Abstract
OBJECTIVES
We performed a mixed-methods study to explore the motivations associated with blood donation by donors with known, but undisclosed HIV-positive status and ARV use (HIV+/ARV+), seeking potential strategies to reduce such donations and mitigate risk for blood recipients. Here we report predominantly the qualitative component.
BACKGROUND
A safe and sustainable blood supply is dependent in part, on effective pre-donation donor assessment. We previously described failure by HIV+/ARV+ blood donors to disclose their status. Such donations may lead to transfusion-transmitted HIV.
METHODS
The Social Ecological Model provided the conceptual framework for this study. Previously identified HIV+/ARV+ donors were invited to complete a survey (including a validated stigma scale) and qualitative interview, which underwent inductive and deductive thematic analysis.
RESULTS
We uncovered two primary motivational paths to HIV+/ARV+ blood donations: privacy and altruism. The latter included a motivation not previously reported in the literature: donating specifically for other people living with HIV (PLWH). The other primary factor was a lack of privacy. These accounts often included donors encountering donation opportunities when accompanied by people to whom they had not and did not plan to disclose their HIV status. Most were highly confident their donations would be identified as HIV-positive and discarded.
CONCLUSION
We demonstrated a complex interaction between individual, social, cultural, and structural/policy factors in blood donations by PLWH who take ARV. Recommendations to limit HIV+ARV+ donations include: 1) Targeted communication strategies to increase knowledge among PLWH of their deferral from blood donation—without increasing stigma, and 2) development of procedures to assist those who feel unable to opt-out of donation due to privacy concerns.
Keywords: Blood donation, motivations, HIV, anti-retroviral agents, health status disclosure
INTRODUCTION
The World Health Organization recently reaffirmed the global need for a safe, sustainable blood supply to support the effective delivery of health services and programs.[1] A key safety component is the reduction of the risk of transfusion-transmitted infections (TTI) such as HIV, Hepatitis B and C.[2] To achieve this, blood transfusion services employ a multi-pronged approach. While laboratory screening is conducted on all donated blood, donors must pass a combination of pre-donation education, donor assessment via a Donor History Questionnaire (DHQ) and potential deferral. Deferral is disqualification from donation, whether indefinitely (e.g. people living with certain infections, including HIV) or for a specified period (e.g. iron deficiency).[3] These efforts have been remarkably successful, decreasing the number of transfusion-related HIV transmissions in the USA from thousands in the early 1980s[4] to an estimated risk of <1 in 1.6 million donations in 2021.[5] Even in South Africa, with its generalized and growing HIV epidemic of more than 8 million people[6] of whom an estimated 70% are on treatment[7], improved screening strategies reduced the risk of HIV transmission from an estimated 22 per million transfusions in 1994[8] to less than 13 per million transfusions in 2015.[9]
The effectiveness of pre-donation donor assessment and deferral, as a blood safety strategy, depends both on asking the right questions (those that address behaviors and health conditions that truly pose risk) and the willingness of would-be donors to disclose such personal information.[10–12] This can pose challenges. Studies in Europe[10, 13], North America[14], Australia[15] and India[16] confirmed higher rates of nondisclosure of risk factors for HIV TTI among donors who tested positive for these than among donors who tested negative. Most studies investigating non-disclosure among blood donors focus on risk behaviors for HIV and other TTI. Non-disclosure of known HIV status or antiretroviral drug (ARV) use among blood donors has been less frequently explored.
Donations from donors with undisclosed, but known HIV-positive status and/or ARV use (HIV+/ARV+), even with undetectable viral loads, may pose a risk to the safety of the blood supply, especially in high HIV prevalence and ARV uptake settings such as South Africa. This is because when people living with HIV (PLWH) are not deferred prior to donation, detection of virus in their blood is dependent on serologic and molecular HIV assays, the efficacy of which may be compromised in persons with early initiation of ARV or those with pre-exposure prophylaxes breakthrough infections.[17–19] It should be noted that while an undetectable viral load is largely protective for sexual transmission of HIV, this is not necessarily true for transfusion associated transmission, as demonstrated by modelling.[20] South Africa implemented a universal “test and treat” strategy in September 2016,[21] so early ARV initiation should now be the norm. With a continued HIV incidence rate greater than 1%, the number of people treated (early) for HIV in the country grows annually[6], and the risk of non-compliant blood donation resulting in HIV transmission to a blood recipient grows along with it.
Failure to disclose HIV+/ARV+ has recently been quantitatively described in both South Africa[22, 23] and the USA.[24] Our group at the South African National Blood Service (SANBS) became aware of anecdotal reports of undisclosed HIV+/ARV+ among South African blood donors. Subsequent investigation revealed detectable ARV in two-thirds of donors who tested HIV antibody positive but negative by individual donation nucleic acid amplification testing, a result that suggests viral suppression from antiretroviral therapy in an HIV-positive person.[22] We found that almost 10% of all HIV-positive donors who donated at SANBS had demonstrable levels of ARV.[23] In the USA, Custer et al[24] demonstrated undisclosed ARV use among 15% of HIV-positive blood donors and in 0.6% of all first-time male blood donors. To our knowledge, qualitative studies of this phenomenon are, as yet, non-existent. To further explore the phenomenon of blood donation by HIV+/ARV+ donors, we designed a mixed-methods study to explore the motivations associated with this behavior. Here we report predominantly the findings of the qualitative component.
METHODS
Institutional review board approval was obtained from both SANBS and the University of Cape Town. This mixed-methods study was conducted at SANBS, a blood service that serves 8 of the 9 provinces in South Africa, and collects approximately 900,000 units of blood from ~450 000 donors. From February to April 2019, eligible, consenting HIV+/ARV+ donors were invited to complete a survey, administered through audio computer-assisted structured interview (ACASI) technology, and an individual, in-depth qualitative interview (“interview”). The Social Ecological Model (SEM) provided the conceptual framework for this study. The SEM is frequently used in health research[25–27] and posits a complex interplay between multiple levels of influences, human behaviour and health outcomes. A version of the SEM adapted specifically to deal with HIV risk[28] guided the development of data collection instruments (Fig. 1). Specifically, survey items and interview questions addressed influences at individual, social, cultural, and policy levels, as well as potential donor motivations entertained by the research team during study conception (e.g., desire for incentives offered for donation, CD4 or viral load test seeking, donor belief they had been cured of HIV).
