Abstract
Lupus nephritis (LN) is one of the main determinants of the severity of systemic lupus erythematosus (SLE). LN flares can lead to organ damage with chronic kidney disease (CKD) or even end-stage kidney disease (ESKD) and impair patients’ survival. The “treat-to-target” strategy, which aims at obtaining and maintaining remission or low disease activity of SLE to alleviate symptoms and prevent organ damage, also refers to the control of residual activity in the kidney. But damage in SLE can also come from treatments, and toxicities related to long-term use of treatments should be prevented. This may contribute to the frequent nonadherence in patients with SLE. The de-escalation or even weaning of treatments whenever possible, or “think-to-untreat” (T2U) strategy, is to be considered in patients with LN. This possibility of treatment weaning in LN was explored in retrospective cohorts, on the basis of long-term clinical remission. It was also proposed prospectively with a kidney-biopsy-based approach, combining clinical and pathologic remission to secure treatment weaning. The WIN-Lupus trial was the first randomized controlled trial comparing the continuation to the discontinuation of maintenance immunosuppressive therapy (IST) after 2 to 3 years in patients with LN in remission. It showed a higher risk of severe SLE flares in patients who discontinued treatment, but also a possibility of weaning without flare in some patients, who need to be better identified. We propose here a narrative review of the available literature on the weaning of treatment in LN and discuss how to secure a T2U strategy.
Keywords: immunosuppressive therapy, lupus nephritis, relapse, systemic lupus erythematosus, treatment de-escalation, treat-to-target
LN is one of the main determinants of the severity of SLE, and has a major impact on morbidity and mortality.1,2 LN flares can induce cumulative kidney damage leading to CKD or even ESKD in 15% to 20% of patients after 10 years3). Nonadherence to treatment is a major risk factor for SLE flares4 and ESKD.5 However, the burden of infectious6 neoplastic,7 and metabolic8 complications of LN treatment, as well as cardiovascular disease associated with LN9 and CKD,10 also heavily impairs patients’ prognosis.11, 12, 13 Furthermore, the drugs burden for patients should be taken in consideration.14,15 In addition to the continued efforts to achieve and maintain remission and/or low disease activity in SLE16, 17, 18, 19 with the development of modern therapeutics, the prevention of toxicities or damages related to long-term use of treatments and the use of optimized nephroprotection strategies is a priority for long-term care of patients with LN. The use of repeat kidney biopsy to assess pathologic remission and reliably differentiate persistent LN activity from the evolution of chronic lesions20 is now being considered in the routine evaluation of patients with LN, pending the development of robust noninvasive biomarkers.
We propose here a narrative review of the available literature on the weaning of treatment in LN, from retrospective cohorts to the randomized controlled trial WIN-Lupus.21 We also discuss the position of repeat biopsy, and propose an algorithm to secure this de-escalation in patients with LN. We propose to name it the "think-to-untreat" (T2U) strategy,22 in reference to its opposite pole, the “treat-to-target” strategy; T2U following treat-to-target in patients with sustained remission of LN.
What are the Current Recommendations in the Maintenance Treatment of LN?
The guidelines for the management of LN have been updated in 2019 by the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA)23 and in 2021 by the Kidney Disease–Improving Global Outcomes (KDIGO) guidelines.24 After the initial (or induction phase) treatment of proliferative LN, the IST must be continued (maintenance treatment) for at least 3 to 5 years, to achieve or consolidate remission and prevent relapses. This continued IST relies on mycophenolate mofetil (MMF) preferably (KDIGO), on MMF or azathioprine (AZA) (EULAR/ERA-EDTA), with AZA in patients with a pregnancy wish. Calcineurin inhibitors (KDIGO) or belimulab (EULAR/ERA-EDTA) can be considered. A low dose of corticosteroids is targeted (<5–7.5 mg/d as per KDIGO, to control extrarenal SLE activity; 2.5–5 mg/d as per EULAR/ERA-EDTA) and can be weaned (in case of complete remission for at least 1 year as per KDIGO; after 3 to 5 years of remission as per EULAR/ERA-EDTA). Hydroxychloroquine (HCQ) is continued in all patients, with a regular ophthalmologic monitoring25 and 50% dose reduction in patients an estimated glomerular filtration rate (eGFR) <30 ml/min.23
The duration of IST after a flare of proliferative LN should not be less than 36 months as per KDIGO,24 and progressive weaning can be discussed in the case of complete remission and clinical quiescence of extrarenal SLE. From the EULAR/ERA-EDTA,23 a progressive weaning of IST can be discussed after the weaning of corticosteroids in patients in complete remission for at least 3 to 5 years. The duration of IST should be individualized according to the speed and quality of remission, duration of flare-free remission, extrarenal lupus activity, and patient wishes. Of note, all these recommendations on duration of maintenance IST were on the basis of a low level of evidence. They are summarized in Table 1. A wish of pregnancy must also be considered to either adapt (azathioprine, calcineurin inhibitors and corticosteroids can be prescribed during pregnancy) or wean IST while continuing HCQ in patients with long-term remission, considering the risk of LN flare favored by hormonal environment.26
Table 1.
