Abstract
Introduction
Lysozyme-associated nephropathy (LyN), a rare cause of kidney injury in patients with chronic myelomonocytic leukemia (CMML), has not been well described to date. We report the clinicopathologic spectrum of LyN from a multi-institutional series.
Method
We identified 37 native kidney biopsies with LyN and retrospectively obtained clinicopathologic data.
Results
Thirty-seven patients had a median age of 74 years and included 78% males. Their most common presentation was acute kidney injury (AKI) or AKI on chronic kidney disease (CKD) (66%) with median estimated glomerular filtration rate (eGFR) of 21.7 ml/min per 1.73 m2, and proteinuria of 1.7 g. A minority (15%) had partial Fanconi syndrome. Serum lysozyme levels were elevated in all tested. Hematologic disorder (n = 28, 76%) was the most common etiology, including CMML (n = 15), acute myeloid leukemia (n = 5), and myelodysplastic syndrome (MDS) (n = 5). Nonhematologic causes (n = 5, 14%), included metastatic neuroendocrine carcinoma (n = 3), sarcoidosis, and leprosy. Etiology was unknown in 4 (11%). Pathology showed proximal tubulopathy with abundant hypereosinophilic intracytoplasmic inclusions, with characteristic staining pattern by lysozyme immunostain. Mortality was high (8/30). However, among the 22 alive, including 85% treated, 7 had improved kidney function, including 1 who discontinued dialysis and 6 with increase in eGFR >15 ml/min per 1.73 m2 compared with eGFR at the time of biopsy.
Conclusion
Increased awareness of the full clinicopathologic spectrum of LyN may lead to prompt diagnosis, earlier treatment, and potentially improved outcome of this rare entity.
Keywords: chronic myelomonocytic leukemia, granulomas, kidney biopsy, lysozyme, lysozyme induced nephropathy, proximal tubulopathy
Graphical abstract
Lysozyme (or muramidase) is a small, nonglycosylated, bacteriocidal enzyme first discovered by Sir Alexander Fleming in 1922.1 Lysozyme is produced predominantly by monocytes and macrophages; however, also by intestinal Paneth cells, salivary gland acinar cells, and other cell types.2 Because of its small size (∼15 kDa) and cationic charge, lysozyme is freely filtered by the glomerulus. Nearly all of the filtered load is reabsorbed by proximal tubular epithelial cells via megalin/cubilin mediated endocytosis.3 Once internalized, lysozyme is subject to endolysosomal proteolysis and thus degraded.4 Given the high absorptive capacity of the kidney for lysozyme, only a minimal amount of lysozyme is excreted in the urine at physiologic levels of production.5
Serum lysozyme levels are known to be elevated to various degrees in several diseases, most notably in CMML and other forms of leukemia with myelomonocytic differentiation6,7; however, to lesser degree in sarcoidosis,8,9 tuberculosis,9,10 and inflammatory bowel diseases.11 Animal models of lysozyme overproduction have demonstrated the kidney’s high absorptive capacity of excess filtered lysozyme and, little, if any, lysozymuria is seen in those with normal kidney.12,13 Lysozyme-associated kidney dysfunction was first described in the late 1960’s6,7,14,15; however, modern reports of LyN are limited to case reports16, 17, 18, 19, 20 or small series6 almost exclusively in patients with CMML. In this report, we build on our prior experience with this entity20,21 to provide a more comprehensive clinical and pathologic description of patients with LyN.
Methods
We identified 37 native kidney biopsies from patients with LyN at Columbia University Irving Medical Center, New York, New York (n = 14, including 1 previously published20), Arkana Laboratories, Little Rock, Arkansas (n = 17, previously reported as an abstract21), Mayo Clinic, Rochester, Minnesota (n = 5), and AmeriPath, Oklahoma City, Oklahoma (n = 1) from 2011 to 2021.
For each patient, clinical and laboratory data at the time of biopsy provided by the submitting nephrologists were recorded. These data include age, self-identified sex, self-identified race, past medical history, indications for kidney biopsy, serum creatinine at baseline and at the time of biopsy, proteinuria (as determined by urine protein-to-creatinine ratio or 24-hour urine protein), proteinuria by dipstick, serum and urine protein electrophoresis, features of Fanconi syndrome (normoglycemic glycosuria, renal tubular acidosis, hypokalemia, hyperchloremia, hypophosphatemia), white blood cell count, differential for monocytes, bone marrow biopsy results, and serum or urine lysozyme levels. Indications for kidney biopsy were recorded as any combination of AKI,22 CKD,23 and AKI superimposed on CKD (AKI on CKD). Nephrotic-range proteinuria was defined as >3.5 g/g or g/d. eGFR was computed using the Chronic Kidney Disease Epidemiology Collaboration equation.24 For each patient, the therapy administered, date of last follow-up, and serum creatinine at follow-up were obtained.
The following variables were recorded for each biopsy: diagnoses other than LyN; the extent of tubulointerstitial scarring, as defined by the Banff criteria25; proximal tubular cell morphology; staining quality of intracytoplasmic inclusions by hematoxylin and eosin, periodic acid-Schiff (PAS), Jones methenamine silver, trichrome, and Congo red stains (because lysozyme is known to bind to Congo red and exhibit a salmon pink color; however, without apple-green birefringence on polarization),26 as well as lysozyme immunohistochemical stain and ultrastructural appearance of the inclusions. In addition, Congo red stain and immunohistochemical stain for lysozyme were performed in 33 non-LyN native kidney biopsies at the Columbia University Irving Medical Center, including 3 cases each of sarcoidosis-associated granulomatous interstitial nephritis, granulomatous interstitial nephritis in patients without history of sarcoidosis, collapsing glomerulopathy, minimal change disease, crystalline and noncrystalline light chain proximal tubulopathy, tenofovir toxicity, acute tubular injury, bacterial infection-related glomerulonephritis, 3 biopsies each from patients with a history of Crohn’s disease and ulcerative colitis (including 2 with minimal change disease and minimal IgA nephropathy, 3 with IgA nephropathy, and 1 with AA-type amyloidosis).
