Abstract
Microglia are the resident innate immune cells in the brain with a major role in orchestrating immune responses. They also provide a frontline of host defense in the central nervous system (CNS) through their active phagocytic capability. Being a professional phagocyte, microglia participate in phagocytic and autophagic clearance of cellular waste and debris as well as toxic protein aggregates, which relies on optimal lysosomal acidification and function. Defective microglial lysosomal acidification leads to impaired phagocytic and autophagic functions which result in the perpetuation of neuroinflammation and progression of neurodegeneration. Reacidification of impaired lysosomes in microglia has been shown to reverse neurodegenerative pathology in Alzheimer’s disease. In this review, we summarize key factors and mechanisms contributing to lysosomal acidification impairment and the associated phagocytic and autophagic dysfunction in microglia, and how these defects contribute to neuroinflammation and neurodegeneration. We further discuss techniques to monitor lysosomal pH and therapeutic agents that can reacidify impaired lysosomes in microglia under disease conditions. Finally, we propose future directions to investigate the role of microglial lysosomal acidification in lysosome–mitochondria crosstalk and in neuron–glia interaction for more comprehensive understanding of its broader CNS physiological and pathological implications.
Keywords: Lysosomal acidification, Autophagy, Phagocytosis, Cytokines, Toxic protein aggregates, Neuroinflammation, Neurodegenerative diseases, Acidic nanoparticles
Background
Microglia are a type of glial cells that represent 5–15% of the adult brain cells [1]. Being professional phagocytes, microglia maintain brain homeostasis under physiological conditions by phagocytosing neuronal synapses, apoptotic cells, and cellular debris, as well as supporting neuronal development and survival by releasing trophic factors [2]. Microglia also form the largest population of resident immune cells which adopt a macrophage-like role in the brain, making them indispensable in cultivating and maintaining the architecture of the central nervous system (CNS) [3, 4]. Under stimulated or diseased conditions, microglia become activated which result in changes in morphology, cytokine production and secretion, and phagocytic capacity [5]. In general, microglia activation and the associated increased cytokine release are intended to be neuroprotective, although chronic microglia activation and excessive neuroinflammatory responses can be detrimental to the brain, leading to the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease (AD) [6]. Microglia can display a spectrum of heterogenous states including homeostatic microglia and those that respond to stimuli with distinct profiles of cytokine expression [2, 7–9]. The specific microglial phenotype invoked is largely a result of environmental signals, not only from neurons and other glial cells, but also from immune cells within the perivascular spaces [2, 10].
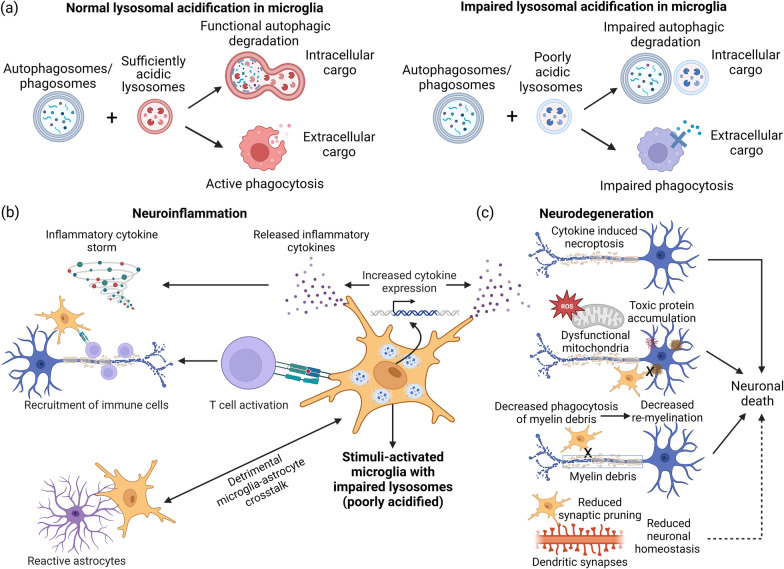
The development of microglia and their responsiveness to stimuli or stress have been reported to be dependent on a functional lysosomal regulatory circuit [11]. Additionally, microglial phagocytosis and autophagy require proper lysosomal acidification and functions to complete the cellular degradation and recycling system [11–13]. Lysosomal acidification is regulated by the membrane-bound vacuolar (H +)-ATPase (V-ATPase) and ion channels [14, 15]. Being the final step of the clearance process, the extent of lysosomal acidification and its enzyme activities play critical roles in substrate metabolism and degradation of endogenous cellular waste (intracellular cargo) and ingested proteins (extracellular cargo) through autophagy and phagocytosis, respectively [12, 16, 17] (Fig. 1a), thus controlling microglial function and determining the degree of their activation under stimulated or diseased conditions.
Fig. 1.
Implications of microglial lysosomal acidification dysfunction in neuroinflammation and neurodegeneration. a In healthy microglia, lysosomes with sufficiently acidic lumen can fuse with autophagosomes or phagosomes to form functional autolysosomes for efficient degradation of both intracellular and extracellular cargoes (left). Under stimulated or diseased conditions, lysosomes with poorly acidic lumen (impaired lysosomal acidification) will have inefficient fusion with autophagosomes or phagosomes, or even no fusion, leading to reduced phagocytic and autophagic functions (right). b In the context of neuroinflammation, stimuli-activated microglia with impaired lysosomal acidification express and secrete more cytokines to perpetuate neuroinflammation. Through releasing more inflammatory cytokines, these dysfunctional microglia recruit and activate immune cells and participate in detrimental crosstalk with astrocytes to propagate inflammatory response. c In the context of neurodegeneration, increased inflammatory cytokine secretion by dysfunctional microglia with lysosomal acidification defect contributes to neuronal death via mechanisms such as necroptosis. In addition, these impaired microglia have reduced phagocytic and autophagic capabilities in the clearance of toxic protein aggregates, damaged organelles such as mitochondria, and myelin debris, as well as in synaptic pruning, leading to eventual neuronal death and neurodegeneration. The figure was created with BioRender.com
Defective lysosomal acidification in the microglia contributes to neuroinflammation and neurodegeneration through a variety of deleterious cellular pathways. Stimuli-activated microglia with defective lysosomal acidification produce and release more inflammatory cytokines [18, 19], recruit and activate immune cells [12, 20], and participate in detrimental crosstalk with astrocytes to exacerbate neuroinflammation [21] (Fig. 1b). On the other hand, dysfunctional microglia with lysosomal acidification impairment and the associated lysosomal membrane permeabilization (LMP) increase inflammatory cytokine secretion and induce neuronal death via mechanisms such as necroptosis [22, 23]. In addition, these impaired microglia have reduced phagocytic and autophagic capabilities in the clearance of damaged organelles such as mitochondria [24, 25], toxic protein aggregates [26], and myelin debris [27], as well as in synaptic pruning [3], leading to eventual neuronal death and neurodegeneration (Fig. 1c). Restoration of microglial autophagy and phagocytosis functions have been shown to be critical for mitigating neuroinflammation [28] and neurodegeneration [12], suggesting the need to modulate these processes, including the modulation of microglial lysosomal acidification. However, the exact mechanisms inducing lysosomal acidification impairment and the consequences of lysosomal pH modulation in microglial function and activation remain unclear and need to be investigated [29].
In this review, we summarize key factors and mechanisms that contribute to lysosomal acidification dysregulation in microglia, including presenilin modification, cytokine and inflammatory stimulation, metabolite and lipid dysregulation, adenosine triphosphate (ATP) level and purinergic receptors signaling, exposure to toxic protein aggregates, lysosome physiology and cathepsin activity, with relevance to the pathogenesis of neuroinflammation and neurodegeneration. In addition, we discuss techniques used for lysosomal pH monitoring, as well as therapeutic agents that have been demonstrated to be effective in restoring lysosomal and autophagic function in CNS cells including microglia. We conclude by proposing future investigations to understand the role of microglial lysosomal acidification in lysosome–mitochondria crosstalk and in the interactions among microglia, astrocytes, and neurons to better elucidate neurodegenerative mechanisms and derive neuroprotective strategies.
Role of presenilins in autolysosomal function of microglia
Presenilin 1 (PSEN1, PS1) and presenilin 2 (PSEN2, PS2) are proteins responsible for the enzymatic activity of γ-secretase, a multi-subunit intramembrane protease complex that plays a significant role in the cleavage of amyloid precursor protein (APP) to generate beta-amyloid (Aβ) [30]. PS1 mutations are commonly associated with early-onset familial AD (FAD) [31]. While PS1 is broadly expressed in the CNS, it is highly expressed in microglia within certain regions of the brain such as the cortex [32]. PS1 plays important roles in modulating microglial activation and inflammatory cytokine release [33]. For instance, in BV2 mouse microglial cell lines, γ‐secretase inhibitors modulated pro‐inflammatory cytokine expression upon lipopolysaccharide (LPS) treatment [34]. Furthermore, PS1 plays a critical role in modulating lysosomal acidification in microglia [32], in addition to its known role in neurons [35], and its malfunction contributes to neuroinflammation and neurodegeneration [33, 36–39].
