Take Home Message
In this study with the longest follow-up to date, an ultrahypofractionated stereotactic boost after external beam radiotherapy showed low genitourinary and gastrointestinal toxicity in patients with intermediate-risk prostate cancer.
Keywords: Intermediate-risk prostate cancer, Prostate cancer, Stereotactic body radiotherapy, Stereotactic body radiotherapy boost
Abstract
Background
Genitourinary (GU) or gastrointestinal (GI) complications and tumor relapse can occur in the long term after radiotherapy for prostate cancer.
Objective
To assess the late tolerance and relapse-free survival (RFS) in patients undergoing hypofractionated stereotactic boost therapy after external beam radiotherapy (EBRT) for intermediate-risk prostate cancer.
Design, setting, and participants
Seventy-six patients with intermediate-risk prostate carcinoma between August 2010 and April 2013 were included. The first course delivered a dose of 46 Gy by conventional fractionation; the second course was a boost of 18 Gy (3 × 6 Gy) within 10 d.
Outcome measurements and statistical analysis
GU and GI toxicities were evaluated as the primary outcomes. The secondary outcomes were overall survival and RFS. The cumulative incidence of toxicity was calculated using a competing-risk approach. Overall survival and RFS were estimated using the Kaplan-Meier method.
Results and limitations
The median follow-up period was 88 mo (range, 81–99 mo). Sixty (79%) patients were treated with the CyberKnife and 16 (21%) using a linear accelerator. The cumulative incidences of GU and GI grade ≥2 toxicities at 120 mo were 1.4% (95% confidence interval [CI]: 0.1–6.6%) and 11.0% (95% CI: 5.1–19.4%), respectively. The overall survival and RFS rates at 8 yr were 89.1% (95% CI: 77–95%) and 76.9% (95% CI: 63.1–86.1), respectively.
Conclusions
A very long follow-up showed low GU and GI toxicities after a hypofractionated stereotactic boost after EBRT for intermediate-risk prostate cancer. Dose escalation of the boost delivered by hypofractionated radiation therapy appears safe for use in future trials.
Patient summary
We found low toxicity and good survival rates after a short and high-precision boost after external beam radiotherapy for intermediate-risk prostate cancer, with a long-term follow-up of 88 mo. This long-term treatment is safe and should be considered in future trials.
1. Introduction
Prostate cancer is the second most common cancer and the fifth most common cancer worldwide in terms of mortality in men [1]. According to the National Comprehensive Cancer Network guidelines, radiation therapy is recommended for men with intermediate-risk prostate cancer [2]. External beam radiation therapy (EBRT) or brachytherapy (BT) alone may be used for favorable intermediate-risk cancer. In cases of unfavorable risk, a low-dose rate (LDR) or a high-dose rate BT boost can be used together with EBRT and androgen deprivation therapy (ADT). However, despite improved biochemical relapse-free survival (RFS) rates, the addition of BT is associated with increased toxicity, especially urinary toxicity [3], [4].
Stereotactic body radiation therapy (SBRT) may provide an alternative to BT. It delivers high doses in a limited number of fractions with high accuracy [5]. Despite higher doses per fraction, in patients treated exclusively by SBRT, the toxicity profile has been shown to be comparable with that with conventionally fractionated radiotherapy [6], [7]. SBRT has been evaluated as a boost after or before EBRT in very few small prospective trials with short follow-up periods [8], [9], [10], [11]. A 5-yr analysis reported favorable gastrointestinal (GI) and genitourinary (GU) toxicity profiles and RFS results [12].
