Abstract
Elevated child and caregiver psychopathology are observed in families of children with cancer, with a subset developing clinically significant symptoms. This study examines whether caregivers’ resting respiratory sinus arrhythmia (RSA) and observed emotion regulation (ER) are protective against caregiver and child psychopathology during the first year of pediatric cancer treatment.
Primary caregivers of children recently diagnosed with cancer (N=159; child Mage=5.6 years; children 48% male, 52% female) completed twelve monthly questionnaires. At month three, primary caregivers were interviewed about their experiences of emotions, and their resting RSA was measured. Data were analyzed using multi-level models.
Observed ER was associated with lower caregiver anxiety, depression, and post-traumatic stress symptoms (PTSS) one year post-diagnosis but was not associated with children’s symptoms. Resting RSA had a significant positive association with child depression/anxiety at the start of treatment and month twelve child PTSS.
Findings suggest that caregivers would benefit from interventions to manage their negative emotions at the start of cancer treatment. Additionally, caregivers who are more physiologically regulated may be more attuned to their children’s negative emotions. Our findings highlight the importance of taking a multi-method approach to understanding how ER impacts functioning.
Keywords: pediatric cancer, psychopathology, emotion regulation, parent-child relationships, RSA
Caregivers of children with cancer juggle several concerns, including financial issues (Bona et al., 2014), worries about their child’s treatment and survival (McGrath & Phillips, 2008), and the daily stresses of parenting and managing the household. Consequently, caregivers show elevated depression, anxiety, and post-traumatic stress symptoms (PTSS), particularly around the time of diagnosis (Compas et al., 2015). For children with cancer, some studies find elevated internalizing symptoms (Pinquart & Shen, 2011), whereas others find no difference (Howard Sharp et al., 2015). There is wide variability in symptom levels among caregivers and children, with a subset developing clinically relevant symptoms (Katz et al., 2018). It is thus important to identify risk and protective factors for psychopathology in families of children with cancer to determine targets for intervention.
Caregivers’ emotion regulation (ER) skills may be one such protective factor. ER refers to the ability to modulate the intensity, quality, and duration of one’s feeling states in the service of external goals (Gross, 2015) and is a transdiagnostic factor in the development of psychopathology (Aldao et al., 2016). Although limited research has been conducted on ER in the context of pediatric cancer, it has been associated with greater resilience to life stressors (Richardson, 2017). Caregivers with stronger ER skills may be better equipped to manage treatment stress, impacting their own and their children’s adjustment. In this paper, we investigated whether caregiver ER is associated with trajectories of caregiver and child psychopathology after diagnosis.
Much of the current research on ER and stress has been conducted with lab-based studies with lower ecological validity (Rottenberg & Johnson., 2007), in clinical samples with diagnosed psychopathology (e.g., Chalmers et al., 2014), or on managing lower-level daily stressors (e.g., Richardson, 2017). Because ER, by definition, involves the modulation of emotional experiences in response to environmental demands (Gross, 2015), it is important to study how individuals regulate in a variety of contexts. In the case of pediatric cancer, families must handle treatment that involves painful, invasive procedures with risks of complications and death. This paper aims to deepen our understanding of the role of ER for families dealing with an intense stressor associated with elevated psychopathology symptoms (Compas et al., 2015).
Caregiver ER as a Protective Factor for Child Psychopathology
Caregivers’ ER shapes children’s ER and risk for psychopathology through modeling, co-regulation, and socialization (Bariola et al., 2011; Calkins & Hill, 2007), so caregivers’ ER may also be protective for their children. More regulated caregivers have greater resources to be supportive and responsive to their children during treatment. To our knowledge, no studies have examined caregiver ER as a potential protective factor for psychopathology during pediatric cancer treatment. Further investigation could help to determine whether ER skills should be addressed in interventions aimed at improving adjustment in families of children with cancer.
Approaches to Studying ER
ER has multiple components, including subjective experience, behavior, and physiology (Gross, 2015), leading to calls to incorporate a multi-method approach to understanding ER (Cole et al., 2004). Different components of ER may play unique roles in helping caregivers to manage stress during cancer treatment, informing the development of targeted interventions that focus on ER components that are most protective for caregivers. The current study incorporated both resting respiratory sinus arrhythmia (RSA), considered to be a physiological component of ER (Beauchaine, 2015), and coder ratings of caregivers’ descriptions of their subjective and behavioral experience of emotions, an observational measure of ER.
RSA measures fluctuations in heart rate that occur due to the vagus nerve, indicative of the body’s level of parasympathetic activation (Porges, 2007). High resting RSA is thought to allow individuals to modulate emotions more flexibly in response to the environment (Porges, 2007) and has been associated with adaptive ER strategies and resilience (El-Sheikh et al., 2001; McLaughlin et al., 2014). Low resting RSA is thought to index emotion dysregulation and is associated with psychopathology (Beauchaine, 2015) and greater psychological distress to stressors (Diamond et al., 2011). Although RSA has not been extensively investigated in cancer contexts, a study conducted with breast cancer patients found that patients with high RSA decreased in anxiety in the year following diagnosis, whereas patients with low RSA increased in anxiety (Kogan et al., 2012). Consequently, similar to other components of ER, high resting RSA may buffer caregivers from the negative psychological effects of treatment.
However, whereas ER measured through self-report and observation has been consistently linked to lower psychopathology (Aldao et al., 2016), findings on resting RSA have been less consistent. A meta-analysis found that adolescents diagnosed with depression had lower resting RSA, but that in non-clinical samples, RSA was not related to depressive symptoms (Koenig et al., 2016). Resting RSA was also positively associated with sadness and non-recovery for individuals with depression (Rottenberg et al., 2002). In community samples, higher resting RSA was associated with the presence of mild depression and anxiety symptoms (Skoranski & Lunkenheimer, 2020), greater state anxiety in a neutral situation (Jönsson, 2007), and more negative affect when discussing a distressing film clip (Butler et al., 2006).
In response to mixed findings on RSA and psychological adjustment, some researchers have argued that, because resting RSA confers more flexible emotional responses to the environment, higher RSA may make individuals more open to the negative impact of stressors (Skoranski & Lunkenheimer, 2020). Those with higher RSA may show symptoms of psychopathology in stressful contexts due to greater engagement and awareness of negative emotions (Vasilev et al., 2009). In the context of cancer, higher resting RSA could confer a more flexible emotional response to the onset of this new stressor and greater processing of the negative emotions that arise after diagnosis. This may lead caregivers with higher RSA to report elevated symptoms of psychopathology as they face the emotional impact of a prolonged, intense stressor. Given the uncertainty of the relation between RSA and psychopathology, the current study examines how the role of resting RSA compares to observed ER in predicting changes in psychopathology over time.
It is also unclear whether caregivers’ physiological regulation is protective for their children. Like other ER measures, RSA is theorized to support positive caregiver-child interactions (Bariola et al., 2011), although current findings are mixed. Higher resting RSA in caregivers has been associated with adaptive emotion socialization practices and children’s knowledge about emotion (Perlman et al., 2008). However, caregivers’ physiological regulation may play a greater role in children’s abilities to recover from stressors than in their emotion expression during the stressor (Shih et al., 2018). By incorporating both observed ER and resting RSA in our study, we investigated how different components of caregiver ER relate to child psychopathology in the midst of an intense stressor.
The Current Study
The current study examined the role of primary caregivers’ resting RSA and observed ER in predicting the trajectory of primary caregiver and child psychopathology during the first year after pediatric cancer diagnosis, the most stressful portion of treatment for families (Leavitt et al., 1999). Given that a subset of caregivers and children develop clinically relevant psychopathology symptoms during this time, it is critical to understand potential protective factors such as ER.
