Abstract
Background
Conflicting findings exist regarding the influence of sex on the development, treatment, course, and outcome of status epilepticus (SE). Our study aimed to investigate sex-related disparities in adult SE patients, focusing on treatment, disease course, and outcome at two Swiss academic medical centers.
Methods
In this retrospective study, patients treated for SE at two Swiss academic care centers from Basel and Geneva from 2015 to 2021 were included. Primary outcomes were return to premorbid neurologic function, death during hospital stay and at 30 days. Secondary outcomes included characteristics of treatment and disease course. Associations with primary and secondary outcomes were assessed using multivariable logistic regression. Analysis using propensity score matching was performed to account for the imbalances regarding age between men and women.
Results
Among 762 SE patients, 45.9% were women. No sex-related differences were found between men and women, except for older age and lower frequency of intracranial hemorrhages in women. Compared to men, women had a higher median age (70 vs. 66, p = 0.003), had focal nonconvulsive SE without coma more (34.9% vs. 25.5%; p = 0.005) and SE with motor symptoms less often (52.3% vs. 63.6%, p = 0.002). With longer SE duration (1 day vs. 0.5 days, p = 0.011) and a similar proportion of refractory SE compared to men (36.9% vs. 36.4%, p = 0.898), women were anesthetized and mechanically ventilated less often (30.6% vs. 42%, p = 0.001). Age was associated with all primary outcomes in the unmatched multivariable analyses, but not female sex. In contrast, propensity score-matched multivariable analyses revealed decreased odds for return to premorbid neurologic function for women independent of potential confounders. At hospital discharge, women were sent home less (29.7% vs. 43.7%, p < 0.001) and to nursing homes more often (17.1% vs. 10.0%, p = 0.004).
Conclusions
This study identified sex-related disparities in the clinical features, treatment modalities, and outcome of adult patients with SE with women being at a disadvantage, implying that sex-based factors must be considered when formulating strategies for managing SE and forecasting outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04592-6.
Keywords: Status epilepticus, Sex, Neurocritical care
Background
Status epilepticus (SE) is a critical and life-threatening neurological condition with ongoing epileptic seizures [1] that comes along with a high morbidity and mortality [2]. This has triggered several studies to explore the impact of various demographic, clinical, epileptological, and treatment characteristics on the course and outcome of critically ill patients with SE. Several factors have been identified that may influence the development, management, course, and outcome of SE, including age, etiology, underlying comorbidities, type of seizures, duration of SE, and treatment modalities. In contrast, studies regarding the role of sex on the emergence of SE, its treatment, course, and outcome are scarce. Although some studies have suggested that female sex may be a risk factor for the development of SE in patients with epilepsy [3], and that women may receive less aggressive care than men regardless of illness severity [4], other studies have reported conflicting findings. For example, early studies suggested that the incidence of SE was lower in women than men, and other studies found no significant sex-related differences regarding the incidence of SE [5–8]. Given these inconsistences and further conflicting data from population-based studies and systematic reviews regarding the influence of sex on course and outcome in patients suffering from SE, the precise role of sex in this regard remains unclear [7, 9–12]. Hence, further research is needed to elucidate the role of sex in the management of SE. In particular, there is a need for more comprehensive studies that examine sex-associated differences in SE patients, including their demographics, clinical characteristics, disease course, and outcome, as well as differences especially when it comes to the development and implementation of treatment strategies. This retrospective observational cohort study aimed to investigate sex-associated differences in adult patients with SE, including treatment, course of disease, complications, and outcomes.
Methods
The retrospective study was performed at the University Hospitals of Basel and Geneva, two Swiss tertiary academic medical care centers. The STROBE-guidelines were followed to enhance the quality of reporting [13]. In accordance with the 1964 Declaration of Helsinki and its subsequent revisions, the local ethics committees (EKNZ 2019–00693 for Basel and CCER 2019–00836 for Geneva) granted approval for the study. The requirement for obtaining patient consent was waived.
Data collection
The clinical data of both medical care centers were collected following the registered STEP-UP study (ClinicalTrials.gov ID: NCT04204863) previously initiated at the University Hospital of Basel and collecting clinical and electrophysiologic data of adult (≥ 18 years of age) patients with SE. From January 1st, 2015 to December 31st, 2021, clinical, laboratory and epileptologic data of all consecutive patients were collected and data were extracted from digital medical records and managed with the password encrypted online browser-based, metadata-driven database organizer REDCAP (Research Electronic Data Capture) [14]. Patients with SE following hypoxic-ischemic encephalopathy (HIE) were excluded from the study as HIE-induced SE has been previously established as a distinct and independent clinical entity known to be associated with elevated mortality rates.
The following data were collected: age, sex, presumed etiology of SE (categorized as potential non-fatal and fatal etiologies as defined elsewhere [15]), and the Glasgow Coma Score (GCS) at SE onset. The types of SE were determined by evaluating the digital EEG reports and/or emergency medical service reports. SE was categorized into the following predefined types as recommended by the current guidelines of the International League Against Epilepsy (ILAE) [1]: focal nonconvulsive without coma (with or without altered consciousness and absences), with motor symptoms (myoclonic and convulsive), and nonconvulsive with coma. Illness severity was quantified by the Status Epilepticus Severity Score (STESS; range 0–6) [16, 17], the Charlson Comorbidity Index (range 0–37) [18], and the Simplified Acute Physiology Score II (SAPS II; range 0–163) [19]. SE duration was defined as the time-period between the diagnosis of SE and the clinical and/or electroencephalographic evidence of seizure termination as previously described [20]. For patients with refractory SE who were treated with anesthesia to achieve an EEG burst-suppression pattern, the duration of SE was determined as the period from seizure onset until the establishment of burst-suppression, if the patient showed no relapse into SE after weaning of anesthetics.
In both centers, patients with SE were monitored with continuous EEGs or intermittent spot EEGs. Continuous EEGs were performed daily for at least 12 h per day and spot EEGs for at least 30 min every 12 h. Thereby, the calculated SE duration represents an approximation with a maximum inaccuracy of 12 h.
The following treatment characteristics were assessed: admission via emergency medical services and time from alarm to hospital admission, duration of mechanical ventilation, the number of administered non-sedating antiseizure drugs, continuously administered anesthetics, and vasopressors administered during SE, as well as duration of in-hospital treatment and ICU stay in days. Finally, complications during SE were noted including infections, arterial hypotension requiring the use of continuously administered vasopressors, and organ failure. In addition, care withdrawal and destination at hospital discharge were extracted from the medical records. To ensure consistency across the datasets all clinical, laboratory, and epileptologic data from patients with non-refractory and refractory SE were collected using the same methodologies.
Antiseizure treatment
During the study period, the antiseizure treatment protocol for SE involved a stepwise approach following the guidelines of the American Epilepsy Society and the Neurocritical Care Society and were guided by the same neurologists and neurointensivists [21, 22]. The first-line treatment consisted of an intravenous benzodiazepine bolus, which was repeated if seizures persisted. For SE not responding to intravenously administered benzodiazepine boluses, second-line treatment was started, which included levetiracetam, lacosamide, valproic acid, or phenytoin. For patients with SE refractory to first- and second-line antiseizure treatment, continuously administered anesthetics were started as the third-line treatment, including propofol and midazolam. In addition, non-sedating antiseizure drugs were added, such as topiramate, zonisamide, oxcarbazepine, pregabalin, sultiame, or perampanel. As part of routine practice, anesthetics were titrated upon the discretion of the treating physicians with the aim of achieving a persistent electrographic proof of either seizure cessation or a burst-suppression pattern for at least 24 h [23]. If SE reoccurred after weaning of third-line anesthetic treatment, barbiturates were started and titrated to induce a complete burst-suppression or an isoelectric EEG.