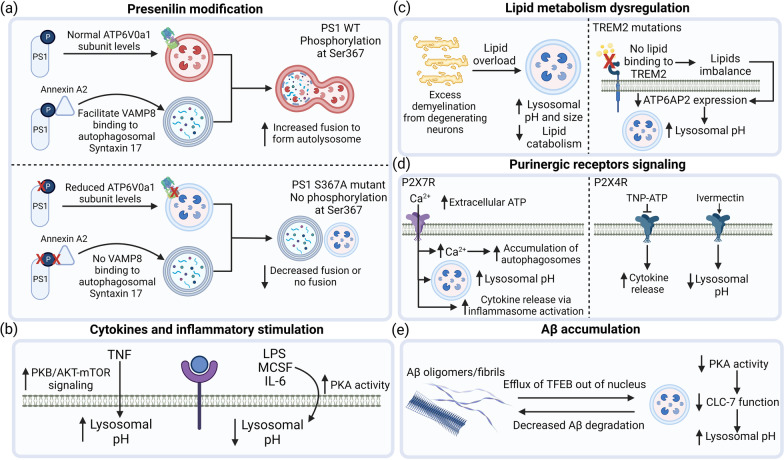
PS1 can be phosphorylated at several sites and the extent of PS1 phosphorylation can affect its function in different ways, including the regulation of cell growth and differentiation [38]. Importantly, phosphorylation of PS1 on its third intracellular loop at Ser367 increases microglial lysosomal acidification and autophagic degradation of APP β-C-terminal fragment (APP-βCTF), the precursor of Aβ [38]. Using a double knock-in mouse model (PS1S367A/S367A), it was shown that there is significant accumulation in levels of APP-βCTF, and inhibition of autophagic flux, together with lysosomal acidification defects in microglia [32]. PS1 Ser367 phosphorylation exerts its effect on microglial lysosomal acidification and associated autophagic function in two ways (Fig. 2a): (1) lack of PS1 phosphorylation at Ser367 reduce lysosomal V-ATPase subunit ATP6V0a1 levels, contributing to impaired assembly of the lysosomal V-ATPase, thereby leading to lysosomal pH elevation [32]; and (2) PS1 phosphorylated at Ser367 specifically binds Annexin A2, which facilitates the binding of lysosomal soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) Vamp8 to the autophagosomal SNARE Syntaxin 17 to modulate the fusion of autophagosomes with lysosomes [38]. Interestingly, PS1 knockout microglia exhibit normal lysosomal acidification with no change in ATP6V0a1 levels [32], indicating that the change in microglial lysosomal pH is not due to loss of PS1 function, which has been shown to be the cause of lysosomal acidification dysfunction in neurons [35, 37], but specifically due to phosphorylation of PS1 at Ser367.
Fig. 2.
Factors affecting microglial lysosomal acidification and associated phagocytic and autophagic function. a Presenilin-1 (PS1) deficient in phosphorylation at Ser367 (S367) leads to reduced ATP6V0a1 levels, impairing lysosomal acidification. Furthermore, PS1 S367A reduces binding to Annexin A2 and decreases VAMP8 binding to autophagosomal Syntaxin 17, thereby preventing autophagosome-lysosome fusion, leading to autophagic inhibition. b Cytokine stimulations induce differential effects of lysosomal acidification alterations in microglia. c Lipids such as myelin debris and TREM2 mediated lipid accumulation impair microglial lysosomal acidification through different mechanisms and decrease lipid catabolic activity. d Purinergic receptors signaling regulate lysosomal acidification. Extracellular ATP activate P2X7R, leading to influx of Ca2+, accumulation of autophagosomes, elevation of lysosomal pH as well as increased cytokine release via inflammasome. On the other hand, inhibition of P2X4R by TNP-ATP results in increased cytokine release, while activation of P2X4R by ivermectin improves lysosomal acidification and promotes microglial function. e Accumulation of Aβ protein has been shown to lead to defective lysosomal acidification, potentially due to efflux of TFEB out of nucleus. Similarly, a decrease in PKA activity along with decreased ClC-7 chloride transporter function leads to lysosomal pH elevation, resulting in reduced degradation of Aβ, further driving neurodegeneration. The figure was created with BioRender.com
Microglial expression of PS2 with N141I mutation resulted in impaired γ-secretase activity as well as increased inflammatory cytokine release. Furthermore, in mice with PS2 N141I mutation, in the absence of inflammatory stimuli, there is enhanced microglial IL-6 and TREM2 expression in brain as well as reduced microglial branch number and length, indicating microglia activation [40]. In the presence of inflammatory insult LPS, a higher inflammatory gene expression of IL-6 and CXCL2, as well as higher nuclear factor kappa B (NF-κB) transcriptional activity is observed in PS2 N141I mouse brain [40]. Lastly, PS1 and PS2 mutants and knockouts have been found to impair microglial phagocytosis of Aβ through either reducing its cytoskeletal structure (PS1) [41] or reduce mitochondrial function (PS2) [42]. Taking into consideration that presenilins play important roles in regulating lysosomal activity and microglial function, a clearer understanding of each of the contributions of PS1 and PS2 as well as their potential interplay, should be further elucidated to clarify the molecular mechanisms regulated by presenilins in microglia.
Differential effects of cytokines and inflammatory stimulation on microglial lysosomal acidification
In the brain, cytokines and inflammatory insults have been shown to regulate immune activation and cell death [29]. As the expression and production of inflammatory cytokines in microglia is much higher than other cell types, microglia plays a major role in driving age-related neuroinflammation [3, 4]. Microglia with a ramified morphology are homeostatic and have little or no phagocytic or inflammatory activity, but are capable of transforming into fully phagocytic and inflammatory activated phenotypes with an amoeboid morphology, upon exposure to different cytokine stimulations [43]. Under different disease states, microglia respond differently to inflammatory stimuli and adopt different cytokine gene profiles [44–50].
Inflammatory stimulations have been shown to lead to microglial lysosomal acidification impairments. However, the contribution of each inflammatory stimulus towards lysosomal acidification remains to be elucidated. For example, while TNF impairs lysosomal acidification in BV2 microglial cells [18], IL-6 promotes lysosomal acidification in primary mouse microglia [51]. LPS exposure in primary mouse microglia lowers lysosomal pH [51], and reduces Aβ burden in APP transgenic mice, potentially through increased microglial phagocytic function [52]. However, in another study it has been shown that while LPS increased lysosomal acidification in N9 mouse microglial cells, autophagic flux is interrupted [53]. This is potentially due to LPS reducing the expression level of VPS34 protein, which is involved in autophagosome biogenesis, leading to autophagosome formation impairment and defective autophagic degradation [53]. Importantly, when conditioned media from activated microglia that has been treated with LPS are treated to neurons, it further induced autophagy inhibition in neurons, leading to neurodegeneration [54]. Hence, different pro-inflammatory cytokines can exhibit differential effects on microglial lysosomal acidification and its associated functions (Fig. 2b).
TNF triggers the phosphorylation of protein kinase B (PKB) or AKT and mTOR signaling, leading to impaired autophagic flux associated with lysosomal acidification dysfunction in mouse primary microglia and BV2 microglial cell lines [18]. In the same study, TNF induces the mouse primary microglia to increase the gene expression of pro-inflammatory genes iNOS, NO, IL-1β, and IL-6 and a decrease in anti-inflammatory genes including Arginase1, Ym1/2, and IL-10. Furthermore, MES23.5 neuronal cells exposed to TNF stimulated microglia conditioned medium exhibit increased cleaved caspase-3 level, indicative of neurotoxicity [18]. This conditioned medium-induced toxicity may derive from an accumulation of pro-inflammatory cytokines or other neurotoxic factors, although no exact characterization of the species was done in this study. Treatment with mTOR inhibitors rapamycin and resveratrol and serum deprivation reversed the deleterious effects via improvement of autophagic function in microglia. Interestingly, the same study also found that TNF promoted transcription factor EB (TFEB) nuclear translocation and increased lysosomal proteins, which is suggested to be due to a compensatory effect of the lysosome to expand in its size to accommodate the accumulated protein loads [18].
