Graphical abstract
Keywords: Second near-infrared region, Fluorescent nanoparticles, Lateral flow test, Drug, Food safety
Abstract
Introduction
Widely used in livestock breeding, residues of antibiotic drugs in milk have become a threat to food safety and human health. Current rapid detection technologies using colorimetric immunochromatographic strip tests (IST) lack the necessary sensitivity for on-site trace monitoring. Fluorescence-based detection in the near-infrared IIa’ (NIR-IIa’) region (1000 ∼ 1300 nm) has enormous potential due to greatly minimized auto-fluorescence and light scattering.
Objectives
The aim of this work is to develop an ultrasensitive IST platform using NIR-IIa’ fluorescent nanoparticles as labels for multiplex antibiotic residues detection in milk.
Methods
NIR-IIa’ fluorescent nanoparticles were assembled by encapsulating synthesized NIR-IIa’ fluorophores into carboxyl - modified polystyrene nanoparticles. The NIR-IIa’ nanoparticles were subsequently used as labels in an IST platform to detect sulfonamides, quinolones, and lincomycin simultaneously in milk. A portable fluorescent reader was fabricated to provide on-site detection. To further validate the developed IST platform, the detection was compared with LC-MS/MS in 22 real milk samples.
Results
Fluorescent nanoparticles were synthesized with low energy emission (1030 nm) and large Stokes shift (>250 nm) showing a much higher signal-to-noise ratio compared with fluorophores emitting in the NIR-I region. The developed IST platform yielded a highly sensitive, simultaneous quantification of sulfonamides, quinolones, and lincomycin in milk with detection limits of 46.7, 27.6 and 51.4 pg/mL, respectively, achieving a wide detection range (up to 50 ng/mL). The IST platform showed good accuracy, reproducibility, and specificity with the portable fluorescent reader which could rapidly quantify in 10 s. These results were better than reported immunochromatographic assays using fluorescent labels, and remarkably, showed a higher recognition ability than LC-MS/MS for real samples.
Conclusion
The utility of NIR-IIa’ fluorescence-based IST platform for the fast, sensitive, and accurate detection of antibiotics in milk was demonstrated, successfully verifying the potential of this platform in detecting trace materials in complex matrices.
Introduction
Antibiotics are widely used for infectious disease prevention and therapy in animal husbandry [1], and have become one of the most common contaminations in bovine milk and other dairy products, due to the failure to follow veterinary instructions and from overuse [2]. With the large-scale consumption of dairy products worldwide, antibiotic residues have been recognized as a threat to food safety and human health, which could lead to allergic and other toxic reactions, and also contributes to the increased drug resistance of microbial strains in humans [3]. Sulfonamides (SA), quinolones (QN), and lincomycin (LIN) are common antibiotics used in dairy cattle management, with set maximum residue limits (MRLs) that are being continually reduced in milk because of their prevalence [4]. Therefore, more sensitive detection methods are urgently required for antibiotic residues monitoring. Various methods have been developed and applied in this field, including microbiological assays [5] and liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) [6]. However, there are problems associated with these technologies, including the length of time required, the expensive equipment, and the complex sample pretreatments required, which limit their application in on-site analysis. Therefore, there is a high demand for a comprehensive antibiotic residues detection method that has the requisite sensitivity, speed, and simplicity to be applied on-site.
Immunochromatographic strip tests (IST) integrate capillary action and antibody recognition to detect a target molecule in a strip form [7], and are a one-step process that minimizes the requirement for technical personnel [8], achieving high specificity and the ability to be used on-site [9]. Acknowledged as the most efficient testing approach, IST technology has been widely used in the detection of biomarkers and hormones in biomedicine, chemical contaminants, and pathogens in environmental monitoring and food safety [10]. However, the traditional colloidal gold nanoparticles (AuNPs) used as labels in IST for naked-eye colorimetric detection result in low sensitivity and suffer strong interference with colored samples [7], [11], [12]. New fluorescent labelling technologies are being developed to increase the sensitivity and quantification ability of IST [13], such as fluorophores [14], fluorescent beads [15], quantum dots [16], and upconverting phosphor nanoparticles [17]. However, these conventional fluorescent labels are excited in the ultraviolet (UV) or visible region, which can lead to photobleaching and instability during storage [9], [11]. Common fluorescent labels also suffer a narrow Stokes shift, causing an overlap between the excitation light and emission fluorescence beam [18]. In addition, the low detection sensitivity of traditional fluorescence-based IST can be attributed, to a certain degree, to the high background signal in UV and visible regions caused by light scattering and autofluorescence of biological components in the matrix, analytes, and nitrocellulose (NC) membranes [11], [19]. Therefore, finding a suitable label that addresses these issues is the key point in improving the sensitivity of IST platforms.
Recently, fluorophores emitting in the second NIR region (NIR-II; 1000–1700 nm) have attracted attention for their potential in biological research and biochemical applications, especially in vivo imaging and diagnosis [20]. The advantage of NIR-II imaging is based on the principle that the signal in this region is largely unaffected by the absorption, autofluorescence, and light scattering of biological tissue components, reducing the imaging background signals and increasing the signal-to-noise ratio [21], [22]. The Stokes shift of NIR-II fluorophores is also much larger than that of conventional fluorescent labels and fluorophores in the NIR-I region, which could significantly reduce the overlap with excitation light. In addition, the relatively robust photostability of NIR-II fluorophores offers tremendous potential for the detection of complex biological samples in different matrices [22]. However, the application of NIR-II fluorescence in IST has, to date, attracted little attention. Therefore, we speculated that better IST detection performance could be achieved using fluorophores emitting in the NIR II region.
In the present study, we synthesized NIR-II fluorescent nanoparticles (NIR-II-FNPs) by encapsulating fluorophores emitting in the NIR-IIa’ range (1000–1300 nm, escaping water absorption) into carboxyl-modified polystyrene nanoparticles and used them as labels for IST to achieve an ultrasensitive platform for simultaneously detecting SA, QN, and LIN residues in milk. The detection results could be obtained quantitatively by our easy-to-use reader which was suitable for on-site analysis within 10 s. The detection sensitivity was improved by more than 10 times compared with commercial IST, and was far below the MRLs for these antibiotic residues in milk. In addition, the ability for the simultaneous detection of three types of antibiotic residues in one strip also improved the efficiency and operational simplicity of the test. To the best of our knowledge, this is the first report of a super-sensitive, rapid, convenient, and accurate NIR-IIa’ fluorescence-based IST detection platform, a technology that we anticipate will lead to considerable improvements in on-site biological detection.