Outcomes
Primary outcomes were return to premorbid neurologic function, death in-hospital and on day 30 after SE onset. Return to premorbid neurologic function was defined as full recovery of all patient’s neurological abilities or restoration to the neurologic functioning present prior to SE based on the physicians’ notes at hospital discharge (as routinely documented in SE patients).
Secondary outcomes included treatment characteristics and course of disease.
Statistics
Patients were categorized according to sex. Chi-square and Fisher exact test, where appropriate, were used for univariable comparisons of proportions. Continuous variables were compared using the Mann–Whitney U test or the Kruskal–Wallis test. Discrete variables were expressed as counts and percentages, and continuous variables were expressed as medians and interquartile ranges (IQR). The level of significance for multiple univariable analyses was adjusted using the Bonferroni correction for multiple comparisons. Uni- and multivariable logistic regression models were performed to identify associations between sex and all primary outcomes (i.e., return to premorbid neurologic function at discharge, death during hospital stay and at 30 days after SE onset). To correct for potential confounding, all baseline characteristics differing between sex and potentially related to outcome, were included into the multivariable logistic regression models. In addition, well-established outcome determinants (based on the current literature [24]), such as SE severity as quantified by the STESS, were included into the multivariable models independent of their distribution between sex in our cohort. To cheque for linearity between our continuous variable “age” and our primary outcomes, we performed the Box-Tidwell test by adding log-transformed interaction terms between age and its corresponding natural log into the model. Insignificance of the p values of the interaction terms implies a linear relation to the logit of the outcome variables confirming that the assumption is satisfied. To further explore associations between sex and differences in SE treatment (defined as secondary outcomes), both uni- and multivariable logistic regression models were performed, the latter including all baseline characteristics differing between sex in univariable comparisons.
To account for the imbalances regarding age between men and women, an additional analysis was performed using propensity score matching. The probability of being female (i.e., propensity score) was calculated with a logistic regression model based on age. Female and male patients were matched on propensity score with use of nearest-neighbor matching. Patients without an eligible match were excluded from additional analyses to reduce the risk of bias from non-exchangeable subjects. The Mann–Whitney U test was used to check for propensity score and age-balances between females and males in the matched cohort. The same uni- and multivariable logistic regression models as performed for the unmatched cohort were repeated to calculate associations with the primary outcomes in the propensity matched cohort. Propensity scores and respective matching was performed using "PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing" (http://ideas.repec.org/c/boc/bocode/s432001.html.), version 4.0.12 30jan2016 by E. Leuven, B. Sianesi.
For multivariable logistic regression models, the Hosmer–Lemeshow chi-square goodness-of-fit tests were performed. These tests provide summary measures of calibration based upon a comparison of observed and estimated outcomes [25]. All multivariable models were adjusted for the potential influence of the participating centers. For all multivariable models, a two-sided p values ≤ 0.05 were considered significant.
Statistical analysis was performed with STATA®16.1 (Stata Corp., College Station, TX, USA).
Results
Univariable comparisons of baseline characteristics
Among 762 SE patients (371 treated in Geneva, 438 treated in Basel), 45.9% were women (flow chart; Fig. 1). Univariable comparisons of demographics and clinical characteristics between men and women are presented in Table 1. Compared to men, women had a higher median age, a higher proportion of focal nonconvulsive SE (NCSE) without coma, and a lower proportion of SE with motor symptoms. EEG data were not available in 9.8% of patients, either because EEG was not performed due to rapid cessation of SE and full recovery (7.7%) or due to recording errors (2.1%). While most presumed underlying etiologies of SE were equally distributed, acute intracerebral hemorrhages was diagnosed as the presumed underlying etiologies of SE less often in women than in men.
Fig. 1.
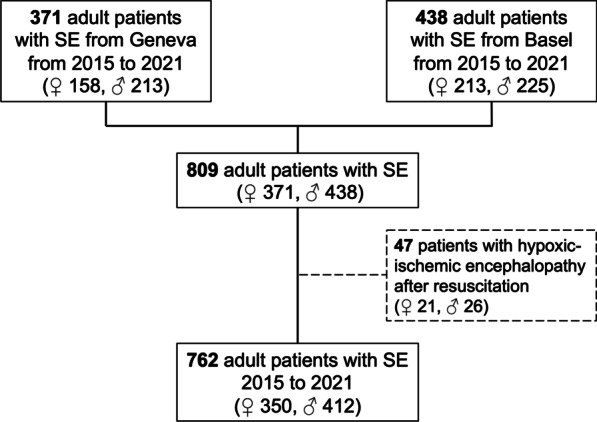
Flow chart. SE = status epilepticus; ♀ = female patients; ♂ = male patients
Table 1.
Univariable comparisons of baseline characteristics between men and women with status epilepticus (n = 762)
| Baseline characteristics | Women (n = 350) |
Men (n = 412) |
|||
|---|---|---|---|---|---|
| Demographics and clinical characteristics | n/median | %/IQR | n/median | %/IQR | p value |
| Age (years; median, IQR) | 70 | 56–81 | 66 | 53–76 | 0.003 |
| Out-of-hospital SE (i.e., admitted for SE; n, %) | 292 | 83.4 | 355 | 86.2 | 0.293 |
| Admitted via other hospital (n, %) | 63 | 18 | 60 | 14.6 | 0.199 |
| SE etiology (n, %) | |||||
| Presumed fatal etiology (not mutually exclusive) | 92 | 26.3 | 107 | 26.0 | 0.921 |
| Fast growing brain tumors | 50 | 14.3 | 60 | 14.6 | 0.914 |
| Acute intracranial hemorrhage | 31 | 8.9 | 63 | 15.3 | 0.007 |
| Infectious (meningo-)encephalitis | 15 | 4.3 | 16 | 3.9 | 0.855 |
| Acute ischemic stroke | 11 | 3.1 | 10 | 2.4 | 0.658 |
| Acute severe traumatic brain injury | 7 | 2.0 | 23 | 5.6 | 0.014 |
| Acute autoimmune encephalitis* | 13 | 3.7 | 9 | 2.2 | 0.278 |
| Presumed non-fatal etiology** | 258 | 73.7 | 305 | 74.0 | 0.921 |
| Known epilepsy | 115 | 32.9 | 150 | 36.4 | 0.305 |
| Unknown etiology | 37 | 10.6 | 29 | 7.0 | 0.084 |
| Consciousness at SE onset | |||||
| GCS at SE onset (median, IQR) | 9 | 5–13 | 8 | 4–12 | 0.073 |
| Coma at SE onset (n, %) | 127 | 36.3 | 174 | 42.2 | 0.094 |
| SE type (n, %) | |||||
| Focal NCSE without coma | 122 | 34.9 | 105 | 25.5 | 0.005 |
| With altered consciousness | 93 | 26.6 | 75 | 18.2 | 0.005 |
| Without altered consciousness | 29 | 8.3 | 30 | 7.3 | 0.005 |
| SE with motor symptoms (convulsive or myoclonic) | 183 | 52.3 | 262 | 63.6 | 0.002 |
| Convulsive SE | 121 | 34.6 | 188 | 45.6 | 0.002 |
| Myoclonic SE | 62 | 17.7 | 74 | 18.0 | 0.929 |
| NCSE with coma | 45 | 12.9 | 45 | 10.9 | 0.410 |
| NCSE with coma (non-subtle) | 32 | 9.1 | 26 | 6.3 | 0.117 |
| Subtle SE | 13 | 3.7 | 19 | 4.6 | 0.538 |
| Illness severity (median, IQR) | |||||
| STESS | 3 | 2–4 | 3 | 2–4 | 0.834 |
| Charlson Comorbidity Index | 4 | 2–6 | 4 | 2–6 | 0.250 |
| SAPS II*** | 45 | 35–56 | 45 | 34–57 | 0.722 |
IQR = interquartile range; GCS = Glasgow Coma Score (range 3–15); SE = status epilepticus; NCSE = nonconvulsive status epilepticus; STESS = Status Epilepticus Severity Score (range 0–6)[16, 17]; Charlson Comorbidity Index (range 0–37)[18]; SAPS II = Simplified Acute Physiology Score II (range 0–163)[19]
*Acute autoimmune encephalitis was defined as the presence of antigen-specific antibodies in the serum and/or cerebrospinal fluid, or cases exhibiting a clinically recognized autoimmune syndrome with supportive histopathologic evidence determined during the diagnostic workup for SE
**Non-fatal etiology of SE encompassed (not mutually exclusive) known epilepsy (n = 265), old remote ischemic or hemorrhagic strokes (n = 127), old remote or mild to moderate traumatic brain injury (n = 18), slowly growing brain tumors (n = 31), intoxications (n = 18), drug withdrawal (n = 75), drug side effects (n = 6), leukoencephalopathy (n = 23), brain surgery (n = 9), acute but small strokes (n = 3),
***available only in patients treated on the ICUs
Bold font indicates statistical significance after Bonferroni correction for multiple comparisons (set at a level of p ≤ 0.01)
Univariable comparisons of outcomes
Table 2 presents the results of the univariable comparisons of primary (upper half) and secondary (lower half) outcomes between female and male patients.
