Abstract
Background
Standard of care treatment options at glioblastoma relapse are still not well defined. Few studies indicate that the combination of trofosfamide plus etoposide may be feasible in pediatric glioblastoma patients. In this retrospective analysis, we determined tolerability and feasibility of combined trofosfamide plus etoposide treatment at disease recurrence of adult glioblastoma patients.
Methods
We collected clinicopathological data from adult progressive glioblastoma patients treated with the combination of trofosfamide and etoposide for more than four weeks (one course). A cohort of patients receiving empiric treatment at the investigators’ discretion balanced for tumor entity and canonical prognostic factors served as control.
Results
A total of n = 22 progressive glioblastoma patients were eligible for this analysis. Median progression-free survival (3.1 vs 2.3 months, HR: 1.961, 95% CI: 0.9724–3.9560, P = .0274) and median overall survival (9.0 vs 5.7 months, HR: 4.687, 95% CI: 2.034–10.800, P = .0003) were significantly prolonged compared to the control cohort (n = 17). In a multivariable Cox regression analysis, treatment with trofosfamide plus etoposide emerged as a significant prognostic marker regarding progression-free and overall survival. We observed high-grade adverse events in n = 16/22 (73%) patients with hematotoxicity comprising the majority of adverse events (n = 15/16, 94%). Lymphopenia was by far the most commonly observed hematotoxic adverse event (n = 11/15, 73%).
Conclusions
This study provides first indication that the combination of trofosfamide plus etoposide is safe in adult glioblastoma patients. The observed survival outcomes might suggest potential beneficial effects. Our data provide a reasonable rationale for follow-up of a larger cohort in a prospective trial.
Keywords: etoposide, glioblastoma, progressive glioblastoma, trofosfamide
Key Points.
Trofosfamide/etoposide treatment is safe in progressive glioblastoma.
Trofosfamide/etoposide may be linked to improved survival in progressive glioblastoma
Importance of the Study.
Despite steady progress in the understanding of glioma biology, treatment options remain scarce when brain cancer relapses. Frequently used therapy options in glioblastoma recurrence include lomustine (CCNU) or bevacizumab. However, standard of care therapy in glioblastoma recurrence has not clearly been defined yet and effective treatment options are urgently needed. Assuming a favorable blood–brain barrier penetrance of trofosfamide and etoposide and previous studies on glioma treatment in children, the combination of both drugs represents a reasonable treatment choice for glioma recurrence treatment in adults. This analysis indicates that the combination of trofosfamide and etoposide is well tolerated. The observed survival outcomes might suggest potential beneficial effects.
In glioblastoma, disease recurrence occurs inevitably and effective treatment options are scarce.1 The nitrosourea compound lomustine (CCNU) is still the most widely used drug and remains the central therapeutic option in glioblastoma recurrence.2 Although multiple studies of bevacizumab have failed to demonstrate a benefit in overall survival in glioblastoma recurrence, bevacizumab is being used to treat symptomatic peritumoral edema and to reduce corticosteroid use.1,3,4 A recent randomized phase II trial (REGOMA trial) of regorafenib in patients with glioblastoma recurrence suggested that regorafenib significantly prolonged overall survival (OS) compared with CCNU.2,5 However, contradictory real-life data, an extended spectrum of unfavorable adverse events as well as poor control arm data raised doubts as to whether regorafenib is indeed superior to CCNU.6,7 Standard of care in glioblastoma recurrence is not well defined and effective treatment options are urgently needed.
Trofosfamide is an alkylating drug and has found application in a broad spectrum of malignancies in medical oncology. Based on previous studies, trofosfamide has a high lipid solubility and can penetrate the blood–brain barrier.8 In addition, due to its oral formulation and favorable toxicity profile—which has been evaluated in multiple studies investigating various cancer types9–13—trofosfamide may prove to be a desirable treatment option for disease recurrence.14 Etoposide is a widely prescribed anticancer drug in medical oncology. Its primary target is the essential enzyme topoisomerase II, which removes knots and tangles from the genome by introducing transient double-stranded DNA breaks.15 Previous reports have demonstrated that etoposide, despite its high protein binding, which may impact bioavailability, reaches drug concentrations comparable to temozolomide in brain and tumor tissue of high-grade gliomas.16 The combination of DNA alkylating drugs with topoisomerase inhibitors may enhance antitumor efficacy by inhibiting the repair mechanism of topoisomerases after DNA alkylation.17,18 Previous reports indicate that the combined treatment with oral trofosfamide and etoposide (T/E) in pediatric newly diagnosed glioblastoma and pontine glioma was well tolerated.17,18 The observed median overall survival (mOS) was in the range of 8–12 months. Due to the small sample sizes and the lack of control arm data in these pediatric investigations, reliable conclusions on antitumor efficacy upon treatment with T/E could not be derived.
