Abstract
Lentivirus vectors based on human immunodeficiency virus (HIV) type 1 (HIV-1) constitute a recent development in the field of gene therapy. A key property of HIV-1-derived vectors is their ability to infect nondividing cells. Although high-titer HIV-1-derived vectors have been produced, concerns regarding safety still exist. Safety concerns arise mainly from the possibility of recombination between transfer and packaging vectors, which may give rise to replication-competent viruses with pathogenic potential. We describe a novel lentivirus vector which is based on HIV, simian immunodeficiency virus (SIV), and vesicular stomatitis virus (VSV) and which we refer to as HIV/SIVpack/G. In this system, an HIV-1-derived genome is encapsidated by SIVmac core particles. These core particles are pseudotyped with VSV glycoprotein G. Because the nucleotide homology between HIV-1 and SIVmac is low, the likelihood of recombination between vector elements should be reduced. In addition, the packaging construct (SIVpack) for this lentivirus system was derived from SIVmac1A11, a nonvirulent SIV strain. Thus, the potential for pathogenicity with this vector system is minimal. The transduction ability of HIV/SIVpack/G was demonstrated with immortalized human lymphocytes, human primary macrophages, human bone marrow-derived CD34+ cells, and primary mouse neurons. To our knowledge, these experiments constitute the first demonstration that the HIV-1-derived genome can be packaged by an SIVmac capsid. We demonstrate that the lentivirus vector described here recapitulates the biological properties of HIV-1-derived vectors, although with increased potential for safety in humans.
Gene therapy is a method under investigation for the treatment of genetic, metabolic, and neurologic diseases, cancer, and AIDS. The primary goals of gene therapy are to deliver a certain gene to a predetermined target cell and to direct the expression of such a gene in a manner which will have therapeutic effects.
A wide variety of methods for gene delivery exist. These are classified into two main groups, viral and nonviral gene transfer methods. Among the virus vectors currently under investigation, lentivirus vectors have unique properties which are attractive with regard to gene therapy (33). These include integration into the host cell chromosome and the ability to infect nondividing cells. Lentivirus vectors have been used for the delivery of transgenes directly into a variety of nondividing cells in vitro and in vivo (1, 17, 37, 47, 48). These cell types include postmitotic neurons, myocytes, liver cells, retinal epithelial cells, and bone marrow-derived CD34+ cells.
The applicability of a safe lentivirus vector in human disease is broad because (i) the host range of lentiviruses can be virtually unlimited when vesicular stomatitis virus (VSV) glycoprotein G (VSV-G) is used to produce envelope pseudotypes; (ii) many relevant targets for gene therapy are nondividing cells (neurons, hepatic cells, hematopoietic stem cells, and myocytes); and (iii) the transgene is highly stable due to chromosomal integration.
Although lentivirus vectors derived from human immunodeficiency virus (HIV) type 1 (HIV-1) offer great promise in the field of gene therapy, concerns regarding safety in humans still exist. We describe here novel lentivirus vectors with a reduced likelihood of recombination and pathogenesis. The construction and characterization of a novel simian immunodeficiency virus (SIV) packaging system, which directs the production of all SIV structural genes except for env, nef, and vpr, are reported. Because the nucleotide homology between HIV-1 and SIV is low, the likelihood of recombination between vector elements should be reduced. In addition, the SIV packaging construct, SIVpack, was derived from SIVmac1A11, a nonvirulent strain of SIV (30). VSV-G is used to produce pseudotype viral particles. We demonstrate that this vector system retains the key features of a lentivirus vector and constitutes a safe alternative to HIV-1-derived systems.
MATERIALS AND METHODS
Plasmid construction.
SIVmac1A11 proviral sequences were obtained from plasmid pSVT3/1A11 (30). A subgenomic fragment of SIVmac1A11 from pSVT3/1A11 was digested with NarI and SalI and subcloned into the simian virus 40 (SV40) expression vector pSVC2 (Paul Luciw, University of California, Davis), previously digested with the same enzymes. The cloning resulted in the complete deletion of the sequences between nucleotides 518 and 806 of SIVmac, which were shown to be responsible for genomic packaging by Rizvi and Panganiban (52). The resulting vector was digested with BspEI and HindIII to excise a 1-kb band, and the ends were filled in with the Klenow polymerase and religated. This process resulted in the deletion of the env gene. This vector was named SIVpack. HIV-thy was derived from vector NLthyΔenv-vprX (44) by introducing between restriction sites SphI and MscI a deletion which eliminates gag and pol and by filling in an NdeI site which inactivates vif. HIV-GFP was generated by subcloning a 1-kb XhoI-to-HpaI restriction fragment from pEGFP-N1 (Clontech, Palo Alto, Calif.) into HIV-thy that had been digested with MluI, filled in with the Klenow polymerase, and digested with XhoI. LNCX-GFP is a murine retrovirus vector which was generated by subcloning the green fluorescent protein (GFP) gene from pEGFP-N1 as a HindIII-to-HpaI fragment into LNCX (34) that had been digested with ClaI, filled in with the Klenow polymerase, and digested with HindIII. HIV-1NL4-3-thyenv(−) (50), HCMV-VSVG (7), and Ψ(−)env(−)ampho (24) were described previously.
Vector production.
Lentivirus vectors were produced by electroporation into COS cells by previously described methods (44, 45). Vectors HIV-GFP/SIVpack/G and HIV-thy/SIVpack/G were generated by cotransfection of plasmids HIV-GFP and HIV-thy, respectively; SIVpack; and HCMV-VSVG. Vector HIV-1NL4-3-thyenv(−)/G was generated by cotransfection of HIV-1NL4-3-thyenv(−) (50) and HCMV-VSVG. Transfection supernatants (36 ml) were precleared by low-speed centrifugation, filtered through 0.2-μm-pore-size filters, and pelleted by ultracentrifugation at 25,000 rpm in a Discovery 100S centrifuge with a Surespin 630 rotor (Sorvall, Newton, Conn.). Virus pellets were resuspended in 0.3 ml of tissue culture medium and frozen at −80°C. Vector titers were measured by infection of HeLa cells as described below, followed by flow cytometric analysis of cells positive for the reporter molecule. For Thy-1, immunological staining is required prior to flow cytometry (45). Vector titers were calculated as follows: titer = [F × C0/V] × D. F is the frequency of Thy-1-positive or GFP-positive cells, determined by flow cytometry; C0 is the total number of target cells at the time of infection; V is the volume of inoculum; and D is the virus dilution factor. The total number of target cells at the time of infection was estimated as twice the number of cells seeded [(2 × 104) × 2], since one cell division occurs between the time of seeding and the time of infection.
Immunologic detection of viral antigens.
