Introduction
Neural epidermal growth factor-like 1 protein (NELL-1) was first identified as a membranous antigen by Sethi et al.1 utilizing laser capture microdissection and mass spectrometry followed by immunostaining. NELL-1 is currently the most common non-phospholipaseA2 receptor (PLA2R) membranous antigen. Segmental membranous changes are well-described in NELL-1 membranous nephropathy (MN).2 NELL-1 MN has been linked to malignancy, use of traditional indigenous medicines, and lipoic acid (LA) supplementation, among other conditions.2, 3, 4, 5 Herein, we report a case of segmental NELL-1 MN associated with tiopronin, a thiol agent used in the treatment of cystinuria.
Case Presentation
Clinical Presentation
A 53-year-old White male presented for evaluation of nephrotic range proteinuria. Past medical history was notable for hypertension, former tobacco use, morbid obesity, type 2 diabetes, and cystinuria, complicated by nephrolithiasis requiring lithotripsy. Medications included tiopronin (initiated approximately 7 months prior), potassium citrate, semaglutide, amlodipine, telmisartan, and chlorthalidone. Physical exam revealed blood pressure 128/92 mm Hg, body mass index of 41.5 kg/m2, and no peripheral edema. Serum creatinine was 1.1 mg/dl (estimated glomerular filtration rate of 80 ml/min per 1.73 m2). Urinalysis revealed 3+ protein, negative blood, and no leukocyturia. The patient had a 24-hour urine protein of 4.6 g, serum albumin of 4.5 g/dl, and normal serum complement levels. Serologic testing was negative for ANA, dsDNA antibody, hepatitis B surface antigen, hepatitis C antibody, MPO-ANCA, PR3-ANCA, anti-PLA2R antibody, anti-glomerular basement membranes (GBM) antibody, and HIV. No M-spike was detected on SPEP or UPEP. Of note, laboratory evaluation 7 months prior, before the initiation of tiopronin, was notable for normal renal function and absence of proteinuria. A kidney biopsy was performed.
Kidney Biopsy Findings
Sampling for light microscopy revealed 23 glomeruli, none of which were globally or segmentally sclerotic. Glomeruli ranged from normal in size to mildly enlarged. Mesangial areas were unremarkable. Glomerular capillary lumina were patent. Glomerular basement membranes (GBM) appeared normal in thickness and contour. On silver-stained sections, some capillary loops had a finely vacuolated texture where cut obliquely; however, no GBM spikes or areas of chain-like thickening were seen. Mild tubular atrophy and interstitial fibrosis involved 10% to 15% of the cortex sampled. There was no significant interstitial inflammation. Nonatrophic proximal tubules had intact brush borders. Arteries and arterioles were unremarkable.
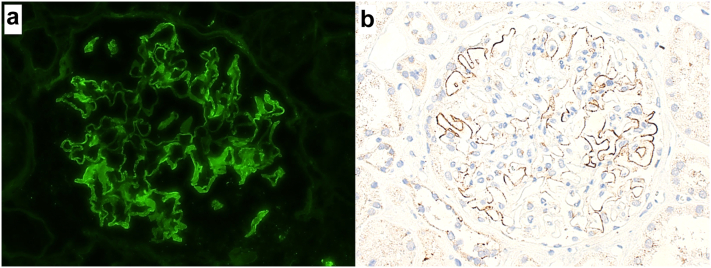
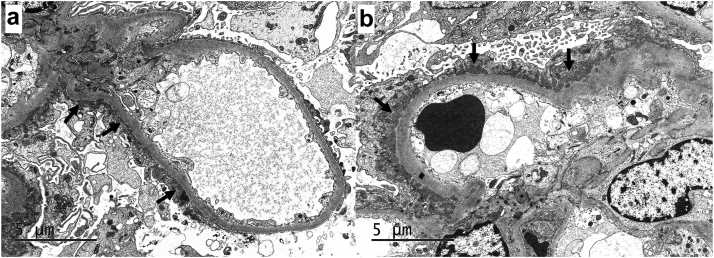
Immunofluorescence microscopy revealed 2-3+ granular to semilinear, segmental glomerular capillary wall staining for IgG (Figure 1a), with trace C3 and 2+ kappa and lambda, involving <50% of the glomerular capillary surface area in a given glomerulus. Electron microscopy revealed small, segmental subepithelial electron dense deposits involving approximately one-third of the examined glomerular capillary surface area (Figure 2a and b). Mesangial, subendothelial, and extraglomerular electron dense deposits were not identified, and endothelial tubuloreticular inclusions were not present. Podocytes displayed 30% to 40% foot process effacement, mainly seen overlying areas of subepithelial immune complex deposition. Immunofluorescence staining PLA2R was negative, whereas immunohistochemical staining for NELL-1 was positive in the distribution of the segmental subepithelial immune deposits (Figure 1b).
