Abstract
Few studies have assessed the association between endogenous steroid hormone levels and a subsequent diagnosis of endometriosis. We prospectively evaluated premenopausal plasma sex hormone levels and the risk of laparoscopically confirmed endometriosis in a nested case-control study within Nurses’ Health Study II. Between blood collection (1996–1999) and 2009, we ascertained 446 women with incident endometriosis and matched them to 878 controls through risk-set sampling. We conducted multivariable conditional logistic regression accounting for matching and confounders to estimate relative risks (RRs) and 95% confidence intervals (CIs). Women with greater early follicular-phase total or free estradiol levels had a nonlinear increased risk of endometriosis (early follicular total estradiol: second quartile vs. first, RR = 2.23 (95% CI: 1.44, 3.47); third quartile, RR = 1.83 (95% CI: 1.16, 2.88); fourth quartile, RR = 1.68 (95% CI: 1.05, 2.68); early follicular free estradiol: second quartile vs. first, RR = 1.63 (95% CI: 1.05, 2.54); third quartile, RR = 2.02 (95% CI: 1.31, 3.12); fourth quartile, RR = 1.04 (95% CI: 0.66, 1.65)). Free testosterone assessed in quartile categories was not associated with endometriosis, although a threshold effect was observed, with a positive association among women in the top 2% of free testosterone levels. Levels of mid–luteal-phase total and free estradiol, follicular and luteal estrone, total testosterone, progesterone, and sex hormone binding globulin were not associated with endometriosis risk. These results support the role of sex steroids in endometriosis etiology, although the relationships suggest complex threshold effects.
Keywords: endometriosis, estradiol, progesterone, prospective studies, testosterone
Abbreviations
- BMI
body mass index
- CI
confidence interval
- NHS II
Nurses’ Health Study II
- RR
relative risk
- SHBG
sex hormone binding globulin
Endometriosis is the presence of endometrial-like tissue in locations outside the uterine cavity, and it affects 6%–10% of reproductive-age women (1, 2). Common signs and symptoms include chronic pelvic pain, dysmenorrhea, dyspareunia, and reduced fertility (1). One of the main theories regarding endometriosis pathogenesis postulates that the disease originates from retrograde menstruation of endometrial tissue into the peritoneal cavity that meets a milieu enhanced for attachment to the peritoneum, invasion of the epithelium, and proliferation (3). However, retrograde menstruation occurs in 76%–90% of all women (4), while endometriosis prevalence is much lower, suggesting that other factors are necessary for endometriosis development (1).
Epidemiologic and biological evidence support the classification of endometriosis as a hormone-dependent disease (5, 6). Endometriosis is prevalent among women of reproductive age but is rarely diagnosed among premenarcheal girls and postmenopausal women (6). Estrogen is the best-defined mitogen for the growth and inflammatory processes in endometriotic tissue, whereas aromatase inhibitors that block estrogen formation lead to lesion regression (6, 7). Progesterone inhibits the mitogenic action of estrogen and enhances endometrium differentiation; however, resistance to progesterone action in the presence of endometriosis is frequently observed (7, 8). Moreover, progestin-based treatments for endometriosis are variably effective (9). Less is known about the role of testosterone in endometriosis pathogenesis. Hyperandrogenic states induce atrophy of the endometrium, leading to the use of androgens in endometriosis treatment (10). Additionally, recent hypotheses have postulated that low testosterone levels in utero may be associated with endometriosis development through changes in the hypothalamic-pituitary-ovarian axis resulting in altered endogenous sex hormone levels in adulthood (11).
Although steroid hormones affect endometriosis tissue growth in experimental models (12, 13) and treatment with exogenous hormones affects lesion volume and endometriosis-related symptoms (14–16), no human data directly relating prediagnosis systemic endogenous levels to endometriosis risk have been reported. We conducted a prospective nested case-control study within the Nurses’ Health Study II (NHS II) cohort to examine the associations of premenopausal early follicular-phase and mid–luteal-phase estrogen, testosterone, progesterone, and sex hormone binding globulin (SHBG) levels with the risk of subsequent laparoscopically confirmed endometriosis.
METHODS
Study population and data collection
NHS II is a prospective cohort study with 116,429 registered female nurses who were aged 25–42 years and resided in 14 US states at enrollment in 1989. Participants completed a detailed questionnaire at baseline, and they complete biennial follow-up questionnaires regarding disease incidence and a variety of biological, reproductive, environmental, dietary, and behavioral factors. This research was approved by the Institutional Review Board of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health (Boston, Massachusetts).
Establishment of the nested blood cohort has been described previously (17). In brief, blood samples were collected between 1996 and 1999 from 29,611 NHS II cohort members who were aged 32–54 years at blood collection. Premenopausal women who had not taken hormones and had not been pregnant or breastfed within the past 6 months (n = 18,521) provided timed blood samples on day 3–5 of their menstrual cycle (early follicular phase) and 7–9 days before the anticipated start of their next cycle (mid–luteal phase). All other women (n = 11,090) provided a single untimed (random) blood sample and may have been using hormones at the time of their blood draw. Samples were shipped with an ice pack via overnight courier to our laboratory, where they were processed and have been stored in continuously monitored liquid nitrogen freezers since collection. Quality control testing has confirmed a lack of degradation across time for plasma sex steroid hormone levels (18).
Participants completed a blood-draw questionnaire that recorded the date and time of blood collection and information on current weight, parity, smoking status, alcohol and medication use, hours since last food intake, recent changes in menstrual cycle characteristics, and the first day of the menstrual cycle in which the blood samples were drawn. Each woman returned a postcard to indicate the first day of her next menstrual cycle following blood collection. We included only the timed blood samples for measurement of estradiol and estrone, levels of which vary through the menstrual cycle; we restricted the data to luteal-phase samples for progesterone, because it is mainly produced during the luteal phase; and we included both luteal and untimed samples for testosterone and SHBG, because cyclical variations in testosterone and SHBG levels are minimal (19).
Case ascertainment and analytical definition
In 1993, participants were first asked whether they had ever had physician-diagnosed endometriosis. If the answer was yes, they reported when the diagnosis had occurred and whether it had been confirmed by laparoscopy. These questions were asked in each subsequent questionnaire. Endometriosis cases were restricted to women who reported laparoscopic confirmation of their diagnosis. As previously reported, medical record case validation for self-reported laparoscopically confirmed endometriosis within this cohort is 97% (20, 21). Among the 29,611 women with blood samples, 525 were diagnosed with laparoscopically confirmed endometriosis at least 1 year after blood draw through 2009 and had plasma samples assayed for endogenous hormones. We excluded women who were not successfully matched to controls due to matching criteria (n = 17), did not have a valid measurement for any hormone (n = 2), or were postmenopausal or had an unknown menopausal status at blood draw (n = 60). The final analytical data set included 446 endometriosis cases.
Control selection
Women with prior cancer (except nonmelanoma skin cancer), women who had had a hysterectomy in any cycle before or during the cycle in which a case was diagnosed, or women who did not have a sufficient plasma volume (≤1.8 mL) were not eligible to serve as controls. For each incident case of laparoscopically confirmed endometriosis, up to 2 controls (n = 878) were randomly selected from the risk set who were of the same race/ethnicity (White, Asian, African-American, Hispanic, other) and age (±1 year) and had the same infertility history (ever/never) and menopausal status at diagnosis (premenopausal/postmenopausal/unknown). Cases and controls were also matched on the month (±1 month) and time of day (±2 hours) of blood draw and fasting status (<2, 2–4, 5–7, 8–11, or >12 hours of fasting) at blood draw.
Assessment of exposure
Estradiol, estrone, and testosterone levels were assayed at Mayo Medical Laboratories (Rochester, Minnesota) by liquid chromatography–tandem mass spectrometry (ThermoFisher Scientific (Franklin, Massachusetts) and Applied Biosystems/MDS Sciex (Foster City, California)). Progesterone and SHBG levels were assayed at the laboratory of Dr. Nader Rifai (Boston Children’s Hospital, Boston, Massachusetts) using a competitive electrochemiluminescence immunoassay on the Roche E Modular system (Roche Diagnostics, Indianapolis, Indiana). The hormone levels of cases and controls ascertained during the 1999–2005 questionnaire cycles (first selection) were measured in 2007, and the hormone levels of cases and controls in the 2007–2009 questionnaire cycles (second selection) were measured in 2009 in the same laboratories using the same methods. The overall intraassay coefficients of variation were 10% or less for each of the hormones in the 2 batches. To account for potential laboratory drift over time, we adjusted all hormone levels for batch using the methods described by Rosner et al. (22).
