Abstract
Background
Olive oil consumption may reduce breast cancer risk, but it is unclear whether olive oil is beneficial for breast cancer prevention in populations outside of Mediterranean regions, namely in the U.S., where the average consumption of olive oil is low compared with Mediterranean populations. We examined whether olive oil intake was associated with breast cancer risk in two prospective cohorts of U.S. women.
Methods
We used multivariable-adjusted time-varying Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence interval (CI) for breast cancer among 71,330 (Nurses’ Health Study, 1990–2016) and 93,295 women (Nurses’ Health Study II, 1991–2017) who were free of cancer at baseline. Diet was assessed by a validated semi-quantitative food frequency questionnaire every 4 years.
Results
During 3,744,068 person-years of follow-up, 9,638 women developed invasive breast cancer. The multivariable-adjusted HR (95% CI) for breast cancer among women who had the highest consumption of olive oil (>1/2 tablespoon/d or >7 g/d) compared with those who never or rarely consumed olive oil, was 1.01 (0.93, 1.09). Higher olive oil consumption was not associated with any subtype of breast cancer.
Conclusion
We did not observe an association between higher olive oil intake and breast cancer risk in two large prospective cohorts of U.S. women, whose average olive oil consumption was low. Prospective studies are needed to confirm these findings and to further investigate whether different varieties of olive oil (e.g., virgin and extra virgin olive oil) may play a role in breast cancer risk.
Subject terms: Cancer epidemiology, Risk factors
Introduction
The Mediterranean Diet is considered one of the dietary patterns with the greatest accumulated scientific evidence regarding its benefits in human health. It is characterized by a high consumption of plant-based foods (vegetables, legumes, fruit, nuts, whole grains), fish and especially the use of olive oil (both for cooking and dressing) [1]. This diet has been associated with excellent health, as seen by a reduction in overall mortality, mortality from cardiovascular disease, incidence of or mortality from cancer, and incidence of other chronic diseases [2, 3]. A question of interest is the role that olive oil, as a major energy source in the Mediterranean diet, plays in the reported health benefits of this dietary pattern. Olive oil is high in monounsaturated fatty acids (MUFA), especially oleic acid, and has more than 200 minor compounds. Some of these are highly bioactive, including phenolic compounds (phenolic alcohols e.g., oleuropein, tyrosol and hydroxytyrosol; the secoiridoids, oleuropein, oleocanthal and oleacein; oleanolic acid, maslinic acid, lignans, eg, pinoresinol; and other bioactive phenols), flavonoids, triterpenes and vitamin E, and may contribute to olive oil’ anti-inflammatory and antioxidant properties [4–6]. Nonetheless, the specific components depend, to a large extent, on the quality of the oil (physicochemical and organoleptic characteristics).
Although most of the evidence linking olive oil consumption and risk of chronic diseases comes from large prospective cohort studies and large clinical trials from Mediterranean or other European countries [7–9], olive oil consumption has also been inversely associated with risk of type 2 diabetes [10], cardiovascular disease risk [11] and total mortality [12] in non-Mediterranean populations. In the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS), we observed inverse associations between olive oil consumption and total cancer mortality in both women and men even though we could not distinguish between the consumption of common olive oil (mostly refined and devoid of phenolic compounds) vs. virgin olive oil (VOO) or extra-virgin olive oil (EVOO), rich in these bioactive compounds, thus resulting in substantial variability in the profile and quality of the oil consumed.
Recent evidence supports a protective role of olive oil against the development of several types of cancer, including breast cancer [13]. In a secondary analysis of the Prevención con Dieta Mediterránea (PREDIMED) randomized trial, although based on a few incident cases, women allocated to a Mediterranean diet supplemented with a free provision of EVOO (which accounted for 22% of total caloric intake or ≥50 g per day) showed a 62% lower risk of invasive breast cancer compared to those allocated to a low-fat control diet [14]. This trial provided first-level scientific evidence on breast cancer protective properties of EVOO within the context of the Mediterranean diet. In a subset of cohorts from Spain, Greece, and Italy belonging to the European Prospective Investigation into Cancer and Nutrition (EPIC) Mediterranean countries, no association was observed between olive oil consumption and risk of estrogen receptor (ER)- or progesterone receptor (PR)-positive tumors among postmenopausal women, but a suggestion of an inverse association with ER- and PR-negative tumors [15]. However, no large prospective cohort studies have been conducted outside the Mediterranean region.
Based on observational studies, the association between olive oil and breast cancer is unclear. A recent systematic review pooled the results of 14 studies and reported an inverse association between olive oil consumption and breast cancer risk, although the association was reproducible in case-control but not in cohort studies [16]. Thus, there is need for additional prospective studies with better assessment of olive oil intake [17].
To our knowledge, no large prospective studies have examined the association between total olive oil consumption and invasive breast cancer risk in the U.S. population, where the average consumption of olive oil is considerably lower than that in Mediterranean countries but has increased in recent years. For example, in the NHS, mean consumption of olive oil increased from 1.6 g/d in 1990 to about 4 g/d in 2010, while the intake of other fats remained stable (except for margarine, which decreased) [12]. Therefore, we longitudinally examined the association between olive oil consumption and breast cancer risk in women from the NHS and NHSII. Moreover, given the paucity of data about differential effects by menopausal status and expression of hormone receptors, we examined the association of olive oil intake with different subtypes of breast cancer.
Methods
Study population
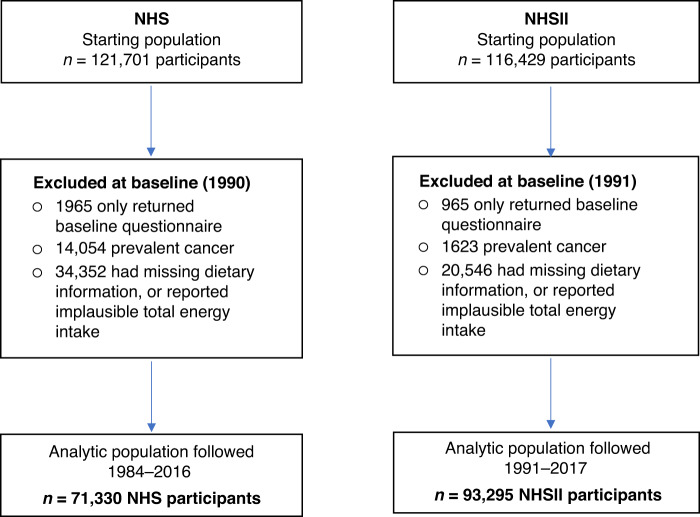
The NHS is an ongoing study of 121,701 female nurses aged 30–55 years in 1976, and NHSII has followed 116,429 female nurses (aged 25–42 years) since 1989. Every two years, participants have provided information on health-related factors and medical history. For this analysis, women in the NHS were followed from 1990 (when olive oil was first included as part of the food frequency questionnaire [FFQ]) to 2016, and in the NHSII women were followed from 1991 when use of olive oil was first asked to 2017. We excluded women who died prior to baseline, had prevalent cancer, had missing dietary information, or reported implausible total energy intake (<600 or >3500 kcal/day); leaving 71,330 women from NHS, and 93,295 from NHSII (Fig. 1). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating tumor registries as required.
