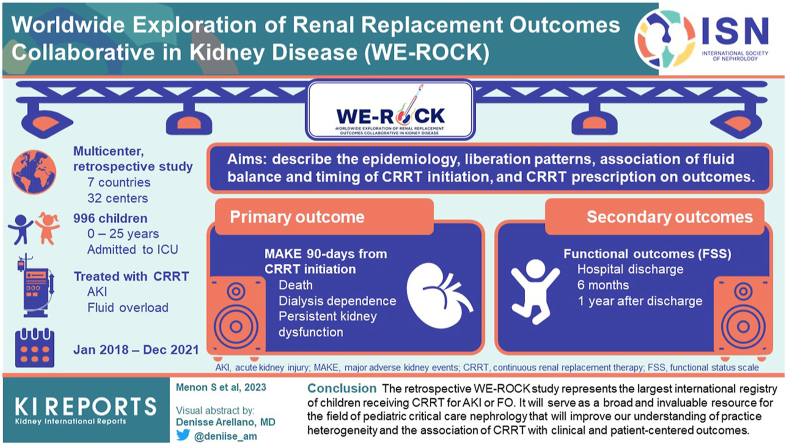
Abstract
Introduction
Continuous renal replacement therapy (CRRT) is used for the symptomatic management of acute kidney injury (AKI) and fluid overload (FO). Contemporary reports on pediatric CRRT are small and single center in design. Large international studies evaluating CRRT practice and outcomes are lacking. Herein, we describe the design of a multinational collaborative.
Methods
The Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) is an international collaborative of pediatric specialists whose mission is to improve short- and long-term outcomes of children treated with CRRT. The aims of this multicenter retrospective study are to describe the epidemiology, liberation patterns, association of fluid balance and timing of CRRT initiation, and CRRT prescription with outcomes.
Results
We included children (n = 996, 0–25 years) admitted to an intensive care unit (ICU) and treated with CRRT for AKI or FO at 32 centers (in 7 countries) from 2018 to 2021. Demographics and clinical characteristics before CRRT initiation, during the first 7 days of both CRRT, and liberation were collected. Outcomes include the following: (i) major adverse kidney events at 90 days (mortality, dialysis dependence, and persistent kidney dysfunction), and (ii) functional outcomes (functional stats scale).
Conclusion
The retrospective WE-ROCK study represents the largest international registry of children receiving CRRT for AKI or FO. It will serve as a broad and invaluable resource for the field of pediatric critical care nephrology that will improve our understanding of practice heterogeneity and the association of CRRT with clinical and patient-centered outcomes. This will generate preliminary data for future interventional trials in this area.
Keywords: acute kidney injury, continuous renal replacement therapy, database, fluid overload, pediatric, WE-ROCK
Graphical abstract
In recent years, our understanding of AKI and the pathologic state of FO in critically ill children and young adults has increased exponentially. Fluid balance (FB) is the difference between total input and output and is often expressed as “daily or cumulative” over a defined duration of time. The 26th Pediatric Acute Disease Quality Initiative defined FO as a pathologic state of FB associated with clinically observable events.1
AKI and pathologic FO have been shown to occur commonly among critically ill children and young adults.2,3 Across 3 international multicenter studies, AKI and the development of FO have been shown to be associated with increased morbidity and mortality.4, 5, 6 As the deleterious impact of AKI and FO has become clear, the field has transitioned toward the prevention, mitigation, and the optimization of interventions to improve outcomes.
Approximately 1% to 5% of critically ill children and young adults with the most severe form of AKI are treated with CRRT. Much of our understanding for prescribing CRRT and the impact on outcomes of critically ill children receiving CRRT are derived from small single center studies and the seminal work of the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) registry. The ppCRRT registry represents the largest registry of pediatric patients treated with CRRT and included 13 centers in the United States.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 This study collected data from 2001 to 2005 and served to set the framework for research and clinical care in pediatric CRRT.7,8,11,13,15, 16, 17, 18 In the nearly 20 years since ppCRRT, there have been significant advances in pediatric and neonatal critical care nephrology technology, practices, education, devices, access, and quality improvement.18,19 A summary of the published CRRT studies that assessed associations with mortality are included in Table 1. To continue to move the field forward, improve practices, and drive research priorities, a contemporary international multicenter cohort evaluating pediatric and neonatal CRRT is greatly needed.
Table 1.
Characteristics and outcomes of single and multicenter CRRT studies in children
| Study | Population | RRT type | Time | Sample size | Incidence of RRT | Survival |
|---|---|---|---|---|---|---|
| Kaddourah et al.4 | Multicenter, prospective N = 73 |
HD, PD CRRT |
3-month period in 2014 | 73 | 1.5% of enrolled ICU patients. 5.8% of patients with AKI |
67% by 28 days after admission |
| Jetton et al.5 | Multicenter, retrospective N = 25 |
HD, PD CRRT |
3-month period in 2014 | 25 | 1.2% of enrolled neonates. 4.1% of neonates with AKI |
76% to hospital discharge |
| Chanchlani et al.a20 | Healthcare administrative database, Ontario N = 375 |
HD, PD CRRT |
2010–2015 | 375 | 0.65 per 1000 person years in 2015 | 81% at 30 days |
| Riley et al.21 | Single center, Texas N = 311 |
CRRT | 2004–2013 | 311 | Not specified | 45% at discharge |
| Al-Ayed et al.22 | Single center, Saudi Arabia N = 96 |
CRRT | 2009–2015 | 96 | 50% at discharge | |
| Holt et al.23 | Single center, Saskatchewan | CRRT | 2007–2020 | 82 | 79% at discharge | |
| Erkol Tuncer et al.24 | Single center, Turkey | CRRT | 2010–2015 | 50 | 42% at discharge | |
| Lee et al.25 | Single center, Korea | CRRT | 2003–2016 | 263 | 30% at discharge | |
| Tain et al.26 | Taiwan’s health care delivery system | HD, PD, CRRT | 2010–2017 | 412 | 6.59% of 23,759 encounters in 412 pediatric patients | 66.4% at discharge |
| Yetimakman et al.27 | Single center, Turkey | CRRT | 2009–2016 | 104 | 51% at discharge |
CRRT, continuous renal replacement therapy; HD, hemodialysis; PD, peritoneal dialysis; RRT, renal replacement therapy.
