Abstract
Objective
Sound touch elastography (STE) and sound touch quantification (STQ) are novel imaging methods to evaluate tissue stiffness. This study aims to investigate renal stiffness in patients with chronic kidney disease (CKD) by STE and STQ, using renal biopsy as ‘gold standard’.
Methods
Between 2019 January and 2022 June, 60 patients who underwent renal biopsy for proteinuria (cases) and 45 healthy volunteers (controls) at our hospital were included in this study. The maximum and mean elastic modulus (Emax, Emean) of region of interest in right kidney were measured by STE and STQ techniques. Biochemical profiles and renal biopsy findings were recorded.
Results
Both Emax and Emean measured by STE were significantly different between cases and controls. ROC analysis of STE measurements revealed using a cutoff of 13.53 kPa for Emax and 10.16 kPa for Emean, the area under the curve (AUC) to distinguish nephropathy from healthy was 0.718 and 0.744. Analysis of ROC for STQ measurements showed that using a cutoff value of 15.87 kPa for Emax and 9.95 kPa for Emean, the AUC for the nephropathy was 0.612 and 0.569. Emax and Emean values were significantly different among CKD patients with mild, moderate and severe pathological stage. The Emax value for STE was positively related to Scr, β2-MG (r = 0.257, 0.292, p < 0.05).
Conclusion
Both STE and STQ are non-invasive, feasible methods to quantitatively evaluate renal stiffness. STE is more effective than STQ in the diagnosis of CKD patients with proteinuria.
Critical relevance statement
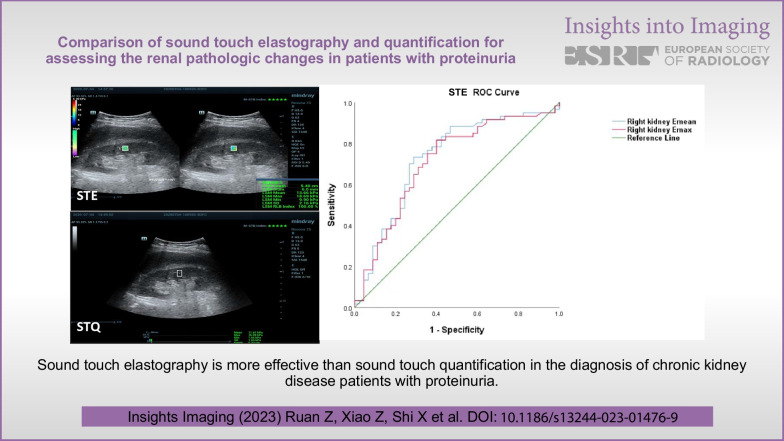
Sound touch elastography is more effective than sound touch quantification in the diagnosis of chronic kidney disease patients with proteinuria.
Key points
• Emax and Emean measured by STE were different between cases and controls.
• Emax and Emean were different among CKD patients with different pathological stages.
• The Emax value for STE was positively related to serum creatinine, β2-microglobulin
Graphical Abstract
Keywords: Sound touch elastography, Sound touch quantification, Shear wave, Ultrasonography, Chronic kidney disease
Introduction
Chronic kidney disease (CKD) is defined as structural or functional abnormalities of the kidney for ≥ 3 months [1]. It is a global health problem, affecting 10.6–13.4% adults [2]. The clinical presentations can vary from asymptomatic or non-specific symptoms such as nausea, fatigue, to edema, proteinuria or even death. Proteinuria can serve as an indicator of early renal disease, and has been used along with the estimated glomerular filtration rate (eGFR) in the classification of CKD. Proteins in the urine can cause glomerulosclerosis and lead to tubulointerstitium disease [3].
The progression of CKD is characterized histologically by glomerulosclerosis, tubulointerstitial fibrosis, and vascular sclerosis [4, 5]. Renal biopsy is currently the gold standard for assessing renal fibrosis and CKD diagnosis. However, even it is a minimally invasive procedure, there are risks of complications with vascular complications. It is believed the risk of bleeding during renal biopsy increased with the severity of renal failure [6]. Therefore, a non-invasive method instead of repeated biopsy is essential in active monitoring of CKD.
Ultrasonography (US) is a safe, affordable and non-invasive procedure to examine the kidneys. Renal length, cortical thickness and echogenicity measured by ultrasound could help to assess renal function impairment in CKD patients [7, 8]. However, US is a highly examiner-dependent, non-quantitative imaging technique. Abnormal US findings usually represent advanced rather than early stage of renal dysfunction [9]. Previous studies have reported that routine US had limited diagnostic utility in CKD classification [10–12].
