Graphical abstract
Keywords: Spontaneous echocardiographic contrast, Normal cardiac anatomy, Chronic lymphocytic leukemia (CLL), COVID-19, Ibrutinib
Highlights
-
•
SEC can occur in a structurally and functionally normal heart.
-
•
SEC formation has a stoichiometric relationship between hematocrit and fibrinogen levels.
-
•
SEC may be underappreciated in hematologic malignancies after chemotherapy initiation.
Introduction
Spontaneous echocardiographic contrast (SEC) is a phenomenon believed to be caused by red blood cell (RBC) aggregation in the setting of abnormal blood flow. Spontaneous echocardiographic contrast is a marker for hypercoagulability, most notably in the left atrium during atrial fibrillation. Therapies for B-cell malignancies, such as ibrutinib and lenalidomide, are associated with atrial fibrillation.1 Here we report a case of new-onset SEC in all 4 cardiac chambers of a patient with a normal heart, observed after recent commencement of ibrutinib for chronic lymphocytic leukemia (CLL) and recovery from COVID-19 infection.
Case Presentation
A 66-year-old man with recently diagnosed CLL, known cerebrospinal fluid involvement, and COVID-19 infection 1 month prior presented to the emergency department with syncope. Treatment for CLL included daily ibrutinib 420 mg orally daily and 2 cycles of weekly intrathecal methotrexate infusion, but the patient had missed a scheduled third cycle.
Orthostatic vitals showed supine heart rate 103 bpm and blood pressure 147/99 mm Hg, which was 107 bpm and 147/97 mm Hg after change in position. Physical examination showed no evidence of jugular venous distension, peripheral edema, or murmurs or additional heart sounds. D-dimer at time of COVID-19 infection was 1,630 ng/mL fibrinogen equivalent units. Repeat COVID-19 testing was negative. Electrolytes, transaminases, and creatinine were within normal range. Sequential high-sensitivity troponin T levels were not significantly elevated at 21, 19, and 24. B-type natriuretic level was not measured. Recent prothrombin time was 11.2 seconds, international normalized ratio, 1.0. Electrocardiogram showed heart rate 104 bpm, sinus rhythm, normal axis, without P or QRS voltage abnormalities, and no ST-T wave changes, unchanged from prior studies. The patient was admitted for further testing.
Transthoracic echocardiogram (TTE) showed left ventricular (LV) ejection fraction of 69%, as measured using two-dimensional biplane modified Simpson’s method. Left ventricular end-diastolic volume (two-dimensional biplane) was measured as 29.8 mL/m2 (normal, 34 ≤ end-diastolic volume index < 75), with the caveat that this may have been an underestimation due to foreshortened apical images. Left ventricular outflow tract stroke volume index was measured at 36 mL/m2 (normal, >35 mL/m2). Left ventricular mass, LV wall thickness and diastolic function (including E/e’), pulmonary pressures, and right ventricular function parameters were within normal range. No valvular abnormalities were noted.
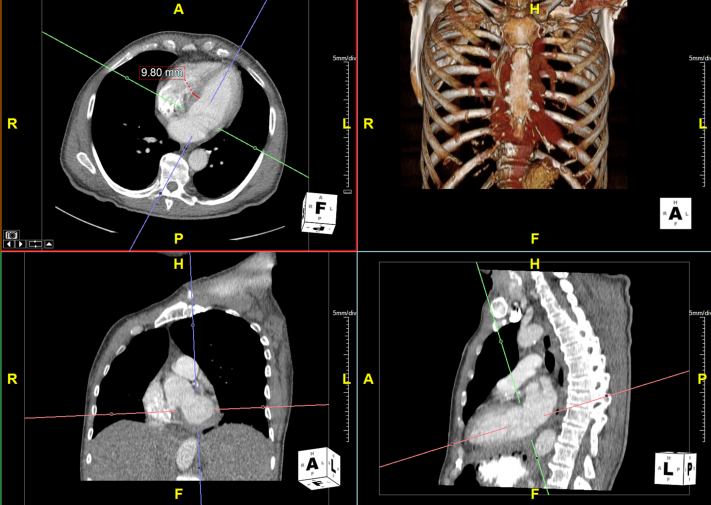
The TTE was unchanged from 4 months prior, with the exception of new, severe SEC observed throughout the study and in all 4 chambers. No perflutren lipid microsphere, agitated saline, or other ultrasound contrast agents were administered at any point during the studies. Institutional experience has noted artefactual, brand-specific disturbances caused by exposure to ultrasound-emitting devices. To rule out technical artifacts, the patient was examined with 2 different cardiovascular ultrasound machines with 2.5 MHz and 3.3 MHz respective transducers, as higher ultrasound frequencies have been described as important for the diagnosis of SEC.2 Both transducers reproduced the findings (Figure 1, Videos 1-3). Normal ventricular wall thickness and LV apex anatomy were confirmed by image analysis of a chest computed tomography study 1 day after the TTE (Figures 2 and 3, Video 4). We were unable to perform strain imaging as our strain software was unable to distinguish myocardial speckles from the blood pool spontaneous contrast.3
Figure 1.
