Graphical abstract
Keywords: Oesophageal cancer, ASR, Trend analysis, Epidemiology
Highlights
-
•
The global incidence and mortality rates of oesophageal cancer in 2020 are revealed.
-
•
The 10-year incidence and mortality trends of oesophageal cancer across age and gender subgroups are decreasing in most countries.
-
•
Compared with other continents, the incidence and mortality trends especially vary in European countries.
-
•
Nations with very high Human Development Index (HDI) have the lowest average incidence and mortality rates, whereas those with high HDI have the highest rates.
Abstract
Introduction
Oesophageal cancer is a prevalent and deadly cancer around the world.
Objectives
We aimed to present a comprehensive analysis of the global geographic patterns and temporal trends in the mortality and incidence of oesophageal cancer.
Methods
The mortality and incidence data of oesophageal cancer in 2020 were obtained from the GLOBOCAN database. Based on World Health Organization (WHO) mortality database and the Cancer Incidence in Five Continents (CI5), we also retrieved the mortality and incidence age-standardized rates (ASRs) of oesophageal cancer. The average annual percentage changes (AAPCs) of mortality and incidence were calculated using the joinpoint regression analysis.
Results
Globally, 0.54 million deaths and 0.6 million new cases were identified in 2020. In the majority of countries of South America and Asia, the mortality and incidence trends have substantially decreased, but trends in European countries have varied. The prevalence in European nations varied, but the incidence in most other continents decreased dramatically. In terms of mortality, the global average rate was 5.6 per 100000, ranging from 16.7 (Malawi) to 0.28 (Belize). European countries varied in mortality, such as Norway (AAPC, male: 0.68; female: 0.89) and Ireland (AAPC, male: −0.96; female: −1.52). Most non-European countries saw large decreases in mortality, such as Singapore (AAPC, male: −4.78; female: −6.89). The elderly had more noticeable trends in mortality and incidence in most countries.
Conclusions
We have identified different trends in mortality and incidence among European countries, whereas declining trends were identified in most non-European countries. However, increasing trends were identified in specific subgroups of some countries, such as men in Thailand. For populations with rising mortality and incidence trends, more preventative efforts are required.
Introduction
In 2020, oesophageal cancer has caused 0.6 million new cases and 0.54 million deaths worldwide, making it a principal cause of cancer death [1], [2]. Using data of the GLOBOCAN database, we estimated that incident cases are expected to rise by about 63.5% by 2040, whereas the number of the death is likely to rise by approximate 68% [1]. As for the histological subtypes, oesophageal squamous cell carcinoma and oesophageal adenocarcinoma are two principal subtypes, with squamous cell carcinoma accounting for over 85% of all occurrences. Furthermore, oesophageal adenocarcinoma is connected to smoking and obesity, whereas oesophageal squamous cell carcinoma is linked to alcohol and cigarette intake. [3], [4]. Changes in the prevalence of the above risk factors may be substantially contributed to the changes in temporal trends. As the 6th leading cause of cancer-related death, oesophageal cancer is considered to be a substantial global health burden [5]. Due to the significant advances in endoscopic diagnosis, basic research and following treatment strategies, detection at an early stage has the potential to increase the survival time of patients with oesophageal cancer [6], [7], [8], [9]. Therefore, comprehensively analyzing the global burden of oesophageal cancer and temporal trends in mortality and incidence could help the allocation of healthcare resources, especially in some developing countries.
Because of the importance of epidemiology for health policy development and the wide variation between regions or populations, many studies have revealed some health characteristics of oesophageal cancer, including risk factors, mortality and incidence [10], [11]. A study using data from the Global Burden of Disease (GBD) project has assessed the burden of oesophageal cancer in different regions from 1990 to 2017, indicating east Asian nations had the greatest burden [12]. Moreover, Wang et al. have demonstrated the age-specific sex differences between different subtypes of oesophageal cancer [13]. Another study initiated by Li et al. have predicted the mortality of oesophageal cancer from 2020 to 2030 in China [14]. In addition to investigating the burden of oesophageal cancer in specific countries, trends among people in subgroups with distinct characteristics should also be analyzed, allowing for more accurate resource allocation for people of various ages. According to a recent study, cancer patterns among the elderly in America differed significantly from those of the younger population [15]. However, the global analysis of oesophageal cancer was only based on the GBD study 2017, which utilized data from estimation and modeling rather than regional registries. And Huang et al. analyzed the global burden of oesophageal cancer using the GLOBOCAN, but the research was conducted several years ago and more supporting data were needed [16]. Moreover, the temporal trends in mortality and incidence among different gender and age subgroups remain unclear, which may make developing health policies for specific populations difficult.
In this research, by integrating data of multiple databases, we provided a global perspective on the mortality and incidence of oesophageal cancer and revealed the current trends in mortality and incidence around the world. For countries included in our study, we found different trends in mortality and incidence among European countries, with more declining trends in mortality being observed for men than women. In addition, declining trends were identified in most non-European countries. In contrast to the majority of non-European countries, mortality and incidence trends for men in Thailand were rising significantly. Moreover, our findings showed that the elderly had more substantial mortality and incidence trends than the young. We also evaluated the differences of mortality and incidence rates among countries with different human development indexes. Our findings may assist to guide health policy decisions and then reduce the future burden of oesophageal cancer.
