Abstract
Background
PD-L1 promotes glycolysis in tumour cells. We observed a correlation between high PD-L1 expression and high 18F-FDG uptake in patients with pancreatic ductal adenocarcinoma (PDAC) in a previous study. This study aims to determine the usefulness of 18F-FDG PET/CT for evaluating the PD-L1 status in PDAC and to elucidate its rationality by integrated analyses.
Methods
For bioinformatics analysis, WGCNA, GSEA and TIMER were applied to analyse the pathways and hub genes associated with PD-L1 and glucose uptake. 18F-FDG uptake assay was used to determine the glucose uptake rate of PDAC cells in vitro. Related genes expression were verified by RT-PCR and western blot. A retrospective analysis was performed on 47 patients with PDAC who had undergone 18F-FDG PET/CT. Maximum standardised uptake values (SUVmax) were determined. The usefulness of SUVmax for evaluating PD-L1 status was determined by receiver operating characteristic (ROC) curve analysis.
Results
Bioinformatics analysis showed that several signalling pathways are associated with both PD-L1 expression and tumour glucose uptake, among which JAK-STAT may be an important one. By in vitro experiments, the regulatory role of PD-L1 on glucose uptake was demonstrated, and its dependency on the JAK-STAT pathway was also verified by the rescue study. The SUVmax of PD-L1-positive patients was significantly higher than PD-L1-negative in tumour cells (TCs) (6.1 ± 2.3 vs. 11.1 ± 4.2; P < 0.001), and in tumour-infiltrating immune cells (TIICs) (6.4 ± 3.2 vs. 8.4 ± 3.5; P < 0.001). In a multivariate analysis, SUVmax was significantly associated with PD-L1 expression in TCs and TIICs (P < 0.001 and P = 0.018, respectively). Using SUVmax cut-off values of 8.15 and 7.75, PD-L1 status in TCs and TIICs could be predicted with accuracies of 91.5% and 74.5%, respectively.
Conclusion
Higher 18F-FDG uptake by PDAC is associated with elevated PD-L1 expression. JAK-STAT is an important pathway that mediates PD-L1 to promote glucose uptake in PDAC.
Subject terms: Cancer imaging, Cellular signalling networks
Introduction
Immune checkpoint (ICP) blocking therapy has brought a new dawn for patients with advanced malignancies in the recent decade [1]. Yet unfortunately, the light has not been shed on pancreatic ductal adenocarcinoma (PDAC), a malignancy with high mortality but few effective therapies [2, 3]. Programmed cell death 1 (PD-1)/ programmed death ligand 1 (PD-L1) immunoblocking therapy is an important immunotherapy method, by blocking the binding of PD-L1 and PD-1, immune cells can be stimulated and thus attack tumour cells. [4]. Several clinical studies have demonstrated that pancreatic ductal adenocarcinoma did not respond well to PD-L1 immunotherapy [5]. PDAC has been reckoned as lacking immune cell infiltration in the tumour microenvironment, which resulted in its insensitivity to ICP inhibitors [6]. The current clinical PD-L1 immunotherapy drugs are monoclonal antibody inhibitors, which play an anti-tumour role by blocking the combination of PD-L1 and PD-1[7]. Our previous study has demonstrated that PD-L1 was highly expressed in part of PDAC, and patients with high PD-L1 expression had a worse prognosis, suggesting that PD-L1 has a tumour-promoting regulatory mechanism independent of PD-1 in PDAC [8].
Glucose is a principle metabolic and biosynthetic nutrient in PDAC [9]. In our recent study, we found that high expression of PD-L1 in PDAC was associated with increased glucose uptake, as well as high expression of a series of enzymes related to glucose metabolism [10]. Research has shown that interference with PD-L1 gene expression can reduce glucose uptake and protein levels of glycolytic enzymes in tumour cells [11]. Using 18F-labelled glucose analogue fludeoxyglucose (FDG) as a tracer, positron emission tomography /computed tomography (PET/CT) provides the glucose metabolic information of the lesions in clinical practice. Our retrospective studies have also reported that 18F-FDG PET/CT can predict PD-L1 expression in bladder cancer and gastric cancer [12, 13]. These studies suggest that PD-L1 has a role in regulating tumour glucose metabolism, but the mechanism is not fully described.