All kidney biopsies were processed by standard techniques for light microscopy, immunofluorescence, and electron microscopy. Immunohistochemical stains for lysozyme were performed using the following antibodies: rabbit monoclonal antibody (clone EP134, prediluted, incubation time 32 min; MilliporeSigma, Burlington, MA) after antigen retrieval using Cell Conditioning Solution 1 (Ventana Medical Systems, Oro Valley, AZ) at Columbia University Irving Medical Center, rabbit polyclonal antibody (catalog#278A-18, prediluted, incubation time 10 min; MilliporeSigma) after antigen retrieval using Proteinase K at Arkana Laboratories, and rabbit polyclonal antibody (catalog #EC 3.2.1.17, dilution 1:2000, incubation time 32 min; Dako, Carpinteria, CA) after antigen retrieval using Cell Conditioning Solution 1 (Ventana Medical Systems) at Mayo Clinic.
Continuous variables were expressed as median (interquartile range, IQR). Categorical variables were shown as frequency and/or percentage. All statistical analyses were performed using R (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
This study was approved by the institutional review boards at the respective institutions and was conducted according to ethical principles and guidelines for the protection of human subjects of research. The authors have completed a STROBE statement provided as supplemental material.
Results
Case Series
Clinical Features
Results are shown in Table 1. Thirty-seven patients had a median age of 74 years (IQR 65–78 years) and included 78% males. Race and ethnicity, available in 32, included 27 (84%) Whites, 3 (9%) Blacks, 1 Hispanic (3%), and 1 (3%) non-White or Black or Hispanic. Twenty-six (76%) patients had a history of CKD before the biopsy, with median baseline serum creatinine of 1.5 mg/dl (IQR 1–1.9 mg/dl, data available in 33) and eGFR of 44 ml/min per 1.73 m2 (IQR 33.4–68.5 ml/min per 1.73 m2). Other reported past medical history included 16 with hypertension, 4 with diabetes mellitus, and 2 with autoimmune disease (1 rheumatoid arthritis and 1 with dermatomyositis associated with essential thrombocythemia).
Table 1.
Clinical and pathologic features of patients with lysozyme-associated nephropathy (N = 37)
| Features | Parametersa | Median (IQR) or N (%) |
|---|---|---|
| Demographics | ||
| Age (yr) | 74 (65–78) | |
| Gendera | ||
| Male | 29 (78) | |
| Female | 8 (22) | |
| Race/Ethnicityb, data available in n = 32 | ||
| White | 27 (84) | |
| Black | 3 (9) | |
| Hispanic | 1 (3) | |
| Other | 1 (3) | |
| Clinical features at the time of biopsy | ||
| Baseline serum creatinine before presentation (mg/dl), data available in n = 33 | 1.5 (1–1.9) | |
| Baseline eGFR by CKD-EPI (ml/min per 1.73 m2), data available in n = 33 | 44 (33.4–68.5) | |
| Chronic kidney disease before biopsy | 26 (76) | |
| Biopsy indication, data available in n = 32 | ||
| AKI or AKI on CKD | 21 (57) | |
| CKD | 16 (43) | |
| Serum creatinine (mg/dl) at the time of biopsy, n = 33 | 2.7 (2.1–3.5) | |
| eGFR by CKD-EPI (ml/min per 1.73 m2), data available in n = 33 | 21.7 (16.8–29.0) | |
| 24-hour urine protein or UPCR (g/g or g/d), data available in n = 31 | 1.7 (0.5–2.0) | |
| Nephrotic range proteinuria | 4 (12) | |
| Presence of proteinuria by semiquantitative dipstick (≥1+) | 14/18 (77) | |
| Microscopic hematuria | 8/29 (28) | |
| Features of partial Fanconi syndrome | 3/20 (15) | |
| Abnormal serum protein electrophoresis | 5/22 (23) | |
| Abnormal urine protein electrophoresis | 3/5 (60) | |
| Elevated serum lysozyme level | 22/22 (100) | |
| Elevated urine lysozyme level | 3/3 (100) | |
| Pathologic features | ||
| Staining characteristics of intracytoplasmic inclusions | ||
| Hypereosinophilic and refractile on H&E | 36/37 (97) | |
| At least focally pale on PAS | 31/37 (84) | |
| Negative for JMS | 35/37 (95) | |
| Fuchsinophilic on trichrome | 37/37 (100) | |
| Pale pink on Congo red | 31/33 (94) | |
| Extent of tubular atrophy & interstitial fibrosis | ||
| None or mild | 20/36 (56) | |
| Moderate | 11/36 (31) | |
| Severe | 5/36 (14) | |
| Vacuoles containing granular electron dense material on electron microscopy | 37/37 (100) | |
| Lysozyme immunostain | ||
| Areas with strong staining | 37/37 (100) | |
| Areas with staining of rim of the inclusions only | 35/37 (95) | |
| Presence of coexisting pathology | 13/37 (35) | |
| Related to underlying etiology | 9/37 (24) | |
| Follow-up information | ||
| Duration of follow-up (months), n = 30 | 8 (3.5–12) | |
| Alive | 22/30 (73) | |
| Last available serum creatinine (mg/dl), n = 21 | 2 (1.3–2.5) | |
| eGFR by CKD-EPI (ml/min per 1.73 m2), n = 21 | 31 (24–44.7) | |
AKI, acute kidney injury; CKD, chronic kidney disease; CKD-EPI equation, Chronic Kidney Disease Epidemiology Collaboration equation; eGFR, estimated glomerular filtration rate; H&E, hematoxylin and eosin; IQR, interquartile range; JMS, Jones methenamine silver; PAS, periodic acid-Schiff; UPCR, urine protein to creatinine ratio.
