Abstract
Background
Patients with heart failure with preserved ejection fraction (HFpEF) exhibit many cardiopulmonary abnormalities that could result in / mismatch, manifesting as an increase in alveolar dead space (VDalveolar) during exercise. Therefore, we tested the hypothesis that VDalveolar would increase during exercise to a greater extent in patients with HFpEF compared with control participants.
Research Question
Do patients with HFpEF develop VDalveolar during exercise?
Study Design and Methods
Twenty-three patients with HFpEF and 12 control participants were studied. Gas exchange (ventilation [E], oxygen uptake [o2], and CO2 elimination [co2]) and arterial blood gases were analyzed at rest, twenty watts (20W), and peak exercise. Ventilatory efficiency (evaluated as the E/co2 slope) also was measured from rest to 20W in patients with HFpEF. The physiologic dead space (VDphysiologic) to tidal volume (VT) ratio (VD/VT) was calculated using the Enghoff modification of the Bohr equation. VDalveolar was calculated as: (VD / VT × VT) – anatomic dead space. Data were analyzed between groups (patients with HFpEF vs control participants) across conditions (rest, 20W, and peak exercise) using a two-way repeated measures analysis of variance and relationships were analyzed using Pearson correlation coefficient.
Results
VDalveolar increased from rest (0.12 ± 0.07 L/breath) to 20W (0.22 ± 0.08 L/breath) in patients with HFpEF (P < .01), whereas VDalveolar did not change from rest (0.01 ± 0.06 L/breath) to 20W (0.06 ± 0.13 L/breath) in control participants (P = .19). Thereafter, VDalveolar increased from 20W to peak exercise in patients with HFpEF (0.37 ± 0.16 L/breath; P < .01 vs 20W) and control participants (0.19 ± 0.17 L/breath; P = .03 vs 20W). VDalveolar was greater in patients with HFpEF compared with control participants at rest, 20W, and peak exercise (main effect for group, P < .01). Moreover, the increase in VDalveolar correlated with the E/co2 slope (r = 0.69; P < .01), which was correlated with peak o2peak (r = 0.46; P < .01) in patients with HFpEF.
Interpretation
These data suggest that the increase in / mismatch may be explained by increases in VDalveolar and that increases in VDalveolar worsens ventilatory efficiency, which seems to be a key contributor to exercise intolerance in patients with HFpEF.
Key Words: dead space, exercise intolerance, gas exchange inefficiency, HFpEF, mismatch
Take-home Points.
Study Question: Do patients with heart failure with preserved ejection fraction (HFpEF) develop alveolar dead space (VDalveolar) during exercise?
Results: VDalveolar was elevated across all conditions (rest, 20W, and peak exercise) and increased during exercise to a greater extent in patients with HFpEF compared with control participants.
Interpretation: Lung regions where the amount of ventilation is high relative to the amount of pulmonary blood flow may develop in patients with HFpEF, resulting in a greater relative distribution of high lung regions (or VDalveolar, or both) during exercise.
FOR EDITORIAL COMMENT, SEE PAGE 1233
Heart failure with preserved ejection fraction (HFpEF) is a major public health concern.1,2 The pathophysiologic features of HFpEF are complex, and this disorder has been characterized as a heterogenous syndrome that exhibits numerous cardiopulmonary abnormalities, including elevated cardiac filling pressures,3, 4, 5 pulmonary edema,6,7 impaired pulmonary vascular recruitment and distension,8, 9, 10 and right ventricle-pulmonary artery uncoupling.11, 12, 13, 14, 15 Although investigations into the cardiac-related consequences of these cardiopulmonary abnormalities are ongoing, these cardiopulmonary abnormalities also could alter pulmonary blood flow distribution considerably and could contribute to mismatch, manifesting as an increase in alveolar dead space (VDalveolar),16 which is characterized by the development of lung regions with a high ratio.16
mismatch typically is estimated by calculating the physiologic dead space (VDphysiologic; the sum of anatomic dead space [VDanatomic] and VDalveolar) to tidal volume (VT) ratio (VD/VT).17 Studies have demonstrated that VD/VT is increased in patients with HFpEF compared with control participants.18, 19, 20 We also demonstrated that VD/VT increased during exercise in patients with HFpEF21 and to a greater extent compared with control participants,22 which we hypothesized could be the result of an increase in VDalveolar secondary to the exacerbation of a variety of cardiopulmonary abnormalities.16 However, VT also can have a major effect on the VD/VT ratio.23 Given that VT was smaller in patients with HFpEF compared with control participants in previous studies,18, 19, 20 one may argue that the smaller VT at least in part could have contributed to the increased VD/VT ratio observed in patients with HFpEF. As such, the subsequent conclusions that can be drawn from the reported data18, 19, 20 regarding the development of VDalveolar as a mechanism of mismatch during exercise in patients with HFpEF are limited.
To circumvent the confounding VT problem, it is possible to estimate VDalveolar from VD/VT, particularly if VD/VT is calculated using the Enghoff modification of the Bohr equation17 because this equation accounts for the mechanical dead space volume of the breathing apparatus, which can influence the VD/VT ratio measurement independently.22 Next, it is recommended that VD/VT be multiplied by VT to estimate VDphysiologic.23 This value should approximate 0.15 L regardless of the VT,23 which theoretically constitutes the VDanatomic in healthy individuals.24 Therefore, any increase in VDphysiologic above the estimated VDanatomic represents an estimate of the VDalveolar.23,25
Although the increase in VD/VT during exercise reported previously indicates worsening mismatch,21,22 the extent to which the increase in VD/VT is the result of an increase in VDalveolar in patients with HFpEF is unclear. Because mismatch is associated with exercise intolerance,22,26,27 such information on the development of VDalveolar as a mechanism of mismatch could have a significant public health outcome by allowing for a better understanding of the mechanism(s) that may contribute to exercise intolerance in patients with HFpEF. This becomes especially important given that exercise intolerance is a dominant symptom of HFpEF and is associated with increased mortality in this population.16 Therefore, the purpose of the present study was to calculate VDalveolar and to compare VDalveolar at rest and during exercise in patients with HFpEF and healthy control participants. We hypothesized that VDalveolar would increase during exercise in patients with HFpEF compared with control participants.
