Abstract
Chronic pain is common among people with human immunodeficiency virus (HIV) and detrimental to quality of life and overall health. It is often underdiagnosed, undertreated, and frankly dismissed in women with HIV, despite growing evidence that it is highly prevalent in this population. Thus, we conducted a systematic review and meta-analysis to estimate the global prevalence of chronic pain in women with HIV. The full protocol can be found on PROSPERO (identifier CRD42022301145). Of the 2984 references identified in our search, 36 were included in the systematic review and 35 in the meta-analysis. The prevalence of chronic pain was 31.2% (95% confidence interval [CI], 24.6%–38.7%; I2 = 98% [95% CI, 97%–99%]; P < .0001). In this global assessment, we found a high prevalence of chronic pain among women with HIV, underscoring the importance of understanding the etiology of chronic pain, identifying effective treatments, and conducting regular assessments in clinical practice.
Keywords: AIDS, chronic pain, HIV, neuropathy, women
This article adressess an existing research gap by investigating global prevalence of chronic pain in women with HIV. Better understanding of chronic pain in this population is critical in light of its interplay with HIV care, general well-being, and aging.
Chronic pain is a concerning comorbidity among people with human immunodeficiency virus (HIV) that can significantly affect medication adherence and retention in care [1], mobility [2], mental/emotional well-being [3], and quality of life [3]. According to the 2017 Infectious Diseases Society of America guidelines, the combination of moderate or higher pain intensity in the last week and bodily pain for >3 months indicates chronic pain syndrome based on the 2-item Brief Chronic Pain Questionnaire. These guidelines suggest that all people with HIV should receive standardized chronic pain screening [4]. Pain prevalence estimates differ depending on country and antiretroviral therapy (ART) era, ranging from 22% to 91% [1, 5–8]. Importantly, the Global Task Force for Chronic Pain in People With HIV has identified “understanding the prevalence of pain” as a research priority [9].
The etiology of pain in people with HIV is likely multifactorial, including biological factors related to HIV, medication side effects, and psychosocial conditions [10]. Prior to the advent of ART, pain among people with HIV was often associated with malignancy, opportunistic infections, or HIV-mediated neuronal damage [11]. Viral proteins can directly damage neurons, resulting in increased pain sensitivity. Immune activation from chronic inflammation further contributes to neuropathy [12]. Opportunistic infections, including tuberculosis (musculoskeletal damage) [13], Cryptococcus (meningitis) [14], and herpes zoster (postherpetic neuralgia) [15], can also lead to persistent pain. With the introduction of first-generation ART, particularly the “d-drugs” (eg, didanosine, stavudine, and zalcitabine), the majority of research and clinical attention was focused on neuropathic pain [12]. While neuropathic pain is much less common with modern ART, the risk is not entirely eliminated [16]. In addition, differences in drug metabolism and body weight may expose women to higher drug concentrations and thus more adverse drug reactions [17], although this has not been specifically linked to chronic pain. More recent studies have focused on nonneuropathic pain subtypes, including headache, myalgia/fibromyalgia, and arthritis. Indeed, recent data suggest that people with HIV frequently experience pain in several body sites [3]. In addition to HIV and ART, psychosocial and behavioral factors likely contribute, including depression [6], posttraumatic stress disorder [18], substance use [19], and HIV-related stigma [20]; these disproportionately impact women with HIV [21–24].
Several reviews have described the prevalence of and risk factors for pain in people with HIV [3, 10, 12, 19, 25], but none to our knowledge has specifically focused on women, even though data suggest that women in the general population experience a higher prevalence of chronic pain than men [26]. This paucity of research is unsurprising given the general lack of women-centered clinical HIV research or study of comorbid disease among women with HIV [27]; nevertheless, it is a stark omission in the literature. Importantly, chronic pain reduces retention in care and medication adherence [1], which are often lower among women with HIV compared to men with HIV [28]. This suggests that understanding and treating chronic pain will be critical to reaching the final 95 (virological suppression) in the Joint United Nations Programme on HIV/AIDS 95-95-95 targets [29]. Furthermore, women with HIV have identified chronic pain as a priority area of research [9], emphasizing the importance of this review for members of the HIV community. Therefore, we assessed the global prevalence of chronic pain among women with HIV and identified gaps in the literature.
METHODS
Search Strategy and Selection Criteria
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 checklist [30]. A research librarian with expertise in systematic reviews was consulted throughout the review process. The full study protocol can be accessed in the International Prospective Register of Systematic Reviews (PROSPERO) repository (ID: CRD42022301145).
Throughout this article, we use the term “women” to characterize persons who identify as women, including in studies that report pain prevalence by biological sex of “female” or gender identity of “woman.” We aimed to include both cisgender and transgender women in this analysis, as evidence suggests that cisgender and transgender women with HIV experience greater pain sensitivity compared to cisgender men [31].
Observational cross-sectional or cohort studies were eligible for inclusion as they are most appropriate for prevalence estimation. Case-control and randomized controlled trials were not included as these study designs intentionally select for participants with certain conditions and are thus not reflective of the general population of people with HIV [32]. Articles were included if they reported the prevalence of chronic pain in women with HIV ≥16 years old. We defined chronic pain as that lasting for at least 3 months in at least 1 anatomical site [4], or diagnosis of a chronic pain disorder (eg, arthritis, neuropathic pain, fibromyalgia, dyspareunia [pain with intercourse]). In our initial search, we selected articles published from the time that HIV was discovered (1983) to 19 January 2022. We then repeated the search on 21 November 2022 to capture newly published articles. Both inpatient and outpatient settings were considered, and data were included from high-, middle-, and low-income countries. In addition, studies that did not include sex/gender-disaggregated prevalence, only assessed pain that was directly attributable to an opportunistic infection (eg, cytomegalovirus meningitis), included <30 women/female participants, or reported on histopathology without subjective symptom experience were also excluded.
Data sources included Medline (Ovid), Embase (Ovid), Evidence-Based Medicine Reviews (Ovid), Cumulative Index to Nursing and Allied Health Literature (EBSCO), and Web of Science Core Collection (see Supplementary Material 1 for the Medline [Ovid] search strategy). We also searched “gray literature” (eg, conference proceedings, HIV/AIDS organization websites), as well as reference lists of relevant review articles. Articles of all languages were eligible for inclusion and translation was sought for those that were in not published in English or French.
The online software Covidence (v.2893) [33] was used for reference screening, full-text review, and data extraction. All abstracts and full texts were screened by 2 authors (T. P. and S. A. S. for English-language references and S. L. A. L. and H. C. F. C. for French-language references). Conflicts were resolved either by consensus or by a third author (A. R. C.).
Data Extraction, Study Quality, and Data Synthesis
Data were extracted in duplicate by T. P. and S. A. S. for English references and S. L. A. L. and H. C. F. C. for French references. Any inconsistencies were resolved as described above. For the primary outcome (pooled prevalence of chronic pain in women with HIV), we extracted data on absolute number of women with chronic pain and total number of women in the study. Data were also extracted for a priori secondary outcomes, including type of pain and country where the study was conducted. Risk of bias was assessed in duplicate using the 9-item Joanna Briggs Institute Prevalence Critical Appraisal Tool (Supplementary Material 2) [34]. We considered studies rated 8–9, 5–7, and ≤4 as high, moderate, and low quality, respectively. Of note, a study may indeed be high quality for its intended objective but rated as low quality based on the risk of bias assessment for systematic reviews. Results of the critical appraisal are represented using GraphPad software (version 9.4.0 for Windows, GraphPad, San Diego, California; www.graphpad.com). Other extracted data included study title and date, design, setting, and sample characteristics (eg, size, inclusion/exclusion criteria, demographic characteristics, HIV medical history). We also extracted data concerning possible confounders or mediators of chronic pain, such as smoking, substance use, diabetes, and exposure to neurotoxic ART or tuberculosis medications, all of which are known risk factors for peripheral neuropathy [35–38]. If 2 references were found to have duplicate data, we selected the one with the most complete and recent dataset.
