Abstract
Objective
Recanalization with balloon angioplasty and/or self-expanding stents (SES) has become the endovascular treatment of choice for symptomatic femoropopliteal occlusive disease. These strategies generate suboptimal clinical results, however, because they fail to expand the artery fully and ineffectively prevent recoil, neointimal hyperplasia, and restenosis. Balloon-expandable stents, given their greater radial force and rigid structure, represent a more effective treatment strategy, but only short lengths can be implanted safely in arteries that deform and bend with skeletal motion. The purpose of this preclinical experiment was to test the hypothesis that simultaneous implantation of a series of short, resorbable, balloon-expandable, paclitaxel-eluting scaffolds would prevent neointimal hyperplasia and stenosis compared with SES in an animal model of percutaneous femoropopliteal intervention.
Methods
We extruded 6 × 60 mm Efemoral Vascular Scaffold Systems (EVSS) from copolymers of poly-L-lactic acid, coated with paclitaxel 3 μg/mm2, crimped onto a single delivery balloon, and implanted percutaneously into the iliofemoral arteries of eight Yucatan mini-swine. We implanted 7- to 8-mm × 60 mm SES into the contralateral experimental arteries. The animals were serially imaged with contrast angiography and optical coherence tomography after 30, 90, 180, 365, and 730 days. The primary end point of this study was neointimal morphometry over time. Secondary end points included acute deformation and angiographic and optical coherence tomography-derived measurements of chronic vascular response.
Results
Over the 2-year study period, one SES was found to be completely occluded at 90 days; all EVSS were widely patent at all time points. Arteries treated with SES exhibited profound neointimal hyperplasia with in-stent stenosis. In contrast, arteries treated with EVSS exhibited only modest vascular responses and minimal stenosis. After 2 years, the mean neointimal thickness (0.45 ± 0.12 vs 1.31 ± 0.91 mm; P < .05) and area (8.41 ± 3.35 vs 21.86 ± 7.37 mm2; P < .05) were significantly decreased after EVSS implantation. By 2 years, all scaffolds in all EVSS-treated arteries had resorbed fully.
Conclusions
In this preclinical animal model of peripheral endovascular intervention, the EVSS decreased neointimal hyperplasia and stenosis significantly compared with SES, then dissolved completely between the first and second years after implantation.
Keywords: Stents, Absorbable stents, Bioresorbable scaffolds, Preclinical testing, Animal model, Peripheral intravascular device
Clinical Relevance
Although generally successful in restoring patency immediately patency and improving arterial blood flow, commercially available peripheral endovascular devices rarely restore the target lesion to its full, original diameter, and often create a pathological environment in a disease artery that is prone to inflammation, cellular activation, proliferation, migration, restenosis, and therapeutic failure. In this preclinical chronic animal study, a novel drug-eluting, bioresorbable, balloon-expandable scaffold system was tested in a validated model of the human femoropopliteal artery.
Article Highlights.
-
•
Type of Research: Chronic, large animal study of an investigational peripheral arterial device
-
•
Key Findings: In this animal model, the treatment of long and mobile peripheral arteries using novel, short, balloon-expandable, serial bioresorbable scaffolds decreased neointimal thickness and stenosis. The scaffolds resorbed fully between the first and second years after implantation.
-
•
Take Home Message: This animal study challenges the perceived limitations of balloon-expandable device implantation in peripheral arteries and suggests a new strategy for reduction of neointimal hyperplasia and preservation of patency after percutaneous peripheral intervention.
Cardiovascular disease is a tremendous burden on human health; by 2030, >400 million people will carry this morbid diagnosis.1 Peripheral arterial occlusive disease (PAOD) is a virtual epidemic afflicting approximately 10% of the population over the age of 50 and 20% of the population over the age of 70.2,3 Its prevalence has increased by >20% over the last decade.4 Symptomatic PAOD causes poor overall health, loss of mobility and independence, decreased quality of life, premature functional decline, and early mortality.5, 6, 7
Revascularization with restoration of in-line flow remains the only reliable treatment of PAOD.8 Although peripheral bypass is an established treatment modality, individual risk and the availability of conduit limit its universal use.9 With the primary goal of achieving effective revascularization with decreased morbidity, endovascular techniques have been developed aggressively. Current paradigms of endovascular therapy use balloon dilatation, mechanical disruption, dilatation with specialty balloons coated with antiproliferative drugs, and/or permanent arterial stenting technology.10,11 These modalities are generally effective in treating short lesions. However, the results of endovascular intervention in the long, chronic occlusions typifying chronic limb-threatening ischemia remain suboptimal. In one contemporary clinical study, the 3-year primary patency of endovascular intervention with paclitaxel-eluting stents or paclitaxel-coated balloons in lesions >10 cm was achieved in only 45% and 26% of patients, respectively.12 In real-world clinical practice, recurrent stenosis complicates ≤50% of all conventional endovascular procedures after only 1 year.13
The purpose of this experiment was to test the hypothesis that the simultaneous implantation of a series of short, resorbable, balloon-expandable, drug-eluting scaffolds would prevent neointimal hyperplasia and stenosis compared with traditional bare stent technology in an animal model of percutaneous femoropopliteal intervention.