Table 2.
Univariable comparisons of primary and secondary and outcomes (n = 762)
| Outcomes | Women (n = 350) |
Men (n = 412) |
|||
|---|---|---|---|---|---|
| Primary outcomes (n, %) | n | % | n | % | p value |
| Persistent seizure termination | 327 | 93.4 | 395 | 95.9 | 0.131 |
| Return to premorbid neurologic function at discharge | 150 | 42.9 | 202 | 49.0 | 0.089 |
| In-hospital death | 29 | 8.3 | 26 | 6.3 | 0.294 |
| Death at 30 days follow-up (loss to follow-up 107 [14%]) | 42 | 12.0 | 36 | 8.7 | 0.085 |
| Outcomes | Women (n = 350) |
Men (n = 412) |
|||
|---|---|---|---|---|---|
| Secondary outcomes | n/median | %/IQR | n/median | %/IQR | p value |
| Treatment characteristics during SE | |||||
| Admitted via emergency medical services with SE (n, % of patients with out-of-hospital SE) | 221 | 75.7 | 274 | 77.2 | 0.361 |
| Seizures suspected by emergency medical services (n, % of cases admitted via emergency teams) | 76 | 34.4 | 99 | 36.1 | 0.706 |
| SE suspected by emergency medical services (n, % of cases admitted via emergency teams) | 82 | 37.1 | 104 | 38.0 | 0.853 |
|
Time from alarm to hospital admission via emergency medical services (minutes; median, IQR) |
52 | 39–67 | 50 | 37–71 | 0.656 |
| Duration of in-hospital treatment (days; median, IQR) | 11 | 6–18 | 10 | 5–18 | 0.124 |
| ICU treatment (n, %) | 185 | 52.9 | 237 | 57.5 | 0.197 |
| Duration of ICU treatment (days; median, IQR) | 3 | 2–8 | 3 | 2–6 | 0.780 |
| Mechanical ventilation (n, %) | 107 | 30.6 | 173 | 42.0 | 0.001 |
| Duration of mechanical ventilation (days; median, IQR) | 3 | 2–8 | 2 | 0.5–4 | 0.094 |
| Number of non-anesthetic antiseizure drugs (median, IQR) | 3 | 2–3 | 2 | 2–3 | 0.572 |
| Patients with benzodiazepines as first-line antiseizure drug (n, %) | 265 | 77.3 | 317 | 77.5 | 0.936 |
| Patients with second-line antiseizure drugs (n, %) | 287 | 83.7 | 327 | 80.0 | 0.189 |
| Patients with continuous anesthetic drugs (n, %) | 89 | 25.4 | 157 | 38.1 | < 0.001 |
| Duration of continuous anesthetics (hours; median, IQR) | 30.0 | 12–112 | 18.0 | 7–48 | 0.036 |
| Overall SE duration (days) | 1 | 0.5–2 | 0.5 | 0.5–1 | 0.011 |
| Treatment refractory SE (n, %) | 129 | 36.9 | 150 | 36.4 | 0.898 |
| Treatment characteristics during RSE | |||||
| Patients with continuous anesthetic drugs (n, %) | 66 | 51.2 | 108 | 72.0 | ≤ 0.001 |
| Duration of continuous anesthetics (hours; median, IQR) | 35.8 | 12–114.5 | 21.6 | 10–66.1 | 0.288 |
| Mechanical ventilation (n, %) | 64 | 49.6 | 105 | 97.2 | 0.001 |
| Duration of mechanical ventilation (days; median, IQR) | 3 | 2–10 | 2 | 2–9 | 0.433 |
| Complications during SE (n, %) | |||||
| Infections/sepsis | 50 | 14.3 | 70 | 17.2 | 0.281 |
| Arterial hypotension requiring vasopressors | 55 | 15.7 | 69 | 16.8 | 0.700 |
| Multiorgan failure | 24 | 6.9 | 37 | 9.0 | 0.282 |
| Care withdrawal (n, %) | |||||
| Care withdrawal | 47 | 13.4 | 45 | 10.9 | 0.290 |
| Withdrawal due to presumed poor prognosis | 38 | 10.9 | 30 | 7.3 | 0.308 |
| Withdrawal following patient directives | 8 | 2.3 | 15 | 3.6 | 0.297 |
| Transfer at discharge (n, %) | |||||
| Home | 104 | 29.7 | 180 | 43.7 | < 0.001 |
| Rehab | 90 | 25.7 | 102 | 24.8 | 0.802 |
| Other hospital | 67 | 19.1 | 63 | 15.3 | 0.176 |
| Nursing home or hospice | 60 | 17.1 | 41 | 10.0 | 0.004 |
IQR = interquartile range; SE = status epilepticus; ICU = intensive care unit; RSE = treatment refractory status epilepticus
Bold font indicates statistical significance after Bonferroni correction for multiple comparisons (set at a level of p ≤ 0.01)
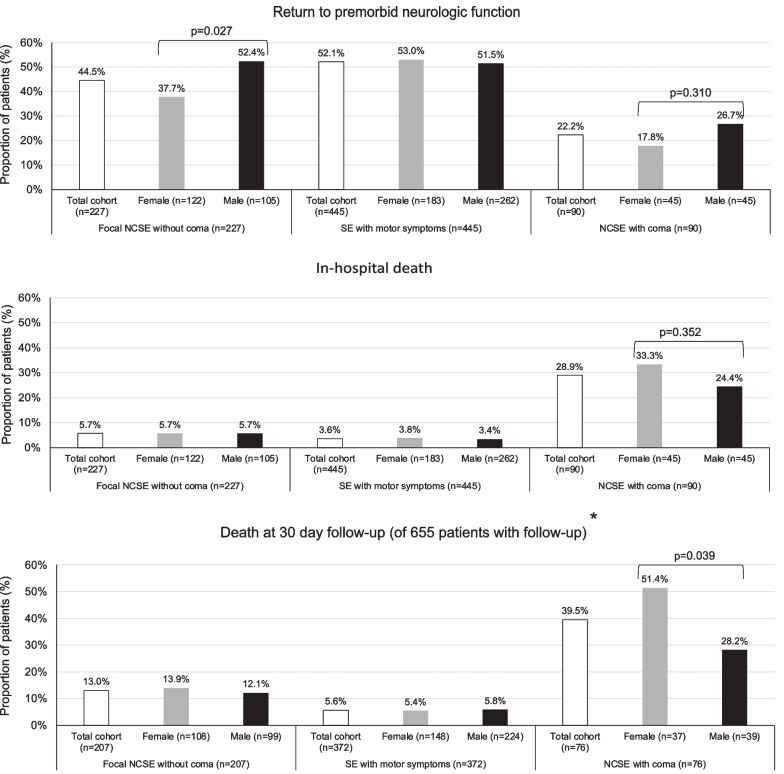
Univariable analyses revealed no significant differences of primary outcomes after Bonferroni correction for multiple comparisons (with a significant p value set at ≤ 0.01). Analyses regarding different primary outcomes of women and men in relation to specific types of SE are presented in Fig. 2. At first glance, these analyses revealed that women with focal NCSE without coma had a lower proportion of return to premorbid neurologic function as compared to men, and women with NCSE with coma had a higher proportion of death at 30 days. However, when correcting for multiple comparisons, these differences lost significance.
