Abstract
Many different strategies can be found in the literature to model organ physiology, tissue functionality, and disease in vitro; however, most of these models lack the physiological fluid dynamics present in vivo. Here, we highlight the importance of fluid flow for tissue homeostasis, specifically in vessels, other lumen structures, and interstitium, to point out the need of perfusion in current 3D in vitro models. Importantly, the advantages and limitations of the different current experimental fluid-flow setups are discussed. Finally, we shed light on current challenges and future focus of fluid flow models applied to the newest bioengineering state-of-the-art platforms, such as organoids and organ-on-a-chip, as the most sophisticated and physiological preclinical platforms.
I. INTRODUCTION
Biological models are continuously evolving, and three-dimensional (3D) in vitro cell cultures have been consolidated as an adequate alternative to overcome the drawbacks of classical biological models. On one hand, 3D in vitro cultures improve the low levels of biological complexity and realism of two-dimensional (2D) in vitro models.1,2 On the other hand, they avoid the ethical implications of in vivo models, allowing a robust control of the experimental parameters, reducing time and cost of experiments,2–4 while closely representing the human pathophysiology.5,6
The extracellular matrix (ECM), essential physical scaffolding that provides key biochemical and biomechanical cues,5 is commonly represented in 3D in vitro models using hydrogels.7 These gels accurately mimic the biological features of the ECM, and they comprise the best option for engineering tissue models in microfluidic devices and bioreactors, including organoids and organ-on-a-chip systems.7,8 Natural materials like collagen,9–11 matrigel,9,12–19 or fibrin17 are widely used, but other synthetic materials, with less degradability and further stability, like polyethylene glycol (PEG)20 are also utilized. In addition, combined materials are employed to form hydrogels with different mechanical properties such as mixtures of gelatin–fibrin,21 matrigel–fibrin,17 matrigel–fibrin–gelatin.22
Importantly, fluid flow has an important role in organ morphogenesis, homeostasis, and pathogenesis.23,24 Flow induces cell mechanical stimulation in luminal structures and the interstitial space,25–27 and it transports fundamental nutrients and signaling molecules,24 and it may alter the ECM.28–30 Many 2D systems have incorporated fluid flow component in their models; however, most 3D cell cultures (i.e., ECM-based cell cultures) still lack the physiological fluid dynamism present in vivo (supplementary material, Fig. 1).
Fluid flow stimulates cells mechanically by inducing shear stress (tangential to cell surface, along the direction of the flow) and pressure stress (normal to the cell surface)31,32 (see Glossary Box). These stresses participate in the regulation of cell proliferation, quiescence, differentiation, or migration processes.33
In vessels, flow sensing plays a critical role in development and maintenance via multiple pathways.34–36 An adequate continuous luminal fluid shear stress (FSS) enhances endothelial cells' (ECs) physiological behavior and cell differentiation,37 while abnormal FSS (generated by altered flow rates and/or flow directions) can result in EC sprouting and endothelial-to-mesenchymal transition (EMT), vascular dysfunction, or atherosclerosis.34,38–40 In addition, luminal fluid pressure produces strain on the endoluminal surface, which, among other effects, regulates the thickness of the vessels, i.e., by increasing smooth muscle cell (SMC) hypertrophy and the content of collagen and elastin.31,41
In the interstitium, fluid forces regulate the activity of fibroblasts (FBs), the most common cell type of connective tissue. Interstitial fluid forces activate/differentiate FBs, augmenting their migration and fibrogenesis-related features.27,28 They increase FB adhesion to the ECM in exposed areas, resulting in cell polarization, actin accumulation, and membrane protrusion in the up-stream direction27 [Fig. 1(a)].
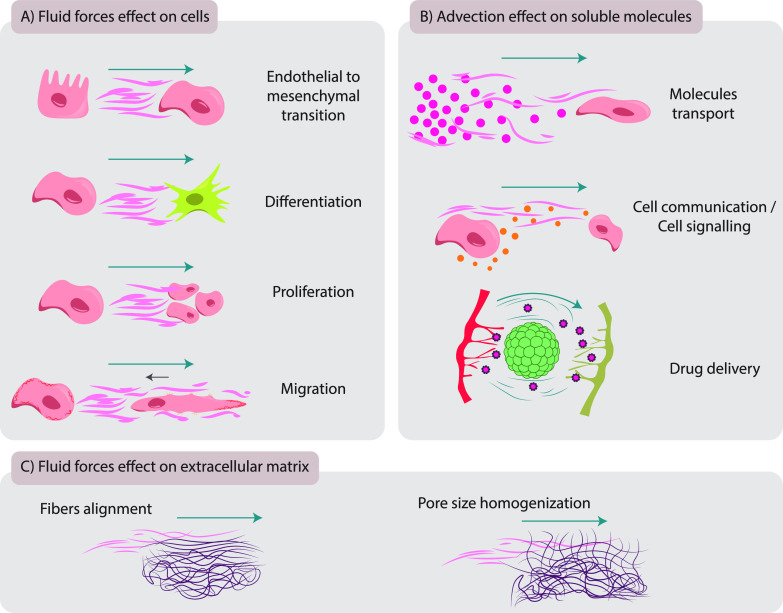
FIG. 1.
The impact of fluid forces on cells, soluble molecules, and extracellular matrix (ECM). (a) The effect of fluid forces on cells affecting endothelial–mesenchymal transition, differentiation, proliferation, and migration. (b) Advection effect on soluble molecules, regulating nutrients transport, generating chemical gradients, and controlling cell communication/cell signaling and drug delivery (fluid flow is represented by blue lines). (c) The effect of fluid forces on the ECM, favoring ECM fibers' alignment and pore size homogenization.
The transport of molecules in a biological fluid, mainly regulated by diffusion (random motion of molecules) and advection (molecules' movement by the fluid's bulk motion), is also affected by flow (see Glossary Box).25,33,42 Flow advection directly impacts on nutrients distribution, cell communication, and drug delivery [Fig. 1(b)]. Thus, the undesired results of the lack of advection can be accentuated in large-scale in vitro cell cultures, where tissue thickness hinders the transport of nutrients and gasses.43 In addition, as diffusion is reduced with increasing molecular size,44 the advection generated by fluid flow is especially relevant for the transport of large molecules like antibodies, nanoparticles, or exosomes, carriers of advanced medicines that harbor a large size in comparison with previous drugs.45
Glossary Box:
The following are the types of stresses induced by fluid flow:
-
•
Shear stress (τ): mechanical force per area exerted tangential to the cell surface, along the direction of flow.
-
•
Pressure stress (p): mechanical force per area induced perpendicular to the cell surface
Transport of molecules in a fluid is mainly regulated by the following:
-
•
Diffusion: net movement of solutes from a region of higher solute concentration to a region of lower solute concentration due to the random motion of the solute and water molecules.
-
•
Advection: molecules movement/transport by a fluid bulk motion.
Other key elements of fluid dynamics in microfluidic devices are as follows:
-
•
Dynamic viscosity or absolute viscosity (μ): refers to internal resistance that a fluid exerts to flow.
-
•
Kinematic viscosity (η): refers to the ratio of dynamic viscosity to density.
-
•
ECM permeability (K): refers to the resistance of a tissue or hydrogel to let the fluid flow through it.
The above-described variables are related in Newtonian fluids by the following equations:
Therefore, in a defined section of a vessel or tissue, and a given flow (Q), the viscosity of a fluid (μ)will directly regulate the shear stress (τ). To achieve the same level of flow (Q) with a highly viscous fluid, the system will require a higher pressure drop (Δp). The permeability of the tissue (k) or the hydraulic resistance of the vessel (Rh) will determine the required pressure drop (Δp).
Conversely, in a defined section of a vessel or tissue, and a given pressure drop (Δp), a high viscosity of the fluid will reduce the flow (Q), also conditioned by the permeability of the tissue (k) or the hydraulic resistance of the vessel (Rh).
Most biological fluids show non-Newtonian behavior (the viscosity is not constant, it is dependent on the shear rate); thus, the above-explained equations are used as an approximation, given the difficulty of studying non-Newtonian behavior.
Regarding the interstitial fluid forces' effect on the ECM, although they have not been studied independently of cells in vivo, in vitro experiments show that fluid forces may promote the homogenization of the pore size and the alignment of ECM fibers 28–30 [Fig. 1(c)], as will be discussed in Sec. II.
This work is organized as follows. First, we describe the role of fluid flow for tissue development, homeostasis, and disease, specifically in vessels, other lumen structures, and interstitium, to highlight the need of perfusable and agitation platforms in current in vitro 3D models (Sec. II). Next, the advantages and limitations of different experimental fluid flow approaches in ECM-containing microfluidic devices and other bioreactors are discussed (Sec. III). Then, we shed light on fluid flow models applied to the newest bioengineering applications, such as, organoids and organ-on-a-chip, as the most sophisticated and physiological preclinical platforms, with the aim of reaching personalized and regenerative medicine (Sec. IV). Finally, we discuss current challenges and future perspectives in the field (Sec. V).
II. FLUID FLOW IN DEVELOPMENT, HOMEOSTASIS, AND DISEASE: ESSENTIALS OF PERFUSED 3D IN VITRO MODELS
As mentioned before, fluid flow is a key regulator for the maintenance of cellular homeostasis, soluble molecules' transport, and ECM structure. In this section, we highlight the importance of fluid forces across the human body to regulate tissue development, homeostasis, and in pathological conditions such as cardiovascular diseases or cancer. We will also comment on the in vitro models used to mimic blood and lymphatic vasculature and other lumen-endothelialized and epithelialized structures.
A. Relevance of flow in blood vasculature
Blood endothelial cells (BECs) sense flow mechanical forces and transduce the signal to different biological outcomes in pivotal processes that will be discussed through this section, such as vascular development, homeostasis, or various disease states.37,46,47
During embryonic development, blood vessels are formed first through vasculogenesis and later through angiogenesis.48 Then, vascular network growth and remodeling takes place when blood circulation has already begun, and the endothelium is exposed to fluid mechanical forces such as shear stress, circumferential stress, and axial stress. Intraluminal shear stress is the force parallel to the tissue surface of the endothelium that arises due to flow of a viscous fluid and depends on the flow rate, viscosity of the blood, as well as on the geometry of the vessel. Circumferential stress (force tangential to the vessel wall) and axial stress (along the longitudinal axis) are governed by the intraluminal pressure. These forces together with transendothelial blood flow (across endothelium) are needed for the expansion of the primary vascular plexus (sprouting angiogenesis).39,49
Adult homeostatic blood vessels (BVs) are formed by ECs lining the inner lumen, which are covered by a basal membrane and pericytes in capillaries, together with smooth muscle cells and adventitia in the case of arteries and veins. The intraluminal shear stress and stretch generated by luminal and transendothelial blood flow, together with intraluminal pressure, govern cell morphology, proliferation, protein expression, and the ability of ECs to attract monocytes.50 Additionally, these forces regulate vessel tone by inducing adaptive dilation of the vessel wall through the activation of the ion channel Piezo1 in ECs and the release of nitric oxide.51
Cardiovascular diseases are the main cause of death globally. One of the most common cardiovascular pathologies is BV occlusion due to atherosclerosis (lipidic plaque formation in arteries) or thrombosis.52,53 Disturbed blood shear stress in vessel branch points or bifurcations is known to induce EC proliferation and activation of inflammatory pathways, thus predisposing these sites to atherosclerosis.51 Turbulent flow shear stress is atherogenic as a result of Piezo1-dependent stimulation of the NF-kB pathway in ECs.54,55 Conversely, the etiology of some vascular malformations such as arteriosus venous malformations is associated to altered blood flow due to vessel enlargement.56
1. 3D in vitro models to mimic blood vasculature
To investigate vessel development, homeostasis, and disease states, tissue engineering approaches have been developed. Tissue engineering models have tried to implement functional vasculature by generating perfusable vascular units.57,58 To this end, preformed vessels can be developed in polydimethylsiloxane (PDMS) by soft lithography59 or microfabricated in plastic58 or hydrogel.60,61 They can also be produced in hydrogels by sacrificial molds62,63 or laser ablation.64
First, with the aim of investigating angiogenesis, different strategies have been used such as ECs monolayers65,66 or endothelialized microvessels,67 where sprouting or angiogenesis processes occur in response to the stimulation with the vascular endothelial growth factor (VEGF).68–70 As discussed above, since the mechanical stimuli exerted by blood flow is a required physiological factor for new vessels formation,71 recent in vitro vascularized models aiming to study angiogenesis recapitulate flow forces to generate more reliable results.65,72,73 When this process starts, ECs begin to remodel the ECM by degrading the matrix via the release of metalloproteinases (MMPs),74,75 which allow EC migration and microvascular network formation.68,76 As an example, Kim et al.57 developed a complete microvascular network of ECs, where they studied EC interaction with pericytes. More recently, 3D bioprinting methods have been used for precise positioning of ECs77–79 to decipher the mechanisms behind vascular morphogenesis processes.80,81
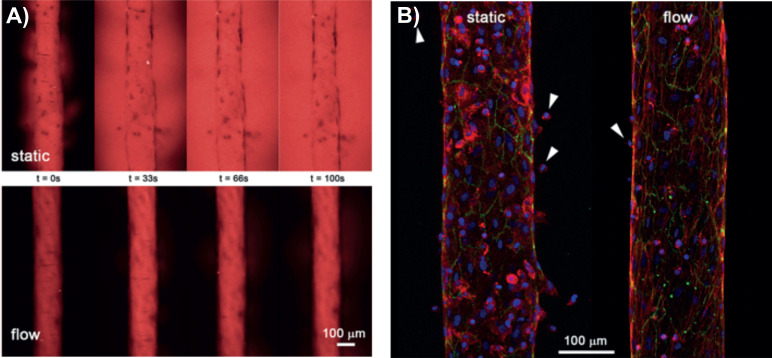
Second, to mimic homeostatic BVs in vitro, lumenized constructs covered with ECs are made by an endothelialization process. The structure, in contact with an ECM, may also include mural cells to recapitulate the in vivo physiology of BVs.82 To achieve flow stimuli in these structures, different perfusion systems including rockers,72 fluid columns,65 or peristaltic pumps73 have been designed, according to the purpose of the investigation (as discussed in Sec. III). These models have served to study physiological events occurring in vivo, highlighting the role of FSS to maintain endothelial cell integrity, while reducing vessel permeability and immune cell extravasation, compared to vessels under static conditions (Fig. 2).67
FIG. 2.
Effect of FSS in blood vessel structure and function. (a) 70 kDa red-fluorescent dextran permeability assay. Vessels generated under flow show high endothelial integrity and reduced permeability compared to vessels under static condition. (b) Reduced monocyte extravasation in vessels formed under flow. Monocytes are indicated with white arrows. Endothelial vessels are stained for F-actin(red), VE-cadherin (green), and DAPI (blue). Adapted with permission from Pérez-Rodríguez et al., J. Biomicrofluidics 15, 0541012 (2021). Copyright 2021 Authors, licensed under a Creative Common Attribution (CC BY) license.67
Third, perfused 3D in vitro models can be effective tools to gain insight into the mechanisms driving the progression of different pathologies. For instance, to study cardiovascular diseases such as atherosclerosis or thrombosis, a microvascular network formation with flow path can be relatively easily implemented in microfluidic devices.83 However, to accurately simulate pathologies in arteries, all relevant stresses and strains should be incorporated into a single setup, and designing such a model is a complex task that requires careful consideration. Capturing the full complexity of in vivo flow is an extremely challenging task in vitro. Therefore, it is crucial to carefully consider the balance between building a system that is limited by over-complexity versus carefully tuning specific flow parameters.
