Abstract
Background
The study aimed to examine the significance of insulin receptor (INSR) expression in predicting resistance to axitinib in clear cell renal cell carcinoma (ccRCC).
Methods
Clinicopathological data were collected from 36 consecutive patients with metastatic RCC who received axitinib. Thirty-three primary tumours were obtained for immunohistochemistry. Patient-derived xenograft (PDX) models were created by transplanting primary tumours into immunodeficient mice, establishing axitinib-resistant PDX models. RCC cell lines were co-cultured with human renal glomerular endothelial cells (HGECs) treated with siRNA of INSR (HGEC-siINSR). Gene expression alteration was analysed using microarray.
Results
The patients with low INSR expression who received axitinib had a poorer outcome. Multivariate analysis showed that INSR expression was the independent predictor of progression-free survival. INSR expression decreased in axitinib-resistant PDX tumours. RCC cell lines showed upregulated interferon responses and highly increased interferon-β levels by co-culturing with HGEC-siINSR. HGECs showed decreased INSR and increased interferon-β after axitinib administration. RCC cell lines co-cultured with HGEC-siINSR showed high programmed death-ligand 1 (PD-L1) expression, which increased after interferon-β administration.
Conclusions
Decreased INSR in RCC could be a biomarker to predict axitinib resistance. Regarding the resistant mechanism, vascular endothelial cells with decreased INSR in RCC may secrete interferon-β and induce PD-L1.
Subject terms: Renal cell carcinoma, Molecular biology
Background
The management of metastatic renal cell carcinoma (mRCC) has changed after introducing immune-oncology (IO) drugs, such as anti-programmed death-1 (PD1) antibodies [1]. Furthermore, the combination therapy of anti-PD-1 and anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) antibodies [2] and recently, the several combination therapies of VEGFR-TKI and anti-PD1 antibody [3–5] or anti-programmed death-ligand 1 (PD-L1) antibody [6] showed significantly higher objective response rate (ORR) and longer progression-free survival (PFS) and overall survival (OS) than sunitinib and are recommended as first-line systemic therapies for mRCC. Therefore, monotherapy with VEGFR-TKIs is rarely used as the first-line therapy presently. However, VEGFR-TKIs, including axitinib, are combined with IO-drug as the first-line therapy and are mostly used as subsequent therapy after IO combination therapy. Furthermore, VEGFR-TKI monotherapy should be selected as the first-line treatment in patients with mRCC with contraindication for IO drugs [7]. Therefore, it is important to identify the factors predicting the efficacy or resistance of VEGFR-TKIs.
Molecular targeted agents, particularly VEGFR-TKIs, provided longer PFS for patients with mRCC in several pivotal clinical trials [8–11] and longer OS than those with cytokine therapy in retrospective studies [12, 13]. Thus, molecular targeted agents have made a substantial impact by providing longer survival to patients with mRCC. However, a certain number of patients with mRCC do not respond to these agents at all, leading to poor outcomes irrespective of subsequent therapies, called primary refractory diseases [14, 15].
Several established risk models are used to predict prognosis and help choose systemic therapy for patients with mRCC, including the Memorial Sloan Kettering Cancer Center (MSKCC) risk model and the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk model. However, few biomarkers to predict the effectiveness of molecular targeted agents for patients with mRCC have been reported [16]. The mechanisms of resistance to VEGFR-TKIs are unclear.
We previously identified the prognostic gene set of patients with clear cell RCC using a cDNA microarray [17]. The results of an additional microarray study with an increased number of patients (data not shown) prompted us to examine further the role of insulin receptor (INSR) expression in the prognostic gene set. After, we sought to elucidate the underlying molecular mechanisms with which the low expression of INSR induces resistance to axitinib using patient-derived xenograft (PDX) mouse models and RCC cell lines. This was because it is essential to observe the interaction between tumour cells and the surrounding stromal tissues, including tumour vessels, in vivo. In addition, we discussed the association between INSR and the inflammatory process since insulin has anti-inflammatory effects [18].
Methods
Bioinformatics analyses
Data on INSR expression and OS of 520 patients with RCC were extracted from publicly available gene expression data sets of The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) and cBioportal (https://www.cbioportal.org/). The study followed the publication guidelines provided by TCGA (http://cancergenome.nih.gov/publications/publicationguidelines). The patients were divided based on the median INSR expression into low- and high-expression groups; OS was compared between the two groups.
Patients’ characteristics
Clinicopathological data were retrospectively collected from the medical records of 36 consecutive patients with metastatic RCC who started to receive axitinib from January 2008 to April 2015 at Tokushima University Hospital (Tokushima, Japan) (Table 1). Tissues were obtained by radical nephrectomy (n = 28) or percutaneous biopsy (n = 5) from 33 primary tumours before administering axitinib. Written informed consent was obtained from all patients. The ethical review board of Tokushima University Hospital (permission number 709) approved the study, which was conducted according to the ethical standards of the Declaration of Helsinki.
Table 1.
Patients’ characteristics.
| Patient age, years, median (range) | 70 (36–84) | |
| Sex (n, %) | Male | 22 (61.1%) |
| Female | 14 (38.9%) | |
| Nephrectomy (n, %) | Yes | 29 (80.6%) |
| No | 7 (19.4%) | |
| Histology (n, %) | Clear cell | 27 (75.0%) |
| Clear cell + sarcomatoid | 3 (8.3%) | |
| Collecting duct | 2 (5.6%) | |
| Papillary | 1 (2.8%) | |
| Sarcomatoid | 1 (2.8%) | |
| MTSCC | 1 (2.8%) | |
| Unknown | 1 (2.8%) | |
| Treatment line (n, %) | First line | 2 (5.6%) |
| Second line | 21 (56.8%) | |
| Third line | 10 (27.8%) | |
| ≥Fourth line | 3 (8.3%) | |
| Prior therapy (n, %) | Sunitinib | 16 (44.4%) |
| Interferon-α | 12 (33.3%) | |
| Sorafenib | 8 (22.2%) | |
| Temsirolimus | 7 (19.4%) | |
| Everolimus | 7 (19.4%) | |
| Interferon-α + IL-2 | 1 (2.8%) | |
| Gemcitabine + Carboplatin | 1 (2.8%) | |
MTSCC mucinous tubular and spindle cell carcinoma, Treatment line treatment line of axitinib.