Macrophage colony-stimulating factor (MCSF), which has the ability to activate the pro-inflammatory character of microglia, has also been shown to promote lysosomal acidification to increase the degradation of fibrillar Aβ in primary mouse microglia [51]. Specifically, MCSF addition increases ClC-7 and osteoporosis-associated transmembrane protein 1 (OSTM1) transcription and interactions in primary mouse microglia, allowing for proper ClC-7 chloride channel trafficking and complete lysosomal acidification [55]. Treatment with either a protein kinase A (PKA) inhibitor or a chloride channel inhibitor partially reversed the acidifying effects of MCSF in primary mouse microglia, indicating that MCSF is acting through PKA-mediated chloride channel phosphorylation [51]. In AD mice, MCSF treatment decreased Aβ plaque density [51], and prevented cognitive decline [56], due to the increased number of microglia as a result of increased infiltration of bone marrow donor cells in response to systemic MCSF treatment and derivation into microglia. As different cytokines have opposing effects on modulating microglial lysosomal acidification and function, it is important for future studies to investigate whether there is a convergence of pathways through which cytokines modulate lysosomal acidification to facilitate future therapeutic targeting [57].
Metabolite and lipid dysregulation alter lysosomal pH
Dysfunctional microglial metabolism has been linked to neuroinflammation [58], as microglia play a key role in multiple cellular processes, including the production and response to signaling molecules [58]. In brains with neurodegenerative pathology, high levels of non-bioavailable iron in the form of ferritin are accumulated in the microglia, and lysosomal acidification has been shown to modulate iron content, thereby affecting microglial functions [59, 60]. On the other hand, there are other metabolites that can affect microglial lysosomal acidification and the associated phagocytic or autophagic functions, including glucose, myelin debris and lipids accumulation (Fig. 2c). Chronic exposure to high levels of glucose and lipids impair lysosomal function and alter microglial function [27, 61–63]. A study evaluating the effects of hyperglycaemia in astrocytes and primary rats microglia showed that chronic exposure to high glucose reduces mitochondrial membrane potential and ATP levels, which results in decreased lysosomal acidification and reduced degradation of Aβ oligomers [64].
Microglia actively participate in lipid metabolism, engaging in both lipid biogenesis and lipid catabolism processes [65]. On one hand, microglia produce essential lipids required for myelin formation and signaling within the brain [65]. On the other hand, microglia exhibit lipid catabolism capabilities through phagocytosis of cellular debris and myelin fragments [65], thereby playing an important role in the protective response to demyelination as they remove the resulting cellular waste, allowing neuronal regenerative processes to proceed [61]. It has been shown that an overload of myelin fragmentation results in the development of enlarged lysosomes and decreased lysosomal degradative function, contributing to microglia senescence and immune dysfunction in the normal aged mouse brain [27]. This suggests that increasing lysosomal acidification and hence microglial lipid catabolic activity is beneficial to myelin debris clearance. Interestingly, recent findings show that in case of severe demyelination in mouse, microglial lysosomal–autophagic pathway becomes overactivated, resulting in increased uptake of lipid into lysosomes, leading to lipid droplet accumulation and failed myelin debris clearance [66]. Lysosomal acidification inhibition with bafilomycin A1 treatment at the acute phase of demyelination is able to improve myelin debris degradation, indicating that an optimal lysosomal acidification in microglia is required to control the myelination process [66].
Triggering receptor expressed on myeloid cells 2 (TREM2) is a transmembrane protein essential for microglia proliferation and survival [67], and has been implicated in lipid imbalance and lysosomal dysfunction in microglia [68]. In an induced pluripotent stem cell (iPSC)-derived microglia (iMGLs) of Nasu–Hakola disease (NHD) with homozygous TREM2 mutations, dysfunctional lysosomal acidification and proteolytic abilities have been reported, together with impaired cholesterol metabolism, defective lipid droplet biogenesis, as well as altered lipid catabolic activity [68]. These changes were evident at the transcriptomic level in iMGLs, where genes implicated in cholesterol pathways (CYP27A1, SOAT1, NCP2, and LPL), lysosomal acidification (ATP6AP2), and autophagy (LAMP2) were downregulated in both iMGLs and NHD brain tissues. There is also a significant increase in genes associated with inflammation and immune activation in NHD patient brains, including inflammatory cytokines (IL8, IL18, IL6R, TGFB1, IL17RA) [68].
In a recent study, it was found that sleep deprivation increases microglial reactivity dependent on TREM2, and there is enhanced TREM2-dependent Aβ plaque deposition compared with mice with regular sleeping patterns [69]. In addition, sleep deprivation in 5xFAD mice with humanized TREM2 common variant led to reduction in lysosomal gene expression, such as ATP6V0a1 and CTSD, indicating that sleep deprivation induced impairment of lysosomal degradation, potentially due to defective lysosomal acidification [69]. In another instance, TMEM106B deficiency has been shown to result in microglial lysosomal dysfunction and reduces the level of TREM2 in mice, leading to microglial dysfunction and myelination defects [70]. The progranulin protein, produced by the GRN gene, has been shown to be essential for maintaining lysosomal acidification in microglia [71]. In progranulin-deficient microglia, there is defective clearance and accumulation of myelin debris [72, 73]. These findings suggest that lipid imbalance and defective lysosomal acidification have a profound effect on modulating microglia inflammatory response and chronic neuroinflammation.
ATP level and purinergic receptors signaling modulate lysosomal pH
P2X purinoceptors are trimeric ATP-gated cation channels formed by homomeric or heteromeric associations from seven different subunits (P2X1–P2X7). Among the seven P2X subunits, P2X4 receptor (P2X4R) and P2X7 receptor (P2X7R) signaling have been shown to modulate microglial function and mediating neuroinflammatory response [74–76]. The activation of P2X7R by ATP reduces lysosomal acidification in MG6 mouse microglial cells and primary microglia [75], resulting in increased lysosomal leakage and cytosolic lysosomal cathepsin B levels, and subsequently activating the NLR family pyrin domain containing 3 (NLRP3) inflammasome [19] (Fig. 2d). NLRP3 inflammasome activation can further lead to caspase-1 cleavage and IL-1β maturation and release from microglia, leading to the propagation of neuroinflammation and induction of neurotoxicity [19].
Within the P2X ion channel family, P2X4R has a unique subcellular distribution, being predominantly found on lysosomes while also being present on the plasma membrane [77]. P2X4R activation with the allosteric modulator ivermectin induces lysosomal acidification and potentiates myelin engulfment and degradation in experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis (MS) [78]. On the other hand, the inhibition of the P2X4R by an antagonist TNP-ATP severely affected the removal and degradation of myelin debris by microglia, and enhanced the release of pro-inflammatory cytokines from microglia in EAE mice [78] (Fig. 2d). Interestingly, it has been shown P2X4R can be negatively regulated by lysosomal pH, where an acidic lysosomal pH inhibit P2X4R activity, while an elevated lysosomal pH in the presence of ATP activates the receptor [79].
Apart from playing an active role in modulating P2X purinoceptors signaling, ATP plays an important role in regulating lysosomal V-ATPase function, where increased ATP activity increases lysosomal acidification [14]. The soluble adenylyl cyclase (sAC) is an extensively expressed intracellular source of cyclic adenosine monophosphate (cAMP) as it catalyzes the conversion of ATP to cAMP and plays a key role in the colocalization of V-ATPase to lysosomes, hence leading to the modulation of lysosomal acidification [80]. Absence of sAC reduces cAMP levels and leads to lysosomal acidification impairments in primary mouse microglia [51]. These findings suggest the heterogenous nature of ATP and P2X purinoreceptors in modulating lysosomal acidification and function, and hence more studies are required to understand the intricate relationship between the different targets, to aid the development of specific therapeutic agents.
Bidirectional relationship between toxic protein aggregates and lysosomal dysfunction
The accumulation of toxic protein aggregates such as tau and Aβ is associated with neurodegeneration [81–86] and microglia have been shown to play an important role in the internalization and degradation of these toxic protein aggregates [36, 87]. However, whether these protein aggregates are responsible for lysosomal deficits, or impaired lysosomal function arising from other insults results in the accumulation of toxic protein aggregates remains unclear. For instance, while some studies support the notion that the build-up of Aβ is a consequence of lysosomal dysfunction [55, 88], others show that Aβ impairs lysosomal functions and lead to deleterious downstream effects [26, 89, 90] (Fig. 2e).
Chronic exposure of BV2 mouse microglial cells to Aβ inhibited autophagic flux and induced lysosomal damage, shown by the presence of the acid hydrolase cathepsin-D in cytoplasm and increased LysoTracker staining in the cytoplasm indicative of lysosomal membrane permeabilization [91]. Prolonged Aβ exposure has also been observed to create an efflux of the nuclear TFEB, a regulator of lysosomal biogenesis, out from the nucleus, resulting in lysosomal acidification impairment and reduced Aβ clearance [26]. A small-molecule activator of TFEB, PF-11, when treated to primary rat microglia enhances TFEB nuclear translocation and mitigates the detrimental effect caused by soluble oligomeric Aβ on lysosomal function [90]. Aβ accumulation in microglia has also been shown to compromise PIEZO1 calcium signaling and reduce lysosomal activity [92]. PIEZO1 is an ion channel and mechanotransducer responsible for communication between the extracellular and intracellular environment. Restoration of PIEZO1 function by small-molecule agonist Yoda1 results in restoration of lysosomal activity and phagocytosis of Aβ [92]. Another type of intrinsically disordered protein, α-synuclein, has also been shown to disrupt microglial lysosomal acidification and inhibit phagocytic function in Parkinson’s disease (PD) [93].
