Graphical abstract
Keywords: Hierarchical, 3D flower, α-Co(OH)2, Supercapacitor, Charge/discharge
Highlights
-
•
Hierarchical nanoflake assembled 3D flower-like cobalt hydroxide was designed.
-
•
Full-cell and half-cell performance were studied using α-Co(OH)2 nanoflake electrodes.
-
•
Excellent specific capacitance and cyclic retention approximately were obtained.
-
•
Cyclic stability of approximately 85% was retained after 5000 repeated cycles.
-
•
Energy density of approximately 67.5 Wh/kg at a power density of 328.94.
Abstract
Introduction
The energy industry has been challenged by the current high population and high energy consumption, forcing the development of effective and efficient supercapacitor devices. The crucial issues until now have been high production cost, deprived cyclic stability, and squat energy density. To resolve these problems, various approaches have been taken, such as the development of long-life electrode materials with high capacity, rapid charging, and slow discharging to overcome poor life cycle stability.
Objectives
In the present work we focus on fabricating cost-effective unique-morphology, high-surface-area alpha-Co(OH)2 for application in an aqueous-electrolyte symmetric supercapacitor.
Methods
In this study, hierarchical nanoflakes assembled in three-dimensional (3D) flower-shaped cobalt hydroxide (HN-3DF-α-Co(OH)2) electrode were synthesized using the solvothermal method with sodium dodecylbenzene sulfonate (SDBS) and methanol as solvents. Spectroscopic and microscopic techniques were used to characterize fabricated HN-3DF-Co(OH)2, which revealed that the materials electrode exhibited the alpha phase with a hierarchical flower-like structure. A half-cell electrochemical assembly (three-electrode assemble cell) and symmetric full cell (two-electrode assemble cell) were examined in an aqueous electrolyte.
Results
In three-electrode assembly cells, HN-3DF-α-Co(OH)2 exhibited 719.5 Fg−1 specific capacitance (Csp) at 1 Ag−1 with excellent cyclic retention stability of approximately 88% after 3000 cycles. In two-electrode symmetric supercapacitive systems, HN-3DF-α-Co(OH)2 achieved a maximum Csp of 70.3 Fg−1 at 0.4 Ag−1 with the highest energy density of approximately 6.25 Wh/kg at a power density of 328.94 W/kg. The fabricated two-electrode assembly cell with the HN-3DF-α-Co(OH)2 electrode retained cyclic stability of approximately 85% after 5000 repeated charge and discharge cycles.
Conclusion
Solvothermally-synthesized, optimized HN-3DF-α-Co(OH)2 showed outstanding electrochemical performance results in three- and two-electrode systems. This unique aqueous symmetric supercapacitor can be used to design cost-effective symmetric capacitors based on metal hydroxide.
Introduction
Portable electronics and microscale energy storage devices with advanced characteristics, such as lightweight, flexible, fast charging, and slow discharging, and small and compact energy storage mechanisms, have attracted considerable research attention [1], [2], [3], [4], [5]. In this study, supercapacitors with longer lifecycles and improved power and energy densities, as compared to conventional capacitive electrodes, were prepared. The performance of the supercapacitive electrode assembled inside an energy storage device depends on the electrode material, its morphology, and its storage mechanism [6], [7], [8], [9], [10], [11], [12], [13]. Electrochemical-based supercapacitors are widely used in all portable devices, memory backup systems, and hybrid electric vehicles because of their high power density and faster charge-discharge capability [8], [9], [10], [11], [12], [13], [14]. However, low energy density and life cycle problems persist. Therefore, the development of advanced supercapacitors with enhanced energy density with long lifecycles is critical for satisfying future energy demand. There are various studies that directly focused on developing inexpensive and novel electrode materials with high power and energy densities [9], [10], [11], [12], [13], [14], [15], [16]. Therefore, various carbonaceous electrode material designs have been proposed for the anode. The use of a double-layered energy storage mechanism in which the charge over the electrode and electrolytes interfaces is used for storage is prevalent. Various metal oxides and hydroxides and conductive polymers with morphology have been used for fabricating cathode materials [10], [11], [12], [13], [14], [15], [16], [17], [18]. These electrode materials primarily function according to the Faradic mechanism, in which the oxidation and reduction reaction occur over the electrode surface. Moreover, metal oxides and metal sulfides have been used as positive electrode materials in pseudocapacitor applications. Because of the cost-effectiveness, excellent electrical conductivity, semiconductor behavior, and lower electronegativity and redox behavior transition metal oxides are used in the pseudocapacitor and battery application [14], [16]. In nickel- and cobalt-based oxide materials, known or morphological diversities such as nanowires, nanotubes, nanorods, and nanoflowers depend on the synthesis method, conditions, and precursor aspects. Moreover, CoO and Co(OH)2 exhibit superior electrochemical performance and electrical conductivity compared to other metal oxides [20]. In particular, three-dimensional (3D) flower-layered metal oxide-hydroxides with distinct morphology has received special attention in supercapacitor application because of their unique properties, such as high surface area and thin nanoflake characters. Specifically, the Co(OH)2 is considered an efficient and active electrode material owing to its low cost high theoretical specific capacitance recoded because of the charge storage mechanism in the presence of the electrolytes, which can be written as Co(OH)2 + OH– ↔ CoOOH + H2O + e- [21]. The redox peak observed because of the ion intercalation and transformation of the Co(OH)2 into CoOOH corresponds to the battery-like characteristic because the 3D morphology and layered structure of Co(OH)2 exists in crystalline phases α or β [22]. In particular, the α phase of Co(OH)2 exhibits a layered, hydrotalcite-based structure. Furthermore, the α phase of Co(OH)2 contains positively-charged layers of Co(OH)2-x with various charge balancing anions, whereas the presence of the water molecules inside the interlayer structure helps to store the charge, causing an expansion in the larger interlayer spacing [22]. Another form of the cobalt hydroxide exhibits a brucite structure that does not allow the intercalation of molecules, whereas the α phase exhibits superior interlayer chemistry with a larger interlayer spacing than those of β-Co(OH)2. This property ensures more electrolyte ion transport during the electrochemical test. In this contest, α-phase Co(OH)2 with the 3D thin nanoflakes exhibits more activity in the electrochemical performance because of its small thickness and 3D structure, which provides a larger specific area during supercapacitive electrochemical performance.