Cell lines
Considering the mechanism of action of axitinib, a selective VEGFR-TKI, various RCC cell lines originating from clear cell RCC were chosen. Caki-1 (RRID: CVCL_0234) and 769-P (RRID: CVCL_1050) cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). The A704 (RRID: CVCL_1065), KMRC-1 (RRID: CVCL_2983), and KMRC-20 (RRID: CVCL_2986) cell lines were purchased from the JCRB cell bank (Osaka, Japan). Human glomerular microvascular endothelial cells (HGECs) were purchased from Cell Systems (Kirkland, WA). All cells were cultured as recommended by the respective sources.
Establishment of a patient-derived xenograft model
Tumour tissues obtained by radical nephrectomy were immediately minced and directly inoculated subcutaneously into the 6–8-week-old SCID male mice (Charles River Laboratories, Yokohama, Japan) in the animal facility at the University of Tokushima. The body weight of the mice used for the experiment was 24.8 ± 1.6 g (mean ± SD). Five mice were housed in one cage under a strict light–dark cycle (light on at 9:00 and off at 21:00 automatically). The animal room was maintained at a constant temperature (22 ± 1 °C) and humidity (50 ± 15%). The mice were fed a standard diet as needed, including filtered tap water using an automatic water supply system. PDX tumours were serially transplanted for tumour expansion. After tumour size on the mice was increased to approximately 200 mm3 [calculated as 1/2 × (length × width2)], 30 mg/kg of axitinib, determined by referring to the previous report [19], were orally administered twice daily. According to a previous report [20], the PDX tumours were resected, showing resistance to axitinib by re-growing after shrinkage, and administered a mixture of medetomidine (0.75 mg/kg body weight), midazolam (4 mg/kg body weight) and butorphanol (5 mg/kg body weight), to relieve the associated pain. The mice were euthanised by cervical dislocation. To establish axitinib-resistant PDX tumour models, five mice were used. INSR expression was compared between axitinib-resistant and axitinib-sensitive PDX tumours using Western blotting.
The ethical review board of Tokushima University Hospital (permission number: 1841) and the Institutional Animal Care and Use Committee (Permission number: T2019-56) of Tokushima University approved the animal experiments. All animal experiments were performed according to the guidelines of the animal facility at the University of Tokushima, which conforms to the Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Immunohistochemistry (IHC)
Sections of paraffin-embedded RCC specimens and PDX tissues were deparaffinised in xylene and rehydrated in a graded ethyl alcohol series (100%, 95%, and 90%). Endogenous peroxide activity was blocked with 0.3% hydrogen peroxide solution in 50% methanol at room temperature for 10 min. Antigen retrieval was performed with the recommended buffer using an autoclave at 120 °C for 10 min followed by cooling down at room temperature for 30 min. After cooling, the slides were rinsed in distilled water and 0.01 mol/l phosphate-buffered saline (PBS, pH 7.4) and incubated with a protein-blocking solution for 5 min. Sections were incubated with primary antibodies against INSR (ab69508, Abcam, Cambridge, MA; diluted 1:1000 in PBS) and CD31 (anti-human: clone JC70A, Agilent Dako Glostrup, Denmark; diluted 1:100 in PBS for human specimens, anti-mouse: clone MEC 13.3, BD Biosciences, Franklin Lakes, NJ; diluted 1:100 in PBS for PDX tumours) overnight at 4 °C. After, they were washed three times in PBS and incubated with 1–2 drops of Dako REALTM EnVisionTM/HRP, Rabbit/Mouse (ENV) reagent (DAKO) for 60 min at room temperature. They were then washed again with PBS and incubated with diaminobenzidine (DAB) and substrate chromogen system for 5 min at room temperature, resulting in brown-coloured precipitates at the antigen site. Sections were then counterstained with haematoxylin for 1 min and mounted.
Immunofluorescence
Sections of PDX tumours were fixed with 2% paraformaldehyde phosphate-buffered saline (PBS) solution (Wako, Osaka, Japan) at 4 °C for 10 min.
The sections were then incubated in 0.1% Triton X-100 in PBS at 4 °C for 2 min and blocked with 5% goat serum. Blocked sections were then incubated with primary antibody at 4 °C overnight and then with Alexa 568- and 488-conjugated secondary antibody (Thermo Fisher Scientific, Waltham, MA) at room temperature for 60 min. Fluorescent images were acquired using a Keyence BZ-9000 fluorescence microscope (Keyence Corporation, Osaka, Japan).
Evaluation of IHC
Two certified pathologists, blinded to clinical features and outcomes, evaluated each slide independently. The IHC expression intensity of INSR was scored on a scale of 0 (no staining), 1 (low), and 2 (high). The INSR staining pattern was scored on a scale of 0–2 (0: focal, 1: intermediate, and 2: diffuse). Specimens analysed using immunohistochemical staining were divided according to the sum of the INSR intensity and the staining pattern score into low (sum score ≤2) or high expression (sum score ≥3).
Quantitative and semi-quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from cultured cells using the RNeasy Mini Kit (QIAGEN, Valencia, CA), according to the manufacturer’s instructions. The appropriate dilutions of each single-stranded cDNA were prepared for subsequent polymerase chain reaction amplification, and reactions were monitored using β-actin as a quantitative control. Each sample was analysed in triplicate for each primer pair. Quantitative RT-PCR was carried out with a Light Cycler 350 S Real-Time PCR System (Roche Diagnostics, Madison, WI). The total volume of the reaction mixture was 20 μl, and it contained 10 μl of 2× SYBR Green Buffer, 0.2 μl of RT Mix, 1 μl of each primer (10 μM) and 1 μl of total RNA (0.01 μg/μl). The reaction mixture was first incubated at 50 °C for 15 min to allow for reverse transcription. PCR was then initiated at 95 °C for 10 min to activate modified Taq polymerase, followed by a 45-cycle amplification (95 °C for 15 s, 55 °C for 20 s and 72°C for 10 s) and 1 cycle (95 °C for 0 s, 65 °C for 15 s and 0.1 °C/s to 95 °C) for melting analysis. The relative expression levels of the target genes including INSR, INF-α, IFN-β, IFN-γ and PD-L1 to β-actin were obtained using Light Cycler Software Ver.3.5 (Roche Diagnostics, Madison, WI) and calculated using the 2 − ΔΔCt method. The sequences of each primer set are shown in Supplementary Table 2.