Free estradiol and free testosterone levels were calculated using the methods of Sodergard et al. (23). When calculating follicular free estradiol values, concentrations of SHBG or testosterone from luteal samples were used if follicular concentrations were missing. A high correlation between follicular free estradiol levels calculated using either timed follicular SHBG and testosterone or luteal SHBG and testosterone was observed in a subset of the NHS II blood cohort (correlation = 0.97; n = 603) (24).
An earlier study was conducted to assess the reproducibility of sex hormone levels within each woman over a period of 3 years (25). Intraclass correlation coefficients for hormones ranged from 0.29 (progesterone) to 0.83 (follicular and luteal SHBG), indicating that a single hormone measurement provides a reasonable representation of hormone levels over at least 3 years.
Statistical analysis
Quartile cutpoints for hormone levels were defined using plasma levels of the controls and were determined separately for the follicular and luteal phases for estrogens.
Conditional logistic regression models accounting for matching were used to estimate relative risks (RRs) and 95% confidence intervals (CIs). We further adjusted for endometriosis risk factors, including birth weight, age at menarche, body mass index (BMI; weight (kg)/height (m)2) at age 18 years and at blood draw, number of pregnancies greater than 6 months in duration, total duration of breastfeeding, time since last birth, age at first birth, smoking history, oral contraceptive use, alcohol consumption, Alternative Healthy Eating Index score (tertiles), physical activity, menstrual cycle length at ages 18–22 years, adult menstrual cycle length, menstrual cycle pattern at ages 18–22 years, and adult menstrual cycle pattern. We additionally considered adjusting for SHBG, testosterone, and progesterone levels in models with follicular total and free estradiol and estrone, luteal total and free estradiol and estrone, total testosterone, and free testosterone (adjusted for SHBG only); however, these adjustments had no substantial impact on the RRs and were not included in the final models (data not shown). Tests for trend were conducted by modeling the quartile median concentrations and calculating the Wald statistic. We used restricted cubic splines with multivariable conditional logistic regression to nonparametrically examine possible nonlinear relationships between levels of each hormone and the RR of endometriosis, deleting data points with hormone levels greater than or equal to the 99th percentile. Spline variables created using 21 knots were chosen through stepwise selection, with the P value for entry and retention set at less than 0.05. We used the likelihood ratio test to evaluate nonlinearity, comparing the model with only the linear term to the model with the linear and cubic spline terms.
We conducted several sensitivity analyses, including exclusion of women with anovulatory menstrual cycles, defined by progesterone levels less than 4 ng/mL, to evaluate heterogeneity by anovulation. We restricted analyses of follicular total and free estradiol to women with follicular estradiol levels below 80 pg/mL, since women with follicular estradiol levels greater than or equal to 80 pg/mL may be in perimenopause. Because the delay between symptom onset and endometriosis diagnosis is 7 years, on average, we excluded endometriosis cases diagnosed within 2, 4, or 6 years after blood collection to address the possibility that undiagnosed endometriosis cases at the time of blood draw might have affected the hormone levels. We examined whether the associations between hormones and endometriosis risk varied by age at blood draw (<40 years vs. ≥40 years), age at endometriosis diagnosis (<45 years vs. ≥45 years), BMI at age 18 years (<20 vs. ≥20), BMI at blood draw (<25 vs. ≥25), infertility history at endometriosis diagnosis (ever vs. never), and parity (parous vs. nulliparous) and smoking history (ever vs. never) reported in 1995. Because we observed that results utilizing unconditional logistic regression adjusting for matching factors (see Web Table 1, available at https://doi.org/10.1093/aje/kwac219) were similar to those for conditional logistic regression, we used unconditional logistic regression adjusting for matching factors in stratified analyses. We tested the statistical significance of the interactions via the likelihood ratio test. All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). All P values presented are 2-sided.
RESULTS
Study population characteristics
From the 1996–1999 blood draw through the 2009 questionnaire cycle, we ascertained 446 incident laparoscopically confirmed endometriosis cases and 878 matched controls. Age-standardized characteristics were compared between cases and controls (Table 1). The mean age at blood collection was 41 years for cases (range, 32–52 years) and 41 years for controls (range, 32–51 years). The median time between blood collection and endometriosis diagnosis was 5 years (interquartile range, 4–8 years; data not shown). The majority of the study population was White. Overall, cases had an earlier age at menarche, were more likely to be nulliparous, had a shorter breastfeeding duration among parous women, were less likely to smoke, and consumed less alcohol compared with controls. Median levels of total and free estradiol, estrone, total and free testosterone, progesterone, and SHBG were in the expected ranges for premenopausal women and were similar between cases and controls (Table 2).
Table 1.
Age-Standardized Characteristics of Endometriosis Case Subjects and Matched Control Subjects in Nurses’ Health Study II, 1996–2009a–c
| Participant Characteristic | Cases (n = 446) | Controls (n = 878) | ||
|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | |
| Age, yearsd | 41.1 (4.1) | 41.4 (4.0) | ||
| White race/ethnicity | 97 | 97 | ||
| BMIe at age 18 years | 21.2 (3.3) | 21.2 (3.2) | ||
| BMI at blood collection | 25.9 (5.9) | 25.5 (6.0) | ||
| History of infertility | 26 | 27 | ||
| Ever use of OCs | 82 | 85 | ||
| Current use of OCsf | 4 | 2 | ||
| Age at menarche, years | ||||
| <12 | 25 | 20 | ||
| 12 | 34 | 32 | ||
| >12 | 42 | 48 | ||
| No. of pregnancies >6 months long | ||||
| 0 (nulliparous) | 27 | 20 | ||
| 1–2 | 51 | 51 | ||
| ≥3 | 22 | 29 | ||
| Duration of breastfeeding, monthsg | ||||
| 0 (none) or <1 | 16 | 12 | ||
| 1–11 | 32 | 32 | ||
| 12–23 | 32 | 28 | ||
| ≥24 | 20 | 27 | ||
| Never smoker | 72 | 67 | ||
| AHEI 2010 scoreh | 48.9 (10.5) | 48.8 (10.8) | ||
| Alcohol intake, g/day | 3.0 (5.3) | 3.8 (7.4) | ||
| Physical activity, MET-hours/weeki | 18.8 (21.0) | 17.9 (21.0) | ||
Abbreviations: AHEI, Alternative Health Eating Index; BMI, body mass index; MET, metabolic equivalent of task; OC, oral contraceptive; SD, standard deviation.
a Values are standardized to the age distribution of the study population.
b Women with laparoscopically confirmed cases of endometriosis were matched 1:2 with controls of comparable age, race/ethnicity, infertility history, and menopausal status at diagnosis, as well as month, time of day, and fasting status at blood draw.
c Some participants were missing information on BMI at age 18 years (4 cases, 7 controls), BMI at blood draw (10 cases, 19 controls), AHEI 2010 score (45 cases, 80 controls), alcohol intake (45 cases, 80 controls), physical activity (4 cases, 16 controls), ever use of OCs (2 cases, 1 control), current use of OCs (3 cases, 1 control), age at menarche (2 cases, 4 controls), number of pregnancies (5 cases, 3 controls), or breastfeeding duration among parous women (1 case, 7 controls).
d Value is not age-adjusted.
e Weight (kg)/height (m)2.
f Current OC users were among women with untimed blood samples. Per eligibility criteria, participants who provided follicular and luteal samples for the measurement of estradiol and estrone levels had not used hormones within the 6 months preceding blood sample collection.
g Among parous women.
h The AHEI 2010 measures adherence to a diet pattern based on foods and nutrients most predictive of disease risk in the literature.
i Metabolic equivalents from recreational and leisure-time activities.
Table 2.