B. Relevance of flow in lymphatic vasculature
The lymphatic system, which is precisely coordinated with the cardiovascular system, transports fluid and cells from the interstitial space to lymph nodes and, then, to the blood circulation.84 Lymphatic endothelial cells (LECs) like BECs are able to mechanosense fluid flow, which is crucial for the development, maintenance, and disease of the lymphatic system, as it will be discussed through this subsection.
Lymphatic vascular development starts by LECs' transdifferentiation from venous ECs (in most cases, though alternative sources have been observed).85 LEC progenitors coalesce and expand to form primitive plexus. Then, under pressure gradient, fluid is transferred from the interstitial space into the lumen of the lymphatic plexus. This process stretches LECs and leads to the expansion of the lymphatic plexus. Mechanical stretching activates integrin ß1, which enhances VEGF-C-dependent phosphorylation of VEGF receptor 3 (R-3). The activation of this signaling pathway potentiates LEC proliferation and expansion of the network.86 Additionally, laminar shear stress induced by intraluminal flow inhibits NOTCH1 and induces Ca2+ signaling and the transcription factors KLF2/4 that promote LEC proliferation and sprouting87–89 till a more mature network consisting in capillaries and collecting lymphatic vessels (LVs) is formed. Indeed, for the formation of collecting LVs and valves, lymph flow sensing by LECs is needed in areas of flow recirculation.37,90,91
Adult homeostatic lymphatic capillaries are formed by LECs lining the inner lumen with discontinuous “button- like” junctions, discontinuous basement membrane, and no supporting mural cells to ensure the absorption of liquid from the interstitium. Whereas adult collecting LVs are formed by LECs with a continuous basement membrane, continuous zipper-like junctions, smooth muscle cell coverage, and intraluminal valves to transport the lymph unidirectionally.92 As transendothelial flow in capillary LVs has been related to junctional maturation,93 LV valve maintenance has been shown to be dependent on luminal FSS.94 Studies conducted on cultured BECs and LECs have demonstrated that VEGFR-3 is involved in regulating the setpoint of FSS, which is the preferred range of shear stress that induces physiological endothelial responses to flow. Therefore, VEGFR-3 can alter cell alignment or suppression of inflammation, and, thus, contribute to the maintenance of vascular homeostasis.95
Dysfunction of the lymphatic system has been related to devastating diseases, such as lymphedema, inflammation (e.g., Crohn's disease), tumor metastasis, obesity, glaucoma, cardiovascular, or neurovascular pathologies.96 In these pathologies, defective LVs induce lymph backflow, which is related with many of the outcomes of the diseases. As an example, in the case of lymphedema, defective lymph drainage frequently leads to tissue fibrosis due to lymph stasis together with fat deposition and local immunodeficiency, caused by the abnormal local chronic inflammatory response, which, in turn, increases susceptibility to infections.97 Additionally, an abnormal cardiac lymph flow has been shown to induce cardiac edema and inflammation, thus affecting heart fluid balance and immune surveillance, which is partially reduced when inducing lymphangiogenesis.98
1. 3D in vitro models to mimic lymphatic vasculature
In the last decade, some engineering studies modeling perfused LVs in vitro have been published. Osaki et al. modeled LVs to study lymphangiogenesis and its interaction with angiogenesis73 using molded microchannels within ECM collagen gel, perfused with a peristaltic pump. Moreover, studying LVs in microfluidic platforms opens new avenues for understanding organ homeostasis and pathology.42 To mimic the transport kinetics of biomolecules and drugs, Zhang and colleagues used bioprinting techniques with very precise bioink flow rate to construct the wall thicknesses of an end-blinded lymphatic capillary next to a blood vessel.99 Though this system lacks cell lining and, thus, active transport, the model could provide insight on how the transport of biomolecules happens by diffusion. On the other hand, since LVs are one of the preferential routes for metastases, Lee et al.100 studied, via syringe pumps, how FSS through a cylindrical lymphatic channel modifies cancer cell motility. Also, breast cancer models with tubular LVs have been generated to study the effect of ECM density on LV morphology, growth, cytokine secretion, and barrier function.101
An additional challenge to increase the biological relevance of microfluidic devices is using more realistic perfusate fluids. The commonly used synthetic (i.e., commercial) cell culture media do not completely correlate with the biochemical composition of the fluids to which cells are exposed in vivo, which can alter the gene expression and relevance of in vitro models.102,103 Furthermore, the biophysical properties of the synthetic media, widely used in perfused in vitro models,45,104 also affect cell behavior.105 Commercial media have reduced viscosity (∼0.8 cP or mPa·s)45,106 in comparison with blood (∼3.4 cP) or lymph viscosity (∼1.8 cP),45 which lowers the resultant shear stress and pressure exerted on cells (in flow-controlled experiments).106
C. Other lumen-endothelialized or epithelialized structures
Our body also contains other tubular structures exposed to fluid flow and shear stress, such as renal convoluted proximal tubules,107,108 renal collecting ducts,109 liver sinusoids,110 or the gut.111 Particularly, their morphology (i.e., cytoskeleton organization, microvilli formation) and function (i.e., cubilin/megalin, albumin, or drug transport),32 as well as their differentiation, are affected by fluid mechanical forces, and 2D static models cannot predict tissue functions that arise in perfused 3D geometries.107,109 For example, FSS in the kidney nephron is thought to vary from ∼1.0 dyne/cm2 in the proximal tubule to <0.5 dyne/cm2 in the collecting duct, while pressure can range between ∼13 mmHg in the proximal tubule to <7 mmHg in the collecting duct.112
1. 3D in vitro models to mimic other lumen or epithelized structures
To engineer the above-mentioned tubular structures in vitro, close-loop perfused 3D models of renal tubules can be built as conduits together with a blood vessel conduit, allowing advanced functional studies with precise tubular-vascular metabolite exchange.113 Similarly, perfusion systems can be incorporated to 3D bile ducts to functionally activate cholangiocytes,114 or to intestines to study their epithelial barrier integrity115 and prolong tissue lifespan by several weeks.116
Different to hydrogel ECM, hydrogel-coated membranes are widely used to simplify the study of flow-induced dynamic lumen–epithelial interactions and facilitate the generation of more complex structures. This is the case of liver sinusoids, the basic structural unit of the liver (composed of liver sinusoidal ECs, holding Kupffer cell, stellate cells, and hepatocytes117), whose functionality is enhanced by flow shear stress118 (in vitro modeling of liver sinusoids is fully reviewed here119). Hydrogel-coated membranes are, as well, used in intestinal crypt-villi structures 120,121 and microbial-gut-vessel interactions,122 also reviewed elsewhere.123–126 However, these membranes, often made of silicone-like PDMS104,127 or polycarbonate and polyester,128,129 poorly mimic the biological and physicochemical properties of the basal membrane and interstitium. This is important to be considered in terms of tissue stiffness,127 soluble molecules transport,128 and cell viability and function.128 Nevertheless, as hydrogel-based membranes lack durability,129 synthetic materials, usually coated with a collagen or Matrigel layer to enhance cell adhesion,104 are still used because of their good visualization, manipulation, and biocompatibility.104,129
D. Relevance of interstitial fluid flow (IFF) and transendothelial flow
In nearly all tissues, plasma leaks out of blood capillaries, flows through the interstitium, and drains into LVs, where it passes through lymph nodes before being returned to the blood circulation. The plasma filtrate that flows between BVs, the interstitium, and LVs is known as interstitial fluid. The interstitial fluid flow (IFF) originates from blood vessels' transendothelial flow, driven by the difference of hydrostatic and osmotic pressure (termed Starling forces) between the blood vessels and the interstitium.130 The resultant transendothelial and IFF highly contribute to tissue development, homeostasis, and pathogenesis, physiologically reaching up to 20% of the body mass,131 moving at 0.1–2.0 μm/s132 and generating a shear stress around 0.1 dynes/cm2.133
The advection of transendothelial and IFF provides nutrient renewal to tissues. Flow regulates via shear stress and the transcellular stress gradient generated, together with other factors, the migration of cells such as FBs, ECs, and mesenchymal stem cells, and specifically, the behavior of focal adhesion kinases (FAKs) and integrins,134–138 as well as MMPs.134,139 Furthermore, interstitial fluid forces can directly impact the alignment of ECM fibers, although not homogeneously across matrices. Current evidence suggests that dense and crosslinked collagen gels 29 are unaffected by IFF, while dense but less crosslinked collagen gels 29 and fibrin gels 140 may slightly align their fibers in the direction of the flow. Matrices of reduced density and stiffness may also result in fiber alignment perpendicular to the flow.140
The aberrant transendothelial and IFF can lead to excessive accumulation of interstitial fluid and augmentation of interstitial fluid pressure (IFP), what is known as edemas. They are especially important in lungs as they increase nutrients' diffusion distance, in intestine compromising the absorptive function, or in the peritoneum (ascites) promoting infections.141
Furthermore, the abnormal transendothelial/IFF and IFP are exacerbated in solid tumor development and wound healing processes.28 The rapid and flawed angiogenesis driven by the fast growth of solid tumors (or the wound healing process), leads to leaky BVs, and together with defective LVs unable to drain interstitial fluid and fibrosis, it causes the increase of IFP.135 In tumors, IFP varies from 10 to 40 mmHg, in contrast with −2–0 mmHg of normal tissue pressures.130,135 Consequently, there is a net convective flow of fluid from the tumor mass into the surrounding tissue, which is recognized as a potential stimulus to promote metastasis by driving tumor cell migration in the direction of flow via autologous chemotaxis.45,142–144 The augmented transendothelial shear stress generated promotes tumor vasculature remodeling,145 and the excessive IFF leads to the mechanical activation of FBs. This increases FBs migration,146 their fibrotic features,28 and transforming growth factor-β (TGF-β) production,135 directly related with immune tolerance, tumor progression, and poor prognosis.28,45 Interestingly, IFF also induces M2 macrophage polarization in the tumor surrounding, altering tumor immune microenvironment toward an invasive phenotype.147 Furthermore, tumoral IFP and IFF generate aberrant transport of metabolites into the tumor surroundings,25 altering nutrients and oxygen arrival and hindering therapeutic drug delivery.130
1. 3D in vitro models to mimic IFF and transendothelial flow
The simplest strategies employed to model transendothelial and IFF in vitro generally involve culturing vascular ECs on a 3D matrix, and/or interstitial cells within the hydrogel. Then, a pressure gradient is applied to drive flow through the gel, for instance, to study the effect of flow on the production of prostaglandins148 or on neointima formation.134
In tumor studies, 3D in vitro models that mimic the in vivo tumor architecture and dynamic fluid conditions are important not only to investigate original tumor microenvironment behavior but also to study drug delivery and toxicology of potential new anti-tumor drugs.149 Thus, some authors used, for example, gravity-driven flow to study the effect of IFF on tumor cell migration 136 and the metastatic capacity of tumorigenic cells142 or peristaltic pumps to prolong the time of experimentation and deepen the knowledge on EMT within the tumor microenvironment.150 Another approach was the use of syringe pumps to investigate the crucial effect of flow in nanoparticle drug delivery to tumor spheroids as predictive of in vivo tumor transport behavior,151 with significantly different results in comparison with static conditions.152 All these studies highlight the relevance of flow for drug screening assays.
In addition, the biochemical composition and biophysical parameters of culture media and perfusates have an impact on interstitial fluids.103 As interstitial fluid usually originates from plasma, the biochemical composition of both fluids is very similar (interstitial fluid contains 40% of plasma protein concentration and has a slightly altered ionic profile).45 Interstitial fluid viscosity (∼1.2 cP)45 is higher than that of commercial media (∼0.8 cP),45,106 and it can affect cancer cell migration, among others effects.105 The alternative of using natural media (from tissue extracts) as perfusates in 3D in vitro models would increase the mimicking of in vivo situation but would reduce the reproducibility of results due to their variable composition.153
III. TYPES OF FLUID FLOW SYSTEMS IN 3D IN VITRO MODELS
This section will traverse the most commonly used setups to apply fluid flow in 3D hydrogel-based in vitro models. The perfusion systems are classified according to the fluid pressure origin in gravity-based (also known as static head, Sec. III A) or pump-based systems (Sec. III B). In addition, agitation systems are also described (Sec. III C). Their different characteristics determine, among other features, the preferential type of flow generated: luminal, interstitial, transendothelial, or simple external agitation. The systems will be described, providing examples of their use, as well as their limitations, improvements, or alternatives.
A. Gravity-based perfusion systems/static head systems
Gravity perfusion systems are the simplest and most economical approaches to generate fluid flow in 3D in vitro models. These systems usually eliminate the issues associated with operating under a complex setup, which may include pumps, and most often also tubing and connectors (all in conjunction with an incubator).154 In addition, these systems allow multiple experiments to be developed in parallel.
1. Fluid columns and Boyden chambers
Fluid column (or reservoir) systems65,146,155 directly connect plastic,156 silicone,65,155 or glass 156 rigid tubes/columns to the commonly used PDMS157 devices. The low cell culture medium volume that columns can hold bias these systems against their use to generate luminal flow. On the contrary, the low permeability of the ECM156 allows the generation of IFF/transendothelial fluid flow over time, as it occurs in angiogenesis studies and investigations of the formation of functional microvascular network,65,138,158,159 wound healing,156 or cancer136,146 (Fig. 3). However, the flow rates generated are not uniform over time, as the difference of pressure evades progressively. Therefore, column refilling of culture medium is needed to prolong the experimental time,146,158,160 or the inclusion of external reservoirs to provide good flow stability to the system (in comparison with basic column systems).139,161 Flow stability can be further enhanced via a siphon effect and resistor, which are able to regulate the flow rates.162,163 To overcome such limitations, the incorporation of external pumps to refill the columns or reservoirs has allowed an automatization of the process. Pumps enable long-term IFF experiments 164 and durable luminal flow assays60,163 (as the luminal flow generated only by fluid columns can last no more than minutes) (Fig. 3).
FIG. 3.