We propose that caregivers higher in observed ER will have lower depression, anxiety, and PTS symptoms across the twelve months. We also hypothesize that their symptoms will decrease more over time. Secondly, we expect that children of caregivers higher in observed ER will similarly have lower depression/anxiety, externalizing, and PTS symptoms at month twelve and that their symptoms will also decrease more over the twelve months. Thirdly, due to mixed evidence, we are testing competing hypotheses on the relation between RSA and psychopathology. Based on the theoretical understanding of high RSA as a protective factor for psychopathology, resting RSA may be associated with lower psychopathology symptoms in both caregivers and children and with greater decreases in both caregiver and child psychopathology over time. On the other hand, based on past findings suggesting that RSA may confer greater engagement with negative emotions, RSA may be associated with higher caregiver and child psychopathology symptoms and less of a decrease in symptoms over time.
Method
Participants
One hundred and fifty-nine families participated in the study. Children were 2 – 17 years old (M = 6.3 years, SD = 3.5 years, 48% male, 52% female), although 89% were aged 10 or younger. All had recently been diagnosed with cancer. Families identified the child’s primary caregiver as their mother (85.7%), father (11.0%), grandmother (1.9%), stepmother (0.6%), or stepfather (0.6%). The average age of primary caregivers was 36.45 years (SD = 7.98). Most primary caregivers were White/Caucasian (88.7%), followed by Black/African American (4.9%), Asian (2.8%), Native Hawaiian/Pacific Islander (0.7%), and other (1.4%). 9.2% of primary caregivers were Hispanic. Children were identified by their primary caregivers as White/Caucasian (84.8%), Black/African American (4.8%), Asian (1.4%), Native American (0.7%), and other (8.3%). 15.2% of children were Hispanic. Children’s cancer diagnoses were leukemia (36.5%), central nervous system (CNS) tumor (22.0%), lymphoma (10.7%), sarcoma (10.7%), Wilm’s tumor (8.2%), neuroblastoma (3.8%), or another form of cancer (8.2%). Families ranged in income from less than $40,000 (31.9%), between $40,000 and $80,000 (26.6%), and over $80,000 (41.5%).
Procedures
We report how we determined our sample size, all data exclusions (if any), all manipulations, and all measures in the study. Families were recruited as part of a larger study from two children’s hospitals in urban areas of the Northwest and Southeast United States. They were approached within two weeks of their child’s cancer diagnosis to take part in the study. Children with neurofibromatosis Type I, relapsed cancer, or secondary malignancies were excluded, as the focus of the study was on families of children newly diagnosed with cancer. Of 502 eligible families, 309 were approached, 176 enrolled, and 159 completed at least one study component. The most common reason cited for declining to participate was being too busy.
Primary caregivers were given an initial questionnaire packet at the time of consent, which was completed between two weeks and four months post-diagnosis (M = 55.23 days, SD = 26.53). After caregivers sent in their initial packet, questionnaires were mailed to primary caregivers every month for a year after diagnosis, yielding up to twelve time points of measurement. Participants had a two-week window to complete each packet. Packets that were not completed within this window were not included in the final dataset, and caregivers continued to receive their packet at the same time every month. Within three months of diagnosis, caregivers were interviewed over the phone, and a home visit was conducted in which primary caregivers’ physiological data was collected.
On average, primary caregivers completed 6.8 packets (SD = 3.84) of the 12 possible assessments. After the initial packet, the highest proportion of primary caregivers were retained at month 6 (68%) and the lowest at month 2 (5%), followed by month 3 (43%). The number of completed packets was not associated with demographic variables, including caregiver age, child age, child gender, diagnosis, marital status, race, or income. Initial caregiver and child psychopathology symptoms were also not associated with the number of completed packets.
Power calculations were used to determine a goal sample size of at least 155 participants at baseline to detect small regression effects (b = .10 - .15), assuming a 65% participation rate and 10% attrition rate. The total number of families who participated in the study (159) exceeded this goal, and coded interview data were available for 64.7% (n = 103) of families, meeting our expected participation rate. However, given that physiological data collection required an at-home visit early in treatment when families tend to be particularly stressed, RSA data were available for fewer families (38.4%, n = 61).
All study procedures were approved by the University of Washington, Seattle Children’s Hospital, and Vanderbilt University institutional review boards. Materials and analysis code for this study are available by emailing the corresponding author. This study’s design and analyses were not pre-registered.
Measures
Caregiver Resting RSA.
Five electrodes were placed on primary caregivers’ torsos to measure cardiac interbeat interval (IBI) via an electrocardiogram (ECG). Mindware software was used to conduct spectral analysis of IBI data, and RSA was calculated by measuring the time between successive R-waves of the ECG. The amount of variance in the IBI spectrum was examined using spectral time-series analysis. The sum of the power densities in the IBI spectrum within the 0.33- to 0.42-Hz band over the total amount of power across all frequency bands was used as the measure of RSA (Behrman & Kliegman, 2002). This protocol and scoring method are well established and highly correlated with output from Porges’s MXEDIT program (r = .96; Gottman et al., 1997;). Resting RSA was assessed as the average RSA while the primary caregiver listened to a neutral story for two minutes.
Caregiver Observed ER.
Primary caregivers’ observed emotion regulation was measured using the Parent Meta Emotion Interview (PMEI; Katz & Gottman, 1986). Caregivers were asked to describe their attitudes, behaviors, and experiences related to their own and their children’s sadness, anger, and fear. Interviews were conducted over the phone and lasted 45–60 minutes on average. Recordings were coded with the Meta-Emotion Coding System (Katz et al., 1994), which yields subscale scores for Awareness, Expressivity, Acceptance, Remediation, and Regulation of caregiver emotions. Observed caregiver ER was calculated from the average of their Regulation subscale scores for sadness, anger, and fear. Scores on this subscale indicated coder inferences of caregivers’ regulatory capacity based on caregivers’ descriptions of their experiences. Sample items in the regulation subscales include: “There is difficulty regulating the intensity of the emotion”, “Caregiver thinks this emotion can be dangerous,” and “This emotion has been a problem/concern”. Interviews were coded by primary coders whose data was used for analyses. 67% of interviews were coded by a second coder to determine inter-rater reliability, and these scores were not used as data. Using one-way random interclass-correlations, reliability was 0.83 for sadness regulation, 0.67 for anger regulation, and 0.71 for fear regulation.
Caregiver Depression Symptoms.
Caregivers reported on their depression using the shortened form of the Center for Epidemiological Studies Depression Scale (CESD-10; Andresen et al., 1994). The CESD-10 has strong predictive validity compared with the original CES-D (Radloff, 1977). The scale has 10 items that ask caregivers to report the frequency of their depression symptoms in the past month, yielding a total sum score from 0–30. Higher scores represent more frequent symptoms. The scale is not intended to be a diagnostic tool, but a score of 10 or higher indicates risk for clinical depression (Andresen et al., 1994). In our sample, reliability was high, with Cronbach’s α ranging from .83-.91 and averaging .87 across the 12 time points.
Caregiver Anxiety Symptoms.
The anxiety subscale of the Depression, Anxiety, and Stress Scale was used to measure anxiety symptoms in primary caregivers (DASS; Lovibond & Lovibond, 1995). Scores on the 7-item subscale range from 0–21, with higher scores indicating more frequent symptoms. In our sample, reliability was acceptable, with Cronbach’s α ranging from .73–.88 and averaging .84 across the 12 time points.