Current recommendations for maintenance immunosuppressive therapy in patients with class III or IV LN with activity, and remission definitions
| Recommendations | EULAR/ERA-EDTA 2019 [Faniourakis, ARD 2020] | KDIGO 2021 [Rovin, Kidney Int 2021] |
|---|---|---|
| Maintenance IST | MMF 1–2 g/d (especially after MMF induction) or AZA 2 mg/kg/d (especially if pregnancy wish) | MMF 1–2 g/d (preferably) or AZA or CNI (if MMF or AZA not tolerated) |
| Maintenance CS | 2.5–5 mg/d for extrarenal SLE | < 5–7.5 mg/d for extrarenal Weaning possible if CCR ≥ 12 mo |
| Weaning of treatment | After > 3–5 yr CCR Gradual tapering CS first, then IST (continue HCQ) |
Total duration of IST ≥ 36 mo Only if extrarenal SLE controlled +/− Repeat biopsy (pathologic remission?) |
AZA, azathioprine; CCR, complete clinical response; CNI, calcineurin inhibitor; CS, corticosteroids; EULAR/ERA-EDTA, Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association; HCQ, hydroxychloroquine IST, immunosuppressive therapy; KDIGO, Kidney Disease–Improving lobal Outcomes; LN, lupus nephritis; MMF, mycophenolate mofetil; PCR, partial clinical remission; UPCR, urinary protein/creatinine ratio.
In patients who have reached ESKD, immunosuppression is guided by the extrarenal manifestations of SLE.23 These manifestations are often reduced in patients on chronic dialysis, and IST can be tapered and weaned in the majority.27,28 Conversely, patients with residual CKD after a first flare or relapse of LN are at higher risk of progressing to EKSD in case of a new LN flare, and particular caution must be taken regarding IST tapering in these patients.
Is it Possible to be Weaned off Treatment After LN Based on Clinical Remission?
The earlier trials from the National Institute of Health in LN showed a higher risk of LN relapse in patients treated with short-course pulses of cyclophosphamide (6 monthly pulses) than in patients treated with long-course pulses of cyclophosphamide (2 additional years of quarterly pulses).29,30 A relapse rate of 45% was observed in 145 patients treated for 1 to 2 years (with pulse cyclophosphamide, pulse methylprednisolone, or both).31 These data justified the need for maintenance IST for the prevention of LN relapses. However, the question of the optimal duration of maintenance IST and the possibility of its weaning remained unresolved.32 Moroni et al. 33 first reported the outcome of patients with IST discontinuation in Milan in 2006, and reported a trial of IST progressive discontinuation in 201334 in 73 patients from a cohort of 161 patients (45%) with LN. To be eligible for IST discontinuation, patients had to be in stable clinical remission (normal serum creatinine, proteinuria <0.5 g/d, absence of hematuria, extrarenal SLE clinically quiescent ≥12 months). A flare was observed in 21 of 73 (29%) patients during IST reduction, who were successfully retreated. The other 52 (71%) patients completely stopped IST and subsequently stopped corticosteroids, among whom 32 did not have to resume IST during the follow-up, and 20 had at least 1 flare and had to resume IST, at least temporarily. Patients were less likely to relapse if they had been treated longer before IST discontinuation and if they continued HCQ. The authors concluded that progressive discontinuation of IST can be proposed in patients who have been treated for ≥5 years after LN and are in complete clinical and kidney remission for ≥3 years.35
Recently, Zen et al.36 reported the outcome of 83 patients who discontinued IST after achievement of stable remission, from a cohort of 238 (34.8%) patients with biopsy-proven LN who were treated with IST during the 1980 to 2020 period in Padova, Italy. A flare of SLE was observed in 19 of 83 (23%) patients, including 8 patients with a relapse of LN. These 19 patients were retreated, with a remission in most patients. The other 64 of the 83 (77%) patients did not experience a flare. Patients were less likely to flare after IST discontinuation if they were older, had been treated more than 3 years, and continued HCQ.
What did the WIN-Lupus Trial Show?
WIN-Lupus21 was the first multicenter randomized controlled trial of IST withdrawal in LN. Patients included had presented a biopsy-proven proliferative LN (class III or IV +/− V, with active lesions), were on maintenance IST with AZA or MMF for 2 to 3 years, were on HCQ for ≥1 year, and were stable in remission for ≥1 year. Patients with a desire to become pregnant within 2 years, patients with an eGFR <30 ml/min per 1.73 m2, who had an extrarenal flare of lupus within 6 months that required corticosteroids >20 mg/d for 7 days or on corticosteroids >10 mg/d, were not included. Patients were randomized (1:1) into 2 groups as follows: (1) continuation of maintenance IST (AZA or MMF) for an additional 2 years or (2) discontinuation of maintenance IST over 3 months.