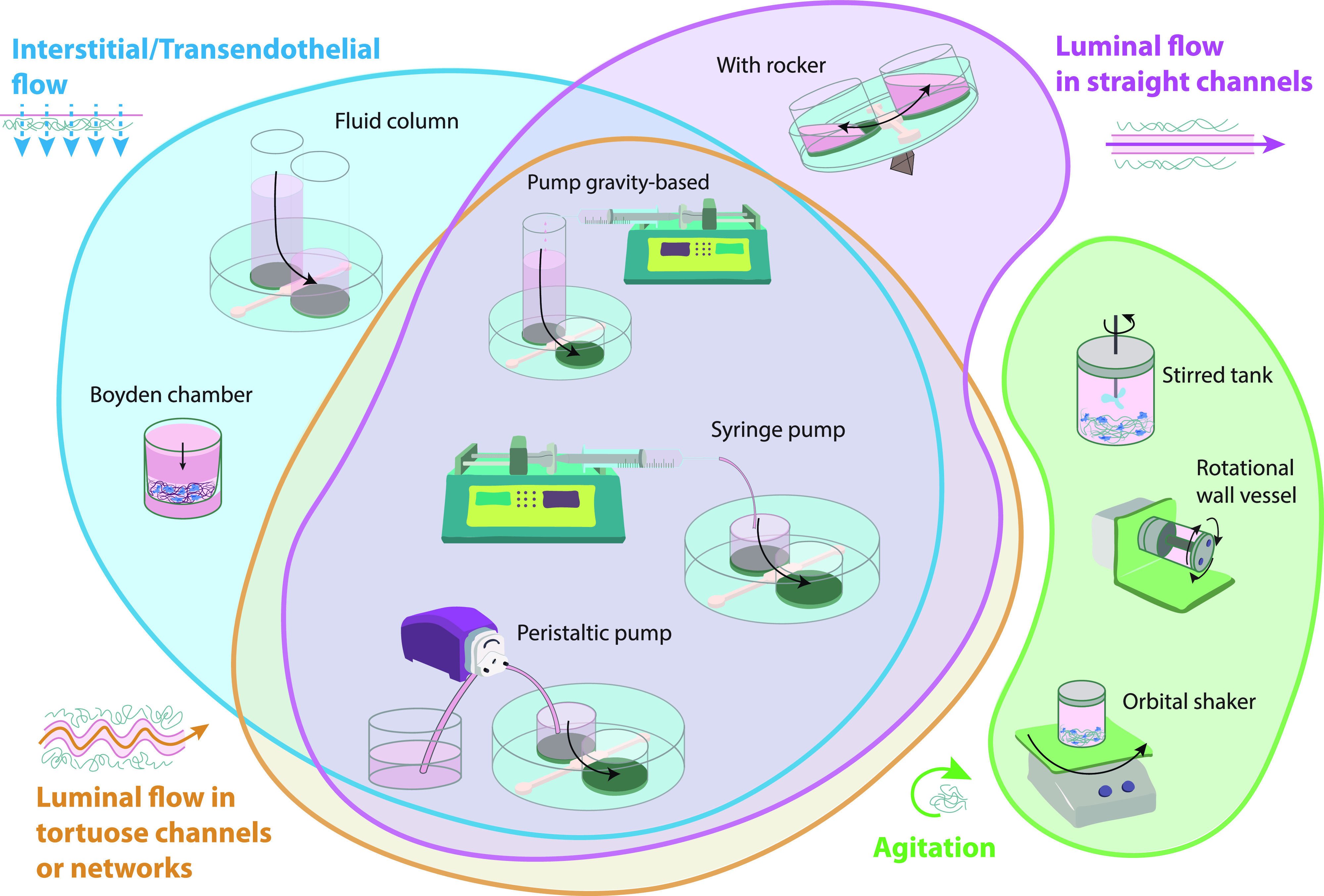
Most commonly used fluid flow setups in 3D in vitro cultures grouped by their preferential type of flow. Transendothelial or IFF (blue) can be achieved by fluid columns and Boyden chambers, with or without associated pump, and pump-based perfusion systems. Straight luminal flow (pink) by rocker-based systems, pump gravity-based systems, and pump-based systems. Tortuous luminal flow (orange) by pump gravity-based systems and pump-based systems. Finally, agitation (green) can be achieved by stirred tanks, RWV, or orbital shaker systems.
As an alternative to microfluidic devices, Boyden chambers (or transwells) are used. Due to their design, they are preferentially chosen to mimic IFF in tissues93,165 or to simulate transendothelial fluid flow effect93 (Fig. 3). Also, they are commonly used in tumor cell migration,139,142,166 invasion,167–171 and epithelial-to-mesenchymal transition172 studies, or to analyze the effect of flow in the tumor microenvironment.173
2. Rocker-based perfusion systems
Unlike previously explained methods that produce unidirectional flow, rocker-based perfusion systems provide bidirectional flow. In adult homeostasis, both in LV and BV, unidirectional flow occurs. However, in contrast to blood vasculature, lymphatic homeostatic vasculature is exposed to reverse/oscillatory flow in lymphatic valve areas.174 In pathological conditions, such as BV obstruction, a bidirectional flow can be induced.175,176 Interestingly, the physiological levels of shear stress generated by bidirectional flow in in vitro microfabricated vessels in comparison with static conditions have proven to enhance cellular structure and function.60
Rocker-based perfusion systems are predominantly used in cell culture to generate shear stress in straight endothelialized (BVs177,178 and LVs179,180) or epithelialized lumens181,182 (Fig. 3). The formed vessels have served as in vitro platforms to study neutrophil183 and monocytes extravasation,67 a process dependent on the properties of the surrounding ECM,67,183 and on the structural integrity of the vessels.67 Such endothelial functional integrity was also developed in blood–brain barrier (BBB) studies under rocker-based fluid flow,184 showing asymmetric transport of substrates,185 and revealing a dual role of astrocyte BBB-regulation after radiation.186
Rocker-based perfusion has also been applied to an in vitro renal proximal tubule model to study drug-induced kidney injury and drug-transporter interaction,187 as well as renal ischemia, being the interrupted flow, together with other parameters, a potent disturbance to the proximal tubule morphology and cell viability.178 And as in the mentioned study, other works have shown the integration of BVs with tissues like mammary ducts to reveal their close interaction.188 Perfused Caco-2 intestine tubes were proven to work as models for drug discovery and transport across intestinal barriers 182,189 or to analyze radical oxygen species (ROS) levels and cell viability.177 On another note, angiogenesis models originating from rocker-perfused vessels177,190 have been used to irrigate complex biological systems, such as in vitro models of respiration191 or organoids,9,192,193 though these models are more commonly perfused with pump-based systems, due to the long-term maturation times required to maintain their physiology.
Alternatively, microfluidic devices can be designed in a way that the fluid is recirculated via a second channel connected to the beginning of the system, as for instance with an endothelialized lumen. Although this is not a continuous flow, this design allows an unidirectional flow within the perfused structure, while avoiding the laborious use of pumps.194
B. Pump-based perfusion systems
Pump-based perfusion systems offer long-term experimentation hardly achieved by any gravity-based system (without pump). Pump-based systems allow long-term IFF,29,140,195 and the generation of physiological luminal flow in straight16,73,163 tortuous tubular structures,22,107,113 or through self-organized152 or previously designed196,197 microvascular networks. This long-term perfusion experimental time helps to overcome the maturation and development limitation of advanced in vitro models,18,21,197–199 and therefore, the use of pumps is extended in complex in vitro systems, like organoids,16,21 organ-on-a-chip,109 body-on-a-chip,200,201 and advanced tissue engineering.202
Mainly, two types of pumps are used in in vitro ECM-based perfused studies: peristaltic/roller pumps203 and syringe pumps.204 Peristaltic pumps can usually move higher volumes than syringe pumps, limited by the volume of the syringes.205 On the contrary, syringe pumps provide consistent physiological flow60 within a large range of flow rates.205–207 Although both peristaltic and syringe pumps can be used to develop IFF29,140,195,208 and luminal flow,18,73,107,113,152,163 peristaltic/roller pumps may generate continuous flow oscillations or waves (also called pulsations)209 capable of producing a more random organization or unalignment of ECs.210
The undesired oscillatory flow, also generated at initial stages of syringe pump flowing,211,212 must be resisted by the system, and it can be dampened to obtain fully steady laminar flow210 by including reservoirs213 or long compliant silicone tubing.21,212 Interestingly, peristaltic-pumps' pulse can be used to model pulsatile blood flow in the arterial vasculature214 or to produce rhythmically aligned stretching and contraction of intestine cells to develop a more physiological peristaltic gut.16 The number of rollers16,215 and the rotation speed209 may introduce flow variabilities that require the characterization of each pulsatile flow.216
Additionally, other alternatives like pressure-controlled systems/pumps or vacuum pumps have been explored to generate fluid flow in 3D cell cultures217–220 or other perfused in vitro microphysiological systems.221–223 Essentially, pressure-controlled systems are formed by an external pressure source (usually controlled by software) and a pressurized reservoir that contains the perfusate. Advantageous to flow-controlled systems, they can exhibit a fast settling of pulseless flow. Also different from flow-controlled systems, pressure-controlled systems must adjust their pressure drop to maintain advection levels when working with different viscous perfusates or different tissue resistances (see Glossary Box).
In comparison to gravity-based perfusion systems, the use of pumps as pressure generators in in vitro models imply an increasing complexity of the system to be set up in number of components, connectivity, and manipulation. This complexity favors potential mechanical strain and detachment of the hydrogel, the generation of bubbles in the system60 (a major challenge224 routinely encountered in microfluidics225), and thus, a substantial reduction of the experimental throughput. To overcome some of these issues, fittings and connectors should be reduced and placed downstream of a (preferably) hydrophilic-made device, designed with rounded and obtuse angles.224 On another note, although infuse pumps generate a more physiological difference of pressure between perfused vessels and its surrounding microenvironment than withdrawing pumps,135 they can aggravate the differences of pressure and temperature within the device, which promote bubble formation,224 and, thus, they may require the use of bubble traps.226–228 Conversely, withdrawing pumps enable the reduction of connectors' use upstream of the device, while preventing pressure decrease within the device. These advantages of withdrawing pumps reduce air trapping and the gaseous saturation of the medium, which are key elements to avoid bubble formation.224 Furthermore, this setup requires upstream medium reservoirs that can be placed within the incubator, providing the necessary thermal stability to prevent gas solubility reduction. The use of two pumps, with infusing and withdrawing modes, respectively, requires a very precise flow coordination,29 and it induces a higher risk of hydrogel destabilization when plugging the tubbing to assemble the device into the system.60
Moreover, to standardize perfused in vitro cell cultures, and, therefore, reduce lab variability, the recently generated fluidic circuit board (FCB) of the translational organ-on-a-chip platform provides an interesting and well characterized tool to develop any kind of perfusion system in 3D in vitro models.229–231 This multi-institutional proposal integrates commercially available components that facilitate connections and reduces the above explained pump-related bubble issues,229 while increasing the throughput of perfusion platforms.231 Additionally, complex liquid-handling instruments that contain pumps are being developed to automate the perfusion process and reduce direct fluidic plumbing and bubble formation troublesome.232 Their use in a multi-organ-chip helped to predict clinical quantitative pharmacokinetic parameters, closing the gap between in vitro and in vivo experimentation.233
Table I groups the types of perfusion systems used to induce flow in 3D (ECM-based) in vitro cell cultures and summarizes the advantages, disadvantages, and examples of the different setup systems.
TABLE I.
Types of perfusion systems to induce flow in in vitro cell cultures. This table groups the different types of perfusion platforms highlighting their advantages, limitations, and potential improvements as well as recommendations and examples of their use.
| Perfusion System | Advantages | Disadvantages | Improvements | When to use (preferentially) | Quantitative data | Examples |
|---|---|---|---|---|---|---|
Columns (or reservoirs) 
|
High throughput - Simple handling - No need of bubble trap |
- Limited in time and speed - Flow decreases over time - Low microscopy visualization in Boyden chambers |
- Repetition over time (although affecting the flow profile) - Including external reservoirs - Including flow resistors (affecting shear stress and advection) |
- Short experiments of interstitial or transendothelial fluid flow preferentially | - Initial pressure drop: ∼1–25 mm H2O - Manual pressure drop renewal for IFF: ∼4–24 h - Flow rate: ∼2–15 μl/min - IFF speed: ∼0.1–5 μm/s Parameters highly dependent on gel permeability, gel dimensions, and reservoir size |
Refs. 65, 136, 138, 139, 146, 155, 156, 158–162, and 164Refs. 93, 142, 165, and 167–173 |
Boyden chamber 
| ||||||
Gravity-based with pump 
|
- Simple handling - Unlimited/long working time - No bubbles formation - Nutrients renewal |
- Low throughput - Limited in speed - Low sterility |
- Including air filters to control sterility | - Very long experiments with renewal of nutrients | - Shear stress: ∼0–2 dyne/cm2 - Nonstop working time: weeks Pressure drop and flow rates are similar to the rest of gravity-based systems. |
Refs. 60, 163, 164, and 166 |
Rocker-based 
|
- High throughput - Simple handing - No need of bubble trap |
- No renewal of nutrients - Low sterility |
- Bypassing design to recirculate flow and perfuse unidirectionallya | - Shear stress cell stimulation without renewal of nutrients | - Angle: ∼±7°to ±37° - Tilt interval: ∼12 s–8 min - Shear stress: ∼0 to 6 dyne/cm2 |
Refs. 60, 67, and 177–194 |
Pump-based via peristaltic pump b
|
- Unlimited working time - Nutrients renewal - Allowed bidirectional flow (although compromising medium renovation) |
- Compromised hydrogel stability when handling - Low throughput - Potential bubble formation |
- Inclusion of bubble traps - Precise design and position of system elements (device, pumps, fittings) - Pulsation dampeners |
- Very long experiments with renewal of nutrients - Peristaltic stimulation |
- Flow rate: ∼0.5–4000 μl/min - Shear stress: ∼ 0 to 20 dyne/cm2 - Nonstop working time: weeks Parameters highly dependent on the pump, tubing, and microphysiological system |
Refs. 16, 21, 22, 73, 107, 113, 140, 150, 196, 197, 208, 213, and 234 |
Pump-based via syringe pump 
|
- Long working time - Nutrients renewal - Allowed bidirectional flow (although compromising medium renovation) |
- Compromised hydrogel stability when handling - Low throughput - Potential bubble formation |
- Inclusion of bubble traps - Precise design and position of system elements (device, pumps, fittings) |
- Long experiments with renewal of nutrients | - Flow rate: ∼1–50 μl/min - Shear stress: ∼ 0 to 2 dyne/cm2 - Nonstop working time: days Parameters highly dependent on the pump, syringe, and microphysiological system |
Refs. 29, 152, 163, 195, 225, and 235 |
Depending on the aim of the experiment, it may just be considered as an alternative.
Depending on the number or rollers and the presence of pulsation dampeners, fluid flow could be either laminar or pulsatile-like.
C. Stirred tank, orbital shaker, or RWV-based agitation systems
In a different scale, there are extra alternatives used to generate fluid flow, as stirred tanks and orbital shakers,236,237 which do not provide perfusion but provide agitation instead. They are generally used to overcome the low nutrient diffusion encountered in tissue engineering238,239 and development of organoids.240,241 Thanks to these systems, fluid flows around the organoid or tissue, facilitating the mass-transfer and exchange of nutrients and waste products with the medium.236 Standard Petri dishes or well plates can also be used as orbital shaking platforms,242 or they can be adapted to be used as stirred tanks for 3D cell culture of organoids.243 Stirred tank or orbital shaker agitation systems are used to help in the maturation of ectoderm tissues like brain organoids240,241,244 and endoderm tissues like liver organoids.245 Nevertheless, except for renal derived organoids246 agitation systems are not commonly applied to mesoderm derived tissues.198,247
On a special note are rotating wall vessel (RWV, or clinostat), bioreactors that provide high mass-transfer while simulating microgravity and enhance cell-cell interaction37 (Fig. 3). RWV showed improved growth and differentiation of retinal organoids in comparison with static culture, recapitulating the spatiotemporal development of the retina in vivo.248 Similarly, cardiac tissue culturing in RWV, compared to static culture, significantly enhanced cardiomyocytes maturation, functionality, and viability.249 Furthermore, these bioreactors reduce the mechanical shear forces and bubbles that can be generated in stirred tanks that can induce cellular damage.250
IV. FLUID FLOW APPLIED TO CURRENT BIOLOGICAL MODELS AND ITS CHALLENGES
In the last decades, bioengineering in vitro 3D models have experimented an unprecedented growth, being nowadays an expanding field with multiple clinical applications. Many of these newest technological models already include perfusable systems, while more sophisticated models, such as those with microvasculature, are being developed. In this section, we discuss important studies that have made use of the newest bioengineering applications such as organ-on-a-chip, organoids, and body-on-a-chip and their importance in drug discovery and in personalized and regenerative medicine.
A. Perfusable bioengineered tissues
The so called “mini-tissues,” also named as organ-on-a-chip (OoC) have been engineered in microfluid devices to reproduce in vitro key aspects of organ development, functionality, or disease.
Since the innovative work of Huh D and colleagues in 2010 who developed a lung-on-a-chip model,251 many other organs, e.g., intestine and kidneys, have been modeled in vitro.252,253 The drawback of OoC models is their simplicity compared to native organs, since they are made almost exclusively on guided microchannels where one or several cell types are cultured in ECM coated membranes to model a particular tissue.251–253 This diminishes the complexity, and, thus, the physiological relevance of OoC models compared to organoids derived from murine or patient stem cells, as explained in Sec. IV B. However, OoC allows understanding cell-to-cell interactions between different cell types in the mechanical/biochemical conditions of the tissue of study, providing useful information on how cells communicate to each other in different conditions.