Ultrasonic shear wave elastography (SWE) is a non-invasive ultrasound elastography technique that uses shear waves to quantitatively measure tissue stiffness and it has been used to determine the stiffness of liver, kidney, thyroid and muscles [13–16]. A recent study reported that SWE was an effective way to evaluate the changes in the stiffness and elasticity of the renal parenchyma in diabetic nephropathy [17]. However, the relationship between renal fibrosis and stiffness still remains uncertain in various studies [18, 19].
Sound touch elastography (STE) and sound touch quantification (STQ) are two modified shear wave elastography methods to evaluate tissue stiffness [13]. STE/STQ use acoustic radiation force impulse (ARFI) elastography techniques to generate shear waves, then tracks the propagation of the shear waves and continuously detects and records the displacement of tissue in the region of interest (ROI) by ultra-wide beam tracking imaging technology. STQ is a point SWE method that uses ARFI to aim for one small ROI to generate a quantitative reading. A larger shear wave velocity (SWV) means a higher stiffness of the tissue. It usually indicates the average rather than maximum elastic modulus of the ROI [20]. In contrast, STE is a bi-dimensional SWE (2D-SWE) method that uses multiple ARFI to target an extended ROI to display a real-time colored stiffness map [21]. It can calculate the SWV and derives the corresponding and maximum elastic modulus [20]. The STE/STQ technology ensures that the shear wave can be recorded more rapidly, accurately, and completely than conventional SWE. There are few studies evaluating renal stiffness using STE or STQ.
The present study aimed to quantitatively analyze renal fibrosis in those patients with unexplained proteinuria using STE and STQ.
Patients and methods
Patient population
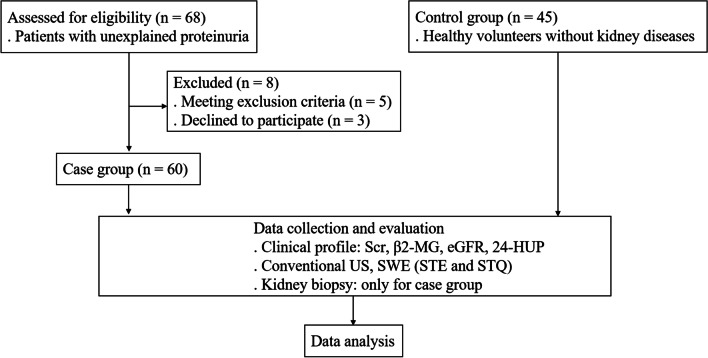
A prospective study was conducted on consecutive patients admitted to the Department of Nephrology in our hospital between 2019 January and 2022 June. Patients with unexplained proteinuria who underwent kidney biopsy were recruited. Those patients with proteinuria who had preexisting hypertension, diabetes, nephrotic syndrome or immunologic nephritis also met inclusion criteria. Patients were excluded if they were not able to attend kidney biopsy or kidney ultrasound elastography; if infection, stone or tumor induced proteinuria; in case the patients had renal replacement therapy, kidney transplant, hydronephrosis, renal calculus or tumor. One hundred and five subjects were finally enrolled and analyzed, which included 60 patients with proteinuria (“cases”) and 45 healthy volunteers (“controls”) from the Physical Examination Center in our hospital. Those individuals with abnormal urinalysis, kidney US or renal function were not eligible in the control group (Fig. 1).
Fig. 1.
Flow chart of the study. Scr serum creatinine, β2-MG β2-microglobulin, eGFR estimated glomerular filtration rate, 24-HUP 24-h urine protein, US ultrasonography, SWE shear wave elastography, STE sound touch elastography, STQ sound touch quantification
This study was approved by the ethics committee of our hospital and was performed in accordance with the ethical guidelines of the Helsinki Declaration and its later amendments. Written informed consent was obtained from all participants.
Demographic data including age, sex, height, weight and body mass index (BMI) were collected. The clinical profile including kidney biopsy, serum creatinine (Scr), β2-microglobulin (β2-MG), glomerular filtration rate (eGFR) and 24-h urine protein (24-HUP) were recorded.
