Abstract
Objectives:
To investigate risk factors and outcomes of cardiogenic shock complicating acute myocardial infarction (AMI-CS) in young patients with AMI.
Background:
AMI-CS is associated with high morbidity and mortality rates. Data regarding AMI-CS in younger individuals are limited.
Methods and Results:
Consecutive patients with type 1 AMI aged 18−50 years admitted to 2 large tertiary-care academic centers were included, and they were adjudicated as having cardiogenic shock (CS) by physician review of electronic medical records using the Society for Cardiovascular Angiography and Interventions CS classification system. Outcomes included allcause mortality (ACM), cardiovascular mortality (CVM) and 1-year hospitalization for heart failure (HHF). In addition to using the full population, matching was also used to define a comparator group in the non-CS cohort. Among 2097 patients (mean age 44 ± 5.1 years, 74% white, 19% female), AMI-CS was present in 148 (7%). Independent risk factors of AMI-CS included ST-segment elevation myocardial infarction, left main disease, out-of-hospital cardiac arrest, female sex, peripheral vascular disease, and diabetes. Over median follow-up of 11.2 years, young patients with AMI-CS had a significantly higher risk of ACM (adjusted HR 2.84, 95% CI 1.68−4.81; P < 0.001), CVM (adjusted HR 4.01, 95% CI 2.17−7.71; P < 0.001), and 1-year HHF (adjusted HR 5.99, 95% CI 2.04−17.61; P = 0.001) compared with matched non-AMI-CS patients. Over the course of the study, there was an increase in the incidence of AMI-CS among young patients with MI as well as rising mortality rates for patients with both AMI-CS and non-AMI-CS.
Conclusions:
Of young patients with AMI, 7% developed AMI-CS, which was associated with a significantly elevated risk of mortality and HHF. (J Cardiac Fail 2022;00:1−12)
Keywords: Myocardial infarction, cardiogenic shock, young, heart failure hospitalization, mortality, risk factors
Cardiogenic shock following acute myocardial infarction (AMI-CS) is a feared complication of acute coronary syndromes and is seen in approximately 5%–10% of patients following AMI1–4; it is the leading cause of AMI-related in-hospital mortality.5 Despite advancements in AMI care, AMI-CS continues to be prevalent and accounts for one-third of all cardiogenic shock (CS); associated mortality rates are reported to be between 35% and 60%.1,4,6
Although multiple studies have examined risk factors and outcomes of AMI-CS, none have focused on this condition in young patients with AMI. Recent analyses have identified older age, female sex, ST-segment elevation myocardial infarction (STEMI), out-of-hospital cardiac arrest (OHCA), peripheral artery disease, and chronic obstructive pulmonary disease as features associated with a higher risk of AMI-CS.1,6 However, it is unknown whether these findings apply to young patients with AMI-CS. Therefore, we sought to investigate risk factors and long-term outcomes, including risk of heart-failure hospitalization and mortality associated with AMI-CS, among patients who experienced an MI at or below the age of 50.
Methods
Study Population
The design of the YOUNG-MI registry has been described previously.7 Briefly, this is a retrospective cohort study from 2000−2016 at 2 large academic medical centers that included all consecutive patients who experienced a first MI at or before the age of 50. All records were adjudicated by physicians. The YOUNG-MI registry is approved by the appropriate institutional review board and is conducted in accordance with institutional guidelines.
Risk Factors and Comorbidities
Electronic medical record review was conducted for each patient to abstract risk factors as well as burden and distribution of coronary artery disease (CAD) on presentation according to previously reported methods.7,8 Multiple troponin assays were used in the study period, so peak standardized troponin, defined as a patient’s peak troponin value divided by the 99th percentile value for the specific assay, was used for comparison.9 The Area Deprivation Index, a measure of neighborhood socioeconomic disadvantage, was used as a surrogate for socioeconomic status with home addresses used to determine this index for each patient.10,11
Patient Identification and Adjudication of Cardiogenic Shock
A computer search algorithm was used to identify patients in the YOUNG-MI registry at risk for AMI-CS by searching for key terms (Supplementary Table 1) in available medical records of previously adjudicated patients with type 1 MI. Additionally, all patients with AMI who presented with cardiac arrest, new-onset systolic heart failure or mechanical complication of MI underwent adjudication for AMI-CS. Subjects identified in this manner underwent adjudication by 2 physicians (HKS, EMD), who independently reviewed each patient’s record to determine whether AMI-CS was present during the AMI admission. Patients were classified into AMI-CS stages based on worst clinical status during hospitalization by using the 2019 Society for Cardiovascular Angiography and Interventions (SCAI) expert consensus classification (Supplementary Table 2).12,13 For this study, stage B–E patients were grouped together to compose the AMI-CS group, as reported in other studies.14 The remainder of the YOUNG-MI cohort composed the comparator group.
Outcomes
Primary outcomes included all-cause mortality (ACM), cardiovascular mortality (CVM), and 1-year hospitalization for heart failure (HHF). Vital status of patients at follow-up was assessed using the Social Security Administration’s Death Master File, Massachusetts Department of Vital Statistics, National Death Index, and longitudinal follow-up in the electronic medical records. Vital status was censored in September 2017. Two independent physicians adjudicated causes of death using all records obtained. In cases of disagreement, consensus was reached by an adjudication committee. Death was classified as in-hospital or postdischarge. Cause of death was categorized as cardiovascular, noncardiovascular or undetermined. If cause of death was undetermined, patients were categorized as having had cardiovascular death. The definition of cardiovascular death was adapted from the 2014 American College of Cardiology/American Heart Association definitions for cardiovascular endpoint events,15 as previously detailed.7 HHF was adjudicated by medical-record review by physicians. To meet criteria for HHF, the subject had to have had a discharge diagnosis of heart failure for an admission following index MI hospitalization at the study hospitals, while also meeting criteria for heart failure by presence of symptoms, signs and escalation of heart-failure therapy during that admission.
Statistical Analysis
Categorical variables are reported as frequencies and proportions and compared using χ2 or Fisher exact tests, as appropriate. Continuous variables are reported as means ± standard deviation or medians (25th–75th percentile) and compared with t tests or Mann-Whitney tests, as appropriate. The proportional hazards assumption was assessed by analyzing Schoenfeld residuals. Survival curves were compared using the log-rank test. A 2-tailed P value less than 0.05 was considered statistically significant.
Cox proportional hazard modeling was used for survival analysis, with corresponding hazard ratios (HR) and 95% confidence intervals (CI) reported. Given the small number of events and modest cohort size, we also conducted a matched analysis. A 3:1 matched cohort was generated using Mahalanobis distance matching16 on age, sex, race, and biologically important risk factors for AMI-CS, including hypertension, smoking status, left main CAD and STEMI. Multivariable Cox models were performed on the matched cohort to examine both univariate and multivariable risk of AMI-CS. Variables that were statistically significant in univariate analyses or were biologically plausible predictors of AMI-CS were included in the multivariable regression. Univariate and multivariable logistic regression was used to determine predictors of AMI-CS, with odds ratio (OR) and 95% CI reported. All analyses were performed using Stata version 15.1 (StataCorp, College Station, TX).