Figure 1:
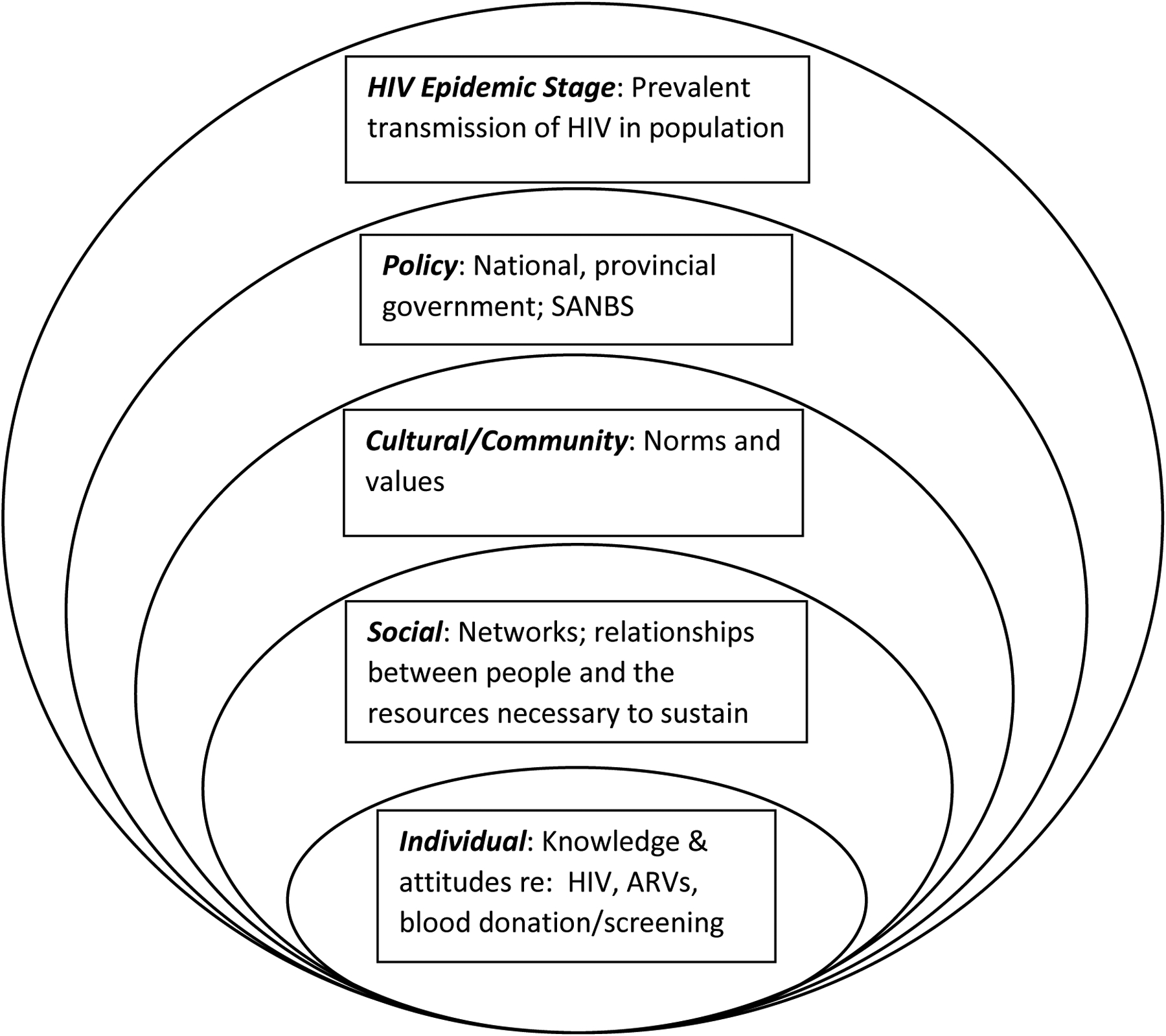
SEM showing levels and potential influences on decision by HIV+/ARV+ individuals to donate blood (adapted from Baral et al. 2013[27]).
Sampling and Recruitment
The 122 HIV-positive donors who tested positive for ARV as part of the HIV+/ARV+ prevalence study[23] were eligible for enrolment. Eligibility criteria included: being aged 18 years or older, conversant in English, residing in the accessible areas of the Gauteng, KwaZulu Natal, Mpumalanga or Eastern Cape Provinces, able to complete data collection procedures and provide consent. Initial outreach followed routine SANBS procedures but was performed by trained research staff with prior experience in HIV counseling and study recruitment. All eligible participants were approached for enrolment and invited to complete the ACASI and interview. Participants had the option to consent to either the ACASI or interview or to both procedures.
Data Collection
The ACASI surveys and interviews were administered in person, at a location mutually acceptable to study participants and staff. The survey instrument included, among other elements, a validated 12-item stigma scale.[29] (Supplementary Table 1) The scale had four domains, each with three items: 1) personalised stigma, 2) disclosure concerns, 3) concerns with public attitudes and 4) negative self-image. All items were answered using a 1–4 point Likert scale, such that higher numbers indicated greater stigma.
The interviews occurred immediately after survey completion, lasted 45–90 minutes, and were audio-recorded with participant consent. These interviews examined the trajectory of HIV testing, diagnosis, and treatment initiation; the impact of HIV; perceptions of HIV transmission risk; perceptions of blood donation and the health care system; timing and context of donation; and motivation to donate. Interview questions were open-ended and designed to produce detailed narratives through a story-telling approach, with interviewers probing as needed for full understanding.
Data Analysis
Survey data were extracted to Excel. Responses from stigma subscales two to four were summed to produce an overall score (subscale 1, which focuses on personalised stigma was of questionable utility within this sample. As noted by the scale’s developers, high levels of secrecy surrounding serostatus, like those present in our sample, may negatively affect the reliability of questions on personalized stigma [30]). Using sub-scales two to four, the lowest possible score was 12 and the highest 36. Scores were averaged (19.1), and then dichotomized into high (above sample mean) or low (below sample mean) stigma categories. These categories were used to segment qualitative data and search for any patterns in motivation related to stigma.
De-identified verbatim transcripts from all interviews were uploaded to Dedoose (a cross-platform application for analysing qualitative and mixed methods data, including text and spreadsheet data)[31] and subjected to thematic analysis.[32] This included inductive, line-by-line analysis to identify themes that emerge from close reading of the text, as well as deductive analysis, which focused on pre-identified themes drawn from the interview guide and relevant academic literature. [33, 34]. A codebook was created following accepted procedures.[35] Data were compared across participant gender and stigma score.[34] In addition, we applied narrative analysis to interviewees’ accounts of their study qualifying donations (SQDs) to better grasp their experience as a whole.[36]
All study procedures were approved by the SANBS and University of Cape Town institutional review boards.
