Abstract
Purpose
To assess the 12-month outcomes of intravitreal faricimab (IVF) in treatment-resistant neovascular age-related macular degeneration (nAMD) subjects recalcitrant to intravitreal aflibercept (IVA).
Methods
This study was conducted as a retrospective interventional case series of nAMD patients receiving treatment at a single private practice institution. All included patients at baseline had undergone six or more IVA injections over the previous 12 months, four or more IVA injections over the previous 6 months, had a central macular thickness (CMT) ≥320 µm on optical coherence tomography (OCT), and were observed to have intraretinal and/or subretinal fluid on OCT assessment. The baseline exam for the purpose of this study was considered the visit in which the patient was switched from IVA to IVF. Patients were managed with a real-world treat-and-extend protocol and followed over a 12-month study period.
Results
A total of 54 eyes of 54 subjects were analyzed. Overall, 31.5% (17/54) of subjects attained a treatment interval ≥8 weeks and had a fluid-free macula on OCT at 12 months. The CMT on OCT decreased from 395.4 (383.5–407.3) µm at baseline to 350.0 (335.1–364.8) µm at the end of the 12-month study interval (p<0.01). There were 16.7% (9/54) of subjects who gained three or more lines of Snellen visual acuity at the end of the study. Visual acuity improved from 0.72 (0.67–0.77) logMAR (Snellen 20/105) at baseline to 0.59 (0.55–0.64) logMAR (Snellen 20/78) at the end of the study (p<0.01).
Conclusion
A clinically significant minority of aflibercept-resistant nAMD subjects may attain longer treatment intervals and improved outcomes at 12 months after switching to IVF when a treat-and-extend treatment protocol is utilized. IVF use may be considered whenever resistance to IVA is encountered in this patient population.
Keywords: faricimab, treatment-resistant, neovascular age-related macular degeneration, recalcitrance
Introduction
The introduction of intravitreal anti–VEGF treatments has resulted in considerable improvements in outcomes for patients with neovascular age-related macular degeneration (nAMD).1,2 However, a variety of limitations to anti-VEGF treatment in the management of nAMD exist, including the constraint of repetitive treatments and insufficient response in a significant subset of patients. Even in a stringent clinical trial setting, such as the CATT trial, 53%–79% of subjects had intraretinal and/or subretinal fluid on optical coherence tomography (OCT) and 13%–17% had lost more than five letters of visual acuity at the 12-month follow-up.3 Therefore, novel delivery systems and treatment modalities continue to be developed to target other mediators implicated in angiogenesis in the hope of further improving treatment response, especially in the subset of nAMD subjects resistant to other anti-VEGF therapies.
One such novel treatment modality is faricimab (Vabysmo; Roche/Genentech, Basel, Switzerland). It is a bispecific antibody that targets both VEGFA and Ang2 and was granted approval by the US Food and Drug Administration in 2022 for the treatment of nAMD based on the outcomes of the two phase III trials LUCERNE and TENAYA.4 In these key trials evaluating treatment-naïve nAMD patients,4 78% of patients undergoing intravitreal faricimab (IVF) injections at ≥12-week treatment intervals had noninferior best-corrected visual acuity compared to patients undergoing intravitreal aflibercept (IVA; Eylea/Regeneron, NY, USA) injections at fixed 8-week treatment intervals at the 12-month follow-up visit. However, a notable weakness in the trial design of LUCERNE and TENAYA is that these studies did not allow patients randomized to the IVA group to extend beyond 8 weeks between injections regardless of the clinical course, whereas patients in the IVF group followed a prespecified treat-and-extend protocol. Consequently, patients undergoing IVF at an interval of ≥12 weeks cannot be compared to patients undergoing IVA at an interval of ≥12 weeks. Furthermore, since LUCERNE and TENAYA excluded all patients that were not treatment-naïve, it would be invalid to generalize the results of these trials to nAMD populations who have formerly undergone treatment with other anti-VEGF agents. The author previously reported the short-term (4-month) benefits of changing nAMD patients from IVA to IVF when treatment resistance was experienced,5 and a few other real-world studies with short-term follow-up (3–6 months) have reported modest benefits with IVF when anti-VEGF resistance was encountered.6,7 In this study, the author reports 1-year outcomes in this same treatment-resistant nAMD patient population using an OCT-guided protocol in a real-world setting.