Results
Baseline Characteristics
The total cohort consisted of 2097 individuals aged ≤ 50 years who experienced a type 1 MI (Visual take-home graphic). The median age was 45.2 years (IQR 41.4−47.8 years); 404 (19.3%) were women, and 1541 (73.5%) were white. Of these, 148 (7.1%) patients were adjudicated to have AMI-CS. Median follow-up was 11.2 years.
Table 1 outlines baseline and AMI characteristics of the cohort by AMI-CS status. At baseline, AMI-CS patients had significantly higher prevalence of peripheral vascular disease (4.7% vs 1.7%), higher Charlson Comorbidity Index (2 vs 1), and less prevalent hypertension (36.5% vs 47.5%) than those without AMI-CS. Patients with AMI-CS had significantly higher standardized troponin (172.7 vs 35.7, P < 0.001) compared with non-AMI-CS patients. At presentation, AMI-CS patients were significantly more likely to present with STEMI (84.5% vs 51.1%, respectively; P < 0.001) and OHCA (26.4% vs 3.1%; P < 0.001) compared with non-AMI-CS patients. The median number of coronary arteries involved in patients with AMI-CS was higher than in the non-AMI-CS group, as was the prevalence of left main CAD on coronary angiography (7.4% vs 2.3%; P < 0.001).
Table 1.
Baseline and Infarction Characteristics of Young Patients With AMI With and Without Cardiogenic Shock
| Total Cohort |
Matched Cohort |
|||||
|---|---|---|---|---|---|---|
| No AMI-CS (n = 1949) | AMI-CS (n = 148) | P value | No AMI-CS (n = 461)) | AMI-CS (n=148) | P value | |
| Demographics | ||||||
| Median age at event (years) | 45.3 (41.4, 47.9) | 44.9 (40.9, 47.3) | 0.13 | 45.0 (41.3, 47.3) | 44.9 (40.9, 47.3) | 0.72 |
| Female sex | 368 (18.9%) | 36 (24.3%) | 0.11 | 101 (21.9%) | 36 (24.3%) | 0.54 |
| Race | ||||||
| White | 1431 (73.4%) | 110 (74.3%) | 0.45 | 352 (76.4%) | 110 (74.3%) | 0.66 |
| Black | 140 (7.2%) | 8 (5.4%) | 34 (7.4%) | 8 (5.4%) | ||
| Latinx | 134 (6.9%) | 13 (8.8%) | 32 (6.9%) | 13 (8.8%) | ||
| Asian | 69 (3.5%) | 3 (2.0%) | 13 (2.8%) | 3 (2.0%) | ||
| Other/unknown | 175 (8.9%) | 14 (9.5%) | 30 (6.5%) | 14 (9.5%) | ||
| Median area deprivation index | 5 (3, 7) | 5.3 (2.5) 5 (3, 7) | 0.04 | 5 (3, 7) | 5 (3, 7) | 0.35 |
| Insurance Classification | ||||||
| Private | 1319 (67.7%) | 77 (52.0%) | 0.002 | 307 (66.6%) | 77 (52.0%) | 0.013 |
| Public | 327 (16.8%) | 38 (25.7%) | 77 (16.7%) | 38 (25.7%) | ||
| None | 154 (7.9%) | 17 (11.5%) | 36 (7.8%) | 17 (11.5%) | ||
| Unknown | 149 (7.6%) | 16 (10.8%) | 41 (8.9%) | 16 (10.8%) | ||
| Family history of premature CAD | ||||||
| Parents | 343 (17.6%) | 22 (14.9%) | 0.57 | 78 (16.9%) | 22 (14.9%) | 0.55 |
| Siblings | 68 (3.5%) | 5 (3.4%) | 22 (4.8%) | 5 (3.4%) | ||
| Both | 72 (3.7%) | 3 (2.0%) | 17 (3.7%) | 3 (2.0%) | ||
| Risk Factors | ||||||
| Diabetes mellitus | 379 (19.4%) | 37 (25.0%) | 0.10 | 86 (18.7%) | 37 (25.0%) | 0.09 |
| Hypertension | 926 (47.5%) | 54 (36.5%) | 0.010 | 176 (38.2%) | 54 (36.5%) | 0.71 |
| Dyslipidemia | 1779 (91.3%) | 135 (91.2%) | 0.98 | 411 (89.2%) | 135 (91.2%) | 0.47 |
| Tobacco use | 1006 (52.2%) | 82 (56.6%) | 0.31 | 270 (58.6%) | 82 (56.6%) | 0.67 |
| Illicit substance use | ||||||
| Cocaine use | 90 (4.6%) | 9 (6.1%) | 0.42 | 17 (3.7%) | 9 (6.1%) | 0.21 |
| Marijuana use | 149 (7.6%) | 15 (10.1%) | 0.28 | 44 (9.5%) | 15 (10.1%) | 0.83 |
| Any substance use | 212 (10.9%) | 21 (14.2%) | 0.22 | 54 (11.7%) | 21 (14.2%) | 0.43 |
| Alcohol use | 133 (6.9%) | 15 (10.2%) | 0.14 | 26 (5.8%) | 15 (10.2%) | 0.06 |
| Obesity | 731 (39.2%) | 49 (33.6%) | 0.18 | 159 (36.6%) | 49 (33.6%) | 0.50 |
| Chronic kidney disease | 11 (0.6%) | 2 (1.4%) | 0.24 | 0 (0.0%) | 2 (1.4%) | 0.01 |
| Peripheral vascular disease | 34 (1.7%) | 7 (4.7%) | 0.01 | 9 (2.0%) | 7 (4.7%) | 0.07 |
| Median Charlson Comorbidity Index | 1 (1, 2) | 2 (1, 2) | <0.001 | 1.0 (1.0, 2.0) | 2.0 (1.0, 2.0) | <0.001 |
| Laboratory Results | ||||||
| Median presentation creatinine (mg/dL) | 1.0 (0.9, 1.1) | 1.1 (0.9, 1.5) | <0.001 | 1.0 (0.8, 1.1) | 1.1 (0.9, 1.5) | <0.001 |
| Standardized troponina | 35.7 (10.0, 133.7) | 172.7 (76.0, 731.2) | <0.001 | 63.8 (18.9, 211.8) | 172.7 (76.0, 731.2) | <0.001 |
| Infarction Characteristics | ||||||
| ST-elevation MI | 996 (51.1%) | 125 (84.5%) | <0.001 | 389 (84.4%) | 125 (84.5%) | 0.98 |
| Out-of-hospital cardiac arrest | 61 (3.1%) | 39 (26.4%) | <0.001 | 21 (4.6%) | 39 (26.4%) | <0.001 |
| Median number of coronary arteries involved | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | <0.001 | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | <0.001 |
| Number of coronary arteries involved | ||||||
| 0 | 266 (14.4%) | 6 (4.2%) | 68 (15.3%) | 6 | 0.001 | |
| 0.001 | (4.2%) | |||||
| 1 | 1029 (55.7%) | 80 (56.3%) | 259 (58.2%) | 80 (56.3%) | ||
| 2 | 366 (19.8%) | 35 (24.7%) | 71 (16.0%) | 35 (24.7%) | ||
| >3 | 187 (10.1%) | 21 (14.8%) | 47 (10.6%) | 21 (14.8%) | ||
| Left main coronary artery disease | 45 (2.3%) | 11 (7.4%) | <0.001 | 24 (5.2%) | 11 (7.4%) | 0.31 |
Values are median (interquartile range), n (%) or mean ± SD.