Various Co(OH)2 nanostructures, such as nanoparticles, nano bars, nanoflakes, sheet-like, and flowered structures, have been synthesized by synthesis methods such as chemical bath deposition, solvothermal/hydrothermal method, chemical precipitation method, and electrodeposition method, have been reported [13], [16]. However, most of these articles reported for the β-phase of the Co(OH)2 formation originate from the metastable state of the α-Co(OH)2 phase, which subsequently likely transforms into the β-Co(OH)2 phase. For example, Janaa et al. designed α- and β-phase Co(OH)2 using the hydrothermal method. Flakes of the α, such as α-Co(OH)2 with interlayer spacing of 8.24 Ã… are larger than the nanorod structure of β-Co(OH)2 interlayer spacing (4.63 Å). These nanostructures are used in numerous supercapacitor applications [22]. Paresh et. al synthesized the nano-bar-like morphology of β-Co(OH)2 film using the CBD method and used such films in supercapacitor applications [23]. Lyons et al. designed two-dimensional Co(OH)2 nanoflakes and used them in supercapacitor and oxygen evaluation catalyst applications. Based on the above discussion, α-phase Co(OH)2 should be produced in excessive amounts. Therefore, we focused on the controlled hydrothermal synthesis of ultrathin hierarchical nanoflakes assembled with 3D flower-like morphology and interlayer spacing of α-phase Co(OH)2. The 3D flower-like morphology of α-Co(OH)2 exhibits nanoflakes with a thickness of approximately 4–6 nm. Based on various reaction times, the morphology of the precursor gradually changed from irregular flakes to a 3D flower-like structure. A novel growth mechanism for the 3D flower-shaped structure with gradual OH– insertion in and outside-inside strategy was proposed. Subsequently, the electrochemical performance of fabricated electrode material was evaluated for the supercapacitor application in full-cell (symmetric) and half-cell assembly. In half-cell assembly, HN-3DF-α-Co(OH)2 exhibited an excellent capacitance value of 719.5 Fg−1 at the current load of 1.0 Ag-1 and retained approximately 88.0% of its initial specific capacitance after 3000 continuous charge/discharge cycles. The two-electrode symmetric cell assembly exhibited the maximum specific capacitance of 70.3 Fg−1 at 0.4 Ag−1 and maintained cyclic stability of approximately 85% after 5000 charge/discharge cycles.
Materials and methods
Materials
The ethylene glycol, cobalt nitrate, ethanol, SDBS, and ammonium hydroxide were acquired from Sigma-Aldrich. The current collector, that is, nickel foam, was acquired from the MTI Corporation (USA). Analytical-grade chemicals were used in these studies.
Methods
PANalytical-X-ray diffractometer (MPD Netherland) equipped with X’Pert-PRO software was used to examine the phase and crystalline behavior of HN-3DF-α-Co(OH)2. The S4800-scanning electron microscope (HITACHI) was used to examine the surface behavior and morphologies of HN-3DF-α-Co(OH)2. The G2-F20-field emission transmission electron microscope (Tecnai, USA) was used to analyze the internal structure, size, and shape of as-prepared HN-3DF-α-Co(OH)2. The ESCALAB-250-X-ray photoelectron spectroscope (UK; TFS) was used to examine the surface composition and chemical interaction of HN-3DF-α-Co(OH)2.
Cell assembly setup and its electrochemical measurements
In this study, 3D nickel foam substrate current collector was used because it functions as an outstanding conductor, has porous behavior, and excellent mechanical stability towards the electrodes. Furthermore, Ni foam provided a large volume of electrolyte ions to the electroactive surface through a short pathway [24], [25]. In this study, a Versa STAT 3, Princeton Research, USA, the electrochemical workstation was used to perform electrochemical measurement of the fabricated HN-3DF-α-Co(OH)2 electrode in the three-electrode system. The working electrode was a Ni foam coated as-synthesized HN-3DF-α-Co(OH)2 electrode; Ag/AgCl was used as reference electrode, and the Pt sheets were used as a counter electrode. The working electrode was fabricated using HN-3DF-α-Co(OH)2 active materials, conductive carbon, and PVDF at the mass ratio of 80:10:10 in the NMP solvent. Subsequently, a paste was coated on the washed Ni foam current collector with an effective electrode area of 1 cm2 add dried electrode at 80 °C for 12 h. The symmetric assembly cell set up with the two equivalent HN-3DF-α-Co(OH)2 electrodes was set up inside the aqueous KOH electrolyte solution.
Synthesis of HN-3DF-α-Co(OH)2 electrode materials
Hierarchical nanoflake-assembled 3D flower-like α-Co(OH)2 was synthesized using the simple solvothermal method. During synthesis, 2.0 mmol of Co(NO3)2·6H2O and 1.0 g of SDBS were dissolved in the 30.0 mL methanol. A few drops of NH3·H2O were added to adjust the pH of the solution around 9, which was stirred for 15 min continuously at room temperature and subsequently transferred to the Teflon-lined assembly and kept in an oven at 160 °C for 12 h. The precipitated solid was collected by centrifuge followed by washing with DI water and ethanol. The obtained product was dried at 80 °C in an oven for 24 h to obtain HN-3DF-α-Co(OH)2 as the final product.
