS Emotional Support 4a v2 (4) | DISC | ||
| MOS Social Support (includes emotional and instrumental support) (19) | DISC | |||
CNS = Central Nervous System; CPAQ = Chronic Pain Acceptance Questionnaire; GAD2 = Generalized Anxiety Disorder screener, 2 item version; FABQ = Fear Avoidance Beliefs Questionnaire; HEAL = Healing Encounters and Attitudes Lists; MAIA-2 = Multidimensional Assessment of Interoceptive Awareness-2; MOS = Medical Outcomes Study; NIH RTF MDS = National Institutes of Health Research Task Force Minimum Data Set; NRS = Numeric Rating Scale; PANAS = Positive and Negative Affect Schedule; PC-PTSD-5 = Primary Care Post-Traumatic Stress Disorder screener for DSM5; PCS = Pain Catastrophizing Scale; PEG = Pain, Enjoyment, General activity; PHQ2 = Patient Health Questionnaire depression screener, 2 item version; PROMIS = Patient Reported Outcomes Measurement Information System; PSEQ = Pain Self-Efficacy Questionnaire; SF = Short Form; TAPS = Tobacco use, Alcohol use, Prescription medication misuse, illicit Substance use.
The PROs/psychosocial questionnaires subgroup aimed for broad coverage of domains and categories relevant to chronic pain and health outcomes, while at the same time choosing validated questionnaires for which concise and precise versions, or “short forms” were available. For example, the PROMIS-29 + 2 health profile [18–25] was recommended because it includes brief (1–4 item) measures of 8 health-related areas: Physical Function, Pain Interference, Pain Intensity, Sleep, Depression, Anxiety, Fatigue, Ability to Participate in Social Roles and Activities, and Cognitive Function. Notably, comprehensive conceptual coverage of all potentially relevant categories was not intended. For instance, patient-reported physical activity is important to measure in cLBP studies [8, 26]. However, an optimally brief physical activity questionnaire for use across BACPAC sites was not identified. Rather, several BACPAC sites assess physical activity objectively via at-home monitoring (described in the BACPAC Biomechanical and Physical Function WG manuscript) and may also administer a patient-report measure of physical activity of their choice.
Domains, Constructs, and Questionnaires
Pain Characteristics and Qualities. Pain intensity and interference with life activities are assessed with the three-item PEG (Pain, Enjoyment, General Activity) [27] and with PROMIS-29 + 2 Pain Intensity and Pain Interference [28]. The duration and frequency of low back pain are assessed directly via two BMD questions. Radicular pain (spread of back pain to the legs) and somatization (bothersome headaches and stomach pain) are assessed via four BMD questions. The Widespread Pain Index [29] and the Michigan Body Map [30] assess additional pain locations and are helpful clues to the presence of nociplastic pain or central nervous system sensitization. For assessing whether pain is of neuropathic origin, we recommend the PainDETECT questionnaire [31]. For projects interested in assessing sensory versus affective pain, the Short-form McGill Pain Questionnaire [32] is a good option. Pain-related disability can be assessed using the Oswestry Disability Index (ODI) [33].
Psychosocial Factors—Pain-Related. Ways of thinking about pain and strategies to cope with pain have an impact on patients’ behavior and quality of life. Pain catastrophizing, or ruminating about and magnifying pain, and having a sense of helplessness, is assessed by the BMD-required Pain Catastrophizing Scale, six-item version [34–36]. The BWG recommends that fear of movement be assessed by the physical activity subscale of the Fear Avoidance Beliefs Questionnaire (FABQ) [37]; however, the Tampa Scale of Kinesiophobia [38, 39] is an alternative option. A similar concept is pain avoidance, which can be assessed via the Pain Anxiety Symptoms Scale short form [40, 41] at a BACPAC project’s discretion. Adaptive coping with pain is also important to assess. Pain acceptance is measured via the Chronic Pain Acceptance Questionnaire-8 (CPAQ) [42, 43]. Pain self-efficacy, or one’s belief in being able to effectively control or cope with pain, is a BWG recommended construct that can be assessed via the four-item version of the Pain Self-Efficacy Questionnaire [36]. If a BACPAC project is particularly interested in assessing a broad range of Pain coping strategies (PCS), the 24-item version of the Coping Strategies Questionnaire [44, 45] is an option. As Pain Catastrophizing is one of the subscales, this subscale can be dropped to avoid redundance with the PCS. For BACPAC projects that assess responses to pain treatment, global satisfaction with treatment is important, and the BMD requires the single-item Patient Global Impression of Change [46] on which patients can rate either the improvement or worsening of symptoms on a seven-point scale. Expectations of improvement is an optional category that can be rated with a single-item expectation of pain relief over a 6-month period, or a single Treatment Expectancy item from the Healing Encounters and Attitudes Lists [47]. There are many other well-validated questionnaires that have significant conceptual overlap with the questionnaires listed above.