Figure 1.
Immunofluorescence microscopy staining for IgG revealed segmental granular to semilinear glomerular capillary wall staining involving <50% of the glomerular capillary surface area in a given glomerulus (a; immunofluorescence microscopy, 200×). Immunohistochemical staining for NELL-1 revealed granular segmental positivity in the same distribution as IgG staining observed by immunofluorescence, supporting a diagnosis of NELL-1 MN (b; immunoperoxidase stain, 200×)
Figure 2.
Ultrastructural examination revealed small subepithelial electron dense deposits (arrow). Note the incomplete distribution of the subepithelial deposits, which involved approximately one-third of the examined glomerular capillary surface area. (a; electron microscopy, 8000×). The subepithelial electron dense deposits were not associated with GBM spike formation, consistent with stage 1 membranous alterations. Note the overlying podocyte foot process effacement (b; electron microscopy, 8000×).
Results
Final Diagnosis
Segmental MN, NELL-1 positive (tiopronin-associated).
Follow-Up
Tiopronin was discontinued and the patient was started on telmisartan and dapagliflozin. He denied the use of LA containing supplements, and he had no known occupational or environmental risk factors for heavy metal exposure. Cancer screening was performed, including computed tomography scan of the chest, abdomen, and pelvis; a screening colonoscopy; and prostate-specific antigen testing; all of which were negative. Immunosuppressive therapies were deferred. Serum testing for anti-NELL-1 antibodies was not performed. Laboratory tests performed approximately 6 months later revealed serum creatinine 1.0 mg/dl, urine protein-to-creatinine ratio of 2.08 g/g, and serum albumin 4 g/dl.
Discussion
Segmental MN (sMN) is a rare, PLA2R-negative variant of MN defined by partial or incomplete subepithelial immune complex deposition, typically involving 25% to 75% of the peripheral capillary walls. The membranous changes tend to be early (stage 1–2), and the degree of glomerulosclerosis and tubulointerstitial scarring are typically mild. sMN can occur in isolation or superimposed on other forms of kidney disease. Isolated sMN usually presents with preserved kidney function and, compared to global MN of the “usual” type, with lower-grade proteinuria and less frequent nephrotic syndrome. In our experience, approximately two-thirds of isolated sMN (19/29) occurred without full nephrotic syndrome, of which most (71%) had either complete or partial remission of proteinuria with conservative management alone.6 Notably, 29% (5/17) of the isolated sMN in our series was NELL-1 positive, none of which had full nephrotic syndrome and all of which had complete (60%) or partial (40%) remission without the use of immunosuppressive therapy. A segmental-to-incomplete distribution of subepithelial deposits has been described in up to 93% of NELL-1-N (Table 1).2
Table 1.
Teaching points
|
|
|
|
NELL-1 MN has been linked to multiple potential secondary etiologies (Table 1). In the original description of NELL-1 MN, malignancy was identified in 12% of patients.1 In the largest series of NELL-1 MN reported to date, malignancy was detected in 33% of patients, compared to 4% of PLA2R MN and 11% of THSD7A MN.2 Of note, 9 of 12 patients who achieved oncologic remission also showed complete or partial remission of proteinuria. A diagnosis of NELL-1 MN merits appropriate cancer screening, which was negative in our patient.
NELL-1 MN was recently linked to use of traditional indigenous medicines (TIMs), which notoriously contain high levels of mercury.3 In this series, NELL-1 MN accounted for 88% of cases of MN in patient’s using TIMs (vs. 5% of MN without TIM use). Sixty-one percent of patients responded to discontinuation of TIMs and conservative therapy alone. Our patient had no known occupational or environmental factors concerning for mercury or other heavy metal exposure.
