Abstract
Background
Several surveys over the past few years have demonstrated that postoperative pain in children is not treated appropriately. One pharmacological treatment option in a multimodal approach for postoperative pain treatment is the systemic administration of opioids. However, opioids are rarely used for postoperative pain treatment in children due to fear of adverse events. One long‐standing opioid for systemic use is nalbuphine, a kappa‐receptor agonist and µ‐receptor antagonist. The efficacy of nalbuphine is believed to be similar to morphine. Increased dosing might result in a ceiling effect, and thus less analgesia than expected. In addition, there might be a lower risk for opioid‐induced side effects (nausea, vomiting) and severe adverse events (respiratory depression) due to the antagonistic effect of the µ‐receptor. Nalbuphine may be an useful opioid for postoperative use in children, but exact efficacy (e.g. compared to other commonly used opioids) has not been determined yet.
Objectives
To assess the efficacy and adverse events of nalbuphine for acute postoperative pain treatment in children undergoing surgery.
Search methods
We systematically searched the following databases: The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 7), MEDLINE via Pubmed (January 1966 to July 2013) and EMBASE via Ovid (January 1947 to July 2013). We did not impose any restrictions regarding language or publication date. We checked all reference lists of retrieved articles for additional references.
Selection criteria
All randomised controlled trials (RCTs) investigating nalbuphine compared with placebo or other opioids were included.
Data collection and analysis
Two review authors independently scanned the retrieved articles and made a decision regarding inclusion or exclusion of studies for this review. The same authors also performed the data extraction and the assessment of risk of bias.
Main results
Ten RCTs including 658 patients were finally included in this systematic review. Five trials compared nalbuphine with placebo. Data from one out of five studies for the outcome moderate/severe pain following nalbuphine compared to placebo gave a risk ratio (RR) 1 hour postoperatively (postop) of 0.1 (95% confidence interval (CI) 0.01 to 0.71; low quality evidence) and a RR 2 hours postop of 0.14 (95% CI 0.02 to 1.06; low quality evidence). The estimated RR based on data from a single study indicated that nalbuphine reduced the requirement for analgesia two hours postop (RR 0.47; 95% CI 0.27 to 0.84; low quality evidence). Two included trials compared nalbuphine with morphine and showed a nonsignificant lower or comparable RR for moderate/severe pain at 1 hour postop (RR 0.84; 95% CI 0.12 to 5.74; low quality evidence), and 2 hours postop (RR 1.09; 95% CI 0.59 to 2.01; low quality evidence) for nalbuphine versus morphine. Four trials compared nalbuphine with tramadol for postoperative pain; data from one trial (per outcome) revealed a lower but nonsignificant RR for the need of additional rescue analgesics in children receiving nalbuphine (RR 2 hours postop 0.75; 95% CI 0.39 to 1.43; low quality evidence) (RR 12 hours postop 0.33; 95% CI 0.04 to 2.77; low quality evidence). One out of three trials comparing nalbuphine with pethidine demonstrated that the RR was not significantly lower following nalbuphine administration compared to pethidine (RR 2 hours postop 1.07; 95% CI 0.52 to 2.23; low quality evidence) (RR 24 hours postop 1.13; 95% CI 0.52 to 2.44; very low quality evidence). The most common adverse event was postoperative nausea and vomiting (PONV). Only one included trial reported that the RR for PONV in the postoperative care unit (PACU) was not significantly higher following nalbuphine compared to placebo (RR 1.00; 95% CI 0.16 to 6.42; low quality evidence) nor to morphine (RR 1.33; 95% CI 0.64 to 2.77; low quality evidence).
Authors' conclusions
Because the overall quality of available evidence was low, this systematic review could not definitively show that the analgesic efficacy of nalbuphine is superior compared to placebo. Furthermore, due to the lack of significant results the comparison with other common opioids is also unclear. The same holds true for the evidence focusing on adverse events following nalbuphine compared to placebo or other opioid administration. The evidence is limited, because studies did not report conclusively all important postoperative pain outcomes (e.g. number of patients with the need for rescue analgesia, postoperative pain scores). Thus, a quantitative analysis was not possible for many major aspects (e.g. rescue analgesia, pain scores) and heterogeneity could not be further explored.
Plain language summary
Does the administration of nalbuphine provide effective and safe postoperative pain treatment in children?
Postoperative pain is still a major problem following surgery in children. There is currently clear evidence that multimodal postoperative pain treatment is the best choice. This approach may involve using nonsteroidal anti‐inflammatory drugs (NSAIDs) and opioids. However, due to the fear of side effects such as respiratory depression (where the lungs cannot provide enough oxygen), opioids are not frequently used for postoperative pain treatment in children. Nalbuphine may provide effective pain relief without causing respiratory depression. In this review, we investigated how well nalbuphine worked, compared to placebo and other opioids, in children with postoperative pain. We also looked at the side effects. We performed a systematic literature search in July 2013. Ten randomised controlled trials with 658 patients were included. The patients were children aged from 0 ‐ 18 years and most did not have any other relevant medical conditions. The overall quality of evidence was low, so this review could not definitively show that nalbuphine is better than placebo. The same holds true for the comparison with other opioids (morphine, tramadol, pethidine, piritramid). We were not able to comment on side effects due to the small numbers of participants in the trials. Future studies need to address these issues, including more robust data for effectiveness and side effects.
Summary of findings
Summary of findings for the main comparison. Nalbuphine compared with placebo.
| Nalbuphine compared with placebo for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 0.3 mg/kg nalbuphine intramuscular/intravenous Comparison: placebo | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients with moderate/severe pain (1 hour postoperatively) | RR 0.1 (0.01 to 0.71) | 40 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with moderate/severe pain (2 hours postoperatively) | RR 0.14 (0.02 to 1.06) | 40 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with the need for rescue analgesia (2 hours postoperatively) | RR 0.47 (0.27 to 0.84) | 76 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with the need for rescue analgesia (12 hours postoperatively) | RR 0.06 (0.00 to 0.94) | 16 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with postoperative nausea and vomiting (postoperative care unit) | RR 1.00(0.16 to 6.42) | 40 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with postoperative nausea and vomiting (24 hours postoperatively) | RR 0.59(0.12 to 2.83) | 37 (1) | ⊕⊕⊝⊝ low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Summary of findings 2. Nalbuphine compared with morphine.
| Nalbuphine compared with morphine for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 0.2/0.3 mg/kg nalbuphine intramuscularly Comparison: 0.2 mg/kg morphine intramuscularly | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients with moderate/severe pain (1 hour postoperatively) | RR 0.84 (0.12 to 5.74) | 90 (2) | ⊕⊕⊝⊝ low | |
| Number of patients with moderate/severe pain (2 hours postoperatively) | RR 1.09 (0.59 to 2.01) | 90 (2) | ⊕⊕⊝⊝ low | |
| Number of patients with the need for rescue analgesia (12 hours postoperatively) | RR 0.61 (0.37 to 1.01) | 50 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with postoperative nausea and vomiting (postoperative care unit) | RR 1.33 (0.64 to 2.77)8 | 90 (2) | ⊕⊕⊝⊝ low | |
| Number of patients with postoperative nausea and vomiting (24 hours postoperatively) | RR 1.20 (0.80 to 1.80) | 50 (1) | ⊕⊕⊝⊝ low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Summary of findings 3. Nalbuphine compared with tramadol.
| Nalbuphine compared with tramadol for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 0.1/0.3 mg/kg nalbuphine intramuscularly/intravenously, 100 μg/kg nalbuphine bolus followed by 0.2 μg/kg/min for 72 hours intravenously Comparison: 0.75/1/2/3 mg/kg tramadol intramuscularly, 1000 μg/kg tramadol bolus followed by 2.0 μg/kg/min for 72 hours intravenously | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients with moderate/severe pain (1 hour postoperatively) | not estimable | 50 (1) | ⊕⊝⊝⊝ very low | |
| Number of patients with the need for rescue analgesia (2 hours postoperatively) | RR 0.75 (0.39 to 1.43) | 76 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with the need for rescue analgesia (12 hours postoperatively) | RR 0.33 (0.04 to 2.77) | 24 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with the need for rescue analgesia (24 hours postoperatively) | RR 1.45 (0.50 to 4.16) | 110 (2) | ⊕⊕⊝⊝ low | |
| Number of patients with postoperative nausea and vomiting (24 hours postoperatively) | RR 0.50 (0.11 to 2.23) | 24 (1) | ⊕⊕⊝⊝ low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Summary of findings 4. Nalbuphine compared with pethidine.
| Nalbuphine compared with pethidine for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 0.1/0.3 mg/kg nalbuphine intramuscularly/intravenously Comparison: 1/1.5 mg/kg pethidine intramuscularly/intravenously | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients with moderate/severe pain (1 hour postoperatively) | not estimable | 50 (1) | ⊕⊝⊝⊝ very low | |
| Number of patients with the need for rescue analgesia (2 hours postoperatively) | RR 1.07 (0.52 to 2.23) | 77 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with the need for rescue analgesia (24 hours postoperatively) | RR 1.13 (0.52 to 2.44) | 50 (1) | ⊕⊝⊝⊝ very low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Summary of findings 5. Nalbuphine compared with piritramid.
| Nalbuphine compared with piritramid for postoperative pain in children | ||||
|
Patient or population: children undergoing surgery Settings: hospital Intervention: 0.1 mg/kg nalbuphine intravenously Comparison: 0.1 mg/kg piritramid intravenously | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Number of patients with the need for rescue analgesia (24 hours postoperatively) | RR 8.17 (0.45 to 147.76) | 37 (1) | ⊕⊕⊝⊝ low | |
| Number of patients with postoperative nausea and vomiting (24 hours postoperatively) | RR 0.59 (0.12 to 2.83) | 37 (1) | ⊕⊕⊝⊝ low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Background
Description of the condition
Although postoperative paediatric pain management has become a recognised topic in research during the last decade, establishing optimal pain treatment strategies in children continues to be a problem (Cummings 1996; Segerdahl 2008; Taylor 2008). Inappropriate pain treatment is often due to an underestimation of pain, difficulties in pain assessment, inadequate prescriptions of analgesics and the restricted number of officially approved analgesic drugs for paediatric care (Howard 2003; Karling 2002; Kart 1996; Walker 2008). Furthermore, there is increasing evidence that an ineffective treatment of postoperative pain is associated with delayed wound healing, a negative development of pain perception and pain behaviour and chronic pain in the future (Anand 2000; Weisman 1998).