RESULTS
Contact details were available for 120 of the 122 potential participants, 62 (52%) of whom were unreachable (either due to incorrect details or not responding to calls); 12 (10%) were ineligible (11 were not conversant in English); 20 (17%) directly or indirectly refused participation; 1 participant enrolled only for the ACASI and was excluded from this manuscript. Hence, a survey and interview were collected from the 25 (21%) consenting participants. (Table I) Participants were predominantly Black African, female, in their 30s, residents of Gauteng province, and had donated at a mobile site. Eight donors (32%) had donated at least one previous donation. Only a third of participants had ever disclosed their HIV status to more than three people. Eleven were classified as perceiving high stigma. Two interviewees (1101M and 2202M) insisted they had not knowingly donated while HIV+/ARV+.
Table I.
Participant demographics, disclosure practices and stigma scores
| N/Median | %/(IQR)1 | |
|---|---|---|
| Total | 25 | 100 |
| Ethnicity | ||
| Black African | 23 | 92 |
| Coloured | 2 | 8 |
| Gender | ||
| Female | 18 | 72 |
| Male | 7 | 28 |
| Donor Type | ||
| First Time | 17 | 68 |
| Lapsed | 5 | 20 |
| Repeat | 3 | 12 |
| Age | ||
| Median | 32 | (24–39) |
| Province | ||
| Eastern Cape | 2 | 8 |
| Free State | 4 | 16 |
| Gauteng | 10 | 40 |
| KwaZulu Natal | 5 | 20 |
| Mpumalanga | 4 | 16 |
| Clinic Type | ||
| Mobile | 22 | 88 |
| Fixed Site | 3 | 12 |
| Disclosure Practice 2 | ||
| Extremely restricted | 3 | 12 |
| Very Highly restricted | 5 | 20 |
| Highly restricted | 8 | 32 |
| Moderately restricted | 8 | 32 |
| Least restricted | 1 | 4 |
| Stigma Scores | ||
| Overall | 22 | (16–29) |
| Disclosure Concerns | 6 | (4–9) |
| Public Attitude | 7 | (3–10) |
| Self-Image | 4 | (3–6) |
Inter quartile range;
Extremely restricted = Disclosed to no one;
Very Highly Restricted = Disclosed to one person; Highly Restricted = disclosed to 2–3 people; Moderately restricted = Disclosed to >3 people, but not outside family and friends; Least restricted = Disclosed to >3 people, including beyond family and friends.
Reviewing interviewees’ reported motivations for their SQDs, we grouped responses into three themes: 1) altruism, expressed both as a general wish to “save lives”, and the specific intention of donating so that blood could be given to other PLWH; 2) a lack of privacy at the donation location, associated with a fear of status disclosure; and 3) other reasons. The latter category included disparate but largely secondary motivations, such as donation to manage a perceived superabundance of blood, or as a way to confirm HIV status. Here we focus on the first two themes, as they heavily predominated among interviewees’ responses. Notably, very few accounts suggested any kind of test-seeking, only one mentioned incentives, and none provided evidence of interviewees believing they had been cured of HIV.
After stratifying interviewees by stigma scale score, and considering the narratives in their entirety, we did not find clear differences in reported motivations. For example, interviewees in both low and high stigma-perceiving categories mentioned: altruistic motivations, privacy-related motivations (including highly restricted serostatus disclosure practices outside of the donation context), and donating as a blood management practice. However, when segmenting interviewees by motivation, those who reported general altruism or privacy concerns as the predominant factor in their donation were evenly split in their stigma perceptions (2 high vs. 2 low; 5 high vs. 5 low, respectively); while those who reported donating blood for other PLWH were more likely to be classified as perceiving low stigma (6 low vs. 2 high). While the overall average stigma score in this data set was 19.2, the averages by motivation were 20.4 for donors concerned with privacy and 18 for those citing altruism. Given the small sample size, the above results are offered in a purely descriptive vein.
As the different stories told by these participants unfold largely along the lines of reported motivations, the following sections segment interviewees by their concern with altruism or privacy to explore the thematic findings in depth.
Altruism-motivated Donors
Overall, altruism was the most commonly reported motivation, mentioned by nearly half of the interviewees (N=12). Frequently framed as the desire to “save lives,” this was reported by interviewees from Gauteng, Free State and Mpumalanga provinces, men and women (6 and 6, respectively), and from both stigma categories.
We identified two distinct sub-themes within these accounts. The first was a general wish to help others (N = 4). Some interviewees talked about friends or family members having previously needed or received a transfusion; others reported awareness of the general need for blood. For instance, one man explained, “I wanted to donate blood because I knew my blood type was the most wanted one” and that traffic accidents had caused “a need for blood” (2202M). A woman shared her wish to donate “because my mother was sick and they donated blood for her. So, I thought if I could donate maybe I could help someone else just like they helped my mother” (4402F).
The wish to engage in a more specific form of altruism was expressed by eight interviewees. They were motivated to donate blood so that it could be given to a recipient also living with HIV. Three other interviewees (1103, 4402 and 4403F) explicitly raised the possibility that a PLWH might donate for other PLWH, although that was not the primary motivation for their SQD. Talk of donating for other PLWH often drew on notions of “matching,” seeming to equate serostatus matching with the matching of blood types required for transfusion. For instance, a male interviewee discussed donating to help “someone else who also has HIV and our blood codes are the same” (4406M). Some interviewees spoke of themselves as being particularly suitable donors for other PLWH, attributing this to their overall health, serological indicators, and/or medication adherence. One interviewee, on ARVs since 2012, said of her blood, “I think it is better than the people who have just found out that they are HIV positive…. because I am drinking the medications regularly and then my health is fine. It will help the people who are HIV positive, especially the ones with low CD4 count” (3306F).
Interviewees’ certainty about the feasibility of donating for another PLWH varied, though most were remarkably confident. Eleven of these 12 interviewees reported having been unaware of the deferral of PLWH at the time they donated and spoke of feeling confusion or remorse when they learned of their ineligibility. One interviewee had been explicitly told he was not eligible but continued to believe his donations could help other PLWH. He explained his reasoning: “Blood is blood, whether infected or not. I still believe it can help other people in need” (3305M). The idea that PLWH would be ineligible to donate rarely surfaced in interviewees’ accounts of the decision-making that led to their donation, and donating was sometimes framed as a duty: “If someone who is HIV-positive needs blood, … I have to [donate] so that I can help” (3302M).