Methods
Resorbable scaffolds
The Efemoral Vascular Scaffold System (EVSS) is a linear series of balloon-expandable, resorbable, drug-eluting scaffolds designed specifically for the endovascular treatment of patients with symptomatic femoropopliteal artery occlusive disease (Fig 1). The individual scaffolds are created through the extrusion of uniform tubes of a co-polymer of the semicrystalline polymer poly-L-lactic acid (PLLA). Extruded tubes are then laser cut in a closed cell pattern designed to achieve radial strength comparable with 316L stainless steel and cobalt-chromium devices.14 In this preclinical study, five individual PLLA-based scaffolds were laser cut, coated with paclitaxel 3 μg/mm2, and crimped onto a standard 6 × 60 mm angioplasty balloon (Fig 1). The EVSS is intended to restore and maintain the patency of long blood vessels by providing transient rigid, radial support while still allowing the unimpeded arterial deformation necessary for effective organismal movement. That this strategy permits the unencumbered motion of long peripheral arteries has previously been demonstrated in an animal model of supraphysiological femoropopliteal artery deformation.15,16
Fig 1.
The Efemoral device. (A) Extruded, laser-cut, spray-coated and sterilized Efemoral bioresorbable individual scaffold. (B) The Efemoral Vascular Scaffold System (EVSS).
Study design
Sixteen iliofemoral arteries in eight Yucatan mini-swine were implanted with the 6-mm diameter EVSS in one hind limb, and a 7- to 8-mm, 60 mm-long, self-expanding nitinol stent (S.M.A.R.T.; Cordis; Hialeah, FL) in the contralateral hind limb via an open carotid approach. This mini-swine was chosen for its size match with the human femoropopliteal artery and the profound bending borne by the hind limb arteries when the quadruped animal crouches and sits. Device laterality was determined by randomization. Devices were placed in the porcine iliac or femoral segments to model physiological and supraphysiological human femoropopliteal arterial deformation, respectively. EVSS were deployed using slow inflation of ≥15 seconds/atm for the first 2 atm and maintaining a 2 atm pressure for approximately 30 seconds. In both limbs, plain balloon angioplasty was used to postdilate the implanted device to a 1.1:1.0 ratio (oversize 10%). Arterial diameters were measured using optical coherence tomography (OCT; Illumien Optis Imaging System; Abbott Laboratories, Abbott Park, IL).
After implantation, angiography was performed with the hind limb in both extension and exaggerated flexion to assess any possible impediment to deformation caused by the implanted devices. OCT was used to facilitate visualization of the nonmetallic experimental scaffolds and clearly delineate the arterial lumen and vascular tissue from the device edges.
Animals received oral acetylsalicylic acid 325 mg and clopidogrel 75 mg daily. At each of the intervals of 30, 90, 180, 365, and 730 days, the animals were reanesthetized and the treated arteries reimaged with both angiography and OCT.
All animal operations were performed in a facility compliant with 21 CFR Part 58 (FDA) Good Laboratory Practice for Nonclinical Laboratory Studies (Charles River Laboratories; Boisbriand, Quebec, Canada). The protocol was reviewed and approved by the Comité Institutionnel de Protection des Animaux d’AccelLAB (the test facility's Institutional Animal Care and Use Committee). The test facility is accredited by both the Association for Assessment and Accreditation of Laboratory Animal Care and the Canadian Council on Animal Care.
Study end points and analyses
Fluoroscopic and OCT images at the time of device implantation and follow-up were assessed quantitively using the OsiriX DICOM Viewer (Pixmeo SARL; Bernex, Switzerland). Lumen diameter and area measurements were taken at the approximate midpoint of each scaffold for the EVSS group and at approximately 1-cm intervals for the SES group (5 measurements). The reference vessel diameter was defined as the mean of lumen diameter proximal and distal to the target artery. Minimum lumen diameter and mean lumen diameter were also measured. Bend angle and axial compression were measured during provocative angiography at implantation. Chronic angiographic end points consisted of maximum and mean percent diameter stenosis, and maximum and mean late lumen loss. OCT end points included mean neointimal thickness and area. The acute and chronic end points used in this experiment are defined in Table I. Results are reported as mean ± standard deviation or standard error of mean. A paired t test was used to compare continuous variables between arteries treated with EVSS vs SES at all time points. Statistical analysis was performed using SPSS, version 20.0 (IBM Corp; Armonk, NY).
Table I.
Acute and chronic angiographic and optical coherence tomographic (OCT) end points and definitions
| End point (unit) | Definition |
|---|---|
| Acute angiographic | |
| Bend angle, ° | Approximate angle between the proximal and distal border of the sampled arterial segment |
| Axial compression, % | = (Arterial segment length in neutral extended position – arterial length in flexed position)/arterial segment length in neutral extended position |
| Diameter reduction during flexion (pinching), % | = [(MLDextended – MLDflexed)/MLDextended] × 100% |
| Chronic angiographic | |
| Maximum % diameter stenosis, % | = (1 – [MLD/RVD]) × 100%) |
| Mean % diameter stenosis, % | = (1 – [mean lumen diameter/RVD]) × 100% |
| Maximum late lumen loss, mm | = [MLDimplant – MLDfollow-up] |
| Mean late lumen loss, mm | = [mean lumen diameterimplant – mean lumen diameterfollow-up] |
| OCT | |
| Mean scaffold/stent area, mm2 | = Σ (scaffold/stent area measurements)/5 |
| Mean lumen area, mm2 | = Σ (lumen area measurements)/5 |
| Mean neointimal area, mm2 | = scaffold/stent area – lumen area |
| Mean scaffold/stent diameter, mm | = 2 × √(scaffold/stent area/π) |
| Mean lumen diameter, mm | = 2 × √(lumen area/π) |
| Mean neointimal thickness, mm | = (scaffold/stent diameter – lumen diameter)/2 |
| Mean area stenosis, % | = (1 – [lumen area/scaffold/stent area]) × 100% |
| Mean diameter stenosis, % | = (1 – [lumen diameter/scaffold/stent diameter]) × 100% |
MLD, Minimum lumen diameter; RVD, reference vessel diameter.