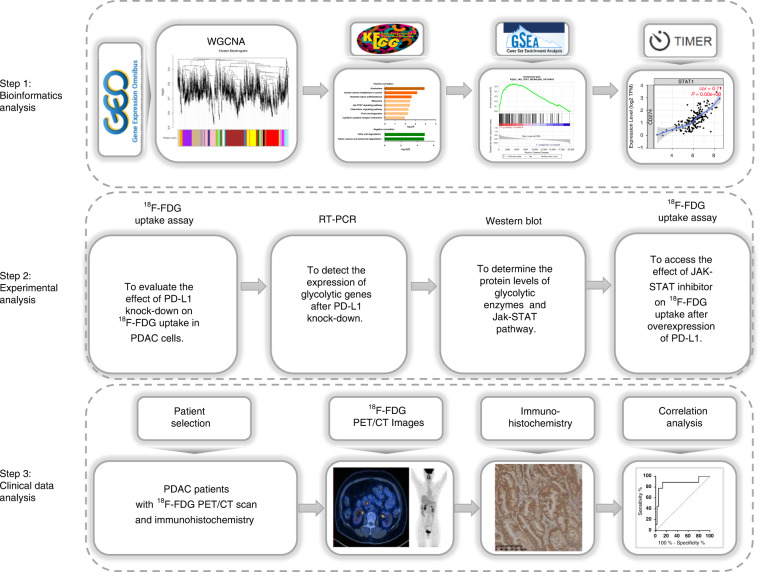
In this study, we designed an integrative study workflow (Fig. 1). We first performed bioinformatics analysis to explore pathways associated with PD-L1 expression and 18F-FDG uptake in pancreatic ductal adenocarcinoma. The role of these pathways in PD-L1-regulated 18F-FDG uptake was also verified by experiments in vitro. Lastly, a retrospective study was conducted to investigate whether the expression of PD-L1 is associated with 18F-FDG uptake and whether 18F-FDG PET/CT imaging can be used to predict the expression of PD-L1 in pancreatic ductal adenocarcinoma. Our study presented evidence that 18F-FDG PET/CT imaging predicts PD-L1 status in pancreatic ductal adenocarcinoma. Besides, we revealed the potential roles of PD-L1 and provided new insights into the exploration of the JAK-STAT regulatory mechanism in PDAC.
Fig. 1. Flowchart of this study.
Step 1: Bioinformatics analysis was applied to reveal the potential pathways between PD-L1 and 18F-FDG uptake. Step 2: Experimental analysis was used to verify the underlying mechanism. Step 3: Retrospective analysis to evaluate the value of 18F-FDG PET/CT in predicting PD-L1 expression in PDAC.
Materials and methods
Weighted correlation network analysis (WGCNA)
The RNA-sequencing data and corresponding patient PET/CT information of pancreatic ductal adenocarcinoma tissue samples were downloaded from the GEO database (GSE107754), among which 8 patients had matching mRNA expression profiles and FDG uptake data. The co-expression gene and enrichment analysis were applied to the WGCNA package of R software, which revealed the correlation between genes [14]. Because the genes with little expression variation usually represent noise, we filter the most variable genes (SD > 0.5) and construct a network. The power of β was set to 6 to ensure a scale-free network. The minimum number of module genes was set to 30. The hierarchical cluster tree summarises the gene modules of different colours. KEGG analysis was performed using KOBAS [15].
Enrichment analysis of GSEA
Gene Set Enrichment Analysis (GSEA) software was used to study the correlation between PD-L1 gene expression level and gene sets in Kyoto Gene and Genome Encyclopedia pathway [16]. A continuous phenotype was established according to the expression level of PD-L1 genes. The Pearson correlation coefficient between PD-L1 and the reference gene set was calculated by the default weighted enrichment statistic, and gene sequencing was conducted according to the correlation coefficient. The number of random combinations was set to 1000, and the normalised enrichment score (ES) and normalised enrichment score (NES) were calculated, with standardised P < 0.01 and false discovery rates (FDR) < 0.05 as significant enrichment.
Correlation between STATs and PD-L1 expression
To analyse the correlation between STATs and PD-L1 expression in pancreatic ductal adenocarcinoma, we employed the Tumour Immune Estimation Resource (TIMER) as a comprehensive resource (https://cistrome.shinyapps.io/timer/). We conducted a series of analyses on the expression of PD-L1 in pancreatic ductal adenocarcinoma and its correlation with the abundance of seven types of STATs. The expression levels of STATs in pancreatic ductal adenocarcinoma were analysed by Gene Expression Profiling Interactive Analysis (GEPIA2; http://gepia2.cancer-pku.cn/).
Cell culture
The pancreatic ductal adenocarcinoma cell lines ASPC-1 and BXPC-3 were purchased from the Cell Bank of the Chinese Academy of Sciences, Shanghai, China. All the cell lines were recently authenticated by STR profiling and tested for mycoplasma contamination. AsPC-1 cells were cultured in RPMI 1640 medium (Cat. No. 11875093, Gibco), and BxPC-3 cells were cultured in high glucose DMEM medium (Cat. No. 11965092, Gibco), supplemented with 10% foetal bovine serum (Cat. No. 16140089, Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin (Cat. No. 10378016, Gibco), and 2 mM l-GlutaMax (Cat. No. 35050061, Gibco) at 37 °C in a humidified 5% CO2 atmosphere.
Materials
The JAK-STAT inhibitor tofacitinib was purchased from Selleck Chemicals (catalogue No. S2789). The antibodies were purchased, including PD-L1 (Proteintech, 28076-1-AP), GLUT1 (Proteintech, 81463-1-RR), HK2 (Proteintech, 22029-1-AP), β-ACTIN (Proteintech, 81115-1-RR), and p-STAT3 (CST, 9145 S). The PD-L1 overexpression plasmid was purchased from Sino Biological Inc. The sequences of PD-L1 shRNA oligos used in this study are as follows: CCGGGCAGGCAATGTGGGACTTAAACTCGAGTTTAAGTCCCACATTGCCTGCTTTTTT; CCGGGGCATTTGCTGAACGCATTTACTCGAGTAAATGCGTTCAGCAAATGCCTTTTTT; CCGGTGGAGGATAAGAACATTATTCCTCGAGGAATAATGTTCTTATCCTCCATTTTTT.
