Abstract
Objective:
A general psychopathology (‘p’) factor captures shared variation across mental disorders. Structural neural alterations have been associated with p concurrently, but less is known about whether these alterations relate to within-person change in p over time, especially during preadolescence, a period of neurodevelopmental changes.
Method:
We examined whether baseline brain structure was prospectively related to the trajectory of p and specific forms of psychopathology over two years in 9,220 preadolescents (aged 9–10 at baseline) from the Adolescent Brain Cognitive Development Study®. We conducted longitudinal multilevel models to determine whether baseline brain structure (volume, surface area, thickness) was associated with between-person differences and within-person change in p (from a higher-order confirmatory factor model) and internalizing, externalizing, neurodevelopmental, somatization, and detachment factor scores (from a correlated factors model) over three study waves.
Results:
Smaller global volume and surface area, but not thickness, were associated with higher between-person levels of p, which persisted over time. None of the brain structure measures related to within-person change in p. Lower baseline cortical thickness was associated with steeper decreases in internalizing psychopathology, which was driven by lower thickness within sensorimotor and temporal regions.
Conclusion:
These novel results identify specific brain structure features that might contribute to transdiagnostic psychopathology development in preadolescence. Children with smaller total brain volume and surface area may be vulnerable to persistent general psychopathology during preadolescence. Cortical thinning reflective of pruning and myelination in sensorimotor and temporal brain regions specifically may protect against increases in internalizing, but not general psychopathology during preadolescence.
Keywords: brain structure, transdiagnostic, general psychopathology, p factor, longitudinal
INTRODUCTION
Factor-analytic models have identified a general psychopathology factor, often termed the ‘p’ factor, which captures shared variation across mental disorder categories1,2. The p factor accounts for comorbidity and severity of psychopathology and has been identified in a range of samples across the lifespan3,4. Individuals high in p experience greater life impairment and distress (i.e., psychiatric hospitalizations, social welfare benefits, violence convictions)1,2, histories of childhood maltreatment1,2, and future psychopathology2,5 and suicidality2,6 than individuals low in p. However, the psychological and neurobiological mechanisms underlying general psychopathology are not yet well-established.
Neuroimaging research has identified global patterns of structural alterations in cortical volume, surface area (SA), and cortical thickness (CT) distributed throughout the brain in individuals high in p, but the type of alteration differed in youth versus adult studies. Prior research in children (aged 9–10) from the Adolescent Brain Cognitive Development (ABCD) study7,8 and adolescents (aged 8–23) from the Philadelphia Neurodevelopmental Cohort9 revealed that global patterns of smaller brain volume and/or SA but not CT were associated with higher levels of p. Adolescent studies also reported widespread negative deviations from normative models of brain volume and SA, with minimal deviations in CT, associated with transdiagnostic psychopathology10,11. Alternatively, research in midlife adults (aged 45) from the Dunedin Longitudinal Study12 found that pervasive patterns of cortical thinning but not smaller SA were associated with higher p. These studies largely have described similar patterns of brain structural alterations associated with both general and specific forms of psychopathology, with a few exceptions (e.g., fear symptoms specifically related to lower CT9). But abnormalities within different features of cortical mantle structure associated with transdiagnostic psychopathology dimensions suggests that differences in developmental stage are important for understanding the structural neural mechanisms underlying general psychopathology.
Cortical volume is the product of SA and thickness. SA and CT develop at different rates throughout the lifespan and are evolutionarily, genetically, and cellularly distinct13. CT increases from birth to early childhood and then decreases linearly throughout childhood and adolescence13–16. Alternatively, volume and SA tend to follow an inverted U-shaped developmental trajectory peaking during preadolescence before plateauing and slightly decreasing throughout adolescence into early adulthood13–15. As CT and SA follow different neurodevelopmental trajectories, they may relate to transdiagnostic psychopathology dimensions differently throughout the lifespan.
Much of the research examining the structural neural correlates of general and specific psychopathology factors has been cross-sectional, which by design, cannot establish the temporal precedence between brain structure and psychopathology. Some studies have examined relations between change in brain structure over two timepoints and mental disorder symptoms17 or whether symptoms prospectively related to change in brain structure18–20. But few studies have examined whether brain structure is associated with change in psychopathology over time11,21, and no known prospective longitudinal investigations of relations between brain morphology and the p factor have been conducted. As a result, we cannot know whether childhood brain structural alterations relate to future levels of or rates of change in p over time. It is especially important to conduct such prospective longitudinal studies during preadolescence, a time marked by extensive neurodevelopmental changes13,13–15, prior to the onset of most mental disorders in adolescence and young adulthood22.
Based on cross-sectional studies of the relations between brain structure and general and specific psychopathology dimensions7–9,12, three primary questions emerge about the role of brain morphology on transdiagnostic psychopathology development in preadolescence: 1) do previously identified inverse cross-sectional relations between brain structure and p persist over time?; 2) does childhood brain morphology prospectively relate to rates of change in p or specific psychopathology factors over time?; 3) and if so, which brain structure components (i.e., volume, SA, or CT) are associated with these psychopathology trajectories? Determining childhood brain structural alterations that prospectively relate to levels and/or rates of change in general and specific forms of psychopathology has implications for early identification of children who may be vulnerable to developing comorbid and severe forms of disorder in adolescence.
In the current study, we used three waves of clinical data and the first wave of structural magnetic resonance imaging (MRI) data from 9,220 preadolescents (aged 9–10 at baseline) from the ABCD study to test whether baseline brain structure is prospectively related to between-person differences and within-person changes in transdiagnostic psychopathology dimensions during preadolescence. We tested whether between-person differences and within-person changes in p over time were associated with structural alterations in volume, SA, or CT. As some forms of psychopathology may develop at different rates than others, we also tested whether baseline brain structure was prospectively associated with the trajectories of externalizing, internalizing, neurodevelopmental, somatization, and detachment symptoms rather than p.
To this end, we calculated unstandardized factor scores from previously identified higher-order and correlated factors confirmatory factor models of the structure of psychopathology using the same clinical scales at each wave23. Using longitudinal multilevel modeling, we tested whether baseline brain structure (volume, SA, CT) was related to the intercept (between-person differences) and slope (within-person rate of change) of the psychopathology factor scores over three waves (2-year timeframe). Baseline brain structure may reflect the extent of volume, SA, and CT maturation that has occurred prior to the child’s age at the baseline MRI. Consistent with prior cross-sectional ABCD studies7,8, we hypothesized that reduced volume and SA but not CT would be associated with higher between-person p factor scores. We considered analyses examining whether brain structure relates to within-person trajectories of p factor scores over time to be exploratory, as no known studies have investigated this question. Finally, we hypothesized that the prospective relations between brain structure and the intercept and slope of psychopathology factor scores would generalize across the specific forms of psychopathology.