Moderately hypofractionated EBRT is the current standard of care for prostate cancer. The median follow-up period in randomized trials ranges from 5 to 6 yr [13], [14], [15]. Similarly, the median follow-up period in randomized trials comparing ultrahypofractionated and conventionally fractionated EBRT ranges from 2 to 5 yr [16], [17]. Very late toxicity is an important issue because some complications, particularly urinary complications, may occur later after hypofractionated radiotherapy for prostate cancer. For example, in a randomized phase 3 study comparing normofractionated (80 Gy/40 fractions) and hypofractionated (62 Gy/20 fractions) radiotherapy, the actuarial incidences of macroscopic hematuria at 8 yr (after censoring hematuria related to bladder cancer) were 4% and 18% after normofractionated and hypofractionated radiotherapy, respectively (hazard ratio: 4.96, 95% confidence interval [CI]: 1.4–17.2, p = 0.012) [18]. Determining late recurrence-free survival is also an important goal, as recurrence can occur >5 yr after radiotherapy. We aimed to assess the long-term tolerance and survival of patients who underwent a hypofractionated stereotactic boost after conventional radiotherapy for intermediate-risk prostate cancer.
2. Patients and methods
2.1. Patient selection
The design and methodology of this national phase 2 multicenter study conducted at four centers in France have been described previously [12]. Patients were included if they had histologically proven intermediate-risk prostate adenocarcinoma according to the D’Amico classification, Eastern Cooperative Oncology Group performance status ≤1, prostatic volume ≤80 cc, no metastasis, no prior prostate cancer treatment (prostatectomy, chemotherapy, hormonotherapy >3 mo) or pelvic irradiation, an International Prostate Symptom Score (IPSS) of ≤10, and life expectancy of ≥10 yr.
The protocol was approved by the local ethics board (Comite de Protection des Personnes Nord Ouest IV; meeting May 11, 2010; reference CPP 10/24), and the study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent prior to participation. This study was registered at ClinicalTrials.gov (NCT01596816).
2.2. Treatment planning and delivery
The first clinical target volume (CTV1) was the prostate and the proximal half of the seminal vesicles, whereas the CTV2 boost was the prostate only. Magnetic resonance imaging (MRI) and computed tomography registration based on intraprostatic fiducials are mandatory to define CTVs. The planning target volume for the first part of the treatment (PTV1) was defined as the addition of a 1-cm margin around the corresponding CTV1 and lowered to 0.5 cm posteriorly to spare the rectum. PTV2 was obtained by expanding 5 mm around the CTV2. During the first part of treatment, 23 fractions (2 Gy/session) were delivered at a total dose of 46 Gy using three-dimensional conformal radiotherapy or intensity-modulated radiation therapy (IMRT).
During the second treatment, a hypofractionated stereotactic boost (three fractions of 6 Gy) was delivered every other day over 5–9 d for a total dose of 18 Gy. The choice of the radiation technique varied according to the center. The planning requirements were the same for the CyberKnife-based and the linear accelerator–based treatments. Of the PTV, 95% received 18 Gy, with a maximum dose of 115% of the prescribed dose. When feasible, the dose to the urethra volume + 3 mm had to be as close as possible to 6 Gy and <6.5 Gy per fraction. Patients did not receive any concomitant or adjuvant ADT.
2.3. Study endpoints and follow-up
The primary outcomes were GI and GU toxicities according to the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) scale. Acute and late toxicities were assessed every 3 mo after boost irradiation for 1 yr, every 6 mo for 2 yr, and annually thereafter. The secondary outcome measures included overall survival (OS), RFS, and time to relapse. Digital rectal examinations and prostate-specific antigen (PSA) assessments were performed every 3 mo for up to 1 yr, every 6 mo for up to 3 yr, every year for up to 5 yr, and then every year.
The follow-up period was extended to 5 yr on an annual schedule, except for the IPSS and the five-item International Index of Erectile Function evaluations. After the end of the follow-up protocol, the patients benefited from yearly follow-up under the same conditions as the study protocol.
2.4. Statistical analyses
The analyses were performed on an intent-to-treat basis. Categorical variables were described as numbers and proportions (95% confidence interval [CI]). Continuous variables were summarized using medians and ranges.
The cumulative toxicity incidence was calculated using a competing-risk approach considering the time interval from the end of radiotherapy to the occurrence of toxicity, with death without toxicity considered a competing event and patients alive without toxicity censored at the date of the last follow-up.