Chanchlani et al.20 describe dialysis over multiple years. Only the recent cohort from 2010-2015 is reported here.
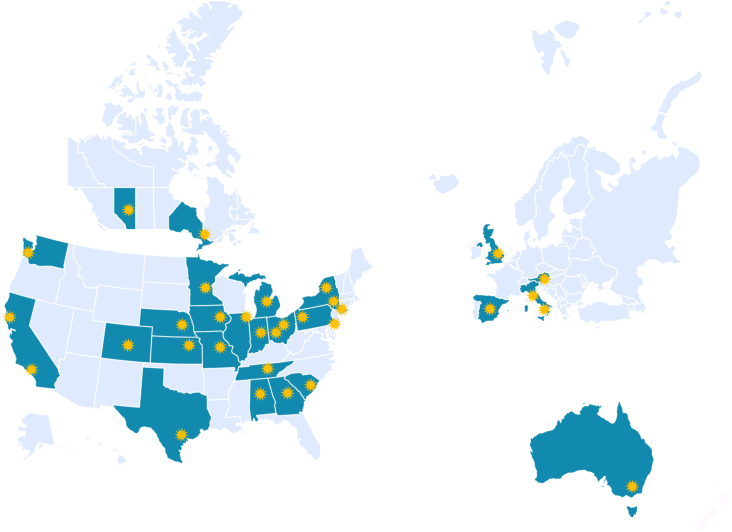
The WE-ROCK investigator group was established in 2020 comprising pediatric intensivists, pediatric cardiac intensivists, advanced practice nurses, and pediatric nephrologists to develop the mission, vision, strategy, and necessary infrastructure for sustained collaboration to drive the field forward. These primary centers, along with several experts in the field, formed the steering committee for what would become a multinational group that expanded to 32 institutions in 7 countries (USA, Canada, United Kingdom, Italy, Spain, Austria, and Australia) (Figure 1). The WE-ROCK study group was developed with the global aims to better understand the epidemiology, practice differences, as well as the short- and long-term clinical and patient-centered outcomes of children receiving CRRT for AKI and FO.
Figure 1.
Map of participating WE-ROCK centers. Symbols denote center. WE-ROCK, Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease.
As the first step to accomplish the global aims, the WE-ROCK investigators collected data from what will be the largest study of children receiving CRRT (n = 996) for AKI or FO. The aims of the initial study from the WE-ROCK investigator group include the following: (i) describe the demographics and predictors of successful CRRT liberation, (ii) understand the current CRRT techniques and practices, (iii) evaluate the association of the timing of CRRT and pathologic FO at initiation with outcomes, and (iv) assess the patient-centered outcomes using the functional status scale (FSS).28 The results of these primary studies will enhance our understanding of CRRT practice to facilitate future large multicenter prospective studies and standardize the delivery of care in this population at high risk for morbidity and mortality. Here, we describe the methods, infrastructure, and the formation of the initial studies from the WE-ROCK investigators, the WE-ROCK registry.
Methods
Design
The WE-ROCK registry is a retrospective multicenter study of pediatric patients receiving CRRT for AKI and FO who were admitted to the pediatric, neonatal, or cardiac ICU from January 1, 2018, to December 31, 2021. Data were entered into the registry from January to August 2022.
Setting
Thirty-two institutions in 7 countries (United States of America, Canada, United Kingdom, Italy, Spain, Austria, and Australia) across 4 continents contributed data to the registry (Figure 1). Site principal investigators are listed in Supplementary Table S1 and represent pediatric nephrologists, intensivists, and advanced practice providers. A summary of the total hospital and ICU bed capacity, excluding the neonatal ICU are also summarized in Supplementary Table S1. Centers were classified into small (≤30 ICU beds), medium (>30–60 ICU beds) and large (>60 ICU beds) (Supplementary Figure S1).
Population
Eligible participants who fulfill all inclusion criteria and meet no exclusion criteria.
Inclusion Criteria
The following inclusion criteria were established to capture as many potential study patients as possible who received CRRT:
-
•
Birth to <25 years of age
-
•
Primary CRRT indication of AKI or FO
Exclusion Criteria
The exclusion criteria are the following:
-
•
Patients with history of end stage kidney disease defined as dialysis dependence before admission.
-
•
Infants with a known diagnosis of severe congenital anomalies of the kidney and urinary tract resulting in end stage kidney disease.
-
•
Patients receiving CRRT for a non-AKI/FO indication even if it developed during the course of treatment (i.e., ingestion, inborn errors of metabolism, and hyperammonemia).
-
•
Patients on extracorporeal membrane oxygenation concurrently with CRRT or within the 7 days before CRRT initiation
-
•
Patients without end stage kidney disease who received peritoneal dialysis (PD) in the same ICU admission before CRRT initiation (i.e., patients in the cardiac ICU who received PD postsurgically)
-
•
Children receiving CRRT with the CARPEDIEM device (Medtronic Inc) because of an existing registry focusing solely on this device.
Variable Collection
A detailed summary of collected variables is included in Supplementary Table S2. A manual of procedures was created and disseminated to all participating sites. Education on data collection was provided during monthly collaboration calls and members of the steering committee and data analysts at Cincinnati Children’s Hospital Medical Center (CCHMC) were available ad hoc for additional queries. Demographic data, including sex, self-reported ethnicity, race, date of birth, date of ICU admission, and date of CRRT initiation were collected for all patients. Race is being included as a variable because of the potential for differences in outcomes.
