Abstract
OBJECTIVE:
To evaluate the association of race–ethnicity and neighborhood socioeconomic status with adherence to National Comprehensive Cancer Network guidelines for endometrial carcinoma.
METHODS:
Data are from the SEER (Surveillance, Epidemiology, and End Results) cancer registry of women diagnosed with endometrial carcinoma for the years 2006–2015. The sample included 83,883 women after inclusion and exclusion criteria were applied. Descriptive statistics, bivariate analyses, univariate, and multivariate logistic regression models were performed to evaluate the association between race–ethnicity and neighborhood socioeconomic status with adherence to treatment guidelines.
RESULTS:
After controlling for demographic and clinical covariates, Black (odds ratio [OR] 0.89, P<.001), Latina (OR .92, P<.001), and American Indian or Alaska Native (OR 0.82, P=.034) women had lower odds of receiving adherent treatment and Asian (OR 1.14, P<.001) and Native Hawaiian or Pacific Islander (OR 1.19 P=.012) women had higher odds of receiving adherent treatment compared with White women. After controlling for covariates, there was a gradient by neighborhood socioeconomic status: women in the high–middle (OR 0.89, P<.001), middle (OR 0.84, P<.001), low–middle (OR 0.80, P<.001), and lowest (OR 0.73, P<.001) neighborhood socioeconomic status categories had lower odds of receiving adherent treatment than the those in the highest neighborhood socioeconomic status group.
CONCLUSIONS:
Findings from this study suggest there are racial–ethnic and neighborhood socioeconomic disparities in National Comprehensive Cancer Network treatment adherence for endometrial cancer. Standard treatment therapies should not differ based on sociodemographics. Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals, regardless of racial–ethnic or socioeconomic background.
Gynecologic cancers represent the third most common malignancies among women.1 Uterine cancer, more specifically endometrial cancer, is the most common gynecologic cancer.2–5 Partly because of its high prevalence, uterine cancer is the second most deadly gynecologic cancer in the United States.1,6–8 Over the past few decades, uterine cancer incidence and mortality have steadily risen for all racial–ethnic groups, with the highest rate of increase observed among racial–ethnic minority women.6,9 There are apparent disparities within uterine cancer, particularly among Black women because they are disproportionately affected by uterine cancer deaths relative to other racial–ethnic groups.6,10 However, research that has focused on disparities relating to the treatment of uterine cancer has been lacking, especially with an inclusion of multiple racial–ethnic minority and socioeconomic status groups.
The National Comprehensive Cancer Network has developed evidenced-based and consensus-driven treatment guidelines that are widely viewed as the standard of care.11,12 Above all, adherence to National Comprehensive Cancer Network treatment guidelines has been associated with improved survival for various cancer sites, which marks it an important metric for treatment and subsequent cancer survival.11–20 To date, however, there are few studies that have looked at endometrial cancer disparities with adherence to National Comprehensive Cancer Network treatment guidelines. These studies have found that identifying as Asian or Pacific Islander was associated with adherence to National Comprehensive Cancer Network guidelines, whereas identifying as Black or Latina was associated with nonadherence to National Comprehensive Cancer Network guidelines.21–23 To our knowledge, there are no studies that have looked at endometrial cancer disparities in National Comprehensive Cancer Network treatment adherence among various socioeconomic status groups.
Racial–ethnic minorities and lower socioeconomic status groups are especially vulnerable to receiving substandard care, which perpetuates poor health outcomes. Substandard care has been associated with social disadvantage (ie, low socioeconomic status, limited access to health care, and increased number of life stressors), structural barriers to health care (ie, geographic proximity to health care facilities), and implicit biases of physicians.24–26 More research is needed to examine disparities in adherence to National Comprehensive Cancer Network endometrial treatment guidelines with inclusion of a comparative analysis of multiple racial–ethnic group backgrounds, because minority racial–ethnic groups experience higher levels of social disadvantage, and greater experiences of biases and racism within the health care system that may influence inequities in health.25 Hence, it is important to examine racial–ethnic and socioeconomic inequities within standard treatment of cancer to identify which groups are most at risk for receiving subpar care. The objective of this study was to evaluate the association of race–ethnicity and neighborhood socioeconomic status with adherence to National Comprehensive Cancer Network treatment guidelines for endometrial cancer and to note where racial–ethnic and socioeconomic disparities exist. We hypothesized that racial–ethnic minorities and women of lower neighborhood socioeconomic status would have lower percentages of adherence to National Comprehensive Cancer Network treatment guidelines.
METHODS
This study used retrospective population-based data from the SEER (Surveillance, Epidemiology, and End Results) database between January 1, 2006, and December 31, 2015, and received approval from the institutional review board of the University of California, Irvine (UCI IRB HS No. 2019–5081). The SEER national cancer registry assembles population based cancer registries throughout the United States and includes incidence and mortality information on demographics, prognostic characteristics, and primary cancer treatment.27
The study population included women who were aged 18 years and older and diagnosed with the first or only endometrial carcinoma. Patients were identified using the endometrial SEER primary site code (C54.1) and histologic subtypes classified as endometrial carcinoma.6,28 A total of 83,883 patients with endometrial carcinoma were included as the final study population (Fig. 1) after excluding those with unknown race–ethnicity, unknown stage of diagnosis, unknown Census tract information, missing or unknown clinical data (surgical treatment, extent of disease, diagnostic confirmation, and surgical staging), and patients or information obtained from autopsy or death certificates.
Fig. 1.
