Abstract
Management of atrial fibrillation (AF) is a clinical conundrum in people with kidney failure. Stroke risk is disproportionately high, but clinicians have a limited armamentarium to improve outcomes in this population in whom there is a concurrently high bleeding risk. Direct oral anticoagulants may have a superior benefit–risk profile compared with vitamin K antagonists in people on hemodialysis. Although research has predominantly focused on identifying a safe and effective oral anticoagulation option to reduce stroke risk in people with kidney failure (and predominantly those on hemodialysis), it remains uncertain how clinicians discriminate between people who would derive net clinical benefit as opposed to net harm. The recommended CHA2DS2-VASc score cutoffs provide poor discriminatory value, and there is an urgent need to identify robust markers of thromboembolic risk in kidney failure.
There is increasing data to challenge the prior dogma of risk equivalence across AF type, and the American Heart Association highlights moving beyond AF as a binary entity to consider the prognostic significance of AF burden. Implantable cardiac monitor studies reveal high rates and varied burden of subclinical and paroxysmal AF in people on hemodialysis. The association between AF burden and the proarrhythmic environment of hemodialysis with cyclical volume loading, offloading, and electrolyte changes is not well studied. We review the significance of AF burden as a contributor to thromboembolic risk, its potential as the missing link in risk assessment, and updated evidence for anticoagulation in people with kidney failure.
Index Words: Anticoagulation, atrial fibrillation, hemodialysis, kidney failure, review, stroke
Introduction
Atrial fibrillation (AF) is a challenging cardiovascular problem in people with kidney failure. Significant practice variation and clinician uncertainty exists as a result of a paucity of evidence to guide decision making in mitigating thromboembolic risk.1 The compelling benefits of vitamin K antagonists (VKAs) for stroke risk reduction in the general population have not been replicated in people with kidney failure,2 and use of VKAs has fallen out of favor. Recent studies report the superior benefit–risk profile of direct oral anticoagulants (DOACs) compared with VKAs in people treated by hemodialysis,3,4 although data for efficacy remain outstanding. Although the availability of safe oral anticoagulation (OAC) options in kidney failure is important, appropriate patient selection for treatment is also imperative—identifying the highest risk patients who may derive net clinical benefit without exposing others to unnecessary bleeding risk.
The prognostic significance of AF burden to thromboembolic risk is an emerging area of research, with new data challenging prior dogma of risk equivalence across AF type. This review examines the current evidence for stroke risk stratification in kidney failure and explores the relevance of AF type and burden to stroke risk. We also provide an update on evidence for OAC and address limitations and future directions in this important area.
Stroke Risk Assessment in Kidney Failure
People with kidney failure experience 5- to 10-fold higher rates of ischemic stroke compared with the general population,5 and those with AF have a 2-fold higher rate than those without AF.6 This risk is traditionally associated with clinical risk factors derived from non-anticoagulated general AF cohorts and randomized controlled trials (RCTs) conducted more than 20 years ago7; this information has resulted in development of stroke risk prediction scores to inform clinical decision making and standardize OAC use. Guidelines recommend the use of CHA2DS2-VASc scores to assess risk of thromboembolic stroke,8, 9, 10 recommending OAC when CHA2DS2-VASc ≥2 in men and ≥ 3 in women.8 There is little data on the predictive performance of the CHA2DS2-VASc score in the kidney failure population.
Given concurrent increased stroke and bleeding risk, risk assessment and careful patient selection is essential in kidney failure.5 Original development cohorts excluded people with chronic kidney disease (CKD), and external validation studies in kidney failure cohorts only report modest discrimination.9, 10, 11, 12 Yet for risk prediction and clinician decision making, calibration (the accuracy of predicted absolute risks) may be more important in weighing the relevant stroke and bleeding risks. The only CHA2DS2-VASc validation study to report calibration found modest discrimination with poor calibration in a prospective Dutch cohort of 2051 people receiving dialysis,11 under-predicting stroke risk with respect to the actual agreement between observed and predicted probabilities. It also highlighted poor predictive performance of another 14 stroke risk models, with only the Framingham Heart Score showing good calibration but poor discrimination.11
De Vriese and Heine13 have proposed an alternative algorithm to estimate net clinical benefit from OAC. The “Dialysis Risk Score” includes stroke history (3 points), diabetes, and age greater than 75 (1 point each) from the CHA2DS2-VASc score (because they were significantly associated with subsequent stroke) while omitting hypertension and heart failure (Box 1).8,13, 14, 15 A history of gastrointestinal bleeding has been shown to predict future events and has been included in the risk score (subtract 1 point).16 It is proposed to consider OAC when the Dialysis Risk Score is ≥2. The authors reported only 44% of Valkyrie trial participants had an indication for OAC based on this alternative method.13
Box 1. The Dialysis Risk Score Compared With the CHA2DS2-VASc Scoring System.
| The Dialysis Risk Score – An Alternative Anticoagulant Strategy in Hemodialysisa |
The CHA2DS2-VASc Scoring Systemb |
||
|---|---|---|---|
| Clinical Characteristic | Score | Clinical Characteristic | Score |
| Prior TIA/ischemic stroke | 3 | Congestive heart failure/LV dysfunction | 1 |
| Diabetes | 1 | Hypertension | 1 |
| Age >75 y | 1 | Age ≥75 y | 2 |
| Gastrointestinal bleeding <1 y | -1 | Diabetes | 1 |
| Stroke/TIA/thromboembolism | 2 | ||
| Vascular disease | 1 | ||
| Age 65-74 y | 1 | ||
| Sex category (female gender) | 1 | ||
Abbreviations: AF, atrial fibrillation; LV, left ventricular; OAC, oral anticoagulant; TIA, transient ischemic attack.