Methods
This retrospective chart review clinical study was undertaken according to the principles of the Helsinki Declaration and complied with the Health Insurance Portability and Accountability Act of 1996. Institutional Review Board approval was obtained from the Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-12). Informed consent was waived because the information was gathered retrospectively and all subject data that could be identifying were omitted. Study subjects received treatment from February 2022 until June 2023 at a single private practice institution in Amarillo, TX.
The inclusion criteria were: 1) the patient was being actively treated for the diagnosis of nAMD, 2) a treat-and-extend protocol was utilized, 3) the patient had received six or more IVA injections during the 12 months prior to baseline, 4) the patient had received four or more IVA injections during the 6 months prior to baseline, 5) the patient had ≥320 µm central macular thickness (CMT) on OCT with identifiable intraretinal and/or subretinal fluid at baseline, 6) the patient’s Snellen visual acuity ranged from 20/25 to 20/200, 7) the patient had 12 months of follow-up after being switched from IVA to IVF, and 8) the patient remained exclusively on IVF during the study period.
The exclusion criteria were: 1) the patient had undergone an ocular procedure other than anti-VEGF treatment within 6 months of baseline or during the research interval (eg, cataract surgery, glaucoma filtration surgery, pars plana vitrectomy), 2) the patient had an underlying condition considered by the examining specialist to be responsible for a reduction of two or more Snellen lines of acuity not related to the diagnosis of nAMD (eg, diabetic retinopathy, glaucoma, cataract, epiretinal membrane), or 3) the patient was lost from intended/scheduled follow-up for a period of ≥4 weeks during the study interval.
For the purpose of this study, the baseline evaluation was the appointment at which the decision was made to change the subject from IVA to IVF. The treat-and-extend protocol utilized during the study period was based principally on the appearance of retinal edema and has been formerly reported by the same author.5 In short, all subjects at baseline received an IVF loading dose consisting of monthly (28–34 days) treatments for a total of three injections. After these had been administered, subjects would be extended if all observable intraretinal and/or subretinal edema on OCT was eliminated. Subjects continued to undergo monthly IVF treatments until the edema was resolved and would continue receiving monthly injections indefinitely if the retinal edema could not be resolved. Once the retinal edema on OCT reabsorbed, patients were extended to intervals of 1–2 weeks until reappearance of the retinal edema on OCT was identifiable. The interval of treatment was then adjusted accordingly to maintain a fluid-free retina. OCT was conducted utilizing the Heidelberg Spectralis system (Heidelberg Engineering, Heidelberg, Germany). Baseline and 12-month follow-up OCT images were assessed by two masked retina specialists for the appearance of intraretinal and/or subretinal edema. If and when an incongruity occurred, a third masked retina specialist made the deciding determination. If both eyes of the same study patient met the above inclusion/exclusion criteria, simple randomization (a random number–generating program) selected which eye would be involved in the study.
Primary and Secondary Outcomes
The main outcome of the study was the number of subjects who had attained a treatment interval ≥8 weeks and had a fluid-free macula on OCT at the end of the 12-month study period. The secondary outcome was the number of subjects gaining three or more lines of Snellen visual acuity at the end of the 12-month study period.
Statistical Analysis
JMP 17 software was used to conduct statistical analysis. One-way ANOVA and likelihood ratios were employed to evaluate for statistical significance at the α<0.05 level. Snellen visual acuity was converted to logMAR for statistical analysis.