While toxic proteins have been shown to result in lysosomal acidification defects, it has been suggested that toxic proteins can accumulate as a result of inherently weak acidification of microglial lysosomes. Compared with the lysosomes of macrophages and neurons, lysosomes of microglia are more weakly acidic, thereby limiting the function of lysosomal enzymes and hence their degradative activity [51, 90]. It was determined that the activities of PKA and ClC-7 chloride transporter, but not V-ATPase, are responsible for the pH difference between macrophage and microglial lysosomes [51, 55]. In addition, it was suggested that the ClC-7 is activated downstream of PKA, increasing lysosomal acidification in microglia [51]. Knockdown of ClC-7 in activated microglia was shown to lead to improper lysosomal acidification, resulting in impaired degradation of fibrillar Aβ [55]. In addition, it was also found that ClC-7 is mistargeted in quiescent microglia, leading to similar cellular and metabolic deficits. This is because delivery of ClC-7 to the lysosome is dependent on association with the OSTM1, the expression of which is decreased in resting microglia [55]. Interestingly, a study has shown that on the contrary, it is the acidic environment of endosome/lysosomal environment that can accelerate toxic Aβ oligomers formation when uptaken from the extracellular environment [94]. However, the same study also mentioned that the accumulation of Aβ oligomers can lead to further impairment of lysosomal function [94].
To monitor Aβ degradation in glial cells with respect to lysosomal pH, AβpH was developed [95]. AβpH is a human Aβ analog that possess similar aggregation characteristics as synthetic Aβ, but is non-fluorescent at physiological condition around pH 7.0–7.5 and fluorescent in mildly acidic environments of pH 4.5–5.0. AβpH has been applied to show that phagocytosis of AβpH was more prominent in microglia relative to astrocytes both in primary mouse microglial cell lines and in wild-type mouse [95]. In future studies, monitoring the rate of AβpH uptake and clearance in microglia with different genetic alterations will allow for insights into the key players responsible for the lysosomal and phagocytic dysfunction in this pathway.
Lysosome physiology and cathepsin activity
Lysosome size and positioning play a role in modulating lysosomal pH in CNS cells including microglia [96]. Enlarged lysosomes in CNS cells display reduced acidity and reduced lysosomal enzyme activity [97]. In microglia, TMEM106B overexpression has been shown to lead to accumulation of large lysosome-associated membrane proteins (LAMP) positive lysosomes, although the lysosomal pH was not quantified [98]. Interestingly, inhibition of lysosomal V-ATPase activity increases TMEM106B expression levels, strongly indicating the association between TMEM106B-induced lysosome enlargement and lysosomal acidification. In Gaucher disease, altered lysosomal localization or positioning has been shown to be early-onset of microglial activation and neuronal loss [99]. Using HeLa cells as a model, it has been shown that lysosomes located at the cell periphery are less acidic than juxtanuclear ones while maintaining similar luminal buffering capacity [96]. The reduced acidification capability of peripheral lysosomes is due to decreased V-ATPase activity and increased passive permeability to protons, thereby decreasing the overall proton concentration in lysosome lumen [96]. Future studies can be conducted to validate if similar regulatory mechanism is conserved in microglia.
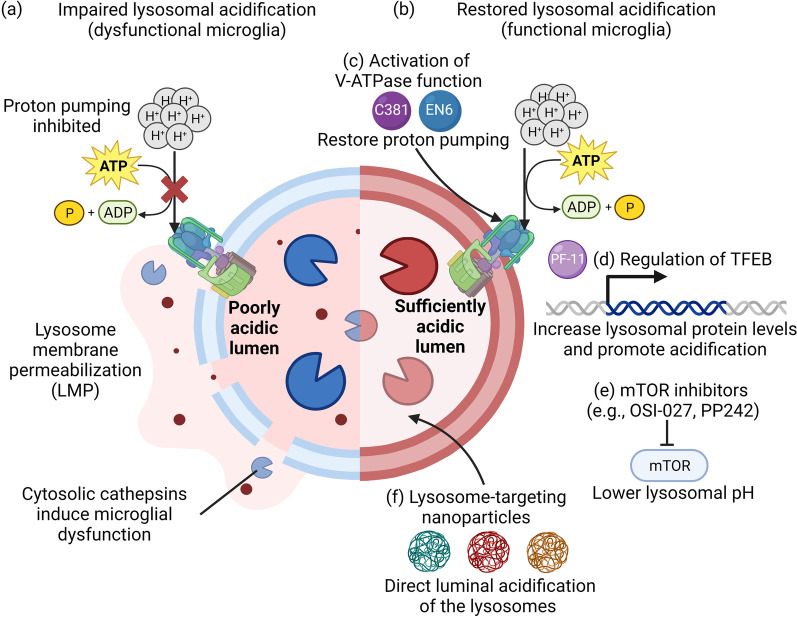
Lysosomal membrane integrity is maintained by highly glycosylated transmembrane proteins, such as LAMP1 and LAMP2, as well as sphingolipids and cholesterol, which all serve to protect the membrane from degradation by lysosomal enzyme [100]. Maintaining lysosomal integrity is essential to preserve lysosomal function, including lysosomal acidification and enzyme activity, and is therefore crucial for cellular homeostasis. Different forms of cellular stress can induce LMP, resulting in the release of intralysosomal components such as cathepsins to the cytoplasm, inducing lysosomal-dependent cell death [91, 100] (Fig. 3a). Furthermore, cathepsins release can increase the release of inflammatory cytokines from microglia which trigger further neuroinflammation [101]. For instance, cathepsin C release has been shown to promote microglial neurotoxic polarization and aggravates neuroinflammation [101]. Cathepsin H could increase the release of IL-1β and interferon-gamma (IFN-γ) from microglial BV2 cells, as well as induce neuronal death [101]. Cathepsin X has been demonstrated to be associated with inflammation-induced neurodegeneration [102]. Maintenance of proper lysosomal and autophagic function through increasing lysosomal biogenesis via TFEB overexpression or by inducing autophagy with rapamycin have been shown to ameliorate LMP induced cell death [103, 104]. Hence, it is imperative for the development of therapeutic agents or strategies that can restore lysosomal acidification to counteract undesired downstream consequences due to LMP (Fig. 3b).
Fig. 3.
An overview of lysosome-targeting therapeutic agents developed to reacidify impaired lysosomes under diseased conditions. a Impaired lysosomal acidification results in lysosomal membrane permeabilization, leading to the release of cathepsins into cytosol that impair microglial function. b Lysosome reacidifying agents can restore lysosomal function to counteract undesired downstream consequences including LMP. Current lysosome-targeting therapeutic strategies have focused on using c small-molecule modulators such as C381 and EN6 to activate V-ATPase activity, d small-molecule activator of TFEB (PF-11) to increase lysosomal protein transcription and expression as well as promote lysosomal acidification, e mTOR inhibitors such as OSI-027 and PP242 to lower lysosomal pH, and f lysosome-targeting acidic nanoparticles to directly acidify the lysosome lumen. The figure was created with BioRender.com
Lysosomal pH monitoring strategies and therapeutic agents for reacidification
Fluorescent probes and reporter plasmids are common methods used for measuring lysosomal pH changes in microglia [105–107]. To increase the specificity to measure pH in the lysosomes organelle component, a more recent development is a tandem fusion of a pHluorin-mCherry linked to the luminal domain of the LAMP1 lysosomal marker protein, along with a cytosolic 3xFLAG tag that can efficiently isolate and purify lysosomes for evaluation of their function [108]. To detect lysosomal pH in vivo, the Nixon group has developed an autophagy reporter mouse expressing mRFP-eGFP-LC3 in neurons [37]. For future applications in lysosomal pH monitoring in microglia, the promoter can be changed from neuronal specific Thy1 promoter to TMEM119 or CXCR3 microglial specific promoter. Recent development of non-invasive lysosomal pH in vivo monitoring probe involve detection with near-infrared light, which only affords a penetration depth of a few millimeters into the tissues [109], making the application of this technique in CNS to be further investigated.
