Abstract
Theiler’s murine encephalomyelitis viruses are picornaviruses that can infect the central nervous system. The DA strain produces an acute polioencephalomyelitis followed by a chronic demyelinating disease in its natural host, the mouse. The ability of DA virus to induce a demyelinating disease renders this virus infection a model for human demyelinating diseases such as multiple sclerosis. Here we describe the generation and characterization of DA virus mutants that contain specific mutations in the viral capsid protein VP1 at sites believed to be important contact regions for the cellular receptor(s). A mutant virus with a threonine-to-aspartate (T81D) substitution in VP1 loop I adjacent to the putative virus receptor binding site exhibited a large-plaque phenotype but had a slower replication cycle in vitro. When this mutant virus was injected into susceptible mice, an altered tropism was seen during the acute stage of the disease and the chronic demyelinating disease was not produced. A virus with a threonine-to-valine substitution (T81V) did not cause any changes in the pattern or extent of disease seen in mice, whereas a virus with a tryptophan substitution at this position (T81W) produced a similar acute disease but was attenuated for the development of the chronic disease. A change in amino acids in a hydrophobic patch located in the wall of the pit, VP1 position 91, to a hydrophilic threonine (V91T) resulted in a profound attenuation of the acute and chronic disease without persistence of virus. This report illustrates the importance of the loop I of VP1 and a site in the wall of the pit in pathogenesis and that amino acid substitutions at these sites result in altered virus-host interactions.
Theiler’s murine encephalomyelitis viruses (TMEVs) belong to the family Picornaviridae and are natural pathogens of mice that can infect the central nervous system (CNS) (13). Based on biological differences, TMEVs are divided into two subgroups. The GDVII subgroup contains neurovirulent strains that produce fatal encephalitis. On intracerebral inoculation of susceptible mice, viruses of the TO subgroup, which contains the BeAn and DA strains, produce a biphasic disease: an acute polioencephalomyelitis which occurs when neurons in the gray matter are infected (acute stage), followed by a chronic demyelinating disease (chronic stage). Here virus persists in glial cells in the white matter (35). The shift seen in tropism from neurons in the gray matter during the acute stage to glial cells in the white matter during the chronic stage may reflect the ability of virus to interact with different receptors. The receptor(s) for TMEV is unknown, but virus-receptor interaction can be studied by altering virus capsid proteins at areas that are believed to interact with a cellular receptor.
The fivefold axis of the virus is surrounded by a depression called the pit. The pit of picornaviruses is believed to be the receptor binding site (7, 14, 17, 18). The three-dimensional structures of TMEVs differ from the structures of other picornaviruses in their unique loop structures that are found in the connections of the β strands (7, 14, 15). These unique loops are located near the fivefold axis at the edge of the pit. There are four large loops between the CD strands of VP1 (loop I and loop II) and the EF strands of VP2 (puff A and puff B) that extend out nearly perpendicular to the surface of the virion (Fig. 1). Exposed amino acids on three of these loops have been shown to be important disease determinants. A change in amino acid 101 of VP1 (loop II) or changes in the VP2 puff (amino acids 141 and 173 of the VP2 puff A and puff B, respectively) have resulted in viruses with altered disease patterns (8, 9, 11, 25, 31, 32, 39). Since changes at sites surrounding the pit affected pathogenesis, it is possible that these sites define a three-dimensional structure that is necessary for the cellular receptor to initiate contact with virus. Furthermore, comparisons of the footprint of human rhinovirus 16 with its receptor intercellular adhesion molecule (ICAM)-1 have shown that in order for the cellular receptor of TMEV to bind in a similar fashion, contact with loop I of VP1 would occur (15, 17).
FIG. 1.
Predicted structure of VP1 and VP2. VP1 is shown in gray; VP2 is shown in yellow with accompanying loops. Loop I of VP1 is shown in red, and loop II of VP1 is in blue. Puff A and puff B found in VP2 are green and purple, respectively.
To further elucidate virus-host interaction and potential receptor contact sites, we have examined loop I and have generated mutant viruses that have amino acid substitutions in loop I and at a site near loop I in the wall of the pit (Fig. 2). We found that substitutions at the tip of loop I of VP1 and a substitution in the wall of the pit resulted in viruses that have altered in vitro characteristics, tropism, and disease patterns. It has been predicted that in order for virus to bind its cellular receptor at the base of the pit, it might make contact with loop I (15). We also predicted that the four hydrophobic amino acids that are equally spaced going down into the canyon could be important contact sites for virus-receptor interaction (Fig. 2D). Since hydrophobic stretches are often observed in protein-protein interactions, we reasoned that these hydrophobic amino acids could be involved in virus-receptor interactions. Here we describe for the first time DA viruses that have alterations in the loop I of VP1 and in a hydrophobic patch near the loop in the wall of the pit causing altered pathogenesis and tropism.
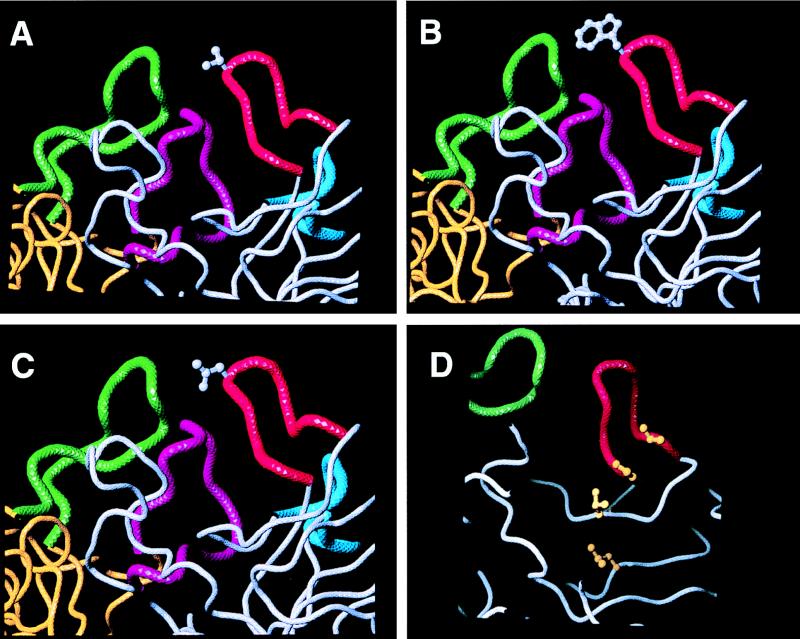
FIG. 2.
Loop I of VP1 with amino acid substitutions. Loop I of VP1 (red) is shown with the substituted amino acids (gray ball-and-stick representations of the residues). (A) Valine at position 81 (T81V); (B) tryptophan at position 81 (T81W); (C) aspartate at position 81 (T81D); (D) hydrophobic patch in the wall of the pit (the four ball-and-stick residues shown in dark yellow). The V91T mutation (valine to threonine at position 91) is the third ball-and-stick residue (light yellow) from the top.