Different microfabrication techniques are being used to form mini-tissues in pre-established device structures, such as soft and photo lithography,254 injection molding,255 micromilling,256 laser photoablation,257 or 3D bioprinting.258 When fluid flow is a requisite in the study, as it is the case for instance to simulate liver sinusoid structures, renal reabsorption, or alveolar-capillary functioning, the incorporation of flow in the system has to be considered during the design of the microfabricated mini tissues/OoCs. Fluid flow, besides its crucial role in transporting nutrients and removing waste metabolites, can provide mechanical cues (laminar stress, transmural) that enhance cell viability and intercellular communication and enable dynamic control of the environment by allowing controlled incorporation of exogenous compounds, e.g., drugs or toxins, in 3D OoC models.80,259 In order to apply fluid flow to OoC models, continuous perfusion to the microchamber is required, and, therefore, to set up such a model is laborious and requires expertise and proper equipment. The advantage of such platform relies on the straightforward readouts and on the control of the tissue microenvironments that allows recapitulating the complex biochemical interactions between different cell types and the inclusion of microenvironmental physical forces, such as shear stress and mechanical traction or compression.260
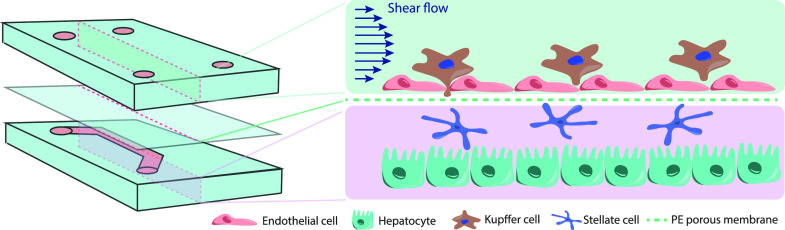
Du et al. engineered a liver-on-a-chip to mimic the interactions between liver sinusoids and blood flow peripheral cells. Their model resembles the liver sinusoid structure and functionality by integrating the four cell types implicated (sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, and hepatocytes), the fenestrated morphology characteristic of liver sinusoids (by using a porous permeable membrane), and shear flow applied to mimic the capillary blood flow pattern in the liver sinusoid118 (Fig. 4). Capillary blood flow in the liver sinusoid is necessary for mass transfer and for nutrient supply and especially for IFF inside the Disse space deriving from capillary flow across the permeable endothelium. However, the model uses murine cells, and it is of use only when short-term analyses are needed, which is valid for innate immune response studies, cytotoxic analysis, or protein secretion.118
FIG. 4.
Liver sinusoid-on-a chip. The model is formed by the four types of hepatic cells, i.e., liver sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, and hepatocytes, distributed layer-by-layer resembling liver sinusoid. Stellate cells are first injected into the basolateral side of the collagen-I pre-coated PE membrane. Sinusoidal endothelial cells are then added on the apical side of the membrane together with Kupffer cells. Hepatocytes are then added to form the bottom layer. Unidirectional flow is incorporated by a syringe pump. Based on Du et al.118
Huh et al. engineered a pioneering model to reproduce lung alveolar-capillary functionality using two microchannels separated by a porous ECM-coated membrane, one channel lined with alveolar epithelial cells and air filled and other channel filled with fluid and lined with lung endothelial cells. Moreover, cyclic mechanical suction was applied to both channels to simulate lung breathing.251 Importantly, the device resembles the architecture, mechanical environment, and functionality of alveolar-capillary unit allowing functionality analysis, e.g., nanoparticles absorption, and pathological studies, e.g., pathogen and immune cell interaction. Nevertheless, the absence of alveolar macrophages (crucial to fight against incoming pathogens and pollutants), the air pressure, and flow values applied, the use of transformed lung endothelial cells, or the thickness of the barrier differ significantly with the in vivo situation and, thus, do not completely mimic lung physiology.251 More recently, a perfusable vascularized epithelial lung high-throughput 64-chip microfluidic plate-based platform was developed. The platform was made with human primary microvascular ECs, fibroblasts, and pericytes with the purpose of studying viral infectivity and viral infection-induced thrombotic events191 in large-scale patient collection of samples in cases as COVID-19 pandemic.
To mimic cardiac physiology, proper propagation and maintenance of mechanical and electrical signaling is fundamental. Several heart-on-a-chip models have been developed providing key signals needed to reproduce mechanical (through cyclic pressure stimulation)261 and electrical (through controlled uniform electric field)262 cues in the heart. However, these systems fail to properly sustain the demand of oxygen and nutrients of such highly metabolically active cells for long periods of time (longer than few days in culture). To overcome this issue, an automated gravity-based controlled flow was implemented in a model that was able to improve the functionality of cardiomyocytes.225
On the other hand, Lin et al. investigated renal reabsorption in a kidney-on-a-chip model where adjacent conduits of epithelial and endothelial cells ECM-embedded presented active reabsorption of solutes (tubular-vascular exchange) in a closed-loop perfusion system.113 The system allows solutes and drugs to be flown and perfusates to be collected at different time points. Also, a high-throughput, 3D-microfluidic platform (Nephroscreen) for the detection of drug-induced nephrotoxicity was generated.143,187 In this platform, FSS was incorporated through passive leveling by gravity. Though immortalized renal cell lines were used, renal cells were able to show a polarized epithelium with functional transporters.187
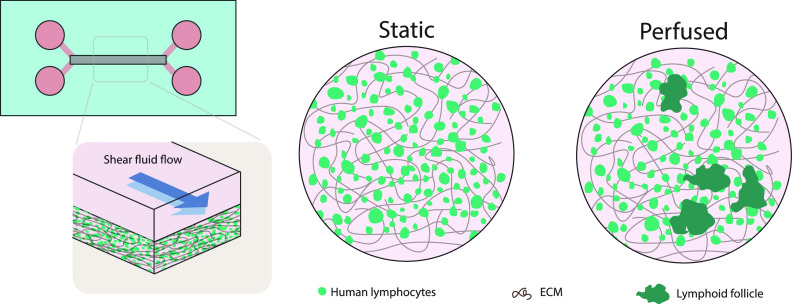
Despite the crucial role of immune cells in shaping tissue homeostasis and their crucial role in disease fighting,263,264 the incorporation of immune components into OoC models is generally unexploited. Recently, an elegant study showed the in vitro formation of lymphoid follicles when culturing B and T lymphocytes in a 3D hydrogel microchip under fluid flow.265 Flow was needed for follicle formation and for preventing autoactivation of lymphocytes. Due to the formation of follicles and the feasible incorporation of dendritic cells to the model, the platform can be useful for preclinical testing of vaccines, adjuvants, or immunotherapy drugs 265 (Fig. 5).
FIG. 5.
Lymph node on-a-chip. An ECM that contains human lymphocytes is stimulated with medium flowed in the upper channel. Differences are observed in the growth of human lymphocytes (in green) between static and flow conditions. Under flow, lymphocytes are grouped, simulating formation of follicles. ECM fibers are shown in brown. Based on Goyal et al.265
Recently, patient derived-induced pluripotent stem cells (iPSC) have been differentiated to be cultured in OoC systems to study the response of patient derived cells to different drugs.266 Together, all the above-mentioned works highlight the potential use of OoC models as platforms for high-throughput personalized drug screening.
Though in the majority of studies using organ-on-a-chip the choice of perfusate has been commercial cell culture medium, some models during the last decade that contain endothelialized hollow channels have been exposed to flowing blood to study thrombosis in vitro.267–269 While these devices are useful for advancing research, they are not used in practical or clinical settings because living cell cultures are not robust and the devices cannot be stored for extended times. For instance, Jain et al.53 studied vascular thrombosis in an endothelialized microfluidic device using whole blood taken from subjects receiving antiplatelet medications. However, only short-time analyses have been performed. To the best of our knowledge, the lymph has not been used in OoC and the inmune and fatty content of lymph (chyle, formed in the intestine) could damage vessels rapidly, if not used at the right concentration and flow. Both perfusates, blood and lymph, are challenging to work with because of their viscosity and complex rheology due to suspended particulates, which results in high shear stresses even in large vessels. To replicate their properties in vitro with cell culture medium, faster flows or increased spatial velocity variability is necessary to maintain the same flow resistance. Especially important and complicated is the selection of perfusate when generating a body-on-a-chip (discussed in Sec. IV C), since the perfusate varies across tissues. Therefore, to simulate faithfully this difference, tissue-specific perfusates with different biochemical and biophysical properties should be used.
B. Perfusable bioengineered organoids
The in vitro differentiation of stem cells to form organoids has enabled researchers to faithfully reproduce in 3D in vitro key structural and functional properties of many organs.270 Since the very first gastric organoids developed by Sato et al.,14 other complex organoids such as brain,240 liver,271 stomach,16 pancreas,12 or lung organoids13 have been generated. Many promising advances have been done using these multi-cellular engineered living systems that allow mimicking organ biology in a Petri dish.272–274
The cellular organization, maturity, and function of organoids has been improved with the implementation of physico-chemical stimuli (e.g., tissue strain/compression or shear stress). In several cases, the inclusion of fluid forces in organoid models has been proven fundamental for their growth, functionality, and long-term maintenance.16,21 Interestingly, the generation of human pancreatic islet organoids in a perfused 3D system exhibited proper growth, differentiation, and maturation compared to static culture, being this platform key for diabetes studies and drug testing.15 However, care should be taken on the flow conditions and incubation time applied to the different tissue of origin organoids since, for example, the extended culture of iPSC-derived kidney organoids in stirred suspension has been proven suboptimal.246
Different from OoC models, organoids-on-a-chip follow intrinsic programs to develop an organ. While an organoid develops, several signaling pathways are activated timely to generate the different cell types of a tissue.275 In this context, biochemical gradients are to be considered for cell type morphogenic differentiation and niche maintenance. For instance, a platform has been designed for neuronal tube development that integrates opposing gradients of bone morphogenic protein (BMP) and Hedgehog signaling molecules to properly form neuronal tube patterning.276 Also, in a gut-on-a-chip model, opposing gradients of WNT and BMP have been achieved mimicking villi and crypt,277 revealing the useful application of scaffold-guided morphogenesis. An excellent example of a spatially arranged organoid-on-a-chip model are the mini-guts generated by Nikolaev and colleagues.116 This model consists of a permeable scaffold that enables the adhesion, growing, and differentiation of intestinal stem cells, and, at the same time, it serves as physical barrier that guides the self-organization of stem cells into a functional epithelium with absorptive and secretory functions. The scaffold is laser-ablated to mimic villus-like and crypt-like region geometry, closely resembling the intestinal epithelium. The mini-guts generated harbor a lumen connected to an external pump for their perfusion, and thanks to the incorporation of flow and removal of dead cells, the mini-guts are able to be fully functional for several weeks and capable of regeneration without the need of organoid passaging116 (Fig. 6).
FIG. 6.
Engineered mini-guts. Microfluidic device with inlet and outlet reservoirs designed to allow intestinal lumen perfusion. Cells were allowed to stay in the cavities and cell seeding medium was changed every day for providing a viable long-term platform (20 days). Based on Nikolaev et al.116
Several organoid models are implementing a different approach, which involves incorporating functional vasculature in the system. Thus, to study brain development and disease, EC reprogramming was induced inside human cortical organoids.18 When functional vasculature-like structures were formed, enhanced maturation of organoids was obtained. This model offers an interesting approach to study the crosstalk of neural and ECs, and a way to reduce hypoxia and apoptosis usually found in avascular organoids.18 Another approach to induce vasculature within cerebral organoids was by adding VEGF (to induce vessel formation) and Wnt7 (to induce development of pericytes) during the generation of cerebral human stem cells. This model allows the formation of BV-like structures with mature blood–brain barrier characteristics in cerebral organoids. The method permitted organoids maintenance up to 4 months in culture;19 however, the development of BVs in cerebral organoids was constrained due to a reduction in the number of vascular-like structures in extended cultures. In addition, the open-circle morphology of endothelial layers in 4-month organoids appeared distorted, potentially resulting from the absence of blood pressure. Consequently, additional investigation is necessary to facilitate the generation of fully functional cerebral organoids with a vascular system capable of supporting the flow of medium.
Besides the relevance of the biochemical composition of the microenvironment, the biomechanical properties of the ECM, e.g., stiffness, permeability, viscoelasticity, porosity, and topography have an important impact on cell behavior; 278 thus, they are crucial to reproduce the in vivo organ functions. For organoid formation, the most commonly used ECM hydrogel for physical support of cells and biochemical signaling is Matrigel.279 However, due to batch-to-batch variability 280 and lack of knowledge about its entire composition, standardized synthetic and natural hydrogels are being designed according to the tissue properties to be reproduced in vitro.
External forces have a crucial effect on organoids formation since the cells can mechanosense these forces by focal adhesion proteins and ion channels and react, activating different mechanotransduction pathways, such as Ras/MAPK, P13K/Akt, RhoA/ROCK, Wnt/β-Catenin, and TGF-β, which are essential for tissue morphogenesis and maintenance.281,282 For example, several organoid-on-a-chip based studies have implemented organ-specific fluid mechanical forces. Thus, Lee K. and colleagues perfused gastric organoids via a peristaltic pump, which allowed organoid stretching and contraction mimicking gastric movement.16 Recently, liver organoids derived from human pluripotent stem cells have been generated in a 3D perfusable micropillar chip model.283 In this system, controllable fluid flow allowed proper growth and differentiation of liver organoids, recapitulating liver formation and cellular heterogeneity; it also enhanced not only cell viability but the expression of key genes for hepatocyte differentiation and metabolism, thus highlighting the relevance of mechanical flow in promoting proper organoid functioning.283 Similarly, Homan et al.21 demonstrated that when partially ECM-embedded kidney organoids derived from human pluripotent stem cells, are perfused, an increased vascularization and maturity of its tubular and glomerular compartments is obtained (Fig. 7). Their proper development was dependent on the flow-induced shear stress generated, revealing that organoids cultured under flow were more mature and polarized than organoids grown in static culture conditions. Thus, this study exemplifies how 3D vascularized organoid models best resemble the structure and function of specific organs.
FIG. 7.
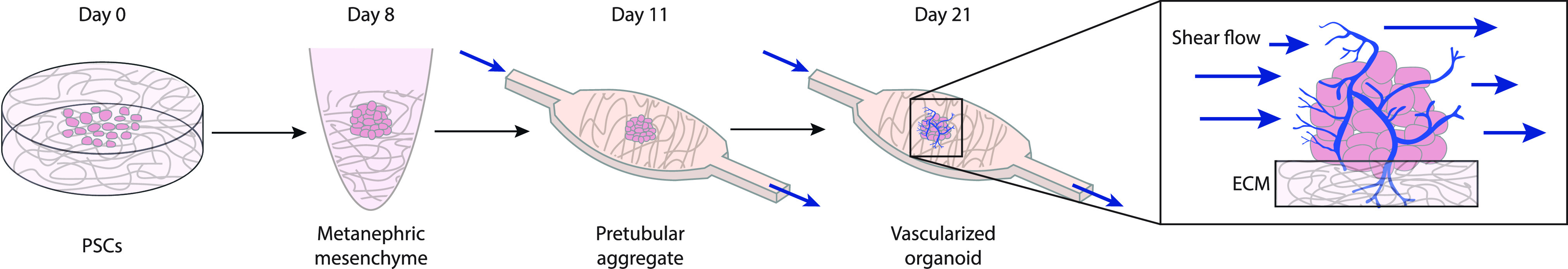
Renal organoids cultured under high fluid flow exhibit robust vascularization and maturation. Renal vascularized organoids are placed on an ECM and subjected to controlled fluidic shear stress. Peripheral vascular network is formed after 21 days under flow and ECM-adherent conditions. Based on Homan et al.21
Ensuring the establishment of vascular networks is crucial for the appropriate engraftment and function of transplanted tissues. Remarkably, Takahashi et al. utilized a dynamic self-condensation method to create tissue organoids from dissociated organ progenitor cells with the presence of vascular and mesenchymal progenitors. This approach facilitated the prompt development of BVs in tissue organoids in the absence of flow.284 To achieve this goal in the pancreas, Takahashi et al. used the above-mentioned approach, co-culturing pancreatic islets with HUVECs and mesenchymal stem cells. When those vascularized islets were co-transplanted in a mouse model of diabetes, mice showed improved pancreatic viability and functionality (insulin secretion capacity). Taken together, this success sheds light on the use of self-condensing cultures for therapeutic transplantation purposes without the requirement of flow in the system.