Methods
Data retrieval
We utilized similar methods of a recent publication analyzing the temporal trends in mortality and incidence of mesothelioma and breast cancer [17], [18], [19]. In our study, we have investigated mortality and incidence trends in the recent ten years, and presented the global burden of oesophageal cancer (ICD-10, C15.0-C15.9) in 2020. Based on the 2020 edition of data from the GLOBOCAN database, we retrieved the mortality and incidence estimates of oesophageal cancer for 185 nations [1]. The Cancer in Five Continents plus (CI5plus) database was used to access the incidence data of oesophageal cancer for most countries [20]. We used the World Population Prospects 2019 to get access to the worldwide population data [21]. Besides, we used the Surveillance, Epidemiology, and End Results (SEER) Program to access the incidence data of the United States, and we used the Nordic Cancer Registries (NORDCAN) to retrieve data of nations of North Europe [22], [23]. A total of 43 countries with incidence data were enrolled and data of 22 countries were at the national level, whereas data from regional registries were used for the other 21 countries without incidence data at the country level. Furthermore, the WHO mortality database was used to obtain the mortality data [24]. The mortality data of the United States and northern European nations were retrieved via the SEER and the NORDCAN database, respectively (Table S1). The mortality data of 44 countries were completely included in this study. According to previous studies in gastrointestinal cancer, patients under 50 years old were considered as a subgroup distinct from older patients, thus we categorized patients using a 50-year-old cutoff [25], [26]. We also used the United Nations (UN) database to obtain the human development index (HDI) data [27]. All countries were divided into four HDI categories based on the quartiles of the distributions: Very High: > 0.799; High: 0.700 to 0.799; Medium: 0.550 to 0.699; Low: < 0.550 [28].
Statistical analysis
We used the Segi-Doll world standard population to adjust the mortality and incidence data by age, and the age-standardized rates (ASRs) were used for the following analysis [29]. ASRs was used to compare the statistics across demographic groups, despite the fact that the size of the groups or the ages of the group members may vary greatly. Next, we analyzed the past 10-year mortality and incidence trends via performing the joinpoint regression analysis [30]. Because the “zero” or “missing” value could not be analyzed by the joinpoint regression, we have excluded all the “zero” and “missing” values in this study. We conducted a logarithmic transformation of the ASRs and calculated standard errors via the binomial approximation. The average annual percentage change (AAPC) and the corresponding 95% confidence interval (CI) were calculated via using the geometric weighting in people of various nations, gender and age groups, which revealed the ten-year trends in mortality and incidence. The length of each segment was assigned weights equal to the specified time interval. The detailed formula or AAPC was: AAPC = x 100. The wis is the length of each segment in the range of years, whereas bis represents the slope coefficient for each segment in the desired range of years. We compared the magnitude of AAPC with zero and we regarded the insignificance as a stable trend, aiming to generate statistical significance. If the AAPC is within one segment, the t-distribution is applied; otherwise, the normal (z) distribution is used. And P < 0.05 was considered statistically significant.
Ethics statement
The use of anonymized, publicly accessible data did not need ethical approval. Access to and use of data from the aforementioned databases did not need the patient consent.
Results
Mortality and incidence rates of oesophageal cancer
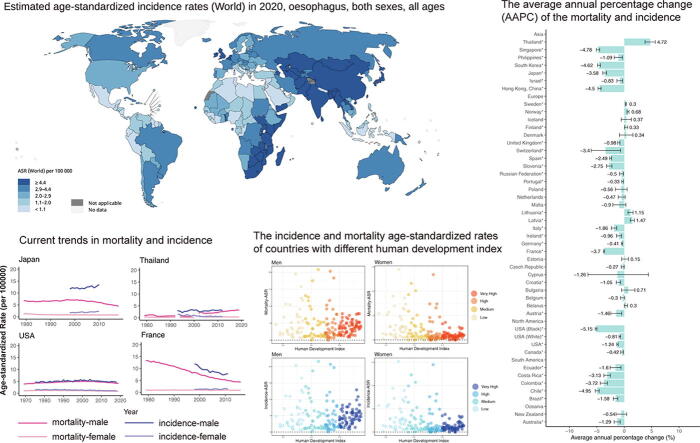
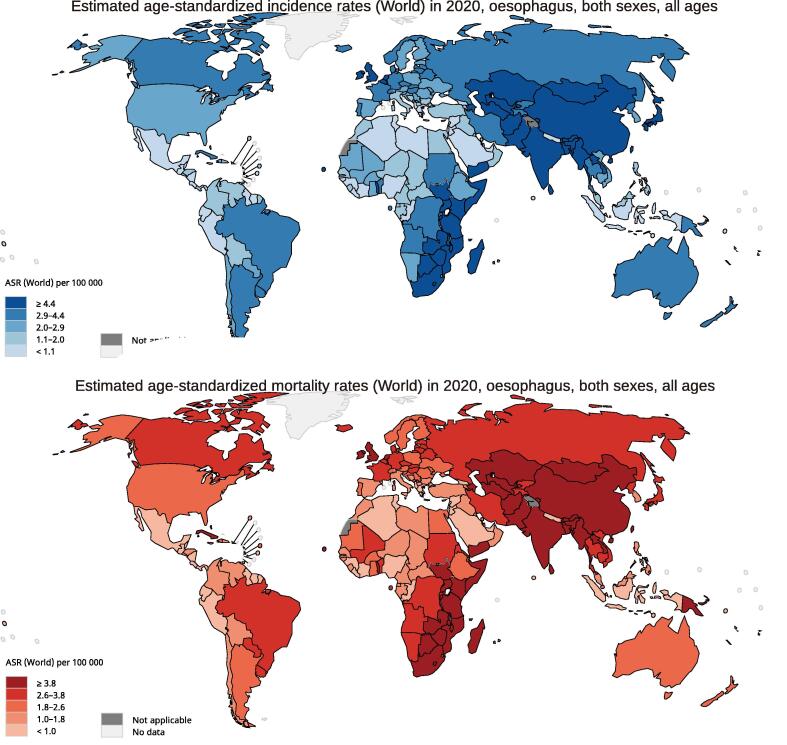
According to the GLOBOCAN 2020 database, there were 604,100 new cases of oesophageal cancer identified worldwide in 2020, with China having the most new cases. The global average incidence was 6.3 per 100000, with rates ranging from 17.5 (Malawi) to 0.28 (Belize). Asia had the greatest incidence of ASR (ASR, 8.5), whereas Latin America and the Caribbean had the lowest (ASR, 2.4). In terms of gender differences, men (ASR, 9.3) had approximately three times the worldwide incidence of oesophageal cancer as women (ASR, 3.6), indicating that oesophageal cancer has gender-specific effects. In terms of mortality, the global average rate was 5.6 per 100000, with rates ranging from 16.7 (Malawi) to 0.28 (Belize). And 544,076 individuals died from oesophageal cancer worldwide in 2020, with China having the largest number of fatalities. When it comes to continents, the highest mortality rates were in Eastern Asia (ASR, 10.7), Eastern Africa (ASR, 7.0), and Central and Southern Africa (ASR, 6.4). Men's mortality (ASR, 8.3) was approximately-three times greater than women's (ASR, 3.2), indicating that gender differences exist in mortality. To display the whole data, we utilized Fig. 1 and Table 1.