Psychosocial Factors—General. Self-report of physical function is assessed via the PROMIS Physical Function 6 b short form, as required by the BMD. This questionnaire adds two items to the PROMIS-29 + 2 health profile, which includes four physical function items. Depression and anxiety are assessed with two-item CDE screening questionnaires, the Patient Health Questionnaire-2 (PHQ2) [48, 49] and Generalized Anxiety Disorder-2 (GAD2) [50]. The BMD added the PROMIS-29 + 2 Depression and Anxiety scales [19], which each includes four items. The BWG recommends assessing stress with the Perceived Stress Scale [51], which has 10-item and 4-item versions. Several discretionary or optional categories include affect, measured by the Positive and Negative Affect Schedule [52, 53], Optimistic attitude (Positive Outlook short form of the Healing Encounters and Attitudes Lists) [47], and general self-efficacy, for which PROMIS has several scales, such as general self-efficacy and self-efficacy for managing symptoms [25]. Cognition can be briefly assessed with 2 items about Memory and Concentration ability from the PROMIS-29 + 2 [19]. Interoceptive awareness, which measures various beliefs and behaviors regarding bodily sensations, including pain, can be assessed optionally using the Multidimensional Assessment of Interoceptive Awareness, version 2 (MAIA-2) [54]. This questionnaire, however, is lengthy and allows for selecting individual scales (e.g., for assessing different habitual attention styles towards pain, including ignoring pain).
Lifestyle Behaviors. Sleep is a BMD-required category and is assessed via the PROMIS Sleep Disturbance 6a short form [55], of which four of the six items are included on the PROMIS-29 + 2 profile, and patient-report of hours and minutes of sleep, on average [56]. PROMIS Sleep-related Impairment 4a or 8a is an additional option. Substance use, assessed with the Tobacco, Alcohol, Prescription Medication (TAPS) [57] screener part 1 and opioid medication (current daily dose) are BMD and CDE requirements. Social participation is an additional recommendation of the BWG and is assessed with the four-item PROMIS Ability to Participate in Social Roles and Activities short form, which is included within the PROMIS-29 + 2. The BWG PROs/psychosocial factors subgroup did not make a specific recommendation for a perceived physical activity questionnaire.
Social Determinants of Health (SDoH). BACPAC projects, particularly those engaging in cLBP phenotyping, may include questions to assess social determinants of health. Adverse life events may be assessed via the Primary Care-Post Traumatic Stress Disorder (PC-PTSD-5) [58] five-item screening instrument or with longer questionnaires such the Life Events Checklist [59]. Financial strain may be assessed with a single item adapted for use in BACPAC MRCs that asks about difficulty paying for basic needs such as food, medical care, and utilities [60]. Alternatively, the THRIVE SDoH tool [61] may be used. Perceived discrimination based upon race, ethnicity, or color, and sexual orientation or gender identity can be included. The BWG developed 1–2 perceived discrimination questions in consultation with the Program Director at the National Institute on Minority Health and Health Disparities (NIMHD). Perceptions of social support can be assessed with PROMIS Emotional Support 4a v2 or the Medical Outcomes Study (MOS) Social Support questionnaire [62], which includes subscales of emotional and instrumental support.