This retrospective analysis focuses on the tolerability and feasibility of T/E treatment in progressive glioblastoma among adult patients.
Materials and Methods
Study Design
We collected canonical clinicopathological data from all patients treated at the Department of Neurology at the University Medicine Essen with T/E since January 2017. In detail, the following selection criteria were required:
(1) Age ≥ 18 years at the time of treatment initiation with T/E.
(2) Diagnosis of progressive glioblastoma according to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System of 2021.4
(3) Treatment with T/E for more than four weeks (one course).
(4) Availability of a magnetic resonance imaging (MRI) scan within 30 days of treatment initiation (baseline MRI) and at least one follow-up MRI scan after treatment initiation with T/E.
Data were collected in an anonymized format within the framework of routine clinical assessments. We evaluated treatment response in accordance with the updated response assessment criteria for high-grade gliomas.19,20 Previous pediatric studies of newly diagnosed glioblastoma and pontine glioma reported administering trofosfamide at a dose of 100 mg/m2 body surface area and etoposide at a dose of 25 mg/m2 body surface area, both in the form of oral daily administration.17,18 The initial studies applied T/E treatment for 21 consecutive days followed by a 7-day rest period before proceeding to the next course.17,18 In this analysis, the authors determined through an evaluation of toxicity that an alternating one week on—one week off schedule with four weeks constituting one full course of therapy was better tolerated. MRI scans were performed every eight weeks after treatment initiation with T/E. We determined toxicity corresponding to the Common Terminology Criteria for Adverse Events (CTCAE - Version 5). We instituted dose reductions in response to any high-grade toxicity (CTCAE grade ≥ III) excluding lymphopenia. Trofosfamide dose was reduced to 75% (and to 50% in repeated instances of high-grade toxicity). The initiation of T/E treatment at reduced dose was deferred until the toxicity grade diminished to ≤ CTCAE grade I.
The decision to administer the T/E combination therapy was based on the discretion of the treating physicians when all approved treatments for progressive glioblastoma had been exhausted and alternative empirical therapies were unavailable due to individual toxicity risk, patient preference, or lack of cost coverage for off-label treatments. Informed consent for T/E treatment was obtained through standard clinical procedures, and the clinicopathological and survival data analyzed in this investigation were retrospectively evaluated.
For estimation of putative treatment efficacy, we identified a control cohort of n = 17 patients with balanced prognostic markers compared to the T/E cohort and treatment with an empiric therapy option instead of T/E. The control cohort was balanced to the T/E cohort regarding canonical clinical features such as histopathologic diagnosis, age, Karnofsky performance score (KPS), sex, MGMT (O6-methylguanine-DNA methyltransferase) promoter status, investigated treatment line and additional treatment in the investigated therapy line. The control cohort consisted of progressive glioblastoma patients treated at the Department of Neurology at the University Medicine Essen since January 2017 and met the following selection criteria:
(1) Age ≥ 18 years at the time of treatment initiation.
(2) Diagnosis of progressive glioblastoma according to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System of 2021.4
(3) Treatment with the investigated empiric recurrence therapy for more than four weeks.
(4) Availability of an MRI scan within 30 days of treatment initiation (baseline MRI) and at least one follow-up MRI scan after treatment initiation with the investigated empiric recurrence therapy.
The control cohort underwent the same data collection, treatment response evaluation, follow-up MRI scans, and toxicity determination processes as the T/E cohort.
This analysis was approved by the local ethics committee at the University Duisburg-Essen (application number: 20-9431-BO).
Statistics
We presented canonical patient characteristics descriptively. We used the Mann–Whitney U test (for continuous variables) and the Fisher’s exact test (for categorical variables) for comparison of feature distribution in the established subgroups.
