Graphical abstract

Keywords: Parkinson's Disease, Gut-Brain Axis, Microbiome, Diet, Oxidative Stress, Neurodegeneration
Highlights
-
•
The crosstalk between the gut and the brain serves as a route for the spread of PD pathology in a bottom-up or top-down manner.
-
•
Gut dysbiosis is evident in PD patients and animal models and is associated with alterations in gut-derived microbial products and immune pathways promoting disease pathology.
-
•
Diet induces changes in the GM, with western diet evoking and Mediterranean diet preventing neuroinflammation and neurodegeneration associated with PD.
-
•
Gut-derived microbial products can be involved in the regulation of the immune system, the inflammation of both the intestine and the brain, and the integrity of intestinal epithelium and blood–brain barrier (BBB).
-
•
Therapeutic strategies that function by reversing gut dysbiosis and mitochondrial dysfunction may prove beneficial to treat PD pathology.
Abstract
Background
Parkinson's disease (PD) is a disease of ⍺-synuclein aggregation-mediated dopaminergic neuronal loss in the substantia nigra pars compacta, which leads to motor and non-motor symptoms. Through the last two decades of research, there has been growing consensus that inflammation-mediated oxidative stress, mitochondrial dysfunction, and cytokine-induced toxicity are mainly involved in neuronal damage and loss associated with PD. However, it remains unclear how these mechanisms relate to sporadic PD, a more common form of PD. Both enteric and central nervous systems have been implicated in the pathogenesis of sporadic PD, thus highlighting the crosstalk between the gut and brain.
Aim
of Review: In this review, we summarize how alterations in the gut microbiome can affect PD pathogenesis. We highlight various mechanisms increasing/decreasing the risk of PD development. Based on the previous supporting evidence, we suggest how early interventions could protect against PD development and how controlling specific factors, including our diet, could modify our perspective on disease mechanisms and therapeutics. We explain the strong relationship between the gut microbiota and the brain in PD subjects, by delineating the multiple mechanisms involved in neuroinflammation and oxidative stress. We conclude that the neurodetrimental effects of western diet (WD) and the neuroprotective effects of Mediterranean diets should be further explored in humans through clinical trials.
Key Scientific Concepts of Review: Alterations in the gut microbiome and associated metabolites may contribute to pathogenesis in PD. In some studies, probiotics have been shown to exert anti-oxidative effects in PD via improved mitochondrial dynamics and homeostasis, thus reducing PD-related consequences. However, there is a significant unmet need for randomized clinical trials to investigate the effectiveness of microbial products, probiotic-based supplementation, and dietary intervention in reversing gut microbial dysbiosis in PD.
Introduction
Parkinson’s Disease (PD) is the second most common neurodegenerative disorder estimated to affect 1–4% of the worldwide population (>60 years) [1]. The age of disease onset is widely variable: onset before 50 years of age, considered early-onset PD; between 20 and 50 years, referred to as young-onset PD; and younger than 20 years known as juvenile PD [2], [3], [4], [5], [6]. A study performed in 2018 documented that the prevalence of PD has increased by ∼50% globally from 1990 to 2017 [7].
The three main hallmarks of PD include: 1) aggregation of the protein ⍺-synuclein in the dopaminergic neurons in substantia nigra pars compacta (SNpc) and brain stem regions [8], 2) prion-like transneuronal propagation [9], and 3) progressive loss of dopaminergic neurons [8]. PD is clinically characterized by motor symptoms, including resting tremor, rigidity, bradykinesia, and non-motor symptoms of gastrointestinal (GI) and urinary dysfunction, constipation, depression, and cognitive dysfunction [10]. The aggregation of ⍺-synuclein is a multistep heterogeneous process. In native conditions, ⍺-synuclein occurs as a soluble monomeric unfolded protein. Misfolding of ⍺-synuclein into β-sheet structures is known to be potentiated by several factors such as environmental toxins [11], oxidative stress [12], and genetic mutations [13]. These β-sheet structures then self-assemble to produce oligomers, protofibrils, and eventually the irreversible and insoluble mature fibrils [14]. Mature fibrils accumulate into Lewy bodies (LBs) and Lewy neurites (LNs) in the dopaminergic neurons of the SNpc and are suggested to be responsible for impairments in the nervous system, resulting in neuronal damage and degeneration [15].
Around 5–10% of PD cases are inheritable and attributable to mutations in genes that cause monogenic forms of the PD disorder [16], [17], [18]. For the last two decades, intense genetic research in Parkinsonism has identified around 28 genes or loci associated with rare monogenic familial forms of PD with Mendelian inheritance [19], [20], [21], [22]. The remaining majority of the PD cases are sporadic, which are likely to be caused by gene-environment interplay [23], [24].
Among various environmental factors, diet, the gut microbiome (GM) and its metabolites have received huge scientific attention. Evidence of a dysfunctional gut-brain axis in PD emerged in the 1980′s, when the first reports of the occurrence of Lewy pathology in the enteric nervous system (ENS) were made [25], [26]. Since then, attempts have been made to model the spread of ⍺-synuclein pathology between the gut and the brain, in both bottom-up and top-down fashions. In this review, we aim to explore the bidirectional communication between the gut and the brain in relation to PD and to summarize the literature explaining the alterations in the GM and microbial products in PD subjects with a focus on diet-induced changes, and their implications on the development, etiology, and pathophysiology of the disease.
Bottom-up transmission of α-synuclein pathology
According to Braak’s hypothesis, sporadic PD can be attributed to an invasive pathogen that travels through nasal and gut routes and triggers ⍺-synuclein aggregation when microbial products from the gut contact ⍺-synuclein in olfactory or enteric neurons [27], [28]. The aggregated ⍺-synuclein spreads to the central nervous system (CNS) via the olfactory bulb and vagus nerve, from where consequently it reaches the SNpc [27], [28]. Furthermore, according to Braak’s staging in PD, ⍺-synuclein pathology first appears in the olfactory bulb and the brainstem regions and later spreads to the cortical regions [27], [29], [30]. Consistent with Braak’s hypothesis, several lines of evidence postulate multiple potential mechanisms for the initiation of ⍺-synuclein pathology in the ENS in PD. LB pathology is detected in vasoactive intestinal polypeptide (VIP) expressing neurons [26], in the ENS and GI tract in humans, and in VIP and cholinergic neurons in rodents [31], [32]. ENS dysfunction has been observed in 60–80% of PD patients resulting in non-motor symptoms such as chronic constipation [33], [34], much prior to the occurrence of motor symptoms [35]. ENS dysfunction is more common in people who develop PD, compared to those who do not develop PD [36] and may fasten PD pathogenesis by ten years prior to the onset of clinical symptoms [37]. Human postmortem investigations suggest that the vagus nerve serves as a route for spreading ⍺-synuclein pathology from the ENS to the CNS [27]. Interestingly, longitudinal human cohort studies from northern Europe reported that undergoing truncal vagotomy is associated with a lower risk of PD development compared to controls [1], [38], [39]. In ⍺-synuclein transgenic mice, ⍺-synuclein aggregates are detected in the ENS even before any pathogenicity is reported in the CNS [40], thus, suggesting the transmission of α-synucleinopathy in a bottom-up fashion.
Top-down transmission of α-synuclein pathology
Investigating whether degeneration of dopaminergic neurons and aggregation of ⍺-synuclein in the brain can affect GI tract functions is necessary, given the bidirectional communication between the gut and the brain [41]. Myenteric plexus is the primary nerve supply to the GI tract, which governs GI motility through motor pathways involving inhibitory neurotransmitters like nitric oxide and VIP, and excitatory neurotransmitters like acetylcholine [42], [43]. Thus, it may serve as a route for transmitting aggregated ⍺-synuclein from the brain to the gut. GI dysfunction associated with PD could result from pathology observed in the CNS inducing changes in the GI tract or alterations in the crosstalk between the brain and the gut. This theory has been comprehensively analyzed using the 6-Hydroxydopamine (6-OHDA) model of PD, where injection of the neurotoxin in medial forebrain bundle (MFB) or substantia nigra induces dopaminergic neuron degeneration in the SNpc. Pellegrini, Fornai [44] reported bowel inflammation in rats that were unilaterally injected with 6-OHDA in two sites of MFB. The enteric inflammation was associated with increase in oxidative stress, levels of pro-inflammatory cytokines, and colonic excitatory tachykininergic motility. Similarly, bilateral injection of 6-ODHA near SNpc in rats caused an elevation in the colonic and inflammatory markers levels and a decrease in acetylcholine levels, which normally enhances gut motility [45]. Mechanistically, it has been suggested that dopaminergic intranigral loss in 6-OHDA animals, significantly polarized intestinal macrophages toward M1-proinflammatory response, characterized by higher expression of inducible nitric oxide synthase (iNOS) over arginase-1, causing abdominal inflammation [44]. Recently, a study showed a preferential route of ⍺-synuclein transmission from the brain to the gut through the afferent and efferent fibers of vagus nerve, wherein overexpression of human ⍺-synuclein in the vagal system of rats resulted in its significant spread to the gastric nerve endings [46]. When a rat model was used in a different study, the overexpression of human ⍺-synuclein in rat midbrain led to its accumulation only within the efferent fibers [46]. Although the aforementioned studies depict the detrimental effect of dopaminergic neuron degeneration on ENS and the bidirectional transmission of ⍺-synuclein between the gut and the brain, they are not sufficient to pinpoint the origin of PD. It is suggested that the pathological features including enteric inflammation, constipation, and the presence of ⍺-synuclein aggregates in the gastric nerves are a consequence of altered neurotransmission and inflammatory pathways triggered by dopaminergic neuronal degeneration resulting in early ENS-related symptoms. The activation of the pro-inflammatory pathway could further exacerbate neuroinflammation both in the ENS and CNS leading to late-stage PD pathologies.
The role of gut microbiome in the neural communication between the gut and the brain
The composition of GM is subjected to significant alterations in response to traumatic brain injury and ischemia [47]. Most of the microbial species hosted in the human body reside in the intestines [48], where they can interfere with nutrient availability and absorption, and consequently affect the host system homeostasis.
The ENS interacts with the autonomic nervous system (ANS) and CNS through the sensory and motor neurons, and neurotransmitters. The neural pathway mediating this interaction depends mainly on afferent and efferent fibers of the vagus nerve [49]. The GM secretes neurotransmitters including, acetylcholine, serotonin, and dopamine, or neuroactive molecules like short-chain fatty acids (SCFAs), that can send signals to the brain [50], [51], [52], through the endocrine pathway.
Colonic biopsies of early PD patients show an accumulation of ⍺-synuclein in the enteric neurons prior to the appearance of motor symptoms [53], [54]. This suggests possible early aggregation of ⍺-synuclein in the ENS of PD patients followed by transmission to the brain in the later stages. Increased gut permeability has also been observed in early stages of PD, which is a major trigger of inflammation and may be associated with increased aggregation of ⍺-synuclein in the gut [55]. An increased oxidative stress is suggested to be a consequence of changes in GM that contributes to the aggregation of ⍺-synuclein in the gut [56]. Aggregated ⍺-synuclein can travel to the brain via the systemic or vagal routes. Considering the systemic route, increased intestinal inflammation and permeability can trigger systemic inflammation, which can enhance the permeability of the blood–brain barrier (BBB), resulting in activation of the microglia in the brain [57]. Considering the vagal route, ⍺-synuclein aggregates can spread in a prion-like manner from the enteric neurons to the brain via the vagus nerve through binding, uptake and internalization of pre-formed fibrils (PFFs) to either Lymphocyte Activation Gene 3 (LAG3) or neurexin1β [58], [59], which can then activate microglia in the brain. Interestingly LAG3 expression is upregulated in activated microglial cells [59]. Microglial activation from either route would result in increased oxidative stress, thus exacerbating neuroinflammation. Furthermore, microglial activation can potentiate aggregation and propagation of ⍺-synuclein, resulting in neurodegeneration and PD progression.
Additionally, altered gut-derived microbial products due to changes in the GM can also lead to oxidative stress and neuroinflammation through maturation of antigen-presenting cells [57]. Microbial peptides and metabolic products are also required for CNS microglia function and changes in microbiota may disturb the inflammatory homeostasis and indirectly affect PD progression and pathogenesis. Further, some GM organisms like Bacteroides vulgatus, Parabacteroides distasonis, Lactobacillus salivarius and Clostridium sps may influence neuroinflammatory signaling and affect PD pathogenesis and brain function [60], [61].
Emerging evidence has suggested that the GM may trigger inflammation by activating CD4+ T cell response. The microbiota-derived antigens may be mediators such as neurotransmitters, SCFAs, and other metabolites [62], [63], or structures that have molecular similarity to self-antigens (i.e. Lewy bodies) [57]. In either circumstance, activated CD4+ T cells would differentiate into T-helper-1 (Th1) and Th17 cells, that would release IFN-g, IL-17, and other mediators that would activate macrophages and neutrophils, and produce chronic inflammation in the gut [64]. The inflammation produced by Th1 and Th17 immunity may further promote ⍺-synuclein aggregation in the ENS and the subsequent spread to the CNS, as described before. Supporting this mechanism, enhanced inflammation with increased levels of pro-inflammatory markers such as IFNg, TNF, and IL-5 has been observed in colonic biopsies of PD patients [65], [66]. Recent studies in mice and humans have shown that oxidized forms of ⍺-synuclein serve as a major antigen for triggering T cell-mediated immune response in PD [67], [68], [69], [70]. Interestingly, Th17 cells also have been shown to kill stem cell-derived dopaminergic neurons by releasing IL-17A [71].
