Abstract
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) causes autoimmune-mediated inflammation of small blood vessels in multiple organs, including the kidneys. The ability to accurately predict kidney outcomes would enable a more personalized therapeutic approach.
Methods
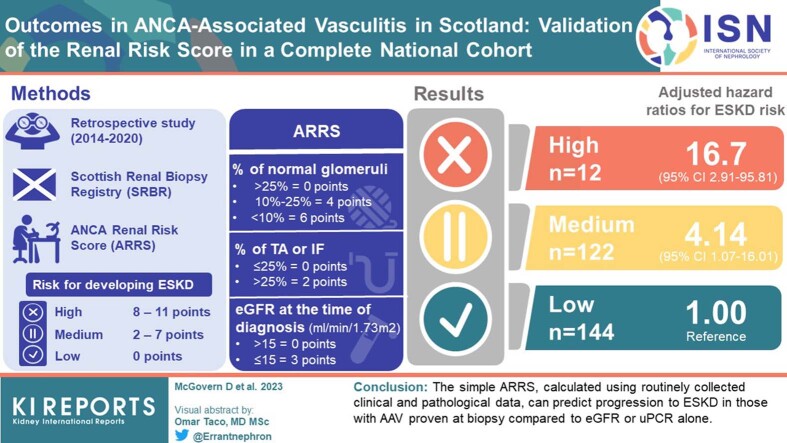
We used our national renal biopsy registry to validate the ability of ANCA Renal Risk Score (ARRS) to predict end-stage kidney disease (ESKD) for individual patients. This score uses histopathological and biochemical data to stratify patients as high, medium, or low risk for developing ESKD.
Results
A total of 288 patients were eligible for inclusion in the study (low risk n = 144, medium risk n = 122, high risk n = 12). Using adjusted Cox proportional hazard models with the low-risk group as reference, we show that outcome differs between the categories: high-risk hazard ratio (HR) 16.69 (2.91–95.81, P = 0.002); medium risk HR 4.14 (1.07–16.01, P = 0.039). Incremental multivariable-adjusted Cox proportional hazards models demonstrated that adding ARRS to a model adjusted for multiple clinical parameters enhanced predictive discrimination (basic model C-statistic 0.864 [95% CI 0.813–0.914], basic model plus ARRS C-statistic 0.877 [95% CI 0.823–0.931]; P <0.01).
Conclusion
The ARRS better discriminates risk of ESKD in AAV and offers clinicians more prognostic information than the use of standard biochemical and clinical measures alone. This is the first time the ARRS has been validated in a national cohort. The proportion of patients with high-risk scores is lower in our cohort compared to others and should be noted as a limitation of this study.
Keywords: ANCA-associated vasculitis (AAV), ANCA Renal Risk Score (ARRS), biopsy registry, end-stage kidney disease (ESKD)
Graphical abstract
Over the past 50 years, there have been substantial advances in the diagnosis and management of AAV. These developments have improved prognosis and provided valuable insights into the pathophysiological mechanisms and epidemiologic associations of this group of diseases.
Renal involvement in AAV is independently associated with poor outcomes.1,2 Contemporary immunosuppression regimens have improved outcomes, but immunosuppression carries a significant side effect burden and should thus be utilized prudently.3, 4, 5, 6, 7, 8, 9, 10, 11 Tailoring of treatment may be guided by diagnostic and prognostic data provided by a kidney biopsy.12, 13, 14 However, one of the challenges in the interpretation of renal biopsy results in AAV is the heterogeneity in histopathological features: a variety of lesions can affect multiple areas of the nephron with differing levels of cellular infiltration, fibrous scarring, and atrophic changes.15
Several classification systems have been proposed to address this issue. The most widely adopted was proposed by Berden et al.16 in 2010, in which kidney biopsies are categorized according to the presence of normal glomeruli, the proportion of cellular crescents (as a marker of active inflammation), and the proportion of globally sclerosed glomeruli (indicative of chronic damage which is less likely to respond to therapy). Biopsies can then be divided into 4 groups as follows: focal (high proportion of normal glomeruli), crescentic (high proportion of cellular crescents), sclerotic (high proportion of globally sclerosed glomeruli), and mixed (a combination of the 3 without any predominance). The authors validated the classification system with a cohort of 100 international patients by demonstrating a correlation between histologic classification and renal function (at presentation and then 1-year and 5- year follow-up). They also demonstrated that ascending histopathological severity (from focal, to crescentic, mixed, and then finally sclerotic) correlated with increasing risk of ESKD.16
Brix et al.17 proposed the ARRS, which utilizes histopathologic and biochemical data to predict outcomes, as an alternative tool to help predict renal survival. The ARRS combines parameters that Brix et al.17 found to influence renal outcomes: most importantly, the percentage of normal glomeruli. The degree of interstitial fibrosis (IF) and tubular atrophy (TA) and the baseline estimated glomerular filtration rate (eGFR) also impact outcomes, albeit not independently. On the basis of an aggregate of individual numerical scores attributed to the percentage of normal glomeruli, presentation eGFR, and degree of IF/TA, patients are then split into high, intermediate, and low-risk groups. Utilizing a total cohort of 205 patients with granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA), they demonstrated that the allocation to 1 of these 3 risk groups was predictive of renal survival; however, they detected no difference in renal outcomes between patients divided into the mixed, sclerotic, and focal classes of the Berden classification system.