Baseline Characteristics
Data at ICU admission included the admitting diagnosis, comorbidities, sepsis, Pediatric Risk of Mortality III (PRISM-III) score,29 height, preillness or dry weight (if available), admission weight, baseline and admission serum creatinine (SCr). Baseline SCr was defined as the lowest SCr (mg/dl) within 90 days before admission. If the baseline was not known or not available, it was back-calculated using the bedside CKiD equation30 using an estimated glomerular filtration rate of 100 ml/min per 1.73 m2 as has been previously described and validated.31
CRRT Initiation
Data at CRRT initiation included SCr and urinary neutrophil gelatinase associated lipocalin immediately before initiation, presence of sepsis, vasoactive-inotrope score,32 Pediatric Logistic Organ Dysfunction 2 (PELOD-2) score33 in the 24 hours prior, FB, and loop diuretic challenge. Hourly urine output was recorded for the 6 hours before, and 6 hours following administration of a loop diuretic. Data on FB were used to calculate percent FO from ICU admission to CRRT initiation, and from dry weight to CRRT initiation using the following equations as previously described.10
Daily CRRT Data
Data were collected from day 0 (defined as the time from CRRT initiation until the start of the next morning shift) until the first CRRT liberation attempt (if before day 7), day 7, or death, whichever came first. A shift was defined as the time at which nursing staff change over. Daily data included weight, FB, urine output, urine neutrophil gelatinase associated lipocalin, SCr, highest serum glucose, phosphorus, and platelet count. Details of CRRT prescription, including device, modality, filter, dose, CRRT fluid type, and anticoagulation were also collected.
CRRT Liberation Data
If CRRT liberation was attempted at any time during CRRT days 0 to 28, additional information was collected on the first liberation attempt only. Successful CRRT liberation was defined as ≥72 hours off renal support therapy, without need for reinitiation of CRRT, hemodialysis, or PD.34 In the event of a successful liberation, daily weight, net FB, urine output, urine neutrophil gelatinase associated lipocalin, SCr, and serum cystatin C were recorded for up to 7 days post CRRT liberation. No liberation data was collected for those who were not successfully liberated at the first attempt. Only data about the first liberation attempt was captured to identify critical metrics that would be used to identify risk factors for failure of the first attempt.
Outcomes Data
Specific variables included total ventilation time, length of stay (ICU and hospital), mortality, dialysis dependence, and SCr for those not dependent on dialysis at hospital discharge, 28 days and 90 days after CRRT initiation. Components of the FSS were obtained at 6 and 12 months after discharge, using any outpatient visits notes that included physicians, nurses, physical therapists, and occupational therapists.
Interventions
No intervention was performed.
Ethics and Dissemination
WE-ROCK was proposed as human subject research. Therefore, each participating site sought ethics or institutional review board approval with a waiver of informed consent or parental permission. Data use agreements were signed between each site and the host site (CCHMC). WE-ROCK investigators will disseminate data through peer reviewed publications and presentations at educational conferences.
Research Electronic Data Capture
Data entry of the variables of interest was performed by participating sites using a web-based database, Research Electronic Data Capture (REDCap)35 hosted by CCHMC. REDCap is a mature, secure web application for building and managing online surveys and databases. Through this system, each participating site was assigned a unique code by the project managers. For sites that were allowed to enter limited protected health information (date of birth and date of admission) by their institutional review board and data use agreement, age and durations were automatically calculated. Sites which were not allowed to enter dates by their data use agreement, entered the ages and durations manually using a separate calculator that was provided to them. All participating sites used the same case report forms. The electronic case report forms were designed by the steering committee. Research personnel from each site are able to access the data from their site for the purposes of using it to generate quality metrics and create dashboards for improvement. Project managers from the Heart Institute Research Core and Center for Acute Care Nephrology at CCHMC could access data from other sites and were responsible for data management, including corresponding with site principal investigators for data queries and communication of missing data elements. The data integrity and cleaning process occurred from September 1 to January 31, 2023. This was performed in an iterative process by creating rules within REDCap which were sent to site investigators for review and completion. All statistical analyses will be conducted at CCHMC.
Primary and Secondary Outcomes
Our primary outcome was major adverse kidney events at 90 days (MAKE-90) from CRRT initiation defined as a composite of death, dialysis dependence, or persistent kidney dysfunction (a >25% decline in estimated glomerular filtration rate).36 The rationale for MAKE-90 is on the basis of the reported estimated median length of stay (42 days, interquartile range 28–71) for children who receive CRRT.21 Secondary outcomes included successful liberation, ICU mortality, hospital mortality, MAKE at 30 days, length of stay, duration of mechanical ventilation, and functional outcomes using the FSS28 at hospital discharge, 6 months and 1 year after discharge (survivors only). MAKE at 30 days has not been rigorously evaluated in children. This large, multicenter collaborative will allow us to evaluate whether MAKE at 30 days is a valid outcome metric in children receiving CRRT.
Analysis
Analysis of data will be conducted independently based on each specific aim using SAS software v9.4 (SAS Institute, Cary, NC, USA). Patients missing data for primary and secondary outcomes will not be included. Multiple imputation will be considered for predictor variables and will be handled separately for each aim as follows:
-
1.
The first aim is to describe liberation patterns among children receiving CRRT and the association with MAKE-90 outcomes. Patients will be divided into the following groups: liberation never attempted, reinstituted (liberation not successful), and liberation successful. We will describe the predictors of liberation success, as well as the risk factors associated with MAKE-90. Liberation success was defined as not needing any dialytic therapy (CRRT, PD, or hemodialysis) within 72 hours of a liberation attempt. We hypothesized as follows: (i) that there would be specific factors associated with liberation success, including only patients who had a trial of liberation; and (ii) that patients with successful liberation would have improved MAKE-90 outcomes compared to those who never had liberation attempted or who failed a liberation attempt. Multivariable logistic regression will be used to assess the factors associated with successful liberation. The model will account for clustering within hospitals and include confounders based on clinical knowledge. Some of the confounders to be included are illness severity, time to CRRT initiation from ICU admission, duration of CRRT to first liberation attempt. A second multivariable logistic regression will be used to assess the association between liberation patterns and MAKE-90. The primary predictors are the liberation patterns (liberated, reinstituted, and never attempted), with the outcome of MAKE-90.