Data Analysis
Our primary outcome of interest was the prevalence of chronic pain in women with HIV. Given the differences in study design and methodologies, we used a random-effects model. Analyses were performed in RStudio using the metafor package after logit transformation [39–41]. Heterogeneity between studies was calculated using the I2 statistic [42], classifying ≤25% as homogenous, 26%–50% as low heterogeneity, 51%–75% as moderate heterogeneity, and 76%–100% as high heterogeneity. We formally tested for outliers and influential studies using leave-one-out analyses, screening of studentized residuals, and diagnostic plots contained within the metafor package. For secondary outcomes, we assessed the pooled prevalence by pain subtype (peripheral neuropathy vs other pain type) and country of study (low-middle vs high-income countries, based on the World Bank classification) [43]. We used funnel plots to visually assess for publication bias and to examine whether sample size impacted the prevalence estimate. Egger regression test was used to assess funnel plot symmetry. All statistical analyses were completed in R version 4.2.1 software. Figures were made in GraphPad (version 9.4.0) and Servier Medical Art (https://smart.servier.com).
RESULTS
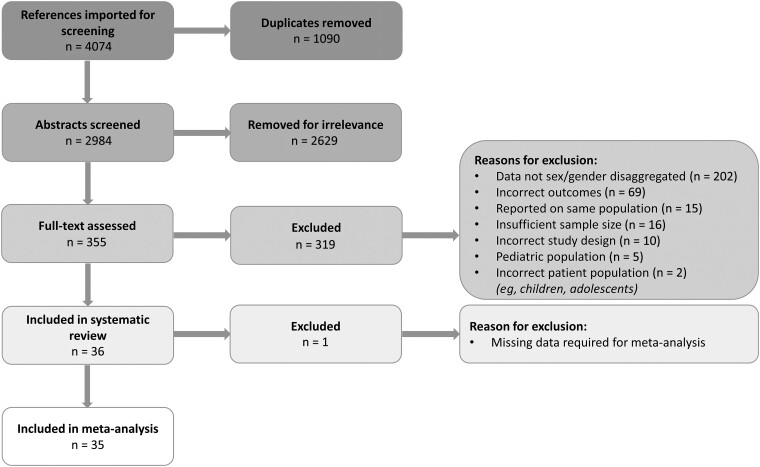
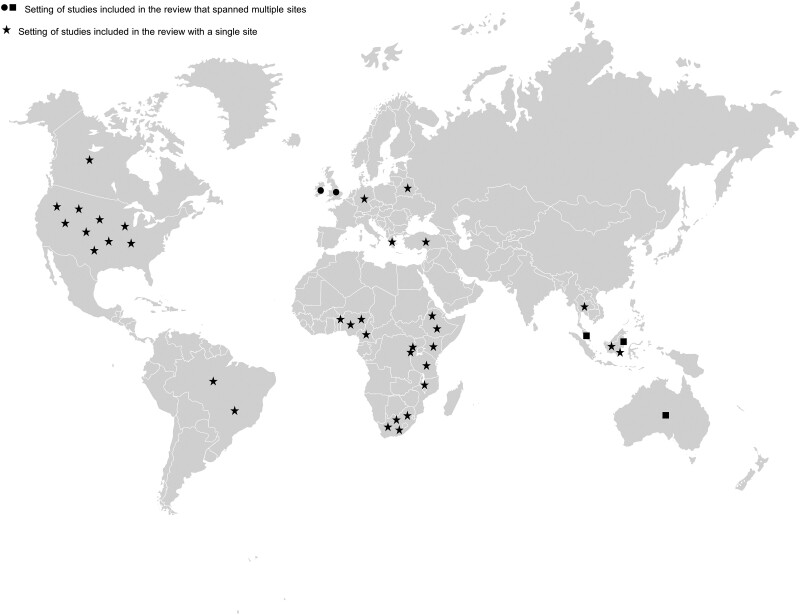
After removal of 1090 duplicates, our search yielded 2984 references, of which 36 were included in the systematic review (Table 1) and 35 in the meta-analysis (Figure 1). These studies provided data from 19 966 participants. One article was excluded from the meta-analysis as its chronic pain prevalence data was reported by CD4 count and ART subgroup [52], which we were unable to combine. The most common reason for article exclusion was lack of sex/gender-disaggregated data. The 36 studies included in the systematic review spanned 22 countries and 5 continents (Figure 2). Two references included data from multiple countries. Most articles (66.7%) were published in the last 10 years. The most commonly reported pain subtype was peripheral neuropathy (n = 22 articles), followed by widespread or other type of chronic pain (n = 6), fibromyalgia/myalgia (n = 3), headache (n = 2), combined chronic pain and peripheral neuropathy (n = 2), and dyspareunia (n = 1). More than half of the articles (n = 25) used validated tools to assess for chronic pain (Table 1). Eight references were rated as high quality, 25 as moderate quality, and 3 as low quality. Missing demographic data and a low number of women enrolled in a study were the most common reasons for lower quality (Supplementary Figure 1).
Table 1.
Characteristics of Selected Studies
| First Author (Year) | Study Type | Study Setting, Recruitment Dates | Inclusion/Exclusion Criteria | Sample Size (Females), No. | Definition of Chronic Pain | Pain Type | Pain Prevalence (%) |
|---|---|---|---|---|---|---|---|
| Evers (2000) [44] | Cross-sectional | Germany, dates NR | Inclusion: age ≥18 y Exclusion: prophylactic drugs for headaches, signs of central neurological manifestations of HIV infection |
131 (30 F) | Headache Classification Committee criteria (migraine headaches) | Other (headache) | 33 |
| Liebschutz (2000) [45] | Cross-sectional | US, Feb 1994–Apr 1996 | Inclusion: adult women seeking primary care for HIV for the first time; fluent in English, Spanish, or Haitian Creole Exclusion: prior medical care for HIV |
50 (50 F) | Abdominal pain, headaches, low back pain, musculoskeletal pain, arthritis, pelvic pain, peripheral neuropathy on review of medical records | Chronic pain | 62 |
| Schifitto (2005) [46] | Prospective cohort | US, dates NR | Inclusion: CD4 count <200 cells/μL with or without cognitive impairment, CD4 count 200–300 cells/μL and evidence of cognitive impairment or neuropsychological testing Exclusion: past or current infection or neoplastic CNS diseases, non-HIV-related neurodegenerative disorders |
376 (111 F) | Neurological examination created for the AIDS Clinical Trial Group, Neuropathic Pain Scale, Part III of the Unified Parkinson’s Disease Rating Scale | Peripheral neuropathy | 37 |
| Onwuegbuzie (2009) [47] | Cross-sectional | Nigeria, Jun–Dec 2004 | Inclusion: ART naive | 100 (58 F) | Standardized Clinical Screening Tool for Sensory Neuropathy | Peripheral neuropathy | 38 |
| Cherry (2009) [48] | Cross-sectional | Australia, Malaysia, Indonesia, 2006 | NR | 294 (39 F) | BPNS | Peripheral neuropathy | 18 |
| Richardson (2009) [49] | Prospective cohort | US, 1996–1998 | Inclusion: adult, CD4 count <200 cells/μL between first and seventh WIHS visit | 339 (339 F) | WIHS structured interview: estimate of frequency and severity of pain over the last 6 mo | Chronic pain | 56 |
| Ellis (2010) [50] | Cross-sectional | US, Sep 2003–Aug 2007 | Exclusion: active opportunistic infection, uncontrolled major psychiatric disorder, inability to cooperate with full day clinical evaluation | 1539 (362 F) | Structured interview to assessed self-reported neuropathic pain | Peripheral neuropathy | 23 |
| Wadley (2011) [51] | Cross-sectional | South Africa, Jul 2008–Apr 2009 | Inclusion: adults, had used d4T for at least 6 mo | 395 (295 F) | BPNS | Peripheral neuropathy | 57 |
| Mullin (2011) [52] | Cross-sectional | Tanzania, Nov 2007–Feb 2008 | Inclusion: ART naive or at least 6 mo on ART Exclusion: random blood sugar >11 mmol/L; current treatment with isoniazid, phenytoin, nitrofurantoin, thalidomide, or metronidazole; a history of high alcohol intake and any other neurological diagnosis other than DSP |