Standardized to times >99th percentile for assay.AMI-CS, cardiogenic shock due to acute myocardial infarction; CAD, coronary artery disease; MI, myocardial infarction; mg, milligrams; dL, deciliter.
After matching for age, sex, race, hypertension, smoking status, left main disease and STEMI, the AMI-CS (n = 148) and non-AMI-CS (n = 461) groups did not have significant differences in baseline comorbidities other than a higher prevalence of chronic kidney disease in the AMI-CS group (Table 1). However, like the total cohort, the AMI-CS group continued to have a higher proportion of uninsured or publicly insured patients as well as higher standardized troponin, prevalence of OHCA and more coronary arteries involved than matched non-AMI-CS patients.
Baseline Characteristics by AMI-CS SCAI Stages
Of the 148 patients with AMI-CS, 31 (20.9%) were at stage B, 76 (51.4%) were at stage C, 17 (11.5%) were at stage D, and 24 (16.2%) were at stage E (Supplementary Table 3). There was a significant trend toward a greater proportion of patients with diabetes with increasing CS stage classification (P for difference across all strata = 0.002). There was a graded increase in presentation creatinine and burden of CAD (by mean number of coronary arteries involved) from stage B to stage E (both P < 0.001).
Risk Factors for Cardiogenic Shock
In univariate analyses, STEMI at presentation, OHCA, number of coronary arteries with CAD, left main disease, and peripheral vascular disease were significantly associated with AMI-CS (Table 2). Patients with hypertension at baseline were less likely to develop AMI-CS (OR 0.62, 95% CI 0.43−0.91; P = 0.015). In multivariate analyses, risk factors independently associated with development of AMI-CS included female sex (OR 1.54, 95% CI 1.01−2.36; P = 0.047); diabetes (OR 1.84, 95% CI 1.19−2.82; P = 0.006); peripheral vascular disease (OR 3.23, 95% CI 1.26−8.28; P = 0.015); STEMI at presentation (OR 5.13, 95% CI 3.19−8.26; P < 0.001); OHCA (OR 10.7, 95% CI 6.63−17.16; P < 0.001); and left main CAD (OR 5.41, 95% CI 2.54−11.53; P < 0.001).
Table 2.
Predictors of AMI-CS in Young Patients
| Characteristic | Univariate Analysis |
Multivariate Analysisa |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| OHCA | 11.07 (7.09−17.30) | <0.001 | 10.67 (6.63−17.16) | <0.001 |
| Left main disease | 3.40 (1.72−6.72) | <0.001 | 5.41 (2.54−11.53) | <0.001 |
| ST-elevation MI | 5.20 (3.30−8.18) | <0.001 | 5.13 (3.19−8.26) | <0.001 |
| Peripheral vascular disease | 2.80 (1.22−6.42) | 0.015 | 3.23 (1.26−8.28) | 0.015 |
| Diabetes | 1.38 (0.94−2.04) | 0.104 | 1.84 (1.19−2.83) | 0.006 |
| Female sex | 1.38 (0.93−2.04) | 0.107 | 1.54 (1.01−2.36) | 0.047 |
| Hypertension | 0.64 (0.45−0.90) | 0.010 | 0.62 (0.43−0.91) | 0.015 |
| Chronic kidney disease | 2.41 (0.53−10.99) | 0.255 | - | - |
| Alcohol use | 1.53 (0.87−2.68) | 0.140 | - | - |
| Marijuana use | 1.36 (0.78−2.38) | 0.278 | - | - |
| Cocaine use | 1.34 (0.66−2.71) | 0.420 | - | - |
| Mean number of vessels involved | 1.31 (1.11−1.54) | 0.001 | - | - |
| Tobacco use | 1.19 (0.85−1.67) | 0.313 | - | - |
| Caucasianb | 1.05 (0.72−1.54) | 0.811 | - | - |
| Dyslipidemia | 0.99 (0.55−1.79) | 0.980 | - | - |
| Age at event | 0.99 (0.95−1.02) | 0.365 | - | - |
| Family history of premature CAD | 0.84 (0.64−1.09) | 0.194 | - | - |
| Obesity | 0.78 (0.56−1.12) | 0.178 | - | - |
Multivariate analyses adjusted for all factors listed with multivariate OR.
Compared to non-CaucasianAMI-CS, cardiogenic shock due to acute myocardial infarction; CAD, coronary artery disease CI, confidence interval; MI, myocardial infarction; OHCA, out-of-hospital cardiac arrest; OR, odds ratio.
In-Hospital Management
All patients in this study had a high rate of cardiac catheterization during admission, regardless of AMI-CS status (Supplementary Table 4). However, a significantly greater proportion of patients with AMI-CS underwent coronary revascularization (percutaneous and/or surgical) than those without AMI-CS (92.6% AMI-CS vs 83.9% non-AMI-CS; P = 0.02). Mechanical circulatory support was used in 71.6% (n = 106) of AMI-CS patients, with intra-aortic balloon pump representing 92.4% (n = 98), followed by extracorporeal membrane oxygenation in 4.7% (n = 7) and percutaneous left ventricular assist device in 3.4% (n = 5). AMI-CS patients had a longer median length of hospital stay than non-AMI-CS patients (8 days vs 3 days; P < 0.001).
Among patients surviving to hospital discharge, patients with AMI-CS (n = 121) were significantly more likely to be prescribed angiotensin converting enzyme inhibitor or angiotensin receptor blocker therapy (71.1% vs 61.1%; P = 0.029) compared with non-AMI-CS patients (n = 1933). Beta-blocker, statin, aspirin, and P2Y12 inhibitor prescription was similar at discharge in the 2 groups. In-hospital management and median length of stay of AMI-CS patients did not differ significantly when stratified by SCAI stages (Supplementary Table 3).