-
2.
The second aim is to describe the patient population and technical aspects of CRRT with regard to modality, filter type, prescription, catheter size and location, and anticoagulation to gain insight into which methods may lead to better outcomes and generate hypotheses for clinical trials in pediatric CRRT. These data will be presented descriptively with consideration of stratification by center-level characteristics.
-
3.
The third aim is to evaluate association of the timing to initiation of CRRT and FO with MAKE-90 outcomes. Time to CRRT start will be assessed as a continuous and categorical variable with early CRRT initiation defined as within ≤48 hours from ICU admission, and late CRRT initiation defined as >48 hours from ICU admission. This time frame was selected on the basis of the work by Buccione and colleagues, where the median time to CRRT start was 40 hours.37 We hypothesized that longer time to CRRT initiation from ICU admission would be associated with worse MAKE-90 outcomes. Multivariable regression will be used to assess the association between time to CRRT initiation measured in days and MAKE-90. Confounding variables will be selected based on known clinical knowledge. The model will also account for clustering between centers. Secondary outcomes will include ventilation duration and ICU length of stay. Regression models will also be used to determine the association between time to CRRT start and duration of ventilation. We will also perform a propensity score analysis and integrate it into the multivariable model.
-
4.
The fourth aim is to evaluate a patient-centered outcome using the FSS.28 The FSS evaluates patient-centered outcomes across 6 domains, namely mental status, sensory, communication, motor function, feeding, and respiratory function. This tool describes functional outcomes in a reliable, reproducible well-defined way for a wide range of pediatric patients and varied inpatient environments.28 We will evaluate the change in FSS from premorbid baseline at ICU discharge and at 6 months and 1 year after ICU discharge using outpatient notes as described above among survivors only. We hypothesize that survivors will have worse functional status than their premorbid baseline at all follow-up time points and there will be specific factors associated with development of a new morbidity. A new morbidity will be defined as an increase in FSS score of ≥3. Multivariable regression will be used to assess these associations.
We will also separately evaluate a subgroup of patients who weigh <10 kg in which we will describe the associations between CRRT dosing and outcomes, and evaluate the relationship between body surface area-based CRRT dosing and weight-based dosing. Other subanalyses will be performed based on the submission, review, and approval of ancillary proposals by the ancillary subcommittee (discussed below). Analytical plans for each ancillary proposal will be developed as it pertains to the research question.
Oversight
A 12-member steering committee comprising physicians (intensivists, cardiac intensivists, and nephrologists), an advance practice provider, and a nurse was established for study oversight and to develop subcommittees. Each of the subcommittees is chaired by a member of the steering committee and the cochair is a member at large. The subcommittees are each made up of 8 to 10 additional members on a volunteer basis who will serve a term of up to 2 years. The subcommittees include protocol development, protocol implementation, ancillary proposal, and manuscript. Ancillary proposal submission opportunities will be available twice yearly and submitted using a protocol and analysis plan template through REDCap and will be reviewed and scored using a rigorous committee driven peer review process based on science and feasibility. All abstracts and manuscripts will undergo deidentified rigorous review by the manuscript committee before submission. The protocol development and implementation committees will review new proposals that would involve new data collection, and this is discussed in more detail in the discussion section.
Discussion
The WE-ROCK investigator group has created the largest international multicenter registry of children treated with CRRT. The WE-ROCK study and resulting registry includes 996 children from 32 centers in 7 countries across 4 continents. The registry includes a diverse mix of centers from across the globe with a broad range of patient populations, representing the largest, most inclusive international pediatric CRRT study to date. This WE-ROCK investigator group and the initial registry will provide invaluable data to improve our understanding of pediatric CRRT practices and outcomes around the world.
Contemporary studies evaluating current CRRT practices and outcomes are greatly needed because current CRRT practice in critically ill children are derived from the seminal work by the ppCRRT registry, which was performed over almost 2 decades ago. Since that time there have been small single center studies that have added important data to the literature, which have also highlighted an increasing heterogeneity in CRRT practice. A recent survey disseminated to WE-ROCK participants using a modified Delphi approach highlighted this significant practice heterogeneity among WE-ROCK study centers,38 which is consistent with findings from a recent survey of European39 and Japanese centers.40 Understanding how these variations in practice associate with outcomes in critically ill children is essential for advancing our knowledge, determining best practice, and improving outcomes of children as was highlighted in a recent commentary on standardizing care in pediatric CRRT.41 The 26th Acute Disease Quality Initiative recently published recommendations and highlighted the need for the development and execution of updated studies in pediatric cohorts investigating AKI outcomes, CRRT practices, and outcomes in children treated with CRRT.1 The WE-ROCK study group is poised through the foundational work described in the design of this international multicenter retrospective study to fill this important void in the literature.
There are several strengths of this registry. This robust international research database captures 325 unique variables beginning with patient demographics, granular data for the first 7 days of both CRRT and the first liberation attempt as well as outcomes. We have captured CRRT practices from 7 countries with plans to expand to several more interested groups that are onboarding, including countries that are not yet represented. With the potential for improved survival, we are evaluating novel patient-centered outcomes using the FSS in children. One of the most important goals and strengths of the WE-ROCK study group is to create a collaborative that would be capable of growing and evolving to include new centers and answer new questions brought forth. As an example, institutional review board and data use agreements are pending from additional regional and international centers. We are actively identifying ways to expand to centers in Asia, Central and South America, Africa, and the Middle East. The repository of data collected will inform critical care nephrologists for many years, and allow for analysis of many epidemiologic associations through subsequent secondary studies. Finally, we have built a robust infrastructure founded on the principles of inclusivity, diversity, and collaboration. Our founding principles will easily extend to collaboration with other existing multicenter and multinational groups, including those which have focused on other acute kidney injury support therapies such as PD, prolonged intermittent renal replacement therapy, and hemodialysis. Indeed, this will allow us to enhance global knowledge together, inclusive of resource limited environments. WE-ROCK has been designed to foster collaboration through participant engagement on subcommittees for the purposes of improvement of care, multidisciplinary research, career development, and academic advancement. We are actively working on expanding the repository to encompass children supported by CRRT for other indications, such as acute liver failure, or with other circulatory therapies such as extracorporeal membrane oxygenation. The results from the aims described will serve as foundational preliminary data for future prospective studies including participating centers that encompass novel patient-centered outcomes. Furthermore, we have already begun to identify ways to develop near real time quality improvement dashboards for which centers will be able to benchmark their own data using the collaborative data. Important demographic predictors of outcomes, such as sex and social determinants of health will also be the subject of future prospective work to delineate why these differences exist.