Western blot
Cells were collected and lysed using RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, complete protease inhibitor cocktail) for 30 min on ice, then centrifuged at 15,000×g for 15 min at 4 °C to remove impurities. Cell lysis was quantified using the BCA Protein Assay Kit (Thermo Fisher Scientific, USA). Equal amounts of the proteins were separated in 10% SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in 5% non-fat milk at room temperature for 1 h followed by incubation at 4 °C overnight with the primary antibodies. Horseradish peroxidase-conjugated secondary antibodies were used as follows. A chemiluminescence detection system was used to detect the immunoreactive protein bands according to the manufacturer’s instructions.
18F-FDG uptake in cells
Pancreatic ductal adenocarcinoma cells were inoculated into 12-well culture plates and cultured overnight. The culture medium was replaced with DMEM (non-glucose) medium containing 18F-FDG (10 μCi/mL), and the culture was continued for 1 h. Discard the culture medium, wash the cells with PBS 3 times, and add 500 μL NaOH solution (100 mmol/L) to fully lysate the cells. The lysate of each well was taken out, and the radioactivity count of each well was determined by a gamma counter.
Patient selection
A retrospective analysis was performed on 47 patients with pancreatic ductal adenocarcinoma who received 18F-FDG PET/CT examination in Renji Hospital from June 2016 to April 2020, including 32 males and 15 females. PD-Ll was detected in all patients by immunohistochemistry, and patients were grouped according to PD-L1 expression. This study was approved by the Institutional Review Board of Ren Ji Hospital and was following the principles of the 2013 revision of the Declaration of Helsinki. Informed consent was obtained from all subjects. For the publication of patient photos, consent to publish was obtained.
Imaging protocol
18F-FDG PET/CT was collected according to standard protocols. All patients avoided exercise outside daily life for 24 h, fasted for 6 h before injection of FDG, sat quietly, and rested for 1 h, and blood glucose was measured lower than 8 mmol/L before injection. 18F-FDG (provided by Shanghai Atomkexing Pharmaceutical Co., LTD., radiochemical purity >95%) 3.7 mbq/kg was injected according to the body weight, and the images were collected by Biograph mCT PET/CT of Siemens. CT scanning parameters: tube voltage 120 Kev, tube current system automatically set; PET scanning parameters: 3D, 2 min/bed, from the crown of the head to mid-thigh; Image reconstruction methods: TrueX, Ultral-HD reconstruction, layer thickness of 1.5 mm.
PET image quantification
Standardised uptake values (SUV) is a semi-quantitative parameter defined as the ratio of the volume of interest (VOI) activity per unit volume to total body volume activity per unit [17]. The maximum standardised uptake value (SUVmax) is defined as the maximum pixel value in the VOI [18]. The use of SUVmax as a measure of tissue/organ uptake facilitates comparison between patients and has been suggested as the basis for diagnosis [19]. For every measurable lesion, the VOI centred on the maximum value pixel was drawn automatically with workstation tools generating the SUVmax. With an SUV cut-off of 2.5, the total lesion glycolysis (TLG) is automatically calculated by the workstation tools. Total SUVmax was calculated as the sum of the SUVmax of all measurable lesions [20]. The investigator was blinded to the group allocation during when assessing the outcome.
Statistical analysis
GraphPad Prism 6 software and R (https://www.r-project.org/) were used to process the data. The quantitative data conforming to the normal distribution is represented by X ± s, and the quantitative data not conforming to the normal distribution is represented by M (P25, P75). Qualitative data are expressed as frequency (percentage). A two-sided t-test was used for comparison between the two groups. Univariate analysis of variance was used for multi-group comparison, and the Newman–Keuls test was used for pair-to-group comparison. The difference between qualitative data was compared by the χ2 test. The area under the curve (AUC) of receiver operating characteristic (ROC) was used to evaluate the efficacy of SUVmax in predicting PD-L1 expression. Pearson correlation coefficient was used for correlation analysis. P < 0.05 means the difference or correlation is statistically significant. All the experiments shown were replicated at least three times in the laboratory.
