Abstract
Background
The aim of this study was to evaluate the efficacy and safety of osimertinib for the treatment of leptomeningeal metastases (LM) from epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC).
Methods
We conducted a systematic review and meta-analysis to aggregate the clinical outcomes of patients with LM from EGFR-mutant NSCLC treated with osimertinib. A comprehensive literature search for published and unpublished studies was implemented in April 2021 of PubMed, EMBASE, the Cochrane Library, and several international conference databases, in accordance with the PRISMA guidelines. Meta-analysis of proportions was conducted to calculate the pooled rate of overall response rate (ORR), disease control rate (DCR), one-year overall survival (OS), and adverse events (AEs).
Results
A total of eleven studies (five prospective and six retrospective) including 353 patients were included. The majority of patients (346/353, 98.0%) received osimertinib as ≥ 2nd-line treatment for LM, either at a dosage of 80 mg (161/353, 45.6%) or 160 mg (191/353, 54.1%). The pooled rates of ORR and DCR were 42% (95% CI 24% to 59%) and 93% (95% CI 88% to 97%), respectively. The pooled one-year OS rate was 59% (95% CI 53% to 65%) in 233 patients from five studies. The highest incidence of AEs of all grades was rash (53%), followed by diarrhea (45%), paronychia (35%), decreased appetite (35%), and dry skin (27%), based on data from four studies.
Conclusions
Our study highlighted and confirmed the meaningful efficacy and a manageable safety profile of osimertinib for the treatment of LM from EGFR-mutant advanced NSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-023-01219-y.
Keywords: Osimertinib, EGFR, NSCLC, Leptomeningeal metastases (LM), Efficacy, Meta-analysis
Introduction
Leptomeningeal metastases (LM), the spread of tumor cells into the leptomeninges and cerebrospinal fluid, represents one of the most deleterious complications of advanced non-small cell lung cancer (NSCLC) [1]. LM is associated with dismal prognosis due to poor response to cytotoxic agents and radiation treatment [2]. Of note, with emergence of modern treatment options such as targeted therapy and immunotherapy, considerably prolonged survival was achieved in patients with NSCLC. Owning to the rapid advances in imaging techniques and particularly tyrosine kinase inhibitors (TKIs) for patients harboring epidermal growth factor receptor (EGFR) mutations, recent years saw increasing incidence of LM from NSCLC [3].
Osimertinib is a third-generation EGFR TKI that inhibits both EGFR sensitizing and T790M mutations in NSCLC. Compared with first- and second-generation EGFR TKIs, osimertinib is more effectively cross the blood–brain barrier (BBB) and therefore with a superior cerebrospinal fluid (CSF) plasma concentration [4]. The phase III FLAURA study demonstrated higher efficacy of osimertinib compared to gefitinib or erlotinib for advanced NSCLC patients harboring EGFR-sensitizing mutations, including patients with central nervous system (CNS) metastases [5, 6]. However, the FLAURA study included only five patients with LM but not the patients with unstable neurological conditions. The AURA LM study reported the activity of osimertinib (80 mg) for the treatment of LM from NSCLC in a retrospective pooled analysis of four prospective studies within the AURA program (AURA extension, AURA2, AURA17, and AURA3) [7]. The included 22 LM patients, and the LM objective response rate (ORR), median LM progression-free survival (PFS) and overall survival (OS) were 55%, 11.1 months, and 18.8 months, respectively. The phase I BLOOM study evaluated the efficacy and safety of double dosage of osimertinib (160 mg qd) in forty-one NSCLC patients with LM [8]. The LM ORR assessed by the Response Assessment in Neuro-Oncology LM (RANO-LM) criteria was 62%. The median investigator-assessed PFS was 8.6 months, and the median OS was 11.0 months. Preliminary conclusions from these studies outlined a promising role of osimertinib for the treatment of LM from EGFR-mutant (EGFRm) NSCLC.
Nevertheless, many published articles or meeting abstracts on osimertinib for the treatment of LM from NSCLC enrolled a relatively small number of patients (< 50 patients in most studies), which weakens the persuasiveness of the conclusions from a single study. Furthermore, those studies vary in study design (prospective versus retrospective), dosages (80 mg versus 160 mg), race (Asian versus Caucasian), or LM response assessment criteria (RANO-LM versus Response Evaluation Criteria in Solid Tumors (RECIST)). Herein, an aggregate analysis of the currently available reports is highly demanded to clarify the role of osimertinib in management of LM from EGFRm NSCLC. In our study, we synthesized the outcomes from different studies with a particular focus on LM ORR, LM DCR, PFS, OS, and AEs, providing more insights for the optimal clinical use of osimertinib. This systematic review and meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [9].