METHOD
Participants
The ABCD sample consists of 11,875 children who participated in a major collaboration between 22 U.S. sites. Complete recruitment details can be found elsewhere24. Exclusion criteria included: not being fluent in English, having a parent not fluent in English or Spanish, major medical or neurological conditions, gestational age <28 weeks or birthweight <1200 g, contraindications to MRI scanning, history of traumatic brain injury, current schizophrenia diagnosis, moderate/severe autism spectrum disorder, intellectual disability, or alcohol/substance use disorder. Institutional review board approval was obtained for each site before data collection. All parents provided written informed consent and children provided assent.
Demographic, clinical, and structural MRI data were accessed from the National Institutes of Mental Health Data Archive (NDA). The current study is based on 9,856 unrelated children (randomly selecting one child per family when more than one participated) from the ABCD 4.0 data release (DOI 10.15154/1523041), including data collected September 1, 2016-February 15, 2021. In response to COVID-19 restrictions, the ABCD study team pivoted to remote or hybrid in-person/remote visits when in-person testing was not feasible from March 2020 on, affecting Wave 3 (Supplement 1, available online). Participants with missing demographic information, complete nonresponse on clinical data, or those who did not pass structural MRI quality assurance measures (Figure S1, available online) were excluded (baseline Wave 1 n=9,220; one-year follow-up Wave 2 n=8,660; two-year follow-up Wave 3 n=8,017).
Psychopathology
Child psychopathology at each wave was assessed with the Child Behavior Checklist (CBCL; age 6–18 form)25, a 119-item parent rating scale describing child behaviors and emotions. Parents rate which behaviors were characteristic of their child over the past six months on a scale of 0 (“Not True (as far as you know)”), 1 (“Somewhat or Sometimes True”), or 2 (“Very True or Often True”) (see Supplement 2, available online, for details on measures).
Covariates
Parents/guardians reported their child’s sex assigned at birth and age (in months). MRI scanner was dummy-coded into Prisma, Discovery, Achieva, and Ingenia variables (Prisma Fit = reference group).
MRI Data Acquisition, Processing, and Quality Control
MRI acquisition and scanning parameters, processing, and quality assurance procedures are described elsewhere26,27 (Supplement 3, available online). Briefly, brain data were collected on 3T scanners (Siemens Prisma and Prisma Fit, General Electric MR 750, Philips Achieva dStream and Ingenia). T1 images were corrected for gradient nonlinearity distortions using scanner-specific, nonlinear transformations. The ABCD Data Analysis, Informatics, and Resources Centre (DAIRC) performed cortical reconstruction and volumetric segmentation using FreeSurfer v7.1.128. We used post-processed volume, SA, and CT data mapped to 34 cortical parcellations per hemisphere based on the Desikan–Killiany brain registration atlas29 and volume of 19 subcortical segmentations30. The DAIRC employed automated and manual approaches to review datasets for quality before sharing data.
Statistical Analyses
Confirmatory Factor Analyses
Previously, higher-order and correlated factors models of CBCL item-level data31 from the ABCD 3.0 release were fit at each of the three waves23 (Supplement 4, available online). These factor models were found to be metric invariant (i.e., equivalent factor loadings across waves)23. Thus, we used unstandardized factor loadings from those baseline factor models to calculate unstandardized p (from the higher-order model) and externalizing (EXT), internalizing (INT), neurodevelopmental (ND), somatization (SOMAT), and detachment (DETACH) factor scores (from the correlated factors model) (see Tables S1–S3, available online, for baseline factor loadings). We calculated factor scores by multiplying each CBCL item by its unstandardized factor loading and then summing the weighted items for each factor.
Longitudinal Multilevel Modeling
We conducted three-level linear growth models to control for nesting within ABCD study sites. Level 1 accounted for the within-subject trajectory of psychopathology factor scores, Level 2 accounted for within-site, between-subject differences, and Level 3 accounted for between-site differences. We included site- and subject-specific random intercepts and slopes for time. Time was coded as wave number (baseline Wave 1=0; Wave 2=1; Wave 3=2).
Analyses were performed in R version 4.1.1 (http://www.r-project.org/) using the lme4 package32. First, we conducted unconditional three-level linear growth models to determine factor score trajectories over wave and the extent of variability in site and subject intercepts and slopes. Second, we examined whether four global brain structure measures (total cortical volume, subcortical volume, cortical SA, mean CT) were associated with site- and subject-level intercepts and slopes of the factor scores over wave, resulting in 24 conditional three-level growth models tested. Baseline brain structure measures were included as time-invariant covariates (TICs). We mean-centered site means for each brain structure variable to capture between-site effects. We site-mean-centered brain structure variables for subjects to capture within-site, between-subjects effects. Sex, age, and MRI scanner model were included as Level 2 TICs.
The conditional three-level growth models were conducted using the following fixed effects formula: Psychopathology factor scores = β1×wave + β2×age + β3×sex + β4×MRI scanner dummies + β5×site brain structure + β6×subject brain structure + β7×site brain structure × wave + β8×subject brain structure × wave. As we were interested in subject-level effects of brain structure on psychopathology factor scores, we only report those relevant regression coefficients here (see Table S4, available online, for site-level results). We assumed that the missing data mechanism was missing-at-random and the likelihood of the growth models based on the observed data was sufficient for inference on the associations of interest. Therefore, all subjects with at least one observation across waves were included in the models.
Third, if any global brain structure measures were associated with the subject-level intercept or slope, we performed follow-up parcel-wise analyses to determine whether results were driven by structural alterations within specific brain regions. Parcel-wise analyses were conducted with the 68 cortical parcellations derived from the surface-based atlas procedure29 and the 19 subcortical regions derived by the automated labeling procedure30. The volume, area, or thickness of each region, hereinafter referred to as “parcels”, were centered and included in the three-level growth models as TICs just as described in the global analyses above. We conducted these parcel-wise analyses both with and without the global brain structure measures included as TICs to evaluate specificity of relations between each parcel and the psychopathology trajectories.
We corrected for multiple comparisons by using a false discovery rate (FDR) procedure33 (q<0.05) for the 48 global tests in one set and follow-up parcel-wise tests in a second set. Analysis code is available at https://github.com/Ageyr13/ABCD_brain_structure_MLM.git. Relationships between brain structure and intercepts would indicate that baseline brain structure is associated with psychopathology at baseline, whereas relationships with slope would indicate that within-person psychopathology trajectories differ as a function of baseline brain structure. If there were significant relations between brain structure and intercepts, but not slopes, we tested whether these relations remained at Waves 2 and 3.