Description of the intervention
Regional analgesia seems to be the most effective way to treat postoperative pain early after surgery and regional analgesia techniques are increasingly used in children after surgery (Ecoffey 2010). However, not all patients and procedures are suitable for regional analgesia techniques. Multimodal analgesia using a combination of nonopioid analgesics (e.g. non‐steroidal anti‐inflammatory drugs, Michelet 2012) and opioids are another perioperative pain management option in children. However, the use of opioids (as a top up method on demand) is used very infrequently, mainly due to fear of side effects. In fact, clinicians are often concerned about their side effects, especially respiratory depression. Nalbuphine is a commonly used kappa‐receptor agonist and µ‐receptor antagonist. Nalbuphine and morphine are considered to be equianalgesic, but due to nalbuphine's own pharmacodynamics, there seems to be a lower risk for opioid‐induced respiratory depression at higher doses, itching, psychological effects (euphoria) and haemodynamic depression (Beaver 1978; Hoskin 1991). Due to the lack of pharmacodynamic data in the paediatric population, the majority of information available to date has been reported in adults. Nalbuphine is a world wide available opioid, but it is more frequently used in French speaking countries. There are currently no data available about average treatment costs of nalbuphine in children, but from adult data it is known that it might be more expensive than morphine or pethidine (Rhodes 1986).
How the intervention might work
Nalbuphine is a synthetic agonist‐antagonist analgesic offering a duration of analgesia of four to five hours (Chen 1993). Recently published pharmacokinetic data in children showed that the total clearance of nalbuphine ‐ mainly via liver enzymes cytochrome P450 3A4 and 2C19 (Stringer 2009) ‐ decreased, while the elimination half‐life increased significantly with increasing age (Bressolle 2011). Furthermore, another trial in neonates (Jacqz‐Aigrain 2003) revealed that birth weight influences the disposition to nalbuphine. In adults, its analgesic potency is about equal to that of morphine while the antagonist potency is about 1/25 of naloxone (Beaver 1978). The dual action as agonist and antagonist could improve common side effects of opioids (addiction, respiratory depression) (Miller 1980). The µ‐antagonist effects of nalbuphine in higher doses antagonise the respiratory depression of opioid agonists, while still maintaining effective analgesia. However, the administration of nalbuphine is associated with sedation, dysphoria, and urinary retention due to its kappa‐agonist actions (Rushton 1997). In adults, nalbuphine can be administrated either orally, parenterally or intramuscularly; however, the bioavailability after an oral administration is very low (20% to 25%) due to intense hepatic first‐pass metabolism (Errick 1983) and therefore not clinically useful. Nevertheless, the systemic administration might be an option in the postoperative period.
Why it is important to do this review
Although opioids are thought to be potent analgesics for postoperative pain management in children, they are still used reluctantly for pain treatment in children due to the fear of severe adverse effects such as respiratory depression or nausea and vomiting (Karling 2002; Kart 1996). Nalbuphine may be a valuable option for the treatment of moderate to severe postoperative pain in children (Miller 1980). However, single studies assessing the efficacy of nalbuphine in children are inconclusive. Thus, this systematic review about the efficacy and potential adverse effects of nalbuphine for postoperative pain treatment in children is needed to assess it's viability in the treatment of these patients.
Objectives
To assess the efficacy and adverse events of nalbuphine for acute postoperative pain treatment in children undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs investigating nalbuphine for postoperative pain in children.
Types of participants
We included children (0 to 18 years old) irrespective of their sex, type of surgery or specific comorbidities (e.g. neuromuscular disorders).
Types of interventions
We included all RCTs investigating nalbuphine for postoperative pain in children in comparison with placebo or any other opoid.
Types of outcome measures
We included studies, when they reported any of the following outcome measures.
Primary outcomes
Postoperative pain scores (assessed by validated pain scores based on a 0 to 10 scale) (1 hour, 2 hours, 12 hours, 24 hours postop).
Number of patients with moderate/severe pain (assessed by using pain score tools) (1 hour, 2 hours, 12 hours, 24 hours postop).
Number of patients with the need for rescue analgesia (1 hour, 2 hours, 12 hours, 24 hours postop).
Secondary outcomes
Number of rescue analgesic doses (postoperative care unit (PACU), 24 hours postop).
Total required dose of rescue analgesia (PACU, 24 hours postop).
Time to first rescue analgesic (hours).
Number of patients with adverse events (postoperative nausea and vomiting (PONV), pruritus, respiratory depression, urinary retention, sedation, bradycardia, hypotension) (PACU, 24 hours postop).
Search methods for identification of studies
The general principle of the systematic search consisted of a combination of indexed and free text terms to reflect the concepts of 'nalbuphine', 'postoperative pain' and 'children'. The search terms were modified according to the constraints of each database (Please see Appendix 1 for the MEDLINE search strategy).
Electronic searches
The following data sources were searched.
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 7)
MEDLINE (Pubmed) (January 1966 to July 2013).
EMBASE (Ovid) (January 1947 to July 2013).
Searching other resources
In addition, we searched reference lists of included and excluded studies.
Data collection and analysis
Two review authors (AS, SR) independently scanned the titles retrieved by the initial search to exclude irrelevant studies. The systematic search was not limited to any specific language. Two review authors (AS, SR) identified studies that were included in this review using a standardised study eligibility form developed by the review authors. If there were any differences or disagreements among the authors, these conflicts were reviewed by a third review author (EPZ).
Selection of studies
Two review authors (AS, SR) independently performed the study quality assessment using the 'Risk of bias' tool provided by The Cochrane Collaboration (Higgins 2011). All differences were resolved by discussion among the review authors.
Data extraction and management
Two review authors (AS, SR) independently extracted the data using standardised data extraction forms developed by the review authors. The number of participants with moderate/severe pain was defined as participants with a pain score of > 3 (Hirschfeld 2013). If different pain scales (Visual Analog Scale (VAS), Numerical Rating Scale (NRS), Faces pain scale revised) were used in the included studies, all pain scales were estimated to be equivalent, as long as they were based on 0 to 10 scale (Standing 2009). Additionally, if the pain degree was assessed with nonvalidated scales (e.g. three point scales (low/moderate/severe pain), we also used these results, but reported this specifically in the text. If there were multi‐arm trials, we extracted the data exclusively for each comparison (e.g. nalbuphine versus tramadol). If necessary, we retrieved additional missing data in published studies by contacting the study authors of the relevant articles. We resolved all differences by discussion among the review authors at each step of the data extraction.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies using The Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). The standard components in the specific domains included adequacy of allocation generation, allocation concealment, blinding, completeness of outcome data, possible selected outcome reporting and any other potential sources of bias. We judged each component as 'low risk' of bias, 'high risk' of bias or 'unclear'. We included 'Risk of bias' tables as part of the Characteristics of included studies tables and risk of bias summary figures (Figure 1 and Figure 2), which detail all of the judgements made for all included studies in the review. Two review authors (AS, SR) independently carried out an assessment of the risk of bias. We resolved any disagreements by discussion between the review authors, with a further review author acting as arbiter (PK).
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
1. Allocation of intervention
We considered allocation of intervention as adequate if it was generated by a random system (e.g. computer, random number table algorithm, tossing of a coin). We considered allocation inadequate if a nonrandom system was used (e.g. names, dates).
2. Concealment of allocation
We considered concealment adequate if an acceptable method, such as a central allocation system, sequentially‐numbered sealed opaque envelopes or an on‐site locked computer was used, ensuring that the group assignment was not revealed to participant recruiters, investigators or participants prior to the final allocation into the respective group. We considered concealment inadequate if it allowed the participant recruiters, investigators or participants to know the treatment allocation in advance or if the concealment procedure was not reported.
3. Blinding of participants and personnel
We considered blinding adequate if participants, persons responsible for participants' care (e.g. nurses) were all blinded to the intervention. We considered blinding inadequate if participants or persons responsible for participants' care were not blinded to the intervention.
4. Blinding of outcome assessment
We considered blinding adequate if the outcome assessors were blinded to the intervention. We considered blinding inadequate if outcome assessors were not blinded to the intervention.
5. Incomplete outcome data reporting
We considered outcome data reporting adequate if all withdrawals and drop‐outs were described. We considered outcome data reporting inadequate if the number of drop‐outs and withdrawals were lacking or if the reason for them was not given.