Privacy-motivated Donors
The other primary factor raised by interviewees as playing a meaningful role in their decision to donate was a lack of privacy. This was described by over a third of interviewees (n =10), across all provinces, and both stigma categories. Nine were female, one male. There was a mix of eligibility beliefs: some were aware of the deferral for HIV; others were uncertain or seemed not to have considered this possibility. In most cases, interviewees reported that when they encountered the opportunity to donate blood, frequently at school or workplace blood drives, they were with other people (co-workers, classmates, friends, romantic partners) to whom they had not planned to disclose their HIV status. The accounts featured a series of decision points, all experienced as threatening to interviewees’ privacy. The first involved presenting for donation. Interviewees generally felt unable to opt-out of attempting to donate without prompting questions and raising suspicion. The second revolved around discussing HIV status or donation eligibility with SANBS staff. Most interviewees reported there was no private place to have such a conversation (though some had gone to the donation site with precisely this intent). The third decision point involved answering the DHQ. Interviewees felt unable to disclose their HIV status on the DHQ, either due to the proximity of co-workers and friends, or because they believed the confidentiality of their answers might be compromised. Overall, in comparison to narratives shared by altruism-motivated donors, these stories were more rooted in the donation context itself. They framed donation as the only safe way out and knowledge of donation eligibility was, in a practical sense, immaterial.
This is well-illustrated by 1106F’s account. She explained that SANBS ran a blood drive at her workplace and “everyone, most of the people in the office, they were going to donate…and yah, so I didn’t have much of an excuse as to why I shouldn’t go” (presenting for donation). She thought that “when I get there, I will [be] able to speak, maybe it will be in private …but it was in the boardroom and you know they had the beds and stuff so we all just filled in the forms in one table” (discussing eligibility). Regarding the screening questions, she “didn’t answer them truthfully” because her co-workers were close at hand (answering the DHQ). In addition, she observed that being deferred from donation attracted undue attention to her as the donor, which was exactly what she was trying to avoid.: “Everyone was looking at each other … it was like a joke because even those who had iron problems and [were] turned away… people were, like, talking like, ‘Oh why have [they] been turned away?’“ Similar stories were shared by interviewees who had donated with classmates at school, a female donor who had donated with a boyfriend, and a domestic worker taken to donate by her employer.
Though a more private environment might have allowed some of these donors to reveal their HIV status to SANBS staff, for others, additional perceived risks would likely still have precluded disclosure. 1107M noted that even having a question about HIV status on the DHQ, “is like, a violation of my privacy.” He elaborated, “If [the form] fall on the wrong hands….my name is there, my ID is there, confidentiality is not there.” Thus, in these narratives, fear of inadvertent HIV status disclosure surfaced in multiple ways, and no interviewee reported disclosing their status on the DHQ. Most were untroubled by having withheld this information because they generally reported confidence that testing done by SANBS would identify their donation as being HIV-positive. One female repeat donor explained, “I know how it works…the blood they are taking, it is going to go through these tests… You know hundred percent sure that this blood that I am giving .…is not going to go anywhere, it is not going to be given to anyone” (1105F).
DISCUSSION
Qualitative research with South African HIV+/ARV+ blood donors revealed that their primary reported motivations were altruism and privacy concerns, with no discernable difference by stigma scores. Among those motivated by altruism, we identified both a general wish to “save lives” and a motivation not previously reported in the literature: the specific desire to donate for other PLWH. Donors motivated by privacy concerns shared quite different accounts, highlighting various elements in the donation process they felt threatened the confidentiality of their HIV status. Far from offering multiple opportunities to exit the process, the experience was framed as a series of decision points in which, in each instance, donation was the only safe option. In this Discussion we slot our research findings into an adapted version of the SEM that grounded our study, discuss the utility of the model, and offer recommendations for reducing the likelihood of future HIV+/ARV+ donation.
Despite the similar way altruism and privacy were described by interviewees (i.e., as “the reason” for their donation), they are different. Altruistic motivations led interviewees to feel drawn to donate, whereas privacy concerns led interviewees to feel pushed to donate. This difference led us to locate these factors in different levels of the SEM.
We categorized altruistic motivations as an individual-level influence because they were always constructed as an expression of personal morality. We recognize, however, that all levels of the model are interconnected (hence the dotted lines around them in Fig. 2). For example, individual altruistic impulses arise within, but are shaped by a cultural context that frames altruism as morally good. Similarly, individual, altruistic motivations may be influenced by blood service policies. For example, blood services implement a policy of minimizing the likelihood of TTIs through multiple strategies. One strategy employed by SANBS is educating potential donors that the blood service should not be used as an HIV testing center, yet to date, however, SANBS has not explicitly disseminated messaging that PLWH are permanently deferred from donation. Considering the U=U (undetectable = untransmissible) campaigns[37] in the media, and successful kidney donations from HIV-positive persons with undetectable HIV viral loads to recipients living with HIV,[38] it should not be surprising that PLWH might consider the same to be possible for blood donation.