Other categories discussed by the PROs/psychosocial questionnaires subgroup of the BWG and that may be of interest to BACPAC or other investigators included: Mindfulness (Five Facet Mindfulness Questionnaire; 63], Pain Behavior (PROMIS Pain Behavior), Personality (60-item NEO short form; 64], and Emotion Regulation.
QST Working Subgroup Recommendations
The QST Subgroup recommended a core set of two QST procedures that would be performed—at minimum—at all BACPAC sites conducting QST (Table 3). These include assessment of pressure pain threshold (PPT) and temporal summation at the lumbar region (primary pain site) and at a remote region as a control site. These QST assessments have demonstrated good to excellent reliability in cLBP [65], neuropathic pain [66, 67], and non-pain samples [68]. There was an understanding that some sites may choose to include additional QST procedures such as conditioned pain modulation (CPM). It was agreed that the order in which these procedures were performed was important and should be standardized across sites. The core set of QST tests are listed below in the recommended order that they should be performed, prior to any additional site-specific QST procedures and with testing of the control site always preceding that of the painful lumbar region.
Table 3.
Minimum set of QST procedures
| QST Procedure | Description | Measurement |
|---|---|---|
| Pain Pressure Threshold (Algometry) | An algometer with a 1-cm2 rubber probe is applied at a rate of 0.5 kgf/second until the participant first reports that the pressure sensation becomes painful. The probe is applied over the primary pain site and the contralateral trapezius as a control site. | Average of three trials at each body site, recorded as pressure intensity in kgf/cm2 |
| Temporal Summation (multiple pinpricks) | Neuropen device with a 40 g Neurotip is used to apply a train of 10 identical pinpricks at a rate of 1 Hz over the primary pain site and the volar forearm as a control site. Participant is asked to rate pain intensity of the 1st and 10th pinprick, and 15- and 30-seconds after the train of pinpricks. | Average of three trials at each body site, recorded as the difference between the 1st and 10th pinprick sensation, and average of pain aftersensation ratings. |
Pressure pain threshold is assessed using a hand-held algometer with a 1-cm2 rubber probe (FPK20 or FPX25, Wagner Instruments, Greenwich, CT, USA). The primary test site is located in the lumbar region by participants’ identification of their most painful site in response to manual over-pressure (springing palpation) performed in the prone position. The control site is located over the contralateral upper trapezius muscle (diagonal from lumbar site). Pressure is manually increased at a rate of rise of 0.5 kgf/cm2/s (10 kgf/cm2 max, metronome guided) until participants first report that the pressure sensation becomes painful. Pressure intensity (in kgf/cm2) read from the algometer at that time is considered the PPT. Measurements are conducted 3×/site with 60-second rest intervals between each pressure application. Probe placement is varied slightly trial to trial to prevent sensitization from repeated testing of the same site. Mean PPT of the three trials is used for analysis.
Temporal summation measures increases in excitatory pain pathways and is thought to reflect the progressive increase in dorsal horn neuronal firing in response to repetitive C-fiber stimulation [69–73]. Enhanced temporal summation is common in chronic pain, including in subsets of patients with cLBP, and is predictive of pain and treatments outcomes [74, 75]. A Neuropen device with a 40-gram Neurotip (Owen Mumford, Oxfordshire, United Kingdom) is used to apply a series of three sets of 10 identical pinprick stimuli applied at the rate of 1 Hz (metronome guided) to both control and primary pain sites. The dominant volar forearm serves as the non-painful control site. The primary pain site is the painful area in the lumbar region, as previously identified in the assessment of PPT done previously. Following each train of 10 stimuli, participants are asked to rate the magnitude of pain sensations of the 1st and 10th pinprick using a 0–10 numerical rating scale (NRS; 0 = no pain, 10 = worst imaginable pain). Temporal summation for each site is calculated as the mean difference in pain ratings evoked by the 1st and 10th stimuli. Participants also rate any ongoing pain aftersensations at 15- and 30-second following each train of stimuli.
Optional—Conditioned Pain Modulation. There is significant controversy in literature over the optimal procedures to elicit a robust CPM, as well as the optimal CPM techniques for predicting responses to treatment and using CPM as a pain treatment biomarker. One of the key scientific gaps that is necessary to fill is the testing of multiple CPM techniques in large populations of patients with chronic pain and relating the CPM findings to treatment outcomes. Therefore, the BWG recommended that the most harmonious approach is to have different sites in BACPAC implement different approaches to measure CPM, with the idea of being able to compare and contrast results from these methods at the study’s conclusion.