326 (225 F) | BPNS | Peripheral neuropathy | ART/CD4 <200 cells/μL: 39; ART/CD4 >200 cells/μL: 39; no ART/CD4 <200 cells/μL: 26; no ART/CD4 >200 cells/μL: 14 |
| Menezes (2011) [53] | Prospective cohort | South Africa, Apr 2004–Dec 2007 | Inclusion: attending HIV clinic, initiating ART with CD4 count ≤200 cells/μL or WHO stage 4 AIDS- defining illness | 9040 (5962 F) | Numbness/dysesthesia after the initiation of ART once other causes were excluded | Peripheral neuropathy | 17 |
| Luma (2012) [54] | Cross-sectional | Cameroon, Jul–Oct 2011 | Inclusion: age >18 y, followed at tertiary hospital | 295 (206 F) | BPNS | Peripheral neuropathy | 18 |
| Evans (2012) [55] | Prospective cohort | South Africa, Jun 2004–May 2010 | Inclusion: age ≥18 y, CD4 count at ART initiation of <200 cells/μL, initiated on standard first-line regimen Exclusion: diabetes, hypertriglyceridemia, hepatitis C |
9399 (5824 F) | Symptom review by a clinician (numbness, dysesthesia, burning/stabbing pain) | Peripheral neuropathy | 4 |
| Robbins (2013) [56] | Cross-sectional | Thailand, Mar–May 2011 | Inclusion: age ≥18 y, ability to speak Thai | 254 (141 F) | “Have you had this pain for more than 3 mo?”, Brief Pain Inventory–Short Form, Thai version of the Self-Administered Leeds Assessment of Neuropathic Symptoms and Sign | Peripheral neuropathy, chronic pain | 27 |
| Valadares (2014) [57] | Cross-sectional | Brazil, dates NR | Inclusion: age 40–60 y, vaginal intercourse in the past month Exclusion: nursing, bilateral oophorectomy, unable to answer questionnaire |
128 (128 F) | Short Personal Experiences Questionnaire | Other (dyspareunia) | 41 |
| Tumusiime (2014) [58] | Cross-sectional | Rwanda, Mar–Jul 2012 | Inclusion: on ART, age 18–60 y Exclusion: active opportunistic infections, CNS disorders, diabetes, vitamin B12 deficiency, renal failure, hypothyroidism, and other pathologies |
507 (366 F) | BPNS | Peripheral neuropathy | 57 |
| Tumusiime (2014) [59] | Cross-sectional | Rwanda, 2005 | Inclusion: >25 y, willing to undergo voluntary counseling and testing for HIV, present in Rwanda since 1994, willing to return for follow-up, ART naive | 704 (704 F) | “In the past 6 mo, have you experienced numbness, tingling, or burning sensations in your arms, legs, hands, or feet that lasted for more than 2 wk?” | Peripheral neuropathy | 52 |
| Uebelacker (2015) [60] | Cross-sectional | US, Oct 2012–Dec 2013 | Inclusion: age ≥18 y, ability to speak English | 238 (89 F) | “Have you had chronic pain for at least the past 6 mo?” | Chronic pain | 62 |
| Sharma (2016) [61] | Prospective cohort | US, 1994–2015 | Not reported | 1412 (1412 F) | Self-report of numbness, tingling, or burning sensations in arms, legs, hands, or feet | Peripheral neuropathy | 21 |
| Jiao (2016) [62] | Chart review | US, Nov 2013–Oct 2014 | Inclusion: age 18–65 y, had at least 3 visits to primary care during study period, had at least 2 prescriptions for ART Exclusion: participants who died during the study period |
638 (274 F) | ICD-9 codes that correspond to chronic pain disorders listed by the International Association for the Study of Pain codes | Chronic pain | 46 |
| Benevides (2017) [35] | Cross-sectional | Brazil, Jan–May 2016 | Inclusion: age 18–70 y Exclusion: cognitive disorder, admitted to the ICU |
147 (60 F) | BPNS | Peripheral neuropathy | 27 |
| Adoukonou (2017) [63] | Cross-sectional | Benin, Apr–July 2011 | Inclusion: people with HIV receiving care at the Parakou University Hospital | 262 (202 F) | Modified BPNS (translated in French) | Peripheral neuropathy | 44 |
| Centner (2017) [64] | Prospective cohort | South Africa, dates NR | Inclusion: age ≥18 y, met criteria for ART initiation Exclusion: diabetes mellitus, serious systemic illness, severe diarrhea, comorbid neurological disease, TB treatment in the last 2 mo, exposure to glucocorticoids within the last 6 mo, pregnancy |
Baseline: 184 (130 F) Follow-up: 102 (72 F) |
BPNS and Total Neuropathy Score | Peripheral neuropathy | Baseline: 15 Follow-up: 15 |
| Navis (2018) [65] | Cross-sectional | US, 2003–2008 | Inclusion: age 18–65 y, at least 3 visits to HIV clinic in last 12 mo | 638 (274 F) | ICD-9/10 codes according to the International Association for the Study of Pain classification of chronic pain | Peripheral neuropathy, chronic pain | 10 |
| Octaviana (2019) [66] | Cross-sectional | Indonesia | Inclusion: adults receiving ART for at least 12 mo (without d4T) Exclusion: history of other conditions linked to neuropathy |
197 (57 F) | The Douleur Neuropathique 4 questionnaire | Peripheral neuropathy | 7 |
| Adem (2019) [67] | Cross-sectional | Ethiopia, Feb–Jun 2017 | Inclusion: aged ≥18 y, attending HIV care clinic Exclusion: TB, cognitive disorders, peripheral nerve injury, chronic renal conditions, active opportunistic infection, leprosy, vitamin B12 deficiency, severe communication impairments |
359 (234 F) | BPNS, neurological exam | Peripheral neuropathy | 25 |
| Demirdal (2019) [68] | Cross-sectional | Turkey, Jun 2018–Jun 2019 | Inclusion: age ≥18 y Exclusion: other severe chronic systemic disease, severe psychiatric disorder, endocrinopathy, uncontrolled hypothyroidism or diabetes mellitus, known history of rheumatic disease, use of antidepressants, use of drugs for treatment of fibromyalgia, recent joint and/or muscle trauma, hepatitis B/C/D infection, malignancy |
225 (33 F) | 2016 American College of Rheumatology Fibromyalgia criteria | Fibromyalgia/myalgia | 27 |
| Sabin (2020) [69] | Cross-sectional | United Kingdom and Ireland, Apr 2013–Feb 2016 | Inclusion: HIV acquisition through sexual transmission, cisgender, White or Black African ethnicity Exclusion: acquiring HIV through other routes |