Study Design and Methods
This was a retrospective analysis of previously collected data. Although some of these data have been published elsewhere,21,22,28 we repeat only the methods and data essential to the new findings presented herein.
Participants
We evaluated 23 patients with HFpEF who were enrolled in our larger ongoing study (ClinicalTrials.gov Identifier: NCT04068844) and 12 control participants who were part of a different study.28 Patients with HFpEF were included if they had signs and symptoms of heart failure based on Framingham criteria,29 an ejection fraction of ≥ 50%, and evidence of volume overload (eg, pulmonary edema) confirmed by a pulmonary capillary wedge pressure of ≥ 25 mm Hg at peak exercise or an increase in pulmonary capillary wedge pressure of ≥ 15 mm Hg from rest to peak exercise. Participants were excluded if they had a diagnosis of severe valvular disease, congenital heart disease, known restrictive or infiltrative cardiomyopathy, acute myocarditis, New York Heart Association class IV chronic heart failure or chronic heart failure that cannot be stabilized with medical therapy, a prior ejection fraction of < 50%, manifest or provocable ischemic heart disease, or significant obstructive lung disease (ie, FEV1 of < 40% predicted). Control participants did not have a history of smoking of > 5 pack-years, asthma, cardiovascular disease, sleep disorders, or musculoskeletal abnormalities that would preclude exercise or a BMI of > 30 kg/m2. Before all testing, written and informed consent was obtained. The experimental procedures were reviewed and approved by the UT Southwestern Institutional Review Board (Identifier: STU2019-0617).
Study Design and Methods
Patients with HFpEF visited the laboratory on two separate occasions. During the first visit, patients with HFpEF underwent preparticipation health screening and performed pulmonary function testing according to American Thoracic Society/European Respiratory Society guidelines30 and a maximum cardiopulmonary exercise test (CPET) to determine participant eligibility. During the second visit, patients with HFpEF underwent pulmonary artery and radial artery catheterizations and performed a 6-min constant-load cycling test and a maximum incremental cycling test on an upright cycle ergometer (Lode BV), as described previously.22 Control participants visited the laboratory on two separate occasions. During the first visit, control participants underwent preparticipation health screening and performed pulmonary function testing according to American Thoracic Society/European Respiratory Society guidelines30 and a maximum CPET to determine participant eligibility. During the second visit, control participants underwent radial artery catheterization and performed a maximum incremental cycling test on an upright cycle ergometer (MedGraphics, model CPE 2000), as described previously.22
Catheterization Protocol
Catheterizations for patients with HFpEF and control participants were performed as described previously.22 Briefly, patients with HFpEF underwent Swan-Ganz catheter placement in the pulmonary artery via brachial or antecubital vein access under fluoroscopic guidance, which was used to measure pulmonary artery pressure, pulmonary capillary wedge pressure, and central blood temperature at rest, during the final minute of constant-load exercise, and at peak exercise. Control participants did not undergo Swan-Ganz catheter placement in the pulmonary artery. Both patients with HFpEF and control participants underwent radial artery catheterization using a modified Seldinger technique. For patients with HFpEF, arterial blood gases were measured at rest, during the final minute of constant-load exercise, and at peak exercise. For control participants, arterial blood gases were measured at rest, during each work rate increment, and at peak exercise. All blood gas samples were withdrawn over a 10-s period to reduce the fluctuations of blood gas tensions over a given respiratory cycle during the same time frame in which pulmonary gas exchange determinations were made. All blood gas samples were temperature corrected and analyzed for Paco2, Pao2, and hemoglobin oxygen saturation (ABL90 Flex; Radiometer).
Measurements Obtained From Patients With HFpEF
Heart rate and rhythm were monitored continuously using 12-lead electrocardiography. BP was monitored via arterial waveform tracings. Invasive central hemodynamic measurements and radial arterial blood gases were measured as described above. Gas exchange, including ventilation (E), oxygen uptake (o2), and CO2 elimination (co2), was measured using a customized breath-by-breath measurement system (Beck Integrative Physiological System; KCBeck, Physiological Consulting) integrated with a mass spectrometer (Perkin-Elmer, model 1100). These measurements were obtained at rest, during the final minute of constant-load cycling (performed at twenty watts, 20W), and at peak exercise. During the maximum incremental cycling test, a maximum effort was determined by observing: (1) a plateau in o2 of < 150 mL/min, (2) the inability to maintain a cycling cadence of > 55 rpm for a duration of 5 s, (3) a respiratory exchange ratio of > 1.05, or a combination thereof.
Measurements Obtained From Control Participants
Heart rate and rhythm were monitored continuously using 12-lead electrocardiography. BP was monitored via an automated system. Radial arterial blood gases were measured as described above, and gas exchange, including E, o2, and co2, was measured using a customized breath-by-breath measurement system that was computerized (NEC 486DX) and integrated with a mass spectrometer (Perkin-Elmer, model 1100). These measurements were obtained at rest, the 20W stage (to facilitate between-group comparisons at a given absolute work rate), and at peak exercise. During the maximum incremental cycling test, a maximum effort was determined by observing: (1) a plateau in o2 of < 150 mL/min, (2) the inability to maintain a cycling cadence of > 55 rpm for a duration of 5 s, (3) a respiratory exchange ratio of > 1.05, or a combination thereof.