Results
All device implants were uncomplicated. All eight experimental animals survived serial repeat open surgical access of the carotid artery with hind limb angiography and OCT throughout the 2-year study.
Acute deformation
The effect of multiple, short, balloon-expandable scaffolds on the ability of the artery to deform during hind limb flexion was examined during the initial implantations. As expected, porcine iliofemoral arteries deformed markedly with hind limb flexion. Bending was not significantly different in arteries treated with the balloon-expandable EVSS or the control contralateral SES (67 ± 29° vs 64 ± 20°; P = .62) (Table II). EVSS implantation resulted in a significantly lower axial compression (1 ± 6% vs 11 ± 8%; P = .029), such that the untoward pinching effect on the minimum lumen diameter was nominally reduced (−5.8 ± 13.2% vs −9.3% ± 22.9%; P = .72).
Table II.
Effects of hind limb flexion on target artery morphology at implantation (mean ± SD)
| EVSS (n = 8) |
SES (n = 8) |
|||
|---|---|---|---|---|
| Extension | Flexion | Extension | Flexion | |
| Bend angle, ° | 10° ± 9° | 67° ± 29°a | 10° ± 5° | 64° ± 20°a |
| Target artery length, cm | 5.8 ± 0.6 | 5.1 ± 0.8 | 5.6 ± 0.9 | 5.5 ± 0.8 |
| Axial compression, % | 1 ± 6% | 11 ± 8%b | ||
| Minimum lumen diameter, mm | 3.90 ± 0.58 | 3.68 ± 0.79 | 4.62 ± 0.68 | 4.12 ± 1.04 |
| Mean lumen diameter, mm | 4.38 ± 0.55 | 4.46 ± 0.63 | 5.19 ± 0.64 | 5.14 ± 0.60 |
EVSS, Efemoral Vascular Scaffold System; SES, self-expanding stents.
Values are mean ± standard deviation.
P < .05 compared with extension.
P < .05 compared with EVSS.
Chronic vascular response
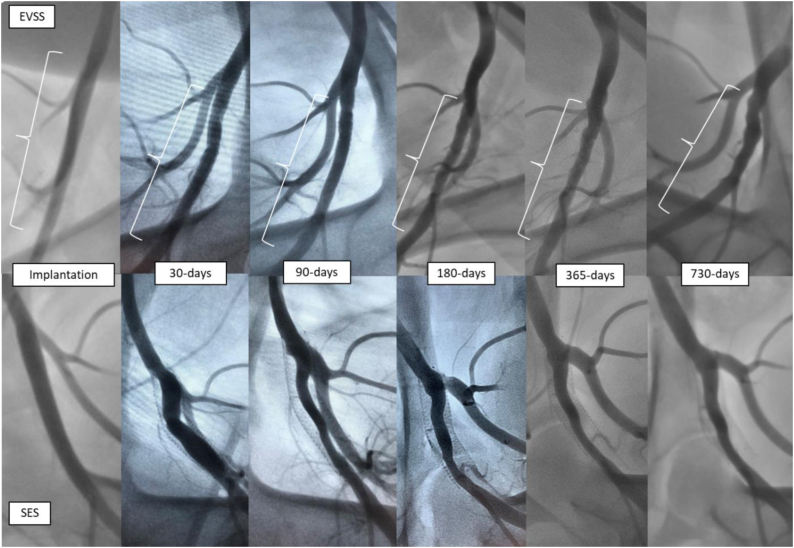
Over the 2-year study period, an analysis of serial angiographic images revealed that arteries treated with control SES exhibited profound neointimal hyperplasia with luminal compromise and in-stent stenosis, whereas arteries treated with EVSS exhibited wide patency with minimal stenosis (Fig 2). One SES placed in the distal femoral artery was found to be occluded at 90 days; all EVSS remained patent at all study time points. There were three instances of proximal migration of scaffolds and one case of proximal SES migration; none resulted in occlusion (although the SES that migrated proximally into the aorta after 30 days had to be traversed repeatedly at subsequent time points). Fracture was not observed in either device.
Fig 2.
Serial angiograms after Efemoral Vascular Scaffold System (EVSS) (top) or self-expanding stents (SES) (bottom) implantation in a porcine femoral artery over two years.
QVA images at implantation revealed that the outward force of the SES created larger lumen diameters as compared with the balloon-expandable EVSS (mean lumen diameter, 5.19 ± 0.64 mm vs 4.38 ± 0.55 mm) (Table III). Thus, the experiment began with the control group having larger diameters. Over time, however, SES generated profound neointimal hyperplasia and in-stent stenosis, which persisted throughout the 2-year study period (Fig 3). In contrast, target arteries treated with the EVSS developed only modest stenosis; this difference was statistically significantly at the 1- and 2-year time points. The net result was that target arteries treated with EVSS eventually exhibited larger arterial lumens for longer time periods (4.98 ± 1.27 mm vs 4.27 ± 1.99 mm) (Table III).
Table III.