Outcomes and Mortality
Patients with AMI-CS had significantly higher unadjusted mortality rates compared with non-AMI-CS patients (Fig. 1). This difference was observed when evaluating in-hospital mortality (18.2% AMI-CS vs 0.8% non-AMI-CS; P < 0.001), 1-year ACM (21.6% AMI-CS vs 1.9% non-AMI-CS; P < 0.001), postdischarge CVM (12.4% AMI-CS vs 5.5% non-AMI-CS; P = 0.002), total ACM (30.4% v. 10.7%; P < 0.001), and total CVM (27% AMI-CS vs 9% non-AMI-CS; P < 0.001). There was no significant difference between the 2 groups in postdischarge ACM (14.9% AMI-CS vs 10% non-AMI-CS; P = 0.086).
Fig. 1.
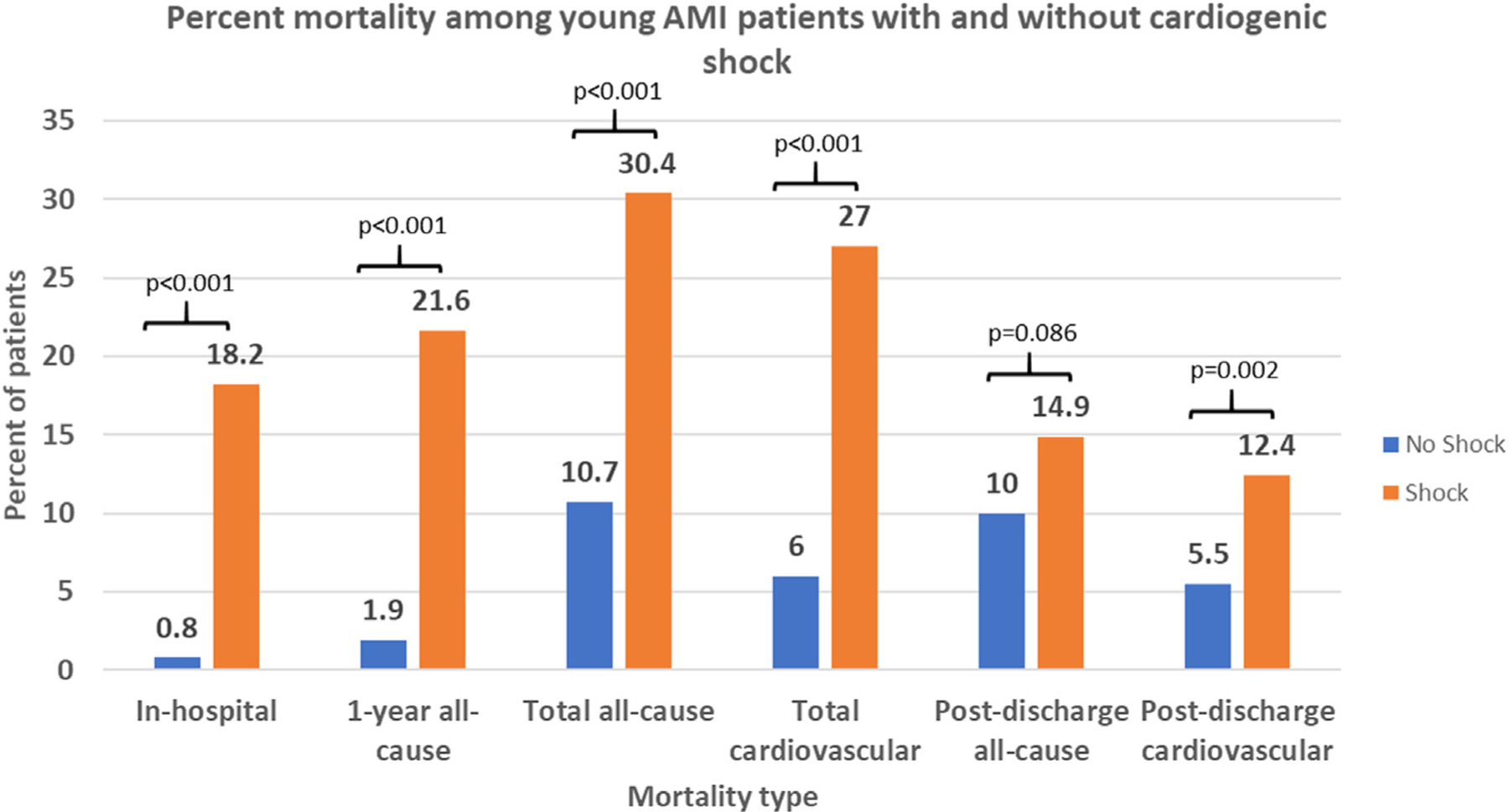
Observed mortality rates among young AMI patients, stratified by AMI-CS status. This figure shows the percent of patients experiencing the differing types of mortality in our cohort, stratified by AMI-CS status, with corresponding P values for each comparison above the bars. AMI-CS patients experienced significantly higher mortality rates in all comparisons relative to non-AMI-CS patients except for postdischarge all-cause mortality.
Patients with AMI-CS had significantly higher incidence-rate ratios for ACM, CVM and 1-year HHF compared with non-AMI-CS patients (Table 3). In the matched comparison, AMI-CS patients had an incidence rate ratio of 4.67 for ACM (95% CI 3.00−7.36; P < 0.001), 7.29 for CVM (95% CI 4.27−12.44; P < 0.001), and 5.68 for 1-year HHF (95% CI 2.08−15.46; P < 0.001). These results remained unchanged in a landmark analysis of the matched cohort including only patients who survived to discharge from the index AMI hospitalization.
Table 3.