The WE-ROCK registry has several limitations. All sites are tertiary or quaternary care centers in high resource countries. Translation of the findings of the proposed analyses will be limited to centers with similar practice models and resources. Centers from low-income and middle-income countries often utilize other methods for acute management of AKI and FO. We did not collect data for other modalities of renal support therapy to limit heterogeneity and capture a robust population of a single dialysis modality. As described above, this limitation can be overcome by our founding principles of collaboration with other groups. The retrospective study design results in missing data points, particularly around details of hourly urine output quantification and response to diuretic challenges, as well as results of specific laboratory tests that may not be considered standard of care, or available at all included centers. It is also possible that we will encounter missing data around MAKE-90 outcomes and FSS reporting at 6 and 12 months post hospitalization. After the database was closed, there were 996 patients in the registry. During the data cleaning process, 16 were identified as being dialysis-dependent or having received CRRT before the start of the study period, thereby leaving 980 patients. MAKE-90 outcome data were not available for 11 patients, thereby leaving 969 (98.8% of the entire cohort) for evaluation of MAKE-90. In subsequent analyses, if MAKE-90 is missing in a large proportion of patients, we will perform a sensitivity analysis to evaluate for differences between groups to limit bias. We will only be able to establish associations, not causality. Because we are only including patients who receive CRRT, we will not be able to identify risk factors for needing CRRT. We are only capturing the first liberation attempt and if it was successful, potentially missing out on other factors that may contribute to recovery and/or survival.
Conclusion
The WE-ROCK investigator group has created a diverse, multidisciplinary research collaborative that will provide an invaluable resource to study CRRT practices and outcomes in a broad cohort of critically ill children. In this initial study performed by the WE-ROCK study group, we will aim to better understand the predictors of successful CRRT liberation, current CRRT techniques and practices, the association of CRRT timing and FB with outcomes; and assess patient-centered outcomes in children treated with CRRT. Most importantly, the WE-ROCK study group has created a robust enduring infrastructure, capable of answering the current questions and performing future prospective interventional and observational studies that will serve to be a driving force to improve outcomes in children treated with CRRT.
Disclosure
All authors declared no competing interests that could affect the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of other authors’ commitments and funding sources that are not directly related to this study:REDCap at Cincinnati Children’s Hospital Medical Center is funded and supported by the Center for Clinical and Translational Science and Training grant support (UL1TR001425). KG is a consultant for Bioporto Diagnostics and Potrero Medical. SM is a consultant for Medtronic, Inc and Nuwellis, Inc. KG and SM receive funding from the Gerber foundation to study CRRT practices and nutritional outcomes in infants <10 kg. TAM is a consultant for Medtronic Inc. MM-L is a consultant for Medtronic Inc. MZ has completed consultant work for Bioporto Diagnostics Inc.
Acknowledgments
We are grateful to the following: T. Christine E. Alvarez MHI RN1, Elizabeth Bixler BS2, Erica Blender Brown MA, CRA3, Cheryl L Brown BS1, Ambra Burrell BA4, Jennifer L Ehrlich RN MHA5, Simrandeep Farma HBSc6, Kim Gahring RN BSN, CCRN7, Barbara Gales RN rn8, Madison R Hilgenkamp9, Sonal Jain MS10, Kate Kanwar BA MS4, Jennifer Lusk BSN RN, CCRN7, Christopher J. Meyer BA AA1, Katherine Plomaritas BSN RN11, Jessica Potts BSN RN12, Alyssa Serratore BNurs, GDipNP(PIC), RN, MsC13, PJ Strack RN,BSN,CCRN14, Sue Taylor RN15, Katherine Twombley MD3, Brynna Van Wyk MSN, ARNP CPNP5, Samantha Wallace MS16, Marcia Zinger RN17
-
1
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
-
2
Baylor College of Medicine, Texas Children's Hospital, Houston, TX, USA
-
3
Medical University of South Carolina, Charleston, SC, USA
-
4
Nationwide Children's Hospital, Columbus, OH, USA
-
5
University of Iowa Stead Family Children's Hospital, Carver College of Medicine, Iowa City, IA, USA
-
6
Hospital for Sick Children, Toronto, ON, Canada
-
7
Children's Hospital Colorado, Aurora, CO, USA
-
8
Mattel Children Hospital at UCLA, Los Angeles, CA, USA
-
9
University of Nebraska Medical Center, Children's Hospital & Medical Center, Omaha, NE, USA
-
10
Seattle Children's Hospital, Seattle, WA, USA
-
11
University of Michigan, C.S. Mott Children's Hospital, Ann Arbor, MI, USA
-
12
Children's of Alabama/University of Alabama at Birmingham, Birmingham, AL, USA
-
13
Royal Children's Hospital, Melbourne, VIC, Australia
-
14
Children's Mercy Hospital, Kansas City, MO, USA
-
15
King's college hospital, London, England
-
16
Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN, USA
-
17
Cohen Children's Medical Center, New Hyde Park, NY, USA
Funding
Cincinnati Children's Hospital heart Institute - date warehourse, cleaning and statistical analysis. This study was funded in part by the Gerber foundation (KG and SM). The funding sources for this study had no role in the design, conduct, collection, management, analysis, and interpretation of the data, nor the preparation, review, or decision to submit the manuscript for publication.