Summary of QVA
| EVSS (n = 8) |
||||||
|---|---|---|---|---|---|---|
| Post-procedure | 30 days | 90 days | 180 days | 365 days | 730 days | |
| Minimum lumen diameter, mm | 3.90 ± 0.58 | 3.78 ± 0.48 | 3.06 ± 0.52 | 3.31 ± 0.61 | 3.57 ± 0.99 | 3.60 ± 0.66 |
| Mean lumen diameter, mm | 4.38 ± 0.55 | 4.27 ± 0.45 | 3.88 ± 0.66 | 3.97 ± 0.47 | 4.49 ± 0.98 | 4.98 ± 1.27 |
| Maximum diameter stenosis, % | 3.2 ± 10.0% | 9.5 ± 5.6% | 24.4 ± 11.7% | 18.3 ± 8.3% | 20.0 ± 9.0% | 19.9 ± 11.5% |
| Mean diameter stenosis, % | −9.1 ± 12.1% | −2.3% ± 5.3% | 4.3% ± 11.7% | 1.5 ± 4.9% | −1.3 ± 7.4% | −0.2 ± 15.4% |
| Maximum late lumen loss, mm | NA | 0.12 ± 1.13 | 0.84 ± 0.48 | 0.59 ± 0.55 | 0.33 ± 0.59 | 0.18 ± 0.51 |
| Mean late lumen loss, mm | NA | 0.11 ± 0.28 | 0.50 ± 0.24 | 0.41 ± 0.35 | −0.11 ± 0.55 | −0.60 ± 0.87 |
| SES (n = 8) |
||||||
|---|---|---|---|---|---|---|
| Post-procedure | 30 Days | 90 Days | 180 days | 365 days | 730 days | |
| Minimum lumen diameter, mm | 4.62 ± 0.68 | 3.49 ± 1.20 | 2.70 ± 1.42 | 2.91 ± 1.67 | 3.19 ± 1.46 | 3.67 ± 1.81 |
| Mean lumen diameter, mm | 5.19 ± 0.64 | 4.49 ± 0.85 | 3.47 ± 1.69 | 3.74 ± 1.69 | 4.07 ± 1.83 | 4.27 ± 1.99 |
| Maximum diameter stenosis, % | −11.8 ± 7.9%a | 20.7 ± 16.2% | 41.4 ± 27.9% | 42.4 ± 25.8%a | 39.1 ± 26.1%a | 33.8 ± 27.7% |
| Mean diameter stenosis, % | −26.1% ± 11.9%a | −4.5 ± 12.1% | 24.2 ± 34.5% | 24.6 ± 32.6% | 22.2 ± 32.9% | 33.4 ± 28.6%a |
| Maximum late lumen loss, mm | NA | 1.13 ± 0.87a | 1.92 ± 1.26 | 1.71 ± 1.42 | 1.42 ± 1.36a | 0.95 ± 1.58 |
| Mean late lumen loss, mm | NA | 0.70 ± 0.68a | 1.72 ± 1.42a | 1.44 ± 1.57 | 1.11 ± 1.60a | 0.92 ± 1.69a |
EVSS, Efemoral Vascular Scaffold System; SES, self-expanding stents.
Values are mean ± standard deviation.
P < .05 compared with EVSS.
Fig 3.
Porcine iliofemoral maximum percent diameter stenosis (A) and mean percent diameter stenosis (B) after implantation with either self-expanding stents (SES) (n = 8) or Efemoral Vascular Scaffold System (EVSS) (n = 8) as measured by QVA. Data points represent mean ± standard error of the mean. ∗P < .05.
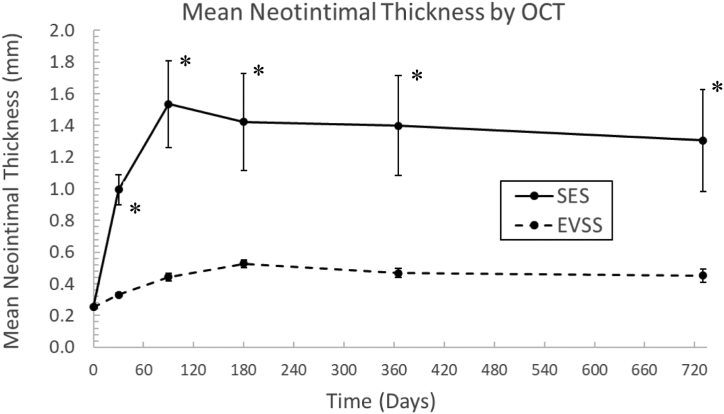
Quantitative OCT analysis results are presented in Table IV and reveal much of the same trends. As expected, the nitinol SES chronically expanded by 36% to reach a mean stent diameter of 7.34 ± 0.47 mm from an initial diameter of 5.41 ± 0.57 mm (P < .01) (Fig 4). These devices generated a profound and sustained neointimal reaction which was evident at 30 days (mean neointimal thickness 0.99 ± 0.27 mm), maximal at 90 days (1.54 ± 0.78 mm), and persistent throughout the 2-year study period (Fig 5). In contrast, the balloon-expandable EVSS generated only a mild vascular response with mean neointimal thickness of <0.6 mm at all time points (Table IV, Fig 5). The generated mean neointimal area was significantly greater at all time points in arteries treated with SES vs EVSS. There was general preservation of device and vascular architecture, with a 22% increase in scaffold diameter that matched the arterial growth of the juvenile animal. Even when implanted more distally into the femoral artery, which is subject to significantly greater mechanical forces, target arteries treated with EVSS exhibited only modest negative vascular remodeling.
Table IV.