944 (128 F) | 2019 American College of Rheumatology Fibromyalgia criteria | Fibromyalgia/myalgia | … |
| Ellis (2020) [70] | Prospective cohort | US, 2003–2019 | Not reported | 253 (54 F) | Self-reported neuropathic pain | Peripheral neuropathy | 35 |
| Mukoma (2020) [71] | Cross-sectional | Kenya, Mar–Apr 2019 | Inclusion: age 20–68 y, on ART | 289 (222 F) | Clinical HIV Associated Neuropathy Tool | Peripheral neuropathy | 55 |
| Safri (2020) [72] | Cross-sectional | Indonesia, dates NR | Inclusion: adults on ART for at least 12 mo Exclusion: exposure to d4T, diabetes, stroke, schizophrenia, vasculitis, deafness, blindness, hyperthyroidism, systemic lupus erythematosus, cytomegalovirus radiculopathy, cancer chemotherapy |
185 (67 F) | BPNS | Peripheral neuropathy |
16 |
| Giani (2020) [73] | Cross-sectional | Malawi, dates NR | Inclusion: age 18–65 y | 498 (359 F) | Structured interview (authors report 1-y prevalence of headache) | Headache | 84 |
| Emorinken (2021) [74] | Cross-sectional | Nigeria, Jun 2016–May 2017 | Inclusion: age ≥18 y, ART naive Exclusion: traumatic musculoskeletal disorders, rheumatic or connective tissue disease, psychological disorders, hypothyroidism, chronic viral infections, pregnancy, using medications to treat fibromyalgia |
160 (110 F) | 2011 modification of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia | Fibromyalgia/myalgia | 12 |
| Kowalski (2021) [75] | Cross-sectional | Poland, Feb 2014–Dec 2016 | Inclusion: age ≥18 y Exclusion: any mental condition |
196 (34 F) | Pain lasting ≥6 mo, Brief Pain Inventory–Short Form, physical exam | Chronic pain | 38 |
| Tu (2021) [76] | Cross-sectional | Canada, 2013–2019 | Inclusion: HIV-1–seropositive adults Exclusion: nonfluency in English, age <18 y, less than grade 9 education, severe psychiatric or neurological disorders, history of brain damage/traumatic brain injury with loss of consciousness, uncorrected vision, or hearing impairments |
519 (75 F) | Review of symptoms, laboratory results, and physical examination | Peripheral neuropathy |
28 |
| Nikolaidis (2022) [77] | Cross-sectional | Greece, dates NR | Inclusion: adults on ART Exclusion: other types of peripheral neuropathy, diabetes, cytomegalovirus, herpes, autoimmune diseases, vitamin B12 deficiency, renal failure, hypothyroidism |
420 (74 F) | BPNS | Peripheral neuropathy |
47 |
| Yitbarek (2022) [78] | Cross-sectional | Ethiopia, Nov–Dec 2020 | Inclusion: receiving care at ART clinic Exclusion: current opportunistic infection, neurological problems, renal failure, hypothyroidism |
555 (297 F) | BPNS | Peripheral neuropathy |
29 |
Abbreviations: ART, antiretroviral therapy; BPNS, Brief Peripheral Neuropathy Screen; CNS, central nervous system; d4T, stavudine; DSP, distal sensory polyneuropathy; HIV, human immunodeficiency virus; ICD, International Classification of Diseases; ICU, intensive care unit; NR, not reported; TB, tuberculosis; US, United States; WIHS, Women's Interagency HIV Study; WHO, World Health Organization.
Figure 1.
Study selection.
Figure 2.
Map of countries where studies included in the review took place. The majority of the studies (n = 34) spanned a single site, while only 2 studies spanned multiple sites.
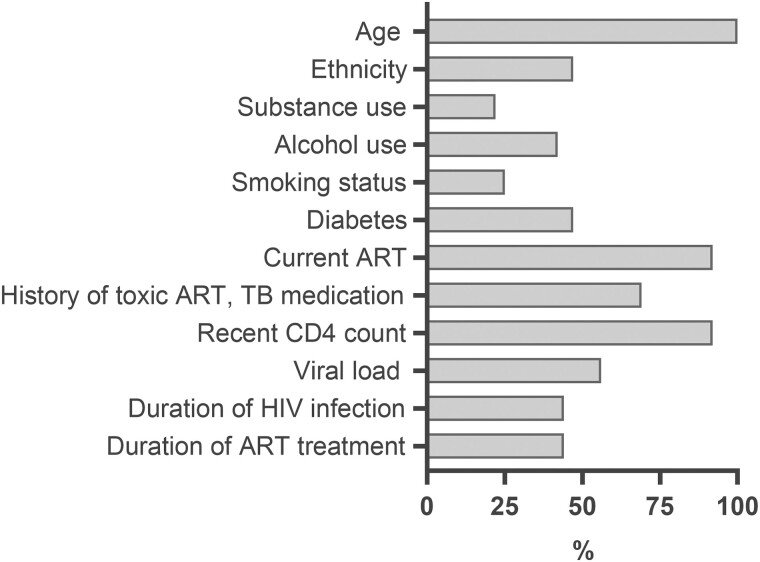
Participant demographics are presented in Supplementary Tables 2 and 3. Eight articles (22%) reported on substance use (Figure 3) and among them, the proportion of participants with a history of illicit substance use ranged from 8% to 77% (Supplementary Table 2). Nine articles (25%) reported on tobacco smoking status, with current or past smoking histories reported for 1%–38% of participants. Fifteen articles (42%) reported on history of alcohol use, with estimates ranging from 6% to 83%.
Figure 3.
Proportion of studies that included data about selected demographic parameters of their study participants. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; TB, tuberculosis.
Included studies spanned the pre-ART era to newer ART; hence, percentage of participants with current ART use varied widely between studies, ranging from 0% to 100% (Supplementary Table 3). Use of neurotoxic HIV or tuberculosis medications was also highly variable. Most articles included data on CD4 counts and HIV plasma viral loads, but few described duration of ART use and/or HIV infection. Several articles either described the prevalence of diabetes among participants (8/35) or identified this as an exclusion criterion (9/35).
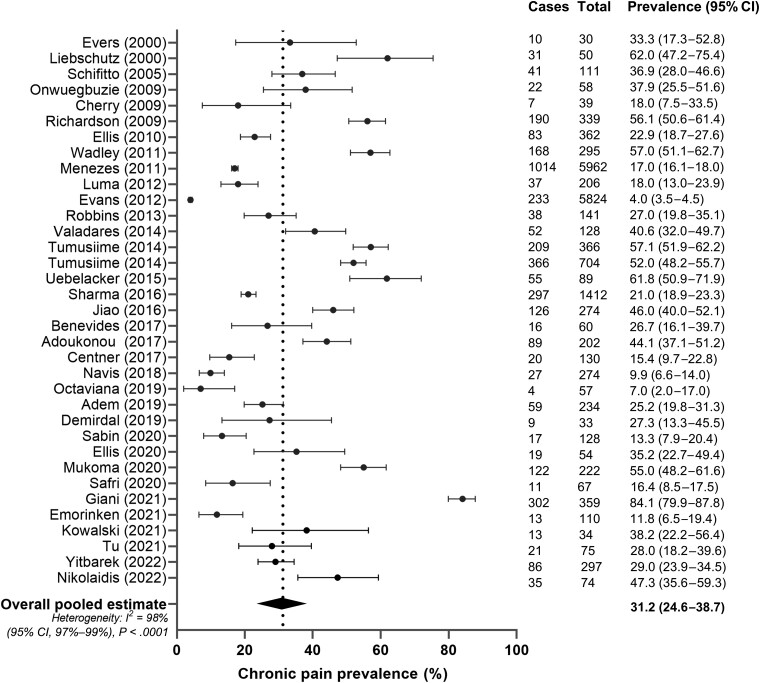
In the primary meta-analysis, the pooled prevalence of chronic pain across all studies was 31.2% (95% confidence interval [CI], 24.6%–38.7%) (Figure 4). The lowest prevalence was reported by Evans et al [55] at 4.0% (95% CI, 3.5%–4.5%) and the highest by Giani et al [73] at 84.1% (95% CI, 79.9%–87.8%). Formal tests for identifying outliers, as well as visual inspection of the leave-one-out plot (Supplementary Figure 2) and studentized residuals (Supplementary Table 1), indicated no truly influential outliers and thus all articles were retained in the final model. Visual inspection of the funnel plots revealed no bias by sample size (Supplementary Figures 3 and 4), and Egger regression test confirmed the absence of significant asymmetry (P = .19). However, the studies showed a high degree of heterogeneity, with an I2 of 98% (95% CI, 97%–99%) (P < .0001), highlighting methodological and demographic differences of the studies analyzed.