Results and discussion
Numerous synthesis methods, such as the deposition method and microwave, hydrothermal, solvothermal, and solid-state syntheses, have been reported for the fabrication/production of metal oxides and hydroxides [26]. Among the aforementioned syntheses, the hydrothermal/solvothermal synthesis process is typically used to fabricate metal oxide or hydroxide nanostructure with various morphologies. In the hydrothermal/solvothermal synthesis metal processor, an aqueous solution of the metal complex is formed. This complex is steadily hydrolyzed by the accumulation of water/solvent [26]. In this study, we selected the solvothermal synthesis process to fabricate 3D nanoflake-assembled nanoflower-like α-Co(OH)2. Fig. 1 displays the schematic of the SDBS-assisted formation of the 3D nanoflower-like HN-3DF-α-Co(OH)2, which reveals that the SDBS ions partially replaced the hydroxyl ions (OH–) from the SDBS intercalated structure in the 003 plane of the few layered and thin nanoflakes-assembled α-Co(OH)2 [27], [28]. No sodium element was observed, which indicated clearly that the surface was free from the physical absorption of the SDBS. Based on the SEM and TEM observation and characterization of the morphology, a possible mechanism of 3D nanoflake-assembled nanoflower-like α-Co(OH)2 was proposed. This mechanism differs from the self-assembling process of nanoflakes aggregation, which is typical and complex process. In this synthesis, SDBS and ammonia work as a directing agent during the solvothermal process of α-Co(OH)2 fabrication in which the sulfonate ions of the SDBS intercalate to the 003 plane of α-Co(OH)2 and form the α-Co(OH)2 and SDBS intercalated structure. Moreover, the redox reaction could be possible at 160 °C to form the thin-layered nanoflakes morphology [27], [28]:
| 4CH3OH + NO3– + H2O → NH4+ + Co(OH)2. | (1) |
| Co2+ + OH– + NH3 + H2O → Co(OH)2 + NH4+. | (2) |
Fig. 1.
Suggested mechanism for the fabrication of HN-3DF-α-Co(OH)2 through the solvothermal process.
Due to the formation of the ions of the OH–, from the results and the aforementioned reaction, we presume that the formation of the nanoflake-assembled 3D α-Co(OH)2 nanoflowers may be induced by the gradual OH– ions incursion inside and outside of the nanoflakes. Therefore, the results of X-ray photoelectron microscopy (XPS) indicates that the gradual OH– ions incursion inside and outside strategy in α-Co(OH)2 nanoflower. During the formation, the OH– ions impose into the precursor particles to form the Co(OH)2 nanoflakes. By contrast, ions of the dodecylbenzene sulfonate transfer off the precursor owing to developing the hierarchical 3D flower-like morphology through ion diffusion from the effects of the thermal condition during the reaction. Therefore, based on the aforementioned results and discussion, the mechanism was revealed and supported the gradual OH– ions incursion inside and outside strategy [27], [28].
X-ray diffraction (XRD) results confirmed the crystalline behavior and phase structure of as-fabricated HN-3DF-α-Co(OH)2 (Fig. 2a). As displayed in Fig. 2a, the diffraction peak observed at 11.78 and 22.82 were attributed to the (0 0 3) and (0 0 6) reflection plane of a hydrotalcite-like α-Co(OH)2 (JCPDF 74–1057) [29], [30]. The interlayer spacing was approximately 0.78 nm, larger than β-Co(OH)2, which revealed the existence of molecule intercalation, such as water between the adjacent host layer of HN-3DF-α-Co(OH)2. In XRD, we did not observe any additional signal related to the cobalt oxide, such as Co3O4, which confirmed the successful fabrication of the HN-3DF-α-Co(OH)2. Moreover, to gain additional chemical structure information of synthesized HN-3DF-α-Co(OH)2, XPS was investigated, and the results are displayed in Fig. 2b and 2c. The Co 2p spectrum revealed a spin–orbit splitting into two main components at approximately 779.90 and 795.5 eV, which correspond to the existence of the Co 2p3/2 and Co 2p1/2 spin orbits, respectively, with splitting energy of approximately 16.2 eV (Fig. 2b). The deconvoluted Co 2p3/2 spectra of the HN-3DF-α-Co(OH)2 exhibited a strong band at approximately 782.20 eV, which was ascribed to the presence of the Co2+ state in the structure. The appearance band at approximately 783.80 eV revealed the existence of the oxyhydroxide of the cobalt with the alpha phase of cobalt hydroxide. The presence of intense shakeup peaks further confirmed the formation of the alpha phase of the Co(OH)2. This result is consistent with previously reported studies [31], [32]. Fig. 2c reveals the O 1 s high resolution spectra of the HN-3DF-α-Co(OH)2. The fitted curves of the spectrum indicate that the sample consists of three main components. The strongest band at approximately 531.6 eV can be assigned to be α-Co(OH)2, which is the dominating band of the fabricated sample. The other smaller shoulder bands indicate the existence of the Co-O bonding at approximately 529.46 eV and the O-H group at approximately 532.80 eV, which details the bonding of water molecules over the electrode active martial surfaces [33].
Fig. 2.
(a) XRD pattern, (b) Co 2p XPS high-resolution spectra, and (c) O 1s XPS high-resolution spectra of HN-3DF-α-Co(OH)2.
The surface and internal morphology of the as-prepared product was investigated through SEM and TEM analysis, and the results are displayed in Fig. 3a-3d. SEM images revealed that HN-3DF-α-Co(OH)2 exhibits 3D nanoflower-like morphology with the uniform surface of nanoflakes (Fig. 3a-3d). The size of nanoflowers was approximately 2.0 to 3.0 μm, which revealed a range of hierarchically 3D nanoflower structures (Fig. 3c and d). High-magnification SEM revealed that the thickness of these ultrathin nanoflakes was approximately 4–6 nm. These flakes were arranged vertically on the surface with an open space forming between the neighboring Co(OH)2 nanoflakes. These numerous nanoflakes with open space in 3D flower structures exhibit several surface active sites, which are favorable during the electrochemical supercapacitor procedure.
Fig. 3.
(a-d) FESEM images of HN-3DF-α-Co(OH)2 at various magnification levels.