Given the wide-ranging cellular implications of lysosomal acidification in microglia, targeting and restoration of defective lysosomal acidification in microglia represents a promising therapeutic avenue for neurodegenerative diseases. Various therapeutic agents including small molecules that activate V-ATPase function (Fig. 3c), regulate TFEB expression (Fig. 3d), and inhibit mTOR signaling (Fig. 3e), as well as lysosome-targeting nanoparticles have been developed (Fig. 3f) [110, 111] and some have been applied for the restoration of impaired microglial lysosomal acidification. In particular, C381 and PF-11 have demonstrated capability to acidify microglial lysosomes [90, 112]. C381 has been reported to stimulate lysosomal acidification, mitigate lysosomal membrane permeabilization, and reduce neuroinflammation and neurodegeneration in mouse models of frontotemporal dementia and PD [112]. Compared to other cell types, it appears that C381 is significantly more effective in restoring lysosomal function in microglia, although whether there is any cell-specific effect remains to be illuminated [112]. By acting through alternative mechanisms, the small-molecule PF-11 has demonstrated the ability to enhance lysosomal acidification and function in microglia by increasing TFEB nuclear translocation and the expression of V-ATPase [90]. Lysosome-directed nanoparticles (NPs) are another type of therapeutic agent that has shown efficacy in improving lysosomal acidification [110, 113–118]. These NPs have been applied in both in vitro and in vivo models to restore lysosomal acidification in various cell types, including neuronal lysosomes, although their effects in modulating microglial lysosomal pH have not yet been characterized. Future screening studies can be conducted using cell-based assays with fluorescent probes and reporter plasmids, as well as artificial lysosomes based on polymersomes loaded with trypsin to discover novel modulators of lysosomal pH [119, 120].
Conclusions
Microglial lysosomal acidification defects have been shown to result in inhibition of phagocytosis and autophagy, leading to the release of pro-inflammatory cytokines and the accumulation of toxic proteins, perpetuating neuroinflammation and exacerbating neurodegeneration. While the above discussion is based on stimuli-induced conditions, it remains important to investigate, under no inflammatory stimulation, whether early-onset genetic mutations or senescent microglia under normal aging would develop lysosomal acidification, and if these lysosomal acidification defects would initiate early neuroinflammation. In addition, while most of the studies adopted strategies to reacidify impaired lysosomes to maintain normal microglial function, some studies have observed that over-acidification of lysosomes can also lead to undesirable effects. Hence, this suggests that there is an optimal lysosomal pH level to be achieved for proper microglial function.
Previously thought to occupy a distinct cellular role, one organelle whose function has come to be recognized as highly intertwined with the lysosome is the mitochondria [97, 121], where the organelle that is damaged first is likely to encourage the subsequent impairment of the other, thereby establishing a complex lysosome–mitochondria crosstalk [59]. Defective lysosomal acidification can induce autophagic dysfunction which may further lead to defective turnover of mitochondria, which results in the accumulation of older mitochondria that generates significant amounts of reactive oxygen species in the microglia [122], which propagates inflammation [122]. On the other hand, mitochondria is the major producer of intracellular ATP, which is an important energy source used by the V-ATPase on lysosomes to maintain proper lysosomal acidification [121]. The contribution of impaired lysosome–mitochondria crosstalk to the pathology of AD, PD, and MS has been widely reported [121, 123, 124], although the mechanisms underlying these deficits remain somewhat elusive and more investigations are required to elucidate this bidirectional relationship [97].
To maintain homeostatic function in the brain, microglia cooperate with neurons and astrocytes, and their functions in turn affect microglia [54, 59]. It has been shown that neuronal damages affect lysosomal acidification in microglia [125]. Microglia and astrocytes both respond to neuronal injury with processes such as phagocytosis, proliferation, and morphological alterations [126–128]. Crosstalk between microglia and astrocytes is maintained in part via secreted factors including cytokines, chemokines, growth factors, neurotransmitters, as well as extracellular vesicles and tunneling nanotubes [129–131]. Hence, the role of microglial lysosomal acidification and function can be further complicated by the crosstalk between CNS cells, and further investigations are needed to clarify this complex relationship for improved understanding of the mechanisms underlying brain health and disease.
Acknowledgements
The authors thank the funding sources for supporting this work.
Abbreviations
- Aβ
β-Amyloid
- AcNPs
Acidic nanoparticles
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- APP-βCTF
APP β-C-terminal fragment
- ATP
Adenosine triphosphate
- BBB
Blood–brain barrier
- cAMP
Cyclic adenosine monophosphate
- CNS
Central nervous system
- EAE
Experimental autoimmune encephalomyelitis
- FAD
Familial Alzheimer’s disease
- IFN-γ
Interferon-gamma
- iMGL
IPSC-derived microglia
- iPSC
Induced pluripotent stem cells
- LAMP
Lysosome-associated membrane proteins
- LMP
Lysosomal membrane permeabilization
- LPS
Lipopolysaccharide
- MCSF
Macrophage colony-stimulating factor
- NF-κB
Nuclear factor kappa B
- NHD
Nasu–Hakola disease
- NLRP3
NLR family pyrin domain containing 3
- NP
Nanoparticle
- OSTM1
Osteoporosis-associated transmembrane protein 1
- P2X4R
P2X4 receptor
- P2X7R
P2X7 receptor
- PaNP
Photo-activated nanoparticle
- PD
Parkinson’s disease
- PKA
Protein kinase A
- PKB
Protein kinase B
- PLGA
Poly(lactic-co-glycolic acid)
- PS1
Presenilin 1
- PS2
Presenilin 2
- sAC
Soluble adenylyl cyclase
- SNARE
Soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- TFEB
Transcription factor EB
- TREM2
Triggering receptor expressed on myeloid cells 2
- UV
Ultraviolet
- V-ATPase
Vacuolar (H+)-ATPase
Author contributions
CHL and JZ conceived the review topic. JDQ, CS, JHW, JZ, and CHL critically reviewed the literatures and wrote the manuscript. KLL, RR, and AMB provided critical comments and edited the manuscript.
Funding
C.H.L. is supported by a Lee Kong Chian School of Medicine Dean’s Postdoctoral Fellowship (021207-00001) from Nanyang Technological University (NTU) Singapore and a Mistletoe Research Fellowship (022522-00001) from the Momental Foundation USA. J.Z. is supported by a Presidential Postdoctoral Fellowship (021229-00001) from NTU Singapore and an Open Fund Young Investigator Research Grant (OF-YIRG) (MOH-001147) from the National Medical Research Council (NMRC) Singapore.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jialiu Zeng, Email: jialiu.zeng@ntu.edu.sg.
Chih Hung Lo, Email: chihhung.lo@ntu.edu.sg.