Sensitivity Analyses
We conducted five sensitivity analyses. First, we included parental education and total combined family income (over past twelve months) as TICs in the growth models as measures of socioeconomic status that may influence brain structure and psychopathology. Second, we controlled for the effects of baseline psychotropic medication use (over past two weeks) by including this as an additional TIC that may influence brain structure and psychopathology. Third, we controlled for the Wave 3 visit setting to ensure that results were not partially driven by remote/hybrid vs. in-person setting. Fourth, we conducted the analyses using factor scores derived from a bifactor model, which imposes an orthogonal structure between the general and specific factors (see Supplement 4 and Table S3, available online, for more details). Fifth, as we randomly selected one sibling per family for inclusion in the main analyses, we subsequently randomly sampled one sibling per family and ran the growth models 100 times to evaluate the robustness of the associations across different combinations of included siblings (one per family).
RESULTS
Descriptive Statistics
Table 1 shows descriptive statistics and missingness information for all study variables (see Table S5 for variable intercorrelations, available online). Of the 9220 included participants at baseline, 1405 (15.31%) had missing CBCL data at Waves 2 or 3. Of those 1405 participants with missing CBCL data, 1047 were missing at Waves 2 or 3, and 358 were missing at both follow-up waves. Baseline differences in all study variables between participants with any missing CBCL data (n=1405) versus those with no missing follow-up data (n=7815) were tested. The sample with no missing follow-up data had a significantly greater proportion of non-Hispanic White participants, a smaller proportion of Black and participants categorized as “Other”, higher parental education and family income, larger volume and SA, and lower baseline p, EXT, and ND factor scores than participants with missing CBCL data. As there are differences between those with and without missing data on baseline factor scores, we conducted an additional sensitivity analysis by removing 358 participants with completely missing CBCL follow-up data.
Table 1.
Descriptive Statistics of Study Variables and Comparisons of Participants With and Without Missing Follow-up Data
| Baseline wave 1 sample | Non-missing sample | Missing sample | X2/t | p | ||||||
|
| ||||||||||
| n | Min | Max | Mean (SD) or % | n | Mean (SD) or % | n | Mean (SD) or % | |||
|
| ||||||||||
| Demographic covariates | ||||||||||
|
| ||||||||||
| Age (months) | 9220 | 107 | 132 | 118.95 (7.40) | 7815 | 118.97 (7.43) | 1405 | 118.84 (7.26) | 0.57 | 0.569 |
| Sex (% Female) | 9220 | 47.7 | 7815 | 47.5 | 1405 | 49.2 | 1.39 | 0.238 | ||
| Non-Hispanic White (%) | 9220 | 51.3 | 7815 | 53.9 | 1405 | 36.6 | 142.87 | <0.001 | ||
| Black (%) | 9220 | 17.7 | 7815 | 15.7 | 1405 | 28.8 | 140.40 | <0.001 | ||
| Asian (%) | 9220 | 4.9 | 7815 | 4.9 | 1405 | 4.7 | 0.12 | 0.729 | ||
| Hispanic (%) | 9220 | 12.0 | 7815 | 11.9 | 1405 | 12.7 | 0.75 | 0.388 | ||
| Other (%) | 9220 | 14.2 | 7815 | 13.6 | 1405 | 17.2 | 12.86 | <0.001 | ||
| Parent Education | 9207 | 1 | 8 | 5.22 (1.65) | 7805 | 5.33 (1.60) | 1402 | 4.63 (1.77) | 13.78 | <0.001 |
| Family Income | 8413 | 1 | 10 | 7.21 (2.43) | 7199 | 7.36 (2.32) | 1214 | 6.32 (2.82) | 12.12 | <0.001 |
| Psychotropic Medication Use (%) | 9220 | 8.8 | 7796 | 8.7 | 1405 | 9.8 | 2.03 | 0.154 | ||
|
| ||||||||||
| Global brain structure | ||||||||||
|
| ||||||||||
| Cortical Volume | 9220 | 394785 | 832508 | 597704.74 (55937.30) | 7815 | 599547.00 (55424.72) | 1405 | 587457.49 (57622.22) | 7.28 | <0.001 |
| Subcortical Volume | 9220 | 36446 | 80975 | 59971.95 (4871.54) | 7815 | 60058.99 (4839.50) | 1405 | 59487.80 (5020.34) | 4.05 | <0.001 |
| Cortical Surface Area | 9220 | 127160 | 275078 | 189473.85 (17987.90) | 7815 | 190007.18 (17823.60) | 1405 | 186507.28 (18605.13) | 6.53 | <0.001 |
| Cortical Thickness | 9220 | 2.42 | 3.06 | 2.73 (0.08) | 7815 | 2.73 (0.08) | 1405 | 2.72 (0.08) | 2.43 | 0.015 |
|
| ||||||||||
| Factor scores | All waves | Non-missing sample | Missing sample | X2/t | p | |||||
|
| ||||||||||
| n | Min | Max | Mean (SD) or % | n | Mean (SD) or % | n | Mean (SD) or % | |||
|
| ||||||||||
| P1 | 9220 | 0 | 57.15 | 7.42 (7.68) | 7815 | 7.29 (7.54) | 1405 | 8.13 (8.42) | 3.50 | <0.001 |
| P2 | 8660 | 0 | 50.63 | 7.16 (7.40) | ||||||
| P3 | 8017 | 0 | 65.72 | 6.73 (7.27) | ||||||
| EXT1 | 9220 | 0 | 40.89 | 3.84 (4.91) | 7815 | 3.76 (4.81) | 1405 | 4.31 (5.40) | 3.58 | <0.001 |
| EXT2 | 8660 | 0 | 39.03 | 3.57 (4.67) | ||||||
| EXT3 | 8017 | 0 | 38.84 | 3.29 (4.55) | ||||||
| INT1 | 9220 | 0 | 14.18 | 1.34 (1.80) | 7815 | 1.34 (1.79) | 1405 | 1.34 (1.85) | 0.06 | 0.952 |
| INT2 | 8660 | 0 | 12.92 | 1.37 (1.82) | ||||||
| INT3 | 8017 | 0 | 13.67 | 1.25 (1.78) | ||||||
| ND1 | 9220 | 0 | 17.89 | 2.65 (3.00) | 7815 | 2.59 (2.95) | 1405 | 2.99 (3.24) | 4.28 | <0.001 |
| ND2 | 8660 | 0 | 17.89 | 2.56 (2.95) | ||||||
| ND3 | 8017 | 0 | 18.27 | 2.42 (2.86) | ||||||
| SOMAT1 | 9220 | 0 | 8.12 | 0.77 (1.10) | 7815 | 0.77 (1.09) | 1405 | 0.78 (1.18) | 0.08 | 0.937 |
| SOMAT2 | 8660 | 0 | 9.17 | 0.76 (1.11) | ||||||
| SOMAT3 | 8017 | 0 | 8.15 | 0.73 (1.07) | ||||||
| DETACH1 | 9220 | 0 | 7.31 | 0.45 (0.82) | 7815 | 0.45 (0.81) | 1405 | 0.48 (0.89) | 1.46 | 0.144 |
| DETACH2 | 8660 | 0 | 7.31 | 0.49 (0.83) | ||||||
| DETACH3 | 8017 | 0 | 7.31 | 0.54 (0.92) | ||||||
Note: Comparisons were made by chi-square tests for categorical variables and independent samples t-tests for continuous variables. P Values are unadjusted; p-values that survived FDR correction for the 20 tests (q<0.01) are indicated in bold. Slight variations in sample size reflect missing data in some variables. Mean parental education of 5.22 is equivalent to an Associate Degree. Mean combined family income of 7.21 is equivalent to between $50,000 and $75,000 income over the past 12 months. 1 = baseline Wave 1; 2 = one-year follow-up Wave 2; 3 = two-year follow-up Wave 3; DETACH = detachment factor scores; EXT = externalizing factor scores; INT = internalizing factor scores; ND = neurodevelopmental factor scores; P = general psychopathology factor scores; SOMAT = somatization factor scores.