The detailed internal structure information obtained from the TEM characterization(Fig. 4) indicates that the hierarchically 3D nanoflower assembled ultrathin nanoflakes flower structure of α-Co(OH)2 with a thickness of approximately 3–6 nm, which is highly correlated with SEM results. The thickness of the wrinkles or stripy lines recognizes the thickness of nanoflakes, which are fabricated with the approximately 3–7 single and automatically grown layer by layer assembling of the thick Co(OH)2 layers, which leads to 033 planes (Fig. 4a-4d). This structure is considerably thinner than that reported in other repotted studies. In 3D flower morphology, the formation of the dark area or stripy lines indicates wrinkle and, overlapping of the surface of the nanoflakes. The high-resolution TEM analysis was used to verify the crystal structure of Co(OH)2. The results are displayed in Fig. 4d. The lattice spacing was observed to be approximately 0.78 nm, which corresponded to the lattice spacing of the 003 plane in the crystal structure of the Co(OH)2. The specific surface rea of the HN-3DF-α-Co(OH)2 electrode was measured uing the adsorption desorption isotherm and results are displayed in Figure S1.The high surface area i.e. 80.50 m2/g of the HN-3DF-α-Co(OH)2 electrode which supported the better diffusion and transportation of the electrolytic ion in the charge–discharge process. The larger surface area is of huge benefit for the increasing the electrolyte/electrode contact area, and provide more active sites for the faradic reaction, which is helpful to achieve excellent electrochemical supercapacitive performance.
Fig. 4.
(a-e) TEM and high-resolution TEM (HRTEM) images of HN-3DF-α-Co(OH)2 at various magnification levels.
Electrochemical analysis of half-cells (three-electrode assembly cell)
The fabricated HN-3DF-α-Co(OH)2 electrode was based on the Faradic redox pseudocapacitance that followed the intercalation or absorption of the electrolyte ions over the surface or over the bulk of the electrodes. The fabricated HN-3DF-α-Co(OH)2 was formed by the interconnected thin nanoflakes, which provided more surface area for the ion and proton intercalation for the fast reaction during the electrochemical process, further supporting the excellent pseudocapacitance and energy density performances. To understand the electrochemical supercapacitive performance of the HN-3DF-α-Co(OH)2 electrode, CV and GCD techniques were employed inside the three electrode assembly cell (Fig. 5, Fig. 6) in the 2 M KOH aqueous electrolyte. The initial behavior of the fabricated HN-3DF-α-Co(OH)2 electrode was examined by the CV analysis within the potential range of 0.0–0.5 V. Fig. 5a displays that the CV profile of the fabricated HN-3DF-α-Co(OH)2 electrode and bare nickel foam at a scan rate of the 10 mV/s, which clearly revealed that the integrated area of the HN-3DF-α-Co(OH)2 electrode was considerably higher than that of the bare nickel foam, indicate a negligible specific capacitance provided by the current collector (nickel foam). The CV curve revealed one oxidation peak at 0.388 V, which supported the oxidation of Co3+ to Co2+. Therefore, in the CV curve, the redox characteristic was observed at the OH– site. Two possible Faradic reactions can be suggested for the electrochemical reaction expressed by the following equation:
| Co(OH)2 + OH– ↔ CoOOH + H2O + e- |
| CoOOH + OH– ↔ CoO2 + H2O + e- |
Fig. 5.
(a) Comparative CV profile of the bare nickel foam and HN-3DF-α-Co(OH)2 electrode at a fixed scan rate, (b) CV curve of HN-3DF-α-Co(OH)2 at various scan rates, (c) comparative GCD curve of bare nickel foam and HN-3DF-α-Co(OH)2 electrode at fixed current density, and (d) GCD curve of the HN-3DF-α-Co(OH)2 electrode at various current densities.
Fig. 6.
(a) Calculated Csp of the HN-3DF-α-Co(OH)2 electrode at various current loads and (b) C/D stability of the HN-3DF-α-Co(OH)2 at a fixed current load of 5 Ag−1 over 3000C/D cycles (inset figure shows the GCD turns at various cycles).
The anodic and cathodic peaks of the HN-3DF-α-Co(OH)2 electrode shifted to the right and left sides with the increase in the scan rate from the 5 to 200 mVs−1. The small peak shifting indicated that a more reversible and faster transfer of the charge occurred over the stance of the electrode during the electrochemical process (Fig. 5b).
The GCD and its corresponding specific capacitance were examined for understanding the charge storage mechanism and potential application of the HN-3DF-α-Co(OH)2 electrode in the energy storage application (Fig. 5c and 5d). Fig. 5c depicts the GCD graph of the bare nickel foam and HN-3DF-α-Co(OH)2 electrode at a fixed current density of 1 Ag−1, which revealed that the discharge time of the HN-3DF-α-Co(OH)2 electrodes was considerably higher than that of the bare nickel foam. Furthermore, the GCD of the HN-3DF-α-Co(OH)2 electrode exhibited a nonlinear behavior region in charge/discharge (C/D) graph, and the voltage plateaus region consisted of a visible redox wave, which is related to the CV curves. This result indicated a Faradic reaction that followed the battery-like behavior because of the redox couple Co3+/Co2+ [21], [17], [18], [19], [20]. The maximum specific capacitance calculated using GCD curves was 719.5 Fg−1 at a fixed current density of 1.0 Ag−1. This phenomenon boosted the performance of the HN-3DF-α-Co(OH)2 electrode possibly because of the following two reasons: first, a broad and large surface area and corresponding pore size originated through the 3D structure of the HN-3DF-α-Co(OH)2 electrode, which facilitated ion intercalation and provided a larger active site volume during the electrochemical process. Second, crystallinity was oriented toward the particular plane with a homogeneous elemental distribution, which may facilitate the intercalation and deintercalation process of the ions between the electrode and electrolytes, boosting the electrochemical supercapacitance performances of the designed electrodes [5], [6], [13]. A comparison of the results with previously published reports revealed that specific capacitance is critical in the energy storage application (Table 1). Additionally, the Csp of the HN-3DF-α-Co(OH)2 was measured at a different current density (Fig. 5d and Fig. 6a). Notably, the tendency of Csp decreased when the current density increased, which is obvious because the limited penetration of the electrolyte in the electrode material (HN-3DF-α-Co(OH)2 electrode) decreases the Csp value.
Table 1.