Several PD mouse model studies have supported the involvement of CD4+ T cells in neurodegeneration. For example, Brochard et al. (2009) showed that CD4+ T cells were majorly responsible for dopaminergic neuronal cell death in MPTP mouse model of PD, and the neuronal cell death was decreased in immunodeficient mice lacking CD4 [72]. Another study, using ⍺-synuclein overexpression model showed that ⍺-synuclein aggregation drives MHCII-dependent CD4+ T cell proliferation, while MHCII depletion produces neuroprotective effects [73]. Liu et al. (2016) has also showed that Th17 cells induce neuroinflammation and dopaminergic neuronal death in the MPTP mouse model of PD [74]. Evidently, CD4+ T cell responses, especially Th17 cells can induce neurodegeneration in PD, however, there are still many unresolved questions. For instance, the involvement of other immune cells and glial cells in this process needs to be explored. Whether the Th17 response occurs due to changes in the composition of the microbiome and microbial products has not yet been studied and identification of the antigen that activates the CD4+ T cell response in PD remains elusive.
PD development and progression are controlled by diet-associated changes in the gut microbiome
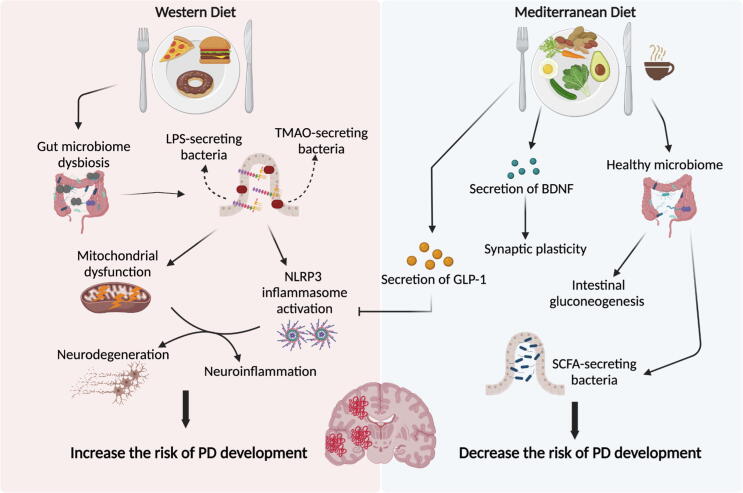
According to previous findings, diet has emerged as a promising potential factor that may prevent PD development or halt its progression. A high-fat and high-sugar diet, also called Western diet (WD), is considered a risk factor for neurodegenerative diseases [75] and it is correlated with the development of PD [76], [77]. Moreover, studies have shown that WD plays a detrimental role in the course of the disease, by aggravating the symptoms in PD patients (Fig. 1) [78]. Evidence suggests that GM dysbiosis or an abnormal microbiome directly promotes PD development [79]. The latter includes consequences such as neuroinflammation and neurodegeneration caused by mitochondrial dysfunction, insulin resistance, NLR family pyrin domain containing 3 (NLRP3) inflammasome activation, an increase in LPS-containing bacteria and a decrease in SCFA-producing bacteria [80].
Fig. 1.
The effects of Western and Mediterranean diet on PD development. Western diet is suggested to increase the risk of PD development through inducing GM dysbiosis, increase in LPS and SCFA production, induction of mitochondrial dysfunction and activation of NLRP3 inflammasome. These events contribute to increased neuroinflammation and neurodegeneration and consequently increase the risk of PD development. By contrast, adhering to a Mediterranean diet plays a neuroprotective role and decreases the risk of PD development. This type of diet, which includes coffee, enhances the production of SCFA and GLP-1, which as a result inhibits the NLRP3 inflammasome activation and prevents inflammation. Moreover, it increases the secretion of BDNF, thus, improving synaptic plasticity and learning. PD: Parkinson’s disease, LPS: lipopolysaccharide, TMAO: trimethyl-amine N-oxide, NLRP3: NLR family pyrin domain containing 3, GLP-1: glucagon-like peptide 1, BDNF: brain-derived neurotrophic factor, SCFA: short-chain fatty acids.
Unlike the WD, adherence to a diet rich in nuts, seeds, fresh fruits and vegetables, olive and coconut oils, and fresh herbs such as tea and coffee, which are all components of a Mediterranean diet (MeDiet), are thought to play a protective role and decrease the risk of PD development [81]. MeDiet promotes healthy microbiome metabolism and induces intestinal gluconeogenesis as well as the production of brain-derived neurotrophic factor (BDNF), thus enhancing synaptic plasticity and learning capacity, and glucagon like peptide 1 (GLP-1) which reduces neuroinflammation by suppressing NLRP3 inflammasome (Fig. 1) [82], [83]. Moreover, previous studies have reported an increase in SCFA levels and a decrease in the levels of trimethyl-amine N-oxide (TMAO), which is a gut microbiota-derived metabolite that is considered a biomarker for severe PD cases when present in high levels [84]. Results from a clinical trial have shown an improvement in cognitive functions including memory, attention and executive functions when following a MeDiet by PD patients [85]. Interestingly, ketogenic diet (KD), which is a low-carb and high-fat diet, ameliorates motor and cognitive sequelae observed in PD patients by inhibiting microglial activation [86], [87]. Given the different roles, targeting healthy dietary patterns, such as MeDiet and KD, would reveal striking outcomes, suggesting diet as a possible therapeutic intervention in PD subjects.
Human and animal studies unraveling the microbiome-gut-brain axis
Thus far, a notable amount of research has been conducted in both humans and animal models to study the involvement of the microbiota-gut-brain axis in PD. Human studies have focused on assessing the abundance of the GM in PD patients in comparison to controls, while murine and Drosophila model studies have attempted to delineate the mechanism behind GM changes and PD progression. The findings of some studies have been mentioned henceforward and reviewed in Table 1, Table 2, Table 3.
Table 1.
A summary of clinical studies showing gut-associated therapeutic approaches to target different PD symptoms.
| Study | Study design/ Sample | Clinical characteristics of PD patients | Therapeutic interventions | Main outcomes | Comments |
|---|---|---|---|---|---|
| Georgescu D. et. al [159] |
A random selection of 40 PD patients (17 males, 23 females; mean age 76.05 ± 2.09 years) between 2013 and 2014, were treated with levodopa or dopamine agonists. | NMS like GI complaints: abdominal pain, bloating, constipation with the sensation of incomplete defecation. | Supplementation of probiotics (60 mg, a mixture of two lactic bacteria: Lactobacillus acidophilus and Bifidobacterium infantis), 2×/day, 1 h after meals for 3 months. trimebutine daily (200 mg/day, 3×/dayN = 20 /group) was used as a positive control and comparative group. |
Decreased bloating and abdominal pain in PD patients when supplemented with probiotics, similar to trimebutine treatment. |
|
| Tamtaji et. al. [161] | A randomized, double-blinded, placebo-controlled clinical trial was done on 60 PD patients, aged 50 to 90 years. | Individuals with PD, diagnosed in accordance with the UK PD Society Brain Bank clinical diagnostic criteria, were included. | Daily 8 × 109 CFU probiotic supplementation or placebo (N = 30 each group) for 12 weeks. |
Probiotic consumption by individuals with PD for 12 weeks had useful impacts on MDS-UPDRS, insulin metabolism and a few metabolic profiles. |
|
| Tan et. al. [186] | A double-blinded, randomized, placebo-controlled, single-center trial. A total of 72 patients were included. | Eligible patients were aged 40 years or older and had a PD diagnosis assessed by movement disorder neurologists according to the Queen Square Brain Bank Criteria with symptom duration of at least six months. The participants matched Rome IV criteria for functional constipation per week for the past three months. | The subjects received either multi-strain probiotics capsules (N = 34) or identical-appearing placebo (N = 38), for 4 weeks. |
Multi-strain probiotics treatment significantly improved constipation-related measures (the number of SBM per week, stool consistency, quality of life-related to constipation, satisfaction with the intervention). |
|
| Xue et. al. [171] | A preliminary study recruited a total of 15 PD patients who received FMT treatment, 11 males and 4 females. The median age was 61 years old. | A thorough assessment of the patient’s conditions was conducted, including disease duration, Hoehn-Yahr classification, treatment drugs. | Healthy donors from patients’ non-relatives (18 to 24 years old) were selected. Fecal microbiota from donors was purified and isolated in the laboratory. The freshly purified fecal microbiota suspension was transplanted into the patient's gut in a short time (less than 1 hr duration between feces release and transplantation). FMT was performed via two routes: the colonic and nasointestinal routes. | Significant decrease in motor symptoms, anxiety, depression, and improved sleep quality in 10 PD patients that received FMT via colonoscopy, but not via nasointestinal tube. |
|
| Kuai et. al. [187] | A prospective, single- study, where 11 PD patients with constipation symptoms participated in the study. | None of the patients had abdominal pain, diarrhea, fever, or other adverse reactions. Baseline characteristics include the Hoehn-Yahr (H-Y) Grade, Unified Parkinson’s Disease Rating Scale (UPDRS) II Score, non-motor symptom scale (NMSS), PAC-QOL score, Wexner constipation score, body mass index (BMI) (mm/kg2). The median disease duration in PD patients was 7.18 ± 3.25 years. | Around 40 to 50 ml of frozen fecal microbiota was suspended, fresh every time, and transplanted into the intestine, within 2–4 min of the suspension, through a nasoduodenal tube. | FMT treatment improved the constipation symptoms in all patients, and changes in the gut microbiota composition. |
|
FMT, Fecal Microbiota Transplant; GI, gastrointestinal; H-Y, Hoehn-Yahr; NMS, nonmotor symptoms; NMSS, non-motor symptom scale; SBM, Spontaneous bowel movement; UPDRS, Unified Parkinson’s Disease Rating Scale.
Table 2.
A summary of some pre-clinical studies showing the induction of various PD models and gut-associated therapeutic approaches.
| References | Animal species and PD model | Sample | Procedure to generate PD model | Therapeutic investigations |
Characteristics of PD model & main outcomes |
Comments |
|---|---|---|---|---|---|---|
| Perez-Pardo et. al. [157] | Intrastriatal rotenone mouse model of PD; seven-week-old C57BL/6J mice. | N = 10/ diet group | Mice underwent stereotaxic surgery and 5.4 μg of freshly prepared rotenone (dissolved in 2 μl DMSO) was infused in their right striatum. | Dietary interventions after clear development of motor symptoms: Diet 1: uridine + DHA Diet 2: uridine + DHA + vitamins + prebiotics |
|
|
| St. Laurent et. al. [128] | Rotenone-induced Drosophila model of PD. | N = 20–25 adult flies | Rotenone (in DMSO) supplemented food was prepared in dry instant fly food (1–5 day old). | Treatment with 10 mM SB-supplemented food. |
|
|
| Rane et. al. [129] | A stepwise 6-OHDA striatal lesion ratmodel of PD in the pre-motor deficit stage (Adult male Long Evans rats (300–350 g)). |
N = 8/group | The 6-OHDA lesion protocol shows 23 % loss of substantia nigra dopamine cells and 27 % loss in striatal dopamine levels at 3 weeks post-lesion. |
Daily IP injections of SB treatment (250 mg/kg/ml in 0.9 % saline) starting 2 weeks post-surgery for 5 days during the dark phase of the light/dark cycle. |
|
|
| Hou et. al. [142] | MPTP Mouse Model of PD Male C57BL/6J mice (8–10 weeks old; 23–28 g weight). | N = 5/group for striatal and serum MPTP levels quantification | Mice received four IP injections of MPTP (14 mg/kg) at 2-h intervals. | Mice received IP injections of H2S slow-releasing compound (GYY) once daily 3 days before and 2 weeks after MPTP. |
|
|
| Kida et. al. [144] | MPTP Mouse Model of PD, Male C57BL/6J mice (8–10 weeks old). | N = 62 in MPTP group | Mice received four IP injections of MPTP (20 mg/kg). | Mice breathed air mixed with H2S (40 ppm; N = 31) for 8 h/day in custom-made plastic chambers. |
|
|
| Wu et. al. [173] | MPTP Mouse Model of PD, Male C57BL/6J mice (8 weeks old). | N = 5–8/ group | For MPTP intoxication, mice received four IP injections of MPTP-HCl (18 or 16 mg/kg) in saline at 2 hr intervals. | Mice were treated with minocycline twice daily (12 hr apart) via IP injections (1.4 to 45 mg/kg) in saline starting 30 min after the first MPTP injection and continuing for 4 more days following the last MPTP injection. |
|
|
| Nurrahma et. al. [160] | 6-OHDA-induced PD rat model. Male Sprague-Dawley (SD) rats (8 weeks old, 280–300 g). | N = 30, divided into 5 per group for therapeutic treatments | Rats received a unilateral 6-OHDA injection (9 μg per rat) into the right medial forebrain bundle. | Supplementation of probiotic Lactobacillus salivarius subsp. salicinius AP-32 (AP-32) and/or the prebiotic residues of microbial culture medium (RM). |
|
|
| Manfredsson et. al. [99] | Young adult (220 g) Sprague-Dawley male α-synuclein PFFs rats Non-human primate (NHP) α-Syn PFFs (Macaca fascicularis). |
N = 15 /group | Direct injection of PFFs into the myenteric layer of either the rat colon or the NHP colon and stomach. |
|
|
|
| Uemura et. al. [98] | Mouse α-synuclein PFFs (C57BL/6 J background). | N = 61 (total sample) | Inoculation of α-Syn PFFs into the gastrointestinal tract of 2-month-old mice. |
|
|
|
| Kim et. al. [100] | Mouse α-synuclein PFFs (C57BL/6 J background). | N = 11–12/ group | Intestinal intramuscular α-synuclein PFF injection for mice at 3 months of age. |
|
|
|
| Kelly et. al. [148] | LPS models of PD, C57BL/6J male mice (7–8 weeks old). | N = 25 in LPS group | Mice received one IP dose of LPS (2.5 mg/kg). |
|
|
|
| Julienne et. al. [101] |
Drosophila PINK1 and Parkin loss-of-function mutants. |
N = 54–57/ group for circadian rhythm analysis | Park25 and PINK1B9 null mutants, PINK1RV revertant allele controls and UAS-PINK1-RNAi Drosophila flies were used in the study. |
|
|
|
| Feltzin et. al. [105] | Parkinsonism models in Drosophila | Size varies depending on different experiment, e.g., 4 independent biological replicates of six individual guts each per age and genotype were used | The autosomal recessive Parkinsonism models parkin1, pink1B9, and a double knockout for the two DJ1 homologs in Drosophila, DJ-1α and DJ-1β (DJ-1 DKO) were used in the study. Males of ages 3 and 20 days were targeted |
|
|
6-OHDA, 6-hydroxydopamine; Akt, protein kinase B; α-syn, alpha synuclein; CNS, Central Nervous System; DAT, Dopamine Transporter; DHA, docosahexaenoic acid; DJ-1, Protein deglycase; DKO, double knockout; dmX, dorsal motor nucleus of the vagus nerve; ENS: Enteric Nervous System; GYY, slow-releasing compound; H2S, hydrogen sulfide; HO-1, heme oxygenase 1; IP, Intraperitoneal; LPS, Lipopolyssacharide; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NHP, Non-human primate; nNOS, neuronal nitric oxide synthase; PD, Parkinson’s Disease; PFFs, Preformed fibrils; Pink-1, PTEN-induced kinase 1; SB, Sodium Butyrate; SD, Sprague-Dawley; SN: substantia nigra; SNpc, substantia nigra pars compacta; SOD, superoxide dismutase; TH, Tyrosine Hydroxylase.