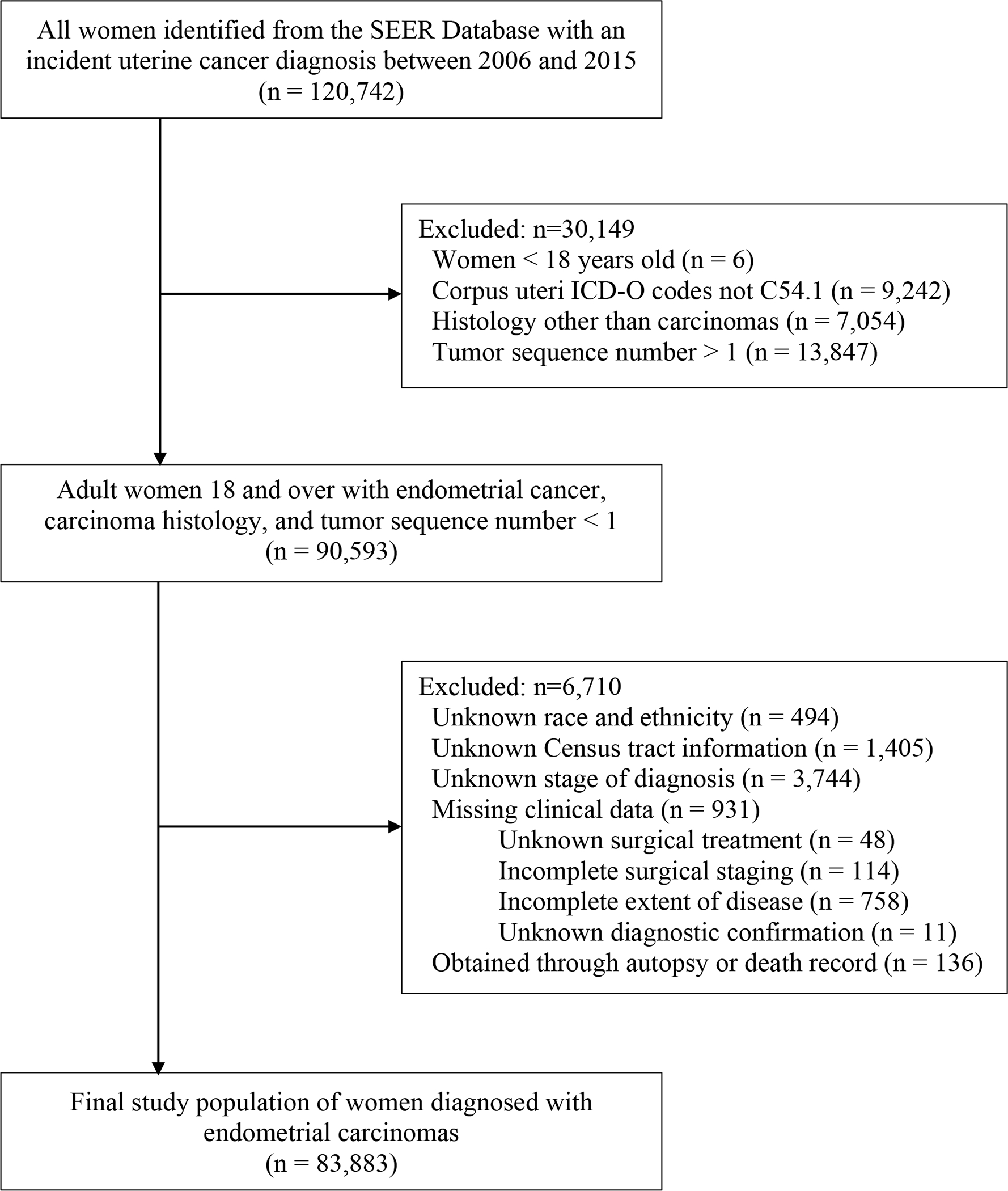
Study population exclusions. This diagram specifies how women diagnosed with endometrial carcinoma between 2006 and 2015 were included in the study. SEER, Surveillance, Epidemiology, and End Results; ICD-O, International Classification of Diseases for Oncology.
Rodriguez. Disparities in Endometrial Cancer Treatment. Obstet Gynecol 2021.
The primary dependent variable was adherence to National Comprehensive Cancer Network guidelines for the first course of treatment, accounting for guideline changes that occurred during the study time period.29–31 National Comprehensive Cancer Network guidelines recommended a combination of therapies dependent on histologic subtype (eg, endometroid carcinomas or other carcinomas) and extent of disease (eg, diseases limited to uterus, suspected or gross cervical involvement, and suspected extrauterine disease).29–31 Based on the National Comprehensive Cancer Network guidelines, we combined the corresponding data to create a binary variable representing adherence to National Comprehensive Cancer Network guidelines (1=adherent treatment, 0=nonadherent treatment). Adherence to National Comprehensive Cancer Network guidelines is further described in Appendices 1–3, available online at http://links.lww.com/AOG/C326. Grade and stage of disease were not considered determinants of treatment adherence for the first course of treatment for endometrial cancer, based on National Comprehensive Cancer Network guidelines.29–31
The main independent variables were race–ethnicity of the patient and neighborhood socioeconomic status. Race–ethnicity was classified into six groups: non-Latina White (reference group), non-Latina Black, Latina; non-Latina Asian, non-Latina Native Hawaiian or Pacific Islander, and non-Latina American Indian or Alaska Native (henceforth White, Black, Latina, Asian, Native Hawaiian or Pacific Islander, and American Indian or Alaska Native, respectively). There were no multiracial groups reported in the SEER data set. Racial–ethnic variables for the SEER data set are ascertained through medical records or administrative information, as opposed to a patient’s self-reported racial–ethnic identity. Neighborhood socioeconomic status was classified into quintiles based on the Yost score, which ranged from highest neighborhood socioeconomic status (reference group) to lowest neighborhood socioeconomic status.32 The Yost score is a composite index of neighborhood socioeconomic status that uses Census tracts, and several indicators of education, income, and occupation.32 Education was represented by an education index; occupation included the proportion of those with blue-collar jobs and the proportion of those older than 16 years of age in the workforce without a job; and income included median household income, proportion of those with incomes below 200% of the poverty level, median rent, and median house value.32 Covariates included demographic and clinical characteristics. Demographic characteristics included age at diagnosis. Age at diagnosis was used as a categorical variable, with four groups based on quartile distribution: younger than 54 years (reference group), 54–61 years, 62–68 years, and 69 years and older. Clinical characteristics included stage of diagnosis, histology, grade of disease, histologic subtype, and year of diagnosis. Stage of diagnosis was a categorical variable with four categories that ranged from stage I (reference group) to stage IV. Histology was coded as a binary variable with the categories that included endometroid carcinomas and other carcinomas. Grade was categorized into five groups: grade 1, well differentiated (reference group); grade 2, moderately differentiated; grade 3, poorly differentiated; grade 4, undifferentiated or anaplastic; and unknown. Year of diagnosis was treated as a continuous variable.
Descriptive statistics for demographic and clinical characteristics by patients’ race–ethnicity and adherence to treatment guidelines were performed. Descriptive statistics for the type of treatment and stage at diagnosis were also performed. Bivariate analyses, univariate, and multivariate logistic regression models were conducted to evaluate the association of race–ethnicity and neighborhood socioeconomic status with the dependent variable–adherence to National Comprehensive Cancer Network guidelines–while controlling for demographic and clinical covariates. We used robust standard errors to adjust for heteroscedasticity in residuals. All statistical analyses were performed using Stata 16.
RESULTS
Demographics of the total sample are shown in Table 1, and demographics of the sample who received adherent treatment are shown in Table 2. Overall, 59.5% of the study population received adherent treatment to National Comprehensive Cancer Network guidelines. The racial–ethnic groups with the lowest percentage of adherent treatment were among Black, Latina, and American Indian or Alaska Native women (57.1%, 54.5%, and 52.7% respectively); the highest percentage of adherent treatment was among Asian, Native Hawaiian and Pacific Islander, and White women (62.3%, 60.6%, and 60.3% respectively). Adherent treatment was higher among the highest neighborhood socioeconomic status groups and gradually decreased as neighborhood socioeconomic status lowered.