Conventional renal ultrasonography and shear wave elastography
Both conventional US and SWE were performed with the convex SC6-1U probe (3.5–5 MHz) on the Mindray ultrasound system (Resona 7s, Shenzhen, China). All sonographic procedures were performed by a senior sonographer who was unaware of group assignments. After emptying bladder participants were scanned in the lateral recumbent position. STE and STQ measurements were obtained in a single region of interest (8 × 6 mm for STE, 6 × 10 mm for STQ), the middle area of right renal parenchyma with specific avoidance of renal pyramids. Three readings were taken per participant and maximum, and mean elastic values (Emax, Emean) were recorded as Young’s modulus in kPa (Fig. 2).
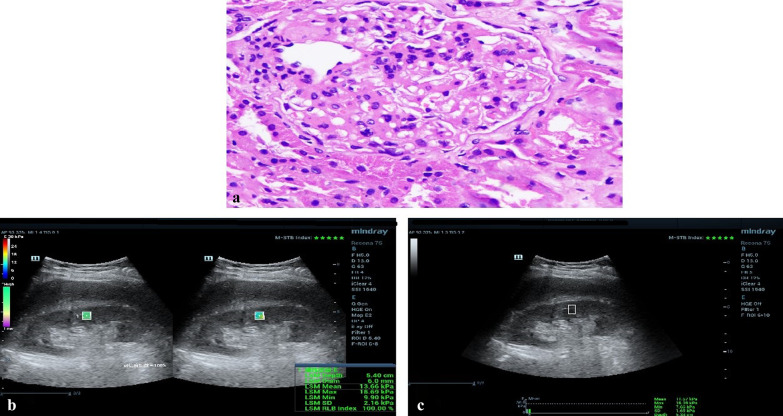
Fig. 2.
A 58-year-old man underwent renal biopsy and shear wave elastography of his right kidney. a The pathological examination revealed IgA nephropathy (H&E staining); b STE examination tested his Emax (18.69 kPa) and Emean (13.66 kPa) values; c STQ examination tested his Emax (16.09 kPa) and Emean (11.67 kPa) values. STE sound touch elastography, STQ sound touch quantification, Emax maximum elastic value, Emean mean elastic value
Kidney biopsy
The percutaneous biopsy was performed under real-time ultrasound guidance, aimed to the lower pole of the right kidney (Fig. 2). The severity of renal impairment was categorized as mild (≤ 9), moderate (10–18), or severe (≥ 19) according to a previously published pathology scoring system [22].
Statistical analysis
Categorical variables were expressed as numbers and percentages. The K–S test was performed to test the normality distribution for continuous data. For normally distributed data, variables were summarized as means (standard deviation, SD); while non-normally distributed, median and (interquartile range, IQR) was reported. Student's t test or One-Way ANOVA was used to determine whether or not there is a statistically significant difference between the means of two or more groups. Mann–Whitney U test or Wilcoxon rank-sum test was used to compare the differences between two or more groups if variables are not normally distributed. Pearson correlation analysis was used to analyze the relationship between two normally distributed variables, while Spearman's correlation was performed on two non-normally distributed variables. The receiver operating characteristic (ROC) curve, and area under the ROC curve (AUC) were calculated to determine the cutoff values for differentiating normal and renal pathologies. Statistical analyses were conducted using SPSS version 26.0 (SPSS Inc, Chicago, IL, USA), setting the statistical significance as a p value < 0.05.
Results
Patients’ characteristics
In this study, there were 60 cases, 37 were male and 23 were female. The mean age was 40.85 ± 15.06 years (range, 8–75 years). Forty-five healthy subjects were included as controls (10 males and 35 females). The mean age was 39.29 ± 15.36 years (range, 23–72 years). Mean height, weight and BMI were 1.68 ± 0.09 m, 71.50 ± 15.00 kg, 25.21 ± 3.99 kg/m2 for cases, and 1.65 ± 0.06 m, 65.93 ± 11.20 kg, 24. 15 ± 3.46 kg/m2 for controls. There were no significant differences between cases and controls in terms of age, height or BMI. The body weight was significantly higher for cases than controls (p < 0.05) (Table 1).
Table 1.