Results
Identification of JAK-STAT as a pathway associated with PD-L1 expression and glucose uptake
To explore the mechanism leading to the correlation between glucose uptake and PD-L1 expression in PDAC, we used WGCNA to analyse the data from the GEO database, and the genes closely related to both glucose uptake and PD-L1 expression were revealed. After reading and preprocessing data with R software, a total of 30,936 expression profile genes were obtained. Then, with SD > 0.5 as the screening criteria, 9284 genes were obtained. When the soft threshold β value is set at 6, the connections between genes are distributed in a scale-free network (Fig. 2a). A total of 43 modules were obtained by WGCNA analysis (Fig. 2b, c). With P.GS.PD-L1 < 0.05 as the limiting criteria, 796 genes were screened, and with P.GS.SUVmean <0.05 as the limiting criteria, 1045 genes were screened. The intersection of the two sets of genes produced a total of 26 genes. Among them, 21 genes were positively correlated with both PD-L1 expression and glucose uptake, whereas 5 genes were negatively correlated with it (Supplementary Table S1). KEGG pathway analysis of these genes showed that they were enriched in pathways of Alcoholism, Central carbon metabolism in cancer, Systemic lupus erythematosus, Ribosome, JAK-STAT signalling pathway, Chemokine signalling pathway, Viral carcinogenesis, Cytokine–cytokine receptor interaction, Fatty acid degradation, Valine, Leucine and isoleucine degradation (Fig. 2d). To determine which of the above ten pathways is most closely associated with PD-L1 expression, we analysed pancreatic ductal adenocarcinoma data from the TCGA database. Using the GSEA method, we verified the correlation between the above ten pathways and PD-L1 expression (Fig. 3a, b). The results showed that 6 of the above ten pathways were correlated with PD-L1 expression, among which the JAK-STAT pathway was the most closely correlated with PD-L1 expression in pancreatic ductal adenocarcinoma (ES = 0.552, NES = 2.038, P < 0.001).
Fig. 2. Weighted correlation network analysis (WGCNA).
a Soft threshold selection in the WGCNA network analysis. b Gene distribution in the WGCNA network analysis. c Relationships between modules and SUVmean as well as PD-L1 gene expression. d KEGG pathway analysis of genes correlated with both SUVmean and PD-L1 gene expression.
Fig. 3. Gene Set Enrichment Analysis (GSEA) of the correlation between PD-L1 gene expression level and gene sets in Kyoto Gene and Genome Encyclopedia (KEGG) pathways.
a The pathways positively correlated with PD-L1 gene expression. b The pathways negatively correlated with PD-L1 gene expression.
The key transcription factors regulating gene expression in the JAK-STAT signal pathway are signal and activator of transcription (STAT). The STAT family consists of seven structurally and functionally related proteins, namely STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6. To determine which STAT is closely related to PD-L1, we used TIMER to analyse the correlation between PD-L1 and STAT expression in pancreatic ductal adenocarcinoma. As shown in Supplementary Fig. S1A, the correlation coefficient between STAT1, STAT2, STAT3 and PD-L1 expression was greater than 0.55, suggesting that they may be closely related to PD-L1. Next, we applied GEPIA2 analysis and found that the expression level of STAT3 in pancreatic ductal adenocarcinoma was higher than that of STAT1 and STAT2 (Supplementary Fig. S1B).
In vitro validation of glucose metabolism regulated by PD-L1 and JAK-STAT pathway
To determine the effect of PD-L1 on glucose metabolism, we used shRNA to interfere with PD-L1 expression in pancreatic ductal adenocarcinoma cells. As shown in Fig. 4a, the knockdown of PD-L1 in pancreatic ductal adenocarcinoma cells was accompanied by a lower glucose metabolic gene expression in the mRNA level compared with the control group. Accordingly, the cell proliferation rate and 18F-FDG uptake were decreased in the PD-L1 knockdown group (Fig. 4b, c). To further verify the changes in glycolysis after PD-L1 knockdown, glucose and lactate in the culture medium and glucose-6-phosphate (G6P) in the cells were measured. As shown in Fig. 4d, e, glucose consumption and lactate production were significantly decreased in the PD-L1 knockdown group. Consistently, the cellular G6P tended to decrease in the PD-L1 knockout group, although the statistical difference was not significant (Fig. 4f).
Fig. 4. The regulation of PD-L1 on 18F-FDG uptake had a dependence on the JAK-STAT pathway.
a Inhibition of PD-L1 decreases the expression of glycolysis-related genes. b Inhibition of PD-L1 reduces cell proliferation. c Inhibition of PD-L1 reduces 18F-FDG uptake. d Inhibition of PD-L1 reduces glucose consumption. e Inhibition of PD-L1 reduces lactate production. f The glucose-6-phosphate levels in the cells. g Inhibition of PD-L1 by shRNA reduces STAT3 phosphorylation. h Overexpression of PD-L1 increases 18F-FDG uptake, which is rescued by tofacitinib, an inhibitor of the JAK-STAT pathway. i Extracellular acid ratio (ECAR) was measured using Seahorse XF. 2-DG 2-deoxyglucose. j The glycolysis was measured as the ECAR rate reached by a given cell after the addition of saturating amounts of glucose. k This glycolytic reserve indicates the capability of a cell to respond to an energetic demand as well as how close the glycolytic function is to the cell’s theoretical maximum. Data are the means ± SD, *P < 0.05, ***P < 0.001. Columns without asterisks indicate insignificant differences.