Endogenous Steroid Hormone Levels for Endometriosis Case Subjects and Matched Control Subjects in Nurses’ Health Study II, 1996–2009a
| Hormone | Cases (n = 446) | Controls (n = 878) | ||
|---|---|---|---|---|
| Median | 10th–90th Percentile Range | Median | 10th–90th Percentile Range | |
| Estradiol, pg/mL | ||||
| Follicular | 40.7 | 22.5–81.5 | 40.4 | 18.2–88.8 |
| Luteal | 131.7 | 80.5–229.5 | 133.5 | 70.4–247.2 |
| Free estradiol, pg/mL | ||||
| Follicular | 0.50 | 0.27–0.89 | 0.49 | 0.24–1.02 |
| Luteal | 1.57 | 0.91–2.74 | 1.63 | 0.90–2.87 |
| Estrone, pg/mL | ||||
| Follicular | 39.8 | 24.8–64.4 | 38.1 | 23.9–65.5 |
| Luteal | 88.4 | 51.6–152.2 | 84.8 | 50.5–155.0 |
| Testosterone, ng/dL | 24.1 | 14.5–40.4 | 24.9 | 15.4–38.6 |
| Free testosterone, ng/dL | 0.19 | 0.09–0.34 | 0.19 | 0.10–0.36 |
| Progesterone, ng/mL | 13.1 | 2.25–22.9 | 13.5 | 1.38–26.2 |
| SHBG, nmol/L | 68.7 | 32.8–125.2 | 68.5 | 33.4–126.0 |
Abbreviation: SHBG, sex hormone binding globulin.
a Some participants were missing information on follicular estradiol (99 cases, 215 controls), luteal estradiol (69 cases, 137 controls), follicular free estradiol (102 cases, 222 controls), luteal free estradiol (69 cases, 138 controls), follicular estrone (99 cases, 211 controls), luteal estrone (68 cases, 137 controls), testosterone (5 cases, 11 controls), free testosterone (21 cases, 45 controls), progesterone (64 cases, 45 controls), or SHBG (19 cases, 126 controls).
Endogenous hormones and endometriosis risk
After adjustment for demographic, anthropometric, reproductive, dietary, and behavioral risk factors, women in the second–fourth quartiles of early follicular total estradiol levels (vs. the first quartile) had higher risk of a subsequent endometriosis diagnosis (second quartile, RR = 2.23 (95% CI: 1.44, 3.47); third quartile, RR = 1.83 (95% CI: 1.16, 2.88); fourth quartile, RR = 1.68 (95% CI: 1.05, 2.68); P for linear trend = 0.26) (Table 3). Women in the second and third quartiles, but not the fourth quartile, of early follicular free estradiol (vs. the first quartile) also had a greater risk of endometriosis (second quartile, RR = 1.63 (95% CI: 1.05, 2.54); third quartile, RR = 2.02 (95% CI: 1.31, 3.12); fourth quartile, RR = 1.04 (95% CI: 0.66, 1.65); P for trend = 0.74). Levels of mid-luteal total and free estradiol, follicular and luteal estrone, total and free testosterone, progesterone, and SHBG were not associated with endometriosis risk in multivariable models (Tables 3 and 4).
Table 3.
Relative Risk of Laparoscopically Confirmed Endometriosis by Quartile of Follicular and Luteal Estradiol, Free Estradiol, and Estrone, and Luteal Progesterone Levels in Nurses’ Health Study II, 1996–2009a
| Follicular Phase | Luteal Phase | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Quartile
Cutpoints |
No. of
Cases |
No. of
Controls |
Base Model b | Multivariate Model c |
Quartile
Cutpoints |
No. of
Cases |
No. of
Controls |
Base Model b | Multivariate Model c | |||||
| Quartile | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||||||
| Estradiol Level, pg/mL | ||||||||||||||
| 1 | <26.6 | 54 | 164 | 1.00 | Referent | 1.00 | Referent | <98.0 | 89 | 186 | 1.00 | Referent | 1.00 | Referent |
| 2 | 26.6–39.9 | 117 | 166 | 2.18 | 1.47, 3.24 | 2.23 | 1.44, 3.47 | 98.0–133.4 | 104 | 180 | 1.24 | 0.86, 1.79 | 1.19 | 0.79, 1.78 |
| 3 | 40.0–58.9 | 93 | 168 | 1.78 | 1.18, 2.67 | 1.83 | 1.16, 2.88 | 133.5–187.1 | 106 | 190 | 1.20 | 0.83, 1.73 | 1.18 | 0.79, 1.77 |
| 4 | ≥59.0 | 83 | 165 | 1.59 | 1.05, 2.40 | 1.68 | 1.05, 2.68 | ≥187.2 | 78 | 185 | 0.90 | 0.61, 1.33 | 0.75 | 0.49, 1.16 |
| P for trendd | 0.28 | 0.26 | 0.38 | 0.11 | ||||||||||
| Free Estradiol Level, pg/mL | ||||||||||||||
| 1 | <0.338 | 64 | 164 | 1.00 | Referent | 1.00 | Referent | <1.22 | 94 | 185 | 1.00 | Referent | 1.00 | Referent |
| 2 | 0.338–0.488 | 98 | 164 | 1.64 | 1.10, 2.44 | 1.63 | 1.05, 2.54 | 1.23–1.62 | 111 | 185 | 1.17 | 0.82, 1.66 | 1.15 | 0.78, 1.69 |
| 3 | 0.489–0.711 | 112 | 164 | 1.87 | 1.27, 2.76 | 2.02 | 1.31, 3.12 | 1.63–2.20 | 84 | 185 | 0.89 | 0.62, 1.29 | 0.76 | 0.50, 1.13 |
| 4 | ≥0.712 | 70 | 164 | 1.11 | 0.73, 1.67 | 1.04 | 0.66, 1.65 | ≥2.21 | 88 | 185 | 0.94 | 0.65, 1.35 | 0.81 | 0.53, 1.22 |
| P for trend | 0.96 | 0.74 | 0.44 | 0.13 | ||||||||||
| Estrone Level, pg/mL | ||||||||||||||
| 1 | <30.0 | 74 | 170 | 1.00 | Referent | 1.00 | Referent | <65.0 | 86 | 184 | 1.00 | Referent | 1.00 | Referent |
| 2 | 30.0–37.9 | 82 | 161 | 1.17 | 0.79, 1.71 | 1.14 | 0.74, 1.75 | 65.0–84.5 | 89 | 185 | 1.05 | 0.72, 1.52 | 1.01 | 0.68, 1.52 |
| 3 | 38.0–48.5 | 87 | 171 | 1.18 | 0.81, 1.71 | 0.97 | 0.63, 1.49 | 84.6–116.9 | 124 | 188 | 1.44 | 1.01, 2.06 | 1.47 | 1.00, 2.17 |
| 4 | ≥48.6 | 104 | 165 | 1.48 | 1.02, 2.15 | 1.36 | 0.90, 2.06 | ≥117.0 | 79 | 184 | 0.95 | 0.64, 1.40 | 0.80 | 0.51, 1.23 |
| P for trend | 0.04 | 0.15 | 0.87 | 0.37 | ||||||||||
| Progesterone Level, ng/mL | ||||||||||||||
| 1 | <7.26 | 106 | 188 | 1.00 | Referent | 1.00 | Referent | |||||||
| 2 | 7.27–13.53 | 92 | 188 | 0.82 | 0.56, 1.21 | 0.79 | 0.52, 1.21 | |||||||
| 3 | 13.54–19.76 | 106 | 188 | 0.92 | 0.63, 1.35 | 0.95 | 0.62, 1.45 | |||||||
| 4 | ≥19.77 | 78 | 188 | 0.67 | 0.45, 1.02 | 0.74 | 0.46, 1.18 | |||||||
| P for trend | 0.10 | 0.31 | ||||||||||||
Abbreviations: CI, confidence interval; MET, metabolic equivalent of task; RR, relative risk.
a Some participants were missing information on follicular estradiol (99 cases, 215 controls), luteal estradiol (69 cases, 137 controls), follicular free estradiol (102 cases, 222 controls), luteal free estradiol (69 cases, 138 controls), follicular estrone (99 cases, 211 controls), luteal estrone (68 cases, 137 controls), or progesterone (64 cases, 45 controls).
b RRs and 95% CIs were estimated by conditional logistic regression conditioned on the matching factors, including age, race/ethnicity, infertility history, and menopausal status at diagnosis, as well as month, time of day, and fasting status at blood draw.
c The multivariate model further adjusted for birth weight (not full-term, <5.5, 5.5–6.9, 7.0–8.4, or ≥8.5 pounds (not full-term, <2.5, 2.5–3.0, 3.1–3.8, or ≥3.9 kg)), age at menarche (<12, 12, or >12 years), body mass index (weight (kg)/height (m)2) at age 18 years (<18.5, 18.5–22.4, 22.5–24.9, or ≥25.0) and at the time of blood collection (<22.5, 22.5–24.9, 25.0–29.9, or ≥30.0), number of pregnancies more than 6 months in duration (0, 1–2, or >2 births), total duration of breastfeeding (nulliparous, 0 or <1, 1–11, 12–23, or ≥24 months), time since last birth (<2, 2–3, 4–5, or ≥6 years), age at first birth (<25, 25–29, 30–34, or ≥35 years), smoking history (never, past, or current smoker), oral contraceptive use (ever or never), alcohol consumption (0, 0–5, or >5 g/day), diet score tertile, physical activity (<3, 3–8, 9–17, or ≥18 MET-hours/week), menstrual cycle length at ages 18–22 years (<26, 26–31, 32–39, or ≥40 days), adult menstrual cycle length (<26, 26–31, or ≥32 days), and menstrual cycle pattern (very regular, regular, or irregular) at ages 18–22 years and in adulthood.
d P values were calculated via a Wald test of a score variable that contained median values of tertiles.
