Abstract
Evidence connects mental illness to other adverse health conditions, including oral health. However, longitudinal associations between mental and oral health remain understudied. We aimed to examine mental health–oral health associations prospectively in a nationally representative US cohort. Data were from the Population Assessment of Tobacco and Health (PATH) Study. The Global Appraisal of Individual Needs–Short Screener measured 3 types of mental health symptoms: internalizing, externalizing, and substance use problems. Six self-reported oral health conditions related to periodontal disease were evaluated: self-rated oral health, bleeding gums, loose teeth, tooth extraction, gum disease, and bone loss around teeth. Cross-sectional analysis within PATH Study wave 4 (2016 to 2018, n = 30,746) compared the survey-weighted prevalence of the 6 oral health outcomes according to severity of mental health problems. Prospectively, oral health outcomes were assessed 2 y later (wave 5, 2018 to 2019) according to wave 4 (baseline) mental health problems (n = 26,168). Survey-weighted logistic regression models controlled for confounders (age, sex, tobacco use, etc.) with imputation for missing values. All 6 adverse oral health conditions were greater in prevalence among participants with severe internalizing problems. Multiple conditions were also associated with severe externalizing or substance use problems. Longitudinally associations attenuated, but multiple associations of meaningful magnitude persisted, most with internalizing problems. For example, the adjusted odds ratio was 1.27 (95% CI, 1.08 to 1.50) for bleeding gums and 1.37 (95% CI, 1.12 to 1.68) for tooth extraction when we compared severe versus none/low internalizing problems. Providers should expect higher levels of oral disease among patients with adverse mental health symptoms. Independent of externalizing and substance use problems, symptoms of internalizing problems (related to depression and/or anxiety) are plausible risk factors for future oral disease. Better integration and coordination of mental and oral health treatment and prevention are recommended.
Keywords: anxiety disorders, dental health surveys, depression, epidemiology, longitudinal studies, periodontal diseases
Introduction
Mental illnesses are among the most common medical conditions globally (GBD 2019 Mental Disorders Collaborators 2022). In the United States, an estimated 21% of adults experience some form of mental illness (Substance Abuse and Mental Health Services Administration 2021). Poor mental health and psychological stress have been associated with multiple adverse health conditions, including cardiovascular disease (Vancheri et al. 2022), insomnia (Sutton 2021), and diabetes (Robinson et al. 2018). Investigations of the links between mental health and oral health have yielded inconsistent results. While several studies have reported meaningful associations between specific mental and oral conditions, including depression and tooth loss (Okoro et al. 2012; Roohafza et al. 2015), depression and dental caries (Delgado-Angulo et al. 2015), and panic disorder and periodontal disease (Khambaty and Stewart 2013), other studies have shown no association, with notably inconsistent findings for depression and periodontal disease (Araújo et al. 2016; Kisely et al. 2016; Ball and Darby 2022).
Despite conflicting epidemiologic results, several plausible pathways exist through which mental health could affect periodontal and overall oral health. Chronic psychosocial stress, potentially exacerbated under mental illness, may impair the host response to periodontal pathogens, increasing disease susceptibility (Decker et al. 2021; Ball and Darby 2022). Xerogenic medications to manage mental conditions may elevate caries risk (Lalloo et al. 2013). Additionally, people with poor mental health may face barriers to access and utilize dental care (Okoro et al. 2012; Tiwari et al. 2021) and potential behavioral risks to oral health, such as tobacco and other substance use (Degenhardt and Hall 2001).
Improved understanding of the association between mental and oral health could inform medical and dental communities in identifying and addressing unmet oral health needs among individuals with from mental illness. Evidence linking mental health to oral health to date has largely drawn from cross-sectional investigations and has often been limited to specific populations, such as clinical patients (Elter et al. 2002) or specific age groups (Persson et al. 2003; Khambaty and Stewart 2013). Additional longitudinal data from large generalizable samples would help to clarify the role of mental illness as a potential risk factor, or risk indicator, for future oral disease.
The present study draws data from the Population Assessment of Tobacco and Health (PATH) Study, an ongoing nationally representative prospective cohort study of US youth and adults designed to assess the impact of tobacco use on health (Hyland et al. 2017). The PATH Study includes self-reported measures of multiple components of mental health and oral health (Conway et al. 2017; Chaffee et al. 2022), allowing generalizable, prospective investigations of the mental health–oral health relationship. The present study first describes the prevalence of adverse oral health conditions among adults with certain mental health problems to inform clinical practice and resource needs; then, longitudinal associations between mental health problems and future oral health are examined to illuminate possible influences of mental health on oral health.