STAT1 and STAT3 have been reported as transcription factors of PD-L1 [21]. Yet interestingly, we found that PD-L1 knockdown inhibited STAT3 phosphorylation, suggesting a regulatory role of PD-L1 on STAT3. In addition, the levels of GLUT1 and HK2 proteins, which are key enzymes in regulating 18F-FDG uptake in the tumour, were decreased (Fig. 4g and Supplementary Fig. S2). To verify the role of the JAK-STAT pathway in PD-L1-regulated FDG uptake, we applied a classic JAK-STAT inhibitor, tofacitinib, to block the JAK-STAT pathway in pancreatic ductal adenocarcinoma cells. PD-L1 overexpressed leads to an increase in FDG uptake rate, which was rescued by tofacitinib incubation (Fig. 4h). To further validate this result, we applied SeaHorse analysis to determine the glycolysis rate. As shown in Fig. 4i, overexpressed PD-L1 was accompanied by increased Extracellular acid ratio (ECAR) levels. Both the glycolysis rate and glycolytic reserve were increased in the PD-L1 overexpression group and were rescued by tofacitinib incubation (Fig. 4j, k). These results suggested that PD-L1 promotes FDG uptake, and has a dependence on the JAK-STAT pathway.
Patient population
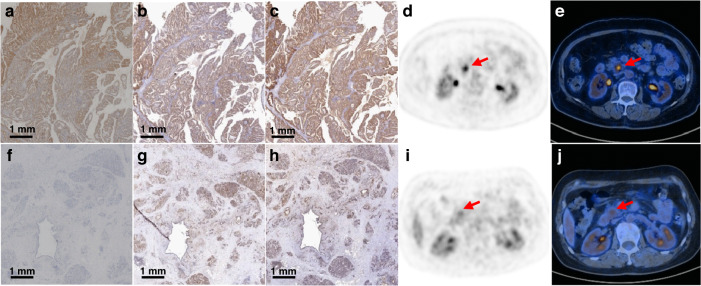
To further verify the above mechanism, we collected clinical patient data. Patient and tumour characteristics are listed in Supplementary Table S2. The median age of the patients was 65 years (range: 41–83 years). Disease in Stages I–II accounted for 31 (66.0%) cases, while Stage III–IV was identified in 16 (34.0%) cases. The SUVmax of the primary tumours ranged from 3.0 to 17.8, with an average of 7.05. Of the 47 primary tumours, 9 (19.1%) were PD-L1 positive in TCs, and 15 (31.9%) were PD-L1 positive in TIICs. Representative PET/CT and IHC staining images of PD-L1 with high- and low expression are shown in Fig. 5. The expression and activation status of STAT3 was also verified.
Fig. 5. Representative images of the IHC and PET/CT.
a IHC of PD-L1 in the PD-L1-positive patient. b IHC of STAT in the PD-L1-positive patient. c IHC of p-STAT in the PD-L1-positive patient. d 18F-FDG PET tomography image in the PD-L1-positive patient. e PET/CT fusion image in the PD-L1-positive patient. f IHC of PD-L1 in the PD-L1-negative patient. g IHC of STAT in the PD-L1-negative patient. h IHC of p-STAT in the PD-L1-negative patient. i 18F-FDG PET tomography image in the PD-L1-negative patient. j PET/CT fusion image in the PD-L1-negative patient.
Correlation between metabolic parameters and PD-L1 expression
The expression of PD-L1 in the 47 tumours was evaluated by immunohistochemical analysis. There was a positive association between SUVmax from PET/CT imaging and expression of PD-L1 in TCs (Fig. 6a). Tumours with PD-L1 positive showed higher values of SUVmax than those with PD-L1 negative (6.1 ± 2.3 and 11.1 ± 4.2, respectively; P < 0.001). Next, the optimal SUVmax thresholds for predicting PD-L1 expression in TCs were determined. Receiver operating characteristic (ROC) curve analysis showed that the highest accuracy (91.5%) for predicting PD-L1 expression in TCs was obtained with a SUVmax cut-off value of 8.15 that resulted in an area under the curve (AUC) of 0.872 ± 0.084. The sensitivity and specificity for predicting PD-L1 expression were 77.8% (7/9) and 94.9% (36/38), respectively (Fig. 6b).
Fig. 6. The association between 18F-FDG accumulation and PD-L1 status in TCs/TIICs of pancreatic ductal adenocarcinoma (n = 47).
a The association between SUVmax and PD-L1 status in TCs. b ROC analysis of SUVmax for predicting PD-L1 status in TCs. c The association between SUVmax and PD-L1 status in TIICs. d ROC analysis of SUVmax for predicting PD-L1 status in TIICs. e TLG in TCs (45.97 ± 73.80 vs. 192.2 ± 183.6, P < 0.001). f ROC of TLG for predicting PD-L1 status in TCs (cut-off = 48.78, AUC = 0.860 ± 0.062). g TLG in TIICs (47.24 ± 76.58 vs. 131.0 ± 163.3, P = 0.020). h ROC of TLG for predicting PD-L1 status in TIICs (cut-off = 39.33, AUC = 0.731 ± 0.082). i Total SUVmax in TCs (8.23 ± 4.92 vs. 24.10 ± 20.38, P < 0.001). j ROC of total SUVmax for predicting PD-L1 status in TCs (Cut-off = 7.70, AUC = 0.819 ± 0.090). k Total SUVmax in TIICs (9.01 ± 6.20 vs. 16.09 ± 17.62, P = 0.048). l ROC of total SUVmax for predicting PD-L1 status in TIICs (cut-off = 9.45, AUC = 0.622 ± 0.091).
