Abstract
Rationale & Objective:
eGFR equations that incorporate a term for race assign a higher value to Black individuals compared to non-Black individuals not attributable to sex, age, or serum creatinine. This difference may contribute to racial disparities in kidney transplant access. We sought to 1) compare time from meeting a transplant eligibility threshold of eGFR ≤20 ml/min/1.73M2 to kidney failure with replacement therapy (KFRT) among Black, Hispanic, and White patients, and 2) assess the impact of incorporation of race into eGFR expressions on establishment of waitlist eligibility and time from eligibility to KFRT.
Study Design:
Retrospective cohort.
Setting & Participants:
Using the OptumLabs® Data Warehouse, we assembled a cohort of 40,042 White, 8,519 Black, and 3,569 Hispanic patients having at least one eGFR value between 20 and 60 mL/min/1.73m2 within the preceding two years and an incident outpatient eGFR of ≤20 ml/min/1.73m2 between 2008–2018, using the CKD-EPI equation that includes a term for race coded as Black or non-Black. We then re-assembled a Black patient cohort based on incident eGFR ≤20 ml/min/1.73m2 (n=11,269) estimated using the same CKD-EPI equation for Black patients but coding patients as non-Black.
Exposure:
Race/ethnicity.
Outcome:
Time to KFRT.
Analytical Approach:
Unadjusted and adjusted Fine-Gray models; linear regression to compute eGFR slopes.
Results:
By 3 years, the cumulative incidence of KFRT was 20.5% among White patients, 40.9% among Hispanic patients, and 36% among Black patients whose eGFR was estimated using a race term coded as Black and 28.7% among Black patients whose eGFR was estimated using a race term coded as non-Black. In fully adjusted analyses including 11,269 Black patients with an eGFR ≤20 ml/min/1.73m2 based on coding them as non-Black, KFRT risk remained greater among Black (HR 1.28; 95% CI, 1.15–1.43) and Hispanic (HR 1.66; 95% CI 1.18–2.31) than among White patients. Based on slopes of eGFR decline, coding Black patients as non-Black would allow earlier waitlist activation by an estimated median of 0.5 years [IQR 0.27–1.23].
Limitations:
Inability to exclude individuals who would not be kidney transplant candidates if comprehensively evaluated.
Conclusions:
A uniform eGFR threshold provides less opportunity for being placed on the transplant waitlist among Black and Hispanic patients. For many Black patients, estimation of GFR as if their race category were non-Black would allow substantially earlier waitlisting but would not eliminate their shorter time to KFRT and reduced opportunity for preemptive transplantation compared to White patients.
Index Words: kidney transplant, racial disparities, kidney failure, CKD progression, estimated glomerular filtration rate
Plain Language Summary
Current US kidney transplant policy requires a GFR of ≤20 ml/min for activation on the waitlist, but current GFR estimating equations assign a higher value to Black patients compared to non-Black patients for the same age, sex, and creatinine values and may disadvantage Black patients regarding time for preemptive transplantation. We examined CKD progression in over 50,000 patients who developed a GFR ≤20 ml/min/1.73m2 based on eGFR estimation that incorporates information about race, finding that Black and Hispanic patients progressed to kidney failure more quickly compared to White patients. We observed that classifying all patients as non-Black for the purpose of GFR estimation would allow Black patients to be eligible for earlier waitlisting; however, a large disparity remains in the time available for pre-emptive transplantation due to faster progression to kidney failure compared to White patients. Additionally, faster progression among Hispanic patients would not be remedied by changes in eGFR calculation.
Introduction
Kidney transplantation is the optimal treatment for individuals with impending kidney failure, and US transplantation policy requires a glomerular filtration rate (GFR) less than or equal to 20 ml/min for activation on the kidney transplant waitlist for all patients.1 This contrasts with a higher rate of chronic kidney disease (CKD) progression among persons of color.2–6 Because commonly used creatinine-based equations for estimated glomerular filtration rate (eGFR) incorporate a race term that assigns higher eGFR to Black patients,7,8 there has been significant concern that use of these equations could lead to delayed waitlisting, thereby contributing to racial disparities in access to kidney transplantation.9–11
The majority of clinical laboratories report eGFR alongside serum creatinine results using either the Modification of Diet in Renal Disease (MDRD) or CKD-Epidemiology Collaboration (CKD-EPI) equation,12 both of which incorporate race terms resulting in 21% or 16% higher eGFR respectively for Black individuals compared to non-Black individuals.7,8 Often, these are reported as two separate estimates: one eGFR for if the patient is Black (eGFRBlack) and another for if the patient is non-Black (eGFRnon-Black). Although the race term was motivated by increased precision and decreased statistical bias in GFR estimation,13 there has been a push to remove race (Black versus non-Black) from the calculation of eGFR out of concern that the higher eGFR may delay access to various aspects of kidney care such as kidney transplantation, as well as the ethical considerations of using race, which is not a biological construct, in a model that overtly drives clinical decisions.9,14 Removal of the race term could lead to earlier activation on the kidney transplant waitlist, allowing Black patients to accrue more waiting time prior to starting dialysis and to have a longer window during which preemptive transplantation could occur, potentially allowing patients to avoid dialysis treatment.