Western blot analysis
Cells were lysed using a Mammalian Cell Extraction kit (Bio Vision, Mountain View, CA). After centrifuging 14,000 × g for 10 min, the supernatant was collected as the total protein extract and stored at –80 °C until use. Equal amounts of protein were separated using SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was then blocked with Block Ace (KAC, Kyoto, Japan) for 60 min and incubated with anti-INSR (Abcam, ab69508; dilution 1:200), anti-β-actin (Sigma-Aldrich Japan KK, A5441, dilution 1:1000), anti-PD-L1 (Cell Signalling Technology, #13684, dilution 1:1000), anti-STAT1 (Cell Signalling Technology, #14994, dilution 1:1000), anti-STAT3 (Cell Signalling Technology, #8768, dilution 1:1000), anti-p-STAT1 (Ser727) (Cell Signalling Technology, #8826, dilution 1:1000), and anti-p-STAT3 (Tyr705) (Cell Signalling Technology, #9145, dilution 1:1000) primary antibodies at 4 °C overnight. After incubating with HRP-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA; dilution 1:1000) for 60 min, blots were developed with an enhanced ECL Prime Kit (GE Healthcare, Piscataway, NJ) and scanned using an Image Reader LAS-500 (Fujifilm, Tokyo, Japan).
RNA interference and co-culture
Small interfering RNA oligonucleotides were used to knock down INSR in HGECs. Non-silencing RNA duplex was used as a negative control. Transfection with 10 nM of two different small interfering RNA (siRNA1: 5072001002F #831, siRNA2: 6157001002F #852, Sigma-Aldrich Japan KK) of INSR to HGECs was performed using Lipofectamine RNAiMAX (Thermo Fisher Scientific) with the forward transfection method. The sequences of siRNAs were shown in Supplementary Table 3. The knockdown effect of siRNA at 72 h after transfection was evaluated by quantitative RT-PCR and Western blot analysis. After transfection, HGECs were seeded into 6-well ThinCert™ cell culture inserts, and 2.5 ml of Caki-1 cell suspension containing 8 × 104 cells/ml in CS-C Complete Medium was placed into a 6-well plate. The inserts were placed in each well of the 6-well plate, and co-cultures were maintained for 24 h.
Microarray analysis
Cyanine-3 (Cy3)-labelled cRNA was prepared from 150 ng RNA using the One-Color Low Input Quick Amp Labeling kit (Agilent, Santa Clara, CA) according to the manufacturer’s instructions; this was followed by RNAeasy column purification (QIAGEN). Dye incorporation and cRNA yield were checked using the NanoDrop ND-1000 Spectrophotometer.
After, 600 ng of Cy3-labelled cRNA (specific activity >6 pmol Cy3/µg cRNA) were fragmented at 60 °C for 30 min in a 25 µl reaction volume containing 25× Agilent fragmentation buffer and 10× Agilent blocking agent according to the manufacturer’s instructions. On completion of the fragmentation reaction, 25 µl of 2× Agilent hybridisation buffer was added and hybridised to Agilent SurePrint GE Unrestricted Microarrays (G2519F) for 17 h at 65 °C in a rotating Agilent hybridisation oven. After hybridisation, microarrays were washed for 1 min at room temperature with GE Wash Buffer 1 (Agilent) and 1 min with GE Wash buffer 2 (Agilent) at 37 °C; they were then dried immediately.
The slides were scanned immediately after washing on the Agilent DNA Microarray Scanner (G2505C) using the one colour scan setting for 8x60K array slides (scan area 61 × 21.6 mm2, scan resolution 3 µm, dye channel set to Green, and Green PMT was set to 100%).
The scanned images were analysed using Feature Extraction Software 10.7.1.1 (Agilent) using default parameters (protocol GE1_107_Sep09 and Grid: 028282_D_F_20110531) to obtain background-subtracted and spatially detrended processed signal intensities. Features flagged in Feature Extraction as Feature Non-uniform outliers were excluded.
Gene set enrichment analysis (GSEA)
The gene set enrichment analysis (GSEA; Broad Institute) was conducted by comparing the observed expression profiles to an a priori gene set with well-defined biological processes (Hallmark gene set) from the Molecular Signatures Database (MSigDB version 6.1) (https://www.ncbi.nlm.nih.gov/pubmed/26771021), using GSEA software version 2.2.3. An enrichment score (ES) was calculated that reflects the degree to which the predefined gene set is overrepresented at the extremes of the entire ranked list of transcripts in the sample. The reported false discovery rate (FDR) is the estimated probability that the observed normalised enrichment score (NES) constitutes a false positive result (https://www.ncbi.nlm.nih.gov/pubmed/16199517).
Statistical analysis
PFS and OS curves in patients with mRCC treated with axitinib were estimated using the Kaplan–Meier method, with significance assessed using the log-rank test. Multivariate Cox regression models were used to examine the significant factors to predict PFS and OS in patients with mRCC administered axitinib. Statistical significance was calculated using two-tailed Welch’s t test to compare interferon or PD-L1 expression between the two groups. Statistical analysis was performed using SPSS Statistics 25 (SPSS, IBM Corp, Armonk, NY). p < 0.05 was considered significant. Regarding the experiments with the PDX tumours, the minimum number of mice was used to confirm the correlation of INSR expression with axitinib resistance; therefore, statistical analysis could not be performed.