Table 1.
Demographic and Cancer Characteristics of Patients With Endometrial Carcinoma by Race-Ethnicity Between 2006 and 2015 in the SEER (Surveillance, Epidemiology, and End Results) Database (N = 83,883)
| Characteristic | White (n=59,626) | Black (n=6,564) | Latina (n=10,065) | Asian (n=6,064) | Native Hawaiian or Pacific Islander (n=1,048) | American Indian or Alaska Native (n=516) | Total Sample (N=83,883) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Adherence to NCCN guidelines | |||||||
| Adherent treatment | 35,971 (60.3) | 3,749 (57.1) | 5,483 (54.5) | 3,775 (62.3) | 635 (60.6) | 272 (52.7) | 49,885 (59.5) |
| Nonadherent treatment | 23,655 (39.7) | 2,815 (42.9) | 4,582 (45.5) | 2,289 (37.7) | 413 (39.4) | 244 (47.3) | 33,998 (40.5) |
| Neighborhood SES | |||||||
| Highest | 15,576 (26.1) | 489 (7.4) | 1,119 (11.1) | 2,025 (33.4) | 166 (15.8) | 44 (8.5) | 19,419 (23.2) |
| Higher-middle | 14,411 (24.2) | 812 (12.4) | 1,684 (16.7) | 1,597 (26.3) | 301 (28.7) | 92 (17.8) | 18,897 (22.5) |
| Middle | 12,507 (21.0) | 1,193 (18.2) | 2,049 (20.4) | 1,168 (19.3) | 263 (25.1) | 104 (20.2) | 17,284 (20.6) |
| Lower-middle | 10,492 (17.6) | 1,522 (23.2) | 2,441 (24.3) | 830 (13.7) | 204 (19.5) | 120 (23.3) | 15,609 (18.6) |
| Lowest | 6,640 (11.1) | 2,548 (38.8) | 2,772 (27.5) | 444 (7.3) | 114 (10.9) | 156 (30.2) | 12,674 (15.1) |
| Age at diagnosis (y) | |||||||
| Younger than 54 | 11,283 (18.9) | 1,208 (18.4) | 3,724 (37.0) | 2,050 (33.8) | 485 (46.3) | 198 (38.4) | 18,948 (22.6) |
| 54–61 | 17,522 (29.4) | 1,807 (27.5) | 2,723 (27.0) | 1,896 (31.3) | 278 (26.5) | 144 (27.9) | 24,370 (29.0) |
| 62–68 | 14,428 (24.2) | 1,813 (27.6) | 1,919 (19.1) | 1,098 (18.1) | 145 (13.8) | 100 (19.4) | 19,503 (23.3) |
| 69 or older | 16,393 (27.5) | 1,736 (26.5) | 1,699 (16.9) | 1,020 (16.8) | 140 (13.4) | 74 (14.3) | 21,062 (25.1) |
| Stage at diagnosis | |||||||
| I | 46,294 (77.6) | 4,395 (66.9) | 7,634 (75.9) | 4,520 (74.5) | 776 (74.0) | 395 (76.6) | 64,014 (76.3) |
| II | 3,452 (5.8) | 515 (7.9) | 651 (6.5) | 340 (5.6) | 63 (6.0) | 24 (4.7) | 5,045 (6.0) |
| III | 7,024 (11.8) | 1,017 (15.5) | 1,178 (11.7) | 840 (13.9) | 138 (13.2) | 72 (13.9) | 10,269 (12.3) |
| IV | 2,856 (4.8) | 637 (9.7) | 602 (5.9) | 364 (6.0) | 71 (6.8) | 25 (4.8) | 4,555 (5.4) |
| Histology | |||||||
| Endometroid carcinomas | 45,787 (76.8) | 3,970 (60.5) | 7,416 (73.7) | 4,517 (74.5) | 805 (76.8) | 405 (78.5) | 62,900 (75.0) |
| Other carcinomas | 13,839 (23.2) | 2,594 (39.5) | 2,649 (26.3) | 1,547 (25.5) | 243 (23.2) | 111 (21.5) | 20,983 (25.0) |
| Grade | |||||||
| 1, well differentiated | 22,889 (38.4) | 1,586 (24.2) | 4,179 (41.5) | 2,407 (39.7) | 467 (44.6) | 207 (40.1) | 31,735 (37.8) |
| 2, moderately differentiated | 14,635 (24.5) | 1,362 (20.7) | 2,218 (22.0) | 1,337 (22.1) | 223 (21.3) | 111 (21.5) | 19,886 (23.7) |
| 3, poorly differentiated | 7,990 (13.4) | 1,537 (23.4) | 1,415 (14.1) | 960 (15.8) | 155 (14.8) | 72 (14.0) | 12,129 (14.5) |
| 4, undifferentiated; anaplastic | 2,482 (4.2) | 645 (9.8) | 387 (3.9) | 300 (4.9) | 56 (5.3) | 14 (2.7) | 3,884 (4.6) |
| Unknown | 11,630 (19.5) | 1,434 (21.9) | 1,866 (18.5) | 1,060 (17.5) | 147 (14.0) | 112 (21.7) | 16,249 (19.4) |
| Year of diagnosis | |||||||
| 2006 | 5,199 (8.7) | 438 (6.7) | 672 (6.7) | 435 (7.2) | 72 (6.9) | 28 (5.4) | 6,844 (8.2) |
| 2007 | 5,421 (9.1) | 520 (7.9) | 729 (7.3) | 478 (7.9) | 91 (8.7) | 44 (8.5) | 7,283 (8.7) |
| 2008 | 5,612 (9.4) | 563 (8.6) | 799 (7.9) | 535 (8.8) | 90 (8.6) | 41 (7.9) | 7,640 (9.1) |
| 2009 | 5,850 (9.8) | 622 (9.5) | 898 (8.9) | 562 (9.3) | 78 (7.4) | 49 (9.5) | 8,059 (9.6) |
| 2010 | 6,110 (10.3) | 640 (9.7) | 929 (9.3) | 612 (10.1) | 83 (7.9) | 56 (10.9) | 8,430 (10.1) |
| 2011 | 5,890 (9.9) | 660 (10.0) | 1,042 (10.4) | 603 (9.9) | 101 (9.6) | 60 (11.6) | 8,356 (9.9) |
| 2012 | 6,265 (10.5) | 752 (11.5) | 1,201 (11.9) | 670 (11.1) | 112 (10.7) | 50 (9.7) | 9,050 (10.8) |
| 2013 | 6,209 (10.4) | 761 (11.6) | 1,161 (11.5) | 699 (11.5) | 157 (15.0) | 70 (13.6) | 9,057 (10.8) |
| 2014 | 6,547 (11.0) | 765 (11.7) | 1,272 (12.6) | 717 (11.8) | 127 (12.1) | 56 (10.9) | 9,484 (11.3) |
| 2015 | 6,523 (10.9) | 843 (12.8) | 1,362 (13.5) | 753 (12.4) | 137 (13.1) | 62 (12.0) | 9,680 (11.5) |
Data are n (%).