Several recent investigations have suggested that removing the race term would allow for substantially earlier waitlist eligibility. Studying the Chronic Renal Insufficiency Cohort (CRIC), a multicenter observational cohort of patients with CKD, Zelnick et al found that among Black study participants starting with an eGFR greater than 20 ml/min/1.73m2, application of the race term (versus its omission) led to a median delay of 1.9 years to achieving an eGFR ≤20 ml/min/1.73m2,15 even if dropping the race term did not necessarily make eGFR estimation more accurate.16 Comparing Black and White participants in CRIC who developed an eGFR ≤20 ml/min/1.73m2 using the CKD-EPI equation, Ku et al found that Black participants had 32% shorter time to reaching kidney failure with replacement therapy (KFRT), and that time to KFRT (i.e., accruable waitlist time before KFRT) might be equalized between White and Black participants by using an adjusted eGFR threshold of 24–25 ml/min/1.73m2 for Black persons.17
Calculating eGFR for Black patients as if they are non-Black would shift an eGFRBlack of 23.2 ml/min/1.73m2 to an eGFRnon-Black of 20 ml/min/1.73m2, thereby allowing transplant listing. However, when the cystatin C-based eGFR, which does not include a race term, was used, Black participants were found to have a 35% shorter time to KFRT, suggesting that disparate rates of CKD progression contributed to the time difference, independent of the use of race in GFR estimation.
To confirm these findings in a larger, real-world population, our objective was to use the OptumLabs® Data Warehouse to examine differences by race on time to KFRT starting from an eGFR threshold of ≤20 ml/min/1.73m2, and to assess the impact of classifying all patients as non-Black for the purpose of GFR estimation on potential waiting time for Black patients.
Methods
Study Design and Population
We conducted a retrospective cohort study using the OptumLabs® Data Warehouse (OLDW), a longitudinal database with de-identified administrative claims and electronic health record (EHR) data.18 The OLDW EHR data asset contains structured data on patient demographics, clinical encounters (including diagnosis codes), clinical observations (e.g., blood pressure), and laboratory results derived from the EHRs of over 55 health systems representing demographically and geographically diverse populations throughout the United States. Because this study involved de-identified, pre-existing data, the University of California, San Francisco Institutional Review Board considered it exempt from approval and requirement for informed consent. We assembled a cohort of patients age 18–75 years having an outpatient eGFR decrease to ≤20 ml/min/1.73m2 from 1/1/2008–12/31/2018, defined as patients having an outpatient eGFR value ≤20 ml/min/1.73m2 during the study period with at least one earlier value between 20 and 60 ml/min/1.73m2 within the preceding two years. We restricted this analysis to eGFR values obtained in the outpatient setting, excluding values obtained during inpatient stays or emergency department visits in order to avoid capturing acute kidney injury episodes. The CKD-EPI equation was used to calculate eGFR using serum creatinine, age, sex, and race.8 Then, using the same criteria but assigning Black persons the non-Black value in the calculation of eGFR, we assembled a cohort of Black patients having a decrease to ≤20 ml/min/1.73m2 calculated as the CKD-EPI eGFRnon-Black value. Patients in this cohort therefore largely overlapped with the subgroup of Black patients of the initially derived cohort (based on eGFRBlack), but would be expected to include more patients who reached an eGFR ≤20 ml/min/1.73m2 earlier during the study period. The date of the incident eGFR ≤20 ml/min/1.73m2 defined the index date for each patient. EHR data from the source health systems may not fully capture clinical information for patients who receive only limited or episodic care within that health system. Thus, we restricted our study to patients having at least two outpatient visits recorded in the data during the year prior to the index date, in order to capture patients likely to have ongoing follow up care within the health system. We then excluded patients having KFRT (dialysis or kidney transplant) prior to their index date. Dialysis and kidney transplant were identified by International Classification of Diseases (ICD) or Current Procedural Terminology (CPT) codes.
Variables
Race/ethnicity was the primary exposure variable and was obtained from electronic health records. We focused our analysis on comparing outcomes for non-Hispanic (NH) White, NH Black, and Hispanic patients. A one-year lookback period was used to identify diagnosis codes, laboratory values, blood pressure measurements, and medication prescriptions to define baseline characteristics. Hypertension was defined as use of anti-hypertensive medications, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg (using the outpatient blood pressure nearest the index date). Diabetes was defined as hemoglobin A1c ≥6.5% (using the value closest to the index date) or use of diabetes medications.
The primary outcome was KFRT, defined as above. In the OLDW, death was ascertained using a combination of electronic health record, death master file, and claims data.
Statistical Analysis
Baseline characteristics were described for the study cohort by race/ethnicity. We computed the cumulative incidence of KFRT by race/ethnicity starting from the incident eGFR ≤20 ml/min/1.73m2 (also computed when classifying Black patients as non-Black), with censoring for loss to follow up, and treating death as a competing risk. In the OLDW, loss to follow up was defined by the date of the most recent EHR data available for each patient. Our primary analysis was unadjusted, given that the focus of our analysis was on actual differences in progression from an eGFR ≤20 ml/min/1.73m2 to KFRT. We included both Black cohorts (using the eGFRnon-Black or eGFRBlack), in order to compare progression to KFRT between the two and assess the impact of classifying Black patients as non-Black for the purpose of GFR estimation on the opportunity for pre-emptive kidney transplantation and pre-dialysis waiting time accrual. Subsequently, in order to determine whether differences persisted after accounting for potential confounding factors, we examined associations between race/ethnicity and time to KFRT using Fine and Gray models adjusted for age and sex (Model 1), then additionally for insurance type, hypertension, diabetes mellitus, coronary artery disease, heart failure, cerebrovascular disease, cancer (excluding non-melanoma skin cancers), and dementia (Model 2).19 Missing data on insurance type (8.8%) was handled using multiple imputation. Because of the substantial overlap between the Black cohorts derived with or without their assignment as non-Black for GFR estimation, for multivariable analyses we included only the Black cohort derived by assigning Black patients as non-Black to assess for persistent disparities in KFRT.