Results
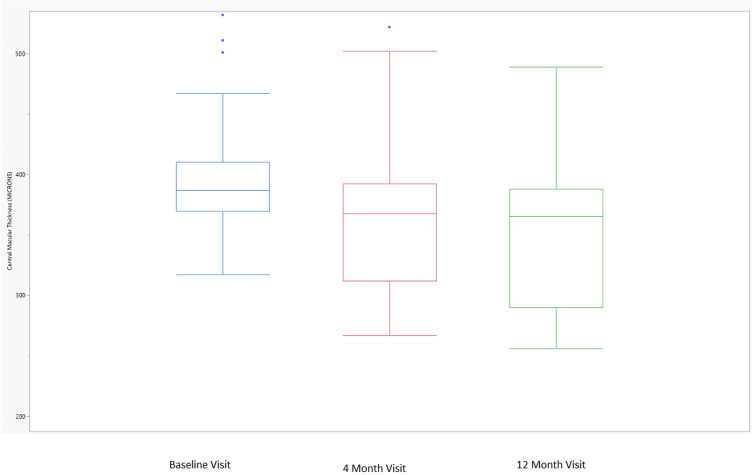
There were a total of 54 eyes of 54 subjects included in the study’s analysis. The research population’s baseline characteristics are presented in Table 1. The agreement rate between the two OCT reviewers was 90.7% (49/54). There were 31.5% (17/54) of subjects who attained a treatment interval ≥8 weeks and had a fluid-free macula on OCT at the end of the study. There were 5.6% (3/54) of subjects on a 12-week treatment interval, 42.6% (23/54) on a treatment interval of 5–7 weeks, and 25.9% (14/54) still on a 4-week treatment interval at the end of the study. Of the 14 subjects still on a 4-week treatment interval at the end of the study, 85.7% (12/14) had received monthly injections for the entire study period without attaining a fluid-free macula on OCT, whilst 14.3% (2/14) at one time had attained a fluid-free macula on OCT but failed to extend beyond 4 weeks without fluid recurrence. The average treatment interval at the end of the study was 6.3 (5.7–6.9) weeks. The overall study population’s CMT on OCT had decreased from 395.4 (383.5–407.3) µm at baseline to 350.0 (335.1–364.8) µm at the end of the study (p<0.01). Figure 1 displays the distributions of CMT on OCT from baseline to 4 months to 12 months. As displayed, the mean 4-month CMT on OCT was similar to the mean 12-month CMT on OCT in the study population. When CMT on OCT decreased by ≥50 µm after the first three IVF treatments, 76.5% (13/17) of subjects were able to attain a treatment interval of ≥8 weeks and have a fluid-free macula on OCT at the end of the study.
Table 1.
Faricimab for aflibercept-resistant neovascular age-related macular degeneration: baseline characteristics of the study population (n=54, means with (95% confidence intervals)
| Age (years) | 75.7 (73.5–77.9) |
| Sex | Female 26 (48.1%) |
| Male 28 (51.9%) | |
| Lens status | Pseudophakic 44 (81.5%) |
| Phakic 10 (18.5%) | |
| Number of anti-VEGF injections prior to the study | 17.3 (15.2–19.4) |
| Central macular thickness on optical coherence tomography (µm) | 395.4 (383.5–407.3) |
| Visual acuity (logMAR) | 0.72 (0.67–0.77) |
Figure 1.
Central macular thickness over time. The box plot displays the median (line inside the box); interquartile range (the 25th percentile is the bottom of the box and the 75th percentile is the top of the box); minimum and maximum observations within 1.5 × the interquartile range (upper and lower fences extending from the box); and outliers larger than 1.5 × the interquartile range (single dots).