Fig. 1.
The global estimated mortality and incidence of oesophageal cancer in 2020, both sexes, all ages. ASR, age-standardized rate. Graph was produced by GLOBOCAN 2020 (https://gco.iarc.fr/).
Table 1.
The incidence and mortality of oesophageal cancer in different regions.
| Incidence |
Mortality |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both sexes |
Male |
Female |
Both sexes |
Male |
Female |
|||||||
| Regions | Cases | ASR per 100,000 | Cases | ASR per 100,000 | Cases | ASR per 100,000 | Cases | ASR per 100,000 | Cases | ASR per 100,000 | Cases | ASR per 100,000 |
| World | 604,100 | 6.3 | 418,350 | 9.3 | 185,750 | 3.6 | 544,076 | 5.6 | 374,313 | 8.3 | 169,763 | 3.2 |
| Africa | ||||||||||||
| Eastern Africa | 16,137 | 7.3 | 8514 | 8.4 | 7623 | 6.4 | 15,188 | 7 | 8028 | 8 | 7160 | 6.1 |
| Middle Africa | 2136 | 2.5 | 1343 | 3.3 | 793 | 1.8 | 2030 | 2.4 | 1275 | 3.2 | 755 | 1.7 |
| Northern Africa | 3254 | 1.5 | 1750 | 1.7 | 1504 | 1.4 | 3152 | 1.5 | 1694 | 1.7 | 1458 | 1.3 |
| Southern Africa | 3610 | 6.7 | 2109 | 9.4 | 1501 | 4.8 | 3449 | 6.4 | 2049 | 9.1 | 1400 | 4.5 |
| Western Africa | 2409 | 1.3 | 1318 | 1.5 | 1091 | 1.1 | 2278 | 1.2 | 1240 | 1.4 | 1038 | 1.1 |
| Americas and Caribbean | ||||||||||||
| Caribbean | 1703 | 2.8 | 1311 | 4.7 | 392 | 1.2 | 1647 | 2.7 | 1267 | 4.6 | 380 | 1.1 |
| Central America | 1779 | 0.93 | 1315 | 1.5 | 464 | 0.43 | 1704 | 0.89 | 1249 | 1.5 | 455 | 0.43 |
| Northern America | 20,806 | 2.9 | 16,298 | 4.8 | 4508 | 1.1 | 18,480 | 2.4 | 14,800 | 4.2 | 3680 | 0.84 |
| South America | 15,529 | 2.8 | 11,779 | 4.7 | 3750 | 1.2 | 14,448 | 2.6 | 10,992 | 4.4 | 3456 | 1 |
| Asia | ||||||||||||
| Eastern Asia | 357,779 | 12.3 | 251,456 | 18.2 | 106,323 | 6.8 | 319,118 | 10.7 | 221,846 | 15.9 | 97,272 | 6 |
| South-Central Asia | 105,034 | 5.6 | 65,281 | 7.1 | 39,753 | 4.2 | 97,436 | 5.2 | 60,429 | 6.5 | 37,007 | 3.9 |
| South-Eastern Asia | 14,439 | 2 | 11,576 | 3.4 | 2863 | 0.72 | 13,695 | 1.9 | 10,999 | 3.3 | 2696 | 0.68 |
| Western Asia | 4300 | 1.7 | 2309 | 2 | 1991 | 1.5 | 4114 | 1.7 | 2194 | 1.9 | 1920 | 1.5 |
| Europe | ||||||||||||
| Northern Europe | 12,968 | 5.3 | 9070 | 8.2 | 3898 | 2.7 | 10,985 | 4.2 | 7717 | 6.6 | 3268 | 2 |
| Southern Europe | 6307 | 1.8 | 4892 | 3.2 | 1415 | 0.63 | 5564 | 1.5 | 4355 | 2.6 | 1209 | 0.5 |
| Western Europe | 18,283 | 4.1 | 13,938 | 6.6 | 4345 | 1.8 | 14,842 | 3 | 11,403 | 5.2 | 3439 | 1.2 |
| Central and Eastern Europe | 15,435 | 2.9 | 12,517 | 5.7 | 2918 | 0.81 | 14,120 | 2.6 | 11,435 | 5.1 | 2685 | 0.73 |
| Oceania | ||||||||||||