Caregiver PTSS Symptoms.
Primary caregivers self-reported on their PTSS using the Impact of Events Scale—Revised (IES-R; Weiss & Marmar, 1997). The 22-item scale assesses PTSS in the past month. Overall distress scores range from 0–88 and are categorized as mild (13–35), moderate (36–61), or severe (>61 onwards). Reliability was high in our sample, with Cronbach’s α ranging from .92–.95, averaging .94 across the 12 time points.
Child Depression/Anxiety Symptoms.
Primary caregivers reported on their children’s internalizing symptoms using the Child Behavior Checklist (CBCL; Achenbach, 1991), a well-validated measure of child functioning. Caregivers of children aged 2–5 completed the preschool form, and those of children aged 6–18 completed the school-age form. Caregivers rated the frequency of symptoms in the past month, and T scores normed for child age and gender were used. The Anxious/Depressed factor score was used as an indicator of children’s internalizing symptoms because use of a T score for somatic complaints, which is included in the Internalizing factor score, may be problematic with chronically ill populations (Perrin et al., 1991). This scale has a cut-off score of 65, with respondents scoring 65–69 considered in the borderline clinical range (93rd percentile), and those with scores of 70 and above considered in the clinical range (97th percentile; Achenbach, 1991).
Child Externalizing Symptoms.
Primary caregivers reported on their children’s externalizing symptoms using the Externalizing Symptoms factor scale of the CBCL (Achenbach, 1991). Caregivers rated the frequency of symptoms in the past month, such as rule-breaking and aggressive behavior, and T scores normed for child age and gender were used. This scale has a cut-off score of 60, with respondents scoring 60–63 considered in the borderline clinical range (83rd percentile), and those with a score of 64 or higher in the clinical range (90th percentile; Achenbach, 1991).
Child Post-Traumatic Stress Symptoms.
PTSS were assessed through primary caregiver report on the UCLA Posttraumatic Stress Disorder Reaction Index (UCLA PTSD-RI; Steinberg et al., 2004). This 21-item questionnaire assesses trauma exposure and PTSS in children within the past month and yields an overall symptom severity score ranging from 0–68. A cut-off score of 40 or greater has been used to indicate severe symptoms for which a clinical PTSD diagnosis may be likely (Cloitre et al., 2009). Reliability was high, with Cronbach’s α ranging from .82–.91 and averaging .87 across the 12 time points.
Data Analytic Strategy
We tested our hypotheses using a multilevel modeling approach with the maximum likelihood method in the nlme package of R version 3.6.3 (Pinheiro et al., 2021). Trajectories of change in caregiver and child psychopathology were estimated from twelve time points of monthly data. All available data were included in our analyses, as multilevel modeling growth models can estimate trajectories from families with different numbers of observations due to missing data. Time points with missing data were handled with listwise deletion.
Since observed ER and RSA data were only available for a subset of families, we examined associations between a variety of participant characteristics (primary caregiver age, child age, child gender, family income, primary caregiver education, primary caregiver race/ethnicity, child diagnosis, primary caregiver relationship status, month 3 negative life events, month 3 treatment-related events, month 3 primary caregiver psychopathology, and hospital site) and missingness in our predictors. We also tested whether the number of questionnaires that primary caregivers completed for each of the psychopathology outcomes was related to their own average psychopathology over the twelve months or to their children’s average psychopathology.
To create the best fitting model for our outcome variables, we constructed growth models of the average trajectory of caregiver and child psychopathology. Time was coded from −11 (month 1) to 0 (month 12) so that the intercept reflected psychopathology at month 12. We first estimated an unconditional growth model with a linear time function. This provided us with an estimated fixed intercept, reflecting the average final level of each outcome, and an estimated fixed slope parameter, reflecting the average rate of change for each outcome. We then added random effects, first for the intercept and then for both the intercept and slope, testing for between family differences in final levels of psychopathology as well as rate of change. To determine the final model, we compared model fit using the log-likelihood, Akaike information criteria, and Bayesian information criteria. We also examined the fit of quadratic models of change in the outcomes over time; however, due to limitations of the data, we were only able to model linear effects of time. The inclusion of random slopes and intercepts best characterized our data, indicating that there was substantial variation in the trajectories of change in psychopathology between families that could potentially be explained by predictors.
Next, we tested caregiver resting RSA and observed ER, measured at month 3, as predictors of the trajectory of caregiver depression, anxiety and PTSS as well as child depression/anxiety, externalizing, and PTSS. Twelve models were run in total. Resting RSA and observed ER were tested separately as predictors to maximize power due to the smaller sample size for RSA. Child age was a covariate in all analyses due to the wide age range in our sample, and child gender was included as a covariate in models predicting child psychopathology, given emerging gender differences in rates of internalizing and externalizing disorders (Hayward & Sanborn, 2002). The main effects of our models represent the predictive association between ER at month 3 and psychopathology at month 12, whereas interactions between our predictors and time reflect the association between ER and the trajectory of psychopathology over the year.
Results
Descriptive Information
Detailed information about trajectories of caregiver and child adjustment can be found in Katz et al. (2018). Average caregiver depression was in the clinical range during the first ten months after diagnosis, average caregiver anxiety was elevated for the first month after diagnosis, and average caregiver PTSS remained in the mild range for the first year after diagnosis. Children’s mean depression/anxiety and externalizing symptoms were within the non-clinical range across all time points. Children’s mean PTSS symptoms were also substantially below the clinical range at all time points. The correlation between resting RSA and observed ER was small and not significant (r = 0.240, p = 0.121).
Associations with Missingness
Missingness in caregiver resting RSA was positively correlated with child age (b = .19, p = 0.01) and the number of concurrent treatment-related events at month 3 (b = 0.28, p = 0.04). Missingness in observed ER was more likely for lower income families (b = −0.19, p = 0.03). Average caregiver depression, anxiety, and PTS symptoms across the twelve months were positively associated with the number of missing measures for all outcome variables (r = 0.17 – 0.22, p = 0.04 – 0.01). Average child psychopathology was not correlated with missingness in the outcome variables (r = 0.05 – 0.16, p = 0.53 – 0.06).
Model Building
Due to the low response rate at month two, models were tested both with and without month two data. Since the pattern of results was similar, all time points were retained in the final models. For all caregiver and child psychopathology outcomes, linear growth models with random intercepts and slopes had the best model fit compared to fully fixed and random intercept models, indicating that there was variability in the rate of change of psychopathology symptoms over time as well as final levels of symptoms. See Supplemental Materials for growth model fit indices. Average caregiver and child psychopathology symptoms decreased significantly over time in the final models. Primary caregiver RSA and observed ER were then separately tested as predictors of primary caregiver and child psychopathology.
Observed ER Predicting Psychopathology
Model fit information is provided in Tables 1 – 6. Observed ER predicted fewer caregiver depression symptoms (b = −6.03, SE = 2.11, p = 0.005; Figure 1) but was not associated with change in caregiver depression. Observed ER also predicted fewer PTS symptoms (b = −12.58, SE = 4.38, p = 0.005; Figure 1) at month twelve but was not associated with change in PTSS over time . There was a non-significant negative association between observed ER and month twelve caregiver anxiety symptoms (b = −1.66, SE = 0.97, p = 0.09; Figure 1). Higher observed ER predicted less of a decrease in caregiver anxiety symptoms over time (b = 0.20, SE = 0.09, p = 0.03; Figure 1). This is because caregivers with lower observed ER had higher anxiety symptoms at the start of treatment (b = −3.89, SE = 0.96, p = 0.001), but dropped to similar symptom levels as those with higher ER twelve months later (b = −1.67, SE = 0.97, p = 0.09), resulting in a greater decrease in anxiety symptoms over time for those with lower ER. Caregiver observed ER did not predict month twelve child psychopathology symptoms or change in child psychopathology over time.