De Vriese and Heine13 propose to initiate anticoagulation in AF and hemodialysis when the Dialysis Risk Score is ≥ 2.
With the potential advent of DOACs, there is an urgent need to better understand the pathophysiologic relationship and establish robust predictors of thromboembolic risk beyond the CHA2DS2-VASc score in people with AF and kidney failure.
AF Type and Burden: A Dose–Response Relationship for Stroke Risk?
Conventionally, AF is regarded as a binary entity (present or absent) and is a risk factor for stroke irrespective of AF type and burden. This is based on the understanding that atrial stasis promotes thrombogenesis and embolism. Recent data have challenged this paradigm of risk equivalence, and it is biologically plausible that spending more time in AF leads to increased thromboembolic risk.
Current guidelines classify AF by pattern—first-diagnosed AF, paroxysmal AF (<7 days duration), persistent AF (≥7 days duration), long-standing persistent AF (>12 months duration where rhythm control strategy is adopted), and permanent AF (AF that is accepted for rate control strategy).8 However, there is significant heterogeneity within these groups. Asymptomatic episodes can go undetected, and paroxysmal AF may include a range of frequency, duration, and overall AF burden. Prognostic significance of AF burden (whether paroxysmal or subclinical) remains unclear17 but may be relevant to stroke risk in the kidney failure population where CHA2DS2-VASc scores are high.
Data from cardiac implantable electronic devices (pacemakers, implantable defibrillators, and cardiac resynchronization devices) provide insight into the association between paroxysmal AF burden and stroke events. A linear increase in thromboembolic events has been reported in a retrospective study of 568 people with an implanted pacemaker and paroxysmal AF. This risk exponentially increased when combining AF duration and CHADS2 score (0.8% vs 5%).18 A similar association was identified with CHA2DS2-VASc for clinical risk scoring.19
Subclinical AF: The Unknown Risk
Subclinical AF is defined as asymptomatic AF detected on monitoring or on interrogation of a cardiac device, detecting even low levels of AF.8 Given the high prevalence of structural heart disease and the proarrhythmic environment of kidney failure and kidney replacement therapy, it is plausible that subclinical AF contributes to disproportionately high rates of stroke in this population. Using implantable cardiac monitors in a cryptogenic stroke cohort, the CRYSTAL-AF study first reported an association between subclinical AF and ischemic stroke risk.20 Meta-analyses of cardiac implantable electronic device cohort studies support this association, although stroke risk was lower than that of clinical AF despite adjustment for CHA2DS2-VASc score.21 However, temporal dissociation has also been reported with <30% of people having an episode of subclinical AF in the 30 days preceding a stroke event, suggesting a more complex relationship.21
Recent data suggest that subclinical AF is prevalent in kidney failure. In a study of 50 people treated by hemodialysis who had implantable cardiac monitors, Wong et al22 first reported AF in 42% of participants monitored over a 12-month period. New AF was identified in 28% with the majority (86%) subclinical or asymptomatic. The Monitoring in Dialysis study23 supported these findings and further reported a median of 7 days with AF but ranging from 1-161 days.24 Subclinical AF has not been characterized in peritoneal dialysis cohorts, although there is no difference in AF incidence between hemodialysis and peritoneal dialysis cohorts beyond 90 days after dialysis commencement.25 In a population where CHA2DS2-VASc scores are high, the interaction between clinical risk factors and AF type or burden may inform stroke risk.26
Relevance of AF Burden to Kidney Failure
The relationship between established structural heart disease and atrial remodeling, vascular risk factors, thrombogenesis, and the proarrhythmic milieu of kidney failure is complex. Chronic volume overload in kidney failure may activate the sympathetic and renin-angiotensin-aldosterone system or induce atrial and ventricular stretch with atrial electrical remodeling. The cyclical volume and electrolyte changes of hemodialysis may precipitate intradialytic or peridialytic AF. Some episodes will self-terminate, but subclinical AF may go unrecognized for extended periods of time.24 The prognostic significance of these episodes is unclear, and there is likely to be significant clinician variability in OAC practice in this population.
Current guidelines do not recommend the use of AF burden in anticoagulation decision making.8,14 Yet this practice would consider a 60-year-old man with kidney failure on hemodialysis with hypertension and diabetes with CHA2DS2-VASC score of 2 to have a modest stroke risk of 2.2% per year, regardless of whether he had permanent AF, 10 paroxysmal episodes a year, or 2 short-lived self-terminating episodes. There is increasing evidence that risk is not equivalent across paroxysmal and nonparoxysmal AF,27 and the American Heart Association concluded that patients with nonparoxysmal AF have a higher risk of stroke than those with paroxysmal AF.17 It is likely there is a significant difference in stroke risk across the 3 scenarios presented with relevance to the kidney failure (and particularly hemodialysis) population who are frequently exposed to arrhythmic precipitants. A study of 40 patients treated by kidney replacement therapy who received a dual-chamber implantable cardioverter defibrillator in the Implantable Cardioverter-Defibrillator in Dialysis Patients (ICD-2) trial demonstrated an association between volume removal and dialysate potassium concentration,28 although this was not replicated in the Monitoring in Dialysis study using implantable cardiac monitors.24 This also raises the potential for stroke risk to be dynamic according to volume status, electrolyte changes, and dialysis prescription. The concept of cardioprotective hemodialysis to minimize cardiovascular harm is not new, but attention to dialysis prescription to modulate AF burden and stroke risk warrants consideration.