To estimate the survival function from lifetime data we used the Kaplan–Meier estimator. To determine independent significant predictors for progression-free survival (PFS) and overall survival (OS), we used the multivariable Cox regression model. PFS was calculated from the date of latest MRI before treatment onset with the investigated therapy option (T/E or an empiric therapy option) until the date of next recurrence-defining MRI or last contact if no recurrence occurred. OS was calculated from the date of latest MRI before treatment onset with the investigated treatment option (T/E or an empiric treatment option) until death or date of last contact. If progression or death had not occurred at the time of data analysis (October 1, 2022), the corresponding patients were considered censored for further survival analysis. Before treatment initiation and recurrence definition upon treatment, we ruled out putative pseudoprogression by subsequent follow-up MRI, positron emission tomography (PET) imaging or surgery according to the updated response assessment criteria for high-grade gliomas.19,20
Results
Patient Cohort
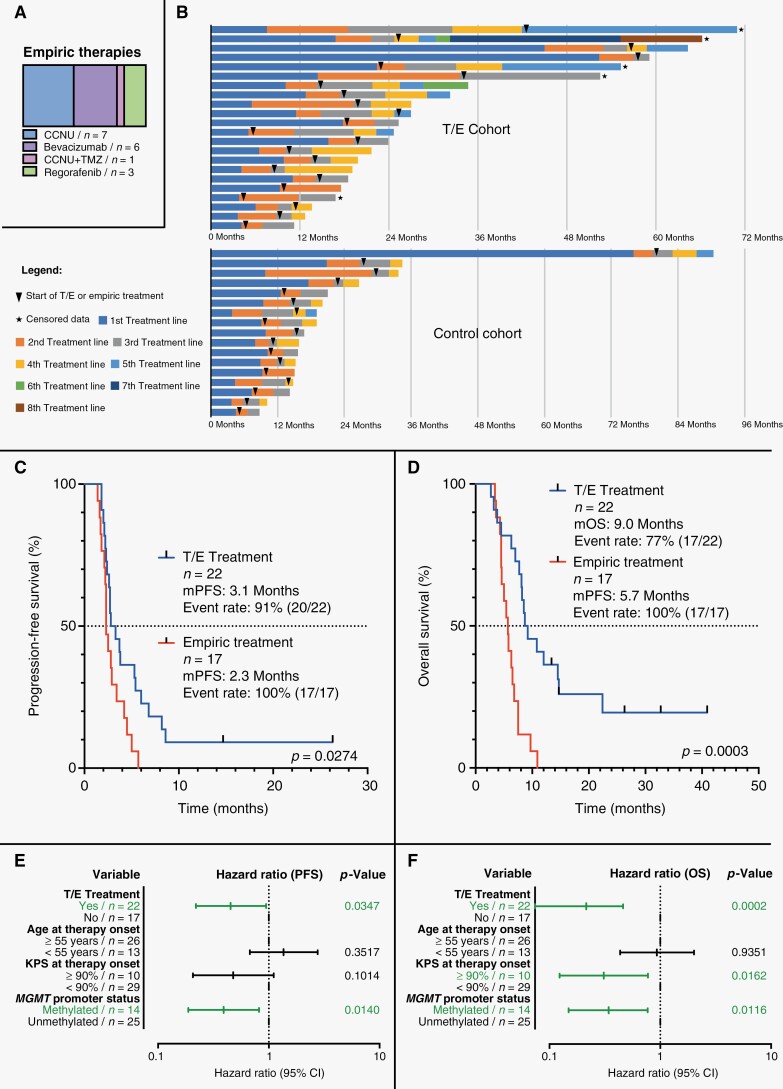
We identified a total of n = 22 patients with progressive glioblastoma who had received T/E (T/E cohort) and a balanced control cohort of n = 17 patients (control cohort) who had received an empiric treatment option. Table 1 comparatively shows detailed patient characteristics of the T/E cohort in comparison to the control cohort. The Flow Diagram in Figure 1a illustrates patient selection for the T/E cohort. In Figure 1b the clinical characteristics of each patient from the T/E cohort and control cohort are comparatively illustrated in a heatmap. In the control cohort n = 7 patients received treatment with CCNU, n = 6 patients received treatment with bevacizumab, n = 3 patients received treatment with regorafenib, and n = 1 patient received treatment with the combination of temozolomide and CCNU (Figure 2a). The rate of pathologic confirmation of recurrence prior to starting therapy was higher in the T/E cohort (n = 6/22, 27%) as compared to the control cohort (n = 3/17, 18%).
Table 1.