References
- 1.Thion MS, Ginhoux F, Garel S. Microglia and early brain development: an intimate journey. Science. 2018;362:185–189. doi: 10.1126/science.aat0474. [DOI] [PubMed] [Google Scholar]
- 2.Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488. doi: 10.3389/fncel.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galloway DA, Phillips AEMM, Owen DRJJ, Moore CS. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 2019;10:790. doi: 10.3389/fimmu.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu F, Wang Y, Stetler AR, Leak RK, Hu X, Chen J. Phagocytic microglia and macrophages in brain injury and repair. CNS Neurosci Ther. 2022;28:1279–1293. doi: 10.1111/cns.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang S, Qin C, Hu Z-W, Zhou L-Q, Yu H-H, Chen M, et al. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol Dis. 2021;152:105290. doi: 10.1016/j.nbd.2021.105290. [DOI] [PubMed] [Google Scholar]
- 6.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139:136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moehle MS, West AB. M1 and M2 immune activation in Parkinson’s disease: foe and ally? Neuroscience. 2015;302:59–73. doi: 10.1016/j.neuroscience.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paolicelli RC, Sierra A, Stevens B, Tremblay M-E, Aguzzi A, Ajami B, et al. Microglia states and nomenclature: a field at its crossroads. Neuron. 2022;110:3458–3483. doi: 10.1016/j.neuron.2022.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor LM, O’Connor BA, Lim SB, Zeng J, Lo CH. Integrative multi-omics and systems bioinformatics in translational neuroscience: a data mining perspective. J Pharm Anal. 2023 doi: 10.1016/j.jpha.2023.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm. 2014;98:1–5. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer H, Shen K, Meireles AM, Talbot WS. A lysosomal regulatory circuit essential for the development and function of microglia. Sci Adv. 2023;8:eabp8321. doi: 10.1126/sciadv.abp8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plaza-Zabala A, Sierra-Torre V, Sierra A. Autophagy and microglia: novel partners in neurodegeneration and aging. Int J Mol Sci. 2017;18:598. doi: 10.3390/ijms18030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Acker ZP, Perdok A, Bretou M, Annaert W. The microglial lysosomal system in Alzheimer’s disease: guardian against proteinopathy. Ageing Res Rev. 2021;71:101444. doi: 10.1016/j.arr.2021.101444. [DOI] [PubMed] [Google Scholar]
- 14.Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification—the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi PC, Bartlett JJ, Pulinilkunnil T. Lysosomal biology and function: modern view of cellular debris bin. Cells. 2020;9:1131. doi: 10.3390/cells9051131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol. 2014;88:594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi F, Yang Y, Kouadir M, Fu Y, Yang L, Zhou X, et al. Inhibition of phagocytosis and lysosomal acidification suppresses neurotoxic prion peptide-induced NALP3 inflammasome activation in BV2 microglia. J Neuroimmunol. 2013;260:121–125. doi: 10.1016/j.jneuroim.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Jin MM, Wang F, Qi D, Liu WW, Gu C, Mao CJ, et al. A critical role of autophagy in regulating microglia polarization in neurodegeneration. Front Aging Neurosci. 2018;10:378. doi: 10.3389/fnagi.2018.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campagno KE, Mitchell CH. The P2X7 receptor in microglial cells modulates the endolysosomal axis, autophagy, and phagocytosis. Front Cell Neurosci. 2021;15:645244. doi: 10.3389/fncel.2021.645244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solé-Domènech S, Cruz DL, Capetillo-Zarate E, Maxfield FR. The endocytic pathway in microglia during health, aging and Alzheimer’s disease. Ageing Res Rev. 2016;32:89–103. doi: 10.1016/j.arr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matejuk A, Ransohoff RM. Crosstalk between astrocytes and microglia: an overview. Front Immunol. 2020;11:1416. doi: 10.3389/fimmu.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci. 2019;116:9714–9722. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaraman A, Reynolds R. Diverse pathways to neuronal necroptosis in Alzheimer’s disease. Eur J Neurosci. 2022;56:5428–5441. doi: 10.1111/ejn.15662. [DOI] [PubMed] [Google Scholar]
- 24.Lin M, Yu H, Xie Q, Xu Z, Shang P. Role of microglia autophagy and mitophagy in age-related neurodegenerative diseases. Front Aging Neurosci. 2023;14:1100133. doi: 10.3389/fnagi.2022.1100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lautrup S, Lou G, Aman Y, Nilsen H, Tao J, Fang EF. Microglial mitophagy mitigates neuroinflammation in Alzheimer’s disease. Neurochem Int. 2019;129:104469. doi: 10.1016/j.neuint.2019.104469. [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Tang P, Chen L, Liu P, Hou C, Zhang X, et al. Amyloid β-induced redistribution of transcriptional factor EB and lysosomal dysfunction in primary microglial cells. Front Aging Neurosci. 2017;9:228. doi: 10.3389/fnagi.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berglund R, Guerreiro-Cacais AO, Adzemovic MZ, Zeitelhofer M, Lund H, Ewing E, et al. Microglial autophagy-associated phagocytosis is essential for recovery from neuroinflammation. Sci Immunol. 2020;5:eabb5077. doi: 10.1126/sciimmunol.abb5077. [DOI] [PubMed] [Google Scholar]
- 29.Udeochu JC, Shea JM, Villeda SA. Microglia communication: Parallels between aging and Alzheimer’s disease. Clin Exp Neuroimmunol. 2016;7:114–125. doi: 10.1111/cen3.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe MS. Structure and function of the γ-secretase complex. Biochemistry. 2019;58:2953–2966. doi: 10.1021/acs.biochem.9b00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledo JH, Liebmann T, Zhang R, Chang JC, Azevedo EP, Wong E, et al. Presenilin 1 phosphorylation regulates amyloid-β degradation by microglia. Mol Psychiatry. 2021;26:5620–5635. doi: 10.1038/s41380-020-0856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Chan SL, Mattson MP. Adverse effect of a presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. NeuroMolecular Med. 2002;2:29–45. doi: 10.1385/NMM:2:1:29. [DOI] [PubMed] [Google Scholar]
- 34.Cao Q, Li P, Lu J, Dheen ST, Kaur C, Ling EA. Nuclear factor-κB/p65 responds to changes in the notch signaling pathway in murine BV-2 cells and in amoeboid microglia in postnatal rats treated with the γ-secretase complex blocker DAPT. J Neurosci Res. 2010;88:2701–2714. doi: 10.1002/jnr.22429. [DOI] [PubMed] [Google Scholar]
- 35.Lee J-H, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CYD, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J-H, Yang D-S, Goulbourne CN, Im E, Stavrides P, Pensalfini A, et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat Neurosci. 2022;25:688–701. doi: 10.1038/s41593-022-01084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustos V, Pulina MV, Bispo A, Lam A, Flajolet M, Gorelick FS, et al. Phosphorylated presenilin 1 decreases β-amyloid by facilitating autophagosome-lysosome fusion. Proc Natl Acad Sci U S A. 2017;114:7148–7153. doi: 10.1073/pnas.1705240114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bustos V, Pulina MV, Kelahmetoglu Y, Sinha SC, Gorelick FS, Flajolet M, et al. Bidirectional regulation of Aβ levels by presenilin 1. Proc Natl Acad Sci U S A. 2017;114:7142–7147. doi: 10.1073/pnas.1705235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fung S, Smith CL, Prater KE, Case A, Green K, Osnis L, et al. Early-onset familial Alzheimer disease variant PSEN2 N141I heterozygosity is associated with altered microglia phenotype. J Alzheimers Dis. 2020;77:675–688. doi: 10.3233/JAD-200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pigino G, Pelsman A, Mori H, Busciglio J. Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation. J Neurosci. 2001;21:834–842. doi: 10.1523/JNEUROSCI.21-03-00834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behbahani H, Shabalina IG, Wiehager B, Concha H, Hultenby K, Petrovic N, et al. Differential role of Presenilin-1 and -2 on mitochondrial membrane potential and oxygen consumption in mouse embryonic fibroblasts. J Neurosci Res. 2006;84:891–902. doi: 10.1002/jnr.20990. [DOI] [PubMed] [Google Scholar]