Unconditional Three-Level Linear Growth Model
Fixed effects indicated a significant decreasing trend of p, EXT, INT, ND, and SOMAT factor scores over the waves, whereas there was a significant increasing trend of DETACH (Table 2). Not surprisingly, the random effects indicate that there was more intraindividual (i.e., wave within subject) and interindividual variability (i.e., subject within site) than between-site variability in the intercept and slope of the factor scores over time. The random effects also show that there was more interindividual variability in the intercepts than the slopes, with minimal variability in the slopes of the factor scores over time. The intrasubject correlations for each of the psychopathology factor scores over wave were: p=0.745; EXT=0.728; INT=0.649; ND=0.747; SOMAT=0.523; DETACH=0.568.
Table 2.
Unconditional Linear Three-Level Growth Models of Psychopathology Factor Scores Over Three Waves
| P | EXT | INT | ND | SOMAT | DETACH | |||||||
|
| ||||||||||||
| β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | |
|
| ||||||||||||
| Fixed Effects | ||||||||||||
|
| ||||||||||||
| Intercept | 7.33*** | 0.25 | 3.78*** | 0.16 | 1.32*** | 0.05 | 2.64*** | 0.09 | 0.77*** | 0.02 | 0.45*** | 0.02 |
| Wave | −0.28*** | 0.06 | −0.24*** | 0.04 | −0.03* | 0.01 | −0.09*** | 0.02 | −0.02* | 0.01 | 0.04*** | 0.01 |
|
| ||||||||||||
| Random Effects | Variance | SD | Variance | SD | Variance | SD | Variance | SD | Variance | SD | Variance | SD |
|
| ||||||||||||
| Level 1 Residual | 12.04 | 3.47 | 5.10 | 2.26 | 1.02 | 1.01 | 1.94 | 1.39 | 0.54 | 0.74 | 0.27 | 0.52 |
| Level 2 Intercept | 46.13 | 6.79 | 18.67 | 4.32 | 2.24 | 1.50 | 7.04 | 2.65 | 0.68 | 0.82 | 0.42 | 0.64 |
| Level 2 Covariance | −0.33 | −0.38 | −0.24 | −0.31 | −0.26 | −0.09 | ||||||
| Level 2 Slope | 2.26 | 1.50 | 0.99 | 0.99 | 0.12 | 0.34 | 0.26 | 0.51 | 0.03 | 0.17 | 0.05 | 0.23 |
| Level 3 Intercept | 1.22 | 1.11 | 0.48 | 0.69 | 0.04 | 0.20 | 0.16 | 0.40 | 0.01 | 0.09 | 0.00 | 0.06 |
| Level 3 Covariance | −0.81 | −0.94 | −0.70 | −0.63 | −0.91 | −1.00 | ||||||
| Level 3 Slope | 0.07 | 0.26 | 0.02 | 0.15 | 0.00 | 0.05 | 0.01 | 0.10 | 0.00 | 0.01 | 0.00 | 0.00 |
Note: Unstandardized estimates are shown. DETACH = detachment factor scores; EXT = externalizing factor scores; INT = internalizing factor scores; Level 1 Residual = intraindividual variability (within-subject repeated measures); Level 2 = interindividual variability (within-site); Level 3 = inter-site variability (between-site); ND = neurodevelopmental factor scores; P = general psychopathology factor scores; SD = standard deviation; SE = standard error; SOMAT = somatization factor scores.