The primary end point was the relapse rate of proliferative LN at 24 months. The main secondary endpoints were the rate of severe SLE flare (renal or extrarenal), survival without LN relapse or severe SLE flare, adverse events, eGFR, corticosteroid consumption, and SLE disease activity, and health-related quality of life. WIN-Lupus was designed as a noninferiority trial.
Between 2011 and 2016, 96 patients were randomized as follows: 48 in the continuation group, and 48 in the discontinuation group. The per-protocol population included 84 patients: 40 in the continuation group and 44 in the discontinuation group. Biopsy-proven renal relapses occurred in 5 of 40 (12.5%) patients in the continuation group and 12 of 44 (27.3%) patients in the discontinuation group (difference 14.8% [95% CI −1.9 to 31.5]). Therefore, noninferiority of IST discontinuation was not demonstrated. However, the superiority of IST continuation was not statistically significant, probably because the study was underpowered. Relapse-free survival did not differ between groups. However, severe SLE flares were less frequent in the treatment continuation group (5/40 vs. 14/44 patients; P = 0.035). Adverse events, eGFR, corticosteroid consumption, SLE disease activity, and quality of life, did not differ between groups.
The main risk factors for renal relapse at inclusion were higher proteinuria-to-creatinuria ratio (P = 0.001), low C3 (P = 0.003), higher SLE disease activity index (P = 0.025), antiphospholipid syndrome (P = 0.041), higher eGFR (P = 0.046). Lower levels of albumin, hemoglobin, leukocytes, lymphocytes, and eosinophils were also associated with the risk of renal relapse.
This study had limitations, including a lack of power (96 patients included out of the 200 planned), an open-label design, no requirement to wean off corticosteroids, study duration possibly too short to identify late relapses or delayed side effects, and possible selection bias (noninclusion of the more severe or more relapsing patients). The strengths of the study include the homogeneity of included patients in terms of renal involvement (biopsy-proven proliferative LN), duration of maintenance IST at inclusion (2–3 years), duration of remission (≥1 year), systematic HCQ prescription, robust primary outcome (biopsy-proven LN relapse), and stable standard of care for LN during the study period.
Overall, the noninferiority of stopping maintenance IST after 2 to 3 years was not demonstrated, and discontinuation of IST was associated with an increased risk of severe SLE flare. However, the majority of patients who discontinued IST did not relapse, which confirms that IST discontinuation is possible in some patients, who must be better identified, possibly with the help of repeat biopsy and/or better stratification of the relapse risk through identification of clinical and/or biological risk factors. Conversely, there is a high need for identifying patients who are at risk of relapse and for whom long-term continuation of IST is needed. The long-term follow-up of the WIN-Lupus cohort is ongoing to describe subsequent therapeutic management, identify late relapses and late toxicities, and provide information on longer-term kidney function.
In the WIN-Lupus trial, IST was tapered down until zero over 3 months, which can be viewed as an abrupt weaning. HCQ was continued in all patients, and corticosteroids could be prescribed (up to 10 mg/d) for the control of extrarenal disease activity or for the prevention of relapses, at the investigator’s discretion. No repeat kidney biopsy was required before IST discontinuation, and some patients may have had persistent pathologic activity, which flared after IST discontinuation.
Can Repeat Kidney Biopsy Guide Treatment Weaning?
There is a discrepancy between clinical remission and pathologic remission in LN.37 Not only can patients with persisting proteinuria have only chronic lesions, but also patients without significant proteinuria can have persisting active lesions. Persistent pathologic activity has been shown to be a risk factor of LN relapse after IST discontinuation,38 and Malvar et al.39 proposed a kidney-biopsy-based strategy for IST discontinuation. Among 220 patients with biopsy-proven class III or IV LN, 75 (35%) patients with a class III or IV LN who had been treated for ≥42 months and had been in clinical remission for ≥12 months underwent a repeat kidney biopsy and were weaned off IST because the repeat biopsy showed no residual pathologic activity. Patients with residual infraclinical pathologic activity were treated with an additional 24-months IST and then rechallenged with a new biopsy with the same procedure for IST continuation or discontinuation. A relapse of LN occurred in only 7 of 75 (9%) patients after a median follow-up of 96 months, which was lower than the 23% to 29% relapse rates previously reported when only clinical remission was considered.
Repeat kidney biopsy is a robust way to assess pathologic remission of LN before allowing IST weaning. It also allows the distinction between active and chronic lesions in patients who do not respond to clinical remission criteria because they display significant proteinuria. However, the absence of current activity of LN does not exclude a relapse after IST weaning. And although pathologic activity is a risk factor for relapse, some patients with ongoing activity do not relapse clinically after IST weaning.38 Overall, kidney biopsy could be proposed to guide IST weaning in priority for patients who wish to discontinue IST relatively early (after 3 years of IST for instance).