- 43.Cai Z, Hussain MD, Yan LJ. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci. 2014;124:307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 44.Orre M, Kamphuis W, Osborn LM, Jansen AHP, Kooijman L, Bossers K, et al. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol Aging. 2014;35:2746–2760. doi: 10.1016/j.neurobiolaging.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Wang W-Y, Tan M-S, Yu J-T, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbas N, Bednar I, Mix E, Marie S, Paterson D, Ljungberg A, et al. Up-regulation of the inflammatory cytokines IFN-ɣ; and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APPSWE transgenic mice. J Neuroimmunol. 2002;126:50–57. doi: 10.1016/s0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 47.Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 48.Urrutia PJ, Bórquez DA, Núñez MT. Inflaming the brain with iron. Antioxidants. 2021;6:1–27. doi: 10.3390/antiox10010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. 2022;22:657–673. doi: 10.1038/s41577-022-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lema Tomé CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and α-Synuclein’s prion-like behavior in Parkinson’s disease—is there a link? Mol Neurobiol. 2013;47:561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, et al. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18:1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herber DL, Roth LM, Wilson D, Wilson N, Mason JE, Morgan D, et al. Time-dependent reduction in Aβ levels after intracranial LPS administration in APP transgenic mice. Exp Neurol. 2004;190:245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Ye X, Zhu M, Che X, Wang H, Liang XJ, Wu C, et al. Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J Neuroinflamm. 2020;17:18. doi: 10.1186/s12974-019-1644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Festa BP, Siddiqi FH, Jimenez-Sanchez M, Won H, Rob M, Djajadikerta A, et al. Microglial-to-neuronal CCR5 signaling regulates autophagy in neurodegeneration. Neuron. 2023;111:2021–2037.e12. doi: 10.1016/j.neuron.2023.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Majumdar A, Capetillo-Zarate E, Cruz D, Gouras GK, Maxfield FR. Degradation of Alzheimer’s amyloid fibrils by microglia requires delivery of CIC-7 to lysosomes. Mol Biol Cell. 2011;22:1664–1676. doi: 10.1091/mbc.E10-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- 57.Lau S-F, Fu AKY, Ip NY. Cytokine signaling convergence regulates the microglial state transition in Alzheimer’s disease. Cell Mol Life Sci. 2021;78:4703–4712. doi: 10.1007/s00018-021-03810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauro C, Limatola C. Metabolic reprograming of microglia in the regulation of the innate inflammatory response. Front Immunol. 2020;11:493. doi: 10.3389/fimmu.2020.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yambire KF, Rostosky C, Watanabe T, Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A, et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife. 2019;8:e51031. doi: 10.7554/eLife.51031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng F, Fleming BA, Jia X, Rousek AA, Mulvey MA, Ward DM. Lysosomal iron recycling in mouse macrophages is dependent upon both LcytB and Steap3 reductases. Blood Adv. 2022;6:1692–1707. doi: 10.1182/bloodadvances.2021005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gabandé-Rodríguez E, Pérez-Cañamás A, Soto-Huelin B, Mitroi DN, Sánchez-Redondo S, Martínez-Sáez E, et al. Lipid-induced lysosomal damage after demyelination corrupts microglia protective function in lysosomal storage disorders. EMBO J. 2019;38:e99553. doi: 10.15252/embj.201899553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muzio L, Viotti A, Martino G. Microglia in neuroinflammation and neurodegeneration: from understanding to therapy. Front Neurosci. 2021;15:742065. doi: 10.3389/fnins.2021.742065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang YC, Hsu SM, Shie FS, Shiao YJ, Chao L-JJ, Chen HW, et al. Reduced mitochondria membrane potential and lysosomal acidification are associated with decreased oligomeric Aβ degradation induced by hyperglycemia: a study of mixed glia cultures. PLoS ONE. 2022;17:e0260966. doi: 10.1371/journal.pone.0260966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loving BA, Bruce KD. Lipid and lipoprotein metabolism in microglia. Front Physiol. 2020;11:393. doi: 10.3389/fphys.2020.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou L-Q, Dong M-H, Hu Z-W, Tang Y, Chu Y-H, Chen M, et al. Staged suppression of microglial autophagy facilitates regeneration in CNS demyelination by enhancing the production of linoleic acid. Proc Natl Acad Sci. 2023;120:e2209990120. doi: 10.1073/pnas.2209990120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ulland TK, Song WM, Huang SC-C, Ulrich JD, Sergushichev A, Beatty WL, et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell. 2017;170:649–663. doi: 10.1016/j.cell.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fabia F, Shih Feng Y, Sidhartha M, Abhirami Kannan I, Rita M, Olena K, et al. Defects in lysosomal function and lipid metabolism in human microglia harboring a TREM2 loss of function mutation. Acta Neuropathol. 2023;145:749–772. doi: 10.1007/s00401-023-02568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parhizkar S, Gent G, Chen Y, Rensing N, Gratuze M, Strout G, et al. Sleep deprivation exacerbates microglial reactivity and Aβ deposition in a TREM2-dependent manner in mice. Sci Transl Med. 2023;15:eade6285. doi: 10.1126/scitranslmed.ade6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang T, Pang W, Feng T, Guo J, Wu K, Nunez Santos M, et al. TMEM106B regulates microglial proliferation and survival in response to demyelination. Sci Adv. 2023;9:eadd2676. doi: 10.1126/sciadv.add2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka Y, Suzuki G, Matsuwaki T, Hosokawa M, Serrano G, Beach TG, et al. Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes. Hum Mol Genet. 2017;26:969–988. doi: 10.1093/hmg/ddx011. [DOI] [PubMed] [Google Scholar]
- 72.Reifschneider A, Robinson S, van Lengerich B, Gnörich J, Logan T, Heindl S, et al. Loss of TREM2 rescues hyperactivation of microglia, but not lysosomal deficits and neurotoxicity in models of progranulin deficiency. EMBO J. 2022;41:e109108. doi: 10.15252/embj.2021109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y, Shao W, Todd TW, Tong J, Yue M, Koga S, et al. Microglial lysosome dysfunction contributes to white matter pathology and TDP-43 proteinopathy in GRN-associated FTD. Cell Rep. 2021;36:109581. doi: 10.1016/j.celrep.2021.109581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montilla A, Mata GP, Matute C, Domercq M. Contribution of P2X4 receptors to CNS function and pathophysiology. Int J Mol Sci. 2020;21:5562. doi: 10.3390/ijms21155562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takenouchi T, Nakai M, Iwamaru Y, Sugama S, Tsukimoto M, Fujita M, et al. The activation of P2X7 receptor impairs lysosomal functions and stimulates the release of autophagolysosomes in microglial cells. J Immunol. 2009;182:2051–2062. doi: 10.4049/jimmunol.0802577. [DOI] [PubMed] [Google Scholar]
- 76.Vázquez-Villoldo N, Domercq M, Martín A, Llop J, Gómez-Vallejo V, Matute C. P2X4 receptors control the fate and survival of activated microglia. Glia. 2014;62:171–184. doi: 10.1002/glia.22596. [DOI] [PubMed] [Google Scholar]
- 77.Kanellopoulos JM, Almeida-da-Silva CLC, Rüütel Boudinot S, Ojcius DM. Structural and functional features of the P2X4 receptor: an immunological perspective. Front Immunol. 2021;12:645834. doi: 10.3389/fimmu.2021.645834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A, et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med. 2018;10:e8743. doi: 10.15252/emmm.201708743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, et al. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J Biol Chem. 2014;289:17658–17667. doi: 10.1074/jbc.M114.552158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahman N, Ramos-Espiritu L, Milner TA, Buck J, Levin LR. Soluble adenylyl cyclase is essential for proper lysosomal acidification. J Gen Physiol. 2016;148:325–339. doi: 10.1085/jgp.201611606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lo CH, Sachs JN. The role of wild-type tau in Alzheimer’s disease and related tauopathies. J life Sci. 2020;2:1–17. doi: 10.36069/jols/20201201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lo CH. Heterogeneous Tau oligomers as molecular targets for Alzheimer’s disease and related tauopathies. Biophysica. 2022;2:440–451. [Google Scholar]
- 83.Lo CH. Recent advances in cellular biosensor technology to investigate tau oligomerization. Bioeng Transl Med. 2021;6:e10231. doi: 10.1002/btm2.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sengupta U, Nilson AN, Kayed R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bloom GS. Amyloid-β and Tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 86.Lo CH, Lim CKW, Ding Z, Wickramasinghe SP, Braun AR, Ashe KH, et al. Targeting the ensemble of heterogeneous tau oligomers in cells: a novel small molecule screening platform for tauopathies. Alzheimer’s Dement. 2019;15:1489–1502. doi: 10.1016/j.jalz.2019.06.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Odfalk KF, Bieniek KF, Hopp SC. Microglia: friend and foe in tauopathy. Prog Neurobiol. 2022;216:102306. doi: 10.1016/j.pneurobio.2022.102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koh J-Y, Kim HN, Hwang JJ, Kim Y-H, Park SE. Lysosomal dysfunction in proteinopathic neurodegenerative disorders: possible therapeutic roles of cAMP and zinc. Mol Brain. 2019;12:18. doi: 10.1186/s13041-019-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Im E, Jiang Y, Stavrides P, Darji S, Erdjument-Bromage H, Neubert TA, et al. Lysosomal dysfunction in Down Syndrome and Alzheimer mouse models is caused by selective v-ATPase inhibition by Tyr682 phosphorylated APP βCTF. bioRxiv. 2022 doi: 10.1101/2022.06.02.494546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao XC, Xue X, Zhang HT, Zhu MM, Yang XW, Wu CF, et al. Pseudoginsenoside-F11 alleviates oligomeric β-amyloid-induced endosome-lysosome defects in microglia. Traffic. 2019;20:61–70. doi: 10.1111/tra.12620. [DOI] [PubMed] [Google Scholar]