Example of CPM with pressure pain as the test stimulus and cold water as the conditioning stimulus. CPM procedures require a conditioning stimulus to induce endogenous analgesic systems and alter pain perception, and a test-stimulus to evaluate the endogenous analgesic response to the conditioning stimulus. CPM is attenuated in the majority of chronic pain participants and its magnitude is predictive of a variety of pain outcomes [76, 77]. Here, immersion of one hand into a circulating cold water bath (4–12°C; NESLAB Digital One RTE 7, Thermo Scientific, Newington, NH, USA, or similar) will serve as the conditioning-stimulus and PPT at the contralateral trapezius will serve as the test-stimulus. This method is consistent with that of Locke [78] and others [79, 80]. Baseline measurement of the test-stimulus will be acquired during the assessment of pressure pain threshold. Conditioning stimulation will begin by immersing the hand to a level 10 cm above the wrist into the water bath. The hand will be immersed for a total of 60–90 seconds of hand immersion; trapezius PPT will be re-measured 1–2 times while the hand is still immersed in the cold water. CPM magnitude will be calculated as the difference in mean PPT measured prior to and during the conditioning stimulus, with increases in PPT during conditioning interpreted as evidence of efficient endogenous pain inhibition.
Discussion
The BWG identified key PRO/psychosocial domains for harmonization and identified well-validated questionnaires to represent the domains. For various reasons specific to the science of each project, not every BACPAC project will include all the same questionnaires. Most of the questionnaires discussed in this paper are required because they are included in the BMD and/or the NIH HEAL CDE. The BWG added several recommended questionnaires and discretionary or optional questionnaires beyond the BMD and HEAL CDE. The BWG psychosocial questionnaires subgroup acknowledges that there is overlap among domains and within the conceptual areas measured by individual questionnaires. We also acknowledge that various professions may have different views about which domains are most important to assess.
One limitation of the work reported here is that the recommendations of the BWG were not based upon an exhaustive systematic review of all possible domains and all existing psychosocial and psychophysical measures that may be relevant to cLBP. Furthermore, the BWG did not comprehensively review all domains and questionnaires that may be important in phenotyping cLBP. For example, the BWG did not identify a questionnaire to assess patient reports of their own physical activity. Furthermore, we did not include all possible domains of SDoH that are assessed in clinical settings but have not yet been validated in research settings. The assessment of psychosocial and behavioral factors affecting pain and influenced by pain could be endless. Likewise, QST batteries can be lengthy and time consuming. It was important to recommend brief assessments where possible to minimize participant burden.
Acknowledgments
The authors would like to acknowledge the BWG members: Candace Floyd, Corey Flynn, Michael Gold, Anna Hoffmeyer, John Jakicic, Anna Kratz, Lanay Mudd, Chris Standaert, Eric Weston, and Ted Zheng. Additionally, the authors would like to thank the UNC DAC for their support with the study.
Funding
The Back Pain Consortium (BACPAC) Research Program is administered by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). This research was supported by the National Institutes of Health through The Helping to End Addiction Long-termSM Initiative, or NIH HEAL Initiative, under award numbers 1U19AR076725-01 (PITT, Sowa), 1UG3AR076568-01 (PITT, Wasan), 1U19AR076737-01 (UCSF, Lotz), and 1U19AR076734-01 (UMICH, Clauw), and by the Early Phase Pain Investigation Clinical Network Data Coordinating Center (EPPIC-Net DCC) grant 3U24NS113844-01 from the National Institutes of Health HEAL Initiative through the National Institute of Neurological Disorders and Stroke, and used the NYU Grossman School of Medicine Center for Biospecimen Research and Development (CBRD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Conflicts of interest: D.A.W.—Consultant to Swing Therapeutics Inc. and Community Health Focus Inc. No other authors have conflicts to disclose.