p<.05
p<.01
p<.001
Global Brain Structure Associations with Psychopathology Trajectories
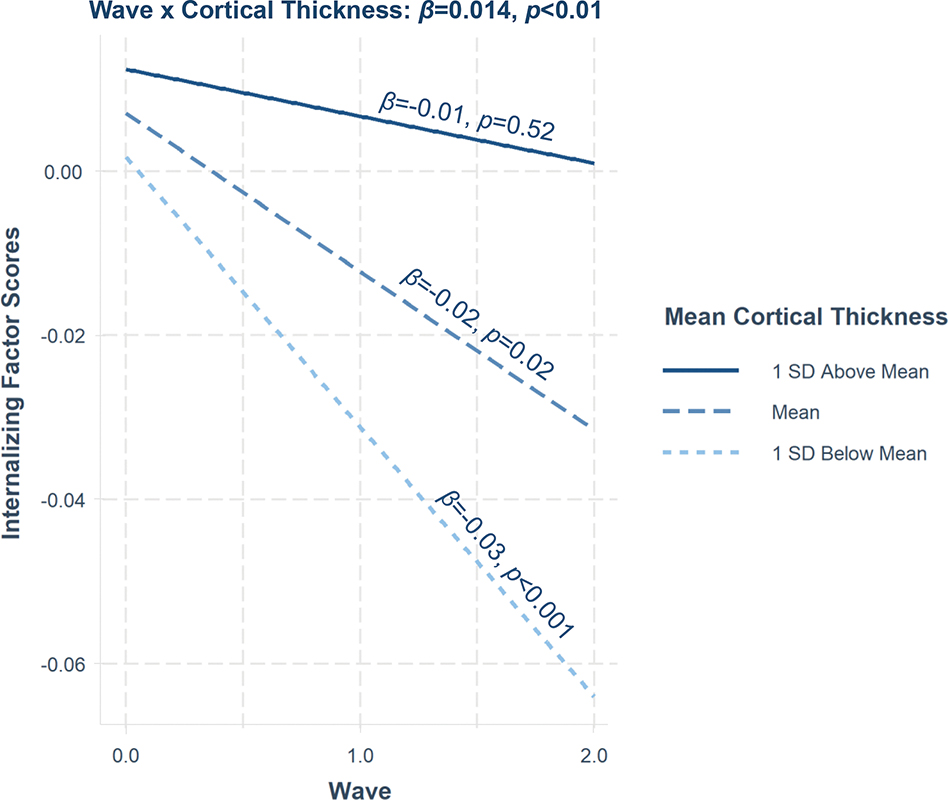
Results show that smaller baseline total cortical volume, subcortical volume, and SA, but not mean CT, were significantly associated with higher p factor scores at baseline (intercept) after FDR correction (Table 3), which remained significant at Waves 2 and 3 (Table S6, available online). These findings are largely consistent across factors except for INT. None of the baseline global brain structure measures was associated with the slope of the psychopathology factor scores over wave, apart from INT. Specifically, baseline mean CT was estimated to have a positive slope over the waves for the INT factor scores, which remained significant even after controlling for p factor scores (β=0.010, p=0.008). We estimated simple slopes at 1 standard deviation (SD) below the mean, the mean, and 1 SD above the mean using the interactions package34 to illustrate this interaction. We found that children with lower baseline mean CT had significantly steeper decreases in INT factor scores over wave (β=−0.03, SE=0.01, p<0.001), followed by children with average mean CT (β=−0.02, SE=0.01, p=0.02). Children with higher mean CT did not show a significant change in INT factor scores over wave (β=−0.01, SE=0.01, p=0.52) (Figure 1).
Table 3.
Global Brain Structure Relations With Intercept and Slope of the Psychopathology Factors Over Wave
| P | EXT | INT | ND | SOMAT | DETACH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Global Brain Structure | Std. β | 95% CI | Std. β | 95% CI | Std. β | 95% CI | Std. β | 95% CI | Std. β | 95% CI | Std. β | 95% CI |
|
| ||||||||||||
| Total Cortical Volume | ||||||||||||
|
| ||||||||||||
| Intercept | −.098 *** | [−.120, −.076] | −.105 *** | [−.128, −.083] | −.003 | [−.024, .019] | −.104 *** | [−.126, −.082] | −.022* | [−.043, −.000] | −.048 *** | [−.069, −.027] |
| Slope | .008 | [−.000, .016] | .005 | [−.004, .013] | .009 | [−.001, .018] | .009* | [.001, .017] | .010 | [−.001, .021] | −.001 | [−.012, .010] |
|
| ||||||||||||
| Total Subcortical Volume | ||||||||||||
|
| ||||||||||||
| Intercept | −.078 *** | [−.100, −.056] | −.080 *** | [−.103, −.058] | −.003 | [−.025, .018] | −.089 *** | [−111, −.067] | −.010 | [−.032, .011] | −.036 ** | [−.057, −.016] |
| Slope | .003 | [−.006, .011] | .003 | [−.006, .011] | .001 | [−.008, .010] | .003 | [−.005, .012] | .004 | [−.007, .015] | −.006 | [−.017, .005] |
|
| ||||||||||||
| Total Cortical Surface Area | ||||||||||||
|
| ||||||||||||
| Intercept | −.103 *** | [−.126, −.081] | −.113 *** | [−.136, −.091] | −.006 | [−.028, .017] | −.106 *** | [−.129, −.084] | −.022* | [−.044, −.001] | −.043 *** | [−.064, −.022] |
| Slope | .006 | [−.003, .014] | .006 | [−.003, .014] | .003 | [−.007, .012] | .006 | [−.002, .014] | .006 | [−.005, .017] | −.003 | [−.014, .007] |
|
| ||||||||||||
| Mean Cortical Thickness | ||||||||||||
|
| ||||||||||||
| Intercept | −.003 | [−.023, .018] | .002 | [−.018, .023] | .005 | [−.015, .025] | −.010 | [−.030, .011] | .002 | [−.018, .022] | −.019 | [−.038, .000] |
| Slope | .005 | [−.003, .014] | −.002 | [−.011, .006] | .014 ** | [.004, .023] | .007 | [−.001, .015] | .009 | [−.002, .020] | .008 | [−.003, .019] |
Note: n=25,897 observations over three waves. Three-level linear growth models were run (Level 1 = within-subject repeated measures of psychopathology factor scores; Level 2 = within-site, between-subject differences; Level 3 = between-site differences). Intercept effects can be interpreted as the main effect of baseline brain structure on between-person differences in the psychopathology factor scores. Slope effects can be interpreted as interactions between baseline brain structure and wave. Site-level intercepts and slopes and covariates age, sex, scanner (dummy-coded) are not shown here (see Table S5, available online). Standardized estimates are shown. Estimates in bold survived FDR correction (q<0.05) for the 48 tests. CI = confidence interval; DETACH = detachment factor scores; EXT = externalizing factor scores; INT = internalizing factor scores; ND = neurodevelopmental factor scores; P = general psychopathology factor scores; SOMAT = somatization factor scores; Std. = standardized.
unadjusted p<.05
unadjusted p<.01
unadjusted p<.001
Figure 1. Mean Cortical Thickness Relations With Rate of Change of Internalizing Factor Scores Over Wave.
Note: The interaction between mean cortical thickness and wave (slope) for internalizing factor scores is shown. Simple slopes analysis revealed that children with higher mean cortical thickness (1 SD above the mean) showed no change in internalizing factor scores over the study waves. Children with lower mean cortical thickness (1 SD below the mean) showed the steepest declines in internalizing factor scores over the waves followed by children with average levels of mean cortical thickness. Standardized estimates are shown.
Parcel-Wise Analyses
Based on results from the global brain structure analyses, we conducted parcel-wise analyses of associations of 68 cortical volume, 68 SA, and 19 subcortical volume parcels with the intercept of p factor scores, and associations of 68 CT parcels with the slope for INT factor scores. Parcel-wise analyses were conducted with and without global brain structure included as a covariate. FDR correction was employed for all 446 parcel-wise tests simultaneously.