Table 3.
A summary of human and animal studies on gut microbiota dysbiosis and PD.
|
Human Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ref | Demographic | Test vs control group | Taxa decreased in PD | Taxa increased in PD | Alpha and Beta diversity | Association of microbiome changes | Functional prediction metagenomics | Comments |
| Hill-Burns et. al. [88] | USA | N = 197 PD patients N = 130 healthy controls of which 54 were spouses |
Lachnospiraceae, Pasteurellaceae, Verrocomicrobiaceae | Bifidobacteriaceae, Lactobacillaceae, Tissierellaceae, Christensenallaceae | Beta diversity differed in weighted and unweighted UniFrac. | PD medications Catechol-O-methyl transferase inhibitors, and anticholinergics (CarbiDopa and LevoDopa to a small extent) showed association with differences. Increased abundance of Ruminococcaceae was seen with disease duration. | Metabolism of plant-derived compounds and xenobiotics degradation. | - Good sample size and strong statistical analysis. - Compares the data with four previous studies with conflicting results. - Confirms some of the reported associations. |
| Barichella et. al., [89] | Italy | N = 350 including de novo drug naïve PD patients and other atypical Parkinsonisms N = 113 healthy controls including spouses, community controls and hospital controls |
Lachnospiraceae | Lactobacillacea, Christensenalliceae | Alpha diversity - PD patients showed higher richness than controls. Beta diversity - both weighted and unweighted UniFrac were significant in PD patients vs controls. |
-These differences were associated with higher cognitive impairment, gait disturbances, and postural instability. -Influence of drug use (catechol-o-methyltransferase inhibitors, proton pump inhibitors), fiber intake, and milk formula feeding on abundance of some of taxa was seen. |
Pathways involved in metabolism of amino acids, energy, glycans, lipids, cofactors, and vitamins. Increased ubiquitin processing and upregulation of xenobiotic degradation pathways. | - Extensive characterization of PD patients was done and have controlled for confounders. - The study investigates and highlights some differences in microbiome with atypical Parkinsonisms. -Their focus on de novo PD patients after adequate follow-up provides relevant insight. |
| Pietrucci et. al. [90] | Central Italy | N = 80 idopathic PD for longer than 6 months N = 72 healthy controls partners and caregivers |
Lachnospiraceae | Lactobacillaceae, Enterobacteriaceae and Enterococcaceae families & Entrococcus, Lactococcus, Klebsiella at the level of genera | Alpha diversity not significantly different for any of the indices. Beta diversity – differed between cases and controls for all the 4 matrices tested, with PD status as significant predictor of gut microbiota composition. | Lower levels of Lachnospiraceae and higher levels of Enterobacteriaceae families also correlated with increased disease severity and motor impairment. Abundance of some of the genera was influenced by drug use (Catechol-O-methyltransferase inhibitors) | Variance in genes involved in metabolism of short chain fatty acids and amino acids, and in lipopolysaccharide biosynthesis. Suggest changes in pathways favoring a pro-inflammatory environment in the gastrointestinal tract, and a reduction in the biosynthesis of amino acids acting as precursors of physiological transmitters. |
- Recruited patients had variable degree of PD and were receiving variable treatments. - Patients’ dietary intake was variable, which may influence microbiome composition. - Whether inflammation causes alterations in microbiome composition or vice versa is still unknown. |
| Qian et. al. [91] | China | N = 45 idiopathic PD patients N = 45 healthy spouses of the patients |
Lactobacillus, Sediminibacterium | Alistipes, Paraprevotella, Klebesiella, Sphingomonas, Acinetobacter, Aquabacterium, Desulfovibrio, Clostridium IV, Lachnospiracea incertae sedis, Butyricicoccus, Clostridium XVIII and Nitrososphaera (Kingdom Archaea) | Alpha diversity - significantly higher in PD than controls. Beta diversity significantly different in the unweighted but not weighted UniFrac between PD and healthy group. |
Correlation between fecal microbiota with disease duration, severity, and medication (LevoDopa equivalent doses, LED) was confirmed with most genera being negatively correlated. Genera Escherichia/Shigella were negatively associated with disease duration. Genera Dorea and Phascolarctobacterium were negatively associated with medication (LED) | Genes involved in metabolism of cofactors and vitamins, porphyrin and chlorophyll metabolism, and biotin metabolism, were higher in the fecal microbiome of the healthy group, whereas the microbial gene functions related to energy metabolism, flavone and flavonol biosynthesis, fatty acid biosynthesis, and apoptosis pathways were higher in the fecal microbiome of the PD group. |
|
| Heintz-Buschart et. al.[92] | Germany | N = 76 PD (DeNoPa cohort) + 21 Rapid Eye movement disorder N = 78 HC (DeNoPa cohort) |
Melainabacteria | Akkermansia, (Verrucomicrobiaceae) Prevotella | – | Within the PD cohort: Anaerotruncus spp., Clostridium cluster XIVa, and Lachnospiracea were related to motor symptoms, and Anaerotruncus, Akkermansia, and other (unclassified) were related to non-motor symptoms | – | - Paper supports that sample collection and preservation should be a standardized method to avoid variabilities. |
| Unger et. al.[123] | Germany | N = 34 PD patients N = 34 age-matched controls |
Bacteroidetes, Prevotellaceae, Faecalibacterium prausnitii, Lactobacillacea and Enterococcaciae | Enterobacteriaceae, Bifidobacterium | – | Drug (Entacapone) use: Changes in abundance of Firmicutes and Faecalibacterium prausnitzii | – | - Small sample size. - Preliminary data on young controls. |
| Keshavarzian et. al., 2015 (96) | USA | N = 38 PD (of which 12 drug naïve) N = 34 HCvolunteers |
At phylum level- Firmicutes At genus level - Roseburia, Blautia, Coprococcus | At phylum level - Bacteroidetes, Proteobacteria and Verrucomicrobia At genus level - Akkermansia, Oscillospira, Bacteroides | Alpha diversity - significant differences were observed at phyla level and significant difference in richness at genera level. | PD duration - Bacteroidetes and Proteobacteria positively correlated. Firmicutes negatively correlated. Significant negative correlation with Lachnospiraceae family. | LPS biosynthesis pathway, ubiquinone, and other terpenoid-quinone biosynthesis pathways were significantly upregulated in PD. Metabolic and biosynthetic pathways downregulated in PD. | - Compares both the sigmoidal mucosa-associated microbiome and the fecal microbiome in PD and controls thus providing more insight into microbiome associated with different locations in the GI tract. -Controls not well-matched. |
| Bedarf et. al.[126] | Germany | N = 31 (short duration, l-DOPA naïve) N = 28 age-matched and general demographics matched non-parkinsonian healthy controls. |
Prevotella copri, Eubacterium biforme, Clostridium saccarolyticum | Akkermansia muciniphila, Alistipes shahii | No difference in alpha or beta diversity. | Slight trend was seen with symptom severity and Eubacteria otherwise no significant associations with clinical data | Decreased Gucouronate degradation and higher tryptophan metabolism in PD | Merits: - Shotgun sequencing provides more detail than 16 s rRNA sequencing. - Viral metagenome was also mapped. Demerits - Low sample size cohort. |
| Petrov et. al. [127] | Russia | N = 89 PD patients N = 66 age matched and weight matched healthy controls |
Genus level - Dorea, Bacteroides, Prevotella, Faecalibaccerium. Species level - Bacteroides massiliensis, Stoquefichus massiliensis, Bacteroides coprocola, Blautia glucerasea, Dorea longicantena, Bacteroides dorei, Bacteroides plebeus, Prevotella copri, Coprococcus eutactus, Rumino coccuscallidus | Genus level - Christensenella, Catabacter, Lactobacillus, Oscillospira, and Bifidobacterium Species level - Christensenella minuta, Catabacter hongkongensis, Lactobacillus mucosae, Ruminocococcus bromii, Papillibacter cinnamivorans | Alpha diversity (chao1 index) reduced in PD group. Beta diversity - significant differences in weighted UniFrac. |
– | – | - Severity and stage of PD in the patients and treatment being received, if any, was not addressed. - Supporting evidence for microbial changes producing inflammation and and α-synuclein aggregation has not been provided. |
| Scheperjans et. al. [131] | Finland | N = 72 PD patients N = 72 age and sex matched controls |
Prevotellaceae | Alpha diversity –no difference. Beta diversity - significantly different in both weighted and unweighted UniFrac. | With disease severity - Prevotellaceae With postural and gait difficulty dominant. phenotype - Enterobacteriaceae was more abundant. |
– | - Temporal and causal relationship between microbiome alterations and PD is not clear. - Dietary habits of recruited subjects was not documented. |
|
| Jin et. al.[139] | China | N = 72 PD with 13 newly diagnosed N = 68 age- and lifestyle- matched healthy controls (family member or spouse) |
Prevotellacea and Lactobacillacea (in older PD group) Lachnospiraceae | Porphyromonadaceae, Christensenellaceae, Clostridialesvadin group, Rikenellaceae (in older PD patients) Turicibacteraceae, Ruminococcaceae, Rikenellaceae (in newly diagnosed) | Alpha diversity - Shannon and Simpson indices were higher in the newly diagnosed group. Beta diversity - significant difference based on unweighted but not weighted UniFrac. |
With disease severity, medication, and non-motor symptoms - Eggerthella, Prevotella, Turicibacter, Lactobacillus, and Enterococcus. With medical treatment - Streptococcus and Prevotella were negatively associated, and Turicibacter was positively correlated. |
Many different pathways involved in bacterial movement, motility, colonization, etc. were increased and pathways involved in biosynthesis and metabolism were lower in the PD. | - Recruited healthy spouses as controls. - Molecular mechanism through which microbiome has changed in PD not delinealted. |
| Animal Model Studies | ||||||||
| Ref | PD model | Test vs control group | Taxa decreased in PD | Taxa increased in PD | Alpha and beta diversity | Association of microbiome changes | Functional prediction metagenomics | Comments |
| Yang et. al. [93] | Rotenone Induced C57BL/6 mice | N = 5 (tested at 1, 2, 3 and 4 weeks of Rotenone treatment) N = 5 (tested at 0 weeks of Rotenone treatment) |
Bacteroidetes (at 3 weeks). Genus: Firmicutes Clostridium (1–4 weeks), Proteobacteria Sutterella (1–4 weeks), Firmicutes Lactococcus (1–3 weeks), Proteobacteria Desulfovibrio 2–4 weeks), Bacteroidetes Paraprevotella (1 and 3 weeks), Actinobacteria Adlercreutzia (2 weeks) |
Firmicutes (at 3 weeks). Genus: Firmicutes Lactobacillus (3 and 4 weeks) | Alpha diversity - No difference with ACE and Chao index. Decrease in Simpson's and Shannon's index at 3 weeks and decrease in Simpson's at 4 weeks. Beta diversity - significant difference at 3 weeks and 4 weeks. |
– | – | - Small sample size per group for 16S rRNA sequencing analysis. - Age of mice used may represent early stage of PD. - Merit: longitudinal study that correlates microbiome changes with motor and non-motor symptoms of PD. |
| Ghaisas et. al.[97] | MitoPark (MP) mice in C57BL/6 background (mitochondrial gene selectively removed from dopaminergic neurons using Cre/Lox system) | MP mice N = 8 (4 males and 4 females) Littermate controls N = 10 (5 males and 5 females) |
– | Prevotella genus (abundance increased with age progression) | Alpha diversity - Chao diversity index was not affected over time. Beta diversity − 36 % nominal difference between groups over time |
– | – | - Small sample size per group for 16S rRNA sequencing analysis. |
| Dehnow et. al.[109] | Rotenone-Induced & transgenic Drosophila melanogaster model | Flies treated with Rotenone and transgenic fly model Healthy controls and flies treated with Creatine (rescue) |
– | Acetobacter (A. pomorum and A. tropicalis) Lactobacillus (L. brevis, L. fructivorans andL. plantarum) | – | – | – | - Mechanism through which creatine rescues rotenone-induced PD is not understood. - Gender of the flies not specified. |
Human studies
One study used 16S rRNA gene sequencing to assess the microbial composition in the stools of 197 PD patients and 130 controls from the US [88]. The study identified 7 taxa at the family level, 8 at the genus level, and 13 at the OTU level that were associated with PD after adjusting for confounders of age, sex, diet, and medication. The results showed a decrease in levels of SCFA-producing bacteria, such as Lachnospiraceae. However, the authors pointed out that SCFA deficiency may be a consequence of PD, rather than a cause or a biomarker; hence, it is not considered unique to PD. Their functional predictions suggest an increase in pathways associated with xenobiotics degradation, which raises the hypothesis that xenobiotics, such as herbicides and insect repellents, could cause gut microbial dysbiosis and subsequent neurodegeneration.