Fig. 1. Flowchart of study population.
NHS Nurses’ Health Study, NHSII Nurses’ Health Study II.
Diet assessment
Diet was assessed with semi-quantitative FFQs with ≥130 items administered in the NHS and NHSII every 4 y. The FFQs included foods with a specified portion size, and participants were asked to indicate the average consumption of each food during the previous year (from among 9 choices ranging from “almost never” to “6 or more/day”).
Participants were asked how often, on average, they had consumed specific foods, as well as types of fats, oils, and brand or type of oils used for cooking and added at the table in the preceding year. Total olive oil intake was calculated from the sum of three items pertaining to consumption of olive oil: olive oil use for salad dressings, olive oil added to food or bread, and olive oil used for baking and frying at home. One tablespoon is equivalent to 13.5 g of olive oil. Other vegetable oil consumption (e.g., corn, safflower, soybean, canola) was calculated based on the participant’s reported oil brand and type of fat used for cooking at home, including frying, sautéing, baking, and salad dressing. Data on homemade baked items and frying fats used at home were also incorporated. Total margarine was calculated based on the reported frequency of stick, tub, or soft margarine consumption and the amount of margarine added from baking and frying at home. The consumption of butter was calculated using the same approach. Intakes of dairy and other fats and nutrients were calculated based on the U.S. Department of Agriculture (USDA) and Harvard University Food Composition Database and our biochemical analyses.
Breast cancer ascertainment
We first identified incident invasive breast cancer cases from biennial questionnaires. We requested permission from women reporting breast cancer to review hospital records and pathology reports for diagnosis confirmation and ascertainment of invasive vs. in situ, and ER, PR, and human epidermal growth factor receptor 2 (HER2) status. Given the high confirmation rate of reported breast cancer cases in the NHS and NHSII (>99%), we included both breast cancer cases confirmed via medical record review and self-reported cases confirmed by the nurse but lacking medical records. For deceased cases, the next of kin was contacted for permission. Deaths were reported by family members or by the postal service in response to follow-up questionnaires, or they were identified through the National Death Index.
Details of breast cancer tissue block collection and tumor microarray (TMA) construction have been reported previously [18]. Briefly, we collected archived formalin-fixed paraffin-embedded breast cancer blocks from participants with incident breast cancer diagnosed up through 2008. For molecular subtype classification, immunohistochemical staining information was available for the markers of ER, PR, HER2, cytokeratin 5/6 and epidermal growth factor receptor. Further staining for the proliferative marker Ki-67 was achieved in NHS cases; Ki-67 data were not available for NHSII cases. Cases with TMAs were very similar to all suitable invasive cases in terms of demographics, breast cancer risk factors and tumor characteristics.
Definitions that correlated with gene expression profile classifications were used for tumor molecular subtyping for a subgroup of cases. If Ki-67 expression data was missing (NHSII tumors), histological grade was used instead. Hence, luminal A tumors were ER-positive and/or PR-positive, HER2-negative, and Ki-67-negative (or histologic grade 1 or 2). Luminal B tumors were either (a) ER-positive and/or PR-positive and HER2-positive or (b) ER-positive and/or PR-positive, HER2-negative, and Ki-67-positive (or histologic grade 3). HER2-enriched tumors were ER-negative, PR-negative, and HER2-positive. Basal-like tumors were ER-negative, PR-negative, HER2-negative and CK 5/6-positive and/or EGFR-positive. For evaluating ER-positive vs. ER-negative tumors, ER status was determined primarily from TMA slides, and if not available, secondarily from pathology reports.
Ascertainment of covariates
Information on lifestyle and other potential risk factors were collected at baseline and updated biennially during follow-up through self-administered questionnaires, including ethnicity (white, non-white), Southern European/Mediterranean ancestry (yes, no), age at menarche (<12, 12, 13, 14, >14 years), menopausal status and age at menopause (premenopausal, <45, 45–49, 50–52, ≥53 years, unknown), postmenopausal hormone use (never user, past user, current user– estrogen only for <5 years, current user – estrogen only for ≥5 years, current estrogen + progestin user for <5 years, current estrogen + progestin user for ≥5 years, current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, 2+ children before age 25, 2+ children ≥25 years of age), breastfeeding history (never, breastfed for ≤6 months, breastfed for >6 months), family history of breast cancer (yes, no), history of biopsy-confirmed benign breast disease (yes, no), height (<1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, ≥1.75 m), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (continuous metabolic equivalents, MET-hours/week), neighborhood-based socioeconomic status indicator (continuous), BMI at age 18 (<20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0 kg/m2), weight change since age 18 (kg, continuous) alcohol consumption (0 g/d, ≥0- < 5 g/d, ≥5.0- < 15 g/d, ≥15.0 g/d), and diet quality (Alternate Healthy Eating Index, AHEI-2010, without the alcohol and polyunsaturated fatty acids components, quintiles).
Statistical analysis
We calculated person-years of follow-up from the date of the return of the baseline questionnaire until the date of breast cancer diagnosis, other cancers (excluding non-melanoma skin cancers), or the end of follow-up (June 2016 for NHS, June 2017 for NHSII), whichever occurred first. To better represent long-term exposures and reduce within-person variation, cumulative averages of total olive oil intake were computed from all previous questionnaires up to the start of each 2-year follow-up interval. Similarly, the cumulative average intake of other covariates, when appropriate, was created to best reflect long-term food intake and lifestyle, and to minimize within-person variation.
To maximize the statistical power, data from the NHS and NHSII were pooled. Age-adjusted and multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CI) were calculated using time-varying Cox proportional hazard models according to categories of cumulative average of olive oil intake. To evaluate whether associations differed by molecular subtype or ER status, we used the Lunn-McNeil approach to derive the p for heterogeneity [19]. All analyses were stratified by cohort and calendar year and age in months was the underlying time scale, enabling the finest possible control of confounding for age and secular trends. In the multivariable-adjusted model, we included race/ethnicity, Mediterranean or Southern European ancestry, socioeconomic status, age at menarche, oral contraceptive use, parity, age at first birth, breastfeeding history, age at menopause, postmenopausal hormone use, family history of breast cancer, benign breast disease, height, BMI at age 18, total caloric intake, diet quality (AHEI-2010), physical activity, alcohol intake and weight change from age 18 years (for categorizations see Ascertainment of Covariates above). We conducted a Wald test for trend, using the median value of each quintile, modeled as a continuous variable.