Number of data points available are provided in this column for continuous variable.
As provided by referring nephrologists.
The majority of patients presented with AKI or AKI on CKD (57%), whereas 43% presented with CKD. At the time of kidney biopsy, median serum creatinine was 2.7 mg/dl (IQR 2.1–3.5 mg/dl, data available in n = 33), median eGFR was 21.7 ml/min per 1.73 m2 (IQR 16.8–29.0 ml/min per 1.73 m2), and median proteinuria was 1.7 g/g or g/d (IQR 0.5–2.0, data available in n = 31), including 4 (12%) with nephrotic range proteinuria. In 1 patient, nonalbumin proteinuria accounted for 80% of the urinary protein and another demonstrated tubular proteinuria, with lysozyme accounting for 17% of the urinary protein. One patient required dialysis at the time of presentation. Among 18 patients with information available, 14 (77%) had proteinuria detected by dipstick. A minority had microscopic hematuria (28%, data available in n = 29). Although 3 (15%) patients had features that could represent partial Fanconi syndrome (1 with hypokalemia, 1 with hyperchloremia, and 1 with normoglycemic glucosuria), none had full Fanconi syndrome among 20 with at least 1 variable available for determination. Among 22 patients with serum electrophoresis results, 5 had abnormal peaks (including 2 characterized as IgGκ, 1 with both IgGκ and IgMλ and 2 not further characterized) without known history of plasma cell neoplasm. Although only 5 patients had urine protein electrophoresis performed, 3 showed abnormal bands, including 1 band at extreme cathodal gamma region, most consistent with lysozyme (Figure 1a and b). The other 2 were reported as a “faint band of limited mobility” and “free κ and λ.”
Figure 1.
Urine protein electrophoresis. (a) The urine sample in lane #10 of this gel (in red brackets) is from a patient with lysozyme-associated nephropathy. The dark band at the top (toward the anode) is albumin. Note the unique band (arrow) corresponding to lysozyme at the extreme cathodal edge of the gel, far below the migration range of the other urine proteins. (b) In the densitometric scan of the same gel, the lysozyme band appears as the shaded peak on the right. The concentration of lysozyme can be estimated by multiplying the total urine protein concentration by the percentage of area under the curve attributable to the peak, as one would measure an M-spike.
The most common etiologies for LyN included CMML (n = 15 [41%], including 2 known to have progressed from MDS and 1 from myelofibrosis), acute myeloid leukemia (AML, n = 5, 14%, including 2 with monocytic differentiation, 1 with myelomonocytic differentiation, and 1 known to have progressed from MDS), and MDS (n = 5, 14%, including 1 with a history of AML in remission). Among those with CMML, their median white blood cell count was 28 K (IQR 20–46 K, data available in n = 14) with median differential for monocyte 24% (IQR 17–29%, data available in n = 12). Among those with AML and MDS, median white blood cell count were 55 K (IQR 11–99 K, data available in n = 4) and 5.7 K (IQR 4.5–18.9, data available in n = 5, with monocyte differential of 8, 21, and 26% in 3 with data available), respectively.
Other less common etiologies included 3 cases of metastatic neuroendocrine carcinoma (with metastasis to liver in all, including 1 with intraglomerular carcinoma), 1 myelofibrosis (JAK2 positive, with myeloproliferative or myelodysplastic features and right lung nodule with caseating necrosis), 1 essential thrombocythemia (JAK2 positive), 1 leukemia not otherwise specified, 1 leprosy, and 1 sarcoidosis (without interstitial nephritis, granulomas, or membranous nephropathy). An underlying etiology was not found in 4 patients, though 2 had splenomegaly, including 1 with elevated angiotensin converting enzyme level and a bone marrow biopsy showing 6% plasma cell neoplasm and the other with transient monocytosis.
Serum lysozyme levels were above the upper limit of normal in all tested patients (100%, 22/22), including 63% (14/22) above the upper limit of quantification. Median serum lysozyme levels could not be reliably calculated, because of differences in reporting practice and reference values among the several reference laboratories. Urine lysozyme levels, though only available in 3 patients, were elevated significantly (73 μg/ml, normal 0–2 μg/ml; 530, and 982 μg/ml, normal 5–11 μg/ml).