Materials and methods
Literature search strategy
We searched for available articles, either published literatures or in conference abstracts with respect to evaluations of the safety and efficacy of osimertinib for EGFR-mutated NSCLC with leptomeningeal metastases. First, a comprehensive literature search on PubMed, EMBASE, and the Cochrane Library was conducted in April 2021 using the following retrieval strategy: (“osimertinib” OR “mereletinib” OR “AZD9291” OR “tagrisso”) AND (“leptomeningeal metastases” OR “leptomeningeal metastasis” OR “carcinomatous meningitis” OR “CNS” OR “central nervous system”). Secondly, meeting abstracts from the three international oncology conferences (American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and World Conference on Lung Cancer (WCLC)) from 2015 to 2020 were screened. Finally, reference lists of retrieved or relevant studies were also inspected to identify additional articles.
Selection criteria
Two authors independently screened titles and abstracts, and disagreement was resolved by a third author. After preliminary screening, the full texts of potentially eligible studies were reviewed to confirm final inclusion using the following selection criteria: (1) prospective clinical trials, retrospective cohort series, or prospective cohort series were available; (2) human studies were performed to evaluate the efficacy and safety of osimertinib for the treatment of patients with LM (diagnosed cytologically and/or radiologically) from EGFRm NSCLC. (3) The studies were published in English, and the most complete and recent report of the trial was used when the same team reported data obtained from the same patients. (4) Regarding the number of patients, due to the relatively low incidence of LM, an enrollment of at least 5 patients from prospectively designed trials or at least 10 patients from retrospective cohorts were required. Case reports, review articles, animal experiments, and duplicate publications were excluded.
Data extraction
Data were extracted and filled in a standardized, predesigned Microsoft Excel form by 2 investigators independently. All relevant data from texts, tables, and figures of each included study were extracted, including first author, publication year, region, number of patients, study design, age, sex, dosage, treatment line of osimertinib, EGFR T790M status, response assessment criteria, follow-up, LM ORR, LM DCR, PFS, OS, and adverse events (AEs), etc. If the response of LM was assessed both by neuroradiologic blinded central in dependent review (BICR) and by investigator, the BICR reported data were preferably recorded. If the prognosis was plotted as a Kaplan–Meier curve in some articles, the software GetData Graph Digitizer 2.24 (http://getdata-graph-digitizer.com/) was applied to digitize and extract the data of one-year OS from the survival curve. Any discrepancies were resolved by discussion or by consulting a third investigator when necessary.
Quality assessment
The modified Newcastle–Ottawa Scale (modified NOS) was used to evaluate the quality of all included articles due to the single arm and non-controlled design [10, 11]. Compared to the NOS, modified NOS eliminates three questions: two relating to selection and comparison of nonexposed patients and one assessing the presence of outcome at study start, and adds one question addressing presence of pharmaceutical industry funding. In brief, the modified NOS includes 6 appraisal items: representativeness of exposed cohort, ascertainment of exposure, assessment of outcome, median follow-up more than 6 months, follow-up completeness, and pharmaceutical industry funding [11].
Statistical analysis
The primary outcome of our study was LM ORR, which was evaluated by the response assessment criteria for LM in the study. Secondary outcomes included LM DCR, one-year OS rate, median LM PFS, median OS, and AEs. The integrated analysis of ORR, DCR, and one-year OS rate was carried out using the generic inverse variance random-effect method, and the effect size was represented by the 95% confidence interval (CI). Heterogeneity between studies was assessed using the Cochran Q statistic and I2. Heterogeneity was considered high, medium or low if ≥ 75%, 50–75%, or < 50%, respectively [12]. A fixed or random effect model was used depending on heterogeneity analysis. AEs of all grades or of grade ≥ III were aggregated separately. Furthermore, funnel plots were used to assess the publication bias of the enrolled studies. All statistical analyses were performed with R version 4.0.4 (R Foundation, Vienna, Austria) using the meta package. A two-sided p-value of < 0.05 was considered as statistically significant.