Patient Characteristics
| T/E cohort (n = 22) |
Control cohort (n = 17) |
P-value | |
|---|---|---|---|
| Age at therapy onset | .74 | ||
| ≥55 years | 14 (64%) | 12 (71%) | |
| <55 years | 8 (36%) | 5 (29%) | |
| KPS at therapy onset, n | .46 | ||
| ≥90% | 7 (32%) | 3 (18%) | |
| <90% | 15 (68%) | 14 (82%) | |
| Sex, n | .74 | ||
| Men | 12 (55%) | 11 (65%) | |
| Women | 10 (45%) | 6 (35%) | |
| MGMT promoter status, n | .74 | ||
| Methylated | 7 (32%) | 7 (41%) | |
| Unmethylated | 15 (68%) | 10 (59%) | |
| Investigated treatment line, n | .73 | ||
| ≥2. recurrence | 16 (73%) | 11 (65%) | |
| 1. recurrence | 6 (27%) | 6 (35%) | |
| Additional treatment in investigated therapy line, n | .73 | ||
| Radiotherapy | 2 (9%) | 2 (12%) | |
| Surgery | 6 (27%) | 3 (18%) | |
| TTFields | 5 (23%) | 6 (35%) | |
| None | 12 (55%) | 7 (41%) | |
| Time from initial glioblastoma diagnosis to start of investigated therapy in months, range (median) | 5–57 (16) | 5–79 (11) | .63 |
| Time from baseline MRI to therapy onset in days, range (median) | 1–30 (16) | 6–30 (21) | .62 |
| Investigated therapy time in weeks, range (median) | 8–64 (12) | 6–22 (8) | .01 |
| Follow-up in months, range (median) | 3–41 (9) | 3–11 (6) | <.01 |
KPS, Karnofsky performance score; MGMT, 06-methylguanine-DNA methyltransferase; MRI, magnetic resonance imaging; T/E, combination of trofosfamide with etoposide; TTFields, tumor treating fields.
Figure 1.
Overview of the T/E cohort and the control cohort. (a) delivers a Flow Diagram of for this analysis selected patients in the T/E cohort. (b) comparatively shows the individual clinical characteristics of every single patient from the T/E cohort and control cohort in a Heatmap. IDH, Isocitrate dehydrogenase; KPS, Karnofsky performance score; MGMT, O6-methylguanine-DNA methyltransferase; MRI, Magnetic resonance imaging; T/E, Combination of trofosfamide with etoposide.
Figure 2.
Survival analysis. (a) shows the administered empiric therapies in the control cohort. (b) illustrates the individual survival of the T/E cohort and the control cohort. Both, median progression-free survival (mPFS) and median overall survival (mOS) were significantly higher in the T/E cohort compared to the control cohort (c and d). In a multivariable Cox regression analysis including all glioblastoma patients (T/E cohort as well as control cohort) T/E treatment as well as methylation of MGMT promoter emerged as statistically significant prognostic markers for PFS; for OS, T/E treatment, methylation of MGMT promoter, and KPS ≥ 90% emerged as statistically significant prognostic markers (e and f). CI, confidence interval; KPS, Karnofsky performance score; MGMT, O6-methylguanine-DNA methyltransferase; mOS, median overall survival; mPFS, median progression-free survival; OS, overall survival; PFS, progression-free survival; T/E, combination of trofosfamide with etoposide; TMZ, temozolomide.
Treatment Efficacy
The swimmer’s plots in Figure 2b show individual times to treatment failure broken down by therapy line for patients in the T/E cohort and in the control cohort. Both, median PFS (mPFS) and median OS (mOS) were significantly higher in the T/E cohort than in the control cohort (mPFS: 3.1 months vs 2.3 months, HR: 1.961, 95% CI: 0.9724–3.9560, P = .0274; mOS: 9.0 months vs 5.7 months, HR: 4.687, 95% CI: 2.034–10.800, P = .0003) as illustrated in Figure 2c and d. PFS-6 and OS-12 were higher in the T/E cohort as compared to the control cohort (PFS-6: 27% vs 0%; OS-12: 41% vs 0%). In the T/E cohort, we observed a response rate of 36% with stable disease in 27% of patients (n = 6) and partial response in 9% (n = 2). In the control cohort, the response rate was 24% with 12% (n = 2) of patients having stable disease and 12% (n = 2) showing a partial response. Progressive disease was noted in 64% and 76% of the T/E cohort and control cohort respectively. In a multivariable Cox regression analysis including all patients (T/E cohort and control cohort) T/E treatment as well as methylation of MGMT promoter emerged as statistically significant prognostic markers regarding PFS; concerning OS, T/E treatment, methylation of MGMT promoter and KPS ≥ 90% emerged as statistically significant prognostic factors (Figure 2e and f).