Table 4.
Relative Risk of Laparoscopically Confirmed Endometriosis by Quartile of Testosterone, Free Testosterone, and Sex Hormone Binding Globulin Levels in Nurses’ Health Study II, 1996–2009a
| Quartile |
Quartile
Cutpoints |
No. of
Cases |
No. of
Controls |
Base Model b | Multivariate Model c | ||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | ||||
| Testosterone Level, ng/dL | |||||||
| 1 | <18.4 | 109 | 212 | 1.00 | Referent | 1.00 | Referent |
| 2 | 18.5–24.9 | 144 | 225 | 1.25 | 0.91, 1.70 | 1.31 | 0.94, 1.83 |
| 3 | 25.0–31.9 | 91 | 214 | 0.83 | 0.59, 1.17 | 0.85 | 0.59, 1.23 |
| 4 | ≥32.0 | 97 | 216 | 0.85 | 0.61, 1.20 | 0.89 | 0.61, 1.29 |
| P for trendd | 0.09 | 0.18 | |||||
| Free Testosterone Level, ng/dL | |||||||
| 1 | <0.131 | 106 | 208 | 1.00 | Referent | 1.00 | Referent |
| 2 | 0.132–0.185 | 106 | 208 | 1.00 | 0.72, 1.40 | 0.99 | 0.69, 1.41 |
| 3 | 0.186–0.265 | 108 | 209 | 1.01 | 0.73, 1.40 | 1.04 | 0.72, 1.52 |
| 4 | ≥0.266 | 105 | 208 | 0.99 | 0.70, 1.39 | 0.94 | 0.63, 1.41 |
| P for trend | 0.95 | 0.77 | |||||
| Sex Hormone Binding Globulin Level, nmol/L | |||||||
| 1 | <47.1 | 110 | 209 | 1.00 | Referent | 1.00 | Referent |
| 2 | 47.2–68.3 | 103 | 209 | 0.95 | 0.68, 1.32 | 1.07 | 0.74, 1.56 |
| 3 | 68.4–94.1 | 113 | 211 | 1.03 | 0.75, 1.42 | 1.15 | 0.78, 1.69 |
| 4 | ≥94.2 | 101 | 209 | 0.91 | 0.66, 1.27 | 1.05 | 0.69, 1.59 |
| P for trend | 0.67 | 0.86 | |||||
Abbreviations: CI, confidence interval; MET, metabolic equivalent of task; RR, relative risk.
a Some participants were missing information on testosterone (5 cases, 11 controls), free testosterone (21 cases, 45 controls), and sex hormone binding globulin (19 cases, 126 controls).
b RRs and 95% CIs were estimated by conditional logistic regression conditioned on the matching factors, including age, race/ethnicity, infertility history, and menopausal status at diagnosis, as well as month, time of day, and fasting status at blood draw.
c The multivariate model further adjusted for birth weight (not full-term, <5.5, 5.5–6.9, 7.0–8.4, or ≥8.5 pounds (not full-term, <2.5, 2.5–3.0, 3.1–3.8, or ≥3.9 kg)), age at menarche (<12, 12, or >12 years), body mass index (weight (kg)/height (m)2) at age 18 years (<18.5, 18.5–22.4, 22.5–24.9, or ≥25.0) and at the time of blood collection (<22.5, 22.5–24.9, 25.0–29.9, or ≥30.0), number of pregnancies more than 6 months in duration (0, 1–2, or >2 births), total duration of breastfeeding (nulliparous, 0 or <1, 1–11, 12–23, or ≥24 months), time since last birth (<2, 2–3, 4–5, or ≥6 years), age at first birth (<25, 25–29, 30–34, or ≥35 years), smoking history (never, past, or current smoker), oral contraceptive use (ever or never), alcohol consumption (0, 0–5, or >5 g/day), diet score tertile, physical activity (<3, 3–8, 9–17, or ≥18 MET-hours/week), menstrual cycle length at ages 18–22 years (<26, 26–31, 32–39, or ≥40 days), adult menstrual cycle length (<26, 26–31, or ≥32 days), and menstrual cycle pattern (very regular, regular, or irregular) at ages 18–22 years and in adulthood.
d P values were calculated via a Wald test of a score variable that contained median values of tertiles.
Restricted cubic splines showed similar curvilinear relationships between follicular total and free estradiol and endometriosis risk as quartile-based regression models (P for nonlinearity: for follicular total estradiol, P = 0.002; for follicular free estradiol, P < 0.001) (Figure 1). As follicular total and free estradiol levels increased, the RR of endometriosis initially increased and then decreased as estradiol levels further increased. The wide confidence interval at the tail of the graphs was due to sparse data at high concentrations (Figure 1). Although free testosterone levels were not associated with endometriosis risk in the quartile analyses, restricted cubic splines showed a curvilinear relationship between free testosterone and endometriosis risk (for free testosterone, P for nonlinearity = 0.003) (Figure 2). The RR for free testosterone levels and endometriosis remained null except for the RR at the highest levels of free testosterone when risk of endometriosis increased.
Figure 1.
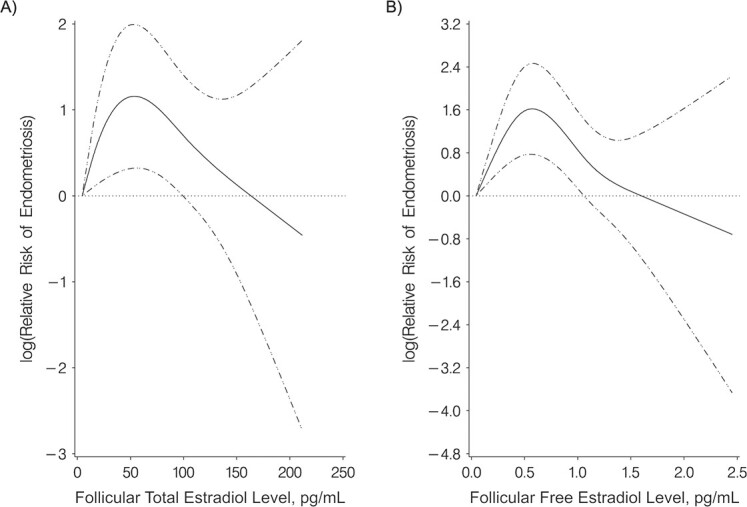
Restricted cubic splines for the relationship of follicular total (A) and free (B) estradiol levels with the risk of laparoscopically confirmed endometriosis (multivariable conditional logistic regression) in Nurses’ Health Study II, 1996–2009. The solid line shows the relative risk of laparoscopically confirmed endometriosis when comparing higher total and free estradiol levels with the minimal levels. Dashed-and-dotted lines show 95% confidence intervals.
Figure 2.
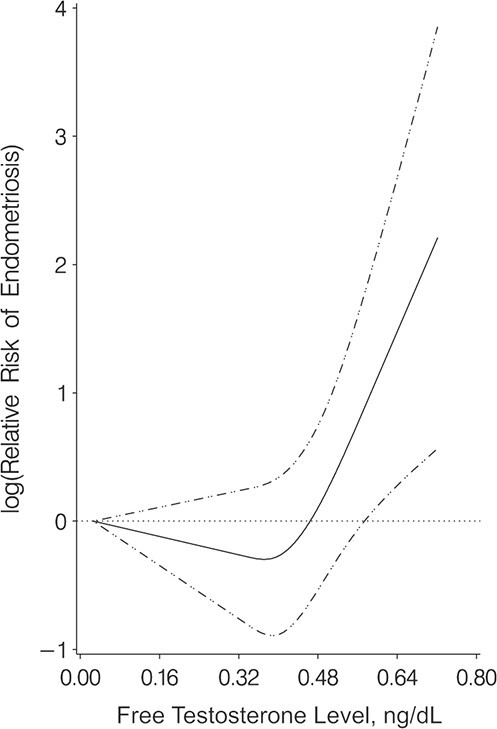
Restricted cubic splines for the relationship between free testosterone level and the risk of laparoscopically confirmed endometriosis (multivariable conditional logistic regression) in Nurses’ Health Study II, 1996–2009. The solid line shows the relative risk of laparoscopically confirmed endometriosis when comparing higher free testosterone levels with the minimal levels. Dashed-and-dotted lines show 95% confidence intervals.