| Australia and New Zealand | 1916 | 3.1 | 1387 | 4.8 | 529 | 1.4 | 1564 | 2.4 | 1164 | 3.9 | 400 | 1 |
| Melanesia | 247 | 3.5 | 161 | 5 | 86 | 2.3 | 234 | 3.4 | 152 | 4.8 | 82 | 2.2 |
| Micronesia | 13 | 2.3 | 10 | 3.8 | 3 | 1.1 | 13 | 2.3 | 10 | 3.8 | 3 | 1.1 |
| Polynesia | 16 | 2.2 | 16 | 4.4 | 0 | 0 | 15 | 2 | 15 | 4.1 | 0 | 0 |
| HDI | ||||||||||||
| High HDI | 362,576 | 9.1 | 250,143 | 13.3 | 112,433 | 5.2 | 337,168 | 8.3 | 232,396 | 12.3 | 104,772 | 4.8 |
| Very high HDI | 111,343 | 3.5 | 87,210 | 6.1 | 24,133 | 1.3 | 85,497 | 2.6 | 66,433 | 4.5 | 19,064 | 0.95 |
| Medium HDI | 111,582 | 5.3 | 70,828 | 6.9 | 40,754 | 3.8 | 103,722 | 4.9 | 65,815 | 6.4 | 37,907 | 3.5 |
| Low HDI | 18,457 | 3.7 | 10,060 | 4.3 | 8397 | 3.2 | 17,558 | 3.6 | 9569 | 4.1 | 7989 | 3.1 |
Trends in mortality
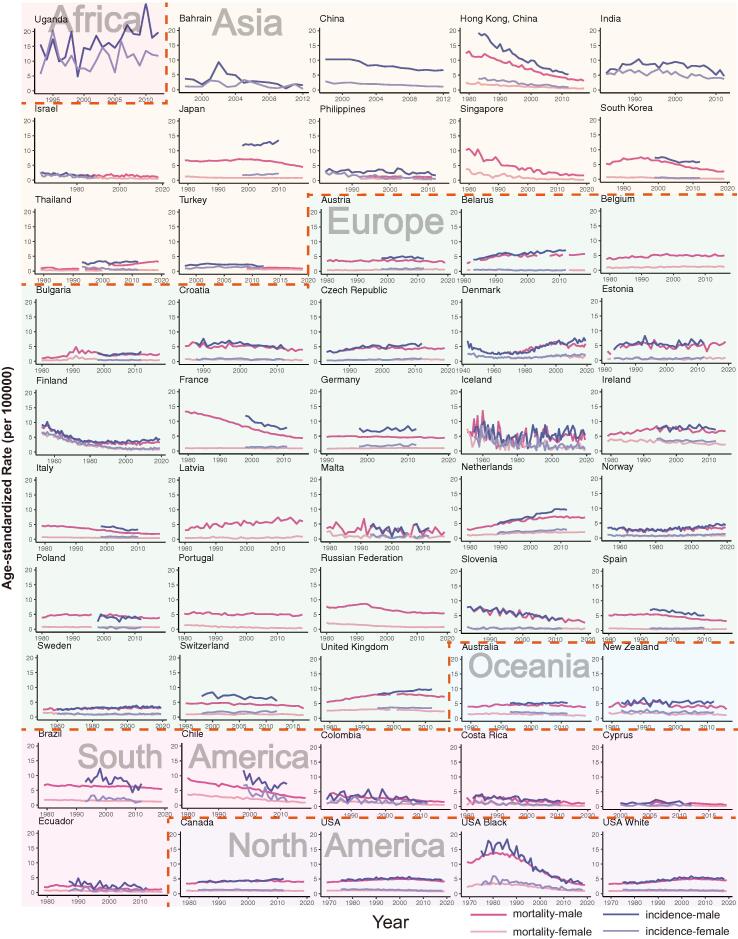
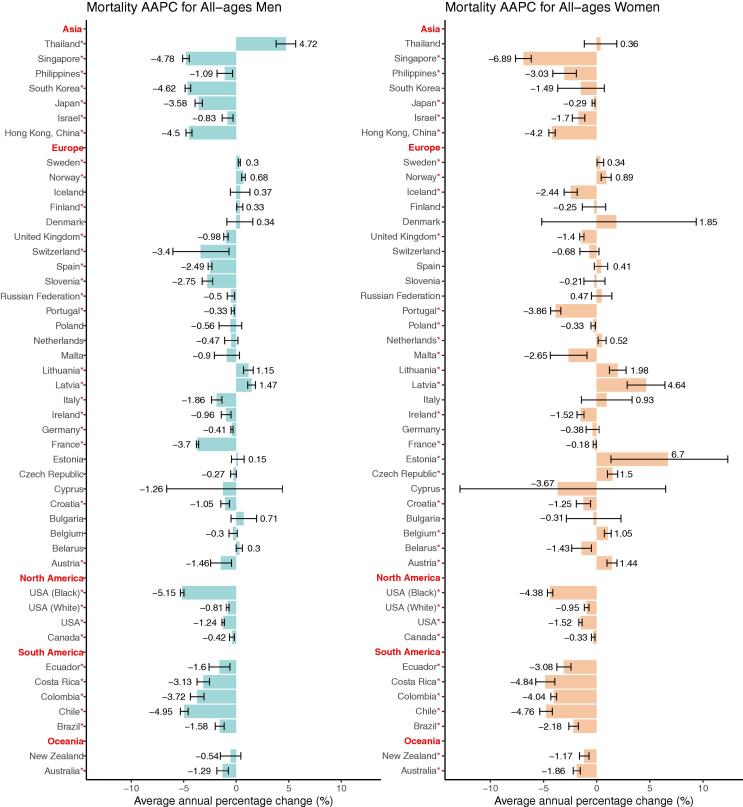
Men's trends in mortality decreased in the majority of nations, ranging from −0.33 (Portugal) to −5.15 (the black population in the United States). All countries in South and North America, as well as the majority of Asian countries, exhibited declining trends, whereas European countries showed substantial variations (Fig. 2, Table S6). Northern European countries had slightly increasing trends, while other European countries had declining or constant trends. In addition, when compared to males in other Asian nations, men in Thailand had an opposite tendency (AAPC, 4.72), which was the highest AAPC and ranked the first among all the countries (Fig. 3, Table S2). In 9 nations, women's trends in mortality were growing, and the trends in women's mortality rates were similar to those of men in most countries (AAPCs, −6.89 to 6.7). The top three populations or nations with the lowest AAPC were Singapore (AAPC, −6.89), Costa Rica (AAPC, −4.84) and Chile (AAPC, −4.76). European countries showed different trends, with three Eastern European countries showing a significant trend of high growth, including Estonia (AAPC, 6.70), Latvia (AAPC, 4.64) and Lithuania (AAPC, 1.98), while other European countries showed a trend of decline or slight increase. In the United States, the decline for blacks is more than four times that for whites, and the country has one of the lowest AAPCs in the world. Similar results were found in the patterns of mortality ASRs.
Fig. 2.
Current trends in mortality and incidence of oesophageal cancer by country or population in men and women.
Fig. 3.
The average annual percentage change (AAPC) of the mortality of oesophageal cancer in all-age female and male. *P values less than 0.05.