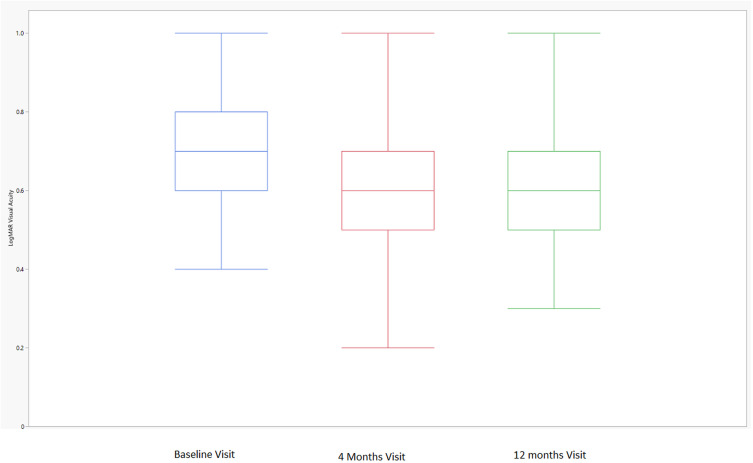
There were 16.7% (9/54) of subjects who gained three or more lines of Snellen visual acuity at the end of the study. Of the nine subjects who gained three or more lines of Snellen visual acuity at 12 months, 88.9% (8/9) of subjects were able to attain a treatment interval of ≥8 weeks and have a fluid-free macula on OCT at the end of the study. Of the nine subjects who had gained three or more lines of Snellen visual acuity at 12 months, only 55.6% (5/9) attained this improvement after the first three IVF injections. There were 3.7% (2/54) of subjects who attained the three or more lines of Snellen visual acuity improvement after the first three IVF injections, but no longer maintained this improvement at the end of the study. The overall study population’s visual acuity improved from 0.72 (0.67–0.77) logMAR (Snellen 20/105) at baseline to 0.59 (0.55–0.64) logMAR (Snellen 20/78) at the end of the study (p<0.01). Figure 2 displays the distributions of visual acuity from baseline to 4 months to 12 months. As displayed, the mean 4-month visual acuity was similar to the mean 12-month visual acuity in the study population. A case example of a subject effectively switched from IVA to IVF is presented in Figure 3.
Figure 2.
LogMAR visual acuity over time. The box plot displays the median (line inside the box); interquartile range (the 25th percentile is the bottom of the box and the 75th percentile is the top of the box); and minimum and maximum observations within 1.5 × the interquartile range (upper and lower fences extending from the box).
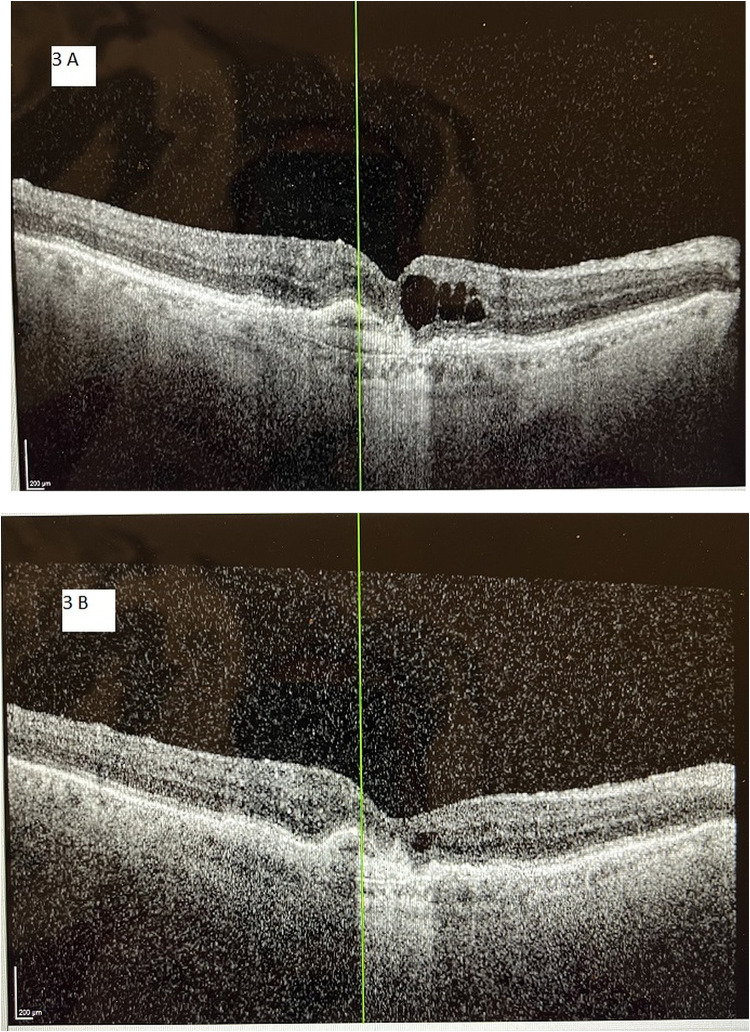
Figure 3.