NCCN, National Comprehensive Cancer Network;SES, socioeconomic status.
Table 2.
Demographic and Cancer Characteristics of Patients With Endometrial Carcinoma With Adherent Treatment Between 2006 and 2015 in the SEER (Surveillance, Epidemiology, and End Results) Database (n=49,885)
| Characteristic | Adherent Treatment (n =49,885) |
|---|---|
|
| |
| Race-ethnicity | |
| White | 35,971 (72.1) |
| Black | 3,749 (7.5) |
| Latina | 5,483 (10.9) |
| Asian | 3,775 (7.6) |
| Native Hawaiian or Pacific Islander | 635 (1.3) |
| American Indian or Alaska Native | 272 (0.6) |
| Neighborhood SES | |
| Highest | 12,258 (24.6) |
| Higher-middle | 11,410 (22.9) |
| Middle | 10,167 (20.4) |
| Lower-middle | 9,017 (18.0) |
| Lowest | 7,033 (14.1) |
| Age at diagnosis (y) | |
| Younger than 54 | 9,776 (19.6) |
| 54–61 | 14,745 (29.6) |
| 62–68 | 12,347 (24.7) |
| 69 or older | 13,017 (26.1) |
| Stage at diagnosis | |
| I | 36,429 (73.0) |
| II | 3,825 (7.7) |
| III | 8,003 (16.0) |
| IV | 1,628 (3.3) |
| Histology | |
| Endometroid carcinomas | 38,552 (77.3) |
| Other carcinomas | 11,333 (22.7) |
| Grade | |
| 1, well differentiated | 15,372 (30.8) |
| 2, moderately differentiated | 14,169 (28.4) |
| 3, poorly differentiated | 8,669 (17.4) |
| 4, undifferentiated; anaplastic | 2,554 (5.1) |
| Unknown | 9,121 (18.3) |
| Year of diagnosis | |
| 2006 | 4,087 (8.2) |
| 2007 | 4,485 (8.9) |
| 2008 | 4,670 (9.4) |
| 2009 | 4,935 (9.9) |
| 2010 | 5,072 (10.2) |
| 2011 | 4,970 (10.0) |
| 2012 | 5,207 (10.4) |
| 2013 | 5,374 (10.8) |
| 2014 | 5,507 (11.0) |
| 2015 | 5,578 (11.2) |
Data are n (%).
SES, socioeconomic status.
Table 3 shows the breakdown of treatment received by stage at diagnosis. Bivariate analyses show a statistically significant association between treatment type and stage at diagnosis (P<.001). Treatment adherence was highest within stage III and stage II cancers. Overall, those with stage I disease had a greater proportion of having a total hysterectomy and bilateral salpingo-oophorectomy. Surgical staging was greatest among patients with stage III and stage II disease. Radiation was higher among those within stage II and stage III disease, and chemotherapy was highest among those with stage III and stage IV disease.
Table 3.
Treatment Received by Stage at Diagnosis Between 2006 and 2015 in the Surveillance, Epidemiology, and End Results (SEER) Database (N = 83,883)
| Treatment | Stage I (n=64,014) | Stage II (n=5,045) | Stage III (n = 10,269) | Stage IV (n=4,555) | Total Sample (N=83,883) | p * |
|---|---|---|---|---|---|---|
|
| ||||||
| Surgically staged | <.001 | |||||
| Yes | 37,895 (59.2) | 3,693 (73.2) | 8,537 (83.1) | 1,939 (42.6) | 52,064 (62.1) | |
| No | 26,119 (40.8) | 1,352 (26.8) | 1,732 (16.9) | 2,616 (57.4) | 31,819 (37.9) | |
| TH-BSO | <.001 | |||||
| Yes | 54,871 (85.7) | 4,054 (80.4) | 8,132 (79.2) | 2,349 (51.6) | 69,406 (82.7) | |
| No | 9,143 (14.3) | 991 (19.6) | 2,137 (20.8) | 2,206 (48.4) | 14,477 (17.3) | |
| Radiation | <.001 | |||||
| Yes | 11,437 (17.9) | 3,086 (61.2) | 5,191 (50.5) | 1,056 (23.2) | 20,770 (24.8) | |
| No | 52,577 (82.1) | 1,959 (38.8) | 5,078 (49.5) | 3,499 (76.8) | 63,113 (75.2) | |
| Chemotherapy | <.001 | |||||
| Yes | 3,538 (5.5) | 861 (17.1) | 6,732 (65.6) | 3,112 (68.3) | 14,243 (17.0) | |
| No | 60,476 (94.5) | 4,184 (82.9) | 3,537 (34.4) | 1,443 (31.7) | 69,640 (83.0) | |
| Adherent treatment | <.001 | |||||
| Yes | 36,429 (56.9) | 3,825 (75.8) | 8,003 (77.9) | 1,628 (35.7) | 49,885 (59.5) | |
| No | 27,585 (43.1) | 1,220 (24.2) | 2,266 (22.1) | 2,927 (64.3) | 33,998 (40.5) | |
Data are n (%) unless otherwise specified.
TH-BSO, total hysterectomy and bilateral salpingo-oophorectomy.
Chi-square test for association between stage at diagnosis and treatment, all P values are two-sided.
Within the study population, there was a higher proportion of Black, Latina, and American Indian or Alaska Native women represented within the lower neighborhood socioeconomic status groups. Additionally, Native Hawaiian and Pacific Islander, American Indian and Alaska Native, Latina, and Asian women had a lower age at diagnosis, with a higher proportion representing those younger than 54 years old. Stage I disease (76.3%) and endometroid carcinomas (75%) represented the majority of patients in the sample. However, Black women represented a higher proportion of later stage at diagnosis (15.5% stage III and 9.7% stage IV) and more aggressive grade of disease (23.4% poorly differentiated and 9.8% undifferentiated or anaplastic) and histologic subtypes (39.5% other carcinomas) relative to the total sample.