The survival function was estimated from inclusion using the Kaplan-Meier method. OS was calculated by considering the time to death from any cause. RFS was estimated based on the time to biochemical progression, local or distant relapse, or death from any cause. Time to relapse was estimated by considering the time to biochemical progression and local or distant relapse. For all survival functions, patients alive and without events were censored at the time of the last news article. Stata version 15.0 (Stata Statistical Software: Release 15, 2017; StataCorp LLC, College Station, TX, USA) was used for statistical analyses.
3. Results
3.1. Patients
Seventy-six patients were prospectively included in this study between August 2010 and April 2013, 79% of whom were treated with CyberKnife and 21% with a linear accelerator. The clinical and pathological characteristics of the patients are presented in Table 1. The median follow-up period was 88 mo (range, 81–99 mo). All patients were included in the long-term toxicity and efficacy analyses. Twelve patients were lost to follow-up before the study date, with no evidence of toxicity or recurrence.
Table 1.
Patient and disease characteristics
| Patients | ||
|---|---|---|
| (n = 76) | ||
| Age, median (range) | 71 | 45–84 |
| BMI (kg/m2) | ||
| Underweight (<18.5) | 1 | 1.4 |
| Normal (18.5–25) | 27 | 37.0 |
| Overweight (25–30) | 32 | 43.8 |
| Obese (≥30) | 13 | 17.8 |
| Unknown | 3 | |
| Diabetes | 10 | 13.2 |
| Smoking history | ||
| Nonsmoker | 40 | 52.6 |
| Previous smoker | 30 | 39.5 |
| Active smoker | 6 | 7.9 |
| T TNM | ||
| T1c | 33 | 43.4 |
| T2a | 16 | 21.1 |
| T2b | 24 | 31.6 |
| T2c | 3 | 3.9 |
| N TNM | ||
| N0 | 75 | 98.7 |
| Nx | 1 | 1.3 |
| M TNM | ||
| M0 | 76 | 100 |
| Gleason score | ||
| 6 | 18 | 23.7 |
| 7 | 58 | 76.3 |
| 3 + 4 | 40 | 52.6 |
| 4 + 3 | 18 | 23.7 |
| Rectal examination | ||
| Normal | 30 | 40.5 |
| Tumoral | 44 | 59.5 |
| Missing | 2 | |
| WHO performance status | ||
| 0 | 67 | 88.2 |
| 1 | 9 | 11.8 |
BMI = body mass index; IQR = interquartile range; TNM = tumor, node, metastasis; WHO = World Health Organization.
Data are median (IQR, range) or n (%).
3.2. Toxicity
Grade ≥2 acute GI and GU toxicities occur in 13.2% and 23.7%, respectively [12].
3.2.1. Late GU toxicity
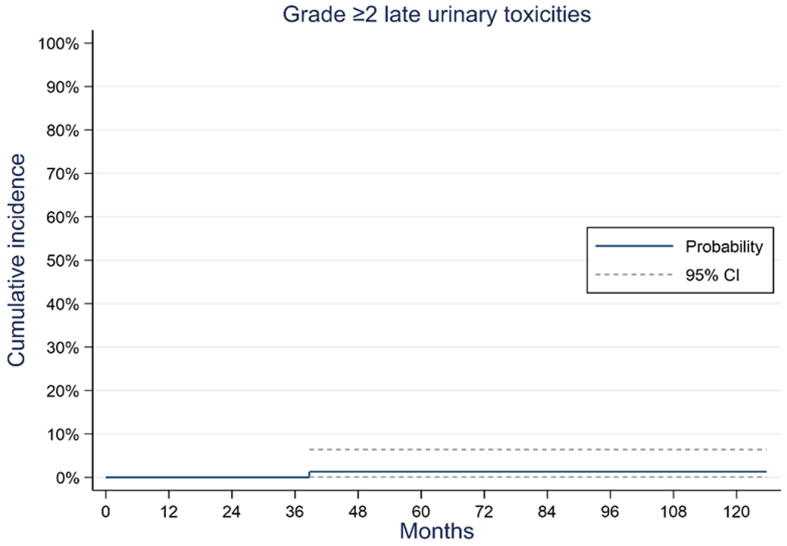
One patient (1%) presented with grade 2 late toxicity of micturition urgency related to the treatment. No late grade >2 toxicity events were observed. The cumulative incidences of urinary grade ≥1 toxicity were 38.8% (95% CI: 27.6–49.3%) and 41.1% (95% CI: 29.5–52.2%) at 72 and 120 mo, respectively. The cumulative incidence of urinary grade ≥2 toxicity at 120 mo after the end of radiotherapy was 1.3% (95% CI: 0.1–6.4%; Fig. 1).