Material and methods
Chemicals and materials
Chemical standards of SA, QN, LIN, chloramphenicol, anti-SA monoclonal antibody (mAb), anti-QN mAb, anti-LIN mAb, SA antigen, QN antigen, and LIN antigen were purchased from Beijing WDWK Biotech Inc. (Beijing, China). Chemical standards of aflatoxin B1, tetracycline, erythromycin, melamine, and gentamicin were purchased from Wuxi JSJK Biotech Inc. (Wuxi, Jiangsu, China). Goat anti-rabbit IgG and rabbit anti-goat IgG were purchased from Changsha BIO Advantage Biotech Inc. (Changsha, Hunan, China). 2-(N-Morpholino)ethanesulfonic acid monohydrate (MES·H2O) was purchased from Aladdin Reagent (Shanghai, China). Bovine serum albumin (BSA), Tween-20, and N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Sulfo-N-hydroxysuccinimide (sulfo-NHS was purchased from Thermo Fisher Scientific Co. (Waltham, MA, USA). Ethanolamine and trehalose were purchased from Adamas-beta Shanghai Luchi Trading Co., ltd. (Shanghai, China). Other chemical reagents were purchased from Tianjin Yongda Chemical Reagent Co. (Tianjin, China). CN135 NC membrane was purchased from Sartorius (Goettingen, Niedersachsen, Germany), the glass-fiber membranes (sample pad), absorption pad, and PVC backing plate SM31-40 were purchased from Shanghai Liangxin Technology Co., ltd. (Shanghai, China). Whole ultra-high-temperature processed milk was purchased from a local grocery. Ultrapure water was produced using a Millipore Milli-Q system (Burlington, MA, USA).
Synthesis and characterization of NIR II-FNPs
Organic molecular fluorophores based a shielding unit-donor–acceptor-donor-shielding (S-D-A-D-S) unit structure emitting in the NIR-IIa’ region were developed by our group, and the fluorophores were then encapsulated into carboxyl-modified polystyrene nanoparticles to form NIR-II-FNPs according to our previous reports [23], [24]. Typically, styrene and acrylic acid were mixed with deionized water (50 mL) under gentle stirring for 15 min at room temperature, then potassium peroxydisulfate aqueous solution (2 mL, 4.5 % (w/v)) was added. After degassing by nitrogen bubbling for 15 min, the mixture was gradually heated to 75 °C in an oil bath and left for 24 h. After being cooled to room temperature and washed 3 times with water, the obtained nanoparticles were dispersed in a 0.25 % (w/v) sodium dodecyl sulfate (SDS) aqueous solution at 3 % (w/v). NIR-II fluorophores in CH2Cl2 solution (2 mL) were added to the nanoparticle dispersion (20 mL) and sonicated for 5 min. The resulting suspension was stirred at 40 °C for 6 h, followed by heating at 50 °C in a water bath overnight to evaporate the CH2Cl2. After ultrasonically washing 3 times with ethanol and water by centrifugation, the resulting NIR-II-FNPs were redispersed in water at 1 % (w/v) and kept at 4 °C.
The inner morphology of the NIR-II-FNPs was observed using a HITACHI HT7700 transmission electron microscope (Tokyo, Japan). The samples were prepared by diluting NIR-II-FNPs to 10 μg/mL in ultrapure water and then bathed them on carbon-stabilized copper grids for 10 min. After drying with absorbent paper, the samples were imaged at an accelerating voltage of 8.0 kV, with a magnification of 80.0 k. The surface morphology of the NIR-II-FNPs was characterized using a HITACHI SU8020 field emission scanning electron microscope (Tokyo, Japan). Powders of the NIR-II-FNPs were fixed on the stage and then processed at an accelerating voltage of 3.0 kV, with a magnification of 100.0 k. Dynamic light scatter (DLS) of the NIR-II-FNPs (10 μg/mL) was performed using a Malvern Zetasizer Nano ZS (Malvern, UK). The strips and NIR-II-FNPs solutions were imaged via a near-infrared fluorescence imaging system (Series III 900/1700), under excitation of a 785 nm laser diode with a 1 ms exposure time (Suzhou Yingrui Optical Technology Co., ltd. Suzhou, Jiangsu, China). The fluorescence excitation spectra of the NIR-II-FNPs (10 μg/mL) was measured with an excitation wavelength of 700–900 nm and emission wavelength of 1100 nm, and the fluorescence emission spectra were measured with an excitation wavelength of 740 nm and emission wavelength of 900–1500 nm (NanoLog FL3-2iHR infrared fluorescence spectrometer, HORIBA Jobin Yvon, Kyoto, Japan).
Preparation of NIR-II-FNPs detection probes
The conjugation of NIR-II-FNPs (possessing carboxyl groups) and mAb (possessing amino groups) was achieved by covalent binding through an EDC/sulfo-NHS-mediated amination reaction according to our previous reports [25]. Briefly, NIR-II-FNPs (100 μL, 10.0 mg/mL) were washed with MES three times (1 mL, 0.1 mol/L, pH 6.5). The resuspended solution (1 mL, 1.0 mg/mL) was mixed with EDC (25 μL, 10 mg/mL) and sulfo-NHS (75 μL, 10 mg/mL) with stirring for 10 min at room temperature to activate the carboxyl groups, and then washed with boric acid buffer solution three times (1 mL, optimized to pH 6.0) (Fig. S1). Thereafter, the optimized amount of 60 μg/mg (60 μg mAb per 1 mg NIR-II-FNPs) of mAb (Fig. S2) was added to the activated nanoparticles, which were resuspended in boric acid buffer solution, and incubated for 3 h at room temperature. Excessive unconjugated mAb was removed by centrifugation (16,900 × g, 15 min) at 4 °C, and then the vacant sites on the NIR-II-FNPs detection probes were blocked by incubation with BSA (1 mL, 1 %) for 3 h. Finally, the detection probes were washed three times with storage buffer (0.5 % BSA and 0.4 % Tween-20 in 0.01 mol/L PBS, pH 7.0) and resuspended in storage buffer (1 mL) in the dark at 4 °C for downstream applications.