Appropriate risk stratification in kidney failure is the missing link in the management of AF and cardiovascular risk. In the general population, Kaplan et al29 demonstrated that AF burden further stratified stroke risk in the intermediate-risk population. In people with CHA2DS2-VASc score 2, stroke risk crossed an actionable threshold of >1% annualized stroke risk with >23.5 hours of maximum daily AF duration.29 People with low CHA2DS2-VASc scores and long duration of AF remained low risk.29 An actionable stroke risk has not been identified in people with kidney failure but would be useful in anticoagulation decision making.
Bleeding Risk in Kidney Failure
Uremic platelet dysfunction, endothelial dysfunction, and anemia may all contribute to the increased bleeding risk in people with kidney failure.30 Bleeding rates are higher in the hemodialysis population at 60.8/1000 person-years compared with people on peritoneal dialysis at 34.6/1000 person-years.31 Bleeding rates are 0.9/1000 person-years in the general population.32 Bleeding risk scores (HAS-BLED, ORBIT, HEMORR2HAGES, and ATRIA) all include CKD as a risk factor and do not provide discriminative value in kidney failure.8,14,33 The strongest predictors of bleeding are a history of prior bleeding or gastrointestinal bleeding in the past 12 months,16,31 and their inclusion in the design of future risk scores will be important.13 Frequent pragmatic assessments are essential in this population.
Oral Anticoagulation in Kidney Failure
Anticoagulation use for AF in people with kidney failure has long been controversial. Yet clinicians continue to endeavor to mitigate the risks of AF-related harm. The US Renal Data System reported 52.1% of people receiving dialysis were receiving OAC in the setting of AF despite uncertainty in the evidence.34
Evidence for VKAs
Observational studies and meta-analyses over the last decade have reported mixed outcomes of VKA use in kidney failure. The SWEDEHEART registry of people with acute myocardial infarction and associated AF reported VKAs were associated with a reduced risk of a composite cardiovascular end point, including ischemic stroke.35 These findings were consistent across CKD strata, including a small kidney failure cohort (n=478) with an estimated glomerular filtration rate <15 mL/min/1.73 m2.35 There was no increased risk of bleeding in this study, but this may reflect the >75% time spent in prothrombin therapeutic range,35,36 which is difficult to reproduce in people treated by hemodialysis.37 Recent kidney failure DOAC trials reported the time in therapeutic range to only be 44%-50.7%.3,4,38
A pooled meta-analysis of 15 studies in kidney failure and with AF did not report a reduction in ischemic stroke or all-cause mortality with VKA therapy but a significantly increased risk of hemorrhagic stroke (without an increase in the overall risk of bleeding).2 Another meta-analysis of 12 studies, including people on peritoneal dialysis, kidney transplant recipients, and people with stage 5 CKD only, reported a nonsignificant trend toward reduction in ischemic stroke with a significant increase in total bleeding risk and no effect on mortality.39
The association between VKAs and accelerated vascular calcification also warrants careful consideration.40 Aside from inhibiting VKA-dependent coagulation factors, off-target effects include interfering with vitamin K-dependent inhibitors of vascular arterial media calcification. Retrospective and cross-sectional studies report an association between VKA therapy and measures of vascular calcification,40 including reduced aortic compliance in hemodialysis cohorts.41 Arterial stiffness may be associated with left ventricular hypertrophy, diastolic dysfunction, and heart failure, further contributing to cardiovascular burden in kidney failure. Another major deterrent is calciphylaxis, which is frequently associated with VKA use,42 characterized by aggressive cutaneous calcification, necrotic ulceration, and infection, and has an annual mortality rate of up to 67%.43
Observational data in this area should be interpreted with caution. Two RCTs comparing VKAs with no OAC and assessing stroke and bleeding risk are ongoing (AKDIAL [Oral Anticoagulation in Haemodialysis Patients], NCT02886962; and DANWARD [The Danish Warfarin-Dialysis Study], NCT03862859) and will provide valuable data.
DOACs: Emerging Evidence for Safety
With minimal renal clearance, rivaroxaban and apixaban are the 2 DOACs potentially suitable for use in people with kidney failure and have been studied in hemodialysis cohorts. Based on pharmacokinetic studies alone, apixaban (5 mg or 2.5 mg twice daily)44 and rivaroxaban (15 mg daily)45 were both approved for use by the US Food and Drug Administration in people with creatinine clearance (CrCl) <25 mL/min, including dialysis-dependence, unless adjustments are indicated by age greater than or equal to 80 years or weight ≤60 kg. The European Medicines Agency and Therapeutic Goods Association in Australia have not, to date, approved them for use in people with CrCl <15 mL/min.
Real world data supports the pharmacokinetic evidence for safety in kidney failure. A retrospective study of people with stage 4 and 5 CKD (88% stage 5 CKD or dialysis-dependent) found that rivaroxaban use <20 mg was associated with a 32% reduction in major bleeding compared with VKA, with no significant difference in stroke incidence.46 A US Renal Data System propensity score-matched cohort study reported apixaban use was associated with a 28% reduction in major bleeding compared with warfarin use with no difference in stroke rates between the 2 cohorts.47 Another study reported apixaban use was associated with at least 32% lower risk of bleeding compared with warfarin.48 Label-concordant dosing may provide a mortality benefit compared with reduced-label dosing.48
Randomized trials of DOACs in the hemodialysis population have recently been completed and provide supportive safety data (Table 1).3,4,38 In 132 people on hemodialysis, the Valkyrie extension study with median 1.88 years of follow-up reported a 63% reduction in fatal and nonfatal cardiovascular events in the pooled rivaroxaban group compared with VKAs. In a high thrombotic risk group (median age 80, median CHA2DS2-VASc score 5, and history of stroke 30%), the event rate was high in both the VKA group (63.8/100 person-years) and pooled rivaroxaban groups (23.8/100 person-years). There were no differences in secondary outcomes of all-cause and cardiovascular mortality or risk of stroke. The risk of major bleeding was reduced by 56% in the pooled rivaroxaban group compared with VKAs.3
Table 1.