Fig. 1.
Cumulative incidence of grade ≥2 late genitourinary toxicities. CI = confidence interval.
3.2.2. Late GI toxicity
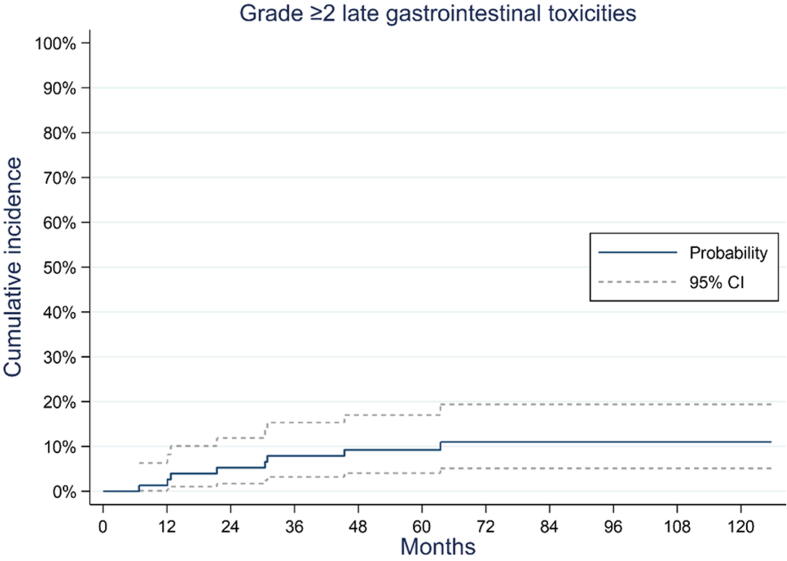
Nine patients (10.5%) presented with grade ≥2 GI toxicity: eight patients had radiation proctitis (n = 5, grade 2; n = 3, grade 3); one of these patients presented with grade 3 proctitis and grade 3 rectal hemorrhage. All toxicities were treatment related. The cumulative incidence of GI grade ≥2 toxicity at 120 mo after the end of radiotherapy was 11.0% (95% CI 5.1–19.4%; Fig. 2).
Fig. 2.
Cumulative incidence of grade ≥2 late gastrointestinal toxicities. CI = confidence interval.
3.3. Survival
Eight patients (10.5 %) died during the study. One death was related to metastatic recurrence of prostate cancer 52 mo after inclusion. The 96-mo OS rate was 89.1% (95% CI: 77–95%). The 96-mo RFS rate was 76.9% (95% CI: 63.1–86.1; Fig. 3). The time to relapse at 96 mo was 85.7% (95% CI: 72.2–92.9).
Fig. 3.
Kaplan-Meier curve for (A) overall survival and (B) relapse-free survival. CI = confidence interval.
4. Discussion
The primary goal of this study was to assess the very long-term safety and efficacy of boosting SBRT after EBRT for intermediate-risk prostate cancer. We showed that an SBRT boost was well tolerated, with very low GU and low GI toxicities at 10 yr. Interestingly, these toxicities did not increase after 5 yr. Our study is a prospective series of an SBRT boost with the longest follow-up.