Footnotes
Figure S1. Histogram summarizing the number of centers by size according to intensive care unit bed capacity. Hospitals were classified by the total intensive care unit bed capacity inclusive of the cardiac intensive care unit and excluding the neonatal intensive care unit. Small is ≤30 beds, medium is >30 to 60 beds and large is >60 beds.
Table S1. Site, city, country, principal investigator of WE-ROCK registry, total hospital bed capacity and intensive care unit bed capacity.
Table S2. Summary of variables collected. This table summarizes each of the variables collected by category, including baseline and demographics, pre-CRRT initiation, Daily CRRT data for 7 days, additional CRRT information (liberation), daily liberation form for 7 days and outcomes.
Contributor Information
Katja M. Gist, Email: katja.gist@cchmc.org.
WE-ROCK Investigators:
Emily Ahern, Ayse Akcan Arikan, Issa Alhamoud, Rashid Alobaidi, Pilar Anton-Martin, Shanthi S. Balani, Matthew Barhight, Abby Basalely, Amee M. Bigelow, Gabriella Bottari, Andrea Cappoli, Eileen A. Ciccia, Michaela Collins, Denise Colosimo, Gerard Cortina, Mihaela A. Damian, Sara De la Mata Navazo, Gabrielle DeAbreu, Akash Deep, Kathy L. Ding, Kristin J. Dolan, Sarah N. Fernandez Lafever, Dana Y. Fuhrman, Ben Gelbart, Katja M. Gist, Stephen M. Gorga, Francesco Guzzi, Isabella Guzzo, Taiki Haga, Elizabeth Harvey, Denise C. Hasson, Taylor Hill-Horowitz, Haleigh Inthavong, Catherine Joseph, Ahmad Kaddourah, Aadil Kakajiwala, Aaron D. Kessel, Sarah Korn, Kelli A. Krallman, David M. Kwiatkowski, Jasmine Lee, Laurance Lequier, Tina Madani Kia, Kenneth E. Mah, Eleonora Marinari, Susan D. Martin, Shina Menon, Tahagod H. Mohamed, Catherine Morgan, Theresa A. Mottes, Melissa A. Muff-Luett, Siva Namachivayam, Tara M. Neumayr, Jennifer Nhan Md, Abigail O'Rourke, Nicholas J. Ollberding, Matthew G. Pinto, Dua Qutob, Valeria Raggi, Stephanie Reynaud, Zaccaria Ricci, Zachary A. Rumlow, María J. Santiago Lozano, Emily See, David T. Selewski, Carmela Serpe, Alyssa Serratore, Ananya Shah, Weiwen V. Shih, H Stella Shin, Cara L. Slagle, Sonia Solomon, Danielle E. Soranno, Rachana Srivastava, Natalja L. Stanski, Michelle C. Starr, Erin K. Stenson, Amy E. Strong, Susan A. Taylor, Sameer V. Thadani, Amanda M. Uber, Brynna Van Wyk, Tennille N. Webb, Huaiyu Zang, Emily E. Zangla, and Michael Zappitelli
Appendix
Members of the WE-ROCK CollaborativeInvestigators
The following individuals served as collaborators and investigators for the WE-ROCK studies. They collaborated in protocol development and review, data analysis, and participated in drafting or review of the manuscript, and their names should be citable by PubMed.
Emily Ahern CPNP, DNP1, Ayse Akcan Arikan MD2, Issa Alhamoud MD3, Rashid Alobaidi MD, MSc4, Pilar Anton-Martin MD, PhD5, Shanthi S Balani MD6, Matthew Barhight MD, MS7, Abby Basalely MD, MS8, Amee M Bigelow MD, MS9, Gabriella Bottari MD10, Andrea Cappoli MD10, Eileen A Ciccia MD11, Michaela Collins BA12, Denise Colosimo MD13, Gerard Cortina MD14, Mihaela A Damian MD, MPH15, Sara De la Mata Navazo MD16, Gabrielle DeAbreu MD8, Akash Deep MD17, Kathy L Ding BS18, Kristin J Dolan MD2, Sarah N Fernandez Lafever MD, PhD16, Dana Y Fuhrman DO, MS19, Ben Gelbart MBBS20, Katja M Gist , DO MSc12, Stephen M Gorga MD, MSc21, Francesco Guzzi MD22, Isabella Guzzo MD10, Taiki Haga MD23, Elizabeth Harvey MD24, Denise C Hasson MD25, Taylor Hill-Horowitz BS8, Haleigh Inthavong BS, MS2, Catherine Joseph MD2, Ahmad Kaddourah MD, MS26, Aadil Kakajiwala MD, MSCI27, Aaron D Kessel MD, MS8, Sarah Korn DO28, Kelli A Krallman BSN, MS12, David M Kwiatkowski MD Msc29, Jasmine Lee MSc24, Laurance Lequier MD4, Tina Madani Kia BS4, Kenneth E Mah MD, MS15, Eleonora Marinari MD10, Susan D Martin MD30, Shina Menon MD31, Tahagod H Mohamed MD9, Catherine Morgan MD MSc4, Theresa A Mottes APRN7, Melissa A Muff-Luett MD32, Siva Namachivayam MBBS20, Tara M Neumayr MD11, Jennifer Nhan Md, MS27, Abigail O'Rourke MD8, Nicholas J Ollberding PhD12, Matthew G Pinto MD33, Dua Qutob MD26, Valeria Raggi MD10, Stephanie Reynaud MD34, Zaccaria Ricci MD13, Zachary A Rumlow DO3, María J Santiago Lozano MD, PhD16, Emily See MBBS20, David T Selewski MD, MSCR35, Carmela Serpe MSc, PhD10, Alyssa Serratore RN, MsC20, Ananya Shah BS18, Weiwen V Shih MD1,18, H Stella Shin MD36, Cara L Slagle MD12, Sonia Solomon DO33, Danielle E Soranno MD37, Rachana Srivastava MD38, Natalja L Stanski MD12, Michelle C Starr MD, MPH37, Erin K Stenson MD1,18, Amy E Strong MD, MSCE3, Susan A Taylor MSc17, Sameer V Thadani MD2, Amanda M Uber DO32, Brynna Van Wyk ARNP, MSN3, Tennille N Webb MD, MSPH39, Huaiyu Zang PhD12, Emily E Zangla DO6, Michael Zappitelli MD, MSc24