Two-dimensional TTE, apical 4-chamber view, diastolic phase, demonstrates diffuse SEC in all heart chambers. (A) Phillips cardiac ultrasound system using a 2.5-MHz transducer. (B) GE cardiac ultrasound system using a 3.3-MHz transducer.
Figure 2.
Chest computed tomography image demonstrating normal ventricular wall thickness and LV apex anatomy in oblique axial, 4-chamber (top left), oblique coronal, short-axis (bottom left), and oblique sagittal, 2-chamber (bottom right) views. Top right image represents the volume-rendered display highlighting the skeletal structures.
Figure 3.
Chest computed tomography (CT) image demonstrating how a rib and the lung track anterior to the apex, helping to explain the noted differences in septal versus lateral wall echogenicity seen on TTE. The CT images are displayed in oblique axial, 4-chamber (top left), oblique coronal, short-axis (bottom left), oblique sagittal, 2-chamber (bottom right), and volume-rendered (top right) displays.
Discussion
Spontaneous echogenic contrast is an echocardiographic finding most commonly seen in the left atrium of patients with atrial fibrillation during transesophageal echocardiography and has prognostic implications as a marker of hypercoagulability. The precise pathophysiology of SEC is still unknown but is generally understood as a phenomenon related to clinical and echocardiographic parameters. Early studies have identified RBC rouleaux formation as a requirement for SEC formation, a concept supported by the correlation between SEC and increased RBC distribution width (RDW).4, 5, 6, 7, 8 Subsequent studies also showed a concentration-dependent relationship between hematocrit and fibrinogen levels in developing SEC, most evident at moderately low hematocrit levels.9 The role of other whole blood components in the formation of SEC is unclear, with some evidence suggesting that activation of leukocytes and platelets drives the formation of echogenic cell aggregates.10,11
The presence of SEC in all 4 chambers despite normal cardiac anatomy has been reported previously. In the prior case, the patient had a notable history of immune thrombocytopenic purpura and presented with autoimmune hemolytic anemia and macrocytosis.12 In comparison, our patient had a long-standing microcytic anemia for the past 2 years and a hematologic malignancy. In both cases it can be postulated that the presence of SEC is a reflection of change in the hematologic milieu. Review of previous and current blood work showed that hemoglobin, hematocrit, and overall white blood cell count were largely unchanged between past and current values, but there were observable increases in the mean corpuscular volume and RDW (Table 1). While progression of CLL may be one explanation for the development of SEC, the lack of significant overall leukocytosis is evidence against a hyperviscous state, as seen in Waldenstrom macroglobulinemia. Increased RDW and mean platelet volume have both been correlated with SEC.8,10,11
Table 1.
Complete blood count with differential values at time of previous and current echocardiogram
| Component (reference range and units) | Previous echocardiogram (– SEC) | Two weeks prior to ibrutinib therapy | Current echocardiogram (+SEC) |
|---|---|---|---|
| White blood cells (3.70-11.00 thousand cells/mcL) | 14.8 | 7.89 | 9.81 |
| RBC (4.20-6.00 million cells/mcL) | 3.85 | 2.77 | 3.36 |
| Hemoglobin (13.0-17.0 g/dL) | 9.1 | 6.8 | 8.8 |
| Hematocrit (39.0%-51.0%) | 27.2 | 20.0 | 26.0 |
| Mean corpuscular volume (80.0-100.0 femtoliter) | 70.8 | 72.2 | 77.4 |
| RDW (11.5%-15.0%) | 17.2 | 16.4 | 19.1 |
| Platelet count (150-400 thousand cells/mcL) | 264 | 485 | 219 |
| Mean platelet volume (9.0-12.7 femtoliter) | 7.6 | 10.1 | 9.2 |
The degree of SEC appears to be notably more severe in our patient compared with the case reported by Kanyal and colleagues.12 “Severe” SEC refers to dense smoke that appeared throughout the entire chambers. A number of different grading systems have been used to assess SEC (mainly with regard to the left atrium). For example, 0 indicates no smoke, 1+, mild smoke visible in some portion of the chamber, and 2+, dense smoke that appears throughout the entire chamber.13 An alternative system describes grade 0 as no SEC, grade 1 as mild, grade 2 as moderate, and grade 3 as severe.14