BT as a boost is an extensively studied technique, and its superiority over EBRT has been reported by several researchers. Randomized trials have tested the benefit of adding BT to EBRT, and boosting provided improved biochemical control [3], [4], [19], [20]. The clinical benefit of adding a BT boost, on the contrary, is not clear, and biochemical control should not be considered a surrogate for survival [21]. Rodda et al. [3] analyzed the GU and GI morbidity profiles of patients treated in the ASCENDE-RT trial. At 5 yr, the cumulative incidence of grade 3 GU toxicity was significantly higher in the LDR-BT arm than in the EBRT arm (18.4% vs 5.2%, p < 0.001). In another randomized trial that included 218 patients, the prevalence of urinary toxicity was significantly higher in the EBRT + high-dose BT arm at 5.5 yr (14% vs 0%, p = 0.02) [20]. According to the current standards, the control arm received a relatively low-dose treatment. Both treatments were equitoxic in terms of long-term grade 3+ late urine and bowel events [22].
An SBRT boost could be a noninvasive alternative to a BT boost after EBRT in intermediate- and high-risk prostate cancer. An SBRT boost has been evaluated in a few early-phase prospective studies, with a short follow-up period of <5 yr [9], [10], [11], except for one [8]. In three of the five series, including ours, a rectal spacer was not used [8], [9]. The SBRT boost was delivered in two to three fractions, except in one phase 1 trial [8].
In our series, a boost of 18 Gy (3 × 6 Gy) was prescribed after the first course of EBRT delivering 46 Gy, and the cumulative incidences of GU and GI grade ≥2 toxicities at 120 mo were 1.3% (95% CI: 0.1–6.4%) and 11.0% (95% CI: 5.1–19.4%), respectively. The incidence of urinary toxicity was lower in our study than that reported in the literature (Table 2). This could be related to the total boost dose, homogeneous dose distribution (maximum of 115% of the prescribed dose), and urethral sparing (dose to the urethra + 3 mm had to be as close as possible to 6 Gy and lower than 6.5 Gy per fraction). Our CTV-to-PTV margins were not small (5 mm) and did not seem to be the cause.
Table 2.
Main prospective trials of external beam radiotherapy (EBRT) + brachytherapy (BT) or stereotactic body radiotherapy (SBRT) boost in prostate cancer
| Type of study | N | D’Amico risk group | Dose/technique | Pelvic radiotherapy/ADT | Median follow-up (yr) | bPFS | Late GU G2 toxicity | Late GU G3 toxicity | Late GI toxicity | Toxicity scale | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rodda et al. [3], Morris et al. [4] | Phase 3 | 398 | High risk 69% | EBRT 46 Gy + LDR BT 115 Gy vs EBRT 78 Gy in 39 fractions | Yes/yes | 6.5 | 86% vs 62% at 9 yr p < 0.001 | NS | 18% vs 5.2% at 5 yr p < 0.001 | Grade 3 8.1% vs 3.2% p = 0.124 | LENT-SOMA |
| Hoskin et al. [20], [22] | Phase 3 | 218 | High risk 53% Intermediate risk 42% | EBRT 35.75 Gy in 13 fractions + HDR BT 2× 8.5 Gy vs EBRT 55 Gy in 20 fractions | No/yes | 10.9 | 71% vs 55% at 6 yr p = 0.008 | “Actuarial incidence of severe adverse events” 38% vs 42% at 12 yr p = 0.6 | Urethral strictures 8% vs 10% at 12 yr p = 0.3 | “Actuarial incidence of severe adverse events” 7% vs 8% at 12 yr | Adapted version of the Dische Scales |
| Sathya et al. [19] | Phase 3 | 104 | High risk 60% Intermediate risk 40% | EBRT 40 Gy + LDR BT 35 Gy vs EBRT 66 Gy in 33 fractions | No/no | 8.2 | 71% vs 39% at 5 yr p = 0.0024 | NS | Grade 3 or 4: 13.7% vs 4% p = 0.09 | Grade 3 or 4: 4% vs 2% p = 0.61 | NCI CCTG CTC |