Data Analysis
VD/VT was calculated using the Enghoff modification of the Bohr equation17: VD/VT = [(Paco2 – Peco2) / Paco2] – (mechanical dead space / VT). Partial pressure of mixed expired CO2 (Peco2) was calculated as: 863 × co2 (standard temperature pressure dry, STPD) / E(body temperature pressure saturated, BTPS) in both groups. Mechanical dead space was estimated to equal 0.18 L and 0.14 L for patients with HFpEF and control participants, respectively.22 VDalveolar was calculated as: VDalveolar = (VD/VT × VT) – VDanatomic.23 VDanatomic was estimated as 2 mL/kg body mass in those with normal BMI and VDanatomic was estimated as 2 mL/kg lean body mass in those with BMI of ≥ 30 kg/m2 as recommended,23 because VDanatomic varies with body size.23 In patients with HFpEF, we also calculated the slope of the relationship between the rest-to-20W change in E and the rest-to-20W change in co2 (ie, the E/co2 slope),31,32 which reflects ventilatory efficiency. Cardiac output was calculated via the direct Fick formula: cardiac output = o2 / arteriovenous oxygen difference.
Statistical Analysis
Data were analyzed using SPSS version 22.0 software. Differences in participant characteristics, cardiorespiratory parameters, and arterial blood gas data (rest, 20W, and peak exercise) were compared using independent t tests, and differences in VDalveolar were compared between groups (patients with HFpEF vs control participants) across conditions (rest, 20W, and peak exercise) using a two-way repeated measures analysis of variance. Where significant interactions were detected, post hoc comparisons using Bonferroni adjustments were performed. Assumptions of normality and homogeneity of variances were assessed using the Shapiro-Wilk test (in addition to an examination of Q-Q plots) and the Levene’s test, respectively. The Shapiro-Wilk test was nonsignificant for independent t tests and the two-way repeated measures analysis of variance when examining all three conditions separately, indicating that the data did not deviate significantly from a normal distribution. The Levene’s test was nonsignificant for the two-way repeated measures analysis of variance, indicating that the variance was not significantly heterogenous. The Levene’s test also allowed us to report the appropriate t test results (ie, the P value for when homogeneity of variances is or is not assumed). Furthermore, relationships between variables were assessed using Pearson correlation coefficient analyses and described as small (0.10 < r < 0.29), medium (0.30 < r < 0.49), and large (r > 0.50).33 Statistical significance was defined as P < .05. All data are presented as mean ± SD.
Results
Participant Characteristics
Participant characteristics and pulmonary function data are displayed in Table 1. All patients with HFpEF were not smoking at the time of the study. Four patients with HFpEF had a history of smoking (approximately a 16 pack-year history on average). Of the patients with HFpEF studied, 15 had a prior diagnosis of obstructive sleep apnea. Participants were similar in age and height, but patients with HFpEF overall were heavier and demonstrated greater BMI compared with control participants. Also, FVC, FEV1, and TLC were lower in patients with HFpEF compared with control participants. Despite these differences, pulmonary function was within normal limits for most patients based on lower limits of normal from 95% CIs. One patient with HFpEF demonstrated moderate obstructive lung disease and one patient had undergone a prior lobectomy. Although diffusion capacity of the lung for carbon monoxide (Dlco) was lower in patients with HFpEF compared with control participants, Dlco normalized to alveolar volume was similar between groups. Cardiorespiratory responses are shown in Table 2, and hemodynamic and arterial blood gas responses are shown in Table 3.
Table 1.
Participant Characteristics
| Characteristic | Control Participants | Patients With HFpEF |
|---|---|---|
| Demographics | ||
| Age, y | 70 ± 3 | 71 ± 6 |
| Sex, male/female, no. | 7/5 | 9/14 |
| Height, m | 1.72 ± 0.1 | 1.70 ± 0.1 |
| Weight, kg | 72.3 ± 11 | 107 ± 15a |
| BMI, kg/m2 | 27.4 ± 1.9 | 37.9 ± 6.8a |
| Pulmonary function | ||
| FVC, % predicted | 115 ± 17 | 86 ± 16a |
| FEV1, % predicted | 104 ± 13 | 85 ± 17a |
| FEV1 to FVC, % | 71 ± 5 | 75 ± 9 |
| TLC, % predicted | 113 ± 13 | 93 ± 15a |
| Dlco, % predicted | 108 ± 14 | 69 ± 19a |
| Dlco normalized to VA, % predicted | 109 ± 16 | 108 ± 25 |
Data are presented as mean ± SD, unless otherwise indicated. Dlco = diffusing capacity of the lung for carbon monoxide; HFpEF = heart failure with preserved ejection fraction; TLC = total lung capacity; VA = alveolar volume.
P < .01.
Table 2.
Cardiorespiratory Parameters at Rest, During 20W, and at Peak Exercise
| Variable | Control Participants | Patients With HFpEF |
|---|---|---|
| Rest | ||
| E, L/min | 11.6 ± 4.0 | 11.9 ± 2.5 |
| o2, L/min | 0.34 ± 0.08 | 0.31 ± 0.07 |
| co2, L/min | 0.28 ± 0.06 | 0.24 ± 0.06 |
| E/co2 ratio | 40.8 ± 9.6 | 50.2 ± 7.4a |
| RER | 0.83 ± 0.09 | 0.79 ± 0.05 |
| 20W | ||
| WR, % peak | 19 ± 7 | 31 ± 11a |
| E, L/min | 19.7 ± 3.0 | 30.1 ± 4.4a |
| o2, L/min | 0.65 ± 0.08 | 0.87 ± 0.16a |
| co2, L/min | 0.54 ± 0.07 | 0.75 ± 0.14a |
| E/co2 ratio | 31.0 ± 5.5 | 40.6 ± 5.3a |
| RER | 0.84 ± 0.08 | 0.87 ± 0.05 |
| Peak exercise | ||
| WR, W | 120 ± 40 | 76 ± 34a |
| WR, % predicted | 120 ± 27 | 82 ± 25a |
| E, L/min | 82.9 ± 24.4 | 58.6 ± 18.9a |
| E, % predicted MVV | 74.7 ± 11.4 | 72.1 ± 8.7 |
| o2, L/min | 1.70 ± 0.51 | 1.27 ± 0.39b |
| o2, % predicted | 106 ± 21 | 73 ± 17a |
| o2, mL/min/kg | 23.35 ± 5.45 | 11.80 ± 3.23a |
| co2, L/min | 2.11 ± 0.58 | 1.39 ± 0.53a |
| E/co2 ratio | 39.6 ± 5.0 | 43.8 ± 7.6 |
| RER | 1.26 ± 0.09 | 1.09 ± 0.12b |
Data are mean ± SD. 20W = 20 watts; HFpEF = heart failure with preserved ejection fraction; MVV = maximal voluntary ventilation; RER = respiratory exchange ratio; E = minute ventilation; o2 = oxygen consumption; co2 = CO2 elimination; WR = work rate.