Figure. 2:
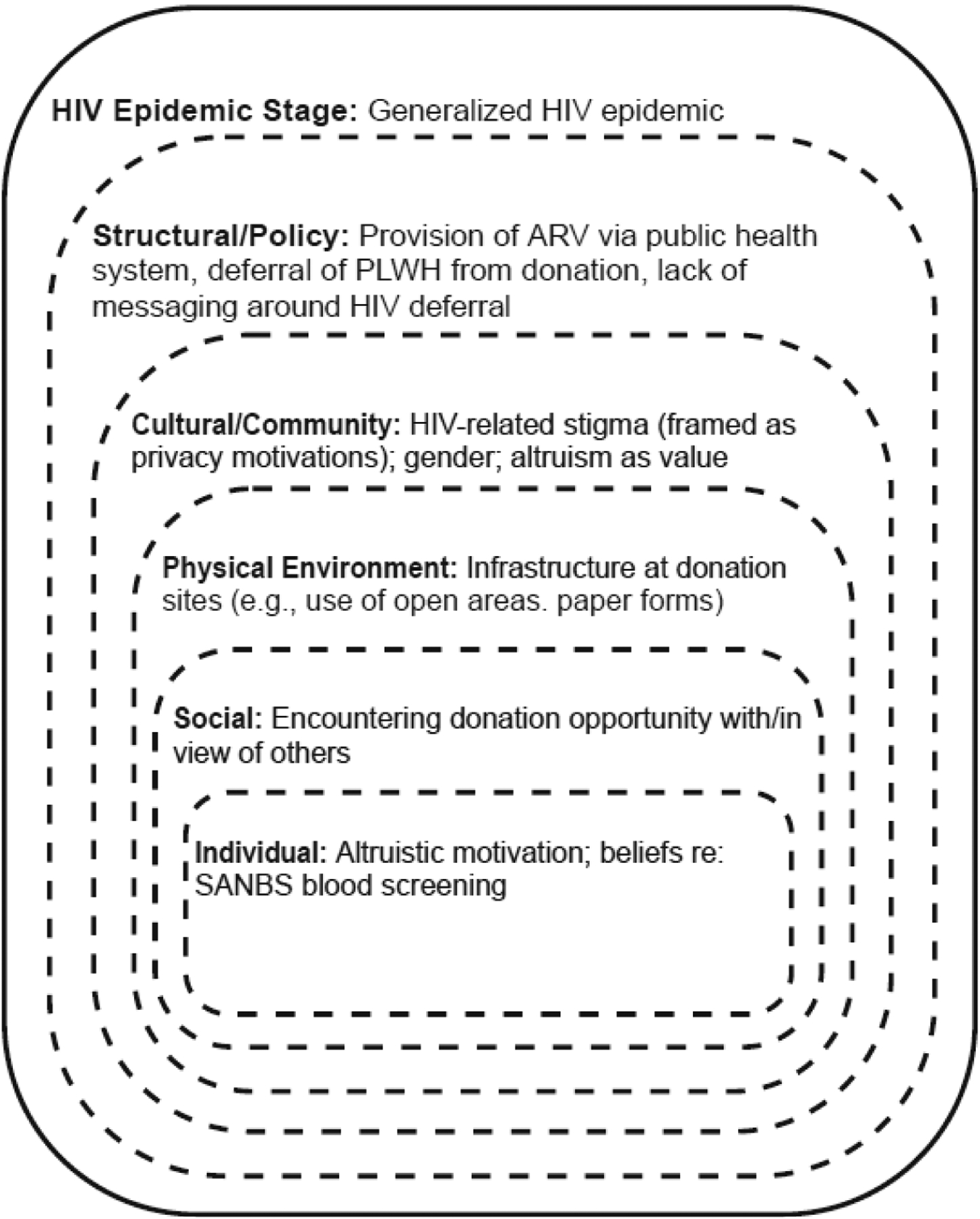
SEM showing levels and influences on decision by HIV+/ARV+ individuals to donate blood (Adapted from Baral et al. 2013[27])
In contrast to individual-level altruism, we classified privacy motivations as a cultural factor. This was because interviewees only raised privacy concerns about their HIV status (vs. other personal information), and linked them to fear of unwanted disclosure given the reported lack of privacy and confidentiality encountered while donating. Privacy concerns are therefore best understood as indexing something beyond individually-varying comfort levels: they are reactions to still-pervasive HIV-related stigma in donor communities. Supporting this, the privacy-motivated group had higher average stigma scores than those donating for altruistic reasons (20.4 vs 18.0), though high and low scores were present in both groups. Furthermore, the overwhelming majority of donors with privacy concerns were female. This is of particular importance in a strongly patriarchal South Africa with its predominantly heterosexual epidemic, which disproportionally affects younger, economically vulnerable, females.[39, 40]
The cultural factor of stigma (expressed as privacy concerns) intersected with a social-level influence on interviewees’ decision-making: HIV+/ARV+ donors often encountered donation opportunities in the presence of other people to whom they had not disclosed and did not wish to disclose, their status. These companions exerted pressure on interviewees’ decision-making beyond the cultural influence of stigma because the imagined potential losses that status disclosure might cause were more concrete and had their own social implications (e.g., loss of a romantic partner, colleagues’ esteem).
A further factor added to this complexity: the physical environment and infrastructure at donation locations, especially mobile blood drives. Many interviewees noted that elements such as the use of paper forms and open spaces lacking privacy constituted an obstacle to disclosure. We created a separate level in the model for such considerations (as done in other ecological work in which the physical environment played an important role[41, 42]). Since interviewees reported experiencing them as highly proximate, but more fixed than social interactions, we placed this level between social and cultural levels. It is worth noting that SANBS policy actually stipulates that screening, even at mobile drives, be conducted in a private environment. Thus, the lack of privacy, in this case, is a question of implementation rather than policy per se.
Policy does influence HIV+/ARV+ blood donation, even if not explicit in the interview data. As mentioned, SANBS’s education efforts, aimed at reducing TTI risk, may influence eligibility perceptions and motivations among donors. At a national level, health policies are catalysts for both the deferral of PLWH from donation and the wide availability of ARVs in South Africa. All of the factors discussed heretofore operate within policy structures that grapple with the generalized South African HIV epidemic, which leaves significant proportions of the potential donor pool affected by HIV.[40] The need to balance supply and safety means deferrals of sub-populations perceived to be at-risk for TTI (e.g., men who have sex with men, as had been done elsewhere), is simply not possible in the South African context.
We accommodated factors this study found relevant for understanding HIV+/ARV+ blood donation in South Africa within the Social Ecological Model, adapting as necessary (Fig. 2). We offer this as a heuristic upon which we will build (e.g., by incorporating findings related to eligibility beliefs and screening experiences), and that others may find useful for considering HIV+/ARV+ donation in other contexts.