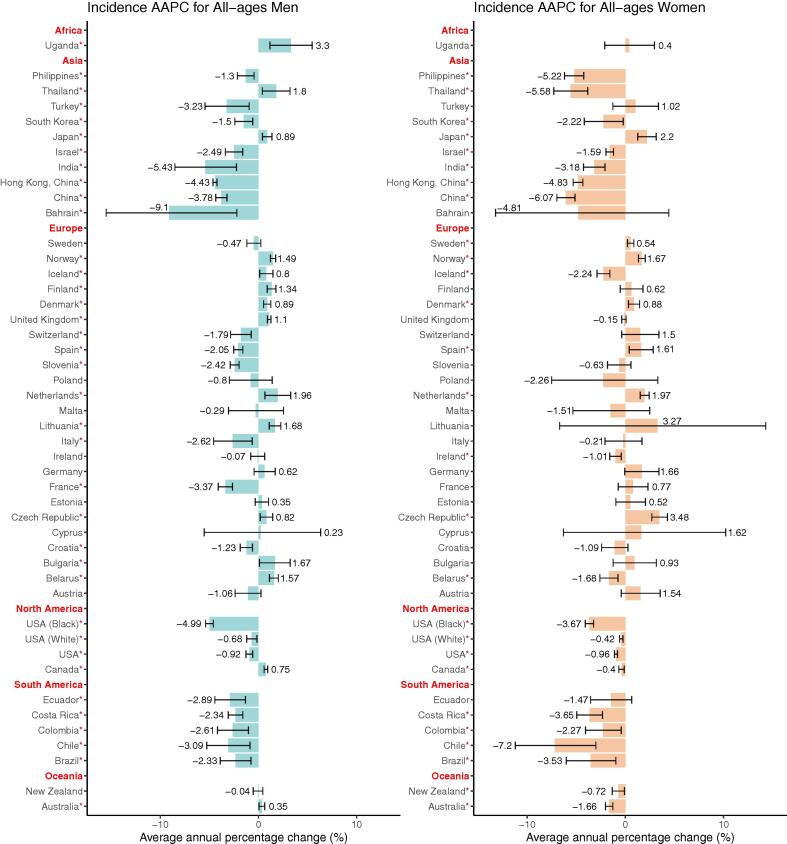
Trends in incidence
Among men, most nations in Asia, South America and North America had a declining trend in incidence, whereas Uganda and a few European countries experienced a considerable rise (AAPCs, −9.1 to 3.3, Fig. 4, Table S3). A total of 20 countries or populations exhibited declining trends, with Bahrain (AAPC, −9.1), India (AAPC, −5.43) and the black in the United States (AAPC, −4.99) having the most dramatic declines. In addition, 15 countries showed increasing trends in incidence, with Uganda (AAPC, 3.3), Netherlands (AAPC, 1.96) and Thailand (AAPC, 1.8) ranking first, second, and third, respectively. Moreover, we divided patients into two subgroups: patients younger than fifty years old and those older than fifty years old. Most nations showed similar trends in these two groups, while certain countries, such as Japan, Norway, and Finland, had opposite tendencies in these two divisions. Thirteen nations had a significant decline in the younger population, while six countries experienced an increase (AAPCs, −6.6 to 6.18, Figure S2, Table S5). Our findings revealed that the young had more significant trends in European countries, whereas trends among the old were more significant in countries of Asia and North America. In terms of the elderly population, we identified that 18 countries showed a considerable drop, with Bahrain (AAPC, −15.45) having the largest drop (Figure S1, Table S4). While 12 countries had a drastic increase, with Uganda having the highest increase (AAPC, 3.3), followed by the Netherlands (AAPC, 2.08, Figure S1, Table S4).
Fig. 4.
The average annual percentage change (AAPC) of the incidence of oesophageal cancer in all-age female and male. *P values less than 0.05.
In most nations, women's incidence patterns were similar to men's. A total of 14 countries had a drastic decline in incidence, the most of which were in Asia, South America, North America and Oceania (AAPCs, −7.2 to −0.4, Fig. 4, Table S3). In Europe, six countries had a growing trend and three countries had a sharp fall, indicating trends in incidence among women were insignificant in most European countries, such as United Kingdom, Italy and France. And countries with the largest increase were the Czech Republic (AAPC, 3.48), Japan (AAPC, 2.2) and Netherlands (AAPC, 1.97). The similar conclusion could be drawn according to the incidence ASRs across the countries (Fig. 2, Table S7). We then compared the trends in incidence among the younger and elder populations, finding that in the majority of nations, rises among the elderly were more substantial (Figure S2, Table S5). Aside from the majority of nations that had a constant trend, 11 countries or populations had a decreasing trend in incidence among younger women, with China having the largest decrease (AAPC, −5.22). Only three countries, Denmark (AAPC, 1.17), Lithuania (AAPC, 4.04) and Estonia (AAPC, 2.22) had significant increases among the young. And most nations, such as Sweden, Germany, and France, had negligible AAPCs, implying that the incidence of young women was steady in most countries. In terms of the elderly population, 18 countries or populations showed a significant drop, with Chile having the largest decrease (AAPC, −7.19), followed by China (AAPC, −5.99, Figure S1, Table S4). Furthermore, 10 countries, consisting of nine European countries and Japan, had an upward trend (AAPCs, 0.54 to 3.37).