Methods
Study Data and Design
The PATH Study is a nationally representative prospective cohort study of US youth and adults. Sampling followed a 4-stage area probability design with oversampling for young adults, tobacco users, and African Americans (Hyland et al. 2017). Sample weights enhance generalizability to the noninstitutionalized civilian household population. Computer-assisted at-home questionnaires include sociodemographic characteristics, behaviors (particularly tobacco use), and health conditions. Baseline (wave 1) took place from September 2013 to December 2014 with annual follow-up through wave 4 and biannual follow-up thereafter. A replenishment sample was added at wave 4 (December 2016 to January 2018) to maintain generalizability and sample size. The present analysis relies on deidentified public use data sets from adult participants (age ≥18 y) at wave 4 (n = 33,822) and wave 5 (December 2018 to November 2019; n = 34,309). Adult participants provided written informed consent and received $35 for participation at wave 4 and $50 at wave 5. The PATH Study received ethical approval from the Westat institutional review board. PATH Study data are available to researchers at no cost (National Addiction & HIV Data Archive Program n.d.). Due to the use of deidentified publicly available data, the University of California San Francisco institutional review board determined the present secondary analysis not to be human subjects research. Reporting the present analysis followed STROBE guidelines (von Elm et al. 2007).
This study featured a cross-sectional and longitudinal analysis (Figure). The cross-sectional analysis was descriptive, whereas the longitudinal analysis aimed to assess potential mental health influences on future oral health. Inclusion in the wave 4 cross-sectional analysis required no missing data on mental health conditions, data for at least 1 oral health condition, no more than 12 self-reported lifetime dental extractions (i.e., ≥20 teeth remaining), and an available survey weight. Inclusion in the longitudinal analysis required meeting cross-sectional inclusion criteria and having data for at least 1 oral health condition at wave 5. Sample sizes varied by the specific oral health outcome assessed.
Figure.
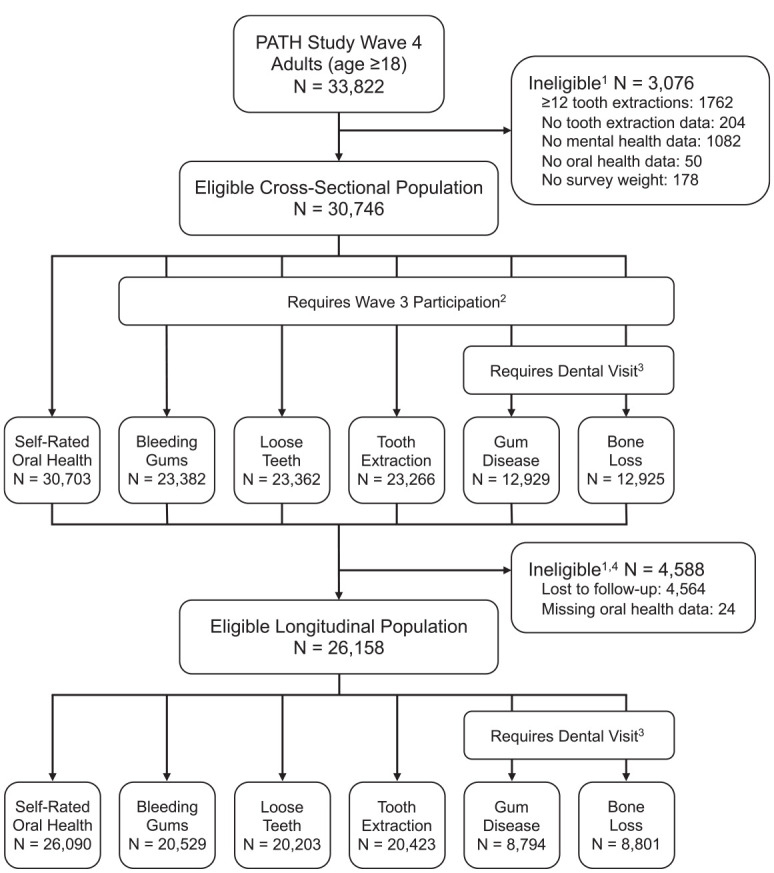
Study population and eligible sample sizes by oral health condition. The Population Assessment of Tobacco and Health (PATH) Study is based on a prospective cohort generalizable to the noninstitutionalized US civilian population. Wave 4 (December 2016 to January 2018) and wave 5 (December 2018 to November 2019) occurred 2 y apart. Due to the survey design, specific oral conditions were measured among different subsets of the study population.1 Listed ineligibility reasons not mutually exclusive.2 For the outcomes of bleeding gums, loose teeth, tooth extraction, gum disease, and bone loss, participants were asked about their experience “in the past 12 mo” only if they had participated in the cohort and were ≥18 y of age at wave 3. These outcomes were first added to the PATH Study at wave 3, when participants were asked about their lifetime history of these conditions (exception: gum disease was included since wave 1). Past–12 mo versions of these items were asked at wave 4.3 For the outcomes of gum disease and bone loss, participants were asked if they had been told by a dental professional that they have these conditions, only if they reported a dental visit in the past 12 mo at the same survey wave.4 For the longitudinal analysis, only participants with measured dental conditions at waves 4 and 5 were included.