Results
Correlation of INSR expression and overall survival using TCGA data
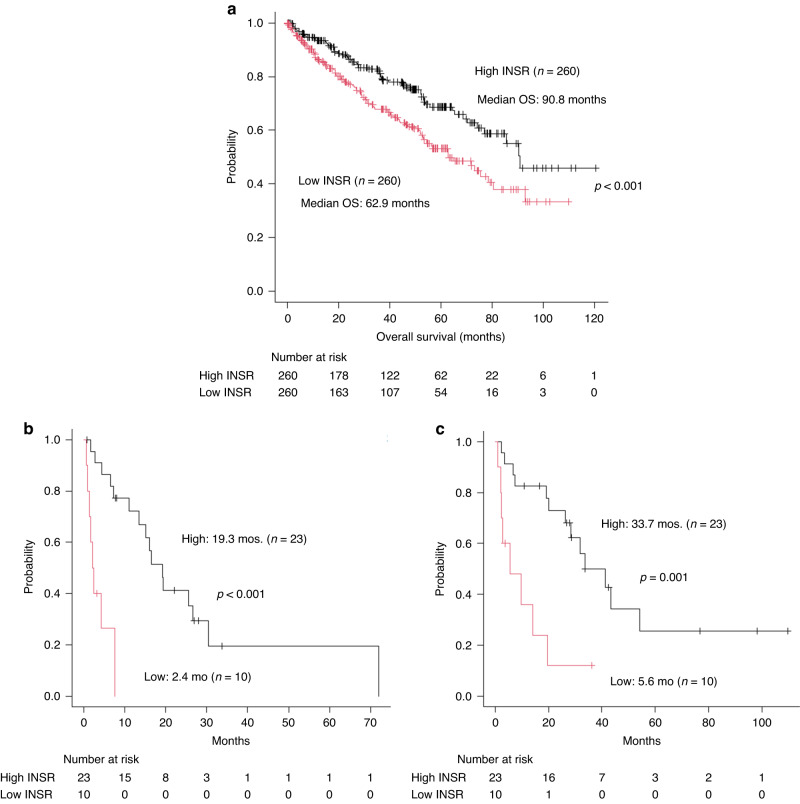
OS was analysed according to INSR expression with the data of 520 patients with RCC who underwent radical nephrectomy in the TCGA data set. The patients with higher INSR expression (n = 260) had significantly longer OS than those with lower INSR expression (n = 260) (median OS 90.8 vs. 62.9 months, p < 0.001) (Fig. 1a).
Fig. 1. Survival of patients with RCC according to INSR expression.
a Survival outcome related with INSR expression according to TCGA data. According to TCGA data, patients with RCC with higher INSR expression (n = 260) have significantly longer overall survival than those with lower INSR expression (n = 260) (median OS 92.1 months vs. 63.8 months, p < 0.001). Kaplan–Meier analysis shows that patients with metastatic renal cell carcinoma (mRCC) with low insulin receptor (INSR) expression who received axitinib have significantly worse progression-free survival (PFS) (median PFS 2.4 vs. 19.3 months, p < 0.0001) (b) and overall survival (OS) (median OS 5.6 vs. 33.7 months, p = 0.001) (c) than those with high INSR expression.
Immunohistochemical evaluation of INSR in mRCC patients with axitinib
INSR expression in the primary tumours (nephrectomy; n = 28, percutaneous biopsy; n = 5) obtained from patients with mRCC who received axitinib was evaluated using IHC. INSR was expressed in vascular endothelial cells inside a tumour, confirmed by the corresponding CD31 positive staining by using human nephrectomy specimens and merged images with immunofluorescence in PDX tumours (Fig. S1). Regarding clinicopathological factors, histology was significantly different between the two groups with low- or high-INSR expression, while other factors are similar (Supplementary Table 1). The group with low-INSR expression consisted of 6 clear cell RCC and 4 non-clear cell RCC (collecting duct carcinoma; n = 2, papillary RCC; n = 1, unclassified; n = 1), while that with high-INSR expression consisted of only clear cell RCC. Kaplan–Meier analysis showed that patients with mRCC with low INSR expression who received axitinib had significantly shorter PFS (median PFS 2.4 vs. 19.3 months, p < 0.0001) (Fig. 1b) and OS (median OS 5.6 vs. 33.7 months, p = 0.001) (Fig. 1c) than those with high INSR expression. Univariate analysis demonstrated that histology (p = 0.034), tumour shrinkage rate by axitinib (p < 0.001), MSKCC risk classification (p = 0.003), IMDC risk classification (p = 0.002), and INSR expression (p < 0.001) were the significant predictors for PFS. Multivariate analysis showed that INSR expression (p = 0.006, HR 6.689, 95% CI: 1.726–25.923) was the only significant independent predictor for PFS (Table 2). Regarding OS, prior nephrectomy (p = 0.006), histology (p = 0.003), tumour shrinkage rate by axitinib (p < 0.001), MSKCC risk classification (p < 0.001), IMDC risk classification (p = 0.004), and INSR expression (p = 0.001) were the significant prognostic factors on univariate analysis. On multivariate analysis, prior nephrectomy (p = 0.04, HR 4.464, 95% CI: 1.072–18.591), histology (p = 0.008, HR 15.809, 95% CI: 2.057–121.510), and MSKCC risk classification (p = 0.029, HR 9.404, 95% CI: 1.262–70.048) were the significant predictors for OS (Table 2).
Table 2.
Univariate and multivariate analyses for progression-free survival and overall survival.
| Univariate | Multivariate | ||
|---|---|---|---|
| p value | p value | HR (95% CI) | |
| Progression-free survival | |||
| Age (≥70 vs. <70 years) | 0.835 | ||
| Sex | 0.393 | ||
| Prior nephrectomy | 0.426 | ||
| Histology (non-clear vs. clear) | 0.034 | 0.196 | 3.086 (0.560–17.007) |
| No. of metastatic organs (≥3 vs. ≤2) | 0.571 | ||
| Treatment line (≥third vs. first + second) | 0.198 | ||
| MSKCC risk classification | 0.003 | 0.983 | 1.107 (0.208–4.983) |
| IMDC risk classification | 0.002 | 0.188 | 2.430 (0.649–9.105) |
| Insulin receptor (low vs. high) | <0.001 | 0.006 | 6.689 (1.726–25.923) |
| Overall survival | |||
| Age (≥70 vs. <70 years) | 0.890 | ||
| Sex | 0.497 | ||
| Prior nephrectomy | 0.006 | 0.040 | 4.464 (1.07–218.591) |
| Histology (non-clear vs. clear) | 0.003 | 0.008 | 15.809 (2.057–121.510) |
| No. of metastatic organs (≥3 vs. ≤2) | 0.289 | ||
| Treatment line (≥third vs. first + second) | 0.139 | ||
| MSKCC risk classification | <0.001 | 0.029 | 9.404 (1–26,270.048) |
| IMDC risk classification | 0.004 | 0.658 | 0.702 (0.147–3.363) |
| Insulin receptor (low vs. high) | 0.001 | 0.311 | 1.857 (0.561–6.151) |
MSKCC Memorial Sloan Kettering Cancer Center, IMDC International Metastatic Renal Cell Carcinoma Database Consortium.