Demographics of patients with proteinuria and control subjects
| Control group (n = 45) | Case group (n = 60) | T value | p value | |
|---|---|---|---|---|
| Gender | – | 0.000** | ||
| Female, n (%) | 35 (77.78) | 23 (38.33) | ||
| Male, n (%) | 10 (22.22) | 37 (61.67) | ||
| Age (year) | 39.29 ± 15.36 | 40.85 ± 15.06 | − 0.521 | 0.603 |
| Height (m) | 1.65 ± 0.06 | 1.68 ± 0.09 | − 1.705 | 0.091 |
| Weight (kg) | 65.93 ± 11.20 | 71.50 ± 15.00 | − 2.089 | 0.039* |
| BMI (kg/m2) | 24.15 ± 3.46 | 25.21 ± 3.99 | − 1.431 | 0.156 |
BMI body mass index
*p<0.05; **p<0.01
Kidney biopsy findings revealed membranous nephropathy in 19 cases, IgA nephropathy in ten, minimal change nephropathy in eight, allergic interstitial nephritis in six, allergic purpura nephritis in six, focal segmental glomerulosclerosis in three, systemic lupus erythematosus with nephritis in two and other pathological types in six.
Maximum and minimum elastic modulus
STE and STQ findings of right kidney were listed in Table 2. It was demonstrated that Emax and Emean were significantly different between cases and controls for STE, but not for STQ.
Table 2.
Comparison of Emax and Emean values measured by STE and STQ between cases and controls
| Control group (n = 45) | Case group (n = 60) | T value | p | |
|---|---|---|---|---|
| STE (kPa) | ||||
| Emean | 9.83 ± 3.21 | 12. 14 ± 3.84 | − 3.271 | 0.001** |
| Emax | 14.70 ± 4.61 | 17.81 ± 5.64 | − 3.020 | 0.003** |
| STQ (kPa) | ||||
| Emean | 10.65 ± 2.86 | 11.47 ± 3.36 | − 1.303 | 0.196 |
| Emax | 17.19 ± 4.98 | 19.10 ± 5.75 | − 1.784 | 0.077 |
STE sound touch elastography, STQ sound touch quantification, Emax maximum elastic value, Emean mean elastic value
*p<0.05; **p<0.01
ROC curve analysis
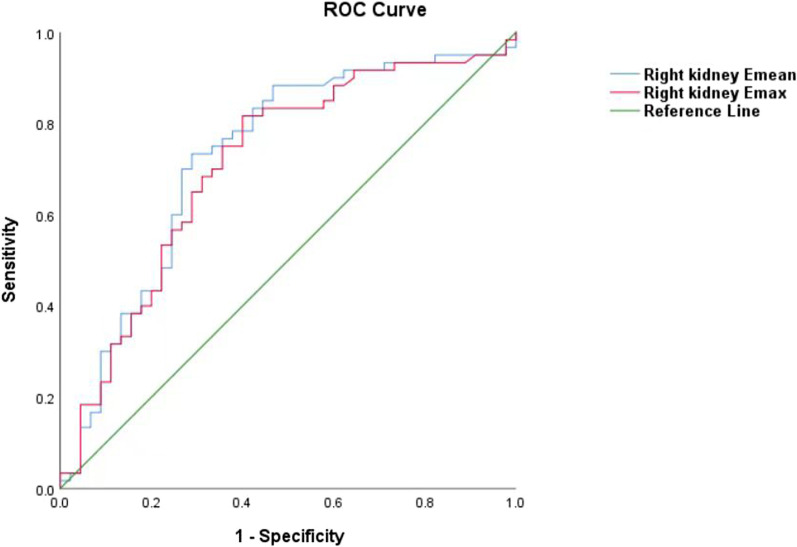
ROC analysis of STE measurements revealed that using a cutoff of 13.53 kPa for Emax, the AUC to distinguish nephropathy from healthy was 0.718 (95% confidence intervel [CI]: 0.615–0.821), with a sensitivity and specificity of 82.76%, 59.09%, respectively (Table 3; Fig. 3). The Youden index was 0.418. While using a cutoff of 10.16 kPa for Emean, a value of 0.744 (95% CI: 0.643–0.846) for AUC diagnose nephropathy from healthy with a sensitivity and specificity of 75.86%, 70.45%, respectively (Table 3; Fig. 3). The Youden index was 0.463.
Table 3.
The optimal diagnostic cut-off values of Emax and Emean measured by STE to diagnose nephropathy
| Cut-off value | Sensitivity (%) | Specificity (%) | Youden index | |
|---|---|---|---|---|
| STE (kPa) | ||||
| Emean | 10.16 | 75.86 | 70.45 | 0.463 |
| Emax | 13.53 | 82.76 | 59.09 | 0.418 |
STE sound touch elastography, Emax maximum elastic value, Emean mean elastic value
Fig. 3.