After accounting for confounders, another study conducted on fecal samples of 193 PD patients and 113 healthy controls in Italy observed a decreased abundance of Lachnospiraceae family in PD patients [89]. The study reports that lower levels of Lachnospiraceae and higher levels of Christensenellaceae and Lactobacillaceae were associated with a poor clinical profile consisting of motor and cognitive deficits. Microbiota functional profiling highlighted alterations in bacterial metabolic pathways in PD patients compared to controls.
Another recent study on an Italian cohort assayed fecal samples from 80 PD patients and 72 healthy controls, in which the confounding factors of age, sex, and weight loss were accounted for [90]. A significant reduction in the levels of Lachnospiraceae family while a significant increase in the levels of Lactobacillaceae, Enterobacteriaceae, and Enterococcaceae families were observed in PD patients in comparison to controls. Altered levels of Enterobacteriaceae and Lachnospiraceae correlated with increase in severity of disease and motor deficits. Functional analysis indicated significant alterations in pathways involved in metabolism of SCFAs and amino acids, and pathways that favor inflammation in the GI tract.
Using 16S rRNA gene sequencing, a study assessed the changes in microbial compositions in the feces of 45 Chinese PD patients compared to their spouses that were considered healthy controls [91]. After adjusting for confounders of gender, age, constipation, and body mass index, a distinct fecal microbiome at the genus level was observed in PD patients in comparison to their healthy spouses. Functional analysis revealed alterations in pathways associated with biosynthesis, metabolism, and apoptosis in PD. The study also reported certain genera that were negatively associated with PD duration, levodopa equivalent doses, and cognitive impairment.
Interestingly, the alterations in GM were 80% similar between PD patients and idiopathic rapid eye movement (REM) sleep behavior disorder (a prodrome of PD) patients according to a study conducted on the DeNoPa cohort [92]. The study highlights the importance of conducting longitudinal studies on idiopathic REM sleep behavior disorder patients, as they are likely to develop ⍺-synuclein pathology. This would also allow characterization of alterations that take place in the GM as they potentially progress to the motor disease. Currently, it is challenging to conclude whether the changes in the GM composition are the cause or an effect of PD. However, as suggested earlier, alterations in the GM and its products may enhance neuronal loss due to increased inflammation and oxidative stress.
Murine studies
A recent study reported GI dysfunction and microbiome dysbiosis in a rotenone-induced mouse model of PD, prior to observing motor deficits and ⍺-synuclein pathology [93]. An overall reduction in the diversity of bacteria was observed in the fecal pellets using 16S rRNA sequencing. After three weeks of rotenone treatment, the ratio of Firmicutes/Bacteroidetes increased the phyla level, where an increase in this ratio has been associated with inflammatory conditions such as inflammatory bowel disease (IBD) [94], type 2 diabetes mellitus [95], and obesity [96]. The model recapitulates the features of exposure to environmental chemicals on the GM in PD.
Another study assayed GI function and GM alterations in the MitoPark transgenic mouse model of PD, which applies the cre/lox system to remove the mitochondrial transcription factor (TFAM) from dopaminergic neurons, resulting in the development of motor deficits associated with PD [97]. Similar to what is seen in PD patients, a decreased GI motility was observed at the age of eight weeks, preceding the onset of motor symptoms. As the condition progressed, constipation, intestinal inflammation and loss of dopaminergic neurons were observed at the age of 24 weeks. A transient increase in only the genus Prevotella was observed in the mice at ages 20 and 24 weeks in comparison to the controls, which is contradictory to other studies. It could be attributed to the fact that rotenone, used to induce PD in other models, can cause extreme colonic inflammation and aggregation of ⍺-synuclein, which is not observed in the MitoPark model. A transient increase in Prevotella could reflect its function in releasing pro-inflammatory factors and promoting intestinal inflammation.
Uemura, Yagi [98] inoculated ⍺-synuclein PFF into the gastrointestinal wall of 2-month-old mice and observed Lewy body-like aggregates in the dorsal motor nucleus of the vagus nerve, 45 days post-inoculation. These aggregates were positive for ⍺-synuclein phosphorylated at Ser129 (pS129-⍺-synuclein), a characteristic of LBs observed in PD. They observed abolishment of this aggregate formation when the mouse ⍺-synuclein PFF was inoculated after vagotomy was performed. The latter suggests that the vagus nerve was the route for retrograde transmission of the ⍺-synuclein aggregates. However, they reported a decrease in the pS129-⍺-synuclein-positive neurons in the dorsal motor nucleus of the vagus nerve, 12 months post-inoculation, after which no progression of the pathology was observed. The study suggests that merely seeding ⍺-synuclein PFF in mice does not completely mimic PD progression, and other factors may be required for further progression of the disease, such as exposure to environmental toxins like rotenone or paraquat, or presence of genetic mutations, such as single nucleotide polymorphisms in SNCA that cause ⍺-synuclein overexpression. Furthermore, the form of ⍺-synuclein PFF injected, and time and site of injection can result in varying outcomes.
Manfredsson, Luk [99] tested the hypothesis of the spread of ⍺-synuclein pathology from the gut to the brain by enteric injections of ⍺-synuclein PFF in young adult rats and non-human primates (NHP). Although they observed GI dysmotility and ⍺-synuclein pathology in rat ENS, it lasted for four months where the pathology was transient in the CNS. Histopathological analysis of NHP 12 months post-injection showed consistent ⍺-synuclein pathology in the ENS; however, no pathology was observed in the brain. More recently, Kim, Kwon [100] presented a novel mouse model of PD that recapitulates the spread of ⍺-synuclein pathology from the gut to the brain, where injection of mouse ⍺-synuclein PFF in the pyloric and duodenal muscularis layers of 3-month old wild-type mice resulted in spread of ⍺-synuclein pathology in a Braak staging-like manner. A crucial result of their study is that the vagus nerve and endogenous ⍺-synuclein mediate gut-to-brain transmission of the ⍺-synuclein pathology, and subsequent motor and non-motor dysfunctions and dopaminergic neurodegeneration. The discrepancy in results compared to the study by Manfredsson, Luk [99] could be attributed to the fact that the latter injected a smaller amount of similar size mouse ⍺-synuclein PFF, per body weight. Additionally, the injection was made at the descending colon, which is not densely innervated by the vagus nerve. Although notable advancements have been made in understanding the association between the microbiota-gut-brain axis and PD pathology, there remains a lack of understanding in origin of the disease, transmission mechanism, and pathways involved in disease progression. Thus, utilizing existing models and developing new robust PD gut-brain axis models are essential to further dissect the molecular pathways.
Drosophila studies
The proteins Pink1 and Parkin act jointly to maintain fidelity of the mitochondria and Drosophila loss-of-function mutations in the respective genes have been shown to display PD phenotypes such as impaired locomotion, dopaminergic neuron degeneration, mitochondrial abnormalities, and decreased longevity [101], [102], [103], [104]. DJ-1, or protein deglycase, detects oxidative stress and plays a role in protecting cells from the detrimental effects of reactive oxidative species. Feltzin [105] used loss-of-function Drosophila mutants for parkin, pink1, and DJ-1, to assess the gut-brain axis in PD. They observed a fivefold increase in microbial load in the guts of 20-day old parkin null flies in comparison to controls, with altered microbial abundance. Cell-type specific knockdown of parkin using RNAi revealed that it is required for maintaining homeostasis of the microbial load in gut enterocytes, but not in neurons or muscles. Additionally, germ-free parkin null flies were more resistant to paraquat in comparison to controls [105], suggesting the detrimental role of the GM in paraquat sensitivity. Evidently, parkin plays a crucial role in maintaining the abundance and composition of the GM, which has implications in PD.
Mutations in pink1 and parkin enhance mitochondrial dysfunction causing the loss of dopaminergic neurons with age [106]. The two mitochondrial proteins, Pink1 and Parkin, regulate the immune system in PD by inhibiting the generation of ROS-induced mitochondrial-derived vesicles and repressing the mitochondrial antigen presentation (MitAP) on MHC Class I Molecules [107]. A study performed using Drosophila highlighted the biological role of Pink1 in regulating mitochondrial dynamics [108]. Interestingly, overexpressing dPink1 promotes mitochondrial fission possibly through interacting with Fis1, a protein involved in the fission pathway. However, long continuous threads of mitochondria were observed in the case of Pink1 inhibition, indicating excessive fusion [109].
Using 16S rRNA sequencing, a recent study reported an increase in the abundance of Lactobacillus species of Lactobacillus brevis, Lactobacillus fructivorans, and Lactobacillus plantarum, and Acetobacter species of Acetobacter pomorum and Acetobacter tropicalis in rotenone-induced Drosophila model of PD in comparison to controls. Additionally, the abundance of bacterial species was reversed in rotenone-induced PD flies supplemented with the potent antioxidant, creatine. The study highlights the influence of diet on Drosophila gut microbial composition and potential benefit of supplementation with creatine in rotenone-induced Drosophila model of PD [109].
The afore-reviewed studies in the different PD animal models highlight the significant progress made in the field, however, they are not short of limitations. So far, there is no “best model” of PD that fully recapitulates all the pathological features of the disease. Additionally, there exists a considerable amount of variability in the approach adopted to induce PD in the various models, such as dosage of neurotoxins, site of injection, and age of the models. Hence, to further the fruitfulness of the pre-clinical research, animal models must be studied in a clinically relevant manner, with standardized and optimized protocols. Most of the clinical studies, which have assessed GM alterations in the context of PD usually follow a case-control design hence no conclusive causal and temporal relationship can be made about microbiome dysbiosis and PD. Currently, there is no consensus on the selection of healthy control groups, while most are age-matched, the recent studies with lifestyle-matched controls especially healthy spouses of the patients could be more reliable as lifestyle and diet have a huge impact on the gut microflora. However, there can be influence by gender and genetics. In these studies, many confounding factors have been controlled and accounted for, however many other uncontrolled confounders may affect results. Further, only a few studies have included de novo, drug naïve patients, and attempted statistical testing with and without inclusion of these patients. While some studies have been able to show a correlation between disease severity, duration and medication use to some of the taxa differences identified but with limited power. Restriction in the geographical area and demographic of the study subjects in each of these studies might explain the inconsistencies and limit the scope for any comprehensive conclusion.
Effect of gut-derived microbial products in PD pathology
The previously mentioned studies have shown the interplay between the alteration in GM composition and PD pathogenesis. The different molecules released by the GM can regulate the immune system, intestinal and neuronal functions, and BBB permeability (Fig. 2). The alterations in levels of some gut-derived microbial products in PD are discussed further henceforth.
Fig. 2.
Neuromodulatory effects of gut-derived microbial products. The GM releases several products that play pivotal roles in regulating the immune system, ENS, CNS, and integrity of the intestinal and blood–brain barrier. Molecules such as short-chain fatty acids (SCFAs), mucin-degrading enzymes, and hydrogen sulfide, through their effects on respective organ systems produce anti-inflammatory and neuroprotective effects. Lipopolysaccharide (LPS) on the other hand is proinflammatory and exacerbates oxidative stress and inflammation, which results in neurodegeneration.
SCFAs
SCFAs, such as acetate, propionate and butyrate, present in a proportion of 60:25:15 [110], are produced by the GM through anaerobic fermentation of dietary fibers and starch [111]. The ratio and amount of SCFAs primarily depends on food substrate; high-fibre diet leads to increased SCFAs and high-fat diet decreases SCFA concentration [112], [113], [114]. The SCFAs produced by microbiome comprises 99% of SCFAs produced in the human body [115], [116]. SCFAs produced by the GM are absorbed by enterocytes via either monocarboxylate transporter1 (MCT1) or sodium coupled monocarboxylate transporter1 (SMCT1) and get metabolized in enterocytes. SCFAs metabolism fulfills ∼5–10% of energy requirement in the gut [117]. A small fraction of un-metabolized SCFAs are transported to hepatocytes and further metabolized, leaving only a minor fraction of SCFA reaching systemic circulation and other tissues.
Although only a minor fraction of microbiota-derived SCFAs reach the systemic circulation and other tissues, their effect on different body organs and tissues has recently been recognized. SCFAs are essential to prevent intestinal diseases [118]. Apart from being shown to regulate inflammation and immune system activation, they also regulate adipose tissue activation, energy homeostasis, appetite, and sleep, and play a pivotal role in the gut-brain axis.
An abundant expression of MCTs in endothelial cells facilitates SCFAs crossing the BBB [119]. Interestingly, leaky and porous BBB in germ-free mice could be rescued by recolonization with SCFA-producing microbiota or direct supplementation with SCFAs, suggesting that SCFAs also regulate BBB integrity [120]. SCFAs are also involved in modulating neurotransmitter synthesis or release and possess antioxidant and anti-inflammatory properties [116]. Moreover, they are suggested to maintain the BBB and blood-intestinal barrier properties and functions [99], [121], [122], [123]. Consequently, decreased abundance of SCFA-producing bacteria would result in deficiency of SCFAs, thus potentiating the detrimental processes of intestinal inflammation and permeability and microglial activation in the brain of PD patients.