Table 1.
Model Fit Indices: Caregiver Depression
| AIC | BIC | Random Intercept Variance (% Change) | Random Slope Variance(% Change) | −2*Log Likelihood | χ2 (df) | p | |
|---|---|---|---|---|---|---|---|
| Random intercept & slope, no predictors | 5569.40 | 5598.62 | 42.33 (59.54) | 0.19 | 5557.40 | 53.11 (6) | <.001 |
| Observed ER (Level 1 n = 654; Level 2 n = 93) | 3685.77 | 3721.64 | 32.04 (−24.31) | 0.19(−.01) | 3669.77 | 20.26 (8) | <.001 |
| Resting RSA (Level 1 n = 386; Level 2 n = 57) | 2196.76 | 2228.41 | 38.35 (−9.40) | .12 (−38.24) | 2180.76 | 2.66 (8) | .26 |
Table 6.
Model Fit Indices: Child PTSS
| AIC | BIC | Random Intercept Variance (% Change) | Random Slope Variance(% Change) | −2*Log Likelihood | χ2 (df) | p | |
|---|---|---|---|---|---|---|---|
| Random intercept & slope, no predictors | 6169.43 | 6198.64 | 63.83 (35.85) | .42 | 6157.43 | 58.16 (6) | <.001 |
| Observed ER (Level 1 n = 650; Level 2 n = 93) | 4116.78 | 4152.60 | 57.80 (−9.45) | .36 (−14.60) | 4100.78 | 5.67 (8) | .06 |
| Resting RSA (Level 1 n = 383; Level 2 n = 57) | 2393.07 | 2424.65 | 52.93 (−17.07) | .06 (−86.54) | 2377.07 | 4.15 (8) | .13 |
Figure 1.
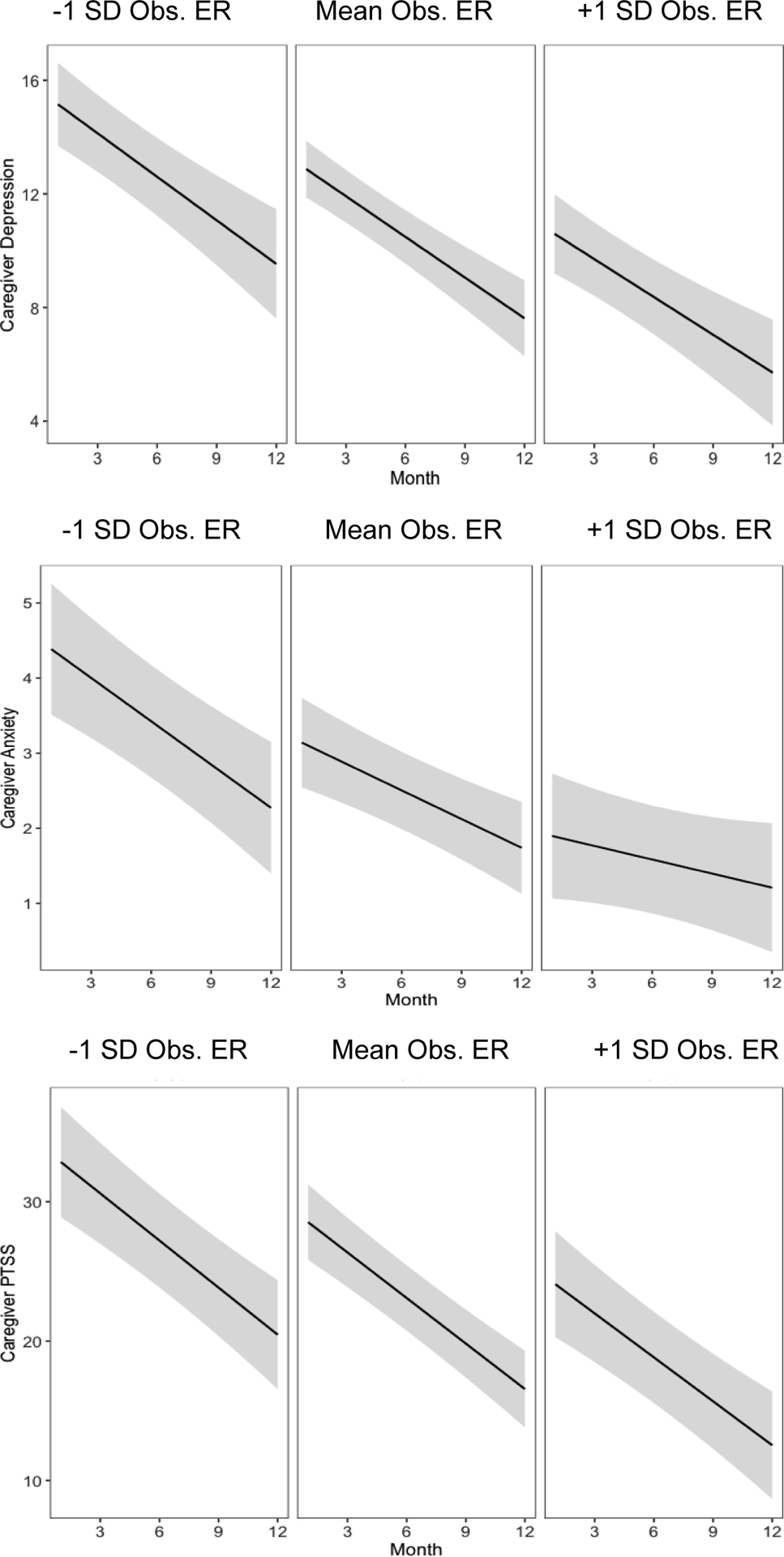
Trajectories of Primary Caregiver Psychopathology Grouped by Levels of Observed ER
Note. Shaded regions indicate 95% confidence intervals.
Caregiver RSA as a Predictor of Psychopathology
RSA did not significantly predict month twelve caregiver depression, anxiety, or PTS symptoms or change in symptoms over time. Caregiver RSA was also not associated with children’s month twelve depression/anxiety. However, children of caregivers with higher RSA showed greater decreases in depression/anxiety symptoms (b = −0.14, SE = 0.06, p = 0.02; Figure 2) because they had higher depression/anxiety symptoms at the start of treatment (b = 2.01, SE = 0.69, p = 0.005) but dropped to similar symptom levels as children of caregivers with lower RSA one year later (b = 0.45, SE = 0.54, p = 0.40). Caregiver RSA was also positively associated with child PTSS at month twelve (b = 2.56, SE = 0.83, p = 0.003; Figure 2), but not with the trajectory of child PTSS. No main or interaction effects of RSA were found for child externalizing symptoms.
Figure 2.