Summary of quantitative optical coherence tomography (OCT) results
| EVSS (n = 8) |
||||||
|---|---|---|---|---|---|---|
| Post-procedure | 30 days | 90 days | 180 days | 365 days | 730 days | |
| Mean scaffold/stent diameter, mm | 5.18 ± 0.68 | 5.50 ± 0.52 | 5.47 ± 0.56 | 5.73 ± 0.63 | 6.21 ± 0.95 | 6.29 ± 0.92 |
| Mean lumen diameter, mm | 4.67 ± 0.65 | 4.84 ± 0.49 | 4.59 ± 0.55 | 4.67 ± 0.67 | 5.28 ± 1.00 | 5.39 ± 0.77 |
| Mean neointimal thickness, mm | 0.25 ± 0.03 | 0.33 ± 0.03 | 0.44 ± 0.07 | 0.53 ± 0.07 | 0.47 ± 0.08 | 0.45 ± 0.12 |
| Mean diameter stenosis, % | 9.9 ± 1.0% | 12.1 ± 1.2% | 16.4 ± 2.8% | 18.7 ± 3.2% | 15.4 ± 3.3% | 14.3 ± 2.7% |
| Mean scaffold/stent area, mm2 | 21.4 ± 5.9 | 24.0 ± 4.7 | 23.9 ± 5.2 | 26.1 ± 5.9 | 31.2 ± 10.3 | 32.1 ± 9.5 |
| Mean Lumen Area, mm2 | 17.5 ± 5.1 | 18.6 ± 4.0 | 16.9 ± 4.4 | 17.6 ± 5.2 | 22.8 ± 9.6 | 23.6 ± 6.8 |
| Mean neointimal area, mm2 | 3.96 ± 0.84 | 5.47 ± 0.92 | 7.00 ± 1.35 | 8.60 ± 1.32 | 8.52 ± 1.53 | 8.41 ± 3.35 |
| Mean area stenosis, % | 18.8 ± 1.9% | 22.7 ± 2.1% | 30.0 ± 4.6% | 33.7 ± 5.2% | 28.3 ± 5.7% | 26.5 ± 4.7% |
| SES (n = 8) |
||||||
|---|---|---|---|---|---|---|
| Post-procedure | 30 Days | 90 Days | 180 days | 365 days | 730 days | |
| Mean scaffold/stent diameter, mm | 5.41 ± 0.57 | 6.49 ± 0.57 | 6.72 ± 0.27 | 6.89 ± 0.40 | 7.18 ± 0.39 | 7.34 ± 0.47 |
| Mean lumen diameter, mm | 4.90 ± 0.56 | 4.50 ± 0.86 | 3.65 ± 1.79 | 4.04 ± 1.95 | 4.39 ± 1.96 | 4.73 ± 2.11 |
| Mean neointimal thickness, mm | 0.26 ± 0.04 | 0.99 ± 0.27a | 1.54 ± 0.78a | 1.42 ± 0.86a | 1.40 ± 0.89a | 1.31 ± 0.91a |
| Mean diameter stenosis, % | 9.6 ± 1.4% | 31.0 ± 9.1%a | 46.5 ± 25.0%a | 42.2 ± 26.0%a | 39.6 ± 25.8%a | 36.5 ± 26.8%a |
| Mean scaffold/stent area, mm2 | 23.3 ± 5.1 | 33.5 ± 5.9a | 35.7 ± 2.9a | 37.5 ± 4.6a | 40.7 ± 4.5a | 42.6 ± 5.6a |
| Mean lumen area, mm2 | 19.1 ± 4.4a | 16.6 ± 6.2 | 12.9 ± 8.9 | 15.8 ± 10.9 | 18.0 ± 10.0 | 20.7 ± 11.6 |
| Mean neointimal area, mm2 | 4.17 ± 0.90 | 16.85 ± 4.25a | 22.79 ± 6.19a | 21.79 ± 7.39a | 22.79 ± 7.52a | 21.86 ± 7.37a |
| Mean area stenosis, % | 18.2 ± 2.6% | 51.1 ± 13.0%a | 65.5 ± 21.4%a | 60.3 ± 22.6% | 57.4 ± 20.9%a | 53.3 ± 22.1%a |
EVSS, Efemoral Vascular Scaffold System; SES, self-expanding stents
Values are mean ± standard deviation.
P < .05 compared with EVSS.
Fig 4.
Mean stent/scaffold diameter after self-expanding stents (SES) (n = 8) or Efemoral Vascular Scaffold System (EVSS) (n = 8) as measured by optical coherence tomography (OCT) after implantation into porcine iliofemoral arteries. Data points represent mean ± standard error of the mean.
Fig 5.
Porcine iliofemoral mean neointimal thickness after implantation with either self-expanding stents (SES) (n = 8) or Efemoral Vascular Scaffold System (EVSS) (n = 8) as measured by quantitative optical coherence tomography (OCT). Data points represent mean ± standard error of the mean. ∗P < .05.
Scaffolded arterial remodeling
Imaging with OCT demonstrated excellent scaffold strut apposition after the procedure, although malapposition was occasionally observed in more distal target arteries at intermediate time points. The scaffold struts were covered by a thin rim of tissue after the first month, fully incorporated into the arterial wall by 6 months, and fully degraded by 2 years (Fig 6).
Fig 6.
Serial optical coherence tomography (OCT) images of the Efemoral Vascular Scaffold System (EVSS) (A) and self-expanding stents (SES) (B) after implantation in the porcine iliofemoral model over 2 years. Note the minimal neointimal response to EVSS, the complete coverage of the struts, and the disappearance of the device over time.