Detection of SIV p27 and HIV-1 p24 was performed by a capture enzyme-linked immunosorbent assay (ELISA) with monoclonal antibodies and protein standards obtained from the NIH AIDS Reagent Repository, polyclonal anti-SIVmac serum donated by Nancy Haigwood and William Sutton (Seattle Biomedical Research Institute, Seattle, Wash.), and polyclonal anti-HIV-1 serum donated by Thomas Evans (University of Rochester Cancer Center, Rochester, N.Y.).
Infections of dividing and growth-arrested HeLa cells.
Exponentially growing HeLa or MAGI (23) cells were detached with 2 mM EDTA in phosphate-buffered saline, irradiated with 2,000 or 5,000 rads or untreated, seeded in 12-well plates at a density of 2 × 104 per well, allowed to attach for 24 h, and subsequently infected with titrated virus stocks. Infections were performed by thawing the virus stocks at 37°C, mixing them with 10 μg of Polybrene (Sigma Chemical Co., St. Louis, Mo.) per ml, and adding the mixtures to adherent cells. Infections were performed for 2 h at 37°C, after which the cells were washed twice with normal medium (Dulbecco’s modified Eagle medium containing 10% fetal calf serum) and cultured until the time of analysis (48 or 72 h) by visual inspection through fluorescence microscopy or flow cytometry.
Isolation and infection of primary mouse neurons.
Primary cortical neurons were harvested from E15 mice and prepared by previously described methods (5). Individual cells were dissociated initially by trypsinization for 15 min at 37°C and washed twice with Hanks balanced salt solution containing Ca2+ and Mg2+. Cells were dissociated further by sequential mechanical dissociation with a serologic pipette and resuspended in serum-free Neurobasal plating medium (Life Technologies, Gaithersburg, Md.) supplemented with 0.5 mM l-glutamine, 25 mM l-glutamic acid, and 2% B-27 (Life Technologies). Cells were plated at 160 cells per mm2 in a 12-well plate precoated with 0.05 mg of poly-d-lysine per ml. Cells were maintained in Neurobasal medium. Cells were characterized by reactivity to mouse anti-neurofilament 200 (Sigma), rabbit anti-tau (Sigma), and rabbit anti-rat neuron-specific enolase (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) antibodies. Primary neurons were infected by removing 700 μl of medium, adding concentrated virus stocks, and incubating the mixtures for 1 h at 37°C. Polybrene was not used for infection of neurons. Cells were then washed and cultured in fresh medium.
Isolation and infection of human peripheral blood macrophages.
Peripheral blood mononuclear cells were isolated on Ficoll, and cell density was adjusted to 3 × 106 cells/ml in RPMI 1640 (GIBCO-BRL) supplemented with 10% human AB serum. Cells were plated in a 12-well plate and incubated for 24 h. Nonadherent cells were discarded by multiple washings performed at days 1, 3, and 5. Adherent cells were maintained for 14 days in RPMI 1640 supplemented with 10% human AB serum and 10% giant cell conditioned medium, which contains granulocyte-macrophage colony-stimulating factor (26). Macrophages were infected as described above for HeLa cells.
Fluorescence microscopy and photography.
Photography was performed with an Olympus BX-70 digital camera, and images were processed with Image Pro Plus (Media Cybernetics, Silver Spring, Md.).
Flow cytometry.
Flow cytometric analysis was performed with an Epics Elite ESP apparatus (Coulter Corp., Hialeah, Fla.). Gates for detection of Thy-1–fluorescein isothiocyanate or GFP were established with mock-infected cells as a background. Because electronic settings varied from experiment to experiment, gates were defined such that the percentage of false-positive events was not higher than 0.3 in the mock-infected population. Cell cycle analysis was performed with Multicycle AV software (Phoenix Flow Systems, San Diego, Calif.).
RESULTS
Construction and characterization of an SIV-based packaging system.
To generate a lentivirus packaging system based on SIV, we chose to use the molecular clone SIVmac1A11 (30, 32). SIVmac1A11 is adapted for growth in human cells; therefore, its ability to complete the viral life cycle and direct gene expression in human cells is optimal. SIVmac1A11 readily infects nondividing cells, such as macrophages (2, 3). In addition, SIVmac1A11 is a nonvirulent molecular clone. When rhesus macaques are experimentally inoculated with SIVmac1A11, the animals display transient viremia and do not show clinical signs of immunodeficiency for observation periods of up to several years (31, 32). Infection by SIVmac1A11 is accompanied by the development of weak humoral immune responses, but viral burden becomes undetectable after 2 months. SIVmac1A11 has a full-length vpx open reading frame and a truncated vpr gene (28). SIVmac vpx is necessary for efficient infection of macrophages (15) and is therefore a desirable gene in a gene transfer vector designed to infect nondividing cells. SIVmac vpr, however, induces cell cycle arrest in G2; therefore, its presence in a lentivirus vector should be deleterious to the host cell (15, 18, 20, 46, 49).
An SIVmac1A11-derived packaging construct, SIVpack (Fig. 1A), was generated by providing transcriptional elements from SV40 in substitution for the SIVmac long terminal repeats (LTR) and by deleting the SIV surface glycoprotein (gp130)-coding region (see Materials and Methods for details). SIVpack has gag, pol, vif, vpx, tat, and rev from SIVmac1A11 (Table 1). The Rev-responsive element is retained to allow the production of unspliced transcripts encoding Gag-Pol. The SIV packaging cis element (52) is not present in SIVpack; therefore, encapsidation of SIVpack-derived mRNAs into lentivirus particles is not expected.
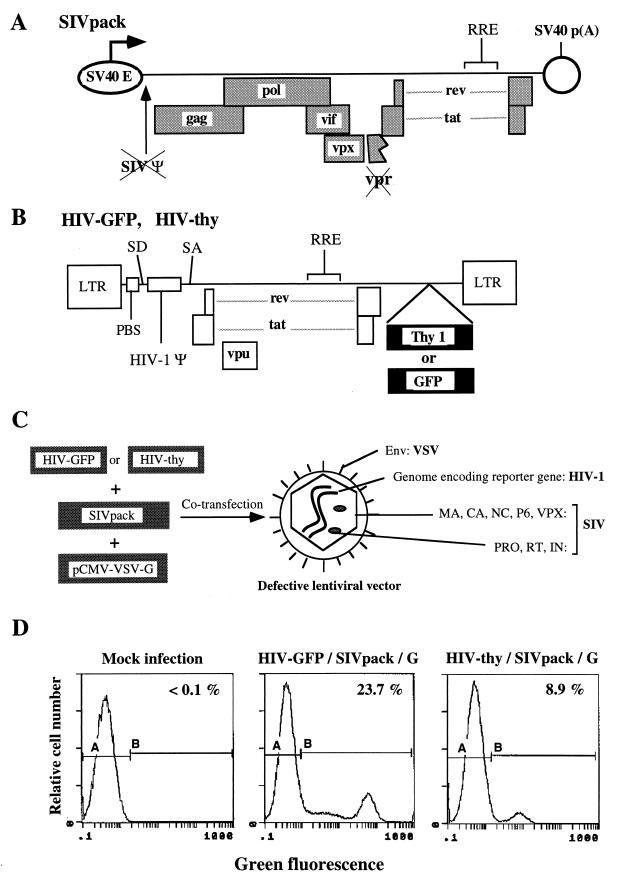
FIG. 1.