An important feature of our case is the sudden onset of SEC. No SEC was present during a stress TTE 4 months prior, despite the confirmed presence of RBC rouleaux formation and anemia at that time (Video 3). Two notable clinical events occurred between the initial TTE and the subsequent study showing diffuse SEC. The patient’s recent history of COVID-19 infection is potentially relevant given the findings reported by Connor-Schuler et al.15 In a retrospective study, SEC was detected in greater than 50% of COVID-infected patients admitted to the intensive care unit, albeit on point-of-care, venous ultrasound examination.15 The presence of SEC was more likely to be present when the d-dimer level was greater than 5,000 ng/mL (specificity, 0.97) and was associated with an odds ratio of 7 for clot development. In our patient, the combination of elevated d-dimer at the time of COVID-19 infection and mild low hematocrit is consistent with the fibrinogen-RBC interdependence on SEC formation.7
Initiation of ibrutinib may be another important contributing factor to the onset of SEC, as ibrutinib therapy has a known association with development of atrial fibrillation.1 While our patient did have an additional risk factor for atrial fibrillation (hypertension), several observations led us to believe that atrial fibrillation was unlikely. Extensive care was taken to inquire about the presence or history of palpitations, which the patient denied. Structural cardiac risk factors for atrial fibrillation were then ruled out with TTE, including the absence of left atrial enlargement and LV hypertrophy. Finally, initial and repeat electrocardiograms showed sinus rhythm without irregularity or concern for inappropriate atrial activity. The absence of atrial fibrillation on continuous cardiac telemetry monitoring for the duration of the admission (7 days total) further supported our decision not to pursue outpatient rhythm monitoring.
No clear relationship was identified between ibrutinib and the onset of SEC in our case, beyond the initiation of therapy. The patient had been prescribed 420 mg orally daily, a standard regimen indicated for CLL. Therapy had been initiated 20 days prior to presentation, continued during admission for a total of 15 days, and received the day of the TTE. Only aspirin was identified as a drug interaction due to antiplatelet properties, and no other CYP3A4 metabolized medications were identified. The mechanism of action for ibrutinib involves inhibiting lymphocyte adhesion and migration.16 Direct testing would be required to determine whether the increased intravascular leukocyte dispersion results in further RBC rouleaux formation and/or viscosity changes that produce SEC. Interestingly, there was a notable and sustained increase in the RDW following initiation of ibrutinib therapy (Figure 4).
Figure 4.
Increase in RDW after commencement of ibrutinib. Day 1 shows the data closest to previous TTE (1 month after study), day 33 (green) is hospital admission for COVID-19 infection, day 60 (red) represents 7 days after starting ibrutinib therapy, and day 76 represents time of TTE with severe diffuse SEC.
Conclusion
Spontaneous echocardiographic contrast is presumed to be a reflection of combined low blood flow and erythrocyte aggregation, as in the left atrium during atrial fibrillation. Here we report a case of SEC in a structurally normal heart, developed after initiation of ibrutinib for CLL, after resolved COVID infection, and in the absence of atrial fibrillation. Spontaneous echocardiographic contrast may be underappreciated in B-cell malignancies after chemotherapy initiation and/or post-COVID infection.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2023.03.005.
Supplementary Data
Two-dimensional TTE, apical 4-chamber view, demonstrates normal chamber dimensions, normal biventricular systolic function, and diffuse SEC in all heart chambers using a Phillips cardiac ultrasound system and a 2.5-MHz transducer.
Two-dimensional TTE, apical 4-chamber view, demonstrates normal chamber dimensions, normal biventricular systolic function, and diffuse SEC in all heart chambers using a GE cardiac ultrasound and a 3.3-MHz transducer.
Two-dimensional TTE, zoomed apical 4-chamber view, demonstrates normal LV size and normal LV systolic function without SEC obtained 4 months prior to this presentation.
Two-dimensional TTE, apical 4-chamber view with exaggerated rib/lung artifact during respiration that created the reduced echogenicity in the lateral wall.