Assembly of immunochromatographic test strips
The immunochromatographic test strips were assembled in the following way: a dried sample pad and absorbent pad were attached onto both sides of an NC membrane on a PVC backing plate. For each part of the strip, the sample pad was treated with a pretreatment solution and dried at 37 °C overnight according to our previous report [25]. Optimized SA antigen (0.9 mg/mL), QN antigen (0.9 mg/mL), LIN antigen (1.2 mg/mL) (Fig. S3), and goat anti-rabbit IgG were diluted by coating with reagents (PBS; 0.01 mol/L with 2 % trehalose and 5 % methanol), and then were dispensed onto the NC membrane using a Dispensing Platform HM3035 (Shanghai Gold Bio Co., ltd. Shanghai, China) as the test lines (T line: T1 line, T2 line, and T3 line) and control line (C line), respectively, and dried at 37 °C overnight. The T line location orders were optimized as described in the Supplementary Materials (Fig. S4). The dispensed volumes were 0.8 μL/cm line and the distance between each line was 3 mm. Finally, the composite strips were cut into 3.9 mm widths using a CNC Guillotine Cutting Module ZQ3500 (Shanghai Gold Bio Co., ltd. Shanghai, China), sealed, and stored in the dark under dry conditions (relative humidity maintained below 30 %) until use.
Fabrication of the strip reader
The fluorescence reader for the NIR-IIa’ region was developed following our previous report [23]. The core optical system included an LED for the excitation and an indium gallium arsenide photodiode for detection. The beam from the LED was reflected perpendicularly using a dichroic mirror to focus on the strip by a glass lens, which formed the exciting light. For detection, the detecting wavelength of the indium gallium arsenide photodiode was 1000–1200 nm. After being collimated by the first lens, emitted light passed through the dichroic mirror and a band-pass filter, so that the fluorescence could be focused by a second lens on the gallium arsenide photodiode detector.
Evaluation of the multiplex NIR-II-FNPs-based IST platform performance
Evaluation of the NIR-II-FNPs-based IST platform was performed using SA, QN and LIN standards, which were diluted to final concentrations of 50, 12.5, 3.125, 0.781, 0.195, 0.049, 0.012, and 0 ng/mL with optimized double-diluted milk (Fig. S5) as spiked samples. The binding rate was defined as the ratio of the T line signal of spiked sample and the blank sample (B/B0). A standard calibration curve was constructed by plotting the logit transformation of (B/B0)/(1 - B/B0) (y) against the logarithm of the added antibiotic residues concentration (x). The limit of detection (LOD) was defined as the concentration yielding a signal exceeding three times the standard deviation of the blank sample signal [26]. The limit of quantitation (LOQ) was defined as the concentration exceeding five times the standard deviation of the blank sample signal. The LOQ was also the lowest point of the linear range. Each spiked sample processed 3 replicates and the blank sample processed 20 replicates.
The detection accuracy was evaluated by spiked recovery experiments in double-diluted milk. Spiked milk samples containing high, medium, and low concentrations of antibiotic residues in the calibration curves were selected to be tested by strips. The recovery (R, %) was calculated according to the equation: R, % = (Cfound / Cadded) × 100 %, where Cfound is the concentration of the analyte calculated from the standard curve, and Cadded is the spiked concentration of the analyte. The reproducibility was evaluated as the coefficient of variation (CV): SD/mean × 100 %, n = 5 (SD: standard deviation).
The specificity of NIR-II-FNPs-based IST platform was evaluated by cross-activity rate of analytes (SA, QN, and LIN) with six other kinds of common antibiotic residues in animal body fluids, including aflatoxin B1, tetracycline, erythromycin, melamine, gentamicin, and chloramphenicol. Cross-activity rate (%) of analyte against each other antibiotic residue was calculated using [27]:
| Cross-activity rate (%) = (IC50 of analyte/IC50 of other antibiotic residue) × 100 %. |
The half-maximal inhibitory concentration (IC50) was calculated from the standard calibration curve when B/B0 was 0.5.
Statistical analysis
All data in this study are shown as mean values (mean) ± SD. The analysis of detection optimization under different conditions was performed using one-way analysis of variance (ANOVA) with Duncan’s multiple range test. The analysis of the measurement of detection specificity was performed using Student’s t-test. All analysis was performed with SPSS software (SPSS 18.0, Armonk, NY, USA), and p values < 0.05 were considered statistically significant.
Results and discussion
Design of the multiplex NIR-II-FNPs-based IST
The design of the multiplex NIR-II-FNPs-based IST detection platform for antibiotic residues analysis is depicted in Scheme 1. After the synthesis of NIR-II-FNPs detection probes (Scheme 1A), to perform a test, the detection probes (with optimized concentrations: 1.5 μg of anti-SA and anti-LIN probes, 2.0 μg of anti-QN probes; Fig. S6) were added to100 μL milk samples and incubated for 10 min at room temperature. This incubation allowed the probes to fully recognize and bind with the antibiotic residues. Then, 100 μL of the mixture was loaded on the sample window of the test strip (Scheme 1B). After 10 min of capillary flow (optimized as shown in Fig. S7), the test strip was placed in a card tray on our strip reader and moved into the interior by the automatic conveying module to conduct a scan (Scheme 1C). The core optical system of our self-designed fluorescent reader comprises an electrical module, optical system, and automatic conveying module all situated inside the device. To avoid beam interference, an LED with a wide Stokes shift (700–900 nm) was employed as the exciting light source, because of their long life and low cost. Traditional fluorescence detectors, like photo-multiplier tubes, avalanche photodiodes, and photon counting modules, are generally complex and cumbersome [28]. Therefore, we used an indium gallium arsenide photodiode detector that substantially reduced the required space and cost, while maintaining a high resolution.
Scheme 1.