Supplement sponsorship
This article appears as part of the supplement entitled “Back Pain Consortium (BACPAC) Research Program” supported by the National Institutes of Health through the NIH HEAL Initiative under award number AR076730-01.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Contributor Information
Carol M Greco, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Department of Physical Therapy, School of Health and Rehabilitation Science, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Ajay D Wasan, Department of Anesthesiology and Perioperative Medicine, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Michael J Schneider, Department of Physical Therapy, School of Health and Rehabilitation Science, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Clinical and Translational Science Institute, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Wolf Mehling, Department of Family and Community Medicine, University of California San Francisco, San Francisco, California, USA.
David A Williams, Chronic Pain and Fatigue Research Center, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan, USA; Department of Psychiatry, University of Michigan Medical School, Ann Arbor, Michigan, USA; Department of Internal Medicine-Rheumatology, University of Michigan Medical School, Ann Arbor, Michigan, USA.
Jessa Darwin, Department of Physical Medicine and Rehabilitation, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Steven E Harte, Chronic Pain and Fatigue Research Center, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan, USA; Department of Internal Medicine-Rheumatology, University of Michigan Medical School, Ann Arbor, Michigan, USA.
References
- 1. Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann Transl Med 2020;8(6):299.doi: 10.21037/atm.2020.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wade DT, Halligan PW.. The biopsychosocial model of illness: A model whose time has come. Clin Rehabil 2017;31(8):995–1004. doi: 10.1177/0269215517709890. [DOI] [PubMed] [Google Scholar]
- 3. Pincus T, Kent P, Bronfort G, Loisel P, Pransky G, Hartvigsen J.. Twenty-five years with the biopsychosocial model of low back pain—is it time to celebrate? A report from the twelfth international forum for primary care research on low back pain. Spine (Phila Pa 1976) 2013;38(24):2118–23. doi: 10.1097/BRS.0b013e3182a8c5d6. [DOI] [PubMed] [Google Scholar]
- 4. Wandner LD, Domenichiello AF, Beierlein J, et al. ; NIH Pain Consortium Institute and Center Representatives. NIH's Helping to End Addiction Long-term(SM) Initiative (NIH HEAL Initiative) Clinical Pain Management Common Data Element Program. J Pain 2022;23(3):370–8. doi: 10.1016/j.jpain.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 5. Dworkin RH, Turk DC, Farrar JT, et al. ; IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1-2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6. Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 2016;157(9):1851–71. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turk DC, Dworkin RH, McDermott MP, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials. Pain 2008;139(3):485–93. doi: 10.1016/j.pain.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 8. Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003;106(3):337–45. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 9. Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain 2006;125(3):208–15. doi: 10.1016/j.pain.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 10. Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: An IMMPACT survey of people with pain. Pain 2008;137(2):276–85. doi: 10.1016/j.pain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11. Turk DC, O'Connor AB, Dworkin RH, et al. Research design considerations for clinical studies of abuse-deterrent opioid analgesics: IMMPACT recommendations. Pain 2012;153(10):1997–2008. doi: 10.1016/j.pain.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH task force on research standards for chronic low back pain. J Pain 2014;15(6):569–85. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Northwestern University. HealthMeasures: Transforming How Health is Measured. Northwestern University. Available at: https://www.healthmeasures.net/index.php (accessed August 2, 2022).
- 14. Northwestern University. Validation (PROMIS). Available at: https://www.healthmeasures.net/explore-measurement-systems/promis/measure-development-research/validation (accessed August 2, 2022).