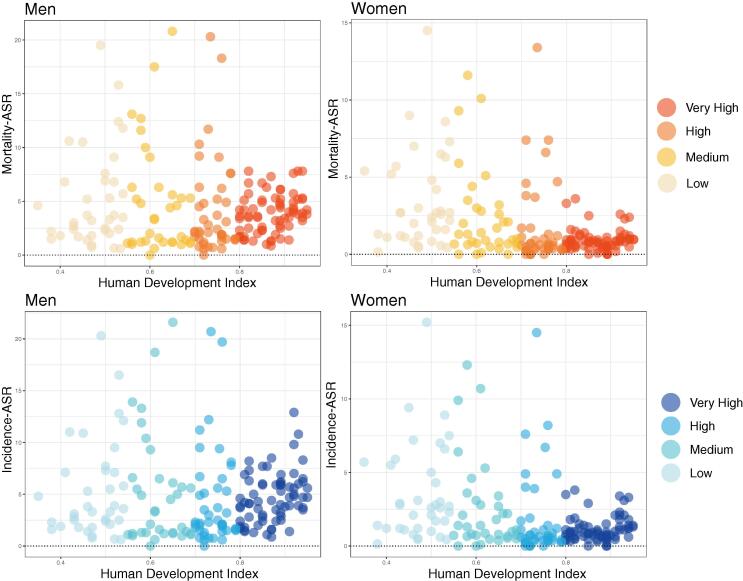
Mortality and incidence rates among countries with different HDIs
We also compared the mortality and incidence rates of men and women in countries with varying HDIs. In terms of incidence, we found that nations with a very high HDI had lower incidence rates for both male and female than other nations, especially among women. Interestingly, nations with very high HDI have the lowest average incidence rates (ASR, 3.5), while nations with high HDI have the highest rates (ASR, 9.1). In addition, the average incidence rates were lower among women than men in countries with different HDI levels. And mortality rates showed a similar trend across HDI levels (Fig. 5). High HDI countries had the highest average mortality (ASR, 8.3), whereas nations with very high HDI has the lowest rates (ASR, 2.6).
Fig. 5.
The incidence and mortality age-standardized rates of countries with different human development index for oesophageal cancer in 2020.
Discussion
The global burden of oesophageal cancer is critical for the prevention and treatment of this disease, which has been studied based on the GBD 2017 study [12]. Some regional studies, such as those conducted in Japan and China, have also investigated the geographic burden of oesophageal cancer [31], [32]. However, no comprehensive study has identified current trends in mortality and incidence of oesophageal cancer according to gender and age. Gender and age are also important variables in carcinogenesis [33], [34]. In our research, we analyzed data from different regions around the world, showing patterns of esophageal cancer mortality and incidence. We found that (1) nations with very high HDI tend to have lower mortality and incidence rates than other countries, especially among women; (2) the majority of Asian, South American, and North American countries have decreasing mortality and incidence trends among men and women, whereas men in Thailand have significant increasing mortality and incidence trends; (3) European countries showed significant variations in mortality and incidence patterns; (4) when compared with the younger populations, the elder ones have a more dramatic trend in mortality and incidence; (5) slight difference was observed in mortality and incidence trends among men and women.
The burden of oesophageal cancer differed significantly among nations with various HDI levels. Previous research has established a link between HDI and cancer incidence and death rates in a variety of cancers. For example, increasing gradients in mortality and incidence were identified with increasing levels of HDI for colorectal cancer [35]. Despite the fact that oesophageal cancer is one of the gastrointestinal cancers, we found that nations with very high HDI had the lowest average mortality and incidence ASRs, whereas countries with high HDI had the highest average ASRs. Oesophageal adenocarcinoma and oesophageal squamous cell carcinoma are two primary subtypes of oesophageal cancer, each has its own set of risk factors. The risk factors of oesophageal adenocarcinoma were male sex, obesity, and reflux [36], whereas the risk factors of oesophageal squamous cell carcinoma were alcohol consumption, cigarette smoking and intake of red meat [12], [37], [38]. Lifestyle variations may contribute to the ASR disparity between countries with very high and high HDI levels. In addition, people in nations with very high HDI may be more health-conscious and can undergo endoscopic examination more easily, resulting in lower mortality and incidence ASRs. Potential patients could be identified at an early stage in countries with very high HDI, then properly examined for diagnosis and treated promptly. Patients with oesophageal cancer might be completely treated at an early stage thanks to the frequency of minimally invasive therapy, and the disease burden could be minimized in nations with very high HDI [7]. Herein, to reduce the burden of oesophageal cancer in the future, more attention should be paid for people other than those in countries with very high HDI.
The mortality and incidence trends differed among European countries, with a little increase in males of Nordic countries and opposing trends in the majority of Southern European countries. People in different parts of Europe have distinct lifestyles, which might result in diverse trends [39]. Different demographic shifts in European countries may potentially be a contributing factor to various patterns. Studies have found that oesophageal adenocarcinoma were more prevalent in European countries than the oesophageal squamous cell carcinoma [40]. Compared with women, our results showed that men in most European countries had much higher mortality and incidence ASRs in the recent decades, which were consistent with prior studies showing that men were more prone to develop oesophageal adenocarcinoma. [36]. In most European countries, the patterns in mortality and incidence were likewise distinct. In almost half of European nations, young men showed decreasing incidence trends and most young women had steady trends, and more declining trends in mortality were observed for men than women. Furthermore, smoking and alcohol consumption have been regarded as possible risk factors for oesophageal cancer, which might be contributing to the gender disparities [41]. Previous publications have revealed that female who smoke are much more sustainable to some respiratory cancer than male [42], [43]. Herein, establishing strict rules on smoking and alcohol consumption may help lower the disease burden of esophageal cancer. In comparison to other malignancies, oesophageal cancer had a lower prevalence in European nations. The trends were less significant than counties in other continents, implying minor changes in individual risk variables, which was consistent with prior research of single countries like Netherlands and Finland [44], [45].
Moreover, we found that the mortality and incidence trends were declining among male and female in most countries we analyzed in Asia, South America and North America. In these continents, the elderly showed more evident mortality and incidence trends than the younger population. The significant decline in mortality and incidence of oesophageal cancer might indicate the development of the treatment approach or the changing patterns of risk factors linked to economic growth. For instance, neoadjuvant chemotherapy and immunotherapy for oesophageal cancer before the surgery were considered novel treatments, and several clinical trials were underway to find the optimum treatment [46], [47]. Moreover, certain low- and middle-income nations have seen a considerable dietary change in recent years, with greater intake of animal-source foods, which may contribute to the drop [48]. In Asia, the majority of countries, such as China, had greater mortality and incidence and showed a drastic decrease in the past few years. As more and more individuals become aware of esophageal cancer, their dietary habits may alter over time, which may be one of causes leading to the remarkable decline. The focus should be on diseases with rising trends in incidence and mortality as we reassessed how to allocate healthcare resources to these populations. Interestingly, in contrast to other Asian nations, men in Thailand showed growing mortality and incidence trends in the last ten years. However, we identified a significant drop in mortality and incidence trends among women in Thailand. With the rapid expansion of economy, men's living patterns in Thailand may alter, resulting in this dramatic increase. More attention should be paid to populations with both increasing trends in incidence and mortality, such as males in Thailand. And specific cohort studies are recommended to be initiated to evaluate risk factors among these people. In the United States, the black people have higher ASRs and are experiencing more dramatic declines in mortality and incidence. The improvement of the living standard of the these people in recent years may result in a drastic decrease [49], [50]. Our findings revealed that mortality and incidence rates of the black population have reached levels similar to those of other ethnic groups in recent years, indicating that the health care system of the United States is working effectively in terms of oesophageal cancer.