INSR expression in the PDX tumour model
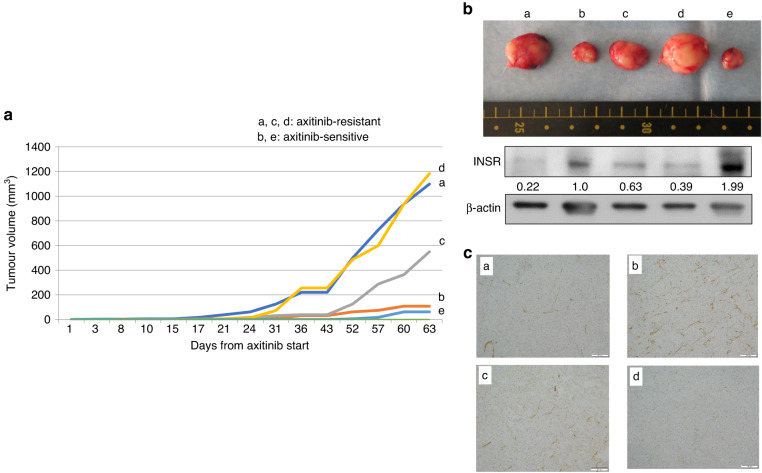
The rate of RCC specimens that could be engrafted and passaged at least once on SCID mice was 34.5% (data not shown). Using locally advanced clear cell RCC specimen (T3bN0M0), we established axitinib-resistant RCC PDX tumours. The growth curves for each PDX tumour are plotted and the axitinib-resistant (a, c, d) and -sensitive (b, e) PDX tumours are demonstrated in Fig. 2a. After, we compared INSR expression between axitinib-resistant and axitinib-sensitive PDX tumours. Axitinib-resistant tumours showed decreased INSR expression, whereas axitinib-sensitive tumours demonstrated substantial INSR expression using Western blot analysis (Fig. 2b). INSR immunohistochemistry showed very weak staining in tumour-a and tumour-d with rapid re-growth with axitinib administration, weak staining in tumour-c with moderate re-growth, and positive staining in tumour-b without re-growth (Fig. 2c). Tumour-e reduced greatly with axitinib and was not available for immunohistochemistry after usage for Western blot analysis. Adverse events associated with axitinib administration were not observed.
Fig. 2. INSR expression in axitinib-resistant or -sensitve PDX tumours.
a Growth curves of PDX tumours treated with axitinib. The growth curves for each PDX tumour are plotted and the axitinib-resistant and -sensitive PDX tumours are demonstrated. b INSR Western blot analysis. Axitinib-resistant PDX tumours (a, c, d) show decreased INSR expression, whereas axitinib-sensitive PDX tumours (b, e) maintain substantial INSR expression on Western blot analysis. Data represents the relative protein expression of INSR/β-actin. c INSR immunohistochemistry. INSR immunohistochemistry showed very weak staining in tumour-a and tumour-d with rapid re-growth with axitinib administration, weak staining in tumour-c with moderate re-growth, and positive staining in tumour-b without re-growth. Tumour-e reduced greatly with axitinib and was not available for immunohistochemistry after usage for Western blot analysis.
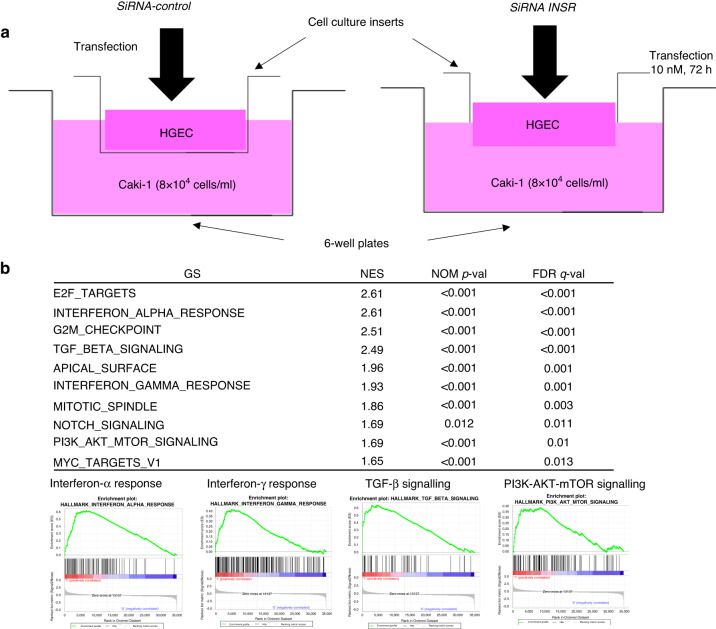
Alteration of gene expression levels in RCC cell lines co-cultured with HGECs with down-regulated INSR
Suppression of mRNA and protein INSR expression in HGECs transfected with siRNA1 of INSR was confirmed by RT-PCR (Fig. S2A) and Western blot analysis (Fig. S2B). A Caki-1 cell line was co-cultured with HGECs with down-regulated INSR (Fig. 3a). Microarray and GSEA analyses showed that several important signalling pathways were up-regulated in the Caki-1 cell line, including inflammation-related signalling pathways such as interferon-α and interferon-γ response, transforming growth factor-β (TGF-β) signalling, and phosphoinositide 3-kinase (PI3K)-AKT-mTOR signalling (Fig. 3b). Microarray profiles (GSE230698) have been registered in the GEO database (Gene Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo/).
Fig. 3. Alteration of gene expression in RCC cell lines cocultured with HGECs with down-regulated INSR.
a Co-culture model with HGECs and RCC cell line. HGECs transfected with siRNA of INSR and those with non-silencing RNA duplex as controls were seeded into 6-well ThinCert™ cell culture inserts, and Caki-1 cells were seeded into a 6-well plate in co-culture models. b GSEA analysis shows that several important signalling pathways, including inflammation-related signalling pathways such as interferon-α and interferon-γ response, TGF-β signalling, and PI3K-AKT-mTOR signalling, are up-regulated in a Caki-1 cell line co-cultured with human renal glomerular endothelial cells (HGECs) with down-regulated INSR.