- 15. Cook KF, Schalet BD, Kallen MA, Rutsohn JP, Cella D.. Establishing a common metric for self-reported pain: Linking BPI Pain Interference and SF-36 Bodily Pain Subscale scores to the PROMIS Pain Interference metric. Qual Life Res 2015;24(10):2305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen CX, Kroenke K, Stump T, et al. Comparative responsiveness of the PROMIS pain interference short forms with legacy pain measures: Results from three randomized clinical trials. J Pain 2019;20(6):664–75. doi: 10.1016/j.jpain.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schalet BD, Revicki DA, Cook KF, Krishnan E, Fries JF, Cella D.. Establishing a common metric for physical function: Linking the HAQ-DI and SF-36 PF subscale to PROMIS((R)) physical function. J Gen Intern Med 2015;30(10):1517–23. doi: 10.1007/s11606-015-3360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dewitt B, Feeny D, Fischhoff B, et al. Estimation of a preference-based summary score for the patient-reported outcomes measurement information system: The PROMIS((R))-Preference (PROPr) scoring system. Med Decis Making 2018;38(6):683–98. doi: 10.1177/0272989X18776637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hays RD, Spritzer KL, Schalet BD, Cella D.. PROMIS(®)-29 v2.0 profile physical and mental health summary scores. Qual Life Res 2018;27(7):1885–91. doi: 10.1007/s11136-018-1842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pilkonis PA, Yu L, Dodds NE, Johnston KL, Maihoefer CC, Lawrence SM.. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS) in a three-month observational study. J Psychiatr Res 2014;56:112–9. doi: 10.1016/j.jpsychires.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cella D, Choi SW, Condon DM, et al. PROMIS(®) adult health profiles: Efficient short-form measures of seven health domains. Value Health 2019;22(5):537–44. doi: 10.1016/j.jval.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deyo RA, Katrina R, Buckley DI, et al. Performance of a Patient Reported Outcomes Measurement Information System (PROMIS) short form in older adults with chronic musculoskeletal pain. Pain Med 2016;17(2):314–24. doi: 10.1093/pm/pnv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pilkonis PA, Yu L, Dodds NE, et al. An item bank for abuse of prescription pain medication from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)). Pain Med 2017;18(8):1516–27. doi: 10.1093/pm/pnw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gruber-Baldini AL, Velozo C, Romero S, Shulman LM.. Validation of the PROMIS(®) measures of self-efficacy for managing chronic conditions. Qual Life Res 2017;26(7):1915–24. doi: 10.1007/s11136-017-1527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor AM, Phillips K, Patel KV, et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain 2016;157(9):1836–50. doi: 10.1097/j.pain.0000000000000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med 2009;24(6):733–8. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stone AA, Broderick JE, Junghaenel DU, Schneider S, Schwartz JE.. PROMIS fatigue, pain intensity, pain interference, pain behavior, physical function, depression, anxiety, and anger scales demonstrate ecological validity. J Clin Epidemiol 2016;74:194–206. doi: 10.1016/j.jclinepi.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 29. Dudeney J, Law EF, Meyyappan A, Palermo TM, Rabbitts JA.. Evaluating the psychometric properties of the Widespread Pain Index and the Symptom Severity scale in youth with painful conditions. Can J Pain 2019;3(1):137–47. doi: 10.1080/24740527.2019.1620097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brummett CM, Bakshi RR, Goesling J, et al. Preliminary validation of the Michigan Body Map. Pain 2016;157(6):1205–12. doi: 10.1097/j.pain.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freynhagen R, Baron R, Gockel U, Tolle TR.. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22(10):1911–20. doi: 10.1185/030079906x132488. [DOI] [PubMed] [Google Scholar]
- 32. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009;144(1-2):35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 33. Fairbank JC, Pynsent PB.. The Oswestry disability index. Spine (Phila Pa 1976) 2000;25(22):2940–52. discussion 2952. [DOI] [PubMed] [Google Scholar]
- 34. Sullivan MJL, Bishop SR, Pivik J.. The pain catastrophizing scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 35. Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E.. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 1997;20(6):589–605. [DOI] [PubMed] [Google Scholar]
- 36. McWilliams LA, Kowal J, Wilson KG.. Development and evaluation of short forms of the Pain Catastrophizing Scale and the Pain Self-efficacy Questionnaire. Eur J Pain (London, England) 2015;19(9):1342–9. doi: 10.1002/ejp.665. [DOI] [PubMed] [Google Scholar]