Pathologic Features
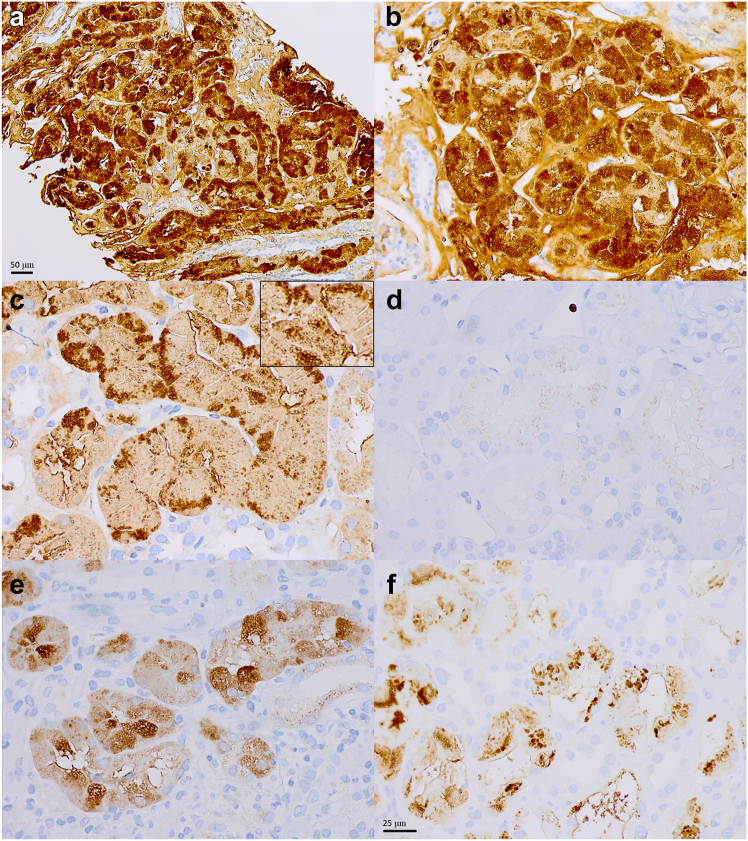
Results are shown in Table 2. Histopathologic evaluation demonstrated proximal tubulopathy. On hematoxylin and eosin-stained sections, proximal tubular epithelial cells appeared hypereosinophilic (Figure 2a and b) and swollen with abundant apical cytoplasm and convex apical membrane. Rare tubular profiles were notable for individual apoptotic cells. Proximal tubular epithelial cells were engorged with round, eosinophilic, refractile protein droplets (97%, 36/37) that extended from apical to basolateral aspect of the affected cells (Figure 2b). These droplets stained variably with PAS, with at least focal PAS-pale areas (as compared to the tubular basement membranes) seen in the majority (84%, 31/37, often in basolateral areas), and were typically nonargyrophilic (95%, 35/37), and fuchsinophilic (100%, 37/37) on trichrome stained sections (Figure 2c–e). The inclusions also exhibited mild to moderate Congophilia (i.e., appearing salmon-pink in color, 94%, 31/33); however, did not produce “apple-green” birefringence typical of amyloid when viewed under polarized light (Figure 2f). Immunohistochemical stain for lysozyme demonstrated a characteristic staining pattern, with areas that exhibit diffuse and strong staining of these droplets (100%, 37/37), often accompanied by patchy areas where only the rim of droplets showed variable immunoreactivity (92%, 34/37) (Figure 3a–c). These staining patterns are distinct from finely granular and apical staining seen in non-LyN cases (Figure 3d). Immunostain for chromogranin A was negative in the distribution of tubular epithelial cell droplets in 3 cases with a history of neuroendocrine carcinoma, including 1 with glomerular metastasis by the tumor, which stained positively for chromogranin but negatively for lysozyme. Tubular atrophy and interstitial fibrosis were mild (56%, 20/36), moderate (31%, 11/36), or severe (14%, 5/36).
Table 2.
Underlying etiology of lysozyme-associated nephropathy (N = 37)
| Categories | N (%) |
|---|---|
| Hematologic disorder | 28 (76) |
| Chronic myelomonocytic leukemia | 15 (41) |
| Acute myeloid leukemia | 5 (14) |
| Myelodysplastic syndrome | 5 (14) |
| Myelofibrosis | 1 (3) |
| Essential thrombocythemia | 1 (3) |
| Leukemia, not otherwise specified | 1 (3) |
| Nonhematologic disorder | 5 (14) |
| Metastatic neuroendocrine carcinoma | 3 (8) |
| Sarcoidosis (without interstitial nephritis, granulomas, or membranous nephropathy) | 1 (3) |
| Leprosy | 1 (3) |
| Unknown | 4 (11) |
Figure 2.
(a) Histologic features of lysozyme-associated nephropathy. Diffuse hypereosinophilia of the proximal tubules at low magnification is the first clue to this diagnosis (original magnification 200×). (b) At higher magnification, these cases demonstrate proximal tubulopathy with abundant and diffuse intracytoplasmic inclusions in proximal tubular epithelial cells. These inclusions are large and round, fill the entire cytoplasm from apex to base, and appear hypereosinophilic on hematoxylin and eosin stain (b). Their granularity can be highlighted by closing the condenser aperture diaphragm and increasing the intensity of light source. The inclusions appear variable; however, they are often at least focally pale on periodic acid-Schiff (c), and are (d) typically negative on Jones methenamine silver, (e) fuchsinophilic on trichrome stain, and (f) pale salmon-color on Congo red stain, without birefringence under polarized light (b–f, original magnification, all 600×).
Figure 3.
Lysozyme immunostain. Lysozyme immunostain (clone EP134) demonstrate characteristic staining patterns, with areas that strongly highlight intracytoplasmic inclusions in lysozyme-associated nephropathy (a, original magnification 200×, and b), accompanied by patchy areas where the immunostain variably highlights only the rim of inclusions (c, inset). (d) These staining patterns are distinct from the finely granular, weak apical staining seen in cases that are not lysozyme-associated nephropathy. (e) Similar, but less intense staining can be seen in some cases of granulomatous interstitial nephritis with or without known history of sarcoidosis. (f) In cases of crystalline variant of light chain proximal tubulopathy, strong staining is seen predominantly in distribution of intracytoplasmic crystals (b–f, original magnification, all 600×).