As a secondary analysis, we examined eGFR slopes surrounding 20 ml/min/1.73m2 by race/ethnicity, given that changing how eGFR is calculated will affect pre-KFRT time in a manner dependent on the rate of eGFR decline near the threshold of interest. We computed the slope of eGFR for each individual based on repeated eGFR measurements surrounding the incident eGFR ≤20 ml/min/1.73m2, using outpatient values within a window of 2 years before and after, and excluding eGFR values obtained after the onset of KFRT. We compared the median and interquartile range (IQR) of eGFR slopes for White, Black, and Hispanic patients, assessing differences using Kruskal-Wallis testing. Subsequently, using eGFR slopes observed for Black patients, we estimated the time delay that would be experienced if waitlist eligibility were based on an eGFRBlack (versus eGFRnon-Black) value of 20 ml/min/1.73m2. This time delay was estimated by dividing 3.2 by the eGFR slope (in ml/min/1.73m2 per year), where 3.2 is the amount by which the inclusion of race raises eGFR (0.16 * 20 = 3.2 ml/min/1.73m2).
All analyses were conducted using R version 4.0 (R Foundation for Statistical Computing).
Results
We identified 52,130 patients (40,042 NH White, 8,519 NH Black, 3,569 Hispanic) meeting inclusion criteria with an outpatient eGFR decline to ≤20 ml/min/1.73m2; derivation of the study population is shown in Figure 1. When we repeated the cohort derivation assigning Black patients the eGFRnon-Black value, we identified a cohort of 11,269 Black patients having outpatient eGFRnon-Black decline to ≤20 ml/min/1.73m2 during the study period.
Figure 1. Derivation of study population.
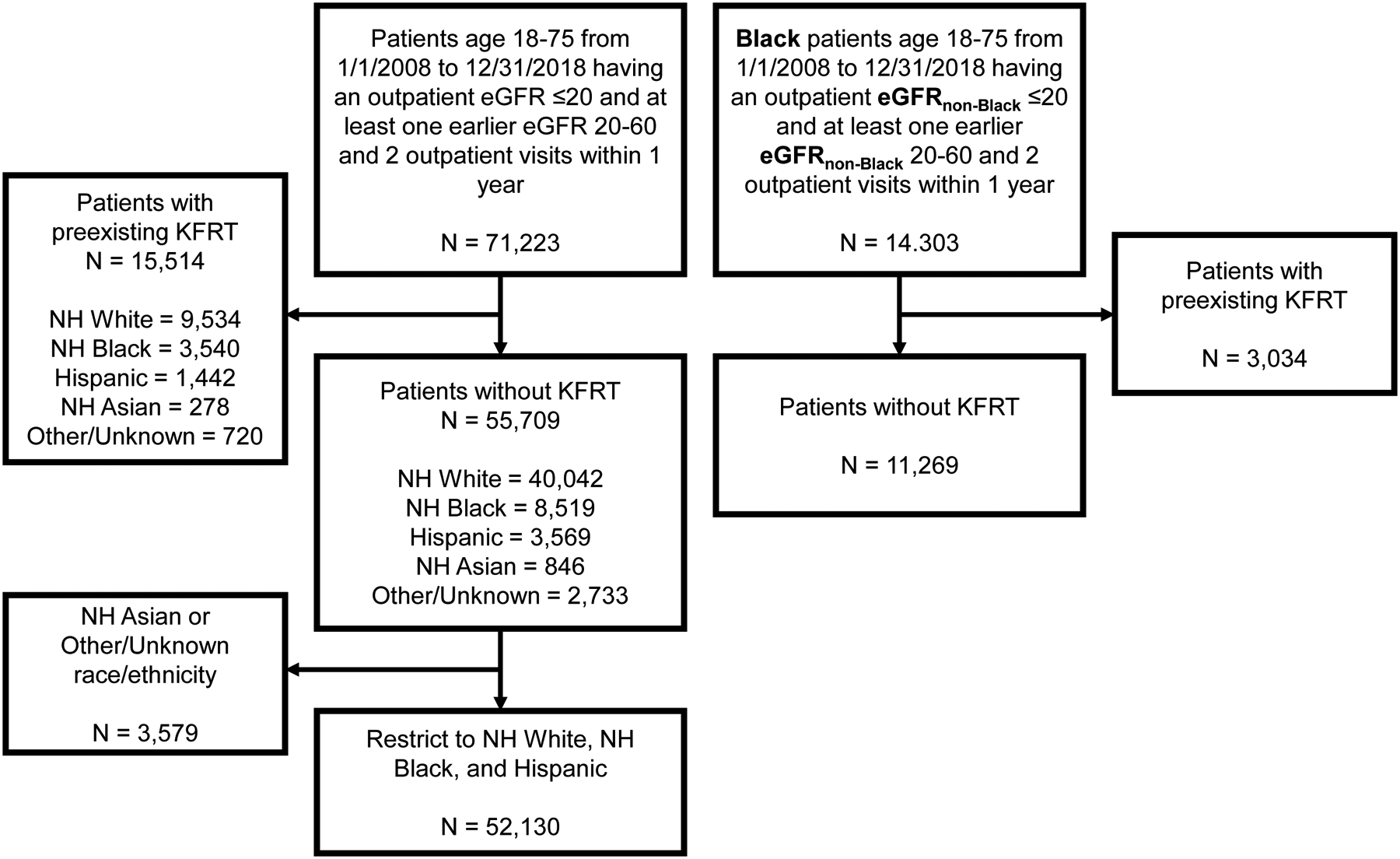
Abbreviations: eGFR = estimated glomerular filtration rate; KFRT = kidney failure with replacement therapy; NH = non-Hispanic.