- 37. Waddell G, Newton M, Henderson I, Somerville D, Main CJ.. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993;52(2):157–68. [DOI] [PubMed] [Google Scholar]
- 38. Hudes K. The Tampa Scale of Kinesiophobia and neck pain, disability and range of motion: A narrative review of the literature. J Can Chiropr Assoc 2011;55(3):222–32. [PMC free article] [PubMed] [Google Scholar]
- 39. Miller RP, Kori SH, Todd DD.. The Tampa Scale: A measure of kinisophobia. Clin J Pain 1991;7(1):51. [Google Scholar]
- 40. McCracken LM, Zayfert C, Gross RT.. The Pain Anxiety Symptoms Scale: Development and validation of a scale to measure fear of pain. Pain 1992;50(1):67–73. doi: 10.1016/0304-3959(92)90113-p. [DOI] [PubMed] [Google Scholar]
- 41. McCracken LM, Dhingra L.. A short version of the Pain Anxiety Symptoms Scale (PASS-20): Preliminary development and validity. Pain Res Manag 2002;7(1):45–50. doi: 10.1155/2002/517163. [DOI] [PubMed] [Google Scholar]
- 42. McCracken LM, Vowles KE, Eccleston C.. Acceptance-based treatment for persons with complex, long standing chronic pain: A preliminary analysis of treatment outcome in comparison to a waiting phase. Behav Res Ther 2005;43(10):1335–46. [DOI] [PubMed] [Google Scholar]
- 43. McCracken LM, Vowles KE, Eccleston C.. Acceptance of chronic pain: Component analysis and a revised assessment method. Pain 2004;107(1-2):159–66. [DOI] [PubMed] [Google Scholar]
- 44. Geisser ME, Robinson ME, Henson CD.. The Coping Strategies Questionnaire and chronic pain adjustment: A conceptual and empirical reanalysis. Clin J Pain 1994;10(2):98–106. [DOI] [PubMed] [Google Scholar]
- 45. Swartzman LC, Gwadry FG, Shapiro AP, Teasell RW.. The factor structure of the Coping Strategies Questionnaire. Pain 1994;57(3):311–6. [DOI] [PubMed] [Google Scholar]
- 46. Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976.
- 47. Greco CM, Yu L, Johnston KL, et al. Measuring nonspecific factors in treatment: Item banks that assess the healthcare experience and attitudes from the patient's perspective. Qual Life Res 2016;25(7):1625–34. doi: 10.1007/s11136-015-1178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kroenke K, Spitzer RL, Williams JBW, Löwe B.. The patient health questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. Gener Hosp Psychiatry 2010;32(4):345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 49. Wang LY, Lin LP, Chen YC, Wang TW, Lin JD.. Correlates of depressive symptoms among middle-aged and older homeless adults using the 9-item patient health questionnaire. Int J Environ Res Public Health 2020;17(13):4754. doi: 10.3390/ijerph17134754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B.. Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146(5):317–25. [DOI] [PubMed] [Google Scholar]
- 51. Cohen S, Kamarck T, Mermelstein R.. A global measure of perceived stress. J Health Soc Behav 1983;24(4):385–96. [PubMed] [Google Scholar]
- 52. Watson D, Clark LA, Tellegen A.. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Person Soc Psychol 1988;54(6):1063–70. [DOI] [PubMed] [Google Scholar]
- 53. Thompson ER. Development and validation of an internationally reliable short-form of the Positive and NEgative Affect Schedule (PANAS). J Cross-Cultural Psychol 2007;38(2):227–42. [Google Scholar]
- 54. Mehling WE, Acree M, Stewart A, Silas J, Jones A.. The Multidimensional Assessment of Interoceptive Awareness, Version 2 (MAIA-2). PLoS One 2018;13(12):e0208034. doi: 10.1371/journal.pone.0208034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33(6):781–92. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McNeely J, Wu L-T, Subramaniam G, et al. Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) tool for substance use screening in primary care patients. Ann Intern Med 2016;165(10):690–9. doi: 10.7326/M16-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prins A, Bovin MJ, Smolenski DJ, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): Development and evaluation within a Veteran primary care sample. J Gen Intern Med 2016;31(10):1206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM.. The Life Events Checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD; 2013. Available at: www.ptsd.va.gov. [Google Scholar]
- 60. Puterman E, Adler N, Matthews KA, Epel E.. Financial strain and impaired fasting glucose: The moderating role of physical activity in the Coronary Artery Risk Development in Young Adults study. Psychosom Med 2012;74(2):187–92. doi: 10.1097/PSY.0b013e3182448d74. [DOI] [PubMed] [Google Scholar]
- 61. Prevention Institute. THRIVE: Tool for Health and Resilience in Vulnerable Environments. Available at: https://www.preventioninstitute.org/tools/thrive-tool-health-resilience-vulnerable-environments (accessed July 29, 2022).