ROC curve of STE measurements for Emax (AUC= 0.718) and Emean (AUC= 0.744) values to diagnose nephropathy. STE sound touch elastography, ROC receiver operating characteristic, Emax maximum elastic value, Emean mean elastic value
Renal pathological features
The kidney biopsy findings showed that 24 patients had mild renal impairment, 27 had moderate and 9 severe. STE measurements of three renal impairment levels demonstrated significantly different Emean and Emax values for right kidney (Table 4); In addition, no significant difference of Emean or Emax values measured by STQ for right kidney was found among those different renal impairment levels.
Table 4.
Comparison of Emax and Emean values measured by STE and STQ in patients with different pathological stages of nephropathy
| Group | STE (kPa) | STQ (kPa) | ||
|---|---|---|---|---|
| Emean | Emax | Emean | Emax | |
| Control group (n = 45) | 9.83 ± 3.21 | 14.70 ± 4.61 | 10.65 ± 2.86 | 17.19 ± 4.98 |
| Renal pathological stage (n = 60) | ||||
| Mild (n = 24) | 11.65 ± 2.36 | 16.79 ± 3.74 | 10.84 ± 2.44 | 19.21 ± 4.76 |
| Moderate (n = 27) | 12.39 ± 3.35 | 18.47 ± 4.97 | 11.85 ± 3.64 | 18.88 ± 4.99 |
| Severe (n = 9) | 12.69 ± 7.43 | 18.58 ± 10.45 | 11.98 ± 4.55 | 19.45 ± 9.85 |
| F value | 3.779 | 3.535 | 1.077 | 1.071 |
| p | 0.013* | 0.017* | 0.362 | 0.365 |
Relationship between SWE values and clinical parameters in proteinuria cases
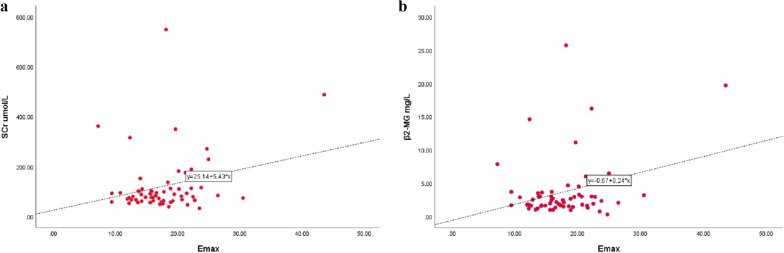
There was a significantly positive correlation between Emax value for STE and Scr, β2-MG (r = 0.257, 0.292, p < 0.05) (Fig. 4). The correlation between Emax value for STE and eGFR (r = −0.135, p = 0.304) or 24-HUP (r = −0.05, p = 0.703) was negative, not significant. Furthermore, a positive insignificant correlation was observed between Emean values for STE and Scr, β2-MG (r = 0.128, 0.240, p = 0.328, 0.065), and a negative insignificant correlation between Emean for STE and eGFR, 24-HUP (r = −0.054, −0.117, p = 0.683, 0.374).
Fig. 4.
The Emax value for STE was positively related to Scr, β2-MG in the case group (r = 0.257, 0.292, p < 0.05). STE sound touch elastography, Emax maximum elastic value, SCr blood creatinine, β2-MG β2-microglobulin
No correlations between Emean value for STQ and Scr, β2-MG, eGFR or 24-HUP. Meanwhile, there was no relationship between Emax values for STQ and Scr, β2-MG, eGFR or 24-HUP.
Discussion and conclusions
In the present study, we found Emax and Emean measured by STE were significantly different between cases and controls. Additionally, both Emax and Emean values gradually increased as the renal impairment progressed from mild, moderate to severe. Our findings are consistent with another study which investigated 75 CKD patients undergoing renal biopsy and SWE [23]. It revealed Young’s modulus was associated with the patient's renal pathological scores, particularly with tubulointerstitial score (ρ = 0.442, p < 0.001) and glomerular score (ρ = 0.375, p = 0.001). The relationship between renal fibrosis and stiffness is still unclear. One study confirmed a positive relationship between renal fibrosis score and SWV [19], while another study showed no association existed [24]. It is likely the decline of renal blood perfusion played a stronger role than tissue fibrosis in renal stiffness [25]. Controversially, another study concluded that there was no relationship between SWV and renal fibrosis or blood perfusion [24].