C. Perfusable bioengineered body-on-a-chip
With the aim of simulating multiorgan interactions and physiological responses at the systemic level, multiple OoCs/organoids-on-a-chip can be connected by fluid flow to construct a body-on-a-chip (as shown in Fig. 8).12,285 To link two or more tissue compartments via a common vasculature, the so called “AngioTube” has been developed. The AngioTube features a central microchannel with sufficient mechanical stability to support a perfusable vascular system and enable the self-assembly of diverse parenchymal tissues.193 Moreover, a platform consisting of compartmentalized microfluidic chips of different cell types connected via fluid flow through micro-engineered porous barriers was engineered to simulate paracrine exchange between cells.286
FIG. 8.
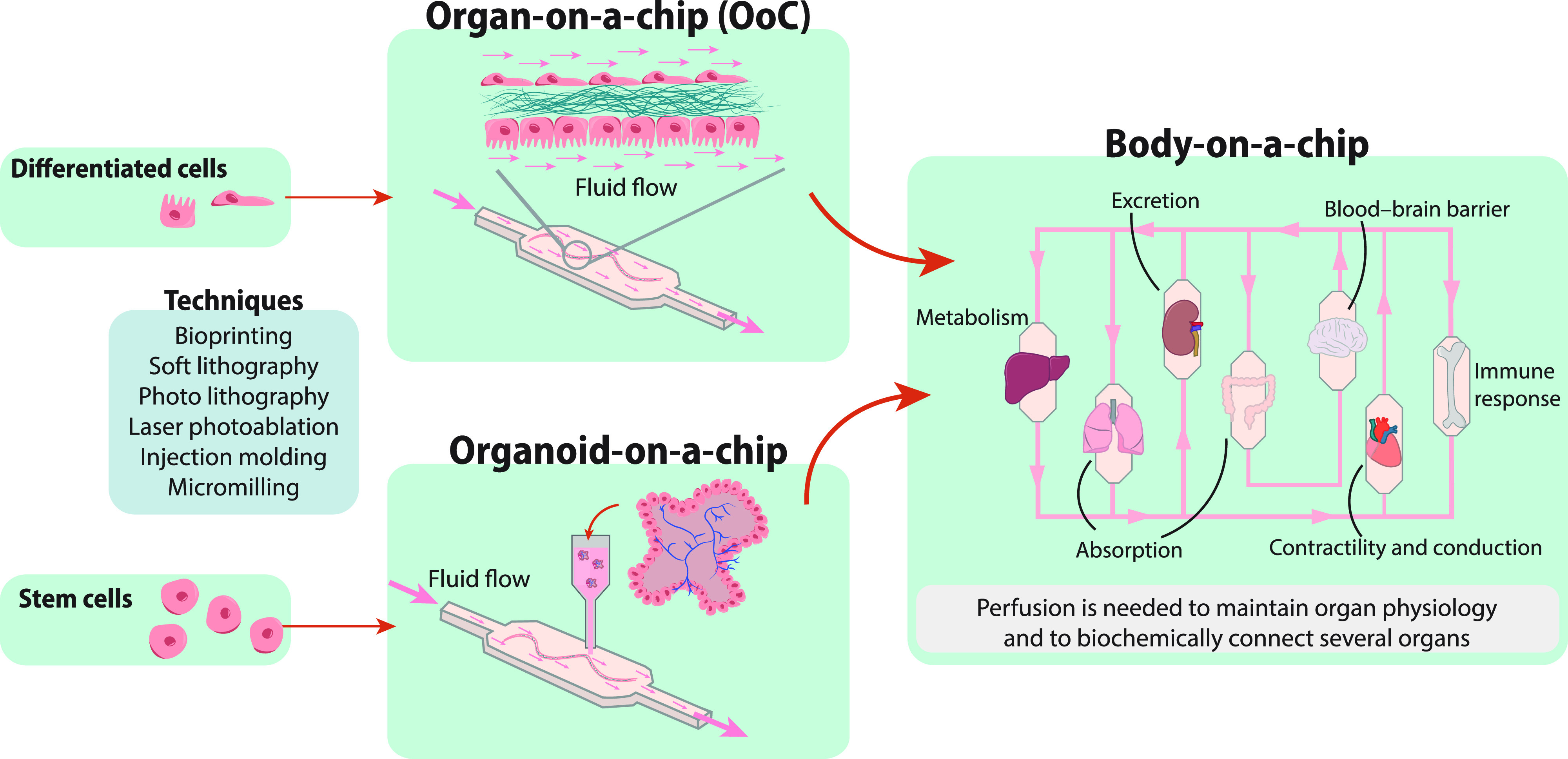
Bioengineered 3D systems to model in vivo organ and body homeostasis or disease. Organ-on-a-chip (OoC) made of differentiated cell types embedded in an ECM are able to mimic some physiological functions of organs. Organoid-on-a-chip is used to model the intrinsic program of an organ. Different techniques are used to make both OoC and organoid-on-a-chip, such as bioprinting, laser ablation, and others. Organ-specific derived organoids or OoC can be connected by flow to establish a body-on-a-chip.
A very interesting 3D-multi-tissue study shed light on the importance of generating body-on-chip platforms for drug screening. The study consisted of multiple microchambers containing different microtissues, interconnected by microchannels and cultured under continuous gravity-driven flow. When culturing liver and colorectal tumor tissues with a chemotherapy agent, the drug impacted tumor growth only after its bio-activation by the liver.287 Another important study was performed by Zhang and colleagues, they generated a perfused body-on-a-chip model using interconnected hepatic and cardiac organoids and a peristaltic pump. They designed an automated multisensory platform for long-term real time monitoring of biophysical and biochemical parameters (oxygen, temperature, and pH).200 However, there are some limitations of the prototype platform such as the use of PDMS, which has been demonstrated to absorb hydrophobic molecules; therefore, the prototype is not valid for acurate biochemical studies. On the other hand, the complex manual assembly of the prototype system is another limitation. Further improvements of the platform would allow for more accurate modeling and analysis of tissues functioning.
All these studies highlight the importance of designing multi-organ platforms for studying the physiology and pathology of organs and for drug screening studies.
D. Perfusable models: key for personalized medicine and drug discovery
Even though personalized cell cultures, as human organ-on-a-chip systems, have not yet been used to take decisions about personalized treatments and precision medicine, their potential to accelerate drug discovery is evident.187,189,288,289 Indeed, such accomplishment is seen close in time288,290 since they have been recently approved by the Food and Drug Administration (FDA) to substitute animal experimentation in pre-clinical assays.
Here, we describe some examples of the potential use of perfused 3D models for patient treatment and drug discovery with a final focus on tumor-on-a-chip technology.
A perfusable 3D kidney-on-chip model using renal organoids108 was developed to understand the impact of flow on drug transport and uptake in kidney organoids. The model mimics the native uptake of nephrotoxic substances in human kidneys and, thus, is a vital stride toward determining their potential for drug screening and disease modeling. The model provides a more precise predictor of nephrotoxicity compared to conventional models, which are based on immortalized proximal tubule epithelial cells.
Fat-on-a-chip models with integrated flow are able to reproduce obesity and its related diseases, e.g., diabetes, osteoarthritis, or fatty liver.291,292 Adipose cells in vivo are protected from shear stresses by the vasculature, which shields the adipose compartment from the bulk flow of fluids. A shortcoming of exerting flow over adipocytes using microfluidic devices is the damaging effect of shear forces.291 Therefore, in vitro approaches should consider the use of vascularized adipocytes instead of directly applying flow. To simulate this in vitro, Loskill et al. created an endothelial-like barrier that connected the media channels and adipose chambers using micropores, allowing them to maintain functional lipid metabolism for a period of weeks.293 Static cultures have the advantage of lower risk of shear stress-induced adipocytes apoptosis, but would need to address the physiological components associated with nutrient delivery through an active and selective vascular barrier to control nutrients transport to the adipose compartment.
On another note, to study infections, such as cerebral malaria, Bernabeu and colleagues generated a new flow-based 3D brain microvessel model. After 3 days in culture, primary human brain microvascular ECs formed fully endothelialized lumens that could be perfused with blood components, such as P. falciparum, to analyze parasite sequestration using a diverse range of flow velocities and wall shear stresses.294 This model can be used to study host cell or pathogen interactions with brain endothelium. Moreover, future 3D microvessel engineering approaches toward a more physiological model could incorporate patient-derived perivascular cells like pericytes and astrocytes. Also, the analysis of other blood components would be interesting to study their role in infection.
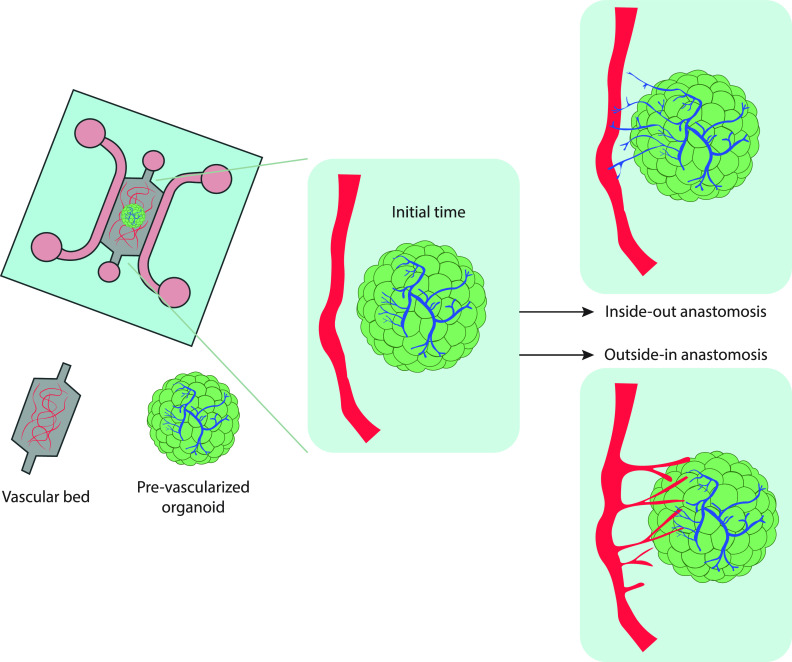
Tumor-on-a-chip technology has become a robust in vitro model for cancer research.295,296 Despite the complexity of mimicking the biochemical and mechanical characteristics of a tumor in vitro, (dense ECM of tumors, immunosuppressive environment given by stromal and immune cells,297–299 hypoxia,300 and abnormal vasculature301) tumor-on-chip platforms closely recreating in vivo tumors have been generated.295,302,303 The first assays using tumor-on-a-chip systems were developed in static conditions, thus ignoring the effects of blood and lymph flow to tumors. However, the most recent models reproduce reliably tumor blood vasculature by integrating pre-vascularized organoids with a capillary bed to achieve functional anastomoses (Fig. 9). When co-culturing organoids and a capillary bed, the type of hydrogel and the cultured media (a mixed of endothelial and organoid growth media) needs to be optimized. Usually, a hydrogel mixture of fibrin and Matrigel allows for the growth of both ECs and organoids, as Rajasekar et al. showed with patient-derived colon organoids.304 The device utilized in the study was equipped with a programmable rocker, which facilitated cyclic luminal flow across the capillary bed that improved growth and functionality of the organoids. While the presence of perfusable vessels near the organoids indicates the potential for establishing connections between the organoid-derived vasculature and the adjacent vasculature, direct evidence of intravascular perfusion within the organoid was lacking. Further investigation and techniques are needed to confirm whether there is functional perfusion within the organoid and if there is direct integration between the organoid vasculature and the surrounding vessels. The same approach could be used with LVs so that both circulatory systems are reproduced with the tumor organoids. Moreover, some tumor-on-a-chip studies have simulated in vivo dynamic flow,101,305 drug efficacy,152,306,307 and tumoral vascular immunosuppressive characteristics.308 Recently, a unique tumor-on-a-chip with perfusable bioprinted blood and LV pair was designed to mimic the transport of drugs in the TME.99 This system is a promising tool to test therapeutic approaches using cancer-derived organoids from biopsies, which could contribute toward personalized medicine treatments.
FIG. 9.
Tumor-on-a chip model. The schematic representation illustrates possible mechanisms for establishing anastomoses (functional connections to form between the vessels) between an in vitro vascular bed and pre-vascularized organoids when co-cultured in a microfluidic chip. In the “inside-out” approach, the organoid-derived vessels extend and merge with the existing vascular bed. The “outside-in” approach relies on the induction of angiogenic sprouts from endothelial cells (ECs) of the vascular bed that penetrate the organoids and establish connections with the organoid-derived vessels. This model allows for the exchange of nutrients, oxygen, and other factors between the vascular bed and organoids. Based on Zhang et al.309
Developing preclinical models using patient biopsies, along with patient tissue sequencing, serves as a fundamental guide for personalized medicine.310 In the study of Pauli et al., patient-derived tumor organoids are being utilized, in conjunction with genomic exome sequencing, to guide precision therapies.311 The study shows great promise in guiding the choice of clinical trials for individual patients.311 Once these type of high-throughput studies are validated, they could be complemented with selected types of human tumor organoids cultured in microfluidic platforms to find the optimal therapeutic strategy to follow.
During metastasis, tumor cells are exposed to mechanical forces such as fluid pressure from their tissue microenvironment. In order to study the role of fluid flow in the metastatic capacity of tumor cells, metastatic processes such as tumor extravasation have been mimicked in in vitro 3D models.312,313 A significant study evaluated in a human microvascular network model the effect of luminal flow induced-shear stress, trans-endothelial flow, and IFF on tumor cell extravasation and migration. The results show that luminal flow enhanced tumor cell extravasation, while transendothelial flow augmented tumor cell transmigration across the endothelium. When subjected to physiological flow, tumor cells were shown to migrate closer to the endothelium, favoring the formation of metastatic foci.220 These data underscore the significance of utilizing in vitro models that can mimic human pathophysiological conditions with high spatio-temporal resolution, as well as applying physiological luminal flow, when investigating extravasation.
E. The importance of flow for tissue engineering and regenerative medicine
The presence of a physiological environment that includes mechanical forces induced by fluid flow and pressure is important for tissue regeneration, morphogenesis, and repair.80,259 This has been demonstrated in various studies, including the mini-guts generated by Nikolaev et al.,116 where luminal exposure to flow helped to regenerate the epithelium. Another study showed that human mesenchymal progenitor cells can differentiate into osteoblasts in a 3D environment with FSS, which makes it a promising model for bone engineering.314
Generally, tissue engineered models harbor poor survival because of their limited mass transfer of oxygen and nutrients. As a consequence, big devices, often called “bioreactors,” were designed to overcome this problem. Bioreactors are crucial for tissue engineering as they allow for the growth of large-scale tissues and organs.315,316 By providing an environment that mimics the in vivo environment as closely as possible, bioreactors can enhance the formation of functional tissues. Bioreactors can induce shear stress and hydrostatic pressure needed for the formation of certain types of tissues such as cartilage and bone.317–319 However, despite the progress made in building tissues, further optimization is required to produce tissues in a timely manner and reach the proper differentiation state of cells needed for clinical use.