Results
Study selection
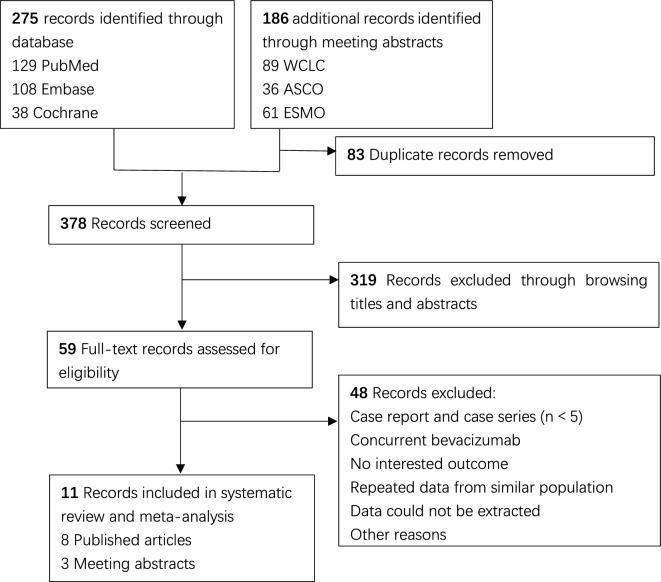
The study flowchart is provided (Fig. 1). In total, 461 studies were identified after performing database searches (275 for PubMed, Embase, and Cochrane Library, 186 for WCLC, ASCO, and ESMO), and 378 studies remained after the removal of duplicates. A total of 319 records were excluded after screening of the titles and abstracts. Finally, another 11 studies (including 8 published articles [6–8, 13–17] and 3 meeting abstracts [18–20]) were included after reading of the full texts.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Flow Diagram
Study characteristics
The 11 included records involving 353 NSCLC patients with LM who received osimertinib. Those records were published between 2017 and 2020 and consisting of 1 phase I study (BLOOM) [8], 3 phase II studies [14, 18, 19], 1 prospective pilot study [15], 1 preplanned exploratory analysis of a phase III study (FLAURA) [6], 1 retrospective pooled analysis of 4 prospective studies [7], and 4 retrospective cohort studies [13, 16, 17, 20]. A majority of patients (346/353, 98.0%) received osimertinib as ≥ 2nd-line treatment for LM, either at a dosage of 80 mg (161/353, 45.6%) or 160 mg (191/353, 54.1%). LM response assessment criteria were available in 7 studies, including 3 that used RANO-LM criteria, 2 that used RECIST 1.1 criteria, and 2 that used radiological response (nonspecific). Follow-up data were available for 5 studies, with a follow-up period between 8.3 and 11.7 months. The patient characteristics of the included studies were summarized in Table 1.
Table 1.
Characteristics of 11 included studies
| Study (year) | Region | No. of patients (female %) | Study design | Age | Therapy line | Dosage of Osimertinib (mg) | T790M status | LM response assessment criteria | Follow-up (mo.) |
|---|---|---|---|---|---|---|---|---|---|
| Yang 2019 | Asian | 41 (71%) | Phase I | 59 (44–75) | ≥ 2 | 160 mg | Positive:22; negative:13; unknown:6 | RANO-LM (BICR) | 9.9 |
| Lee 2020 | Asian | 110 (62%) | Retrospective | 58 (39–75) | ≥ 2 |
80 mg (n = 67); 160 mg (n = 43) |
Positive:60; negative:37; unknown:13 | NA | NA |
| Ahn 2020 | Global | 22 (59%) | Retrospective pooled analysis of 4 prospective studies | 58 (36–80) | ≥ 2 | 80 mg | 100% positive | RANO-LM (BICR) | 11.7 |
| Park 2020 | Asian | 40 (60%) | Phase II | 59 (38–77) | ≥ 2 | 160 mg | 100% positive | RECIST 1.1 | 9.6 |
| Nanjo 2017 | Asian | 13 (62%) | Prospective pilot study | 67 (54–79) | ≥ 5 | 80 mg | 100% positive | CNS radiographic change | NA |
| Zheng 2020 | Asian | 45 (60%) | Retrospective | 54 (22–82) | 1(16%); ≥ 2(84%) | 80 mg | Positive:9; negative:36 | RANO-LM | NA |
| Saboundji 2018 | Europe | 20 (70%) | Retrospective | 61.2 (11.2) | ≥ 2 |
80 mg (17); 160 mg (2); 40 mg (1) |
65% positive | NA | 11.1 |
| Reungwetwattana 2018 | Global | 5 (NA) | Preplanned, exploratory analysis of FLAURA | NA | 1 | 80 mg | 0% | RECIST 1.1 | NA |