Using a large national cohort of patients who have had AAV confirmed on kidney biopsy, we aimed to validate the ARRS proposed by Brix et al.17
Methods
Patient Identification
The Scottish Renal Biopsy registry is a complete national data set of all kidney biopsies performed in adults within Scotland since January 1, 2014. The registry is compiled from data collected prospectively by the 9 adult kidney units across the country and includes demographics, investigation results (including biopsy histopathology), diagnoses, extrarenal disease manifestations, treatments, and outcomes. From the Scottish Renal Biopsy Registry, 302 patients were identified with AAV diagnosed on kidney biopsy between 2014 and 2020 inclusive. In patients who had undergone repeat biopsy, only the first biopsy was included. All patients had a record of renal function at the time of biopsy. Fourteen patients with dual positivity for autoantibodies to myeloperoxidase-ANCA and proteinase 3-ANCA were excluded, as were patients with dual ANCA and anti-glomerular basement membrane autoantibody positivity. All patients had a diagnosis of either GPA or MPA made by the treating nephrologist.
ARRS
ARRS was calculated for all patients included within the registry. The score is calculated by 3 separate parameters, which are each assigned numerical points as follows: percentage of normal glomeruli (<10% = 6, 10–25% = 4, >25% = 0); percentage of TA or IF (>25% = 2, ≤25% = 0); and eGFR (≤15 ml/min per 1.73 m2 = 3, >15 ml/min per 1.73 m2 = 0). eGFR was calculated from the presentation serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.18 These points were aggregated, and patients were then divided into the following 3 renal risk categories, as defined by Brix et al.17: high (8–11 points), medium (2–7 points) and low (0 points).
Socioeconomic Deprivation
Socioeconomic deprivation is prevalent in Scotland and is recognized as an important contributing factor to morbidity and mortality.19,20 It was therefore accounted for in this study as a baseline variable, as described above. The Scottish Index of Multiple Deprivation 2016 tool21 was used to establish relative levels of deprivation within this national cohort. The Scottish Index of Multiple Deprivation is the official Scottish Government tool for measuring deprivation across the population using an amalgamation of metrics representing the following 7 domains: income, employment, education, health, access to services, crime and housing. The domain metrics are then used to create a relative ranking based on postcode, from 1 (most deprived) to 6976 (least deprived) based on postcode. The Scottish Index of Multiple Deprivation data zones are then grouped into quintiles of deprivation, from 1 (most deprived) to 5 (least deprived). The patient’s postcode at the time of biopsy was utilized.
Outcomes
Adverse outcomes were documented, including all-cause mortality with death at 90 days following biopsy and at any time point during the study period. Our prespecified primary outcome of interest was ESKD, defined as irrevocable loss of native kidney function, necessitating kidney replacement therapy or conservative kidney management for at least 90 days. Disease relapse was defined as the first recurrence of disease following remission induction that changed clinical management.
Statistical Analysis
Demographic and clinical data were collated and presented as mean and standard deviation or median and interquartile range (IQR) for normally and non-normally distributed data respectively. Normality of distribution was determined by visual inspection of histograms and quantile-quantile plots. Data were missing for urinary protein-to-creatinine ratio (uPCR, 12%) and requirement for kidney replacement therapy (0.3%); these data were multiply-imputed by chained equations using the average of 5 separately imputed datasets.
Unadjusted survival analysis was used to compare survival to the 4 outcomes of interest according to ARRS category. Between-group comparison was assessed using log-rank test. Incremental multivariable-adjusted Cox proportional hazards models were created for each of the following outcomes of interest - survival to first relapse, ESKD, and death overall:
-
•
Model 1: adjusted for age, sex, ANCA subtype, uPCR and eGFR.
-
•
Model 2: model 1 plus ARRS category.
-
•
Model 3: model 2 plus treatment modalities (plasma exchange, need for kidney replacement therapy at presentation, cyclophosphamide, and rituximab).
-
•
Model 4: model 3 plus SIMD category.
The low-risk group was considered the reference for all analyses. eGFR and uPCR were log-transformed before analysis.
Area under the receiver operating curves were used to test predictive discrimination of eGFR, uPCR, and ARRS as individual variables for the outcomes of interest. C-statistics were used to test the predictive discrimination of incremental models for the outcomes of interest. Comparisons between the nested models were performed using likelihood ratio tests. All analyses were conducted using R statistical software (version 4.1.1) using tidyverse, mice, mitools, survival, pROC, and survminer packages. Results with P < 0.05 were regarded as significant.
Results
Of the 288 patients included in the study, 108 were diagnosed with GPA and 180 with MPA. Baseline demographics by ARRS category are presented in Table 1. Mean age at biopsy was 66.9 ± 12.3 years and 149 (51.7%) patients were male with 106 (36.8%) positive for autoantibody to proteinase 3-ANCA. For ARRS, the patients were stratified into low (n = 144, 50.0%), medium (n = 132, 45.8%), and high (n = 12, 4.2%) risk groups respectively. Of the 288 patients eligible for inclusion, 39 (13.5%) patients relapsed, 39 (13.5%) progressed to ESKD and 67 (23.2%) patients died. Mean duration of follow-up was 1131 ± 582 days, from presentation to the date of data collection. Median times to relapse, ESKD, and death were 2.6 (IQR 0.8–3.6), 3.0 (IQR 1.5–4.0) and 3.2 (IQR 2.2–4.3) years, respectively.
Table 1.