Bivariate analysis using a χ2 test revealed a statistically significant association between race–ethnicity and adherence to National Comprehensive Cancer Network treatment guidelines (P<.001). Univariate logistic regression results of the association between race–ethnicity and adherence to National Comprehensive Cancer Network treatment guidelines show that Black (odds ratio [OR] 0.88, P<.001, 95% CI 0.83–0.92), Latina (OR 0.79, P<.001, 95% CI 0.75–0.82), and American Indian or Alaska Native (OR 0.73, P<.001, 95% CI 0.62–0.87) women have a statistically significant lower probability of receiving adherent treatment when compared with White women. On the other hand, Asian (OR 1.08, P=.003, 95% CI 1.03–1.15) women have a statistically significant higher probability of receiving adherent treatment when compared with White women. Within neighborhood socioeconomic status, we see a gradient in adherence with the probability of receiving adherent treatment decreasing as neighborhood socioeconomic status decreases relative to women in the highest neighborhood socioeconomic status group. Post hoc power analysis determined that this study was not adequately powered to detect significant racial–ethnic differences in treatment for Asian, Native Hawaiian or Pacific Islander, and American Indian or Alaska Native women relative to White women when conducting two-tailed analyses at α=0.05 and 80% power indicating the importance of future studies that include larger sample sizes of these groups. This study was adequately powered at α=0.05 and 80% power to detect differences in all neighborhood socioeconomic status groups.
The multivariate logistic regression models (Table 4) indicate that the association between race–ethnicity and adherence to National Comprehensive Cancer Network treatment guidelines kept the same pattern when controlling for demographic and clinical covariates. When compared with White women, Black (OR 0.88, P<.001, 95% CI 0.84–0.94), Latina (OR 0.92, P<.001, 95% CI 0.88–0.96), and American Indian and Alaska Native (OR 0.82, P=.034, 95% CI 0.69–0.98) women had statistically significantly lower odds of receiving adherent treatment, after controlling for neighborhood socioeconomic status, age at diagnosis, stage at diagnosis, histology, grade of disease, and year of diagnosis. Asian (OR 1.14, P<.001, 95% CI 1.08–1.21) women and Native Hawaiian and Pacific Islander (OR 1.19, P=.012, 95% CI 0.69–0.98) women continued to have statistically significantly higher odds of receiving adherent treatment when compared with White women after all covariates were included. We continue to see the gradient pattern for neighborhood socioeconomic status after controlling for race–ethnicity and all covariates relative to women in the highest neighborhood socioeconomic status group: the high–middle neighborhood socioeconomic status group (OR 0.89, P<.001, 95% CI 0.86–0.93), the middle neighborhood socioeconomic status group (OR 0.84, P<.001, 95% CI 0.80–0.88), the low–middle neighborhood socioeconomic status group OR 0.80, P<.001, 95% CI 0.78–0.86), and the lowest neighborhood socioeconomic status group (OR 0.73, P<.001, 95% CI 0.69–0.77).
Table 4.
Univariate and Multivariate Logistic Regression of Predictors of Adherence to National Comprehensive Cancer Network Treatment Guidelines Among Patients With Endometrial Carcinoma Between 2006 and 2015 in the SEER (Surveillance, Epidemiology, and End Results) Database (N=83,883)
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|
| |||
| Race-ethnicity | |||
| White | Referent | Referent | |
| Black | 0.88 (0.83–0.92)* | 0.89 (0.84–0.94)* | |
| Latina | 0.79 (0.75–0.82)* | 0.92 (0.88–0.96)* | |
| Asian | 1.08 (1.03–1.15)† | 1.14 (1.08–1.21)* | |
| Native Hawaiian or Pacific Islander | 1.01 (0.89–1.15) | 1.19 (1.04–1.35)† | |
| American Indian or Alaska Native | 0.73 (0.62–0.87)* | 0.82 (0.69–0.98)† | |
| Neighborhood SES | |||
| Highest | Referent | Referent | |
| High-middle | 0.89 (0.85–0.93)* | 0.89 (0.86–0.93)* | |
| Middle | 0.83 (0.80–0.87)* | 0.84 (0.80–0.88)* | |
| Low-middle | 0.80 (0.77–0.83)* | 0.82 (0.78–0.86)* | |
| Lowest | 0.73 (0.70–0.76)* | 0.73 (0.69–0.77)* | |
| Age at diagnosis (y) | |||
| Younger than 54 | Referent | ||
| 54–61 | 1.37 (1.32–1.43)* | ||
| 62–68 | 1.54 (1.47–1.61)* | ||
| 69 or older | 1.33 (1.27–1.38)* | ||
| Stage at diagnosis | |||
| I | Referent | ||
| II | 2.06 (1.93–2.21)* | ||
| III | 2.14 (2.04–2.26)* | ||
| IV | 0.31 (0.29–0.33)* | ||
| Histology | |||
| Endometroid carcinomas | Referent | ||
| Other carcinomas | 0.56 (0.54–0.58)* | ||
| Grade | |||
| 1, well differentiated | Referent | ||
| 2, moderately differentiated | 2.58 (2.48–2.68)* | ||
| 3, poorly differentiated | 3.45 (3.28–3.64)* | ||
| 4, undifferentiated; anaplastic | 3.25 (3.00–3.53)* | ||
| Unknown | 1.48 (1.42–1.54)* | ||
| Year of diagnosis | 0.99 (0.98–0.99)* | ||
SES, socioeconomic status.
Model 1 includes only race and ethnicity, model 2 includes only neighborhood SES, and model 3 includes race and ethnicity, neighborhood SES, and all demographic and clinical covariates (age at diagnosis, stage at diagnosis, histology, grade, and year of diagnosis).
P<.001.
P< .05.
DISCUSSION
Our findings suggest racial–ethnic and neighborhood socioeconomic disparities in the receipt of adherent care, which aligns with prior studies on endometrial cancer and other cancer sites. There are limited studies that have examined adherence to National Comprehensive Cancer Network guidelines for endometrial cancer and even fewer that have looked at racial–ethnic and neighborhood socioeconomic disparities in treatment adherence.21–23 Because adherence to National Comprehensive Cancer Network guidelines is associated with improved survival for many cancer sites, including uterine cancer, it is important to explore disparities and deviations in these guidelines among various racial–ethnic groups and neighborhood socioeconomic status groups to ensure that all groups are receiving equitable treatment.
There are three key findings from our study. First, Black, Latina, and American Indian and Alaska Native women had the lowest percentages of treatment that was adherent to National Comprehensive Cancer Network guidelines compared with White women. Similarly, Kaspers et al21 found that percentages of adherent treatment to National Comprehensive Cancer Network guidelines for endometrial cancer were lowest among Black and Latina women. Additional research that has looked at treatment patterns in endometrial cancer, aside from those that are National Comprehensive Cancer Network guideline concordant, have found that Black and Latina women are less likely to receive definitive surgical treatment and lymph node sampling when compared with White women.33 Other studies have found that Black, Latina, and American Indian and Alaska Native women with endometrial cancer were less likely to have minimally invasive hysterectomies and were more likely to receive open surgery when compared with White women.34,35 This is important because minimally invasive surgeries have been deemed an appropriate quality measure for the primary treatment of endometrial cancer.35 Additional research on treatment patterns found that American Indian and Alaska Native women with endometrial cancer had appropriate lymph node assessments, indicating at least partial adherence to National Comprehensive Cancer Network guidelines.36 Further research is needed to examine treatment adherence with larger sample sizes of American Indian and Alaska Native women.