MATERIALS AND METHODS
Construction of mutants.
pDAFL3 is a transcription vector that contains the entire cDNA of the DA strain of TMEV and was kindly provided by Raymond P. Roos (University of Chicago) (23). An Altered Sites in vitro mutagenesis system (Promega, Madison, Wis.) was used to introduce the mutations. pALTER-1 was cut with KpnI (Gibco, Gaithersburg, Md.), gel purified, and dephosphorylated. pDAFL3 was also digested with KpnI (Gibco), and the 2.3-kb fragment that contains portions of the sequence of VP1 was ligated into pALTER-1. Mutagenesis procedures were performed as recommended by the manufacturer (Promega) with the following oligonucleotides: for the threonine-to-valine substitution at position 81, the tip of loop I, the oligonucleotide started at position 3230 (of the DA virus genome) (5′-TTTGTCCAGATAGCGTTTCTGGACCGGTCA-3′); for the threonine-to-tryptophan substitution at position 81 of VP1, the oligonucleotide started at position 3231 (5′-TTGTCCAGATAGCTGGTCTGGACCGGTCA-3′); for the threonine-to-aspartate substitution at position 81 of VP1, the oligonucleotide started at position 3230 (5′-TTTGTCCAGATAGCGATTCTGGACCGGTCA-3′); and the oligonucleotide for the valine-to-threonine substitution in the wall of the canyon beneath loop I of VP1 started at position 3261 (5′-AACAAAGGCTCCAACTCAGTGGAGATGG-3′). The nucleotide substitutions corresponding to the codon changes are in boldface. The oligonucleotides were synthesized at the DNA/Peptide Facility, Huntsman Cancer Center, University of Utah. Plasmids were sequenced (DNA Sequencing Core Facility, University of Utah) to confirm the presence and accuracy of the mutations. The 2.3-kb fragments that contained the correct mutations were excised from pALTER-1 with KpnI (Gibco) and ligated back into KpnI-cleaved dephosphorylated pDAFL3.
Cells, transfection, and virus.
BHK-21 cells and astrocytes (SJL/J primary culture derived in this laboratory) were maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco). BSC-1 and Vero (African green monkey kidney cell lines) and Neuro-2a (mouse neuroblastoma) cells were maintained in complete minimal essential medium (MEM; Gibco). SF9 cells (fall armyworm ovary) were maintained in Grace’s medium (Gibco) at room temperature with rotation. All cell lines except astrocytes were purchased from the American Type Culture Collection (Rockville, Md.).
The mutagenized pDAFL3 plasmids were linearized with XbaI (Gibco), which cleaves downstream of the cDNA of DA virus. The AmpliScribe T7 transcription system (Epicentre Technologies, Madison, Wis.) was used to generate transcripts of the mutant RNA. Subconfluent BHK-21 cells were transfected with 5 μg of the mutant RNA, using DMREC-reagent (Gibco) as recommended by the manufacturer. After 24 h, cells were transferred to 25-cm2 flasks. Transfected cells were observed for cytopathic effects every 24 h for 5 days.
Transfected (infected) cells and their supernatants were harvested and stored in 100-μl aliquots at −70°C (original stocks). Virus pools were titered on BHK-21 cells by plaque assay (10). To generate a working virus pool for each virus, subconfluent BHK-21 cells in 60-mm-diameter dishes were infected with virus (original stocks) at a multiplicity of infection (MOI) of 0.05 to 0.1 in 6 ml of DMEM supplemented with 2% fetal bovine serum (FBS). The infection was allowed to proceed until extensive cytopathic effects were seen. The infected cells with supernatant were harvested, titered, frozen, and stored at −70°C. GDVII virus was propagated in BHK-21 cells.
We had also generated a mutant infectious DNA clone that had the entire loop I of VP1 deleted (amino acids 79 to 84 [DSTSGP]). On transfection of the mutant viral RNA, no infectious virus was isolated. Western blot analysis revealed that VP1 was translated (data not shown). Likely explanations for the fact that no infectious virus could be detected are that virus particles were not being assembled because the deletion exerted an affect on viral assembly and that they were being assembled but were noninfectious.
In vitro virus replication.
Subconfluent monolayers in 35-mm-diameter wells of BHK-21, BSC-1, Vero, and Neuro-2a, astrocytes, and SF9 cells were infected with each of the different viruses and allowed to absorb for 1 h at 37°C. Cells were washed twice with phosphate-buffered saline (PBS), and 1 ml of medium containing 2% FBS was added. At different times postinfection, the supernatants and infected cells were harvested. Samples were stored at −70°C and were titrated by plaque assay. Virus titration was performed in duplicate. Results are representative of two independent experiments.
Thermal stability studies.
Each virus was diluted in 2 ml of DMEM (2% FBS) and divided into four tubes. Two of the tubes of each virus were incubated for 5 min in water baths calibrated at 42°C. The other two tubes remained on ice. The virus-containing fluids heated or unheated were then plated onto subconfluent BHK-21 cells to determine viral titers. The percent viability was calculated as follows: (average titer of heated virus/average titer of unheated virus) × 100.
Animals.
Four to six-week-old male SJL/J mice (purchased from the National Cancer Institute, Bethesda, Md.) were used. Virus pools were diluted and 2 × 105 PFU in 20 μl of DMEM were injected intracerebrally in the right hemisphere of anesthetized (methoxyfluorane; Pitman-Moore, Inc., Mundelein, Ill.) mice. Mice were weighed three times for the first week postinfection and once weekly for the subsequent weeks and were observed for any clinical signs.
Histological evaluation.
For histological and immunohistochemical studies, mice were euthanized with an overdose of halothane (Halocarbon Laboratories, River Edge, N.J.) 1, 4, or 8 weeks postinfection (six mice per mutant virus per time point). Blood was removed and mice were perfused with 4% paraformaldehyde in PBS. Brains and spinal cords were paraffin embedded, and 4-μm sections were cut. Sections were stained with luxol fast blue to determine the extent of demyelination and degree of inflammation. Brain sections were scored for the presence of meningitis (none = 0, slight = 1, and severe = 2), inflammation (none = 0, 1 to 20 lesions = 1, 21 to 50 lesions = 2, and >50 lesions = 3), and the presence (=1) or absence (=0) of demyelination (2, 28). For evaluation of the spinal cord, each cross-section on a slide (with 10 cross-sections) was divided into four quadrants (dorsal, ventral, and two laterals). Each quadrant was examined for either the presence or absence of demyelination, inflammation, and meningitis and then scored (20, 22, 28). The total score was then divided over the total number of quadrants and multiplied by 100 to generate a percent disease value.
Immunohistochemistry.
To quantify the number of cells in the CNS that contained viral proteins and to evaluate the distribution of virus, immunohistochemistry was performed on adjacent sections. Deparaffinized and blocked (3% sheep serum in PBS) sections were incubated with hyperimmune rabbit polyclonal anti-DA virus antiserum at 4°C for 16 h (39). The secondary antibody was a sheep anti-rabbit immunoglobulin G-biotin conjugate (1:250; Boehringer Mannheim, Indianapolis, Ind.), and the signal was amplified by using Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, Calif.) and developed with 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, Mo.). The sections were counterstained with hematoxylin. Antigen-positive cells were counted as described in Tsunoda et al. (29).
CNS viral titers.
Amounts of infectious virus in the brains and spinal cords of infected mice were determined as follows. Mice injected as described above were euthanized and perfused with PBS at 1, 2, 4, and 8 weeks postinfection (three mice per time point). The brains and spinal cords were aseptically removed and placed into preweighed tubes containing DMEM. Ten to twenty percent homogenates were made of brains and spinal cords. After the homogenates were frozen and thawed three times, they were plaqued on BHK-21 cell monolayers. The amount of virus per gram of tissue was calculated.
RT-PCR.