Fig. 2.
Sex-associated differences of primary outcomes among different types of status epilepticus (n = 762). SE = status epilepticus; NCSE nonconvulsive status epilepticus. *Loss to follow-up in 14% of patients
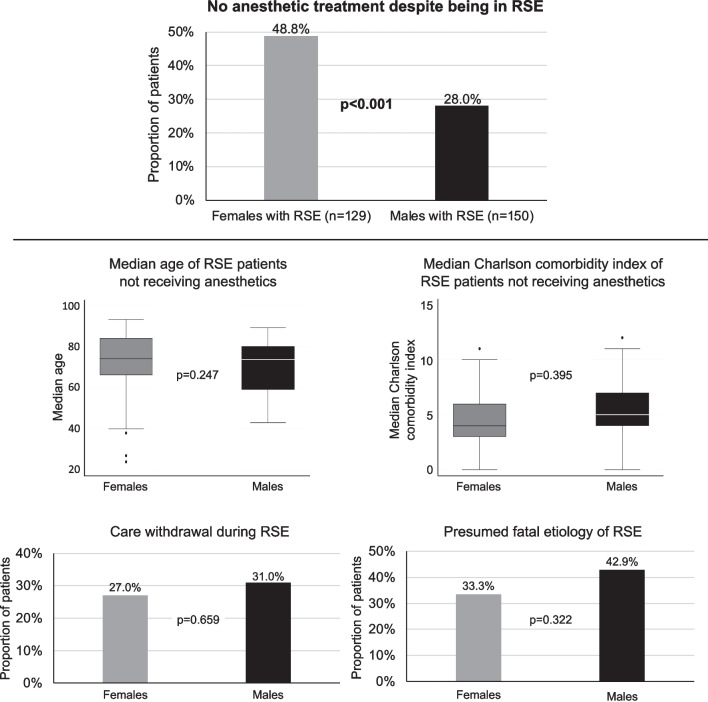
Comparisons regarding secondary outcomes revealed that women had a longer duration of SE but similar proportions of treatment refractory SE. As compared to men, women were anesthetized and mechanically ventilated less often (Table 2). Figure 3 shows subgroup analyses of patients with treatment refractory SE. These analyses revealed that women received guideline-conforming therapeutic escalation with anesthetics less often compared to men, even though there were no significant differences between men and women in terms of median age, Charlson comorbidity index, care withdrawal, and presumed fatal etiologies of the SE among patients not receiving anesthetics. Further univariable comparisons of treatment characteristics between men and women with different types of SE are presented in the Additional file 1: Table S1.
Fig. 3.
Clinical characteristics of patients with treatment refractory status epilepticus and different use of anesthetics (n = 279). RSE = treatment refractory status epilepticus; bold font indicates statistical significance after Bonferroni correction for multiple comparisons (set at a level of ≤ 0.01)
The proportion of patients with care withdrawal was comparable between men and women (Table 2). Nevertheless, women were transferred to nursing homes or hospices more frequently. Of women discharged to nursing homes or hospices 42 (70%) were > 65 years of age, 25 (41.7%) were > 80 years of age, and only 4 (6.7%) had potentially fatal etiologies and 16 (26.7%) were in treatment refractory SE that could not be terminated in 6 (10%) women.
Uni- and multivariable logistic regression analyses regarding primary outcomes
Table 3 (upper half) shows the results of uni- and multivariable logistic regression analyses investigating the potential association between sex and outcomes while adjusting for differences between men and women as identified by the univariable comparisons and for well-established outcome predicting characteristics, such as SE severity as quantified by the STESS. These analyses found increasing age to consistently be the strongest predictor of poor outcomes, including no return to premorbid function, death during hospital stay, and death at 30 days post-SE onset independently of potential confounding factors. However, the analyses did not find an independent association between female sex and outcome. The Box-Tidwell test revealed insignificant p values for the interaction term of our continuous variable “age” with its corresponding natural log indicating its linear relation to all primary outcomes. The Hosmer–Lemeshow goodness-of-fit tests were all insignificant indicating adequate model fits.
Table 3.
