Abstract
Gastrointestinal angiodysplasias (GIADs) are rare disorder but can cause noticeable issue clinically. Their clinical characteristics can range from being an asymptomatic incidental finding to causing life-threatening bleeding. Many modalities are applied for treating bleeding GIADs include endoscopic therapies, angiography with embolization, surgical resection, and pharmacologic therapy. However, since patients with GIADs are often aged and have many comorbidities, endoscopic therapies may not be the best initial option. Angiography is suitable method for hemodynamically unstable patients with active bleeding, patients with an unknown active bleeding source, and patients who are poor surgical candidates. Angiography not only diagnose the bleeding point but also provide therapeutic endovascular intervention at the same time. We report a case of endovascular management of severe lower gastrointestinal bleeding from a GIAD in the cecum using a mixture of n-butyl cyanoacrylate and lipiodol to embolize the bleeding source. Clinical symptoms improved without prominent complications.
Keywords: Angiodysplasia, Lower gastrointestinal bleeding, Selective transarterial embolization
Introduction
Gastrointestinal angiodysplasias (GIADs) are pathologically dilated communications between mucosal capillaries and submucosal veins. This condition also acknowledge as angioectasias, vascular ectasias, or arterio-venous malformations, these lesions account for most diagnosed vascular lesions of the gastrointestinal (GI) tract [1]. Histologically, GIADs are the collection of abnormal, thin-walled arteries, venous and capillaries in the mucosa and submucosa layer which are lined by endothelium. The majority of GIAD lesions were found in the cecum and ascending colon. The clinical presentation of bleeding GIADs can vary from an asymptomatic to a life-threatening event requiring emergent hemostatic treatment [2]. Endovascular management plays an essential role for those who suffer GI hemorrhage that cannot be controlled by endoscopic treatment or in patients who cannot undergo endoscopic hemostatic due to active bleeding causing hemodynamic instability. GIADs characteristic is blush sign of slowly filling veins on angiography, which can be recognized in >90% of cases [3]. We report a case of successful endovascular management of severe lower GI bleeding from a GIAD in the cecum and review the literature of this rare condition.
Case report
An 87-year-old female was admitted to the hospital for severe hematochezia. They had hypovolemic shock symptoms, including hypotension, tachycardia, pale skin color, and confusion. The patient received immediate resuscitation by intravenous fluid and blood transfusion. A thorough history and careful examination were taken to determine the etiology of the hemorrhage. We noted that the patient had endured several minor hematochezia approximately a week before admission, and she had a cardiovascular issue called dilated cardiomyopathy. On clinical examination, the patient's blood pressure was 90/60 mm Hg and hemoglobin level was 6.2 g/dL. Emergent multislice computed tomography (MSCT) was taken and identified an active extravasation point in the cecum (Fig. 1). Later, the patient was quickly moved to emergency intervention room.
Fig. 1.
MSCT axial images in the arterial (A) and venous (B) phase show a hyperattenuated spot in the cecum (arrow) that faded into an enlarged, enhanced collection in the delay phase (C), a sign of active extravasation.
We used a 5Fr sheath to access femoral artery under local anesthesia. After a comprehensive study of the superior mesenteric and ileocecal arteries, selective catheterization of the ileocecal artery was conducted with a microcatheter (Asahi Parkway Soft 1.98F) and microguidewire (Asahi Chikai 0.014″) to confirm the diagnosis. We detected angiodysplasia characteristics corresponding to the bleeding point in the MSCT image. We performed superselective embolization by injecting a mixture of n-butyl cyanoacrylate (NBCA) and lipiodol at a 1:1 ratio. Postprocedural angiography confirmed complete embolization of the angiodysplasia (Fig. 2). The patient's clinical symptoms improved, including achieving hemodynamic stability. Their hemoglobin level after 3 days follow-up had increased to 9.2 g/dL. No complications were recorded when we performed a check-up MSCT before the patient was discharged (Fig. 3).
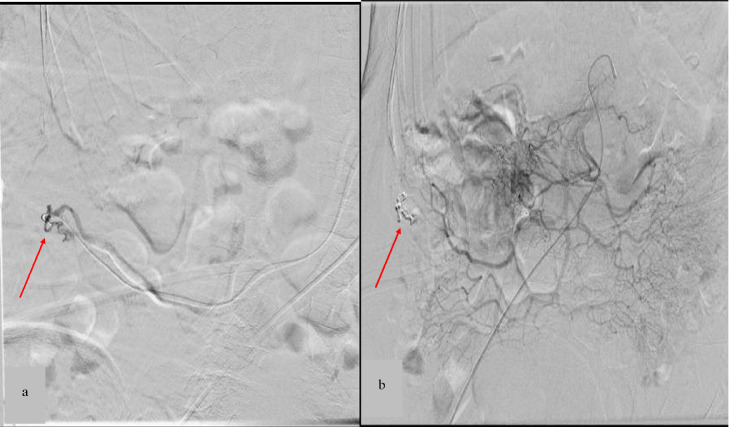
Fig. 2.