Production of an RNA pseudotype lentivirus vector. (A) SIVpack is a packaging construct based on SIVmac1A11. SIVpack was constructed by subcloning a subgenomic fragment of SIVmac1A11 into an SV40-derived expression vector and subsequently deleting gp120 envelope sequences; SIVmac1A11 contains a frameshift mutation which inactivates vpr. (B) Transfer vectors. HIV-GFP and HIV-thy are transfer vectors based on HIV-1 and contain all the cis-acting elements needed for reverse transcription, integration, and expression. PBS, primer binding site; SD, splice donor; SA, splice acceptor; RRE, Rev-responsive element. (C) Production of lentivirus vectors. HIV-GFP or HIV-thy, SIVpack, and a VSV-G expression construct (7) were transfected by electroporation into COS cells, and supernatants were harvested and frozen at 48 h. Infection was quantitated on the basis of GFP or Thy-1 expression, depending on the transfer vector used. (D) Flow cytometric analysis of HeLa cells infected with lentivirus vectors. HeLa cells were infected with the indicated lentivirus vectors at a dilution of 1:10. At 48 h postinfection, cells were analyzed by flow cytometry for expression of GFP or Thy-1. See Table 2 for the resulting titers.
TABLE 1.
Description of principal genetic features of an SIVmac1A11-based lentivirus packaging construct
| Molecular determinant | Feature(s) | Reference(s) | Maintained (M) or deleted (D) |
|---|---|---|---|
| gag/pol | Encodes indispensable structural proteins | M | |
| vif | Necessary for infectivity in certain cell types; dispensable in HIV-1 packaging construct | 9, 27, 41, 61–63 | M |
| vpx | Infection of nondividing cells | 15, 22, 42 | M |
| vpr | Induces cell cycle arrest in CD4+ lymphocytes; dispensable in HIV-1 packaging construct | 15, 46, 49, 63 | D |
| tat | Necessary for high-level expression from the viral LTR; may be dispensable in HIV-1 packaging construct | 55 | M |
| rev | Necessary for expression of Gag-Pol | 4, 12, 25, 39, 60 | M |
| env | Replaced in trans by VSV-G | 7 | D |
| nef | Key determinant of pathogenesis in vivo; dispensable in HIV-1 packaging construct | 21, 63 | D |
| RRE | cis-Acting element necessary for Rev function | 4, 12, 25, 39 | M |
Generation of infectious vector stocks was accomplished by cotransfection of SIVpack, a transfer construct (HIV-GFP or HIV-thy; Fig. 1A and B), and a VSV-G-expressing construct (7). HIV-GFP and HIV-thy contain multiple-deletion HIV-1 genomes which express HIV-1 tat, rev, vpu, and a reporter gene, either GFP or thy-1 (45). Supernatants from these transfections were used to infect HeLa cells, and the extent of infection was measured by monitoring GFP or surface Thy-1 expression (Fig. 1D and Table 2).
TABLE 2.
Comparison of titers from retrovirus vectorsa
| Virus | Transfection constructs
|
Titer (IU/ml) | Core protein (ng/ml) | ||
|---|---|---|---|---|---|
| Transfer vector | Packaging vector | Envelope | |||
| HIV-GFP/SIVpack/G | HIV-GFP | SIVpack | HCMV-VSVG | 2.3 × 106 | 2.7 × 103 |
| HIV-GFP/Ψ(−)env(−)ampho/G | HIV-GFP | Ψ(−)env(−)ampho | HCMV-VSVG | <100 | NA |
| HIV-GFP/SIVpack | HIV-GFP | SIVpack | None | <100 | 5.1 × 102 |
| HIV-GFP/G | HIV-GFP | None | HCMV-VSVG | <100 | <0.1 |
| HIV-thy/SIVpack/G | HIV-Thy | SIVpack | HCMV-VSVG | 8.9 × 105 | 1.2 × 103 |
| LNCX-GFP/SIVpack/G | LNCX-GFP | SIVpack | HCMV-VSVG | <100 | 1.7 × 103 |
| LNCX-GFP/Ψ(−)env(−)ampho/G | LNCX-GFP | Ψ(−)env(−)ampho | HCMV-VSVG | 1.3 × 105 | NA |
| HIV-GFP/HIVpack/G | HIV-GFP | pCMVΔR8.2 | HCMV-VSVG | 2.0 × 107 | 1.4 × 103 |
HeLa cells (106) were infected with vectors at a dilution of 1:10 in tissue culture medium, and at 48 h postinfection, GFP- or Thy-1-positive cells were quantitated by flow cytometry. Vector titers were calculated as described in Materials and Methods and expressed in infectious units (IU). One IU corresponds to one GFP- or Thy-1-positive cell. Detection of core proteins (SIV p27 and HIV-1 p24) was performed by a capture ELISA; the lower limit of detection for this ELISA was 0.1 ng/ml. NA, not applicable. The transduction efficiency of HIV-GFP/SIVpack/G for infection of HeLa cells with an undiluted virus preparation was 40%.
The SIVmac-packaged vectors HIV-GFP/SIVpack/G and HIV-thy/SIVpack/G displayed titers of 2.3 × 106 and 8.9 × 105 infectious units (IU)/ml, respectively (Table 2). Transfections performed in the absence of the envelope (HIV-GFP/SIVpack) or the packaging construct (HIV-GFP/G) produced no detectable infectious vector (<100 IU/ml). To our knowledge, the above experiment constitutes the first demonstration that the HIV-1-derived genome can be packaged by an SIVmac capsid. Retrovirus particles which package a heterologous genomic RNA have been described earlier and are referred to as RNA pseudotypes (8, 11, 14, 52). Since HIV-GFP/SIVpack/G and HIV-thy/SIVpack/G contain a heterologous envelope (VSV-G), these vectors are also considered envelope pseudotypes. Encapsidation of an HIV-1-derived genome by SIVmac proteins probably occurs due to the relative phylogenetic proximity of SIVmac and HIV-1. A more distantly related virus, such as murine leukemia virus, would not be expected to efficiently encapsidate an HIV-1-derived genome. To verify this idea, HIV-GFP was cotransfected with HCMV-VSVG and a murine leukemia virus-derived packaging construct, Ψ(−)env(−)ampho (24), to produce the hypothetical vector HIV-GFP/Ampho-pack/G (Table 2). Infection with HIV-GFP/Ampho-pack/G produced no detectable GFP expression (<100 IU/ml). In contrast, a murine leukemia virus-derived transfer vector, LNCX-GFP, was efficiently packaged by a murine packaging construct [LNCX-GFP/Ψ(−)env(−)ampho/G; 1.3 × 105 IU/ml] but was not packaged by SIVpack (LNCX-GFP/SIVpack/G; <100 IU/ml). Thus, encapsidation of a heterologous transfer vector by SIVpack-derived virus particles is specific because it occurs when the transfer vector is from a closely related virus (i.e., HIV-1) but not when it is from a murine retrovirus.