Second, we found that Asian and Native Hawaiian or Pacific Islander women had significantly higher odds of receiving treatment that was adherent to National Comprehensive Cancer Network guidelines, when compared with White women. Kaspers et al21 also found higher percentages of adherent treatment to National Comprehensive Cancer Network guidelines for Asian and Pacific Islander women, which are aggregated together, compared with White women. Our findings are aligned with this study, which together contributes to small but growing literature on racial–ethnic disparities in endometrial cancer, including an examination of patterns for Asian and Pacific Islander women.37 Further studies should be done involving Asian women and women who are Native Hawaiian or Pacific Islander to explore social and clinical factors that contribute to higher adherence. More importantly, these studies should disaggregate Asian women and women who are Native Hawaiian or Pacific Islander to examine whether patterns differ among these groups. Additionally, larger sample sizes of these populations are needed.
Although our findings are aligned with some of the past research on racial–ethnic disparities in treatment adherence for endometrial cancer, not all studies have observed such disparities. For instance, one study conducted by using the National Cancer Database did not observe any racial–ethnic disparities in its analysis of treatment adherence among nonendometrioid endometrial cancers.23 This may indicate that racial–ethnic disparities might differ based on histologic subtypes of endometrial cancer. Another study conducted by using the Women’s Health Initiative that explored National Comprehensive Cancer Network treatment adherence among endometrial cancer did not note any racial–ethnic disparities.22 However, the sample used for this study was predominately White, so it is possible that there was not a large enough sample size of racial–ethnic minority groups to allow for such analyses on racial–ethnic disparities. More research on racial–ethnic minority groups and standard treatment guidelines for endometrial cancer are warranted to further our understanding of these racial–ethnic disparities.
Third, we found that there is a neighborhood socioeconomic status gradient in treatment adherence, such that women in the lower neighborhood socioeconomic status groups have lower odds of receiving adherent treatment relative to women in the highest neighborhood socioeconomic status group. Our study is novel in that it examines neighborhood socioeconomic disparities in the understudied context of treatment adherence for endometrial cancer. However, there are prior studies that have looked at disparities in National Comprehensive Cancer Network treatment adherence among other gynecologic cancer sites. For instance, one study that looked at adherence to National Comprehensive Cancer Network guidelines for cervical cancer found that individuals with lower socioeconomic status were more likely to receive nonadherent treatment.14 Similarly, another study that looked at adherence to treatment guidelines for ovarian cancer found that socioeconomic status is a risk factor for receiving nonstandard care, even after adjustment for other factors such as medical comorbidities.13 Additionally, a research review on disparities within gynecologic cancer have shown that women within lower socioeconomic status groups are less likely to receive standard treatment therapies.38
The results presented here indicate that historically marginalized and socially disadvantaged groups receive substandard care when compared with higher status groups (ie, White women and those of higher neighborhood socioeconomic status). This disparity in receipt of National Comprehensive Cancer Network adherent care may be aligned with limited health care resources and unequal access to factors associated with quality of care. For instance, studies on gynecologic cancer have found that health care accessibility measured through insurance type may affect care, because patients with Medicaid insurance have lower adherence to treatment guidelines compared with those with Medicare or private insurance.38,39 Additionally, prior studies on ovarian cancer have indicated that residential location and geographic proximity to specialized facilities influence the likelihood of receiving adherent treatment.40–42 Although not explicitly examined in the literature described above, residential location may reflect race-based residential segregation and its effect on access to high quality medical care for low-income and racial–ethnic minority communities.43 Future research should explicitly explore race-based segregation and its association with adherent treatment for cancer. Additionally, other structural factors, such as experiences of discrimination or biases within the health care system and with health care professionals, should be explored because they may be related to deviance from standard treatment regimens for some groups.44,45 Future research should explore what factors may mitigate or exacerbate these disparities, such as insurance type, geographical location, access and barriers to treatment, physician specialty, physician bias, hospital volume, and hospital type (eg, academic vs community).
This study has several strengths that should be noted. First, the SEER registry involves a large sample size and allows for inclusion of multiple years in the study period in which treatment guidelines did not substantially change during the study period. Because this data set is a population-based data set with inclusion of multiple racial–ethnic categories, our findings are generalizable to the U.S. population. Second, the registry allowed for inclusion of a diverse population of women from several racial–ethnic groups and socioeconomic backgrounds. The inclusion of multiple racial–ethnic groups is especially important because much of the research on inequities on endometrial cancer to date focuses on White women compared with Black women.14,21,46,47 Our study examines not only disparities between Black and White women, but also includes a focus on patterning for Latina women, Asian women, women who are Native Hawaiian or Pacific Islander, and American Indian and Alaska Native women within the sample, which provides us a more nuanced understanding of racial–ethnic patterning of adherent treatment for endometrial cancer. Third, our study disaggregated Asian women and women who are Native Hawaiian or Pacific Islander to view each of these groups separately instead of together as they are many times in literature.48,49 Hence, our study provides greater insight into racial–ethnic disparities in endometrial cancer treatment, because we have included a range of racial–ethnic groups into our analysis that are reflective of the racial–ethnic diversity in the U.S. and works toward the disaggregation of racial–ethnic groups.
Despite these strengths, this study does have limitations that should be acknowledged. First, the retrospective population-based cohort study design is subject to potential selection and reporting bias owing to the nature of the methodology. In addition, this observational study has the possibility for unmeasured confounding. For instance, the SEER registry does not have granular information related to access to care and hospital or physician characteristics (ie, insurance coverage and type, geographic location, facility type, and physician specialty). Prior research has indicated that measures associated with access to and hospital or physician characteristics affect treatment adherence and outcomes for various cancer sites.21–23 Additionally, the reasons for nonadherence are not available in this data set. However, prior research that has explored reasons for nonadherent treatment in ovarian cancer among all racial–ethnic groups has indicated that the most common reasons are comorbidities, disease progression, or consideration of individual cancer characteristic risks.11,18,19,50 Future research should qualitatively explore reasons for nonadherent treatment within endometrial cancer and other cancer sites among various racial–ethnic groups and socioeconomic status groups, with special attention to low-income women of color. Further, the SEER database does not include any indicators for medical comorbidities. The available literature has pointed to comorbidities such as obesity and diabetes, which has high prevalence in Black and Latina communities, influencing available treatment modalities.14,19,26,50 Future research should include medical comorbidities as a covariate to account for any deviations in the patient’s ability to receive guideline-adherent care. This may point to whether medical comorbidities should be included as considerations in National Comprehensive Cancer Network guidelines to safeguard deviations in the standard therapies that are established and not inversely benefit some groups that may have more comorbidities, which may vary systematically by race–ethnicity. Of equal importance, SEER treatment data are limited to the first course of treatment and do not report hormonal therapy or specific dose of radiation or chemotherapy. This additional treatment information and inclusion of adjuvant therapies would be helpful to examine National Comprehensive Cancer Network treatment adherence for both first course and adjuvant treatment. Additionally, because race–ethnicity of the patient was registry reported, there may be some misclassification that is not accurate or aligned with the patient’s self-reported racial–ethnic identity. Lastly, we did not have access to individual levels of socioeconomic status, so we used neighborhood socioeconomic status as a proxy for community-level socioeconomic status, which may not account fully for the role of individual level socioeconomic status on these associations.