Study Variables
Oral Health
Six oral health outcomes were evaluated: self-rated oral health (“Overall, how would you rate the health of your teeth and gums?” dichotomized for analysis as “poor” or “fair” vs. “good,” “very good,” or “excellent”; Locker 2001), bleeding gums (“In the past 12 mo, have you observed any bleeding after brushing or flossing, or due to other conditions in your mouth?”), loose teeth (“In the past 12 mo, have you had any teeth become loose on their own, without an injury?”), tooth extraction (“In the past 12 mo, how many of your permanent teeth have been removed because of tooth decay or gum disease?” dichotomized for analysis as any vs. none), gum disease, and bone loss around teeth. The latter 2 outcomes were asked of participants who had visited a dentist in the past 12 mo and followed the format “In the past 12 mo, have you been told by a dentist, hygienist, or other health professional that you [have gum disease | lost bone around your teeth]?” Most oral health survey items were first added to the PATH Study at wave 3 (gum disease was included since wave 1). Participants were asked about their past–12 mo oral health status at wave 4 if they were continuing from wave 3. Noncontinuing participants (i.e., newly enrolled at wave 4) were instead asked about their lifetime history of these conditions. PATH Study oral health measures were derived from existing validated instruments, such as those from the Centers for Disease Control and Prevention’s Periodontal Disease Surveillance Project (Eke et al. 2012).
Mental Health
Three mental health conditions were evaluated with the Global Appraisal of Individual Needs–Short Screener (GAIN-SS; Dennis et al. 2006): internalizing problems (internally directed behaviors; e.g., feelings of depression or anxiety), externalizing problems (outwardly directed behaviors; e.g., difficulty focusing or feelings of aggression), and substance use problems (e.g., withdrawal, reduced involvement in work or school, or other social problems). Each GAIN-SS mental health condition was measured with a 4- to 7-item scale assessing experiences over the past 12 mo and later categorized as none/low, moderate, or high levels of mental health problems, following previous specifications (Conway et al. 2017).
Covariables
Sociodemographic covariables included sex, age, annual household income, race/ethnicity, educational attainment, marital status, and health insurance. Health and behavioral covariables included body mass index, diabetes history, alcohol use, and tobacco use (cigarettes, electronic cigarettes, cigars, pipes, hookah, and smokeless tobacco). Table 1 displays the covariable specifications used in the analysis. To describe the sample, covariables were measured at wave 4; for covariable adjustment in the analytic models, time-varying covariables were measured at wave 3 (i.e., prior to mental health and oral health variables).
Table 1.
Population Characteristics: Cross-sectional and Longitudinal Samples.
| Sample, % | ||
|---|---|---|
| Characteristic, % | Cross-sectional (n = 30,756) | Longitudinal (n = 26,158) |
| Sex | ||
| Male | 47.9 | 47.9 |
| Female | 52.1 | 52.1 |
| Age, y | ||
| 18 to 24 | 13.2 | 13.2 |
| 25 to 34 | 18.9 | 18.9 |
| 35 to 44 | 16.9 | 17.1 |
| 45 to 54 | 16.9 | 16.8 |
| 55 to 64 | 16.7 | 16.8 |
| ≥65 | 17.4 | 17.1 |
| Race/ethnicity | ||
| Non-Hispanic White | 63.8 | 63.9 |
| Non-Hispanic Black | 11.4 | 11.4 |
| Non-Hispanic other | 8.5 | 8.5 |
| Hispanic/Latinx | 16.2 | 16.3 |
| Annual income, $ | ||
| <10,000 | 10.8 | 10.7 |
| 10,000 to 24,999 | 17.5 | 17.4 |
| 25,000 to 49,999 | 22.6 | 22.5 |
| 50,000 to 99,999 | 27.3 | 27.6 |
| ≥100,000 | 21.8 | 21.9 |
| Educational attainment | ||
| Below high school | 14.3 | 14.1 |
| High school or GED | 23.1 | 23.0 |
| Some college | 31.6 | 31.7 |
| College degree | 30.9 | 31.1 |
| Marital status | ||
| Married | 52.7 | 52.9 |
| Widowed, divorced, or separated | 19.2 | 19.2 |
| Never married | 28.1 | 27.9 |
| Body mass index | ||
| <25 | 33.1 | 32.8 |
| 25 to 29.99 | 33.6 | 33.5 |
| ≥30 | 33.3 | 33.7 |
| Health insurance | ||
| Yes | 88.1 | 88.3 |
| No | 11.9 | 11.7 |
| Diabetes history a | ||
| Yes | 82.5 | 82.6 |
| No | 17.5 | 17.4 |
| Alcohol use (past 30 d) b | ||
| None | 45.7 | 45.1 |
| Light | 31.6 | 31.9 |
| Moderate | 14.9 | 15.1 |
| Heavy | 7.9 | 8.0 |
| Cigarette smoking | ||
| Never | 60.7 | 61.0 |
| Former | 22.6 | 22.4 |
| Current light (1 to 9 cigarettes/d) | 9.9 | 9.8 |
| Current heavy (≥10 cigarettes/d) | 6.9 | 6.8 |
| Current use c | ||
| Electronic cigarettes | 3.1 | 3.1 |
| Other combustibles | 3.6 | 3.6 |
| Smokeless tobacco | 2.7 | 2.7 |
| Internalizing problems | ||
| None/low | 66.8 | 66.3 |
| Moderate | 19.6 | 19.9 |
| High | 13.7 | 13.8 |
| Externalizing problems | ||
| None/low | 69.8 | 69.3 |
| Moderate | 19.5 | 19.8 |
| High | 10.7 | 10.9 |
| Substance use problems | ||
| None/low | 84.3 | 84.2 |
| Moderate | 12.2 | 12.3 |
| High | 3.4 | 3.5 |
The population characteristics of the wave 4 sample as measured at wave 4. All percentages are weighted to be representative of the PATH Study wave 4 sample; thus, the weights applied to the longitudinal sample additionally account for attrition from wave 4 to wave 5. Sample sizes for individual covariables may be less than the total due to missing data.