Figure 4.
Pooled prevalence of chronic pain in women with human immunodeficiency virus. Cases: number of women with chronic pain in the study. Total: number of women enrolled in the study. Black circles indicate chronic pain prevalence in each study (with 95% confidence interval [CI]); diamond and vertical dashed line indicate the pooled estimate of chronic pain.
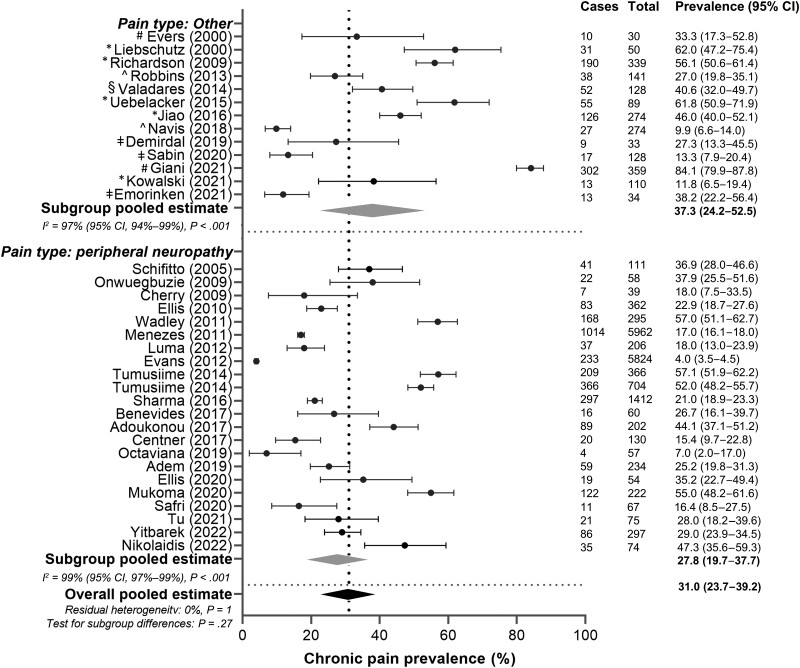
In the subgroup analysis, the prevalence of peripheral neuropathy was compared to all other chronic pain subtypes (Figure 5). Twenty-two articles were included in the peripheral neuropathy subgroup, with a pooled prevalence estimate of 27.8% (95% CI: 19.7%–37.7%) and an I2 of 99% (95% CI: 97%–99%) (P < .001). For all other pain subtypes, the pooled prevalence was 37.3% (95% CI: 24.2%–52.5%) and I2 of 97% (95% CI: 94%–99%) (P < .001), which was not significantly different from the peripheral neuropathy subgroup (P = .27).
Figure 5.
Subgroup analysis of chronic pain prevalence in women with human immunodeficiency virus based on chronic pain type (peripheral neuropathy vs other). Cases: number of women with chronic pain in the study. Total: number of women enrolled in the study. Black circles indicate chronic pain prevalence in each study (with 95% confidence interval [CI]); gray diamonds indicate pooled prevalence of chronic pain within the subgroups; black diamond and vertical dashed line indicate pooled estimate of chronic pain. #Headache. *Chronic pain. ^Chronic pain and peripheral neuropathy. §Dyspareunia. ǂFibromyalgia/myalgia.
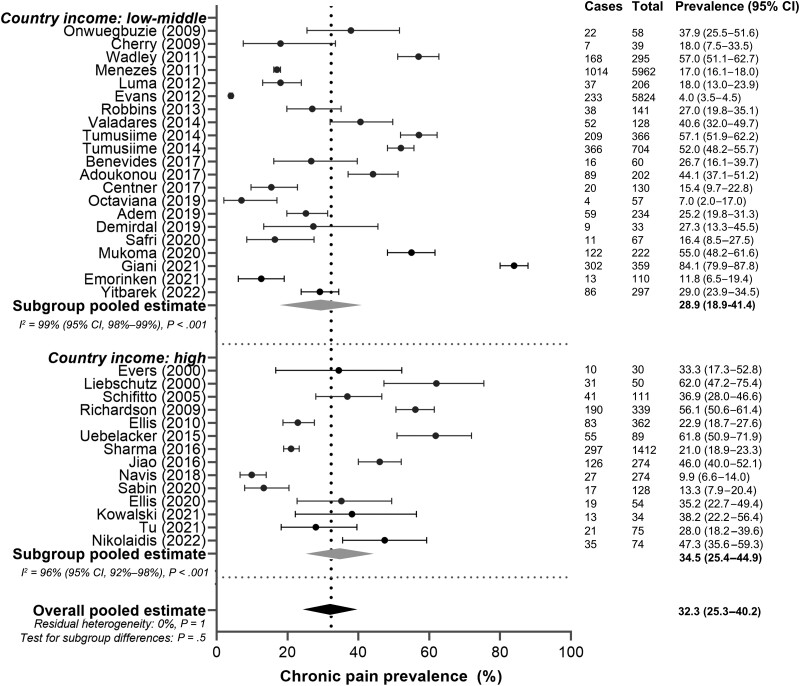
The subgroup analysis comparing countries indicated that the prevalence of chronic pain in high-income countries of 34.5% (95% CI: 25.4%–44.9%) was similar to that in low- and middle-income countries 28.9% (95% CI: 18.9%–41.4%) (P = .5; Figure 6). Again, there was significant heterogeneity in both the high-income and middle/low-income subgroups, with I2 values of 96% (95% CI: 92%–98%) (P < .001) and 99% (95% CI: 98%–99%) (P < .001), respectively.
Figure 6.
Subgroup analysis of chronic pain prevalence in women with human immunodeficiency virus based on country where the study was conducted (low-middle vs high income). Cases: number of women with chronic pain in the study. Total: number of women enrolled in the study. Black circles indicate chronic pain prevalence in each study (with 95% confidence interval [CI]); gray diamonds indicate pooled prevalence of chronic pain within the subgroups; black diamond and a vertical dashed line indicate pooled estimate of chronic pain.
Three studies compared the prevalence of chronic pain between persons with HIV and HIV-negative controls. Sharma et al [61] reported on peripheral neuropathy among middle-aged women and reported a higher prevalence in women with HIV compared to HIV-negative controls (20.6% vs 14.0%; P = .0003). Similarly, Tumusiime et al [59] found that 52% of women with HIV experienced neuropathic pain symptoms, compared with 44% of HIV-negative women, although this difference was not statistically significant (P = .06). Finally, Valadares et al [57] examined the prevalence of dyspareunia and reported no difference between these 2 groups (41.4% in women with HIV vs 34.8% in HIV-negative women; P = .24).
Only 1 study reported on the severity of chronic pain among women with HIV and various pain subtypes, where the majority (65%) of participants had AIDS-defining illnesses [49]. Half of the women (50.0%) reported high levels of pain, including 34.5% with extreme pain.
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis to describe the prevalence of chronic pain among women with HIV. In our global assessment across 22 countries and 19 966 participants, we identified that at least one-third of women with HIV experience chronic pain, underscoring its pervasiveness and signaling the importance of regularly assessing for pain. This is similar to the global prevalence of chronic pain among HIV-negative persons in middle- and low-income countries at 33%, but higher than the prevalence in the United States at 20.4% [79, 80]. However, few studies have directly compared the prevalence of chronic pain in women with and without HIV. Our results indicate that, although most research attention has been focused on peripheral neuropathy, women commonly experience other pain subtypes. Hence, further research is needed to holistically understand these pain experiences. Our analysis did not detect any difference between pain subtypes or country of study. To our knowledge, no previous meta-analyses have estimated the prevalence of chronic pain in people with HIV, although 1 meta-analysis exists including both acute and chronic pain [25]. That meta-analysis reported a pooled prevalence of 54%, which is higher than what we report here, but expected for data collected on both acute and chronic pain types. A scoping review of chronic pain in people with HIV suggested that the prevalence ranges from 25% to 90% [12], whereas we observed a range of 4.0% to 84.1%. This wider range is likely related to differences in search methodology and inclusion/exclusion criteria used by Addis et al [12], the details of which are difficult to ascertain.