P < .01.
P < .05.
Table 3.
Hemodynamic and Arterial Blood Gas Data at Rest, During 20W, and at Peak Exercise
| Variable | Control Participants | Patients With HFpEF |
|---|---|---|
| Rest | ||
| HR, beats/min | 75 ± 11 | 74 ± 16 |
| Qc, L/min | — | 5.2 ± 1.3 |
| Pao2, mm Hg | 99.2 ± 8.6 | 83.6 ± 5.7a |
| Paco2, mm Hg | 36.2 ± 4.2 | 40.5 ± 4.0a |
| HbO2, % | 96 ± 1 | 94 ± 2a |
| 20W | ||
| HR, beats/min | 109 ± 18 | 93 ± 16 |
| Qc, L/min | — | 8.9 ± 2.0 |
| Pao2, mm Hg | 99.4 ± 9.1 | 85.1 ± 12.9b |
| Paco2, mm Hg | 36.5 ± 4.1 | 40.6 ± 4.2b |
| HbO2, % | 97 ± 1 | 95 ± 3b |
| Peak exercise | ||
| HR, beats/min | 157 ± 9 | 122 ± 19a |
| HR, % predicted maximum | 99 ± 6 | 79 ± 12a |
| Qc, L/min | — | 10.5 ± 2.7 |
| Pao2, mm Hg | 108.5 ± 8.1 | 88.3 ± 15.1a |
| Paco2, mm Hg | 31.6 ± 2.5 | 38.9 ± 4.7a |
| HbO2, % | 97 ± 1 | 94 ± 3b |
Data are presented as mean ± SD. 20W = 20 watts; HbO2 = hemoglobin oxygen saturation; HFpEF = heart failure with preserved ejection fraction; HR = heart rate; Qc = cardiac output; — = no data available.
P < .01.
P < .05.
VDalveolar
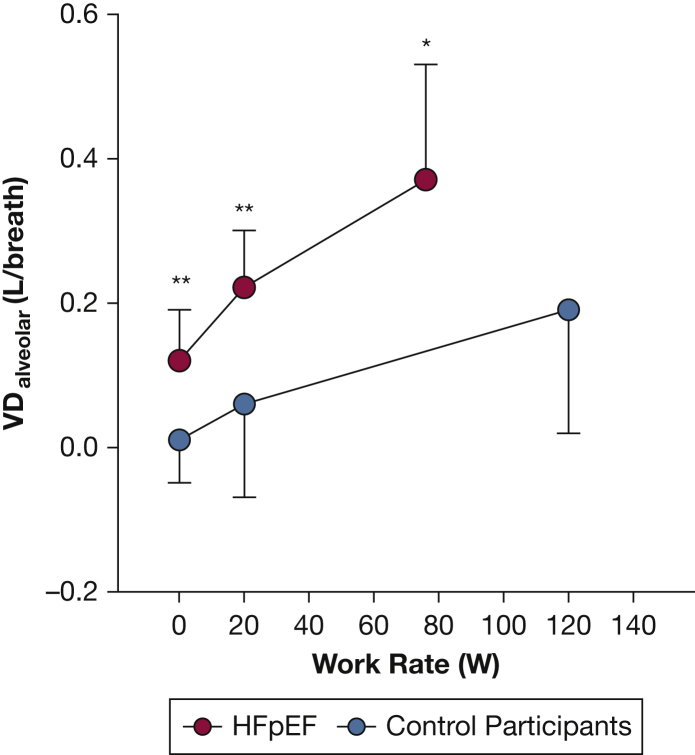
VDalveolar increased from rest to 20W in patients with HFpEF (P < .01) (Fig 1), whereas VDalveolar did not change significantly from rest to 20W in control participants (P = .19) (Fig 1). Thus, the increase in VDalveolar was greater in patients with HFpEF compared with control participants from rest to 20W (patients with HFpEF, 0.11 ± 0.05 L/breath; control participants, 0.04 ± 0.08 L/breath; P = .03). Thereafter, VDalveolar increased from 20W to peak exercise in both patients with HFpEF (P < .01) (Fig 1) and control participants (P = .03) (Fig 1), the increase of which was similar between groups (patients with HFpEF, 0.13 ± 0.12 L/breath; control participants, 0.13 ± 0.16 L/breath; P = .94). Furthermore, VDalveolar was greater in patients with HFpEF compared with control participants at rest, 20W, and peak exercise (P < .01, main effect for group) (Fig 1). Furthermore, the change in VDalveolar from rest to 20W significantly correlated with the E/co2 slope (r = 0.69; P < .01) (Fig 2A). Finally, the E/co2 slope significantly correlated inversely with peak o2peak (r = 0.46; P < .01) (Fig 2B).
Figure 1.
Graph showing mean ± SD values for VDalveolar measured at rest and during exercise (20W and peak exercise) in control participants (blue circles) and patients with HFpEF (red circles). Differences in VDalveolar measurements were compared between groups (control participants vs patients with HFpEF) across condition (rest, 20W, and peak exercise) using a two-way repeated measures analysis of variance. ∗Significantly different between groups at P < .05. ∗∗Significantly different between groups at P < .01. 20W = 20 watts; HFpEF = heart failure with preserved ejection fraction; VDalveolar = alveolar dead space.