This version of the SEM reveals a complex interaction between individual, social, cultural, and structural/policy factors and multiple pathways to donations by PLWH who take ARVs. Indeed, the nuances of the decision-making in interviewees’ narratives cannot be adequately grasped, or responded to, without a multi-level model. In particular, the SEM is helpful in understanding that addressing factors related to HIV+/ARV+ donation at one level, such as individual motivation to help others, may not eliminate the behavior, as influences at other levels (HIV-related stigma and privacy concerns) will still be operant if measures are not taken to mitigate them. For example, someone who was initially motivated to donate for other PLWH might learn this is not possible and no longer wish to donate, but still feel compelled to if encountering a blood drive at their workplace. Thus, an “altruism-motivated” donor could “transform” into a “privacy-motivated” donor if blood collection infrastructure/procedures and HIV-related stigma have not changed. Furthermore, a more private environment and screening experience might have made a meaningful difference in the comfort with status disclosure for some HIV+/ARV+ donors, but others clearly stated little could be done to mitigate the perceived threat posed by potential status revelation in a broader context of HIV-related stigma
The foregoing notwithstanding, we must mention a major conclusion of our analysis and note that reaching it required us to think beyond the adapted SEM that grounded this study. Adapting a model is typically seen as an appropriate way to attend to research context.[43, 44] In this research, adapting the SEM allowed us to focus on HIV risk, which was both helpful and somewhat obfuscatory. This tailoring allowed us, for example, to consider the nature of South Africa’s HIV epidemic, but it also led us to implicitly conceptualize “HIV+/ARV+ donors” as unique, rather than prompting us to ask what they might share with other donors, or how their behavior might be similar to that exhibited in other contexts. Despite this, commonalities emerged. For example, a large group of our interviewees reported altruistic motivations for donation. Activators for altruism were varied and included donating because of loved ones, knowledge of blood shortages, and a moral duty to donate, including specifically for other PLWH. Though donating for other PLWH is, as far as we know, a novel finding, the other reasons are indistinguishable from those offered by many donors not living with HIV, both in South Africa[45, 46], and elsewhere[47]. We came to realize that the expectation that HIV+/ARV+ donors’ motivations would be different derived from assumptions that, in some cases, were not supported by data (e.g., HIV+/ARV+ donors know they are ineligible to donate; such donors would not consider their blood helpful to others). For those donors who donated prior to HIV acquisition, it makes little sense to expect their donation motivations, post-HIV diagnosis and treatment initiation, to be different than they had been historically, especially given the messaging around “U=U” and HIV being just “another chronic disease”.[48, 37]
In addition, though reports of HIV+/ARV+ blood donations were initially surprising, looking beyond the specific context of blood donation suggests perhaps they should not have been. Non-disclosure of health information, including HIV status and ARV use, in other medical settings, is well described. Failure to disclose general medication use, even upon direct questioning by their clinicians, was reported in up to 15% of patients in the USA.[49] Furthermore, non-disclosure of known HIV status and ARV use have been confirmed in several African household surveys[50, 51] and in HIV and ARV research programs.[52–54] ARV denial was reported in as many as one in three participants in a study validating self-reported ARV use in rural South Africa.[55] These trends should have led us to expect status disclosure in a semi-public setting to be problematic, even though other HIV+/ARV+ donors have disclosed their status.[56, 57] What may warrant more investigation is the conditions under which some would-be donors living with HIV do disclose their status.
From the discussions with the participants and the main themes identified in this study, certain recommendations to limit HIV+ARV+ donations emerged. Specifically, these include: 1) Improve the likelihood that PLWH are aware of their permanent deferral from blood donation, as a way to reduce the potency of altruism as a motivator. Historical blood donor messaging relating to HIV centered on the risk of donation during the HIV “window period” and donation sites not being used as HIV-testing sites. This should be augmented with clear communication on the ineligibility of PLWH as blood donors. It is crucial that this be conveyed in a manner that will not further stigmatize those living with the virus. Crafting effective messaging and identifying appropriate channels for dissemination should be done in collaboration with PLWH. 2) Develop procedures to assist those who feel unable to opt-out of donation due to peer pressure and privacy concerns. These could include systems for donors to confidentially withdraw their donations directly after donation or providing donors with a “palatable” option to explain their potential deferral to those observing their donation process. The latter would still require improved privacy infrastructure conducive to confidential discussion at donation sites. Enforcement and compliance monitoring of existing privacy policies, especially at often-used facilities need to be further strengthened.
Our study had several limitations, including the potential for selection bias. We recruited participants from a relatively small pool of HIV+/ARV+, English conversant, South African blood donors. Those who were not interviewed may have had meaningfully different experiences that are not represented here. Furthermore, we tried to reduce potential social desirability bias through assurance of anonymity, personal safety and the use of open-ended questions. While these measures might not have been entirely successful (two interviewees refused to acknowledge awareness of HIV+/ARV+ status at the time of donation), interviewees did recount behavior often considered socially undesirable, suggesting they felt sufficiently comfortable at some level to share such responses.
To our knowledge, this is the first attempt at investigating the motivations driving donations by HIV+/ARV+ donors. As appropriate for highly exploratory, qualitative research, we make no claims of exhaustiveness and instead offer the significant convergence of themes we found around altruism and privacy as a starting point on which to build. Researchers and professionals should critically consider how these findings may apply in different contexts (national, cultural, and types of epidemics). We believe our findings may well have utility in other settings, as HIV is a relatively stigmatized infection globally, blood donation requires fundamentally similar processes regardless of national context, and our findings dovetail with those from other research on disclosure of healthcare information in general.[52, 50, 49, 53, 51]
CONCLUSION
The phenomenon of blood donation by HIV+/ARV+ has been documented in two contexts.[22, 24] Though its global prevalence is unknown, there is little reason to assume that it is not occurring more widely. Our research uncovered complex, diverse motivations related to privacy and altruism leading to HIV+/ARV+ blood donations. The growing HIV+/ARV+ populations both in South Africa and elsewhere and the increasing uptake of pre-exposure HIV prophylaxis may well result in increasing numbers of such donations unless actively managed. To reduce such donations, we need a better understanding of why they are occurring. Here we have reported only on the main donor motivations associated with these non-compliant donations, which is but a small component of this complex phenomenon. We urge other scholars to assess the occurrence of this phenomenon in other settings and further elucidate the motivations and contexts leading or contributing thereto.
Supplementary Material
Acknowledgments:
We gratefully acknowledge the willingness of our study participants to share their stories with us, the SANBS field staff: Cynthia Nyoni, Debbie Strydom; Wendy Ntaka and Cecilia Nomsobo for their commitment to the execution of this study and the SANBS data analytics staff: Ronel Swanevelder and Tinus Brits without whom none of this work would be possible.
This work was supported by research contracts from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) for the Recipient Epidemiology and Donor Evaluation Study-III International program: HHSN268201100009I (to UCSF and the South African National Blood Service), HHSN268201100002I (to RTI International) and HHSN2682011-00001I (to Vitalant Research Institute); and NIH Fogarty International Center grant D43-TW010345 (to ELM).