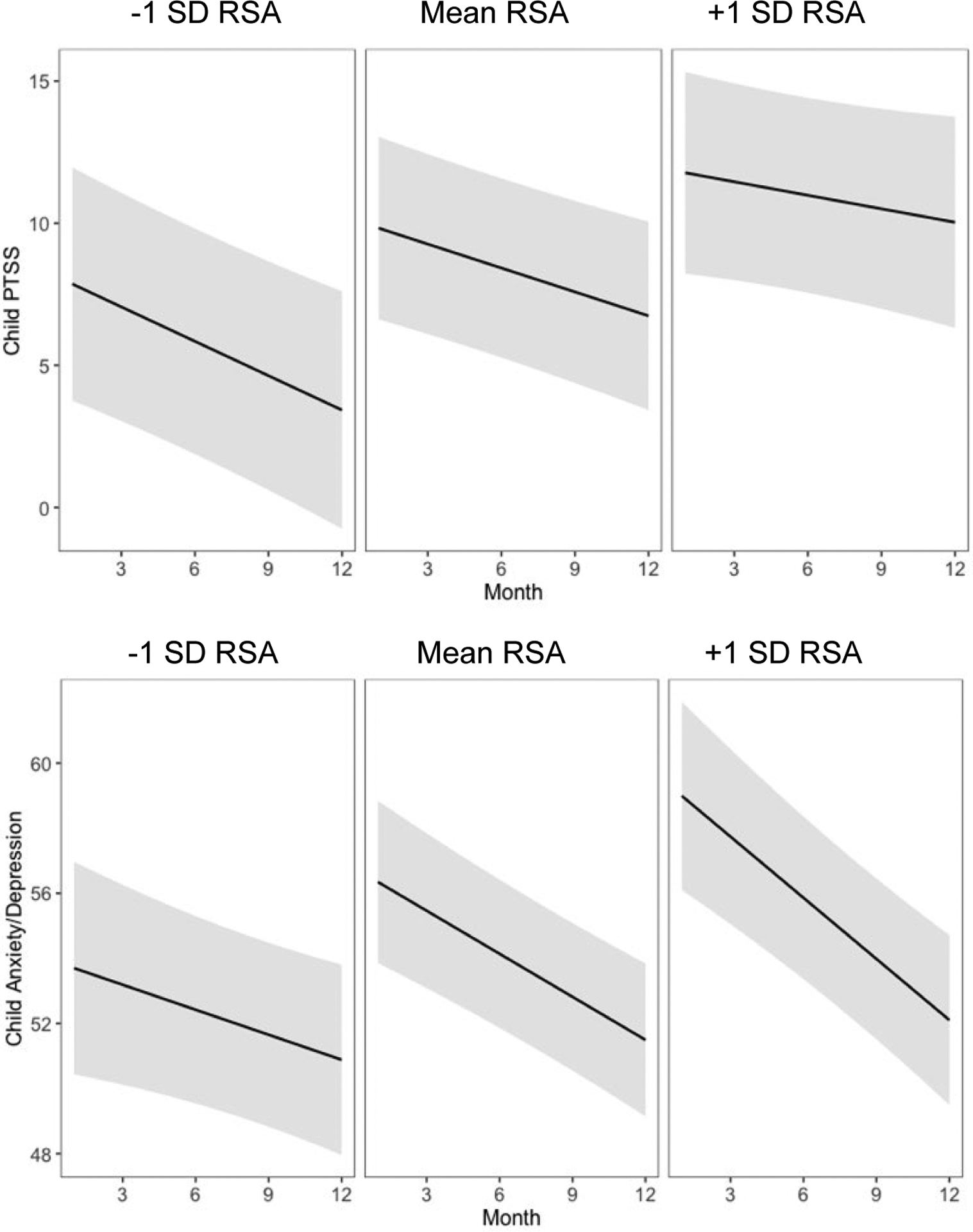
Estimated Trajectories of Child PTSS and Anxiety/Depression Symptoms Grouped by Levels of Caregiver Resting RSA
Note. Shaded regions indicate 95% confidence intervals.
Discussion
This study examined ER and the trajectory of primary caregiver and child psychopathology during the first year of pediatric cancer treatment, a highly stressful time during which a subset of caregivers and children develop clinically relevant psychopathology symptoms (Compas et al., 2015; Pinquart & Shen, 2011). Caregiver ER was proposed as a potential protective factor for caregivers and children. Subjective measures of ER have been consistently linked with lower psychopathology (Aldao et al., 2016), whereas findings on RSA, a physiological component of ER, have been mixed (Chalmers et al., 2014; Skoranski & Lunkenheimer, 2020). To understand the function of different aspects of ER in managing the stress of cancer treatment, the study examined both coder ratings of caregivers’ descriptions of their emotional experiences and caregivers’ resting RSA. We hypothesized that primary caregiver observed ER would be negatively associated with caregiver and child psychopathology at month twelve and greater decreases in psychopathology over time, whereas resting RSA may be either negatively or positively associated with caregiver and child psychopathology.
There was a small, nonsignificant positive correlation between resting RSA and observed ER. The lack of a strong association between the measures suggests that they tap into different aspects of ER. Whereas observed ER may indicate caregivers’ direct experiences of negative emotions, resting RSA may reflect physiological relaxation at baseline that caregivers are less consciously aware of. These forms of ER may be linked to risk for psychopathology in different ways, with observed ER being more closely associated with emotional experiences and resting RSA being more indirectly associated with psychopathology. Our findings highlight the importance of examining different interlocking facets of ER to elucidate their unique effects.
Consistent with our expectations, higher observed ER in caregivers predicted lower caregiver depression and PTSS one year post-diagnosis. Higher observed ER was also associated with lower caregiver anxiety early in treatment, and there was a non-significant association with lower anxiety twelve months later. These findings align with literature linking ER with resilience to life stressors (Richardson, 2017). In our study, coders rated caregivers high in ER if they described ease in managing the frequency, intensity, and duration of sadness, anger, and fear. Greater competency in regulating negative emotions may help caregivers feel more prepared to tackle the challenges of a cancer diagnosis. Additionally, adaptive ER skills may help caregivers to maintain relational support systems that are protective against psychopathology (McShall & Johnson, 2015). Future research should examine whether interventions that build skills to manage negative emotions (e.g., Hajal & Paley, 2020) can be utilized to promote psychological adjustment in caregivers of children with cancer.
Caregivers’ observed ER was not associated with children’s psychopathology symptoms, contrary to study hypotheses and past research (Calkins & Hill, 2007). Our study may not have had enough variability in child symptoms to observe an effect of caregiver ER, as there was relatively good psychological adjustment in our sample of children, which has also been observed in other studies (Howard Sharp et al., 2015). This could be due to high connectedness to caregivers and peers (Norberg & Steneby, 2009), childhood resilience to stress (Masten, 2001), and support from medical care teams. Furthermore, even caregivers who are struggling with regulation may hide their negative feelings from their child, as suggested by findings that caregivers try to stay strong in front of their child with cancer (Kars et al., 2008), so regulation difficulties may be more apparent in other areas of caregivers’ lives instead. Subsequent studies could examine the impact of caregiver ER in various contexts, such as relationships and career functioning. Additionally, although observed ER was not directly linked to child psychopathology, it may have an indirect effect via caregivers’ parenting behaviors and socialization of child emotions. Caregiver ER may also influence child psychopathology by moderating the bidirectional association between caregiver and child psychopathology, a potential avenue for future research.
In contrast to the theoretical understanding of higher resting RSA as indicative of adaptive ER (Beauchaine, 2015), caregivers’ RSA was not associated with their own psychopathology symptoms in our study. Several studies have similarly found no association between resting RSA and psychopathology (e.g., LeMoult et al., 2016; Moser et al., 1998). In a meta-analysis, only 6 of the 39 comparisons conducted found a significant difference in the resting RSA of individuals with and without depression (Rotteburg, 2007). The authors concluded that the link between RSA and depression may be overstated in the literature due to publication bias.