We performed several sensitivity analyses to test the robustness of our findings. First, we used the average of the last two most recent measure of the diet instead of using cumulative intakes of olive oil. Second, we tested whether olive oil consumption and risk of breast cancer varied by selected traditional risk factors: BMI; physical activity; menopausal status; ancestry (Southern European/Mediterranean vs. other) and dietary quality (AHEI-2010 and Alternate Mediterranean Diet Score, AMED). Third, to take advantage of repeated diet assessments in these cohorts and evaluate the latency between olive oil consumption and breast cancer incidence, we conducted separate Cox models at different lag periods, with the risk of invasive breast cancer. In the simple update model, olive oil consumption reported on the most recent FFQ before each follow-up interval was used; in the latency models, we used olive oil consumption reported at different latencies (i.e., 4–8, 8–12, 12–16, 16–20 y) before breast cancer diagnosis.
Statistical tests were two-sided with P-values < 0.05 indicating statistical significance. All analyses were performed using SAS for UNIX version 9.4 (SAS Institute, Cary, NC).
Results
During 3,744,068 person-years of follow-up, we documented 9,638 incident breast cancer cases in the Nurses’ Health Studies. The baseline characteristics of the participants according to total olive oil consumption in NHS and NHSII are shown in Table 1. Compared with women who never consumed olive oil, those who had higher olive oil intake were slightly older, tended to exercise more, had a lower BMI, had lower weight gain since age 18, and were more likely to have Southern European/Mediterranean ancestry. They also had higher total energy intakes and higher diet quality (as measured by AHEI-2010 and AMED).
Table 1.
Age-standardized characteristics of the study population by olive oil consumption in the Nurses’ Health Study (1990) and Nurses’ Health Study II (1991).
| Nurses’ Health Study | Nurses’ Health Study II | |||||||
|---|---|---|---|---|---|---|---|---|
| Never or < 1 per month | >0– ≤ 4.5 g/d (>0 to ≤ 1 teaspoon) | >4.5– ≤7 g/d > 1 teaspoon to ≤ 1/2 TBS) | >7 g/d (>1/2 TBS) | Never or < 1 per month | >0– ≤ 4.5 g/d (>0 to ≤ 1 teaspoon) | >4.5– ≤ 7 g/d > 1 teaspoon to ≤ 1/2 TBS) | >7 g/d (>1/2 TBS) | |
| Number of participants | 38,003 | 26,730 | 2843 | 3754 | 54,989 | 33,569 | 2454 | 2283 |
| Age, y | 57 (7) | 57 (7) | 57 (7) | 57 (7) | 37 (5) | 37 (5) | 37 (5) | 38 (4) |
| Total olive oil (g/d) | 0 (0) | 1.5 (1.2) | 5.8 (0.5) | 9 (4.6) | 0 (0) | 1.4 (1.1) | 5.8 (0.4) | 8.9 (4.6) |
| Butter (g/d) | 1.1 (3) | 1.3 (3.1) | 1.6 (3.3) | 1.7 (3.8) | 0.8 (2.3) | 1.3 (2.8) | 1.7 (3.1) | 1.8 (3.4) |
| Body mass index, kg/m2 | 26 (5) | 25.5 (4.7) | 25.4 (4.6) | 25.4 (4.6) | 24.8 (5.5) | 24.1 (5) | 23.9 (5) | 24.1 (5) |
| Body mass index at age 18 years, kg/m2 | 21.5 (3.1) | 21.2 (2.8) | 21.2 (2.7) | 21.3 (2.9) | 21.4 (3.4) | 21.1 (3.2) | 21.2 (3.2) | 21.3 (3.2) |
| Weight change from age 18 years, kg | 11.9 (12.3) | 11.6 (11.5) | 11.3 (11.1) | 11.1 (11.2) | 9.3 (11.8) | 8 (10.8) | 7.4 (11.1) | 7.4 (11) |
| Height, m | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) |
| Ethnicity, white, % | 97.4 | 98.1 | 98.6 | 98.8 | 96.1 | 96.8 | 97.8 | 97.4 |
| Southern European or Mediterranean ancestry, % | 14.7 | 18.9 | 24.8 | 28.2 | 15.5 | 20.6 | 27.2 | 31.3 |
| Former smoker, % | 36.0 | 41.5 | 45.7 | 48.1 | 19.3 | 25.9 | 30.3 | 32.5 |
| Current smoker, % | 16.8 | 16.8 | 16.1 | 13.6 | 12.2 | 12.1 | 13.6 | 12.1 |
| Self-reported history of diabetes (%) | 5.2 | 4.2 | 4.3 | 4.4 | 1.1 | 0.8 | 1.0 | 0.8 |
| Family history of breast cancer (%) | 10.5 | 10.4 | 10.8 | 10.9 | 5.7 | 6.2 | 6.2 | 7.7 |
| Biopsy-confirmed benign breast disease (%) | 29.3 | 30.7 | 30.9 | 31.6 | 9.6 | 9.6 | 10.2 | 10.5 |
| Age at menarche <12 years (%) | 22.2 | 22.5 | 23.3 | 23.6 | 24.6 | 24.1 | 27.0 | 27.3 |
| Oral contraceptive use, ever (%) | 48.9 | 50.7 | 51.5 | 48.7 | 84.9 | 84.5 | 83.0 | 81.5 |
| Parity, na | 3.2 (1.5) | 3.2 (1.5) | 3.1 (1.5) | 3.1 (1.5) | 2.2 (0.9) | 2.1 (0.9) | 2 (0.9) | 2.1 (0.9) |
| Nulliparous, % | 5.3 | 5.6 | 6.6 | 6.4 | 22.6 | 29.3 | 31.4 | 30.4 |
| Breastfeeding, >6 months (%)a | 27.4 | 28.9 | 30.4 | 28.4 | 60.4 | 65.8 | 68.1 | 68.3 |
| Postmenopausal (%) | 69.7 | 69.8 | 70.1 | 69.7 | 3.5 | 2.8 | 2.0 | 2.3 |
| Postmenopausal hormone use, never (%)b | 40.6 | 38.3 | 37.3 | 39.6 | 6.9 | 7.6 | 10.1 | 9.5 |
| Physical activity, MET-h/week | 14.4 (19.8) | 16.6 (22.7) | 18.4 (23.1) | 18.9 (26.8) | 19.1 (25.2) | 22.7 (29) | 26.7 (31.7) | 27.9 (36.1) |
| Alcohol intake, g/d | 4.1 (8.6) | 5.9 (10.1) | 7.2 (10.9) | 7.2 (10.9) | 2.4 (5.3) | 4 (6.7) | 5.1 (7.3) | 5.3 (7.9) |
| Total calories, kcal/d | 1701 (506) | 1760 (510) | 1909 (519) | 1991 (538) | 1751 (542) | 1821 (549) | 1982 (547) | 2070 (548) |
| AHEI-2010 score | 41.8 (10.3) | 44.3 (10.2) | 46.5 (10.2) | 47.4 (10.2) | 37.1 (9.8) | 40.9 (10) | 44 (10.2) | 45.8 (10.3) |
| AMED score | 3.2 (1.7) | 3.8 (1.7) | 4.3 (1.7) | 4.4 (1.6) | 3.6 (1.6) | 4.2 (1.6) | 4.8 (1.6) | 4.9 (1.5) |
AHEI Alternative Healthy Eating Index (without the alcohol and polyunsaturated fatty acids components, scored as 0–90, with higher scores representing healthier diets), AMED Alternate Mediterranean diet (without MUFA/SFA ratio), MET metabolic equivalent task, TBS tablespoon. Values are presented as mean (SD) or percentage, standardized to the age distribution of the study population.
aAmong parous women only.
bAmong postmenopausal women.