Demographic and clinical characteristics of the included study population at baseline are shown in Table 1. At the time of incident eGFR ≤20 ml/min/1.73m2, NH White patients were older (mean age 64 years) compared to NH Black or Hispanic patients, who were 3–6 years younger on average. NH White patients on average had lower blood pressure compared to NH Black and Hispanic patients (mean blood pressure 127/70 vs 135/76 and 135/73 mmHg, respectively). The median UACR was higher among NH Black (672 mg/g; IQR [76, 2303]) and Hispanic patients (247 mg/g; IQR [6, 2413]) compared to NH White patients (133 mg/g; IQR [26, 1096]).
Table 1.
Baseline characteristics of patients with eGFR decline to ≤20 ml/min/1.73m2 by race/ethnicity
| Characteristic | Non-Hispanic White | Non-Hispanic Black | Hispanic | |
|---|---|---|---|---|
| Based on eGFRBlack | Based on eGFRnon-Black | |||
| n | 40042 | 8519 | 11269 | 3569 |
| Age (years) | 64 (9) | 61 (11) | 61 (11) | 58 (11) |
| Female Sex | 21873 (54.6) | 4945 (58.0) | 6586 (58.4) | 1831 (51.3) |
| Insurance type | ||||
| Commercial | 26393 (73.2) | 5685 (71.1) | 7545 (71.3) | 1624 (50.7) |
| Medicare | 6763 (18.8) | 1071 (13.4) | 1408 (13.3) | 429 (13.4) |
| Medicaid | 2663 (7.4) | 1176 (14.7) | 1537 (14.5) | 923 (28.8) |
| Uninsured | 95 (0.3) | 40 (0.5) | 50 (0.5) | 177 (5.5) |
| Other | 125 (0.3) | 27 (0.3) | 42 (0.4) | 47 (1.5) |
| Hypertension | 36183 (90.4) | 8010 (94.0) | 10589 (94.0) | 3343 (93.7) |
| Systolic blood pressure (mmHg) | 127 (23) | 135 (26) | 134 (26) | 135 (27) |
| Diastolic blood pressure (mmHg) | 70 (13) | 76 (15) | 76 (15) | 73 (14) |
| ACEi or ARB use | 23545 (58.8) | 5531 (64.9) | 7549 (67.0) | 2547 (71.4) |
| Diabetes mellitus | 21678 (54.1) | 5171 (60.7) | 6752 (59.9) | 2621 (73.4) |
| Hemoglobin A1c (%) | 7.0 (2.1) | 7.2 (1.9) | 7.2 (2.0) | 7.5 (2.0) |
| Hyperlipidemia | 21580 (53.9) | 4409 (51.8) | 5819 (51.6) | 2026 (56.8) |
| Statin use | 23927 (59.8) | 5487 (64.4) | 7275 (64.6) | 2420 (67.8) |
| eGFR (ml/min/1.73m2) | 17 [15, 19] | 17 [15, 19] | 17 [15, 19] | 17 [15, 19] |
| Median creatinine measurements per year* | 4 [2, 8] | 3 [2, 6] | 3 [2, 6] | 4 [2, 7] |
| UACR (mg/g) | 133 [26, 1096] | 672 [76, 2303] | 396 [42, 1942] | 247 [6, 2413] |
| Coronary artery disease | 11527 (28.8) | 1866 (21.9) | 2528 (22.4) | 801 (22.4) |
| Heart failure | 10719 (26.8) | 2491 (29.2) | 3316 (29.4) | 776 (21.7) |
| Cerebrovascular disease | 2190 (5.5) | 737 (8.7) | 927 (8.2) | 219 (6.1) |
| Cirrhosis | 1785 (4.5) | 250 (2.9) | 337 (3.0) | 245 (6.9) |
| Dementia | 486 (1.2) | 107 (1.3) | 124 (1.1) | 22 (0.6) |
| Cancer, excluding non-melanoma skin cancer | 5492 (13.7) | 879 (10.3) | 1242 (11.0) | 246 (6.9) |
Values for categorical variables are given as n (%) and for continuous variables as mean (standard deviation) or median [interquartile range].
Data were missing for insurance type in n = 5,579 (8.8%); blood pressure in n = 6,782 (10.7%); hemoglobin A1c in n = 18,469 (29.1%); UACR in n = 41,358 (65.2%).
Median creatinine measurements per year is based on the number of outpatient values each patient had in the year prior to their index date.
Abbreviations: ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; eGFR = estimated glomerular filtration rate; UACR = urine albumin/creatinine ratio.
Over the follow up period (median 24 months), there were 18,002 KFRT events (9,401 among NH White patients, 3,411 among NH Black patients with the eGFRBlack value, 3,718 among NH Black patients with the eGFRnon-Black value, and 1,472 among Hispanic patients). The number of deaths was 13,532 among NH White patients, 1,849 among NH Black patients derived with the eGFRBlack value, 2,577 among NH Black patients derived with the eGFRnon-Black value, and 490 among Hispanic patients. Outcome event rates are shown in Table S1.