Nevertheless, there were still some limitations to this research. First, due to the restrictions of the databases, we lacked the mortality and incidence data in many underdeveloped nations, leading to some bias in our research. And all the databases lacked the histological data that allowed us to stratify patients with adenocarcinoma and squamous cell carcinoma. Second, because we did not consider the data from less developed regions of these countries, we might overestimate the data for countries with only one cancer registry. In addition, because of the suboptimal reporting mechanism and limited resources, we might underestimate data from less developed countries. Third, due to the difference of cancer registries in multiple countries and over time, it might be hard to compare the mortality and incidence rate directly. However, the comparison of gender and age subgroups within the same nation was unaffected.
Conclusions
To conclude, we comprehensively analyzed the geographical disparities of mortality and incidence of oesophageal cancer by gender and age, as well as current trends in multiple countries. Regardless of the decreasing trends in most countries included in our study, the worldwide patient population remained high. The trends in mortality and incidence of oesophageal cancer varied among European countries, but decreased in countries across other continents, such as China and the United States. The decease might be attributable to the implementation of earlier preventative strategies and a more straightforward approach to advanced therapeutic interventions among countries included in our analysis. Compared with the majority of non-European countries, mortality and incidence trends for men in Thailand were rising significantly. And mortality and incidence trends were also identified among individuals in Nordic countries. In addition, the elderly had more substantial mortality and incidence trends than the young. To diminish the impact on society, potential risk factors are expected to be monitored in populations with increasing trends. Further studies are needed to determine the causes behind populations with increased trends in mortality and incidence of oesophageal cancer. And detailed information of many developing and underdeveloped countries not included in our study should be collected and analyzed.
Compliance with ethics requirements ethical statement
This article does not contain any studies with human or animal subjects.
Funding
The study was funded by the Medical and Health Science-Technology Innovation Project of Chinese Academy of Medical Sciences (2021-I2M-1-015), National Key R&D Program of China (2021YFC2500900), National Natural Science Foundation of China (82273129), Central Health Research Key Projects (2022ZD17).
Compliance with Ethics Requirements.
Not applicable.
Ethics approval and consent to participate.
Not applicable.
CRediT authorship contribution statement
Bolun Zhou: Formal analysis, Conceptualization, Data curation, Investigation, Writing – original draft. Fenglong Bie: Formal analysis, Investigation, Software. Ruochuan Zang: Formal analysis, Investigation. Moyan Zhang: Formal analysis, Investigation. Peng Song: Resources. Lei Liu: Resources. Yue Peng: Methodology, Software. Guangyu Bai: Methodology, Software. Qilin Huai: Methodology, Software. Yuan Li: Methodology, Software. Liang Zhao: Conceptualization, Supervision. Shugeng Gao: Conceptualization, Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.10.007.
Contributor Information
Liang Zhao, Email: drzhaoliang@126.com.
Shugeng Gao, Email: gaoshugeng@cicams.ac.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M., Laversanne M., Brown L.M., Devesa S.S., Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol. 2017;112(8):1247–1255. doi: 10.1038/ajg.2017.155. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J., Smyth E., Cunningham D., Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. doi: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed] [Google Scholar]
- 5.Smyth E.C., Lagergren J., Fitzgerald R.C., Lordick F., Shah M.A., Lagergren P., et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lao-Sirieix P., Fitzgerald R.C. Screening for oesophageal cancer. Nat Rev Clin Oncol. 2012;9(5):278–287. doi: 10.1038/nrclinonc.2012.35. [DOI] [PubMed] [Google Scholar]
- 7.di Pietro M., Canto M.I., Fitzgerald R.C. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology. 2018;154(2):421–436. doi: 10.1053/j.gastro.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W., Zhou B., Yang Z., Liu X., Huai Q., Guo L., et al. Integrating microarray-based spatial transcriptomics and single-cell RNA-sequencing reveals tissue architecture in esophageal squamous cell carcinoma. EBioMedicine. 2022;84 doi: 10.1016/j.ebiom.2022.104281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hossen S., Hossain M.K., Basher M.K., Mia M.N.H., Rahman M.T., Uddin M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J Adv Res. 2019;15:1–18. doi: 10.1016/j.jare.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrift A.P. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18(6):432–443. doi: 10.1038/s41575-021-00419-3. [DOI] [PubMed] [Google Scholar]
- 11.Arnold M., Soerjomataram I., Ferlay J., Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 12.The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(6):582–97. [DOI] [PMC free article] [PubMed]
- 13.Wang S., Zheng R., Arnold M., Abnet C., Zeng H., Zhang S., et al. Global and national trends in the age-specific sex ratio of esophageal cancer and gastric cancer by subtype. Int J Cancer. 2022;151(9):1447–1461. doi: 10.1002/ijc.34158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N., Wu P., Shen Y., Yang C., Zhang L., Chen Y., et al. Predictions of mortality related to four major cancers in China, 2020 to 2030. Cancer Commun (Lond) 2021;41(5):404–413. doi: 10.1002/cac2.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sung H., Siegel R.L., Rosenberg P.S., Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3) doi: 10.1016/S2468-2667(18)30267-6. e137–e47. [DOI] [PubMed] [Google Scholar]
- 16.Huang J., Koulaouzidis A., Marlicz W., Lok V., Chu C., Ngai C.H., et al. Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries. Cancers (Basel) 2021;13(1) doi: 10.3390/cancers13010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heer E., Harper A., Escandor N., Sung H., McCormack V., Fidler-Benaoudia M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8) doi: 10.1016/S2214-109X(20)30215-1. e1027–e37. [DOI] [PubMed] [Google Scholar]
- 18.Zhai Z., Ruan J., Zheng Y., Xiang D., Li N., Hu J., et al. Assessment of Global Trends in the Diagnosis of Mesothelioma From 1990 to 2017. JAMA Netw Open. 2021;4(8):e2120360. doi: 10.1001/jamanetworkopen.2021.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N., Deng Y., Zhou L., Tian T., Yang S., Wu Y., et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1):140. doi: 10.1186/s13045-019-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray F CM, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J. Cancer Incidence in Five Continents, vol XI (electronic version). Lyon: International Agency for Research on Cancer. 2017.