6. Selective reporting
We considered data reporting as adequate, if all relevant pain outcomes (at least validated pain scores) were reported. We considered outcome data reporting as inadequate if only selected pain outcomes or not validated measurements were used.
Measures of treatment effect
For binary data (dichotomous outcomes), we calculate the risk ratio (RR) with 95% confidence interval (CI), while for continuous data, we estimated the mean difference (MD) with 95% CI. To estimate the statistical significance of the results, we calculated the 95% CI for each item. Furthermore, we calculated the number needed to treat to benefit (NNT) for the efficacy outcome, and the number needed to treat to harm (NNH) for adverse events.
Dealing with missing data
If there were doubts about missing data (patient drop‐outs, selective outcome reporting, etc.), we contacted the relevant authors to obtain further information. If full information could be obtained, we intended to perform intention‐to‐treat (ITT) analyses. If full information could not be obtained, we performed complete‐case analyses. We planed to perform sensitivity analyses by imputing missing data (best case/worst case) in order to test the robustness of retrieved results.
Assessment of heterogeneity
We assessed clinical and methodological differences of included studies in order to decide if studies were sufficiently homogeneous to be combined. We analysed statistical heterogeneity using the I2 statistic. We used this test to calculate each of the outcomes listed above for the comparison with sham interventions. We assumed there was heterogeneity, if the I2 was ≧ 30%.
Data synthesis
We used either an inverse variance fixed‐effect or random‐effects model to analyse the data in Review Manager 5.2 (Review Manager 2012). If the test for heterogeneity (I2 statistic ≧ 30%) was positive, we undertook the analysis using a random‐effects model. However, taking into account that random‐effects models sometimes do not deliver more conservative results, especially in the presence of a small‐study effect, we reported the obtained summary statistics in conjunction with a sensitivity analysis (results obtained with a fixed‐effect model). If the random‐effects estimate demonstrated a larger effect size, we discussed whether it was reasonable to conclude that the intervention was more effective in smaller studies. We reported summary RRs and MDs with 95% CIs. We considered RRs with the range of the lower and upper bounds of the 95% CI not crossing one, and MDs with the range of the lower and upper bounds of the 95% CI not crossing zero, as statistically significant. To determine if studies were of different methodological quality, we performed sensitivity analyses (high quality studies versus low quality studies).
Subgroup analysis and investigation of heterogeneity
Consideration was given to the magnitude of clinical and methodological heterogeneity. To evaluate the effects of clinical heterogeneity, we planed to perform subgroup analyses calculating the RR or MD in conjunction with the corresponding CI for each subgroup. We used a fixed‐effect heterogeneity Chi2 statistic to compare subgroups. Additionally, we considered nonoverlapping subgroup CIs as consistent with a statistically significant difference.
We planned to analyse the following subgroups.
Type of surgery (head and neck, thoracic, abdominal, extremity).
Different drug doses and application types (orally, intravenous) of nalbuphine.
Different age groups of the included children.
However, if the overall number of included trials was low no subgroup analyses were performed. (Differences between protocol and review)
Summary of findings tables
We used the principles of the GRADE system (Schunemann 2008) to assess the quality of the body of evidence associated with specific outcomes (number of patients with moderate/severe pain, number of participants requiring rescue) in our review and constructed 'Summary of findings’ (SoF) tables (Table 1, Table 2, Table 3, Table 4, Table 5) using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one could be confident that an estimate of effect or association reflected the item being assessed.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
From the systematic literature search we identified 24 potentially relevant publications. After further review of title and abstract, we identified eleven studies as being potentially eligible for inclusion in the study. Finally, we included ten RCTs in the present meta‐analysis after reading the full‐text. There were no significant disagreements between review authors during the extraction process.
Included studies
Summary details of the ten included studies are given in the Characteristics of included studies tables.
Design
All ten included studies were parallel group RCTs and so met the inclusion criteria of this review. No trial was funded by any drug company. Nalbuphine was administrated either intramuscularly (Barsoum 1995; Krishnan 1987; Schäffer 1986; Wandless 1987) or intravenously (Büttner 1990; Galy 1991; Habre 1997; Littlejohn 1996; Moyado‐Garcia 2009; van den Berg 1999). In one study nalbuphine was administered before induction of general anaesthesia (Galy 1991). Four authors noted that nalbuphine was given directly after induction (Habre 1997; Littlejohn 1996; van den Berg 1999; Wandless 1987), whereas five studies administered nalbuphine before or after the end of surgery (Barsoum 1995; Büttner 1990; Krishnan 1987; Moyado‐Garcia 2009; Schäffer 1986). Only one trial used a combination of intravenous nalbuphine bolus with subsequent continuous infusion (Moyado‐Garcia 2009).
Sample sizes
The number of children analysed in the ten studies ranged from 24 to 152 (658 in total). Sample size calculations and ITT analysis were not reported in any of the included studies.
Setting
Four studies were carried out in the UK (Habre 1997; Krishnan 1987; Littlejohn 1996; Wandless 1987), two in Germany (Büttner 1990; Schäffer 1986), one in France (Galy 1991), one in the United Arab emirates (Barsoum 1995), one in Saudia Arabia (van den Berg 1999) and one in Canada (Moyado‐Garcia 2009).
Participants
Studies involved children aged from 11 months to 21 years of age mainly without any relevant comorbidities (only one trial specifically investigated children with obstructive sleeping disorder (Habre 1997)), with each study having a different age range. In one trial, young adults (up to 21 years) were included; however, a specific analysis of the demographic data of the different study groups showed that the mean age in all study groups was below 18 years (van den Berg 1999). Therefore, the reviewers decided to include the data of this RCT. Common paediatric surgical procedures were performed in all trials: Ear‐nose‐throat (ENT) surgery or teeth extraction (Habre 1997; Krishnan 1987; Littlejohn 1996; van den Berg 1999) and lower abdominal surgery (Barsoum 1995; Büttner 1990; Schäffer 1986; Wandless 1987). The surgical procedures were not explicitly described in two studies (Galy 1991; Moyado‐Garcia 2009).
Interventions
Six trials were multi‐arm trials (Barsoum 1995; Büttner 1990; Galy 1991; Littlejohn 1996; van den Berg 1999; Littlejohn 1996). Five included trials investigated postoperative analgesic efficacy of nalbuphine versus placebo treatment (Büttner 1990; Galy 1991; Krishnan 1987; Littlejohn 1996; van den Berg 1999). Eight studies compared patients receiving nalbuphine or other different opioid treatments: morphine (Krishnan 1987; Wandless 1987), pethidine (Barsoum 1995; Habre 1997; van den Berg 1999), tramadol (Barsoum 1995; Moyado‐Garcia 2009; Schäffer 1986; van den Berg 1999) or piritramid (Büttner 1990).
Outcomes
As described in the methods section we tried to contact the authors for further relevant data, but we did not receive any response. Therefore, a ITT analysis was not possible. All trials investigated the efficacy and adverse effects following perioperative (before or after anaesthesia induction, before or after the end of surgery) administration of nalbuphine in comparison with placebo or other opioid treatments (morphine, pethidine, tramadol or piritramid). Three studies reported the number of patients with moderate/severe pain (assessed by using three/four point postoperative pain severity scales (low/moderate/severe pain) (1 hour, 2 hours, 12 hours, 24 hours postop) (Barsoum 1995; Krishnan 1987; Wandless 1987). The number of patients requiring rescue analgesia was recorded in six studies (Barsoum 1995; Büttner 1990; Galy 1991; Moyado‐Garcia 2009; van den Berg 1999; Wandless 1987). Outcome variables were either ordinal (e.g. severity of pain) or nominal in nature (e.g. presence of postoperative nausea and vomiting (PONV)).
Excluded studies
Reasons for exclusions of the six studies are given in the Characteristics of excluded studies table. One trial was excluded, because it analysed retrospective data (Nascimento 2002), while two trials were not included, because they were only published as abstracts (Quaki 2007; Velegrakis 1998). Another trial included also data from adults (van den Berg 1994), whereas in two trials nalbuphine was administered either only as rescue medication (Bazin 2010) or patient controlled IV analgesia (Dongmin 2001).
Risk of bias in included studies
We assessed the methodological quality of included trials by using The Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011), which can be seen within Figure 1 and Figure 2.
Allocation
All included trials (not Barsoum 1995) reported that they used a randomised study design and were rated as being at low risk of selection bias. In contrast only two studies described explicitly the concealment of allocation and were rated as being at low risk of bias (Moyado‐Garcia 2009; Schäffer 1986). The method of allocation concealment was not reported in the remaining studies and therefore they were assessed as being at unclear risk of bias, whereas one study was an open label trial and was rated at high risk of bias (Barsoum 1995).
Blinding
Nine trials were performed as double‐blinded studies, in which the participant and provider of investigation were blinded to therapy (Barsoum 1995; Büttner 1990; Habre 1997; Krishnan 1987; Littlejohn 1996; Moyado‐Garcia 2009; Schäffer 1986; van den Berg 1999; Wandless 1987). These trials were rated at low risk of performance bias. Only one trial did not state explicitly that a double‐blind design was used and was rated as being at unclear risk (Galy 1991). In contrast only two trials were assessed as being at low risk for detection bias, because they reported that the outcome assessors were blinded (Barsoum 1995; Littlejohn 1996). All other studies were assessed as being at unclear risk of bias.