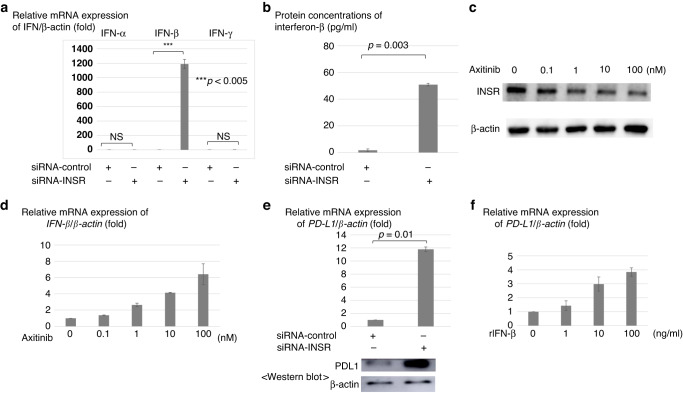
Interferon production by HGECs with down-regulated INSR
Based on the results of the above co-culture experiments, interferon expression of HGECs with down-regulated INSR were further examined. Among interferons, mRNA interferon-β expression in HGECs with down-regulated INSR was significantly increased by approximately 1200-fold, while mRNA interferon-α and γ expression was not increased (Fig. 4a). This phenomenon was confirmed by additional experiments with another siRNA of INSR (siRNA2), showing that this is not an off-target effect (Fig. S2C–E). Protein secretion of interferon-β in the HGECs supernatant with down-regulated INSR was also increased compared to control (Fig. 4b).
Fig. 4. Interferon-β and PD-L1 expressions associated with HGECs with down-regulated INSR or axitinib administration.
a Among interferons, mRNA interferon-β expression in HGECs with down-regulated INSR is greatly elevated by approximately 1200-fold more than the control. b Protein secretion of interferon-β in HGEC supernatant with down-regulated INSR is remarkably elevated than in the control. c In HGECs with axitinib, INSR protein expression of HGECs is decreased in a concentration-dependent manner on Western blot analysis. d In HGECs with axitinib, mRNA interferon-β expression of HGECs is increased concentration-dependently. e When a Caki-1 cell line is co-cultured with HGECs with down-regulated INSR, mRNA PD-L1 expression of the Caki-1 cell line is increased by more than ten times compared to the control along with increased protein PD-L1 expression. f When recombinant interferon-β is directly administered to a Caki-1 cell line, mRNA expression of PD-L1 in the Caki-1 cell line is increased concentration-dependently.
INSR and interferon-β expression of HGECs treated with axitinib
In axitinib-administered HGEC, INSR protein on Western blot analysis (Fig. 4c) and mRNA interferon-β (Fig. 4d) expression levels were decreased and increased concentration-dependently, respectively.
PD-L1 expression in RCC cell lines co-cultured with HGECs with down-regulated INSR or by direct interferon-β administration
In a Caki-1 cell line co-cultured with HGECs with down-regulated INSR, PD-L1 protein expression was increased by more than ten times (p = 0.01) (Fig. 4e). In addition, when recombinant interferon-β was directly administered to a Caki-1 cell line, mRNA PD-L1 expression in the Caki-1 cell line was increased in a concentration-dependent manner (Fig. 4f). Increased mRNA PD-L1 expression by recombinant interferon-β was also observed in other clear cell RCC cell lines, including KMRC1, KMRC20, A704, and 769-P (Fig. S3). Increased phosphorylation of STAT1 and STAT3, which are key molecules in the interferon-induced PD-L1 expression pathway, was also observed in a Caki-1 cell line after administering recombinant interferon-β (Fig. S4). Increased STAT1 and STAT3 phosphorylation were also observed in other clear cell RCC cell lines, including KMRC1, KMRC20, A704, and 769P, although STAT1 phosphorylation in 769P was slightly increased (Fig. S5).
Discussion
The mainstay of systemic therapy has shifted from molecular targeted therapy, including VEGFR-TKI, to IO drug combination therapy, leading to higher ORR and longer PFS and OS [2–6]. However, VEGFR-TKIs, including axitinib, are combined with an IO-drug as the first-line therapy, and playing a central role as the subsequent therapy after IO combination therapy. Furthermore, patients with mRCC with a contraindication for IO drugs should be administered VEGFR-TKI monotherapy [7]. VEGFR-TKIs monotherapy has several disadvantages, including a low complete response (CR) rate owing to an inability to eradicate tumour cells by their mechanism of action, primary refractory disease, and acquired resistance. Therefore, it is essential to determine the factors predicting the efficacy or resistance of VEGFR-TKIs.
Few biomarkers to predict the effectiveness of molecular targeted agents for patients with mRCC have been reported [16]. High expression levels of hypoxia-inducible factor 2α (HIF2α), platelet-derived growth factor β (PDGFRβ), and VEGF receptor 3 (VEGFR3) as hypoxia markers on IHC were associated with better objective response or longer PFS in patients with clear cell RCC after administering sunitinib. In contrast, PD-L1 expression, reported to predict a worse prognosis in patients with mRCC [21–23], may predict less treatment efficacy by sunitinib, confirmed by the randomised phase 3 clinical trial, which compared nivolumab plus ipilimumab with sunitinib for previously untreated advanced clear cell RCC (CheckMate214) [2]. In this pivotal clinical trial, in patients with mRCC who received sunitinib, those with higher PD-L1 expression (≥1%) had worse OS than those with lower PD-L1 expression (<1%). However, resistance mechanisms to VEGFR-TKIs remain unclear.
Our previous microarray study [17] shows that decreased INSR expression in the prognostic gene set could predict poor outcomes in patients with RCC. This was confirmed by the analyses with the TCGA data; therefore, further examination of the role of INSR in patients with mRCC was necessary.
First, to examine the correlation of INSR expression and efficacy of axitinib, the specimens from patients with RCC who received axitinib for metastasis or recurrence after nephrectomy was examined immunohistochemically. Patients with RCC with low INSR expression who received axitinib had poorer PFS and OS than those with high INSR expression. INSR expression was an independently significant prognostic factor for PFS, but not for OS by multivariate analyses. It may be plausible that INSR expression in the primary tumour before systemic therapy could not predict OS because of sequential therapy with various drugs and other confounding factors, such as age, performance status, and co-morbidity.