The association between baseline global volume and SA and the intercept of p factor scores was distributed throughout the brain with 66 cortical volume, 68 SA, and 19 subcortical volume parcels demonstrating significant associations with intercepts (Figure 2A–C). These parcel-wise relations largely were no longer significant after controlling for global brain structure (see Table S7, available online, for a few exceptions). Alternatively, the association between mean CT and the slope for INT factor scores was driven by 16 of the 68 CT parcels, and remained significant after controlling for global CT. Baseline CT within the bilateral paracentral lobule, left precentral gyrus, bilateral postcentral gyrus, bilateral superior parietal lobule, left inferior temporal gyrus, left middle temporal gyrus, bilateral parahippocampal gyrus, right cuneus, bilateral lateral occipital cortex, and bilateral lingual gyrus parcels significantly predicted the slope for INT over wave (Figure S2 and Table S8, available online).
Figure 2. Parcel-Wise Cortical Volume, Surface Area, and Subcortical Volume Relations With the Intercept of p Factor Scores.
Note: Statistical parametric maps from parcel-wise analyses are shown to illustrate significant negative associations of (A) cortical volume and (B) cortical surface area with the intercept of p factor scores. (C) shows a forest plot of significant negative associations between subcortical volume parcels and the intercept of p factor scores. All associations shown are false discovery rate corrected for all parcel-wise tests (q<0.05). Color bars reflect effect sizes (standardized betas).
Sensitivity Analyses
The above results largely remained after controlling for parental education and family income, psychotropic medication use, visit setting, employing factor scores from a bifactor model, and excluding participants with completely missing follow-up data (Tables S9–13, available online). Exceptions were due to slight changes in unadjusted p-values, which either no longer met the FDR significance threshold (q<0.05) or now met this threshold, despite effect sizes remaining similar (Supplement 5, available online). Critically, we also found that the above estimates were stable across inclusion of 100 different combinations of randomly selected siblings (Figure S3, available online).
DISCUSSION
In the ABCD study of preadolescents, we examined prospective relations between baseline brain structure and transdiagnostic psychopathology trajectories over a two-year period. Consistent with prior ABCD research7,8, we found that smaller global baseline volume and SA, but not CT, were associated with higher between-person p factor scores, and now show that these relations persisted over wave, independent of sex, age, scanner, site, socioeconomic status, psychotropic medication, visit setting, type of confirmatory factor model, and inclusion of different randomly selected siblings. Between-person effects largely generalized across all forms of psychopathology except for INT. None of the brain structure measures were prospectively related to within-person trajectories of p. However, lower baseline mean CT was associated with steeper declines in INT factor scores over time, driven by lower CT within visual, somatomotor, and temporal regions.
We found that smaller volume and SA was associated with between-person levels of p. These findings both replicate and extend prior ABCD research, which found that global patterns of smaller volume and/or SA was cross-sectionally associated with higher p7,8. This internal replication is particularly of note given that each of these investigations, including the present study, used different combinations of psychopathology measurements (i.e., diagnostic interview vs. CBCL) and confirmatory factor models (i.e., bi-factor vs. higher-order/correlated factors). Our results further show that these inverse relations between volume and SA and the p factor are stable during preadolescence.
However, we did not find evidence of prospective relations between brain structure and within-person rates of change in p or the specific psychopathology factors, except for INT. Lower average CT (i.e., thinner cortex) was associated with within-person rates of change in INT over time. This finding is consistent with prior cross-sectional research in the Philadelphia Neurodevelopmental Cohort, which found CT was associated with fears but not p factor scores9. Cortical thinning is thought to reflect myelination (i.e., increasing proportion of myelinated axons) and pruning (i.e., synapse, dendrite, or cell body loss/ remodeling)35,36. Baseline CT may reflect the degree to which pruning and myelination have occurred thus far in development. Therefore, the CT by wave interaction can be interpreted as children with thinner cortices at ages 9–10 (putatively reflecting greater pruning and myelination) showed the steepest decrease in INT symptoms during preadolescence. This interaction was driven by CT within visual, somatomotor, and temporal regions involved in sensation-, perception-, and action-related processes. Why might this interaction be driven by these specific regions? Developmental neuroimaging studies have shown that cortical maturation occurs on a gradient from lower-order, unimodal sensorimotor cortices to higher-order, heteromodal association cortices (i.e., prefrontal and parietal regions)16. Sensorimotor brain regions show peak volume and thickness first, and thin more rapidly at an earlier age in childhood than heteromodal association cortices16,35,37. As a result, these sensorimotor and temporal regions should have undergone extensive thinning by ages 9–10. Thus, our findings suggest that children who have undergone normative age-related thinning in sensorimotor and temporal brain regions at ages 9–10 may be most protected from developing INT symptoms, as compared to children with thicker sensorimotor and temporal regions.
Variability in cortical maturation may be due to some combination of genetic, molecular, and environmental processes. Although speculative, our results suggest that childhood environments optimized for sensorimotor and temporal cortical maturation may protect children from developing INT symptoms. For example, Rosen et al. (2019)38 posit that early cognitive stimulation from caregivers supporting maturation of visual sensory cortices may scaffold healthy prefrontal cortex and executive function skills development, which is dysfunctional in most mental disorders, and has been shown to prospectively predict later psychopathology in the ABCD sample23. Results from cross-sectional adult studies have shown that smaller visual association cortex volume (specifically within lingual gyrus), which supports executive functioning through its prefrontal cortex connections, is associated with higher levels of p and specific factors, including INT39–41. We are reminded that although often overlooked, it is important to consider the role of sensorimotor cortices in psychopathology risk in addition to typically studied heteromodal association cortices.
Why having thinner sensorimotor and temporal cortices might protect against development of INT, but not general psychopathology is less clear. Although speculative, given that lower global CT is associated with higher levels of p in adulthood12, one hypothesis is that alterations in CT may first be associated with change in specific INT psychopathology in preadolescence before generalizing to all forms of disorder (i.e., p) over the course of development. This interpretation would be consistent with a dynamic mutualism theory, which proposes that symptom comorbidity and severity, as captured by p, may increase over time42. In other words, childhood alterations in cortical thinning could initially relate to the trajectory of INT psychopathology, but as symptom comorbidity and severity increases throughout youth development, CT may predict the trajectory of p. As this finding was not hypothesized in advance, it will be important to determine its replicability in other studies. Nevertheless, the current findings suggest that prospective relations between childhood levels of CT and subsequent within-person change in psychopathology do not generalize across all forms of disorder during preadolescence.