Toxicity
In both cohorts (T/E cohort and control cohort) no treatment-related death occurred. We observed high-grade adverse events (CTCAE grade III or higher) in n = 16 (73%) patients of the T/E cohort and in n = 11 (65%) patients of the control cohort. Ninety-four percent (n = 15) of the n = 16 patients with high-grade adverse events in the T/E cohort experienced high-grade hematotoxicity with lymphopenia (73%, n = 11) being by far the most common hematotoxicity. Sixty-four percent (n = 7) of the n = 11 patients with high-grade adverse events in the control cohort experienced high-grade hematotoxicity. Six percent (n = 1) of the n = 16 patients with high-grade adverse events in the T/E cohort had nonhematotoxic high-grade events and 36% (n = 4) of the n = 11 patients with high-grade adverse events in the control cohort had nonhematotoxic high-grade events. Table 2 comparatively shows the toxicity data of the T/E cohort and the control cohort in detail.
Table 2.
Toxicity According to the Common Terminology Criteria for Adverse Events (CTCAE–Version 5).
| T/E cohort (n = 22) | Control cohort (n = 17) | |
|---|---|---|
| Treatment-related deaths, n | 0 | 0 |
| Patients with events CTCAE ≥ III, n | 16 (73%) | 11 (65%) |
| Patients with hematotoxicity CTCAE ≥ III, n | 15 (94%) | 7 (64%) |
| Lymphopenia | 11 (73%) | 4 (57%) |
| Neutropenia | 1 (7%) | 0 (-) |
| Pancytopenia | 3 (20%) | 0 (-) |
| Thrombopenia | 0 (-) | 3 (43%) |
| Patients with nonhematotoxicity CTCAE ≥ III, n | 1 (6%) | 4 (36%) |
| Fatigue | 0 (-) | 1 (25%) |
| Pneumonia | 0 (-) | 1 (25%) |
| Pulmonary embolism | 1 (100%) | 0 (-) |
| Seizure | 0 (-) | 2 (50%) |
T/E, combination of trofosfamide with etoposide.
Discussion
Our study, while retrospective, provides initial evidence that T/E treatment is well tolerated and could potentially be associated with a survival benefit in adult patients with progressive glioblastoma, when compared to a balanced control cohort treated with available empirical therapy options.
Our finding is important as treatment options for glioblastoma recurrence are scarce and a clear-cut standard remains to be defined. Nonetheless, CCNU is considered first choice for glioblastoma recurrence in many neuro-oncology centers. CCNU for the treatment of glioblastoma recurrence was associated with a median PFS of 1.0–2.7 months, a median OS of 5.6–9.8 months and a response rate of 0%–13.9% in established phase II or III trials.3,5,21–24 Among the substances tested against CCNU monotherapy in glioblastoma recurrence, only bevacizumab and regorafenib were shown to significantly prolong PFS.3,5 Given bevacizumab was not associated with OS prolongation at glioblastoma recurrence3 and anti-VEGF (vascular endothelial growth factor) treatment is related to a high degree of pseudoresponse,25,26 treatment with bevacizumab at glioblastoma recurrence is generally not considered superior to CCNU. Similarly, although the REGOMA trial indicated regorafenib might be a reasonable choice at glioblastoma recurrence, regorafenib is not widely accepted superior to CCNU in the neuro-oncological community for its undesirable toxicity profile and failure to entirely convince under real-life conditions.5,6,27 Our study shows that exposure to T/E treatment was associated with a median PFS of 3.1 months in glioblastoma recurrence and falls on the higher end compared to established phase II or III trials. When juxtaposing the median progression-free survival (mPFS) observed in our analysis with the outcomes of previous phase II and III glioblastoma recurrence trials, comparable mPFS durations exceeding our observed 3.1 months following T/E treatment were found only in the REGAL trial with the combination of cediranib and CCNU (mPFS: 4.2 months),24 and in the BELOB and EORTC 26101 trials, which combined bevacizumab and CCNU (mPFS: 4.0 and 4.2 months, respectively).21 Comparatively, when analyzing the median overall survival (mOS) from our study alongside the outcomes of prior phase II or III glioblastoma recurrence trials, mOS durations surpassing our observed 9.0 months following T/E treatment were demonstrated in the REGAL trial with cediranib and CCNU (mOS: 9.4 months) and CCNU alone (mOS: 9.8 months),24 in the BELOB and EORTC 26101 trials with bevacizumab and CCNU (mOS: 11.0 and 9.1 months, respectively),3,21 in the AxiG trial with a combination of axitinib and CCNU (mOS: 11.7 months) as well as axitinib alone (mOS: 12.4 months),28 and in the M12-356 trial with the combination of depatuximab mafodotin and temozolomide (mOS: 9.6 months).29 Notably, the comparison of survival times from our analysis with those from earlier phase II or III glioblastoma recurrence trials is skewed due to several factors, including methodological issues. For instance, most patients in the aforementioned trials received study treatment at their first glioblastoma recurrence, whereas in our analysis, only about a third of patients received T/E or control treatment at their first glioblastoma recurrence, with the majority receiving treatment at the second or subsequent recurrences. Given T/E treatment was overall well tolerated including in patients with low KPS (< 70%, n = 3, 14%), and most patients starting T/E treatment at advanced recurrence (≥ second recurrence, n = 16, 73%), T/E treatment may represent a reasonable treatment option at glioblastoma recurrence.