Completed Randomized Controlled Trials of DOACs in People Receiving Hemodialysis
| Study | Recruited Cohort | Intervention | Follow-up | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|
| Valkyrie study3 | 132 prevalent HD with AF and CHA2DS2VASc ≥2; Median age 80, median CHA2DS2-VASc 5, stroke history 30% | Rivaroxaban 10 mg or rivaroxaban 10 mg with vitamin K2 compared with VKA | 1.88 y (IQR 1.01-3.38) | Composite fatal and nonfatal cardiovascular events; rivaroxaban: HR, 0.41 (95% CI, 0.25-0.68); rivaroxaban with vitamin K2: HR, 0.34 (95% CI, 0.19-0.61) | Efficacy end points: symptomatic limb ischemia: 19 in pooled rivaroxaban group compared with 20 in VKA (P = 0.008); no differences in other individual components of the fatal and nonfatal cardiovascular events Safety end point (life-threatening, major, and minor bleeding): pooled rivaroxaban group: HR, 0.44 (95% CI, 0.23-0.85) |
| RENAL-AF38 | 154 prevalent HDa with AF and CHA2DS2-VASc ≥2; Median age 68 y, median CHA2DS2VASc 4, stroke history 19% | Apixaban 5 mg or 2.5 mg twice daily (per label recommendations) vs VKA | 0.9 y (IQR not available) | Composite of all-cause death, major bleeding, and clinically relevant nonmajor bleeding (HR, 1.20; 95% CI, 0.63-2.30) | Stroke: apixaban: 3.0% (95% CI, 0.5-9.7); VKA: 3.3% (95% C,I 0.6-10.5) Death: apixaban: 21 events (26%); VKA: 13 events (18%) |
| AXADIA-AFNET 84 | 97 prevalent HDb with AF and CHA2DS2VASc ≥2; Median age 77, median CHA2DS2-VASc, stroke history not available | Apixaban 2.5 mg twice daily vs VKA | 1.27 y (IQR 0.48-1.92) | Composite of all-cause death, major bleeding and clinically relevant nonmajor bleeding: HR, 0.93 (95% CI 0.53-1.65) | Composite of myocardial infarction, ischemic stroke, all-cause death and DVT/PE: HR, 0.76 (95% CI 0.34-1.70) |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; HD, hemodialysis; HR, hazard ratio; IQR, interquartile range; PE, pulmonary embolism; VKA, vitamin K antagonist.
Trial stopped prematurely due to enrollment challenges. Initial targeted sample size 762 participants to test a noninferiority hypothesis.
Initial recruitment target of 222 participants, but this was amended to test a noninferiority null hypothesis.
The investigator-initiated AXADIA-AFNET 8 (A Randomised Controlled Trial Comparing Apixaban to the Vitamin K antagonist Phenprocoumon in Patients on Chronic Hemodialysis) and prematurely terminated RENAL-AF (Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation) trials compared apixaban with a VKAs in a hemodialysis cohort with AF and CHA2DS2-VASc ≥2.4,38 The AXADIA-AFNET 8 trial randomized 97 participants to either apixaban 2.5 mg twice daily or the VKA phenprocoumon.4 There were no apparent differences in safety and efficacy between the apixaban and VKA arms over a mean of 1.27 years. Noninferiority could not be shown according to prespecified hierarchical testing procedures for the primary safety outcome (all-cause death and major bleeding events or clinically relevant nonmajor bleeding) but there were no differences in observed event rates (apixaban 22 [45.8%] vs VKA 25 [51.0%] p[NI]=0.16]. Overall safety event rate was high at 36.4/100 person-years with 48.5% of participants experiencing an event during the trial period. There were no differences in the composite outcome of cardiovascular and thrombotic events hazard ratio 0.76 (95% confidence interval, 0.34-1.70)—the majority of events were cardiovascular deaths, and only 1 ischemic stroke/transient ischemic attack was reported.4
The RENAL-AF trial aimed to recruit 760 participants but was prematurely terminated due to enrollment challenges.38 A total of 154 participants were randomized to either apixaban 5 mg twice daily or 2.5 mg twice daily (as per label recommendations) or the VKA warfarin. After a median follow-up of just under 1 year, there were no significant differences in the primary safety or secondary efficacy outcomes between the apixaban and VKA group, with inadequate power to draw any conclusions. Gastrointestinal bleeding was the main contributor to major bleeding with hemodialysis access site bleeding responsible for the majority of clinically relevant nonmajor bleeding. Similar to the AXADIA-AFNET 8 trial, ischemic stroke was infrequent with only 3 events reported overall, and death was the most common key secondary outcome.38
Importantly, RENAL-AF reported pharmacokinetics data at steady state in 49 participants.38 The observed exposures (12-hour area under the curve) of apixaban 5 mg and 2.5 mg twice daily doses were similar to exposures in participants from the original ARISTOTLE trial49 with mild to advanced CKD (CrCl 15-90 mL/min). The 12-hour area under the curve in the apixaban 5 mg twice daily dose in RENAL-AF was significantly higher than those with normal kidney function (CrCl ≥90 mL/min). However, the one-year incidence of major or clinically relevant nonmajor bleeding in the apixaban arm was not significantly different to the VKA arm (31.5% vs 25.5%; hazard ratio, 1.25; 95% confidence interval, 0.63-2.30).38