Butyrate
Butyrate, butter in latin, is a widely known and extensively studied SCFA that exerts its anti-inflammatory properties by inhibition of histone deacetylase (HDAC), hence regulating the expression of transcription factors that play key roles in immune and inflammatory responses. For instance, its HDAC inhibitory activity has been shown to inhibit the activation of nuclear factor kB (NF-kB) [124] or upregulate Foxp3 [125] in intestinal cells, thus producing anti-inflammatory effects. Furthermore, the HDAC inhibitory activity also allows butyrate to strengthen both intestinal barrier and BBB integrity [56]. Butyrate through regulation of gap junction proteins, preserves intestine and BBB barrier integrity and delays the inflammation process [32].
A decrease in the abundance of butyrate producing Firmicutes bacteria has been observed in the stool of PD patients [88], [121], [126], [127]. A multitude of studies have demonstrated the protective effects of butyrate treatment in various PD models. A study showed that dietary supplementation of butyrate in the rotenone-induced Drosophila model of PD, rescued locomotor impairment and early mortality in the flies [128]. Another study showed that sodium butyrate treatment alleviated pre-motor cognitive deficits, such as attention, cognitive flexibility, and troubleshooting, in a rat model of PD [129]. A recent study showed that intragastric injection of sodium butyrate in MPTP-induced mouse model of PD improved their coordination and cognitive behavior [130]. These results suggest a crucial role of butyrate and butyrate-producing bacteria in preventing the progression of PD.
Propionate
Propionate is also an HDAC inhibitor, which possesses anti-inflammatory properties and contributes to the BBB integrity [56]. There are no studies singly evaluating propionate in PD, however, several studies have reported a decrease in the propionate-producing bacteria, Prevotella, in the feces of PD patients [126], [127], [131]. Interestingly, both butyrate and propionate can regulate catecholamine production by controlling the expression of the tyrosine hydroxylase gene[132]. The latter could explain the decrease in dopamine synthesis observed in PD patients, since tyrosine hydroxylase is an enzyme which is required for the synthesis of dopamine[133].
Acetate
Acetate is used as a substrate by butyrate- and propionate-producing bacteria to produce butyrate and propionate, respectively [134]. A significant decrease in acetate concentration has been reported in the fecal [123] and plasma [135] samples of PD patients in comparison to healthy age-matched controls. Mechanistically, considering that bacteria utilize acetate to produce butyrate and propionate, alterations in the concentration of acetate would have implications on their concentrations and functioning.
SCFAs improve gut health through several mechanisms ranging from maintenance of intestinal barrier integrity, mucus production, and they also possess antioxidant and anti-inflammatory properties. Although signaling pathways triggered by SCFAs are not fully understood, there are reports that SCFAs bind to GPCRs including, Free Fatty Acid Receptor 2 and 3 (FFAR2 and FFAR3), and G protein–coupled receptor 109A (GPR109a) /niacin receptor1(NIACR1)/Hydroxycarboxylic Acid Receptor 2 (HCAR2) [136], [137]. SCFAs can modulate secretion of GLP-1 and their binding in hepatocytes regulate insulin secretion [111]. It is worth noting that increased expression of GPR109a in both blood and SNpc of PD patients has been reported [138], that may be indicative of inflammatory response in PD.
Mucin-degrading enzymes
The mucus lines the interior of the GI tract and acts as the first line of defense against microorganisms, microbial end-products, digestive acids and enzymes, and toxins. It also serves as a source of energy and carbon for the bacteria residing in its layers. Consequently, these bacteria release enzymes that degrade mucin (chief component of mucus), to break down the mucus network and utilize it as an energy source. At the genus level, studies have reported a decrease in Prevotella [121], [123] and Ruminococcus [139] bacteria, and an increase in Akkermansia [121], [123] in the fecal samples of PD patients, which are examples of bacteria that secrete several or all mucin-degrading enzymes. The discrepancy in the abundance of different genus of bacteria that secrete mucin-degrading enzymes can be hypothesized to play a compensatory role. A decrease in Prevotella and Ruminococcus bacteria would result in increase in mucosal permeability due to decrease in mucin synthesis, as degradation of the mucus layer aids its turnover and replenishment. The increase in Akkermansia could act as a compensation mechanism to maintain the integrity of the mucosal layer. However, failure in generating this compensatory response could in turn result in increased intestinal permeability and inflammation [140].
Hydrogen sulfide
Hydrogen sulfide (H2S) has neuromodulatory effects on the intestinal epithelial cells, which not only play a role in transportation of nutrients but also form a barrier to prevent invasion of pathogens [141]. Studies showed neuroprotective effects of H2S, where its administration in different murine models of PD showed decreased motor deficits and dopaminergic neuronal loss [142], [143], [144], [145]. Prevotella is a hydrogen-producing genus of bacteria [141], which as reported previously, is decreased in the feces of PD patients. Thus, decreased abundance of Prevotella could result in decreased production of H2S causing interference with its neuroprotective function. Cakmak [146] suggested that decreased levels of Prevotella in PD could trigger a compensatory mechanism resulting in increased intestinal permeability to allow H2S uptake.
Lipopolysaccharide
Lipopolysaccharide (LPS) is a proinflammatory endotoxin released from gram-negative bacteria, which are considered to initiate oxidative stress and inflammation, resulting in the ⍺-synuclein pathology in the ENS. LPS has been noted to disrupt the BBB integrity causing inflammation in the brain. LPS-mediated progressive mouse models of PD have been generated that display ⍺-synuclein aggregation and dopaminergic neuronal loss. Intranasal administration of LPS in mice induced ⍺-synuclein aggregation in the SNpc and dopaminergic neuronal loss [147]. Intraperitoneal dose of LPS in mice increased accumulation of ⍺-synuclein in the intestine and intestinal permeability [148]. A recent study showed that LPS injection in the striatum of mice resulted in generation of self-renewable, distinct ⍺-synuclein fibrils that induced pathological phenotypes such as the occurrence of phosphorylated ⍺-synuclein [149]. Another recent study showed increased abundance of LPS-producing Gammaproteobacteria in the stool of PD patients [150]. Furthermore, the study showed that LPS administration in ⍺-synuclein overexpressing mice caused early emergence of motor deficits in comparison to untreated mice, who remained asymptomatic at that stage. Thus, gut-derived LPS can potentially play a crucial role in ⍺-synuclein aggregation and transmission, and progression of PD.
Therapeutic strategies for combatting altered gut microbiome in PD
Current therapies for PD alleviate symptoms with limited efficiency and lack any substantial prophylactic effect. The delivery of levodopa, the chemical precursor of dopamine, is currently the gold standard for motor symptom treatment, but it comes with its own disadvantages like long absorption time, side effects, decreased efficacy overtime, and inability to treat all patients [151]. Deep brain stimulation (DBS) is another treatment in which neurostimulator electrodes are inserted through the skull to stimulate particular brain areas at frequency of 60–130Hz depending on symptoms that need to be treated. Usually DBS of subthalamic nuclei or basal ganglionic structure like globus pallidus, is effective for all major movement symptoms including resting tremors, rigidity and trouble walking [152]. DBS further aids in reducing the dose of levodopa intake [153]. However, the surgery for DBS involves risks such as infections, stroke, or brain hemorrhage or emergence of psychiatric problems like impulsivity. Gait worsening may happen in some patients after DBS, that is attributable to misplaced neurostimulating electrodes. Evidently, the current symptomatic therapies do not alleviate the late-stage symptoms, and cannot prevent dopaminergic neuron degeneration and disease progression.
Use of MRI-guided focused ultrasound might resolve above mentioned issues [154]. Notably, researchers have achieved long lasting effects by stimulation of basal ganglia using optogenetics, when compared with effect duration using DBS [155]. Optogenetics, with its cellular specificity and precise neuronal control, offers a unique opportunity for precise and targeted dopaminergic neuron modulation in PD patients. Mastro, Zitelli [156] showed restoration of movement in 6-ODHA mouse model of PD upon enhancing the activity of parvalbumin (PV)-expressing external globus pallidus (GPe) neurons over that of Lim homeobox 6 (Lhx6)-expressing GPe neurons in the basal ganglia using optogenetics. The prokinetic effects persisted for at least four hours after stimulation, which is significantly longer than current treatments. Such studies that help understand the neural circuitry involved in PD are essential to develop more efficient DBS protocols.
Although targeting the gut-brain axis cannot directly halt PD progression, dietary interventions possess the capacity to reinstate the gut microbial composition, which could improve disease prognosis. Perez-Pardo, de Jong [157] reported neuroprotective effect of dietary intervention with a combination of two precursors of membrane synthesis, uridine and docosahexaenoic acid (DHA), nutrients that increase phospholipid synthesis, and prebiotic fibers, in a rotenone model of PD. They showed partial alleviation of motor and non-motor PD symptoms induced by rotenone as well as restoration of striatal dopamine transporter levels. Gut-associated interventions that could prevent and/or treat PD, such as probiotics supplementation, fecal microbiota transplantation, and antibiotics have been further discussed henceforth and reviewed in Table 1, Table 2.
Probiotics
Probiotics have the potential to reverse PD-related alteration in gut microbial composition, resulting in restored GI function, and decreased gut leakiness and ENS inflammation. Bacterial strains such as Bifidobacteria and Lactobacilli produce vitamins, antioxidants, and bioactive molecules [158], which can decrease oxidative stress thus preventing further neuroinflammation and ⍺-synuclein aggregation. Another study showed decreased bloating and abdominal pain in PD patients when supplemented with Lactobacillus acidophilus and Bifidobacterium infantis for 3 months in tablet form [159]. Of interest, a study reported the neuroprotective effect of supplementing probiotic [Lactobacillus salivarius subsp. salicinius AP-32 (AP-32)], prebiotic residual medium (RM) obtained from the AP-32 culture medium, and combining AP-32 and RM (A-RM) in PD [160]. These supplementations improved mitochondrial functions by exerting an antioxidative effect, prevented motor deficits caused by muscle atrophy via increasing energy metabolism in the brain and muscle, and decreased dopaminergic neuron loss in 6-OHDA-induced PD rat model.
A recent double-blind, randomized, placebo-controlled study evidenced that consumption of 12 weeks of probiotic supplementation (combination of Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum in tablet form) decreased MDS-UPDRS score (Movement Disorders Society-Unified Parkinson’s Disease Rating Scale) in comparison to placebo [161]. The metabolic profiles of high-sensitivity C-reactive protein and malondialdehyde were decreased, while those of glutathione levels were enhanced. Collectively, their findings support the potential beneficiary effect of probiotics on oxidative stress and lipid profiles. Many clinical trials with FDA-defined phases that evaluate the efficacy of probiotics on PD-associated symptoms have been conducted or are in process. For example, a phase 3, double-blinded, randomized, placebo-controlled trial assessed constipation and motor symptoms in 55 idiopathic PD patients either receiving multi-strain probiotic or placebo twice daily for eight weeks (ClinicalTrials.gov Identifier: NCT04451096). They observed improvement in bowel opening frequency and gut transit time in PD patients having constipation [162](PMID: 33382780). In another, a phase 4 clinical, multicenter, randomized, double-blinded, placebo-controlled trial will assess motor symptoms, constipation, and sleep in 240 PD patients either receiving Bifidobacterium triple viable capsules(BIFICO)or placebo over a period of 24 weeks (ClinicalTrials.gov Identifier: NCT04871464).
Despite the promising outcomes from different preclinical and clinical studies, several issues and concerns with probiotic supplementation still need to be addressed [163], [164]. It is clear that there is a long way to go before revealing the complex mechanism underlying the impact of probiotics on the gut microbiota; the process includes investigating: 1) the risk of developing fungaemia or bacteraemia, 2) the possibility of small intestinal bacterial overgrowth, 3) the risk of contamination, 4) the need of a personalized assessment when prescribing probiotics 4) the ubiquity of probiotic products as supplements sold by manufacturers, and 5) the possible interactions with levodopa in the small intestine, which in turn reduces the amount of drug reaching the brain. Hence, rigorous clinical trials are absolutely necessary to determine the most efficient combinations of strains, dosage and treatment duration and to evaluate efficacy and safety in the long-term supplementation.
Fecal microbiota transplantation (FMT)
FMT has been used to restore the GM for treating GI infections and disorders such as irritable bowel syndrome and IBD [165], [166], [167], and can be a useful strategy for regulating the GM in PD patients. As the name suggests, in FMT the feces from a healthy donor are transplanted into the GI tract of a patient. The technique is being applied to treat autism, multiple sclerosis, and other CNS disorders with the aim to regulate immunological mechanisms[168]. Several recent studies have explored the use of FMT for treating GI dysfunction in PD in murine models and human subjects. Sun, Zhu [169] reported motor impairments and decreased concentration of striatal dopamine and serotonin in normal mice treated with the GM from MPTP-induced PD mice. 16S rRNA sequencing showed decrease in phylum Firmicutes and order Clostridiales, increase in phylum Proteobacteria, order Turicibacterales and Enterobacteriales, and increase in SCFAs in feces of PD mice. Interestingly, FMT from normal control mice into PD mice elicited decreased gut dysbiosis, decreased fecal SCFAs, increased striatal dopamine and serotonin concentrations, and improved motor function. In a case study, a 71-year-old male PD patient dealing with intractable constipation showed improved defecation and almost vanished leg tremors when treated with FMT from a 26-year-old healthy male [170]. A preliminary study reported significantly decreased motor symptoms, anxiety, depression, and improved sleep quality in ten PD patients that received FMT via colonoscopy, but not via nasal-jejunal tube [171]. Two clinical trials with FDA-defined phases are underway, testing the efficacy of FMT in PD patients. A phase 2/3 pilot study will assess the motor symptoms and constipation level in PD patients after receiving FMT (n = 15) with a half-year follow-up, compared to PD patients without FMT (n = 35), and healthy controls (n = 50) (ClinicalTrials.gov Identifier: NCT03876327). A phase 1/2 randomized study will assess motor symptoms, changes in bowel movements, and change of levodopa bioavailability in 40 PD patients who will either receive FMT or placebo treatment, with a 12 month follow-up (ClinicalTrials.gov Identifier: NCT05204641). Thus, the FMT approach has huge potential in being beneficial for PD, and more well-designed placebo-controlled, randomized trials, with larger cohort size, longer follow-up durations, and detailed analysis of the GM composition will help to assess its safety, side effects, and underlying mechanisms.