Our analysis identified key gaps in the literature. First, more research is needed to understand nonneuropathic chronic pain subtypes, especially those that are specific to women, such as breast, pelvic, vaginal, and menstrual pain. We identified only 1 article that assessed dyspareunia and none that evaluated other female-specific pain manifestations. Similarly, only 1 article discussed pain severity. This is a critical component of understanding the pain experience of women with HIV and its effect on quality of life. Furthermore, there was a paucity of studies discussing how chronic pain interferes with daily activities or contributes to stigma. Few studies included an HIV-negative control group, although we do acknowledge the difficulty of recruiting HIV-negative controls that share comparable psychosocial identities with women with HIV. We also note that although pain prevalence was reported across a diverse array of countries, some regions were missing, including South America, Asia, and Eastern Europe. Furthermore, we were unable to find any articles describing chronic pain among transgender women with HIV, which speaks to the importance of inclusion of this key population in research. This is especially concerning given that cisgender and transgender women with HIV have similar pain sensitivity, which is greater than that of cisgender men [31]. Also, much of the existing literature is in younger or middle-aged people, highlighting the need for more research into the chronic pain experience of older women. Last, we agree with Madden et al [3] that the literature lacks research into effective pain management, which should be noted as a critical area of further study.
We identified several important characteristics that were inconsistently reported across studies, limiting meaningful interpretation of the results and likely contributing to the high I2 values. For instance, several studies omitted data on ethnicity, substance use, alcohol, smoking, diabetes, HIV viral loads, duration of HIV infection, and duration of ART use (Figure 3). We also noted that articles used a diverse array of tools or questions to assess chronic pain (Table 1), which further limits comparability. This emphasizes the need for consistent and validated tools to assess chronic pain in both clinical and research settings.
Our study should be interpreted in light of its limitations. First, there was a very high level of heterogeneity between articles, which likely reflects the differences in how chronic pain is defined and measured, diversity in the study samples, type of ART exposure, and time or location of data collection. This high heterogeneity was not explained by subgroup analyses. This suggests that careful consideration of geographic and individual-level factors are important when considering the risk for chronic pain in a patient population. However, Migliavaca et al [81] emphasize that a high I2 is not unexpected for this study type, as they found a median I2 of 96.9% (interquartile range, 90.5%–98.7%) among 235 systematic reviews of prevalence. Given the sample size, we were unable to perform a meta-regression to account for important confounders, including age, use of neurotoxic medications, years with HIV, comorbid diabetes, or psychosocial conditions. Additionally, we were not able to conduct some important subgroup analyses, such as stratifying studies based on history of AIDS or opportunistic infections, duration of HIV/ART, or exposure to newer versus older ART regimens due to heterogeneity in data reporting and/or missing data. Finally, it should be acknowledged that most articles in this analysis were of moderate quality based on the risk of bias assessment, highlighting the need for more high-quality research in this area.
Overall, our results suggest that chronic pain is highly prevalent among women with HIV and warrants regular assessment at healthcare visits. Several clinical tools exist to assess for the presence of chronic pain, as well as severity and subjective experiences, and should be used frequently [82–84]. We concur with recommendations from the Global Task Force for Chronic Pain in People With HIV [9], including the need for further research into effective methods of pain management, etiologies of chronic pain, and chronic pain prevention. Our results highlight that it is especially important to consider these factors from a women-centered lens [85]. Of note, this research was motivated by discussions with women with HIV and their interest in this topic. We therefore wish to emphasize the importance of sharing research results about chronic pain with women with HIV to validate their experiences, increase opportunities for meaningful dialogue, identify knowledge gaps, and encourage co-learning between clinical, academic, and community partners.
Supplementary Material
Contributor Information
Tetiana Povshedna, Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada; Centre for Blood Research, University of British Columbia, Vancouver, British Columbia, Canada; Edwin S. H. Leong Healthy Aging Program, University of British Columbia, Vancouver, British Columbia, Canada.
Shayda A Swann, Edwin S. H. Leong Healthy Aging Program, University of British Columbia, Vancouver, British Columbia, Canada; Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; Women's Health Research Institute, British Columbia Women’s Hospital and Health Centre, Vancouver, British Columbia, Canada.
Sofia L A Levy, Faculty of Science, University of British Columbia, Vancouver, British Columbia, Canada.
Amber R Campbell, Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada; Women's Health Research Institute, British Columbia Women’s Hospital and Health Centre, Vancouver, British Columbia, Canada; Oak Tree Clinic, British Columbia Women’s Hospital and Health Centre, Vancouver, British Columbia, Canada.
Manon Choinière, Department of Anesthesiology and Pain Medicine, Faculty of Medicine, Université de Montréal, Montreal, Quebec, Canada.
Madeleine Durand, Department of Medicine, Faculty of Medicine, Université de Montréal, Montreal, Quebec, Canada.
Colleen Price, Canadian HIV/AIDS and Chronic Pain Society, Global Pain and HIV Task Force, Ottawa, Ontario, Canada.
Prubjot Gill, Woodward Library, University of British Columbia, Vancouver, British Columbia, Canada.
Melanie C M Murray, Edwin S. H. Leong Healthy Aging Program, University of British Columbia, Vancouver, British Columbia, Canada; Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; Women's Health Research Institute, British Columbia Women’s Hospital and Health Centre, Vancouver, British Columbia, Canada; Oak Tree Clinic, British Columbia Women’s Hospital and Health Centre, Vancouver, British Columbia, Canada.
Hélène C F Côté, Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada; Centre for Blood Research, University of British Columbia, Vancouver, British Columbia, Canada; Edwin S. H. Leong Healthy Aging Program, University of British Columbia, Vancouver, British Columbia, Canada; Women's Health Research Institute, British Columbia Women’s Hospital and Health Centre, Vancouver, British Columbia, Canada.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. A. S. and T. P. led the conceptualization of the research aims. S. A. S., with input from T. P., S. L. A. L., A. R. C., M. C., M. D., C. P., P. G., M. C. M. M., and H. C. F. C., created the study protocol. S. A. S. developed and executed the search strategy. S. A. S. and T. P., with input from S. L. A. L., A. R. C., and H. C. F. C., led article selection. S. A. S. and T. P. conducted the data extraction. T. P. led the data analysis and figure preparation. S. A. S. wrote the manuscript. T. P., S. L. A. L., A. R. C., M. C., M. D., C. P., P. G., M. C. M. M., and H. C. F. C. provided critical review of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing. Primary data were not collected for this study and thus are not available for sharing. The study protocol can be accessed on PROSPERO (ID number CRD42022301145).
Patient consent. This review utilized previously published summary-level data and therefore did not necessitate patient consent.
Disclaimer. The funder(s) of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Financial support . T. P. holds funds from the University of British Columbia Four Year Fellowship. S. A. S. receives funding from the Canadian Institutes of Health Research (CIHR) Vanier Graduate Scholarship. M. C. M. M. receives Health Professional Investigator salary funding from the Michael Smith Foundation for Health Research. M. D. received a clinician-researcher salary award from the Fonds de recherche du Québec-Santé. This work is partially supported by the CIHR Canadian HIV Trials Network (CTN 335), 2 CIHR project grants (BCA-408242 and 175006), and a CIHR HIV/AIDS Community-Based Research Grant (170103).