Additionally, although lower RSA has been observed in individuals with diagnosed psychopathology (Chalmers et al., 2014), in community samples, the degree of association between RSA and psychopathology symptoms may be less consistent (Koenig et al., 2016; Skoranski & Lunkenheimer, 2020). Caregivers of children with cancer face an intense, prolonged stressor with a sudden onset and possible life threat. Although RSA is thought to confer flexible responses to stressors (Porges, 2007), it is unclear how exactly RSA may impact responses to such a context. Individuals with higher RSA may report symptoms of psychopathology due to greater emotional engagement with stressors and awareness of their negative emotions (Skoranski & Lunkenheimer, 2020; Vasilev et al., 2009). One year may also not have been sufficient time for caregivers to develop effective coping methods and thus for differences to emerge for those who had greater physiological resources to manage stress.
Unexpectedly, resting RSA predicted greater PTSS in children at month twelve and greater child depression/anxiety symptoms at the start of treatment. Although resting RSA has been linked to greater resilience to stressors (El-Sheikh et al., 2001; McLaughlin et al., 2014), it has also been related to greater self-reported awareness of emotions (Vasilev et al., 2009), compassion (Stellar et al., 2015), and consideration of others’ mental states (Borelli et al., 2018). Thus, caregivers with higher RSA may be more sensitive to their child’s mental states, leading them to report greater internalizing symptoms.
It is also possible that the unique context of cancer treatment places children of parents with higher RSA at greater risk for developing psychopathology symptoms during the initial period after diagnosis. Given that caregivers with higher RSA may be more likely to acknowledge negative emotions (Vasilev et al., 2009), they may also encourage their children to draw more focus to the negative emotions that arise during cancer treatment, heightening PTSS and internalizing symptoms. Greater engagement with negative feelings may be typically adaptive but have an adverse effect during this early period of cancer treatment. In fact, lower engagement with treatment stress could help to minimize distress in the short term, as strategies that turn attention away from negative feelings, such as distraction, have been found to reduce distress during medical procedures (Manne et al., 1993). Future research could investigate the relation between caregivers’ resting RSA and emotion socialization in stressful contexts as well as the long-term impact on child adjustment. Furthermore, research involving multiple reporters of child adjustment, such as teachers and the child themselves, could elucidate whether the positive association between caregiver RSA and child psychopathology is due to poorer child adjustment or to greater caregiver awareness of their children’s emotions.
The study builds upon past research on ER by examining its role in the context of pediatric cancer, providing insight into ER’s role in managing responses to a sudden, intense, and prolonged stressor that has been associated with elevated psychopathology symptoms. The multi-method study design allowed for comparison between observed and physiological components of ER, and the study’s longitudinal nature allowed us to examine changes in caregiver and child psychopathology symptoms over the first year of treatment.
There are also some limitations to the study. It is unclear whether the association between ER and psychopathology might change later during treatment or after cancer remission, as high RSA could be linked to greater recovery and post-traumatic growth over time as families process their experiences. Our sample is also predominantly White (88.7%), partially because White children are at higher risk for cancer diagnosis (Chow et al., 2010), so we were unable to examine racial and cultural variation in how caregivers respond and regulate during treatment.
Given the intensive nature of cancer treatment, there was a large amount of missing data, particularly for RSA, limiting our power to detect significant effects. Fewer families participated in ECG data collection, likely because it required a home visit during the particularly stressful early period of treatment. We found more missing RSA data in caregivers of older children and those who reported more treatment-related events at month three, when RSA was measured. Although we have no theoretical reason to suspect that our findings on resting RSA would differ for these families, this limits our ability to understand the role of ER in families handling more intensive treatment during this time. For observed ER, missingness was more likely for lower income families. Thus, we are also limited in our ability to generalize our findings on observed ER and psychopathology to lower income families, who handle greater financial and overall chronic stress (Baum et al., 1999).
When examining missingness in our outcomes, we found that primary caregivers who reported higher average psychopathology completed fewer questionnaires. Nevertheless, the average proportion of caregivers in the clinical range each month was high (53.6% for depression, 30.3% for anxiety, and 20.6% for PTSS). Although our data may not capture some caregivers at time points when they were experiencing higher psychopathology, many caregivers with high psychopathology symptoms still completed the questionnaires, informing our model.
Since missingness is linked to our caregiver psychopathology outcomes, some data may be missing not at random (MNAR), introducing potential bias to our parameter estimates. Despite this pattern of missingness, observed ER still predicted aspects of caregiver psychopathology. Since low ER was associated with greater caregiver psychopathology in our sample, we expect that having more data from caregivers who reported higher psychopathology would likely have further strengthened this finding. Furthermore, having less data from caregivers with higher average psychopathology may have contributed to the lack of association between resting RSA and caregiver psychopathology. Past research has found RSA to be more consistently linked with psychopathology in clinical samples than in community samples (Koenig et al., 2016), suggesting that there may be a stronger association with RSA among individuals with higher psychopathology symptoms. Thus, stronger effects may have emerged if we had more data from caregivers who reported greater symptoms.
Lastly, it is important to consider alternative explanations for our findings. Since we ran twelve models, there is a higher possibility that our findings contain a false positive, underscoring the need for further replication. Additionally, the association between observed ER and caregiver psychopathology could be influenced by the fact that caregivers experiencing fewer stressors, such as lower treatment intensity or less financial strain, would experience less negative emotion. Such caregivers may find it easier to manage their negative emotions and experience fewer psychopathology symptoms. Caregivers with low emotional awareness or a minimizing response style may have also reported low negative emotions in both the interview measuring ER and in questionnaires measuring psychopathology. To address potential third variables, future studies could experimentally manipulate different forms of ER to study their effects on psychopathology.
Conclusion
Examining ER in the context of pediatric cancer can deepen our understanding of its role in dealing with an intense real-life stressor that has been associated with elevated psychopathology symptoms (Compas et al., 2015). Our findings for observed ER and resting RSA highlight the importance of taking a multi-method approach to understanding how different aspects of ER impact functioning in a variety of settings. Our findings on observed ER indicate that caregivers who feel more equipped to manage negative emotions experience better psychological adjustment in the first year of their child’s treatment. Thus, caregivers could benefit from interventions to manage the frequency, intensity, and duration of their negative emotions at the start of treatment. Our unexpected findings on caregivers’ resting RSA and child psychopathology may indicate that caregivers who are more physiologically regulated are more attuned to their children’s negative emotions or that higher RSA caregivers may encourage greater engagement with negative emotions for their children, with more research needed to understand the role of caregiver resting RSA during cancer treatment.
Supplementary Material
Table 2.
Model Fit Indices: Caregiver Anxiety
| AIC | BIC | Random Intercept Variance (% Change) | Random Slope Variance(% Change) | −2*Log Likelihood | χ2 (df) | p | |
|---|---|---|---|---|---|---|---|
| Random intercept & slope, no predictors | 4492.48 | 4521.64 | 10.55 (21.93) | .05 | 4480.48 | 24.05 (6) | <.001 |
| Observed ER (Level 1 n = 647; Level 2 n = 93) | 2932.33 | 2968.10 | 6.12 (−41.99) | .03 (45.83) | 2916.33 | 13.39 (8) | .001 |
| Resting RSA (Level 1 n = 385; Level 2 n = 57) | 1813.30 | 1844.93 | 8.37 (−20.61) | .05 (0) | 1797.30 | 4.54 (8) | .10 |
Table 3.