Primers were designed to regions of the DA virus genome for reverse transcriptase-PCR (RT-PCR) as follows: for the reverse transcription reaction starting at position 3509, 5′-CGCGTCTCGCCAAGGCTG-3′; for the forward reaction of PCR starting at position 2863, 5′-GGATGGGTGACTGTCTGGC-3′; and for the reverse reaction starting at position 3509, 5′-CGCGTCTCGCCAAGGCTG-3′. RNA was extracted from infected samples (BHK-21 cells, brains, and spinal cords) was immediately used in the reverse transcription reaction using Ready-To-Go-You-Prime First-Strand Beads (Pharmacia Biotech, Piscataway, N.J.), using the recommended protocol. When samples were prepared for sequencing, the following protocol was used. After cDNA synthesis, the entire reaction was used for PCR amplification with added Taq DNA polymerase (Gibco) and 40 pmol of each forward and reverse primer. The cycling conditions were as follows: denaturation at 92°C for 1 min, annealing at 58°C for 1 min, and extension at 72°C for 1.5 min. After 30 cycles, there was a final extension period for 10 min and cooling to 4°C. PCR products were resolved on a 0.8% TAE agarose gel. To determine whether spinal cord homogenates of infected mice contained viral RNA, nested PCR was used with the following modifications. Five-microliter aliquots of the reverse transcription reaction products were amplified by using the same forward and reverse primers as described above, with the following cycling conditions: 45 s of melting at 94°C, 45 s of annealing at 58°C, and 1.5 min of extension at 72°C for 25 cycles, with a final extension at 72°C for 5 min. From this reaction, 0.2 μl was used for the nested PCR using the same cycling conditions for 20 cycles with the forward primer starting at position 2915, (5′-CTGTCAACTCTGACATCCTCAC-3′) and the reverse primer starting at position 3405 (5′-CAGAGCGCTGACTGTAACCTC-3′). PCR products were resolved on an 0.8% Tris-acetic acid-EDTA (TAE) agarose gel.
Enzyme-linked immunosorbent assays (ELISA).
Mice were bled from the tail vein upon arrival and at the time of sacrifice. Sera were prepared and assayed for content of anti-DA virus antibodies. Ninety-six well plates (Nunc-Immuno Plate Maxi Sorp; Nunc, Rochester, N.Y.) were coated with 50 μl of a 10-μg/ml DA virus solution (10) in PBS overnight at 4°C. After blocking with diluent (PBS, 10% FBS, 0.2% Tween 20), twofold serial dilutions of the mouse sera beginning at 1:100 were added to the plates and incubated at room temperature for 2 h. After washes with PBS containing 0.1% Tween 20, the plates were incubated with a goat anti-mouse peroxidase-labeled antibody (1 mg/ml, 1:3,000; Gibco) in diluent. After 90 min of incubation at room temperature, the plates were washed and incubated with o-phenylenediamine dihydrochloride (4 μg/ml; Sigma) and H2O2 (0.01%) in a citrate buffer (pH 5.0) in the dark for 30 min. The reaction was stopped by the addition of 50 μl of 1 N HCl. The optical density of the reaction product was determined with a Titertek Multiskan Plus MK II spectophotometer at a wavelength of 492 nm. The endpoint of the assay was determined as the reciprocal of the highest dilution that gave an optical density reading that was 3 standard deviations above the control baseline.
RESULTS
Generation of mutant viruses.
Mutations in pDAFL3 corresponding to the amino acid substitutions were made and sequenced. This confirmed the presence and accuracy of the mutations. In vitro transcription reactions produced infectious RNA that was transfected into BHK-21 cells. The infected cells and supernatants were harvested and titered by plaque assay. pDAFL3-derived virus is hereafter referred to as pDA virus, and the viruses with amino acid substitutions in VP1 were named as shown in Table 1.
TABLE 1.
Mutations in virusesa
| Virus name | VP1 position, change | Sequence change |
|---|---|---|
| pDA | None | None |
| T81V | 81, threonine to valine | ACT to GTT |
| T81W | 81, threonine to tryptophan | ACT to TGG |
| T81D | 81, threonine to aspartate | ACT to GAT |
| V91T | 91, valine to threonine | GTT to ACT |
pDA is the wild-type DA virus. Mutations were created as described in Materials and Methods.
T81D virus produces large plaques.
DA and GDVII viruses have different plaque morphologies. DA virus produces small plaques on BHK cells. In contrast, GDVII virus makes large plaques (12). Interestingly, T81D virus produced large plaques (2.36 ± 0.14 mm) similar in size to plaques produced by GDVII virus (3.20 ± 0.19 mm), a neurovirulent TMEV that belongs in a separate subgroup, rather than small plaques seen with the parental virus, pDA (0.6 ± 0.04 mm), in BHK cells. In addition, large plaques were produced in primary mouse astrocytes (pDA virus, 0.21 ± 0.02 mm; T81D virus, 1.04 ± 0.05 mm; GDVII virus, 1.33 ± 0.13 mm). The T81V, T81W, and V91T viruses all produced only small plaques in both BHK cells and astrocytes.
T81D virus has altered replication kinetics.
To determine whether the changes made in the virus capsid proteins influenced viral replication, one-step growth curves were performed (Fig. 3). The replication kinetics of the mutant viruses were compared with those for pDA virus. At 8 h postinfection, all but the T81D virus had an increase in titer. The T81D virus had a longer lag phase. Growth kinetics similar to those for pDA virus were found with the T81V, T81W, and V91T mutant viruses. At 24 h postinfection, the pDA, T81V, T81W, and V91T viruses had reached their peak titers; however, the T81D virus did not peak until 36 h postinfection. At 48 h, there was no further increase in T81D virus titer (data not shown).
FIG. 3.
T81D virus has an increased lag during its replication cycle. Virus was added at an MOI of 5 to BHK-21 cells; at indicated time points, infected cells were harvested and plaqued to determine viral titers. The T81D virus has an increased lag phase and replicates to lower titers.
T81D virus produces less virus in various cell lines.
To assess whether T81D virus infection also produced less virus in different cell lines, we infected various cell lines with viruses and compared their titers 24 h postinfection (Fig. 4). The T81D virus produced less virus in BHK-21 cells, and this correlated with the data obtained with the one-step growth curves. Replication of T81D virus was also reduced in two CNS-derived cell lines, Neuro-2a and astrocytes, and decreased in two African Green monkey kidney cell lines, BSC-1 and Vero. Replication in SF9 cells also resulted in a reduction of viral titer, but infection of SF9 cells with all viruses resulted in little virus production. With the other mutant viruses, we found no variations in the ability to replicate in these cell lines.
FIG. 4.
T81D replicates to lower titers in various cell lines. Virus was added at an MOI of 1 to BHK-21 (BHK), Neuro-2a (Neu), astrocyte (Astr), BSC-1 (BSC), Vero, and SF9 cell lines. Twenty-four hours later, the infected cells were harvested and plaque assays were performed.
T81D virus is thermostable.
There is a difference in temperature sensitivity found between GDVII and DA viruses (3, 19, 32). GDVII virus is less sensitive to elevated temperatures than DA and other TO subgroup viruses. To determine whether differences exist in stability among the different mutants and pDA virus, we assessed thermostability. Noticeable differences were found at 42°C (Table 2). As expected, GDVII virus was stable at 42°C. In contrast, pDA, T81V, T81W, and V91T viruses were labile at this temperature. However, T81D virus, similar to GDVII virus, was stable at 42°C.