Discussion
In a porcine model of peripheral vascular intervention, the simultaneous, serial implantation of five balloon-expandable, PLLA-based scaffolds did not affect the ability of the target artery to deform during skeletal motion. Chronically, implantation of the drug-coated EVSS generated a mean neointimal thickness of only 0.44 ± 0.07 mm, which remained essentially unchanged over the 2-year study period. In contrast, the SES generated a mean neointimal thickness of 1.54 ± 0.78 mm at 90 days (P < .05) which, by OCT, resulted in 65 ± 21% area stenosis in the 4.14 ± 0.68-mm porcine peripheral artery. This result was expected; the chronic outward forces of nitinol are known to stimulate gross cellular reactions, especially in highly deformable arterial segments. The control group of this experiment redemonstrated conclusively the pathological relationship between continued SES expansion and vascular proliferation.17
The experiment reported herein represents the first long-term test of the novel EVSS, which was designed specifically to address the shortcomings of currently available peripheral devices and historic bioresorbable vascular scaffolds (BVS). The EVSS is purposefully designed for the long, occlusive, atherosclerotic lesions typical of the human peripheral arterial tree and addresses the pitfalls of prior generations of BVS by (1) targeting the significant unmet clinical needs of peripheral vascular intervention (as opposed to the well-established field of coronary intervention), (2) exhibiting the high radial force typical of balloon-expandable metal stents (instead of weak, nitinol SES), (3) using a novel biocopolymer of PLLA to enhance strength and ductility, (4) formulation with a polymer that degrades promptly within 2 years of implantation, and (5) serially mounting multiple, independent scaffolds over the device length to mitigate fracture and facilitate the endovascular treatment of long, occlusive lesions.
In the current era, the treatment of choice for patients with symptomatic PAOD includes some combination of transluminal balloon angioplasty, percutaneous atherectomy, drug-coated balloons (DCB), bare nitinol SES, covered nitinol stents, and/or drug-eluting nitinol stents (DES). The field has grown precipitously with the conduct and reporting of six international pivotal clinical trials of DCB and DES, each of which led to the release of a novel device into the US marketplace.18, 19, 20, 21, 22 DCBs, although effective in treating short segments, rarely provide sustained patency in the long lesions encountered in clinical practice. In a real-world registry of DCB deployed in long femoropopliteal lesions (mean, 24 cm), bailout stenting was required in 23% of cases and a discouraging 54% primary patency was noted at 2 years.23 A major reason for this shortcoming is the inability of balloon angioplasty to dilate stiff plaque-laden arteries fully and overcome the inevitable acute recoil that follows balloon deflation. This result is illustrated in a recent study in which residual stenosis ≥30% after DCB angioplasty was observed in 43% of patients.12,24
To expand target arteries fully, treat traumatic dissections, minimize residual stenosis, and provide more sustainable patency, intravascular stents made of stainless steel were invented.25 An equiatomic alloy of nickel-titanium (nitinol) was developed subsequently to enhance flexibility and facilitate the treatment of longer lesions.26 Unfortunately, nitinol SES present several critical drawbacks. First, their poor radial strength dilates arteries that are chronically hardened by atherosclerosis incompletely.27 Second, the chronic outward force exerted by these ever-expanding, permanent devices continually induces mural inflammation, foreign body reaction, smooth muscle cell proliferation, and restenosis. Even when used in conjunction with antiproliferative agents, the implantation of DES results in restenosis in 37% of patients after the first year.28
It has long been theorized that a nonpermanent stent that provides effective scaffolding after percutaneous angioplasty and then dissolves as the artery heals might be the ideal device for intravascular intervention. BVS offer the theoretical advantages of effective scaffolding that enhances postprocedure lumen diameter and prevents postdilation elastic recoil, avoiding chronic foreign body reaction and in-stent restenosis, allowing adaptive remodeling, preserving vasoactive function, and facilitating follow-up intravascular imaging and surveillance.29,30 To date, the most studied BVS is the coronary Absorb device (Abbott Laboratories) which consisted of a pure PLLA polymer scaffold and a poly-D, L-lactide coating containing everolimus. This BVS was tested in the pivotal ABSORB III trial.31 Despite showing promising results in the short term, by 2 years this first-generation BVS demonstrated a significantly higher rate of target lesion failure (11% vs 7.9%; P = .03) when compared with the best-in-class, third-generation coronary DES. However, several recent reports have suggested that its abandonment may have been premature.32, 33, 34 The long-term results of arteries and patients after the dissolution of Absorb suggests that the period of excess risk (observed during the first 3 years) resolves after the device disappears and may yield superior long-term outcomes. It was hypothesized that a prolonged degradation time may have caused continued chronic inflammation, leading to scaffold thrombosis. This critical shortcoming was addressed in the design of the novel device tested herein; the EVSS was created using a biocopolymer with a far faster degradation time such that it disappears between the first and second years after implantation.