Repeat biopsy has also been proposed in the routine management of patients with LN, to assess pathologic remission and possibly adjust therapy if persistent pathologic activity is confirmed as a risk factor for LN relapse and/or kidney damage. In a study of 42 patients with active proliferative LN from Leuven,40 a per-protocol biopsy was systematically performed after 24 months of IST. Eleven (26%) patients relapsed despite IST continuation, after a median of 17.9 months after repeat biopsy. High pathologic activity index was predictive of subsequent clinical relapse (defined by urinary protein-to-creatinine ratio >1 g/g), independently of urinary protein-to-creatinine ratio levels at the time of biopsy, whereas chronicity index score and tubulointerstitial lesions on repeat biopsy were predictive of long-term kidney function.
An ongoing prospective multicenter trial (REBIOLUP, NCT04449991)20 in incident patients with LN aims at the following: (i) determining the proportion of patients in pathologic remission after 12 months of standard treatment; (ii) correlating pathologic response to clinical response; (iii) evaluating whether therapeutic adaptation guided by repeat biopsy improves kidney outcomes compared to the standard strategy without repeat biopsy; (iv) characterize response to therapy in pure membranous (class V) LN and determine how repeat biopsy modifies long-term prognosis.
Because kidney biopsy is an invasive procedure, with bleeding complications,41 the development of noninvasive biomarkers to monitor disease activity (including in the kidney) and accompany therapeutic de-escalation is particularly important.42, 43, 44 The ongoing LUMIER2 study (LUpus Molecular Immuno-monitoring to Evaluate the Risk of Relapse, NCT02811094) will assess prospectively the value of blood transcriptomic signatures to predict SLE flares in clinically quiescent patients.
How Should We Monitor Treatment De-escalation in LN?
The possibility of a future treatment de-escalation should be discussed with patients at diagnosis of LN. This will make it possible to address the fact that the treatment will be long, and may initially include many medications, but that it will not necessarily be lifelong, and that under certain conditions, it may be possible later to consider gradually reducing or even stopping it. This approach may also reduce the risk of the patient becoming discouraged and stopping the treatment on their own at a time that would not be medically appropriate.35 This can be called the T2U strategy.
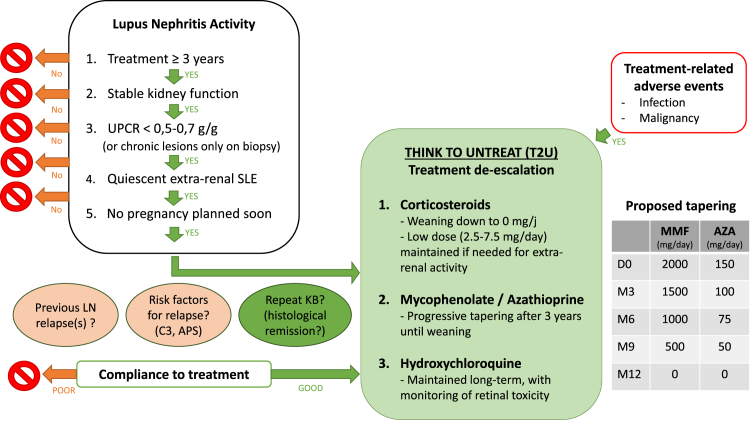
The algorithm described in Figure 1, could accompany and structure treatment de-escalation.22 The first question to address is: "In which patients is IST weaning possible?” Eligible patients must have been treated with IST for at least 3 years, they must be in renal remission of LN (stable renal function and urinary protein-to-creatinine ratio <0.5–0.7 g/g), with a sustained quiescence of extrarenal SLE manifestations (for at least 12 months in our opinion, but the optimal duration remains to be defined), and with no short-term pregnancy plan. In case of doubt, or systematically according to local practices, a kidney biopsy should be performed to ascertain renal remission. In addition, other risk factors for LN relapse should be included in the assessment of the feasibility of the weaning plan. These risk factors could include history of LN relapse, persistent abnormalities related to subclinical lupus immunologic activity such as low C3,21 and poor compliance to treatment. Finally, the discussion should include the risk of worsening CKD and ESKD in case of LN relapse.
Figure 1.
Proposed algorithm to discuss treatment de-escalation. APS, antiphospholipid syndrome; AZA, azathioprine; LN, lupus nephritis; MMF, mycophenolate mofetil; UPCR, urinary protein-to- creatinine ratio.
The regular monitoring of this de-escalation (for instance, outpatient visit with blood and urine analyses scheduled every 3 months) is crucial for the early detection of SLE flares or LN relapses and for the prompt readjustment of treatment.