Schematic illustration of the multiplex NIR-II-FNPs-based immunochromatographic strip test (IST) platform. (A) Schematic synthesis of the NIR-II-FNPs detection probes. (B) Detection process of the platform. (C) The designed portable reader with internal structure. (D) Quantitative detection principle of the platform.
After each scan, a smooth curve with peaks corresponding to fluorescent signals of the T line and the C line was obtained, and the quantitative signal values were given within 10 s from the integrals of the peak areas. Every test result (curve and values) was shown on the touch screen and could be saved and printed. The test mechanism and corresponding results of the competitive immunoassay are displayed in Scheme 1D. When NIR-II-FNPs detection probes were mixed with negative samples, the probes would combine with the corresponding different coating antigens on the T lines (T1 line with SA antigen, T2 line with QN antigen, and T3 line with LIN antigen) on the NC membrane to produce a detectable fluorescent signal. Conversely, the antibiotic residues in positive samples would combine competitively with these probes to diminish and even completely inhibit the fluorescence signal of the T lines. Therefore, the signal intensity on the T lines is inversely proportional to the antibiotic concentration in the samples. The NIR-II-FNPs conjugated with rabbit anti-goat lgG would combine with goat anti-rabbit lgG on the C line to indicate the validity of the strips. Overall, the whole detection process of our platform requires only a few simple steps, takes approximately 20 min on-site, and can provide quantitative results for three antibiotic residues simultaneously.
Synthesis and characterization of NIR-II-FNPs
The fluorophores we synthesized, which emitted in the NIR-IIa’ region, showed remarkable fluorescence quantum yields in toluene solution (31 %) and aqueous solution (2.0 %) in our previous report [24]. To ensure accurate detection, the NIR-IIa’ fluorophores were encapsulated in surface-enriched, carboxyl group-containing nanoparticles as fluorescent labels (NIR-II-FNPs) for IST (Scheme 1A). This method was designed to overcome the limitations of low solubility and self-aggregation that occur in direct application of NIR-IIa’ fluorophores in aqueous phases and lead to low quantum yields [29]. Transmission electron microscopy (TEM) (Fig. 1A) and scanning electron microscopy (SEM) (Fig. 1B) images of the NIR-II-FNPs indicated that they possess consistent shape and a smooth surface with high hydrophilicity and monodispersity. The DLS measurements (Fig. 1C) further demonstrated their remarkable dispersity and homogeneity with an average diameter of 359.6 ± 2.3 nm and a PDI value of 0.021. As shown in Fig. 1D, the NIR-II-FNPs were physically stable with good suspensibility in water under natural light and with NIR-II excitation (785 nm), with no apparent aggregation or sedimentation observed during storage. The excitation spectrum of NIR-II-FNPs is shown in Fig. 1E, and the maximum excitation wavelength was approximately 785 nm. Fig. 1F shows the emission spectrum with the maximum wavelength of 1030 nm. The large Stokes shift (∼250 nm) was a result of the excited-state geometric reorganization which allows for planarization of the π-conjugated backbone [24]. Nanoparticle-based fluorescent labels have shown outstanding advantages over free fluorophores, including enhanced photostability, improved fluorescence signals, and easy conjugation of biomolecules [30].
Fig. 1.
Optical characterization of NIR-II-FNPs. (A) TEM image. (B) SEM image. (C) DLS measurement. (D) Optical images of NIR-II-FNPs in water under natural light (left) and NIR excitation (right). (E) Fluorescence excitation spectrum. (F) Fluorescence emission spectrum.
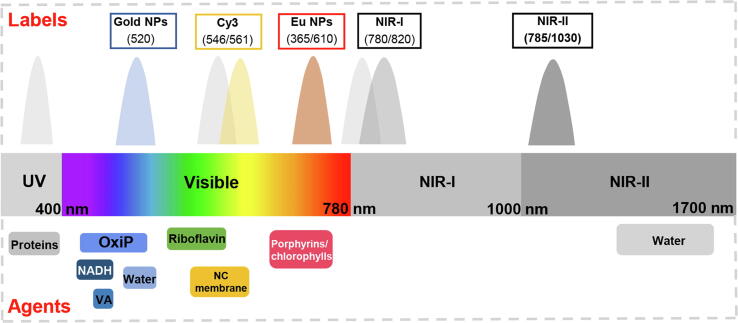
Given that the analytical sensitivity of IST is limited by the high background noise from light scattering and autofluorescence of complex matrices (such as milk), the analytes, and the NC membrane in the UV and visible regions, traditional labels, such as gold nanoparticles, Cy3, and even lanthanide chelated nanoparticles, which emit in these regions, are not suitable for IST detection (Fig. 2). In contrast, the fluorescent labels that emit at both NIR-I and NIR-II regions avoid fluorescence overlap with background autofluorescence and have the potential to increase IST detection sensitivity.
Fig. 2.
Representative spectra of fluorescent labels and autofluorescent agents in milk in the UV, visible, and near infrared (NIR) regions. Gold NPs: gold nanoparticles, Cy3: Cy3 fluorescent dye, Eu NPs: lanthanide chelated NPs, VA: vitamin A, OxiP: fluorescent oxidation products, NADH: reduced nicotinamide adenine dinucleotide, NC membrane: nitrocellulose membrane.
To compare the signal-to-noise ratio of NIR-I fluorophores (IR-Dye 800CW) and NIR-II-FNPs in the IST platform with a milk matrix, we tested these two labels by spotting the NC membrane with PBS, the two kinds of labels, pure milk (100 % milk), and milk doubly diluted by PBS (50 % milk), and then measured the fluorescence signal at excitation/emission peaks of 785/820 nm (NIR-I) and 785/1030 nm (NIR-IIa’) (Fig. 3A). The NC membrane demonstrated obviously higher background autofluorescence at 785/820 nm compared with 785/1030 nm. Furthermore, the signal-to-noise ratio of 785/1030 nm was significantly higher than that of 785/820 nm (Fig. 3B) and was not affected by the milk matrix. In contrast, the signal-to-noise ratio of 785/820 nm in 50 % milk and 100 % milk were much lower than that in PBS, indicating that the fluorescent signal of NIR-I dyes in milk could be obscured by the matrix background, which may be associated with the scattering of milk lipids at the NIR-I region [20]. Therefore, the NIR-II-FNPs demonstrated better IST detection capability and were more adaptable than NIR-I dyes; a result of the larger Stokes shift which eliminated the interference from excitation light, and the larger distance of the emission wavelength from the region of background autofluorescence and light scattering.