Recently, multiphoton ablation,320 3D bioprinting,321,322 and microchamber-based approaches323 have emerged as promising technologies for tissue engineering. Multiphoton ablation, a technique that uses laser light to selectively remove material from a sample in a highly precise manner, has been used to create channels of specific size and shape within a scaffold material such as collagen. The scaffold can then be seeded with ECs to create functional BVs.320 The use of pre-formed microvessels as a guide for endothelial cell growth can help ensure that the resulting vessels are properly aligned and connected, which is important for their proper function. 3D Bioprinting technology has the potential to revolutionize tissue engineering and regenerative medicine. By precisely depositing cells and biomaterials in a controlled manner, it is possible to create complex, 3D structures that mimic the architecture and function of natural tissues and organs. This has opened up new avenues for the formation of functional organs and large-scale growth of artificial tissues.79 Bioprinting of organoid-derived stem cells into ECM matrices has been shown to promote self-organization of cells into tissue-like structures, which could be used for drug discovery, disease modeling, and regeneration. Moreover, the recent development of multicell-type bioprinted lumenized intestinal epithelium is a promising step toward creating functional tissues for transplantation.79 Microchamber-based approaches, on the other hand, allow for the formation of organoids with defined cell numbers and spatial arrangements, which can be further assembled into larger structures. These technologies offer new opportunities for the development of effective tissue engineering approaches for regenerative medicine.
In vivo engraftment approaches provide important validation for the functionality and regenerative potential of in vitro engineered tissues. They demonstrate that these tissues can integrate with the surrounding tissue, support vascularization, and perform their intended functions. For instance, kidney organoid transplantation under the kidney capsule of immunodeficient mice has allowed mouse ECs to infiltrate the transplanted kidney organoid and promote vascularization, urine production, and renal recovery.21,324–326 Also, cardiac tissue engineering has been applied using human embryonic stem cell-derived ECs, generating in vitro perfusable microvessels. When implanted on infarcted rat hearts, the microvessels are able to be engrafted with rat cardiac vasculature being fully functional.327 Tissue engineering techniques also have the potential to pave the way for future regenerative medicine therapies, where in vitro engineered tissues could be transplanted into patients to treat a variety of diseases and injuries. However, more research is needed to further improve the functionality, safety, and long-term viability of these engineered tissues before they can be applied in a clinical setting.
V. CONCLUSIONS, CURRENT CHALLENGES, AND FUTURE PERSPECTIVES
Microfluidic-based cell culture technologies offer a high degree of control on the experimental design, system parameters, and system modeling characterization. Thus, they hold great promise for the development of relevant preclinical models (OoC and organoids), drug discovery, as well as personalized and regenerative medicine. In this scenario, the implementation of fluid flow in 3D perfusable microfluidic devices is fundamental to mimic human physiopathology in many organs. Though several vascularized organ 3D models have already been developed,17,152,307,328 current challenges consist of reproducing the complex organ's microenvironment and vessel complex geometry considering the strong coupling between biology and mechanics. Despite the fact that the implementation of 3D organ vascularization is progressing, lymphatic vasculature is lacking from many current applications and should be considered due to its crucial physiological role. Another challenge is the in situ analysis of the perfused microfluidic devices at the transcriptome and metabolome level (key for translational applications) due to the low volume of cells and secreted metabolites in the chips. For that, the use of biosensors incorporating real-time monitoring is essential, as well as the methods to scale up properly designed 3D cell growing to allow the implementation of high-throughput methods (such as single cell RNA sequencing). In addition, the choice of defined perfusate together with human-biocompatible derived matrices is to be considered.
Despite the existing limitations, OoC and organoids are already being used for drug discovery studies, and show significant potential for personalized treatment decisions in diseases like cancer. The above-mentioned progressing construction of tissues vascularization carry high expectations for subsequent transplantation of large-scale prevascularized constructs in regenerative medicine. These constructs would enhance grafts viability and would favor host tissue regeneration.
Precise microvascular network can be provided with 3D bioprinting technology,77–79,329 a field in expansion, which will allow, together with fluid flow, the formation of functional organs and long-term and large-scale growth of artificial tissues. Engineering strategies combined with computational models for biophysical and topological parameters will help in reducing variability, while increasing the degree of automation. Especially important will be future implementation of engineering personalized “humans-on-chips” in which several organs derived from a patient are integrated in the presence of vascular, immune, and microbiome compartments. Altogether, bringing accurately perfused vascular biology to bioengineering approaches is necessary to advance drug discovery and personalized and regenerative medicine in which the collaboration between engineers, clinicians, chemists and biologists is fundamental.
SUPPLEMENTARY MATERIAL
See the supplementary material for Fig. 1, which shows the evolution of 3D cell culture and perfused 3D cell culture tendencies in publications for the last 30 years. Specifically, the percentage of cell culture publications including 3D ECM and perfused 3D ECM terms is analyzed, as well as the fold increase of publications including 3D ECM and perfused 3D ECM terms (compared with 1993–2002 decade), and the percentage of 3D ECM cell culture publications that include fluid flow term.
ACKNOWLEDGMENTS
This work was supported by the European Research Council (ERC-Advanced Grant CADENCE No. 74268), the Spanish Ministry of Science and Innovation (No. PID2021–122409OB-C21), the Spanish Ministry of Universities (Y. J-L FPU17/03867 and A.G-L 2021 Funding Programme for attracting international talent to the Spanish academic system MZ-240621), and the Government of Aragon (Grant No. 2019–23). We are grateful for the insightful discussion and comments of Professor Lütolf (EPFL, Switzerland), and we thank Daniel Camacho (University of Zaragoza, Spain) for technical support.
Contributor Information
José Manuel García-Aznar, Email: mailto:jmgaraz@unizar.es.
Alejandra González-Loyola, Email: mailto:a.gonzalez@unizar.es.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Ethics Approval
Ethics approval is not required.
Author Contributions
Yago Juste-Lanas: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – original draft (equal); Writing – review & editing (equal). Silvia Hervas-Raluy: Investigation (equal); Visualization (equal); Writing – review & editing (equal). Jose Manuel García-Aznar: Conceptualization (equal); Funding acquisition (equal); Writing – review & editing (equal). Alejandra Gonzalez-Loyola: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing – original draft (equal); Writing – review & editing (equal).
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Alhaque S., Themis M., and Rashidi H., “ Three-dimensional cell culture: From evolution to revolution,” Philos. Trans. R. Soc. B 373, 20170216 (2018). 10.1098/rstb.2017.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boussommier-Calleja A., Li R., Chen M. B., Wong S. C., and Kamm R. D., “ Microfluidics: A new tool for modeling cancer–immune interactions,” Trends Cancer 2, 6–19 (2016). 10.1016/j.trecan.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan A. J., Brougham C. M., Garciarena C. D., Kerrigan S. W., and O'Brien F. J., “ Towards 3D in vitro models for the study of cardiovascular tissues and disease,” Drug Discov. Today 21, 1437–1445 (2016). 10.1016/j.drudis.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 4. Wolf K. J., Chen J., Coombes J. D., Aghi M. K., and Kumar, S , “ Dissecting and rebuilding the glioblastoma microenvironment with engineered materials,” Nat. Rev. Mater. 4, 651–668 (2019). 10.1038/s41578-019-0135-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frantz C., Stewart K. M., and Weaver V. M., “ The extracellular matrix at a glance,” J. Cell. Sci. 123, 4195–4200 (2010). 10.1242/jcs.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. González-Díaz E. C. and Varghese S., “ Hydrogels as extracellular matrix analogs,” Gels 2, 20 (2016). 10.3390/gels2030020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu H. et al. , “ Advances in hydrogels in organoids and organs-on-a-chip,” Adv. Mater. 31, 1902042 (2019). 10.1002/adma.201902042 [DOI] [PubMed] [Google Scholar]
- 8. Kelava I. and Lancaster M. A., “ Stem cell models of human brain development,” Cell Stem Cell 18, 736–748 (2016). 10.1016/j.stem.2016.05.022 [DOI] [PubMed] [Google Scholar]
- 9. Bonanini F. et al. , “ In vitro grafting of hepatic spheroids and organoids on a microfluidic vascular bed,” Angiogenesis 25, 455 (2022). 10.1007/s10456-022-09842-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz N. M. et al. , “ Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease,” Nat. Mater. 16(11), 1112–1119 (2017). 10.1038/nmat4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linnemann J. R. et al. , “ Quantification of regenerative potential in primary human mammary epithelial cells,” Development (Cambridge) 142, 3239–3251 (2015). 10.1242/dev.123554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koike H. et al. , “ Engineering human hepato-biliary-pancreatic organoids from pluripotent stem cells,” Nat. Protoc. 16, 919–936 (2021). 10.1038/s41596-020-00441-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller A. J. et al. , “ Generation of lung organoids from human pluripotent stem cells in vitro,” Nat. Protoc. 14, 518–540 (2019). 10.1038/s41596-018-0104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato T. et al. , “ Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche,” Nature 459, 262–265 (2009). 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- 15. Tao T. et al. , “ Engineering human islet organoids from iPSCs using an organ-on-chip platform,” Lab Chip 19, 948–958 (2019). 10.1039/C8LC01298A [DOI] [PubMed] [Google Scholar]
- 16. Lee K. K. et al. , “ Human stomach-on-a-chip with luminal flow and peristaltic-like motility,” Lab Chip 18, 3079–3085 (2018). 10.1039/C8LC00910D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shin N. et al. , “ Vascularization of iNSC spheroid in a 3D spheroid-on-a-chip platform enhances neural maturation,” Biotechnol. Bioeng. 119, 566–574 (2022). 10.1002/bit.27978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cakir B. et al. , “ Engineering of human brain organoids with a functional vascular-like system,” Nat. Methods 16, 1169–1175 (2019). 10.1038/s41592-019-0586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ham O., Jin Y. B., Kim J., and Lee M. O., “ Blood vessel formation in cerebral organoids formed from human embryonic stem cells,” Biochem. Biophys. Res. Commun. 521, 84–90 (2020). 10.1016/j.bbrc.2019.10.079 [DOI] [PubMed] [Google Scholar]
- 20. Li C. Y. et al. , “ Micropatterned cell-cell interactions enable functional encapsulation of primary hepatocytes in hydrogel microtissues,” Tissue Eng. Part A 20, 2200–2212 (2014). 10.1089/ten.tea.2013.0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Homan K. A. et al. , “ Flow-enhanced vascularization and maturation of kidney organoids in vitro,” Nat. Methods 16, 255–262 (2019). 10.1038/s41592-019-0325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu M. et al. , “ Integrated genome and tissue engineering enables screening of cancer vulnerabilities in physiologically relevant perfusable ex vivo cultures,” Biomaterials 280, 121276 (2022). 10.1016/j.biomaterials.2021.121276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daems H. M., Peacock M., and Jones E. A. V., “ Fluid flow as a driver of embryonic morphogenesis,” Development 147, dev185579 (2020). 10.1242/dev.185579 [DOI] [PubMed] [Google Scholar]
- 24. Hall J. E. and Hall M. E., Guyton and Hall Textbook of Medical Physiology E-Book (Elsevier, 2020). [Google Scholar]
- 25. Chim L. K. and Mikos A. G., “ Biomechanical forces in tissue engineered tumor models this review comes from a themed issue on tissue engineering and regenerative medicine: The future of tissue engineering,” Curr. Opin. Biomed. Eng. 6, 42–50 (2018). 10.1016/j.cobme.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roux E., Bougaran P., Dufourcq P., and Couffinhal T., “ Fluid shear stress sensing by the endothelial layer,” Front Physiol. 11, 861 (2020). 10.3389/fphys.2020.00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polacheck W. J., German A. E., Mammoto A., Ingber D. E., and Kamm R. D., “ Mechanotransduction of fluid stresses governs 3D cell migration,” Proc. Natl. Acad. Sci. U. S. A. 111, 2447–2452 (2014). 10.1073/pnas.1316848111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ng C. P., Hinz B., and Swartz M. A., “ Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro,” J. Cell Sci. 118, 4731–4739 (2005). 10.1242/jcs.02605 [DOI] [PubMed] [Google Scholar]
- 29. del Amo C. et al. , “ Matrix architecture plays a pivotal role in 3D osteoblast migration: The effect of interstitial fluid flow,” J. Mech. Behav. Biomed. Mater. 83, 52–62 (2018). 10.1016/j.jmbbm.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 30. Prendergast M. E. et al. , “ Local extensional flows promote long-range fiber alignment in 3D collagen hydrogels.” Biofabrication 14, 035019 (2022). 10.1088/1758-5090/ac7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehoux S., “ Molecular mechanisms of the vascular responses to hemodynamic forces,” in Biomechanics of Coronary Atherosclerotic Plaque: From Model to Patient ( Elsevier, 2021), pp. 49–83. [Google Scholar]
- 32. Birdsall H. H. and Hammond T. G., “ Role of shear stress on renal proximal tubular cells for nephrotoxicity assays,” J. Toxicol. 2021, 6643324. 10.1155/2021/6643324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riehl B. D., Donahue H. J., and Lim J. Y., “ Fluid flow control of stem cells with investigation of mechanotransduction pathways,” in Biology and Engineering of Stem Cell Niches ( Elsevier Inc., 2017). pp. 257–272. [Google Scholar]
- 34. Souilhol C. et al. , “ Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes,” Nat. Rev. Cardiol. 17, 52–63 (2020). 10.1038/s41569-019-0239-5 [DOI] [PubMed] [Google Scholar]
- 35. Nauli S. M. et al. , “ Non-motile primary cilia as fluid shear stress mechanosensors,” Methods Enzymol. 525, 1 (2013). 10.1016/B978-0-12-397944-5.00001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferreira R. R., Fukui H., Chow R., Vilfan A., and Vermot J., “ The cilium as a force sensor-myth versus reality,” J. Cell Sci. 132, jcs213496 (2019). 10.1242/jcs.213496 [DOI] [PubMed] [Google Scholar]
- 37. Hahn C. and Schwartz M. A., “ Mechanotransduction in vascular physiology and atherogenesis,” Nat. Rev. Mol. Cell Biol. 10, 53–62 (2009). 10.1038/nrm2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson B. D., Mather K. J., and Wallace J. P., “ Mechanotransduction of shear in the endothelium: Basic studies and clinical implications,” Vasc. Med. 16, 365–377 (2011). 10.1177/1358863X11422109 [DOI] [PubMed] [Google Scholar]