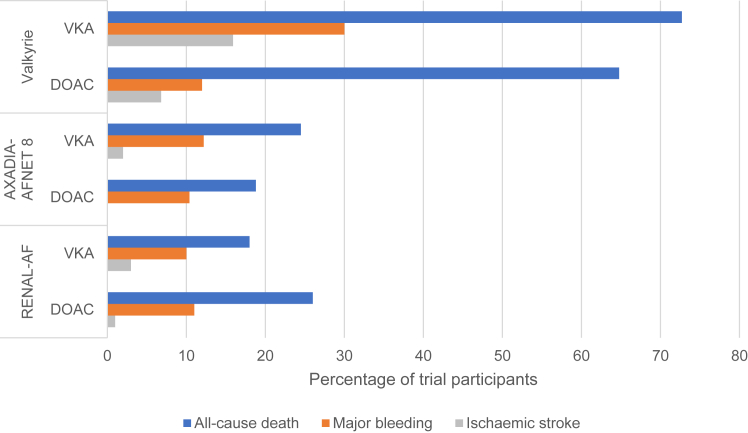
Randomized trial data now clearly demonstrate the disproportionately high mortality and bleeding risk on anticoagulation (Fig 1); 22%-26% of RENAL-AF participants had a bleeding event in under 1 year of follow-up38 even in excess of observational data.47 This needs to be carefully weighed against reported ischemic stroke (5.2/100 person-years) and mortality rates (26.9/100 person-years).6 In the absence of an RCT with a nonanticoagulated control arm, it is uncertain what proportion of bleeding is attributable to anticoagulation. The Strategies for the Management of Atrial Fibrillation in patiEnts receiving Dialysis (SAFE-D; NCT03987711) trial comparing VKA with apixaban and no anticoagulation is ongoing. The upcoming Stroke Prophylaxis With Apixaban in Chronic Kidney Disease Stage 5 Patients with Atrial Fibrillation (SACK; NCT05679024) trial comparing apixaban 2.5 mg twice daily with no oral anticoagulation will include people on kidney replacement therapy and those with estimated glomerular filtration rate <15 mL/min/1.73 m2 not on kidney replacement therapy.
Figure 1.
Comparison of stroke, major bleeding, and all-cause death ratesa in randomized controlled trials of DOACs in people receiving hemodialysis. Abbreviations: DOAC, direct oral anticoagulant; VKA, vitamin K antagonist. aEvent rates have been reported to allow for available data. The event rates may reflect the median follow-up across the Valkyrie (1.88 years), AXADIA-AFNET 8 (1.27 years), and RENAL-AF (0.9 years) trials.
Clinical Challenges and Implications for Management
Limited data in advanced CKD allow shared decision making between the clinician and patient, particularly in cases of declining kidney function where treatment decisions may be difficult (Table 2).2, 3, 4,8,10,13,50,51 However, guideline recommendations in kidney failure are inconsistent given clinical equipoise (Table 3)8,26,33 and have typically been directed by studies in the hemodialysis population. It is important to note the absence of data to guide management of people on peritoneal dialysis. The anticoagulation threshold for net clinical benefit in people with kidney failure and AF has not been established and may be different for people treated by hemodialysis or peritoneal dialysis. Some clinicians may consider overall bleeding risks to be prohibitively high, considering competing risks of mortality in the elderly and comorbid. Identifying robust markers of thromboembolic risk will be imperative to establish if there is any role for anticoagulation in people with kidney failure with AF.
Table 2.
Summary Practice Points Across the Spectrum of Chronic Kidney Disease
| Practice Points | |
|---|---|
| General population8 |
|
| Advanced CKD (15-29 mL/min/1.73 m2) |
|
| Kidney failure (eGFR <15 mL/min/1.73 m2) |
|
Abbreviations: AF, atrial fibrillation; CKD, chronic kidney disease; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; OAC, oral anticoagulation; VKA, vitamin K antagonist.
Table 3.
Clinical Guideline Recommendations on the Utility of Anticoagulation in Chronic Kidney Disease Categories
| AHA14 | ESC8 | KDIGO33 | |
|---|---|---|---|
| CrCl 30-49 mL/min | DOACs are recommended over warfarin in DOAC-eligible patients. Use apixaban, dabigatran, or rivaroxaban according to dose reduction recommendation. | DOACs are recommended over warfarin in DOAC-eligible patients. Use apixaban, dabigatran, or rivaroxaban according to dose reduction recommendation. | DOACs are recommended over warfarin in DOAC-eligible patients. Use apixaban, dabigatran, or rivaroxaban according to dose reduction recommendation. |
| CrCl 15-29 mL/min | DOACs are recommended over warfarin in DOAC-eligible patients. Use apixaban, dabigatran, or rivaroxaban according to dose reduction recommendation. | Consider warfarin based on limited clinical data. Use apixaban 2.5 mg twice daily or rivaroxaban 15 mg daily with caution. Do not use dabigatran. | Consider warfarin, apixaban 2.5 mg twice daily or rivaroxaban 15 mg daily. No dabigatran recommendation. |
| CrCl <15 mL/min including on kidney replacement therapy | Consider warfarin based on limited clinical data; consider apixaban 2.5 mg twice daily or rivaroxaban 15 mg daily with limited clinical safety data. | No specific recommendation regarding warfarin. Do not use DOAC. | Equipoise for warfarin use based on observational and meta-analysis data; consider apixaban 2.5 mg twice daily or rivaroxaban 15 mg daily with limited clinical safety data. |
Abbreviations: AHA, American Heart Association; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; ESC, European Society of Cardiology; KDIGO, Kidney Disease: Improving Global Outcomes.