Fig. 3.
Comparison of NIR-I and NIR-IIa’ labels. (A) Dot-blot images of PBS, NIR-I (785/820 nm) and NIR-IIa’ (785/1030 nm) dyes, pure milk (100 % milk), milk doubly diluted by PBS (50 % milk), and dyes mixed with milk on a nitrocellulose (NC) membrane. (B) The ratio of the fluorescence intensity of NIR-I and NIR-IIa’ dyes in PBS, 50 % milk, and 100 % milk to the corresponding matrix intensity (signal to noise ratio). ** represents significant differences at p < 0.01 (mean ± SD, n = 3).
Multiplex NIR-II-FNPs-based IST platform performance evaluation
Spiked double-diluted milk samples with SA, QN, and LIN standards were detected by our NIR-II-FNPs-based IST platform. After recognition by our strip reader, the fluorescent signals were observed to decrease with increasing antibiotics concentration, which can be seen from the image of the test strips (Fig. 4A). The recorded fluorescent signals curves presented in Fig. 4B indicated that the detection platform could detect three antibiotics simultaneously with strong signals for each. The standard curves (Fig. 4C) showed a good linear relationship over the detection range for these three antibiotics. The LOD of SA was 46.7 pg/mL, and the LOQ was 0.119 ng/mL with a detection range from 0.119 to 50 ng/mL. The LOD of QN was 27.6 pg/mL, and the LOQ was 0.054 ng/mL with a detection range from 0.054 to 50 ng/mL. For LIN, the LOD was also as low as 51.4 pg/mL, and the LOQ was 0.136 ng/mL with a detection range from 0.136 to 50 ng/mL. Significantly, the sensitivity of the method for the three antibiotics was over 1000-times below the MRLs [4].
Fig. 4.
Performance evaluation of the multiplex NIR-II-FNPs-based immunochromatographic strip test (IST) platform. (A) Typical optical images obtained using the platform for the detection of sulfonamides (SA) – T1, quinolones (QN) – T2, and lincomycin (LIN) – T3. (B) Fluorescence intensity curves of the strips. (C) Calibration curves of the three antibiotics (mean ± SD, n = 3). For 1–8, the concentrations of the three antibiotics were 0, 0.012, 0.048, 0.195, 0.781, 3.125, 12.5, and 50 ng/mL, respectively.
This remarkably high sensitivity was because of the NIR-II-FNPs labels that led to less background interference. Moreover, NIR-IIa’ light has low absorption and scattering on the NC membrane, which led to more of the signal reaching the detector [21]. The signal enhancement by encapsulation of the NIR-IIa’ fluorophore into nanoparticles also decreased the analyte lower detection limit. The brightness and low background of the NIR-II-FNPs, even at high concentrations, yields a high signal-to-noise ratio, enabling a wide antibiotic residues detection range, covering all possible levels of contamination in milk [31]. Our sensitive fluorescent strip reader also enabled the identification of a wide range of emission signals from NIR-II-FNPs in the NIR-IIa’ region. Therefore, this NIR-II-FNPs-based ultrasensitive IST platform possesses excellent detection performance and is expected to expand the application of IST technologies to rapid on-site detection of samples with complex matrices and low analyte concentrations.
High accuracy was demonstrated throughout all the samples (Table 1) analyzed using our NIR-II-FNPs-based IST platform. Recoveries in 0.2, 3.0, 7.0, and 10.0 ng/mL solutions of SA ranged from 102.6 % to 112.3 %, and in 1.0, 5.0, 7.0, and 10.0 ng/mL solutions of QN ranged from 105.6 % to 113.0 %. For LIN, recoveries in 0.2, 3.0, 7.0, and 10.0 ng/mL solution ranged from 103.7 % to 116.3 %. In addition, high reproducibility, with a CV of < 10.0 % (n = 5), was achieved. Thus, the performance of the platform has met the high requirements for quantitative analysis.
Table 1.
Recovery results of the NIR-II-FNPs-based IST platform for milk samples.
| Analyte | Spiked concentration (ng/mL) | Found concentration (ng/mL) | CV (%) a | Recovery (%) |
|---|---|---|---|---|
| Sulfonamides | 0.2 | 0.2051 | 4.60 | 102.6 |
| 3.0 | 3.3440 | 6.88 | 111.5 | |
| 7.0 | 7.8584 | 5.18 | 112.3 | |
| 10.0 | 11.0287 | 3.92 | 110.3 | |
| Quinolones | 1.0 | 1.0562 | 5.82 | 105.6 |
| 5.0 | 5.5773 | 8.86 | 111.5 | |
| 7.0 | 7.9101 | 9.59 | 113.0 | |
| 10.0 | 10.6253 | 9.92 | 107.4 | |
| Lincomycin | 0.2 | 0.2142 | 6.96 | 107.1 |
| 3.0 | 3.1125 | 8.36 | 103.7 | |
| 7.0 | 8.0243 | 6.88 | 114.6 | |
| 10.0 | 11.6306 | 5.8 | 116.3 |
CV is the acronym for the coefficient of variation (n = 5).
The cross-reactivity rates of three analytes (SA, QN, and LIN) with six other kinds of common antibiotic residues were all below 2.51 % detected by the NIR-II-FNPs-based IST platform, which indicated that the NIR-II-FNPs probes possess high specificity (Table 2). The fluorescent signals of the IST also showed that when comparing the signals of blank samples with 50 ng/mL solutions of six other kinds antibiotic residues, we found no significant differences among them, indicating there was no observed cross-reactivity (Fig. S8). The strong anti-interference was a function of the antibody specificity and confirmed that the developed method could be applied in specificity analysis for multiple residual samples.
Table 2.