It remains to be elucidated to what extent the observed survival benefit is to be attributed to trofosfamide and to etoposide. Trofosfamide—being an alkylating drug—may not be as efficacious in MGMT promoter unmethylated patients (as compared to MGMT promoter methylated patients) and it remains unclear whether trofosfamide is the most suitable combination partner for etoposide and whether the combination of T/E has its justification in MGMT promoter methylated patients only. This notion could not be developed further in our cohort for sample size restrictions. It would be of interest to combine etoposide with drugs already established in glioblastoma treatment such as temozolomide and CCNU.
Nonetheless, T/E treatment performed superior to the control group after accounting for prognostically relevant factors (age, KPS, MGMT promoter methylation status) justifying follow-up on T/E in progressive glioblastoma in a prognostic and controlled trial. Noteworthy, however, T/E treatment was given as monotherapy and in 45% (n = 10) of the patients in combination with radiotherapy, Tumor Treating Fields (TTFields) or repeat maximum-safe resection limiting conclusions on putative efficacy. Compared to the control cohort, the T/E cohort displayed a higher distribution of MGMT promoter unmethylated patients (68% vs 59%), higher rates of ≥ 2 recurrences (73% vs 65%), and a higher proportion of patients receiving no additional treatment in the investigated therapy line (55% vs 41%). However, the T/E cohort was comprised of slightly younger patients (64% vs 71% aged ≥ 55 years) with a better KPS (32% vs 18% ≥ 90%). Moreover, in the T/E cohort, 59% of patients (n = 13) underwent at least one additional systemic therapy post-T/E, compared to 71% (n = 12/17) in the control cohort. This prevalence of subsequent systemic treatments potentially constrains the interpretation of the investigated treatment’s impact on overall survival. Furthermore, it has to be mentioned that some patients in both cohorts began treatments long after the typical median survival times reported in literature. Conversely, other patients started treatments relatively early post-diagnosis. This reflects the clinical reality that off-label treatments like T/E are considered when approved treatments have been exhausted, which may inherently bias the patient selection. While the implications of these observed discrepancies remain ambiguous, it is imperative to factor in these considerations during the interpretation of the presented data.
In the previously performed pediatric trials data on adverse events were not systematically assessed. The reported rate of high-grade adverse events seemed to be comparatively low and was in the range of 31%–38%.17,18 The rate of high-grade adverse events observed in our T/E cohort slightly exceeded that of the CCNU control arm data of the REGOMA trial5 (n = 16/22, 73% vs n = 21/33, 64%). In other established phase II or III trials the rate of high-grade adverse events of CCNU were in the range of 26%–64%.21–24 However, by far the most common high-grade adverse event during T/E treatment in our analysis was lymphopenia, which usually does not reflect treatment-limiting toxicity. Noteworthy, in n = 5/11 patients (45%) lymphopenia was present even before treatment initiation of T/E and persisted after treatment giving rise to uncertainty as to what extent T/E induces lymphopenia. It remains to be determined to what extent etoposide, owing to its limited bioavailability,30 may primarily contribute to the observed toxicities without achieving therapeutic concentrations within the tumor. The absence of documented single-agent response rates for progressive glioblastomas additionally makes it difficult to evaluate distinct single-drug contributions on treatment efficacy; we also want to note that the frequent breakdown of the blood–brain barrier in glioblastoma complicates the estimation of how well specific drugs can reach therapeutic concentrations in the tumor tissue—ideally this is to be dealt with in a phase 0 trial. Compared to the control cohort in our analysis, the rate of nonhematotoxic high-grade adverse events was lower in the T/E cohort (36% vs 6%). However, the rate of high-grade adverse events (hematotoxic as well as nonhematotoxic) in the T/E cohort was comparable to the rate of all high-grade adverse events (hematotoxic as well as nonhematotoxic) in the control cohort (73% vs 64%).