Although the EVSS is the first BVS potentially applicable to long lesions, it is not the first absorbable stent to be specifically developed for the peripheral vasculature. The Esprit BVS (Abbott) consisted of a larger and longer Absorb and was tested in a small, prospective, single-arm, open-label, multicenter study of 35 external iliac and superficial femoral arterial interventions. After 2 years, the binary restenosis rate was only 16% and the incidence of target lesion revascularization was 12%.35 Although these results were favorable, enthusiasm for BVS had been tempered severely by the coronary experience and its further development was halted unfortunately.36, 37, 38
Of course, this preclinical study suffers from several important limitations, most notably the absence of atherosclerosis in the wild-type experimental animal; the vascular responses of human atherosclerotic arteries can only be inferred from this nondiseased porcine model. Also, because the two devices under study differed in many critical aspects (balloon expandable vs SES, drug vs bare, resorbable vs metal, segmented vs continuous), no single variable can be isolated to explain the significant differences in vascular reaction. Finally, the choice of bare SES as control may be problematic as it may not necessarily represent the standard of care in this rapidly evolving field.
Conclusions
Neointimal hyperplasia remains the Achilles' heel of successful and durable percutaneous endovascular intervention. In this preclinical animal model of femoropopliteal endovascular intervention, the EVSS was associated with significantly less neointimal hyperplasia and stenosis compared with SES, and completely dissolved between the first and second years after implantation. A transient device that effectively scaffolds the artery and decreases arterial inflammation has the potential to shift the paradigm of endovascular treatment delivery. The hypothesis that this new device will improve the results of endovascular intervention in patients with symptomatic PAOD is being tested in the EFEMORAL I First-in-Human Clinical Trial (ClinicalTrials.gov Identifier: NCT04584632).
Author Contributions
Conception and design: REK, IT, EAE, EMC, ML, LBS
Analysis and interpretation: REK, IT, EAE, EMC, JGB, LGG, ML, LBS
Data collection: REK, EMC, LGG, ML, LBS
Writing the article: REK, LBS
Critical revision of the article: IT, EAE, EMC, JGB, LGG, ML, LBS
Final approval of the article: REK, IT, EAE, EMC, JGB, LGG, ML, LBS
Statistical analysis: REK, LBS
Obtained funding: REK, IT, LBS
Overall responsibility: LBS
Footnotes
Efemoral Medical, Inc., is a for-profit research and development medical device company. This study was supported by an NIHSBIR grant (NIH1-R43-HL145671 and 1-R44-HL165972).
Author conflict of interest: I.T., E.A.E., and E.M. serve as consultants for Efemoral Medical. R.E.K. serves as Science Director for Efemoral Medical. L.B.S. serves as Chief Medical Officer for Efemoral Medical.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Video Commentary on this article included.
Additional material for this article may be found online at www.jvascsurg.org.
Supplementary data
References
- 1.Bansilal S., Castellano J.M., Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201(Suppl 1):S1–S7. doi: 10.1016/S0167-5273(15)31026-3. [DOI] [PubMed] [Google Scholar]
- 2.Criqui M.H., Denenberg J.O., Langer R.D., Fronek A. The epidemiology of peripheral arterial disease: importance of identifying the population at risk. Vasc Med. 1997;2:221–226. doi: 10.1177/1358863X9700200310. [DOI] [PubMed] [Google Scholar]
- 3.Criqui M.H., Fronek A., Barrett-Connor E., Klauber M.R., Gabriel S., Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 4.Fowkes F.G., Rudan D., Rudan I., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 5.Diehm C., Allenberg J.R., Pittrow D., et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 6.McDermott M.M., Liu K., Greenland P., et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 7.McDermott M.M., Liu K., Ferrucci L., et al. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol. 2011;57:962–970. doi: 10.1016/j.jacc.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte M.S., Bradbury A.W., Kolh P., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e140. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farber A., Menard M.T., Conte M.S., et al. Surgery or endovascular therapy for chronic limb-threatening ischemia. N Engl J Med. 2022;387:2305–2316. doi: 10.1056/NEJMoa2207899. [DOI] [PubMed] [Google Scholar]
- 10.Kokkinidis D.G., Armstrong E.J. Emerging and future therapeutic options for femoropopliteal and infrapopliteal endovascular intervention. Interv Cardiol Clin. 2017;6:279–295. doi: 10.1016/j.iccl.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Shishehbor M.H., Jaff M.R. Percutaneous therapies for peripheral artery disease. Circulation. 2016;134:2008–2027. doi: 10.1161/CIRCULATIONAHA.116.022546. [DOI] [PubMed] [Google Scholar]
- 12.Bausback Y., Wittig T., Schmidt A., et al. Drug-eluting stent versus drug-coated balloon revascularization in patients with femoropopliteal arterial disease. J Am Coll Cardiol. 2019;73:667–679. doi: 10.1016/j.jacc.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Sabeti S., Mlekusch W., Amighi J., Minar E., Schillinger M. Primary patency of long-segment self-expanding nitinol stents in the femoropopliteal arteries. J Endovasc Ther. 2005;12:6–12. doi: 10.1583/04-1359.1. [DOI] [PubMed] [Google Scholar]
- 14.Duda S.H., Wiskirchen J., Tepe G., et al. Physical properties of endovascular stents: an experimental comparison. J Vasc Interv Radiol. 2000;11:645–654. doi: 10.1016/s1051-0443(07)61620-0. [DOI] [PubMed] [Google Scholar]