Baseline demographic data presented by ARRS category
| ARRS Category | Total | Low | Medium | High | P |
|---|---|---|---|---|---|
| N | 288 | 144 | 132 | 12 | - |
| Sex – Female n (%) | 139 (48.3) | 68 (47.2) | 65 (49.2) | 6 (50.0) | 0.94 |
| Age (yrs): mean (SD) | 66.9 (12.3) | 66.1 (12.1) | 68.3 (11.9) | 61.0 (16.0) | 0.07 |
| Creatinine (μmol/l): median (IQR) | 216 [142, 342] | 153 [105, 210] | 318 [217, 465] | 566 [523, 772] | <0.001 |
| eGFR (CKD-EPI, ml/min per 1.73 m2): median (IQR) |
22 [13, 37] | 34 [22, 54] | 13 [9, 23] | 7 [4, 8] | <0.001 |
| Urinary PCR (mg/mmol): median (IQR) | 136 [69, 269] | 91 [45, 167] | 190 [106, 366] | 494 [211, 697] | <0.001 |
| PR3 (%) | 106 (36.8) | 54 (37.5) | 43 (32.6) | 9 (75.0) | <0.01 |
| MPO (%) | 182 (63.2) | 90 (62.5) | 89 (67.4) | 3 (25.0) | - |
| Corticosteroid (%) | 286 (99.3) | 143 (99.3) | 131 (99.2) | 12 (100.0) | 0.95 |
| Cyclophosphamide (%) | 215 (74.7) | 104 (72.2) | 101 (76.5) | 10 (83.3) | 0.56 |
| Rituximab (%) | 45 (15.6) | 17 (11.8) | 25 (18.9) | 3 (25.0) | 0.17 |
| Plasma exchange (%) | 56 (19.4) | 9 (6.2) | 38 (28.8) | 9 (75.0) | <0.01 |
| KRT at baseline (%) | 34 (11.8) | 0 (0) | 25 (18.9) | 9 (75) | <0.001 |
| Pulmonary hemorrhage (%) | 21 (7.3) | 9 (6.2) | 8 (6.1) | 4 (33.3) | <0.01 |
| SIMD 1st quintile n (%) | 58 | 32 (22.2) | 25 (18.9) | 1 (8.3) | 0.254 |
| SIMD 2nd quintile n (%) | 57 | 25 (17.4) | 26 (19.7) | 6 (50) | - |
| SIMD 3rd quintile n (%) | 51 | 30 (20.8) | 20 (15.2) | 1 (8.3) | - |
| SIMD 4th quintile n (%) | 60 | 29 (20.1) | 29 (22.0) | 2 (16.7) | - |
| SIMD 5th quintile n (%) | 62 | 28 (19.4) | 32 (24.2) | 2 (16.7) | - |
ARRS, ANCA Renal Risk Score; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; MPO, positive autoantibody to myeloperoxidase; PCR, protein-to-creatinine ratio; PR3, positive autoantibody to proteinase 3; SIMD, Scottish Index of Multiple Deprivation, quintile 1 = most deprived.
ARRS Comparisons
Baseline demographic and outcome data, stratified by risk group, are demonstrated in Table 1. Median eGFR was lower (CKD-EPI, ml/min per 1.73 m2, median [IQR]: high 7 [4–8]; medium 13 [9–23], and low 34 [22–54], P < 0.001) and uPCR was higher (mg/mmol, median [IQR]: high 494 [211–697], medium 318 [217–465], and low 152 [105–210], P < 0.001) in the high-risk group compared to the other risk groups (Table 1). In the high-risk group, proteinase 3-ANCA positivity and pulmonary hemorrhage were more common (P < 0.01 for both). High-risk patients were more likely to receive plasma exchange and/or hemodialysis at presentation (P <0.01 and <0.001, respectively). Relapse was progressively less common with increasing ARRS (no relapses in high risk group: medium 9.3% and low 18.4%; P = 0.02).
Risk Prediction of ESKD
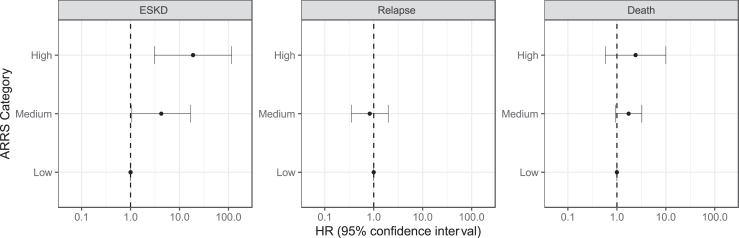
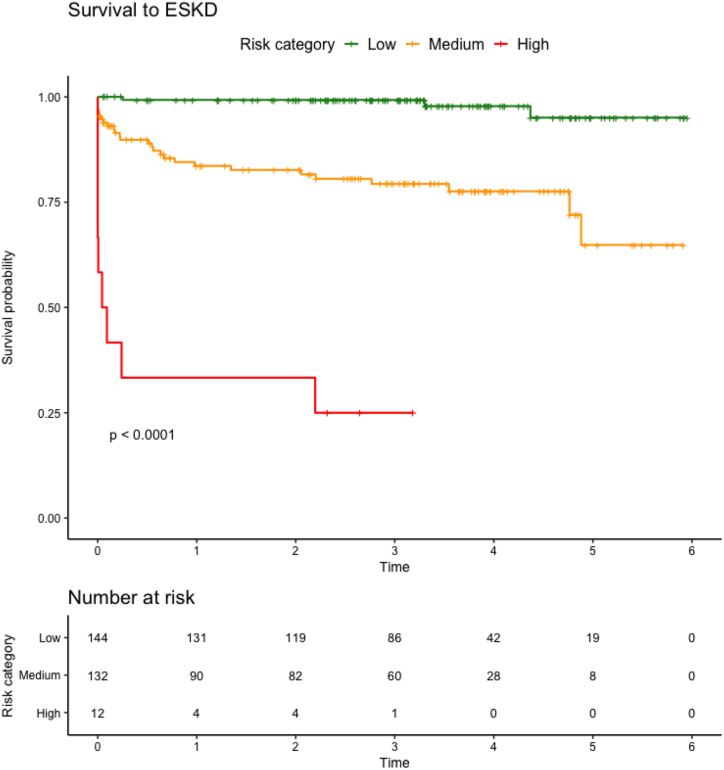
On unadjusted survival analysis, higher ARRS category was associated with higher hazards of progression to ESKD (log-rank P < 0.001; Figure 2, Figure 1).