Ovulatory menstrual cycles
When we excluded women with anovulatory menstrual cycles, that is, those with mid-luteal progesterone levels less than 4 ng/mL, the associations for follicular total and free estradiol were attenuated (follicular total estradiol: second quartile, RR = 1.56 (95% CI: 0.94, 2.58); third quartile, RR = 1.25 (95% CI: 0.75, 2.09); fourth quartile, RR = 1.09 (95% CI: 0.63, 1.90) (P for trend = 0.26); follicular free estradiol: second quartile, RR = 1.36 (95% CI: 0.82, 2.25); third quartile, RR = 1.64 (95% CI: 1.00, 2.69); fourth quartile, RR = 0.70 (95% CI: 0.40, 1.21) (P for trend = 0.74); Web Table 2). However, higher progesterone levels were inversely associated with endometriosis risk after the exclusion of anovulatory menstrual cycles (second quartile, RR = 0.48 (95% CI: 0.27, 0.86); third quartile, RR = 0.61 (95% CI: 0.34, 1.10); fourth quartile, RR = 0.44 (95% CI: 0.24, 0.82); P for trend = 0.31).
Stratification
While most of the tests for heterogeneity were nonsignificant, notable differences were observed for the association of luteal total and free estradiol with endometriosis risk by subgroup. A decreasing trend across quartiles of luteal total estradiol was observed among women with a BMI less than 20 at age 18 years (second quartile, RR = 1.62 (95% CI: 0.89, 2.95); third quartile, RR = 1.03 (95% CI: 0.57, 1.88); fourth quartile, RR = 0.67 (95% CI: 0.36, 1.26); P for trend = 0.05); however, no trend was observed among women with a BMI greater than or equal to 20 (P for trend = 0.52; P for heterogeneity = 0.06; Table 5). Similar heterogeneity was observed for luteal free estradiol stratified by BMI at age 18 years (P for heterogeneity = 0.05). Heterogeneity was also observed for free testosterone by BMI at age 18 years, with a suggestion of increased risk of endometriosis among participants with BMI less than 20 at age 18 years but no clear association for BMI greater than or equal to 20 (P for heterogeneity = 0.03). Among women aged 40 years or more at blood draw, associations with endometriosis risk were stronger for follicular total estradiol compared with women aged less than 40 years (P for heterogeneity = 0.07). For luteal free estradiol, heterogeneity was noted for infertility history; however, no discernible patterns were observed across the subgroups (P for heterogeneity = 0.03). We observed heterogeneity for total follicular estrone and smoking status, with the risk of endometriosis being stronger among ever smokers than among never smokers (P for heterogeneity = 0.03), and for luteal total estradiol, with risk of endometriosis being stronger among parous women than among nulliparous women (P for heterogeneity = 0.01; Web Table 3). Results for BMI at blood draw were similar to those for BMI at age 18 years, with the exception of heterogeneity for progesterone, with a decreased risk of endometriosis with increasing levels of progesterone for BMI less than 25 at blood draw (P for trend = 0.02) and no clear association for BMI greater than or equal to 25 (P for trend = 0.09; P for heterogeneity = 0.002; Web Table 3).
Table 5.
Relative Riska of Laparoscopically Confirmed Endometriosis by Quartile of Plasma Sex Steroid Levels Among Subgroups in Nurses’ Health Study II, 1996–2009
| Hormone | Quartile 2 | Quartile 3 | Quartile 4 |
P for
Trendb |
Quartile 2 | Quartile 3 | Quartile 4 |
P for
Trendb |
P From LRT c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||||
| Age at Blood Draw <40 Years d | Age at Blood Draw ≥40 Years e | ||||||||||||||
| Follicular estradiol | 1.14 | 0.58, 2.25 | 1.11 | 0.55, 2.21 | 1.36 | 0.65, 2.84 | 0.43 | 4.10 | 2.39, 7.02 | 2.87 | 1.64, 5.01 | 2.24 | 1.29, 3.91 | 0.22 | 0.07 |
| Luteal estradiol | 0.95 | 0.50, 1.81 | 1.57 | 0.85, 2.87 | 1.03 | 0.54, 1.95 | 0.74 | 1.43 | 0.91, 2.25 | 1.13 | 0.71, 1.80 | 0.85 | 0.52, 1.38 | 0.25 | 0.23 |
| Follicular free estradiol | 1.15 | 0.58, 2.25 | 1.42 | 0.73, 2.75 | 0.98 | 0.45, 2.15 | 1.00 | 1.94 | 1.17, 3.24 | 2.50 | 1.49, 4.19 | 1.37 | 0.82, 2.31 | 0.6 | 0.50 |
| Luteal free estradiol | 1.67 | 0.90, 3.08 | 1.16 | 0.62, 2.16 | 1.10 | 0.58, 2.10 | 0.87 | 1.12 | 0.71, 1.76 | 0.80 | 0.49, 1.30 | 1.03 | 0.65, 1.63 | 0.88 | 0.88 |
| Follicular estrone | 1.55 | 0.81, 2.99 | 1.50 | 0.78, 2.91 | 1.49 | 0.75, 2.98 | 0.38 | 1.04 | 0.62, 1.74 | 1.21 | 0.73, 2.01 | 1.72 | 1.06, 2.77 | 0.01 | 0.62 |
| Luteal estrone | 1.04 | 0.55, 1.98 | 1.53 | 0.85, 2.75 | 1.66 | 0.87, 3.18 | 0.07 | 1.10 | 0.69, 1.75 | 1.38 | 0.87, 2.18 | 0.71 | 0.44, 1.16 | 0.16 | 0.17 |
| Testosterone | 1.06 | 0.58, 1.94 | 1.22 | 0.66, 2.24 | 0.81 | 0.43, 1.50 | 0.47 | 1.55 | 1.05, 2.28 | 0.80 | 0.51, 1.25 | 1.01 | 0.65, 1.58 | 0.32 | 0.13 |
| Free testosterone | 0.99 | 0.54, 1.83 | 0.80 | 0.43, 1.48 | 1.02 | 0.56, 1.85 | 0.95 | 1.11 | 0.73, 1.70 | 1.29 | 0.85, 1.97 | 1.12 | 0.72, 1.76 | 0.58 | 0.66 |
| Progesterone | 0.93 | 0.49, 1.77 | 1.06 | 0.57, 1.99 | 0.63 | 0.32, 1.26 | 0.24 | 0.84 | 0.53, 1.33 | 1.10 | 0.70, 1.73 | 0.95 | 0.57, 1.58 | 0.91 | 0.52 |
| SHBG | 0.94 | 0.52, 1.71 | 0.91 | 0.52, 1.61 | 1.07 | 0.61, 1.89 | 0.81 | 1.00 | 0.65, 1.54 | 1.06 | 0.70, 1.63 | 0.89 | 0.58, 1.38 | 0.63 | 0.82 |
| BMI f <20 at Age 18 Yearsg | BMI ≥20 at Age 18 Years h | ||||||||||||||
| Follicular estradiol | 2.66 | 1.24, 5.68 | 2.40 | 1.15, 5.02 | 2.13 | 1.01, 4.48 | 0.33 | 2.71 | 1.64, 4.48 | 1.84 | 1.07, 3.18 | 1.69 | 0.97, 2.93 | 0.40 | 0.86 |
| Luteal estradiol | 1.62 | 0.89, 2.95 | 1.03 | 0.57, 1.88 | 0.67 | 0.36, 1.26 | 0.05 | 1.05 | 0.65, 1.69 | 1.52 | 0.95, 2.46 | 1.11 | 0.67, 1.81 | 0.52 | 0.06 |
| Follicular free estradiol | 2.01 | 0.99, 4.08 | 3.22 | 1.61, 6.45 | 1.74 | 0.83, 3.63 | 0.33 | 1.50 | 0.91, 2.48 | 1.63 | 0.97, 2.72 | 1.03 | 0.60, 1.75 | 0.78 | 0.48 |
| Luteal free estradiol | 1.71 | 0.98, 2.99 | 0.88 | 0.48, 1.61 | 0.80 | 0.43, 1.49 | 0.16 | 0.92 | 0.57, 1.49 | 0.94 | 0.57, 1.54 | 1.20 | 0.75, 1.93 | 0.36 | 0.05 |
| Follicular estrone | 1.01 | 0.53, 1.92 | 1.33 | 0.71, 2.49 | 1.88 | 0.99, 3.56 | 0.02 | 1.38 | 0.83, 2.30 | 1.25 | 0.74, 2.09 | 1.57 | 0.96, 2.57 | 0.12 | 0.73 |
| Luteal estrone | 1.18 | 0.66, 2.11 | 1.44 | 0.82, 2.53 | 0.59 | 0.31, 1.12 | 0.10 | 0.96 | 0.59, 1.58 | 1.44 | 0.90, 2.31 | 1.30 | 0.80, 2.12 | 0.17 | 0.10 |
| Testosterone | 1.87 | 1.10, 3.18 | 1.25 | 0.71, 2.20 | 1.11 | 0.61, 2.02 | 0.69 | 1.10 | 0.72, 1.68 | 0.78 | 0.50, 1.24 | 0.80 | 0.50, 1.26 | 0.17 | 0.43 |