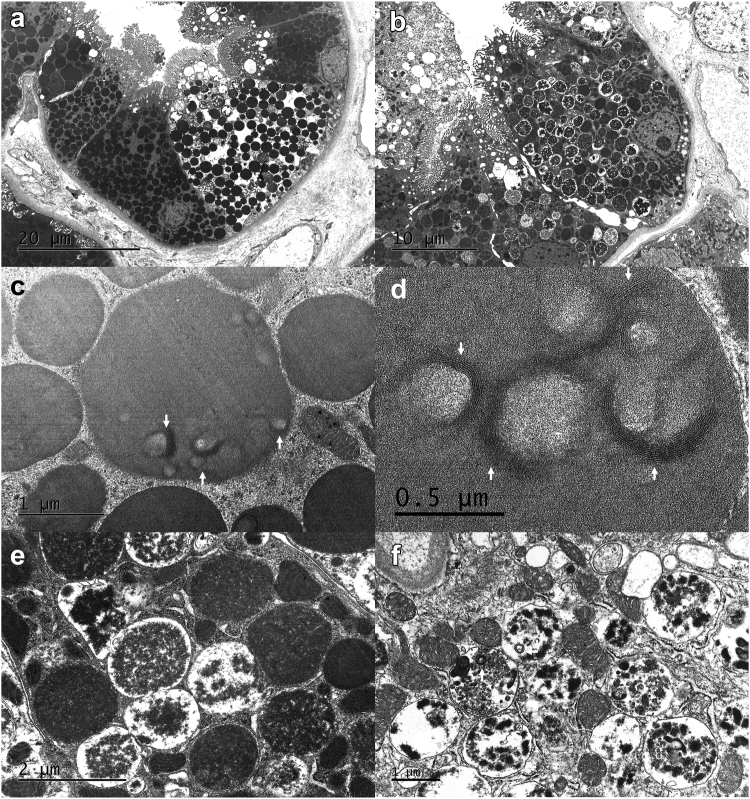
Ultrastructural examination demonstrated abundant intracytoplasmic, membrane-bound vacuoles containing homogenous or granular electron dense material (100%, Figure 4a and b). These 2 types of vacuoles sometimes coexisted in a single cell. Vacuoles containing homogeneous material sometimes demonstrated small admixed curvilinear substructures at high magnification (Figure 4c and d). Vacuoles containing granular electron dense material sometimes demonstrated admixed cellular debris in the form of degenerating phospholipid-type material (Figure 4e and f). In exceptional cases, membrane-bound vacuoles containing degenerating organelles (consistent with autophagolysosomes) were identified.
Figure 4.
Ultrastructural appearance of inclusions in lysozyme-associated nephropathy. The content of intracytoplasmic vacuoles/inclusions in proximal tubular epithelial cells in lysozyme-associated nephropathy demonstrate variable morphology, perhaps reflecting the varying stages of degradation of lysozyme in lysosomes. (a) Some inclusions demonstrate uniform electron density without definitive outer membranes (original magnification 3000×). (b) Others appear more granular or mottled, with peripheral areas of electron lucency and appear to be contained in membrane-bound vacuoles (original magnification 4000×). (a and b) These inclusions are present predominantly in proximal tubular epithelial cells (note the presence of brush borders). Some inclusions that appear uniformly electron dense focally exhibit more electron dense curvilinear material often surrounding the central electron lucent areas (c, arrows, original magnification 40,000×). (d) At higher magnification, this curvilinear material is composed of multiple layers of electron dense curved bands, somewhat resembling the fingerprint pattern occasionally seen in patients with lupus or cryoglobulinemia (original magnification 60,000×). (e) In contrast, inclusions that appear granular demonstrate variable electron density and often demonstrate electron lucent periphery. No substructures are apparent in vacuoles containing granular inclusions (original magnification 25,000×). (f) Some vacuoles containing granular material demonstrate membrane-bound material, likely representing degenerating phospholipid-type material (original magnification, 25,000×).
Aside from nonspecific chronic changes attributable to arterionephrosclerosis, 13 (35%) biopsies demonstrated coexisting pathology including 4 with direct involvement of the kidney by underlying neoplasm (2 AML, 1 CMML, and 1 with intraglomerular neuroendocrine carcinoma), 3 with extramedullary hematopoiesis, 2 with myeloproliferative neoplasm associated glomerulopathy,27 2 with immune complex mediated glomerulonephritis (1 mild IgA nephropathy and 1 endocapillary proliferative glomerulonephritis with “full-house” staining in a patient with an uncharacterized autoimmune disease), 1 idiopathic nodular glomerulosclerosis (without history of smoking or diabetes), and 1 with diffuse diabetic glomerulosclerosis. In 2 cases, given its severity, arterionephrosclerosis was felt to be the finding that best correlated with the clinical presentation.
Staining Characteristic of Non-LyN Cases for Lysozyme and Congo Red
Among 33 non-LyN cases at Columbia University Irving Medical Center, only 3 of 6 cases with granulomatous interstitial nephritis, including 1 with known history of sarcoidosis, demonstrated a staining pattern for lysozyme and Congo red stains akin to those seen in LyN cases, though the intensity of lysozyme staining was less than that of cases diagnosed as LyN (Figure 3e).
Of the remaining 30 cases, 3 cases of crystalline light chain proximal tubulopathy showed strong staining for lysozyme immunostain and weak Congo red staining (without “apple-green” birefringence) in the distribution of intracytoplasmic crystals (which may represent nonspecific staining of crystals or entrapment of lysozyme in dysfunctional lysosomes, Figure 3f); 2 cases of noncrystalline light chain proximal tubulopathy showed focally strong and diffuse weak staining for lysozyme and minimal focal Congo red staining; and 1 case of bacterial infection-related glomerulonephritis had focal strong and diffuse weak finely granular lysozyme staining with negative Congo red staining. The remaining 24 cases had either weak, finely granular apical staining for lysozyme in the distribution of apical protein droplets (n = 14) or no significant staining for lysozyme (n = 10). These 24 cases did not demonstrate significant Congo red staining. Fourteen cases with weak, finely granular apical staining for lysozyme included 3 with diffuse staining (1 with sarcoidosis, 1 with acute tubular injury and history of recent sepsis, and 1 with bacterial infection-related glomerulonephritis) and 11 with focal staining.