Figure 2.
Forest plot for hazards of survival to ESKD, relapse and death
Forest plot with outputs of Cox proportional hazards ratios (HR with 95% confidence intervals) for survival to end-stage kidney disease (ESKD), relapse and death. No relapse events in group with high ANCA Renal Risk Score (ARRS) category, therefore no data displayed. ARRS, ANCA Renal Risk Score; ESKD, end-stage kidney disease.
Figure 1.
Unadjusted survival to ESKD by ARRS category. Unadjusted survival analysis to end-stage kidney disease (ESKD). Log-rank P displayed for difference between ANCA Renal Risk Score (ARRS) category. ARRS, ANCA Renal Risk Score; ESKD, end-stage kidney disease.
In the simplest multivariable-adjusted Cox proportional hazard model, higher uPCR and lower eGFR were associated with progression to ESKD (Table 2). In progressively more complex models, medium or high ARRS was associated with progression to ESKD. ARRS category was not associated with increased hazards of relapse or death (Figure 2), though among those with high ARRS, there were no recorded relapses during the follow-up period.
Table 2.
Multivariable-adjusted hazard ratios for risk of ESKD for models 1–4
| Variable | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Age | 0.97 (0.95–1.00, P = 0.031) | 0.98 (0.95–1.00, P = 0.100) | 0.98 (0.95–1.01, P = 0.227) | 0.98 (0.96–1.01, P = 0.308) |
| Sex | 0.73 (0.38–1.39, P = 0.336) | 0.66 (0.33–1.29, P = 0.220) | 0.63 (0.31–1.29, P = 0.208) | 0.52 (0.25–1.08, P = 0.080) |
| MPO positive | 0.53 (0.28–1.01, P = 0.055) | 1.42 (0.66–3.09, P = 0.371) | 1.69 (0.78–3.68, P = 0.186) | 1.70 (0.78–3.70, P = 0.184) |
| eGFR (log) | 0.30 (0.19–0.47, P < 0.001) | 0.43 (0.25–0.74, P = 0.002) | 0.53 (0.27–1.05, P = 0.068) | 0.52 (0.25–1.07, P = 0.077) |
| uPCR (log) | 1.60 (1.04–2.44, P = 0.031) | 1.43 (0.94–2.19, P = 0.098) | 1.34 (0.88–2.03, P = 0.177) | 1.31 (0.88–1.97, P = 0.186) |
| ARRS category medium | 3.80 (1.00–14.34, P = 0.049) | 4.14 (1.07–16.01, P = 0.039) | 4.24 (1.06–16.89, P = 0.040) | |
| ARRS category high | 12.97 (2.50–67.28, P = 0.002) | 16.69 (2.91–95.81, P = 0.002) | 18.91 (3.07–116.34, P = 0.002) | |
| CYC | 3.61 (0.68–19.26, P = 0.133) | 4.28 (0.69–26.45, P = 0.118) | ||
| KRT at baseline | 2.80 (1.03–7.66, P = 0.044) | 2.61 (0.97–6.97, P = 0.056) | ||
| PEX | 0.49 (0.17–1.44, P = 0.193) | 0.52 (0.19–1.45, P = 0.213) | ||
| SIMD 1st Quintile | 2.15 (0.59–7.82, P = 0.246) | |||
| SIMD 2nd Quintile | 2.68 (0.80–8.98, P = 0.110) | |||
| SIMD 3rd Quintile | 3.16 (0.82–12.27, P = 0.096) | |||
| SIMD 4th Quintile | 3.47 (1.01–11.96, P = 0.049) |
ARRS, ANCA Renal Risk Score; CYC, cyclophosphamide; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; MPO, myeloperoxidase; PEX, plasma exchange; SIMD, Scottish Index of Multiple Deprivation; uPCR, urinary protein-to- creatinine ratio.
Adjusted hazard ratios for the risk of developing ESKD for models 1–4. Model 1 – adjusted for age, sex, ANCA subtype, eGFR and uPCR. Model 2 – model 1 plus ARRS category. Model 3 – model 2 plus treatment modalities (CYC, RTX, PEX, baseline KRT). Model 4 – model 3 plus SIMD quintile. All data are displayed as hazard ratio (confidence intervals, P-value). Hazard ratios calculated for MPO-ANCA subtype with PR3-ANCA subtype group as reference. Hazard ratios calculated for ARRS Category Medium and ARRS Category High, using ARRS Category Low as reference. Hazard ratios calculated for SIMD quintiles 1–4 using quintile 5 (i.e., least deprived quintile) as reference.