- 62. Sherbourne CD, Stewart AL.. The MOS social support survey. Soc Sci Med 1991;32(6):705–14. [DOI] [PubMed] [Google Scholar]
- 63. Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L.. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006;13(1):27–45. [DOI] [PubMed] [Google Scholar]
- 64. Maples-Keller JL, Williamson RL, Sleep CE, Carter NT, Campbell WK, Miller JD.. Using item response theory to develop a 60-item representation of the NEO PI-R using the international personality item pool: Development of the IPIP-NEO-60. J Pers Assess 2019;101(1):4–15. doi: 10.1080/00223891.2017.1381968. [DOI] [PubMed] [Google Scholar]
- 65. Vuilleumier PH, Biurrun Manresa JA, Ghamri Y, et al. Reliability of quantitative sensory tests in a low back pain population. Reg Anesth Pain Med 2015;40(6):665–73. doi: 10.1097/AAP.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 66. Wasan AD, Alter BJ, Edwards RR, et al. Test-retest and inter-examiner reliability of a novel bedside quantitative sensory testing battery in postherpetic neuralgia patients. J Pain 2020;21(7-8):858–68. doi: 10.1016/j.jpain.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Geber C, Klein T, Azad S, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): A multi-centre study. Pain 2011;152(3):548–56. doi: 10.1016/j.pain.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 68. Nothnagel H, Puta C, Lehmann T, et al. How stable are quantitative sensory testing measurements over time? Report on 10-week reliability and agreement of results in healthy volunteers. J Pain Res 2017;10:2067–78. doi: 10.2147/JPR.S137391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Graven-Nielsen T, Kendall SA, Henriksson KG, et al. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain 2000;85(3):483–91. doi: 10.1016/S0304-3959(99)00308-5. [DOI] [PubMed] [Google Scholar]
- 70. Price DD, Mao J, Frenk H, Mayer DJ.. The Symbol receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain 1994;59(2):165–74. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 71. Price DD, Mao J, Frenk H, Mayer DJ.. The N-methyl-d-aspartate receptor antagonist destromethorphan selectively reduces temporal summation of second pain in man. Pain 1994;59(2):165–74. [DOI] [PubMed] [Google Scholar]
- 72. Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ.. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002;99(1-2):49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 73. Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD.. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001;91(1-2):165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 74. Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB.. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015;156(1):55–61. doi: 10.1016/j.pain.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 75. Weissman-Fogel I, Granovsky Y, Crispel Y, et al. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain 2009;10(6):628–36. doi: 10.1016/j.jpain.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 76. Nir RR, Yarnitsky D.. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9(2):131–7. doi: 10.1097/SPC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 77. O'Brien AT, Deitos A, Triñanes Pego Y, Fregni F, Carrillo-de-la-Peña MT.. Defective endogenous pain modulation in fibromyalgia: A meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain 2018;19(8):819–36. doi: 10.1016/j.jpain.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 78. Locke D, Gibson W, Moss P, Munyard K, Mamotte C, Wright A.. Analysis of meaningful conditioned pain modulation effect in a pain-free adult population. J Pain 2014;15(11):1190–8. doi: 10.1016/j.jpain.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 79. Goodin BR, McGuire L, Allshouse M, et al. Associations between catastrophizing and endogenous pain-inhibitory processes: Sex differences. J Pain 2009;10(2):180–90. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 80. Oono Y, Nie H, Matos RL, Wang K, Arendt-Nielsen L.. The inter- and intra-individual variance in descending pain modulation evoked by different conditioning stimuli in healthy men. Scand J Pain 2011;2(4):162–9. doi: 10.1016/j.sjpain.2011.05.006. [DOI] [PubMed] [Google Scholar]