Incidence Rates for All-Cause Mortality, Cardiovascular Mortality and Heart Failure Hospitalization Amongst Patients With AMI-CS Compared to Those Without AMI-CS
| Incidence Rate |
||||
|---|---|---|---|---|
| No AMI-CS (95% CI) | AMI-CS (95% CI) | Incidence Rate Ratioa (95% CI) | P value | |
| Full Cohort | ||||
| All-cause mortality (per 100 py) | 1.07 (0.93 – 1.23) | 4.28 (3.20 – 5.73) | 3.91 (2.82 – 5.43) | <0.001 |
| Cardiovascular mortality (per 100 py) | 0.60 (0.50−0.72) | 3.81 (2.79−5.19) | 6.28 (4.35−9.08) | <0.001 |
| One-year heart failure hospitalizations (per 100 pd) | 0.01 (0.01−0.02) | 0.05 (0.03−0.09) | 4.35 (2.20−8.59) | <0.001 |
| Matchedb Cohort | ||||
| All-cause mortality (per 100 py) | 0.89 (0.66−1.20) | 4.28 (3.20−5.73) | 4.67 (3.00−7.26) | <0.001 |
| Cardiovascular mortality (per 100 py) | 0.50 (0.33−0.74) | 3.81 (2.79−5.19) | 7.29 (4.27−12.44) | <0.001 |
| One-year heart failure hospitalizations (per 100 pd) | 0.01 (0.00−0.02) | 0.05 (0.03−0.09) | 5.68 (2.08−15.46) | <0.001 |
| Full Cohort that Survived to Discharge | ||||
| All-cause mortality (per 100 py) | 0.98 (0.85−1.13) | 1.72 (1.08−2.73) | 1.77 (1.09−2.87) | 0.019 |
| Cardiovascular mortality (per 100 py) | 0.55 (0.45−0.66) | 1.43 (0.86−2.38) | 2.67 (1.55−4.60) | <0.001 |
| One-year heart failure Hospitalizations (per 100 pd) | 0.01 (0.01−0.02) | 0.05 (0.03−0.09) | 4.32 (2.12−8.78) | <0.001 |
| Matchedb Cohort That Survived to Discharge | ||||
| All-cause mortality (per 100 py) | 0.80 (0.58−1.09) | 1.72 (1.08−2.73) | 2.23 (1.26−3.94) | 0.005 |
| Cardiovascular mortality (per 100 py) | 0.42 (0.27−0.65) | 1.43 (0.86−2.38) | 3.56 (1.80−7.04) | <0.001 |
| One-year heart failure hospitalizations (per 100 pd) | 0.01 (0.00−0.02) | 0.05 (0.03−0.09) | 5.45 (1.96−15.15) | <0.001 |
Incidence rate ratio for shock group compared to no-shock group.
Matched for age, sex, race, hypertension, smoking status, left main coronary artery disease, STEMI.pd, person-days; py, person-years.
After adjustment, AMI-CS patients had a significantly elevated risk of ACM, CVM and 1-year HHF compared with matched non-AMI-CS patients (Fig. 2A). In Kaplan-Meier analyses, there was early separation of the curves, with persistently higher risk of ACM, CVM and HHF for the AMI-CS group. After adjustment for key covariates, AMI-CS patients continued to have a significantly higher risk of ACM (adjusted hazard ratio [aHR] 2.84 [95% CI 1.68−4.81]; P < 0.001), CVM (aHR 4.09 (95% CI 2.17−7.71; P < 0.001) and 1-year HHF (aHR 5.99 [95% CI 2.04−17.61]; P = 0.001) compared with matched non-AMI-CS patients (Table 4).
Fig. 2.
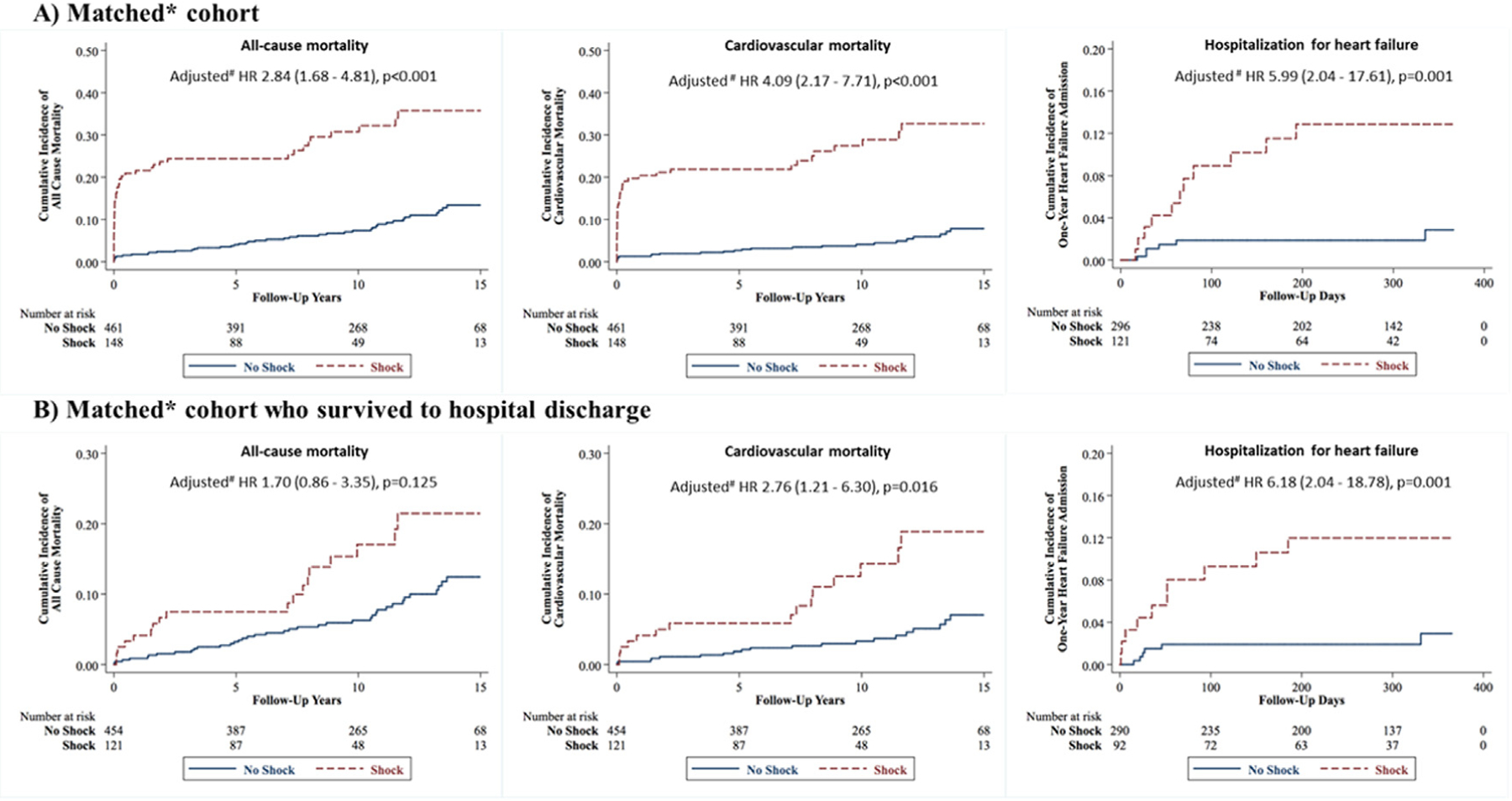
Kaplan-Meier analysis of all-cause mortality, cardiovascular mortality and 1-year hospitalization for heart failure in young MI patients. Kaplan-Meier curves for all-cause mortality, cardiovascular mortality and 1-year hospitalization for heart failure for (A) the matched cohort and (B) those in the matched cohort who survived to discharge, stratified by AMI-CS status. AMI-CS patients in all comparisons experienced greater adverse outcomes, except for all-cause mortality in those who survived to discharge. HR, hazard ratio. *Matched for age, sex, race, hypertension, smoking status, left main coronary artery disease, ST elevation myocardial infarction (STEMI). #Multivariate analyses adjusted for age, sex, area deprivation index (ADI), out-of-hospital cardiac arrest (OHCA), ST elevation myocardial infarction (STEMI).