Antibiotics
Antibiotics like tetracycline that are used to fight bacterial infections have been repurposed to treat neurodegenerative diseases such as PD. Their properties of inhibiting mitochondrial dysfunction, production of proinflammatory molecules, microglial activation, and protein misfolding/aggregation, make them an excellent candidate to prevent the neuropathologies of PD [172]. Two semi-synthetic derivatives of tetracycline, minocycline, and doxycycline, have been widely tested in various models of PD, yielding neuroprotective effects. Minocycline has been reported to prevent: microglial activation and nigrostriatal dopaminergic neuron degeneration in MPTP mouse model of PD [173]; loss of tyrosine hydroxylase immunoreactive neurons in rotenone in vitro model of PD [174]; dopaminergic neuronal loss in Drosophila DJ-1A familial model of PD [175] and loss of tyrosine hydroxylase positive neurons in 6-OHDA-lesioned rats [176]. Doxycycline has been reported to mitigate nigrostriatal dopaminergic neuronal loss in 6-ODHA PD mouse model [177], inhibit LPS-induced dopaminergic neuronal degeneration in rats by downregulating the microglial MHC II protein expression [178], and reshape α-synuclein oligomers concomitantly preventing α-synuclein aggregation and further seeing in vitro [179]. The neuroprotective effects of tetracyclines in various PD models warrant further research to test their efficacy as a viable treatment for PD symptoms and to assess occurrence of side effects. Several clinical trials are registered to explore the modification of GM using antibiotics to improve PD-related symptoms. A phase 2, randomized, double-blinded, placebo-controlled study will assess the effect of doxycycline on motor symptoms and cognitive functions in 60 PD patients treated with levodopa over a period of eight weeks (ClinicalTrials.gov Identifier: NCT05492019). A phase 1/2, randomized, placebo-controlled trial will assess motor symptoms in 86 PD patients receiving rifaximin over a period of two weeks (ClinicalTrials.gov Identifier: NCT03575195). A phase 2, randomized, placebo-controlled study will assess the efficacy and safety of a broad-spectrum cephalosporin antibiotic, ceftriaxone, on the motor, cognitive, and pathological features of PD in 106 PD patients for a period of 17 weeks (ClinicalTrials.gov Identifier: NCT03413384). Studies that extensively assess the GM alterations in humans and PD models are essential to determine the target species for antibiotics, and further investigations on dosage and treatment duration are required to test the efficacy and safety of the antibiotics in humans.
Conclusion and future perspectives
PD is a multifactorial disease that has contributions from both genetics and environment. Findings from studies based on the well-known Parkinsonian neuroxins such as MPTP, paraquat (a herbicide commonly used in Taiwan in rice fields) and rotenone [180], [181], suggest that environmental factors may be more important than genetic factors in development of PD. Internal organs constantly exchange signals and change in size or function in response to environmental or internal challenges. Specifically, the intestine and brain are two internal organs which are suggested to be exchanging many signals and factors. Many of these factors may be derivatives of microbial byproducts.
It is vivid how the gut microbiota is increasingly acknowledged as contributing to the pathogenesis of different neurodegenerative diseases, including PD [84], [182]. This review summarized the possible mechanisms through which GM and microbial products can influence the development of PD or its progression. Therapeutic strategies that restore GM composition, GI function, and reverse mitochondrial dysfunction have also been reviewed, which have considerable potential to alleviate the motor and non-motor symptoms of PD. However, further studies are needed to understand the bidirectional communication between the gut and the brain in the setting of PD, and how diet can affect inflammation and neurodegeneration through altering the microbiome. It is still unknown whether PD begins from the gut or the brain, and emerging evidence has suggested that the gut-brain propagation of PD may be a subtype of the disease [183]. Although research using animal models has provided useful insight into the pathophysiology of PD, several limitations are still acting as an obstacle to complete understanding of the story of PD. The latter includes the small sample size used in different studies and the inability of the generated animal models to maximally mimic the symptoms and characteristics of the disease in humans. Unfortunately, there has been no substantial evidence for the development of new strategies to investigate the underlying disease mechanisms.
Given the heterogeneity of microbiome among different individuals [184], a concern is raised about the possibility to account for gut microbiota variability in PD patients and sheds light on the importance of individualized therapy or medicine when performing the clinical assessment and diagnosis and when choosing the potential treatment. There are a lot of factors that can affect the microbiome [185], however, not all of them are controllable. Among the controllable factors are lifestyle, probiotics, antibiotics, and diet. The proverbial saying “you are what you eat” is very true when it comes to shaping our health by defining our microbiome composition, in terms of quantity and quality. Current pre-clinical and clinical studies exploring the gut-associated therapeutic strategies to target different PD symptoms have limitations such as small sample/cohort size, variable patient disease severity, insufficient intervention time, lack of appropriate outcome measures such as assessment of GM composition and load before and after treatment and determination of identity of associated bacterial strains, and high variation in the key readout variables. More studies are required to identify the mechanisms underlying the effects of diet modification, probiotic supplementation and fecal transplantation following the development of PD in humans. The aforementioned gaps also warrant the development of more robust gut-brain axis models of PD, that recapitulate all the neuropathological hallmarks of PD. This will not only allow to explore the disease mechanisms and signaling pathways involved, but also will allow to identify modifiers that enhance or suppress the disease phenotypes and to assess the efficacy of novel therapeutic strategies.
Conflict of Interest
The authors have declared no conflict of interest.
CRediT authorship contribution statement
Safa Salim: Writing, Visualization, Editing and Proofreading. Fatima Ahmad: Writing, Visualization, Editing and Proofreading. Ayesha Banu: Writing, Editing and Proofreading. Farhan Mohammad: Writing, Editing, Proofreading, Funding and Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the members of the Farhan lab for their comments. This work received no external funding and was supported by intramural funding to Dr. Mohammad Farhan from the College of Health and Life Sciences (CHLS), Hamad Bin Khalifa University, Qatar Foundation. S.S, F.A. A.B. and F.M. were further supported by the College of Health and Life Sciences (CHLS), Hamad Bin Khalifa University (HBKU), Qatar Foundation. All figures were prepared using Biorender.
Biographies

Ms. Safa Salim: Safa Salim graduated with a major in Biological Sciences and a minor in Psychology from Carnegie Mellon University Qatar in 2017. She obtained her Master's in Biological and Biomedical Sciences from Hamad Bin Khalifa University, Qatar in 2019 and then joined the Ph.D. program. Currently, Safa is working on her dissertation project with Dr. Mohammad Farhan, who is an Assistant Professor at the College of Health and Life Sciences. The project focuses on understanding the role of gut-brain axis in Drosophila models of Parkinson’s Disease, to uncover the molecular mechanisms underlying the disease.”

Ms. Fatima Ahmad: Fatima acquired master’s degree in Neuropsychology at the College of Medical Science, Lebanese University in September 2020. Fatima Ahmad is a Ph.D. student in Dr. Mohammad Farhan at the College of Health and Life Sciences, HBKU and currently working on a project to assess serotonin levels in Drosophila and how it is associated with mitochondrial biogenesis, aging and mental health.

Ms Ayesha Banu Ayesha acquired her master's degree in Biochemistry from Osmania University, Hyderabad, India in 2012. She joined the Ph.D. program in Biological and Biomedical Sciences at Hamad Bin Khalifa University and is currently working with Dr. Mohammad Farhan on her dissertation project focused on the role of Serotonin in feeding behavior in Drosophila and understanding the neural circuit involved in the control of feeding.

Dr. Mohammad Farhan: Dr. Mohammad Farhan obtained his master’s in Biotechnology from Hamdard University, Delhi, India and Ph.D. from Institute of Genomics & Integrative Biology (IGIB), Delhi, India in 2009. Currently, Dr. Mohammad Farhan is working as an assistant professor at the College of Health and Life Sciences and supervising many Ph.D., Masters, and Postdocs. Dr. Farhan’s lab focusses on understanding the role of gut-brain axis in various neurodevelopmental and neurodegenerative models.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Tysnes O.-B., Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 2.Dauer W., Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 3.Glatt S.L., Hubble J.P., Lyons K., Paolo A., Tröster A.I., Hassanein R.E.S., et al. Risk factors for dementia in parkinson’s disease: Effect of education. Neuroepidemiology. 1996;15(1):20–25. doi: 10.1159/000109885. [DOI] [PubMed] [Google Scholar]
- 4.Hubble JP, Cao T, Hassanein RES, Neuberger JS, Roller WC. Risk factors for parkinson’s disease. Neurology 1993;43. https://doi.org/10.1212/wnl.43.9.1693. [DOI] [PubMed]
- 5.Giovannini P., Piccolo I., Genitrini S., Soliveri P., Girotti F., Geminiani G., et al. Early-onset Parkinson’s disease. Mov Disord. 1991;6(1):36–42. doi: 10.1002/mds.870060107. [DOI] [PubMed] [Google Scholar]
- 6.Schrag A., Schott J.M. Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. Lancet Neurol. 2006;5(4):355–363. doi: 10.1016/S1474-4422(06)70411-2. [DOI] [PubMed] [Google Scholar]
- 7.Vos T., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14(2):223–236. doi: 10.1176/jnp.14.2.223. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003;110. https://doi.org/10.1007/s00702-002-0808-2. [DOI] [PubMed]
- 10.Jankovic J. Parkinson’s disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Yang H. Environmental toxins and α-synuclein in Parkinson’s disease. Mol Neurobiol. 2005;31(1-3):273–282. doi: 10.1385/MN:31:1-3:273. [DOI] [PubMed] [Google Scholar]
- 12.Puspita L., Chung S.Y., Shim J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain. 2017:10. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasten M., Klein C. The many faces of alpha-synuclein mutations. Mov Disord. 2013;28(6):697–701. doi: 10.1002/mds.25499. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh D., Mehra S., Sahay S., Singh P.K., Maji S.K. α-synuclein aggregation and its modulation. Int J Biol Macromol. 2017;100:37–54. doi: 10.1016/j.ijbiomac.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Irwin D.J., Lee V.-Y., Trojanowski J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14(9):626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krüger R., Sharma M., Riess O., Gasser T., Van Broeckhoven C., Theuns J., et al. A large-scale genetic association study to evaluate the contribution of Omi/HtrA2 (PARK13) to Parkinson’s disease. Neurobiol Aging. 2011;32(3):548.e9–548.e18. doi: 10.1016/j.neurobiolaging.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesage S, Brice A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum Mol Genet 2009;18. https://doi.org/10.1093/hmg/ddp012. [DOI] [PubMed]
- 18.Pirkevi C., Lesage S., Brice A., Başak A.N. From genes to proteins in Mendelian Parkinson’s disease: An overview. Anat Rec. 2009;292 doi: 10.1002/ar.20968. [DOI] [PubMed] [Google Scholar]
- 19.Gasser T. Genomic and proteomic biomarkers for Parkinson disease. Neurology. 2009;72(Issue 7, Supplement 2):S27–S31. doi: 10.1212/WNL.0b013e318198e054. [DOI] [PubMed] [Google Scholar]
- 20.Nalls M.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M., et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46(9):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spataro N, Calafell F, Cervera-Carles L, Casals F, Pagonabarraga J, Pascual-Sedano B, et al. Mendelian genes for Parkinson’s disease contribute to the sporadic forms of the disease†. Hum Mol Genet 2014;24. https://doi.org/10.1093/hmg/ddu616. [DOI] [PubMed]
- 22.van Heesbeen H.J., Smidt M.P. Entanglement of Genetics and Epigenetics in Parkinson’s Disease. Front Neurosci. 2019:13. doi: 10.3389/fnins.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormack A.L., Thiruchelvam M., Manning-Bog A.B., Thiffault C., Langston J.W., Cory-Slechta D.A., et al. Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10(2):119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 24.Uversky V.N. Neurotoxicant-induced animal models of Parkinson’s disease: Understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318(1):225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 25.Qualman S.J., Haupt H.M., Yang P., Hamilton S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87(4):848–856. [PubMed] [Google Scholar]
- 26.Wakabayashi K., Takahashi H., Ohama E., Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79(6):581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 27.Braak H., Tredici K.D., Rüb U., de Vos R.A.I., Jansen Steur E.N.H., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 28.Rietdijk C.D., Perez-Pardo P., Garssen J., van Wezel R.J.A., Kraneveld A.D. Exploring Braak’s hypothesis of parkinson’s disease. Front Neurol. 2017:8. doi: 10.3389/fneur.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braak H., Del Tredici K. Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process? Acta Neuropathol. 2014;128(6):767–772. doi: 10.1007/s00401-014-1356-1. [DOI] [PubMed] [Google Scholar]
- 30.Postuma R.B., Aarsland D., Barone P., Burn D.J., Hawkes C.H., Oertel W., et al. Identifying prodromal Parkinson’s disease: Pre-Motor disorders in Parkinson’s disease. Mov Disord. 2012;27(5):617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 31.Swaminathan M., Fung C., Finkelstein D.I., Bornstein J.C., Foong J.P.P. α-Synuclein Regulates Development and Function of Cholinergic Enteric Neurons in the Mouse Colon. Neuroscience. 2019;423:76–85. doi: 10.1016/j.neuroscience.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Magen I., Yuan P.-Q., Subramaniam S.R., Richter F., Chesselet M.-F., et al. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24(9):e425–e436. doi: 10.1111/j.1365-2982.2012.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui M.F., Rast S., Lynn M.J., Auchus A.P., Pfeiffer R.F. Autonomic dysfunction in Parkinson’s disease: A comprehensive symptom survey. Park Relat Disord. 2002;8(4):277–284. doi: 10.1016/s1353-8020(01)00052-9. [DOI] [PubMed] [Google Scholar]