- 15.El Khoury R., Nikanorov A., McCarroll E., et al. An animal model of human peripheral arterial bending and deformation. J Surg Res. 2019;241:240–246. doi: 10.1016/j.jss.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 16.El Khoury R., Tzvetanov I., Estrada E.A., et al. Intravascular treatment of long segments of experimental peripheral arteries with multiple, serial, balloon-expandable, resorbable scaffolds. JVS Vasc Sci. 2022;3:205–210. doi: 10.1016/j.jvssci.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H.Q., Nikanorov A., Virmani R., Jones R., Pacheco E., Schwartz L.B. Late stent expansion and neointimal proliferation of oversized Nitinol stents in peripheral arteries. Cardiovasc Intervent Radiol. 2009;32:720–726. doi: 10.1007/s00270-009-9601-z. [DOI] [PubMed] [Google Scholar]
- 18.Dake M.D., Ansel G.M., Jaff M.R., et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 19.Tepe G., Laird J., Schneider P., et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaff M.R., Rosenfield K., Scheinert D., et al. Drug-coated balloons to improve femoropopliteal artery patency: rationale and design of the LEVANT 2 trial. Am Heart J. 2015;169:479–485. doi: 10.1016/j.ahj.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan P., Faries P., Niazi K., et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic Studies. Circulation. 2017;136:1102–1113. doi: 10.1161/CIRCULATIONAHA.117.028893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray W.A., Keirse K., Soga Y., et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. 2018;392:1541–1551. doi: 10.1016/S0140-6736(18)32262-1. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt A., Piorkowski M., Gorner H., et al. Drug-coated balloons for complex femoropopliteal lesions: 2-year results of a real-world registry. JACC Cardiovasc Interv. 2016;9:715–724. doi: 10.1016/j.jcin.2015.12.267. [DOI] [PubMed] [Google Scholar]
- 24.Kuntz R.E., Safian R.D., Carrozza J.P., Fishman R.F., Mansour M., Baim D.S. The importance of acute luminal diameter in determining restenosis after coronary atherectomy or stenting. Circulation. 1992;86:1827–1835. doi: 10.1161/01.cir.86.6.1827. [DOI] [PubMed] [Google Scholar]
- 25.Palmaz J.C., Sibbitt R.R., Reuter S.R., Tio F.O., Rice W.J. Expandable intraluminal graft: a preliminary study. Work in progress. Radiology. 1985;156:73–77. doi: 10.1148/radiology.156.1.3159043. [DOI] [PubMed] [Google Scholar]
- 26.Stoeckel D., Pelton A., Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004;14:292–301. doi: 10.1007/s00330-003-2022-5. [DOI] [PubMed] [Google Scholar]
- 27.Chang I.S., Park K.B., Do Y.S., et al. Heavily calcified occlusive lesions of the iliac artery: long-term patency and CT findings after stent placement. J Vasc Interv Radiol. 2011;22:1131–1137.e1. doi: 10.1016/j.jvir.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Iida O., Takahara M., Soga Y., et al. 1-Year results of the ZEPHYR registry (Zilver PTX for the femoral artery and proximal popliteal artery): predictors of restenosis. JACC Cardiovasc Interv. 2015;8:1105–1112. doi: 10.1016/j.jcin.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Oberhauser J.P., Hossainy S., Rapoza R.J. Design principles and performance of bioresorbable polymeric vascular scaffolds. EuroIntervention. 2009;5(Suppl F):F15–F22. doi: 10.4244/EIJV5IFA3. [DOI] [PubMed] [Google Scholar]
- 30.Berglund J., Guo Y., Wilcox J.N. Challenges related to development of bioabsorbable vascular stents. EuroIntervention. 2009;5(Suppl F):F72–F79. doi: 10.4244/EIJV5IFA12. [DOI] [PubMed] [Google Scholar]
- 31.Ellis S.G., Kereiakes D.J., Metzger D.C., et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015;373:1905–1915. doi: 10.1056/NEJMoa1509038. [DOI] [PubMed] [Google Scholar]
- 32.Kereiakes D.J., Ellis S.G., Metzger C., et al. 3-Year clinical outcomes with everolimus-eluting bioresorbable coronary scaffolds: the ABSORB III trial. J Am Coll Cardiol. 2017;70:2852–2862. doi: 10.1016/j.jacc.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Stone G.W., Kimura T., Gao R., et al. Time-varying outcomes with the Absorb bioresorbable vascular scaffold during 5-year follow-up: a systematic meta-analysis and individual patient data pooled study. JAMA Cardiol. 2019;4:1261–1269. doi: 10.1001/jamacardio.2019.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kereiakes D.J., Ellis S.G., Metzger D.C., et al. Clinical outcomes before and after complete everolimus-eluting bioresorbable scaffold resorption: five-year follow-up from the ABSORB III trial. Circulation. 2019;140:1895–1903. doi: 10.1161/CIRCULATIONAHA.119.042584. [DOI] [PubMed] [Google Scholar]
- 35.Lammer J., Bosiers M., Deloose K., et al. Bioresorbable everolimus-eluting vascular scaffold for patients with peripheral artery disease (ESPRIT I): 2-year clinical and imaging results. JACC Cardiovasc Interv. 2016;9:1178–1187. doi: 10.1016/j.jcin.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 36.Henriques J.P.S., Elias J. The first generation ABSORB BVS scaffold; to be or not to be? Neth Heart J. 2017;25:416–418. doi: 10.1007/s12471-017-1007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeRubertis B.G., Kum S., Varcoe R.L. The demise of the Absorb BVS: lessons learned from the discontinuation of a disappearing stent. J Endovasc Ther. 2018;25:706–709. doi: 10.1177/1526602818799735. [DOI] [PubMed] [Google Scholar]
- 38.Raber L., Ueki Y. Bioresorbable scaffolds: unfulfilled prophecies. Circulation. 2019;140:1917–1920. doi: 10.1161/CIRCULATIONAHA.119.043773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.