TABLE 2.
Thermostability of TMEVa
| Virus | PFU/ml
|
% Viability | |
|---|---|---|---|
| 0°C | 42°C | ||
| GDVII | 8.1 × 105 | 7.8 × 105 | 96.3 |
| pDA | 1.5 × 106 | 1.3 × 105 | 8.7 |
| T81V | 3.7 × 106 | 2.8 × 105 | 7.6 |
| T81W | 3.9 × 106 | 6.9 × 105 | 17.7 |
| T81D | 1.7 × 106 | 1.6 × 106 | 94.1 |
| V91T | 1.9 × 106 | 1.6 × 105 | 8.4 |
Viruses were incubated at 0 or 42°C for 5 min and plaqued on BHK-21 cell monolayers.
Clinical signs.
Although no obvious clinical signs were noted in any groups during the acute stage of the disease, the mice infected with T81W, T81D, and V91T viruses gained more weight than the pDA and T81V virus-infected mice during the first 3 weeks postinfection (data not shown). During the chronic stage of the disease, all six mice infected with wild-type pDA virus and four of six mice infected with T81V virus exhibited waddling gait and spastic paralysis. In contrast, none of the mice infected with T81W, T81D, and V91T viruses showed clinical signs. This correlated well with the histological and CNS viral titer data described below.
Histopathology.
After 1, 4, and 8 weeks postinfection, CNS tissue from infected mice was processed for paraffin embedding. During the acute stage, pDA virus preferentially produced disease in the hippocampus, cerebral cortex, thalamus, and brainstem. Mice infected with T81V and T81W viruses exhibited a pattern of disease similar to that seen with pDA virus-infected mice. However, the overall disease seen with the T81D and V91T virus-infected mice was markedly reduced, with little or no disease seen in the hippocampus (Fig. 5). The total pathological score of the brain 1 week postinfection is illustrated in Fig. 6. The extent and pattern of disease produced by T81V virus was similar to those produced by pDA virus. Less disease was found in mice infected with the T81W virus, but this was not significant. However, significantly less disease was observed in mice infected with the T81D and V91T viruses (compared with wild-type pDA virus, P < 0.05 by analysis of variance [ANOVA]). These viruses induced less perivascular cuffing and less meningitis in the brains 1 week postinfection. Little or no disease was seen in the spinal cords of T81D or V91T virus-infected mice.
FIG. 5.
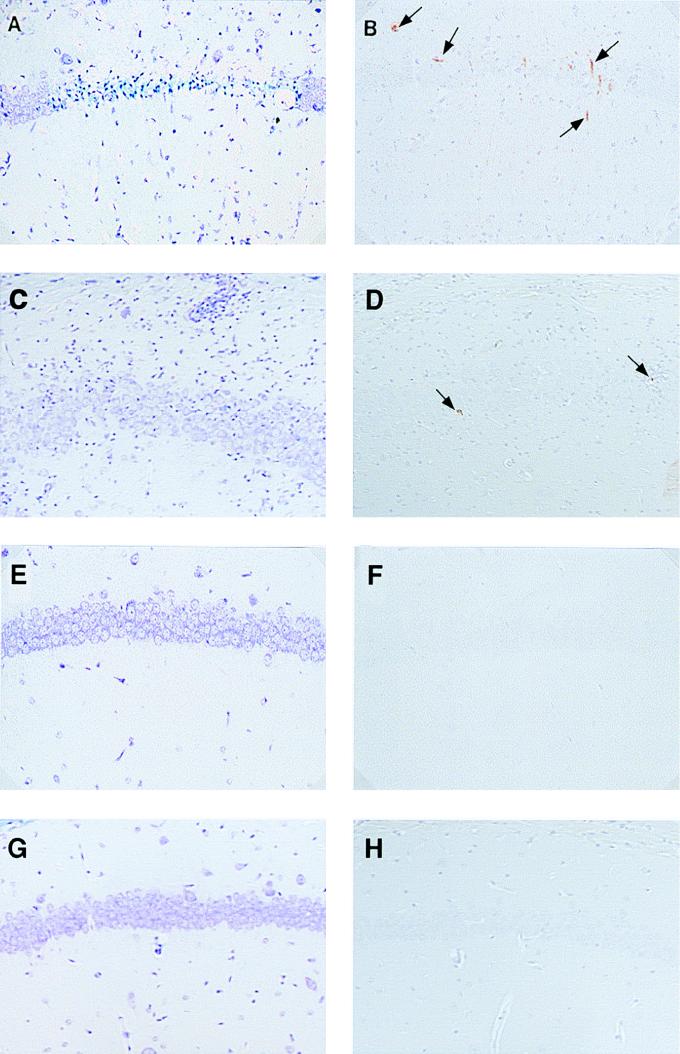
Histopathology and immunohistochemistry of the brain during the acute stage (1 week postinfection) of TMEV infection. We analyzed consecutive hippocampal sections of SJL/J mice infected with wild-type pDA (A and B) or T81V (C and D), T81D (E and F), or V91T mutant (G and H) virus by luxol fast blue staining (A, C, E, and G) and immunohistochemistry detection for viral antigen (B, D, F, and H). Pyramidal cell loss and inflammation were noted in pDA (A) and T81V (C) virus infection, respectively, while the pyramidal cell layers of T81D (E) and V91T (G) virus-infected mice were normal. We could detect viral antigen-positive cells (arrow) in pDA (B) and T81V (D) virus infection but not in T81D (F) and V91T (H) virus infection. Magnification is ×166.
FIG. 6.
Mice infected with T81D or V91T virus have decreased disease in the brain during the acute stage. Brains of infected mice were scored for the extent of perivascular cuffing and meningitis and for the presence or absence of demyelination. The highest possible score is 6. The T81D and V91T virus-infected mice exhibited significantly less disease (∗, P < 0.05, ANOVA).
At 4 weeks postinfection and thereafter, TMEV-induced disease was primarily located in the spinal cords (chronic stage) (Fig. 7). At 4 weeks postinfection, the extent and pattern of disease in mice infected with the T81V virus were similar to those in mice infected with wild-type pDA virus. Large areas of demyelination accompanied by inflammatory infiltrates and meningitis were seen at the ventral root zones, and in some cases, the entire white matter of the spinal cord was involved. Less disease was seen in mice infected with the T81W, T81D, and V91T viruses (Fig. 7C, E, and G). The same pattern was observed at 8 weeks postinfection. The T81W, T81D, and V91T viruses produced attenuated disease, whereas the T81V virus was similar to wild-type virus in the extent and pattern of disease production.
FIG. 7.
Histopathology and immunohistochemistry of the spinal cords during the chronic stage (4 weeks postinfection) of TMEV infection. Ventral root entry zones of the mice infected with wild-type pDA (A and B) or T81W (C and D), T81D (E and F), or V91T (G and H) mutant virus. Sections were stained with luxol fast blue (A, C, E, and G) and analyzed with immunohistochemistry for viral antigen (B, D, F, and H). In pDA virus infection, severe inflammatory demyelinating lesion (A) with virus persistence (B; arrows) was conspicuous. In T81W infection, mild cell infiltrate was noted (C), while no antigen-positive cells were detected (D). In T81D (E and F) and V91T (G and F) virus infection, few lesions were noted without viral persistence. Magnification is ×166.