Cross-reactivity of sulfonamides (SA), quinolones (QN) and lincomycin (LIN) with other antibiotic residues commonly found in animal body fluids.
| Analytes | Antibiotic residues | IC50 (ng/mL)a | Cross-activity rate (%) |
|---|---|---|---|
| SA (T1)b | 2.51 | 100 | |
| QN | >100 | <2.51 | |
| LIN | >100 | <2.51 | |
| 6 other antibiotic residuesc | >100 | <2.51 | |
| QN (T2)d | 0.85 | 100 | |
| SA | >100 | <0.85 | |
| LIN | >100 | <0.85 | |
| 6 other antibiotic residues | >100 | <0.85 | |
| LIN (T3)e | 1.37 | 100 | |
| SA | >100 | <1.37 | |
| QN | >100 | <1.37 | |
| 6 other antibiotic residues | >100 | <1.37 |
IC50: Half-maximal inhibitory concentration, which was calculated from the standard calibration curve when B/B0 was 0.5; b T1: Test-line 1; c 6 other antibiotic residues: including aflatoxin B1, tetracycline, erythromycin, melamine, gentamicin, and chloramphenicol; d T2: Test-line 2; e T3: Test-line 3.
Validation of the NIR-II-FNPs-based IST platform in real samples
To further validate our NIR-II-FNPs-based IST platform, 22 milk samples collected from local markets were measured with our platform and laboratory standard LC-MS/MS, and the results were shown in Table 3. Samples 1–3, 8–10, 15 and 16 were found to be positive for antibiotic residues with both methods, and the detection values were in good agreement. Samples 4, 5, 11, 12, and 17–19, with lower concentrations of antibiotic residues, were determined as positive by our platform, but negative by LC-MS/MS. Other samples were detected to be negative by both methods. These results demonstrated that our platform was consistent with LC-MS/MS in antibiotic residual detection, and could even recognize residual antibiotics at concentrations lower than the LOD of LC-MS/MS.
Table 3.
Results of the detection of three antibiotic residues in real samples by the NIR-II-FNPs-based IST platform and LC-MS/MS.
| Sample No. | NIR-FNPs-based IST (ng/mL) | LC-MS/MS (ng/mL) |
Result |
|---|---|---|---|
| 1 | SA, 6.50a | SA, 6.75 | Positive |
| 2 | SA, 5.34 | SA, 5.65 | Positive |
| 3 | SA, 2.91 | SA, 3.08 | Positive |
| 4 | SA, 1.09 | -b | Positive |
| 5 | SA, 0.88 | – | Positive |
| 6 | – | – | Negative |
| 7 | – | – | Negative |
| 8 | QN, 5.03c | QN, 5.10 | Positive |
| 9 | QN, 3.22 | QN, 3.13 | Positive |
| 10 | QN, 1.79 | QN, 1.57 | Positive |
| 11 | QN, 0.41 | – | Positive |
| 12 | QN, 0.22 | – | Positive |
| 13 | – | – | Negative |
| 14 | – | – | Negative |
| 15 | LIN, 4.52d | LIN, 4.15 | Positive |
| 16 | LIN, 2.17 | LIN, 2.05 | Positive |
| 17 | LIN, 1.16 | – | Positive |
| 18 | LIN, 0.85 | – | Positive |
| 19 | LIN, 0.52 | – | Positive |
| 20 | – | – | Negative |
| 21 | – | – | Negative |
| 22 | – | – | Negative |
SA: sulfonamides; b “-” represents a negative result; c QN: quinolones; d LIN: lincomycin.
Table 4 summarizes the characteristics of multiplex IST platforms using our NIR-II-FNPs compared with other labels reported for antibiotic residues detection in literature. The sensitivities toward SA, QN and LIN to the NIR-II-FNPs were higher than all other labels, including latex beads, quantum dots, time-resolved fluorescent europium microspheres, and colloidal gold which is the most commonly used label in commercial IST. Notably, the sensitivities of SA and QN were both higher than those observed with IST detection based on an IR-Dye 800CW label with an emissive wavelength in the NIR Ⅰ region. This is because of the higher signal-to-noise ratio of the NIR-II-FNPs compared to IR-Dye 800CW on NC membranes, which is consistent with our comparison results described above. The inferior performance of visible and other fluorescence labels is possibly due to the matrix, which requires improvements in the optical properties of these labels [32]. Nevertheless, the wider quantitative range of the NIR-II-FNPs-based IST platform guarantees its potential reliability for antibiotic residues detection in complex samples.
Table 4.
Comparison of the NIR-II-FNPs-based immunochromatographic assay with other literature reported labels for antibiotic residues detection.
| Mode of detection | Label | Analyte | LOD a (ng/ml) |
Linear range (ng/mL) |
References |
|---|---|---|---|---|---|
| Visible light | Colloidal gold | Sulfonamide | 0.12 | 0.19–0.92 | Wang et al. [33] |
| Colloidal gold | Quinolone | 0.21 | 0.40–3.65 | Wang et al. [33] | |
| Colloidal gold | Lincomycin | 0.40 | Not mentioned | Bartosh et al. [34] | |
| Latex bead | Sulfonamide | 0.08 | 0.12–0.59 | Wang et al. [33] | |
| Latex bead | Quinolone | 0.14 | 0.24–1.75 | Wang et al. [33] | |
| Visible fluorescence | Quantum dot | Sulfonamide | 3.00 | Not mentioned | Hu et al. [35] |
| Quantum dot | Fluoroquinolone | 0.60 | Not mentioned | Hu et al. [35] | |
| Time-resolved fluorescent europium microspheres |
Lincomycin | 0.06 | Not mentioned | Ashuo et al. [36] | |
| NIR-I region | IR-Dye 800CW | Sulfadimidine | 0.10 | 0.1–3.98 | Chen et al. [11] |
| IR-Dye 800CW | Enrofloxacin | 0.08 | 0.08–2.0 | Chen et al. [11] | |
| NIR-IIa’ region | NIR-II-FNPs | Sulfonamide | 0.047 | 0.119–50 | This work |
| NIR-II-FNPs | Quinolone | 0.028 | 0.054–50 | This work | |
| NIR-II-FNPs | Lincomycin | 0.051 | 0.136–50 | This work |
LOD, Limit of Detection.