GED, general educational development (alternative to high school diploma); PATH, Population Assessment of Tobacco and Health.
Lifetime (ever) diagnosis.
Light, moderate, or heavy alcohol use categories differed by sex (female: 1 to 9, 10 to 29, ≥30 drinks; male: 1 to 19, 20 to 59, ≥60 drinks).
For each listed product, percentage of participants now using that product “some days” or “every day.” Electronic cigarettes: all e-cigarettes and electronic nicotine delivery systems. Other combustibles: cigars, pipes, and hookah. Smokeless tobacco: snuff, chewing tobacco, and snus pouches.
Analytic Approach
Weighted prevalence estimates for each oral health outcome (wave 4 in the cross-sectional analysis and wave 5 in the longitudinal analysis) were compared at each level of wave 4 internalizing, externalizing, and substance use problems (chi-square tests). Separate survey-weighted logistic regression models were fitted for each oral health outcome. Wave 4 internalizing, externalizing, and substance use problems were included in all models, as were all Table 1 covariables (time-varying covariables from wave 3 and sex, race/ethnicity, and age from wave 4). Longitudinal models also adjusted for oral health status at wave 4. Missing covariable data were multiply imputed by chained equations via the mi: command suite in Stata 16 (StataCorp). Adjusted odds ratios were considered statistically significant if 95% CIs excluded 1.
Secondary Analyses
Several alternative model specifications were fitted to explore secondary objectives (Appendix). Internalizing, externalizing, and substance use problems were specified in separate models (i.e., rather than mutually adjusted for one another in the same model). Rather than define internalizing and externalizing problems as measured at wave 4 alone, longitudinal models were refitted with mental health problems defined as change in status from wave 4 to wave 5 (e.g., increase or decrease in severity between time points). Finally, to assess the incidence of oral health problems not already present at baseline, longitudinal models were restricted to participants without each condition at wave 4.
Results
Population Characteristics
The weighted characteristics of the cross-sectional and longitudinal study samples were nearly identical (Table 1). Approximately one-third of the samples experienced a moderate or high level of internalizing problems; externalizing problems were nearly as prevalent. Over 15% of the samples experienced a moderate or high level of substance use problems.
Prevalence and Risk of Oral Conditions
Experience of mental health problems was strongly associated with greater prevalence of current adverse oral conditions and, for several conditions, greater risk of adverse oral conditions 2 y later (Table 2). In the descriptive cross-sectional analysis, for all 3 measures of mental health and all 6 oral conditions, there was a consistent stepwise increase in oral condition prevalence at more severe levels of mental health problems. Only for substance use problems and gum disease was there a slight deviation from the stepwise pattern. This pattern of worse oral health over more severe levels of mental health problems largely persisted in the longitudinal analysis, particularly for internalizing problems and for self-rated oral health, gum bleeding, and loose teeth. However, longitudinal associations departed from a stepwise pattern for some mental health and oral health measures, such as tooth extraction. Further details, including unweighted counts and univariate odds ratios, can be found in Appendix Table 1.
Table 2.
Oral Conditions by Mental Health Status: Cross-sectional and Longitudinal Associations.