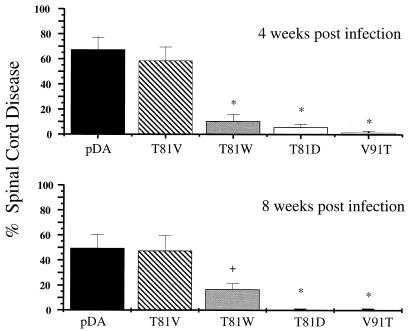
Coronal spinal cord sections were scored for the presence or absence of either demyelination, inflammation, or meningitis at 4 and 8 weeks postinfection (Fig. 8). The T81V virus produced levels of disease comparable to those produced by pDA virus, whereas significantly less involvement was seen in the T81W, T81D, and V91T virus-infected mice at 4 and 8 weeks postinfection (Fig. 8; determined by ANOVA).
FIG. 8.
Histological score in the spinal cord at 4 and 8 weeks postinfection. The percent disease was calculated by counting the quadrants that contained either demyelination, inflammation, or meningitis. The T81W, T81D, and V91T virus mutant-infected mice exhibited significantly less disease at 4 and 8 weeks postinfection.
Viral antigen positive cells in the CNS.
To determine the distribution and number of virus infected cells, immunohistochemistry for viral antigens was performed. At 1 week postinfection, the T81V virus-infected mice exhibited a pattern of antigen-positive cells in the pyramidal cell layer of the hippocampus, cerebral cortex, thalamus, and brainstem similar to that seen with pDA virus-infected mice in the brain (Fig. 5B and D). However, the T81W, T81D, and V91T virus-infected mice contained fewer viral antigen-positive cells in the cerebral cortex, thalamus, and brainstem and few (T81W) or none (T81D and V91T) in the hippocampus (Fig. 5F and H). Most antigen-positive cells in pDA, T81V, T81W, and V91T virus-infected mice were neurons, based on their morphology.
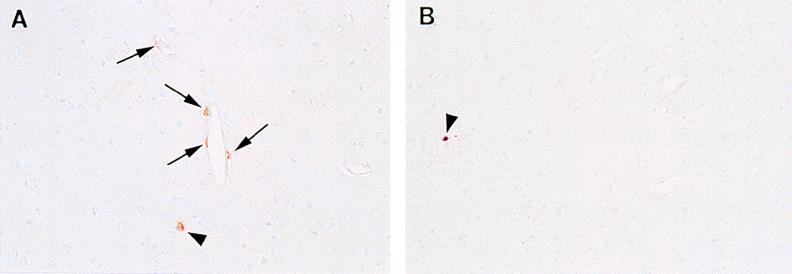
However, the T81D mutant exhibited an altered tropism. While no neurons in the pyramidal cell layer in the hippocampus were seen to contain viral antigens, antigen-positive neurons were scattered throughout the thalamus. More interestingly, antigen-positive cells were also found to be associated with small vessels in the thalamus (Fig. 9A). This was not found with infections of wild-type pDA virus or any of the other mutant viruses (Fig. 9B). The T81D mutant displayed an altered tropism to endothelial cells or perivascular cells (macrophages).
FIG. 9.
T81D mutant virus-infected mice have viral antigen-positive cells associated with small vessels. (A) Coronal section of the thalamus of a T81D virus-infected animal; (B) section of the thalamus of a pDA virus-infected animal. Note the viral antigen-positive cells (arrows) as detected by immunohistochemistry associated with small vessels (A), which is not seen with the pDA virus-infected animal (B). Viral antigen-positive neurons (arrowheads) were seen in both T81D and pDA virus infection.
During the chronic stage, 4 and 8 weeks postinfection, the white matter of the spinal cord contained antigen-positive cells in the demyelinating lesions of mice infected with wild-type pDA (Fig. 7B) and T81V mutant viruses. We found few viral antigen-positive cells in the T81W and T81D-infected mice and none in the V91T-infected mice at 4 or 8 weeks postinfection (Fig. 7D, F, and H). Morphologically, the viral antigen-positive cells were glial cells or macrophages in all mutant and wild-type DA virus infections (13, 35). The antigen-positive cells in all five coronal brain sections and all spinal cord sections of all infected mice were counted (Table 3). We found that the overall number of antigen-positive cells in the T81V virus-infected mice was similar to that in wild-type pDA virus-infected mice in the brains but was lower during the chronic stage of the spinal cord. Nevertheless, abundant viral antigen-positive cells were found in the spinal cords of mice infected with both viruses. In contrast, the T81W and T81D virus-infected mice contained a reduced number of viral antigen-positive cells in the brain and few or none in the spinal cord in both acute and chronic stages. In addition, the V91T virus-infected mice contained few antigen-positive cells in the brain and none in the spinal cord at all time points. Overall, there appears to be a relationship between increased demyelination and inflammation with an elevated number of antigen-positive cells.
TABLE 3.
Virus antigen-positive cells in the CNSa
| Virus | Time (wk) post-infection | No. of antigen-positive cells (avg ± SEM)
|
|
|---|---|---|---|
| Brain | Spinal cord | ||
| pDA | 1 | 36.17 ± 10.15 | 0.17 ± 0.17 |
| 4 | 26.33 ± 5.75 | 117.50 ± 23.03 | |
| 8 | 18.00 ± 7.48 | 64.67 ± 19.70 | |
| T81V | 1 | 26.67 ± 9.80 | 1.50 ± 1.15 |
| 4 | 23.00 ± 8.25 | 56.33 ± 14.15 | |
| 8 | 18.00 ± 4.65 | 19.83 ± 3.60 | |
| T81W | 1 | 15.67 ± 8.00 | 0.83 ± 0.83 |
| 4 | 7.00 ± 4.40 | 0 | |
| 8 | 7.97 ± 3.00 | 4.00 ± 2.97 | |
| T81D | 1 | 12.50 ± 2.95 | 0 |
| 4 | 0.67 ± 0.49 | 0.50 ± 0.50 | |
| 8 | 3.00 ± 1.63 | 0 | |
| V91T | 1 | 0.50 ± 0.22 | 0 |
| 4 | 1.33 ± 0.84 | 0 | |
| 8 | 0.83 ± 0.48 | 0 | |
The brains and spinal cords were cut into 5 and 10 coronal sections, respectively. The same regions for each mouse were examined. The viral antigen-positive cells were detected by immunohistochemistry, and the total numbers of TMEV antigen-positive cells in the brain and spinal cord of each mouse were enumerated in a light microscope at a magnification of ×200.
CNS viral titers.
To determine the amount of infectious virus in the CNS of virus-infected mice, we performed plaque assays of CNS tissue homogenates. Twelve mice per group were injected with each virus; at 1, 2, 4, and 8 weeks postinfection three mice were sacrificed, their brains and spinal cords were homogenized, and plaque assays were performed to determine viral titers.
At 1 week postinfection, the viral titers in the brain were high in wild-type pDA, T81V and T81W virus-infected mice but were considerably lower in T81D and V91T virus-infected mice (Table 4). Over time, at 8 weeks postinfection, the titers decreased in the brain in wild-type pDA virus and T81V virus-infected mice. The viral titers in the brain of T81W virus-infected mice decreased even further at 8 weeks postinfection, when in one of the three mice no infectious virus was detected. The brains of T81D and V91T virus-infected mice contained very little or no virus at 8 weeks postinfection.
TABLE 4.