Conclusion
In conclusion, we have developed a multiplex ultrasensitive immunochromatographic detection platform using NIR-II-FNPs, together with a custom-designed fluorescence strip reader for SA, QN, and LIN detection in milk. NIR-II-FNPs were successfully synthesized with a large Stokes shift and strong emission in the NIR-IIa’ region (1030 nm), representing a new type of label capable of achieving stronger fluorescence intensity. The signal-to-noise ratio of our NIR-II-FNPs in complex matrices was significantly higher than the fluorophores emitting in the NIR-I region. For simple and rapid detection, a portable self-designed reader was developed to report and analyze the tested fluorescent signal, enabling facile use on-site. After inserting the strip into the reader and pressing a single button, the quantitative results are shown on the screen and could be saved and printed within 10 s. The multiplex approach, conducting three analyses all in one sample, decreased the dosage of samples and reduced the operation time. We obtained quantitative detection of SA, QN and LIN with limits of 46.7 pg/mL, 27.6 pg/mL and 51.4 pg/mL with a wide detection range (up to 50 ng/mL) in milk, all of which values were much lower (over 1000-times) than the MRLs established by the European Union, and the entire detection process time was within 20 min. Our platform also showed high accuracy with recoveries of 102.6 % to 116.3 %, high reproducibility (CV < 10.0 %), and high specificity with less than 2.51 % cross-reactivity rates with other common antibiotic residues. Various milk samples were collected to test using our platform, and the detection ability for antibiotic residues was demonstrated to be superior to LC-MS/MS, indicating the practical reliability and utility of the NIR-IIa’ fluorescence-based approach. Our IST platform offers an easy-to-use, fast, and accurate method for simultaneous analytes detection in a complex matrix, and we anticipate it will enable the further application of IST technologies in environmental monitoring and clinical fields.
Compliance with Ethics Requirement
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Yunyue Zhang: Investigation, Methodology, Writing – original draft. Tao Liao: Software, Methodology, Writing – original draft. Guoxin Wang: Funding acquisition, Resources, Software. Juan Xu: Investigation, Software. Mohan Wang: Writing – original draft, Resources. Fazheng Ren: Resources, Project administration. Hao Zhang: Conceptualization, Project administration, Supervision, Validation, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the earmarked fund for CARS-38, Shenzhen Science and Technology Plan Project (Grant No. JSGG20191231141403880), Guangdong Province Key Research and Development Program (Grant No. 2020B1111160003), 2115 Talent Development Program of China Agricultural University (Grant No. 00109024) and the National Natural Science Foundation of China (Grant No. 31972068).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.10.008.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chafer-Pericas C., Maquieira A., Puchades R. Fast screening methods to detect antibiotic residues in food samples, Trends. Analyt Chem. 2010;29:1038–1049. doi: 10.1016/j.trac.2010.06.004. [DOI] [Google Scholar]
- 3.Jank L., Martins M.T., Arsand J.B., Motta T.M.C., Feijó T.C., dos Santos Castilhos T., et al. Liquid chromatography-tandem mass spectrometry multiclass method for 46 antibiotics residues in milk and meat: Development and validation. Food Anal Methods. 2017;10(7):2152–2164. [Google Scholar]
- 4.European Commission, 2010. Official Journal of the European Union.20.1. 2010, L15/1.
- 5.Virolainen N.E., Pikkemaat M.G., Elferink J.W.A., Karp M.T. Rapid detection of tetracyclines and their 4-epimer derivatives from poultry meat with bioluminescent biosensor bacteria. J Agric Food Chem. 2008;56:11065–11070. doi: 10.1021/jf801797z. [DOI] [PubMed] [Google Scholar]
- 6.Koesukwiwat U., Jayanta S., Leepipatpiboon N. Validation of a liquid chromatography-mass spectrometry multi-residue method for the simultaneous determination of sulfonamides, tetracyclines, and pyrimethamine in milk. J Chromatogr A. 2007;1140:147–156. doi: 10.1016/j.chroma.2006.11.099. [DOI] [PubMed] [Google Scholar]
- 7.Kim J., Kwon J.H., Jang J., Lee H., Kim S., Hahn Y.K., et al. Rapid and background-free detection of avian influenza virus in opaque sample using NIR-to-NIR upconversion nanoparticle-based lateral flow immunoassay platform. Biosens Bioelectron. 2018;112:209–215. doi: 10.1016/j.bios.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Choi J.R., Tang R.H., Wang S.Q., Abas W.A.W., Pingguan-Murphy B., Xu F. Paper-based sample-to-answer molecular diagnostic platform for point-of-care diagnostics. Biosens Bioelectron. 2015;74:427–439. doi: 10.1016/j.bios.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 9.You MinLi, Lin M., Gong Y., Wang S., Li A., Ji L., et al. Household fluorescent lateral flow strip platform for sensitive and quantitative prognosis of heart failure using dual-color upconversion nanoparticles. ACS Nano. 2017;11(6):6261–6270. doi: 10.1021/acsnano.7b02466. [DOI] [PubMed] [Google Scholar]
- 10.Huang X.L., Aguilar Z.P., Xu H.Y., Lai W.H., Xiong Y.H. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens Bioelectron. 2016;75:166–180. doi: 10.1016/j.bios.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Chen Q., Han M., Liu J., Zhao P., He L., et al. Near-infrared fluorescence-based multiplex lateral flow immunoassay for the simultaneous detection of four antibiotic residue families in milk. Biosens Bioelectron. 2016;79:430–434. doi: 10.1016/j.bios.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 12.Nash M.A., Waitumbi J.N., Hoffman A.S., Yager P., Stayton P.S. Multiplexed enrichment and detection of malarial biomarkers using a stimuli-responsive iron oxide and gold nanoparticle reagent system. ACS Nano. 2012;6:6776–6785. doi: 10.1021/nn3015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin M., Zhao Y., Wang ShuQi, Liu M., Duan ZhenFeng, Chen YongMei, et al. Recent advances in synthesis and surface modification of lanthanide-doped upconversion nanoparticles for biomedical applications. Biotechnol Adv. 2012;30(6):1551–1561. doi: 10.1016/j.biotechadv.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Song C.M., Liu J.X., Li J.W., Liu Q. Dual FITC lateral flow immunoassay for sensitive detection of Escherichia coli 0157:H7 in food samples. Biosens Bioelectron. 2016;85:734–739. doi: 10.1016/j.bios.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 15.Plouffe B.D., Murthy S.K. Fluorescence-based lateral flow assays for rapid oral fluid roadside detection of cannabis use. Electrophoresis. 2017;38:501–506. doi: 10.1002/elps.201600075. [DOI] [PubMed] [Google Scholar]