| Percentage | ||||||
|---|---|---|---|---|---|---|
| Associations | Fair/Poor Self-rated Oral Health | Bleeding Gums | Loose Teeth | Tooth Extraction | Gum Disease | Bone Loss Around Teeth |
| Cross-sectional a | ||||||
| Internalizing problems | ||||||
| None/low | 14.2 | 20.6 | 3.4 | 8.3 | 5.2 | 6.5 |
| Moderate | 25.6 | 36.9 | 6.3 | 11.8 | 8.9 | 9.4 |
| High | 32.8 | 43.7 | 10.9 | 15.0 | 12.6 | 12.4 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Externalizing problems | ||||||
| None/low | 16.5 | 20.6 | 4.2 | 9.3 | 5.6 | 7.1 |
| Moderate | 21.9 | 36.9 | 6.2 | 10.3 | 8.0 | 8.5 |
| High | 29.7 | 50.7 | 7.8 | 13.4 | 12.3 | 10.5 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 |
| Substance use problems | ||||||
| None/low | 17.7 | 24.9 | 4.5 | 9.3 | 6.5 | 7.4 |
| Moderate | 22.2 | 34.0 | 6.0 | 11.3 | 6.3 | 9.0 |
| High | 38.5 | 48.7 | 13.3 | 18.3 | 13.9 | 13.6 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Longitudinal b | ||||||
| Internalizing problems | ||||||
| None/low | 15.3 | 19.7 | 3.8 | 11.5 | 5.2 | 7.6 |
| Moderate | 24.3 | 34.5 | 6.6 | 13.6 | 6.6 | 8.8 |
| High | 31.3 | 38.7 | 8.7 | 16.9 | 8.8 | 10.4 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.071 |
| Externalizing problems | ||||||
| None/low | 17.4 | 19.4 | 4.6 | 12.7 | 5.2 | 7.7 |
| Moderate | 21.5 | 35.3 | 5.6 | 12.3 | 7.0 | 9.5 |
| High | 27.5 | 44.8 | 6.7 | 13.0 | 8.2 | 7.7 |
| P value | <0.001 | <0.001 | 0.002 | 0.773 | 0.014 | 0.200 |
| Substance use problems | ||||||
| None/low | 18.1 | 23.6 | 4.7 | 12.6 | 5.8 | 7.9 |
| Moderate | 23.0 | 32.7 | 6.1 | 11.4 | 5.1 | 9.0 |
| High | 36.0 | 38.0 | 9.3 | 19.4 | 8.5 | 11.2 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.284 | 0.304 |
All percentages were weighted to be representative of the PATH Study wave 4 sample. All P values were based on the survey-weighted chi-square test.
PATH, Population Assessment of Tobacco and Health.
Prevalence of each oral condition at wave 4 according to wave 4 mental health variables.
Risk of each oral condition at wave 5 according to wave 4 mental health variables.
Adjusted Longitudinal Associations
Associations between mental health and oral health attenuated in a longitudinal setting, but internalizing problems remained a meaningful predictor of several future adverse oral conditions (Table 3). With the exceptions of gum disease and bone loss around teeth, a high level of internalizing problems was associated with higher odds of adverse oral conditions 2 y later—1.3 times higher for bleeding gums and 1.4 times for fair/poor self-rated oral health, loose teeth, and tooth extractions—versus none or a low level of internalizing problems. Externalizing problems remained meaningfully associated with greater odds of bleeding gums, loose teeth, and gum disease, albeit statistically significant for bleeding gums and not with other oral outcomes. Substance use problems did not have meaningful or statistically significant associations with any of the assessed oral conditions.
Table 3.
Relative Odds of Oral Conditions by Mental Health Status: Longitudinal Analysis.
| Adjusted Odds Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Problems | Fair/Poor Self-rated Oral Health | Bleeding Gums | Loose Teeth | Tooth Extraction | Gum Disease | Bone Loss Around Teeth |
| Internalizing | ||||||
| None/low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 1.24 (1.06, 1.45) | 1.38 (1.21, 1.59) | 1.50 (1.14, 1.97) | 1.15 (0.96, 1.38) | 1.02 (0.71, 1.46) | 0.96 (0.69, 1.35) |
| High | 1.36 (1.12, 1.63) | 1.27 (1.08, 1.50) | 1.39 (1.02, 1.89) | 1.37 (1.12, 1.68) | 0.94 (0.55, 1.58) | 1.00 (0.66, 1.51) |
| Externalizing | ||||||
| None/low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 1.15 (0.99, 1.33) | 1.42 (1.23, 1.63) | 1.18 (0.88, 1.58) | 1.04 (0.86, 1.25) | 1.17 (0.78, 1.76) | 1.34 (0.96, 1.85) |
| High | 1.17 (0.95, 1.44) | 1.49 (1.26, 1.77) | 1.21 (0.87, 1.67) | 0.95 (0.76, 1.19) | 1.24 (0.69, 2.22) | 0.99 (0.60, 1.65) |
| Substance use | ||||||
| None/low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 1.04 (0.89, 1.22) | 1.10 (0.95, 1.27) | 1.11 (0.85, 1.44) | 0.77 (0.64, 0.94) | 0.82 (0.52, 1.30) | 0.96 (0.65, 1.43) |
| High | 1.03 (0.81, 1.32) | 0.81 (0.63, 1.03) | 0.90 (0.63, 1.30) | 1.10 (0.85, 1.41) | 1.02 (0.54, 1.92) | 1.17 (0.59, 2.30) |
All odds ratios were weighted to be representative of the PATH Study wave 4 sample. A separate logistic regression model was fitted for each wave 5 oral health outcome and included all Table 1 covariables. Covariables sex, age, and race/ethnicity were measured at wave 4; all other covariables were measured at wave 3. Each model additionally adjusted for the oral health condition at the wave 4 baseline (e.g., the wave 5 past 12-mo bleeding gum model included wave 4 past 12-mo bleeding gum status as a covariable).
PATH, Population Assessment of Tobacco and Health.