-
1
Children's Hospital Colorado, University of Colorado School of Medicine, Aurora, CO, USA
-
2
Baylor College of Medicine, Texas Children's Hospital, Houston, TX, USA
-
3
University of Iowa Stead Family Children's Hospital, Carver College of Medicine, Iowa City, IA, USA
-
4
Univeristy of Alberta, Edmonton, Canada
-
5
Le Bonheur Children's Hospital, Memphis, TN, USA
-
6
University of Minnesota, Minneapolis, MN, USA
-
7
Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, USA
-
8
Cohen Children's Medical Center, Zucker School of Medicine, New Hyde Park, NY, USA
-
9
Nationwide Children's Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
-
10
Bambino Gesù Children Hospital, IRCCS, Rome, Italy
-
11
Washington University School of Medicine, St. Louis Children's Hospital, St. Louis, MO, USA
-
12
Cincinnati Children's Hospital Medical Center; University of Cincinnati College of Medicine, Cincinnati, OH, USA
-
13
Meyer Children's Hospital, IRCCS, Florence, Italy
-
14
Medical University of Innsbruck, Innsbruck, Austria
-
15
Stanford University School of Medicine, Palo Alto, CA, USA
-
16
Gregorio Marañón University Hospital; School of Medicine, Madrid, Spain
-
17
King's College Hospital, London, England
-
18
University of Colorado, School of Medicine, Aurora, CO, USA
-
19
University of Pittsburgh Medical Center Children's Hospital of Pittsburgh, Pittsburgh, PA, USA
-
20
Royal Children's Hospital, University of Melbourne, Murdoch Children's Research Institute, Melbourne, Victoria, Australia
-
21
University of Michigan Medical School, C.S. Mott Children's Hospital, Ann Arbor, MI, USA
-
22
Santo Stefano Hospital, Prato, Italy
-
23
Osaka City General Hospital, Osaka, Japan
-
24
Hospital for Sick Children, Toronto, Ontario, Canada
-
25
NYU Langone Health, Hassenfeld Children’s Hospital, New York, NY, USA
-
26
Sidra Medicine and Weil Cornel Medicine, Qatar, Doha, Qatar
-
27
Children's National Hospital, Washington, DC, USA
-
28
Westchester Medical Center, Westchester, NY, USA
-
29
Lucile Packard Children's Hospital, Palo Alto, CA, USA
-
30
Golisano Children's Hospital at University of Rochester Medical Center, Rochester, NY, USA
-
31
Seattle Children's Hospital, University of Washington, Seattle, WA, USA
-
32
University of Nebraska Medical Center, Children's Hospital & Medical Center, Omaha, NE, USA
-
33
Maria Fareri Children's Hospital at Westchester Medical Center, Valhalla, NY, USA
-
34
Hopital Bicetre, APHP Université Paris-Saclay, Kremlin-Bicetre, Val de Marne, France
-
35
Medical University of South Carolina, Charleston, SC, USA
-
36
Children's Healthcare of Atlanta, Emory University, Atlanta, GA, USA
-
37
Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN, USA
-
38
Mattel Children's Hospital at UCLA, Los Angeles, Ca, USA
-
39
Children's of Alabama/University of Alabama at Birmingham, Birmingham, AL, USA
Supplementary Material
Figure S1. Histogram summarizing the number of centers by size according to intensive care unit bed capacity. Hospitals were classified by the total intensive care unit bed capacity inclusive of the cardiac intensive care unit and excluding the neonatal intensive care unit. Small is ≤30 beds, medium is >30 to 60 beds and large is > 60 beds.
Table S1. Site, city, country, principal investigator of WE-ROCK registry, total hospital bed capacity and intensive care unit bed capacity.
Table S2. Summary of variables collected. This table summarizes each of the variables collected by category, including baseline and demographics, pre-CRRT initiation, Daily CRRT data for 7 days, additional CRRT information (liberation), daily liberation form for 7 days and outcomes.