Compared to White patients, NH Black (regardless of how their race was classified in GFR estimation) and Hispanic patients were substantially more likely to progress to KFRT (Figure 2). By 3 years of follow up, the risk of KFRT among NH Black (with the eGFRBlack value) and Hispanic cohorts was 36.0% (95% CI 34.9–37.0%) and 40.9% (95% CI 39.0–42.7%), respectively, compared to NH White patients with a KFRT risk of 20.5% (95% CI 20.0–20.9%). This disparity was modestly attenuated by using the eGFRnon-Black value, with KFRT risk of 28.7% (95% CI 27.8–29.6%) over 3 years. In unadjusted analyses, NH Black (based on eGFRnon-Black) and Hispanic patients respectively had a 1.51-fold (95% CI, 1.46 to 1.56) and 2.25-fold (95% CI, 2.13 to 2.38) increased hazard of KFRT (Table 2). After multivariable adjustment, the increased risk of KFRT was attenuated but remained statistically significantly among NH Black (HR 1.28; 95% CI, 1.15 to 1.43) and Hispanic patients (HR 1.66; 95% CI, 1.18 to 2.31). In the secondary analysis examining eGFR decline, the median eGFR slope for White patients was −4.2 ml/min/1.73m2 per year [IQR −8.9 to −0.6], which was slower relative to CKD progression in NH Black (−6.4 ml/min/1.73m2 per year; IQR [−11.8 to −2.6]) and Hispanic patients (−7.6 ml/min/1.73m2 per year; IQR [−14.3 to −3.2]). Differences between racial/ethnic groups were statistically significant (p <0.001). For Black patients, the potential time delay between an eGFRnon-Black versus an eGFRBlack of 20 ml/min/1.73m2 based on the eGFR slopes was a median of 0.5 years [IQR 0.27, 1.23].
Figure 2. Cumulative incidence of kidney failure with replacement therapy (dialysis or transplant) by race/ethnicity starting from first outpatient eGFR ≤20 ml/min/1.73m2.
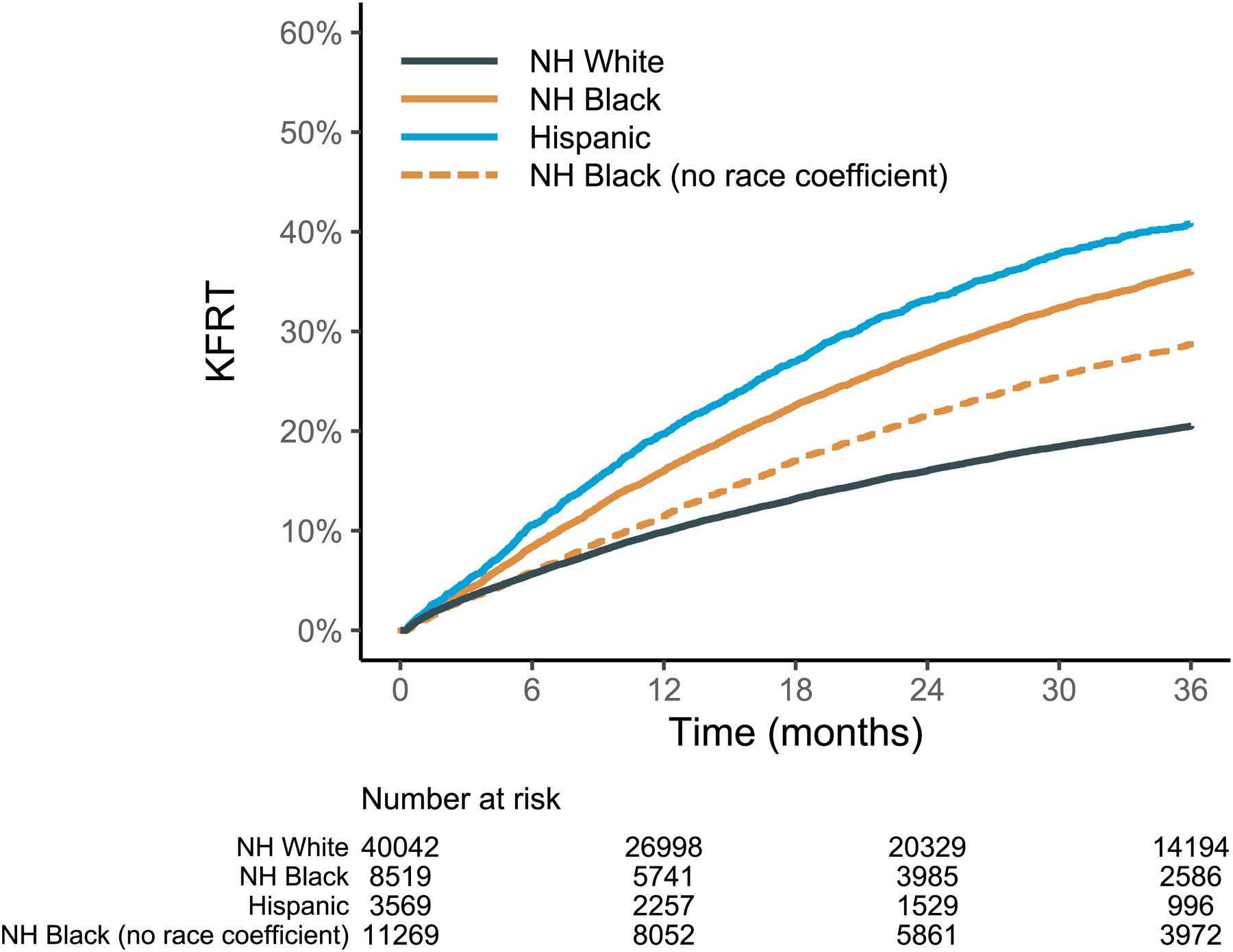
Abbreviations: eGFR = estimated glomerular filtration rate; KFRT = kidney failure with replacement therapy; NH = non-Hispanic.