Secondary Analyses
Alternative model specifications largely yielded results consistent with the primary findings (Appendix). Associations strengthened when each mental health variable was assessed in a separate model, albeit not enough for substance use problems as a predictor of future oral health (Appendix Table 2). Worsening mental health over time was associated with most of the assessed oral conditions for internalizing problems but only with fair/poor self-rated oral health and bleeding gums for externalizing problems (Appendix Table 3). Restricted to participants without the oral condition at wave 4, internalizing problems were associated with greater odds of fair/poor self-rated oral health, bleeding gums, loose teeth, and tooth extraction at wave 5; externalizing problems were associated with greater odds of wave 5 bleeding gums (Appendix Table 4).
Discussion
In this study, symptoms of mental health problems were associated with multiple measures of adverse oral health, even after adjustment for a bevy of shared socioeconomic and demographic risk factors. In general, associations were strongest and most consistent for symptoms of internalizing problems but weaker and largely limited to the cross-sectional setting for externalizing and substance use problems. These results provide robust, generalizable evidence of greater prevalence of poor oral health among individuals with mental illness and suggest that at least some aspects of mental health may affect oral health over time.
Internalizing problems comprise a number of symptoms common to disorders such as depression, anxiety, and traumatic stress (Dennis et al. 2006). The associations between internalizing symptoms and oral health observed in our study are consistent with previous findings linking symptoms of depression and/or anxiety with tooth loss (Okoro et al. 2012), caries (Delgado-Angulo et al. 2015), and other oral conditions (Kisely et al. 2016). A recent study of >500,000 UK Biobank participants found a higher prevalence of periodontal disease (defined by self-reported symptoms) among people with a history of psychosis, regardless of whether psychosis was clinically diagnosed or self-reported (Kang et al. 2022). Dental anxiety, while not measured in the PATH Study, has been associated with greater caries experience and may deter dental care utilization (Wisløff et al. 1995). Indeed, barriers to dental care could explain at least part of the relationship observed in the present study (Slack-Smith et al. 2017). Mechanisms related to psychosocial stress, inflammation, and immune response also remain plausible pathways connecting poor mental health to oral health (Decker et al. 2021).
Externalizing problems, comprising symptoms related to attention deficient and impulsivity, and substance use problems, which did not directly measure substance intake but rather social disruptions caused by drug use or drug seeking, were not as strongly or reliably associated with adverse oral conditions in this study. As a contextual consideration, these associations were from statistical models mutually adjusted for all 3 problems: internalizing, externalizing, and substance misuse. When analyzed separately, externalizing and substance use problems appeared to be stronger risk indicators for poor oral health, suggesting that co-occurrence with internalizing problems may confound these associations. Relatedly, all models were adjusted for tobacco and alcohol use. Thus, these models assessed substance use problems independent of actual tobacco and alcohol intake. Therefore, these findings do not conflict with the established role of tobacco use in oral disease (Johnson and Bain 2000) or with further evidence implicating alcohol (Genco and Borgnakke 2013) and illicit drugs (Baghaie et al. 2017).
Some caution is required when comparing associations across oral health outcomes in the present analysis. Outcomes are not independent. As a group, several share a common link to symptoms of chronic periodontal disease. Thus, we consider consistency of associations across multiple oral health outcomes, as observed for internalizing problems, as stronger evidence of an underlying influence on oral health. However, there were important differences in analytic samples. Notably, gum disease and alveolar bone loss survey questions were posed only to participants with a recent dental visit. While this restriction has potential to introduce selection bias, prior analysis of PATH Study data suggested that such bias is likely minimal (Chaffee et al. 2020). Of the 6 outcomes assessed, bleeding gums was the most prevalent and the only outcome longitudinally associated with internalizing and externalizing problems.
Mental and oral health variables in this study were evaluated by self-report, not clinical examination. GAIN-SS measures are strongly correlated with common mental illnesses but are not diagnostic (Dennis et al. 2006). Self-reported oral health measures, in general, have relatively low sensitivity but good specificity for periodontal disease, leading to underestimates of actual disease prevalence (Blicher et al. 2005). If misclassification of oral health status is nondifferential by mental health status, the associations presented could be overly conservative and a reason for future studies with clinically assessed outcomes. Differential reporting of oral health by mental health status cannot be ruled out and is worthy of further research. Measurement differences might explain inconsistencies between the present results and prior studies showing no associations between mental health and clinical periodontal parameters (Persson et al. 2003; Castro et al. 2006; Solis et al. 2014). However, differences in sample size, geography, and participant characteristics should also be considered.
Among other study limitations, unmeasured confounding could contribute to the observed associations; for example, no measure of diet was available. Additionally, many mental and oral diseases are chronic conditions that develop and progress over time. The 2-y interval between survey waves may be insufficient for more severe periodontal outcomes to develop, such as loss of alveolar bone and teeth. However, an outcome such as bleeding gums may develop over a shorter period. The observed longitudinal associations of gum bleeding with internalizing and externalizing problems, even among those without gum bleeding at baseline, suggests a possible causal role. Yet, despite the longitudinal structure of the data set, some overall uncertainty remains regarding the exact temporal sequence in which conditions were initiated. This study was designed to assess mental health status as a risk factor for future adverse oral health conditions, as supported in other studies (Cademartori et al. 2018), but additional evidence suggests a possible bidirectional relationship that merits further research. For example, tooth loss may contribute to depression symptoms among older adults (Kusama et al. 2021). Key study strengths include the large nationally representative sample, prospective design, ability to assess multiple dimensions of mental and oral health, and robustness of statistical findings to alternative model specifications.