Performance comparison of the designed HN-3DF-α-Co(OH)2 electrode with reported studies.
| Electrode Material | Synthesis Method | Morphology | Electrolyte | Specific Capacitance | Retention % | Ref |
|---|---|---|---|---|---|---|
| Co2P | Decomposition | flower-like | 6 M KOH | 416 Fg−1@1 Ag−1 | 97% after 6000 cycles | [34] |
| Co3N | liquid-phase | nanoparticles | 6 M KOH | 112.3 Fg−1@0.5 Ag−1 | 66.7 % after 10,000 cycles | [35] |
| Co2P | Hydrothermal | rod-like | 1 M LiCl | 156.1 Fg−1 @1mAcm−2 | 82% after 5000 cycles | [36] |
| CoSe2 | salinization | hollow core-branch | 3 M KOH | 759.5 Fg−1@1mAcm−2 | 94.5% after 5000 | [37] |
| CoWO4 | Hydrothermal | nanoparticles | 2 M KOH | 764.4 Fg−1@1Ag−1 | 6000 cycles | [38] |
| CoV2O6 | co-precipitation | sponge-like | 2 M KOH | 306.6 Fg−1@1Ag−1 | 83% after 20,000 cycles | [39] |
| Co3V2O8 | Hydrothermal | nanoplates | 3 M KOH | 739 Fg−1@ 0.5 Ag−1 | 95.3% after 2000 cycles | [40] |
| Co11(HPO3)8(OH)6 | hydrothermal | ultralong nanoribbons | 3 M KOH | 312 Fg−1@1.25 Ag−1 | 89.4% after 3000 cycles | [41] |
| NH4CoPO4·H2O | chemical precipitation | micro flowers | 3 M KOH | 369.4 Fg−1@ 0.62 Ag−1 | 99.7% after 400 cycles | [42] |
| CoHPO4·3H2O | Solvothermal | nanosheet | 3 M KOH | 413 Fg−1@1.5A g−1 | 85.1% after 3000 | [43] |
| CoOOH | CBD | porous films | 2 M KOH | 386 Fg−1@ 1mAcm−2 | 90% after 5000 | [44] |
| α-Co(OH)2 | hydrothermal | nanowire | 2 M KOH | 642.5 Fg−1@1 A g−1 | NA | [45] |
| HN-3DF-α-Co(OH)2 | Hydrothermal | 3D flower like | 2 M KOH | 719.5 Fg−1@1 Ag−1 | 88% after 5500 | Present work |
The electrode stability life is a major concern in terms of the potential application in various energy storage applications due to the degradation of the active materials during the long C/D process. The cyclic stability test of the HN-3DF-α-Co(OH)2 electrode was performed by continuous GCD measurement and calculation of the specific capacitance (Fig. 6b) at fixed current densities of 5.0 Ag−1 over continuous 3000 charge–discharge cycles. As displayed in Fig. 6b, the HN-3DF-α-Co(OH)2 electrode exhibited excellent cyclic stability of 88%. The excellent electrochemical performance of the fabricated HN-3DF-α-Co(OH)2 electrodes is due to its rational 3D hierarchical structure. Thus, the sheet-like structure and open space between the adjacent sheets facilitate superior ion diffusion, rapid ion transportation, and excellent electrode–electrolyte contact during the electrochemical processes. Thus, the supercapacitor performances of the HN-3DF-α-Co(OH)2 electrodes were improved. This phenomenon revealed that the HN-3DF-α-Co(OH)2 electrode may exhibit excellent potential for energy storage application.
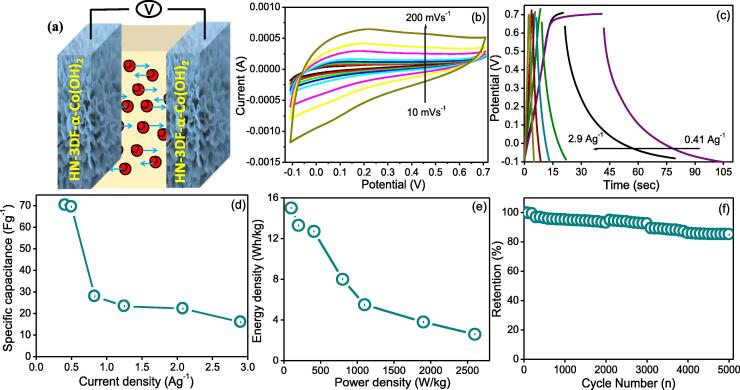
Symmetric supercapacitive performance of the HN-3DF-α-Co(OH)2 electrode in full cell
The energy density of the supercapacitor is relative to the square of the applied potential voltage. Therefore, we applied the solvothermal synthesized HN-3DF-α-Co(OH)2 electrode assembly in the symmetric cell to boost the energy density and enhance the potential window (Fig. 7a). The CV graph of the HN-3DF-α-Co(OH)2 electrode in the symmetric assembly cell had a scan rate of 10–100 mVs−1 with the potential ranging from − 0.1 to 0.8 V. The shape of the CV curves were rectangular without anodic and cathodic peaks (Fig. 7b), which differed from the behavior of the CV voltammograms observed in the three-electrode assembly cell. The absence of the oxidation and reduction peaks in the voltammograms of the 3 HN-3DF-α-Co(OH)2 electrode symmetric assembly cell could be attributed to the pseudo constant rate of the C/D process occurring over the electrode during the voltammetry cycles, which is typical in the metal-oxide-based hybrid electrode assembly [46], [47]. The C/D measurement of the HN-3DF-α-Co(OH)2 electrode symmetric assembly cell was measured (Fig. 7c), and the corresponding specific capacitance was calculated. The highest capacitance values based on the entire volume of the assembly were 70.30, 69.50, 28.0, 23.40, 22.30, and 16.04 Fg−1 at a current load of 0.4, 0.5, 0.83, 1.25, 2.08, and 2.9 Ag−1 (Fig. 7d).
Fig. 7.
(a) Schematic of the symmetric cell assembled with the HN-3DF-α-Co(OH)2 electrode, (b) CV graph of the symmetric assembly cell at different scan rates, (c) GCD graph of the symmetric assembly cell at various current densities, (d) calculated specific capacitance of the HN-3DF-α-Co(OH)2 electrode at different current loads, (e) power density and energy density graph of the HN-3DF-α-Co(OH)2, and (f) C/D stability test graph of the HN-3DF-α-Co(OH)2 electrode at a fixed current load over 5000 cycles.