Incomplete outcome data
All trials explicitly stated that all patients were included and there were no drop‐outs; therefore all trials were assessed as being at low risk of attrition bias.
Selective reporting
In contrast to the latter finding, all included trials reported only a low number of relevant outcomes and were therefore rated as being at high risk for reporting bias.
Other potential sources of bias
There were no other potential sources of bias within the included trials.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
In general, due to different outcome data reporting, the overall number of pooled outcomes was very low.
Nalbuphine versus Placebo (Comparison 1)
Five included trials reported data for the comparison 'nalbuphine versus placebo' (Büttner 1990; Galy 1991; Krishnan 1987; Littlejohn 1996; van den Berg 1999)(Table 1).
Postoperative pain outcomes
Only one trial including 40 children (Krishnan 1987) investigated the number of children with moderate/severe postoperative pain following tonsillectomy; postoperative pain was assessed with a four point numeric scale (no pain, mild, moderate, severe pain) 1 hour and 2 hours postop. The RR for moderate/severe pain in children treated with 0.3 mg/kg nalbuphine at the end of surgery following tonsillectomy was lower compared to those receiving only placebo (RR 1 hour postop 0.10; 95% CI 0.01 to 0.71 (Analysis 1.1); RR 2 hours postop 0.14; 95% CI 0.02 to 1.06 (Analysis 1.2)). Furthermore, two included trials (Galy 1991; van den Berg 1999) investigated the outcome 'number of patients requiring rescue analgesia': 2 hours postop (Analysis 1.3; van den Berg 1999) and 12 hours postop (Analysis 1.4; Galy 1991); the RRs for the need for rescue analgesia was significantly lower in children receiving 0.3 mg/kg nalbuphine compared to placebo in the beginning of surgery. There were no data available focusing on secondary postoperative pain outcomes.
1.1. Analysis.
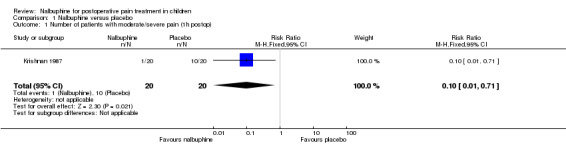
Comparison 1 Nalbuphine versus placebo, Outcome 1 Number of patients with moderate/severe pain (1h postop).
1.2. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 2 Number of patients with moderate/severe pain (2h postop).
1.3. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 3 Number of patients with the need for rescue analgesia (2h postop).
1.4. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 4 Number of patients with the need for rescue analgesia (12h postop).
Adverse events
All included trials reported data on adverse events following nalbuphine administration compared to placebo. The RRs for PONV in the postoperative care unit (PACU) or 24 hours postop were not significantly different (RR PACU 1.00; 95% CI 0.16 to 6.42; Analysis 1.5; Krishnan 1987) or lower (RR 24 hours postop 0.59; 95% CI 0.12 to 2.83; Analysis 1.6; Büttner 1990) between children receiving intraoperative nalbuphine or placebo at the end of surgery. Comparable results were seen for the more specific outcome 'number of patients with vomiting': van den Berg 1999 reported a nonsignificantly lower RR for postoperative vomiting in the PACU in children treated with nalbuphine at the beginning of surgery (RR 0.24; 95% CI 0.03 to 2.03; Analysis 1.7). At 24 hours postop the RR for vomiting in children treated with nalbuphine was nonsignificantly higher (RR 5.6; 95% CI 0.34 to 93.35; Analysis 1.8; Galy 1991). The RR for pruritus 24 hours postop was similar in children receiving nalbuphine compared to placebo (RR 0.78; 95% CI 0.06 to 10.37; Analysis 1.9). One trial reported that children treated with 0.3 mg/kg nalbuphine in the beginning of surgery were more sedated in the first 24 hours postop (Analysis 1.12; Galy 1991). No children suffered from respiratory depression (Analysis 1.10), urinary retention (Analysis 1.11) or bradycardia (Analysis 1.13) in four trials assessing these side effects (Galy 1991; Krishnan 1987; Littlejohn 1996; van den Berg 1999).
1.5. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 5 Number of patients with PONV (PACU).
1.6. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 6 Number of patients with PONV (postop 24h).
1.7. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 7 Number of patient with vomiting (PACU).
1.8. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 8 Number of patients with vomiting (postop 24h).
1.9. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 9 Number of patients with pruritus (postop 24h).
1.12. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 12 Number of patients with sedation (24h postop) ).
1.10. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 10 Number of patients with respiratory depression.
1.11. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 11 Number of patients with urinary retention.
1.13. Analysis.

Comparison 1 Nalbuphine versus placebo, Outcome 13 Number with patients with bradycardia (PACU).
Nalbuphine versus Morphine (Comparison 2)
The comparison 'nalbuphine versus morphine' was investigated in two included trials (Krishnan 1987; Wandless 1987) (Table 2).
Postoperative pain outcomes
Two included trials (Krishnan 1987; Wandless 1987) investigated the number of patients with moderate/severe postoperative pain following tonsillectomy (assessed on a four point numeric scale (no pain, mild, moderate, severe pain)) (Krishnan 1987) and minor abdominal surgery (assessed on a three point scale (no pain, moderate, severe pain)) (Wandless 1987). The RR for moderate/severe pain in children treated with 0.2/0.3 mg/kg nalbuphine was not significantly lower at 1 hour (RR 0.84; 95% CI 0.12 to 5.74; I2= 66%; Analysis 2.1) and 2 hours postop (RR 1.09; 95% CI 0.59 to 2.01; I2= 0%; Analysis 2.2) following nalbuphine compared to morphine. The observed heterogeneity might be related to the different application times of the study drugs in both trials (postop (Krishnan 1987) versus after induction of anaesthesia (Wandless 1987)). Additionally, in one trial (Wandless 1987), the RR for the need for rescue analgesia was not significantly reduced (RR 0.61; 95% CI 0.37 to 1.01; Analysis 2.3), when the children were treated with 0.2 mg/kg nalbuphine instead of 0.2 mg/kg morphine intraoperatively.
2.1. Analysis.

Comparison 2 Nalbuphine versus morphine, Outcome 1 Number of patients with moderate/severe pain (1h postop).
2.2. Analysis.

Comparison 2 Nalbuphine versus morphine, Outcome 2 Number of patients with moderate/severe pain (2h postop).
2.3. Analysis.

Comparison 2 Nalbuphine versus morphine, Outcome 3 Number of patients with the need for rescue analgesia (12h postop).
Adverse events
Data were only available for a low number of adverse events. The RR for PONV in the PACU was similar in the group of children treated with nalbuphine compared to those receiving morphine (RR 1.33; 95% CI 0.64 to 2.77; I2= 0%; Analysis 2.4; Krishnan 1987; Wandless 1987). The same holds true for PONV 24 hours postop (RR 1.2; 95% CI 0.8 to 1.8; Analysis 2.5; Wandless 1987). The number of patients with respiratory depression (Analysis 2.6) or bradycardia (Analysis 2.7) in the PACU was low in both groups ‐ only one child receiving 0.2 mg/kg morphine after induction suffered respiratory depression in the PACU (Wandless 1987).
2.4. Analysis.

Comparison 2 Nalbuphine versus morphine, Outcome 4 Number of patients with PONV (PACU).
2.5. Analysis.

Comparison 2 Nalbuphine versus morphine, Outcome 5 Number of patients with PONV (24h postop).
2.6. Analysis.

Comparison 2 Nalbuphine versus morphine, Outcome 6 Number of patient with respiratory depression.
2.7. Analysis.

Comparison 2 Nalbuphine versus morphine, Outcome 7 Number with patient with bradycardia (PACU).
Nalbuphine versus Tramadol (Comparison 3)
Four trials were available for the comparison 'nalbuphine versus tramadol' (Barsoum 1995; Moyado‐Garcia 2009; Schäffer 1986; van den Berg 1999) (Table 3).
Postoperative pain outcomes
Only one included trial reported data on the outcome number of patients with moderate/severe pain (1 hour postop) (assessed on a four point verbal scale (no pain, mild, moderate, severe pain) after lower abdominal surgery (Barsoum 1995); however, there were no children in either group complaining about moderate/severe pain following 0.1 mg/kg nalbuphine or 2 mg/kg tramadol (Analysis 3.1). The number of patients with the need for rescue analgesia was assessed in all four included trials. At 2 hours and 12 hours postop the RR for the need for rescue analgesia was nonsignificantly lower in children treated with nalbuphine 0.1 to 0.3 mg/kg (RR 2 hours postop 0.75; 95% CI 0.39 to 1.43; Analysis 3.2; van den Berg 1999); RR 12 hours postop 0.33; 95% CI 0.04 to 2.77; Analysis 3.3; Moyado‐Garcia 2009). However, 24 hours postop the RR was nonsignificantly higher in the nalbuphine group (RR 1.45; 95% CI 0.50 to 4.16; I2= 34%; Analysis 3.4; Barsoum 1995; Schäffer 1986).
3.1. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 1 Number of patients with moderate/severe pain (1h postop).
3.2. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 2 Number of patients with the need for rescue analgesia (2h postop).
3.3. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 3 Number of patients with the need for rescue analgesia (12h postop).
3.4. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 4 Number of patients with the need for rescue analgesia (24h postop).