Follow-up
Thirty (81%, 30/37) patients had follow-up information available, with median duration of 8 months (IQR 3.5–12 months). Among 8 deceased (27%, 8/30); 3 had CMML; 2 had AML; and of the remaining, each had metastatic neuroendocrine carcinoma, myelofibrosis, and MDS. Notably, 3 who died had involvement of the kidney parenchyma by the underlying neoplasm (2 AML and 1 neuroendocrine carcinoma). Six of these 8 patients received chemotherapy (60%, Table 3).
Table 3.
Therapy for patients with lysozyme-associated nephropathy among those with follow-up (N = 30)
| Categories | N | Therapy |
|---|---|---|
| Alive | 22 | |
| Chronic myelomonocytic leukemia | 10 | 3 azacitidine 3 decitabine, including 1 with venetoclax, and 1 with hydroxyurea and ruxolitinib 1 ruxolitinib 1 hydroxyurea and corticosteroid 2 no treatment |
| Acute myeloid leukemia | 3 | 1 decitabine and venetoclax 1 no therapy 1 unknown |
| Myelodysplastic syndrome | 2 | 1 azacitidine 1 hydroxyurea |
| Neuroendocrine carcinoma | 2 | 2 chemotherapy and radiation therapy, including 1 with lanreotide |
| Essential thrombocythemia | 1 | Ruxolitinib |
| Leprosy | 1 | Azithromycin, dapsone, minocycline |
| Sarcoidosis | 1 | Corticosteroid |
| Unknown | 2 | 1 no therapy 1 unknown |
| Deceased | 8 | |
| Chronic myelomonocytic leukemia | 3 | 1 decitabine and cedazuridine 1 venetoclax 1 chemotherapy NOS |
| Acute myeloid leukemia | 2 | 1 decitabine 1 no chemotherapy |
| Neuroendocrine carcinoma | 1 | Chemotherapy NOS |
| Myelodysplastic syndrome | 1 | No chemotherapy |
| Myelofibrosis | 1 | Ruxolitinib, then palliative care |
NOS, not otherwise specified.
Twenty-two alive patients (73%, 22/30) had the following etiologies: 10 CMML, 3 AML, 2 MDS, 2 neuroendocrine carcinoma, 1 essential thrombocythemia, 1 leprosy, 1 sarcoidosis, and 2 with unknown etiology. Among 20 with therapeutic information available, 17 were treated for underlying etiology (85%, 17/20). Therapy provided is summarized in Table 3. One patient with AML who had required hemodialysis became dialysis-independent after therapy. The remaining 21 patients had median serum creatinine of 2 mg/dl (IQR 1.3–2.5 mg/dl) and eGFR of 31 ml/min per 1.73 m2 (24.0–47.7 ml/min per 1.73 m2) at the last follow-up. Among 20 patients with eGFR available at the time of biopsy and follow-up, 6 (30%, 6/20) had increase in eGFR greater than 15 ml/min per 1.73 m2 above the baseline glomerular filtration rate at time of biopsy, 8 (40%, 8/20) had increase less than 15 ml/min per 1.73 m2, 1 (5%. 1/20) had stable eGFR, and 5 (25%, 5/20) had decrease in eGFR less than 15 ml/min per 1.73 m2. Follow-up serum lysozyme levels, available in 2 patients (1 with CMML and 1 with essential thrombocythemia), were within normal limits.
Discussion
We report the first large multi-institutional series describing the clinical and pathologic features of patients with LyN. Given that previous reports of this entity are limited,16, 17, 18, 19, 20, 21 we hope that our description will serve as a practical guide for both nephrologists and pathologists to diagnose and treat patients with this rare and likely under-recognized entity.
Patients with LyN are elderly and present with AKI or AKI on CKD, often accompanied by subnephrotic range proteinuria. Incomplete Fanconi syndrome is seen in a minority of patients and has been previously reported.6 Proteinuria in these patients appears to be due to a combination of lysozymuria and albuminuria. Concomitant albuminuria may reflect tubular albuminuria,28 reduction of negative charge of the glomerular filtration barrier by lysozyme,29 or presence of coexisting kidney pathology. Conceptually, a discrepancy between urine dipstick protein measurements and quantified urine protein levels, typical of nonalbuminuric proteinuria, would be expected in patients with LyN. In our series, the lack of urine albumin measurement and the presence of age-related and glomerular pathology may have masked this phenomenon. In suspected cases of LyN, skilled interpretation of urine protein electrophoresis may be useful to identify a characteristic “cationic peak.”7
Consistent with prior reports,16, 17, 18, 19, 20, 21 CMML is the most commonly identified etiology. However, we observed a much broader range of underlying conditions, including other hematologic malignancies with myelomonocytic differentiation (i.e., AML or MDS), granulomatous diseases (i.e., sarcoidosis and leprosy), and nonhematologic neoplasms (i.e., metastatic neuroendocrine carcinoma). These underlying conditions reflect the variety of conditions known to be associated with lysozyme overproduction and lysozymuria. The origin of lysozyme overproduction may or may not be due to overexpression by the tumor, as demonstrated in 1 case of metastatic neuroendocrine carcinoma-associated LyN, and, in such cases, may be related to the inflammatory response against the tumor.