Figure 2.
A, B, Scatterplots showing the relationships between the change in VDalveolar from rest to 20W and the E/co2 slope (A) and the E/co2 slope and o2peak (B) in patients with heart failure with preserved ejection fraction. Relationships were analyzed using Pearson correlation coefficients. The dashed lines indicate linear regression. 20W = 20 watts; E = ventilation; co2 = CO2 elimination; VDalveolar = alveolar dead space; o2peak = peak oxygen uptake; twenty watts = 20W.
Discussion
To our knowledge, this is the first study to examine VDalveolar in patients with HFpEF. The novel findings from this study are: (1) VDalveolar was elevated across all conditions (rest, 20W, and peak exercise) and increased during exercise to a greater extent in patients with HFpEF compared with control participants, (2) the increase in VDalveolar correlated significantly with the E/co2 slope in patients with HFpEF, and (3) the E/co2 slope correlated significantly with O2peak in patients with HFpEF.
Patients with HFpEF exhibit many cardiopulmonary abnormalities that could result in mismatch, particularly during exercise when cardiopulmonary abnormalities are exacerbated and the degree of mismatch is likely to worsen. Indeed, we previously demonstrated that mismatch increased during exercise in patients with HFpEF (as evidenced by an increase in VD/VT),21,22 which we hypothesized could be the result of an increase in VDalveolar secondary to the exacerbation of a variety of cardiopulmonary abnormalities. Consistent with our hypothesis are the findings of the present study: an increase in VDalveolar from rest to exercise in patients with HFpEF, the magnitude of which was greater than that observed in control participants. Therefore, the results of the present study extend our previous work21,22 by providing insight into the mechanism(s) that may be responsible for the increased VD/VT ratio during exercise in patients with HFpEF. Our findings also support those of others17,34,35 who suggest that a VD/VT ratio that increases, or fails to decrease from rest to exercise, indicates worsening mismatch possibly because of increasing VDalveolar. Sullivan et al36 also reported similar results in patients with heart failure with reduced ejection fraction, whereby an elevated VD/VT ratio primarily was the result of an increase in VDphysiologic. Collectively, these findings,17,34, 35, 36 combined with that of the present study, provide strong evidence that patients with HFpEF develop profound gas exchange abnormalities during exercise, secondary to an increase in VDalevolar.
It is well documented that an increase in VDalevolar contributes to ventilatory inefficiency, which typically is defined as an increase in the E/co2 slope.37 A growing body of evidence shows that patients with HFpEF exhibit an increased E/co2 slope when compared with control participants.9,18 Van Iterson et al19 also demonstrated that an increased E/co2 slope can be ascribed primarily to an increased VD/VT ratio in patients with HFpEF.19 The findings of the present study extend those of Van Iterson et al19 by showing that the increase in VDalevolar (from rest to 20W) also was related to the E/co2 slope in patients with HFpEF. After a retrospective analysis of alveolar E, which was calculated as Ex(1 – VD/VT),36 no significant difference was found in alveolar E between groups at 20W (patients with HFpEF, 15.98 ± 3.38 L/min; control participants, 14.68 ± 2.14 L/min; P = .25). Therefore, the increased ventilatory demand and thus the E/co2 slope in patients with HFpEF must be in response to an increase in VDalveolar to maintain appropriate alveolar E and blood gas homeostasis. These findings are similar to what has been observed in patients with heart failure with reduced ejection fraction in whom exercise E is elevated, yet arterial blood gases are maintained.36 Moreover, multiple studies have observed a link between ventilatory inefficiency and exercise intolerance in patients with a range of cardiopulmonary conditions.38, 39, 40, 41 Herein, we also showed, for the first time m that those patients who exhibited a greater magnitude of ventilatory inefficiency demonstrated a lower exercise tolerance. This was evidenced by the fact that the E/co2 slope was related inversely to o2peak in patients with HFpEF. Although the association between the E/co2 slope and O2peak was relatively weak, the strength of the relationship is similar to what has been observed previously in patients with heart failure with reduced ejection fraction.42,43 Overall, the results of the present study extend that of previous studies and suggest that elevations in VDalveolar contribute to ventilatory inefficiency in patients with HFpEF, which seems to contribute to exercise intolerance in these patients.
The increase in VDalveolar has not been shown before in patients with HFpEF. Indeed, it is well known that VDalveolar is characterized by the development of lung regions with a high ratio.44 Thus, it is conceivable that during exercise, lung regions develop where the amount of ventilation is high relative to the amount of pulmonary blood flow in patients with HFpEF, resulting in a greater relative distribution of high lung regions (or VDalveolar, or both).45,46 Although the mechanism(s) governing a greater relative distribution of high lung regions during exercise in patients with HFpEF is unclear, it is possible that the aforementioned cardiopulmonary abnormalities associated with HFpEF could alter the distribution of or limit pulmonary perfusion, or both, which ultimately could lead to a greater proportion of alveoli that are underperfused yet are otherwise well ventilated. Moreover, it must be acknowledged that we cannot completely exclude airway-related causes of mismatch; yet, the lack of spirometric abnormities in the patients with HFpEF studied seems to point to abnormalities relating to pulmonary circulation, rather than the airways, as the primary origin of mismatch. Finally, we also observed a mild reduction in Dlco at rest in patients with HFpEF relative to control participants. These findings are consistent with those of Olson et al9 and likely are the manifestation of pulmonary vascular remodeling, the development of pulmonary edema, a decrease in alveolar volume resulting from obesity, or a combination thereof. Given that a mild reduction in Dlco also can impact alveolar-arterial O2 and CO2 exchange, the extent to which these factors contribute to measurements of VDalveolar during exercise is unclear. However, it should be noted that the relationships and lung diffusion capacity of patients with HFpEF have not yet been analyzed by means of the multiple inert gas elimination technique,47 which remains the only way to quantify the distribution of ventilation and perfusion alongside simultaneous measurements of lung diffusion capacity at rest and during exercise. Separating the roles of mismatch and diffusion limitation in determining gas exchange abnormalities is beyond the scope of this study, but represents an interesting avenue for further research to determine the mechanism(s) potentially responsible for the development of VDalveolar in patients with HFpEF.