Perioperative anticoagulation management will be an important consideration to transplant nephrologists and surgeons. Reversal of VKAs is relatively simple and has been managed safely for decades, but evidence for DOAC reversal is predominantly in elective surgery or in major bleeding and emergent invasive procedures. Anti-Xa assays can be used to exclude clinically relevant concentrations of factor Xa, but DOAC assays are not readily available. Prothrombin complex concentrate (plasma-derived concentrate of the vitamin K-dependent clotting factors in their inactive form) and andexanet alfa (recombinant factor Xa) have both been evaluated in rivaroxaban and apixaban-associated major bleeding cohorts,52, 53, 54 but not in emergency surgery.55
Left Atrial Appendage Occlusion
Left atrial appendage occlusion is a device-based, nonpharmacologic option that may be an attractive option in kidney failure. The left atrial appendage is believed to be the source of thrombi in >90% of patients with AF-related strokes, and left atrial appendage occlusion is a reasonable alternative in people with contraindications to systemic anticoagulation.56,57 Data on safety and efficacy remain outstanding, but early reports are promising. A prospective study of 92 people receiving dialysis comparing left atrial appendage occlusion with OAC and no anticoagulation did not report any stroke events in the left atrial appendage occlusion cohort after 2 years of follow-up, with reduced bleeding rates noted when compared with OAC.58 The WatchAFIB (NCT02039167) and STOPHARM (NCT02885545) trials of left atrial appendage occlusion involving people with stage 4/5 CKD and those on hemodialysis were both terminated because of slow recruitment. A prospective observational study of the Watchman left atrial appendage occlusion device (WATCH-HD; NCT03446794) and an RCT comparing standard care with the Amplatzer Cardiac Plug (LAA-KIDNEY; NCT05204212) in people with kidney failure is ongoing.
Factor XI Inhibitors
There is increasing evidence that factor XI is essential for thrombosis but may be less relevant to hemostasis. Factor XI inhibitors may be appealing anticoagulants in populations who are at high risk of bleeding, including those with kidney failure.59 Thrombosis is an intravascular process where factor XI is essential for thrombus expansion and stabilization via the intrinsic coagulation pathway. In contrast, hemostasis is believed to be predominately mediated by the extrinsic pathway where vessel injury triggers rapid thrombin generation without need for feedback to factor XI.59 People with congenital factor XI deficiency rarely experience spontaneous bleeding and have a lower risk of thrombosis, which is correlated with factor XI levels without an associated increase in bleeding events.60
Phase 2 factor XI inhibitors studies have shown proof-of-concept, with a 40%-50% reduction in venous thromboembolism and 59% reduction in bleeding compared with enoxaparin as standard of care in the elective hip and knee replacement population.61 In hemodialysis, phase 2 RCTs of factor XI antisense inhibitors fesomersen (BAY 2976217; NCT04534114) and IONIS-FXI RX (ISIS 416858; NCT03358030) are recently completed and reportedly are well tolerated and safe, although the results are yet to be published. Phase 1 (NCT03787368) and phase 2 (NCT04523220) studies of intravenous and subcutaneous osocimab (human monoclonal antibody) are ongoing in the hemodialysis population.
Conclusion
Management of AF-related complications in people with kidney failure is a common clinical conundrum. Thromboembolic risk is high, but the extent to which AF influences stroke risk is uncertain, and clinicians have a limited armamentarium to improve outcomes for this population. Recent randomized trial data report an improved safety profile for DOACs compared with VKA anticoagulation in people on hemodialysis, although overall bleeding risk is high. It follows that dedicated risk prediction to identify people at high thromboembolic risk in kidney failure is vitally important. AF burden has been shown to refine stroke risk in general AF cohorts. Variability in AF burden may translate to a dynamic stroke risk in people receiving dialysis, further challenging clinical decision making, and is a promising avenue of investigation in kidney failure.