HCQ should probably be the last medication to be weaned,45,46 and remains the cornerstone of SLE treatment even in patients with LN,47 with reduced doses in case of CKD23 and appropriate monitoring for retinal toxicity.25 Whether corticosteroids or IST (AZA or MMF, or newly biologics) should be discontinued first is not fully answered, and may depend on the profile of each patient, in particular the level of dependence on corticosteroids for extrarenal manifestations of SLE. After the first year of treatment, we consider that corticosteroids are mostly dedicated to the control of the extrarenal activity of SLE. In general, corticosteroids are associated with cumulative damage in SLE,48, 49, 50 and the ideal recommended target is 0 mg/d,23,51,52 which implies that the progressive weaning of corticosteroids in patients with LN53 may precede that of IST. In a patient requiring low-dose corticosteroid (≤5 mg/d), for example for joint pain, tapering AZA or MMF should not be systematically discouraged provided that SLE symptoms remain controlled. But IST may also need to be maintained, or reintroduced, to spare corticosteroids in patients requiring higher doses for extrarenal manifestations. The presence of type 2 symptoms (fibromyalgia, chronic pain, and fatigue), not directly related to SLE activity and not improved by IST, should also be considered when evaluating the need for classical medications.54, 55, 56
There is no clear recommendation and several protocols have been proposed in the literature for IST weaning. Malvar et al.39 proposed a tapering of MMF of 500 mg/mo. De Rosa et al.38 proposed a tapering of MMF by reducing the dose by 50% during the first 3 months after the second biopsy, 50% during the next 3 months, and then stopping IST (prednisolone was weaned below 10 mg/d as dictated by extrarenal symptoms). In the WIN-lupus trial,21 patients randomized in the discontinuation group were weaned off IST over 3 months. Here, we propose a slow tapering protocol over 12 months in Figure 1.
In case of SLE flare during treatment de-escalation, de-escalation should be suspended. In case of mild extrarenal flare, corticosteroids may be increased transiently. In case of moderate flare, of persistent corticosteroid requirement, IST may be increased again or resumed. In case of suspected LN flare, a new kidney biopsy should be performed, and the patient treated according to pathologic involvement.
Nephroprotective Strategies: Not to Be Forgotten
LN is not the only factor implicated in the development of CKD and ESKD in patients with SLE, who are also at increased risk for hypertension,57 diabetes,58 and obesity,59 and may be smokers. Non-LN related kidney damage is to be considered and prevented through the control of these risk factors in patients with LN. 60
In this perspective, nephroprotective drugs such as angiotensin converting enzyme inhibitors or angiotensin receptor blockers are already part of the recommended therapies in patients with LN.23,24 Patients with LN were excluded from the pioneer DAPA-CKD trial,61 which showed the benefit of sodium glucose cotransporter inhibitors (SGLT2i) on CKD progression in patients with nondiabetic kidney diseases. This caution was probably related to the risk of genital infection favored by glycosuria. Yet, a benefit was demonstrated for patients with other glomerulonephritis such as IgA nephropathy in several trials of SGLT2i,62 and in the recently published EMPA-KIDNEY trial63 from which patients with LN were not excluded. The SGLT2i, dapagliflozin is already recommended in patients with nondiabetic CKD (eGFR between 20 and 75 ml/min per 1.73 m2) with proteinuria (urinary protein-to-creatinine ratio ≥200 mg/d).64 Dedicated trials on the specific benefit/risk balance of SGLT2i in patients with recent LN are needed,65 but once kidney damage is established, with chronic lesions, the combination of angiotensin converting enzyme inhibitors or angiotensin receptor blockers with SGLT2i to prevent hyperfiltration of remnant glomeruli and further chronic damage are likely to be beneficial66 whether the initial cause of kidney injury is LN or not.
Conclusion
Weaning of maintenance therapy is possible in some patients with LN, provided they have been treated for at least 3 years, are on sustained remission, are compliant to HCQ, are not planning a pregnancy in the following months, and are willing to undergo close monitoring during treatment de-escalation. Repeat kidney biopsy is a valuable tool to assess pathologic remission in patients for whom the T2U discussion is open. Nephroprotective strategies should not be overlooked in these patients. This review also highlights the need to improve and standardize the strategy for IST weaning in LN. A constructive suggestion is to build a reliable relapse risk score specific to the T2U strategy. This score should be generated by combining data from prospective cohorts and data from randomized clinical trials such as WIN-Lupus or other studies dedicated to IST weaning.
Disclosure
NJC reports expertise and lecture fees from Otuska and GSK in the field of LN. SB Reports expertise and lecture fees from Astra-Zeneca and Borhinger-Ingelleim in the field of SGLT2i. LC reports expertise and lecture fees from GSK and Novartis, and research grants from Astra-Zeneca and GSK, in the field of SLE. ED reports expertise and lecture fees from Otsuka, GSK, Novartis, Amgen, Astra-Zeneca in the field of LN.