- 34.Ueki A., Otsuka M. Life style risks of Parkinson’s disease: Association between decreased water intake and constipation. J Neurol. 2004;251(S7):vii18–vii23. doi: 10.1007/s00415-004-1706-3. [DOI] [PubMed] [Google Scholar]
- 35.Adams-Carr K.L., Bestwick J.P., Shribman S., Lees A., Schrag A., Noyce A.J. Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87(7):710–716. doi: 10.1136/jnnp-2015-311680. [DOI] [PubMed] [Google Scholar]
- 36.Chen H., Zhao E.J., Zhang W., Lu Y.i., Liu R., Huang X., et al. Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl Neurodegener. 2015;4(1) doi: 10.1186/2047-9158-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott R.D., Petrovitch H., White L.R., Masaki K.H., Tanner C.M., Curb J.D., et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 38.Liu B., Fang F., Pedersen N.L., Tillander A., Ludvigsson J.F., Ekbom A., et al. Vagotomy and Parkinson disease A Swedish register-based matched-cohort study) Neurology. 2017;88(21):1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svensson E., Horváth-Puhó E., Thomsen R.W., Djurhuus J.C., Pedersen L., Borghammer P., et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 40.Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated α-synuclein gene mutations precede central nervous system changes. Hum Mol Genet 2010;19. https://doi.org/10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed]
- 41.Santos S.F., De Oliveira H.L., Yamada E.S., Neves B.C., Pereira A. The gut and Parkinson’s disease - A bidirectional pathway. Front Neurol. 2019:10. doi: 10.3389/fneur.2019.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colucci M., Cervio M., Faniglione M., De Angelis S., Pajoro M., Levandis G., et al. Intestinal dysmotility and enteric neurochemical changes in a Parkinson’s disease rat model. Auton Neurosci Basic Clin. 2012;169(2):77–86. doi: 10.1016/j.autneu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Harsanyiova J., Buday T., Kralova T.A. Parkinson’s Disease and the Gut: Future Perspectives for Early Diagnosis. Front Neurosci. 2020:14. doi: 10.3389/fnins.2020.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellegrini C., Fornai M., Colucci R., Tirotta E., Blandini F., Levandis G., et al. Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J Neuroinflammation. 2016;13(1) doi: 10.1186/s12974-016-0608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrido-Gil P., Rodriguez-Perez A.I., Dominguez-Meijide A., Guerra M.J., Labandeira-Garcia J.L. Bidirectional Neural Interaction Between Central Dopaminergic and Gut Lesions in Parkinson’s Disease Models. Mol Neurobiol. 2018;55(9):7297–7316. doi: 10.1007/s12035-018-0937-8. [DOI] [PubMed] [Google Scholar]
- 46.Ulusoy A., Phillips R.J., Helwig M., Klinkenberg M., Powley T.L., Di Monte D.A. Brain-to-stomach transfer of α-synuclein via vagal preganglionic projections. Acta Neuropathol. 2017;133(3):381–393. doi: 10.1007/s00401-016-1661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice M.W., Pandya J.D., Shear D.A. Gut microbiota as a therapeutic target to ameliorate the biochemical, neuroanatomical, and behavioral effects of traumatic brain injuries. Front Neurol. 2019:10. doi: 10.3389/fneur.2019.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hooper L.V., Gordon J.I. Commensal Host-Bacterial Relationships in the Gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 49.Forsythe P., Bienenstock J., Kunze W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. 2014:817. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 50.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015:28. [PMC free article] [PubMed] [Google Scholar]
- 51.Dinan T.G., Stilling R.M., Stanton C., Cryan J.F. Collective unconscious: How gut microbes shape human behavior. J Psychiatr Res. 2015;63:1–9. doi: 10.1016/j.jpsychires.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hilton D., Stephens M., Kirk L., Edwards P., Potter R., Zajicek J., et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127(2):235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 54.Shannon K.M., Keshavarzian A., Dodiya H.B., Jakate S., Kordower J.H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s Disease? Evidence from 3 cases. Mov Disord. 2012;27(6):716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 55.Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE. 2011;6(12):e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bullich C., Keshavarzian A., Garssen J., Kraneveld A., Perez‐Pardo P. Gut Vibes in Parkinson’s Disease: The Microbiota-Gut-Brain Axis. Mov Disord Clin Pract. 2019;6(8):639–651. doi: 10.1002/mdc3.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campos-Acuña J., Elgueta D., Pacheco R. T-cell-driven inflammation as a mediator of the gut-brain axis involved in Parkinson’s disease. Front Immunol. 2019:10. doi: 10.3389/fimmu.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birol M., Wojcik S.P., Miranker A.D., Rhoades E., Bates G.P. Identification of n-linked glycans as specific mediators of neuronal uptake of acetylated α-synuclein. PLoS Biol. 2019;17(6):e3000318. doi: 10.1371/journal.pbio.3000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao X., Ou M.T., Karuppagounder S.S., Kam T.-I., Yin X., Xiong Y., et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353(6307) doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kverka M, Zakostelska Z, Klimesova K, Sokol D, Hudcovic T, Hrncir T, et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin Exp Immunol 2011;163. https://doi.org/10.1111/j.1365-2249.2010.04286.x. [DOI] [PMC free article] [PubMed]
- 61.Wang C., Xiao Y., Yu L., Tian F., Zhao J., Zhang H., et al. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J Adv Res. 2022;36:27–37. doi: 10.1016/j.jare.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pacheco R. Targeting dopamine receptor D3 signalling in inflammation. Oncotarget. 2017:8. doi: 10.18632/oncotarget.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ostanin D.V., Bao J., Koboziev I., Gray L., Robinson-Jackson S.A., Kosloski-Davidson M., et al. T cell transfer model of chronic colitis: Concepts, considerations, and tricks of the trade. Am J Physiol - Gastrointest Liver Physiol. 2009;296(2):G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villumsen M., Aznar S., Pakkenberg B., Jess T., Brudek T. Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977–2014. Gut. 2019;68(1):18–24. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 66.Sun M., He C., Cong Y., Liu Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015;8(5):969–978. doi: 10.1038/mi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benner E.J., Banerjee R., Reynolds A.D., Sherman S., Pisarev V.M., Tsiperson V., et al. Nitrated α-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;;3(1):e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reynolds A.D., Stone D.K., Hutter J.A.L., Benner E.J., Mosley R.L., Gendelman H.E. Regulatory T Cells Attenuate Th17 Cell-Mediated Nigrostriatal Dopaminergic Neurodegeneration in a Model of Parkinson’s Disease. J Immunol. 2010;184(5):2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christiansen J.R., Olesen M.N., Otzen D.E., Romero-Ramos M., Sanchez-Guajardo V. α-Synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology. J Neuroinflammation. 2016:13. doi: 10.1186/s12974-016-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sulzer D., Alcalay R.N., Garretti F., Cote L., Kanter E., Agin-Liebes J., et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546(7660):656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sommer A., Marxreiter F., Krach F., Fadler T., Grosch J., Maroni M., et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell. 2018;23(1):123–131.e6. doi: 10.1016/j.stem.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 2009;119. https://doi.org/10.1172/JCI36470. [DOI] [PMC free article] [PubMed]
- 73.Harm A.S., Cao S., Rowse A.L., Thome A.D., Li X., Mangieri L.R., et al. MHCII is required for α-Synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013:33. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Z., Huang Y., Cao B.-B., Qiu Y.-H., Peng Y.-P. Th17 Cells Induce Dopaminergic Neuronal Death via LFA-1/ICAM-1 Interaction in a Mouse Model of Parkinson’s Disease. Mol Neurobiol. 2017;54(10):7762–7776. doi: 10.1007/s12035-016-0249-9. [DOI] [PubMed] [Google Scholar]
- 75.Więckowska-Gacek A., Mietelska-Porowska A., Wydrych M., Wojda U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. 2021:70. doi: 10.1016/j.arr.2021.101397. [DOI] [PubMed] [Google Scholar]
- 76.Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson’s disease: The role of food groups and specific foods. Mov Disord 1999;14. https://doi.org/10.1002/1531-8257(199901)14:1<21::AID-MDS1006>3.0.CO;2-Y. [DOI] [PubMed]
- 77.Liddle R.A. Parkinson’s disease from the gut. Brain Res. 2018;1693:201–206. doi: 10.1016/j.brainres.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mischley L.K., Lau R.C., Bennett R.D. Role of diet and nutritional supplements in Parkinson’s disease progression. Oxid Med Cell Longev. 2017;2017:1–9. doi: 10.1155/2017/6405278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rohr M.W., Narasimhulu C.A., Rudeski-Rohr T.A., Parthasarathy S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv Nutr. 2020:11. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maraki M.I., Yannakoulia M., Stamelou M., Stefanis L., Xiromerisiou G., Kosmidis M.H., et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov Disord. 2019;34(1):48–57. doi: 10.1002/mds.27489. [DOI] [PubMed] [Google Scholar]
- 82.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 83.Shi A., Shi H., Wang Y., Liu X., Cheng Y., Li H., et al. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int Immunopharmacol. 2018;54:125–130. doi: 10.1016/j.intimp.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 84.Jackson A., Forsyth C.B., Shaikh M., Voigt R.M., Engen P.A., Ramirez V., et al. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front Neurol. 2019:10. doi: 10.3389/fneur.2019.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paknahad Z., Sheklabadi E., Derakhshan Y., Bagherniya M., Chitsaz A. The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complement Ther Med. 2020:50. doi: 10.1016/j.ctim.2020.102366. [DOI] [PubMed] [Google Scholar]
- 86.Phillips M.C.L., Murtagh D.K.J., Gilbertson L.J., Asztely F.J.S., Lynch C.D.P. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov Disord. 2018;33(8):1306–1314. doi: 10.1002/mds.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang X., Cheng B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci. 2010;42(2):145–153. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- 88.Hill-Burns E.M., Debelius J.W., Morton J.T., Wissemann W.T., Lewis M.R., Wallen Z.D., et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017:32. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barichella M., Severgnini M., Cilia R., Cassani E., Bolliri C., Caronni S., et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov Disord. 2019;34(3):396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 90.Pietrucci D., Cerroni R., Unida V., Farcomeni A., Pierantozzi M., Mercuri N.B., et al. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Park Relat Disord. 2019;65:124–130. doi: 10.1016/j.parkreldis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 91.Qian Y., Yang X., Xu S., Wu C., Song Y., Qin N., et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun. 2018;70:194–202. doi: 10.1016/j.bbi.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 92.Heintz-Buschart A., Pandey U., Wicke T., Sixel-Döring F., Janzen A., Sittig-Wiegand E., et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33(1):88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang X., Qian Y., Xu S., Song Y., Xiao Q. Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Front Aging Neurosci. 2018:9. doi: 10.3389/fnagi.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frank D.N., St. Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Remely M., Aumueller E., Jahn D., Hippe B., Brath H., Haslberger A.G. Microbiota and epigenetic regulation of inflammatory mediators in type 2 diabetes and obesity. Benef Microbes. 2014;5(1):33–43. doi: 10.3920/BM2013.006. [DOI] [PubMed] [Google Scholar]
- 96.Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghaisas S., Langley M.R., Palanisamy B.N., Dutta S., Narayanaswamy K., Plummer P.J., et al. MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson’s disease. Neurotoxicology. 2019;75:186–199. doi: 10.1016/j.neuro.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uemura N., Yagi H., Uemura M.T., Hatanaka Y., Yamakado H., Takahashi R. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener. 2018:13. doi: 10.1186/s13024-018-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manfredsson F.P., Luk K.C., Benskey M.J., Gezer A., Garcia J., Kuhn N.C., et al. Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiol Dis. 2018;112:106–118. doi: 10.1016/j.nbd.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim S., Kwon S.-H., Kam T.-I., Panicker N., Karuppagounder S.S., Lee S., et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 2019;103(4):627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Julienne H., Buhl E., Leslie D.S., Hodge J.J.L. Drosophila PINK1 and parkin loss-of-function mutants display a range of non-motor Parkinson’s disease phenotypes. Neurobiol Dis. 2017;104:15–23. doi: 10.1016/j.nbd.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greene J.C., Whitworth A.J., Kuo I., Andrews L.A., Feany M.B., Pallanck L.J. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100(7):4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S., et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441(7097):1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 104.Yang Y., Gehrke S., Imai Y., Huang Z., Ouyang Y., Wang J.-W., et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103(28):10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feltzin V., Wan K.H., Celniker S.E., Bonini N.M. Role and impact of the gut microbiota in a Drosophila model for parkinsonism. BioRxiv. 2019 [Google Scholar]
- 106.Pickrell A., Youle R. The roles of PINK1, Parkin, and mitochondrial fidelity in parkinson’s disease. Neuron. 2015;85(2):257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matheoud D., Sugiura A., Bellemare-Pelletier A., Laplante A., Rondeau C., Chemali M., et al. Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell. 2016;166(2):314–327. doi: 10.1016/j.cell.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 108.Yang Y., Ouyang Y., Yang L., Beal M.F., McQuibban A., Vogel H., et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105(19):7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dehnow A.H., Krishna M.S. Gut Microbial Diversity in Rotenone Induced and Transgenically Created PD (Parkinson Disease) Flies of Drosophila Melanogaster. azb. 2020;8(2):45–51. [Google Scholar]