References
- 1.Goldstein S.L., Akcan-Arikan A., Alobaidi R., et al. Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.29442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alobaidi R., Morgan C., Basu R.K., et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:257–268. doi: 10.1001/jamapediatrics.2017.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messmer A.S., Zingg C., Muller M., Gerber J.L., Schefold J.C., Pfortmueller C.A. Fluid overload and mortality in adult critical care patients-a systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48:1862–1870. doi: 10.1097/CCM.0000000000004617. [DOI] [PubMed] [Google Scholar]
- 4.Kaddourah A., Basu R.K., Bagshaw S.M., Goldstein S.L., Investigators A. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jetton J.G., Boohaker L.J., Sethi S.K., et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–194. doi: 10.1016/S2352-4642(17)30069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alten J.A., Cooper D.S., Blinder J.J., et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: A report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med. 2021;49:e941–e951. doi: 10.1097/CCM.0000000000005165. [DOI] [PubMed] [Google Scholar]
- 7.Askenazi D.J., Goldstein S.L., Koralkar R., et al. Continuous renal replacement therapy for children ≤10 kg: a report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr. 2013;162:587–592.e3. doi: 10.1016/j.jpeds.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores F.X., Brophy P.D., Symons J.M., et al. Continuous renal replacement therapy (CRRT) after stem cell transplantation. A report from the prospective pediatric CRRT Registry Group. Pediatr Nephrol. 2008;23:625–630. doi: 10.1007/s00467-007-0672-2. [DOI] [PubMed] [Google Scholar]
- 9.Foland J.A., Fortenberry J.D., Warshaw B.L., et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein S.L., Currier H., Graf C., Cosio C.C., Brewer E.D., Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 11.Hackbarth R., Bunchman T.E., Chua A.N., et al. The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs. 2007;30:1116–1121. doi: 10.1177/039139880703001212. [DOI] [PubMed] [Google Scholar]
- 12.Lombel R.M., Kommareddi M., Mottes T., et al. Implications of different fluid overload definitions in pediatric stem cell transplant patients requiring continuous renal replacement therapy. Intensive Care Med. 2012;38:663–669. doi: 10.1007/s00134-012-2503-6. [DOI] [PubMed] [Google Scholar]
- 13.Selewski D.T., Cornell T.T., Blatt N.B., et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40:2694–2699. doi: 10.1097/CCM.0b013e318258ff01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selewski D.T., Cornell T.T., Lombel R.M., et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37:1166–1173. doi: 10.1007/s00134-011-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland S.M., Goldstein S.L., Alexander S.R. The prospective pediatric continuous renal replacement therapy (ppCRRT) registry: a critical appraisal. Pediatr Nephrol. 2014;29:2069–2076. doi: 10.1007/s00467-013-2594-5. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland S.M., Zappitelli M., Alexander S.R., et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Symons J.M., Chua A.N., Somers M.J., et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. 2007;2:732–738. doi: 10.2215/CJN.03200906. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein S.L., Vidal E., Ricci Z., et al. Survival of infants treated with CKRT: comparing adapted adult platforms with the carpe diem. Pediatr Nephrol. 2022;37:667–675. doi: 10.1007/s00467-021-05180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon S., Broderick J., Munshi R., et al. Kidney support in children using an ultrafiltration device: A multicenter, retrospective study. Clin J Am Soc Nephrol. 2019;14:1432–1440. doi: 10.2215/CJN.03240319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanchlani R., Nash D.M., McArthur E., et al. Secular trends in incidence, modality and mortality with dialysis receiving AKI in children in Ontario: a population-based cohort study. Clin J Am Soc Nephrol. 2019;14:1288–1296. doi: 10.2215/CJN.08250718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley A.A., Watson M., Smith C., et al. Pediatric continuous renal replacement therapy: have practice changes changed outcomes? A large single-center ten-year retrospective evaluation. BMC Nephrol. 2018;19:268. doi: 10.1186/s12882-018-1068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Ayed T., Rahman N.U., Alturki A., Aljofan F. Outcome of continuous renal replacement therapy in critically ill children: a retrospective cohort study. Ann Saudi Med. 2018;38:260–268. doi: 10.5144/0256-4947.2018.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt T., Griffin O., Cyr A., Brockman R., Wihak L., Hansen G. Lessons learned from a small pediatric continuous renal replacement therapy program. Crit Care Res Pract. 2021;2021 doi: 10.1155/2021/6481559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erkol Tuncer G.H., Ekim M., Okulu E., Atasay B., Kendirli T. Continuous renal replacement therapy in critically ill children: single-center experience. Turk J Med Sci. 2021;51:188–194. doi: 10.3906/sag-2006-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K.H., Sol I.S., Park J.T., et al. Continuous renal replacement therapy (CRRT) in children and the specialized CRRT team: a 14-year single-center study. J Clin Med. 2019;9:110. doi: 10.3390/jcm9010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tain Y.L., Kuo H.C., Hsu C.N. Changing trends in dialysis modalities utilization and mortality in children, adolescents and young adults with acute kidney injury, 2010–2017. Sci Rep. 2021;11 doi: 10.1038/s41598-021-91171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yetimakman A.F., Kesici S., Tanyildiz M., Bayrakci U.S., Bayrakci B. A report of 7-year experience on pediatric continuous renal replacement therapy. J Intensive Care Med. 2019;34:985–989. doi: 10.1177/0885066617724339. [DOI] [PubMed] [Google Scholar]
- 28.Pollack M.M., Holubkov R., Glass P., et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124:e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollack M.M., Patel K.M., Ruttimann U.E. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz G.J., Munoz A., Schneider M.F., et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zappitelli M., Parikh C.R., Akcan-Arikan A., Washburn K.K., Moffett B.S., Goldstein S.L. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaies M.G., Gurney J.G., Yen A.H., et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 33.Leteurtre S., Duhamel A., Salleron J., et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41:1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 34.Liu C., Peng Z., Dong Y., et al. Predicting successful continuous renal replacement therapy liberation in critically ill patients with acute kidney injury. J Crit Care. 2021;66:6–13. doi: 10.1016/j.jcrc.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billings F.T., Shaw A.D. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract. 2014;127:89–93. doi: 10.1159/000363725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buccione E., Guzzi F., Colosimo D., et al. Continuous renal replacement therapy in critically ill children in the pediatric Intensive Care Unit: a retrospective analysis of real-life prescriptions, complications, and outcomes. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.696798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuhrman D.Y., Gist K.M., Akcan-Arikan A. Current practices in pediatric continuous kidney replacement therapy: a systematic review-guided multinational modified Delphi consensus study. Pediatr Nephrol. 2023 doi: 10.1007/s00467-022-05864-z. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daverio M., Cortina G., Jones A., et al. Continuous kidney replacement therapy practices in pediatric intensive care units across Europe. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.46901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haga T., Ide K., Tani M. Characteristics of pediatric continuous renal replacement therapies in hospitals with pediatric intensive care units in Japan. Ther Apher Dial. 2022;27:562–570. doi: 10.1111/1744-9987.13958. [DOI] [PubMed] [Google Scholar]
- 41.Gist K.M., Fuhrman D.Y., Akcan-Arikan A. Standardizing care in pediatric continuous kidney replacement therapy-can we reach consensus without adequate evidence? JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.46909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.