References
- 1.Yap D.Y.H., Tang C.S.O., Ma M.K.M., et al. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. 2012;27:3248–3254. doi: 10.1093/ndt/gfs073. [DOI] [PubMed] [Google Scholar]
- 2.Murimi-Worstell I.B., Lin D.H., Nab H., et al. Association between organ damage and mortality in systemic lupus erythematosus: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-031850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tektonidou M.G., Dasgupta A., Ward M.M. Risk of end-stage renal disease in patients with lupus nephritis, 1971–2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol. 2016;68:1432–1441. doi: 10.1002/art.39594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman C.H., Yazdany J., Guan H., et al. Medication nonadherence is associated with increased subsequent acute care utilization among Medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2015;67:1712–1721. doi: 10.1002/acr.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costedoat-Chalumeau N., Houssiau F.A. Improving medication adherence in patients with lupus nephritis. Kidney Int. 2021;99:285–287. doi: 10.1016/j.kint.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Feldman C.H., Hiraki L.T., Winkelmayer W.C., et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67:1577–1585. doi: 10.1002/art.39070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiss E., Kovacs L., Szodoray P. Malignancies in systemic lupus erythematosus. Autoimmun Rev. 2010;9:195–199. doi: 10.1016/j.autrev.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce I. Lupus: the new diabetes. SLE and chronic disease management. Lupus. 2013;22:1203–1204. doi: 10.1177/0961203313505690. [DOI] [PubMed] [Google Scholar]
- 9.Conrad N., Verbeke G., Molenberghs G., et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet. 2022;400:733–743. doi: 10.1016/S0140-6736(22)01349-6. [DOI] [PubMed] [Google Scholar]
- 10.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 11.Thomas G., Mancini J., Jourde-Chiche N., et al. Mortality associated with systemic lupus erythematosus in France assessed by multiple-cause-of-death analysis. Arthritis Rheumatol. 2014;66:2503–2511. doi: 10.1002/art.38731. [DOI] [PubMed] [Google Scholar]
- 12.Hermansen M.L., Lindhardsen J., Torp-Pedersen C., et al. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatol (Oxf Engl) 2017;56:709–715. doi: 10.1093/rheumatology/kew475. [DOI] [PubMed] [Google Scholar]
- 13.Levy B., Couchoud C., Rougier J.-P., et al. Outcome of patients with systemic lupus erythematosus on chronic dialysis: an observational study of incident patients of the French National Registry 2002–2012. Lupus. 2015;24:1111–1121. doi: 10.1177/0961203315578763. [DOI] [PubMed] [Google Scholar]
- 14.Ali A.Y., Abdelaziz T.S., Behiry M.E. The prevalence and causes of non-adherence to immunosuppressive medications in patients with lupus nephritis flares. Curr Rheumatol Rev. 2020;16:245–248. doi: 10.2174/1573397115666190626111847. [DOI] [PubMed] [Google Scholar]
- 15.Aim M.A., Queyrel V., Tieulié N., et al. Importance of temporality and context in relation to life habit restrictions among patients with systemic lupus erythematosus: a psychosocial qualitative study. Lupus. 2022;31:1423–1433. doi: 10.1177/09612033221115966. [DOI] [PubMed] [Google Scholar]
- 16.Zen M., Iaccarino L., Gatto M., et al. Lupus low disease activity state is associated with a decrease in damage progression in Caucasian SLE patients, but overlaps with remission. Ann Rheum Dis. 2018;77:104–110. doi: 10.1136/annrheumdis-2017-211613. [DOI] [PubMed] [Google Scholar]
- 17.Ugarte-Gil M.F., Hanly J., Urowitz M., et al. Remission and low disease activity (LDA) prevent damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis. 2022;81:1541–1548. doi: 10.1136/ard-2022-222487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatto M., Zen M., Iaccarino L., Doria A. New therapeutic strategies in systemic lupus erythematosus management. Nat Rev Rheumatol. 2019;15:30–48. doi: 10.1038/s41584-018-0133-2. [DOI] [PubMed] [Google Scholar]
- 19.van Vollenhoven R.F., Bertsias G., Doria A., et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med. 2021;8 doi: 10.1136/lupus-2021-000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamirou F., Houssiau F.A. Management of lupus nephritis. J Clin Med. 2021;10:670. doi: 10.3390/jcm10040670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jourde-Chiche N., Costedoat-Chalumeau N., Baumstarck K., et al. WIN-Lupus study group. Weaning of maintenance immunosuppressive therapy in lupus nephritis (WIN-Lupus): results of a multicentre randomised controlled trial. Ann Rheum Dis. 2022;81:1420–1427. doi: 10.1136/annrheumdis-2022-222435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiche L., Jousse-Joulin S., Jourde-Chiche N. From “Treat to Target” to “Think to Untreat”: therapeutic de-implementation as a new paradigm in systemic lupus erythematosus. Rev Med Intern. 2022;44:101–104. doi: 10.1016/j.revmed.2022.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Fanouriakis A., Kostopoulou M., Cheema K., et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713–723. doi: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 24.Rovin B.H., Adler S.G., Barratt J., et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:753–779. doi: 10.1016/j.kint.