- 110.Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 111.Puddu A., Sanguineti R., Montecucco F., Viviani G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. 2014;2014:1–9. doi: 10.1155/2014/162021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jakobsdottir G., Xu J., Molin G., Ahrné S., Nyman M., Bassaganya-Riera J. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE. 2013;8(11):e80476. doi: 10.1371/journal.pone.0080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mortensen P.B., Clausen M.R. Short-chain fatty acids in the human colon: Relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl. 1996;31(sup216):132–148. doi: 10.3109/00365529609094568. [DOI] [PubMed] [Google Scholar]
- 114.Rosenberg E.W., Laurenzo G.D. Audit criteria from the city of Memphis hospital. J Tenn Med Assoc. 1975;68 [PubMed] [Google Scholar]
- 115.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020;11 doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr 1984;39. https://doi.org/10.1093/ajcn/39.2.338. [DOI] [PubMed]
- 118.Feng W., Ao H., Peng C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L., et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5(1) doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263) doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 122.Li W., Wu X., Hu X.u., Wang T., Liang S., Duan Y., et al. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China. Life Sci. 2017;60(11):1223–1233. doi: 10.1007/s11427-016-9001-4. [DOI] [PubMed] [Google Scholar]
- 123.Unger M.M., Spiegel J., Dillmann K.-U., Grundmann D., Philippeit H., Bürmann J., et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 124.Inan M.S., Rasoulpour R.J., Yin L., Hubbard A.K., Rosenberg D.W., Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118(4):724–734. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 125.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 126.Bedarf J.R., Hildebrand F., Coelho L.P., Sunagawa S., Bahram M., Goeser F., et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9(1) doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Petrov V.A., Saltykova I.V., Zhukova I.A., Alifirova V.M., Zhukova N.G., Dorofeeva Y.B., et al. Analysis of gut microbiota in patients with parkinson’s disease. Bull Exp Biol Med. 2017;162(6):734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 128.St. Laurent R., O’Brien L.M., Ahmad S.T. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rane P., Shields J., Heffernan M., Guo Y., Akbarian S., King J.A. The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacology. 2012;62(7):2409–2412. doi: 10.1016/j.neuropharm.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 130.Liu J., Wang F., Liu S., Du J., Hu X., Xiong J., et al. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. 2017;381:176–181. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- 131.Scheperjans F., Aho V., Pereira P.A.B., Koskinen K., Paulin L., Pekkonen E., et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 132.DeCastro M., Nankova B.B., Shah P., Patel P., Mally P.V., Mishra R., et al. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Mol Brain Res. 2005;142(1):28–38. doi: 10.1016/j.molbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 133.Westfall S., Lomis N., Kahouli I., Dia S.Y., Singh S.P., Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74(20):3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 135.Ahmed S.S., Santosh W., Kumar S., Christlet H.T.T. Metabolic profiling of Parkinson’s disease: Evidence of biomarker from gene expression analysis and rapid neural network detection. J Biomed Sci. 2009;16 doi: 10.1186/1423-0127-16-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bolognini D., Tobin A.B., Milligan G., Moss C.E. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol. 2016;89(3):388–398. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- 137.Mohajeri M.H., Brummer R.J.M., Rastall R.A., Weersma R.K., Harmsen H.J.M., Faas M., et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57(S1):1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wakade C., Chong R., Bradley E., Thomas B., Morgan J., Block M.L. Upregulation of GPR109A in Parkinson’s disease. PLoS ONE. 2014;9(10):e109818. doi: 10.1371/journal.pone.0109818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jin M., Li J., Liu F., Lyu N.a., Wang K., Wang L.u., et al. Analysis of the Gut Microflora in Patients With Parkinson’s Disease. Front Neurosci. 2019;13 doi: 10.3389/fnins.2019.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reunanen J., Kainulainen V., Huuskonen L., Ottman N., Belzer C., Huhtinen H., et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81(11):3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Linden D.R., Levitt M.D., Farrugia G., Szurszewski J.H. Endogenous production of H2S in the gastrointestinal tract: Still in search of a physiologic function. Antioxidants Redox. Signal. 2010;12(9):1135–1146. doi: 10.1089/ars.2009.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hou X., Yuan Y., Sheng Y., Yuan B., Wang Y., Zheng J., et al. GYY4137, an H2S slow-releasing donor, prevents nitrative stress and α-synuclein nitration in an MPTP mouse model of parkinson’s disease. Front Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu L.-F., Lu M., Tiong C.X., Dawe G.S., Hu G., Bian J.-S. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell. 2010;9(2):135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 144.Kida K., Yamada M., Tokuda K., Marutani E., Kakinohana M., Kaneki M., et al. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxidants Redox. Signal. 2011;15(2):343–352. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie L.i., Hu L.-F., Teo X.Q., Tiong C.X., Tazzari V., Sparatore A., et al. Therapeutic Effect of Hydrogen Sulfide-Releasing L-Dopa Derivative ACS84 on 6-OHDA-Induced Parkinson’s Disease Rat Model. PLoS ONE. 2013;8(4):e60200. doi: 10.1371/journal.pone.0060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cakmak YO. Provotella-derived hydrogen sulfide, constipation, and neuroprotection in Parkinson’s disease. Mov Disord 2015;30. https://doi.org/10.1002/mds.26258. [DOI] [PubMed]
- 147.He Q., Yu W., Wu J., Chen C., Lou Z., Zhang Q., et al. Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS ONE. 2013;8(11):e78418. doi: 10.1371/journal.pone.0078418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kelly L.P., Carvey P.M., Keshavarzian A., Shannon K.M., Shaikh M., Bakay R.A.E., et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord. 2014;29(8):999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim C., Lv G., Lee J.S., Jung B.C., Masuda-Suzukake M., Hong C.-S., et al. Exposure to bacterial endotoxin generates a distinct strain of α-synuclein fibril. Sci Rep. 2016;6(1) doi: 10.1038/srep30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gorecki A.M., Preskey L., Bakeberg M.C., Kenna J.E., Gildenhuys C., MacDougall G., et al. Altered gut microbiome in Parkinson’s disease and the influence of lipopolysaccharide in a human α-synuclein over-expressing mouse model. Front Neurosci. 2019;13 doi: 10.3389/fnins.2019.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Poewe W., Antonini A., Zijlmans J.C., Burkhard P.R., Vingerhoets F. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging. 2010:5. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perlmutter J.S., Mink J.W. Deep brain stimulation. Annu Rev Neurosci. 2006;29(1):229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Savitt J.M., Dawson V.L., Dawson T.M. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J Clin Invest. 2006:116. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martínez-Fernández R., Rodríguez-Rojas R., del Álamo M., Hernández-Fernández F., Pineda-Pardo J.A., Dileone M., et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol. 2018;17(1):54–63. doi: 10.1016/S1474-4422(17)30403-9. [DOI] [PubMed] [Google Scholar]
- 155.Steinbeck J.A., Choi S.J., Mrejeru A., Ganat Y., Deisseroth K., Sulzer D., et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol. 2015;33(2):204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mastro K.J., Zitelli K.T., Willard A.M., Leblanc K.H., Kravitz A.V., Gittis A.H. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat Neurosci. 2017;20(6):815–823. doi: 10.1038/nn.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perez-Pardo P., de Jong E.M., Broersen L.M., van Wijk N., Attali A., Garssen J., et al. Promising effects of neurorestorative diets on motor, cognitive, and gastrointestinal dysfunction after symptom development in a mouse model of Parkinson’s disease. Front Aging Neurosci. 2017:9. doi: 10.3389/fnagi.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.LeBlanc J.G., Milani C., de Giori G.S., Sesma F., van Sinderen D., Ventura M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 159.Georgescu D., Ancusa O.E., Georgescu L.A., Ionita I., Reisz D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin Interv Aging. 2016:11. doi: 10.2147/CIA.S106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nurrahma B.A., Tsao S.P., Wu C.H., Yeh T.H., Hsieh P.S., Panunggal B., et al. Probiotic Supplementation Facilitates Recovery of 6-OHDA-Induced Motor Deficit via Improving Mitochondrial Function and Energy Metabolism. Front Aging Neurosci. 2021:13. doi: 10.3389/fnagi.2021.668775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tamtaji O.R., Taghizadeh M., Daneshvar Kakhaki R., Kouchaki E., Bahmani F., Borzabadi S., et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(3):1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 162.Ibrahim A., Ali R.A.R., Manaf M.R.A., Ahmad N., Tajurruddin F.W., Qin W.Z., et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS ONE. 2020;15(12):e0244680. doi: 10.1371/journal.pone.0244680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rekdal V.M., Bess E.N., Bisanz J.E., Turnbaugh P.J., Balskus E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;80-:364. doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fasano A., Visanji N.P., Liu L.W.C., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14(6):625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 165.Carlucci C., Petrof E.O., Allen-Vercoe E. Fecal Microbiota-based Therapeutics for Recurrent Clostridium difficile Infection, Ulcerative Colitis and Obesity. EBioMedicine. 2016;13:37–45. doi: 10.1016/j.ebiom.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pamer E.G. Fecal microbiota transplantation: Effectiveness, complexities, and lingering concerns. Mucosal Immunol. 2014;7(2):210–214. doi: 10.1038/mi.2013.117. [DOI] [PubMed] [Google Scholar]
- 167.Konturek P.C., Koziel J., Dieterich W., Haziri D., Wirtz S., Glowczyk I., et al. Successful therapy of Clostridium difficile infection with fecal microbiota transplantation. J Physiol Pharmacol. 2016:67. doi: 10.1016/s0016-5085(17)31403-8. [DOI] [PubMed] [Google Scholar]
- 168.Evrensel A., Ceylan M.E. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci. 2016;14(3):231–237. doi: 10.9758/cpn.2016.14.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sun M.-F., Zhu Y.-L., Zhou Z.-L., Jia X.-B., Xu Y.-D., Yang Q., et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 170.Huang H, Xu H, Luo Q, He J, Li M, Chen H, et al. Fecal microbiota transplantation to treat Parkinson’s disease with constipation: A case report. Med (United States) 2019;98. https://doi.org/10.1097/MD.0000000000016163. [DOI] [PMC free article] [PubMed]
- 171.Xue L.-J., Yang X.-Z., Tong Q., Shen P., Ma S.-J., Wu S.-N., et al. Fecal microbiota transplantation therapy for Parkinson’s disease: A preliminary study. Medicine (Baltimore) 2020;99(35):e22035. doi: 10.1097/MD.0000000000022035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bortolanza M., Nascimento G.C., Socias S.B., Ploper D., Chehín R.N., Raisman-Vozari R., et al. Tetracycline repurposing in neurodegeneration: focus on Parkinson’s disease. J Neural Transm. 2018;125(10):1403–1415. doi: 10.1007/s00702-018-1913-1. [DOI] [PubMed] [Google Scholar]
- 173.Wu D.C., Jackson-Lewis V., Vila M., Tieu K., Teismann P., Vadseth C., et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22(5):1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Radad K., Moldzio R., Rausch W.-D. Minocycline protects dopaminergic neurons against long-term rotenone toxicity. Can J Neurol Sci. 2010;37(1):81–85. doi: 10.1017/s0317167100009690. [DOI] [PubMed] [Google Scholar]
- 175.Faust K., Gehrke S., Yang Y., Yang L., Beal M.F., Lu B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009:10. doi: 10.1186/1471-2202-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Quintero E.M., Willis L., Singleton R., Harris N., Huang P., Bhat N., et al. Behavioral and morphological effects of minocycline in the 6-hydroxydopamine rat model of Parkinson’s disease. Brain Res. 2006;1093(1):198–207. doi: 10.1016/j.brainres.2006.03.104. [DOI] [PubMed] [Google Scholar]
- 177.Lazzarini M., Martin S., Mitkovski M., Vozari R.R., Stühmer W., Bel E.D. Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia. 2013;61(7):1084–1100. doi: 10.1002/glia.22496. [DOI] [PubMed] [Google Scholar]
- 178.Zhang G.B., Feng Y.H., Wang P.Q., Song J.H., Wang P., Wang S.A. A study on the protective role of doxycycline upon dopaminergic neuron of LPS-PD rat model rat. Eur Rev Med Pharmacol Sci. 2015;19 [PubMed] [Google Scholar]
- 179.González-Lizárraga F., Socías S.B., Ávila C.L., Torres-Bugeau C.M., Barbosa L.R.S., Binolfi A., et al. Repurposing doxycycline for synucleinopathies: Remodelling of α-synuclein oligomers towards non-toxic parallel beta-sheet structured species. Sci Rep. 2017;7(1) doi: 10.1038/srep41755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Betarbet R., Sherer T.B., Di D.A., Greenamyre J.T. Mechanistic approaches to Parkinson’s disease pathogenesis. Brain Pathol. 2002;12(4):499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bové J., Prou D., Perier C., Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2(3):484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Romano S., Savva G.M., Bedarf J.R., Charles I.G., Hildebrand F., Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. Npj Park Dis. 2021:7. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Klann E.M., Dissanayake U., Gurrala A., Farrer M., Shukla A.W., Ramirez-Zamora A., et al. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front Aging Neurosci. 2022;13 doi: 10.3389/fnagi.2021.782082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ma Z.S. Heterogeneity-disease relationship in the human microbiome-associated diseases. FEMS Microbiol Ecol. 2020:96. doi: 10.1093/femsec/fiaa093. [DOI] [PubMed] [Google Scholar]
- 185.Hasan N., Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019; 2019. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tan A.H., Lim S.Y., Chong K.K., Manap A., MAA, Hor JW, Lim JL,, et al. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology. 2021:96. doi: 10.1212/WNL.0000000000010998. [DOI] [PubMed] [Google Scholar]
- 187.Kuai X.-y., Yao X.-H., Xu L.-J., Zhou Y.-Q., Zhang L.-P., Liu Y.i., et al. Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb Cell Fact. 2021;20(1) doi: 10.1186/s12934-021-01589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]