In age-adjusted models, total olive oil intake was associated with increased invasive breast cancer risk in the NHSII and in the pooled analysis (Table 2). Multivariate adjustment for covariates (especially hormonal and reproductive factors) attenuated the association which became non-significant. The HR (95% CI) was 1.01 (0.93, 1.09) for participants consuming >7 g olive oil/d compared with those who never/almost never consumed olive oil (P-trend = 0.81). When the models for total olive oil were adjusted for weight change since age 18, the estimates for breast cancer risk were consistent (data not shown). Furthermore, olive oil intake was not associated with the risk of breast cancer when we used an average of the last two most recent measurements instead of using the cumulative average intake.
Table 2.
HR (95% CI) of invasive breast cancer according to categories of total olive oil in the Nurses’ Health Study (cases = 5725), Nurses’ Health Study II (cases = 3913) and pooled data (cases = 9638).
| Never or <1 per month | >0– ≤ 4.5 g/d ( > 0 to ≤1 teaspoon) | >4.5– ≤ 7 g/d( > 1 teaspoon to ≤1/2 TBS) | >7 g/d ( > 1/2 TBS) | P-trend | HR (95% CI) for 5 g increase in olive oil intake | |
|---|---|---|---|---|---|---|
| Cumulative average intake | ||||||
| NHS | ||||||
| N° cases/Person-years | 1,546/421869 | 2,935/752465 | 536/126727 | 708/179293 | ||
| Age-adjusted model | 1 (ref.) | 1.06 (1.00, 1.13) | 1.14 (1.03, 1.26) | 1.06 (0.97, 1.17) | 0.22 | 1.01 (0.99, 1.03) |
| Multivariable model | 1 (ref.) | 1.02 (0.95, 1.09) | 1.07 (0.96, 1.19) | 0.99 (0.89, 1.09) | 0.89 | 1.00 (0.98, 1.02) |
| NHSII | ||||||
| N° cases/Person-years | 835/677172 | 2,200/1177561 | 393/185729 | 485/223253 | ||
| Age-adjusted model | 1 (ref.) | 1.20 (1.11, 1.31) | 1.24 (1.09, 1.40) | 1.23 (1.09, 1.38) | 0.03 | 1.03 (1.01, 1.05) |
| Multivariable model | 1 (ref.) | 1.08 (0.99, 1.18) | 1.08 (0.94, 1.23) | 1.04 (0.91, 1.18) | 0.81 | 1.01 (0.98, 1.03) |
| Pooled data | ||||||
| N° cases/Person-years | 2,381/1099042 | 5,135/1930025 | 929/312456 | 1,193/402546 | ||
| Age-adjusted model | 1 (ref.) | 1.11 (1.06, 1.17) | 1.17 (1.08, 1.27) | 1.12 (1.04, 1.21) | 0.02 | 1.02 (1.00, 1.04) |
| Multivariable model | 1 (ref.) | 1.04 (0.99, 1.10) | 1.07 (0.99, 1.16) | 1.01 (0.93, 1.09) | 0.81 | 1.00 (0.99, 1.02) |
| Average level of the last two measurements | ||||||
| NHS | ||||||
| N° cases/Person-years | 1,860/504057 | 2,446/622335 | 445/116566 | 974/237395 | ||
| Age-adjusted model | 1 (ref.) | 1.08 (1.01, 1.15) | 1.03 (0.92, 1.14) | 1.10 (1.01, 1.19) | 0.14 | - |
| Multivariable model | 1 (ref.) | 1.03 (0.97, 1.10) | 0.97 (0.87, 1.08) | 1.04 (0.95, 1.13) | 0.83 | - |
| NHSII | ||||||
| N° cases/Person-years | 1,106/837476 | 1,487/805866 | 402/204783 | 918/415590 | ||
| Age-adjusted model | 1 (ref.) | 1.23 (1.13, 1.33) | 1.16 (1.03, 1.30) | 1.24 (1.13, 1.36) | 0.004 | - |
| Multivariable model | 1 (ref.) | 1.14 (1.05, 1.23) | 1.04 (0.92, 1.17) | 1.10 (0.99, 1.21) | 0.81 | - |
| Pooled | ||||||
| N° cases/Person-years | 2,966/1341533 | 3,933/1428201 | 847/321350 | 1892/652984 | ||
| Age-adjusted model | 1 (ref.) | 1.13 (1.08, 1.19) | 1.08 (1.00, 1.16) | 1.16 (1.09, 1.23) | <0.01 | - |
| Multivariable model | 1 (ref.) | 1.07 (1.02, 1.13) | 1.00 (0.92, 1.08) | 1.06 (0.99, 1.13) | 0.70 | - |
Results are expressed as Hazard Ratios (HR) and 95% Confidence Intervals (95% CI).
Multivariable model: adjusted for ethnicity (white, non-white), Southern European/Mediterranean ancestry (yes, no), age at menarche ( < 12, 12, 13, 14, >14 years), menopausal status and age at menopause (premenopausal, <45, 45–49, 50–52, 53 + , unknown), postmenopausal hormone use (never user, past user, current user– estrogen only for <5 years, current user – estrogen only for ≥5 years, current estrogen + progestin user for < 5 years, current estrogen + progestin user for ≥5 years, current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, 2+ children before age 25, 2+ children ≥25 years of age), breastfeeding history (never, breastfed for ≤ 6 months, breastfed for > 6 months), family history of breast cancer (yes or no), history of biopsy-confirmed benign breast disease (yes or no), height ( < 1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, 1.75 + m), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (continuous MET-hours/week), neighborhood-based socioeconomic status indicator (continuous), BMI at age 18 ( < 20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0) and AHEI-2010 (without alcohol and polyunsaturated fatty acids components, quintiles).
NHS Nurses’ Health Study, NHSII Nurses’ Health Study II.
There was no association between olive oil and the risk of ER-negative (HR, 1.01; 95% CI, 0.81, 1.25; P-trend = 0.69) or positive tumors (HR, 1.03; 95% CI, 0.93, 1.13; P-trend = 0.71), or tumor molecular subtypes (luminal A, luminal B, basal-like or HER2-enriched) when comparing extreme categories of consumption (Table 3). In sensitivity analyses, we further adjusted for weight change since age 18 years (data not shown), but the results remained unchanged.