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Jorge A.M., Mancini C., Zhou B., et al. Hydroxychloroquine dose per ophthalmology guidelines and the risk of systemic lupus erythematosus flares. JAMA. 2022;328:1458–1460. doi: 10.1001/jama.2022.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakhouri F., Schwotzer N., Cabiddu G., et al. Glomerular diseases in pregnancy: pragmatic recommendations for clinical management. Kidney Int. 2022;103:264–281. doi: 10.1016/j.kint.2022.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Coplon N.S., Diskin C.J., Petersen J., Swenson R.S. The long-term clinical course of systemic lupus erythematosus in end-stage renal disease. N Engl J Med. 1983;308:186–190. doi: 10.1056/NEJM198301273080403. [DOI] [PubMed] [Google Scholar]
- 28.Nossent H.C., Swaak T.J., Berden J.H. Systemic lupus erythematosus: analysis of disease activity in 55 patients with end-stage renal failure treated with hemodialysis or continuous ambulatory peritoneal dialysis. Dutch working party on SLE. Am J Med. 1990;89:169–174. doi: 10.1016/0002-9343(90)90295-o. [DOI] [PubMed] [Google Scholar]
- 29.Boumpas D.T., Austin H.A., 3rd, Vaughn E.M., et al. Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet. 1992;340:741–745. doi: 10.1016/0140-6736(92)92292-n. [DOI] [PubMed] [Google Scholar]
- 30.Illei G.G., Austin H.A., Crane M., et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001;135:248–257. doi: 10.7326/0003-4819-135-4-200108210-00009. [DOI] [PubMed] [Google Scholar]
- 31.Illei G.G., Takada K., Parkin D., et al. Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: long-term followup of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum. 2002;46:995–1002. doi: 10.1002/art.10142. [DOI] [PubMed] [Google Scholar]
- 32.Moroni G., Gatto M., Raffiotta F., et al. Can we withdraw immunosuppressants in patients with lupus nephritis in remission? An expert debate. Autoimmun Rev. 2018;17:11–18. doi: 10.1016/j.autrev.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Moroni G., Gallelli B., Quaglini S., et al. Withdrawal of therapy in patients with proliferative lupus nephritis: long-term follow-up. Nephrol Dial Transplant. 2006;21:1541–1548. doi: 10.1093/ndt/gfk073. [DOI] [PubMed] [Google Scholar]
- 34.Moroni G., Longhi S., Giglio E., et al. What happens after complete withdrawal of therapy in patients with lupus nephritis. Clin Exp Rheumatol. 2013;31(4 suppl 78):S75–S81. [PubMed] [Google Scholar]
- 35.Moroni G., Frontini G., Ponticelli C. When and how is it possible to stop therapy in patients with lupus nephritis. A narrative review. Clin J Am Soc Nephrol. 2021;16:1909–1917. doi: 10.2215/CJN.04830421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zen M., Fuzzi E., Loredo Martinez M., et al. Immunosuppressive therapy withdrawal after remission achievement in patients with lupus nephritis. Rheumatology (Oxford) 2022;61:688–695. doi: 10.1093/rheumatology/keab373. [DOI] [PubMed] [Google Scholar]
- 37.Malvar A., Pirruccio P., Alberton V., et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant. 2017;32:1338–1344. doi: 10.1093/ndt/gfv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Rosa M., Azzato F., Toblli J.E., et al. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int. 2018;94:788–794. doi: 10.1016/j.kint.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Malvar A., Alberton V., Lococo B., et al. Kidney biopsy–based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int. 2020;97:156–162. doi: 10.1016/j.kint.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Parodis I., Adamichou C., Aydin S., et al. Per-protocol repeat kidney biopsy portends relapse and long-term outcome in incident cases of proliferative lupus nephritis. Rheumatology (Oxford) 2020;59:3424–3434. doi: 10.1093/rheumatology/keaa129. [DOI] [PubMed] [Google Scholar]
- 41.Halimi J.M., Gatault P., Longuet H., et al. Major bleeding and risk of death after percutaneous native kidney biopsies: A French nationwide cohort study. Clin J Am Soc Nephrol. 2020;15:1587–1594. doi: 10.2215/CJN.14721219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banchereau R., Hong S., Cantarel B., et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. 2016;165:551–565. doi: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jourde-Chiche N., Whalen E., Gondouin B., et al. Modular transcriptional repertoire analyses identify a blood neutrophil signature as a candidate biomarker for lupus nephritis. Rheumatol (Oxf Engl) 2017;56:477–487. doi: 10.1093/rheumatology/kew439. [DOI] [PubMed] [Google Scholar]