Table 4.
Risk of All-Cause Mortality, Cardiovascular Mortality and Hospitalization for Heart Failure in Young Patients With AMI-CS
| Cohort | Univariate |
Multivariatea |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| All-cause mortality | ||||
| Full cohort | 3.84 (2.78−5.30) | <0.001 | 2.20 (1.40−3.44) | 0.001 |
| Matched cohortb | 4.40 (2.89−6.70) | <0.001 | 2.84 (1.68−4.81) | <0.001 |
| Full cohort that survived to hospital discharge | 1.77 (1.09−2.87) | 0.021 | 1.54 (0.83−2.85) | 0.170 |
| Matched cohort that survived to hospital discharge | 2.21 (1.26−3.89) | 0.006 | 1.70 (0.86−3.35) | 0.125 |
| Cardiovascular Mortality | ||||
| Full cohort | 6.09 (4.24−8.73) | <0.001 | 3.13 (1.88−5.21) | <0.001 |
| Matched cohort | 6.80 (4.09−11.32) | <0.001 | 4.09 (2.17−7.71) | <0.001 |
| Full cohort that survived to hospital discharge | 2.67 (1.55−4.58) | <0.001 | 2.29 (1.13−4.66) | 0.022 |
| Matched cohort that survived to hospital discharge | 3.52 (1.79−6.89) | <0.001 | 2.76 (1.21−6.30) | 0.016 |
| One-Year Hospitalization for Heart Failure | ||||
| Full cohort | 4.34 (2.20−8.57) | <0.001 | 5.40 (2.42−12.07) | <0.001 |
| Matched cohort | 5.62 (2.08−15.22) | 0.001 | 5.99 (2.04−17.61) | 0.001 |
| Full cohort that survived to hospital discharge | 4.30 (2.12−8.72) | <0.001 | 5.57 (2.39−12.95) | <0.001 |
| Matched cohort that survived to hospital discharge | 5.37 (1.95−14.78) | 0.001 | 6.18 (2.04−18.78) | 0.001 |
Multivariate analyses adjusted for age, sex, ADI, STEMI, OHCA, DM, HTN, dyslipidemia, tobacco use, cocaine use, cardiac catheterization, presentation creatinine, standardized troponin.
Matched for age, sex, race, HTN, smoking status, left main coronary artery disease, STEMI.ADI, area deprivation index; DM, diabetes mellitus; HTN, hypertension; OHCA, out-of-hospital cardiac arrest; STEMI, ST elevation myocardial infarction.
Landmark Analysis After Hospital Discharge
To examine long-term outcomes of AMI-CS independent of in-hospital and acute AMI mortality, we performed a landmark analysis including only patients who survived index AMI hospitalization (Fig. 2B). AMI-CS patients had a significantly higher risk of CVM (aHR 2.76 [95% CI 1.21−6.30 P = 0.016]) and 1-year HHF (aHR 6.18 [95% CI 2.04 – 18.78]; P = 0.001) compared to non-AMI-CS patients after adjustment for baseline characteristics (Table 4). AMI-CS was not associated with a significantly higher risk of ACM (aHR 1.70 [95% CI 0.86−3.35]; P = 0.125) compared with non-AMI-CS patients who survived to hospital discharge. When examining outcomes by SCAI CS stage, AMI-CS patients had incremental ACM and CVM with higher SCAI CS stages (Supplementary Fig. 1, Fig. 2).
Era Effect: Incidence and Outcomes of AMI-CS
When the study period was divided into 4 eras with a similar number of subjects in each (2000−2003, 2004−2006, 2007−2010, 2011−2016), there was increasing AMI-CS incidence in the most recent era (P for trend = 0.02) (Supplementary Fig. 3a). Similarly, there was increasing in-hospital ACM among all patients (both AMI-CS and non-AMI-CS) when comparing the most recent era to preceding ones (Supplementary Fig. 3b). In-hospital ACM increased from 0.5% in non-AMI-CS patients in 2000−2003 to 1.7% in 2011−2016, whereas among AMI-CS patients, in-hospital ACM increased from 16.2% in 2000−2003 to 21.7% in 20112016 (P for trend in full cohort = 0.016). However, there was no significant difference in mechanical circulatory support use among AMI-CS patients over the eras (Supplementary Fig. 3c) (P for trend for mechanical circulatory support use = 0.15).
Discussion
Using the YOUNG-MI registry, we investigated risk factors and long-term outcomes associated with AMI-CS among young patients. Our main findings are as follows. First, AMI-CS was diagnosed in 7% of the young AMI population in this study, with increasing incidence in the contemporary era. Second, factors independently associated with incident AMI-CS in young patients included female sex, diabetes, peripheral vascular disease, STEMI at presentation, OHCA, and left main disease. Third, young AMI-CS patients had a significantly higher risk of ACM, CVM and 1-year HHF than those without AMI-CS, even after adjustment for important covariates. These data show that similar to older cohorts, AMI-CS in younger individuals is associated with poor short- and long-term outcomes. Our findings emphasize the need for prevention, early detection and appropriate therapy for AMI-CS in young patients to improve morbidity and mortality rates.
Epidemiology and Risk Factors of AMI-CS in Young Patients
Most studies of AMI-CS risk factors and incidence have been representative of older patients. Among these older populations, AMI-CS is thought to occur in 5%–10% of cases.1 The incidence of AMI-CS in our cohort was 7%, suggesting that the rate of AMI-CS is similar in young patients with AMI when compared with older populations.
With regard to risk factors, a French registry profiled 9951 patients with AMI (median age > 70 years) and showed that age, STEMI, OHCA, family history of CAD, peripheral vascular disease, and chronic obstructive pulmonary disease were all independently associated with a higher risk of AMI-CS.1 Although several risk factors appear to be common between our study of young patients and prior studies primarily of older patients,1,17 3 important differences are worth discussing.
First, age was not significantly associated with incident AMI-CS in patients restricted to age ≤ 50 years, whereas in older populations, increasing age is associated with a greater risk. The younger age of our cohort likely explains this discordance, whereas increasing age in a younger cohort may not confer incremental risk as it does in older populations.