| Free testosterone | 1.79 | 1.07, 2.96 | 1.49 | 0.85, 2.60 | 1.24 | 0.66, 2.32 | 0.68 | 0.64 | 0.39, 1.05 | 0.82 | 0.52, 1.28 | 0.90 | 0.58, 1.41 | 0.86 | 0.03 |
| Progesterone | 0.99 | 0.54, 1.84 | 0.98 | 0.55, 1.76 | 0.93 | 0.50, 1.74 | 0.82 | 0.77 | 0.48, 1.24 | 1.07 | 0.67, 1.71 | 0.64 | 0.37, 1.11 | 0.28 | 0.67 |
| SHBG | 1.47 | 0.79, 2.74 | 1.35 | 0.74, 2.47 | 0.92 | 0.50, 1.68 | 0.34 | 0.77 | 0.50, 1.19 | 0.88 | 0.57, 1.34 | 1.05 | 0.67, 1.63 | 0.74 | 0.13 |
| No History of Infertility i | History of Infertility j | ||||||||||||||
| Follicular estradiol | 2.91 | 1.74, 4.86 | 1.89 | 1.12, 3.21 | 2.08 | 1.22, 3.53 | 0.18 | 1.99 | 0.98, 4.02 | 2.44 | 1.17, 5.07 | 1.43 | 0.66, 3.08 | 0.57 | 0.36 |
| Luteal estradiol | 1.13 | 0.73, 1.75 | 1.26 | 0.81, 1.96 | 0.91 | 0.57, 1.44 | 0.66 | 1.74 | 0.88, 3.42 | 1.37 | 0.70, 2.68 | 0.95 | 0.48, 1.87 | 0.48 | 0.76 |
| Follicular free estradiol | 1.93 | 1.17, 3.18 | 2.26 | 1.38, 3.68 | 1.31 | 0.78, 2.20 | 0.77 | 1.28 | 0.64, 2.55 | 1.71 | 0.83, 3.51 | 1.24 | 0.57, 2.69 | 0.61 | 0.72 |
| Luteal free estradiol | 1.42 | 0.93, 2.16 | 0.74 | 0.46, 1.19 | 1.19 | 0.75, 1.87 | 0.99 | 0.75 | 0.37, 1.50 | 1.20 | 0.63, 2.31 | 0.74 | 0.38, 1.42 | 0.49 | 0.03 |
| Follicular estrone | 1.16 | 0.72, 1.88 | 1.37 | 0.85, 2.21 | 1.75 | 1.09, 2.79 | 0.01 | 1.32 | 0.65, 2.67 | 1.23 | 0.61, 2.50 | 1.62 | 0.80, 3.29 | 0.22 | 0.97 |
| Luteal estrone | 1.07 | 0.69, 1.67 | 1.38 | 0.90, 2.13 | 0.80 | 0.50, 1.27 | 0.41 | 0.98 | 0.47, 2.02 | 1.55 | 0.81, 2.99 | 1.21 | 0.61, 2.43 | 0.45 | 0.54 |
| Testosterone | 1.36 | 0.92, 2.01 | 0.95 | 0.62, 1.44 | 0.89 | 0.58, 1.38 | 0.27 | 1.24 | 0.69, 2.25 | 0.80 | 0.41, 1.56 | 0.86 | 0.44, 1.67 | 0.36 | 1.00 |
| Free testosterone | 1.18 | 0.77, 1.79 | 1.08 | 0.72, 1.62 | 1.20 | 0.78, 1.84 | 0.51 | 0.90 | 0.49, 1.67 | 1.15 | 0.41, 1.56 | 0.84 | 0.44, 1.59 | 0.66 | 0.65 |
| Progesterone | 0.78 | 0.49, 1.23 | 1.11 | 0.72, 1.72 | 0.87 | 0.53, 1.43 | 0.96 | 1.04 | 0.55, 1.95 | 0.84 | 0.43, 1.63 | 0.60 | 0.29, 1.21 | 0.13 | 0.51 |
| SHBG | 0.84 | 0.56, 1.27 | 0.99 | 0.66, 1.48 | 0.83 | 0.55, 1.25 | 0.50 | 1.26 | 0.67, 2.36 | 1.04 | 0.57, 1.92 | 1.11 | 0.59, 2.08 | 0.91 | 0.60 |
Abbreviations: BMI, body mass index; CI, confidence interval; LRT, likelihood ratio test; RR, relative risk; SHBG, sex hormone binding globulin.
a All RRs and 95% CIs were estimated by conditional logistic regression conditioned on matching factors, including age, race/ethnicity, infertility history, and menopausal status at diagnosis, as well as month, time of day, and fasting status at blood draw. Quartile 1 was used as the reference group.
b P values were calculated via a Wald test of a score variable that contained median values of the quartiles.
c P values were calculated from LRTs of the interaction terms.
d Analyses of age at blood draw <40 years included 20–52 cases and 41–85 controls.
e Analyses of age at blood draw ≥40 years included 28–102 cases and 93–155 controls.
f Weight (kg)/height (m)2.
g Analyses of BMI <20 at age 18 years included 17–64 cases and 46–110 controls.
h Analyses of BMI ≥20 at age 18 years included 37–82 cases and 89–149 controls.
i Analyses of no history of infertility included 34–97 cases and 102–155 controls.
j Analyses of history of infertility included 19–47 cases and 38–75 controls.
Sensitivity analyses
When restricting the analysis to women with follicular estradiol levels less than 80 pg/mL, the associations for follicular total and free estradiol became slightly stronger and associations for the remaining steroid hormones remained largely unchanged (Web Table 4). When excluding endometriosis cases diagnosed within 2, 4, or 6 years after the blood draw, the associations for follicular total and free estradiol remained essentially unchanged (Web Table 5). However, higher progesterone levels were associated with decreased endometriosis risk when excluding endometriosis cases diagnosed within 4 and 6 years after the blood draw (top vs. bottom quartile: for the 4-year exclusion, RR = 0.43 (95% CI: 0.25, 0.74), P for trend = 0.01; for the 6-year exclusion, RR = 0.38 (95% CI: 0.18, 0.82), P for trend = 0.02).
DISCUSSION
To our knowledge, this is the first study to have prospectively investigated the association between circulating hormone levels and subsequent risk of an endometriosis diagnosis at least 1 year after blood collection. We observed that higher plasma levels of total and free estradiol in the early follicular phase of the menstrual cycle were associated with increased risk of endometriosis. Higher mid-luteal plasma progesterone levels were associated with decreased endometriosis risk among women whose samples were collected during an ovulatory cycle. A curvilinear relationship with free testosterone was observed, with endometriosis risk being increased among women with the highest free testosterone levels, who comprised approximately 2% of our study population.
Strong circumstantial evidence indicates that the development and progression of endometriosis depend on estrogen (5, 6). Endometriosis-related pelvic pain improves when estrogen production is reduced via bilateral oophorectomy or gonadotropin-releasing hormone agonist treatment (26, 27). We observed that higher plasma levels of follicular total and free estradiol were associated with a greater risk of endometriosis diagnosis, including a suggestion of a curvilinear relationship, which remained after exclusion of cases diagnosed within 2, 4, and 6 years after blood draw. Most previous case-control studies have found no difference in follicular estradiol levels between endometriosis cases and controls (28–30); however, in one study, Takahashi et al. (30) observed higher estradiol levels in the menstrual effluent of endometriosis cases compared with controls. Additionally, in a small case-control study, Shan et al. (31) reported higher follicular estradiol levels among endometriosis cases compared with controls. The small sample sizes of these studies (n < 75 cases) make the results difficult to interpret with respect to random error. Additionally, hormone measurements in these studies were performed after diagnosis and thus may not reflect the pertinent earlier etiological window, and may have been influenced by endometriosis treatments, often including exogenous hormone therapy.