When HIV-GFP was transfected with a previously described HIV-1-derived packaging construct, pCMVΔR8.2 (38), the vector titer obtained was 2.0 × 107 IU/ml, approximately 1 order of magnitude higher than the titer obtained with SIVpack. The higher infectivity of an HIV-1-packaged vector may reflect more efficient genome encapsidation by HIV-1 proteins than by SIV proteins.
Transduction of nondividing cells with HIV-GFP/SIVpack/G.
One key property of lentiviruses is their ability to infect nondividing cells. This ability stems from the fact that lentiviruses contain multiple nuclear localization determinants (6). These determinants, for HIV-1, consist of the matrix (MA), integrase (IN), and Vpr proteins (6). SIVmac (36) and, in particular, SIVmac1A11 (3, 30) efficiently infect macrophages. Although the presence of the MA and IN determinants of nuclear transport has not been formally demonstrated for SIV, it is presumed that such determinants are conserved between SIV and HIV-1 (6). The potential role of Vpr in nuclear localization is more complex in SIVmac than in HIV-1 because SIVmac contains two related genes, vpr and vpx (54, 58). SIVmac vpx, but not vpr, has retained the nuclear transport function (15).
Based on the above facts, we hypothesized that HIV-GFP/SIVpack/G should be competent for infection of nondividing cells. This hypothesis was tested by infecting radiation-arrested cells. Radiation-arrested cells were produced by subjecting MAGI (23) cells to 2,000 or 5,000 rads of gamma radiation. After irradiation, cells were plated and, at days 1 through 5 postirradiation, stained with propidium iodide to analyze DNA contents. Irradiated cells accumulated and remained in the G2 phase of the cell cycle for the duration of the experiment (data not shown). We confirmed the lack of proliferation of irradiated cells by plating cells in multiple replicate wells and counting viable and nonviable cells daily from days 1 to 4. Irradiated cell numbers and viability remained unchanged for the duration of the experiment (data not shown).
MAGI cells are HeLa cell transfectants containing an integrated, silent LTR–β-galactosidase cassette which is induced when Tat is produced upon infection by HIV (23). Untreated (nonirradiated) or radiation-arrested MAGI cells (2,000 or 5,000 rads) were exposed to HIV-GFP/SIVpack/G at a multiplicity of infection (MOI) of 0.02 or 0.002; 48 h later, infections were quantitated by measurement of GFP fluorescence (Fig. 2) and staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (data not shown). The number of GFP-positive (i.e., infected) cells was not significantly different between nonirradiated cells and cells irradiated with 2,000 or 5,000 rads. When infections were quantitated on the basis of β-galactosidase instead of GFP expression, identical results were obtained (data not shown). Thus, the ability of HIV-GFP/SIVpack/G to transduce cells is independent of the proliferation state of the target cells.
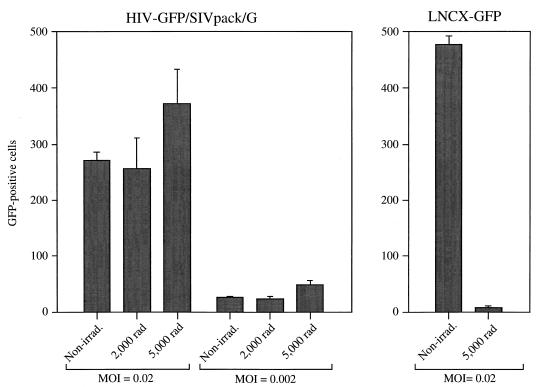
FIG. 2.
HIV-GFP/SIVpack/G transduces nondividing cells with a high efficiency. MAGI cells (23) were treated with 0 (Non-irrad.), 2,000, or 5,000 rads of gamma radiation. After 24 h, cells were infected with the indicated retrovirus vectors. Infections were analyzed on the basis of GFP expression. Infections were performed in triplicate. Mean values are reported. Error bars represent standard deviations.
In contrast to infection by lentiviruses, infection by murine oncoviruses is highly dependent on cell division (16, 19). Therefore, irradiated MAGI cells, which can be infected with HIV-GFP/SIVpack/G, should be resistant to infection with a murine oncovirus. To confirm this expectation, irradiated and nonirradiated MAGI cells were infected in parallel with the murine vector LNCX-GFP/Ψ(−)env(−)ampho/G (Table 2 and Fig. 2). Infection with LNCX-GFP/Ψ(−)env(−)ampho/G was heavily dependent on the cycling state of the target cells, as evidenced by a 110-fold decrease in the number of infected irradiated cells relative to nonirradiated cells (Fig. 2).
Transduction with HIV-GFP/SIVpack/G is stable.
Retrovirus-mediated gene therapy is intended as a means of permanent genetic modification of target cells. Thus, the genetic modification introduced should not affect the growth properties and viability of the transduced cells. We previously demonstrated that defective HIV-1-derived genomes compromised the viability of the target cells, even when deleterious genes such as nef and env were eliminated from the viral genome (44). We therefore decided to test whether cells transduced with HIV-GFP/SIVpack/G would maintain expression of the reporter gene several weeks after infection. HeLa cells were infected with HIV-GFP/SIVpack/G as described in the legend to Fig. 1 and Table 2. At 48 h postinfection, flow cytometric analysis demonstrated a level of infection of 8.6%. We wished to evaluate the stability of the retrovirus construct under conditions in which transduced cells would have no selective growth advantage with respect to nontransduced ones. To accomplish this, bulk transduced cells were seeded in microtiter wells at a density of one cell per well in the absence of drug selection. A total of 221 cell clusters were visually inspected for GFP expression. Since we did not use drug selection, we expected that 8.6% of the 221 cell clusters (19 clusters) would be positive. One week after plating of the infected cells, 221 cell clusters were counted; 26 (11.7%) were GFP positive. After 19 days in culture, 25 of the 26 GFP-positive cell clusters remained positive and showed growth properties and morphology indistinguishable from those of nontransduced (GFP-negative) cell clusters. Therefore, transduction with HIV-GFP/SIVpack/G led to integration and stable expression of the reporter gene without compromising the division or viability of the target cells.