The Csp of the symmetric capacitor decreases with the increasing value of the current, which is consistent with the aforementioned three-electrode assembly cell performance. This consistency was apparent at a higher current rate because of the insufficient contact time of the active electrode materials toward the redox reaction [46]. The calculated Csp of the current study is better and higher than the reported literature capacitance value of the symmetric capacitor. For example, nanoflakes α-(CoOH)2 at 44.0 Fg−1 [48] and SnS/S doped G hybrid at 2.98 mF/cm2 [49]. The enhanced performance and good rate capability could be due to the rational 3D design of the HN-3DF-α-Co(OH)2 electrode. The 3D morphological analysis revealed a large accessible surface area during electrochemical performance and pseudo capacitance nature of the HN-3DF-α-Co(OH)2 electrode.
Long-term stability played a major role towards the potential application of the electrode materials in the design of the energy storage devices. This study evaluated the performance of the electrode over the 5000 consecutive charge–discharge cycles at 0.83 Ag−1. The results of the experiment are displayed in Fig. 7f.
The symmetric assembly cell revealed 85.03 % capacitance retention after long numbers of cycles, which confirmed the suitability of the α-Co(OH)2 electrode-based supercapacitor assembly used for energy storage application (Fig. 7f). The power and energy densities are the main two major parameters for evaluating the practicability of symmetric supercapacitors. The power and energy densities of the Hn-3DF-α-Co(OH)2 electrodes were calculated through the equation mentioned in the supplementary information at various current densities illustrated in Fig. 7e. Various energy densities were observed at the different power densities, as follows: 6.25 Wh/Kg @ 328.94 W/Kg, 6.17 Wh/Kg @ 385.62 W/Kg, 2.40 Wh/Kg @ 648.64 W/Kg, 2.08 Wh/Kg @ 1000 W/Kg, and 1.40 Wh/Kg @ 2295.08 W/Kg. The maximum energy density 6.25 Wh/Kg was reached at the power density of 328.94 W/Kg.
Overall, the assembled symmetric cell results proved that the HN-3DF-α-Co(OH)2 electrode is feasible for practical application in the field of energy storage application. The higher Cs and excellent cyclic stability ratio of the HN-3DF-α-Co(OH)2 electrode could be attributed to its unique interlinked nanoflakes. Analysis of the 3D porous morphology revealed a large exposed surface area with a large number of the electroactive sites for the oxidation and reduction reaction and conductive current collector nickel foam skeleton boosting the ionic transport, effective charge transfer pathways, superior electrode–electrolyte contact, and short diffusion path, which enhanced the overall supercapacitive performance of the electrodes.
Conclusion
In this study, a facile one-step solvothermal method was used for the synthesis of the HN-3DF-α-Co(OH)2 electrode morphology. Furthermore, the possible mechanism was discussed in detail. The interconnected sheet-like morphology of the HN-3DF-α-Co(OH)2 electrode assembled in the three-electrode and two-electrode systems revealed excellent electrochemical performance in a 2 M KOH electrolyte. In the three-electrode system, the highest specific capacitance of 719.5 Fg−1 was obtained at 1.0 Ag−1, with excellent capacitance retention of 88% after 3000 cycles. Next, the symmetric cells were successfully assembled with the HN-3DF-α-Co(OH)2 electrode as positive and negative electrodes in the 2 M KOH electrolyte. The fabricated symmetric cell exhibited 85% capacitance retention after continuous 5000 charge–discharge cycles in addition to the cyclic stability. It also delivered a maximum energy density of 6.25 Wh/kg at a power density of 328.94 W/kg. The performance of the current study of the aqueous symmetric supercapacitor generated a novel method of design for fabricating cost-effective symmetric capacitors based on metal hydroxide.
CRediT authorship contribution statement
Sajid Ali Ansari: Conceptualization, Methodology, Data curation, Supervision, Funding acquisition. Nazish Parveen: Writing – original draft, Investigation, Validation, Resources. Mohd Al Saleh Al Othoum: Writing – review & editing, Visualization, Software, Resources. Mohammad Omaish Ansari: Writing – review & editing, Visualization, Software, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported through the Annual Funding track by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Project No. GRANT827].
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Sajid Ali Ansari, Email: sansari@kfu.edu.sa.
Nazish Parveen, Email: nislam@kfu.edu.sa.
Mohd Al Saleh Al-Othoum, Email: malothoum@kfu.edu.sa.