Table 3.
HRs and 95% CIs for the association categories of cumulative average intake of total olive oil and breast cancer tumor subtypes in the NHS and NHSII.
| Never or <1 per month | >0– ≤ 4.5 g/d ( >0 to ≤1 teaspoon) | >4.5– ≤7 g/d ( >1 teaspoon to ≤1/2 TBS) | >7 g/d ( >1/2 TBS) | P- trend | |
|---|---|---|---|---|---|
| ER-negative breast cancer (case n = 1416) | |||||
| N° cases/ Person-years | 386/1100933 | 759/1934091 | 127/313200 | 144/403532 | |
| Age-adjusted model | 1 (Ref.) | 1.16 (1.02, 1.32) | 1.19 (0.97, 1.46) | 1.03 (0.84, 1.25) | 0.80 |
| Multivariable model | 1 (Ref.) | 1.13 (0.98, 1.28) | 1.16 (0.93, 1.44) | 1.01 (0.81, 1.25) | 0.69 |
| ER-positive breast cancer (case n = 6860) | |||||
| N° cases/ Person-years | 1,646/1099737 | 3,638/1931419 | 679/312702 | 897/402820 | |
| Age-adjusted model | 1 (Ref.) | 1.13 (1.06, 1.20) | 1.22 (1.11, 1.33) | 1.19 (1.09, 1.30) | <0.01 |
| Multivariable model | 1 (Ref.) | 1.03 (0.97, 1.10) | 1.07 (0.97, 1.18) | 1.03 (0.93, 1.13) | 0.71 |
| p-heterogeneitya = 0.99 | |||||
| Luminal A breast cancer (case n = 2668) | |||||
| N° cases/ Person-years | 765/972970 | 1,361/1336018 | 248/194625 | 294/236045 | |
| Age-adjusted model | 1 (Ref.) | 1.07 (0.97, 1.17) | 1.19 (1.03, 1.38) | 1.10 (0.95, 1.26) | 0.13 |
| Multivariable model | 1 (Ref.) | 1.00 (0.91, 1.10) | 1.08 (0.92, 1.26) | 0.97 (0.83, 1.12) | 0.88 |
| Luminal B breast cancer (case n = 1220) | |||||
| N° cases/ Person-years | 305/973399 | 654/1336630 | 116/194737 | 145/236181 | |
| Age-adjusted model | 1 (Ref.) | 1.21 (1.05, 1.39) | 1.30 (1.04, 1.61) | 1.24 (1.01, 1.51) | 0.11 |
| Multivariable model | 1 (Ref.) | 1.14 (0.99, 1.32) | 1.22 (0.97, 1.53) | 1.15 (0.92, 1.44) | 0.38 |
| HER2-enrichedb (case n = 226) | |||||
| N° cases/ Person-years | 58/973632 | 127/1337102 | 23/194816 | 18/236290 | |
| Age-adjusted model | 1 (Ref.) | 1.28 (0.93, 1.76) | 1.42 (0.87, 2.33) | 0.86 (0.50, 1.48) | 0.53 |
| Multivariable model | 1 (Ref.) | 1.17 (0.84, 1.62) | 1.27 (0.76, 2.13) | 0.77 (0.43, 1.37) | 0.33 |
| Basal-like tumorsb (case n = 260) | |||||
| N° cases/ Person-years | 100/973596 | 121/1337109 | 18/194823 | 21/236288 | |
| Age-adjusted model | 1 (Ref.) | 0.99 (0.75, 1.30) | 1.02 (0.61, 1.70) | 1.00 (0.62, 1.62) | 0.96 |
| Multivariable model | 1 (Ref.) | 1.01 (0.76, 1.33) | 1.05 (0.62, 1.78) | 1.03 (0.61, 1.72) | 0.88 |
| p-heterogeneitya = 0.54 | |||||
Results are expressed as Hazard Ratios (HR) and 95% Confidence Intervals (95% CI).
Multivariable model: adjusted for ethnicity (white, non-white), Southern European/Mediterranean ancestry (yes, no), age at menarche ( <12, 12, 13, 14, >14 years), menopausal status and age at menopause (premenopausal, <45, 45–49, 50–52, 53 + , unknown), postmenopausal hormone use (never user, past user, current user– estrogen only for <5 years, current user – estrogen only for ≥5 years, current estrogen + progestin user for <5 years, current estrogen + progestin user for ≥5 years, current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, 2+ children before age 25, 2+ children ≥25 years of age), breastfeeding history (never, breastfed for ≤ 6 months, breastfed for > 6 months), family history of breast cancer (yes or no), history of biopsy-confirmed benign breast disease (yes or no), height ( <1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, 1.75 + m), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (continuous MET-hours/week), neighborhood-based socioeconomic status indicator (continuous), BMI at age 18 ( <20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0) and AHEI-2010 (without alcohol and polyunsaturated fatty acids components, quintiles).
NHS Nurses’ Health Study, NHSII Nurses’ Health Study II.
aFor testing heterogeneity by subtype, we used the Lunn–McNeil approach, for multivariable model 1.
bDue to smaller sample sizes in analyses, to ensure that models would run, covariate categorizations were simplified.
When we examined olive oil consumption over specific lag times (Table 4), we did not identify any association of olive oil consumption with breast cancer risk at any of the periods (0–4, 4–8, 8–12, 12–16, 16–20 y lag). Stratified analysis (Table 5) showed no significant effect modification by menopausal status, ancestry, current BMI, or AHEI-2010 for olive oil consumption in relation to overall invasive breast cancer. In ancillary analyses, we cross-classified olive oil consumption with Mediterranean diet (AMED) with risk of invasive breast cancer (Table 6). We found no statistically significant association for olive oil cross-classified with AMED.
Table 4.
Risk of invasive breast cancer according to olive oil categories with different lag periods using pooled data from NHS and NHSII.