In TIICs, the SUVmax was higher in patients with high PD-L1 expression than in those with low expression (6.4 ± 3.2 and 8.4 ± 3.5, respectively; P < 0.001) (Fig. 6c). ROC curve analysis showed that the highest accuracy (74.5%) for predicting PD-L1 expression in TIICs was obtained with a SUVmax cut-off value of 7.75 which resulted in an area under the curve of 0.691 ± 0.084. The sensitivity and specificity for predicting PD-L1 expression in TIICs were 53.3% (6/15) and 84.9% (29/31), respectively (Fig. 6d).
To further evaluate the association between PD-L1 and metabolic burden, we applied additional parameters, including TLG and total SUVmax. As shown in Fig. 4e–l, both TLG and total SUVmax were higher in PD-L1 positive lesions, regardless PD-L1 was expressed in TCs or TIICs. These results suggest that 18F-FDG PET/CT may be useful in the assessment of the expression of PD-L1 in pancreatic ductal adenocarcinoma.
Correlations between patient characteristics and expression of PD-L1
Correlations between the clinical characteristics of the 47 patients and the expression of PD-L1 in TCs/TIICs were evaluated by univariate analysis (Table 1). No significant differences in gender, age, tumour size, lymph node metastasis, or distant metastasis were found between the PD-L1-positive and PD-L1-negative groups in TCs/ TIICs. However, the groups differed significantly in terms of SUVmax, TLG, and total SUVmax from PET/CT imaging (Table 1). In multivariate analysis, SUVmax and TLG were significantly correlated with PD-L1 expression in TCs, whereas TLG was correlated with PD-L1 expression in TIICs (Supplementary Table S3).
Table 1.
Univariate analysis of the relationship between PD-L1 expression in TC and TIIC with disease variables (n = 47).
| Variable | PD-L1 expression in TCs | PD-L1 expression in TIICs | |||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | P value | Negative | Positive | P value | ||
| Gender | 0.919 | 0.597 | |||||
| Female | 12 | 3 | 11 | 4 | |||
| Male | 26 | 6 | 21 | 11 | |||
| Age (years) | 65.3 ± 9.2 | 68.9 ± 5.9 | 0.273 | 66.8 ± 9.6 | 64.2 ± 6.7 | 0.346 | |
| T stage | 0.464 | 0.465 | |||||
| T1/T2 | 26 | 5 | 20 | 11 | |||
| T3/T4 | 12 | 4 | 12 | 4 | |||
| N stage | 0.184 | 0.377 | |||||
| N0 | 22 | 3 | 18 | 7 | |||
| N1/N2 | 16 | 6 | 14 | 8 | |||
| M stage | 0.060 | 0.749 | |||||
| M0 | 29 | 4 | 22 | 11 | |||
| M1 | 9 | 5 | 10 | 4 | |||
| Clinical stage | 0.130 | 0.944 | |||||
| I/II | 27 | 4 | 21 | 10 | |||
| III/IV | 11 | 5 | 11 | 5 | |||
| SUVmax | <0.001 | 0.006 | |||||
| >8.15 | 2 | 7 | >7.75 | 5 | 8 | ||
| ≤8.15 | 36 | 2 | ≤7.75 | 27 | 7 | ||
| TLG | 0.002 | 0.003 | |||||
| >48.78 | 10 | 8 | >39.33 | 9 | 11 | ||
| ≤48.78 | 28 | 1 | ≤39.33 | 23 | 4 | ||
| Total SUVmax | <0.001 | 0.058 | |||||
| >7.70 | 13 | 8 | >9.45 | 8 | 7 | ||
| ≤7.70 | 25 | 1 | ≤9.45 | 24 | 8 | ||
Discussion
PD-L1 is a transmembrane protein and can be expressed in tumour cells. Previous studies mostly focused on the PD-L1/PD-1 axis, believing that the function of PD-L1 is mainly to bind to PD-1 and act on T cells. When PD-L1 on the cell membrane binds with PD-1 on T cells and other immune cells, tumour cells s end out inhibitory signals, so T cells cannot recognise tumour cells and cannot kill tumour cells, leading to immune escape of tumour [22]. Due to the short intracellular segment of PD-L1, few scholars have studied the signal transduction in tumour cells mediated by PD-L1 [22]. Until a report by Cell in 2015, tumour cells were treated with PD-L1 mab in vitro, and it was found that PD-L1 originally located in the cell membrane entered the cytoplasm, and Akt and other signalling pathways were subsequently inhibited [11]. This literature points out a new direction for PD-L1 research. In recent years, a series of studies on PD-L1 as a signal molecule have been published, and the signal conduction function of PD-L1 has become a new research hotspot [23, 24].