| Akazawa 2019 | Asian | 6 (50%) | Phase II | 61.5 (50–75) | ≥ 2 | 80 mg | 100% | Radiological response | NA |
| Ahn 2019 | Asian | 40 (NA) | Phase II | NA | ≥ 2 |
80 mg: 16; 160 mg: 24 |
100% | NA | 8.3 |
| Andrew 2020 | American | 11 | Retrospective | NA | ≥ 2 | 160 mg | NA | NA | NA |
BICR: blinded central in dependent review; CNS: central nervous system; LM: leptomeningeal metastases; mo.: months; NA: not available; RANO: Response Assessment in Neuro-Oncology; RECIST: Response Evaluation Criteria in Solid Tumors
Pooled LM ORR and DCR
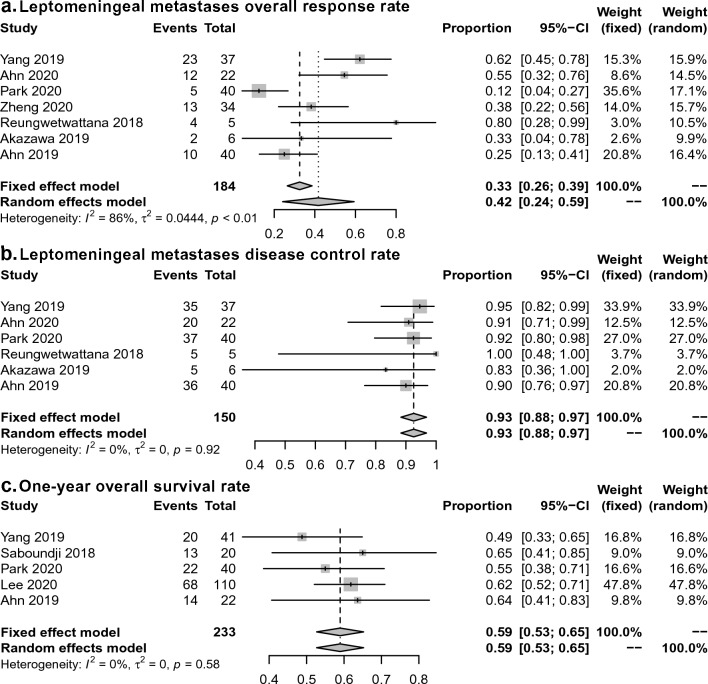
LM ORR was available in 7 studies [6–8, 14, 16, 18, 19], and LM DCR was available in 6 studies [6–8, 14, 18, 19]. The pooled LM ORR was 42% (95% CI 24% to 59%; I2 = 86% according to the random-effects model; Fig. 2a), and the pooled LM DCR was 93% (95% CI 88% to 97%; I2 = 0% according to the fixed-effects model; Fig. 2b).
Fig. 2.
Meta-analysis of ORR, DCR, and one-year OS of osimertinib for the treatment of LM from EGFR-mutant NSCLC. a ORR; b DCR; c one-year OS
The pooled LM ORR and DCR were similar when excluding studies published in abstract form, at 47% (95% CI 23% to 72%; I2 = 90%) for ORR and 95% (95% CI 91% to 100%; I2 = 0%) for DCR. When excluding 2 studies with a small number of patients (n < 10), the pooled LM ORR and DCR were found to be 38% (95% CI 19% to 57%; I2 = 88%) and 92% (95% CI 88% to 97%; I2 = 0%), respectively.
In the subgroup analysis, the pooled LM ORR was 33% (95% CI 5% to 60%; I2 = 93%) in three prospective phase I/II studies, which was lower than the 50% (95% CI 32% to 67%; I2 = 46%) of the four retrospective cohort studies. With respect to the dosage of osimertinib, the pooled LM ORR was 47% (95% CI 36% to 59%; I2 = 46%) for patients treated with 80 mg osimertinib and 37% (95% CI 0% to 86%; I2 = 96%) for patients treated with 160 mg osimertinib. The funnel plots were roughly symmetric for ORR and DCR (Additional file 1: Fig. S1). This indicated no publication bias and objectively reporting by the included studies.
1 year OS rate, median PFS, and OS
The pooled one-year OS was 59% (95% CI 53% to 65%; I2 = 0%) in 233 patients from five studies (Fig. 3) [8, 13, 14, 17, 19]. Median PFS and OS were not aggregated and are presented narratively (Table 2). The median PFS was reported in seven studies [7, 8, 14, 16–18, 20], ranging from 3.7 to 17.3 months. The median OS was available in 5 studies [7, 8, 13, 14, 17], ranging from 11.0 to 18.8 months. In one study, osimertinib-treated patients were associated with improved OS compared with osimertinib-untreated patients (17.0 versus 5.0 months, HR = 0.36, 95% CI 0.28 to 0.47) [13]. In another study, the median iPFS was significantly longer in T790M-positive than T790M-negative patients who received osimertinib (15.6 vs. 7.0 months, P = 0.04) [16].