Standard treatment therapies should not differ based on one’s race–ethnicity or socioeconomic status. Our study found that even after accounting for demographic and clinical characteristics, racial–ethnic and neighborhood socioeconomic disparities are apparent in receipt of National Comprehensive Cancer Network guideline-adherent treatment for women who are Black, Latina, American Indian or Alaska Native, and are of low socioeconomic status. Interventions are needed to ensure that equitable cancer treatment practices are available for all individuals regardless of their racial–ethnic or socioeconomic backgrounds.
Supplementary Material
Acknowledgments
Victoria E. Rodriguez was supported in part by a grant from the National Cancer Institute R25CA11238308 Cancer Epidemiology Education in Special Populations, City University of New York, School of Medicine, Amr Soliman P.I. Additional support for this project was provided by the Faculty Mentor Program Fellowship from the University of California, Irvine Graduate Division.
The authors thank Dr. Annie Ro for all of her guidance and comments on earlier versions of this work.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
PEER REVIEW HISTORY
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C327.
REFERENCES
- 1.Faubion SS, MacLaughlin KL, Long ME, Pruthi S, Casey PM. Surveillance and care of the gynecologic cancer survivor. J Womens Health 2015;24:899–906. doi: 10.1089/jwh.2014.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskovic M, Lichtensztajn DY, Nguyen T, Karam A, English DP. Racial disparities in outcomes for high-grade uterine cancer: a California Cancer Registry study. Cancer Med 2018;7:4485–95. doi: 10.1002/cam4.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birmann BM, Barnard ME, Bertrand KA, Bao Y, Crous-Bou M, Wolpin BM, et al. Nurses’ health study contributions on the epidemiology of less common cancers: endometrial, ovarian, pancreatic, and hematologic. Am J Public Health 2016;106:1608–15. doi: 10.2105/AJPH.2016.303337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook LS, Nelson HE, Cockburn M, Olson SH, Muller CY, Wiggins CL. Comorbidities and endometrial cancer survival in Hispanics and non-Hispanic Whites. Cancer Causes Control 2013;24:61–9. doi: 10.1007/s10552-012-0090-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez AM, Schmeler KM, Kuo YF. Disparities in endometrial cancer outcomes between non-Hispanic White and Hispanic women. Gynecol Oncol 2014;135:525–33. doi: 10.1016/j.ygyno.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 6.Henley SJ, Miller JW, Dowling NF, Benard VB, Richardson LC. Uterine cancer incidence and mortality - United States, 1999–2016. Morb Mortal Wkly Rep 2018;67:1333–8. doi: 10.15585/mmwr.mm6748a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363–85. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER*Explorer. Accessed September 15, 2020. https://seer.cancer.gov/explorer/application.php
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Accessed September 15, 2020. https://seer.cancer.gov/statfacts/html/corp.html
- 11.Lee JY, Kim TH, Suh DH, Kim JW, Kim HS, Chung HH, et al. Impact of guideline adherence on patient outcomes in early-stage epithelial ovarian cancer. Eur J Surg Oncol 2015;41:585–91. doi: 10.1016/j.ejso.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 12.Levinson KL, Riedel DJ, Ojalvo LS, Chan W, Angarita AM, Fader AN, et al. Gynecologic cancer in HIV-infected women: treatment and outcomes in a multi-institutional cohort. AIDS 2018;32:171–7. doi: 10.1097/QAD.0000000000001664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol 2015;125:833–42. doi: 10.1097/AOG.0000000000000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaendler KS, Chang J, Ziogas A, Bristow RE, Penner KR. Disparities in adherence to National Comprehensive Cancer Network treatment guidelines and survival for stage IB–IIA cervical cancer in California. Obstet Gynecol 2018;131:899–908. doi: 10.1097/AOG.0000000000002591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hines RB, Barrett A, Twumasi-Ankrah P, Broccoli D, Engel-man KK, Baranda J, et al. Predictors of guideline treatment nonadherence and the impact on survival in patients with colorectal cancer. J Natl Compr Canc Netw 2015;13:51–60. doi: 10.6004/jnccn.2015.0008 [DOI] [PubMed] [Google Scholar]
- 16.Erickson Foster J, Velasco JM, Hieken TJ. Adverse outcomes associated with noncompliance with melanoma treatment guidelines. Ann Surg Oncol 2008;15:2395–402. doi: 10.1245/s10434-008-0021-0 [DOI] [PubMed] [Google Scholar]
- 17.Worhunsky DJ, Ma Y, Zak Y, Poultsides GA, Norton JA, Rhoads KF, et al. Compliance with gastric cancer guidelines is associated with improved outcomes. J Natl Compr Canc Netw 2015;13:319–25. doi: 10.6004/jnccn.2015.0044 [DOI] [PubMed] [Google Scholar]
- 18.Jaap K, Fluck M, Hunsinger M, Wild J, Arora T, Shabahang M, et al. Analyzing the impact of compliance with national guidelines for pancreatic cancer care using the National Cancer Database. J Gastrointest Surg 2018;22:1358–64. doi: 10.1007/s11605-018-3742-9 [DOI] [PubMed] [Google Scholar]
- 19.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Impact of National Cancer Institute comprehensive cancer centers on ovarian cancer treatment and survival. J Am Coll Surg 2015;220:940–50. doi: 10.1016/j.jamcollsurg.2015.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis CM, Hessel AC, Roberts DB, Guo YZ, Holsinger FC, Ginsberg LE, et al. Prereferral head and neck cancer treatment: compliance with National Comprehensive Cancer Network treatment guidelines. Arch Otolaryngol Head Neck Surg 2010;136:1205–11. doi: 10.1001/archoto.2010.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaspers M, Llamocca E, Quick A, Dholakia J, Salani R, Felix AS. Black and Hispanic women are less likely than White women to receive guideline-concordant endometrial cancer treatment. Am J Obstet Gynecol 2020;223:398.e1–18. doi: 10.1016/j.ajog.2020.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felix AS, McLaughlin EM, Caan BJ, Cohn DE, Anderson GL, Paskett ED. Guideline-concordant endometrial cancer treatment and survival in the Women’s Health Initiative Life and Longevity after Cancer study. Int J Cancer 2020;147:404–12. doi: 10.1002/ijc.32740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dholakia J, Llamocca E, Quick A, Salani R, Felix AS. Guideline-concordant treatment is associated with improved survival among women with non-endometrioid endometrial cancer. Gynecol Oncol 2020;157:716–22. doi: 10.1016/j.ygyno.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doll KM. Investigating Black-White disparities in gynecologic oncology: theories, conceptual models, and applications. Gynecol Oncol 2018;149:78–83. doi: 10.1016/j.ygyno.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 25.Fiscella K, Sanders MR. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health 2016;37:375–94. doi: 10.1146/annurev-publhealth-032315-021439 [DOI] [PubMed] [Google Scholar]