| Alayed et al. [8] | Phase 1 | 30 | Intermediate risk 100% | 10, 12.5, and 15 Gy in 1 SBRT fraction before EBRT 37.5 Gy in 15 fractions | No/no | 6 | Cumulative incidence of biochemical failure: 7.7% at last follow-up | 43% (95% CI: 25–62) | 3.3% (95% CI: 0.1–17) | Grade 2: 26.6% (95% CI: 12.3–45.9%) No grade 3 Grade 4: 3.3% (95% CI: 0.1–17.2%) | CTCAE |
| Eade et al. [11] | Phase 1 | 36 | High risk 64% Intermediate risk 36% | 20, 22, and 24 Gy in 2 SBRT fractions combined with EBRT 46 Gy in 23 fractions | No/yes in 61% of the patients | 2 | 93% at 3 yr | 19.3% at 2 yr | 0 | No late grade 2+ | CTCAE |
| Pryor et al. [10] | Phase 2 | 135 | High risk 24% Intermediate risk 76% | 19–20 Gy in 2 SBRT fractions before EBRT 46 Gy in 23 fractions | No/yes in 36% of the patients | 2 | 98% at 2 yr | 23% at 2 yr | 2% at 2 yr | Grade 2+: 4.5% | CTCAE |
| Kim et al. [9] | Phase 1/2a | 26 | High risk 100% | EBRT 44 Gy in 20 fractions before 18 or 21 Gy in 3 SBRT fractions | Yes/yes | 3 | 88% at 3 yr | 4% at 2 yr | 0 | Grade 2: 4% at 2 yr | CTCAE |
| Our series | Phase 2 | 76 | Intermediate risk 100% | EBRT 46 Gy in 20 fractions before 18 Gy in 3 SBRT fractions | No/no | 7.3 | Relapse-free survival 76.9% (95% CI: 63.1–86.1) at 8 yr | 1.3% (95% CI: 0.1–6.4%) at 10 yr | 0 | Grade 2+: 11% (95% CI: 5.1–19.4%) at 10 yr | CTCAE |
ADT = androgen deprivation therapy; bPFS = biological progression–free survival; CI = confidence interval; CTCAE = Common Terminology Criteria for Adverse Events; GI = gastrointestinal; GU = genitourinary; HDR = high dose rate; LDR = low dose rate; NCI CCTG CTC = National Cancer Institute of Canada Clinical Trials Group expanded common toxicity criteria; NS = not specified.
In the phase 1 trial delivering a single-fraction SBRT boost, 30 patients were accrued to 10, 12.5, or 15 Gy single fraction before EBRT (37.5 Gy in 15 fractions). Two of the ten patients in the 15 Gy cohort developed late grade ≥3 GI and GU toxicities, with a median follow-up of 72 mo [8]. Another phase 1 sequential dose escalation evaluated 20, 22, and 24 Gy to the prostate in two SBRT fractions, combined with 46 Gy in 23 fractions of EBRT. Thirty-six men with intermediate- and high-risk prostate cancer were enrolled. Late grade 2 GU toxicity at 2 yr was 19.3%, with a median follow-up of 24 mo [11]. The largest study was that of a phase 2 trial, which included 135 patients (76% intermediate risk and 24% high risk) and evaluated 19–20 Gy in two fractions delivered 1 wk apart, followed by conventionally fractionated EBRT (46 Gy in 23 fractions). The median follow-up duration was 24 mo. The cumulative incidences of late grade ≥2 and grade 3 GU toxicities were 24.9% and 2.2%, respectively [10].
In accordance with our series, but with a shorter follow-up and only 26 patients, Kim et al. [9] evaluated a scheme combining whole pelvic EBRT of 44 Gy in 20 fractions and two boost doses of 18 Gy and 21 Gy in three fractions. The median follow-up duration was 35 mo, and the incidences of late grade 1–2 GU and GI toxicities were 12% and 8%, respectively. According to the authors, a boost dose of 21 Gy in three fractions seems appropriate for further studies [9]. Indeed, in the light of the excellent very long-term safety of the regimen evaluated in our series, a slightly higher dose seems possible for the boost in further studies, particularly in patients with high-risk cancers.