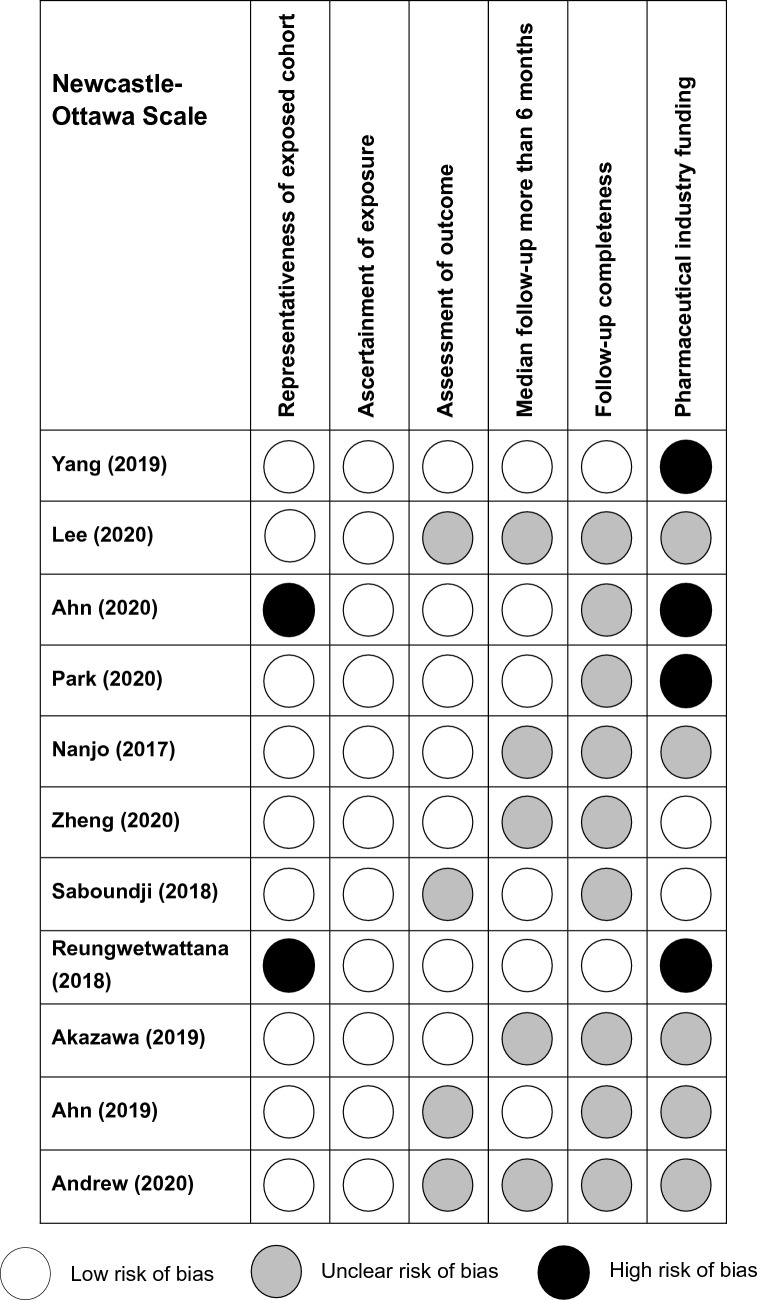
Fig. 3.
Modified Newcastle–Ottawa Scale of the included studies
Table 2.
Summary of extracted outcome data and pooled analysis
| Study | LM ORR (%) | LM DCR (%) | PFS (moths) | OS (moths) | 1-y OS rate (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | |||||
| Yang 2019 | 62 (23/37) | 45–78 | 95 (35/37) | 82–99 | 8.6 | 5.4–13.7 | 11.0 | 8.0–18.0 | 48.8 | |||
| Lee 2020 | NA | NA | NA | NA | NA | NA | 17.0 | 15.1–18.9 | 61.8 | |||
| Ahn 2020 | 55 (12/22) | 32–76 | 91 (20/22) | NA | 11.1 | 4.6-NR | 18.8 | 6.3-NR | 63.6 | |||
| Park 2020 | 12.5 (5/40) | NA | 92.5 (37/40) | NA | 8.0 | 7.2-NR | 13.3 | 9.1-NR | 55.0 | |||
| Nanjo 2017 | NA) | NA | NA | NA | NA | NA | NR | NA | NA | |||
| Zheng 2020 | 38.2 (13/34) | NA | NA | NA | 15.6a | 4.0–17.2a | NA | NA | NA | |||
| 7.0b | 4.0–10.0b | |||||||||||
| Saboundji 2018 | NA | NA | NA | NA | 17.3 | 2.7–20.8 | 18.0 | 4.4–23.8 | 65.0 | |||
| Reungwetwattana 2018 | 80 (4/5) | NA | 100 (5/5) | NA | NA | NA | NA | NA | NA | |||
| Akazawa 2019 | 33.3 (2/6) | NA | 83.3 (5/6) | NA | 3.7 | NA | NR | NA | NA | |||
| Ahn 2019 | 25 (10/40) | NA | 90 (36/40) | NA | NA | NA | 13.2 | NA | NA | |||
| Andrew 2020 | NA | NA | NA | NA | 5.8 | 1.7–9.0 | NA | NA | NA | |||
| Meta-analysis | 42 | 24–59 | 90 | 85–94 | NA | NA | NA | NA | 59 (53–65) | |||
CI: confidence interval; DCR: disease control rate; LM: leptomeningeal metastases; NA: not available; ORR: overall response rate; OS: overall survival; PFS: progression free survival
aT790M + patients
bT790M- patients
Adverse events
Of the 11 included studies, AEs were reported in 4 prospective studies including 116 patients [7, 8, 14, 15]. The highest incidence of AEs of all grades was rash (53%, 95% CI 25% to 81%), followed by diarrhea (45%, 95% CI 29% to 61%), paronychia (35%, 95% CI 25% to 44%), decreased appetite (35%, 95% CI 13% to 57%), and dry skin (27%, 95% CI 18% to 35%). The pooled data of pneumonia with any grade and grade ≥ 3 were 5% (95% CI 0 to 9%) and 4% (95% CI 0 to 7%), respectively. AEs that led to osimertinib discontinuation were reported in 9 of 41 cases (22%) in one study and 1 of 22 cases (5%) in another study. There were no osimertinib-related deaths reported in the 4 studies. The details of common toxicities are presented in Table 3.