| Simple update (0–4 y lag) | 4–8 y lag | 8–12 y lag | 12–16 y lag | 16–20 y lag | |
|---|---|---|---|---|---|
| Invasive breast cancer | |||||
| Cases/Person-Years | 9,540/3743346 | 8,370/3075767 | 6,838/2434444 | 4,989/1812433 | 3,427/1234703 |
| Never or <1 per month | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| >0– ≤ 4.5 g/d | 1.07 (1.01, 1.12) | 1.06 (1.00, 1.11) | 0.99 (0.94, 1.05) | 1.00 (0.94, 1.07) | 1.00 (0.92, 1.07) |
| >4.5– ≤ 7 g/d | 1.06 (0.98, 1.14) | 1.05 (0.96, 1.14) | 1.06 (0.96, 1.16) | 1.06 (0.95, 1.19) | 1.05 (0.91, 1.21) |
| >7 g/d | 1.03 (0.97, 1.10) | 0.99 (0.92, 1.06) | 0.95 (0.88, 1.03) | 0.96 (0.87, 1.06) | 0.94 (0.82, 1.08) |
| P-trend | 0.70 | 0.32 | 0.34 | 0.55 | 0.53 |
Results are expressed as Hazard Ratios (HR) and 95% Confidence Intervals (95% CI). Multivariable model adjusted for ethnicity (white, non-white), Southern European/Mediterranean ancestry (yes, no), age at menarche ( <12, 12, 13, 14, >14 years), menopausal status and age at menopause (premenopausal, <45, 45–49, 50–52, 53 + , unknown), postmenopausal hormone use (never user, past user, current user– estrogen only for <5 years, current user – estrogen only for ≥5 years, current estrogen + progestin user for <5 years, current estrogen + progestin user for ≥5 years, current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, 2+ children before age 25, 2+ children ≥25 years of age), breastfeeding history (never, breastfed for ≤6 months, breastfed for >6 months), family history of breast cancer (yes or no), history of biopsy-confirmed benign breast disease (yes or no), height (<1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, 1.75 + m), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (continuous MET-hours/week), neighborhood-based socioeconomic status indicator (continuous), BMI at age 18 ( <20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0) and AHEI-2010 (without alcohol and polyunsaturated fatty acids components, quintiles).
NHS Nurses’ Health Study, NHSII Nurses’ Health Study II.
Table 5.
Subgroup analyses for risk of invasive breast cancer according to categories of cumulative total olive oil intake using pooled data from NHS and NHSII.
| Never or <1 per month | >0– ≤4.5 g/d ( >0 to ≤1 teaspoon) | >4.5– ≤7 g/d >1 teaspoon to ≤1/2 TBS) | >7 g/d (>1/2 TBS) | P for trend | P for interaction | |
|---|---|---|---|---|---|---|
| Subgroup | ||||||
| Body mass index, kg/m2 | ||||||
| <25 kg/m2 | 1 (Ref.) | 1.03 (0.95, 1.12) | 1.06 (0.93, 1.20) | 1.03 (0.92, 1.17) | 0.73 | 0.67 |
| ≥25 kg/m2 | 1 (Ref.) | 1.02 (0.95, 1.09) | 1.05 (0.94, 1.17) | 0.94 (0.85, 1.05) | 0.26 | |
| AHEI-2010 | ||||||
| Below median | 1 (Ref.) | 1.02 (0.95, 1.09) | 1.03 (0.90, 1.17) | 1.03 (0.90, 1.16) | 0.76 | 0.68 |
| Above median | 1 (Ref.) | 1.06 (0.97, 1.15) | 1.09 (0.97, 1.22) | 1.00 (0.89, 1.11) | 0.43 | |
| AMED | ||||||
| Below median | 1 (Ref.) | 1.06 (0.95, 1.19) | 1.14 (0.94, 1.39) | 1.09 (0.89, 1.33) | 0.37 | 0.08 |
| Above median | 1 (Ref.) | 1.10 (0.95, 1.26) | 1.05 (0.87, 1.26) | 1.01 (0.84, 1.20) | 0.31 | |
| Ancestry | ||||||
| Southern European/Mediterranean | 1 (Ref.) | 1.00 (0.88, 1.15) | 1.11 (0.92, 1.34) | 0.93 (0.78, 1.11) | 0.51 | 0.40 |
| Other ancestry | 1 (Ref.) | 1.05 (0.99, 1.11) | 1.06 (0.96, 1.16) | 1.04 (0.95, 1.14) | 0.76 | |
| Menopausal status | ||||||
| Premenopausal | 1 (Ref.) | 1.04 (0.76, 1.41) | 0.85 (0.43, 1.69) | 0.98 (0.54 1.78) | 0.79 | 0.97 |
| Postmenopausal | 1 (Ref.) | 1.01 (0.95, 1.09) | 1.09 (0.97, 1.21) | 0.99 (0.89, 1.09) | 0.99 | |
Results are expressed as Hazard Ratios (HR) and 95% Confidence Intervals (95% CI). Multivariable model adjusted for ethnicity (white, non-white), Southern European/Mediterranean ancestry (yes, no), age at menarche (<12, 12, 13, 14, >14 years), menopausal status and age at menopause (premenopausal, <45, 45–49, 50–52, 53 + , unknown), postmenopausal hormone use (never user, past user, current user– estrogen only for <5 years, current user – estrogen only for ≥5 years, current estrogen + progestin user for <5 years, current estrogen + progestin user for ≥5 years, current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, 2+ children before age 25, 2+ children ≥25 years of age), breastfeeding history (never, breastfed for ≤ 6 months, breastfed for >6 months), family history of breast cancer (yes or no), history of biopsy-confirmed benign breast disease (yes or no), height ( <1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, 1.75 + m), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (continuous MET-hours/week), neighborhood-based socioeconomic status indicator (continuous) and BMI at age 18 ( <20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0).
AHEI Alternate Healthy Eating Index, AMED Alternate Mediterranean Diet Score, NHS Nurses’ Health Study, NHSII Nurses’ Health Studies II.
Table 6.
Cross-classified analysis of olive oil consumption and Mediterranean Diet (AMED, based on the median) with invasive breast cancer risk in NHS and NHSII.
| No OO consumption | Low OO consumption | High OO consumption | |
|---|---|---|---|
| Low AMED adherence | 1.00 (ref.) | 1.05 (0.93, 1.18) | 0.97 (0.86, 1.09) |
| High AMED adherence | 0.90 (0.83, 0.98) | 0.99 (0.93, 1.05) | 0.92 (0.84, 1.01) |
Results are expressed as Hazard Ratios (HR) and 95% Confidence Intervals (95% CI). Multivariable model adjusted for ethnicity (white, non-white), Southern European/Mediterranean ancestry (yes, no), age at menarche ( <12, 12, 13, 14, >14 years), menopausal status and age at menopause (premenopausal, <45, 45–49, 50–52, 53 + , unknown), postmenopausal hormone use (never user, past user, current user– estrogen only for <5 years, current user – estrogen only for ≥5 years, current estrogen + progestin user for <5 years, current estrogen + progestin user for ≥5 years, current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, 2+ children before age 25, 2+ children ≥25 years of age), breastfeeding history (never, breastfed for ≤ 6 months, breastfed for > 6 months), family history of breast cancer (yes or no), history of biopsy-confirmed benign breast disease (yes or no), height ( <1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, 1.75 + m), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (continuous MET-hours/week), neighborhood-based socioeconomic status indicator (continuous) and BMI at age 18 ( <20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0).
AMED Alternate Mediterranean Diet Score, NHS Nurses’ Health Study, NHSII Nurses’ Health Studies II, OO Olive Oil.
Discussion
In these two large prospective cohorts of U.S. women, using repeated dietary assessments, consumption of olive oil was not associated with the risk of overall breast cancer, nor any specific subtype. Because this is the first evaluation of olive oil consumption and subtype-specific breast tumors in the U.S., confirmation is needed. Our overall results align with meta-analyses of prospective studies, which generally show that consuming olive oil is not related to breast cancer risk [16].