Aside from an increased VDalveolar and ventilatory inefficiency, a secondary observation is that most patients with HFpEF seemed to show an inadequate ventilatory response, which is typically defined as Paco2 of ≥ 38 mm Hg at peak exercise.48 Indeed, this was evidenced by patients with HFpEF exhibiting Paco2 on average of 39 mm Hg at peak exercise. Although E for a given co2 (ie, E/co2 ratio) at peak exercise was slightly higher in patients with HFpEF compared with control participants, it is likely that the E achieved in patients with HFpEF was not sufficiently high to compensate for the increased VDalveolar and decrease Paco2 adequately, that is, to 35 mm Hg, which reflects the upper limit of an adequate ventilatory response.48 To confirm this hypothesis, we estimated the E required to decrease Paco2 to 35 mm Hg using the following equation: (863 × co2) / (Paco2 × [1 – VD/VT]).49 By using this equation, we determined that patients with HFpEF would have had to increase E at peak exercise on average by approximately 9% (ie, from 58.63 ± 18.86 L/min to 63.55 ± 16.54 L/min) to decrease Paco2 to 35 mm Hg, which would constitute an adequate ventilatory response.48 Currently, the reason why patients with HFpEF were unable to generate an adequate ventilatory response is unclear. However, it could be related to a blunted drive to breathe resulting from either the presence of sleep-disordered breathing, obesity hypoventilation syndrome, mechanical ventilatory constraints resulting from increases in fat mass surrounding the chest wall, particularly because the patients with HFpEF in the present study had obesity with a mean BMI of approximately 38 kg/m2, or a combination thereof. In support of the latter, we recently showed that the ventilatory response to exercise is reduced in adults with obesity50 and that a reduced ventilatory response could be the result of the presence of obesity-related mechanical ventilatory constraints that are imposed on breathing. Therefore, not only do patients with HFpEF exhibit ventilatory inefficiency, but they also exhibit an inadequate ventilatory response, which also could be an important contributor to exercise intolerance in these patients.
The results of the present study have important clinical implications, particularly for patients who are referred for CPET for unexplained dyspnea. CPET is a valuable clinical tool, and assessing mismatch often is an important reason for completing CPET. Currently, mismatch typically is estimated by calculating the VD/VT ratio.17 Although a VD/VT ratio that fails to decrease, or increases from rest to exercise, indicates worsening mismatch, the mechanism(s) responsible for an increase in VD/VT often are not detectable or assessed during CPET. That is, rarely is it determined whether an elevated VD/VT ratio is ascribed to an increase in VDalveolar resulting from the potential exacerbation of cardiopulmonary abnormalities or a small VT resulting from mechanical ventilatory constraints that limit VT expansion and blunt the ventilatory response to exercise. Based on the findings of the present study, which suggest that the increase in VD/VT21,22 largely may be explained by VDalveolar, we propose that clinicians and physiologists consider deriving VDalveolar from the VD/VT ratio to obtain a measurement that may provide insight into the potential (patho)physiologic mechanisms governing the development of mismatch during exercise in patients with HFpEF. Given that mismatch has been linked with ventilatory inefficiency and exercise intolerance, both of which are used widely as prognostic indicators for heart failure, it is important for clinicians and physiologists to understand the potential (patho)physiologic mechanism(s) responsible for the increase in mismatch so that novel therapeutics can be developed to improve clinical outcomes in patients with HFpEF.
Moreover, and from a symptomatology perspective, whether VDavleolar-related increases in ventilatory demand (evidenced by an increase in the E/co2 slope) contribute to exertional dyspnea in this population is unknown. However, it is possible that an increased ventilatory demand resulting from increased VDalveolar may provoke greater mechanical ventilatory constraints (as has been shown in patients with heart failure with reduced ejection fraction51), which could predispose these patients to a greater potential for exertional dyspnea at a given exercise work rate and consequently result in the premature termination of exercise. Furthermore, we demonstrated that VDalveolar was elevated (relative to control participants) even at low levels of exercise, which may explain in part why these patients often experience dyspnea and exercise intolerance at low levels of physical activity (eg, activities of daily living). Of note, it must be emphasized that the underlying mechanism(s) responsible for the development of VDalveolar still need to be uncovered in patients with HFpEF, particularly because these patients exhibit many cardiopulmonary abnormalities that could alter pulmonary blood flow distribution considerably and could contribute to the development of VDalveolar (eg, elevated cardiac filling pressures,3, 4, 5 pulmonary edema,6,7 impaired pulmonary vascular recruitment or distension,8, 9, 10 and right ventricle-pulmonary artery uncoupling11, 12, 13, 14, 15). We propose that this is a critical area that deserves further investigation so that clinicians are able to understand how best to improve gas exchange efficiency and, thus, potentially to manage patients’ symptoms effectively.
We examined patients with HFpEF older than 60 years, which limits the generalization of our findings to only older individuals with HFpEF. Moreover, the submaximal exercise conditions in the present study were different between groups. Also noteworthy is that mechanical dead space was estimated as the effective dead space, rather than volumetric dead space. This is because previous studies suggest that VD/VT calculations that take into account the entire volumetric dead space underestimate VDphysiologic (and thus, VDalveolar).52 As such, we estimated the effective dead space of the breathing apparatus using methods based on Bradley and Younes.52 Finally, it should be acknowledged that two patients in the present study harbored concomitant pulmonary abnormalities; that is, one patient had moderate obstructive lung disease and one patient had undergone a prior lobectomy. It is unclear whether these pulmonary abnormalities contribute to VDalveolar or provoke greater VDalveolar beyond what is observed in patients with HFpEF with normal lung function (when assessed based on spirometric values). Notably, the VDalveolar measurements made in these two patients were similar to that measured in the other patients studied and the outcomes of the present study did not change even after removing these two patients from the analysis. Although some pulmonary abnormalities could contribute to the development of VDalveolar, we do not have the capacity to inform outcomes based on the additive effects of pulmonary abnormalities and HFpEF on the development of VDalveolar during exercise. This is an area that deserves further investigation.