To further examine the association of RCC resistance to axitinib and INSR expression, PDX mouse models were established with locally advanced clear cell RCC specimens. Unlike the experiments with cell lines, the PDX model, including tumour stroma, reflects the real tumour environment, representing a faithful modelling system [24]. The principle of using mice to understand human biology is based on the physiological similarities between the species. The PDX models also supported the findings that decreased INSR expression in RCC indicates resistance to axitinib.
After, we sought to elucidate the underlying mechanism of INSR in axitinib resistance. On IHC, INSR was expressed in vascular endothelial cells inside renal tumours but not in the tumour cells. Since VEGFR-TKIs, including axitinib or sunitinib, act on VEGFR of vascular endothelial cells in renal tumours [25], the association of INSR expression in vascular endothelial cells with RCC cells was examined. Co-culture experiments with HGECs whose INSR were knocked down and the RCC cell lines to mimic tumour microenvironment showed that important signalling pathways, including interferon-α and interferon-γ response-related genes, as well as E2F target, TGF-β signalling, and PI3K-AKT-mTOR signalling, were up-regulated in the RCC cell lines.
Furthermore, we focused on the association between INSR and the interferons in HGECs. We newly found that mRNA and the secreted protein interferon-β level in HGECs with down-regulated INSR were greatly increased among the interferons. When axitinib was administered to HGECs, the same phenomenon of decreased INSR expression with increased interferon-β expression in HGECs was reproduced in a concentration-dependent manner. Several reports have described the association of interferon-β and AKT, a downstream molecule of the INSR. Seo et al. reported that interferon-β production was augmented when AKT was inhibited, indirectly supporting our findings [26].
Finally, we sought to figure out the correlation of interferon-β with PD-L1 expression, which is regarded as a predictor of poor response to VEGFR-TKIs in patients with mRCC. It is known that interferon-γ, secreted by T cells, induces PD-L1 expression in cancer cells and tumour-infiltrating macrophages [27, 28]. In addition, PD-L1 expression is induced by interferon-α, interferon-β, and interferon-γ exposure in melanoma cells. Importantly, we have shown that the increased interferon-β secretion by HGECs with down-regulated INSR induced RCC PD-L1 expression. This significant finding may help us understand axitinib’s resistance mechanism in RCC. Furthermore, insulin may have anti-inflammatory effects, and interference with insulin signalling may promote inflammation. Increased inflammatory mediators, including tumour necrosis factor-α and interleukin-6, reported to be poor prognostic factors in patients with RCC, may also be related to axitinib’s resistance mechanism [18].
This study had several limitations. First, the clinical data were retrospectively collected with possible selection biases; a small number of patients with mRCC treated with axitinib were analysed for survival outcomes according to INSR expression. Most tissues were obtained from nephrectomy (84.8%), while some were obtained from percutaneous biopsy (5.2%). Since it was reported that significant intra-tumour heterogeneity exists in RCC tumours [29], the examined tissue may not be representative of the whole tumour, particularly in the tissues obtained from a biopsy. The group of low-INSR expression included four patients with non-clear cell RCC who usually demonstrate poorer response to VEGFR-TKIs such as axitinib than those with clear cell RCC [30], while there were no patients with non-clear cell RCC in that of high-INSR expression. That may have affected the difference of PFS between the two groups although histological subtype was not a significant factor for PFS by multivariate analyses. Second, results on PDX models were not statistically evaluated because the number of axitinib-resistant and -sensitive PDX tumours was small. However, as the PDX models were only used to confirm our assumption that decreased INSR expression correlates with axitinib resistance in vivo, the number of mice was minimised for experiments. Third, HGECs were used in the co-culture experiment; however, they may not act like vascular endothelial cells in a renal tumour. Fourth, we have shown that interferon-β induced the increased PD-L1 expression through increased STAT1 and STAT3 phosphorylation. However, the experiment to confirm that interferon-β blocking should decrease STAT1 and STAT3 phosphorylation and PD-L1 expression will be required. Fifth, we did not conduct the study to examine the correlation of INSR expression with vessel density to show that INSR is not a surrogate marker of tumour angiogenesis. However, we observed the decreased INSR expression in RCC tumours with maintained vessel density. Sixth, axitinib was used for the experiments in this study, but the results may not apply to other multi-kinase inhibitors, including sunitinib, pazopanib or cabozantinib. However, since axitinib selectively targets VEGFR, it is an appropriate drug to examine the effect of VEGFR inhibition exclusively. Recently, the combination of IO drugs and VEGFR-TKIs, including axitinib, cabozantinib, and lenvatinib, has shown significant superiority to sunitinib regarding ORR, PFS and OS and has been approved as the first-line therapy for metastatic RCC. This combination therapy may overcome the resistance to axitinib induced by decreased INSR expression. In the subsequent therapy, cabozantinib, which inhibits Met, AXL, and VEGFR [31]; and the combination of lenvatinib and everolimus [32] demonstrated better improvement in OS than everolimus. Cabozantinib or lenvatinib and everolimus combination may be less associated with the resistant mechanism by decreased INSR expression. Conversely, as nivolumab and ipilimumab combination therapy is reported to be more effective in patients with PD-L1-positive mRCC [2], decreased INSR expression may be a predictive marker for those who receive nivolumab and ipilimumab combination therapy if it is confirmed that decreased INSR expression may induce PD-L1 expression. The above issues should be further examined and clarified.
Irrespective of the limitations mentioned above, in the present circumstance, where few biomarkers are available to predict resistance to VEGFR-TKIs, and VEGFR-TKIs still play an important role as a part of the first-line combination therapy or as the subsequent therapy after IO combination therapy; the findings that INSR might be a predictive marker for axitinib resistance, and interferon-β, secreted from vascular endothelial cells with down-regulated INSR inside renal tumours, may play a key role in the mechanism of axitinib resistance by inducing PD-L1 expression are novel and significant.