Table 3.
Meta-analysis of common adverse events
| Adverse events | No. of studies | Any grade | ≥ Grade 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Patients | Rates % (95% CI) |
Heterogenicity (I2) (%) | Patients | Rates % (95% CI) |
Heterogenicity (I2) (%) | |||
| Rash | 3 | 45/94 | 48 (25–81) | 88 | 1/94 | 1 (0–3) | 0 | |
| Diarrhea | 3 | 49/103 | 48 (29–61) | 66 | 3/103 | 3 (0–5) | 11 | |
| Paronychia | 3 | 33/94 | 35 (25–44) | 0 | 0/94 | 0 (0–2) | 0 | |
| Decrease Appetite | 3 | 38/103 | 37 (13–57) | 84 | 3/103 | 3 (0–5) | 17 | |
| Dry skin | 3 | 28/103 | 27 (18–35) | 0 | 0/103 | 0 (0–2) | 0 | |
| Pneumonia | 3 | 6/94 | 6 (0–8) | 6 | 4/94 | 4 (0–7) | 0 | |
| Nausea | 2 | 22/81 | 27 (8–45) | 75 | 1/81 | 1 (0–4) | 0 | |
| Stomatitis | 2 | 11/81 | 14 (6–21) | 0 | 1/81 | 1 (0–4) | 0 | |
Study quality assessment
The quality of the included studies was evaluated by modified NOS (Fig. 3). Four studies reported pharmaceutical industry funding [6–8, 14]. The FLAURA [6] and AURA studies [7] were considered to have low representativeness (high risk) because both studies only enrolled LM patients with stable neurological conditions. All studies ascertained the usage of osimertinib. Median follow-up was more than 6 months in 6 studies [6–8, 14, 17, 19]. Only 2 studies [6, 8] reported the percentage of patients lost to follow-up. Overall, 2 studies [8, 14] were at high risk of bias in 1 criterion, and 2 studies were at high risk of bias in 2 criteria [6, 7].
Discussion
In this pooled analysis, we included 11 studies consisting of 353 patients, and the pooled results showed that the ORR, DCR, and one-year OS rate of osimertinib for the treatment of EGFR-mutant NSCLC with LM were 42% (95% CI 24% to 59%), 93% (95% CI 88% to 97%), and 59% (95% CI 53% to 65%), respectively. The ORR data were numerically lower than another pooled study of osimertinib for advanced NSCLC with metastasis to any site (ORR 79%, 95% CI 75–84%) [21], and lower than two meta-analyses of osimertinib for NSCLC with CNS metastases (mainly brain metastases, ORR was 64% and 70%, respectively) [11, 22]. Nonetheless, considering the refractory characteristics of LM, an ORR of 42% was acceptable or even satisfactory when compared with cytotoxic agents, radiotherapy or first- and second-generation EGFR TKIs [23–25].
The incidence of LM is considerably higher in EGFR-mutated NSCLC patients than EGFR wild-type ones (9.4% vs. 1.7%, P < 0.001) [26]. The third-generation TKI osimertinib was a favorable treatment option for patients with LM after resistance to first- or second-generation EGFR TKIs for two reasons. First of all, osimertinib is effective against the EGFR T790M mutation, which accounts for approximately 50% of the resistance from first- or second-generation EGFR TKIs [27]. Secondly, osimertinib is associated with a superior blood–brain barrier penetration capacity than other EGFR TKIs [6, 28]. Notably, disease progression in many patients was confined to LM with stable extracranial disease attributing to the poor penetration ability of gefitinib or erlotinib to the CNS system; whereas osimertinib is particularly highlighted for these patients. For treatment-naïve NSCLC LM patients, osimertinib is also recommended as an effective first-line treatment. In the FLAURA study, five patients in the osimertinib arm with LM, four of whom had a complete radiographic response and one had radiographic non-CR, non-PD response [6].