References
- 1. Denis CM, Morales KH, Wu Q, Metzger DS, Cheatle MD. Association between diagnoses of chronic noncancer pain, substance use disorder, and HIV-related outcomes in people living with HIV. J Acquir Immune Defic Syndr 2019; 82:S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merlin JS, Westfall AO, Chamot E, et al. Pain is independently associated with impaired physical function in HIV-infected patients. Pain Med 2013; 14:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madden VJ, Parker R, Goodin BR. Chronic pain in people with HIV: a common comorbidity and threat to quality of life. Pain Manag 2020; 10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruce RD, Merlin J, Lum PJ, et al. 2017 HIVMA of IDSA clinical practice guideline for the management of chronic pain in patients living with HIV. Clin Infect Dis 2017; 65:e1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Surratt HL, Kurtz SP, Levi-Minzi MA, Cicero TJ, Tsuyuki K, O’Grady CL. Pain treatment and antiretroviral medication adherence among vulnerable HIV-positive patients. AIDS Patient Care STDS 2015; 29:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merlin JS, Westfall AO, Raper JL, et al. Pain, mood, and substance abuse in HIV: implications for clinic visit utilization, ART adherence, and virologic failure. J Acquir Immune Defic Syndr 2012; 61:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain 2011; 12:1004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mwesiga EK, Kaddumukasa M, Mugenyi L, Nakasujja N. Classification and description of chronic pain among HIV positive patients in Uganda. Afr Health Sci 2019; 19:1978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merlin JS, Hamm M, de Abril Cameron F, et al. The Global Task Force for Chronic Pain in People with HIV (PWH): developing a research agenda in an emerging field. AIDS Care 2023; 35:1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merlin JS, Zinski A, Norton WE, et al. A conceptual framework for understanding chronic pain in patients with HIV. Pain Pract 2014; 14:207–16. [DOI] [PubMed] [Google Scholar]
- 11. Penfold J, Clark AJM. Brief review pain syndromes in HIV infection. Can J Anaesth 1992; 39:724–30. [DOI] [PubMed] [Google Scholar]
- 12. Addis DR, DeBerry JJ, Aggarwal S. Chronic pain in HIV. Mol Pain 2020; 16:174480692092727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leone A, Cerase A, Costantini A. Musculoskeletal tuberculosis. Microbiol Spectr 2017; 7:227–37. [Google Scholar]
- 14. Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol 2016; 13:13–24. [DOI] [PubMed] [Google Scholar]
- 15. Hadley GR, Gayle JA, Ripoll J, et al. Post-herpetic neuralgia: a review. Curr Pain Headache Rep 2016; 20:17. [DOI] [PubMed] [Google Scholar]
- 16. Vecchio AC, Marra CM, Schouten J, et al. Distal sensory peripheral neuropathy in human immunodeficiency virus type 1–positive individuals before and after antiretroviral therapy initiation in diverse resource-limited settings. Clin Infect Dis 2020; 71:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Floridia M, Giuliano M, Palmisano L, Vella S. Gender differences in the treatment of HIV infection. Pharmacol Res 2008; 58:173–82. [DOI] [PubMed] [Google Scholar]
- 18. Smith MY, Egert J, Winkel G, Jacobson J. The impact of PTSD on pain experience in persons with HIV/AIDS. Pain 2002; 98(1–2):9–17. [DOI] [PubMed] [Google Scholar]
- 19. Scott W, Arkuter C, Kioskli K, et al. Psychosocial factors associated with persistent pain in people with HIV: a systematic review with meta-analysis. Pain 2018; 159:2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shacham E, Rosenburg N, Önen NF, Donovan MF, Turner Overton E. Persisting HIV-related stigma among an outpatient US clinic population. Int J STD AIDS 2015; 26:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yousuf A, Musa R, Isa MLM, Arifin SRM. Anxiety and depression among women living with HIV: prevalence and correlations. Clin Pract Epidemiol Ment Health 2020; 16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner AC, Jaworsky D, Logie CH, et al. High rates of posttraumatic stress symptoms in women living with HIV in Canada. PLoS One 2018; 13:e0200526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shokoohi M, Bauer GR, Kaida A, et al. Substance use patterns among women living with HIV compared with the general female population of Canada. Drug Alcohol Depend 2018; 191:70–7. [DOI] [PubMed] [Google Scholar]
- 24. Logie C, James L, Tharao W, Loutfy M. Associations between HIV-related stigma, racial discrimination, gender discrimination, and depression among HIV-positive African, Caribbean, and Black women in Ontario, Canada. AIDS Patient Care STDS 2013; 27:114–22. [DOI] [PubMed] [Google Scholar]
- 25. Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. J Int AIDS Soc 2014; 17:18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9:883–91. [DOI] [PubMed] [Google Scholar]
- 27. Raffe S, Sabin C, Gilleece Y. Comorbidities in women living with HIV: a systematic review. HIV Med 2022; 23:331–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puskas CM, Forrest JI, Parashar S, et al. Women and vulnerability to HAART non-adherence: a literature review of treatment adherence by gender from 2000 to 2011. Curr HIV/AIDS Rep 2011; 8:277–87. [DOI] [PubMed] [Google Scholar]
- 29. Joint United Nations Programme on HIV/AIDS (UNAIDS) . Understanding fast-track: accelerating action to end the AIDS epidemic by 2030. Geneva, Switzerland: UNAIDS; 2015. [PubMed]
- 30. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021; 18:e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strath LJ, Sorge RE, Owens MA, et al. Sex and gender are not the same: why identity is important for people living with HIV and chronic pain. J Pain Res 2020; 13:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gordis L. Case-Control studies and other study designs. In: Gordis L, ed. Epidemiology. 5th ed. Philadelphia, PA: Elsevier: 2013:191. [Google Scholar]
- 33. Veritas Health Innovation. Covidence systematic review software. Available at: www.covidence.org. Accessed 22 November 2022.
- 34. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015; 13:147–53. [DOI] [PubMed] [Google Scholar]
- 35. Benevides MLACSE, Filho SB, Debona R, Bergamaschi ENC, Nunes JC. Prevalence of peripheral neuropathy and associated factors in HIV-infected patients. J Neurol Sci 2017; 375:316–20. [DOI] [PubMed] [Google Scholar]
- 36. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep 2019; 19:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Evans SR, Ellis RJ, Chen H, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS 2011; 25:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mafukidze AT, Calnan M, Furin J. Peripheral neuropathy in persons with tuberculosis. J Clin Tuberc Other Mycobact Dis 2016; 2:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide. 1st ed. London: Chapman & Hall/CRC Press; 2021. [Google Scholar]
- 40. RStudio Team . RStudio: integrated development environment for R. Boston, MA: RStudio Team; 2021. [Google Scholar]
- 41. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36:1–48. [Google Scholar]
- 42. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–58. [DOI] [PubMed] [Google Scholar]
- 43. World Bank . World Bank country and lending groups—country classification. 2021. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 26 August 2022.