It is also possible that brain structure may not have been associated with within-person trajectories of p because the waves occurred over a short two-year timeframe during preadolescence, prior to the onset of most mental disorders in adolescence22. Consequently, there was not much change or interindividual variability in the slopes observed in the psychopathology dimensions over the waves. Effect sizes of relations between brain structure and psychopathology factors over time were small (but not smaller than is typical43), possibly because the influence of brain structure on future psychopathology may increase with time as psychopathology emerges in later years. A second possibility is that a static brain structure measure at one timepoint may not provide enough information to relate to within-person psychopathology change. One study found that longitudinal relations between brain structure and youth EXT symptoms were driven by change in brain structure rather than structure at one timepoint19. Third, although brain structural abnormalities often are conceptualized as preceding the onset of symptoms, it is possible that they may be a consequence of p rather than a prospective predictor of changes in p. Indeed, a prospective youth longitudinal study found that INT and EXT symptoms were associated with changes in brain structure, but not the other way around21. Fourth, brain connectivity alterations may be more likely to relate to within-person psychopathology trajectories than brain morphology. Cross-sectional ABCD research has shown concurrent relations between general and specific psychopathology factors and alterations in structural44 and functional connectivity45–47. Future ABCD studies should investigate these questions as these children are followed into adolescence.
Interestingly, we found that the trajectories of p, EXT, INT, ND, and SOMAT factor scores decreased, whereas the trajectory of DETACH factor scores increased over the waves. Child psychopathology may truly decrease during this preadolescent period before the rise of many forms of psychopathology in adolescence22. Anxiety and impulse-control disorders typically onset in preadolescence and then begin to decline before the onset of substance use, mood, and thought disorders in adolescence and young adulthood48. It is less clear why DETACH symptoms (i.e., being withdrawn, underactive, low energy) increased over the waves. Wave 3 occurred during the COVID-19 pandemic, so parents may have rated their children as more withdrawn and underactive because children were spending most of their time at home, not in school. However, one might expect the effects of the pandemic to similarly lead to increases in other forms of psychopathology given that recent studies have found that rates of anxiety and depression increased from pre- to during the pandemic49. Regardless, it will be important to continue to examine psychopathology trajectories in later adolescent timepoints.
Our study has the following limitations. First, ~15% of the included sample had missing follow-up data and there were significant differences between those with complete versus missing data. However, sensitivity analyses that removed participants with completely missing follow-up data yielded the same results as the main analyses. Second, there were only three waves of data available at the time of analysis, which limited our analysis approach to tests of linear changes in the transdiagnostic psychopathology dimensions over time. Third, our clinical assessments relied on parent reports of child symptoms, which could be subject to reporting biases. However, maternal psychopathology was not found to bias parent reporting of child symptoms in the ABCD study50.
Despite these limitations, this study is the first to examine prospective relations between brain structure and between-person differences and within-person changes in the p and specific psychopathology factors over time in the large ABCD sample of preadolescents aged 9–10 followed for two years. Our novel findings identified specific brain structure features that might contribute to transdiagnostic psychopathology development in preadolescence. Smaller total brain volume and cortical SA may be risk markers for future persistent preadolescent levels of general psychopathology. Future studies should continue to investigate whether cortical thinning reflective of pruning and myelination in sensorimotor and temporal brain regions may specifically protect against increases in INT, but not general psychopathology during preadolescence.
Supplementary Material
Acknowledgments
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI 10.15154/1524711. DOIs can be found at http://dx.doi.org/10.15154/1524711. Authors received funding support from the National Institutes of Health: Dr. Romer (grant F32 MH124409); Dr. Ren (grants 5R01 AG066670-02, 5R01 MH120400-03, 1R01 AT011002-01A1, 1R01 MH125852, 3R01 HD093680-04S1, 5R33 DA042847-05); and Dr. Pizzagalli (grants R01 MH101521 and R37 MH068376).
Footnotes
Dr. Ren served as the statistical expert for this research.
Disclosure: Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Concert Pharmaceuticals, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka Pharmaceuticals, Sunovion Pharmaceuticals, and Takeda Pharmaceuticals; honoraria from the Psychonomic Society and the American Psychological Association (for editorial work) and Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, Millennium Pharmaceuticals, and Wellcome Leap. He has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), and Neuroscience Software, over the past 3 years. Drs. Romer and Ren have confirmed they have no disclosures to make in association with the work presented in this manuscript, and there are no conflicts of interest with the work conducted in this study. All views expressed are solely those of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adrienne L. Romer, Harvard Medical School, Boston, Massachusetts.; Center for Depression, Anxiety and Stress Research, McLean Hospital, Belmont, Massachusetts.
Boyu Ren, Harvard Medical School, Boston, Massachusetts.; Laboratory for Psychiatric Biostatistics, McLean Hospital, Belmont, Massachusetts.
Diego A. Pizzagalli, Harvard Medical School, Boston, Massachusetts.; Center for Depression, Anxiety and Stress Research, McLean Hospital, Belmont, Massachusetts. McLean Imaging Center, McLean Hospital, Belmont, Massachusetts.
References
- 1.Caspi A, Houts RM, Belsky DW, et al. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2(2):119–137. doi: 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology. 2012;121(4):971–977. doi: 10.1037/a0028355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. AJP. 2018;175(9):831–844. doi: 10.1176/appi.ajp.2018.17121383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychological Bulletin. 2017;143(2):142–186. doi: 10.1037/bul0000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettersson E, Lahey BB, Larsson H, Lichtenstein P. Criterion Validity and Utility of the General Factor of Psychopathology in Childhood: Predictive Associations With Independently Measured Severe Adverse Mental Health Outcomes in Adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2018;57(6):372–383. doi: 10.1016/j.jaac.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 6.Hoertel N, Franco S, Wall MM, et al. Mental disorders and risk of suicide attempt: a national prospective study. Mol Psychiatry. 2015;20(6):718–726. doi: 10.1038/mp.2015.19 [DOI] [PubMed] [Google Scholar]