- 44.Tailliar M., Schanstra J.P., Dierckx T., et al. Urinary peptides as potential non-invasive biomarkers for lupus nephritis: results of the Peptidu-LUP study. J Clin Med. 2021;10:1690. doi: 10.3390/jcm10081690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez-Ruiz R., Bornkamp N., Kim M.Y., et al. Discontinuation of hydroxychloroquine in older patients with systemic lupus erythematosus: a multicenter retrospective study. Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almeida-Brasil C.C., Hanly J.G., Urowitz M., et al. Flares after hydroxychloroquine reduction or discontinuation: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis. 2022;81:370–378. doi: 10.1136/annrheumdis-2021-221295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha C., Alexander S., Ashby D., et al. Hydroxycloroquine blood concentration in lupus nephritis: a determinant of disease outcome? Nephrol Dial Transplant. 2018;33:1604–1610. doi: 10.1093/ndt/gfx318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sada K.E., Katayama Y., Asano Y., et al. Association of one-point glucocorticoid-free status with chronic damage and disease duration in systemic lupus erythematosus: a cross-sectional study. Lupus Sci Med. 2022;9 doi: 10.1136/lupus-2022-000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tani C., Elefante E., Signorini V., et al. Glucocorticoid withdrawal in systemic lupus erythematosus: are remission and low disease activity reliable starting points for stopping treatment? A real-life experience. RMD Open. 2019;5 doi: 10.1136/rmdopen-2019-000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji L., Xie W., Fasano S., Zhang Z. Risk factors of flare in patients with systemic lupus erythematosus after glucocorticoids withdrawal. A systematic review and meta-analysis. Lupus Sci Med. 2022;9 doi: 10.1136/lupus-2021-000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji L., Xie W., Zhang Z. Low-dose glucocorticoids should be withdrawn or continued in systemic lupus erythematosus? A systematic review and meta-analysis on risk of flare and damage accrual. Rheumatol (Oxf Engl) 2021;60:5517–5526. doi: 10.1093/rheumatology/keab149. [DOI] [PubMed] [Google Scholar]
- 52.Ji L., Gao D., Hao Y., et al. Low-dose glucocorticoids withdrawn in systemic lupus erythematosus: a desirable and attainable goal. Rheumatol (Oxf Engl) 2022;62:181–189. doi: 10.1093/rheumatology/keac225. [DOI] [PubMed] [Google Scholar]
- 53.Nakai T., Fukui S., Ikeda Y., et al. Glucocorticoid discontinuation in patients with SLE with prior severe organ involvement: a single-center retrospective analysis. Lupus Sci Med. 2022;9 doi: 10.1136/lupus-2022-000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moazzami M., Strand V., Su J., Touma Z. Dual trajectories of fatigue and disease activity in an inception cohort of adults with systemic lupus erythematosus over 10 years. Lupus. 2021;30:578–586. doi: 10.1177/0961203320983892. [DOI] [PubMed] [Google Scholar]
- 55.Eudy A.M., Reeve B.B., Coles T., et al. The use of patient-reported outcome measures to classify type 1 and 2 systemic lupus erythematosus activity. Lupus. 2022;31:697–705. doi: 10.1177/09612033221090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arcani R., Jouve E., Chiche L., Jourde-Chiche N. Categorization of patients with systemic lupus erythematosus using disease activity, patient-reported outcomes and transcriptomic signatures. Clin Rheumatol. 2023;42:1555–1563. doi: 10.1007/s10067-023-06525-8. [DOI] [PubMed] [Google Scholar]
- 57.Taylor E.B., Ryan M.J. Understanding mechanisms of hypertension in systemic lupus erythematosus. Ther Adv Cardiovasc Dis. 2016;11:20–32. doi: 10.1177/1753944716637807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang M.Y., Hwang J.C., Feng I.J. Impact of diabetes mellitus on the risk of end-stage renal disease in patients with systemic lupus erythematosus. Sci Rep. 2018;8:6008. doi: 10.1038/s41598-018-24529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun C., Qin W., Zhang Y.H., et al. Prevalence and risk of metabolic syndrome in patients with systemic lupus erythematosus: a meta-analysis. Int J Rheum Dis. 2017;20:917–928. doi: 10.1111/1756-185X.13153. [DOI] [PubMed] [Google Scholar]
- 60.Falasinnu T., O’Shaughnessy M.M., Troxell M.L., et al. A review of non-immune mediated kidney disease in systemic lupus erythematosus: a hypothetical model of putative risk factors. Semin Arthrol Rheum. 2020;50:463–472. doi: 10.1016/j.semarthrit.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Committees and investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 62.Nuffield Department of Population Health Renal Studies Group SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–1801. doi: 10.1016/S0140-6736(22)02074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.EMPA-KIDNEY Collaborative Group. Herrington W.G., Staplin N., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cherney D.Z.I., Dekkers C.C.J., Barbour S.J., et al. DIAMOND investigators. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (diamond): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8:582–593. doi: 10.1016/S2213-8587(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 65.Wang H., Li T., Sun F., et al. Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: a phase I/II trial. RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vart P., Vaduganathan M., Jongs N., et al. Estimated lifetime benefit of combined RAAS and SGLT2 inhibitor therapy in patients with albuminuric CKD without diabetes. Clin J Am Soc Nephrol. 2022;17:1754–1762. doi: 10.2215/CJN.08900722. [DOI] [PMC free article] [PubMed] [Google Scholar]