In conclusion, dental and medical practitioners should anticipate a higher prevalence of oral disease among individuals with symptoms of common mental illnesses. In particular, internalizing problems, typical of depression and anxiety, are not only associated with diminished oral health but may contribute to enhanced oral disease risk over time. These findings suggest a need for improved coordination of mental and oral health care, such as better communication among care providers and efforts to reduce access barriers to appropriate treatment for mental and oral health needs.
Author Contributions
A. Kalaigian, contributed to conception and design, data analysis, drafted and critically revised the manuscript; B.W. Chaffee, contributed to conception and design, data analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345231171108 for Mental Health and Oral Health in a Nationally Representative Cohort by A. Kalaigian and B.W. Chaffee in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported with institution funding from the University of California San Francisco as part of a student research fellowship. The PATH Study is supported with federal funds from the National Institute on Drug Abuse, National Institutes of Health, and from the Center for Tobacco Products, Food and Drug Administration, Department of Health and Human Services, under contract to Westat. Westat and the funders had no role in the present article.
References
- Araújo MM, Martins CC, Costa LC, Cota LO, Faria RL, Cunha FA, Costa FO. 2016. Association between depression and periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 43(3):216–228. [DOI] [PubMed] [Google Scholar]
- Baghaie H, Kisely S, Forbes M, Sawyer E, Siskind DJ. 2017. A systematic review and meta-analysis of the association between poor oral health and substance abuse. Addiction. 112(5):765–779. [DOI] [PubMed] [Google Scholar]
- Ball J, Darby I. 2022. Mental health and periodontal and peri-implant diseases. Periodontol 2000. 90(1):106–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blicher B, Joshipura K, Eke P. 2005. Validation of self-reported periodontal disease: a systematic review. J Dent Res. 84(10):881–890. [DOI] [PubMed] [Google Scholar]
- Cademartori MG, Gastal MT, Nascimento GG, Demarco FF, Corrêa MB. 2018. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta-analysis. Clin Oral Investig. 22(8):2685–2702. [DOI] [PubMed] [Google Scholar]
- Castro GD, Oppermann RV, Haas AN, Winter R, Alchieri JC. 2006. Association between psychosocial factors and periodontitis: a case-control study. J Clin Periodontol. 33(2):109–114. [DOI] [PubMed] [Google Scholar]
- Chaffee BW, Lauten K, Sharma E, Everard CD, Duffy K, Park-Lee E, Taylor E, Tolliver E, Watkins-Bryant T, Iafolla T, et al. 2022. Oral health in the Population Assessment of Tobacco and Health Study. J Dent Res. 101(9):1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BW, Persai D, Vora MV. 2020. Interdental cleaning and oral health status in an adult cohort, 2015 to 2018. J Dent Res. 99(10):1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Green VR, Kasza KA, Silveira ML, Borek N, Kimmel HL, Sargent JD, Stanton C, Lambert E, Hilmi N, et al. 2017. Co-occurrence of tobacco product use, substance use, and mental health problems among adults: findings from wave 1 (2013-2014) of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend. 177:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker AM, Kapila YL, Wang HL. 2021. The psychobiological links between chronic stress-related diseases, periodontal/peri-implant diseases, and wound healing. Periodontol 2000. 87(1):94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. 2001. The relationship between tobacco use, substance-use disorders and mental health: results from the national survey of mental health and well-being. Nicotine Tob Res. 3(3):225–234. [DOI] [PubMed] [Google Scholar]
- Delgado-Angulo EK, Sabbah W, Suominen AL, Vehkalahti MM, Knuuttila M, Partonen T, Nordblad A, Sheiham A, Watt RG, Tsakos G. 2015. The association of depression and anxiety with dental caries and periodontal disease among Finnish adults. Community Dent Oral Epidemiol. 43(6):540–549. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Chan YF, Funk RR. 2006. Development and validation of the GAIN–Short Screener (GSS) for internalizing, externalizing and substance use disorders and crime/violence problems among adolescents and adults. Am J Addict. 15 Suppl 1:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans G, Dye B, Genco R. 2012. Advances in surveillance of periodontitis: the centers for disease control and prevention periodontal disease surveillance project. J Periodontol. 83(11):1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elter JR, White BA, Gaynes BN, Bader JD. 2002. Relationship of clinical depression to periodontal treatment outcome. J Periodontol. 73(4):441–449. [DOI] [PubMed] [Google Scholar]