Adverse events
Adverse events were reported in all included trials. The number of patients with PONV 24 hours postop was nonsignificantly lower in the nalbuphine group compared to the tramadol group (Analysis 3.5; Moyado‐Garcia 2009). The same holds true for the more specific outcome 'vomiting' 24 hours postop (RR 0.33; 95% CI 0.01 to 7.81; Analysis 3.7; Barsoum 1995), while in the PACU the RR was completely comparable (RR 1.0; 95% CI 0.50 to 2.0; Analysis 3.6; Schäffer 1986; van den Berg 1999). The RR for sedation was not significantly higher in the group of children treated with 0.1 mg/kg nalbuphine compared to those receiving 1 mg/kg tramadol for postoperative pain (RR 2.0; 95% CI 0.21 to 19.23; Analysis 3.9; Moyado‐Garcia 2009). No patients with bradycardia (Analysis 3.10) or with respiratory depression (Analysis 3.8) were mentioned in two included trials (Barsoum 1995; van den Berg 1999).
3.5. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 5 Number of patients with PONV (24h postop).
3.7. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 7 Number of patients with vomiting (24h postop).
3.6. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 6 Number of patients with vomiting (PACU).
3.9. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 9 Number of patients with sedation (24h postop).
3.10. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 10 Number of patients with bradycardia (PACU).
3.8. Analysis.

Comparison 3 Nalbuphine versus tramadol, Outcome 8 Number of patients with respiratory depression (PACU).
Nalbuphine versus Pethidine (Comparison 4)
Three RCTs investigated 'nalbuphine versus pethidine' for postoperative pain (Barsoum 1995; Habre 1997; van den Berg 1999) (Table 4).
Postoperative pain outcomes
Barsoum and colleagues noted that no children complained about moderate/severe pain 1 hour postop following 0.1 mg/kg nalbuphine or 1 mg/kg pethidine (Analysis 4.1; Barsoum 1995). Furthermore, the RR for the need for rescue analgesia (Barsoum 1995; van den Berg 1999) was almost comparable between both groups (RR 2 hours postop 1.07; 95% CI 0.52 to 2.23; Analysis 4.2; RR 24 hours 1.12; 95% CI 0.52 to 2.44; Analysis 4.3). The time to first rescue analgesic was around 1.02 hours longer following 0.1 mg/kg nalbuphine compared to 1 mg/kg tramadol administered after induction in children undergoing tonsillectomy (Analysis 4.4; Habre 1997).
4.1. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 1 Number of patients with moderate/severe pain (1h postop).
4.2. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 2 Number of patients with the need for rescue analgesia (2h postop).
4.3. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 3 Number of patients with the need for rescue analgesia (24h postop).
4.4. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 4 Time to first rescue analgesic (h).
Adverse events
All three trials reported data on adverse events. In the PACU the RR for vomiting was not significantly lower in the nalbuphine group mentioned in one trial comparing 0.3 mg/kg nalbuphine with 3.0 mg/kg tramadol administered after induction (RR 0.32; 95% CI 0.04 to 2.99; Analysis 4.5). However, 24 hours postop the RR for vomiting was comparable (RR 1.00; 95% CI 0.7 to 1.42; Analysis 4.6). Furthermore, the RR for respiratory depression was not significantly lower in children treated with nalbuphine compared to pethidine (RR 0.3; 95% CI 0.01 to 7.74; Analysis 4.7; Habre 1997; van den Berg 1999). No children suffered bradycardia in the PACU in either group (Analysis 4.8; Barsoum 1995; Habre 1997).
4.5. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 5 Number of patients with vomiting (PACU).
4.6. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 6 Number of patients with vomiting (24h postop).
4.7. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 7 Number of patients with respiratory depression (PACU).
4.8. Analysis.

Comparison 4 Nalbuphine versus pethidine, Outcome 8 Number of patients with bradycardia (PACU).
Nalbuphine versus Piritramid (Comparison 5)
Only one trial investigated 'nalbuphine versus piritramid' in 54 children (Büttner 1990) (Table 5).
Postoperative pain outcomes
Only the number of patients requiring rescue analgesia was assessed in the study. The RR for the need for rescue analgesia was not significantly higher in children treated with 0.1 mg/kg nalbuphine compared to children treated with 0.1 mg/kg piritramid 24 hours postop (RR 8.17; 95% CI 0.45 to 147.76; Analysis 5.1).
5.1. Analysis.

Comparison 5 Nalbuphine versus piritramid, Outcome 1 Number of patients with the need for rescue analgesia (24h postop).
Adverse events
The RR for PONV 24 hours postop was nonsignificantly lower in the nalbuphine group (RR 0.59; 95% CI 0.12 to 2.83; Analysis 5.2).
5.2. Analysis.

Comparison 5 Nalbuphine versus piritramid, Outcome 2 Number of patients with PONV (24h postop).
Discussion
Summary of main results
This quantitative systematic review included ten RCTs with 658 paediatric patients focusing on the analgesic efficacy and safety of nalbuphine compared to placebo and other opioids for postoperative pain treatment. However, the overall evidence was only of low quality so we could not definitively show a superior analgesic efficacy of nalbuphine compared to placebo or other opioids. The currently existing data suggested that patients treated with nalbuphine in comparison with placebo may have a lower RR for moderate/severe postoperative pain in the PACU and consecutively a lower RR for the need for additional rescue analgesics. The evidence regarding adverse events following nalbuphine compared to placebo or other opioids is also very limited. Generally, the results should be interpreted with great caution due to the small number of usable data, heterogeneous studies (procedures, time of administration and drug doses used) and a high risk of reporting bias.
Overall completeness and applicability of evidence
Several surveys in recent years demonstrated that postoperative pain in children is still a relevant problem (Groenewald 2012; Segerdahl 2008). Furthermore, despite the increasing use of regional anaesthetic techniques (Ecoffey 2010; Rabbitts 2010), a recently published database analysis showed that despite effective regional analgesia, 60% of children required an opioid as rescue medication at least once (Dadure 2009). This finding highlights the important role of opioids as possible rescue analgesics for an effective multimodal postoperative pain treatment.
Our review could not definitively show that nalbuphine compared to placebo might improve analgesic efficacy in children, because only data from a low number of included trials could be pooled for this comparison. The comparison of nalbuphine with opioids was also influenced by the small amount of usable data. Only nonsignificant results were retrieved so that the evidence is currently not clear. The same holds true for analysis of adverse events following nalbuphine versus placebo or other opioids, so that the overall evidence regarding efficacy and adverse events following nalbuphine administration in children is currently unclear.
Quality of the evidence
Although the overall number of included trials (10) and patients (658 paediatric patients) was reasonable, only a low number of relevant outcomes and heterogenous assessments were reported. Therefore, the presented results might be influenced by the high risk of selective reporting. Additionally, the included studies were only small so that the observed CIs were wide (imprecision of results). Apart from that, there was large clinical heterogeneity (pain scores, drug administration) in the study design, which might limit the results. As in other reviews focusing on postoperative pain therapy in children, different pain assessments with various observational or self reporting postoperative pain scales were a specific problem and might have influenced the retrieved results; more specifically the trials included in our review did not use validated pain scoring systems, which might have influenced the outcome 'number of patients with moderate/severe pain'. Furthermore, the study design was limited, because we observed a large heterogeneity concerning the application times (before surgery versus at the end of surgery), types of administration (intravenous versus intramuscular) and administered drug doses. Additionally, due to the low number of reported outcomes, subgroup and sensitivity analyses (especially focusing on the influence of surgery) were not possible. So we could not adequately analyse heterogeneity. Finally, the overall methodological quality of included trials was only rated as moderate, because only selected outcomes were reported, allocation concealment was only seldom described in detail and a blinding of outcome assessment was not performed. To conclude, according to the GRADE approach (Schunemann 2008), we double‐downgraded almost all RCTs and we triple‐downgraded one RCT because it was an open label trial (Barsoum 1995). Therefore, we rated the overall evidence as low quality.
Authors' conclusions
Implications for practice.
Because the overall evidence was limited, mainly by selective outcome reporting and imprecision of results due to lack of data, this quantitative review could not definitively demonstrate that 0.1 to 0.3 mg/kg nalbuphine compared to placebo might be an effective postoperative analgesic. The same holds true for the comparison with other opioids. Due to limited data we could not perform a subgroup analysis focusing on the influence of age, which might be an influencing factor, because the elimination half‐life of nalbuphine is significantly shorter in young children compared to young adults (Jaillon 1989). Thus, younger infants might need earlier additional drug doses than older children for effective pain treatment. Finally, again due to limited data, we were not able to show a benefit, by a lower number of adverse events following nalbuphine administration compared to placebo or other opioids. However, the number of studied patients does not allow a definite conclusion yet.
Implications for research.
Based on the findings of this review we determined the following implications for research.
Due to the low amount of available data, further RCTs comparing nalbuphine with other postoperative opioids (tramadol, morphine, and piritramid in Germany) are needed. This would enable an appropriate risk benefit analysis. Trials should use a clearly defined age group of children, comparable procedures and specific validated observational and self reported pain assessment scales in order to get validated and comparable results.
Nalbuphine administration in children should be studied following different surgical procedures in order to detect possible procedure‐specific efficacy and dosing.
Additionally, nalbuphine administration should be studied in children with specific comorbidities, like obstructive sleep apnoea, who are at higher risk for opioid‐related adverse events. Nalbuphine might be a useful drug, because it might offer analgesia without causing respiratory depression.