Univariable and multivariable logistic regression analyses regarding primary and secondary outcomes (n = 762)
| Primary outcomes | ||||||
|---|---|---|---|---|---|---|
| Univariable model | Multivariable model* corrected for the influence of both centers |
|||||
| Potential predictors of outcome/confounders (as identified in Table 1 and as established in the literature) |
OR | 95% CI | p value | OR | 95% CI | p value |
| Return to premorbid neurologic function | ||||||
| Female sex | 0.78 | 0.59–1.04 | 0.089 | 0.75 | 0.55–1.02 | 0.070 |
| Age (per every additional year of age) | 0.97 | 0.97–0.98 | < 0.001 | 0.99 | 0.98–0.99 | 0.011 |
| Acute intracranial hemorrhage | 0.27 | 0.16–0.46 | < 0.001 | 0.28 | 0.16–0.48 | < 0.001 |
| SE severity (as quantified by the STESS) | 0.36 | 0.27–0.49 | < 0.001 | 0.49 | 0.33–0.73 | < 0.001 |
| SE type | 0.90 | 0.73–1.11 | 0.313 | 1.07 | 0.83–1.38 | 0.584 |
| In-hospital death | ||||||
| Female sex | 1.34 | 0.77–2.32 | 0.295 | 1.40 | 0.77–2.53 | 0.268 |
| Age (per every additional year of age) | 1.04 | 1.02–1.06 | < 0.001 | 1.04 | 1.01–1.06 | 0.002 |
| Acute intracranial hemorrhage | 2.13 | 1.08–4.20 | 0.030 | 1.87 | 0.89–3.90 | 0.097 |
| SE severity (as quantified by the STESS) | 5.35 | 2.49–11.49 | < 0.001 | 1.64 | 0.64–4.23 | 0.304 |
| SE type | 2.85 | 1.92–4.23 | < 0.001 | 2.77 | 1.78–4.32 | < 0.001 |
| Death at 30 days follow-up | ||||||
| Female sex | 1.34 | 0.77–2.32 | 0.295 | 1.27 | 0.90–1.80 | 0.177 |
| Age (per every additional year of age) | 1.04 | 1.02–1.06 | < 0.001 | 1.04 | 1.03–1.05 | < 0.001 |
| Acute intracranial hemorrhage | 2.13 | 1.08–4.20 | 0.030 | 1.19 | 0.72–1.97 | 0.492 |
| SE severity (as quantified by the STESS) | 2.05 | 1.45–2.89 | < 0.001 | 0.96 | 0.60–1.52 | 0.849 |
| SE type | 1.26 | 0.99–1.59 | 0.059 | 1.44 | 1.09–1.90 | 0.010 |
| Secondary outcomes | ||||||
|---|---|---|---|---|---|---|
| Univariable model | Multivariable model* corrected for the influence of both centers |
|||||
| Potential influences on treatment decisions (as identified in Table 1) |
OR | 95% CI | p value | OR | 95% CI | p value |
| Use of anesthetics | ||||||
| Female sex | 0.55 | 0.41–0.76 | < 0.001 | 0.64 | 0.45–0.91 | 0.013 |
| Age (per every additional year of age) | 0.97 | 0.97–0.98 | < 0.001 | 0.98 | 0.97–0.99 | < 0.001 |
| Acute intracranial hemorrhage | 1.50 | 0.96–2.34 | 0.073 | 1.32 | 0.78–2.21 | 0.299 |
| SE type | 2.77 | 2.18–3.52 | < 0.001 | 3.35 | 2.57–4.37 | < 0.001 |
| Use of mechanical ventilation | ||||||
| Female sex | 0.61 | 0.45–0.82 | 0.001 | 0.72 | 0.52–1.00 | 0.050 |
| Age (per every additional year of age) | 0.98 | 0.97–0.99 | < 0.001 | 0.98 | 0.97–0.99 | < 0.001 |
| Acute intracranial hemorrhage | 2.39 | 1.54–3.70 | < 0.001 | 2.46 | 1.50–4.03 | < 0.001 |
| SE type | 2.84 | 2.25–3.60 | < 0.001 | 3.11 | 2.42–3.99 | < 0.001 |
OR = odds ratio; CI = confidence interval; SE = status epilepticus; STESS = status epilepticus severity score
*All Hosmer–Lemeshow goodness-of-fit tests insignificant indicating adequate model fit
Bold font indicates statistical significance (with a p value set at ≤0.05)
Subsequently, patients were matched according to their propensity scores generated with age to account for the imbalances regarding age between men and women. Figure 4A and B presents the balanced cohort regarding the propensity score distribution and age after matching females with males according to their propensity score generated by age. While the propensity score-matched multivariable analyses revealed no association between female sex and in-hospital death or death at 30 days after seizure onset, female sex was associated with decreased odds for return to premorbid neurologic function independently of potential confounders, such as age, acute intracranial hemorrhage, STESS, and type of SE (Table 4).
Fig. 4.
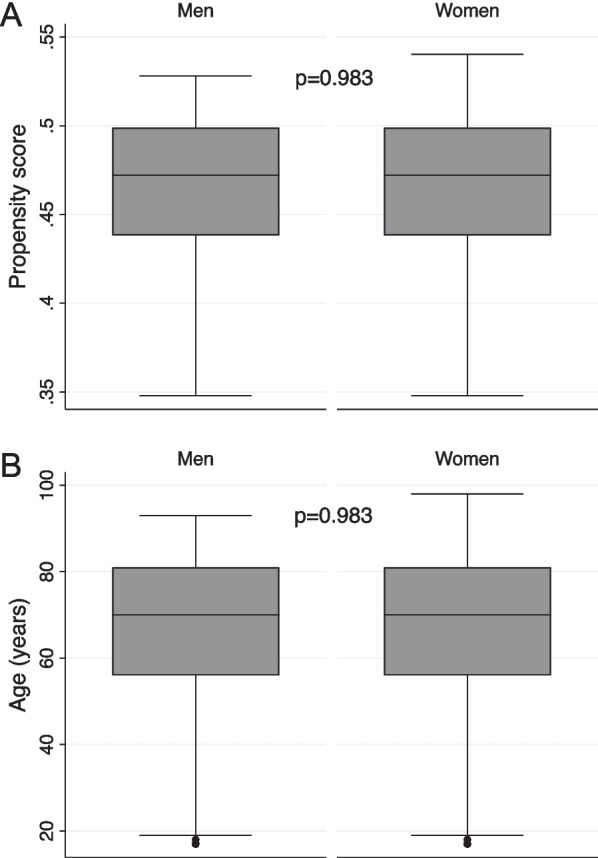
Propensity score (A) and age (B) distribution in the propensity score-matched cohort (n = 417)
Table 4.
Univariable and multivariable logistic regression analyses regarding primary outcomes within the propensity score-matched cohort (n = 417)
| Primary outcomes | ||||||
|---|---|---|---|---|---|---|
| Univariable model | Multivariable model* corrected for the influence of both centers |
|||||
| Potential predictors of outcome/confounders (as identified in Table 1 and as established in the literature) |
OR | 95% CI | p value | OR | 95% CI | p value |
| Return to premorbid neurologic function | ||||||
| Female sex | 0.62 | 0.46–0.84 | 0.002 | 0.48 | 0.33–0.68 | < 0.001 |
| Age (per every additional year of age) | 0.97 | 0.96–0.98 | < 0.001 | 0.97 | 0.96–0.98 | < 0.001 |
| Acute intracranial hemorrhage | 0.07 | 0.03–0.16 | < 0.001 | 0.07 | 0.03–0.15 | < 0.001 |
| SE severity (as quantified by the STESS) | 0.44 | 0.33–0.61 | < 0.001 | 0.99 | 0.62–1.56 | 0.949 |
| SE type | 0.89 | 0.72–1.09 | 0.262 | 0.90 | 0.70–1.16 | 0.406 |
| In-hospital death | ||||||
| Female sex | 2.34 | 1.20–4.59 | 0.013 | 2.00 | 0.93–4.26 | 0.074 |
| Age (per every additional year of age) | 1.04 | 1.02–1.07 | < 0.001 | 1.04 | 1.01–1.07 | 0.005 |
| Acute intracranial hemorrhage | 1.15 | 0.47–2.82 | 0.753 | 1.05 | 0.41–2.70 | 0.913 |
| SE severity (as quantified by the STESS) | 3.59 | 1.57–8.20 | 0.002 | 1.55 | 0.55–4.37 | 0.403 |
| SE type | 1.92 | 1.26–2.94 | 0.003 | 1.95 | 1.24–3.07 | 0.004 |
| Death at 30 days follow-up | ||||||
| Female sex | 1.05 | 0.75–1.46 | 0.788 | 0.74 | 0.49–1.10 | 0.136 |
| Age (per every additional year of age) | 1.04 | 1.02–1.05 | < 0.001 | 1.06 | 1.04–1.07 | < 0.001 |
| Acute intracranial hemorrhage | 0.96 | 0.58–1.58 | 0.866 | 0.65 | 0.38–1.13 | 0.124 |
| SE severity (as quantified by the STESS) | 1.73 | 1.22–2.46 | 0.002 | 0.45 | 0.27–0.76 | 0.003 |
| SE type | 1.65 | 1.30–2.10 | < 0.001 | 2.19 | 1.65–2.89 | < 0.001 |
OR = odds ratio; CI = confidence interval; SE = status epilepticus; STESS = status epilepticus severity score
*All Hosmer–Lemeshow goodness-of-fit tests insignificant indicating adequate model fit
Bold font indicates statistical significance (with a p value set at 0.05)
Uni- and multivariable logistic regression analyses regarding secondary outcomes
Uni- and multivariable analyses for the use of anesthetics and mechanical ventilation (defined as secondary outcomes) are presented in Table 3 (lower half). These analyses revealed that increasing age and female sex were both independently associated with decreased odds for the continuous administration of anesthetics and the use of mechanical ventilation. The Hosmer–Lemeshow goodness-of-fit tests all indicated adequate model fits.