Transduction of various cell types with HIV-GFP/SIVpack/G.
We tested the ability of HIV-GFP/SIVpack/G to transduce various cell types which may be representative of potential targets for gene therapy. The results are summarized in Table 3. We first infected immortalized CD4-positive lymphocytes, CEMX174 (NIH AIDS Research and Reference Reagent Program). Infection of CEMX174 cells at an MOI of 1.0 resulted in a frequency of GFP-positive cells of 11% (Table 3 and Fig. 3A and B).
TABLE 3.
Infection of various cell types with HIV-GFP/SIVpack/G and LNCX-GFP/Ψ(−)env(−)ampho/Ga
| Target cells | % of GFP-positive cells after exposure to:
|
|
|---|---|---|
| HIV-GFP/ SIVpack/G | LNCX-GFP/Ψ(−) env(−)ampho/G | |
| CEMX174 | 11 | ND |
| Primary human macrophages | 15 | <0.1 |
| Primary mouse neurons | 15 | <0.1 |
The indicated cells were exposed to HIV-GFP/SIVpack/G and LNCX-GFP/Ψ(−)env(−)ampho/G at an MOI of 1.0, chosen based on vector titrations on HeLa cells. Except for mouse neurons, cells were infected in the presence of Polybrene at 10 μg/ml. At 48 or 72 h postinfection, fluorescent cells were visually counted under a microscope. Mock infections of the same cell types were performed in parallel and resulted in no detectable fluorescent cells. ND, not determined.
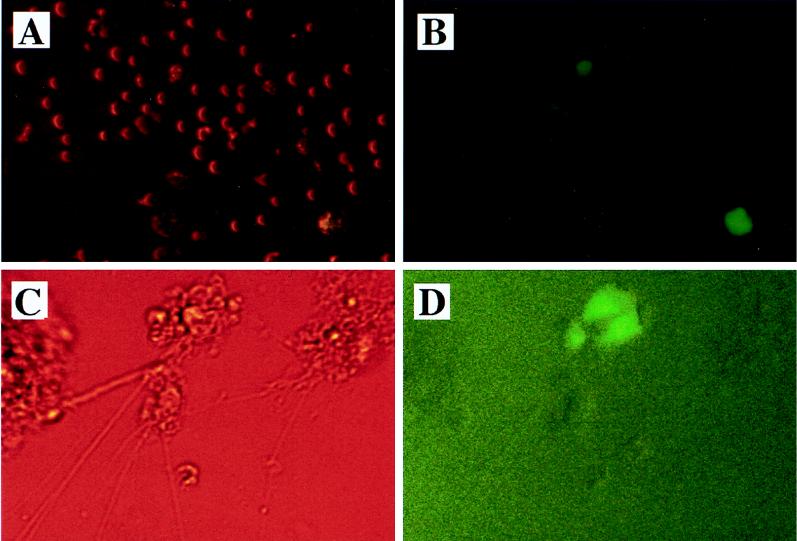
FIG. 3.
Infection of CD4+ lymphocytes and primary neurons with HIV-GFP/SIVpack/G. Cells were infected with HIV-GFP/SIVpack/G at an MOI of 0.2 and visualized by fluorescence microscopy at day 3 postinfection. (A and B) CEMX174 cells. (C and D) Mouse neuronal cells. (A and C) Phase-contrast micrographs. (B and D) Fluorescence micrographs. Mouse neuronal cells were positive for reactivity to the following antibodies: mouse anti-neurofilament 200, rabbit anti-tau, mouse anti-MAP2, and rabbit anti-rat neuron-specific enolase.
Two types of primary cells, mouse neurons from fetal brain tissue (Fig. 3C and D and Table 3) and human peripheral blood macrophages (Table 3), were transduced with HIV-GFP/SIVpack/G. Primary cells were infected at an MOI of 1.0. These infections resulted in 15% GFP-positive cells for both macrophages and neurons. The MOI is calculated based on the infectivity of lentivirus vectors in HeLa cells as described above. In the above and other experiments not shown, we found that all tested primary and nonprimary cells of mammalian origin can be infected with HIV-GFP/SIVpack/G. This result is consistent with previous reports indicating that lentivirus vectors pseudotyped with VSV-G display broad tropism (7, 38). We routinely find, however, that most retrovirus vectors infect HeLa cells efficiently, but they infect primary cells less efficiently.
Infection of mouse neurons and human macrophages with the murine vector LNCX-GFP/Ψ(−)env(−)ampho/G produced no detectable GFP expression, consistent with the lack of infectivity of oncovirus-derived vectors in postmitotic cells (16, 19).
Lack of induction of cell cycle arrest.
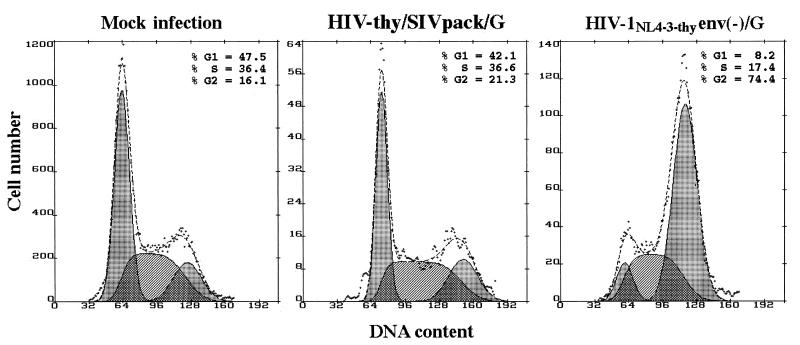
The vpr genes from HIV-1, HIV-2, SIVmac, and SIVagm were shown to cause cell cycle arrest (15, 46). Vpr-induced cell cycle arrest is followed by apoptosis (57) and is therefore deleterious to the target cell. We predicted that an SIV-packaged lentivirus vector which contains Vpx but not Vpr would not cause cell cycle arrest. To test this prediction, cycling HeLa cells were infected with HIV-thy/SIVpack/G. At 48 h postinfection, cells were simultaneously stained for cell surface expression of Thy-1 and DNA contents as described previously (20). Infected cells expressing Thy-1 were electronically gated and analyzed for DNA contents (Fig. 4). A control infection with HIV-1NL4-3-thyenv(−)/G (50), an HIV-1-derived vector which has full-length vpr, resulted in dramatic cell cycle arrest in G2 (G2 plus M, 74.4%), whereas infection with HIV-thy/SIVpack/G produced no significant cell cycle arrest (G2 plus M, 21.9%) compared to data for mock-infected cells (G2 plus M, 16.1%).
FIG. 4.