Whether increased doses (e.g., 160 mg) could enhance the efficacy of osimertinib for LM is indeed a question deserves further discussions. In a phase II study including forty NSCLC patients with LM treated with 160 mg osimertinib, a 92.5% disease control rate and 12.5% complete response rate according to RECIST 1.1, a median PFS of 8.0 months, and median OS of 13.3 months were reported [14]. These data were encouraging and repeated by the BLOOM phase I study (ORR 62%, DCR 78%, PFS 8.6 months, and OS 11.0 months). The clinical use of osimertinib 80 mg once daily in patients with LM is effective and widely recommended. A retrospective study from Zheng et al. reported an LM ORR of 38.2% (13/34), and the median intracranial PFS was 15.6 months for EGFR T790M mutation patients who received 80 mg Osimertinib [16]. The AURA phase I study compared a wide range of osimertinib doses (20–240 mg) but did not reveal significant difference in efficacy, e.g., 80 mg vs. 160 mg [29]. In our subgroup analysis, the pooled ORR was 47% for patients treated with 80 mg osimertinib, compared with 37% for patients treated with 160 mg osimertinib. To be noticed, studies of osimertinib 80 mg were either retrospective cohorts or studies with a small number of subjects (n = 13), or retrospective analyses of FLAURA and AURA series, which included only LM patients with stable neurological conditions. Further prospective studies for dosage specified efficacy and safety profiles of osimertinib are highly warranted.
The tolerance and safety are critical issues for NSCLC patients with LM since many patients have neurological impairment symptoms and progressively worsening. In our pooled analysis, rash (53%) and diarrhea (45%) of any grades were the most frequently reported AEs induced by osimertinib. This is in accordance with another meta-analysis on osimertinib for the treatment of advanced NSCLC with metastasis to any site (42% rash, 44% diarrhea). In the present study, pneumonia (5%) was the most frequently reported grade 3 or higher AE. No new safety concerns were identified in this study. No osimertinib-related death event was reported in the 4 included studies. The BLOOM study reported that all patients had at least 1 AE, 27 patients (66%) had an AE grade of 3 or higher, and 10 (24%) had AEs possibly causally related to osimertinib, as judged by the investigator. EGFR TKI-induced intestinal lung disease and pneumonia were frequent fatal AEs in a study based on 53 cohorts of 9569 participants [30].
Several limitations must be appreciated in this study. Among the first was the relatively small patient size in most included studies. Ten out of eleven included studies enrolled less than 50 patients. Second, all included studies were retrospective (n = 6) or phase I/II prospective (n = 5) studies, and no phase III randomized control trials were currently available. Third, the criteria of LM response assessment differed with different reports. Among the 11 studies, 3 studies defined treatment response by RANO-LM, 4 studies by RECIST or radiological response, and 4 studies did not report definitions of LM response. There is still a lack of suitable response assessment criteria for LM. RECIST is widely used for solid tumors; however, it is not appropriate for LM assessment since the presence of LM in imaging is usually linear but not bulk. RANO-LM was proposed to specifically evaluate the response assessment of LM in 2016 by incorporating several factors: standardized neurological examination, cerebral spinal fluid (CSF) cytology or flow cytometry, and radiographic evaluation [31]. RANO-LM is superior to RECIST for the response assessment of LM however still needs to be optimized [32]. Fourth, a meta-analysis of median PFS and OS was not conducted due to insufficient data available. Although we have previously shown that EGFR-mutated LM patients are likely to gain limited benefits from WBRT [2], the potential role of osimertinib in combinations with other treatment modalities such as WBRT or intrathecal chemotherapy for specific EGFR-mutated patients was yet to be elucidated, i.e., co-existed with brain metastasis [33] or recurrent LM patients.
In conclusion, our systematic review and meta-analysis found that osimertinib showed meaningful CNS efficacy in terms of radiologic response and a manageable safety profile in patients with LM from EGFR-mutant NSCLC.
Supplementary Information
Additional file 1: Figure S1. Funnel plot of potential publication bias of ORR, DCR and one-year survival.
Acknowledgements
None.
Abbreviations
- AEs
Adverse events
- BBB
Blood–brain barrier
- CI
Confidence interval
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- EGFR
Epidermal growth factor receptor
- TKIs
Tyrosine kinase inhibitors
- LM
Leptomeningeal metastases
- NOS
Newcastle–Ottawa Scale
- ORR
Objective response rate
- OS
Overall survival
- RECIST
Response Evaluation Criteria in Solid Tumors
- NSCLC
Non-small cell lung cancer
- PFS
Progression-free survival
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- RANO
Response Assessment in Neuro-Oncology
Author contributions
Conception and design: LW, JZ, CS, ZX, CZ. Administrative support: ZX, LC, MC, LC. Provision of study materials or patients: none. Collection and assembly of data: all authors. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province [No. 2019A1515011943], China Postdoctoral Science Foundation [No. 2019M662974] and Science and Technology Program of Guangzhou [No. 202002030445, No. 202002030086], and Medical Scientific Research Foundation of Guangdong Province [No. A2020505, No. A2020499, No. B2021203, No. B2021139]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Wen, Junjie Zhen and Changguo Shan contributed equally to this work.