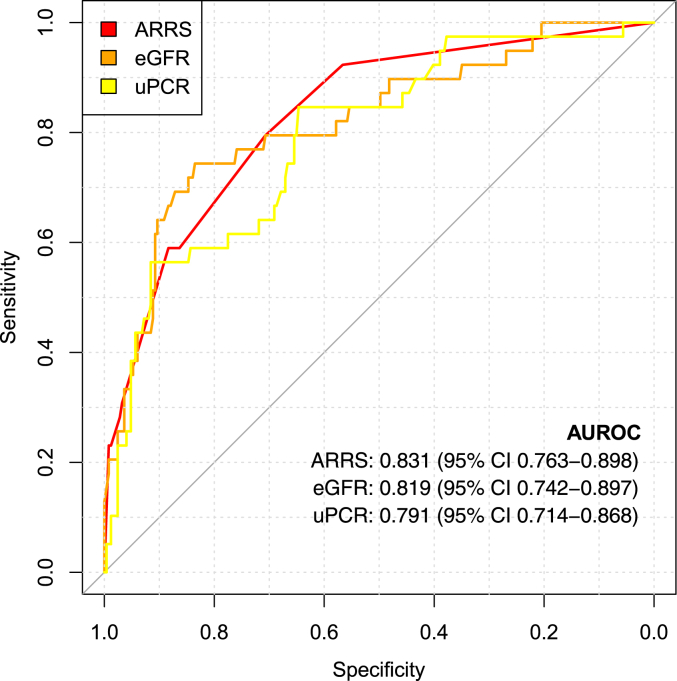
ARRS showed better discrimination for ESKD than either eGFR or uPCR alone (Figure 3). The simplest model (eGFR, uPCR, age, sex, and ANCA subtype) allowed very good or excellent risk prediction of ESKD (C-statistic 0.864, 95% CI 0.813–0.914); however, this was substantially improved by addition of ARRS (C-statistic 0.877, 95% CI 0.823–0.931), and improved again by inclusion of treatment modalities (C-statistic 0.894 (0.853–0.935) (Table 3). The addition of deprivation category did not further improve discrimination of ESKD.
Figure 3.
Receiver operating curves for discrimination of end-stage kidney disease ARRS, antineutrophil cytoplasmic antibody (ANCA) renal risk score; AUROC, area under the receiver operating curve; eGFR, estimated glomerular filtration rate; uPCR, urinary protein-to-creatinine ratio.
Table 3.
Model fit comparison with and without ARRS included
| Model | Comparator | C-statistic (95% CI) | P-value |
|---|---|---|---|
| Model 1 | n/a | 0.864 (0.813–0.914) | n/a |
| Model 2 | Model 1 | 0.877 (0.823–0.931) | <0.001 |
| Model 3 | Model 2 | 0.894 (0.853–0.935) | 0.028 |
| Model 4 | Model 3 | 0.902 (0.865–0.938) | 0.867 |
ARRS, Antibody Renal Risk Score.
C-statistics to test the predictive discrimination of antinuclear cytoplasmic Antibody Renal Risk Score (ARRS) for end-stage kidney disease (ESKD). P-value is for likelihood ratio tests.
Model 1: adjusted for age, sex, ANCA subtype, uPCR and eGFR.
Model 2: model 1 plus ARRS category.
Model 3: model 2 plus treatment modalities (plasma exchange, need for KRT at presentation, cyclophosphamide, and rituximab).
Model 4: model 3 plus the SIMD category.
Discussion
Although outcomes have improved significantly in AAV with the use of immunosuppression therapy, these treatments are associated with a significant burden of side effects. Establishing accurate and objective ways of predicting outcomes such as ESKD is paramount; unsurprisingly, patients place great importance on avoiding this outcome.22 Knowing ESKD risk can help patients and clinicians to plan for ESKD and to tailor AAV treatment. For example, some experts recommend adding plasma exchange to induction therapy when the risk of ESKD is high.22 Furthermore, if clinicians identify a patient as being at high risk for developing ESKD, a considered, early decision may be made to limit exposure to aggressive immunosuppressive therapies if there is clear indication it is likely to be futile. In addition, being able to predict ESKD could also be advantageous in the accurate and appropriate recruitment of patients into clinical trials.
Our study aims to validate the ARRS proposed by Brix et al.,17 which utilizes a novel combination of histopathological and biochemical data to accurately predict the likelihood of ESKD in kidney biopsy-proven AAV. Our data suggest that the ARRS is a highly accurate tool for the prediction of ESKD in AAV. Our statistical approach in the creation of multiple Cox proportional hazard models has allowed us to demonstrate that the addition of data regarding the treatments delivered to patients (but not the addition of data regarding socioeconomic deprivation) strengthens the ability of the ARRS to predict ESKD.
The ARRS has been proposed as an alternative to the established Berden classification system. Multiple international validation studies have been performed for the Berden classification with mixed results.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Several of these studies did not demonstrate significant differences between the mixed and crescentic classes for kidney outcomes and this lack of distinction has been confirmed in 2 meta-analyses.40,41 Furthermore, although Berden et al.42 did note that more extensive tubulointerstitial disease was associated with worse renal outcomes, they felt including extra-glomerular pathology in their classification system added complexity without prognostic improvement. They also noted that though patients with crescentic and sclerotic classifications of disease had similar kidney function at presentation, there was a more substantial renal recovery in those with crescentic disease, because of the reversibility of active inflammation compared to fibrosis, thereby delineating the importance of fibrosis in prognostication.16 The same group now plans on reassessing whether tubulointerstitial pathology should be included in their future classification systems.40 It has been highlighted by other groups that the degree of IF and/or TA is highly predictive of renal function at 12 months and 18 months,13,42 and an independent predictor of ESKD (although only on univariate analyses).35,36 The inclusion of extraglomerular disease in the ARRS is one of the key features that distinguishes it from the Berden classification.
It should also be noted that the Berden classification does not incorporate laboratory parameters, whereas Brix et al.17 incorporate a biochemical measurement of kidney function into their score - the eGFR at time of biopsy. In our first hazard model for ESKD we noted a higher eGFR was associated with lower risk, but the addition of the ARRS in model 2 really improved the predictive capabilities of the model. This suggests that the histopathological features included in the ARRS - the percentage of normal glomeruli and the percentage of IF/TA - are themselves significant predictors of ESKD and a kidney biopsy offers clinicians much more prognostic information than biochemical data alone.