Article Information
Authors’ Full Names and Academic Degrees
Mandy M. Law, MBBS, Sven-Jean Tan, PhD, Michael C.G. Wong, PhD, and Nigel D. Toussaint, PhD
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received March 13, 2023 as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Editor-in-Chief. Accepted in revised form April 22, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Halperin L.F., Lee M.K., Liew J., et al. Anticoagulation for patients with atrial fibrillation and end-stage renal disease on dialysis: a national survey. Can J Cardiol. 2021;37(6):924–928. doi: 10.1016/j.cjca.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Randhawa M.S., Vishwanath R., Rai M.P., et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vriese A.S., Caluwé R., Van Der Meersch H., De Boeck K., De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32(6):1474–1483. doi: 10.1681/ASN.2020111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinecke H., Engelbertz C., Bauersachs R., et al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation. 2023;147(4):296–309. doi: 10.1161/CIRCULATIONAHA.122.062779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seliger S.L., Gillen D.L., Longstreth W.T., Jr., Kestenbaum B., Stehman-Breen C.O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64(2):603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman D., Sood M.M., Rigatto C., Holden R.M., Hiremath S., Clase C.M. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27(10):3816–3822. doi: 10.1093/ndt/gfs416. [DOI] [PubMed] [Google Scholar]
- 7.Pisters R., Lane D.A., Marin F., Camm A.J., Lip G.Y. Stroke and thromboembolism in atrial fibrillation. Circ J. 2012;76(10):2289–2304. doi: 10.1253/circj.cj-12-1036. [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 9.Chao T.F., Liu C.J., Wang K.L., et al. Incidence and prediction of ischemic stroke among atrial fibrillation patients with end-stage renal disease requiring dialysis. Heart Rhythm. 2014;11(10):1752–1759. doi: 10.1016/j.hrthm.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 10.de Jong Y., Fu E.L., van Diepen M., et al. Validation of risk scores for ischaemic stroke in atrial fibrillation across the spectrum of kidney function. Eur Heart J. 2021;42(15):1476–1485. doi: 10.1093/eurheartj/ehab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong Y., Ramspek C.L., van der Endt V.H.W., et al. A systematic review and external validation of stroke prediction models demonstrates poor performance in dialysis patients. J Clin Epidemiol. 2020;123:69–79. doi: 10.1016/j.jclinepi.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Wang T.K., Sathananthan J., Marshall M., Kerr A., Hood C. Relationships between anticoagulation, risk scores and adverse outcomes in dialysis patients with atrial fibrillation. Heart Lung Circ. 2016;25(3):243–249. doi: 10.1016/j.hlc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 13.De Vriese A.S., Heine G. Anticoagulation management in haemodialysis patients with atrial fibrillation: evidence and opinion. Nephrol Dial Transplant. 2022;37(11):2072–2079. doi: 10.1093/ndt/gfab060. [DOI] [PubMed] [Google Scholar]
- 14.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Wizemann V., Tong L., Satayathum S., et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77(12):1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 16.Sood M.M., Larkina M., Thumma J.R., et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: results from the DOPPS. Kidney Int. 2013;84(3):600–608. doi: 10.1038/ki.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L.Y., Chung M.K., Allen L.A., et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137(20):e623–e644. doi: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botto G.L., Padeletti L., Santini M., et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20(3):241–248. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 19.Boriani G., Botto G.L., Padeletti L., et al. Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke. 2011;42(6):1768–1770. doi: 10.1161/STROKEAHA.110.609297. [DOI] [PubMed] [Google Scholar]
- 20.Sanna T., Diener H.C., Passman R.S., et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan R., Perera T., Elliott A.D., et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39(16):1407–1415. doi: 10.1093/eurheartj/ehx731. [DOI] [PubMed] [Google Scholar]
- 22.Wong M.C., Kalman J.M., Pedagogos E., et al. Temporal distribution of arrhythmic events in chronic kidney disease: highest incidence in the long interdialytic period. Heart Rhythm. 2015;12(10):2047–2055. doi: 10.1016/j.hrthm.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Roy-Chaudhury P., Tumlin J.A., Koplan B.A., et al. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93(4):941–951. doi: 10.1016/j.kint.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Koplan B.A., Winkelmayer W.C., Costea A.I., et al. Implantable loop recorder monitoring and the incidence of previously unrecognized atrial fibrillation in patients on hemodialysis. Kidney Int Rep. 2022;7(2):189–199. doi: 10.1016/j.ekir.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu J., Shah M.K., Perez J.J., et al. Dialysis modality and incident atrial fibrillation in older patients with ESRD. Am J Kidney Dis. 2019;73(3):324–331. doi: 10.1053/j.ajkd.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiver K.D., Quah J., Lahiri A., Ganesan A.N., McGavigan A.D. Atrial fibrillation burden: an update-the need for a CHA2DS2-VASc-AFBurden score. Europace. 2021;23(5):665–673. doi: 10.1093/europace/euaa287. [DOI] [PubMed] [Google Scholar]
- 27.Ganesan A.N., Chew D.P., Hartshorne T., et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591–1602. doi: 10.1093/eurheartj/ehw007. [DOI] [PubMed] [Google Scholar]
- 28.Buiten M.S., de Bie M.K., Rotmans J.I., et al. The dialysis procedure as a trigger for atrial fibrillation: new insights in the development of atrial fibrillation in dialysis patients. Heart. 2014;100(9):685–690. doi: 10.1136/heartjnl-2013-305417. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan R.M., Koehler J., Ziegler P.D., Sarkar S., Zweibel S., Passman R.S. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140(20):1639–1646. doi: 10.1161/CIRCULATIONAHA.119.041303. [DOI] [PubMed] [Google Scholar]