CNS viral titers
| Virus | Time (wk) postinfection | Avg CNS titer (PFU/g)
|
|
|---|---|---|---|
| Brain | Spinal cord | ||
| pDA | 1 | 6.3 × 105 | 8.2 × 104 |
| 2 | 4.3 × 104 | 8.1 × 105 | |
| 4 | 5.0 × 104 | 5.0 × 105 | |
| 8 | 1.1 × 104 | 1.1 × 105 | |
| T81V | 1 | 3.7 × 105 | 2.4 × 105 |
| 2 | 4.8 × 104 | 2.5 × 105 | |
| 4 | 1.2 × 105 | 6.3 × 104 | |
| 8 | 1.2 × 104 | 1.0 × 104a | |
| T81W | 1 | 1.3 × 105 | 9.8 × 104 |
| 2 | 5.3 × 103 | 3.6 × 104 | |
| 4 | 7.9 × 103a | 8.5 × 104 | |
| 8 | 1.5 × 103a | 3.0 × 103b | |
| T81D | 1 | 3.5 × 103a | 2.7 × 103b |
| 2 | 4.2 × 101a | 1.7 × 101b | |
| 4 | 0 | 0 | |
| 8 | 6.7 × 102a | 0 | |
| V91T | 1 | 5.1 × 104 | 0 |
| 2 | 9.3 × 102 | 0 | |
| 4 | 2.5 × 102 | 0 | |
| 8 | 9.0 × 103b | 0 | |
One mouse of three contained no infectious virus.
Two of three mice contained no infectious virus.
In the spinal cord, the viral titers remained high over time in wild-type pDA virus-infected mice (Table 4). There was a slight decrease seen at 8 weeks postinfection with T81V-infected mice. The viral titers in T81W infected mice largely decreased over time. At 8 weeks postinfection, no infectious virus could be isolated in two of three mice. In contrast, the spinal cords of T81D mutant-infected mice contained few virus particles at 1 and 2 weeks postinfection, and no infectious virus was isolated at 4 and 8 weeks postinfection from any mice. The V91T virus-infected mice never contained demonstrable amounts of infectious virus in the spinal cord (limit of detection is 25 PFU/g of tissue).
At 8 weeks postinfection, samples of spinal cord homogenates (pDA, T81V, T81W, and T81D virus-infected mice) or brain (V91T virus-infected mouse) were processed for RT-PCR. These were sequenced to determine the presence of the correct mutation and to eliminate possible reversions to wild-type and/or contamination during housing or handling of animals. In all instances, the virus sequenced was the correct virus injected and still contained the original mutation (data not shown).
Antibody titers correlate with virus persistence.
Prior to infection and at the time of sacrifice, sera were taken from the mice that were used for the histological evaluation. Anti-TMEV antibody titers of these mice were determined (Fig. 10). In all groups, anti-TMEV antibodies were detectable even 1 week postinfection. Over time, a significant increase in antibody titers was seen with the wild-type pDA and T81V virus-infected mice. This was not evident with the T81W, T81D, and V91T virus-infected mice, suggesting that anti-TMEV antibody titers correlate with viral antigen-positive cells.
FIG. 10.
Antibody titers to wild-type and mutant viruses correlate with histopathology. Sera were collected prior to infection and at 1, 2, 4, and 8 weeks postinfection (pi) from mice that were used for the histological scoring. Anti-TMEV titers were determined by ELISA. Anti-TMEV titers increased in the mice infected with the wild-type pDA and T81V viruses and remained low for the T81W, T81D, and V91T virus-infected mice.
DISCUSSION
We have targeted loop I of VP1 for mutagenesis in an attempt to delineate virus tropism and interaction with the host. Loop I is 1 of the four exposed loops adjacent to the putative binding site for the cellular receptor for TMEV (7, 14, 17, 18).
The T81V and T81W viruses both have hydrophobic substitutions at the top of loop I of VP1. The growth kinetics and thermal stability of the T81V and T81W viruses were similar to those for wild-type pDA virus. Therefore, a hydrophobic substitution did not significantly alter the phenotype of these viruses in vitro.
In vivo, the T81V virus, which has a threonine replaced with a valine (a substitution of a hydroxyl group with a methyl group at the β-carbon, hydrophobic), produced a pattern and disease similar to that of wild-type pDA virus. This small change in hydrophobicity at the top of the loop of VP1 did not have an apparent effect on virus-host interactions, whereas when a hydrophobic change with the introduction of two aromatic rings was introduced at this position, the T81W virus infection resulted in a similar acute disease but an attenuated chronic disease. We hypothesize that the large hydrophobic residue, tryptophan, at the tip of loop I of VP1 in T81W virus could have distorted the conformation of the loop in such a manner that it might have hindered virus-receptor interactions in vivo.
Both T81V and T81W viruses could replicate in astrocytes in vitro as well as wild-type pDA virus (Fig. 4). However, in vivo, no viruses efficiently infected glial cells or macrophages during the acute stage. During the chronic persistent stage, pDA and T81V viruses but not T81W virus infection resulted in infected glial cells and/or macrophages. In CNS inflammatory disease including TMEV infection (13), major histocompatibility complex and adhesion molecules, potential candidates of virus receptors (17), on glial cells and macrophages have been reported to be induced or up-regulated during the course of disease. Taken together, during the chronic stage, pDA and T81V viruses could use the induced- or up-regulated molecule(s) on glial cells or macrophages as virus receptor; T81V might bind less efficiently to these molecules. This remains to be determined.
Therefore, the results can be interpreted to mean that the affinity and tropism remained the same during the acute stage for neurons, but that the alteration in the loop had an effect on infection of glial cells in the spinal cord during the transition to the chronic stage, leading to reduced persistence and demyelination. Although smaller amounts of T81W virus were detected during the chronic stage, this was not sufficient to cause significant disease in the spinal cord as was seen with wild-type pDA virus and the T81V mutant virus.
The V91T virus, which had a valine in the wall of the pit replaced with a threonine (uncharged polar), also exhibited an altered disease pattern, whereas minor differences were seen with in vitro analyses. The acute disease was attenuated and the chronic demyelinating disease and viral persistence were absent. Although there were a few antigen-positive cells and small histopathological changes in the brain during the acute stage, significant amounts of infectious virus could be isolated from the mice infected with V91T virus. Furthermore, infectious V91T virus could be isolated from the brain even at 8 weeks postinfection, which indicated that the virus inoculum was not defective. In contrast, in the spinal cord, no infectious virus or disease was evident at any time point, and in only one instance could viral genome be detected by RT-PCR during the chronic stage (data not shown). It is possible that this substitution in the wall of the canyon interrupted virus-receptor interactions and that this resulted in a lower affinity for the receptor or lack of interaction with the receptor. Thus, our results can be interpreted to mean that the threonine substitution in the wall of the canyon rendered it unable to infect glial cells because of loss of affinity for receptor. Alternatively, early clearance by immune system could negate viral persistence. However, this is unlikely, as discussed below.