NELL-1 MN has been described complicating LA supplementation, first in a kidney allograft occurring after LA and dimercaptopropane sulfonate use, and later in 5 patients, including 3 who developed high-grade proteinuria while enrolled in a clinical trial for multiple sclerosis.4,7 Discontinuation of LA and supportive measures resulted in clinical remission within the first year of follow-up. Larsen et al. 8 subsequently described 15 patients with NELL-1 MN who were on LA, accounting for 13% of their NELL-1 MN cases. In this series, 11 patients achieved clinical remission (6 complete, 5 partial) after a mean follow-up of 438 ± 345 days.8 Interestingly, LA was reportedly discontinued in only 1 patient, and none received immunosuppressive therapy. Our patient denied use of LA supplements.
Tiopronin (α-mercaptopropionyl glycine), marketed as “thiola,” is an orphan drug used as second-line therapy in patients with cystinuria.9 It prevents cysteine stone formation by reducing the disulfide bond of cystine and binding the sulfhydryl group of the resultant cysteine monomers, thus forming a water soluble tiopronin-cysteine mixed disulfide. Nephrotic proteinuria is a rare but well-described complication of tiopronin treatment, with biopsy-proven minimal change disease and MN both having been previously reported.S1–S4 No target membranous antigen was reported in the previously reported cases of tiopronin-associated MN.
Tiopronin is a thiol containing derivative of glycine. It is structurally similar to D-penicillamine and bucillamine but lacks anti-inflammatory properties. The reduced form of LA (dihydrolipoic acid) also contains 2 thiol groups. A thiol group (aka “sulfhydryl group”) consists of a sulfur atom with 2 lone pairs, bonded to hydrogen (--SH). Thiol compounds have been linked to several autoimmune-related adverse events, including drug-induced MN, drug-induced pemphigus, and drug-induced lupus.S5
There are several potential mechanisms for thiol-induced MN, mainly related to the ability of thiol groups to disrupt disulfide bonds. Thiol-induced changes in tertiary and quaternary protein structure may result in epitope unmasking. A similar pathogenic mechanism has been described in thiol-induced pemphigus, where drug-induced acantholysis may induce an autoantibody response.S6 Alternatively, thiol-induced protein modifications may induce a hapten-like effect, promoting immunoglobulin affinity and/or T-cell receptor epitope recognition. A similar mechanism has been proposed for mercury-induced autoimmunity.S7 The shared affinity of thiol agents and mercury for disulfide bonds in endogenous proteins is intriguing given the association of NELL-1 MN with both thiol agents (LA and tiopronin) and mercury-containing TIMs. Notably, drug-induced MN is also known to occur in the setting of other thiol agents including D-penicillamine, captopril, and bucillamine. Miyazaki et al.S8 recently reported 4 Japanese patients with NELL-1 MN complicating rheumatoid arthritis, 3 of which were linked to bucillamine use. Data on NELL-1 expression in MN associated with D-penicillamine and captopril is lacking. Further studies are needed to investigate the potential of a broader association between thiol compounds and the development of NELL-1 MN (Table 1).
Disclosure
The authors declared no competing interests.
Patient Consent
Appropriate patient consent was obtained.
Footnotes
Supplementary References.
Supplementary Material
Supplementary References.
References
- 1.Sethi S., Debiec H., Madden B., et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–174. doi: 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Caza T.N., Hassen S.I., Dvanajscak Z., et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99:967–976. doi: 10.1016/j.kint.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurien A.A., Ks P., Walker P.D., Caza T.N.J., walker P.D., Caza T.N. Traditional indigenous medicines are an etiologic consideration for NELL1-positive membranous nephropathy. Kidney Int. 2022;102:1424–1426. doi: 10.1016/j.kint.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Spain R.I., Andeen N.K., Gibson P.C., et al. Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy. Kidney Int. 2021;100:1208–1213. doi: 10.1016/j.kint.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S. The many faces of NELL1 MN. Clin Kidney J. 2022;16:442–446. doi: 10.1093/ckj/sfac237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudose S., Santoriello D., Debiec H., et al. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. 2021;99:247–255. doi: 10.1016/j.kint.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Munch J., Kruger B.M., Weimann A., et al. Posttransplant nephrotic syndrome resulting from NELL1-positive membranous nephropathy. Am J Transplant. 2021;21:3175–3179. doi: 10.1111/ajt.16610. [DOI] [PubMed] [Google Scholar]
- 8.Caza T.N., Larsen C.P. Lipoic acid in neural epidermal growth factor-like 1-associated membranous nephropathy: more than a coincidence? Kidney Int. 2022;101:418–419. doi: 10.1016/j.kint.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Chillaron J., Font-Llitjos M., Fort J., et al. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6:424–434. doi: 10.1038/nrneph.2010.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.