References:
- 1.World Health Organisation. Action framework to advance universal access to safe, effective and quality-assured blood products 2020–2023. Geneva: World Health Organisation; 2020. [Google Scholar]
- 2.World Health Organisation. Availability, safety and quality of blood products In: Sixty-Third World Health Assembly, editor. Resolution WHA6312. Geneva: World Health Organisation; 2010. [Google Scholar]
- 3.World Health Organisation ROftEM. Strategic framework for blood safety and availability 2016–2025. Geneva: World Health Organisation; 2017. [Google Scholar]
- 4.Ward JW. Transfusion-associated (T-A)-AIDS in the United States. Developments in biological standardization. 1993;81:41–3. [PubMed] [Google Scholar]
- 5.Steele WR, Dodd RY, Notari EP, Haynes J, Anderson SA, Williams AE, et al. HIV, HCV, and HBV incidence and residual risk in US blood donors before and after implementation of the 12-month deferral policy for men who have sex with men. Transfusion. 2021. Mar;61(3):839–50. [DOI] [PubMed] [Google Scholar]
- 6.Statistics South Africa. Mid-year population estimates, 2021. Pretoria: Statistics South Africa; 2021. [Google Scholar]
- 7.Marinda E, Simbayi L, Zuma K, Zungu N, Moyo S, Kondlo L, et al. Towards achieving the 90-90-90 HIV targets: results from the south African 2017 national HIV survey. BMC Public Health. 2020. Sep 9;20(1):1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitas F, Fleming AF, Morris J. Residual risk of transmission of HIV through blood transfusion in South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1994. Mar;84(3):142–4. [PubMed] [Google Scholar]
- 9.Vermeulen M, Lelie N, Coleman C, Sykes W, Jacobs G, Swanevelder R, et al. Assessment of HIV transfusion transmission risk in South Africa: a 10-year analysis following implementation of individual donation nucleic acid amplification technology testing and donor demographics eligibility changes. Transfusion. 2019;59(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slot E, Janssen MP, Marijt-van der Kreek T, Zaaijer HL, van de Laar TJ. Two decades of risk factors and transfusion-transmissible infections in Dutch blood donors. Transfusion (Philadelphia, Pa). 2016;56(1):203–14. [DOI] [PubMed] [Google Scholar]
- 11.Willson S, Miller K, Seem D, Kuehnert MJ. Cognitive evaluation of the AABB Uniform Donor History Questionnaire. Transfusion. 2016;56(6pt2):1662–67. [DOI] [PubMed] [Google Scholar]
- 12.Preußel K, Offergeld R. Which Infectious Blood Donors Could Be Identified by the Donor History Questionnaire? - Comparison of Blood Donors Infected with HIV or HCV with Notified Cases from General Population in Germany. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2018. Apr;45(2):108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duquesnoy A, Danic B, Santos A, Martinaud C, Woimant G, Laperche S, et al. Context and social perceptions of blood donation in donors found positive for human immunodeficiency virus in France. Transfusion (Philadelphia, Pa). 2017;57(9):2240–47. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien SF, Xi G, Yi Q-L, Goldman M. Understanding non-disclosure of deferrable risk: a study of blood donors with a history of intravenous drug use. Transfusion Medicine. 2010;20(1):15–21. [DOI] [PubMed] [Google Scholar]
- 15.Lucky TTA, Seed CR, Waller D, Lee JF, McDonald A, Wand H, et al. Understanding noncompliance with selective donor deferral criteria for high-risk behaviors in Australian blood donors. Transfusion (Philadelphia, Pa). 2014;54(7):1739–49. [DOI] [PubMed] [Google Scholar]
- 16.Sachdev S, Mittal K, Patidar G, Marwaha N, Sharma RR, Duseja AK, et al. Risk Factors for Transfusion Transmissible Infections Elicited on Post Donation Counselling in Blood Donors: Need to Strengthen Pre-donation Counselling. Indian J Hematol Blood Transfus. 2015;31(3):378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hare CB, Pappalardo BL, Busch MP, Karlsson AC, Phelps BH, Alexander SS, et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis. 2006;42(5):700–8. Epub 2006 Jan 23. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. New England Journal of Medicine. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Souza MS, Pinyakorn S, Akapirat S, Pattanachaiwit S, Fletcher JL, Chomchey N, et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin Infect Dis. 2016;63(4):555–61. doi: 10.1093/cid/ciw365. Epub 2016 Jun 17. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen M, Coleman C, Mitchel J, Reddy R, Drimmelen H, Fickett T, et al. Comparison of human immunodeficiency virus assays in window phase and elite controller samples: viral load distribution and implications for transmission risk. Transfusion. 2013;53(10pt2):2384–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pillay Y, Pillay A. IMPLEMENTATION OF THE UNIVERSAL TEST AND TREAT STRATEGY FOR HIV POSITIVE PATIENTS AND DIFFERENTIATED CARE FOR STABLE PATIENTS. In: National Department of Health SA, editor.: Pretoria, South Africa; 2016. [Google Scholar]
- 22.Sykes W, van den Berg K, Jacobs G, Jauregui A, Roubinian N, Wiesner L, et al. Discovery of “false Elite Controllers”: HIV antibody-positive RNA-negative blood donors found to be on antiretroviral treatment. J Infect Dis. 2019. Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Berg K, Vermeulen M, Louw VJ, Murphy EL, Maartens G. Undisclosed HIV status and antiretroviral therapy use among South African blood donors. Transfusion. 2021. Aug;61(8):2392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Custer B, Quiner C, Haaland R, Martin A, Stone M, Reik R, et al. HIV antiretroviral therapy and prevention use in US blood donors: a new blood safety concern. Blood. 2020;136(11):1351–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gombachika BC, Fjeld H, Chirwa E, Sundby J, Malata A, Maluwa A. A Social Ecological Approach to Exploring Barriers to Accessing Sexual and Reproductive Health Services among Couples Living with HIV in Southern Malawi. ISRN Public Health. 2012;2012:13 pages. [Google Scholar]
- 26.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s Experiences with Oral and Vaginal Pre-Exposure Prophylaxis: The VOICE-C Qualitative Study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLean R Impacting attitudes and values: Reducing stigma and discrimination and improving STBBI prevention. Canadian Public Health Association Sex and Stigma Matters Conference. Winnipeg, Canada: 2015. [Google Scholar]