Table 2.
Hazard ratios (95% confidence intervals) for time to KFRT and mortality
| Kidney Failure with Replacement Therapy | |||
|---|---|---|---|
| Cohort | Unadjusted | Model 1 | Model 2 |
| NH White | Reference | ||
| NH Black | 1.51 (1.46, 1.56) | 1.34 (1.31, 1.39) | 1.28 (1.15, 1.43) |
| Hispanic | 2.25 (2.13, 2.38) | 1.80 (1.71, 1.90) | 1.66 (1.18, 2.31) |
Model 1 is adjusted for age and sex.
Model 2 is adjusted for age, sex, hypertension, diabetes mellitus, coronary artery disease, heart failure, cirrhosis, dementia, cancer (not including non-melanoma skin cancer), and insurance type.
P for all hazard ratios was <0.001.
Abbreviations: KFRT = kidney failure with replacement therapy; NH = non-Hispanic.
Discussion
In a large cohort of patients with incident eGFR ≤20 ml/min/1.73m2, we found that Black and Hispanic patients had substantially faster progression to KFRT compared to White patients. In the cohort of Black patients with incident eGFRnon-Black ≤20 ml/min/1.73m2, we still observed a greater hazard of KFRT compared to White patients, a disparity that persisted even when adjusted for age and comorbidities. While using the eGFRnon-Black value could lead to substantially earlier waitlist eligibility for many Black patients, a large disparity remains in the time window available for transplantation to pre-empt dialysis due to disparities in the rate of CKD progression.
The finding of faster CKD progression to KFRT among Black and Hispanic patients compared to White patients starting from incident eGFR ≤20 ml/min/1.73m2 suggests that even if there were no delays in any steps in the pretransplant process leading up to waitlisting, Black and Hispanic patients (compared to White patients) would still have less time on average to receive a kidney transplant before starting dialysis, and would have less waiting time accrued upon starting dialysis. This is particularly concerning as it represents only the “tip of the iceberg”, compounding well-documented racial disparities occurring at various steps in the process of attaining a kidney transplant, including elicitation of patient preferences, identification of potential living donors, transplant referral, transplant evaluation, and preemptive waitlisting—in addition to the disparities that persist after dialysis initiation, when the majority of transplants occur.20–23
Our results showing disparities in CKD progression from an eGFR ≤20 ml/min/1.73m2, as well as meaningfully earlier waitlisting for many patients that would result from use of the eGFRnon-Black value for Black patients, confirm the findings of the prior studies using CRIC in a broader cohort of over 50,000 patients and shed additional insight on recent investigations of the impact of incorporating race into GFR estimation.15,17 Our findings suggest that while classifying all patients as non-Black for the purpose of GFR estimation would not completely equalize the time from reaching an eGFR of 20 ml/min/1.73m2 to KFRT, it would still lead to earlier waitlisting for Black patients: a difference estimated to be a median of 6 months, and for 25% of Black patients, a difference of greater than 1.23 years (14.8 months). Policies and interventions to narrow this disparity in pre-dialysis accruable waiting time would represent major progress towards equity in transplant access, though we should note that disparities in kidney transplant are complex and multifactorial, and not likely to be fully remedied by any single change in isolation. Furthermore, we found substantial disparities in CKD progression among Hispanic patients—a group who would not derive the benefit of earlier waitlisting from a change in the use of race in eGFR equations.
The recent reassessment of the use of race in eGFR equations has underscored kidney transplantation as a longstanding and major healthcare disparity for patients with CKD. While the use (and misuse) of race in clinical prediction equations has rightly been under intense scrutiny,14,24 our results suggest that with respect to barriers to kidney transplant, the impact of race in eGFR equations is outweighed by more alarming disparities in CKD progression, a disparity that also impacts Hispanic populations who are not ostensibly disadvantaged by variables used in eGFR calculation. Given the disparities in the rate of CKD progression, it is unlikely that any purely GFR-based approach to the timing of pre-emptive waitlisting, including race-neutral ones such as cystatin C or measured GFR, will effectively remedy disparities in transplantation. For example, allowing patients receiving dialysis to backdate their waitlist time to when they had an eGFR of ≤15 ml/min/1.73m2 may have the effect of adding more time for White patients than Black or Hispanic patients due to differences in CKD progression.11 In addition, Ku et al found in CRIC that time to KFRT starting from a cystatin C-based eGFR of 20 ml/min/1.73m2 was 35% shorter for Black participants compared to White, suggesting that the time disparity is not solely attributable to the incorporation of race into GFR estimation.17 Furthermore, a potential consequence of classifying all patients as non-Black for the purpose of GFR estimation could be earlier dialysis initiation among Black patients, as the timing of KFRT may be partly based on eGFR in clinical practice. Persistent disparities in other steps of transplant access raise the concern that Black patients may not receive the full benefit of earlier waitlisting, as national data from 2019 show that only 22% of waitlisted Black patients were listed preemptively, compared to 48% of waitlisted White patients.11 Meanwhile, the change in eGFR calculation may stimulate earlier dialysis initiation among Black patients, counteracting the added time from earlier achievement of the 20 ml/min/1.73m2 threshold. These issues highlight more general concerns about unintended consequences stemming from using eGFR for decision making when the intended basis of the decision is risk of kidney failure and not glomerular filtration. As an alternative to eGFR, use of risk thresholds based on the Kidney Failure Risk Equation, a widely validated prediction model for kidney failure,25,26 has been shown to provide more precise estimates of time to KFRT compared to eGFR.27 As the nephrology community now has well-validated prognostic models for prediction of KFRT risk,28–30 there should be less reason for continued reliance on eGFR alone as a surrogate for risk. However, a challenge for application of prediction models will be how to accurately capture the sizable racial/ethnic differences in CKD progression while not using race/ethnicity as an input. As race is a social and not a biological construct, its application in clinical prediction models is highly problematic (e.g., assignment of race which often depends on individual interpretation and setting, handling of mixed race, and the potential for systematic discrimination).31,32 Aside from prognostic models for kidney failure risk, using individuals’ observed rate of eGFR decline would be a potential alternative for improving equitable pre-KFRT waiting time. While this approach has the advantage of being highly individualized, being based on patients’ actual clinical data, it also requires that adequate historical data are available. An additional challenge is that eGFR decline may not reliably predict time to KFRT because of non-linear eGFR trajectories of CKD progression.33,34 We note that while no criteria (eGFR, kidney failure prediction model, or eGFR decline) will perfectly predict time to KFRT, some criteria might allow for more equitable estimation of time to KFRT, which should be a goal in and of itself in policy implementation.