Interpretation
The results of the present study confirm our original hypothesis by demonstrating that VDalveolar increased during exercise in patients with HFpEF, the magnitude of which was greater when compared with control participants. We also demonstrated that VDalveolar correlated with the Eco2 slope, the latter of which was associated inversely with o2peak in patients with HFpEF. These data have important clinical implications and suggest that the increase in mismatch may be explained by increases in VDalveolar and that increases in VDalveolar worsens ventilatory inefficiency, which seems to be a key contributor to exercise intolerance in patients with HFpEF. Further investigation is warranted to determine the underlying mechanism(s) governing the development of VDalveolar in patients with HFpEF and subsequently how VDalveolar (and mismatch) might be targeted therapeutically potentially to improve exercise tolerance in these patients.
Acknowledgments
Author contributions: B. N. B., A. R. T., J. P. M., L. S. H., B. D. L., S. S., and T. G. B. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Each of the authors also contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Funding/support: B.N.B is supported by an American Heart Association Postdoctoral Fellowship (grant number: 826064). This research was also supported by the National Institutes of Health (grant number: 1P01HL137630), King Charitable Foundation Trust, Susan Lay Atwell Gift for Pulmonary Research, Cain Foundation, and Texas Health Presbyterian Hospital Dallas.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors wish to thank, Raksa B. Moran, BSN, Margot Morris, BS, Cindi Foulk, BS, Marcus Payne, MS, Mitchell Samels, MS, and Rebekah Summerall, MPH, for their help with data collection and processing for this project. We would also like to extend our sincere gratitude to Dr Peter D. Wagner, MD, for his assistance with the interpretation of these data.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Shah S.J., Kitzman D.W., Borlaug B.A., et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borlaug B.A., Nishimura R.A., Sorajja P., Lam C.S., Redfield M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorfs S., Zeh W., Hochholzer W., et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35(44):3103–3112. doi: 10.1093/eurheartj/ehu315. [DOI] [PubMed] [Google Scholar]
- 5.Eisman A.S., Shah R.V., Dhakal B.P., et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11(5) doi: 10.1161/CIRCHEARTFAILURE.117.004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fermoyle C.C., Stewart G.M., Borlaug B.A., Johnson B.D. Effects of exercise on thoracic blood volumes, lung fluid accumulation, and pulmonary diffusing capacity in heart failure with preserved ejection fraction. Am J Physiol Regul Integr Comp Physiol. 2020;319(5):R602–R609. doi: 10.1152/ajpregu.00192.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy Y.N.V., Obokata M., Wiley B., et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40:3721–3730. doi: 10.1093/eurheartj/ehz713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayyaz A.U., Edwards W.D., Maleszewski J.J., et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation. 2018;137(17):1796–1810. doi: 10.1161/CIRCULATIONAHA.117.031608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson T.P., Johnson B.D., Borlaug B.A. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4(6):490–498. doi: 10.1016/j.jchf.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis G.D. The role of the pulmonary vasculature in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;53(13):1127–1129. doi: 10.1016/j.jacc.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Borlaug B.A., Kane G.C., Melenovsky V., Olson T.P. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37(43):3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guazzi M., Dixon D., Labate V., et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10(10 pt B):1211–1221. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Melenovsky V., Hwang S.J., Lin G., Redfield M.M., Borlaug B.A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen M.J., Hwang S.J., Kane G.C., et al. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8(3):542–550. doi: 10.1161/CIRCHEARTFAILURE.114.002114. [DOI] [PubMed] [Google Scholar]
- 15.Ghio S., Guazzi M., Scardovi A.B., et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19(7):873–879. doi: 10.1002/ejhf.664. [DOI] [PubMed] [Google Scholar]
- 16.Del Buono M.G., Arena R., Borlaug B.A., et al. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(17):2209–2225. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 17.Lewis D.A., Sietsema K.E., Casaburi R., Sue D.Y. Inaccuracy of noninvasive estimates of VD/VT in clinical exercise testing. Chest. 1994;106(5):1476–1480. doi: 10.1378/chest.106.5.1476. [DOI] [PubMed] [Google Scholar]
- 18.Obokata M., Olson T.P., Reddy Y.N.V., Melenovsky V., Kane G.C., Borlaug B.A. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39(30):2810–2821. doi: 10.1093/eurheartj/ehy268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Iterson E.H., Johnson B.D., Borlaug B.A., Olson T.P. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail. 2017;19(12):1675–1685. doi: 10.1002/ejhf.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fermoyle C.C., Stewart G.M., Borlaug B.A., Johnson B.D. Simultaneous measurement of lung diffusing capacity and pulmonary hemodynamics reveals exertional alveolar-capillary dysfunction in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.120.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balmain B.N., Tomlinson A.R., MacNamara J.P., et al. Estimating exercise Paco2 in patients with heart failure with preserved ejection fraction. J Appl Physiol (1985) 2022;132(1):36–45. doi: 10.1152/japplphysiol.00474.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balmain B.N., Tomlinson A.R., MacNamara J.P., et al. Physiological dead space during exercise in patients with heart failure with preserved ejection fraction. J Appl Physiol. 2022;132:632–640. doi: 10.1152/japplphysiol.00786.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner P.D. The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial blood gases. Eur Respir J. 2015;45(1):227–243. doi: 10.1183/09031936.00039214. [DOI] [PubMed] [Google Scholar]