For clinical applications, two treatment strategies can be considered although it should be difficult to restore the decreased INSR. We have shown the upregulation of PI3K-AKT-mTOR signalling in a RCC cell line cocultured with HGECs with down-regulated INSR. Temsirolimus and everolimus are a mTOR inhibitor, already approved drugs for patients with mRCC and may be chosen for patients with mRCC with decreased INSR expression. In addition, we observed increased STAT-3 phosphorylation after administering recombinant interferon-β in RCC cell lines. As STAT-3 is central in regulating the anti-tumour immune response and plays important roles in inhibiting the expression of crucial immune activation regulators and promoting the production of immunosuppressive factors [33], combination of existing IO drugs and STAT-3 inhibitors may be considered for those patients with mRCC with decreased INSR expression.
In conclusion, decreased INSR expression in RCC may predict resistance to axitinib, resulting from increased PD-L1 expression induced by interferon-β secreted by vascular endothelial cells with down-regulated INSR in the RCC. Further examination of the role of INSR expression in RCC is warranted in the IO combination therapy era.
Supplementary information
Author contributions
M Takahashi planned the study, collected the patients’ data and tumour samples, obtained informed consent from the patients, analysed the results, and wrote the manuscript. KD planned and performed the experiments, collected tumour samples, obtained informed consent, analysed the results, and helped write the manuscript. TF planned the experiments, collected tumour samples, obtained informed consent, and helped write the manuscript. YF performed the experiments and helped write the manuscript. YB and HU performed immunohistochemical staining and evaluation. MK performed the bioinformatic analysis for the microarray data. T-OD performed immunohistochemical staining and animal experiments. YS, RT, YU, M Tsuda, YK, KY, and YY collected tumour samples, obtained informed consents, and helped write the manuscript. HK supervised the study as a whole and helped write the manuscript.
Funding
Funding was provided by a research grant from Novartis Pharma (grant numbers not applicable) and MSD (grant numbers not applicable).
Data availability
The data sets analysed during this study are available from the corresponding author upon reasonable request. Publicly available data sets were extracted from The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) and cBioportal (https://www.cbioportal.org/).
Competing interests
M Takahashi received honoraria from Pfizer Inc. and a research grant from Novartis Pharma and MSD. HK received honoraria from Pfizer Inc. Other authors have no conflict of interest.
Ethics approval and consent to participate
The study was approved by the ethical review board of Tokushima University Hospital (permission number 709) and conducted according to the ethical standards of the Declaration of Helsinki. The ethical review board of Tokushima University Hospital (permission number: 1841) and the Institutional Animal Care and Use Committee (Permission number: T2019-56) of Tokushima University approved the animal experiments. All of the animal experiments were performed according to guidelines of the animal facility at the University of Tokushima and conform to Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Consent for publication
Written informed consent for data usage and publication was obtained from all patients.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02325-8.
References
- 1.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–41. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aeppli S, Schmaus M, Eisen T, Escudier B, Grünwald V, Larkin J, et al. First-line treatment of metastatic clear cell renal cell carcinoma: a decision-making analysis among experts. ESMO Open. 2021;6:100030. doi: 10.1016/j.esmoop.2020.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (axis): a randomised phase 3 trial. Lancet. 2011;378:1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase iii trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 12.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium Prognostic Model: a population-based study. Lancet Oncol. 2013;14:141–8. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindskog M, Wahlgren T, Sandin R, Kowalski J, Jakobsson M, Lundstam S, et al. Overall survival in swedish patients with renal cell carcinoma treated in the period 2002 to 2012: Update of the rencomp study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35:541.e515–e522. doi: 10.1016/j.urolonc.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Busch J, Seidel C, Erber B, Issever AS, Hinz S, Kempkensteffen C, et al. Retrospective comparison of triple-sequence therapies in metastatic renal cell carcinoma. Eur Urol. 2013;64:62–70. doi: 10.1016/j.eururo.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Heng DY, Mackenzie MJ, Vaishampayan UN, Bjarnason GA, Knox JJ, Tan MH, et al. Primary anti-vascular endothelial growth factor (vegf)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol. 2012;23:1549–55. doi: 10.1093/annonc/mdr533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Donas J, Leandro-Garcia LJ, Gonzalez Del Alba A, Morente M, Alemany I, Esteban E, et al. Prospective study assessing hypoxia-related proteins as markers for the outcome of treatment with sunitinib in advanced clear-cell renal cell carcinoma. Ann Oncol. 2013;24:2409–14. doi: 10.1093/annonc/mdt219. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Rhodes DR, Furge KA, Kanayama H, Kagawa S, Haab BB, et al. Gene expression profiling of clear cell renal cell carcinoma: gene identification and prognostic classification. Proc Natl Acad Sci USA. 2001;98:9754–9. doi: 10.1073/pnas.171209998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Wilmes LJ, Pallavicini MG, Fleming LM, Gibbs J, Wang D, Li KL, et al. Ag-013736, a novel inhibitor of vegf receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging. 2007;25:319–27. doi: 10.1016/j.mri.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Saito Y, Takeuchi T. Anesthetic effects of a three-drugs mixture–comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp Anim. 2015;64:39–47. doi: 10.1538/expanim.14-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor b7-h1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RH, Dong H, Kwon ED. Implications of b7-h1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 23.Xu F, Xu L, Wang Q, An G, Feng G, Liu F. Clinicopathological and prognostic value of programmed death ligand-1 (pd-l1) in renal cell carcinoma: a meta-analysis. Int J Clin Exp Med. 2015;8:14595–603. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173:515.e7–28.e7. doi: 10.1016/j.cell.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27:3225–34. doi: 10.1200/JCO.2008.19.9836. [DOI] [PubMed] [Google Scholar]
- 26.Seo GJ, Yang A, Tan B, Kim S, Liang Q, Choi Y, et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep. 2015;13:440–9. doi: 10.1016/j.celrep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated b7-h1 promotes t-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 28.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of b7-h1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 29.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osterman CK, Rose TL. A systematic review of systemic treatment options for advanced non-clear cell renal cell carcinoma. Kidney Cancer. 2020;4:15–27. doi: 10.3233/KCA-190078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–23. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motzer RJ, Hutson TE, Ren M, Dutcus C, Larkin J. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol. 2016;17:e4–5. doi: 10.1016/S1470-2045(15)00543-4. [DOI] [PubMed] [Google Scholar]
- 33.Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting stat3 in cancer immunotherapy. Mol Cancer. 2020;19:145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analysed during this study are available from the corresponding author upon reasonable request. Publicly available data sets were extracted from The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) and cBioportal (https://www.cbioportal.org/).