Model Fit Indices: Caregiver PTSS
| AIC | BIC | Random Intercept Variance (% Change) | Random Slope Variance(% Change) | −2*Log Likelihood | χ2 (df) | p | |
|---|---|---|---|---|---|---|---|
| Random intercept & slope, no predictors | 7117.77 | 7146.90 | 179.38 (2.44) | 1.00 | 7105.77 | 40.85 (6) | <.001 |
| Observed ER (Level 1 n = 638; Level 2 n = 93) | 4624.34 | 4660.00 | 134.58 (−24.97) | .72 (−28.29) | 4608.34 | 16.19 (8) | <.001 |
| Resting RSA (Level 1 n = 380; Level 2 n = 58) | 2854.99 | 2886.51 | 177.93(−.81) | .98(−2.05) | 2838.99 | 4.05 (8) | 0.13 |
Table 4.
Model Fit Indices: Child Depression/Anxiety
| AIC | BIC | Random Intercept Variance (% Change) | Random Slope Variance(% Change) | −2*Log Likelihood | χ2 (df) | p | |
|---|---|---|---|---|---|---|---|
| Random intercept & slope, no predictors | 5624.55 | 5653.65 | 28.24 (−.75) | 0.28 | 5612.55 | 75.59 (6) | <.001 |
| Observed ER (Level 1 n = 639; Level 2 n = 93) | 3785.02 | 3820.70 | 32.51 (14.25) | .30 (6.59) | 3769.02 | 4.37 (8) | 0.11 |
| Resting RSA (Level 1 n = 381; Level 2 n = 57) | 2243.02 | 2274.57 | 16.52 (−41.93) | .14 (−50.87) | 2227.02 | 4.74 (8) | 0.09 |
Table 5.
Model Fit Indices: Child Externalizing
| AIC | BIC | Random Intercept Variance (% Change) | Random Slope Variance(% Change) | −2*Log Likelihood | χ2 (df) | p | |
|---|---|---|---|---|---|---|---|
| Random intercept & slope, no predictors | 6110.36 | 6139.46 | 125.62 (20.33) | .37 | 6098.36 | 51.93 (6) | <.001 |
| Observed ER (Level 1 n = 639; Level 2 n = 93) | 4109.52 | 4145.20 | 101.37 (−19.30) | .37 (.11) | 4093.52 | 5.64 (8) | .06 |
| Resting RSA (Level 1 n = 381; Level 2 n = 57) | 2459.55 | 2491.09 | 146.04 (16.25) | .42 (13.73) | 2443.55 | 4.58 (8) | .10 |
Author Note
This research was supported by grant R01-CA134794 from the National Cancer Institute to Lynn Fainsilber Katz, PhD. A portion of the manuscript findings was presented as a poster at the Society for Research in Child Development Conference in March 2021. Materials and analysis code for this study are available by emailing the corresponding author. This study’s design and its analysis were not pre-registered.
References
- Achenbach TM (1991). Child Behavior Checklist/4–18. University of Vermont, Psychiatry. [Google Scholar]
- Aldao A, Gee D, De Los Reyes A, & Seager I (2016). Emotion regulation as a transdiagnostic factor in the development of internalizing and externalizing psychopathology: Current and future directions. Development and Psychopathology, 28(4pt1), 927–946. 10.1017/S0954579416000638 [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, & Patrick DL (1994). Screening for depression in well older adults: Evaluation of a short form of the CES-D. American Journal of Preventive Medicine, 10(2), 77–84. 10.1016/S0749-3797(18)30622-6 [DOI] [PubMed] [Google Scholar]
- Bai S & Repetti RL (2015). Short-term resilience processes in the family. Family Relations, 64, 108–119. 10.1111/fare.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariola E, Gullone E, & Hughes EK (2011). Child and adolescent emotion regulation: The role of parental emotion regulation and expression. Clinical Child and Family Psychology Review, 14(2), 198–212. 10.1007/s10567-011-0092-5 [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, & Yali AM (1999). Socioeconomic status and chronic stress: Does stress account for SES effects on health? In Adler NE, Marmot M, McEwen BS, & Stewart J (Eds.), Socioeconomic status and health in industrial nations: Social, psychological, and biological pathways (pp. 131–144). New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. In Current Opinion in Psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman RE, & Kliegman RM (2002). Nelson essentials of pediatrics. (4th ed.) W.B. Saunders. [Google Scholar]
- Bona K, Dussel V, Orellana L, Kang T, Geyer R, Feudtner C, & Wolfe J (2014). Economic impact of advanced pediatric cancer on families. Journal of Pain and Symptom Management, 47(3), 594–603. 10.1016/j.jpainsymman.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli JL, Ensink K, Hong K, Sereno AT, Drury R, & Fonagy P (2018). School-aged children with higher reflective functioning exhibit lower cardiovascular reactivity. Frontiers in Medicine, 5, 196. 10.3389/fmed.2018.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, & Gross JJ (2006). Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology, 43(6), 612–622. 10.1111/j.1469-8986.2006.00467.x [DOI] [PubMed] [Google Scholar]
- Calkins SD, & Hill A (2007). Caregiver influences on emerging emotion regulation: Biological and environmental transactions in early development. In Gross JJ (ed). Handbook of Emotion Regulation, First Edition, 229–248. The Guilford Press. [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJA, & Kemp AH (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 80. 10.3389/fpsyt.2014.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EJ, Puumala SE, Mueller BA, Carozza SE, Fox EE, Horel S, … & Spector LG (2010). Childhood cancer in relation to parental race and ethnicity: A 5‐state pooled analysis. Cancer, 116(12), 3045–3053. 10.1002/cncr.25099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloitre M, Stolbach BC, Herman JL, van der Kolk B, Pynoos R, Wang J, & Petkova E (2009). A developmental approach to complex PTSD: Childhood and adult cumulative trauma as predictors of symptom complexity. Journal of Traumatic Stress, 22, 399–408. 10.1002/jts.20444 [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, & Dennis TA (2004). Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development, 75(2), 317–333. 10.1111/j.1467-8624.2004.00673.x [DOI] [PubMed] [Google Scholar]
- Compas BE, Bemis H, Gerhardt CA, Dunn MJ, Rodriguez EM, Desjardins L, Preacher KJ, Manring S, & Vannatta K (2015). Mothers and fathers coping with their children’s cancer: Individual and interpersonal processes. Health Psychology, 34(8), 783–793. 10.1037/hea0000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, & Otter-Henderson KD (2011). Individual differences in vagal regulation moderate associations between daily affect and daily couple interactions. Personality and Social Psychology Bulletin, 37(6), 731–744. 10.1177/0146167211400620 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J & Whitson SM (2001), Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development, 72, 1617–1636. 10.1111/1467-8624.00369 [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF, & Hooven C (1997). Meta-emotion: How families communicate emotionally. Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Gross JJ (2015). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26(1), 1–26. 10.1080/1047840X.2014.940781 [DOI] [Google Scholar]
- Hajal NJ & Paley B (2020). Parental emotion and emotion regulation: A critical target of study for research and intervention to promote child emotion socialization. Developmental Psychology, 56(3), 403–417. 10.1037/dev0000864 [DOI] [PubMed] [Google Scholar]
- Hayward C, & Sanborn K (2002). Puberty and the emergence of gender differences in psychopathology. Journal of Adolescent Health, 30(4), 49–58. 10.1016/S1054-139X(02)00336-1 [DOI] [PubMed] [Google Scholar]