References
- 1.Guo W., Dun C., Yu C., Song X., Yang F., Kuang W., et al. Mismatching integration-enabled strains and defects engineering in LDH microstructure for high-rate and long-life charge storage. Nat Commun. 2022;13 doi: 10.1038/s41467-022-28918-0. 1409 (1–10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q., Chen Y., Jiang X., Qiao X., Wang Y., Zhao H., et al. Self-assembly defect-regulating superstructured carbon. Energy Storage Mater. 2022;48:164–171. [Google Scholar]
- 3.Wang Q., Zhou Y., Zhao X., Chen K., Bingni G., Yang T., et al. Tailoring carbon nanomaterials via a molecular scissor. Nano Today. 2021;36 [Google Scholar]
- 4.Wang Q., Liu F., Jin Z., Qiao X., Huang H., Chu X., et al. Hierarchically divacancy defect building dual-activated porous carbon fibers for high-performance energy-storage devices. Adv Funct Mater. 2020;30:2002580. [Google Scholar]
- 5.Wang G., Zhang L., Zhang J. A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev. 2012;41:797–828. doi: 10.1039/c1cs15060j. [DOI] [PubMed] [Google Scholar]
- 6.Parveen N., Ansari S.A., Ansari M.Z., Ansari M.O. Manganese oxide as an effective electrode material for energy storage: a review. Environ Chem Lett. 2022;283–309 [Google Scholar]
- 7.Deng T., Zhang W., Arcelus O., Kim J.-G., Carrasco J., Yoo S.J., et al. Atomic-level energy storage mechanism of cobalt hydroxide electrode for pseudocapacitors. Nat Commun. 2017;8:15194. doi: 10.1038/ncomms15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng T., Lu Y., Zhang W., Sui M., Shi X., Wang D., et al. Inverted design for high-performance supercapacitor via Co(OH)2-derived highly oiented MOF electrodes. Adv Energy Mater. 2018;8:1702294. [Google Scholar]
- 9.Parveen N., Ansari S.A., Al-Abawi B.T., Ansari M.O. Fabrication of binder-free hierarchical three dimensional NiO nanoflakes@carbon nanofibers for superior symmetric supercapacitor application. J Storage Mater. 2022;55 [Google Scholar]
- 10.Adeel M., Canzonieri V., Daniele S., Rizzolio F., Rahman M.M. Organobase assisted synthesis of Co(OH)2 nanosheets enriched with oxygen vacancies for nonenzymatic glucose sensing at physiological pH. J Ind Eng Chem. 2021;103:165–174. [Google Scholar]
- 11.Barai H.R., Rahman M.M., Adeel M., Joo S.W. MnSn(OH)6 derived Mn2SnO4@Mn2O3 composites as electrode materials for high-performance supercapacitors. Mater Res Bull. 2022;148 [Google Scholar]
- 12.Adeel M., Parisi S., Mauceri M., Asif K., Bartoletti M., Puglisi F., et al. Self-therapeutic cobalt hydroxide nanosheets (Co(OH)2 NS) for Ovarian cancer therapy. ACS Omega. 2021;6(43):28611–28619. doi: 10.1021/acsomega.1c03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yina H., Tang Z. Ultrathin two-dimensional layered metal hydroxides: an emerging platform for advanced catalysis, energy conversion and storage. Chem Soc Rev. 2016;45:4873–4891. doi: 10.1039/c6cs00343e. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez A.S., Izquierdo M.T., Mathieu S., Ghanbaja J., Celzard A., Fierro V. Structure and electrochemical properties of carbon nanostructures derived from nickel(II) and iron(II) phthalocyanines. J Adv Res. 2020;22:85–97. doi: 10.1016/j.jare.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansari M.Z., Parveen N., Nandi D.K., Ramesh R., Ansari S.A., Cheon T., et al. Enhanced activity of highly conformal and layered tin sulfide (SnSx) prepared by atomic layer deposition (ALD) on 3D metal scaffold towards high performance supercapacitor electrode. Sci Rep. 2019;9(1):10225. doi: 10.1038/s41598-019-46679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee D.P., Nandi A.K. A review on the recent advances in hybrid supercapacitors. J Mater Chem A. 2021;9:15880–15918. [Google Scholar]
- 17.Ansari S.A., Parveen N., Kotb H.M., Alshoaibi A. Hydrothermally derived three-dimensional porous hollow double-walled Mn2O3 nanocubes as superior electrode materials for supercapacitor applications. Electrochim Acta. 2020;355 [Google Scholar]
- 18.Wang Y., Huang H., Choi W.M. Polypyrrole decorated cobalt carbonate hydroxide on carbon cloth for high performance flexible supercapacitor electrodes. J Alloys Compd. 2021;886 [Google Scholar]
- 19.Li X., He H. Hydrous RuO2 nanoparticles coated on Co(OH)2 nanoflakes as advanced electrode material of supercapacitors. Appl Surf Sci. 2019;470:306–317. [Google Scholar]
- 20.Wang P., Zhou H., Meng C., Wang Z., Akhtar K., Yuan A. Cyanometallic framework-derived hierarchical Co3O4-NiO/graphene foam as high-performance binder-free electrodes for supercapacitors. Chem Eng J. 2019;369:57–63. [Google Scholar]
- 21.Suksomboon M., Srimuk P., Krittayavathananon A., Luanwuthia S., Sawangphruk M. Effect of alkaline electrolytes on the charge storage capacity and morphology of porous layered double cobalt hydroxide-coated graphene supercapacitor electrodes. RSC Adv. 2014;4:56876–56882. [Google Scholar]
- 22.Janaa M. Periyasamy Sivakumara, Manikantan Kotaa, Min Gyu Junga, Ho Seok Park, Phase- and interlayer spacing-controlled cobalt hydroxides for high performance asymmetric supercapacitor applications. J Power Sources. 2019;422:9–17. [Google Scholar]
- 23.P.S. Gaikar, S.L. Gaikwad., R.K. Mahadule, G.C. Wakde, A.P. Angre,; A. S. Bandekar, et al., β-Cobalt hydroxide as an efficient electrode for electrochemical supercapacitor application, J. Nanoeng. Nanomanuf. 6(2):157-160.