Second, our analysis identifies female sex as an independent risk factor for AMI-CS in the young population with AMI. Prior work in this cohort has reported that young women with MI were less likely than men to undergo coronary revascularization or to be discharged on guideline-directed medical therapy.18 Furthermore, young women hospitalized for an MI who survived to discharge had significantly higher ACM than men.18 A prior study examined sex disparities in AMI-CS in a young cohort (age ≤ 55 years).19 This study reported higher rates of AMI-CS in men compared to women, despite women having a higher risk of in-hospital mortality than men. In the same study, women were also less likely to undergo angiography and interventions, findings reproduced in an older population (age ≥ 75 years) with AMI-CS.19,20 The higher risk of AMI-CS in young women reported in our study may be related to these prior findings of less aggressive therapy and diagnostic studies or to potentially delayed identification of AMI-CS. There are also other well-described sex-based differences and disparities in the care of young patients with AMI.18,21–24
Third, patients with AMI and with hypertension were at significantly lower risk of AMI-CS compared with those without hypertension. However, hypertension was independently associated with worse outcomes in AMI-CS patients. These seemingly contradictory results may be a result of several factors. Patients already diagnosed with hypertension prior to their MI may have had better access to medical care, potentially reducing their risk for AMI-CS. Additionally, these patients may have been on more baseline antihypertensive and cardioprotective medications, which could have improved their AMI outcomes. Finally, because medical record diagnosis of hypertension was used for analysis, there may be under-reporting of this diagnosis in this younger population.
Outcomes of Young Patients With AMI-CS
Prior studies have reported in-hospital mortality rates of AMI-CS ranging from 35%–60%, with a high degree of intrastudy variability, likely due to heterogeneity in AMI-CS definitions and data capture.1,2,6,23,25,26 Our cohort had an in-hospital mortality rate of 18.2% among young AMI-CS patients. This lower in-hospital mortality may reflect our rigorous adjudication of AMI-CS using the recent SCAI definitions,12 which has not been used in most prior studies. Furthermore, a lower burden of comorbidities and healthier baseline health status in the younger population than in older patients may lead to the lower AMI-CS in-hospital mortality rates seen in our study. This has been reported in contemporary studies, where increasing age is associated with worse outcomes in AMI-CS, regardless of SCAI stage, with younger patients having better outcomes.27,28
An important long-term consequence of AMI-CS is heart failure (HF). In an older cohort, AMI-CS was associated with 3.25 and 2.9 times higher risk of HHF at 1 and 5 years, respectively, compared to patients without AMI-CS.29 In our study, young AMI-CS patients were at 6 times the risk of 1-year HHF compared to non-AMI-CS patients, with a very early separation of curves in risk analyses. Therefore, the risk of HHF in the young AMI-CS population seems to be at least similar to that in older populations, which highlights an important opportunity for intervention to prevent HF in a young population.
SCAI Classification in Young Patients With AMI-CS
Analyses stratifying AMI-CS patients by SCAI stage showed several important findings. First, patients with higher SCAI CS stages had a greater burden of diabetes but not other CV comorbidities at baseline and presented with worse renal function. Second, there was a graded worsening of mortality with each step of the SCAI CS stages. These findings are in keeping with other observations, which have shown increasing mortality rates with higher SCAI stage in older CS patients.13,30 A recent study using data from the Critical Care Cardiology Trials Network database showed similar findings of a graded increase of in-hospital mortality rates with higher SCAI stages in older patients.30 Interestingly, the study reported lower stage E in-hospital mortality rates (62.1%) compared to our cohort (88%), but higher stage D and C in-hospital mortality rates compared to our study.30 This suggests that the sickest young AMI-CS patients (stage E) have extremely poor outcomes at par with older stage E AMI-CS patients, whereas less severe AMI-CS stages might be associated with lower mortality rates in younger patients. In landmark analyses restricted to patients surviving to hospital discharge, there was no significant difference in long-term ACM or CVM based on SCAI stage. Thus, SCAI stages were particularly good at identifying individuals with poor in-hospital outcomes, but they did not seem to further risk-stratify those who survived to discharge. These findings further emphasize the theme of the recently developed 3-axis model of CS evaluation and prognostication from SCAI that takes into account shock severity (SCAI stages, as done in our study) along with phenotype, etiology and risk modifiers, so as to better understand patient trajectory.13
Era Effect
An important observation is that incidence and outcomes of AMI-CS did not improve in the contemporary era. There was increasing AMI-CS among young AMI patients as well as rising in-hospital mortality rates in both AMI-CS and non-AMI-CS patients over time. These findings are unique when compared with prior studies. An Italian study aggregating 28,217 primarily older patients (median age > 70 years) with AMI reported no significant change in incident AMI-CS from 2001−2014, with an overall 4.3% incidence of AMI-CS.2 Thus, future studies have to examine whether advances in AMI care translate into better outcomes in the young population with AMI-CS. There was high use of mechanical circulatory support in the AMI-CS patients from the earliest era (81% in 2000−2003), and no significant change was seen over the study period, which was, therefore, unlikely to account for the change in mortality rates over this period.
Limitations
This study must be assessed in the context of its limitations. This was a retrospective observational study and, thus, was susceptible to biases inherent in the study design. Adjudication of AMI-CS was made by 2 physicians using predefined criteria. However, due to the retrospective nature of this study, there may have been misclassification of AMI-CS status. There may also have been increasing recognition and diagnosis of AMI-CS in the recent eras due to greater use of diagnostics such as pulmonary artery catheters. However, differences in AMI-CS recognition in the earlier eras would not fundamentally change the direction of the results. Furthermore, potentially relevant clinical variables such as Killip class, vital signs during hospitalization, mortality risk scores, infarct-related artery, ejection fraction, and revascularization details were not available to incorporate in the analyses. The data in this study ranged from 2000−2016 and, thus, represent heterogeneity and transitions in therapies of AMI and AMI-CS, given advances in the field during the study period. However, despite improving AMI outcomes in recent decades,31 our study did not find improvement in outcomes across time. The study was based at 2 large academic medical centers and, thus, the generalizability of these findings may be limited. Pre-hospital deaths were not included in this study, so we cannot account for the effect of AMI-CS in those patients. The in-hospital course of illness and other organs affected were not available for analysis. Additionally, although we attempted to reduce confounding by pursuing both a matched analysis and statistical adjustment for covariates, there may be unmeasured confounders that could affect our results. Furthermore, echocardiographic data was not available for incorporation in our analyses. Also, the inclusion of SCAI AMI-CS stage B in the AMI-CS group may have led to less critically ill patients’ being included in this group, potentially lowering the event rate in the CS group. However, this would have biased the results toward the null and would not explain the key differences seen in the analyses. Finally, the smaller size of the AMI-CS group limits the study’s power to detect small differences among subgroups.
Conclusion
AMI-CS is prevalent among young patients with AMI (7% of cohort), with increasing incidence over the study period and is associated with increased hospitalizations due to HF and high mortality rates despite use of guideline-recommended therapies. In addition, the SCAI classification system accurately stratifies mortality risk in young patients with AMI-CS. This study identifies a pressing need to focus on the prevention, diagnosis and treatment of young patients with AMI-CS.