- 21.United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Prospects 2019, Online Edition. Rev. 1.
- 22.Danckert B FJ, Engholm G , Hansen HL, Johannesen TB, Khan S, Køtlum JE, Ólafsdóttir E, Schmidt LKH, Virtanen A and Storm HH. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2 (26.03.2019). Association of the Nordic Cancer Registries. Danish Cancer Society. Available from http://www.ancr.nu, Accessed August 10, 2021.
- 23.SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. [Cited 2021 September 27]. Available from https://seer.cancer.gov/explorer/.
- 24.Mathers C.D., Fat D.M., Inoue M., Rao C., Lopez A.D. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83(3):171–177. [PMC free article] [PubMed] [Google Scholar]
- 25.Kalliala I., Athanasiou A., Veroniki A.A., Salanti G., Efthimiou O., Raftis N., et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: a systematic review and meta-analysis of the literature. Ann Oncol. 2020;31(2):213–227. doi: 10.1016/j.annonc.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low E.E., Demb J., Liu L., Earles A., Bustamante R., Williams C.D., et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology. 2020;159(2):492–501.e7. doi: 10.1053/j.gastro.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United Nations. 2018 Statistical Update: Human Development Indices and Indicators. Available at: http://hdr.undp.org/en/2018-update. Accessed September 10, 2021.
- 28.United Nations. Human development classification, 2018 Statistical Update. Available at: http://hdr.undp.org/sites/default/files/2018_human_development_statistical_update.pdf. Accessed September 30, 2021.
- 29.Segi M., Fujisaku S., Kurihara M. Geographical observation on cancer mortality by selected sites on the basis of standardised death rate. Gan. 1957;48(2):219–225. [PubMed] [Google Scholar]
- 30.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y., Totsuka Y., He Y., Kikuchi S., Qiao Y., Ueda J., et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23(4):233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roerecke M., Shield K.D., Higuchi S., Yoshimura A., Larsen E., Rehm M.X., et al. Estimates of alcohol-related oesophageal cancer burden in Japan: systematic review and meta-analyses. Bull World Health Organ. 2015;93(5):329–338. doi: 10.2471/BLT.14.142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y., Liu L., Chen H., Wang Y., Xu Y., Mao H., et al. Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell. 2016;29(5):711–722. doi: 10.1016/j.ccell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou B., Zang R., Zhang M., Song P., Liu L., Bie F., et al. Worldwide burden and epidemiological trends of tracheal, bronchus, and lung cancer: A population-based study. EBioMedicine. 2022;78 doi: 10.1016/j.ebiom.2022.103951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 36.Lagergren J., Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63(4):232–248. doi: 10.3322/caac.21185. [DOI] [PubMed] [Google Scholar]
- 37.Prabhu A., Obi K.O., Rubenstein J.H. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109(6):822–827. doi: 10.1038/ajg.2014.71. [DOI] [PubMed] [Google Scholar]
- 38.Qu X, Ben Q, Jiang Y. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Epidemiol. 2013;23(12):762–70.e1. [DOI] [PubMed]
- 39.Vokinger K.N., Hwang T.J., Grischott T., Reichert S., Tibau A., Rosemann T., et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost-benefit analysis. Lancet Oncol. 2020;21(5):664–670. doi: 10.1016/S1470-2045(20)30139-X. [DOI] [PubMed] [Google Scholar]
- 40.Whiteman D.C., Sadeghi S., Pandeya N., Smithers B.M., Gotley D.C., Bain C.J., et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57(2):173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 41.Coleman H.G., Xie S.H., Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology. 2018;154(2):390–405. doi: 10.1053/j.gastro.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 42.Powell H.A., Iyen-Omofoman B., Hubbard R.B., Baldwin D.R., Tata L.J. The association between smoking quantity and lung cancer in men and women. Chest. 2013;143(1):123–129. doi: 10.1378/chest.12-1068. [DOI] [PubMed] [Google Scholar]
- 43.Harris R.E., Zang E.A., Anderson J.I., Wynder E.L. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol. 1993;22(4):592–599. doi: 10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- 44.van Putten M., de Vos-Geelen J., Nieuwenhuijzen G.A.P., Siersema P.D., Lemmens V., Rosman C., et al. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer. 2018;94:138–147. doi: 10.1016/j.ejca.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 45.Helminen O., Sihvo E., Gunn J., Sipilä J.O.T., Rautava P., Kytö V. Trends and results of oesophageal cancer surgery in Finland between 2004 and 2014. Eur J Cardiothorac Surg. 2020;57(1):107–113. doi: 10.1093/ejcts/ezz189. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Pan YM, Wang TT, Gao DJ, Hu B. Endotherapy versus surgery for early neoplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc. 2014;79(2):233–41.e2. [DOI] [PubMed]
- 47.Huang J., Xu J., Chen Y., Zhuang W., Zhang Y., Chen Z., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 48.Popkin B.M., Adair L.S., Ng S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Black-white disparities in health care. Jama. 1990;263(17):2344–6. [DOI] [PubMed]
- 50.Taylor L.A., Udeagbala O., Biggs A., Lekas H.M., Ray K. Should a Healthcare System Facilitate Racially Concordant Care for Black Patients? Pediatrics. 2021;148(4) doi: 10.1542/peds.2021-051113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.