Noteworthy, limitations of our study include its retrospective nature, the potential for unintentional selection bias, a relatively small sample size, the heterogeneity in disease stages among participants, and the lack of randomization. In addition, a lack of pathologic verification of disease recurrence prior to the initiation of the therapy under investigation in certain patients further constrains the study. The diversity in therapeutic agents administered within the control cohort, as well as potential influence of subsequent alternative systemic therapies following T/E and control treatments in both cohorts, impede the comprehensive evaluation of the study and formulation of robust conclusions. In the T/E cohort, 59% of the patients (n = 13) underwent at least one further systemic treatment following T/E, relative to 71% (n = 12/17) in the control cohort. This aspect potentially constrains definitive conclusions about the effect of the investigated treatments on overall survival. While the precise implications of these observed variations remain indeterminate, their potential influence necessitates careful consideration during the interpretation of the presented data.
Nevertheless, to partially address these limitations, we did our best to assemble a valid control cohort and observed that the combination of T/E is safe and feasible in the treatment of adult patients with progressive glioblastoma. Moreover, our study found not only a potential survival benefit in terms of PFS but also noted significantly prolonged OS times after initiating T/E treatment. The OS data, therefore, might serve as a more robust endpoint, somewhat immune to the fact that patients treated with more aggressive approaches frequently may remain on the corresponding therapy longer than those receiving treatments which are known to have minimal efficacy due to optimism by physicians and patients (optimism bias). It is tempting to speculate that this combination could potentially result in a promising survival benefit. The observed provocative survival signal warrants further evaluation and provides a reasonable rationale for follow-up of a larger cohort in a prospective controlled trial.
Supplementary Material
Acknowledgments
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Contributor Information
Teresa Schmidt, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Sarina Agkatsev, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Jonas Feldheim, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Christoph Oster, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Tobias Blau, DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany; Institute of Neuropathology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Ulrich Sure, Department of Neurosurgery and Spine Surgery, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Kathy Keyvani, Institute of Neuropathology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Christoph Kleinschnitz, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Martin Stuschke, Department of Radiotherapy, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Ken Herrmann, Department of Nuclear Medicine, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Cornelius Deuschl, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Björn Scheffler, DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Sied Kebir, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Martin Glas, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Lazaros Lazaridis, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Funding
None declared.
Conflict of Interest
Teresa Schmidt received honoraria and travel support from Novocure.
Björn Scheffler is supported by the German Cancer Consortium (DKTK) and the DFG-CRU337.
Sied Kebir received honoraria and travel support from Novocure.
Martin Glas reports honoraria from Roche, Novartis, UCB, Abbvie, Daiichi Sankyo, Novocure, Bayer, Janssen-Cilag, Medac, Merck, Kyowa Kirin, travel support from Novocure and Medac, research grant from Novocure.
Lazaros Lazaridis received honoraria and travel support from Novocure.
All remaining authors have declared no conflicts of interest.
Authorship
Writing and reviewing of the manuscript: Teresa Schmidt, Sarina Agkatsev, Jonas Feldheim, Christoph Oster, Tobias Blau, Ulrich Sure, Kathy Keyvani, Christoph Kleinschnitz, Martin Stuschke, Ken Herrmann, Cornelius Deuschl, Björn Scheffler, Sied Kebir, Martin Glas, Lazaros Lazaridis.
Statistical analysis: Teresa Schmidt, Lazaros Lazaridis.
Medical data assessment: Teresa Schmidt, Sied Kebir, Lazaros Lazaridis.
Conceptualization: Teresa Schmidt, Sarina Agkatsev, Sied Kebir, Martin Glas, Lazaros Lazaridis.