- 7.Durham EL, Jeong HJ, Moore TM, et al. Association of gray matter volumes with general and specific dimensions of psychopathology in children. Neuropsychopharmacol. 2021;46(7):1333–1339. doi: 10.1038/s41386-020-00952-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mewton L, Lees B, Squeglia LM, et al. The relationship between brain structure and general psychopathology in preadolescents. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaczkurkin AN, Park SS, Sotiras A, et al. Evidence for Dissociable Linkage of Dimensions of Psychopathology to Brain Structure in Youths. AJP. 2019;176(12):1000–1009. doi: 10.1176/appi.ajp.2019.18070835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkes L, Moore TM, Calkins ME, et al. Transdiagnostic dimensions of psychopathology explain individuals’ unique deviations from normative neurodevelopment in brain structure. Translational Psychiatry. 2021;11(1):1–13. doi: 10.1038/s41398-021-01342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blok E, Geenjaar EPT, Geenjaar EAW, Calhoun VD, White T. Neurodevelopmental Trajectories in Children With Internalizing, Externalizing and Emotion Dysregulation Symptoms. Frontiers in Psychiatry. 2022;13. Accessed March 20, 2022. https://www.frontiersin.org/article/10.3389/fpsyt.2022.846201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romer AL, Elliott ML, Knodt AR, et al. Pervasively thinner neocortex as a transdiagnostic feature of general psychopathology. AJP. 2021;178(2):174–182. doi: 10.1176/appi.ajp.2020.19090934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamnes CK, Herting MM, Goddings AL, et al. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J Neurosci. 2017;37(12):3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Mills KL, Siegmund KD, Tamnes CK, et al. Inter-individual variability in structural brain development from late childhood to young adulthood. NeuroImage. 2021;242:118450. doi: 10.1016/j.neuroimage.2021.118450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sydnor VJ, Larsen B, Bassett DS, et al. Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron. 2021;109(18):2820–2846. doi: 10.1016/j.neuron.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whittle S, Vijayakumar N, Simmons JG, Allen NB. Internalizing and Externalizing Symptoms Are Associated With Different Trajectories of Cortical Development During Late Childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2020;59(1):177–185. doi: 10.1016/j.jaac.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 18.Bos MGN, Peters S, van de Kamp FC, Crone EA, Tamnes CK. Emerging depression in adolescence coincides with accelerated frontal cortical thinning. Journal of Child Psychology and Psychiatry. 2018;59(9):994–1002. doi: 10.1111/jcpp.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bos MGN, Wierenga LM, Blankenstein NE, Schreuders E, Tamnes CK, Crone EA. Longitudinal structural brain development and externalizing behavior in adolescence. Journal of Child Psychology and Psychiatry. 2018;59(10):1061–1072. doi: 10.1111/jcpp.12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducharme S, Albaugh MD, Hudziak JJ, et al. Anxious/Depressed Symptoms are Linked to Right Ventromedial Prefrontal Cortical Thickness Maturation in Healthy Children and Young Adults. Cerebral Cortex. 2014;24(11):2941–2950. doi: 10.1093/cercor/bht151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muetzel RL, Blanken LME, van der Ende J, et al. Tracking Brain Development and Dimensional Psychiatric Symptoms in Children: A Longitudinal Population-Based Neuroimaging Study. AJP. 2018;175(1):54–62. doi: 10.1176/appi.ajp.2017.16070813 [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 23.Romer AL, Pizzagalli DA. Is executive dysfunction a risk marker or consequence of psychopathology? A test of executive function as a prospective predictor and outcome of general psychopathology in the adolescent brain cognitive development study®. Developmental Cognitive Neuroscience. 2021;51:100994. doi: 10.1016/j.dcn.2021.100994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achenbach TM. The Achenbach System of Emprically Based Assessment (ASEBA): Development, Findings, Theory and Applications. University of Vermont Research Center for Children, Youth, and Families; 2009. [Google Scholar]
- 26.Casey BJ, Cannonier T, Conley MI, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagler DJ, Hatton Sean N, Cornejo MD, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 29.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Salat DH, Busa E, et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- 31.Michelini G, Barch DM, Tian Y, Watson D, Klein DN, Kotov R. Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Translational Psychiatry. 2019;9(1):1–15. doi: 10.1038/s41398-019-0593-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Soft. 2015;67(1). doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 34.Long JA. Interactions: Comprehensive, user-friendly toolkit for probing interactions. R package version 1.1.0. Retrieved from https://CRAN.R-project.org/packageinteractions [Google Scholar]
- 35.Sowell ER. Longitudinal Mapping of Cortical Thickness and Brain Growth in Normal Children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitaker KJ, Vértes PE, Romero-Garcia R, et al. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proceedings of the National Academy of Sciences. 2016;113(32):9105–9110. doi: 10.1073/pnas.1601745113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental Trajectories of the Human Cerebral Cortex. Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen ML, Amso D, McLaughlin KA. The role of the visual association cortex in scaffolding prefrontal cortex development: A novel mechanism linking socioeconomic status and executive function. Developmental Cognitive Neuroscience. 2019;39:100699. doi: 10.1016/j.dcn.2019.100699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romer, Knodt AR, Houts R, et al. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Molecular Psychiatry. 2018;23(4):1084–1090. doi: 10.1038/mp.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romer AL, Knodt AR, Sison ML, et al. Replicability of structural brain alterations associated with general psychopathology: evidence from a population-representative birth cohort. Mol Psychiatry. 2021;26(8):3839–3846. doi: 10.1038/s41380-019-0621-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romer AL, Pizzagalli DA. Associations between brain structural alterations, executive dysfunction, and general psychopathology in a healthy and cross-diagnostic adult patient sample. Biological Psychiatry Global Open Science. 2022;2(1):17–27. doi: 10.1016/j.bpsgos.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McElroy E, Belsky J, Carragher N, Fearon P, Patalay P. Developmental stability of general and specific factors of psychopathology from early childhood to adolescence: dynamic mutualism or p-differentiation? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2018;59(6):667–675. doi: 10.1111/jcpp.12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardenas-Iniguez C, Moore TM, Kaczkurkin AN, et al. Direct and Indirect Associations of Widespread Individual Differences in Brain White Matter Microstructure with Executive Functioning and General and Specific Dimensions of Psychopathology in Children. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. Published online November 2020:S2451902220303499. doi: 10.1016/j.bpsc.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sripada C, Angstadt M, Taxali A, et al. Widespread attenuating changes in brain connectivity associated with the general factor of psychopathology in 9- and 10-year olds. Transl Psychiatry. 2021;11(1):1–7. doi: 10.1038/s41398-021-01708-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karcher NR, Michelini G, Kotov R, Barch DM. Associations Between Resting-State Functional Connectivity and a Hierarchical Dimensional Structure of Psychopathology in Middle Childhood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2021;6(5):508–517. doi: 10.1016/j.bpsc.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lees B, Squeglia LM, McTeague LM, et al. Altered Neurocognitive Functional Connectivity and Activation Patterns Underlie Psychopathology in Preadolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2021;6(4):387–398. doi: 10.1016/j.bpsc.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th Ed.).; 2013. https://doi-org.ezproxy.frederick.edu/10.1176/appi.books.9780890425596 [Google Scholar]
- 49.Hawes MT, Szenczy AK, Klein DN, Hajcak G, Nelson BD. Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol Med Published online January 13, 2021:1–9. doi: 10.1017/S0033291720005358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olino TM, Michelini G, Mennies RJ, Kotov R, Klein DN. Does maternal psychopathology bias reports of offspring symptoms? A study using moderated non-linear factor analysis. Journal of Child Psychology and Psychiatry. 2021;62(10):1195–1201. doi: 10.1111/jcpp.13394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.