Metabolic reprogramming is one of the hallmarks of cancer and has been a hot research topic in the past decade [25]. Aerobic glycolysis is reckoned as the main metabolic pathway which exhibits plasticity in PDAC [26]. Increased glycolysis rates provide cancer cells with the energy and carbon sources they need to sustain rapid proliferation [9]. Accumulating evidence suggests that PD-L1 promotes glycolysis and regulates glucose metabolism in human cervical cancer, bladder cancer, leukaemia and other tumours [27–29]. In this study, short hairpin RNA (shRNA) interferes with PD-L1 gene expression, resulting in decreased glucose uptake and protein level of glycolysis-related enzymes in PDAC cells. These pieces of evidence suggest that PD-L1 plays a certain role in tumour glucose metabolism.
The WGCNA is a comprehensive collection of R functions for seeking coexpressed gene modules, and exploring the association between gene network and the phenotype of concern, as well as the core genes in the network [30]. We applied this method to analyse the data set from GEO and found a series of signalling pathways associated with glucose uptake and PD-L1 expression in PDAC. Further verification by GSEA identified JAK-STAT as one of the key signalling pathways.
JAK-STAT signalling is an important pathway in cancer progression, both an internal driver of tumour growth/metastasis and a regulator of immune monitoring [31]. Gene expression of glucose transporters, hexokinases, pyruvate kinases, and other key glycolysis enzymes are regulated by the JAK-STAT pathway [32]. As transcription factors in the JAK-STAT signalling pathway, there are six proteins in the STATs family [33]. Using TIMER analysis, we found that STAT1 and STAT3 had a high correlation with PD-L1 mRNA levels, and had a considerable expression amount in PDAC. In one of our previous studies, it has been confirmed that STAT1 acts as a transcription factor of PD-L1 [10]. In this study, we found that the protein level of STAT3 was regulated by PD-L1 knockdown. Moreover, by blocking the JAK-STAT3 pathway with inhibitor, the function of PD-L1 to promote glucose uptake of PDAC was rescued. These results indicate that PD-L1 regulates tumour glucose uptake through JAK-STAT3 pathway (Fig. 7).
Fig. 7. Mechanism diagram of this study.
PD-L1 promotes the expression of GLUT1 and HK2, the key enzymes of glycolysis, by activating the JAK-STAT pathway, thereby promoting glycolysis and ultimately leading to the increased glucose uptake in pancreatic ductal adenocarcinoma cells.
To further verify the validity of this conclusion, we analysed the 18F-FDG PET/CT and pathological immunohistochemical data of 47 patients with PDAC. We found that PD-L1 was correlated with 18F-FDG uptake, and PET/CT could be used to predict PD-L1 expression of PDAC in TCs and TIICs (The sensitivity and specificity for predicting PD-L1 expression in TCs were 77.8% (7/9) and 94.9% (36/38); the sensitivity and specificity for predicting PD-L1-TIICs expression were 53.3% (6/15) and 84.9% (29/31)). 18F-FDG PET/CT may be a useful alternative to PD-L1 protein expression in pancreatic ductal adenocarcinoma patients. 18F-FDG PET/CT as a noninvasive diagnostic tool has been widely used in the preoperative evaluation and prognosis of pancreatic ductal adenocarcinoma. As no PD-L1 imaging agent has been formally introduced into clinical practice, this study provides new implications for the grouping of immunotherapy-related clinical studies on PDAC, that is, pre-grouping of patients according to the metabolic status of 18F-FDG PET/CT, and then re-evaluating the effectiveness of immunotherapy.
Conclusion
PD-L1 regulates the expression of GLUT1 and HK2, the key enzymes of glycolysis, by activating the JAK-STAT pathway, thereby promoting glycolysis of pancreatic ductal adenocarcinoma cells and ultimately leading to the reorganisation of pancreatic ductal adenocarcinoma glucose metabolism (Fig. 7). This study revealed the mechanism of PD-L1 regulation of glucose metabolism recombination in pancreatic ductal adenocarcinoma from the perspective of the tumour cell signalling pathway, and the research results will provide a new theoretical basis for the selection of combined immunotherapy targets for pancreatic ductal adenocarcinoma.
Supplementary information
Acknowledgements
We are grateful to the physicians, nurses, and all the patients who participated in this research.
Author contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by Jiajin Li, XiaQing, Cheng Wang, Liangrong Wan and Haiqin Bao. The first draft of the manuscript was written by Jiajin Li, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The study was sponsored by the National Key Research and Development Program of China (2021YFA0910000 and 2020YFA0909000), the Interdisciplinary Program of Shanghai Jiao Tong University (ZH2018QNA41), and the National Natural Science Foundation of China (Nos. 81771858 and 82171972).
Data availability
All analysed and derivative raw data are available on request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were approved by the Institutional Review Board of Shanghai Jiao Tong University-affiliated Ren Ji Hospital and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not describe any studies with animals performed by any of the authors.
Consent for publication
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiajin Li, Ruohua Chen, Yumei Chen.
Contributor Information
Gang Huang, Email: huang2802@163.com.
Jianjun Liu, Email: nuclearj0817@126.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02297-9.