Although the ARRS has been validated in other retrospective cohorts in the UK, Mexico, Turkey, and the US,43, 44, 45, 46 the major strength of this study is the size and completeness of the cohort: it is the largest study in which the ARRS has been validated. A further key strength is that the patients are from a complete national cohort, in which all patients with AAV diagnosed on kidney biopsy in Scotland are included. Therefore, there is no international variation in practice nor regional omissions in data collection.
Scotland has high levels of socioeconomic deprivation and worse life expectancy rates than neighboring countries in Western Europe; deprivation is likely to be a crucial contributing factor.19,20 It has been previously demonstrated that the incidence of AAV is unrelated to deprivation in Scotland.47 This study has demonstrated that in Scotland, the inclusion of deprivation data in ESKD hazard models does not improve their predictive power. This is an encouraging finding that suggests that regardless of the recognized socioeconomic inequalities that exist in Scotland, diagnosis and treatment in AAV are equitable.
The main limitation of our study is that our cohort differs from others because of the low proportion of high-risk patients identified by the score. In the training and validation cohorts of Brix et al.,17 18.5% of their patients were categorized as high risk. Other large validation studies in European cohorts have identified 20% to 25% of their patients as high risk.48,49 In our study, only 4.2% of patients scored as “high risk.”
One possible explanation for why this discrepancy exists is that it could be a consequence of Scotland’s population distribution – 91% of Scotland’s population live in 2.3% of the country’s land area50 and within these conurbations, patients have ready access to 1 of the country’s 9 renal units, which could conceivably result in patients being diagnosed more rapidly and at an earlier stage of disease. Furthermore, we have previously shown that rurality does not impact the incidence of MPA, and that the incidence of GPA is higher in rural populations.47 Although these data hint at environmental trigger(s) for GPA, it also shows that living rurally in Scotland is not a barrier to AAV diagnosis. Another potential explanation for our cohort having fewer patients at high risk of ESKD is the higher proportion of MPA patients in Scotland, when compared to similar cohorts. It is well described that AAV patients at higher latitudes are more likely to have GPA than MPA.51 However, in our cohort, 63% were diagnosed with MPA, whereas in the German cohort of Brix et al.,17 55% had MPA.17 Other cohort studies in the UK and Sweden demonstrated only 34% and 51% of their respective cohorts had MPA.52,53 Although kidney involvement is more common in MPA than GPA, there is a recognized distinct phenotype of MPA involving slowly progressive nephritis.54,55 An overrepresentation of this slowly progressive MPA phenotype in our cohort may have contributed to the unique risk profile. When considering other factors that may have impacted the number of patients with a high ARRS score, we considered underreporting of IFTA by pathologists; however, such a discrepancy would seem unlikely to occur at a national level. In addition, we also considered whether larger-than-average biopsy cores, containing large numbers of glomeruli, may have impacted the percentage of diseased glomeruli, resulting in lower risk scores. However, the mean glomeruli count for our biopsy cohort was 17, so this would also seem unlikely.
Our findings could be strengthened further by additionally categorizing patients according to the Berden classification system. This would allow for direct comparison between the 2 classification systems and is an opportunity for future work, alongside establishing why our Scottish cohort have a lower percentage of patients at high risk of ESKD.
In summary, the simple ARRS, calculated using routinely collected clinical and pathologic data, can predict progression to ESKD in those with AAV proven at biopsy. That the ARRS has now been validated in a large, multicenter, national cohort should allow clinicians to deploy it as a valuable prognostic tool in the management of AAV with kidney involvement.
Disclosure
JSL has received personal honoraria from AstraZeneca, Bristol Myers Squibb, and Pfizer. MK has received personal honoraria from AstraZeneca and has received support from Napp Pharmaceuticals for attending a meeting. SB is the recipient of grants from Wellcome Accelerator and Kidney for Life and has received consulting fees/personal honoraria/support for attending meetings from Vifor. EM has received personal honoraria from AstraZeneca and Pharmacosmos. KIS has consulted for Bayer AG and Boehringer Ingelheim. All the other authors have declared no competing interests.