A 76-year-old male with neovascular age-related macular degeneration and persistent macular fluid, despite 11 aflibercept injections during the previous 12 months and a total of 35 anti-VEGF treatments over the previous 3 years. The vertical line (green) in each image orients the reader to the same position in the macula between images. (A) Optical coherence tomography image at baseline demonstrates diffuse intraretinal macular fluid. Central macular thickness was 340 µm and Snellen visual acuity was 20/100. (B) Optical coherence tomography image of the same subject after switching from aflibercept to faricumab and receiving seven faricumab treatments over 12 months. Central macular thickness decreased to 268 µm and Snellen visual acuity improved to 20/60. The intraretinal macular edema is notably reduced from baseline, and now is only a trace amount. The subject was able to be extended to a 7-week treatment interval at the end of the 12-month study period.
Discussion
Repetitive anti-VEGF injections over long periods of time may result in immunoreactivity and tachyphylaxis with a diminishing therapeutic effect,8–10 and changing to another medication may sometimes overcome this problem.11 Aflibercept became an alternative treatment option in nAMD patients recalcitrant to other anti-VEGF medications since its introduction >10 years ago. A meta-analysis confirmed that switching to aflibercept from other anti-VEGF agents can result in improved outcomes when treatment resistance is experienced.12 However, little guidance currently exists on what to do if and when aflibercept recalcitrance occurs in this patient population.
To the author’s awareness, this is the first study to report outcomes as far out as 1 year in recalcitrant nAMD patients switched to faricimab from aflibercept (or any other anti-VEGF agent) due to treatment resistance. We assessed functional and anatomical responses by measuring Snellen visual acuity and CMT on OCT before and after the switch from aflibercept to faricimab, and these factors directly influenced the patient’s interval of treatment as per the treat-and-extend protocol employed. In the recalcitrant nAMD subjects meeting our study’s primary outcome (about 30%), fewer intravitreal injections were able to be given over 12 months without compromising the subject’s functional and/or anatomical outcomes. Of particular note, the mean reduction in CMT on OCT and the mean improvement in Snellen visual acuity in the study population was observed by 4 months and remained steady throughout the 12-month study period. This study employed a real-world treat-and-extend protocol typical of what most specialists who treat nAMD prefer,13,14 thereby allowing our results to be clinically useful to other specialists considering use of faricimab when aflibercept resistance is encountered.
In comparison to other anti-VEGF medications, aflibercept has an increased affinity for binding VEGF and it binds placental growth factor.15 However, faricimab has advantages over aflibercept, which include a higher affinity for binding VEGFA and additionally blocking Ang2, which is integral in the process of neovascularization.16,17 Although only about 30% of subjects were able to be extended to ≥8 weeks and just about 17% had gained three or more lines of Snellen visual acuity at 12 months, all subjects included in this study had been actively treated for ≥12 months and on average had received 17 anti-VEGF treatments prior to switching to faricumab. With this in mind, it may be inferred that the lack of a clinically meaningful response in a majority of the subjects after the switch to faricumab could have been the result of permanent retinal atrophic changes and photoreceptor loss, even before the switch in anti-VEGF agents occurred. Published research does imply that long-term functional outcomes are best when all lingering and residual retinal edema is dried up.18 In that circumstance, a shorter delay before switching to faricumab may have led to better anatomical and functional outcomes in our aflibercept-recalcitrant nAMD subjects. However, in the 30% of aflibercept-resistant nAMD subjects who were able to extend to ≥8 weeks between faricumab injections using the study’s treat-and-extend protocol, all three of the involved parties benefited: the patient (fewer doctor appointments), the physician (freeing up availability to treat other patients needing care), and society (reduced expense to the public insurance payer).