- 30.Lutz J., Menke J., Sollinger D., Schinzel H., Thürmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29–40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 31.van Eck van der Sluijs A., Abrahams A.C., Rookmaaker M.B., et al. Bleeding risk of haemodialysis and peritoneal dialysis patients. Nephrol Dial Transplant. 2021;36(1):170–175. doi: 10.1093/ndt/gfaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández-Díaz S., Rodríguez L.A. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002;55(2):157–163. doi: 10.1016/s0895-4356(01)00461-9. [DOI] [PubMed] [Google Scholar]
- 33.Turakhia M.P., Blankestijn P.J., Carrero J.J., et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018;39(24):2314–2325. doi: 10.1093/eurheartj/ehy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2020. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. [Google Scholar]
- 35.Carrero J.J., Evans M., Szummer K., et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311(9):919–928. doi: 10.1001/jama.2014.1334. [DOI] [PubMed] [Google Scholar]
- 36.Szummer K., Gasparini A., Eliasson S., et al. Time in therapeutic range and outcomes after warfarin initiation in newly diagnosed atrial fibrillation patients with renal dysfunction. J Am Heart Assoc. 2017;6(3) doi: 10.1161/JAHA.116.004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang F., Hellyer J.A., Than C., et al. Warfarin utilisation and anticoagulation control in patients with atrial fibrillation and chronic kidney disease. Heart. 2017;103(11):818–826. doi: 10.1136/heartjnl-2016-309266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pokorney S.D., Chertow G.M., Al-Khalidi H.R., et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146(23):1735–1745. doi: 10.1161/CIRCULATIONAHA.121.054990. [DOI] [PubMed] [Google Scholar]
- 39.Van Der Meersch H., De Bacquer D., De Vriese A.S. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: a systematic review and meta-analysis. Am Heart J. 2017;184:37–46. doi: 10.1016/j.ahj.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Chatrou M.L., Winckers K., Hackeng T.M., Reutelingsperger C.P., Schurgers L.J. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012;26(4):155–166. doi: 10.1016/j.blre.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Mac-Way F., Poulin A., Utescu M.S., et al. The impact of warfarin on the rate of progression of aortic stiffness in hemodialysis patients: a longitudinal study. Nephrol Dial Transplant. 2014;29(11):2113–2120. doi: 10.1093/ndt/gfu224. [DOI] [PubMed] [Google Scholar]
- 42.Ruderman I., Toussaint N.D., Hawley C.M., et al. The Australian Calciphylaxis Registry: reporting clinical features and outcomes of patients with calciphylaxis. Nephrol Dial Transplant. 2021;36(4):649–656. doi: 10.1093/ndt/gfz256. [DOI] [PubMed] [Google Scholar]
- 43.Chinnadurai R., Huckle A., Hegarty J., Kalra P.A., Sinha S. Calciphylaxis in end-stage kidney disease: outcome data from the United Kingdom Calciphylaxis Study. J Nephrol. 2021;34(5):1537–1545. doi: 10.1007/s40620-020-00908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mavrakanas T.A., Samer C.F., Nessim S.J., Frisch G., Lipman M.L. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28(7):2241–2248. doi: 10.1681/ASN.2016090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Vriese A.S., Caluwé R., Bailleul E., et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66(1):91–98. doi: 10.1053/j.ajkd.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Coleman C.I., Kreutz R., Sood N.A., et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132(9):1078–1083. doi: 10.1016/j.amjmed.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Siontis K.C., Zhang X., Eckard A., et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519–1529. doi: 10.1161/CIRCULATIONAHA.118.035418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wetmore J.B., Weinhandl E.D., Yan H., Reyes J.L., Herzog C.A., Roetker N.S. Apixaban dosing patterns versus warfarin in patients with nonvalvular atrial fibrillation receiving dialysis: a retrospective cohort study. Am J Kidney Dis. 2022;80(5):569–579.e1. doi: 10.1053/j.ajkd.2022.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Granger C.B., Alexander J.H., McMurray J.J.V., et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 50.Stanifer J.W., Pokorney S.D., Chertow G.M., et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141(17):1384–1392. doi: 10.1161/CIRCULATIONAHA.119.044059. [DOI] [PubMed] [Google Scholar]
- 51.Weir M.R., Ashton V., Moore K.T., Shrivastava S., Peterson E.D., Ammann E.M. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and stage IV-V chronic kidney disease. Am Heart J. 2020;223:3–11. doi: 10.1016/j.ahj.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Connolly S.J., Crowther M., Eikelboom J.W., et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326–1335. doi: 10.1056/NEJMoa1814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majeed A., Ågren A., Holmström M., et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130(15):1706–1712. doi: 10.1182/blood-2017-05-782060. [DOI] [PubMed] [Google Scholar]
- 54.Schulman S., Gross P.L., Ritchie B., et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118(5):842–851. doi: 10.1055/s-0038-1636541. [DOI] [PubMed] [Google Scholar]
- 55.Cuker A., Burnett A., Triller D., et al. Reversal of direct oral anticoagulants: guidance from the Anticoagulation Forum. Am J Hematol. 2019;94(6):697–709. doi: 10.1002/ajh.25475. [DOI] [PubMed] [Google Scholar]
- 56.Boersma L.V., Schmidt B., Betts T.R., et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reddy V.Y., Möbius-Winkler S., Miller M.A., et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) J Am Coll Cardiol. 2013;61(25):2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 58.Genovesi S., Porcu L., Slaviero G., et al. Outcomes on safety and efficacy of left atrial appendage occlusion in end stage renal disease patients undergoing dialysis. J Nephrol. 2021;34(1):63–73. doi: 10.1007/s40620-020-00774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eikelboom J., Floege J., Thadhani R., Weitz J.I., Winkelmayer W.C. Anticoagulation in patients with kidney failure on dialysis: factor XI as a therapeutic target. Kidney Int. 2021;100(6):1199–1207. doi: 10.1016/j.kint.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 60.Preis M., Hirsch J., Kotler A., et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129(9):1210–1215. doi: 10.1182/blood-2016-09-742262. [DOI] [PubMed] [Google Scholar]
- 61.Nopp S., Kraemmer D., Ay C. Factor XI inhibitors for prevention and treatment of venous thromboembolism: a review on the rationale and update on current evidence. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.903029. [DOI] [PMC free article] [PubMed] [Google Scholar]