Unlike the STE findings, Emax and Emean measured by STQ were not significantly different between cases and controls in this study. We speculated this discrepancy was due to different STE and STQ mechanisms. Compared with STE, STQ usually indicates the average rather than maximum elastic modulus of the ROI. Conversely, STE provides the maximum elastic modulus of ROI, displays a real-time colored stiffness map and reduces the influence of reverberation artifact [20, 21].Thus STE is superior to STQ in reflecting renal stiffness.
Elasticity can be measured from an ROI and can be displayed as the maximum (Emax), and mean (Emean) of Young’s modulus elasticity measurements. One study found that both Emax and Emean were effective to investigate breast lesions. Two other studies demonstrated that Emax was a better SWE parameter than Emean to distinguish benign from malignant breast lesions [26, 27]. To the best of our knowledge, this is the first study to compare STE performance with that of STQ for renal stiffness. We performed STE measurements and found Emax had a higher sensitivity than Emean (STE: 82.76 vs. 75.86%). However, the Youden index was low (STE: 0.418). The Emax cut-off value (STE: 13.53 kPa) suggested a considerable diagnostic accuracy and high false negative rate.
In this study, the association between elastic and clinical parameters was complicated, making it difficult to draw a definitive conclusion. Similarly, both positive and negative relationship between SWV and eGFR was found in various papers [28, 14]. It is reasonable to assume that renal stiffness is not dramatically correlated with Scr, β2-MG, eGFR, or 24hUP, which probably results from diverse pathological changes. Thus it’s essential to subgroup pathological types in further research.
There are some limitations to this study. Breathing and excess belly fat might affect the reliability of SWE results; we didn’t analyze the impact of age, blood perfusion and pressure or cardiovascular diseases on renal stiffness; the sample size is small. It is necessary to compare different pathological types in a larger population in future work.
In conclusion, both STE and STQ are promising, non-invasive, feasible methods to quantitatively evaluate renal stiffness for CKD patients with proteinuria. STE is more effective than STQ to assess renal stiffness.
Abbreviations
- ARFI
Acoustic radiation force impulse
- AUC
Area under the receiver operating characteristic curve
- BMI
Body mass index
- CI
Confidence interval
- CKD
Chronic kidney disease
- eGFR
Estimated glomerular filtration rate
- Emax
Maximum elastic modulus
- Emean
Mean elastic modulus
- 24-HUP
24-Hour urine protein
- IQR
Interquartile range
- ROC
Receiver operating characteristic curves
- ROI
Region of interest
- SCr
Serum creatinine
- SD
Standard deviation
- STE
Sound touch elastography
- STQ
Sound touch quantification
- SWE
Shear wave elastography
- SWV
Shear wave velocity
- US
Ultrasonograhy
- β2-MG
β2-Microglobulin
Author contributions
RZ and WM conceived the study and designed the experimental process. RZ, XZ, SX and WT carried out sample collection. RZ, HL and LY carried out data analysis and supervised this study. RZ and WM wrote the manuscript and interpretation of data.
Funding
This work was financially supported by the Clinical Medical Science and Technology Innovation Plan of Jinan Municipal Bureau of Science and Technology (202019042).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent participate
This study was approved by the Ethics Committee of Second Hospital of Shandong University. Informed consent was obtained from each participant or guardian.