HIV-thy/SIVpack/G does not induce cell cycle arrest. HeLa cells were mock infected or infected with HIV-thy/SIVpack/G or HIV-1NL4-3-thyenv(−)/G (50) as indicated. At 48 h, cells were stained with fluorescein isothiocyanate-conjugated anti-Thy-1 antibody, fixed, permeabilized, and stained for DNA contents with propidium iodide. Histograms show the cell cycle profiles of Thy-1-positive cells for HIV-thy/SIVpack/G and HIV-1NL4-3-thy env(−)/G infections and untreated cells for mock infection. Cell cycle analysis was performed with Multicycle AV software. The G1, S, and G2 peaks (left, middle, and right shaded areas, respectively) are shown below the DNA profile (dotted line).
Detection of replication-competent viruses.
We investigated the potential for the emergence of replication-competent viruses in the lentivirus system. We used CEMX174 cells (56) as indicator cells because they are susceptible to infection by a broad range of SIVmac and HIV-1 strains (2, 56). COS cells which had been transfected to produce HIV-GFP/SIVpack/G or supernatants thereof were cocultured with CEMX174 cells (first vector passage) for 48 h. CEMX174 cells became infected with defective retrovirus vectors, as evidenced by GFP expression, as described in the legend to Fig. 3 (data not shown). Supernatants from vector-infected CEMX174 cells were used to infect fresh CEMX174 cells (second vector passage). CEMX174 cells infected with the second vector passage were then cultured for 14 days. The presence of replication-competent viruses was evaluated by use of GFP fluorescence and a p27 capture ELISA at days 7 and 14 after exposure to the second vector passage. At both times, no virus could be detected by use of GFP fluorescence or the p27 capture ELISA (data not shown). In addition, supernatants from indicator CEMX174 cells at the same times were used to infect MAGI cells for detection of any potential recombinant viruses which may have retained the expression of Tat. No blue foci could be identified in these cells at 3 days postexposure. Visual examination of second-passage CEMX174 cells and MAGI cells failed to reveal any cytopathic effects which might have been expected in the presence of replication-competent viruses.
DISCUSSION
The most urgent issue regarding the safety of lentivirus vectors is the potential for recombination leading to replication-competent or “helper” virus. The generation of helper virus in preparations of retrovirus vectors has been documented in numerous instances involving oncoviruses (10, 13, 29, 51, 59). In later generations of vectors in which viral protein-coding regions were split in the packaging cells, requiring multiple crossover events to generate replication-competent recombinant virus, the frequency of recombination leading to helper virus was decreased but not eliminated (40). Helper virus has the potential for inducing pathogenesis, as demonstrated by studies in which monkeys were infused with transduced bone marrow cells after ablation of endogenous marrow with gamma irradiation (13, 51, 59). In these studies, helper virus gave rise to lymphoma in the monkeys.
We introduce a new lentivirus vector system containing genetic elements from three different viruses, SIVmac, HIV-1, and VSV. The packaging construct is derived from SIVmac1A11, a nonvirulent SIV isolate previously described (30). The transfer vectors are derived from HIV-1 and are engineered to express either thy-1 or GFP as reporter genes. A heterologous envelope, VSV-G, is supplied in trans as previously described (1, 7, 37) to provide broad cellular tropism. Because VSV-G is a heterologous component of this viral system, the vectors HIV-thy/SIVpack/G and HIV-GFP/SIVpack/G are envelope pseudotypes (7, 38). In addition, since HIV-1-derived genomic RNA is incorporated by heterologous Gag-Pol components derived from SIVmac, these vectors are also RNA pseudotypes (8, 11, 14, 52).
Our studies demonstrate that an RNA and envelope pseudotype vector is functional in gene transduction. The potential safety of this vector system is based on the existence of low nucleotide sequence homology between HIV-1 and SIVmac. A second property of this vector with important implications for safety is that the packaging construct is derived from a nonvirulent SIVmac isolate. Thus, the potential for pathogenicity with this vector system should be minimal.
HIV-1 vpr was shown to induce arrest in the G2 phase of the cell cycle (20, 46, 49, 53). Induction of G2 arrest by vpr is thought to lead to apoptosis (57) and is thus a deleterious function. An ideal lentivirus vector for nondividing cells would encode the former but not the latter function of vpr. The HIV-1 vpr gene is represented in SIVmac by two different genes, vpx and vpr. The two functions of HIV-1 vpr are segregated such that SIVmac vpx participates in infection of nondividing cells via nuclear transport of preintegration complexes (15) and vpr induces cell cycle arrest (46). We have developed a lentivirus vector based on SIVmac which expresses vpx but not vpr. We demonstrate that this vector is able to infect nondividing cells but is unable to cause cell cycle arrest in proliferating cells.
The influence of lentivirus accessory genes involved in virulence (vpr, vpu, nef, and vif) on the ability to transduce nondividing and primary cells was recently addressed in the context of an HIV-1-derived lentivirus vector (63). The study by Zufferey et al. (63) showed that deletion of vif, vpr, vpu, and nef from an HIV-1-derived lentivirus vector does not affect its ability to infect irradiated 293T cells. When primary macrophages were used, deletion of vpr decreased infection by 50%, but deletion of vif, vpu, or nef had no effect. In the experiments presented here, the individual roles of the SIVmac accessory genes were not directly evaluated. We conclude that SIVmac nef and vpr are not essential for the function of the vectors HIV-GFP/SIVpack/G and HIV-thy/SIVpack/G, although it is formally possible that nef and vpr, if present, may modulate vector efficiency in a quantitative fashion. In addition, vif and vpx are present in the SIVmac packaging system, although their individual contributions remain to be evaluated by comparing constructs which differ in the presence of single genes, as was done by Zufferey et al. (63). Thus, future experiments involving the SIVmac packaging system will address the potential contributions of SIVmac vpr, vpx, nef, and vif.
The genetic engineering experiments described in this work cover a rather small spectrum of the possibilities for the development and improvement of lentivirus vectors. An additional safety feature which may be incorporated into future lentivirus systems is the use of self-inactivating transfer vectors (33, 35). In these vectors, the LTR are engineered such that following proviral integration, a viral promoter is not regenerated. Expression is then limited to an internal promoter specific for the gene of interest.
The lentivirus vector that we propose here provides proof of the feasibility of heterologous packaging. However, additional changes will have to be made before such a vector can be considered useful in vivo. These changes include deletion of tat, rev, and vpu from the transfer vector, as was described earlier for an HIV-1-derived system with homologous packaging (38). Deletion of tat will require the inclusion of a strong promoter downstream from the 5′ LTR. Deletion of rev will require the inclusion of a constitutive transport element of Mason-Pfizer monkey virus (43). This element works in cis to allow unspliced and singly spliced mRNAs to be expressed at high levels in a Rev-independent manner.