Conversely, we observed no association of luteal total and free estradiol and luteal estrone levels with endometriosis risk, which is consistent with earlier case-control studies, although the results may differ by revised American Society for Reproductive Medicine endometriosis stage (29, 32, 33). Two studies in which more than 75% of participants had stage I or II disease observed no difference in luteal estradiol levels between endometriosis cases and controls (29, 32). However, in one small case-control study, Donnez et al. (33) reported lower estradiol levels for women with stage III or IV endometriosis (n = 15) on cycle days 14–19 as compared with a reference curve of normal ovulatory women, but no difference in estradiol levels for women with stage I (n = 26) or stage II (n = 17) endometriosis. The retrospective nature of these case-control studies precludes extrapolation of observations to etiology or causality, since the endogenous hormone measurements reflect the active disease state, which may be confounded by or a marker of symptom treatment and thus unrelated to the pathophysiology of endometriosis initiation or promotion.
Differences observed for follicular versus luteal estrogen levels and endometriosis risk may be explained by differences in the correlation between peripheral and peritoneal cavity hormone levels throughout the menstrual cycle. Among 158 women with regular menstrual cycles who were not using hormones, Donnez et al. observed that peritoneal fluid and serum estradiol concentrations were similar during the follicular phase but that peritoneal fluid levels were higher than serum levels during the luteal phase (33). This difference in estradiol levels in the luteal phase is most likely due to the high estradiol level of follicular fluid that empties into the peritoneal cavity during ovulation. Thus, circulating follicular estradiol levels may be more relevant to the underlying endometriosis pathogenesis than circulating luteal estradiol, for which peritoneal fluid levels may be more relevant. Finally, it is possible that the observed elevated estradiol levels in the follicular phase resulted from endometriosis that was present but not diagnosed at the time of blood collection. However, after exclusion of cases diagnosed within 2, 4, and 6 years after the blood draw, the associations between follicular estradiol levels and endometriosis remained unchanged, suggesting that undiagnosed endometriosis at blood draw was not influencing the association between follicular estradiol levels and endometriosis risk.
We observed that higher progesterone levels were associated with a lower risk of endometriosis when excluding anovulatory cycles. Because progesterone levels during anovulatory cycles are negligible, they do not reflect women’s longer-term progesterone exposure. Previous case-control studies have noted lower progesterone peritoneal fluid levels among endometriosis cases compared with controls, particularly for severe disease (32, 33). Conversely, no differences have been observed between endometriosis cases and controls for serum progesterone levels (29, 32); however, small sample sizes may have reduced those studies’ statistical power to detect modest differences. Progesterone exerts antiestrogenic effects in the endometrium, partly by inducing 17β-hydroxysteroid dehydrogenase 2 that catalyzes the biologically potent estradiol to less estrogenic estrone (34, 35). However, endometriotic tissue fails to respond to progesterone because of extremely low progesterone receptor levels in ectopic endometrium (36, 37), which leads to diminished inactivation of estradiol (7). Additionally, we observed an inverse association between progesterone and endometriosis risk when excluding endometriosis cases diagnosed within 4 and 6 years after blood draw, suggesting that progesterone levels may be important earlier in the etiology of endometriosis. However, due to the large within-person variation (intraclass correlation coefficient = 0.29) of progesterone measurement over 3 years (25), it is difficult to draw conclusions on associations between progesterone and endometriosis risk.
Endometriosis lesions express androgen receptors and atrophy when exposed to androgens (10, 38). Danazol, a modified testosterone, was the first drug approved specifically for endometriosis treatment (39). The potential mechanism of danazol is inhibition of ovarian steroidogenesis, resulting in decreased secretion of estradiol and increased androgen levels (40). Recently, evolutionary biologists have postulated that low testosterone in utero, and potentially throughout adulthood, may be associated with a greater risk of endometriosis (11). Nonhuman primate studies have shown that exposure to androgens in utero leads to higher levels of testosterone later in life (41, 42); however, no human data exist for the relationship between testosterone levels in utero and later in life. Few studies have assessed the association between circulating testosterone levels and endometriosis, and none have examined the relationship between testosterone levels and subsequent risk of endometriosis. Results from cross-sectional studies have been mixed, with some studies finding lower serum testosterone levels among endometriosis patients compared with controls (43, 44), one study finding no association (45, 46), and one study finding higher free testosterone levels among endometriosis patients compared with controls (46). However, none of these studies were prospective or accounted for confounders, and all were restricted to endometriosis patients who were currently experiencing infertility and thus were not representative of the majority of women with endometriosis (47). Contrary to the hypothesized protective role of higher testosterone levels in endometriosis risk (11), we observed in nonparametric restricted cubic spline analyses that as free testosterone increased, the risk of endometriosis increased. However, these results should be interpreted with caution because the increase in endometriosis risk was observed only above 0.57 ng/dL of free testosterone, which comprised 2% of cases and controls with testosterone measurements. The quartile analysis that minimized the influence of but also detection of influential tails was null, although there was a suggestion of an inverse trend.
This study had several limitations. The mean age at endometriosis diagnosis within this study population was approximately 5 years higher than the mean age at diagnosis in the entire cohort through 2009 (46 years vs. 41 years) and is attributable to the timing of NHS II blood collection. Associations between the hormones and endometriosis risk did not appear to be modified by age at endometriosis diagnosis, suggesting that the associations may have been similar if the study population were younger at blood collection. Another limitation is that we lacked information on revised American Society for Reproductive Medicine stage for endometriosis and could not evaluate whether associations varied by disease stage, although, as we described previously (20), approximately 60% of endometriosis cases within NHS II were categorized as stage I or II at diagnostic laparoscopy. In addition, it is likely that a proportion of our controls had asymptomatic disease or were symptomatic but had not reached the point of diagnosis. However, this nondifferential misclassification is likely to have biased our estimated effect estimates toward the null.
Our study had many strengths. Blood samples were collected on average 5 years prior to endometriosis diagnosis. The prospective study design uniquely allows for evaluation of the temporality of the association between hormone levels and risk of endometriosis, or at least a window earlier in the disease process than at or after the time of surgical diagnosis, and is essential for etiological hypothesis-testing and causal inference. The carefully timed collection of samples allowed us to study both follicular- and luteal-phase estrogen levels. All matched case-control plasma sets were shipped and assayed blinded to case/control status. The large sample size enabled the exploration and detection of important modifiers for the hormones in relation to endometriosis risk. Finally, infertility history at diagnosis was matched between cases and controls, minimizing the possibility that our results are explained by underlying conditions that caused infertility.
In conclusion, in this large prospective nested case-control study, higher circulating levels of follicular total and free estradiol and lower progesterone levels were prospectively associated with an increased risk of laparoscopically confirmed endometriosis. More investigations are warranted to evaluate and explain the observed curvilinear relationship between follicular total and free estradiol and free testosterone and endometriosis. Additionally, the influence of higher estradiol levels and lower progesterone levels leading to endometriosis development could be an interesting hypothesis to test in nonhuman primates or autologous rodent models to mimic long-term low-dose estrogen exposure to see how this alters the establishment of endometriosis. These results suggest that steroid hormones are involved in endometriosis pathogenesis, as many believe; however, their influence appears to be nonlinear and more complex than suggested by experimental models or cross-sectional studies of women well along on their endometriosis journey.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Adolescent/Young Adult Medicine, Department of Pediatrics, Boston Children’s Hospital and Harvard Medical School, Boston, Massachusetts, United States (Amy L. Shafrir, Stacey A. Missmer); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts, United States (Fan Mu, A. Heather Eliassen, Kathryn L. Terry, Stacey A. Missmer); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, United States (A. Heather Eliassen); Department of Nutrition, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts, United States (A. Heather Eliassen); Department of Obstetrics, Gynecology, and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan, United States (Madhavi Thombre Kulkarni, Stacey A. Missmer); Department of Obstetrics and Gynecology, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, United States (Kathryn L. Terry); and Department of Biostatistics and Epidemiology, School of Public Health and Health Sciences, University of Massachusetts, Amherst, Massachusetts, United States (Susan E. Hankinson).