All these studies evaluated a whole-gland SBRT boost in combination with EBRT. A focal boost to the intraprostatic tumor, identified using multiparametric MRI, has been evaluated or is currently being investigated using conventional or hypofractionated IMRT or SBRT [23], [24], [25]. Nevertheless, in locally advanced cancers that largely invade the two lobes and/or seminal vesicles, for example, or in patients with intraprostatic lesions that are difficult to delineate, a whole-gland boost could still be used.
The long-term efficacy was satisfactory in our series using exclusive radiotherapy without ADT for intermediate-risk prostate cancer. Nevertheless, the Zumsteg et al.’s [26] classification was not used during the implementation of this trial, and the number of positive biopsies was not documented. According to the Zumsteg et al.’s [26] classification, based on the Gleason score, PSA level, and clinical stage, at least 40 patients (52.6%) had unfavorable intermediate-risk cancer in our series.
The main limitation of this study is the collection of data on long-term toxicities following the framework of the initial clinical trial. Nevertheless, after the end of the follow-up protocol, the patients benefited from yearly follow-up under the same conditions as the study protocol. The loss-to-follow-up rate was comparable with that seen in trials with a long follow-up [27] and did not seem to be related to poor study execution.
5. Conclusions
The very long-term results of the CKNO-PRO trial demonstrated that this combination of EBRT and SBRT was well tolerated and yielded good efficacy results. Considering the excellent very long-term safety of this regimen, a slightly higher boost dose seems possible. To our knowledge, no prospective study has been published that compares a brachy boost with an SBRT boost combined with EBRT. Our data provide a good basis to compare these schedules in future prospective studies on patients presenting with unfavorable intermediate- and high-risk prostate cancer.
Author contributions: David Pasquier had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Nickers, Lartigau.
Acquisition of data: Pasquier, Nickers, Peiffert, Maingon, Pommier, Lacornerie, Tresch, Barthoulot, Lartigau.
Analysis and interpretation of data: Tresch, Barthoulot.
Drafting of the manuscript: Pasquier, Peiffert, Barthoulot.
Critical revision of the manuscript for important intellectual content: Pasquier, Nickers, Peiffert, Maingon, Pommier, Lacornerie, Tresch, Barthoulot, Lartigau.
Statistical analysis: Barthoulot.
Obtaining funding: Lartigau.
Administrative, technical, or material support: None.
Supervision: None.
Other: None.
Financial disclosures: David Pasquier certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: The trial was funded by the Institut National du Cancer, PHRC-K 2009.
Acknowledgements: M Vanseymortier from the sponsorship unit at Centre Oscar Lambret, Lille, involved in the trial management - The data managers from Centre de Traitement des Données du Cancéropôle Nord-Ouest (CTD-CNO), who were in charge of the trial data management - The funders (PHRC-K 2009).
Data sharing: The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.