Discussion
This observational study investigated sex-associated differences of SE, its treatment practices, course of disease, complications, and outcomes in a large cohort of adult patients treated at two well equipped Swiss academic tertiary medical care centers. Besides the large cohort of 762 adult SE patients with similar clinical characteristics to those in other adult SE studies including age [26–30], outcome [6, 27, 31], etiologies [6, 27–29], complications [30, 31], SE severity [27, 28], and types of SE [6, 26], our study carefully accounted for the withdrawal of care due to patients’ directives or presumed poor prognosis, and for many additional potential confounders that were frequently neglected in previous studies but can significantly affect outcomes. An additional strength of the study is the utilization of propensity score-matched analyses, which account for imbalances in age distribution between men and women. While our study revealed an independent association of age with all primary outcomes, female sex was not independently associated with the primary outcomes after correcting for potential confounders in our multivariable models at first glance. However, propensity score-matched analyses accounting for potential imbalances regarding age between men and women revealed that female sex was associated with decreased odds for return to premorbid neurologic outcome independently of potential confounders. This comes along with an independent association of female sex with decreased odds for being treated with anesthesia and mechanical ventilation aside from other factors, such as age, intracranial hemorrhage, and types of SE.
The slightly lower number of women admitted to our care centers during the study period is in line with some early studies suggesting a lower incidence of SE in women than men [5–8, 32]. To what degree this lower number of women represents a true lower incidence, under-detection, or undertreatment remains unclear. While women had a higher median age, and a longer median duration of SE, most treatment characteristics did not markedly differ except for a lower percentage receiving anesthesia and mechanical ventilation. When focusing on patients who experienced treatment refractory SE, analyses revealed that women received guideline-conforming treatment escalation with anesthetics less often compared to men, even though there were no significant differences between men and women in terms of median age, Charlson comorbidity index, care withdrawal, and presumed fatal etiologies (except for intracranial hemorrhage seen more frequently in men) in the subgroup of patients with treatment refractory SE (as presented in Fig. 1). The low percentage of non-survivors who received anesthetics appears in line with previous studies from our group demonstrating how early anesthesia, in particular if directly introduced after benzodiazepines, reduced SE duration and hospital stay, improving outcome especially in the absence of potentially fatal etiologies [33]. The findings of our study regarding undertreatment of women are, unfortunately, consistent with previous studies reporting undertreatment of critically ill women in intensive care medicine [4, 34]. In a recent meta-analysis by Modra et al. including 545′538 critically ill patients admitted to ICU, women received less invasive ventilation, renal replacement therapies and had a shorter ICU stay [34]. Sociocultural differences more than biology may account for these findings. While previous studies have revealed that advanced directives and treatment limitations are more prevalent in women [35] and that female sex is a risk factor for care withdrawal or limited ICU treatments [36], we found no differences in terms of care withdrawal in our study. However, although documented care withdrawal in women did not markedly differ from withdrawal in men, women in our cohort were transferred to rehabilitation centers less often but were discharged to nursing homes or hospices more frequently. These results are all the more worrying when considering that women discharged to nursing homes or hospices in our study were > 80 years of age in only 42%, had potentially fatal etiologies in only 7%, and had refractory SE that could not be terminated in only up to 10%. The exact reasons for the higher proportion of women being transferred to nursing homes or hospices could not be clarified due to the retrospective nature of our study. However, less intensive care and/or withdrawal of care may also be a possible explanation, why in an earlier study using the Taiwan National Health Insurance Research Database, the in-hospital mortality for women with SE increased rapidly after the age of 40–45 years [32]. A rather provocative hypothesis for these findings may be that especially in older age and earlier generations, men are still less able to take care of their wives at home than vice versa, as women are still often more involved in caregiving and household chores or men where simply deceased. Effort has been made in cardiovascular research, but also during the COVID-19 pandemic, to assess the impact of sociocultural (gender) variables (e.g. marital status, responsibility in household, caregiving duties) on disease manifestations and outcomes [37, 38]. Unfortunately, studies of SE or epilepsy patients in this context are scarce and further information on sociocultural variables were not available in our study.
ICU admission is a prerequisite for treatment of patients with SE. Although women outlive men worldwide, women remain underrepresented in ICU patients [39]. Sex and gender differences in ICU admission have been reported, mostly revealing a disadvantage for women [4, 40, 41]. If the focus is shifted away from SE or epilepsy patients and toward neurocritically ill patients in general, a study of 450′948 adult patients revealed that critically ill women with cardio- and neurovascular diagnoses had a lower likelihood for ICU admission compared to men, despite being more severely ill [4]. In our study, illness severity as assessed by the SAPS II and Charlson Comorbidity Score did not differ between sexes. Not surprisingly, biological sex and sex-specific thresholds as estimates of organ dys/function (SAPS II) are not included in ICU risk assessment tools, and may thus underestimate illness severity in women, given their lower thresholds in most biomarkers. Accordingly, earlier studies have described female sex as an important promotor of the emergence of SE in patients with epilepsy [3] and that female sex is associated with a higher mortality in SE [32]. This is a serious and worrisome hypothesis which warrants further studies, including careful reassessment of contemporary risk assessment tools in order to provide equal opportunities to men and women [42].
Finally, our study revealed that women had focal NCSE without coma more, and SE with motor symptoms less often. As focal NCSE without coma represents a less severe SE type than convulsive types or NCSE with coma [16], the decreased odds for return to premorbid function in women when accounting for potential imbalances regarding age and adjusting for potential confounders are even more worrisome.
Considering our additional results, that female sex is independently associated with decreased odds for the use of anesthetics and mechanical ventilation it seems more than plausible that this undertreatment takes its toll.
The fact that women in our cohort were older than men further offers the hypothesis that young women may be hormonally protected against seizures and that protection may vanish with the age-related decrease in sexual hormones. Studies have focused on the influence of gonadal hormones on the evolution of seizures in women with epilepsy [43]. The prevailing view is that estrogen may promote the emergence of seizures whereas progesterone is thought to prohibit seizures. However, sound studies on hormonal influences regarding SE are lacking and the retrospective nature of our study did not allow for further analyses in this regard.
The only rather reassuring result in our study is the finding that, in the multivariate analysis, female sex was not an independently associated with the other primary outcomes under investigation such as in-hospital death and death at 30 days after seizure onset. The extent to which this finding downplays the aforementioned results warrants critical consideration by the reader.
The independent association of female sex and disparities regarding specific treatment and outcome after multivariable adjustments and after accounting for imbalances regarding age between men and women strongly suggest that our findings are unlikely solely explainable by age or the well-known potential confounders. However, it is essential to recognize the inherent limitations of retrospective studies when examining sex-related differences in SE management and outcomes. Although intriguing, the retrospective study design with a Swiss two center cohort does not allow firm conclusions regarding a causal relation between undertreatment and the lower odds of return to premorbid function of women.