On the other hand, T81D virus has a mutation (negatively charged, acidic) that may largely alter the conformation of the loop and/or surrounding features. T81D virus has a threonine residue replaced by an aspartate residue on the most exposed region of loop I of VP1. This reflects a change from a β-branched hydrophilic side chain to a γ-branched acidic side chain. In all characterization experiments, the data indicate that T81D virus had an altered phenotype. In vitro T81D virus produced large plaques in two cell lines, had a slower replication cycle, and was more thermally stable than the other mutant viruses or wild-type virus. The plaque phenotype and thermal stability of T81D virus approximated that of the neurovirulent strain GDVII virus. Traditionally, plaque size has often been correlated with neurovirulence for many viruses, including TMEV (12, 27). However, the data indicate that there is no correlation between plaque size and neurovirulence and that there is a correlation between plaque size and thermal stability. A similar finding was described by Oleszak et al. (16) when two plaque size variants were analyzed from DA virus. The small variant was thermally sensitive, whereas the slightly larger variant was less sensitive. However, the in vitro growth characteristics of the small- and large-plaque variants varied from the data obtained for pDA and T81D viruses. The small-plaque variant analyzed by Oleszak et al. (16) grew more slowly and to lower titers, whereas our large-plaque variant exhibited this phenotype. Unfortunately, the small- or large-plaque variants examined by Oleszak et al. (16) were not sequenced, and therefore it remains difficult to associate amino acids in the genome with their influence on thermal stability and plaque size as well as determine their location and structural relationship. We propose a correlation between thermal stability and plaque size and believe that the aspartate substitution at the top of loop I of VP1 renders the virus particle more stable and resistant to denaturation at elevated temperatures, presumably due to altered structure.
Significantly, the T81D mutant virus exhibited an altered tropism in the brain during the acute stage. Cells associated with small vessels, presumably endothelial cells or perivascular cells (macrophages), in the thalamus stained positive for viral antigen, whereas few if any pyramidal neurons in the hippocampus were involved. There are reports suggesting that endothelial cells might play a role in TMEV infection both in vitro and in vivo. Endothelium and/or perivascular cells of TMEV-infected nude mice contained viral RNA but not viral antigen (38). In situ hybridization demonstrated viral RNA in cells associated with the vascular endothelium away from demonstrable lesions. In addition, there have been studies involving endothelial cell lines that were infected with TMEV in vitro. TMEV infection of cloned cerebral endothelial cell lines from susceptible and nonsusceptible mice indicated that all cell lines were equally permissive (34). Further studies with these endothelial cell lines demonstrated that these cells could be persistently infected (24). Virus replication occurred, but viral titers were lower than in acutely infected cells and titers from endothelial cells of susceptible mice were lower than titers from cells from nonsusceptible mice. These authors hypothesized that during natural infection the endothelial cells become persistently infected and could initiate a series of events that leads to virus dissemination to the CNS or, alternatively, that the endothelial cell acts as a reservoir of TMEV in infected mice. The virus could then continue to reinfect the CNS and cause the demyelination and infiltration present in the CNS. However, cells associated with small vessels were never found to contain viral antigen or viral RNA during the acute stage of wild-type virus infection in susceptible mice (1, 21, 26). Furthermore, only a mutant virus (T81D) exhibited a tropism for cells associated with small vessels, and infectious virus did not persist; this altered tropism is attributed to the change in the capsid protein. We conclude that although cells associated with small vessels (presumably endothelial or perivascular cells) might play a role in disease, it is more likely that they play an important immunological role in presenting antigen rather than a role in virus persistence.
Although cerebral endothelial cells are known to express Ia antigen in some particular instances, such as experimental allergic encephalomyelitis, these cells are not professional antigen-presenting cells and lack efficient costimulatory signals. Recent studies have shown that antigen presentation without costimulatory signals induces anergy rather than proliferation of T cells (33). Thus, in T81D virus infection, antigen presentation by endothelial cells might anergize TMEV-specific T cells, which are believed to play an important role in causing demyelinating disease. This could lead to suppression of TMEV-induced chronic demyelinating disease.
Viruses are known to evade the immune response by several mechanisms that can lead to their persistence. The differences in persistence seen among the mutant viruses in this experiment might be caused not only by altered virus-cell interaction, such as binding, uncoating, and penetration, but also by different immune responses to the viruses. Serum neutralizing antibody has been shown to be important in TMEV clearance (5, 10). In our experiments, however, we detected higher anti-TMEV antibody titers in pDA and T81V virus-infected mice than in the other mutant virus-infected mice, which had decreased viral persistence (Table 4). Therefore, lack of a humoral immune response did not correlate with persistence of pDA and T81V virus infection. In addition, major T-cell epitopes have been found in VP133–47 (30), VP1233–244 (37), VP274–86 (6), VP2122–130 (4), and VP324–37 (36) but not in loop I of VP1 in TMEV infection. Thus, altered virus-cell interactions are more likely to contribute to the changes in phenotype seen with the TMEV mutants rather than the host immune responses against these viruses. Experiments that have analyzed these functions of the T81D virus and wild-type virus have determined that the T81D virus binding to permissive cell lines is the same as wild-type virus, but that the T81D virus exhibits a delay in the entry of the infectious genome (unpublished data).
This is the first report of in vivo analyses of capsid mutants of TMEV with site-directed mutations in loop I of VP1. Mice infected with virus mutants with three different amino acid substitutions at 1 position (81 of loop I) have led to markedly different disease patterns. One of these mutants exhibited an altered tropism, whereas a disruption of a hydrophobic patch in the wall of the canyon resulted in a profound attenuation of the acute and chronic disease.
ACKNOWLEDGMENTS
We thank Jane E. Libbey, Li-Qing Kuang, Sheri Williams, and Kris Hudson for technical assistance. We thank Kathleen Borick for preparation of the manuscript.
This work was supported by National Institutes of Health grant NS 34497.