Strengths of this study included a large, diverse study population with longitudinal laboratory data enabling identification of incident eGFR ≤20 ml/min/1.73m2 in a manner representative of typical clinical testing patterns. Limitations included inability to exclude individuals who would not be kidney transplant candidates if comprehensively evaluated. Episodes of acute kidney injury could have been misclassified as incident eGFR ≤20 ml/min/1.73m2 events, even with our restriction to outpatient laboratory values. KFRT was identified by diagnostic and procedure codes that while likely specific, may not be fully sensitive.35,36 EHR data may be incomplete if patients do not exclusively receive care within one health system; under-ascertainment of outcomes for this reason would bias our results to overestimate delay in eligibility. Our analysis estimating the potential delay in waitlisting assumed that eligibility would be based on the eGFRBlack for Black patients. However, some transplant centers accept the eGFRnon-Black value for waitlisting of Black patients. We did not examine a re-expressed eGFR equation where race is not considered. Finally, we did not examine the impact of disparate CKD progression surrounding other eGFR thresholds pertinent to advanced CKD care, such as dialysis initiation.
In summary, in a large cohort of patients with incident eGFR ≤20 ml/min/1.73m2, we found substantially more rapid progression to KFRT among Black and Hispanic patients compared to White patients, suggesting that using a threshold of eGFR ≤20 ml/min/1.73m2 may contribute to inequitable opportunity for pre-emptive transplant and pre-dialysis waiting time accrual among Black and Hispanic patients. While classifying all patients as non-Black for the purpose of GFR estimation is associated with a substantially earlier waitlist eligibility for many Black patients, a large disparity remained in the window available for pre-emptive transplantation due to disparities in the rate of CKD progression. These disparities are unlikely to be remedied by better eGFR equations. Future work should investigate the role of waitlisting eligibility based on alternative criteria such as kidney failure risk, rather than eGFR, as a means to advance equity in access to kidney transplantation.
Supplementary Material
Table S1. Unadjusted event rates by race/ethnicity
Support:
Dr. Chu was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award (F32DK122629). The funders of this study had no role in the design of this study; collection, analysis, or interpretation of data; writing the report; or the decision to submit this report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Supplemental Materials Table of Contents
References
- 1.Organ Procurement and Transplantation Network Policies. Accessed December 13, 2020. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
- 2.Peralta CA, Katz R, DeBoer I, et al. Racial and Ethnic Differences in Kidney Function Decline among Persons without Chronic Kidney Disease. J Am Soc Nephrol. 2011;22(7):1327–1334. doi: 10.1681/ASN.2010090960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Beukel TO, de Goeij MCM, Dekker FW, Siegert CEH, Halbesma N, PREPARE Study Group. Differences in progression to ESRD between black and white patients receiving predialysis care in a universal health care system. Clin J Am Soc Nephrol. 2013;8(9):1540–1547. doi: 10.2215/CJN.10761012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derose SF, Rutkowski MP, Crooks PW, et al. Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis. 2013;62(2):236–244. doi: 10.1053/j.ajkd.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsa A, Kao WHL, Xie D, et al. APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease. N Engl J Med. 2013;369(23):2183–2196. doi: 10.1056/NEJMoa1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suarez J, Cohen JB, Potluri V, et al. Racial Disparities in Nephrology Consultation and Disease Progression among Veterans with CKD: An Observational Cohort Study. J Am Soc Nephrol. 2018;29(10):2563–2573. doi: 10.1681/ASN.2018040344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann Intern Med. 1999;130(6):461. doi: 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA. 2019;322(2):113. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed S, Nutt CT, Eneanya ND, et al. Examining the Potential Impact of Race Multiplier Utilization in Estimated Glomerular Filtration Rate Calculation on African-American Care Outcomes. J Gen Intern Med. 2020;36(2):464–471. doi: 10.1007/s11606-020-06280-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reese PP, Mohan S, King KL, et al. Racial disparities in preemptive wait-listing and deceased donor kidney transplantation: Ethics and solutions. Am J Transplant. 21(3):958–967. doi: 10.1111/ajt.16392 [DOI] [PubMed] [Google Scholar]
- 12.Miller WG, Jones GRD. Estimated Glomerular Filtration Rate; Laboratory Implementation and Current Global Status. Adv Chronic Kidney Dis. 2018;25(1):7–13. doi: 10.1053/j.ackd.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Tighiouart H, Titan SM, Inker LA. Estimation of Glomerular Filtration Rate With vs Without Including Patient Race. JAMA Intern Med. 2020;180(5):793–795. doi: 10.1001/jamainternmed.2020.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powe NR. Black Kidney Function Matters: Use or Misuse of Race? JAMA. 2020;324(8):737–738. doi: 10.1001/jama.2020.13378 [DOI] [PubMed] [Google Scholar]