Several epidemiological studies have been conducted to evaluate the role of the Mediterranean diet, olive oil consumption, and intake of some olive oil components such as polyphenols on the risk of breast cancer. However, these investigations are mostly based on case-control studies with fewer prospective and intervention studies and, consequently, the results are not fully consistent and inconclusive. A recent meta-analysis of ten studies [17] (two prospective and eight case–control studies), including 81,436 women, evaluated the association between olive oil intake and breast cancer risk. Women in the highest category of olive oil consumption (compared to the lowest) had a suggestive lower risk of breast cancer, although the association was not significant in either prospective or case-control analyses. In EPIC, one of the two prospective studies, olive oil consumption during adult life was not associated with risk of breast cancer after following 62,284 postmenopausal women for a mean of 9 years [15]. Consistent with our results, this null association persisted for ER-positive or PR-positive tumors, although a suggestive association was observed for ER- and PR-negative tumors. The other observational study was a per protocol analysis within the PREDIMED trial [14], which aimed to compare (1) Mediterranean diet plus EVOO and (2) Mediterranean diet plus nuts to a low-fat dietary pattern (control group). A protective effect was observed for a Mediterranean diet supplemented with EVOO on breast cancer risk. However, these results are not directly comparable with our results from the Nurse’s Health Studies. First, the amount of olive oil consumed by the Spanish and U.S. populations varied remarkably. In the PREDIMED trial, women assigned to a Mediterranean diet plus EVOO had a mean baseline EVOO consumption of 21 ± 23 g/d (8.6% of total calories), which substantially increased at year three to 50.7 ± 17.0 (22.0% of total caloric intake), whereas the consumption of refined-common olive oil was substantially reduced to less than 1% of caloric intake. In our U.S. study, the mean baseline consumption of any olive oil was 2.5 g/d and 8.9 g/d in the highest category. Second, in the PREDIMED trial, participants were supplied with polyphenol-rich EVOO. In our study, we could not distinguish between different olive oil varieties or accurately measure the consumption of EVOO where phenolic contents and other bioactive compounds are found in higher concentrations. The distinction is important because refined olive oil has much lower levels of phenolic compounds than EVOO and may therefore have fewer health benefits, specifically on breast cancer risk [20]. The single category which is devoid of these bioactive components may have contributed to the lack of significant results. Of note, our study showed that benefits of olive oil cannot be observed when consumed in lower average amounts than in Mediterranean countries.
Our overall results conflict with previous findings from case–control studies [17, 21], conducted in traditional Mediterranean countries (i.e., Greece, Spain, Italy and France), which generally show that consuming olive oil is associated with lower breast cancer risk. Only two case-control studies have specifically stratified by menopause status [22, 23]. Trichopoulou et al. found a stronger association among postmenopausal women [22], while La Vecchia et al. did not find that the association between olive oil and breast cancer varied by menopausal status [23] which aligns with our results. However, as case–control studies can be prone to information and selection bias, and olive oil is an indicator of a healthy Mediterranean diet and possibly other favorable lifestyle behaviors, study design and residual confounding might explain part of the differences observed. In addition, most epidemiological studies do not distinguish between the consumption of common, lower quality, olive oil and EVOO, thus resulting in great variability in the profile and quality of the oil consumed.
Oleic acid is the primary fatty acid in olive oil, and there is some evidence to suggest that it may have potential effects on breast cancer risk. Possible mechanisms by which oleic acid could affect breast cancer risk include altering gene expression, inhibiting tumor growth, reducing inflammation, or promoting cell differentiation. Nonetheless, in the most recent meta-analysis, no association was found between dietary oleic acid intake [n = 4, 1.11 (0.83–1.48), I2 = 51.79%] and risk of breast cancer [24]. In the Nurses’ Health Study II, neither total monounsaturated fatty acids nor oleic acid (18:1n-9c), measured in blood erythrocytes, were associated with risk of breast cancer [25]. These results align with a recent Spanish nested-case control study [26]. While epidemiological evidence is still sparse, extensive experimental in vivo and in vitro data have shown a protective effect of olive oil and its compounds (hydroxytyrosol, oleuropein and squalene, among others) on mammary carcinogenesis. Such effects act through complex and multiple mechanisms, including changes in epigenetics, transcriptome, and protein expression that modulate several signaling pathways [27].
To our knowledge, this is the first large prospective study to assess the relationship between olive oil and breast cancer risk in a non-Mediterranean population. Our study strengths include the number of breast cancer cases, repeated dietary assessments during a long follow-up period, information on hormone receptor subtypes, and comprehensive adjustment for potentially confounding variables. Higher olive oil consumption could be a marker of a globally healthier diet and higher socioeconomic status. However, when we adjusted for diet quality (i.e., AHEI-2010), and socioeconomic indicator variables, the results remained substantially unchanged. Additionally, our study was conducted among a predominantly non-Hispanic white population of nurses, which minimizes potential confounding by socioeconomic factors but may limit generalizability. Further, given the observational nature of our study, we cannot completely exclude the possibility of residual confounding. Nonetheless, no association was observed after adjusting for known and suspected predictors of breast cancer risk. In our study, dietary assessment was conducted using validated self-reported FFQs, which unavoidably may include some degree of measurement error. However, the use of cumulatively averaged measurements reduced random measurement errors caused by within-person variation. We evaluated “olive oil” which certainly could be a blend of refined and virgin olive oils or virgin olive oils that preserve their minor compounds. Unfortunately, we could not distinguish between olive oil varieties containing different amounts of polyphenols and other nonlipid bioactive compounds. This fact may explain our negative results, and further studies are needed to answer this question. Nonetheless, we previously found significant inverse associations between olive oil intake and total cardiovascular disease and coronary heart disease in these U.S. cohorts, which could be possibly attributed to the lipid profile (high in MUFA, especially oleic acid,) rather than the bioactive minor compounds [11, 12].
In conclusion, olive oil consumption during adult life was not associated with the risk of overall breast cancer in two large prospective cohort studies of U.S. women. Further research is needed to understand the reasons for the difference in these findings and the inverse associations found in the PREDIMED trial. Future studies should delineate the amount and type of olive oil and whether any potential effect is related to the fatty acid composition of olive oil or its content of phenolic compounds.
Acknowledgements
The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming.