- 39. Song J. W. and Munn L. L., “ Fluid forces control endothelial sprouting,” Proc. Natl. Acad. Sci. U. S. A. 108, 15342–15347 (2011). 10.1073/pnas.1105316108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morbiducci U. et al. , “ On the importance of blood rheology for bulk flow in hemodynamic models of the carotid bifurcation,” J. Biomech. 44, 2427–2438 (2011). 10.1016/j.jbiomech.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 41. Stewart R. H., “ A modern view of the interstitial space in health and disease,” Front Vet. Sci. 7, 924 (2020). 10.3389/fvets.2020.609583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Henderson A. R., Choi H., and Lee E., “ Blood and lymphatic vasculatures on-chip platforms and their applications for organ-specific in vitro modeling,” Micromachines (Basel) 11, 147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mekala N. K., Baadhe R. R., and Potumarthi R., “ Mass transfer aspects of 3D cell cultures in tissue engineering,” Asia-Pacific J. Chem. Eng. 9, 318–329 (2014). 10.1002/apj.1800 [DOI] [Google Scholar]
- 44. Ray L., Iliff J. J., and Heys J. J., “ Analysis of convective and diffusive transport in the brain interstitium,” Fluids Barriers CNS 16, 6 (2019). 10.1186/s12987-019-0126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swartz M. A. and Fleury M. E., “ Interstitial flow and its effects in soft tissues,” Annu. Rev. Biomed. Eng. 9 229–256 (2007). 10.1146/annurev.bioeng.9.060906.151850 [DOI] [PubMed] [Google Scholar]
- 46. Mammoto T., Mammoto A., and Ingber D. E., “ Mechanobiology and developmental control,” Annu. Rev. Cell Dev. Biol. 29, 27–61 (2013). 10.1146/annurev-cellbio-101512-122340 [DOI] [PubMed] [Google Scholar]
- 47. Davis M. J. and Hill M. A., “ Signaling mechanisms underlying the vascular myogenic response,” Physiol. Rev 79, 387–423 (1999). 10.1152/physrev.1999.79.2.387 [DOI] [PubMed] [Google Scholar]
- 48. Olsson A. K., Dimberg A., Kreuger J., and Claesson-Welsh L., “ VEGF receptor signalling? in control of vascular function,” Nat. Rev. Mol. Cell Biol. 7(5), 359–371 (2006). 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- 49. Ghaffari S., Leask R. L., and Jones E. A. V., “ Flow dynamics control the location of sprouting and direct elongation during developmental angiogenesis,” Development 142, 4151–4157 (2015). 10.1242/dev.128058 [DOI] [PubMed] [Google Scholar]
- 50. Kutys M. L. and Chen C. S., “ Forces and mechanotransduction in 3D vascular biology,” Curr. Opin. Cell Biol. 42, 73–79 (2016). 10.1016/j.ceb.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baeyens N., Bandyopadhyay C., Coon B. G., Yun S., and Schwartz M. A., “ Endothelial fluid shear stress sensing in vascular health and disease,” J. Clin. Invest. 126, 821–828 (2016). 10.1172/JCI83083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Esmon C. T., “ Basic mechanisms and pathogenesis of venous thrombosis,” Blood Rev. 23, 225 (2009). 10.1016/j.blre.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jain A. et al. , “ Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium,” Biomed. Microdevices 18, 73 (2016). 10.1007/s10544-016-0095-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davies P. F., Spaan J. A., and Krams R., “ Shear stress biology of the endothelium,” Ann. Biomed. Eng. 33, 1714–1718 (2005). 10.1007/s10439-005-8774-0 [DOI] [PubMed] [Google Scholar]
- 55. Albarrán-Juárez J. et al. , “ Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation,” J. Exp. Med. 215, 2655–2672 (2018). 10.1084/jem.20180483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corti P. et al. , “ Interaction between alk1 and blood flow in the development of arteriovenous malformations,” Development 138, 1573–1582 (2011). 10.1242/dev.060467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim S., Lee H., Chung M., and Jeon N. L., “ Engineering of functional, perfusable 3D microvascular networks on a chip,” Lab Chip 13, 1489–1500 (2013). 10.1039/c3lc41320a [DOI] [PubMed] [Google Scholar]
- 58. van Duinen V. et al. , “ 96 perfusable blood vessels to study vascular permeability in vitro,” Sci. Rep. 7, 18071 (2017). 10.1038/s41598-017-14716-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Funamoto K. et al. , “ Endothelial monolayer permeability under controlled oxygen tension,” Integr. Biol. 9, 529–538 (2017). 10.1039/C7IB00068E [DOI] [PubMed] [Google Scholar]
- 60. Polacheck W. J., Kutys M. L., Tefft J. B., and Chen C. S., “ Microfabricated blood vessels for modeling the vascular transport barrier,” Nat. Protoc. 14, 1425–1454 (2019). 10.1038/s41596-019-0144-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zheng Y. et al. , “ In vitro microvessels for the study of angiogenesis and thrombosis,” Proc. Natl. Acad. Sci. U. S. A. 109, 9342–9347 (2012). 10.1073/pnas.1201240109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller J. S. et al. , “ Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues,” Nat. Mater. 11, 768–774 (2012). 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang X. Y. et al. , “ Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template,” Lab Chip 14, 2709–2716 (2014). 10.1039/C4LC00069B [DOI] [PubMed] [Google Scholar]
- 64. Brandenberg N. and Lutolf, M. P. , “ In situ patterning of microfluidic networks in 3D cell-laden hydrogels,” Adv. Mater. 28, 7450–7456 (2016). 10.1002/adma.201601099 [DOI] [PubMed] [Google Scholar]
- 65. Chan J. M. et al. , “ Engineering of in vitro 3D capillary beds by self-directed angiogenic sprouting,” PLoS One 7, e50582 (2012). 10.1371/journal.pone.0050582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamamoto K. et al. , “ Construction of continuous capillary networks stabilized by pericyte-like perivascular cells,” Tissue Eng. Part A 25, 499–510 (2019). 10.1089/ten.tea.2018.0186 [DOI] [PubMed] [Google Scholar]
- 67. Pérez-Rodríguez S., Huang S. A., Borau C., García-Aznar J. M., and Polacheck W. J., “ Microfluidic model of monocyte extravasation reveals the role of hemodynamics and subendothelial matrix mechanics in regulating endothelial integrity,” Biomicrofluidics 15, 054102 (2021). 10.1063/5.0061997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carmeliet P. and Jain R. K., “ Molecular mechanisms and clinical applications of angiogenesis,” Nature 473, 298–307 (2011). 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Herbert S. P. and Stainier D. Y. R., “ Molecular control of endothelial cell behaviour during blood vessel morphogenesis,” Nat. Rev. Mol. Cell Biol. 12, 551–564 (2011). 10.1038/nrm3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Papetti M. and Herman I. M., “ Mechanisms of normal and tumor-derived angiogenesis,” Am. J. Physiol. Cell Physiol. 282, C947 (2002). 10.1152/ajpcell.00389.2001 [DOI] [PubMed] [Google Scholar]
- 71. Gebala V., Collins R., Geudens I., Phng L. K., and Gerhardt H., “ Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo,” Nat. Cell Biol. 18, 443–450 (2016). 10.1038/ncb3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nguyen D. H. T. et al. , “ Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro,” Proc. Natl. Acad. Sci. U. S. A. 110, 6712–6717 (2013). 10.1073/pnas.1221526110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Osaki T., Serrano J. C., and Kamm R. D., “ Cooperative effects of vascular angiogenesis and lymphangiogenesis,” Regen. Eng. Transl. Med. 4, 120–132 (2018). 10.1007/s40883-018-0054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heissig B., Hattori K., Friedrich M., Rafii S., and Werb Z., “ Angiogenesis: Vascular remodeling of the extracellular matrix involves metalloproteinases,” Curr. Opin. Hematol. 10, 136–141 (2003). 10.1097/00062752-200303000-00007 [DOI] [PubMed] [Google Scholar]
- 75. Davis G. E. and Senger, D. R. , “ Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization,” Circ. Res. 97, 1093–1107 (2005). 10.1161/01.RES.0000191547.64391.e3 [DOI] [PubMed] [Google Scholar]
- 76. Potente M., Gerhardt H., and Carmeliet P., “ Basic and therapeutic aspects of angiogenesis,” Cell 146, 873–887 (2011). 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- 77. Zhang Y. S. et al. , “ Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip,” Biomaterials 110, 45–59 (2016). 10.1016/j.biomaterials.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhu W. et al. , “ Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture,” Biomaterials 124, 106–115 (2017). 10.1016/j.biomaterials.2017.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brassard J. A., Nikolaev M., Hübscher T., Hofer M., and Lutolf M. P., “ Recapitulating macro-scale tissue self-organization through organoid bioprinting,” Nat. Mater. 20, 22–29 (2021). 10.1038/s41563-020-00803-5 [DOI] [PubMed] [Google Scholar]
- 80. Vianello S. and Lutolf M. P., “ Understanding the mechanobiology of early mammalian development through bioengineered models,” Dev. Cell 48, 751–763 (2019). 10.1016/j.devcel.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 81. Duchemin A. L., Vignes H., Vermot J., and Chow R., “ Mechanotransduction in cardiovascular morphogenesis and tissue engineering,” Curr. Opin. Genet. Dev. 57, 106–116 (2019). 10.1016/j.gde.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 82. van Dijk C. G. M. et al. , “ A new microfluidic model that allows monitoring of complex vascular structures and cell interactions in a 3D biological matrix,” Lab Chip 20, 1827–1844 (2020). 10.1039/D0LC00059K [DOI] [PubMed] [Google Scholar]
- 83. Diamond S. L., “ Systems analysis of thrombus formation,” Circ. Res. 118, 1348–1362 (2016). 10.1161/CIRCRESAHA.115.306824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. González-Loyola A. and Petrova T. v., “ Development and aging of the lymphatic vascular system,” Adv. Drug Deliv Rev. 169, 63–78 (2021). 10.1016/j.addr.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 85. Frye M. et al. , “ Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program,” Nat. Commun. 9, 1511 (2018). 10.1038/s41467-018-03959-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hägerling R. et al. , “ A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy,” EMBO J. 32, 629–644 (2013). 10.1038/emboj.2012.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jerafi-Vider A. et al. , “ VEGFC induced cell cycle arrest mediates sprouting and differentiation of venous and lymphatic endothelial cells,” bioRxiv 2020.06.17.155028 (2020). [DOI] [PMC free article] [PubMed]
- 88. Planas-Paz L. et al. , “ Mechanoinduction of lymph vessel expansion,” EMBO J. 31, 788–804 (2012). 10.1038/emboj.2011.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Choi D. et al. , “ ORAI1 activates proliferation of lymphatic endothelial cells in response to laminar flow through Krüppel-like factors 2 and 4,” Circ. Res. 120, 1426–1439 (2017). 10.1161/CIRCRESAHA.116.309548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sabine A. et al. , “ Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation,” Dev. Cell 22, 430–445 (2012). 10.1016/j.devcel.2011.12.020 [DOI] [PubMed] [Google Scholar]
- 91. Kampmeier O. F., “ The genetic history of the valves in the lymphatic system of man,” Am. J. Anatomy 40, 413–457 (1928). 10.1002/aja.1000400302 [DOI] [Google Scholar]
- 92. Baluk P. et al. , “ Functionally specialized junctions between endothelial cells of lymphatic vessels,” J. Exp. Med. 204, 2349–2362 (2007). 10.1084/jem.20062596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Miteva D. O. et al. , “ Transmural flow modulates cell and fluid transport functions of lymphatic endothelium,” Circ. Res. 106, 920–931 (2010). 10.1161/CIRCRESAHA.109.207274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sabine A. et al. , “ FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature,” J. Clin. Invest. 125, 3861–3877 (2015). 10.1172/JCI80454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baeyens N. et al. , “ Vascular remodeling is governed by a vegfr3-dependent fluid shear stress set point,” Elife 4, 1–35 (2015). 10.7554/eLife.04645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Oliver G., Kipnis J., Randolph G. J., and Harvey N. L., “ The lymphatic vasculature in the 21st century: Novel functional roles in homeostasis and disease,” Cell 182, 270–296 (2020). 10.1016/j.cell.2020.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Alitalo K., “ The lymphatic vasculature in disease,” Nat. Med. 17, 1371–1380 (2011). 10.1038/nm.2545 [DOI] [PubMed] [Google Scholar]
- 98. Henri O. et al. , “ Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction,” Circulation 133, 1484–1497 (2016). 10.1161/CIRCULATIONAHA.115.020143 [DOI] [PubMed] [Google Scholar]
- 99. Cao X. et al. , “ A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair,” Adv. Funct. Mater. 29, 1807173 (2019). 10.1002/adfm.201807173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee H. J. et al. , “ Fluid shear stress activates YAP1 to promote cancer cell motility,” Nat. Commun. 8, 14122 (2017). 10.1038/ncomms14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lugo-Cintrón K. M. et al. , “ Matrix density drives 3D organotypic lymphatic vessel activation in a microfluidic model of the breast tumor microenvironment,” Lab Chip 20, 1586–1600 (2020). 10.1039/D0LC00099J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. McKee T. J. and Komarova S. V., “ Is it time to reinvent basic cell culture medium?” Am. J. Physiol.-Cell Physiol. 312, C624–C626 (2017). 10.1152/ajpcell.00336.2016 [DOI] [PubMed] [Google Scholar]
- 103. Ackermann T. and Tardito S., “ Cell culture medium formulation and its implications in cancer metabolism,” Trends Cancer 5, 329–332 (2019). 10.1016/j.trecan.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Leung C. M. et al. , “ A guide to the organ-on-a-chip,” Nat. Rev. Methods Primers 2, 33 (2022). 10.1038/s43586-022-00118-6 [DOI] [Google Scholar]
- 105. Gonzalez-Molina, J. et al. , “ Extracellular fluid viscosity enhances liver cancer cell mechanosensing and migration,” Biomaterials 177, 113–124 (2018). 10.1016/j.biomaterials.2018.05.058 [DOI] [PubMed] [Google Scholar]
- 106. Poon C., “ Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices,” J. Mech. Behav. Biomed. Mater 126, 105024 (2022). 10.1016/j.jmbbm.2021.105024 [DOI] [PubMed] [Google Scholar]
- 107. Homan K. A. et al. , “ Bioprinting of 3D convoluted renal proximal tubules on perfusable chips,” Sci. Rep. 6, 34845 (2016). 10.1038/srep34845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Aceves J. O. et al. , “ 3D proximal tubule-on-chip model derived from kidney organoids with improved drug uptake,” Sci. Rep. 12, 14997 (2022). 10.1038/s41598-022-19293-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rein J. L. et al. , “ Effect of luminal flow on doming of mpkCCD cells in a 3D perfusable kidney cortical collecting duct model,” Am. J. Physiol. Cell Physiol. 318, C136–C147 (2020). 10.1152/ajpcell.00405.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hilscher M. B. et al. , “ Mechanical stretch increases expression of CXCL1 in liver sinusoidal endothelial cells to recruit neutrophils, generate sinusoidal microthombi, and promote portal hypertension,” Gastroenterology 157, 193–209.e9 (2019). 10.1053/j.gastro.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kim S. W. et al. , “ Shear stress induces noncanonical autophagy in intestinal epithelial monolayers,” Mol. Biol. Cell. 28, 3043–3056 (2017). 10.1091/mbc.e17-01-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Verschuren E. H. J. et al. , “ Sensing of tubular flow and renal electrolyte transport,” Nat. Rev. Nephrol. 16 337–351 (2020). 10.1038/s41581-020-0259-8 [DOI] [PubMed] [Google Scholar]
- 113. Lin N. Y. C. et al. , “ Renal reabsorption in 3D vascularized proximal tubule models,” Proc. Natl. Acad. Sci U. S. A. 116, 5399–5404 (2019). 10.1073/pnas.1815208116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Du Y. et al. , “ A bile duct-on-a-chip with organ-level functions,” Hepatology 71, 1350–1363 (2020). 10.1002/hep.30918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Trietsch S. J. et al. , “ Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes,” Nat. Commun. 8, 262 (2017). 10.1038/s41467-017-00259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nikolaev M. et al. , “ Homeostatic mini-intestines through scaffold-guided organoid morphogenesis,” Nature 585, 574–578 (2020). 10.1038/s41586-020-2724-8 [DOI] [PubMed] [Google Scholar]