Brief Lay Summary
The epidemiology, characteristics and outcomes of young patients with acute myocardial infarction (AMI) who develop cardiogenic shock (AMI-CS) is unknown. In this detailed analysis of 2097 patients in the YOUNG-MI registry, AMI patients under the age of 50 years had a 7% incidence of AMI-CS, a condition that was associated with poor short- and long-term outcomes in this population. ST-elevation myocardial infarction, left main disease, cardiac arrest, female sex, peripheral vascular disease, and diabetes were all associated with risk of developing AMI-CS. The incidence of AMI-CS has been increasing despite advances in medical and surgical therapies.
Supplementary Material
Footnotes
Disclosures
Sanjay Divakaran: Grant funding: Joint KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst and the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679-10). James Januzzi: Trustee, American College of Cardiology; Research Support/Funding, Applied Therapeutics, Innolife, Novartis Pharmaceuticals, and Abbott Diagnostics; Consulting: Abbott, Janssen, Novartis, and Roche Diagnostics; Clinical Endpoint Committees/Data Safety Monitoring Boards: Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia, and Takeda. Garrick Stewart: Consulting: Abbott Laboratories, Procyrion. Deepak L. Bhatt: Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, DRS.LINQ (stock options), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee, American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. Ron Blankstein: Research support: Amgen, Novartis; Consulting: Roivant Sciences, Caristo Diagnostics, Silence Therapeutics. All other authors have no disclosures.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2022.08.012.
References
- 1.Aissaoui N, Puymirat E, Delmas C, Ortuno S, Durand E, Bataille V, et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail 2020;22:664–72. [DOI] [PubMed] [Google Scholar]
- 2.De Luca L, Olivari Z, Farina A, Gonzini L, Lucci D, Di Chiara A, et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes: management changes in cardiogenic shock. Eur J Heart Fail 2015;17:1124–32. [DOI] [PubMed] [Google Scholar]
- 3.Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS. NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448. [DOI] [PubMed] [Google Scholar]
- 4.Berg DD, Bohula EA, Morrow DA. Berg DD, Bohula EA, Morrow DA. Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care 2021;27:401–8. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med 1999;340:1162–8. [DOI] [PubMed] [Google Scholar]
- 6.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. JAHA 2014;3:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Collins B, Qamar A, Gupta A, Fatima A, Divakaran S, et al. Study of young patients with myocardial infarction: design and rationale of the YOUNG-MI Registry. Clin Cardiol 2017;40:955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, et al. Cardiovascular risk and statin eligibility of young adults after an MI. J Am Coll Cardiol 2018;71:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Gupta A, DeFilippis EM, Qamar A, Biery DW, Almarzooq Z, et al. Cardiovascular mortality after type 1 and type 2 myocardial infarction in young adults. J Am Coll Cardiol 2020;75:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman AN, Biery DW, Ginder C, Singh A, Baek J, Wadhera RK, et al. Association of socioeconomic disadvantage with long-term mortality after myocardial infarction: the Mass General Brigham YOUNG-MI Registry. JAMA Cardiol 2021;6:880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kind AJH, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med 2014;161:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 13.Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Soc Cardiovasc Angiogr Intervent 2022;79:933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thayer KL, Zweck E, Ayouty M, Garan AR, Hernandez-Montfort J, Mahr C, et al. Invasive hemodynamic assessment and classification of in-hospital mortality risk among patients with cardiogenic shock. Circ: Heart Fail 2020;13:334–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. J Am Coll Cardiol 2015 2014;66:403–69. [DOI] [PubMed] [Google Scholar]
- 16.Stuart EA. Matching methods for causal inference: a review and a look forward. Statist Sci 2010;25:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auffret V, Cottin Y, Leurent G, Gilard M, Beer J-C, Zabalawi A, et al. , ORBI and RICO Working Groups. Predicting the development of in-hospital cardiogenic shock in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: the ORBI risk score. Eur Heart J 2018;39:2090–102. [DOI] [PubMed] [Google Scholar]
- 18.DeFilippis EM, Collins BL, Singh A, Biery DW, Fatima A, Qamar A, et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the Mass General Brigham YOUNG-MI registry. Eur Heart J 2020;41:4127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallabhajosyula S, Ya’Qoub L, Singh M, Bell MR, Gulati R, Cheungpasitporn W, et al. Sex disparities in the management and outcomes of cardiogenic shock complicating acute myocardial infarction in the young. Circ: Heart Fail 2020;13:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallabhajosyula S, Dunlay SM, Hayes SN, Best PJM, Brenes-Salazar JA, Lerman A, et al. Sex and gender disparities in the management and outcomes of acute myocardial infarction–cardiogenic shock in older adults. Mayo Clin Proceed 2020;95:1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty-year trends and sex differences in young adults hospitalized with acute myocardial infarction: the ARIC Community Surveillance Study. Circulation 2019;139:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangalore S, Fonarow GC, Peterson ED, Hellkamp AS, Hernandez AF, Laskey W, et al. Age and gender differences in quality of care and outcomes for patients with ST-segment elevation myocardial infarction. Am J Med 2012;125:1000–9. [DOI] [PubMed] [Google Scholar]
- 23.Kolte D, Khera S, Dabhadkar KC, Agarwal S, Aronow WS, Timmermans R, et al. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non-ST-elevation myocardial infarction. Am J Cardiol 2016;117:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Gupta T, Kolte D, Khera S, Agarwal N, Villablanca PA, Goel K, et al. Contemporary Sex-based differences by age in presenting characteristics, use of an early invasive strategy, and inhospital mortality in patients with non ST-segment elevation myocardial infarction in the United States. Circ Cardiovasc Interv 2018;11:1–9. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 2009;119:1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harjola V-P, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, et al. , for the CardShock study investigators and the GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock: clinical picture and outcome of cardiogenic shock. Eur J Heart Fail 2015;17:501–9. [DOI] [PubMed] [Google Scholar]
- 27.Padkins M, Breen T, Anavekar N, Diepen S, Henry TD, Baran DA, et al. Age and shock severity predict mortality in cardiac intensive care unit patients with and without heart failure. ESC Heart Fail 2020;7:3971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanwar M, Thayer KL, Garan AR, Hernandez-Montfort J, Whitehead E, Mahr C, et al. Impact of age on outcomes in patients with cardiogenic shock. Front Cardiovasc Med 2021;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauridsen MD, Rorth R, Butt JH, Kristensen SL, Schmidt M, Moller JE, et al. Five-year risk of heart failure and death following myocardial infarction with cardiogenic shock: a nationwide cohort study. Eur Heart JAcute Cardiovasc Care 2021;10:40–9. [DOI] [PubMed] [Google Scholar]
- 30.Lawler PR, Berg DD, Park J-G, Katz JN, Baird-Zars VM, Barsness GW, et al. The range of cardiogenic shock survival by clinical stage: data from the Critical Care Cardiology Trials Network Registry. Crit Care Med 2021;49:1293–302. [DOI] [PubMed] [Google Scholar]
- 31.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS,Callaway CW,et al. ,onbehalfof the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2021 update: a report from the American Heart Association. Circulation 2021;143:e254–743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