- Henry JD & Crawford JR (2005). The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 44, 227–239. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- Howard Sharp KM, Willard VW, Okado Y, Tillery R, Barnes S, Long A, & Phipps S (2015). Profiles of connectedness: Processes of resilience and growth in children with cancer. Journal of Pediatric Psychology, 40(9), 904–913. 10.1093/jpepsy/jsv036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson P (2007). Respiratory sinus arrhythmia as a function of state anxiety in healthy individuals. International Journal of Psychophysiology, 63(1), 48–54. 10.1016/j.ijpsycho.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Kars MC, Duijnstee MS, Pool A, van Delden JJ, & Grypdonck MH (2008). Being there: parenting the child with acute lymphoblastic leukemia. Journal of Clinical Nursing, 17(12), 1553–1562. 10.1111/j.1365-2702.2007.02235.x [DOI] [PubMed] [Google Scholar]
- Katz LF, Fladeboe K, King K, Gurtovenko K, Kawamura J, Friedman D, Compas B, Gruhn M, Breiger D, Lengua L, Lavi I, & Stettler N (2018). Trajectories of child and caregiver psychological adjustment in families of children with cancer. Health Psychology, 37(8), 725–735. 10.1037/hea0000619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LF, & Gottman JM (1986). The meta-emotion interview. Unpublished manual. University of Washington, Department of Psychology. [Google Scholar]
- Katz LF, & Gottman JM (1997). Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology, 26(2), 157–171. 10.1207/s15374424jccp2602_4 [DOI] [PubMed] [Google Scholar]
- Katz LF, Mittman A, & Hooven C (1994). The meta-emotion coding system. Unpublished manuscript. University of Washington, Department of Psychology. [Google Scholar]
- Koenig J, Kemp AH, Beauchaine TP, Thayer JF, & Kaess M (2016). Depression and resting state heart rate variability in children and adolescents — A systematic review and meta-analysis. Clinical Psychology Review, 46, 136–150. 10.1016/j.cpr.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Kogan AV, Allen JJB, & Weihs KL (2012). Cardiac vagal control as a prospective predictor of anxiety in women diagnosed with breast cancer. Biological Psychology, 90(1), 105–111. 10.1016/j.biopsycho.2012.02.019 [DOI] [PubMed] [Google Scholar]
- Leavitt M, Martinson IM, Liu CY, Armstrong V, Hornberger L, Zhang JQ, & Han XP (1999). Common themes and ethnic differences in family caregiving the first year after diagnosis of childhood cancer: Part II. Journal of Pediatric Nursing, 14(2), 110–122. 10.1016/S0882-5963(99)80045-1 [DOI] [PubMed] [Google Scholar]
- LeMoult J, Yoon KL, & Joormann J (2016). Rumination and cognitive distraction in Major Depressive Disorder: An examination of respiratory sinus arrhythmia. Journal of Psychopathology and Behavioral Assessment, 38(1), 20–29. 10.1007/s10862-015-9510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, & Lovibond PF (1995). Manual for the depression anxiety stress scales. (2nd Edition) Sydney: Psychological Foundation. [Google Scholar]
- Manne SL, Jacobsen PB, Gorfinkle K, Gerstein F, & Redd WH (1993). Treatment adherence difficulties among children with cancer: The role of parenting style. Journal of Pediatric Psychology, 18(1), 47–62. 10.1093/jpepsy/18.1.47 [DOI] [PubMed] [Google Scholar]
- Masten AS (2001). Ordinary magic: Resilience processes in development. American Psychologist, 56(3), 227–238. 10.1037//0003-066x.56.3.227 [DOI] [PubMed] [Google Scholar]
- McGrath P & Phillips E (2008). “It is very hard”: Treatment for childhood lymphoma from the parents’ perspective. Issues in Comprehensive Pediatric Nursing, 31(1), 37–54. DOI: 10.1080/01460860701877209 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Alves S, & Sheridan MA (2014). Vagal regulation and internalizing psychopathology among adolescents exposed to childhood adversity. Developmental Psychobiology, 56(5), 1036–1051. 10.1002/dev.21187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShall JR, & Johnson MD (2015). The association between relationship distress and psychopathology is consistent across racial and ethnic groups. Journal of Abnormal Psychology, 124(1), 226–231. 10.1037/a0038267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Lehofer M, Hoehn-Saric R, McLeod DR, Hildebrandt G, Steinbrenner B, Voica M, Liebmann P, & Zapotoczky HG (1998). Increased heart rate in depressed subjects in spite of unchanged autonomic balance? Journal of Affective Disorders, 48(2), 115–124. 10.1016/S0165-0327(97)00164-X [DOI] [PubMed] [Google Scholar]
- Norberg AL and Steneby S (2009). Experiences of parents of children surviving brain tumour: a happy ending and a rough beginning. European Journal of Cancer Care, 18: 371–380. 10.1111/j.1365-2354.2008.00976.x [DOI] [PubMed] [Google Scholar]
- Perlman SB, Camras LA, & Pelphrey KA (2008). Physiology and functioning: Parents’ vagal tone, emotion socialization, and children’s emotion knowledge. Journal of Experimental Child Psychology, 100(4), 308–315. 10.1016/j.jecp.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Perrin EC, Stein RE, & Drotar D (1991). Cautions in using the Child Behavior Checklist: Observations based on research about children with a chronic illness. Journal of Pediatric Psychology, 16(4), 411–421. 1941423 [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2021). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–153, https://CRAN.R-project.org/package=nlme.
- Pinquart M & Shen Y (2011). Behavior problems in children and adolescents with chronic physical illness: A meta-analysis, Journal of Pediatric Psychology, 36(9), 1003–1016. 10.1093/jpepsy/jsr042 [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Richardson CME (2017). Emotion regulation in the context of daily stress: Impact on daily affect. Personality and Individual Differences, 112, 150–156. 10.1016/j.paid.2017.02.058 [DOI] [Google Scholar]
- Rottenberg J, & Johnson SL (Eds.). (2007). Emotion and psychopathology: Bridging affective and clinical science. American Psychological Association. 10.1037/11562-000 [DOI] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, & Gotlib IH (2002). Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. Journal of Affective Disorders, 71(1–3), 265–272. 10.1016/S0165-0327(01)00406-2 [DOI] [PubMed] [Google Scholar]
- Shih EW, Quiñones-Camacho LE, & Davis EL (2018). Parent emotion regulation socializes children’s adaptive physiological regulation. Developmental Psychobiology, 60(5), 615–623. 10.1002/dev.21621 [DOI] [PubMed] [Google Scholar]
- Skoranski AM, & Lunkenheimer E (2020). Person-centered profiles of parasympathetic physiology, anxiety symptoms, and depressive symptoms in mothers and fathers of young children. Developmental Psychobiology, 63(4). 10.1002/dev.22043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, & Pynoos RS (2004). The University of California at Los Angeles Post-Traumatic Stress Disorder Reaction Index. Current Psychiatry Reports, 6(2), 96–100. 10.1007/s11920-004-0048-2 [DOI] [PubMed] [Google Scholar]
- Stellar JE, Cohen A, Oveis C, & Keltner D (2015). Affective and physiological responses to the suffering of others: Compassion and vagal activity. Journal of Personality and Social Psychology, 108(4), 572–585. 10.1037/pspi0000010 [DOI] [PubMed] [Google Scholar]
- Vasilev CA, Crowell SE, Beauchaine TP, Mead HK and Gatzke-Kopp LM (2009). Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. Journal of Child Psychology and Psychiatry, 50, 1357–1364. 10.1111/j.1469-7610.2009.02172.x [DOI] [PubMed] [Google Scholar]
- Weiss DS, & Marmar CR (1997). The Impact of Event Scale-Revised. In Wilson JP & Keane TM (Eds.), Assessing Psychological Trauma and PTSD, New York: Guilford, 399–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