(A) Digital subtraction angiography of the ileocecal artery confirms a GIAD (arrow). (B) Angiography shows complete embolization of the lesion after superselective embolization.
Fig. 3.
An MSCT coronal image in arterial (A) and axial images in venous (B) and delay (C) phases 3 days after the embolization procedure showed a mixture of NBCA and lipiodol at the GIAD site (arrow). There was no sign of extravasation or bowel infraction.
Discussion
Angiodysplasia is among one of the most common vascular abnormality that found in GI tract in general population. The prevalence of angiodysplasias increases with age, so patients who aged >60 years are most affected. They may be asymptomatic or present with GI bleeding. The colon is the most common site of angiodysplasias in the GI tract [4]. While the etiology of this lesion remains unclear, many hypotheses have been proposed in the literature. Among them, hypoperfusion of microvasculature mechanism which lead to ischemic necrosis of abnormal vascular lesions is widely accepted, this condition is common in elderly population or those who have cardiovascular/pulmonary disease [5]. Pate et al. reported that the cause of angiodysplasia was mucosal hypoperfusion affiliated with cardiac disease [6]. Our patient suffered from dilated cardiomyopathy, a progressive heart muscle disease. It compromises heart function and causes hypoperfusion of the cecum wall. Therefore, it is a risk factor for angiodysplasia.
The clinical presentation of GIADs can vary from asymptomatic to a life-threatening event that required emergent hemostatic intervention. At first presentation, 90% of GIADs will stop bleeding spontaneously [2]. However, GIADs are inclined to repetitive bleeding, estimated approximately 70%. The signs and symptoms of GIADs can be diverse and influenced by lesion position in GI tract. Our patient has GIAD in the cecum which is a lower GI tract, so she presented with hematochezia. The outcome of bleeding from GIADs is varied between patients. However, many researches show that the most affected population are likely to be elderly (aged >65 years) and suffer from multiple comorbidities, because their status can easily be decompensated by recurrent GIADs bleeding [3].
According to characteristics of bleeding and the suspicion of the bleeding location, the initial diagnostic modality will be decided. Upper endoscopy and colonoscopy are common initial diagnostic methods. The visualization of angiodysplasias is 5-10 mm flat cherry-red fern-like projecting vessels originating from a central artery under endoscopy or colonoscopy [5]. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are also useful investigative tools for diagnosis. In this case, we use CTA to detect the active bleeding point in the cecum. Wells et al. reported that the sensitivity and specificity of CTA in GI bleeding are 85.2% and 92.1%, respectively [7].
GIADs account for 30%-40% of lower GI hemorrhages. While most patients with GIADs can be treated conservatively because the acute bleeding will spontaneously stop in 90% of cases, angiodysplasia tends to be multiple, with a high possibility of recurrent bleeding. Therefore in clinical practice, the treatment should be applied individually . Asymptomatic patients are treated conservatively in general. Endoscopic thermal therapy is currently the treatment of choice in patients with an ongoing transfusion requirement, however abundant bleeding or bezoar could worsen visualization in some cases and make it impossible to locate bleeding source. Moreover, the endoscope diagnosis and treatment success rate only around 70% because of different operation experiences [1,8].
Hemostatic by endovascular embolization should be calculated in cases of conservative therapy failure or an unstable patient with active bleeding in which colonoscopy may be impossible. This method was first described by Bookstein et al. in 1974 and has quickly become an effective treatment for GI bleeding from vascular lesions [9]. In our case, endovascular management was necessary because the patient had severe blood loss and hemodynamic instability. We used a microcatheter that could superselectively facilitate into targeted vessels. Because active extravasation (with a minimum flow rate of 0.5-1 mL/min) is detected in just 6%-20% of cases on digital subtraction angiography (DSA) [9], we only found densely opacified, dilated and slowly emptying intramural veins at the same active bleeding site on CTA (Fig. 2), which is specific to GIAD. Therefore, we decided to use NBCA.
The widely used embolic agents for GIAD are microcoils, polyvinyl alcohol particles, and gelfoam. NBCA glue is a permanent agent that polymerizes when exposed to blood, normal saline, or any other ionic solution. Moreover, it can provide distal occlusion depend on the mixture percentage with lipiodol, so the doctor can easily control the embolization of the bleeding point and the collateral circulation. To date, success rates have reached 80%-100%; failure (20%) can be encountered by following problems such as vessel perforation, dissection, spasm, bleeding cessation, or vessel tortuosity. Early rebleeding (<30 days) occurs in 10%-30% of cases as the result of the recanalization of previously embolized vessels or new sources of bleeding [10].