References
- 1.Bear AS, Vonderheide RH, O’Hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020;38:788–802. doi: 10.1016/j.ccell.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds EE, Doubeni CA, Sawhney MS, Kanjee Z. Should this patient be screened for pancreatic cancer?: grand rounds discussion from Beth Israel deaconess medical center. Ann Intern Med. 2020;173:914–21.. doi: 10.7326/M20-6384. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Dang F, Ren J, Wei W. Biochemical aspects of PD-L1 regulation in cancer immunotherapy. Trends Biochem Sci. 2018;43:1014–32.. doi: 10.1016/j.tibs.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–8. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 2021;12:108–33. [DOI] [PMC free article] [PubMed]
- 7.Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. [DOI] [PMC free article] [PubMed]
- 8.Li J, Yin L, Chen Y, An S, Xiong Y, Huang G, et al. PD-L1 correlated gene expression profiles and tumor infiltrating lymphocytes in pancreatic cancer. Int J Med Sci. 2021;18:3150–7. doi: 10.7150/ijms.61771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell. 2017;31:5–19. doi: 10.1016/j.ccell.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Xia Q, Jia J, Hu C, Lu J, Li J, Xu H, et al. Tumor-associated macrophages promote PD-L1 expression in tumor cells by regulating PKM2 nuclear translocation in pancreatic ductal adenocarcinoma. Oncogene. 2021;41:865–77. doi: 10.1038/s41388-021-02133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C-H, Qiu J, O’Sullivan D, Buck Michael D, Noguchi T, Curtis Jonathan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Zhou X, Liu J, Huang G. Relationship between the expression of PD-1/PD-L1 and (18)F-FDG uptake in bladder cancer. Eur J Nucl Med Mol imaging. 2019;46:848–54.. doi: 10.1007/s00259-018-4208-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Chen Y, Huang G, Liu J. Relationship between PD-L1 expression and (18)F-FDG uptake in gastric cancer. Aging. 2019;11:12270–7. doi: 10.18632/aging.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi R, Bao X, Unger K, Sun J, Lu S, Manapov F, et al. Identification and validation of hypoxia-derived gene signatures to predict clinical outcomes and therapeutic responses in stage I lung adenocarcinoma patients. Theranostics. 2021;11:5061–76.. doi: 10.7150/thno.56202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–22. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Z, Cao Z, Zhang E, Huang H, Tang Y. Elevated CDK5R1 predicts worse prognosis in hepatocellular carcinoma based on TCGA data. Biosci Rep. 2021;41:BSR20203594. [DOI] [PMC free article] [PubMed]
- 17.Lucignani G, Paganelli G, Bombardieri E. The use of standardized uptake values for assessing FDG uptake with PET in oncology: a clinical perspective. Nucl Med Commun. 2004;25:651–6. doi: 10.1097/01.mnm.0000134329.30912.49. [DOI] [PubMed] [Google Scholar]
- 18.Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 19.Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR. 2010;31:496–505. doi: 10.1053/j.sult.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Zhang R, Gong W, Wang C, Zeng C, Zhai Y, et al. Positron emission tomography-computed tomography parameters predict efficacy of immunotherapy in head and neck squamous cell carcinomas. Front Oncol. 2021;11:728040. doi: 10.3389/fonc.2021.728040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359–70.. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52.. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Qin B, Moyer AM, Nowsheen S, Tu X, Dong H, et al. Regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res. 2020;30:590–601. doi: 10.1038/s41422-020-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, Nihira NT, Bu X, Chu C, Zhang J, Kolodziejczyk A, et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat Cell Biol. 2020;22:1064–75.. doi: 10.1038/s41556-020-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50. doi: 10.1186/s12943-020-01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y, et al. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin beta4/SNAI1/SIRT3 signaling pathway. Oncogene. 2018;37:4164–80.. doi: 10.1038/s41388-018-0252-x. [DOI] [PubMed] [Google Scholar]
- 28.Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J, et al. Retinoic acid-related orphan receptor C regulates proliferation, glycolysis, and chemoresistance via the PD-L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer Res. 2019;79:2604–18.. doi: 10.1158/0008-5472.CAN-18-3842. [DOI] [PubMed] [Google Scholar]
- 29.Ma P, Xing M, Han L, Gan S, Ma J, Wu F, et al. High PDL1 expression drives glycolysis via an Akt/mTOR/HIF1alpha axis in acute myeloid leukemia. Oncol Rep. 2020;43:999–1009. doi: 10.3892/or.2020.7477. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Ma T, Zou D. Identification of unique transcriptomic signatures and hub genes through RNA sequencing and integrated WGCNA and PPI network analysis in nonerosive reflux disease. J Inflamm Res. 2021;14:6143–56.. doi: 10.2147/JIR.S340452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks AJ, Putoczki T. JAK-STAT signalling pathway in cancer. Cancers. 2020;12:1971. doi: 10.3390/cancers12071971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valle-Mendiola A, Soto-Cruz I. Energy metabolism in cancer: the roles of STAT3 and STAT5 in the regulation of metabolism-related genes. Cancers. 2020;12:124. doi: 10.3390/cancers12010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodington DW, Desai HR, Woo M. JAK/STAT—emerging players in metabolism. Trends Endocrinol Metab: TEM. 2018;29:55–65. doi: 10.1016/j.tem.2017.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analysed and derivative raw data are available on request.