Associate Editor: Guillaume Ploussard
References
- 1.Gandaglia G., Leni R., Bray F., et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4:877–892. doi: 10.1016/j.euo.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffer E., Srinivas S., Antonarakis E.S., et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Cancer Netw. 2021;19:134–143. doi: 10.6004/jnccn.2021.0008. [DOI] [PubMed] [Google Scholar]
- 3.Rodda S., Tyldesley S., Morris W.J., et al. ASCENDE-RT: an analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:286–295. doi: 10.1016/j.ijrobp.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Morris W.J., Tyldesley S., Rodda S., et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Jackson W.C., Silva J., Hartman H.E., et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. 2019;104:778–789. doi: 10.1016/j.ijrobp.2019.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King C.R., Freeman D., Kaplan I., et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217–221. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 7.King C.R., Collins S., Fuller D., et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys. 2013;87:939–945. doi: 10.1016/j.ijrobp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Alayed Y., Loblaw A., Chu W., et al. Stereotactic body radiation therapy boost for intermediate-risk prostate cancer: a phase 1 dose-escalation study. Int J Radiat Oncol Biol Phys. 2019;104:1066–1073. doi: 10.1016/j.ijrobp.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y.J., Ahn H., Kim C.S., Kim Y.S. Phase I/IIa trial of androgen deprivation therapy, external beam radiotherapy, and stereotactic body radiotherapy boost for high-risk prostate cancer (ADEBAR) Radiat Oncol. 2020;15:234. doi: 10.1186/s13014-020-01665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pryor D., Sidhom M., Arumugam S., et al. Phase 2 multicenter study of gantry-based stereotactic radiotherapy boost for intermediate and high risk prostate cancer (PROMETHEUS) Front Oncol. 2019;9:217. doi: 10.3389/fonc.2019.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eade T., Hruby G., Booth J., et al. Results of a prospective dose escalation study of linear accelerator-based virtual brachytherapy (BOOSTER) for prostate cancer; virtual HDR brachytherapy for prostate cancer. Adv Radiat Oncol. 2019;4:623–630. doi: 10.1016/j.adro.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquier D., Peiffert D., Nickers P., et al. A multicenter phase 2 study of hypofractionated stereostatic boost in intermediate risk prostate carcinoma: a 5-year analysis of the CKNO-PRO trial. Int J Radiat Oncol Biol Phys. 2020;106:116–123. doi: 10.1016/j.ijrobp.2019.09.039. [DOI] [PubMed] [Google Scholar]
- 13.Dearnaley D., Syndikus I., Mossop H., et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catton C.N., Lukka H., Gu C.-S., et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 15.Lee W.R., Dignam J.J., Amin M.B., et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–2332. doi: 10.1200/JCO.2016.67.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tree A.C., Ostler P., van der Voet H., et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2022;23:1308–1320. doi: 10.1016/S1470-2045(22)00517-4. [DOI] [PubMed] [Google Scholar]
- 17.Widmark A., Gunnlaugsson A., Beckman L., et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 18.Sanguineti G., Arcidiacono F., Landoni V., et al. Macroscopic hematuria after conventional or hypofractionated radiation therapy: results from a prospective phase 3 study. Int J Radiat Oncol Biol Phys. 2016;96:304–312. doi: 10.1016/j.ijrobp.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Sathya J.R., Davis I.R., Julian J.A., et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23:1192–2119. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 20.Hoskin P.J., Rojas A.M., Bownes P.J., Lowe G.J., Ostler P.J., Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103:217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Xie W., Regan M.M., Buyse M., et al. Event-free survival, a prostate-specific antigen-based composite end point, is not a surrogate for overall survival in men with localized prostate cancer treated with radiation. J Clin Oncol. 2020;38:3032–3041. doi: 10.1200/JCO.19.03114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoskin P.J., Rojas A.M., Ostler P.J., Bryant L., Lowe G.J. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: mature 12-year results. Radiother Oncol. 2021;154:214–229. doi: 10.1016/j.radonc.2020.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Kerkmeijer L.G.W., Groen V.H., Pos F.J., et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: results from the FLAME randomized phase III trial. J Clin Oncol. 2021;39:787–796. doi: 10.1200/JCO.20.02873. [DOI] [PubMed] [Google Scholar]
- 24.Draulans C., van der Heide U.A., Haustermans K., et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother Oncol. 2020;147:92–98. doi: 10.1016/j.radonc.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Hannan R., Salamekh S., Desai N.B., et al. SABR for high-risk prostate cancer: a prospective multilevel MRI-based dose escalation trial. Int J Radiat Oncol Biol Phys. 2022;113:290–301. doi: 10.1016/j.ijrobp.2021.10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zumsteg Z.S., Spratt D.E., Pei I., et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Shahid N., Loblaw A., Chung H.T., et al. Long-term toxicity and health-related quality of life after single-fraction high dose rate brachytherapy boost and hypofractionated external beam radiotherapy for intermediate-risk prostate cancer. Clin Oncol. 2017;29:412–420. doi: 10.1016/j.clon.2017.01.042. [DOI] [PubMed] [Google Scholar]