- 24.Weibel E. Springer; 1963. Morphometry of the Human Lung. [Google Scholar]
- 25.Severinghaus J.W., Stupfel M. Alveolar dead space as an index of distribution of blood flow in pulmonary capillaries. J Appl Physiol. 1957;10(3):335–348. doi: 10.1152/jappl.1957.10.3.335. [DOI] [PubMed] [Google Scholar]
- 26.Cross T.J., Kim C.-H., Johnson B.D., Lalande S. The interactions between respiratory and cardiovascular systems in systolic heart failure. J Appl Physiol. 2019;128(1):214–224. doi: 10.1152/japplphysiol.00113.2019. [DOI] [PubMed] [Google Scholar]
- 27.Lalande S., Cross T.J., Keller-Ross M.L., Morris N.R., Johnson B.D., Taylor B.J. Exercise intolerance in heart failure: central role for the pulmonary system. Exerc Sport Sci Rev. 2019;48(1):11–19. doi: 10.1249/JES.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babb T.G. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol. 1997;82(3):746–754. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 29.Lofstrom U., Hage C., Savarese G., et al. Prognostic impact of Framingham heart failure criteria in heart failure with preserved ejection fraction. ESC Heart Fail. 2019;6(4):830–839. doi: 10.1002/ehf2.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller M.R., Hankinson J., Brusasco V., et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 31.Balmain B.N., Wilhite D.P., Bhammar D.M., Babb T.G. External dead space explains sex-differences in the ventilatory response to submaximal exercise in children with and without obesity. Respir Physiol Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood H.E., Mitchell G.S., Babb T.G. Short-term modulation of the exercise ventilatory response in young men. J Appl Physiol (1985) 2008;104(1):244–252. doi: 10.1152/japplphysiol.00820.2007. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Academic Press; 1977. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 34.Zimmerman M.I., Miller A., Brown L.K., Bhuptani A., Sloane M.F., Teirtein A.S. Estimated vs actual values for dead space/tidal volume ratios during incremental exercise in patients evaluated for dyspnea. Chest. 1994;106:131–136. doi: 10.1378/chest.106.1.131. [DOI] [PubMed] [Google Scholar]
- 35.Wasserman K., Hanse J.E., Sue D.Y., Stringer W.W., Whipp B.J. Lippincott Williams & Wilkins; 2005. Principles of Exercise Testing and Interpreation. [Google Scholar]
- 36.Sullivan M.J., Higginbotham M.B., Cobb F.R. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988;77(3):552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 37.Phillips D.B., Collins S.E., Stickland M.K. Measurement and interpretation of exercise ventilatory efficiency. Front Physiol. 2020;11:659. doi: 10.3389/fphys.2020.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen J.E., Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest. 1996;109(6):1566–1576. doi: 10.1378/chest.109.6.1566. [DOI] [PubMed] [Google Scholar]
- 39.Neder J.A., Arbex F., Alencar M.C., et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J. 2015;45:377–387. doi: 10.1183/09031936.00135514. [DOI] [PubMed] [Google Scholar]
- 40.Cundrle I., Jr., Olson L.J., Johnson B.D. Pulmonary limitations in heart failure. Clin Chest Med. 2019;40(2):439–448. doi: 10.1016/j.ccm.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kee K., Stuart-Andrews C., Ellis M.J., et al. Increased dead space ventilation mediates reduced exercise capacity in systolic heart failure. Am J Respir Crit Care Med. 2016;193(11):1292–1300. doi: 10.1164/rccm.201508-1555OC. [DOI] [PubMed] [Google Scholar]
- 42.Clark A.L., Davies L.C., Francis D.P., Coats A.J. Ventilatory capacity and exercise tolerance in patients with chronic stable heart failure. Eur J Heart Fail. 2000;2:47–51. doi: 10.1016/s1388-9842(99)00060-4. [DOI] [PubMed] [Google Scholar]
- 43.Clark A.L., Volterrani M., Swan J.W., Coats A.J.S. The increased ventilatory response to exercise in chronic heart failure: relation to pulmonary pathology. Heart. 1997;77:138–146. doi: 10.1136/hrt.77.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner P.D. Ventilation-perfusion matching during exercise. Chest. 1992;101(5 suppl):192S–198S. doi: 10.1378/chest.101.5_supplement.192s. [DOI] [PubMed] [Google Scholar]
- 45.Petersson J., Glenny R.W. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J. 2014;44(4):1023–1041. doi: 10.1183/09031936.00037014. [DOI] [PubMed] [Google Scholar]
- 46.Robertson H.T. Dead space: the physiology of wasted ventilation. Eur Respir J. 2015;45:1704–1716. doi: 10.1183/09031936.00137614. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins S.R., Wagner P.D. Springer; 2017. The Multiple Inert Gas Elimination Technique (MIGET) [DOI] [PubMed] [Google Scholar]
- 48.Dempsey J.A., Wagner P.D. Exercise-induced arterial hypoxemia. J Appl Phsyiol. 1999;87(6):1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- 49.Ward S.A. Ventilatory control in humans: constraints and limitations. Exp Physiol. 2007;92(2):357–366. doi: 10.1113/expphysiol.2006.034371. [DOI] [PubMed] [Google Scholar]
- 50.Balmain B.N., Halverson Q.M., Tomlinson A.R., Edwards T., Ganio M.S., Babb T.G. Obesity blunts the ventilatory response to exercise in men and women. Ann Am Thorac Soc. 2021;18(7):1167–1174. doi: 10.1513/AnnalsATS.202006-746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson B.D., Beck K.C., Olson L.J., et al. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000;117:321–332. doi: 10.1378/chest.117.2.321. [DOI] [PubMed] [Google Scholar]
- 52.Bradley P.W., Younes M. Relation between respiratory valve dead space and tidal volume. J Appl Physiol. 1980;49(3):528–532. doi: 10.1152/jappl.1980.49.3.528. [DOI] [PubMed] [Google Scholar]