- 16.Ren M., Xu H., Huang X., Kuang M., Xiong Y., Xu H., et al. Immunochromatographic assay for ultrasensitive detection of aflatoxin B-1 in maize by highly luminescent quantum dot beads. ACS Appl Mater Interfaces. 2014;6(16):14215–14222. doi: 10.1021/am503517s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F., Liu X.G. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem Soc Rev. 2009;38:976–989. doi: 10.1039/b809132n. [DOI] [PubMed] [Google Scholar]
- 18.Liu J.W., Yin Z. A novel NIR-emissive probe with large Stokes shift for hypochlorite detection and imaging in living cells. Talanta. 2019;196:352–356. doi: 10.1016/j.talanta.2018.12.086. [DOI] [PubMed] [Google Scholar]
- 19.Swanson C., D'Andrea A. Lateral flow assay with near-infrared dye for multiplex detection. Clin Chem. 2013;59:641–648. doi: 10.1373/clinchem.2012.200360. [DOI] [PubMed] [Google Scholar]
- 20.Li C., Chen G., Zhang Y., Wu F., Wang Q. Advanced fluorescence imaging technology in the near-Infrared-II window for biomedical applications. J Am Chem Soc. 2020;142:14789–14804. doi: 10.1021/jacs.0c07022. [DOI] [PubMed] [Google Scholar]
- 21.Jin Y., Chen Q., Luo S., He L., Fan R., Zhang S., et al. Dual near-infrared fluorescence-based lateral flow immunosensor for the detection of zearalenone and deoxynivalenol in maize. Food Chem. 2021;336:127718. doi: 10.1016/j.foodchem.2020.127718. [DOI] [PubMed] [Google Scholar]
- 22.Hong G., Zou Y., Antaris A.L., Diao S., Wu D.i., Cheng K., et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat Commun. 2014;5(1) doi: 10.1038/ncomms5206. [DOI] [PubMed] [Google Scholar]
- 23.Liao T., Yuan F., Shi C., He C.X., Li Z.G. Lanthanide chelate-encapsulated polystyrene nanoparticles for rapid and quantitative immunochromatographic assay of procalcitonin. RSC Adv. 2016;6:103463–103470. doi: 10.1039/c6ra23816e. [DOI] [Google Scholar]
- 24.Yang Q., Ma Z., Wang H., Zhou B., Zhu S., Zhong Y., et al. Rational design of molecular fluorophores for biological imaging in the NIR-II window. Adv Mater. 2017;29(12):1605497. doi: 10.1002/adma.201605497. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Ren F., Wang G., Liao T., Hao Y., Zhang H. Rapid and sensitive pathogen detection platform based on a lanthanide-labeled immunochromatographic strip test combined with immunomagnetic separation. Sens Actuators B Chem. 2021;329:129273. [Google Scholar]
- 26.Taranova N.A., Berlina A.N., Zherdev A.V., Dzantiev B.B. 'Traffic light' immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens Bioelectron. 2015;63:255–261. doi: 10.1016/j.bios.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Wang Z., Guo L., Xu X., Wu A., Kuang H., et al. Sensitive lateral flow immunoassay for the residues of imidocarb in milk and beef samples. ACS Omega. 2021;6(4):2559–2569. doi: 10.1021/acsomega.0c04422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak L., Neuzil P., Pipper J., Zhang Y., Lee S.H. An integrated fluorescence detection system for lab-on-a-chip applications. Lab Chip. 2007;7:27–29. doi: 10.1039/b611745g. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y.H., Ye F.M., Zeigler M., Wu C.F., Chiu D.T. Near-infrared fluorescent dye-doped semiconducting polymer dots. ACS Nano. 2011;5:1468–1475. doi: 10.1021/nn103304m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S., Pellach M., Kam Y., Grinberg I., Corem-Salkmon E., Rubinstein A., et al. Synthesis and characterization of near IR fluorescent albumin nanoparticles for optical detection of colon cancer. Mater Sci Eng C Mater Biol Appl. 2013;33(2):923–931. doi: 10.1016/j.msec.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Lee L.G., Nordman E.S., Johnson M.D., Oldham M.F. A low-cost, high-performance system for fluorescence lateral flow assays. Biosensors (Basel) 2013;3:360–373. doi: 10.3390/bios3040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giust D., Lucío M.I., El-Sagheer A.H., Brown T., Williams L.E., Muskens O.L., et al. Graphene oxide-upconversion nanoparticle based portable sensors for assessing nutritional deficiencies in crops. ACS Nano. 2018;12(6):6273–6279. doi: 10.1021/acsnano.8b03261. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Li X., Peng T., Wang Z., Wen K., Jiang H. Latex bead and colloidal gold applied in a multiplex immunochromatographic assay for high-throughput detection of three classes of antibiotic residues in milk. Food Control. 2017;77:1–7. doi: 10.1016/j.foodcont.2017.01.016. [DOI] [Google Scholar]
- 34.Bartosh A.V., Sotnikov D.V., Hendrickson O.D., Zherdev A.V., Dzantiev B.B. Design of multiplex lateral flow tests: A case study for simultaneous detection of three antibiotics. Biosensors (Basel) 2020;10(3):17. doi: 10.3390/bios10030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu G., Sheng W., Li S., Zhang Y., Wang J., Wang S. Quantum dot based multiplex fluorescence quenching immune chromatographic strips for the simultaneous determination of sulfonamide and fluoroquinolone residues in chicken samples. RSC Adv. 2017;7:31123–31128. doi: 10.1039/c7ra01753g. [DOI] [Google Scholar]
- 36.Ashuo A., Zou W., Fu J., Yang T., Yu L., Liu W., et al. High throughput detection of antibiotic residues in milk by time-resolved fluorescence immunochromatography based on QR code, Food Addit. Contam Part A Chem Anal Control Expo Risk Assess. 2020;37(9):1481–1490. doi: 10.1080/19440049.2020.1778192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.