REFERENCES
- 1.Aubert C, Chamorro M, Brahic M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- 2.Barnett L A, Whitton J L, Wada Y, Fujinami R S. Enhancement of autoimmune disease using recombinant vaccinia virus encoding myelin proteolipid protein. J Neuroimmunol. 1993;44:15–25. doi: 10.1016/0165-5728(93)90263-x. . (Erratum, 48:120.) [DOI] [PubMed] [Google Scholar]
- 3.Calenoff M A, Faaberg K S, Lipton H L. Genomic regions of neurovirulence and attenuation in Theiler murine encephalomyelitis virus. Proc Natl Acad Sci USA. 1990;87:978–982. doi: 10.1073/pnas.87.3.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dethlefs S, Escriou N, Brahic M, van der Werf S, Larsson-Sciard E-L. Theiler’s virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J Virol. 1997;71:5361–5365. doi: 10.1128/jvi.71.7.5361-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujinami R S, Rosenthal A, Lampert P W, Zurbriggen A, Yamada M. Survival of athymic (nu/nu) mice after Theiler’s murine encephalomyelitis virus infection by passive administration of neutralizing monoclonal antibody. J Virol. 1989;63:2081–2087. doi: 10.1128/jvi.63.5.2081-2087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerety S J, Karpus W J, Cubbon A R, Goswami R G, Rundell M K, Peterson J D, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- 7.Grant R A, Filman D J, Fujinami R S, Icenogle J P, Hogle J M. Three-dimensional structure of Theiler virus. Proc Natl Acad Sci USA. 1992;89:2061–2065. doi: 10.1073/pnas.89.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarousse N, Martinat C, Syan S, Brahic M, McAllister A. Role of VP2 amino acid 141 in tropism of Theiler’s virus within the central nervous system. J Virol. 1996;70:8213–8217. doi: 10.1128/jvi.70.11.8213-8217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jnaoui K, Michiels T. Adaptation of Theiler’s virus to L929 cells: mutations in the putative receptor binding site on the capsid map to neutralization sites and modulate viral persistence. Virology. 1998;244:397–404. doi: 10.1006/viro.1998.9134. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz C I B, Sun X, Fujinami R S. Protection of SJL/J mice from demyelinating disease mediated by Theiler’s murine encephalomyelitis virus. Microb Pathog. 1995;18:11–27. doi: 10.1016/s0882-4010(05)80009-9. [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Sato S, Patick A K, Pease L R, Roos R P, Rodriguez M. Molecular characterization of a nondemyelinating variant of Daniel’s strain of Theiler’s virus isolated from a persistently infected glioma cell line. J Virol. 1998;72:1262–1269. doi: 10.1128/jvi.72.2.1262-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipton H L. Persistent Theiler’s murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980;46:169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- 13.Lipton H L. Theiler’s viruses. In: Webster R G, Granoff A, editors. Encyclopedia of virology plus CD-ROM product. London, England: Academic Press; 1996. [Google Scholar]
- 14.Luo M, He C, Toth K S, Zhang C X, Lipton H L. Three-dimensional structure of Theiler murine encephalomyelitis virus (BeAn strain) Proc Natl Acad Sci USA. 1992;89:2409–2413. doi: 10.1073/pnas.89.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo M, Toth K S, Zhou L, Pritchard A, Lipton H L. The structure of a highly virulent Theiler’s murine encephalomyelitis virus (GDVII) and implications for determinants of viral persistence. Virology. 1996;220:246–250. doi: 10.1006/viro.1996.0309. [DOI] [PubMed] [Google Scholar]
- 16.Oleszak E L, Leibowitz J L, Rodriguez M. Isolation and characterization of two plaque size variants of Theiler’s murine encephalomyelitis virus (DA strain) J Gen Virol. 1988;69:2413–2418. doi: 10.1099/0022-1317-69-9-2413. [DOI] [PubMed] [Google Scholar]
- 17.Olson N H, Kolatkar P R, Oliveira M A, Cheng R H, Greve J M, McClelland A, Baker T S, Rossmann M G. Structure of a human rhinovirus complexed with its receptor molecule. Proc Natl Acad Sci USA. 1993;90:507–511. doi: 10.1073/pnas.90.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pevear D C, Fancher M J, Felock P J, Rossmann M G, Miller M S, Diana G, Treasurywala A M, McKinlay M A, Dutko F J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989;63:2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchard A E, Calenoff M A, Simpson S, Jensen K, Lipton H L. A single base deletion in the 5′ noncoding region of Theiler’s virus attenuates neurovirulence. J Virol. 1992;66:1951–1958. doi: 10.1128/jvi.66.4.1951-1958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez M, Lafuse W P, Leibowitz J, David C S. Partial suppression of Theiler’s virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens) Neurology. 1986;36:964–970. doi: 10.1212/wnl.36.7.964. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez M, Leibowitz J L, Lampert P W. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez M, Quddus J. Effect of cyclosporin A, silica quartz dust, and protease inhibitors on virus-induced demyelination. J Neuroimmunol. 1986;13:159–174. doi: 10.1016/0165-5728(86)90062-7. [DOI] [PubMed] [Google Scholar]
- 23.Roos R P, Stein S, Ohara Y, Fu J, Semler B L. Infectious cDNA clones of the DA strain of Theiler’s murine encephalomyelitis virus. J Virol. 1989;63:5492–5496. doi: 10.1128/jvi.63.12.5492-5496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapatino B V, Petrescu A D, Rosenbaum B A, Smith R, Piedrahita J A, Welsh C J. Characteristics of cloned cerebrovascular endothelial cells following infection with Theiler’s virus. II. Persistent infection. J Neuroimmunol. 1995;62:127–135. doi: 10.1016/0165-5728(95)00094-4. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Zhang L, Kim J, Jakob J, Grant R A, Wollmann R, Roos R P. A neutralization site of DA strain of Theiler’s murine encephalomyelitis virus important for disease phenotype. Virology. 1996;226:327–337. doi: 10.1006/viro.1996.0660. [DOI] [PubMed] [Google Scholar]
- 26.Stroop W G, Baringer J R, Brahic M. Detection of Theiler’s virus RNA in mouse central nervous system by in situ hybridization. Lab Investig. 1981;45:504–509. [PubMed] [Google Scholar]
- 27.Takemoto K K. Plaque mutants of animal viruses. Prog Med Virol. 1966;8:314–348. [PubMed] [Google Scholar]
- 28.Tsunoda I, Kuang L-Q, Tolley N D, Whitton J L, Fujinami R S. Enhancement of experimental allergic encephalomyelitis (EAE) by DNA immunization with myelin proteolipid protein (PLP) plasmid DNA. J Neuropathol Exp Neurol. 1998;57:758–767. doi: 10.1097/00005072-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Tsunoda I, Kurtz C I B, Fujinami R S. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology. 1997;288:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- 30.Usherwood E J, Johnston I C, Lovelidge L J, Tonks P, Nash A A. Lymphocyte recognition elements on the VP1 protein of Theiler’s virus. Immunology. 1995;85:190–197. [PMC free article] [PubMed] [Google Scholar]
- 31.Wada Y, McCright I J, Whitby F G, Tsunoda I, Fujinami R S. Replacement of loop II of VP-1 of the DA strain with loop II of the GDVII strain of Theiler’s murine encephalomyelitis virus alters neurovirulence, viral persistence and demyelination. J Virol. 1998;72:7557–7562. doi: 10.1128/jvi.72.9.7557-7562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wada Y, Pierce M L, Fujinami R S. Importance of amino acid 101 within capsid protein VP1 for modulation of Theiler’s virus-induced disease. J Virol. 1994;68:1219–1223. doi: 10.1128/jvi.68.2.1219-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warrens A N, Zhang J Y, Sidhu S, Watt D J, Lombardi G, Sewry C A, Lechler R I. Myoblasts fail to stimulate T cells but induce tolerance. Int Immunol. 1994;6:847–853. doi: 10.1093/intimm/6.6.847. [DOI] [PubMed] [Google Scholar]
- 34.Welsh C J, Sapatino B V, Rosenbaum B A, Smith R. Characteristics of cloned cerebrovascular endothelial cells following infection with Theiler’s virus. I. Acute infection. J Neuroimmunol. 1995;62:119–125. doi: 10.1016/0165-5728(95)00093-2. [DOI] [PubMed] [Google Scholar]
- 35.Yamada M, Zurbriggen A, Fujinami R S. Pathogenesis of Theiler’s murine encephalomyelitis virus. Adv Virus Res. 1991;39:291–320. doi: 10.1016/S0065-3527(08)60798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yauch R L, Kerekes K, Saujani K, Kim B S. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler’s virus in demyelination-susceptible SJL/J mice. J Virol. 1995;69:7315–7318. doi: 10.1128/jvi.69.11.7315-7318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yauch R L, Kim B S. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler’s virus is located within VP1(233–244) J Immunol. 1994;153:4508–4519. [PubMed] [Google Scholar]
- 38.Zurbriggen A, Fujinami R S. Theiler’s virus infection in nude mice: viral RNA in vascular endothelial cells. J Virol. 1988;62:3589–3596. doi: 10.1128/jvi.62.10.3589-3596.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zurbriggen A, Hogle J M, Fujinami R S. Alteration of amino acid 101 within capsid protein VP-1 changes the pathogenicity of Theiler’s murine encephalomyelitis virus. J Exp Med. 1989;170:2037–2049. doi: 10.1084/jem.170.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]