References
- 1. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020; 22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weller M, Le Rhun E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat Rev. 2020; 87:102029. [DOI] [PubMed] [Google Scholar]
- 3. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017; 377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019; 20(1):110–119. [DOI] [PubMed] [Google Scholar]
- 6. Kebir S, Rauschenbach L, Radbruch A, et al. Regorafenib in patients with recurrent high-grade astrocytoma. J Cancer Res Clin Oncol. 2019; 145(4):1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glas M, Kebir S. Regorafenib in glioblastoma recurrence: how to deal with conflicting “real-life” experiences? Ther Adv Med Oncol. 2019; 11:1758835919887667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yule SM, Price L, Pearson AD, Boddy AV. Cyclophosphamide and ifosfamide metabolites in the cerebrospinal fluid of children. Clin Cancer Res. 1997; 3(11):1985–1992. [PubMed] [Google Scholar]
- 9. Reichardt P, Pink D, Tilgner J, et al. Oral trofosfamide: an active and well-tolerated maintenance therapy for adult patients with advanced bone and soft tissue sarcomas. Results of a retrospective analysis. Onkologie. 2002; 25(6):541–546. [DOI] [PubMed] [Google Scholar]
- 10. Kroiss M, Deutschbein T, Schlotelburg W, et al. ; German Adrenocortical Carcinoma Study Group. Salvage treatment of adrenocortical carcinoma with trofosfamide. Horm Cancer. 2016; 7(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartmann JT, Oechsle K, Mayer F, Kanz L, Bokemeyer C. Phase II trial of trofosfamide in patients with advanced pretreated soft tissue sarcomas. Anticancer Res. 2003; 23(2C):1899–1901. [PubMed] [Google Scholar]
- 12. Hartmann JT, Kopp HG, Gruenwald V, et al. ; German Sarcoma Group within the Working Group Medical Oncology (AIO) of the German Cancer Society/AIO-STS-002, Arbeitsgemeinschaft Internistische Onkologie der Deutschen Krebsgesellschaft e.V. Randomised phase II trial of trofosfamide vs. doxorubicin in elderly patients with untreated metastatic soft-tissue sarcoma. Eur J Cancer. 2020; 124:152–160. [DOI] [PubMed] [Google Scholar]
- 13. Schelker RC, Herr W, Reichle A, Vogelhuber M. Low-dose trofosfamide plus rituximab is an effective and safe treatment for diffuse large B-cell lymphoma of the elderly: a single center experience. BMC Cancer. 2018; 18(1):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Latz D, Nassar N, Frank R. Trofosfamide in the palliative treatment of cancer: a review of the literature. Onkologie. 2004; 27(6):572–576. [DOI] [PubMed] [Google Scholar]
- 15. Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr Med Chem Anticancer Agents. 2005; 5(4):363–372. [DOI] [PubMed] [Google Scholar]
- 16. Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011; 104(3):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolff JE, Molenkamp G, Westphal S, et al. Oral trofosfamide and etoposide in pediatric patients with glioblastoma multiforme. Cancer. 2000; 89(10):2131–2137. [DOI] [PubMed] [Google Scholar]
- 18. Wolff JE, Westphal S, Molenkamp G, et al. Treatment of paediatric pontine glioma with oral trophosphamide and etoposide. Br J Cancer. 2002; 87(9):945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 20. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017; 14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014; 15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 22. Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010; 28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brandes AA, Carpentier AF, Kesari S, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016; 18(8):1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013; 31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007; 67(2):323–326. [DOI] [PubMed] [Google Scholar]
- 26. Pope WB, Young JR, Ellingson BM. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep. 2011;11(3):336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzaridis T, Gepfner-Tuma I, Hirsch S, et al. Regorafenib in advanced high-grade glioma: a retrospective bicentric analysis. Neuro Oncol. 2019; 21(7):954–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duerinck J, Du Four S, Bouttens F, et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J Neurooncol. 2018; 136(1):115–125. [DOI] [PubMed] [Google Scholar]
- 29. Lassman AB, van den Bent MJ, Gan HK, et al. Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial. Neuro Oncol. 2019; 21(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei HJ, Upadhyayula PS, Pouliopoulos AN, et al. Focused ultrasound-mediated blood-brain barrier opening increases delivery and efficacy of etoposide for glioblastoma treatment. Int J Radiat Oncol Biol Phys. 2021; 110(2):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.