Footnotes
STROBE Checklist
Supplementary Material
STROBE Checklist
References
- 1.Luqmani R.A., Bacon P.A., Beaman M., et al. Classical versus non-renal Wegener’s granulomatosis. Q J Med. 1994;87:161–167. [PubMed] [Google Scholar]
- 2.Mahr A., Girard T., Agher R., Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatol (Oxf Engl) 2001;40:492–498. doi: 10.1093/rheumatology/40.5.492. [DOI] [PubMed] [Google Scholar]
- 3.Fauci A.S., Haynes B.F., Katz P., Wolff S.M. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 4.Fauci A.S., Katz P., Haynes B.F., Wolff S.M. Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med. 1979;301:235–238. doi: 10.1056/NEJM197908023010503. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman G.S., Kerr G.S., Leavitt R.Y., et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 6.Flossmann O., Berden A., de Groot K., et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 7.Langford C.A. Cyclophosphamide as induction therapy for Wegener’s granulomatosis and microscopic polyangiitis. Clin Exp Immunol. 2011;164(suppl 1):31–34. doi: 10.1111/j.1365-2249.2011.04364.x. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones R.B., Furuta S., Tervaert J.W., et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis. 2015;74:1178–1182. doi: 10.1136/annrheumdis-2014-206404. [DOI] [PubMed] [Google Scholar]
- 9.Guillevin L., Pagnoux C., Karras A., et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 10.Smith R.M., Jones R.B., Specks U., et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis. 2020;79:1243–1249. doi: 10.1136/annrheumdis-2019-216863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayne D.R.W., Merkel P.A., Schall T.J., Bekker P., ADVOCATE Study Group Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384:599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- 12.Bajema I.M., Hagen E.C., Hermans J., et al. Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int. 1999;56:1751–1758. doi: 10.1046/j.1523-1755.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 13.Hauer H.A., Bajema I.M., Van Houwelingen H.C., et al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int. 2002;62:1732–1742. doi: 10.1046/j.1523-1755.2002.00605.x. [DOI] [PubMed] [Google Scholar]
- 14.Neumann I., Kain R., Regele H., Soleiman A., Kandutsch S., Meisl F.T. Histological and clinical predictors of early and late renal outcome in ANCA-associated vasculitis. Nephrol Dial Transplant. 2005;20:96–104. doi: 10.1093/ndt/gfh563. [DOI] [PubMed] [Google Scholar]
- 15.Joh K., Muso E., Shigematsu H., et al. Renal pathology of ANCA-related vasculitis: proposal for standardization of pathological diagnosis in Japan. Clin Exp Nephrol. 2008;12:277–291. doi: 10.1007/s10157-008-0052-7. [DOI] [PubMed] [Google Scholar]
- 16.Berden A.E., Ferrario F., Hagen E.C., et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 17.Brix S.R., Noriega M., Tennstedt P., et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018;94:1177–1188. doi: 10.1016/j.kint.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. Ann Intern Med. 2011;155:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannegieter S., Leon D., Morton S., McKee M. Understanding the health of Scotland’s population in an international context part 2. http://www.healthscotland.com/documents/1300.aspx Published 2003.
- 20.Hanlon P., Lawder R.S., Buchanan D., et al. Why is mortality higher in Scotland than in England and Wales? Decreasing influence of socioeconomic deprivation between 1981 and 2001 supports the existence of a ‘Scottish Effect’. J Public Health (Oxf) 2005;27:199–204. doi: 10.1093/pubmed/fdi002. [DOI] [PubMed] [Google Scholar]
- 21.Gov.scot Scottish index of multiple deprivation 2020. https://www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020/
- 22.Zeng L., Walsh M., Guyatt G.H., et al. Plasma exchange and glucocorticoid dosing for patients with ANCA-associated vasculitis: a clinical practice guideline. BMJ. 2022;376 doi: 10.1136/bmj-2021-064597. [DOI] [PubMed] [Google Scholar]
- 23.Nohr E., Girard L., James M., Benediktsson H. Validation of a histopathologic classification scheme for antineutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol. 2014;45:1423–1429. doi: 10.1016/j.humpath.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Chang D.Y., Wu L.H., Liu G., Chen M., Kallenberg C.G., Zhao M.H. Re-evaluation of the histopathologic classification of ANCA-associated glomerulonephritis: a study of 121 patients in a single center. Nephrol Dial Transplant. 2012;27:2343–2349. doi: 10.1093/ndt/gfr643. [DOI] [PubMed] [Google Scholar]
- 25.Iwakiri T., Fujimoto S., Kitagawa K., et al. Validation of a newly proposed histopathological classification in Japanese patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. BMC Nephrol. 2013;14:125. doi: 10.1186/1471-2369-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muso E., Endo T., Itabashi M., et al. Evaluation of the newly proposed simplified histological classification in Japanese cohorts of myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in comparison with other Asian and European cohorts. Clin Exp Nephrol. 2013;17:659–662. doi: 10.1007/s10157-012-0755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Togashi M., Komatsuda A., Nara M., et al. Validation of the 2010 histopathological classification of ANCA-associated glomerulonephritis in a Japanese single-center cohort. Mod Rheumatol. 2014;24:300–303. doi: 10.3109/14397595.2013.854068. [DOI] [PubMed] [Google Scholar]
- 28.Naidu G.S., Sharma A., Nada R., et al. Histopathological classification of pauci-immune glomerulonephritis and its impact on outcome. Rheumatol Int. 2014;34:1721–1727. doi: 10.1007/s00296-014-3041-z. [DOI] [PubMed] [Google Scholar]
- 29.Tanna A., Guarino L., Tam F.W., et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant. 2015;30:1185–1192. doi: 10.1093/ndt/gfu237. [DOI] [PubMed] [Google Scholar]
- 30.Ellis C.L., Manno R.L., Havill J.P., Racusen L.C., Geetha D. Validation of the new classification of pauci-immune glomerulonephritis in a United States cohort and its correlation with renal outcome. BMC Nephrol. 2013;14:210. doi: 10.1186/1471-2369-14-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Córdova-Sánchez B.M., Mejía-Vilet J.M., Morales-Buenrostro L.E., Loyola-Rodríguez G., Uribe-Uribe N.O., Correa-Rotter R. Clinical presentation and outcome prediction of clinical, serological, and histopathological classification schemes in ANCA-associated vasculitis with renal involvement. Clin Rheumatol. 2016;35:1805–1816. doi: 10.1007/s10067-016-3195-z. [DOI] [PubMed] [Google Scholar]