Accordingly, we advise against drawing overly hasty conclusions or providing premature reassurances based solely on the results of this one study. Until further research provides more certainty, physicians are urged to be heighten their awareness of age-, sex- or gender-related unequal treated of such critically ill patients and to actively strive toward a more equitable and inclusive medicine, ensuring optimal SE outcomes for all individuals, irrespective of gender or age.
Limitations
Our study has additional limitations to the ones discussed above that must be acknowledged. Firstly, our study was conducted at only two Swiss academic tertiary care centers, which limits the generalizability of our findings to other settings. Secondly, the observational study design is prone to several biases that may have been missed but have influenced our results. Thirdly, SE duration represents an approximation, particularly in cases of missing EEG data due to recording errors (in 2.1%) and/or nonconvulsive SE with unwitnessed seizure onset. In addition, potentially delayed EEG confirmation of SE termination caused by the time needed to organize and interpret the EEG may have led to an overestimation of SE duration, and the opposite is true when it comes to estimating seizure onset without motor symptoms thus requiring confirmation of suspected SE by EEG. Fourth, analyses regarding sex-related delay of treatment could not be performed as reliable data regarding the onset of SE is often unavailable. The latter is explained by the fact that the exact timepoint at which especially nonconvulsive seizures start is not precisely known (a shortcoming even hardly avoidable in prospective studies).
Conclusion
This study has uncovered concerning sex-related disparities in the clinical characteristics, therapeutic interventions, and outcomes of adult patients experiencing SE, with women exhibiting a relative disadvantage. To what extent these results are explained by undetected and unexplored sex-specific differences in a systemic response to SE remains unclear. Nevertheless, this does not make them any less concerning. Until further studies provide more explanations in this context, these findings indicate that sex must be taken into account when formulating strategies for managing SE and forecasting specific outcomes.
Supplementary Information
Additional file 1. Univariable comparison of treatment characteristics between men and women with different types of status epilepticus.
Acknowledgements
Not applicable
Abbreviations
- CI
Confidence interval
- GCS
Glasgow coma scale
- HIE
Hypoxic-ischemic encephalopathy
- ICU
Intensive care unit
- ILAE
International league against epilepsy
- IQR
Interquartile range
- NCSE
Nonconvulsive status epilepticus
- OR
Odds ratio
- REDCAP
Research electronic data capture
- SAPS II
Simplified acute physiology scale II
- SE
Status epilepticus
- STESS
Status epilepticus Severity score
Author contributions
SMB, PDS and RS planned and designed the study. PDS, SMB, and RS acquired and interpreted the data. RS analyzed the data and wrote the manuscript. SMB, PDS, PSCK, PG, CEG, OES, GMDM, SH, SR, AK, JP, HQ, SM, and MS interpreted the data, revised the manuscript and substantially contributed to the inaugural draft. All authors approved the final submitted version.
Funding
Open access funding provided by University of Basel. The funder (University Hospital Basel) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. This study was performed and designed without the input or support of any pharmaceutical company, or other commercial interest.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
In accordance with the 1964 Declaration of Helsinki and its subsequent revisions, the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz) granted approval for the study. The requirement for obtaining patient consent was waived.
Consent for publication
Not applicable.
Competing interests
SMB declares no competing interests. PDS was supported by the Swiss National Science Foundation (163398, CRS115-180365) and is supported by the 2022 Swiss League Against Epilepsy Research Support Prize. PSCK declares no competing interests. PG declares no competing interests. CEG has received speakers’ fees from Sanofi Genzyme, travel support from Siemens Healthineers, and research support from the Novartis Foundation, Switzerland, and Bayer Pharmaceuticals. OEG declares no competing interests. GMDM has been receiving support from the Swiss National Science Foundation (Nr 32003B_200573, Nr. PBBEP3_139388); Spezialprogramm Nachwuchsförderung Klinische Forschung, University of Basel; Science Funds (Wissenschaftspool) of the University Hospital Basel; Swiss Heart Foundation; ProPatient Foundation Basel; Bangerter-Rhyner-Stiftung; Swisslife Jubiläumsstiftung for Medical Research; Swiss Neurological Society; Fondazione Dr Ettore Balli; De Quervain research grant; Thermo Fisher GmbH; Novartis grant; travel honoraria by Bayer and BMS/Pfizer; speaker honoraria by Bayer and Medtronic; consultant honoraria by Bayer and Novartis. He is member of the Steering Committee of PACIFIC Stroke (NCT04304508). Industry payments are made to the research fund of the University Hospital Basel. SH is supported by the Swiss National Foundation (SNF) (Ref 10001C_192850/1 and 10531C_182422), the Gottfried Julia Bangerter-Rhyner Foundation (8472/HEG-DSV), and the Swiss Society of General Internal Medicine (SSGIM). SR received unconditional research grants from UCB-pharma. He received honoraria from serving on the scientific advisory boards of Angellini/Arvelle, Bial, Eisai, GW, and UCB-pharma, and from serving as a consultant for Angellini/Arvelle, Eisai, Pfizer, Novartis, Sandoz, and UCB-pharma. He does not hold any stocks of any pharmaceutical industries or manufacturers of medical devices. He received funding from UCB-pharma, and Swiss National Science Foundation Grants: Grant Number 320030_169379/1 and coapplicant for grants numbers 33CM30_125115/1 and 33CM30_140338/1; he disclosed that he is the past-president of the Swiss League against Epilepsy (no payments). AK has received honoraria for consulting from Abbvie, EliLilly, Lundbeck, Mitsubishi Tanabe, Novartis, and TEVA that were paid to a teaching and research fund at the University Hospital Geneva. JP declares no competing interests. HQ declares no competing interests. SM declares no competing interests. MS is a shareholder of Epilog NV (Ghent, Belgium). She received speaker's fees from Philips and Desitin. She received grants from the Swiss National Science Foundation (163398, CRS115-180365). RS received research grants from the Swiss National Foundation (Ref 320030_169379), the Research Fund of the University Basel, the Scientific Society Basel, and the Gottfried Julia Bangerter-Rhyner Foundation. He received personal grants from UCB-pharma and holds stocks from Novartis, Roche, Alcon, and Johnson & Johnson.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sira M. Baumann and Pia De Stefano are equally contributing first authors.