Lentivirus vectors offer potential for the treatment of a wide variety of syndromes, including genetic and metabolic deficiencies, viral infection, and cancer. Inherited genetic defects, such as adenosine deaminase deficiency, familial hypercholesterolemia, cystic fibrosis, mucopolysaccharidosis type VII, type I and II diabetes, classical phenylketonuria, and Gaucher disease, may be overcome by lentivirus vector-mediated gene therapy because they constitute single-gene deficiencies for which the involved genes are known.
Certain types of cancer may also benefit from the use of lentivirus vectors. Hypoxia and lack of vascularization lead to the generation of tumor cells which exhibit limited or no proliferation. Partly because of the lack of growth, these cells are highly resistant to genotoxic agents. A lentivirus vector may prove to be a useful vehicle for delivery of a “lethal” gene (such as herpesvirus thymidine kinase) to such quiescent tumor cells.
Viral diseases may also constitute appropriate targets for lentivirus gene delivery. In particular, a number of gene therapy approaches have been proposed for the treatment of HIV-1 infection. Preliminary studies have used defective murine oncoviruses for the delivery of antisense RNAs, ribozymes, and trans-dominant proteins against HIV-1 replication. The usefulness of an HIV-1-derived vector for delivery of an anti-HIV-1 strategy would be limited by inhibition of the vector itself. Lentivirus vectors based on SIVmac would overcome such a limitation because sequence and functional disparities between HIV-1 and SIVmac would likely prevent anti-HIV-1 reagents from inhibiting the SIV vector.
ACKNOWLEDGMENTS
We thank W. Sutton, N. Haigwood, and S. Mossman for the generous contribution of antibodies and technical assistance for the detection of SIV p27. We also thank M. Sacco for providing assistance with digital imaging. We thank P. Challita-Eid, R. Bambara, and E. Schwarz for critical reading of the manuscript.
This work was supported by NIH research grants to V.P. (R29-AI41407) and J.D.R. (R01-AI41957).
REFERENCES
- 1.Akkina R K, Walton R M, Chen M L, Li Q-X, Planelles V, Chen I S Y. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banapour B, Marthas M L, Munn R J, Luciw P A. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac) Virology. 1991;183:12–19. doi: 10.1016/0042-6822(91)90113-p. [DOI] [PubMed] [Google Scholar]
- 3.Banapour B, Marthas M L, Ramos R A, Lohman B L, Unger R E, Gardner M B, Pedersen N C, Luciw P A. Identification of viral determinants of macrophage tropism for simian immunodeficiency virus SIVmac. J Virol. 1991;65:5798–5805. doi: 10.1128/jvi.65.11.5798-5805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berchtold S, Hornung U, Aepinus C. The activation domain of simian immunodeficiency virus SIVmac239 Rev protein is structurally and functionally analogous to the HIV-1 Rev activation domain. Virology. 1995;211:285–289. doi: 10.1006/viro.1995.1403. [DOI] [PubMed] [Google Scholar]
- 5.Brewer G J, Torricelli J R, Evege E K, Price P J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 6.Bukrinsky M I, Haffar O K. HIV-1 nuclear import: in search of a leader. Front Biosci. 1997;2:578–587. doi: 10.2741/a213. [DOI] [PubMed] [Google Scholar]
- 7.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Certo J L, Shook B F, Yin P D, Snider J T, Hu W S. Nonreciprocal pseudotyping: murine leukemia virus proteins cannot efficiently package spleen necrosis virus-based vector RNA. J Virol. 1998;72:5408–5413. doi: 10.1128/jvi.72.7.5408-5413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Ido E, Jin M, Kuwata T, Igarashi T, Mizuno A, Koyanagi Y, Hayami M. Replication of human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus strain mac (SIVmac) and chimeric HIV-1/SIVmac viruses having env genes derived from macrophage-tropic viruses: an indication of different mechanisms of macrophage-tropism in human and monkey cells. J Gen Virol. 1998;79:741–745. doi: 10.1099/0022-1317-79-4-741. [DOI] [PubMed] [Google Scholar]
- 10.Chong H, Vile R G. Replication-competent retrovirus produced by a ‘split-function’ third generation amphotropic packaging cell line. Gene Ther. 1996;3:624–629. [PubMed] [Google Scholar]
- 11.Corbeau P, Kraus G, Wong-Staal F. Transduction of human macrophages using a stable HIV-1/HIV-2-derived gene delivery system. Gene Ther. 1998;5:99–104. doi: 10.1038/sj.gt.3300563. [DOI] [PubMed] [Google Scholar]
- 12.Dillon P J, Nelbock P, Perkins A, Rosen C A. Function of the human immunodeficiency virus type 1 and 2 Rev proteins is dependent on their ability to interact with a structured region present in env gene mRNA. J Virol. 1990;64:4428–4437. doi: 10.1128/jvi.64.9.4428-4437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donahue R E, Kessler S W, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, et al. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Embretson J E, Temin H M. Lack of competition results in efficient packaging of heterologous murine retroviral RNAs and reticuloendotheliosis virus encapsidation-minus RNAs by the reticuloendotheliosis virus helper cell line. J Virol. 1987;61:2675–2683. doi: 10.1128/jvi.61.9.2675-2683.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher T M, Brichacek B, Sharova N, Newman M A, Stivahtis G, Sharp P M, Emerman M, Hahn B H, Stevenson M. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) EMBO J. 1996;15:6155–6165. [PMC free article] [PubMed] [Google Scholar]
- 16.Fritsch E F, Temin H M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977;24:461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman M J, Lee P S, Yang J S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 18.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphries E H, Temin H M. Cell cycle-dependent activation of Rous sarcoma virus-infected stationary chicken cells: avian leukosis virus group-specific antigens and ribonucleic acid. J Virol. 1972;10:82–87. doi: 10.1128/jvi.10.1.82-87.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kestler H W, Ringler D J, Panicaly D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus load and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 22.Kewalramani V N, Emerman M. Vpx association with mature core structures of HIV-2. Virology. 1996;218:159–168. doi: 10.1006/viro.1996.0176. [DOI] [PubMed] [Google Scholar]
- 23.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landau N R, Littman D R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le S Y, Malim M H, Cullen B R, Maizel J V. A highly conserved RNA folding region coincident with the Rev response element of primate immunodeficiency viruses. Nucleic Acids Res. 1990;18:1613–1623. doi: 10.1093/nar/18.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liesveld J L, Rush S, Kempski M C, Turner A R, Brennan J K, Gasson J C, Abboud C N. Phenotypic characterization of the human fibrous histiocytoma giant cell tumor (GCT) cell line and its cytokine repertoire. Exp Hematol. 1993;21:1342–1352. [PubMed] [Google Scholar]