What's new
| Date | Event | Description |
|---|---|---|
| 25 May 2016 | Review declared as stable | See Published notes. |
Notes
A restricted search in May 2016 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We would like to thank the peer reviewers for their valuable comments and the editorial office of the Cochrane Pain, Palliative and Supportive Care Group for their support. This review was not registered in any kind of database.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Nalbuphine/
nalbuphine.mp.
(en2234a or en 2234a or nubain).mp.
1 or 2 or 3
Pain, Postoperative/
(pain* and (postoperative or post operative or surg*)).mp.
5 or 6
exp Infant/
exp Child/
Adolescent/
(infant* or child* or adolesc*).mp.
8 or 9 or 10 or 11
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
4 and 7 and 12 and 21
key:
mp=title, original title, abstract, name of substance word, subject heading word, unique identifier
pt=publication type
ab=abstract
fs=floating subheading
Data and analyses
Comparison 1. Nalbuphine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with moderate/severe pain (1h postop) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 0.71] |
| 2 Number of patients with moderate/severe pain (2h postop) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.06] |
| 3 Number of patients with the need for rescue analgesia (2h postop) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.84] |
| 4 Number of patients with the need for rescue analgesia (12h postop) | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.94] |
| 5 Number of patients with PONV (PACU) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.42] |
| 6 Number of patients with PONV (postop 24h) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.12, 2.83] |
| 7 Number of patient with vomiting (PACU) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.03] |
| 8 Number of patients with vomiting (postop 24h) | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.6 [0.34, 93.35] |
| 9 Number of patients with pruritus (postop 24h) | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.06, 10.37] |
| 10 Number of patients with respiratory depression | 3 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Number of patients with urinary retention | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Number of patients with sedation (24h postop) ) | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.2 [1.03, 223.37] |
| 13 Number with patients with bradycardia (PACU) | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Nalbuphine versus morphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with moderate/severe pain (1h postop) | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.12, 5.74] |
| 2 Number of patients with moderate/severe pain (2h postop) | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.59, 2.01] |
| 3 Number of patients with the need for rescue analgesia (12h postop) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.37, 1.01] |
| 4 Number of patients with PONV (PACU) | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.77] |
| 5 Number of patients with PONV (24h postop) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.80, 1.80] |
| 6 Number of patient with respiratory depression | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 7 Number with patient with bradycardia (PACU) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Nalbuphine versus tramadol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with moderate/severe pain (1h postop) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of patients with the need for rescue analgesia (2h postop) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.39, 1.43] |
| 3 Number of patients with the need for rescue analgesia (12h postop) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.77] |
| 4 Number of patients with the need for rescue analgesia (24h postop) | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.50, 4.16] |
| 5 Number of patients with PONV (24h postop) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.11, 2.23] |
| 6 Number of patients with vomiting (PACU) | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 2.00] |
| 7 Number of patients with vomiting (24h postop) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 8 Number of patients with respiratory depression (PACU) | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Number of patients with sedation (24h postop) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.21, 19.23] |
| 10 Number of patients with bradycardia (PACU) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Nalbuphine versus pethidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with moderate/severe pain (1h postop) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of patients with the need for rescue analgesia (2h postop) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.52, 2.23] |
| 3 Number of patients with the need for rescue analgesia (24h postop) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.52, 2.44] |
| 4 Time to first rescue analgesic (h) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐0.20, 2.24] |
| 5 Number of patients with vomiting (PACU) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.04, 2.99] |
| 6 Number of patients with vomiting (24h postop) | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.42] |
| 7 Number of patients with respiratory depression (PACU) | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.74] |
| 8 Number of patients with bradycardia (PACU) | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Nalbuphine versus piritramid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with the need for rescue analgesia (24h postop) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.17 [0.45, 147.76] |
| 2 Number of patients with PONV (24h postop) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.12, 2.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barsoum 1995.
| Methods | Open trial Parallel design |
|
| Participants | Mainly lower abdominal surgery (appendectomy, hernia repair, testicular or urethral surgery), n = 75 (61 males, 14 females), children aged 2 to 12 years Group 1: 22 males, 9 females; mean age 5.4 years (standard deviation (SD) 2.6) Group 2: 20 males, 5 females; mean age 6.3 years (SD 1.9) Group 3: 19 males, 6 females; mean age 6.2 years (SD 2.8) |
|
| Interventions | Group 1: 0.1 mg/kg Nalbuphine intramuscularly (IM), (n = 25) Group 2: 2 mg/kg Tramadol IM, (n = 25) Group 3: 1 mg/kg Pethidine IM, (n = 25) Administration time: after the end of surgery (first expression of pain) An additional injection of half the initial dose was administrated 30 and 60 min if inadequate analgesia |
|
| Outcomes | ‐ Postoperative pain scales (VRS) (0.5, 1, 3, 6, 12, 24 hours postoperatively) ‐ Number of patiens with no/slight pain 1 hour postoperatively ‐ Total analgesic consumption 24 hours postoperatively ‐ Frequency of administration 24 hours postoperatively ‐ Investigators assessment of overall pain relief (excellent, very good, good, satisfactory, poor) at 24 hours postoperatively ‐ Number, nature, time of onset of adverse events (vomiting, haemodynamic, respiratory parameters) ‐ Overall assessment of tolerability 24 hours postoperatively |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Open label trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study was intended to be a double‐blind (observer blinded),.." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The study was intended to be a double‐blind (observer blinded),.." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Büttner 1990.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Small urological surgery (herniotomy, circumcision, orchidopexy), n = 54 Children aged 1 to 4 years |
|
| Interventions | Group 1: 0.1 mg/kg Nalbuphine intravenously (IV) (n = 20), Group 2: 0.1 mg/kg Piritramid IV (n = 17) Group 3: Placebo IV (n = 17) Administration time: after the end of surgery |
|
| Outcomes | ‐ Observational pain scale (0 to 15 points) ‐ Number of patients with pain ‐ Number of patients with need for additional analgesics ‐ Number of adverse events (vomiting) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... was tested in a randomized double‐blind trial..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "... was tested in a randomized double‐blind trial..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Galy 1991.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Small surgeries, n = 33, children aged 11 months to 9 years | |
| Interventions | Group 1: 0.3 mg/kg Nalbuphine intravenously (IV) + caudal anaesthesia (with lidocaine) (n = 9) Group 2: Placebo IV + caudal anaesthesia (with lidocaine) (n = 7) Group 3: 0.3 mg/kg Nalbuphine supp + caudal anaesthesia (with lidocaine) (n = 10) Group 4: Placebo + caudal anaesthesia (with lidocaine) (n = 7) Administration time: premedication |
|
| Outcomes | ‐ Postoperative pain (0, 0.5, 1, 2, 3, 4, 5, 6 hours after surgery) ‐ Number of patients with need for additional analgesics ‐ Duration of analgesia ‐ Number of adverse events (vomiting, pruritus) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | " randomized allocation" (in the French original: "...sont tirès au sort pour recevoir...") |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Habre 1997.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Adenotonsillectomy, n = 90, children aged 2 to 12years Group 1: 26 males, 19 females; mean age 67 months (standard deviation (SD) 26) Group 2: 18 males, 27 females; mean age 76 months (SD 29) |
|
| Interventions | Group 1: 0.1 mg/kg Nalbuphine intravenously (IV) (n = 45) Group 2: 1 mg/kg Pethidine IV (n = 45) Administration time: after induction All children were given 15 mg/kg paracetamol orally 1 hour before surgery. |
|