- 15.Zelnick LR, Leca N, Young B, Bansal N. Association of the Estimated Glomerular Filtration Rate With vs Without a Coefficient for Race With Time to Eligibility for Kidney Transplant. JAMA Netw Open. 2021;4(1). doi: 10.1001/jamanetworkopen.2020.34004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu C-Y, Yang W, Go AS, Parikh RV, Feldman HI. Analysis of Estimated and Measured Glomerular Filtration Rates and the CKD-EPI Equation Race Coefficient in the Chronic Renal Insufficiency Cohort Study. JAMA Netw Open. 2021;4(7):e2117080. doi: 10.1001/jamanetworkopen.2021.17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku E, McCulloch CE, Adey DB, Li L, Johansen KL. Racial Disparities in Eligibility for Preemptive Waitlisting for Kidney Transplantation and Modification of eGFR Thresholds to Equalize Waitlist Time. J Am Soc Nephrol. 2021;32(3):677–685. doi: 10.1681/ASN.2020081144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OptumLabs. OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. Eden Prairie, MN: n.p., July 2020. PDF. Reproduced with permission from OptumLabs [Google Scholar]
- 19.Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 20.King KL, Husain SA, Jin Z, Brennan C, Mohan S. Trends in Disparities in Preemptive Kidney Transplantation in the United States. Clin J Am Soc Nephrol. 2019;14(10):1500–1511. doi: 10.2215/CJN.03140319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Melanson TA, Plantinga LC, et al. Racial/Ethnic Disparities in Waitlisting for Deceased Donor Kidney Transplantation One year after Implementation of the New National Kidney Allocation System. Am J Transplant. 2018;18(8):1936–1946. doi: 10.1111/ajt.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gander JC, Zhang X, Plantinga L, et al. Racial Disparities in Preemptive Referral for Kidney Transplantation in Georgia. Clin Transplant. 2018;32(9):e13380. doi: 10.1111/ctr.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monson RS, Kemerley P, Walczak D, Benedetti E, Oberholzer J, Danielson KK. Disparities in completion rates of the medical pre-renal transplant evaluation by race/ethnicity and gender. Transplantation. 2015;99(1):236–242. doi: 10.1097/TP.0000000000000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diao JA, Inker LA, Levey AS, Tighiouart H, Powe NR, Manrai AK. In Search of a Better Equation - Performance and Equity in Estimates of Kidney Function. N Engl J Med. 2021;384(5):396–399. doi: 10.1056/NEJMp2028243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangri N, Stevens LA, Griffith J, et al. A Predictive Model for Progression of Chronic Kidney Disease to Kidney Failure. JAMA. 2011;305(15):1553–1559. doi: 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 26.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grams ME, Li L, Greene TH, et al. Estimating Time to ESRD Using Kidney Failure Risk Equations: Results From the African American Study of Kidney Disease and Hypertension (AASK). Am J Kidney Dis. 2015;65(3):394–402. doi: 10.1053/j.ajkd.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramspek CL, Evans M, Wanner C, et al. Kidney Failure Prediction Models: A Comprehensive External Validation Study in Patients with Advanced CKD. J Am Soc Nephrol. 2021;32(5):1174–1186. doi: 10.1681/ASN.2020071077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramspek CL, de Jong Y, Dekker FW, van Diepen M. Towards the best kidney failure prediction tool: a systematic review and selection aid. Nephrol Dial Transplant. 2019;35(9):1527–1538. doi: 10.1093/ndt/gfz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Inker LA, Goyal N. Promoting Equity in Eligibility for Registration on the Kidney Transplantation Waiting List: Looking beyond eGFRcr. J Am Soc Nephrol. 2021;32(3):523–525. doi: 10.1681/ASN.2020121802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulus JK, Kent DM. Predictably unequal: understanding and addressing concerns that algorithmic clinical prediction may increase health disparities. NPJ Digit Med. 2020;3(1):1–8. doi: 10.1038/s41746-020-0304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulus JK, Kent DM. Race and Ethnicity: A Part of the Equation for Personalized Clinical Decision Making. Circ Cardiovasc Qual Outcomes. 2017;10(7):e003823. doi: 10.1161/CIRCOUTCOMES.117.003823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caravaca-Fontán F, Azevedo L, Luna E, Caravaca F. Patterns of progression of chronic kidney disease at later stages. Clin Kidney J. 2018;11(2):246–253. doi: 10.1093/ckj/sfx083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang A, Kramer H. CKD progression: a risky business. Nephrology Dialysis Transplantation. 2012;27(7):2607–2609. doi: 10.1093/ndt/gfs095 [DOI] [PubMed] [Google Scholar]
- 35.Taneja C, Berger A, Inglese GW, et al. Can Dialysis Patients Be Accurately Identified Using Healthcare Claims Data? Perit Dial Int. 2014;34(6):643–651. doi: 10.3747/pdi.2012.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grams ME, Plantinga LC, Hedgeman E, et al. Validation of CKD and Related Conditions in Existing Datasets: A Systematic Review. Am J Kidney Dis. 2011;57(1):44–54. doi: 10.1053/j.ajkd.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Unadjusted event rates by race/ethnicity