- 26.Temkin SM, Rimel BJ, Bruegl AS, Gunderson CC, Beavis AL, Doll KM. A contemporary framework of health equity applied to gynecologic cancer care: a Society of Gynecologic Oncology evidenced-based review. Gynecol Oncol 2018;149:70–7. doi: 10.1016/j.ygyno.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER. Accessed September 9, 2020. https://seer.cancer.gov/index.html
- 28.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER ICD-O-3 coding materials. Accessed March 11, 2020. https://seer.cancer.gov/icd-o-3/index.html
- 29.Greer BE, Koh W-J, Abu-Rustum N, Bookman MA, Bristow RE, Campos SM, et al. Uterine cancers: clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006;4:438–62. doi: 10.6004/jnccn.2006.0037 [DOI] [PubMed] [Google Scholar]
- 30.Greer BE, Koh W-J, Abu-Rustum N, Bookman MA, Bristow RE, Campos SM, et al. Uterine neoplasms: clinical practice guidelines in oncology. J Natl Compr Canc Netw 2009;7:498–531. doi: 10.6004/jnccn.2009.0035 [DOI] [PubMed] [Google Scholar]
- 31.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw 2014;12:248–80. doi: 10.6004/jnccn.2014.0025 [DOI] [PubMed] [Google Scholar]
- 32.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12:703–11. doi: 10.1023/A:1011240019516 [DOI] [PubMed] [Google Scholar]
- 33.Bregar AJ, Alejandro Rauh-Hain J, Spencer R, Clemmer JT, Schorge JO, Rice LW, et al. Disparities in receipt of care for high-grade endometrial cancer: a National Cancer Data Base analysis. Gynecol Oncol 2017;145:114–21. doi: 10.1016/j.ygyno.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 34.Esselen KM, Vitonis A, Einarsson J, Muto MG, Cohen S. Health care disparities in hysterectomy for gynecologic cancers: data from the 2012 National Inpatient Sample. Obstet Gynecol 2015;126:1029–39. doi: 10.1097/AOG.0000000000001088 [DOI] [PubMed] [Google Scholar]
- 35.Mannschreck D, Matsuno RK, Moriarty JP, Borah BJ, Dowdy SC, Tanner EJ, et al. Disparities in surgical care among women with endometrial cancer: Obstet Gynecol 2016;128:526–34. doi: 10.1097/AOG.0000000000001567 [DOI] [PubMed] [Google Scholar]
- 36.Bruegl AS, Joshi S, Batman S, Weisenberger M, Munro E, Becker T. Gynecologic cancer incidence and mortality among American Indian/Alaska Native women in the Pacific Northwest, 1996–2016. Gynecol Oncol 2020;157:686–92. doi: 10.1016/j.ygyno.2020.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Von Behren J, Abrahão R, Goldberg D, Gomez SL, Setiawan VW, Cheng I. The influence of neighborhood socioeconomic status and ethnic enclave on endometrial cancer mortality among Hispanics and Asian Americans/Pacific Islanders in California. Cancer Causes Control 2018;29:875–81. doi: 10.1007/s10552-018-1063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee S, Gupta D, Caputo TA, Holcomb K. Disparities in gynecological malignancies. Front Oncol 2016;6:36. doi: 10.3389/fonc.2016.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temkin SM, Fleming SA, Amrane S, Schluterman N, Terplan M. Geographic disparities amongst patients with gynecologic malignancies at an urban NCI-designated cancer center. Gynecol Oncol 2015;137:497–502. doi: 10.1016/j.ygyno.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 40.Bristow RE, Chang J, Ziogas A, Anton-Culver H, Vieira VM. Spatial analysis of adherence to treatment guidelines for advanced-stage ovarian cancer and the impact of race and socioeconomic status. Gynecol Oncol 2014;134:60–7. doi: 10.1016/j.ygyno.2014.03.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dottino JA, Cliby WA, Myers ER, Bristow RE, Havrilesky LJ. Improving NCCN guideline-adherent care for ovarian cancer: value of an intervention. Gynecol Oncol 2015;138:694–9. doi: 10.1016/j.ygyno.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 42.Villanueva C, Chang J, Bartell SM, Ziogas A, Bristow R, Vieira VM. Contribution of geographic location to disparities in ovarian cancer treatment. J Natl Compr Canc Netw 2019;17:1318–29. doi: 10.6004/jnccn.2019.7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 2001;116:404–16. doi: 10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casagrande SS, Gary TL, LaVeist TA, Gaskin DJ, Cooper LA. Perceived discrimination and adherence to medical care in a racially integrated community. J Gen Intern Med 2007;22:389–95. doi: 10.1007/s11606-006-0057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penner LA, Dovidio JF, Gonzalez R, Albrecht TL, Chapman R, Foster T, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol 2016;34:2874–80. doi: 10.1200/JCO.2015.66.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC. Disparities in treatment and survival for women with endometrial cancer: a contemporary National Cancer Database registry analysis. Gynecol Oncol 2016;143:98–104. doi: 10.1016/j.ygyno.2016.07.107 [DOI] [PubMed] [Google Scholar]
- 47.Rauh-Hain JA, Melamed A, Schaps D, Bregar AJ, Spencer R, Schorge JO, et al. Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies. Gynecol Oncol 2018;149:4–11. doi: 10.1016/j.ygyno.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 48.Chen MS, Hawks BL. A debunking of the myth of healthy Asian Americans and Pacific Islanders. Am J Health Promot 1995;9:261–8. doi: 10.4278/0890-1171-9.4.261 [DOI] [PubMed] [Google Scholar]
- 49.Srinivasan S, Guillermo T. Toward improved health: disaggregating Asian American and Native Hawaiian/Pacific Islander data. Am J Public Health 2000;90:1731–4. doi: 10.2105/AJPH.90.11.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erickson BK, Martin JY, Shah MM, Straughn JM, Leath CA. Reasons for failure to deliver National Comprehensive Cancer Network (NCCN)-adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol 2014;133:142–6. doi: 10.1016/j.ygyno.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