- 28.Baral S, Logie CH, Grosso A, Wirtz AL, Beyrer C. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health. 2013;13(1):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinius M, Wettergren L, Wiklander M, Svedhem V, Ekström AM, Eriksson LE. Development of a 12-item short version of the HIV stigma scale. Health and quality of life outcomes. 2017;15(1):115–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindberg MH, Wettergren L, Wiklander M, Svedhem-Johansson V, Eriksson LE. Psychometric evaluation of the HIV stigma scale in a Swedish context. PLoS One. 2014;9(12):e114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dedoose. 9.0.17 ed. Los Angeles, CA: SocioCultural Research Consultants, LLC; 2021. p. web application for managing, analyzing, and presenting qualitative and mixed method research data. [Google Scholar]
- 32.Bernard HR. Research Methods in Anthropology: Qualitative and Quantitative Approaches. 4th ed. Oxford: AltaMira Press; 2006. [Google Scholar]
- 33.Ryan GW, Bernard HR. Techniques to Identify Themes. Field Methods. 2003;15:85–109. [Google Scholar]
- 34.Bernard HR, Ryan GW. Analyzing Qualitative Data:Systematic Approaches. Thousand Oaks, CA: SAGE Publications, Inc.; 2010. [Google Scholar]
- 35.MacQueen KM, McLellan E, Kay K, Milstein B. Codebook Development for Team-Based Qualitative Analysis. Cultural Anthropology Methods. 1998;10(2):31–36. [Google Scholar]
- 36.Mattingly C, Garro LC, editors. Narrative and the cultural construction of illness and healing. Berkeley, California: University of California Press; 2001. [Google Scholar]
- 37.Okoli C, Van de Velde N, Richman B, Allan B, Castellanos E, Young B, et al. Undetectable equals untransmittable (U = U): awareness and associations with health outcomes among people living with HIV in 25 countries. Sexually transmitted infections. 2021;97(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werbel WA, Durand CM. Solid Organ Transplantation in HIV-Infected Recipients: History, Progress, and Frontiers. Current HIV/AIDS reports. 2019;16(3):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. The Lancet. 2010;376(9734):41–48. [DOI] [PubMed] [Google Scholar]
- 40.Statistics South Africa. Mid-year population estimates, 2020. Pretoria: Statistics South Africa; 2020. [Google Scholar]
- 41.Mama SK, Diamond PM, McCurdy SA, Evans AE, McNeill LH, Lee RE. Individual, social and environmental correlates of physical activity in overweight and obese African American and Hispanic women: A structural equation model analysis. Preventive Medicine Reports. 2015. 2015/01/01/;2:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essiet IA, Baharom A, Shahar HK, Uzochukwu B. Application of the Socio-Ecological Model to predict physical activity behaviour among Nigerian University students. The Pan African medical journal. 2017;26:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health education quarterly. 1988. Winter;15(4):351–77. [DOI] [PubMed] [Google Scholar]
- 44.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Social science & medicine (1982). 1994. Oct;39(7):887–903. [DOI] [PubMed] [Google Scholar]
- 45.Muthivhi TN, Olmsted MG, Park H, Sha M, Raju V, Mokoena T, et al. Motivators and deterrents to blood donation among Black South Africans: a qualitative analysis of focus group data. Transfusion medicine (Oxford, England). 2015. Aug;25(4):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swanevelder R, Reddy R, Chowdhury D, Olmsted M, Brambilla D, Jentsch U, et al. Using a motivator and deterrent questionnaire to predict actual donation return behavior among first-time African-origin blood donors. Transfusion. 2019;59(9):2885–92. [DOI] [PubMed] [Google Scholar]
- 47.Bednall TC, Bove LL. Donating blood: a meta-analytic review of self-reported motivators and deterrents. Transfusion medicine reviews. 2011. Oct;25(4):317–34. [DOI] [PubMed] [Google Scholar]
- 48.Hughes SD. HIV Serodiscordant Couples and the Discourse of Normality: Reconciling the Biomedical and the Social in Porto Alegre, Brazil. In: Persson A, Hughes SD, editors. Cross-Cultural Perspectives on Couples with Mixed HIV Status: Beyond Positive/Negative. Cham: Springer International Publishing; 2017. p. 55–69. [Google Scholar]
- 49.Levy A, Scherer AM, Zikmund-Fisher BJ, Larkin K, Barnes GD, Fagerlin A. Prevalence of and factors associated with patient nondisclosure of medically relevant information to clinicians. JAMA Network Open. 2018;1(7):e185293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim AA, Mukui I, Young PW, Mirjahangir J, Mwanyumba S, Wamicwe J, et al. Undisclosed HIV infection and antiretroviral therapy use in the Kenya AIDS indicator survey 2012: relevance to national targets for HIV diagnosis and treatment. Aids. 2016;30(17):2685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dietrich C, Moyo S, Briggs-Hagen M, Ochieng A, Porter S, Marinda E, et al. Sensitivity and specificity of self-reported ARV use in a South African national household survey. . AIDS Impact. London, United Kingdom 2019. [Google Scholar]
- 52.Fogel JM, Wang L, Parsons TL, Ou SS, Piwowar-Manning E, Chen Y, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052). The Journal of infectious diseases. 2013. Nov 15;208(10):1624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moyo MS, Gaseitsiwe KS, Powis EK, Pretorius Holme EM, Mohammed ET, Zahralban-Steele EM, et al. Undisclosed antiretroviral drug use in Botswana: implication for national estimates. AIDS. 2018;32(11):1543–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fogel JM, Sandfort T, Zhang Y, Guo X, Clarke W, Breaud A, et al. Accuracy of Self-Reported HIV Status Among African Men and Transgender Women Who Have Sex with Men Who were Screened for Participation in a Research Study: HPTN 075. AIDS and behavior. 2019. Jan;23(1):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manne-Goehler J, Rohr J, Montana L, Siedner M, Harling G, Gomez-Olive FX, et al. ART Denial: Results of a Home-Based Study to Validate Self-reported Antiretroviral Use in Rural South Africa. AIDS and behavior. 2019. Aug;23(8):2072–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glatt TN, Swanevelder R, Prithvi Raj S, Mitchel J, van den Berg K. Donor deferral and return patterns – a South African perspective. ISBT Science Series.n/a(n/a). [Google Scholar]
- 57.Rapodile T, Mitchel J, Swanevelder R, Murphy EL, van den Berg K. Re-engineering the medical assessment of blood donors in South Africa: The balance between supply and safety. Transfusion. 2021. Oct 13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