This work was supported by research grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grants HD52473, HD96358, and HD96033) and from the National Cancer Institute (grants CA50385, CA176726, and CA67262).
The Brigham and Women’s Hospital Institutional Review Board and the National Institutes of Health/National Cancer Institute have approved data-sharing in this ongoing cohort study via requests to the Nurses’ Health Study Data Access Committee. Requests for data access can be submitted by filling in a form on the study’s public website (http://www.nurseshealthstudy.org/researchers). For more information regarding data access, please contact the Data Access Committee at 181 Longwood Avenue, Channing Laboratory, Room 351, Boston, MA 02115 (phone: 617-525-2279; e-mail: snhsaccess@channing.harvard.edu).
This work was presented in poster form at the 51st Annual Meeting of the Society for Epidemiologic Research, Baltimore, Maryland, June 19–22, 2018.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–1256. [DOI] [PubMed] [Google Scholar]
- 2. Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- 3. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14(4):422–469. [Google Scholar]
- 4. D’Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update. 2002;8(1):84–88. [DOI] [PubMed] [Google Scholar]
- 5. Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30(1):1–19. [DOI] [PubMed] [Google Scholar]
- 6. Zondervan KT, Becker CM, Koga K, et al. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. [DOI] [PubMed] [Google Scholar]
- 7. Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103. [DOI] [PubMed] [Google Scholar]
- 8. Ma L, Andrieu T, McKinnon B, et al. Epithelial-to-mesenchymal transition contributes to the downregulation of progesterone receptor expression in endometriosis lesions. J Steroid Biochem Mol Biol. 2021;212:105943. [DOI] [PubMed] [Google Scholar]
- 9. Chwalisz K, Perez MC, DeManno D, et al. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26(3):423–438. [DOI] [PubMed] [Google Scholar]
- 10. Selak V, Farquhar C, Prentice A, et al. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068. [DOI] [PubMed] [Google Scholar]
- 11. Dinsdale N, Nepomnaschy P, Crespi B. The evolutionary biology of endometriosis. Evol Med Public Health. 2021;9(1):174–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiong W, Zhang L, Yu L, et al. Estradiol promotes cells invasion by activating β-catenin signaling pathway in endometriosis. Reproduction. 2015;150(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huhtinen K, Ståhle M, Perheentupa A, et al. Estrogen biosynthesis and signaling in endometriosis. Mol Cell Endocrinol. 2012;358(2):146–154. [DOI] [PubMed] [Google Scholar]
- 14. Vercellini P, Buggio L, Berlanda N, et al. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril. 2016;106(7):1552–1571. [DOI] [PubMed] [Google Scholar]
- 15. Brown J, Pan A, Hart R. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev. 2010;(12):CD008475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barra F, Grandi G, Tantari M, et al. A comprehensive review of hormonal and biological therapies for endometriosis: latest developments. Expert Opin Biol Ther. 2019;19(4):343–360. [DOI] [PubMed] [Google Scholar]
- 17. Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66(4):2476–2482. [DOI] [PubMed] [Google Scholar]
- 18. Bolelli G, Muti P, Micheli A, et al. Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiol Biomarkers Prev. 1995;4(5):509–513. [PubMed] [Google Scholar]
- 19. Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415. [DOI] [PubMed] [Google Scholar]
- 20. Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–796. [DOI] [PubMed] [Google Scholar]
- 21. Shafrir AL, Wise LA, Palmer JR, et al. Validity of self-reported endometriosis: a comparison across four cohorts. Hum Reprod. 2021;36(5):1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosner BA, Cook N, Portman R, et al. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–666. [DOI] [PubMed] [Google Scholar]
- 23. Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. [DOI] [PubMed] [Google Scholar]
- 24. Bauer SR, Fortner RT, Gates MA, et al. Analgesic use in relation to sex hormone and prolactin concentrations in premenopausal women. Cancer Causes Control. 2013;24(6):1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Missmer SA, Spiegelman D, Bertone-Johnson ER, et al. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15(5):972–978. [DOI] [PubMed] [Google Scholar]
- 26. Namnoum AB, Hickman TN, Goodman SB, et al. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64(5):898–902. [DOI] [PubMed] [Google Scholar]
- 27. Tekin YB, Dilbaz B, Altinbas SK, et al. Postoperative medical treatment of chronic pelvic pain related to severe endometriosis: levonorgestrel-releasing intrauterine system versus gonadotropin-releasing hormone analogue. Fertil Steril. 2011;95(2):492–496. [DOI] [PubMed] [Google Scholar]
- 28. Pedachenko N, Anagnostis P, Shemelko T, et al. Serum anti-Mullerian hormone, prolactin and estradiol concentrations in infertile women with endometriosis. Gynecol Endocrinol. 2021;37(2):162–165. [DOI] [PubMed] [Google Scholar]
- 29. Michaud N, Al-Akoum M, Akoum A. Blood soluble interleukin 1 receptor accessory protein levels are consistently low throughout the menstrual cycle of women with endometriosis. Reprod Biol Endocrinol. 2014;12(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi K, Nagata H, Kitao M. Clinical usefulness of determination of estradiol level in the menstrual blood for patients with endometriosis. Nippon Sanka Fujinka Gakkai Zasshi. 1989;41(11):1849–1850. [PubMed] [Google Scholar]
- 31. Shan J, Ni Z, Cheng W, et al. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch Gynecol Obstet. 2021;304(5):1363–1373. [DOI] [PubMed] [Google Scholar]
- 32. Barry-Kinsella C, Sharma SC, Cottell E, et al. Mid to late luteal phase steroids in minimal stage endometriosis and unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 1994;54(2):113–118. [DOI] [PubMed] [Google Scholar]
- 33. Donnez J, Langerock S, Thomas K. Peritoneal fluid volume and 17β-estradiol and progesterone concentrations in ovulatory, anovulatory, and postmenopausal women. Obstet Gynecol. 1982;59(6):687–692. [PubMed] [Google Scholar]
- 34. Tseng L, Gurpide E. Estradiol and 20α-dihydroprogesterone dehydrogenase activities in human endometrium during the menstrual cycle. Endocrinology. 1974;94(2):419–423. [DOI] [PubMed] [Google Scholar]
- 35. Yang S, Fang Z, Gurates B, et al. Stromal PRs mediate induction of 17β-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelium: a paracrine mechanism for inactivation of E2. Mol Endocrinol. 2001;15(12):2093–2105. [DOI] [PubMed] [Google Scholar]
- 36. Igarashi TM, Bruner-Tran KL, Yeaman GR, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84(1):67–74. [DOI] [PubMed] [Google Scholar]
- 37. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 38. Massarotti C, Mirabelli Badenier I, Paudice M, et al. Steroids receptors immunohistochemical expression in different sites of endometriosis. J Gynecol Obstet Hum Reprod. 2021;50(3):101861. [DOI] [PubMed] [Google Scholar]
- 39. Dmowski WP, Scholer HF, Mahesh VB, et al. Danazol—a synthetic steroid derivative with interesting physiologic properties. Fertil Steril. 1971;22(1):9–18. [DOI] [PubMed] [Google Scholar]
- 40. Floyd WS. Danazol: endocrine and endometrial effects. Int J Fertil. 1980;25(1):75–80. [PubMed] [Google Scholar]
- 41. Eisner J, Barnett M, Dumesic D, et al. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77(1):167–172. [DOI] [PubMed] [Google Scholar]
- 42. Dumesic D, Abbott D, Eisner J, et al. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67(1):155–163. [DOI] [PubMed] [Google Scholar]
- 43. Ono YJ, Tanabe A, Nakamura Y, et al. A low-testosterone state associated with endometrioma leads to the apoptosis of granulosa cells. PLoS One. 2014;9(12):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barbieri RL, Sluss PM, Powers RD, et al. Association of body mass index, age, and cigarette smoking with serum testosterone levels in cycling women undergoing in vitro fertilization. Fertil Steril. 2005;83(2):302–308. [DOI] [PubMed] [Google Scholar]
- 45. Pellicer A, Valbuena D, Bauset C, et al. The follicular endocrine environment in stimulated cycles of women with endometriosis: steroid levels and embryo quality. Fertil Steril. 1998;69(6):1135–1141. [DOI] [PubMed] [Google Scholar]
- 46. Evsen M, Sak ME, Soydinc HE, et al. Serum levels of androgens and prostate-specific antigen in endometriosis. Clin Exp Obstet Gynecol. 2014;16(4):432–435. [PubMed] [Google Scholar]
- 47. Prescott J, Farland LV, Tobias DK, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod. 2016;31(7):1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