On kidney biopsy, LyN is characterized by engorgement of proximal tubular cells by refractile, eosinophilic protein droplets. These droplets demonstrate variable PAS staining; however, they are often PAS-pale, and are generally nonargyrophilic, and appear bright red on trichrome stained sections. Ultrastructurally, these droplets correspond to intracytoplasmic, membrane-bound vacuoles containing homogenous and/or granular electron dense material, sometimes with admixed cellular debris. LyN must be distinguished from other proximal tubulopathies characterized by accumulation of excess intracytoplasmic noncrystalline proteins, including chromogranin tubulopathy30 and noncrystalline form light chain proximal tubulopathy.31,32 Immunohistochemical staining for chromogranin and immunofluorescence staining for kappa and lambda light chains (including with pronase digestion) are useful in excluding these entities.33 Because lysozyme is normally filtered through the glomeruli and reabsorbed in proximal tubules, accurate interpretation of lysozyme immunostain requires careful validation, familiarity with the staining patterns seen in non-LyN conditions, and recognition of the salient light and ultrastructural features of LyN. Correlation with serum lysozyme levels and urine protein electrophoresis, may be helpful because serum lysozyme levels are typically above the upper limit of quantification (63%) in these cases. Notably, because lysozyme can also be identified on urine protein electrophoresis (if interpreted by a skilled clinical pathologist), communication with the clinical chemistry laboratory may also be beneficial. Lastly, coexisting pathology related to underlying neoplasms, seen in approximately 25% of cases, may also be a helpful diagnostic feature.
Although excess circulating lysozyme appears necessary to the pathogenesis of LyN, the precise mechanism by which lysozyme induces injury to proximal tubular epithelial cells is unknown. In animal models, serial urinary protein electrophoresis shows that tubular proteinuria precedes lysozymuria, suggesting that tubular injury may occur early in the disease course.12 The presence of intracytoplasmic membrane-bound vacuoles containing the residua of degenerated membranes is also evidence of lysozyme-mediated tubular cell injury. Similar to noncrystalline light chain proximal tubulopathy,34 impaired endolysosomal function may result from direct cytotoxicity by nonamyloid aggregation (variously referred to as “amorphous aggregates”35 or mesoscopic clusters36) of native and, possibly, partially denatured lysozyme induced by supersaturation. Such a mechanism may explain the lack of reactivity at the center of some intracytoplasmic inclusions and the rare presence of curvilinear substructure on ultrastructural examination. Superimposed ischemic and/or toxic tubular insults may also exacerbate lysozyme-mediated tubular injury.
In our series, the prognosis of LyN is guarded. The high mortality (27%) is likely due in large part to the strong association with hematologic malignancy. In particular, those with direct involvement of the kidney by an underlying neoplasm have poor prognosis (3 of 4 died, with the other placed on palliative care). However, because treatment of the underlying etiology appears to result in improvement of kidney function in some (including 33% with increase in eGFR greater than 15 ml/min per 1.73 m2), prompt diagnosis and treatment of the underlying etiology is essential.
There are several limitations to our study. Given the multi-institutional and retrospective design, we do not have complete clinical information, and data obtained are not standardized. In particular, concurrent urine albumin measurement, urine protein electrophoresis, and complete follow-up information regarding hematologic response could not be obtained. In addition, small sample size and the variety of etiologies precludes meaningful statistical analysis of factors associated with outcome. Lastly, the clinical significance of lysozyme accumulation in cases of sarcoidosis and granulomatous interstitial nephritis, also observed by others,37 in which coexisting pathology likely better correlates with clinical presentation, is unclear and merits further study, though this is likely dependent on the severity of both processes.
In summary, we describe and expand the clinicopathologic spectrum of patients with LyN. Although rare, greater awareness of this entity and the wide range of etiologies has the potential to lead to prompt diagnosis and treatment, as well as improved clinical outcomes.
Disclosure
SK is being supported by the Young Investigator Grant of the National Kidney Foundation. All other authors have declared no conflicting interest.
Acknowledgments
Author Contributions
SK, NC, and DS designed the study; SK performed statistical analysis; all authors contributed to the acquisition and interpretation of data; SK and DS made the figures; SK, NC, and DS drafted the paper; and all authors revised the paper and approved the final version of the manuscript.
Footnotes
STROBE Statement.
Supplementary Material
STROBE Statement.
References
- 1.Fleming A., Wright A.E. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond S B Containing Pap Biol Character. 1922;93:306–317. doi: 10.1098/rspb.1922.0023. [DOI] [Google Scholar]
- 2.Mason D.Y., Taylor C.R. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975;28:124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seliverstova E.V., Prutskova N.P. Receptor-mediated endocytosis of lysozyme in renal proximal tubules of the frog Rana temporaria. Eur J Histochem. 2015;59:2482. doi: 10.4081/ejh.2015.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen E.I., Maunsbach A.B. Intralysosomal digestion of lysozyme in renal proximal tubule cells. Kidney Int. 1974;6:396–407. doi: 10.1038/ki.1974.125. [DOI] [PubMed] [Google Scholar]
- 5.Osserman E.F., Canfield R.E., Beychok S., Columbia University, Institute of Cancer Research . Academic Press; 1974. Lysozyme; [Proceedings] [Google Scholar]