| Outcomes | ‐ Observational pain score (facial expression, position in bed, vocalisation, nurse assessment; 0 to 8 points) at different time points ‐ Number of analgesic doses in the recovery area ‐ Number of patients with more than 3 doses of opioids ‐ Time to first analgesic ‐ Sedation score ‐ Number of adverse events (vomiting) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Children were then randomly assigned to receive on induction .." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Recovery nursing staff were therefore blinded to the choice of opioid" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Krishnan 1987.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Tonsillectomy, n = 60, children aged 4 to 12 years Group 1: males 11, females 9, mean age 6.579 years (standard error of mean (SEM) 0.528) Group 2: males 10, females 10, mean age 6.469 years (SEM 0.629) Group 3: males 8, females 12, mean age 7.016 years (SEM 0.488) |
|
| Interventions | Group 1: 0.3 mg/kg Nalbuphine intramuscularly (IM) (n = 20) Group 2: 0.2 mg/kg Morphin IM (n = 20) Group 3: Placebo IM (n = 20) Administration time: 5 minutes before the end of surgery |
|
| Outcomes | ‐ Observational pain and restlessness scale (none, mild, moderate, severe) ‐ Number of patients with pain ‐ Number of adverse events (vomiting, cardiovascular, respiratory effects) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | |
Littlejohn 1996.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Teeth extraction, n = 60, children aged 3 to 7 years Group 1: 12 males, 9 females; mean age 6.7 years (standard deviation (SD) 2.6) Group 2: 10 males, 9 females; mean age 6.7 years (SD 3.0) Group 3: 8 males, 12 females; mean age 6.9 years (SD 2.8) |
|
| Interventions | Group 1: 0.3 mg/kg Nalbuphine intravenously (IV) (n = 21) Group 2: 1 to 2 mg/kg Diclofenac rectally (n =19) Group 3: control (no analgesic) (n = 20) Administration time: after induction |
|
| Outcomes | ‐ Hannallah observational pain scale (0 to 10 points) ‐ Number of patients with pain ‐ Number of patients with need for additional analgesics ‐ Number of adverse events (vomiting) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "In a randomized, double‐blind study ..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "In a randomized, double‐blind study ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Pain related behaviour was evaluated in all patients by the same blinded observer..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Moyado‐Garcia 2009.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Various surgical procedures with expected moderate to severe postoperative pain, n = 24, children aged 1 to 10 years Group 1: 7 males, 5 females; mean age 6.2 years (range 2.5 to 10.0) Group 2: 7 males, 5 females; mean age 4.4 years (range 1.6 to 10.0) |
|
| Interventions | Group 1: Nalbuphine (bolus 100 μg/kg, 0.2 μg/kg/min for 72 hours) intravenously (IV) (n = 12) Group 2: Tramadol (bolus 1,000 μg/kg, 2.0 μg/kg/min for 72 hours) IV (n = 12) Administration time: immediately before the closure of the surgical incision |
|
| Outcomes | ‐ Children's Hospital of Eastern Ontario Pain Scale (CHEOPS), facial pain intensity scale (0 to 5 points), Visual Analogue Scale (VAS) (every hour until 24 hours postoperatively) ‐ Number of patients with need for additional analgesics ‐ Sedation score (1 to 5 points) ‐ Number of adverse events (vomiting, cardiovascular, respiratory effects) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... a scheduled surgical procedure were randomly allocated to receive either ..." |
| Allocation concealment (selection bias) | Low risk | "By means of a pre designed table of random numbers, children were allocated to receive either ..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "In a double blind design ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Schäffer 1986.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Herniotomy, orchidopexy, circumcision, hydrocelectomy, n = 60, children aged 1 to 9 years Group 1: 25 males, 5 females; mean age 4.5 years (standard deviation (SD) 1.9) Group 2: 26 males, 4 females; mean age 4.87 years (SD 2.4) |
|
| Interventions | Group 1: 0.15 to 0.2 mg/kg Nalbuphine intramuscularly (IM) Group 2: 0.75 to 1 mg/kg Tramadol IM Administration time: after surgery (at the arrival in the recovery area) |
|
| Outcomes | ‐ Visual Analogue Scale (VAS) (1, 2, 3, 4, 6, 8, 24 hours postoperatively) ‐ Number of adverse events (vomiting, headache, singultus, hallucination, dry mouth) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...each group received in a randomized and double‐blind manner ..." |
| Allocation concealment (selection bias) | Low risk | "... received according to the plan of randomisation..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...each group received in a randomized and double‐blind manner ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
van den Berg 1999.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Adenotonsillectomy, n = 152, children aged 8 to 21 years Group 1: 23 males, 16 females; mean age 15 years (standard deviation (SD) 14) Group 2: 25 males, 13 females; mean age 14 years (SD 15) Group 3: 26 males, 12 females; mean age 16 years (SD 14) Group 4: 25 males, 12 females; mean age 13 years (SD 15) |
|
| Interventions | Group 1: 0.3 mg/kg Nalbuphine intravenously (n = 39) Group 2: 3.0 mg/kg Tramadol IV (n = 38) Group 3: 1.5 mg/kg Pethidine IV (n = 38) Group 4: Placebo IV (n = 37) Administration time: at induction All children received 1 mg/kg diclofenac rectally/intramuscularly 1 to 2 hours before surgery. |
|
| Outcomes | ‐ Observational restlessness‐pain score (0 to 3 points) ‐ Sedation score (1 to 4 points) ‐ Number of patients with need for additional analgesics ‐ Recovery time (minutes) ‐ Awakening time (minutes) ‐ Number of adverse events (vomiting) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each patient was block randomized on arrival ..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A prospective, double‐blind, randomized, controlled study .. " |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Wandless 1987.
| Methods | Randomised controlled trial Parallel design |
|
| Participants | Orchidopexy, n = 50, children aged < 11 years Group 1: 25 males; mean age 6.1 years (standard deviation (SD) 2.7) Group 2: 25 males; mean age 4.1 years (SD 2.2) |
|
| Interventions | Group 1: 0.2 mg/kg Nalbuphine intramuscularly (IM) (n = 25) Group 2: 0.2 mg/kg Morphine IM (n = 25) Administration time: immediately after induction |
|
| Outcomes | ‐ Observational postoperative pain scale (none, moderate, severe) (1, 2, 4, 24 hours postoperatively) ‐ Number of patients with need for additional analgesics ‐ Number of patients with moderate/severe pain ‐ Number of adverse events (vomiting, sweating, respiratory depression) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Fifty boys under 11 years of age were allocated randomly to receive ..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A double‐blind investigation was conducted..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients evaluated |
| Selective reporting (reporting bias) | High risk | Only selective outcome reporting |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bazin 2010 | Nalbuphine as rescue medication |
| Dongmin 2001 | Intravenous patient‐controlled‐analgesia |
| Nascimento 2002 | Retrospective study |
| Quaki 2007 | Only published as abstract |
| van den Berg 1994 | Adult patients (> 18 years) |
| Velegrakis 1998 | Only published as abstract |
Differences between protocol and review
Due to inconsistent outcome data reporting with regard to pain scores, we could not extract data for the primary outcome postoperative pain scores. Therefore, the authors decided to assess the outcome 'number of patients with the need for rescue analgesia' as the primary outcome. Additionally, the planned subgroup analysis focusing on different durations of postoperative care (PACU/24 hours/48 hours) was deleted, because we primarily analysed the outcomes at different times (primary outcomes at 1 hour, 2 hours, 12 hours, 24 hours postop; adverse events within the PACU and 24 hours postop).
Contributions of authors
| Drafted the protocol | AS |
| Developed a search strategy | AS |
| Searched for studies (usually 2 authors) | AS, SR |
| Obtained copies of studies | AS, SR |
| Selected which studies to include (2 + 1 arbiter) | AS, SR, PZ |
| Extracted data from studies (2 authors) | AS, SR |
| Entered data into RevMan | AS, SR |
| Carried out the analysis | AS, SR |
| Interpreted the analysis | AS, PZ |
| Drafted the final write‐up of the review | AS, SR, PZ, EPZ |
| Update the review | AS, SR |
| Content expert name | AS, PZ |
| Methodologist name | AS |
Declarations of interest
Alexander Schnabel: No conflicts of interest.
Sylvia Reichl: No conflicts of interest.
Peter Zahn: No conflicts of interest.
Esther Pogatzki‐Zahn: No conflicts of interest.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Barsoum 1995 {published data only}
- Barsoum MW. Comparison of the efficacy and tolerability of tramadol, pethidine and nalbuphine in children with postoperative pain. Clinical Drug Investigation 1995;9(4):183‐90. [Google Scholar]
Büttner 1990 {published data only}
- Büttner W, Finke W, Schwanitz M, Pfisterer M. Nalbuphine and piritramid in the postoperative phase in young children. 1. General condition. Der Anaesthesist 1990;39(4):211‐6. [PubMed] [Google Scholar]
Galy 1991 {published data only}
- Galy A, Rochette A, Beauvoir C, Chardon P, D´Athis F. Intravenous and rectal postoperative nalbuphine analgesia in children. Controlled placebo study. Annals Francaises d'anesthesie et de Reanimation 1991;10 Suppl 1:R104. [Google Scholar]
Habre 1997 {published data only}
- Habre W, McLeod B. Analgesic and respiratory effect of nalbuphine and pethidine for adenotonsillectomy in children with obstructive sleep disorder. Anaesthesia 1997;52(11):1101‐6. [DOI] [PubMed] [Google Scholar]