- 27.Liu H, Wu X, Newman M, Shaw G M, Hahn B H, Kappes J C. The Vif protein of human and simian immunodeficiency viruses is packaged into virions and associates with viral core structures. J Virol. 1995;69:7630–7638. doi: 10.1128/jvi.69.12.7630-7638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luciw P, Shaw K, Unger R, Planelles V, Stout M, Lackner J, Pratt-Lowe E, Leung N, Banapour B, Marthas M. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac) AIDS Res Hum Retroviruses. 1992;8:395–402. doi: 10.1089/aid.1992.8.395. [DOI] [PubMed] [Google Scholar]
- 29.Markowitz D, Goff S, Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 30.Marthas M L, Banapour B, Sutjipto S, Siegel M E, Marx P A, Gardner M B, Pedersen N C, Luciw P A. Rhesus macaques inoculated with molecularly cloned simian immunodeficiency virus. J Med Primatol. 1989;18:311–319. [PubMed] [Google Scholar]
- 31.Marthas M L, Ramos R A, Lohman B L, Van Rompay K K, Unger R E, Miller C J, Banapour B, Pedersen N C, Luciw P A. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67:6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marthas M L, Sutjipto S, Higgins J, Lohman B, Torten J, Luciw P A, Marx P A, Pedersen N C. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990;64:3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller A D. Development and application of retroviral vectors. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 437–473. [PubMed] [Google Scholar]
- 34.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–982. , 984–986, 989–990. [PMC free article] [PubMed] [Google Scholar]
- 35.Miyoshi H, Blomer U, Takahashi M, Gage F H, Verma I M. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori K, Ringler D J, Kodama T, Desrosiers R C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992;66:2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naldini L, Blomer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 39.Olsen H S, Beidas S, Dillon P, Rosen C A, Cochrane A W. Mutational analysis of the HIV-1 Rev protein and its target sequence, the Rev responsive element. J Acquired Immune Defic Syndr. 1991;4:558–567. [PubMed] [Google Scholar]
- 40.Otto E, Jones-Trower A, Vanin E F, Stambaugh K, Mueller S N, Anderson W F, McGarrity G J. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum Gene Ther. 1994;5:567–575. doi: 10.1089/hum.1994.5.5-567. [DOI] [PubMed] [Google Scholar]
- 41.Park I W, Myrick K, Sodroski J. Effects of vif mutations on cell-free infectivity and replication of simian immunodeficiency virus. J Acquired Immun Defic Syndr. 1994;7:1228–1236. [PubMed] [Google Scholar]
- 42.Park I W, Sodroski J. Functional analysis of the vpx, vpr, and nef genes of simian immunodeficiency virus. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:335–344. [PubMed] [Google Scholar]
- 43.Pasquinelli A E, Ernst R K, Lund E, Grimm C, Zapp M L, Rekosh D, Hammarskjold M L, Dahlberg J E. The constitutive transport element (CTE) of Mason-Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J. 1997;16:7500–7510. doi: 10.1093/emboj/16.24.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Planelles V, Bachelerie F, Jowett J B M, Haislip A, Xie Y, Banooni P, Masuda T, Chen I S Y. Fate of the human immunodeficiency virus type 1 provirus in infected cells: a role for vpr. J Virol. 1995;69:5883–5889. doi: 10.1128/jvi.69.9.5883-5889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Planelles V, Haislip A, Withers-Ward E S, Stewart S A, Xie Y, Shah N P, Chen I S Y. A new reporter system for detection of retroviral infection. Gene Ther. 1995;2:369–376. [PubMed] [Google Scholar]
- 46.Planelles V, Jowett J B M, Li Q X, Xie Y, Hahn B, Chen I S Y. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poeschla E, Gilbert J, Li X, Huang S, Ho A, Wong-Staal F. Identification of a human immunodeficiency virus type 2 (HIV-2) encapsidation determinant and transduction of nondividing human cells by HIV-2-based lentivirus vectors. J Virol. 1998;72:6527–6536. doi: 10.1128/jvi.72.8.6527-6536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poeschla E M, Wong-Staal F, Looney D J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 49.Poon B, Grovit-Ferbas K, Stewart S A, Chen I S Y. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science. 1998;281:266–269. doi: 10.1126/science.281.5374.266. [DOI] [PubMed] [Google Scholar]
- 50.Poon B, Jowett J B, Stewart S A, Armstrong R W, Rishton G M, Chen I S. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA-alkylating agent nitrogen mustard. J Virol. 1997;71:3961–3971. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purcell D F, Broscius C M, Vanin E F, Buckler C E, Nienhuis A W, Martin M A. An array of murine leukemia virus-related elements is transmitted and expressed in a primate recipient of retroviral gene transfer. J Virol. 1996;70:887–897. doi: 10.1128/jvi.70.2.887-897.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizvi T A, Panganiban A T. Simian immunodeficiency virus RNA is efficiently encapsidated by human immunodeficiency virus type 1 particles. J Virol. 1993;67:2681–2688. doi: 10.1128/jvi.67.5.2681-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharp P M, Bailes E, Stevenson M, Emerman M, Hahn B H. Gene acquisition in HIV and SIV. Nature. 1996;383:586–587. doi: 10.1038/383586a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shibata R, Sakai H, Ogawa K, Ishimoto A, Adachi A. Comparative studies on tat mutants of three primate lentiviruses. Arch Virol. 1990;114:243–250. doi: 10.1007/BF01310753. [DOI] [PubMed] [Google Scholar]
- 56.Stefano K A, Collman R, Kolson D, Hoxie J, Nathanson N, Gonzalez-Scarano F. Replication of a macrophage-tropic strain of human immunodeficiency virus type 1 (HIV-1) in a hybrid cell line, CEMx174, suggests that cellular accessory molecules are required for HIV-1 entry. J Virol. 1993;67:6707–6715. doi: 10.1128/jvi.67.11.6707-6715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart S A, Poon B, Jowett J B, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanin E F, Kaloss M, Broscius C, Nienhuis A W. Characterization of replication-competent retroviruses from nonhuman primates with virus-induced T-cell lymphomas and observations regarding the mechanism of oncogenesis. J Virol. 1994;68:4241–4250. doi: 10.1128/jvi.68.7.4241-4250.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viglianti G A, Sharma P L, Mullins J I. Simian immunodeficiency virus displays complex patterns of RNA splicing. J Virol. 1990;64:4207–4216. doi: 10.1128/jvi.64.9.4207-4216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto Y, Saito Y, Iida S, Asano J, Sone S, Adachi A. Functional analysis of vif genes derived from various primate immunodeficiency viruses. Virus Genes. 1997;14:195–200. doi: 10.1023/a:1007931826241. [DOI] [PubMed] [Google Scholar]
- 62.Zou J X, Luciw P A. The requirement for Vif of SIVmac is cell-type dependent. J Gen Virol. 1996;77:427–434. doi: 10.1099/0022-1317-77-3-427. [DOI] [PubMed] [Google Scholar]
- 63.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]