- 44. Evers S, Wibbeke B, Reichelt D, Suhr B, Brilla R, Husstedt IW. The impact of HIV infection on primary headache. Unexpected findings from retrospective, cross-sectional, and prospective analyses. Int Assoc Study Pain 2000; 85:191–200. [DOI] [PubMed] [Google Scholar]
- 45. Liebschutz JM, Feinman G, Sullivan L, Stein M, Samet J. Physical and sexual abuse in women infected with the human immunodeficiency virus: increased illness and health care utilization. Arch Intern Med 2000; 160:1659–64. [DOI] [PubMed] [Google Scholar]
- 46. Schifitto G, McDermott MP, McArthur JC, et al. Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology 2005; 64:842–8. [DOI] [PubMed] [Google Scholar]
- 47. Onwuegbuzie G, Isamade E, Idoko J, Ogunniyi A. Prevalence of distal symmetrical polyneuropathy among drug naive HIV/AIDS patients in Jos, Nigeria. Afr J Neurol Sci 2009; 28. [Google Scholar]
- 48. Cherry CL, Affandi JS, Imran D, et al. Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology 2009; 73:315–20. [DOI] [PubMed] [Google Scholar]
- 49. Richardson JL, Heikes B, Karim R, Weber K, Anastos K, Young M. Experience of pain among women with advanced HIV disease. AIDS Patient Care STDS 2009; 23:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ellis RJ, Rosario D, Clifford DB, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus–associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER study. Arch Neurol 2010; 67:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wadley AL, Cherry CL, Price P, Kamerman PR. HIV Neuropathy risk factors and symptom characterization in stavudine-exposed South Africans. J Pain Symptom Manage 2011; 41:700–6. [DOI] [PubMed] [Google Scholar]
- 52. Mullin S, Temu A, Kalluvya S, Grant A, Manji H. High prevalence of distal sensory polyneuropathy in antiretroviral-treated and untreated people with HIV in Tanzania. Trop Med Int Health 2011; 16:1291–6. [DOI] [PubMed] [Google Scholar]
- 53. Menezes CN, Maskew M, Sanne I, Crowther NJ, Raal FJ. A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects. BMC Infect Dis 2011; 11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luma HN, Tchaleu BCN, Doualla MS, et al. HIV-associated sensory neuropathy in HIV-1 infected patients at the Douala General Hospital in Cameroon: a cross-sectional study. AIDS Res Ther 2012; 9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Evans D, Takuva S, Rassool M, Firnhaber C, Maskew M. Prevalence of peripheral neuropathy in antiretroviral therapy naïve HIV-positive patients and the impact on treatment outcomes—a retrospective study from a large urban cohort in Johannesburg, South Africa. J Neurovirol 2012; 18:162–71. [DOI] [PubMed] [Google Scholar]
- 56. Robbins NM, Chaiklang K, Supparatpinyo K. Undertreatment of pain in HIV+ adults in Thailand. J Pain Symptom Manage 2013; 45:1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Valadares ALR, Pinto-Neto AM, Gomes DDC, et al. Dyspareunia in HIV-positive and HIV-negative middle-aged women: a cross-sectional study. Open 2014; 4:e004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tumusiime DK, Venter F, Musenge E, Stewart A. Prevalence of peripheral neuropathy and its associated demographic and health status characteristics, among people on antiretroviral therapy in Rwanda. BMC Public Health 2014; 14:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tumusiime DK, Musabeyezu E, Mutimurah E, et al. Over-reported peripheral neuropathy symptoms in a cohort of HIV infected and uninfected Rwandan women: the need for validated locally appropriate questionnaires. Afr Health Sci 2014; 14:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, Stein MD. Chronic pain in HIV-infected patients: relationship to depression, substance use, and mental health and pain treatment. Pain Med 2015; 16:1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sharma A, Hoover DR, Shi Q, et al. Falls among middle-aged women in the Women's Interagency HIV Study. Antivir Ther 2016; 21:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jiao JM, So E, Jebakumar J, George MC, Simpson DM, Robinson-Papp J. Chronic pain disorders in HIV primary care: clinical characteristics and association with healthcare utilization. Pain 2016; 157:931–7. [DOI] [PubMed] [Google Scholar]
- 63. Adoukonou TA, Kouna-Ndouongo P, Kpangon A, et al. Distal sensory polyneuropathy among HIV-infected patients at Parakou University Hospital, Benin, 2011. Med Sante Trop 2017; 27:190–4. [DOI] [PubMed] [Google Scholar]
- 64. Centner CM, Little F, Van Der Watt JJ, et al. Evolution of sensory neuropathy after initiation of antiretroviral therapy. Muscle Nerve 2017; 57:371–9. [DOI] [PubMed] [Google Scholar]
- 65. Navis A, Jiao J, George MC, Simpson D, Robinson-Papp J. Comorbid pain syndromes in HIV-associated peripheral neuropathy. Pain Med 2018; 29:1445–50. [DOI] [PubMed] [Google Scholar]
- 66. Octaviana F, Safri AY, Setiawan DD, et al. Neuropathic pain in HIV patients receiving ART without stavudine in an Indonesia referral hospital. J Neurol Sci 2019; 397:146–9. [DOI] [PubMed] [Google Scholar]
- 67. Adem KS, Janakiraman B, Gebremeskel BF, Chala MB, Gelaw AY, Alemu K. Epidemiology and factors associated with peripheral neuropathy among HIV infected patients in Gondar, Ethiopia: a cross-sectional study. PLoS One 2019; 14:e0211354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Demirdal US, Bilir N, Demirdal T. The effect of concomitant fibromyalgia in HIV infected patients receiving antiretroviral therapy: a prospective cross-sectional study. Ann Clin Microbiol Antimicrob 2019; 18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sabin CA, Harding R, Bagkeris E, et al. The predictors of pain extent in people living with HIV. AIDS 2020; 34:2071–9. [DOI] [PubMed] [Google Scholar]
- 70. Ellis RJ, Diaz M, Sacktor N, et al. Predictors of worsening neuropathy and neuropathic pain after 12 years in people with HIV. Ann Clin Transl Neurol 2020; 7:1166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mukoma JN, Matheri JM, Tawa N. Prevalence and clinical characteristics associated with peripheral neuropathy amongst persons on HAART in Busia County, Kenya. South African J Physiother 2020; 76:1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Safri AY, Gaff J, Octaviana F, et al. Brief report: demographic and genetic associations with markers of small and large fiber sensory neuropathy in HIV patients treated without stavudine. J Acquir Immune Defic Syndr 2020; 85:612–6. [DOI] [PubMed] [Google Scholar]
- 73. Giani L, Mwazangati M, Uluduz D, et al. Headache burden in a HIV population of sub-Saharan Africa: scope and implications from a pilot study. Neurol Sci 2020; 42:493–4. [DOI] [PubMed] [Google Scholar]
- 74. Emorinken A, Dic-Ijiewere MO, Erameh CO, Ugheoke AJ, Agbebaku FO, Agbadaola OR. Fibromyalgia in HIV-positive patients in Nigeria: a cross-sectional prospective study. Int J Rheum Dis 2021; 24:1273–81. [DOI] [PubMed] [Google Scholar]
- 75. Kowalski M, Horban A, Slomka B, Shahnazaryan K, Rongies W. Is age and not antiretroviral therapy the strongest risk factor for chronic pain in HIV-infected population? BMC Infect Dis 2021; 21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tu W, Johnson E, Fujiwara E, Gill MJ, Kong L, Power C. Predictive variables for peripheral neuropathy in treated HIV type 1 infection revealed by machine learning. AIDS 2021; 2021:1785–93. [DOI] [PubMed] [Google Scholar]
- 77. Nikolaidis I, Karakasi MV, Bakirtzis C, et al. Epidemiology of HIV-associated peripheral neuropathy in people living with human immunodeficiency virus infection in Greece. Int J STD AIDS 2022; 33:978–86. [DOI] [PubMed] [Google Scholar]
- 78. Yitbarek GY, Addis WD, Dagnaw FT, et al. Magnitude of peripheral sensory neuropathy and associated factors among HIV/AIDS clients receiving care at public health institutions, northwest Ethiopia. Res Artic Mol Pain 2022; 18:17448069221089593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jackson T, Thomas S, Stabile V, Han X, Shotwell M, McQueen K. Prevalence of chronic pain in low-income and middle-income countries: a systematic review and meta-analysis. Lancet 2015; 385:S10. [DOI] [PubMed] [Google Scholar]
- 80. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morb Mortal Wkly Rep 2018; 67:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Migliavaca CB, Stein C, Colpani V, Munn Z, Falavigna M. Quality assessment of prevalence studies: a systematic review. J Clin Epidemiol 2020; 127:59–68. [DOI] [PubMed] [Google Scholar]
- 82. Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of chronic pain: domains, methods, and mechanisms. J Pain 2016; 17:T10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol 2011; 38:1113–22. [DOI] [PubMed] [Google Scholar]
- 84. Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology 2005; 65:1778–81. [DOI] [PubMed] [Google Scholar]
- 85. O’Brien N, Greene S, Carter A, et al. Envisioning women-centered HIV care: perspectives from women living with HIV in Canada. Womens Health Issues 2017; 27:721–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.