- 24.Feng G., Jiangying Q., Zongbin Z., Quan Z., Beibei L., Jieshan Q. A green strategy for the synthesis of graphene supported Mn3O4 nanocomposites from graphitized coal and their supercapacitor application. Carbon. 2014;80:640–650. [Google Scholar]
- 25.Zhang J., Zang J., Wang Y., Xin G., Zhang Y. One-pot synthesis of a Mn(MnO)/ Mn5C2/carbon nanotube nanocomposite for supercapacitors. RSC Adv. 2014;4:64162–64168. [Google Scholar]
- 26.Safarpour M., Arefi-Oskoui S., Khataee A. A review on two-dimensional metal oxide and metal hydroxide nanosheets for modification of polymeric membranes. Ind Eng Chem Res. 2020;82:31–41. [Google Scholar]
- 27.Lai C. Construction of atomically ultrathin 3D flower-like a-Co(OH)2 hierarchical microspheres with gradually “OH-incursion” outside inside strategy. J Alloys Compd. 2019;777:492–498. [Google Scholar]
- 28.Chen H., Hu L., Yan Y., Che R., Chen M., Wu L. One-step fabrication of ultrathin porous nickel hydroxide-manganese dioxide hybrid nanosheets for supercapacitor electrodes with excellent capacitive performance. Adv Energy Mater. 2013;3:1636–1646. [Google Scholar]
- 29.Liu Z., Ma R., Osada M., Takada K., Sasaki T. Selective and controlled synthesis of α- and β-cobalt hydroxides in highly developed hexagonal platelets. Am Chem Soc. 2005;127(40):13869–13874. doi: 10.1021/ja0523338. [DOI] [PubMed] [Google Scholar]
- 30.Dixit M., Subbanna G.N., Kamath P.V. Homogeneous precipitation from solution by urea hydrolysis: a novel chemical route to the α-hydroxides of nickel and cobalt. J Mater Chem. 1996;6:1429–1432. [Google Scholar]
- 31.Yang J., Liu H., Martens W.N., Frost R.L. Synthesis and Characterization of Cobalt Hydroxide, Cobalt Oxyhydroxide, and Cobalt Oxide Nanodiscs. J Phys Chem C. 2009;114:111–119. [Google Scholar]
- 32.McIntyre N.S., Cook M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal Chem. 1975;47(13):2208–2213. [Google Scholar]
- 33.Chang J.K., Wu C.M., Sun I.W. Nano-architectured Co(OH)2 electrodes constructed using an easily-manipulated electrochemical protocol for high-performance energy storage applications. J Mater Chem. 2010;20:3729–3735. [Google Scholar]
- 34.X. Chen, M. Cheng†, D. Chen‡, R. Wang, Shape-controlled synthesis of Co2P nanostructures and their application in supercapacitors, ACS Appl. Mater. Interfaces, 2016, 8, 6, 3892–3900. [DOI] [PubMed]
- 35.Hou J., Gao J., Kong L. Liquid phase reduction synthesis of a cobalt boride–activated carbon composite with improved specific capacitance and retention rate as a new positive electrode material for supercapacitors. NJC. 2019;43:14475–14484. [Google Scholar]
- 36.Zheng Z., Retana M., Hu X., Luna R., Ikuhara Y., Zhou W. Three-dimensional cobalt phosphide nanowire arrays as negative electrode material for flexible solid-state asymmetric supercapacitors. ACS Appl Mater Interfaces. 2017;9(20):16986–16994. doi: 10.1021/acsami.7b01109. [DOI] [PubMed] [Google Scholar]
- 37.Chen T., Li S., Wen J., Gui P., Guo Y., Guan C., et al. Rational construction of hollow core-branch CoSe2 nanoarrays for high-performance asymmetric supercapacitor and efficient oxygen evolution. Small. 2018;14:1700979. doi: 10.1002/smll.201700979. [DOI] [PubMed] [Google Scholar]
- 38.He G., Li J., Li W., Li B., Noor N., Xu K., et al. One-pot synthesis of nickel foam supported self-assembly of NiWO4 and CoWO4 nanostructures that act as high performance electrochemical capacitor electrodes. J Mater Chem A. 2015;3:14272–14278. [Google Scholar]
- 39.He X., Jiang J., Tian H., Niu Y., Li Z., Hu Y., et al. A facile method to synthesize CoV2O6 as a high-performance supercapacitor cathode. RSC Adv. 2019;9:9475–9479. doi: 10.1039/c8ra10041a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Liu Y., Chen J., Guo Q., Wang T., Pang H. Cobalt vanadium oxide thin nanoplates: primary electrochemical capacitor application. Sci Rep. 2014;4:5687. doi: 10.1038/srep05687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang H., Liu Y., Li J., Ma Y., Li G., Ai Y., et al. Cobalt phosphite microarchitectures assembled by ultralong nanoribbons and their application as effective electrochemical capacitor electrode materials. Nanoscale. 2013;5:503–507. doi: 10.1039/c2nr32597g. [DOI] [PubMed] [Google Scholar]
- 42.Pang H., Yan Z., Wang W., Chen J., Zhang J., Zheng H. Facile fabrication of NH4CoPO4.H2O nano/microstructures and their primarily application as electrochemical supercapacitor. Nanoscale. 2012;4:5946. doi: 10.1039/c2nr31208e. [DOI] [PubMed] [Google Scholar]
- 43.Pang H., Wang S., Shao W., Zhao S., Yan B., Li X., et al. Few-layered CoHPO4$3H2O ultrathin nanosheets for high performance of electrode materials for supercapacitors. Nanoscale. 2013;5:5752. doi: 10.1039/c3nr01460f. [DOI] [PubMed] [Google Scholar]
- 44.Dhawale D.S., Kim S., Park D., Choy J., Al-deyab S.S., Ariga K., et al. Hierarchically ordered porous CoOOH thin-film electrodes for high-performance supercapacitors. ChemElectroChem. 2015;2:497–502. [Google Scholar]
- 45.Jiang J., Liu J., Ding R., Zhu J., Li Y., Hu A., et al. Large-scale uniform α-Co(OH)2 long nanowire arrays grown on graphite as pseudocapacitor electrodes. ACS Appl Mater Interfaces. 2011;3(1):99–110. doi: 10.1021/am1009887. [DOI] [PubMed] [Google Scholar]
- 46.Sun C., Ma M., Yang J., Zhang Y., Chen P., Huang W., et al. Phase-controlled synthesis of a-NiS nanoparticles confined in carbon nanorods for high performance supercapacitors. Sci Rep. 2014;4:7054. doi: 10.1038/srep07054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W., Gao Z., Wang J., et al. Solvothermal one-step synthesis of Ni-Al layered double hydroxide/carbon nanotube/reduced graphene oxide sheet ternary nanocomposite with ultrahigh capacitance for supercapacitors. ACS Appl Mater Interfaces. 2013;5:5443–5454. doi: 10.1021/am4003843. [DOI] [PubMed] [Google Scholar]
- 48.Jagadale D., Kumbhar V.S., Dhawale D.S., Lokhande C.D. Performance evaluation of symmetric supercapacitor based on cobalt hydroxide [Co(OH)2] thin-film electrodes. Electrochim Acta. 2013;98:32–38. [Google Scholar]
- 49.Huang X., Zhang H., Li N. Symmetric transparent and flexible supercapacitor based on bio-inspired graphene-wrapped Fe2O3 nanowire networks. Nanotechnology. 2017;28 doi: 10.1088/1361-6528/aa542a. [DOI] [PubMed] [Google Scholar]