References
- 1.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 2.Mevius A, Joeres L, Gille P, Molzan M, Foskett N, Wilke T, et al. Epidemiology, real-world treatment and mortality of patients with status epilepticus in Germany: insights from a large healthcare database. Brain Commun. 2023;5(3):fcad145. doi: 10.1093/braincomms/fcad145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langenbruch L, Strippel C, Gorlich D, Elger CE, Moddel G, Meuth SG, et al. Occurrence of status epilepticus in persons with epilepsy is determined by sex, epilepsy classification, and etiology: a single center cohort study. J Neurol. 2021;268(12):4816–4823. doi: 10.1007/s00415-021-10600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todorov A, Kaufmann F, Arslani K, Haider A, Bengs S, Goliasch G, et al. Gender differences in the provision of intensive care: a Bayesian approach. Intensive Care Med. 2021;47(5):577–587. doi: 10.1007/s00134-021-06393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. 1998;50(3):735–741. doi: 10.1212/wnl.50.3.735. [DOI] [PubMed] [Google Scholar]
- 6.Knake S, Rosenow F, Vescovi M, Oertel WH, Mueller HH, Wirbatz A, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001;42(6):714–718. doi: 10.1046/j.1528-1157.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. 2002;58(7):1070–1076. doi: 10.1212/wnl.58.7.1070. [DOI] [PubMed] [Google Scholar]
- 8.Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR) Neurology. 2000;55(5):693–697. doi: 10.1212/wnl.55.5.693. [DOI] [PubMed] [Google Scholar]
- 9.Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long-term mortality after a first episode of status epilepticus. Neurology. 2002;58(4):537–541. doi: 10.1212/wnl.58.4.537. [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse EJ, Garnett LK, Towne AR, Morton LD, Barnes T, Ko D, et al. Prospective population-based study of intermittent and continuous convulsive status epilepticus in Richmond. Virginia Epilepsia. 1999;40(6):752–758. doi: 10.1111/j.1528-1157.1999.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 11.Chin RF, Neville BG, Scott RC. A systematic review of the epidemiology of status epilepticus. Eur J Neurol Offic J Eur Fed Neurol Soc. 2004;11(12):800–810. doi: 10.1111/j.1468-1331.2004.00943.x. [DOI] [PubMed] [Google Scholar]
- 12.Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Short-term mortality after a first episode of status epilepticus. Epilepsia. 1997;38(12):1344–1349. doi: 10.1111/j.1528-1157.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumann SM, Semmlack S, Rybitschka A, Kliem PSC, De Marchis GM, Ruegg S, et al. Prolonged mechanical ventilation in patients with terminated status epilepticus and outcome: an observational cohort study. Epilepsia. 2021;62(12):3042–3057. doi: 10.1111/epi.17100. [DOI] [PubMed] [Google Scholar]
- 16.Rossetti AO, Logroscino G, Bromfield EB. A clinical score for prognosis of status epilepticus in adults. Neurology. 2006;66(11):1736–1738. doi: 10.1212/01.wnl.0000223352.71621.97. [DOI] [PubMed] [Google Scholar]
- 17.Sutter R, Kaplan PW, Rüegg S. Independent external validation of the status epilepticus severity score. Crit Care Med. 2013;41:e475–e479. doi: 10.1097/CCM.0b013e31829eca06. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 20.Sutter R, Semmlack S, Spiegel R, Tisljar K, Ruegg S, Marsch S. Distinguishing in-hospital and out-of-hospital status epilepticus: clinical implications from a 10-year cohort study. Eur J Neurol Offi J Eur Fed Neurol Soc. 2017;24(9):1156–1165. doi: 10.1111/ene.13359. [DOI] [PubMed] [Google Scholar]
- 21.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 22.Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the american epilepsy society. Epilepsy Curr. 2016;16(1):48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroeger D, Amzica F. Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci Offi J Soc Neurosci. 2007;27(39):10597–10607. doi: 10.1523/JNEUROSCI.3440-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutter R, Kaplan PW, Rüegg S. Outcome predictors for status epilepticus—what really counts. Nat Rev Neurol. 2013;9(9):525–534. doi: 10.1038/nrneurol.2013.154. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S. A goodness of-fit test for the multiple logistic regression model. Commu in Stat. 1980;A9:1043–1069. [Google Scholar]
- 26.Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51(2):251–256. doi: 10.1111/j.1528-1167.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 27.Fatuzzo D, Novy J, Rossetti AO. Use of newer antiepileptic drugs and prognosis in adults with status epilepticus: comparison between 2009 and 2017. Epilepsia. 2018;59(7):e98–e102. doi: 10.1111/epi.14434. [DOI] [PubMed] [Google Scholar]
- 28.Beuchat I, Novy J, Rossetti AO. Newer antiepileptic drugs in status epilepticus: prescription trends and outcomes in comparison with traditional agents. CNS Drugs. 2017;31(4):327–334. doi: 10.1007/s40263-017-0424-1. [DOI] [PubMed] [Google Scholar]
- 29.Strzelczyk A, Ansorge S, Hapfelmeier J, Bonthapally V, Erder MH, Rosenow F. Costs, length of stay, and mortality of super-refractory status epilepticus: a population-based study from Germany. Epilepsia. 2017;58(9):1533–1541. doi: 10.1111/epi.13837. [DOI] [PubMed] [Google Scholar]
- 30.Belluzzo M, Furlanis G, Stragapede L, Monti F. Role of comorbidities and in-hospital complications in short-term status epilepticus outcome. Clin Neurol Neurosurg. 2017;154:13–18. doi: 10.1016/j.clineuro.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Zelano J, Moller F, Dobesberger J, Trinka E, Kumlien E. Infections in status epilepticus: a retrospective 5-year cohort study. Seizure. 2014;23(8):603–606. doi: 10.1016/j.seizure.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Ong CT, Sheu SM, Tsai CF, Wong YS, Chen SC. Age-dependent sex difference of the incidence and mortality of status epilepticus: a twelve year nationwide population-based cohort study in Taiwan. PLoS ONE. 2015;10(3):e0122350. doi: 10.1371/journal.pone.0122350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Stefano P, Baumann SM, Semmlack S, Ruegg S, Marsch S, Seeck M, et al. Safety and efficacy of coma induction following first-line treatment in status epilepticus: a 2-center study. Neurology. 2021;97(6):e564–e576. doi: 10.1212/WNL.0000000000012292. [DOI] [PubMed] [Google Scholar]
- 34.Modra LJ, Higgins AM, Abeygunawardana VS, Vithanage RN, Bailey MJ, Bellomo R. Sex differences in treatment of adult intensive care patients: a systematic review and meta-analysis. Crit Care Med. 2022;50(6):913–923. doi: 10.1097/CCM.0000000000005469. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann M, Perren A, Cerutti B, Dysli C, Rothen HU, Swiss Society of Intensive Care M Severity-adjusted ICU mortality only tells half the truth-the impact of treatment limitation in a nationwide database. Crit Care Med. 2020;48(12):e1242–e1250. doi: 10.1097/CCM.0000000000004658. [DOI] [PubMed] [Google Scholar]
- 36.Block L, Petzold M, Syrous AN, Lindqvist B, Odenstedt Herges H, Naredi S. Age, SAPS 3 and female sex are associated with decisions to withdraw or withhold intensive care. Acta Anaesthesiol Scand. 2019;63(9):1210–1215. doi: 10.1111/aas.13411. [DOI] [PubMed] [Google Scholar]
- 37.Gebhard CE, Hamouda N, Gebert P, Regitz-Zagrosek V, Gebhard C, Investigators C. Sex versus gender-related characteristics: which predicts clinical outcomes of acute COVID-19? Intensive Care Med. 2022;48(11):1652–1655. doi: 10.1007/s00134-022-06836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, et al. Sex versus gender-related characteristics: Which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. 2016;67(2):127–135. doi: 10.1016/j.jacc.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 39.Thornton J. WHO report shows that women outlive men worldwide. BMJ. 2019;365:l1631. doi: 10.1136/bmj.l1631. [DOI] [PubMed] [Google Scholar]
- 40.Fowler RA, Sabur N, Li P, Juurlink DN, Pinto R, Hladunewich MA, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007;177(12):1513–1519. doi: 10.1503/cmaj.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill A, Ramsey C, Dodek P, Kozek J, Fransoo R, Fowler R, et al. Examining mechanisms for gender differences in admission to intensive care units. Health Serv Res. 2019. [DOI] [PMC free article] [PubMed]
- 42.Jacobson S, Liedgren E, Johansson G, Ferm M, Winso O. Sequential organ failure assessment (SOFA) scores differ between genders in a sepsis cohort: Cause or effect? Ups J Med Sci. 2012;117(4):415–425. doi: 10.3109/03009734.2012.703255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47(9):1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Univariable comparison of treatment characteristics between men and women with different types of status epilepticus.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.