- 32.Ford S.L., Polkinghorne K.R., Longano A., et al. Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis. 2014;63:227–235. doi: 10.1053/j.ajkd.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Hilhorst M., Wilde B., van Breda Vriesman P., van Paassen P., Cohen Tervaert J.W. Limburg Renal Registry. Limburg renal registry. Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol. 2013;24:1371–1375. doi: 10.1681/ASN.2012090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintana L.F., Peréz N.S., De Sousa E., et al. ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol Dial Transplant. 2014;29:1764–1769. doi: 10.1093/ndt/gfu084. [DOI] [PubMed] [Google Scholar]
- 35.Moroni G., Binda V., Leoni A., et al. Predictors of renal survival in ANCA-associated vasculitis. Validation of a histopatological classification schema and review of the literature. Clin Exp Rheumatol. 2015;33(2 suppl 89):S-56–63. [PubMed] [Google Scholar]
- 36.Diaz-Crespo F., Villacorta J., Acevedo M., et al. The predictive value of kidney biopsy in renal vasculitis: a multicenter cohort study. Hum Pathol. 2016;52:119–127. doi: 10.1016/j.humpath.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen T., Gregersen J.W., Krag S.R., Ivarsen P. The relation between histopathological classification and renal outcome, ANCA subtype and treatment regimens in ANCA-associated vasculitis. Clin Exp Rheumatol. 2016;34(3 suppl 97):S105–S110. [PubMed] [Google Scholar]
- 38.Andreiana I., Stancu S., Avram A., Taran L., Mircescu G. ANCA positive crescentic glomerulonephritis outcome in a Central east European cohort: a retrospective study. BMC Nephrol. 2015;16:90. doi: 10.1186/s12882-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjørneklett R., Sriskandarajah S., Bostad L. Prognostic value of histologic classification of ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol. 2016;11:2159–2167. doi: 10.2215/CJN.04800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Daalen E.E., Wester Trejo M.A.C., Göçeroğlu A., et al. Developments in the histopathological classification of ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol. 2020;15:1103–1111. doi: 10.2215/CJN.14561119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y.X., Xu J., Pan X.X., et al. Histopathological classification and renal outcome in patients with antineutrophil cytoplasmic antibodies-associated renal vasculitis: a study of 186 patients and metaanalysis. J Rheumatol. 2017;44:304–313. doi: 10.3899/jrheum.160866. [DOI] [PubMed] [Google Scholar]
- 42.Berden A.E., Jones R.B., Erasmus D.D., et al. Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody-associated glomerulonephritis after rituximab therapy. J Am Soc Nephrol. 2012;23:313–321. doi: 10.1681/ASN.2011040330. [DOI] [PubMed] [Google Scholar]
- 43.Li A.S., Saleh C., Denley H., Patel M., Brix S.R. ANCA renal risk score predicts outcome in the Manchester cohort. Kidney Int. 2019;96:246–247. doi: 10.1016/j.kint.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Mejía-Vilet J.M., Martín-Nares E., Cano-Verduzco M.L., Pérez-Arias A.A., Sedano-Montoya M.A., Hinojosa-Azaola A. Validation of a renal risk score in a cohort of ANCA-associated vasculitis patients with severe kidney damage. Clin Rheumatol. 2020;39:1935–1943. doi: 10.1007/s10067-020-04936-5. [DOI] [PubMed] [Google Scholar]
- 45.Gercik O., Bilgin E., Solmaz D., et al. Histopathological subgrouping versus renal risk score for the prediction of end-stage renal disease in ANCA-associated vasculitis. Ann Rheum Dis. 2020;79:675–676. doi: 10.1136/annrheumdis-2019-216742. [DOI] [PubMed] [Google Scholar]
- 46.Kant S., Costigliolo F., Brix S.R., Fenaroli P., Rosenberg A., Geetha D. Application of the ANCA renal risk score in the United States: a single-center experience. Kidney Med. 2021;3:686–688. doi: 10.1016/j.xkme.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aiyegbusi O., Frleta-Gilchrist M., Traynor J.P., et al. ANCA-associated renal vasculitis is associated with rurality but not seasonality or deprivation in a complete national cohort study. RMD Open. 2021;7 doi: 10.1136/rmdopen-2020-001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan P.G., O’Brien J., Pusey C.D., McAdoo S.P. Validation of the ANCA renal risk score in a London cohort: potential impact of treatment on prediction outcome. Kidney Int. 2021;99:488–489. doi: 10.1016/j.kint.2020.04.061. [DOI] [PubMed] [Google Scholar]
- 49.Villacorta J., Diaz-Crespo F., Guerrero C., Acevedo M., Cavero T., Fernandez-Juarez G. Long-term validation of the renal risk score for vasculitis in a southern European population. Clin Kidney J. 2020;14:220–225. doi: 10.1093/ckj/sfaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Records of Scotland Mid-2020 population estimates for settlements and localities in Scotland. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/population/population-estimates/settlements-and-localities/mid-2020 Published 2022.
- 51.Kitching A.R., Anders H.J., Basu N., et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6:71. doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 52.Watts R.A., Mooney J., Skinner J., Scott D.G., Macgregor A.J. The contrasting epidemiology of granulomatosis with polyangiitis (Wegener’s) and microscopic polyangiitis. Rheumatol (Oxf Engl) 2012;51:926–931. doi: 10.1093/rheumatology/ker454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohammad A.J., Jacobsson L.T., Westman K.W., Sturfelt G., Segelmark M. Incidence and survival rates in Wegener’s granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatol (Oxf Engl) 2009;48:1560–1565. doi: 10.1093/rheumatology/kep304. [DOI] [PubMed] [Google Scholar]
- 54.Moiseev S., Novikov P., Jayne D., Mukhin N. End-stage renal disease in ANCA-associated vasculitis. Nephrol Dial Transplant. 2017;32:248–253. doi: 10.1093/ndt/gfw046. [DOI] [PubMed] [Google Scholar]
- 55.Trivioli G., Gopaluni S., Urban M.L., et al. Slowly progressive anti-neutrophil cytoplasmic antibody-associated renal vasculitis: clinico-pathological characterization and outcome. Clin Kidney J. 2020;14:332–340. doi: 10.1093/ckj/sfaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.