Zhao et al. successfully performed transarterial embolization with NBCA in 7 patients with persistent GI hemorrhages whose conservative treatment failed; only 1 (14%) experienced rebleeding (as a consequence of the presence of a second artery supplying the lesion), and none suffered from bowel infarction [8]. Usual complications (5%-9%) such as hematomas, thrombosis, pseudoaneurysm formation, and bowel infarction can occur. The most significant risk of embolization is bowel infarction. However, the development of superselective catheterization has reduced the rate of this complication significantly [11]. Pitrone et al. reported successfully treating GIAD with NBCA in 4 patients with no sign of bowel infarction or clinical recurrence within the first 2 years postoperatively [10]. In our case, we performed abdominal MSCT 3 days after the procedure and did not detect any sign of bowel infarction.
Conclusions
In short, endovascular embolization is a safe and efficacious treatment option for managing low GI hemorrhage due to GIADs. It is a rescue therapy for hemodynamically unstable patients who cannot undergo endoscopy and for patients who are poor surgery candidates.
Authors’ contributions
Phan HVP, HHP, and Thi VG: Case file retrieval and case summary preparation. Phan HVP and Nguyen MD: preparation of manuscript and editing. All authors read and approved the final manuscript.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our institution does not require ethical approval for reporting individual cases or case series. Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Patient consent
Informed consent for patient information to be published in this article was obtained.
Footnotes
Competing Interests: The authors do not report any conflicts of interest.
References
- 1.Beg S, Ragunath K. Review on gastrointestinal angiodysplasia throughout the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2017;31(1):119–125. doi: 10.1016/j.bpg.2016.11.004. Epub 2017 Jan 12. [DOI] [PubMed] [Google Scholar]
- 2.Jackson CS, Strong R. Gastrointestinal angiodysplasia: diagnosis and management. Gastrointest Endosc Clin N Am. 2017;27(1):51–62. doi: 10.1016/j.giec.2016.08.012. Epub 2016 Mar 13. [DOI] [PubMed] [Google Scholar]
- 3.Holleran G, McNamara D. An overview of angiodysplasia: management and patient prospects. Expert Rev Gastroenterol Hepatol. 2018;12(9):863–872. doi: 10.1080/17474124.2018.1503532. Epub 2018 Aug 3. [DOI] [PubMed] [Google Scholar]
- 4.Ueno S, Nakase H, Kasahara K, Uza N, Kitamura H, Inoue S, et al. Clinical features of Japanese patients with colonic angiodysplasia. J Gastroenterol Hepatol. 2008;23(8 Pt 2):e363–e366. doi: 10.1111/j.1440-1746.2007.05126.x. Epub 2007 Aug 27. [DOI] [PubMed] [Google Scholar]
- 5.Aghighi M, Taherian M, Sharma A. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL: 2023. Angiodysplasia. 2023 Apr 23. [PubMed] [Google Scholar]
- 6.Pate GE, Chandavimol M, Naiman SC, Webb JG. Heyde's syndrome: a review. J Heart Valve Dis. 2004;13(5):701–712. Epub 2004 Oct 12. [PubMed] [Google Scholar]
- 7.Wells ML, Hansel SL, Bruining DH, Fletcher JG, Froemming AT, Barlow JM, et al. CT for evaluation of acute gastrointestinal bleeding. Radiographics. 2018;38(4):1089–1107. doi: 10.1148/rg.2018170138. Epub 2018 Jun 8. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Li G, Yu X, Xie P. Evaluation of superselective transcatheter arterial embolization with n-butyl cyanoacrylate in treating lower gastrointestinal bleeding: a retrospective study on seven cases. Gastroenterol Res Pract. 2016;2016 doi: 10.1155/2016/8384349. Epub 2016 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira Leite TF. Superselective transcatheter arterial embolization of iatrogenic inferior epigastric artery after paracentesis: unusual manifestation of hemoperitoneum. Int J Surg Case Rep. 2020;74:32–35. doi: 10.1016/j.ijscr.2020.07.001. Epub 2020 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitrone P, Stagno A, Cattafi A, Caloggero S, Silipigni S, Ascenti V, et al. The use of cyanoacrylate in the treatment of angiodysplasias: a safe and cheap alternative to coils. BJR Case Rep. 2022;8(5) doi: 10.1259/bjrcr.20210130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AR, Jala V, Arshad H, Bilal M. Evaluation and management of lower gastrointestinal bleeding. Dis Mon. 2018;64(7):321–332. doi: 10.1016/j.disamonth.2018.02.002. Epub 2018 Mar 7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.