Krishnan 1987 {published data only}
- Krishnan A, Tolhurst‐Cleaver CL, Kay B. Controlled comparison of nalbuphine and morphine for post‐tonsillectomy pain. Anaesthesia 1985;40(12):1178‐81. [DOI] [PubMed] [Google Scholar]
Littlejohn 1996 {published data only}
- Littlejohn IH, Tarling MM, Flynn PJ, Ordman AJ, Aiken A. Post‐operative pain relief in children following extraction of carious deciduous teeth under general anaesthesia: a comparison of nalbuphine and diclofenac. European Journal of Anaesthesiology 1996;13(4):359‐63. [DOI] [PubMed] [Google Scholar]
Moyado‐Garcia 2009 {published data only}
- Moyao‐García D, Hernández‐Palacios JC, Ramírez‐Mora JC, Nava‐Ocampo AA. A pilot study of nalbuphine versus tramadol administered through continuous intravenous infusion for postoperative pain control in children. Acta Biomedica 2009;80(2):124‐30. [PubMed] [Google Scholar]
Schäffer 1986 {published data only}
- Schäffer J, Piepenbrock S, Kretz FJ, Schönfeld C. Nalbuphine and tramadol for the control of postoperative pain in children. Der Anaesthesist 1986;35(7):408‐13. [PubMed] [Google Scholar]
van den Berg 1999 {published data only}
- Berg AA, Montoya‐Pelaez LF, Halliday EM, Hassan I, Baloch MS. Analgesia for adenotonsillectomy in children and young adults: a comparison of tramadol, pethidine and nalbuphine. European Journal of Anaesthesiology 1999;16(3):186‐94. [DOI] [PubMed] [Google Scholar]
Wandless 1987 {published data only}
- Wandless JG. A comparison of nalbuphine with morphine for post‐orchidopexy pain. European Journal of Anaesthesiology 1987;4(2):127‐32. [PubMed] [Google Scholar]
References to studies excluded from this review
Bazin 2010 {published data only}
- Bazin V, Bollot J, Asehnoune K, Roquilly A, Guillaud C, Windt A, et al. Effects of perioperative intravenous low dose of ketamine on postoperative analgesia in children. European Journal of Anaesthesiology 2010;27:47‐52. [DOI] [PubMed] [Google Scholar]
Dongmin 2001 {published data only}
- Dongmin S, Sukwha K, Chong Sung K, Hee‐Soo K. Postoperative pain management using intravenous patient‐controlled analgesia for pediatric patients. The Journal of craniofacial surgery 2001;12(2):129‐33. [DOI] [PubMed] [Google Scholar]
Nascimento 2002 {published data only}
- Nascimento Junior Pd, Módolo NS, Rodrigues Junior GR. Postoperative analgesia in children less than 1 year of age: a retrospective analysis. Revista Brasileira de Anestesiologia 2002;52:739‐46. [PubMed] [Google Scholar]
Quaki 2007 {published data only}
- Ouaki J, Rochette A, Raux O, Dadure Ch, Capdevila X. Tramadol vs nalbuphine: analgesic efficacy and side effects. A prospective, randomized, double‐blinded study in children. European Journal of Anaesthesiology 2007;24:138‐9. [Google Scholar]
van den Berg 1994 {published data only}
- Berg AA, Honjol NM, Rama Prabhu NV, Datta S, Rozario CJ, Muraleedaran R, et al. Analgesics and ENT surgery. British Journal of Clinical Pharmacology 1994;38:533‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velegrakis 1998 {published data only}
- Velegrakis D, Loizou C, Papageorgiou M, Malissiova A. Comparison of postoperative analgesia in children after continuous i.v. infusion of morphine or nalbuphine through a single‐use infuser (Baxter). British Journal of Anaesthesia 1998;80:A495. [Google Scholar]
Additional references
Anand 2000
Beaver 1978
- Beaver WT, Feise GA. A comparison of the analgesic effect of intramuscular nalbuphine and morphine in patients with postoperative pain. The Journal of Pharmacology and Experimental Therapeutics 1978;204(2):487‐96. [PUBMED: 340643] [PubMed] [Google Scholar]
Bressolle 2011
- Bressolle F, Khier S, Rochette A, Kinowski JM, Dadure C, Capdevila X. Population pharmacokinetics of nalbuphine after surgery in children. British Journal of Anaesthesia 2011;106(4):558‐65. [PUBMED: 21310722] [DOI] [PubMed] [Google Scholar]
Chen 1993
- Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sciences 1993;52(4):389‐96. [PUBMED: 8093631] [DOI] [PubMed] [Google Scholar]
Cummings 1996
- Cummings EA, Reid GJ, Finley GA, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain 1996;68(1):25‐31. [PUBMED: 9251995] [DOI] [PubMed] [Google Scholar]
Dadure 2009
- Dadure C, Bringuier S, Raux O, Rochette A, Troncin R, Canaud N, et al. Continuous peripheral nerve blocks for postoperative analgesia in children: feasibility and side effects in a cohort study of 339 catheters. Canadian Journal of Anaesthesia 2009;56(11):843‐50. [PUBMED: 19697092] [DOI] [PubMed] [Google Scholar]
Ecoffey 2010
- Ecoffey C, Lacroix F, Giaufre E, Orliaguet G, Courreges P, Association des Anesthésistes Réanimateurs Pédiatriques d'Expression Française (ADARPEF). Epidemiology and morbidity of regional anesthesia in children: a follow‐up one‐year prospective survey of the French‐Language Society of Paediatric Anaesthesiologists (ADARPEF). Paediatric Anaesthesia 2010;20(12):1061‐9. [PUBMED: 21199114] [DOI] [PubMed] [Google Scholar]
Errick 1983
- Errick JK, Heel RC. Nalbuphine. A preliminary review of its pharmacological properties and therapeutic efficacy. Drugs 1983;26(3):191‐211. [PUBMED: 6137354] [DOI] [PubMed] [Google Scholar]
Groenewald 2012
- Groenewald CB, Rabbitts JA, Schroeder DR, Harrison TE. Prevalence of moderate‐severe pain in hospitalized children. Paediatric anaesthesia 2012;22(7):661‐8. [PUBMED: 22332912] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, March 2011. [Google Scholar]
Hirschfeld 2013
- Hirschfeld G, Zernikow B. Variability of "optimal" cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain 2013;154(1):154‐9. [PUBMED: 23182623] [DOI] [PubMed] [Google Scholar]
Hoskin 1991
- Hoskin PJ, Hanks GW. Opioid agonist‐antagonist drugs in acute and chronic pain states. Drugs 1991;41(3):326‐44. [PUBMED: 1711441] [DOI] [PubMed] [Google Scholar]
Howard 2003
- Howard RF. Current status of pain management in children. JAMA 2003;290(18):2464‐9. [PUBMED: 14612483] [DOI] [PubMed] [Google Scholar]
Jacqz‐Aigrain 2003
- Jacqz‐Aigrain E, Debillon T, Daoud P, Boithias C, Hamon I, Rayet I, et al. Population pharmacokinetics of nalbuphine in neonates. Paediatric and Perinatal Drug Therapy 2003;5:190‐8. [Google Scholar]
Jaillon 1989
- Jaillon P, Gardin ME, Lecocq B, Richard MO, Meignan S, Blondel Y, et al. Pharmacokinetics of nalbuphine in infants, young healthy volunteers, and elderly patients. Clinical Pharmacology and Therapeutics 1989;46(2):226‐33. [PUBMED: 2758732] [DOI] [PubMed] [Google Scholar]
Karling 2002
- Karling M, Renstrom M, Ljungman G. Acute and postoperative pain in children: a Swedish nationwide survey. Acta Paediatrica 2002;91(6):660‐6. [PUBMED: 12162598] [DOI] [PubMed] [Google Scholar]
Kart 1996
- Kart T, Laan K, Crombach J, Rasmussen M, Moller KB, Hole P, et al. Postoperative pain management in children has been improved, but can be further optimized. European Journal of Pediatric Surgery: official journal of the Austrian Association of Pediatric Surgery 1996;6(5):259‐64. [PUBMED: 8933127] [DOI] [PubMed] [Google Scholar]
Michelet 2012
- Michelet D, Andreu‐Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, et al. A meta‐analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesthesia and analgesia 2012;114(2):393‐406. [PUBMED: 22104069] [DOI] [PubMed] [Google Scholar]
Miller 1980
- Miller RR. Evaluation of nalbuphine hydrochloride. American Journal of Hospital Pharmacy 1980;37(7):942‐9. [PUBMED: 6994499] [PubMed] [Google Scholar]
Rabbitts 2010
- Rabbitts JA, Groenewald CB, Moriarty JP, Flick R. Epidemiology of ambulatory anesthesia for children in the United States: 2006 and 1996. Anesthesia and Analgesia 2010;111(4):1011‐5. [PUBMED: 20802051] [DOI] [PubMed] [Google Scholar]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan) [Computer program]. Version 5.2.. Copenhagen: The Nordic Cochrane Centre, 2012.
Rhodes 1986
- Rhodes RA. A cost analysis of nalbuphine versus meperidine used in oral surgery procedures. Clinical Therapeutics 1986;8(4):415‐9. [PUBMED: 3524842] [PubMed] [Google Scholar]
Rushton 1997
- Rushton AR, Sneyd JR. Opioid analgesics. British Journal of Hospital Medicine 1997;57(3):105‐6. [PUBMED: 9196589] [PubMed] [Google Scholar]
Schunemann 2008
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evidence‐Based Medicine 2008;13(6):162‐3. [PUBMED: 19043023] [DOI] [PubMed] [Google Scholar]
Segerdahl 2008
- Segerdahl M, Warren‐Stomberg M, Rawal N, Brattwall M, Jakobsson J. Children in day surgery: clinical practice and routines. The results from a nation‐wide survey. Acta Anaesthesiologica Scandinavica 2008;52(6):821‐8. [PUBMED: 18498436] [DOI] [PubMed] [Google Scholar]
Standing 2009
- Standing JF, Savage I, Pritchard D, Waddington M. Diclofenac for acute pain in children. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005538.pub2] [DOI] [PubMed] [Google Scholar]
Stringer 2009
- Stringer RA, Strain‐Damerell C, Nicklin P, Houston JB. Evaluation of recombinant cytochrome P450 enzymes as an in vitro system for metabolic clearance predictions. Drug Metabolism and Disposition: the Biological Fate of Chemicals 2009;37(5):1025‐34. [PUBMED: 19196847] [DOI] [PubMed] [Google Scholar]
Taylor 2008
- Taylor EM, Boyer K, Campbell FA. Pain in hospitalized children: a prospective cross‐sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Research & Management: the Journal of the Canadian Pain Society 2008;13(1):25‐32. [PUBMED: 18301813] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2008
- Walker SM. Pain in children: recent advances and ongoing challenges. British Journal of Anaesthesia 2008;101(1):101‐10. [PUBMED: 18430745] [DOI] [PubMed] [Google Scholar]
Weisman 1998
- Weisman SJ, Bernstein B, Schechter NL. Consequences of inadequate analgesia during painful procedures in children. Archives of Pediatrics and Adolescent Medicine 1998;152(2):147‐9. [PUBMED: 9491040] [DOI] [PubMed] [Google Scholar]