- GBD 2019 Mental Disorders Collaborators. 2022. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 9(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco RJ, Borgnakke WS. 2013. Risk factors for periodontal disease. Periodontol 2000. 62(1):59–94. [DOI] [PubMed] [Google Scholar]
- Hyland A, Ambrose BK, Conway KP, Borek N, Lambert E, Carusi C, Taylor K, Crosse S, Fong GT, Cummings KM, et al. 2017. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NW, Bain CA; EU–Working Group On Tobacco and Oral Health. 2000. Tobacco and oral disease. Br Dent J. 189(4):200–206. [DOI] [PubMed] [Google Scholar]
- Kang J, Palmier-Claus J, Wu J, Shiers D, Larvin H, Doran T, Aggarwal VR. 2022. Periodontal disease in people with a history of psychosis: results from the UK Biobank population-based study. Community Dent Oral Epidemiol [epub ahead of print 18 Oct 2022] in press. doi: 10.1111/cdoe.12798 [DOI] [PubMed] [Google Scholar]
- Khambaty T, Stewart JC. 2013. Associations of depressive and anxiety disorders with periodontal disease prevalence in young adults: analysis of 1999–2004 National Health and Nutrition Examination Survey (NHANES) data. Ann Behav Med. 45(3):393–397. [DOI] [PubMed] [Google Scholar]
- Kisely S, Sawyer E, Siskind D, Lalloo R. 2016. The oral health of people with anxiety and depressive disorders—a systematic review and meta-analysis. J Affect Disord. 200:119–132. [DOI] [PubMed] [Google Scholar]
- Kusama T, Kiuchi S, Umehara N, Kondo K, Osaka K, Aida J. 2021. The deterioration of oral function and orofacial appearance mediated the relationship between tooth loss and depression among community-dwelling older adults: a JAGES cohort study using causal mediation analysis. J Affect Disord. 286:174–179. [DOI] [PubMed] [Google Scholar]
- Lalloo R, Kisely S, Amarasinghe H, Perera R, Johnson N. 2013. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 21(4):338–342. [DOI] [PubMed] [Google Scholar]
- Locker D. 2001. Oral health indicators and determinants for population health surveys: a report for Health Canada. Toronto (Canada): University of Toronto. [Google Scholar]
- National Addiction & HIV Data Archive Program. n.d. Population Assessment of Tobacco and Health (PATH) Study series [accessed 2023 Feb 17]. https://www.icpsr.umich.edu/web/nahdap/series/606
- Okoro CA, Strine TW, Eke PI, Dhingra SS, Balluz LS. 2012. The association between depression and anxiety and use of oral health services and tooth loss. Community Dent Oral Epidemiol. 40(2):134–144. [DOI] [PubMed] [Google Scholar]
- Persson GR, Persson RE, MacEntee CI, Wyatt CC, Hollender LG, Kiyak HA. 2003. Periodontitis and perceived risk for periodontitis in elders with evidence of depression. J Clin Periodontol. 30(8):691–696. [DOI] [PubMed] [Google Scholar]
- Robinson DJ, Coons M, Haensel H, Vallis M, Yale JF. 2018. Diabetes and mental health. Can J Diabetes. 42 Suppl 1:S130–S141. [DOI] [PubMed] [Google Scholar]
- Roohafza H, Afghari P, Keshteli AH, Vali A, Shirani M, Adibi P, Afshar H. 2015. The relationship between tooth loss and psychological factors. Community Dent Health. 32(1):16–19. [PubMed] [Google Scholar]
- Slack-Smith L, Hearn L, Scrine C, Durey A. 2017. Barriers and enablers for oral health care for people affected by mental health disorders. Aust Dent J. 62(1):6–13. [DOI] [PubMed] [Google Scholar]
- Solis AC, Marques AH, Pannuti CM, Lotufo RF, Lotufo-Neto F. 2014. Evaluation of periodontitis in hospital outpatients with major depressive disorder. J Periodontal Res. 49(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2021. Key substance use and mental health indicators in the United States: results from the 2020 National Survey on Drug Use and Health. HHS publication Pep21-07-01-003, NSDUH series H-56. Rockville (MD): Center for Behavioral Health Statistics and Quality; [accessed 2023 Feb 17]. https://www.samhsa.gov/data/ [Google Scholar]
- Sutton EL. 2021. Insomnia. Ann Intern Med. 174(3):ITC33–ITC48. [DOI] [PubMed] [Google Scholar]
- Tiwari T, Kelly A, Randall CL, Tranby E, Franstve-Hawley J. 2021. Association between mental health and oral health status and care utilization. Front Oral Health. 2:732882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancheri F, Longo G, Vancheri E, Henein MY. 2022. Mental stress and cardiovascular health—part I. J Clin Med. 11(12):3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 335(7624):806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisløff TF, Vassend O, Asmyhr O. 1995. Dental anxiety, utilisation of dental services, and DMFS status in Norwegian military recruits. Community Dent Health. 12(2):100–103. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345231171108 for Mental Health and Oral Health in a Nationally Representative Cohort by A. Kalaigian and B.W. Chaffee in Journal of Dental Research