Author contributions
ARN, MGF and AHE designed research. ARN, MGF, WCW, WYC, MDH, BAR, MAM and AHE conducted the research; ARN analyzed the data; ARN and AHE drafted the manuscript; All the authors made critical revisions to the manuscript for important intellectual content; ARN, MGF and AHE had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
This study was supported by grants UM1 CA186107, U01 CA176726, P01 CA87969, and R01 CA50385 from the National Institutes of Health, the Breast Cancer Research Foundation and Susan G Komen Foundation and Ramon Areces Foundation. The funding sources did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Data availability
The data described in the article, code book, and analytic code will be made available upon application and approval. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (e-mail: nhsaccess@channing.harvard.edu).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Completion of the questionnaire implied informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 2.Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72:30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 3.Galbete C, Schwingshackl L, Schwedhelm C, Boeing H, Schulze MB. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: an umbrella review of meta-analyses. Eur J Epidemiol. 2018;33:909–31. doi: 10.1007/s10654-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaforio JJ, Visioli F, Alarcón-de-la-Lastra C, Castañer O, Delgado-Rodríguez M, Fitó M, et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients. 2019;11:2039.. doi: 10.3390/nu11092039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucciantini M, Leri M, Nardiello P, Casamenti F, Stefani M. Olive polyphenols: antioxidant and anti-inflammatory properties. Antioxidants. 2021;10:1044.. doi: 10.3390/antiox10071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano-Castellón J, López-Yerena A, Domínguez-López I, Siscart-Serra A, Fraga N, Sámano S, et al. Extra virgin olive oil: a comprehensive review of efforts to ensure its authenticity, traceability, and safety. Compr Rev Food Sci Food Saf. 2022;21:2639–64. doi: 10.1111/1541-4337.12949. [DOI] [PubMed] [Google Scholar]
- 7.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-González MA, Dominguez LJ, Delgado-Rodríguez M. Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case–control, cohort and intervention studies. Br J Nutr. 2014;112:248–59. doi: 10.1017/S0007114514000713. [DOI] [PubMed] [Google Scholar]
- 9.Morze J, Danielewicz A, Przybyłowicz K, Zeng H, Hoffmann G, Schwingshackl L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur J Nutr. 2021;60:1561–86. doi: 10.1007/s00394-020-02346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guasch-Ferré M, Hruby A, Salas-Salvadó J, Martínez-González MA, Sun Q, Willett WC, et al. Olive oil consumption and risk of type 2 diabetes in US women. Am J Clin Nutr. 2015;102:479–86. doi: 10.3945/ajcn.115.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guasch-Ferré M, Liu G, Li Y, Sampson L, Manson JE, Salas-Salvadó J, et al. Olive oil consumption and cardiovascular risk in U.S. adults. J Am Coll Cardiol. 2020;75:1729–39. doi: 10.1016/j.jacc.2020.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guasch-Ferré M, Li Y, Willett WC, Sun Q, Sampson L, Salas-Salvadó J, et al. Consumption of olive oil and risk of total and cause-specific mortality among U.S. adults. J Am Coll Cardiol. 2022;79:101–12. doi: 10.1016/j.jacc.2021.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battino M, Forbes-Hernández TY, Gasparrini M, Afrin S, Cianciosi D, Zhang J, et al. Relevance of functional foods in the Mediterranean diet: the role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit Rev Food Sci Nutr. 2019;59:893–920. doi: 10.1080/10408398.2018.1526165. [DOI] [PubMed] [Google Scholar]
- 14.Toledo E, Salas-Salvadó J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern Med. 2015;175:1752.. doi: 10.1001/jamainternmed.2015.4838. [DOI] [PubMed] [Google Scholar]
- 15.Buckland G, Travier N, Agudo A, Fonseca-Nunes A, Navarro C, Lagiou P, et al. Olive oil intake and breast cancer risk in the Mediterranean countries of the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2012;131:2465–9. doi: 10.1002/ijc.27516. [DOI] [PubMed] [Google Scholar]
- 16.Markellos C, Ourailidou ME, Gavriatopoulou M, Halvatsiotis P, Sergentanis TN, Psaltopoulou T. Olive oil intake and cancer risk: A systematic review and meta-analysis. Caruso C, ed. PLOS ONE. 2022;17:e0261649. 10.1371/journal.pone.0261649. [DOI] [PMC free article] [PubMed]
- 17.Sealy N, Hankinson SE, Houghton SC. Olive oil and risk of breast cancer: a systematic review and dose–response meta-analysis of observational studies. Br J Nutr. 2021;125:1148–56. doi: 10.1017/S0007114520003499. [DOI] [PubMed] [Google Scholar]
- 18.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, et al. Comparison of molecular phenotypes of ductal carcinoma in situand invasive breast cancer. Breast Cancer Res. 2008;10:R67.. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. doi: 10.2307/2532940. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Lopez C, Carpena M, Lourenço-Lopes C, Gallardo-Gomez M, Lorenzo JM, Barba FJ, et al. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods. 2020;9:1014.. doi: 10.3390/foods9081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donat-Vargas C, Guerrero-Zotano Á, Lope V, Bermejo B, Casas A, Baena-Cañada JM, et al. Use VIRGIN olive oil as your preferred fat to reduce your risk of breast cancer: case-control EpiGEICAM study. Eur J Clin Nutr. 2022. Published online February 22, 10.1038/s41430-022-01101-w. [DOI] [PubMed]
- 22.Trichopoulou A, Katsouyanni K, Stuver S, Tzala L, Gnardellis C, Rimm E, et al. Consumption of Olive Oil and Specific Food Groups in Relation to Breast Cancer Risk in Greece. JNCI J Natl Cancer Inst. 1995;87:110–6. doi: 10.1093/jnci/87.2.110. [DOI] [PubMed] [Google Scholar]
- 23.La Vecchia C, Negri E, Franceschi S, Decarli A, Giacosa A, Lipworth L. Olive Oil, Other Dietary Fats, and the Risk of Breast Cancer (Italy) Cancer Causes Control. 1995;6:545–50. doi: 10.1007/BF00054164. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Hou L, Wang W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: a meta-analysis of prospective cohort studies: fat, fatty acids and breast cancer. Int J Cancer. 2016;138:1894–904. doi: 10.1002/ijc.29938. [DOI] [PubMed] [Google Scholar]
- 25.Hirko KA, Chai B, Spiegelman D, Campos H, Farvid MS, Hankinson SE, et al. Erythrocyte membrane fatty acids and breast cancer risk: a prospective analysis in the nurses’ health study II: fatty acids and breast cancer risk. Int J Cancer. 2018;142:1116–29. doi: 10.1002/ijc.31133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lope V, Guerrero-Zotano Á, Casas A, Baena-Cañada JM, Bermejo B, Pérez-Gómez B, et al. Serum phospholipids fatty acids and breast cancer risk by pathological subtype. Nutrients. 2020;12:3132.. doi: 10.3390/nu12103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moral R, Escrich E. Influence of olive oil and its components on breast cancer: molecular mechanisms. Molecules. 2022;27:477.. doi: 10.3390/molecules27020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data described in the article, code book, and analytic code will be made available upon application and approval. Further information including the procedures to obtain and access data from the Nurses’ Health Studies is described at https://www.nurseshealthstudy.org/researchers (e-mail: nhsaccess@channing.harvard.edu).