Consent for publication
A consent to publication form has been signed by each participant or guardian.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kipp R, Kellerman PS. Chronic kidney disease. In: Moorthy AV, editor. Pathophysiol Kidney dis hypertens. Philadelphia: Elsevier; 2009. pp. 145–157. [Google Scholar]
- 2.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/S0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 4.Fogo AB, Kon V (2001) Pathophysiology of progressive renal diseases—an overview. In: Immunologic renal diseases, 2nd edn. LippincottWilliams & Wilkins, pp 55–72
- 5.López-Novoa JM, Rodríguez-Peña AB, Ortiz A, et al. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications. J Transl Med. 2011;9(1):13. doi: 10.1186/1479-5876-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen J, Lindequist S, Knudsen DU, et al. Ultrasound-guided renal biopsy with biopsy gun technique–efficacy and complications. Acta Radiol. 1995;36(3):276–279. doi: 10.1177/028418519503600313. [DOI] [PubMed] [Google Scholar]
- 7.Tuma J. The focal renal lesions. Praxis. 2013;102(12):731–740. doi: 10.1024/1661-8157/a001319. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita SR, von Atzingen AC, Iared W, et al. Value of renal cortical thickness as a predictor of renal function impairment in chronic renal disease patients. Radiol Bras. 2015;48:12–16. doi: 10.1590/0100-3984.2014.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin HY, Lee YL, Lin KD, et al. Association of renal elasticity and renal function progression in patients with chronic kidney disease evaluated by real-time ultrasound elastography. Sci Rep. 2017;7:43303. doi: 10.1038/srep43303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sicari R, Gargani L, Wiecek A, et al. The use of echocardiography in observational clinical trials: the EURECA-m registry. Nephrol Dial Transplant. 2013;28(1):19–23. doi: 10.1093/ndt/gfs399. [DOI] [PubMed] [Google Scholar]
- 11.Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 12.KDOQI Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Xia SJ, Ren XP, Ni ZX, et al. A noninvasive method-shear-wave elastography compared with transient elastography in evaluation of liver fibrosis in patients with chronic hepatitis B. Ultrasound Q. 2019;35:147–152. doi: 10.1097/RUQ.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 14.Leong SS, Wong JHD, Md Shah MN et al (2018) Shear wave elastography in the evaluation of renal parenchymal stiffness in patients with chronic kidney disease. Br J Radiol 91(1089):20180235 [DOI] [PMC free article] [PubMed]
- 15.Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95:5281–5288. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 16.Zhou WC, Ma XJ, Pan L, et al. Application of conventional ultrasound coupled with virtual touch tissue imaging and quantification in the assessment of muscle strength. Ann Palliat Med. 2020;9:3402–3409. doi: 10.21037/apm-20-1715. [DOI] [PubMed] [Google Scholar]
- 17.Hassan K, Loberant N, Abbas N, et al. Shear wave elastography imaging for assessing the chronic pathologic changes in advanced diabetic kidney disease. Ther Clin Risk Manag. 2016;12:1615–1622. doi: 10.2147/TCRM.S118465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui G, Yang Z, Zhang W, et al. Evaluation of acoustic radiation force impulse imaging for the clinicopathological typing of renal fibrosis. Exp Ther Med. 2014;71:233–235. doi: 10.3892/etm.2013.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Q, Wang XY, He HG, et al. Acoustic radiation force impulse imaging for noninvasive assessment of renal histopathology in chronic kidney disease. PLoS One. 2014;912:e115051. doi: 10.1371/journal.pone.0115051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song P, Zhao H, Manduca A, et al. Comb-push ultrasound shear elastography (CUSE): a novel method for two-dimensional shear elasticity imaging of soft tissues. IEEE Trans Med Imaging. 2012;31(9):1821–1832. doi: 10.1109/TMI.2012.2205586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigrist R, Liau J, Kaffas AE, et al. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7(5):1303–1329. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katafuchi R, Kiyoshi Y, Oh Y, et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clin Nephrol. 1998;49:1–8. [PubMed] [Google Scholar]
- 23.Leong SS, Wong J, Md SM, et al. Shear wave elastography accurately detects chronic changes in renal histopathology. Nephrology (Carlton) 2021;26(1):38–45. doi: 10.1111/nep.13805. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Xia P, Lv K, et al. Assessment of renal tissue elasticity by acoustic radiation force impulse quantification with histopathological correlation: preliminary experience in chronic kidney disease. Eur Radiol. 2014;24(7):1694–1699. doi: 10.1007/s00330-014-3162-5. [DOI] [PubMed] [Google Scholar]
- 25.Asano K, Ogata A, Tanaka K, et al. Acoustic radiation force impulse elastography of the kidneys: is shear wave velocity affected by tissue fibrosis or renal blood flow? J Ultrasound Med. 2014;33(5):793–801. doi: 10.7863/ultra.33.5.793. [DOI] [PubMed] [Google Scholar]
- 26.Shi XQ, Li JL, Li QY, et al. Performance of ultrasonic shear wave elastography in assessing benign and malignant breast lesions. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:294–299. doi: 10.3881/j.issn.1000-503X.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Berg WA, Cosgrove DO, Dore CJ, et al. BE1 Investigators. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435–449. doi: 10.1148/radiol.11110640. [DOI] [PubMed] [Google Scholar]
- 28.Grosu I, Bob F, Sporea I, et al. Correlation of point shear wave velocity and kidney function in chronic kidney disease. J Ultrasound Med. 2018;37(11):2613–2620. doi: 10.1002/jum.14621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.