Contributor Information
Zhanhong Xie, Email: 13556184135@163.com.
Cheng Zhou, Email: czhou.rob@gmail.com.
References
- 1.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19(1):e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 2.Zhen J, et al. Whole brain radiotherapy (WBRT) for leptomeningeal metastasis from NSCLC in the era of targeted therapy: a retrospective study. Radiat Oncol. 2020;15(1):185. doi: 10.1186/s13014-020-01627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao BC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. Journal of Thorac Oncol. 2015;10(12):1754–1761. doi: 10.1097/JTO.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 4.Ballard P, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam SS, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 6.Reungwetwattana T, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018 doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 7.Ahn MJ, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15(4):637–648. doi: 10.1016/j.jtho.2019.12.113. [DOI] [PubMed] [Google Scholar]
- 8.Yang JCH, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38(6):538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Erickson AW, Brastianos PK, Das S. Assessment of effectiveness and safety of osimertinib for patients with intracranial metastatic disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(3):e201617. doi: 10.1001/jamanetworkopen.2020.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, et al. osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol. 2020;15(11):1758–1766. doi: 10.1016/j.jtho.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Park S, et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol. 2020;31(10):1397–1404. doi: 10.1016/j.annonc.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Nanjo S, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118(1):32–37. doi: 10.1038/bjc.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng MM, et al. Genotyping of cerebrospinal fluid associated with osimertinib response and resistance for leptomeningeal metastases in EGFR-mutated NSCLC. J Thorac Oncol. 2021;16(2):250–258. doi: 10.1016/j.jtho.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Saboundji K, et al. Efficacy of osimertinib in EGFR-mutated non-small cell lung cancer with leptomeningeal metastases pretreated with EGFR-tyrosine kinase inhibitors. Target Oncol. 2018;13(4):501–507. doi: 10.1007/s11523-018-0581-2. [DOI] [PubMed] [Google Scholar]
- 18.Akazawa Y, et al. EP1.01–13 a phase 2 trial assessing osimertinib activity against leptomeningeal carcinomatosis in EGFR-mutant lung cancer. J Thorac Oncol. 2019;14(10):S918. doi: 10.1016/j.jtho.2019.08.1992. [DOI] [Google Scholar]
- 19.Ahn M, et al. MA21.10 phase II study of 160mg of osimertinib in EGFR T790M positive NSCLC with brain or leptomeningeal metastases who progressed on prior EGFR TKI. J Thorac Oncol. 2019;14(10):S338. doi: 10.1016/j.jtho.2019.08.680. [DOI] [Google Scholar]
- 20.Piper-Vallillo A, et al. High-dose osimertinib for CNS progression in EGFR+ non-small cell lung cancer (NSCLC): a multi-institutional experience. J Clin Oncol. 2020;38:9586. doi: 10.1200/JCO.2020.38.15_suppl.9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi L, et al. Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: a meta-analysis. Int J Cancer. 2019;145(1):284–294. doi: 10.1002/ijc.32097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, et al. Osimertinib for EGFR-mutant lung cancer with central nervous system metastases: a meta-analysis and systematic review. Ann Palliat Med. 2020;9(5):3038–3047. doi: 10.21037/apm-20-605. [DOI] [PubMed] [Google Scholar]
- 23.Yan W, et al. Whole brain radiation therapy does not improve the overall survival of EGFR-mutant NSCLC patients with leptomeningeal metastasis. Radiat Oncol. 2019;14(1):168. doi: 10.1186/s13014-019-1376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umemura S, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama lung cancer study group. Lung Cancer. 2012;77(1):134–139. doi: 10.1016/j.lungcan.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Kuiper JL, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer. 2015;89(3):255–261. doi: 10.1016/j.lungcan.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Li YS, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11(11):1962–1969. doi: 10.1016/j.jtho.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colclough N, et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27(1):189–201. doi: 10.1158/1078-0432.CCR-19-1871. [DOI] [PubMed] [Google Scholar]
- 29.Jänne PA, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 30.Xie X, et al. Fatal toxic effects related to EGFR tyrosine kinase inhibitors based on 53 cohorts with 9569 participants. J Thorac Dis. 2020;12(8):4057–4069. doi: 10.21037/jtd-19-4000A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamberlain M, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–492. doi: 10.1093/neuonc/now183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Rhun E, et al. The RANO leptomeningeal metastasis group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol. 2019;21(5):648–658. doi: 10.1093/neuonc/noz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou C, et al. Individualized nomogram for predicting survival in patients with brain metastases after stereotactic radiosurgery utilizing driver gene mutations and volumetric surrogates. Front Oncol. 2021 doi: 10.3389/fonc.2021.659538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Funnel plot of potential publication bias of ORR, DCR and one-year survival.
Data Availability Statement
Not applicable.