References
- 1.Essa H., Lodhi T., Dobson R., Wright D., Lip G.Y.H. How to manage atrial fibrillation secondary to ibrutinib. JACC CardioOncol. 2021;3:140–144. doi: 10.1016/j.jaccao.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savéry D., Cloutier G. High-frequency ultrasound backscattering by blood: analytical and semianalytical models of the erythrocyte cross section. J Acoust Soc Am. 2007;121:3963–3971. doi: 10.1121/1.2715452. [DOI] [PubMed] [Google Scholar]
- 3.Ito T., Suwa M. Left atrial spontaneous echo contrast: relationship with clinical and echocardiographic parameters. Echo Res Pract. 2019;6:R65–R73. doi: 10.1530/ERP-18-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X.F., Liu L., Cheng T.O., Li Z.A., Deng Y.B., Wang J.E. The relationship between intracardiovascular smoke-like echo and erythrocyte rouleaux formation. Am Heart J. 1992;124:961–965. doi: 10.1016/0002-8703(92)90979-6. [DOI] [PubMed] [Google Scholar]
- 5.Reeder G.S., Charlesworth J.E., Moore S.B. Cause of spontaneous echocardiographic contrast as assessed by scanning electron microscopy. J Am Soc Echocardiogr. 1994;7:169–173. doi: 10.1016/s0894-7317(14)80123-5. [DOI] [PubMed] [Google Scholar]
- 6.Gerede D.M., Kaya C.T., Vurgun V.K., Acbuca A., Tak B.T., Ongun A., et al. Red cell distribution width as a predictor of left atrial spontaneous echo contrast in echocardiography. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peverill R.E., Graham R., Gelman J., Yates L.A., Harper R.W., Smolich J.J. Haematologic determinants of left atrial spontaneous echo contrast in mitral stenosis. Int J Cardiol. 2001;81:235–242. doi: 10.1016/s0167-5273(01)00572-1. [DOI] [PubMed] [Google Scholar]
- 8.Providência R., Ferreira M.J., Gonçalves L., Faustino A., Paiva L., Fernandes A., et al. Mean corpuscular volume and red cell distribution width as predictors of left atrial stasis in patients with non-valvular atrial fibrillation. Am J Cardiovasc Dis. 2013;3:91–102. [PMC free article] [PubMed] [Google Scholar]
- 9.Rastegar R., Harnick D.J., Weidemann P., Fuster V., Coller B., Badimon J.J., et al. Spontaneous echo contrast videodensity is flow-related and is dependent on the relative concentrations of fibrinogen and red blood cells. J Am Coll Cardiol. 2003;41:603–610. doi: 10.1016/s0735-1097(02)02898-x. [DOI] [PubMed] [Google Scholar]
- 10.Zotz R.J., Müller M., Genth-Zotz S., Darius H. Spontaneous echo contrast caused by platelet and leukocyte aggregates? Stroke. 2001;32:1127–1133. doi: 10.1161/01.str.32.5.1127. [DOI] [PubMed] [Google Scholar]
- 11.Akpek M., Kaya M.G., Yarlioglues M., Dogdu O., Ardic I., Sahin O., et al. Relationship between platelet indices and spontaneous echo contrast in patients with mitral stenosis. Eur J Echocardiogr. 2011;12:865–870. doi: 10.1093/ejechocard/jer159. [DOI] [PubMed] [Google Scholar]
- 12.Kanyal R., Brugger J., Ramoutar A., Arshad W., Kurbaan A.S., Xiao H.B. Spontaneous contrast in all cardiac chambers in a patient with a normal heart: case report with literature review. Int J Cardiol. 2014;175:e19–e20. doi: 10.1016/j.ijcard.2014.04.234. [DOI] [PubMed] [Google Scholar]
- 13.Sadanandan S., Sherrid M.V. Clinical and echocardiographic characteristics of left atrial spontaneous echo contrast in sinus rhythm. J Am Coll Cardiol. 2000;35:1932–1938. doi: 10.1016/s0735-1097(00)00643-4. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T., Shinoda Y., Ikeoka K., Inui H., Fukuoka H., Sunaga A., et al. Dabigatran exhibits low intensity of left atrial spontaneous echo contrast in patients with nonvalvular atrial fibrillation as compared with warfarin. Heart Vessels. 2017;32:326–332. doi: 10.1007/s00380-016-0871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor-Schuler R., Daniels L., Coleman C., Harris D., Herbst N., Fiza B. Presence of spontaneous echo contrast on point-of-care vascular ultrasound and the development of major clotting events in coronavirus disease 2019 patients. Crit Care Explor. 2021;3 doi: 10.1097/CCE.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Rooij M.F., Kuil A., Geest C.R., Eldering E., Chang B.Y., Buggy J.J., et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, apical 4-chamber view, demonstrates normal chamber dimensions, normal biventricular systolic function, and diffuse SEC in all heart chambers using a Phillips cardiac ultrasound system and a 2.5-MHz transducer.
Two-dimensional TTE, apical 4-chamber view, demonstrates normal chamber dimensions, normal biventricular systolic function, and diffuse SEC in all heart chambers using a GE cardiac ultrasound and a 3.3-MHz transducer.
Two-dimensional TTE, zoomed apical 4-chamber view, demonstrates normal LV size and normal LV systolic function without SEC obtained 4 months prior to this presentation.
Two-dimensional TTE, apical 4-chamber view with exaggerated rib/lung artifact during respiration that created the reduced echogenicity in the lateral wall.