- 6.Muggia F.M., Heinemann H.O., Farhangi M., Osserman E.F. Lysozymuria and renal tubular dysfunction in monocytic and myelomonocytic leukemia. Am J Med. 1969;47:351–366. doi: 10.1016/0002-9343(69)90219-8. [DOI] [PubMed] [Google Scholar]
- 7.Osserman E.F., Lawlor D.P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966;124:921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pascual R.S., Gee J.B., Finch S.C. Usefulness of serum lysozyme measurement in diagnosis and evaluation of sarcoidosis. N Engl J Med. 1973;289:1074–1076. doi: 10.1056/NEJM197311152892007. [DOI] [PubMed] [Google Scholar]
- 9.Ota H., Yasuma A. Lysozyme activity in hematologic and non-hematologic disorders with special reference to reactive monocytosis associated with chronic infections and inflammatory reactions. Tohoku J Exp Med. 1974;114:15–26. doi: 10.1620/tjem.114.15. [DOI] [PubMed] [Google Scholar]
- 10.Perillie P.E., Khan K., Finch S.C. Serum lysozyme in pulmonary tuberculosis. Am J Med Sci. 1973;265:297–302. doi: 10.1097/00000441-197304000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Peeters T.L., Vantrappen G., Geboes K. Serum lysozyme levels in Crohn’s disease and ulcerative colitis. Gut. 1976;17:300–305. doi: 10.1136/gut.17.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klockars M., Azar H.A., Hermida R., et al. The relationship of lysozyme to the nephropathy in chloroleukemic rats and the effects of lysozyme loading on normal rat kidneys. Cancer Res. 1974;34:47–60. [PubMed] [Google Scholar]
- 13.Prockop D.J., Davidson W.D. A study of urinary and serum lysozyme in patients with renal disease. N Engl J Med. 1964;270:269–274. doi: 10.1056/NEJM196402062700602. [DOI] [PubMed] [Google Scholar]
- 14.Pruzanski W., Platts M.E. Serum and urinary proteins, lysozyme (muramidase), and renal dysfunction in mono and myelomonocytic leukemia. J Clin Invest. 1970;49:1694–1708. doi: 10.1172/JCI106387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osserman EF, Azar HA. Renal tubular lesions secondary to lysozyme in human and rat monocytic leukemia [abstract] Fed Proc. 1969;28:619. [Google Scholar]
- 16.Maraj A., MacEneaney O., Doyle B., Quinn J. Lysozyme-induced nephropathy: a rare manifestation of chronic myelomonocytic leukaemia. Br J Haematol. 2020;189:393. doi: 10.1111/bjh.16455. [DOI] [PubMed] [Google Scholar]
- 17.Mohamadou I., Buob D., Rabant M., et al. The case | Acute kidney injury associated with chronic myelomonocytic leukemia. Kidney Int. 2021;99:495–496. doi: 10.1016/j.kint.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Patel A.B., Miles R.R., Deininger M.W. Lysozyme nephropathy in chronic myelomonocytic leukemia. Clin Case Rep. 2019;7:1263–1264. doi: 10.1002/ccr3.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel T.V., Rennke H.G., Sloan J.M., DeAngelo D.J., Charytan D.M. A forgotten cause of kidney injury in chronic myelomonocytic leukemia. Am J Kidney Dis. 2009;54:159–164. doi: 10.1053/j.ajkd.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santoriello D., Andal L.M., Cox R., D’Agati V.D., Markowitz G.S. Lysozyme-induced nephropathy. Kidney Int Rep. 2017;2:84–88. doi: 10.1016/j.ekir.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cossey L., Larsen C.P. Kidney Week; 2019. Lysozyme-induced AKI: a case series. [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(Suppl):1–138. [Google Scholar]
- 23.Levey A.S., Eckardt K.U., Tsukamoto Y., et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 24.Delgado C., Baweja M., Crews D.C., et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–288.e1. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Roufosse C., Simmonds N., Clahsen-van Groningen M., et al. 2018 Reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertzog A.J. The Paneth cell. Am J Pathol. 1937;13:351–360. [PMC free article] [PubMed] [Google Scholar]
- 27.Said S.M., Leung N., Sethi S., et al. Myeloproliferative neoplasms cause glomerulopathy. Kidney Int. 2011;80:753–759. doi: 10.1038/ki.2011.147. [DOI] [PubMed] [Google Scholar]
- 28.Dickson L.E., Wagner M.C., Sandoval R.M., Molitoris B.A. The proximal tubule and albuminuria: really. J Am Soc Nephrol. 2014;25:443–453. doi: 10.1681/ASN.2013090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caulfield J.P., Farquhar M.G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976;73:1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekulic M., Waikar S., Motwani S.S., Weins A., Rennke H.G. Chromogranin A tubulopathy: differing histopathologic patterns of acute tubular injury in the setting of neuroendocrine neoplasms. Kidney Int Rep. 2019;4:1085–1093. doi: 10.1016/j.ekir.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes M.B., Valeri A.M., Herlitz L., et al. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27:1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen C.P., Bell J.M., Harris A.A., Messias N.C., Wang Y.H., Walker P.D. The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod Pathol. 2011;24:1462–1469. doi: 10.1038/modpathol.2011.104. [DOI] [PubMed] [Google Scholar]
- 33.Nasr S.H., Galgano S.J., Markowitz G.S., Stokes M.B., D’Agati V.D. Immunofluorescence on pronase-digested paraffin sections: a valuable salvage technique for renal biopsies. Kidney Int. 2006;70:2148–2151. doi: 10.1038/sj.ki.5001990. [DOI] [PubMed] [Google Scholar]
- 34.Sirac C., Batuman V., Sanders P.W. The proximal tubule toxicity of immunoglobulin light chains. Kidney Int Rep. 2021;6:1225–1231. doi: 10.1016/j.ekir.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al Adem K., Lukman S., Kim T.Y., Lee S. Inhibition of lysozyme aggregation and cellular toxicity by organic acids at acidic and physiological pH conditions. Int J Biol Macromol. 2020;149:921–930. doi: 10.1016/j.ijbiomac.2020.01.267. [DOI] [PubMed] [Google Scholar]
- 36.Safari M.S., Byington M.C., Conrad J.C., Vekilov P.G. Polymorphism of lysozyme condensates. J Phys Chem B. 2017;121:9091–9101. doi: 10.1021/acs.jpcb.7b05425. [DOI] [PubMed] [Google Scholar]
- 37.Sanada S., Yoda S., Sato T. Pathological value of lysozyme staining for renal sarcoidosis. Nephrol Dial Transplant. 2020;35:1638–1641. doi: 10.1093/ndt/gfaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.