Limitations of this study include the retrospective design, the lack of a control group, and the application of logMAR vision rather than ETDRS scoring. Study strengths comprise the moderately large number of subjects included considering faricimab’s newness, the relatively long follow-up (12 months) considering faricimab’s newness, and the real-world environment utilizing a treat-and-extend protocol familiar to most treating physicians. In conclusion, a significant minority of aflibercept-resistant nAMD subjects who switch to faricimab may achieve longer treatment intervals without compromising anatomical and/or visual outcomes after 1 year when a real-world treat-and-extend protocol is employed. Additional research is warranted to corroborate this conclusion.
Funding Statement
There is no funding to report.
Abbreviations
nAMD, neovascular age-related macular degeneration; CMT, central macular thickness; OCT, optical coherence tomography; VA, visual acuity.
Data Sharing
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics
The study was approved by the Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-12) in accordance with the ethical standards laid down in the Declaration of Helsinki. Informed consent from study participants was waived because this was a retrospective study with no identifying patient information presented.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 3.Martin DF, Maguire MG, Ying GA, et al; CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi: 10.1016/S0140-6736(22)00010-1 [DOI] [PubMed] [Google Scholar]
- 5.Rush RB, Rush SW. Intravitreal faricimab for aflibercept-resistant neovascular age-related macular degeneration. Clin Ophthalmol. 2022;16:4041–4046. doi: 10.2147/OPTH.S395279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung EH, Oh DJ, Alderson SE, et al. Initial real-world experience with in treatment-resistant neovascular age-related macular degeneration. Clin Ophthalmol. 2023;17:1287–1293. doi: 10.2147/OPTH.S409822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanani AM, Aziz AA, Khan H, et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: the TRUCKEE study - 6 month results. Eye. 2023. doi: 10.1038/s41433-023-02553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina. 2009;29(6):723–731. doi: 10.1097/IAE.0b013e3181a2c1c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keane PA, Liakopoulos S, Ongchin SC, et al. Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(7):3115–3120. doi: 10.1167/iovs.08-1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008;115(12):2199–2205. doi: 10.1016/j.ophtha.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 11.Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012;96(1):14–20. [DOI] [PubMed] [Google Scholar]
- 12.Seguin-Greenstein S, Lightman S, Tomkins-Netzer O. A meta-analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant neovascular age-related macular degeneration following switching to treatment with aflibercept. J Ophthalmol. 2016;2016:1–8. doi: 10.1155/2016/4095852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh A, Lanzetta P, Lee WK, et al. Recommended guidelines for use of intravitreal aflibercept with a treat-and-extend regimen for the management of neovascular age-related macular degeneration in the Asia-Pacific region: report from a consensus panel. Asia Pacific J Ophthalmol. 2017;6(3):296–302. [DOI] [PubMed] [Google Scholar]
- 14.Ross AH, Downey L, Devonport H, et al. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye. 2020;34(10):1825–1834. doi: 10.1038/s41433-019-0747-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferro Desideri L, Traverso CE, Nicolò M. The emerging role of the Angiopoietin-Tie pathway as therapeutic target for treating retinal diseases. Expert Opin Ther Targets. 2022;26(2):145–154. doi: 10.1080/14728222.2022.2036121 [DOI] [PubMed] [Google Scholar]
- 17.Sahni J, Dugel PU, Patel SS, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: the AVENUE Phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138(9):955–963. doi: 10.1001/jamaophthalmol.2020.2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DM, Tuomi L, Shapiro H. Anatomical measures as predictors of visual outcomes in ranibizumab-treated eyes with neovascular age-related macular degeneration. Retina. 2013;33:23–34. doi: 10.1097/IAE.0b013e318263cedf [DOI] [PubMed] [Google Scholar]