- 91.Pomilio C, Gorojod RM, Riudavets M, Vinuesa A, Presa J, Gregosa A, et al. Microglial autophagy is impaired by prolonged exposure to β-amyloid peptides: evidence from experimental models and Alzheimer’s disease patients. GeroScience. 2020;42:613–632. doi: 10.1007/s11357-020-00161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jäntti H, Sitnikova V, Ishchenko Y, Shakirzyanova A, Giudice L, Ugidos IF, et al. Microglial amyloid beta clearance is driven by PIEZO1 channels. J Neuroinflamm. 2022;19:147. doi: 10.1186/s12974-022-02486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haenseler W, Zambon F, Lee H, Vowles J, Rinaldi F, Duggal G, et al. Excess α-synuclein compromises phagocytosis in iPSC-derived macrophages. Sci Rep. 2017;7:9003. doi: 10.1038/s41598-017-09362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schützmann MP, Hasecke F, Bachmann S, Zielinski M, Hänsch S, Schröder GF, et al. Endo-lysosomal Aβ concentration and pH trigger formation of Aβ oligomers that potently induce Tau missorting. Nat Commun. 2021;12:4634. doi: 10.1038/s41467-021-24900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prakash P, Jethava KP, Korte N, Izquierdo P, Favuzzi E, Rose IVLL, et al. Monitoring phagocytic uptake of amyloid β into glial cell lysosomes in real time. Chem Sci. 2021;12:10901–10918. doi: 10.1039/d1sc03486c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol. 2016;212:677–692. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deus CM, Yambire KF, Oliveira PJ, Raimundo N. Mitochondria–lysosome crosstalk: from physiology to neurodegeneration. Trends Mol Med. 2020;26:71–88. doi: 10.1016/j.molmed.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Stagi M, Klein ZA, Gould TJ, Bewersdorf J, Strittmatter SM. Lysosome size, motility and stress response regulated by fronto-temporal dementia modifier TMEM106B. Mol Cell Neurosci. 2014;61:226–240. doi: 10.1016/j.mcn.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zigdon H, Meshcheriakova A, Farfel-Becker T, Volpert G, Sabanay H, Futerman AH. Altered lysosome distribution is an early neuropathological event in neurological forms of Gaucher disease. FEBS Lett. 2017;5:774–783. doi: 10.1002/1873-3468.12591. [DOI] [PubMed] [Google Scholar]
- 100.Wang F, Gómez-Sintes R, Boya P. Lysosomal membrane permeabilization and cell death. Traffic. 2018;19:918–931. doi: 10.1111/tra.12613. [DOI] [PubMed] [Google Scholar]
- 101.Nakanishi H. Cathepsin regulation on microglial function. Biochim Biophys Acta Proteins Proteom. 2020;1868:140465. doi: 10.1016/j.bbapap.2020.140465. [DOI] [PubMed] [Google Scholar]
- 102.Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28:1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 103.Rodríguez-Muela N, Hernández-Pinto AM, Serrano-Puebla A, García-Ledo L, Latorre SH, de la Rosa EJ, et al. Lysosomal membrane permeabilization and autophagy blockade contribute to photoreceptor cell death in a mouse model of retinitis pigmentosa. Cell Death Differ. 2015;22:476–487. doi: 10.1038/cdd.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.García-Sanz P, Orgaz L, Bueno-Gil G, Espadas I, Rodríguez-Traver E, Kulisevsky J, et al. N370S-GBA1 mutation causes lysosomal cholesterol accumulation in Parkinson’s disease. Mov Disord. 2017;32:1409–1422. doi: 10.1002/mds.27119. [DOI] [PubMed] [Google Scholar]
- 105.Chikte S, Panchal N, Warnes G. Use of LysoTracker dyes: a flow cytometric study of autophagy. Cytometry A. 2014;85:169–178. doi: 10.1002/cyto.a.22312. [DOI] [PubMed] [Google Scholar]
- 106.Yapici NB, Bi Y, Li P, Chen X, Yan X, Mandalapu SR, et al. Highly stable and sensitive fluorescent probes (LysoProbes) for lysosomal labeling and tracking. Sci Rep. 2015;5:8576. doi: 10.1038/srep08576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chin MY, Ang K-H, Davies J, Alquezar C, Garda VG, Rooney B, et al. Phenotypic screening using high-content imaging to identify lysosomal pH modulators in a neuronal cell model. ACS Chem Neurosci. 2022;13:1505–1516. doi: 10.1021/acschemneuro.1c00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ponsford AH, Ryan TA, Raimondi A, Cocucci E, Wycislo SA, Fröhlich F, et al. Live imaging of intra-lysosome pH in cell lines and primary neuronal culture using a novel genetically encoded biosensor. Autophagy. 2020;17:1–19. doi: 10.1080/15548627.2020.1771858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim M, Chen C, Yaari Z, Frederiksen R, Randall E, Wollowitz J, et al. Nanosensor-based monitoring of autophagy-associated lysosomal acidification in vivo. Nat Chem Biol. 2023 doi: 10.1038/s41589-023-01364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lo CH, Zeng J. Defective lysosomal acidification: a new prognostic marker and therapeutic target for neurodegenerative diseases. Transl Neurodegener. 2023;12:29. doi: 10.1186/s40035-023-00362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeng J, Shirihai OS, Grinstaff MW. Modulating lysosomal pH: a molecular and nanoscale materials design perspective. J life Sci. 2020;2:25–37. doi: 10.36069/jols/20201204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vest RT, Chou C-C, Zhang H, Haney MS, Li L, Laqtom NN, et al. Small molecule C381 targets the lysosome to reduce inflammation and ameliorate disease in models of neurodegeneration. Proc Natl Acad Sci U S A. 2022;119:e2121609119. doi: 10.1073/pnas.2121609119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arotcarena MM-L, Soria FN, Cunha A, Doudnikoff E, Prévot G, Daniel J, et al. Acidic nanoparticles protect against α-synuclein-induced neurodegeneration through the restoration of lysosomal function. Aging Cell. 2022;21:e13584. doi: 10.1111/acel.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trudeau KM, Colby AH, Zeng J, Las G, Feng JH, Grinstaff MW, et al. Lysosome acidification by photoactivated nanoparticles restores autophagy under lipotoxicity. J Cell Biol. 2016;214:25–34. doi: 10.1083/jcb.201511042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeng J, Martin A, Han X, Shirihai O, Grinstaff M. Biodegradable PLGA nanoparticles restore lysosomal acidity and protect neural PC-12 cells against mitochondrial toxicity. Ind Eng Chem Res. 2019;58:13910–13917. doi: 10.1021/acs.iecr.9b02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang B, Martini-Stoica H, Qi C, Lu T-C, Wang S, Xiong W, et al. TFEB-vacuolar ATPase signaling regulates lysosomal function and microglial activation in tauopathy. bioRxiv. 2023 doi: 10.1101/2023.02.06.527293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng J, Acin-Perez R, Assali EA, Martin A, Brownstein AJ, Petcherski A, et al. Restoration of lysosomal acidification rescues autophagy and metabolic dysfunction in non-alcoholic fatty liver disease. Nat Commun. 2023;14:2573. doi: 10.1038/s41467-023-38165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lo CH, O’Connor LM, Loi GWZ, Saipuljumri EN, Indajang J, Lopes KM, et al. Acidic nanoparticles restore lysosomal acidification and rescue metabolic dysfunction in pancreatic β-cells under lipotoxic condition. bioRxiv. 2023 doi: 10.1101/2023.07.11.548395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu X, Moreno S, Boye S, Wang P, Voit B, Appelhans D. Artificial organelles with digesting characteristics: imitating simplified lysosome- and macrophage-like functions by trypsin-loaded polymersomes. Adv Sci. 2023;10:2207214. doi: 10.1002/advs.202207214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lo CH, Zeng J. Application of polymersomes in membrane protein study and drug discovery: progress, strategies, and perspectives. Bioeng Transl Med. 2023;8:e10350. doi: 10.1002/btm2.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Audano M, Schneider A, Mitro N. Mitochondria, lysosomes, and dysfunction: their meaning in neurodegeneration. J Neurochem. 2018;147:291–309. doi: 10.1111/jnc.14471. [DOI] [PubMed] [Google Scholar]
- 122.Nakanishi H, Wu Z. Microglia-aging: Roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res. 2009;201:1–7. doi: 10.1016/j.bbr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 123.Pitt D, Lo CH, Gauthier SA, Hickman RA, Longbrake E, Airas LM, et al. Toward precision phenotyping of multiple sclerosis. Neurol - Neuroimmunol Neuroinflamm. 2022;9:e200025. doi: 10.1212/NXI.0000000000200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fairley LH, Lai KO, Wong JH, Chong WJ, Vincent AS, D’Agostino G, et al. Mitochondrial control of microglial phagocytosis by the translocator protein and hexokinase 2 in Alzheimer’s disease. Proc Natl Acad Sci. 2023;120:e2209177120. doi: 10.1073/pnas.2209177120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ziegenfuss JS, Doherty J, Freeman MR. Distinct molecular pathways mediate glial activation and engulfment of axonal debris after axotomy. Nat Neurosci. 2012;15:979–987. doi: 10.1038/nn.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Di Benedetto G, Burgaletto C, Bellanca CM, Munafò A, Bernardini R, Cantarella G. Role of microglia and astrocytes in Alzheimer’s disease: from neuroinflammation to Ca2+ homeostasis dysregulation. Cells. 2022;11:2728. doi: 10.3390/cells11172728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lo CH, Skarica M, Mansoor M, Bhandarkar S, Toro S, Pitt D. Astrocyte heterogeneity in multiple sclerosis: current understanding and technical challenges. Front Cell Neurosci. 2021;15:726479. doi: 10.3389/fncel.2021.726479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sen MK, Mahns DA, Coorssen JR, Shortland PJ. The roles of microglia and astrocytes in phagocytosis and myelination: insights from the cuprizone model of multiple sclerosis. Glia. 2022;70:1215–1250. doi: 10.1002/glia.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark GT, Ingelsson M, et al. Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes. J Neurosci. 2017;37:11835. doi: 10.1523/JNEUROSCI.0983-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Delpech J-C, Herron S, Botros MB, Ikezu T. Neuroimmune crosstalk through extracellular vesicles in health and disease. Trends Neurosci. 2019;42:361–372. doi: 10.1016/j.tins.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rueda-Carrasco J, Martin-Bermejo MJ, Pereyra G, Mateo MI, Borroto A, Brosseron F, et al. SFRP1 modulates astrocyte-to-microglia crosstalk in acute and chronic neuroinflammation. EMBO Rep. 2021;22:e51696. doi: 10.15252/embr.202051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.