- 117. Ewart L. et al. , “ Performance assessment and economic analysis of a human liver-chip for predictive toxicology,” Commun. Med. 2, 154 (2022). 10.1038/s43856-022-00209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Du Y. et al. , “ Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip,” Lab Chip 17, 782–794 (2017). 10.1039/C6LC01374K [DOI] [PubMed] [Google Scholar]
- 119. Lee S. Y., Kim D., Lee S. H., and Sung J. H., “ Microtechnology-based in vitro models: Mimicking liver function and pathophysiology,” APL Bioeng. 5, 041505 (2021). 10.1063/5.0061896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bein A. et al. , “ Nutritional deficiency in an intestine-on-a-chip recapitulates injury hallmarks associated with environmental enteric dysfunction,” Nat. Biomed. Eng. 6, 1236–1247 (2022). 10.1038/s41551-022-00899-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fois C. A. M., Schindeler A., Valtchev P., and Dehghani F., “ Dynamic flow and shear stress as key parameters for intestinal cells morphology and polarization in an organ-on-a-chip model,” Biomed. Microdevices 23, 55 (2021). 10.1007/s10544-021-00591-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jing B. et al. , “ Establishment and application of peristaltic human gut-vessel microsystem for studying host–microbial interaction,” Front Bioeng. Biotechnol 8, 272 (2020). 10.3389/fbioe.2020.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Thomas D. P., Zhang J., Nguyen N.-T., and Ta H. T., “ Microfluidic gut-on-a-chip: Fundamentals and challenges,” Biosensors 13, 136 (2023). 10.3390/bios13010136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xiang Y. et al. , “ Gut-on-chip: Recreating human intestine in vitro,” J. Tissue Eng. 11, 204173142096531 (2020). 10.1177/2041731420965318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ashammakhi N. et al. , “ Gut-on-a-chip: Current progress and future opportunities,” Biomaterials 255, 120196 (2020). 10.1016/j.biomaterials.2020.120196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Marrero D. et al. , “ Gut-on-a-chip: Mimicking and monitoring the human intestine,” Biosens. Bioelectron. 181, 113156 (2021). 10.1016/j.bios.2021.113156 [DOI] [PubMed] [Google Scholar]
- 127. Zamprogno P. et al. , “ Mechanical properties of soft biological membranes for organ-on-a-chip assessed by bulge test and AFM,” ACS Biomater Sci. Eng. 7, 2990–2997 (2021). 10.1021/acsbiomaterials.0c00515 [DOI] [PubMed] [Google Scholar]
- 128. Wang C., Tanataweethum N., Karnik S., and Bhushan A., “ Novel microfluidic colon with an extracellular matrix membrane,” ACS Biomater. Sci. Eng. 4, 1377–1385 (2018). 10.1021/acsbiomaterials.7b00883 [DOI] [PubMed] [Google Scholar]
- 129. Salimbeigi G., Vrana N. E., Ghaemmaghami A. M., Huri P. Y., and McGuinness G. B., “ Basement membrane properties and their recapitulation in organ-on-chip applications,” Mater. Today Bio 15, 100301 (2022). 10.1016/j.mtbio.2022.100301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Heldin C. H., Rubin K., Pietras K., and Östman A., “ High interstitial fluid pressure—An obstacle in cancer therapy,” Nat. Rev. Cancer 4 806–813 (2004). 10.1038/nrc1456 [DOI] [PubMed] [Google Scholar]
- 131. Wittkowske C., Reilly G. C., Lacroix D., and Perrault C. M., “ In vitro bone cell models: Impact of fluid shear stress on bone formation,” Front Bioeng. Biotechnol. 4, 87 (2016). 10.3389/fbioe.2016.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shieh A. C. and Swartz M. A., “ Regulation of tumor invasion by interstitial fluid flow,” Phys. Biol. 8, 015012 (2011). 10.1088/1478-3975/8/1/015012 [DOI] [PubMed] [Google Scholar]
- 133. Shieh A. C., “ Biomechanical forces shape the tumor microenvironment,” Ann. Biomed. Eng. 39, 1379–1389 (2011). 10.1007/s10439-011-0252-2 [DOI] [PubMed] [Google Scholar]
- 134. Shi Z. D., Ji X. Y., Qazi H., and Tarbell J. M., “ Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1,” Am. J. Physiol Heart Circ Physiol. 297, H1225 (2009). 10.1152/ajpheart.00369.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Swartz M. A. and Lund A. W., “Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity,” Nat. Rev. Cancer 12, 210–219 (2012). 10.1038/nrc3186 [DOI] [PubMed] [Google Scholar]
- 136. Polacheck W. J., Charest J. L., and Kamm R. D., “ Interstitial flow influences direction of tumor cell migration through competing mechanisms,” Proc. Natl. Acad. Sci. U. S. A. 108, 11115–11120 (2011). 10.1073/pnas.1103581108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Vickerman V., Blundo J., Chung S., and Kamm R., “ Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging,” Lab Chip 8, 1468–1477 (2008). 10.1039/b802395f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Abe Y. et al. , “ Balance of interstitial flow magnitude and vascular endothelial growth factor concentration modulates three-dimensional microvascular network formation,” APL Bioeng. 3, 036102 (2019). 10.1063/1.5094735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Qazi H., Shi Z. D., and Tarbell J. M., “ Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression,” PLoS One 6, e20348 (2011). 10.1371/journal.pone.0020348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ng C. P. and Swartz M. A., “ Mechanisms of interstitial flow-induced remodeling of fibroblast–collagen cultures,” Ann. Biomed. Eng. 34, 446–454 (2006). 10.1007/s10439-005-9067-3 [DOI] [PubMed] [Google Scholar]
- 141. Scallan J., Huxley V. H., and Korthuis R. J., “ Capillary fluid exchange: Regulation, functions, and pathology,” Colloq. Ser. Integr. Syst. Physiol.: From Mol. Funct. 2, 1–94 (2010). 10.4199/C00006ED1V01Y201002ISP003 [DOI] [PubMed] [Google Scholar]
- 142. Shields J. D. et al. , “ Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling,” Cancer Cell 11, 526–538 (2007). 10.1016/j.ccr.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 143. Li A., Sun M., Spill F., Sun R., and Zaman M. H., “ Are the effects of independent biophysical factors linearly additive? A 3D tumor migration model,” Biophys. J. 117, 1702–1713 (2019). 10.1016/j.bpj.2019.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stylianopoulos T., “ The solid mechanics of cancer and strategies for improved therapy,” J. Biomech. Eng. 139, 021004 (2017). 10.1115/1.4034991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zanotelli M. R. and Reinhart-King C. A., “ Mechanical forces in tumor angiogenesis,” Adv. Exp. Med. Biol. 1092, 91–112 (2018). 10.1007/978-3-319-95294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yeon J. H. et al. , “ Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts,” Acta Biomater. 76, 146–153 (2018). 10.1016/j.actbio.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 147. Li R. et al. , “ Interstitial flow promotes macrophage polarization toward an M2 phenotype,” Mol. Biol. Cell 29, 1927–1940 (2018). 10.1091/mbc.E18-03-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wang S. and Tarbell J. M., “ Effect of fluid flow on smooth muscle cells in a 3-dimensional collagen gel model,” Arterioscler., Thromb., Vasc. Biol. 20, 2220–2225 (2000). 10.1161/01.ATV.20.10.2220 [DOI] [PubMed] [Google Scholar]
- 149. Bhise N. S. et al. , “ Organ-on-a-chip platforms for studying drug delivery systems,” J. Controlled Release 190, 82–93(2014). 10.1016/j.jconrel.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mina S. G., Huang P., Murray B. T., and Mahler G. J., “ The role of shear stress and altered tissue properties on endothelial to mesenchymal transformation and tumor-endothelial cell interaction,” Biomicrofluidics 11, 044104 (2017). 10.1063/1.4991738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Albanese A., Lam A. K., Sykes E. A., Rocheleau J. V., and Chan W. C. W., “ Tumour-on-a-chip provides an optical window into nanoparticle tissue transport,” Nat. Commun. 4, 2718 (2013). 10.1038/ncomms3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nashimoto Y. et al. , “ Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid,” Biomaterials 229, 119547 (2020). 10.1016/j.biomaterials.2019.119547 [DOI] [PubMed] [Google Scholar]
- 153. Arora M., “ Cell culture media: A review,” Mater. Methods 3, 175 (2013). 10.13070/mm.en.3.175 [DOI] [Google Scholar]
- 154. Bourn M. D. et al. , “ High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures,” J. Controlled Release 326, 13–24 (2020). 10.1016/j.jconrel.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 155. Sudo R. et al. , “ Transport‐mediated angiogenesis in 3D epithelial coculture,” FASEB J. 23, 2155–2164 (2009). 10.1096/fj.08-122820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Moreno-Arotzena O., Meier J. G., del Amo C., and García-Aznar J. M., “ Characterization of fibrin and collagen gels for engineering wound healing models,” Materials 8, 1636–1651 (2015). 10.3390/ma8041636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Miranda I. et al. , “ Properties and applications of PDMS for biomedical engineering: A review,” J. Funct. Biomater. 13, 2 (2022). 10.3390/jfb13010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Haase K. et al. , “ Physiologic flow-conditioning limits vascular dysfunction in engineered human capillaries,” Biomaterials 280, 121248 (2022). 10.1016/j.biomaterials.2021.121248 [DOI] [PubMed] [Google Scholar]
- 159. Seiler K. M. et al. , “ Patient-derived small intestinal myofibroblasts direct perfused, physiologically responsive capillary development in a microfluidic gut-on-a-chip model,” Sci. Rep. 10, 3842 (2020). 10.1038/s41598-020-60672-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kothapalli C. R. et al. , “ A high-throughput microfluidic assay to study neurite response to growth factor gradients,” Lab Chip 11, 497–507 (2011). 10.1039/C0LC00240B [DOI] [PubMed] [Google Scholar]
- 161. Gerigk M. et al. , “ On-chip perivascular niche supporting stemness of patient-derived glioma cells in a serum-free, flowable culture,” Lab Chip 21, 2343–2358 (2021). 10.1039/D1LC00271F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Jeong G. S. et al. , “ Siphon-driven microfluidic passive pump with a yarn flow resistance controller,” Lab Chip 14, 4213–4219 (2014). 10.1039/C4LC00510D [DOI] [PubMed] [Google Scholar]
- 163. Dessalles C. A., Ramón-Lozano C., Babataheri A., and Barakat A. I., “ Luminal flow actuation generates coupled shear and strain in a microvessel-on-chip,” Biofabrication 14, 015003 (2021). 10.1088/1758-5090/ac2baa [DOI] [PubMed] [Google Scholar]
- 164. Ng C. P., Helm C. L. E., and Swartz M. A., “ Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro,” Microvasc. Res. 68, 258–264 (2004). 10.1016/j.mvr.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 165. Tomei A. A., Siegert S., Britschgi M. R., Luther S. A., and Swartz M. A., “ Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment,” J. Immunol. 183, 4273–4283 (2009). 10.4049/jimmunol.0900835 [DOI] [PubMed] [Google Scholar]
- 166. Haessler U., Teo J. C. M., Foretay D., Renaud P., and Swartz M. A., “ Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber,” Integr. Biol. 4, 401–409 (2012). 10.1039/c1ib00128k [DOI] [PubMed] [Google Scholar]
- 167. Cornelison R. C., Brennan C. E., Kingsmore K. M., and Munson J. M., “ Convective forces increase CXCR4-dependent glioblastoma cell invasion in GL261 murine model,” Sci. Rep. 8, 17057 (2018). 10.1038/s41598-018-35141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kingsmore K. M. et al. , “ Interstitial flow differentially increases patient-derived glioblastoma stem cell invasion via CXCR4, CXCL12, and CD44-mediated mechanisms,” Integr. Biol. 8, 1246–1260 (2016). 10.1039/c6ib00167j [DOI] [PubMed] [Google Scholar]
- 169. Kingsmore K. M. et al. , “ MRI analysis to map interstitial flow in the brain tumor microenvironment,” APL Bioeng. 2, 031905 (2018). 10.1063/1.5023503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Shah A. D., Bouchard M. J., and Shieh A. C., “ Interstitial fluid flow increases hepatocellular carcinoma cell invasion through CXCR4/CXCL12 and MEK/ERK signaling,” PLoS One 10, e0142337 (2015). 10.1371/journal.pone.0142337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tchafa A. M., Shah A. D., Wang S., Duong M. T., and Shieh A. C., “ Three-dimensional cell culture model for measuring the effects of interstitial fluid flow on tumor cell invasion,” J. Visualized Exp. 65, 4159 (2012). 10.3791/4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Tchafa A. M., Ta M., Reginato M. J., and Shieh A. C., “ EMT transition alters interstitial fluid flow-induced signaling in ERBB2-positive breast cancer cells,” Mol. Cancer Res. 13, 755–764 (2015). 10.1158/1541-7786.MCR-14-0471 [DOI] [PubMed] [Google Scholar]
- 173. Shieh A. C., Rozansky H. A., Hinz B., and Swartz M. A., “ Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts,” Cancer Res. 71, 790–800 (2011). 10.1158/0008-5472.CAN-10-1513 [DOI] [PubMed] [Google Scholar]
- 174. Sweet D. T. et al. , “ Lymph flow regulates collecting lymphatic vessel maturation in vivo,” J. Clin. Invest. 125, 2995–3007 (2015). 10.1172/JCI79386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Johnsena S., Schreiberb S. J., Connollyb F., Schepelmanna K., and Valduezac J. M., “ Pitfall of vertebral artery insonation: Bidirectional flow without subclavian artery pathology,” Perspect. Med. 1, 449–451 (2012). 10.1016/j.permed.2012.04.003 [DOI] [Google Scholar]
- 176. Kozak M. F., Yoo S. J., Mertens L., Ho A., and Grosse-Wortmann L., “ Assessment of ductal blood flow in newborns with obstructive left heart lesions by cardiovascular magnetic resonance,” J. Cardiovasc. Magn. Reson. 15, 45 (2013). 10.1186/1532-429X-15-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Soragni C. et al. , “ A versatile multiplexed assay to quantify intracellular ROS and cell viability in 3D on-a-chip models,” Redox Biol. 57, 102488 (2022). 10.1016/j.redox.2022.102488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Vormann M. K. et al. , “ Modelling and prevention of acute kidney injury through ischemia and reperfusion in a combined human renal proximal tubule/blood vessel-on-a-chip,” Kidney360 3, 217–231 (2022). 10.34067/KID.0003622021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lee G. H. et al. , “ Multilayer microfluidic platform for the study of luminal, transmural, and interstitial flow,” Biofabrication 14, 025007 (2022). 10.1088/1758-5090/ac48e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Henderson I. S., A. R., Ilan , and Lee E., “ A bioengineered lymphatic vessel model for studying lymphatic endothelial cell-cell junction and barrier function,” Microcirculation 28, e12730 (2021). 10.1111/micc.12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Vriend J. et al. , “ Screening of drug-transporter interactions in a 3D microfluidic renal proximal tubule on a chip,” AAPS J. 20, 87 (2018). 10.1208/s12248-018-0247-0 [DOI] [PubMed] [Google Scholar]
- 182. Weller A. et al. , “ Quantifying the transport of biologics across intestinal barrier models in real-time by fluorescent imaging,” Front Bioeng. Biotechnol. 10, 1424 (2022). 10.3389/fbioe.2022.965200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Riddle R. B., Jennbacken K., Hansson K. M., and Harper M. T., “ Endothelial inflammation and neutrophil transmigration are modulated by extracellular matrix composition in an inflammation-on-a-chip model,” Sci. Rep. 12, 6855 (2022). 10.1038/s41598-022-10849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wevers N. R. et al. , “ A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport,” Fluids Barriers CNS 15, 23 (2018). 10.1186/s12987-018-0108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kurosawa T. et al. , “ Construction and functional evaluation of a three-dimensional blood-brain barrier model equipped with human induced pluripotent stem cell-derived brain microvascular endothelial cells,” Pharm. Res. 39, 1535–1547 (2022). 10.1007/s11095-022-03249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Verma S. D. et al. , “ Astrocytes regulate vascular endothelial responses to simulated deep space radiation in a human organ-on-a-chip model,” Front Immunol. 13, 4990 (2022). 10.3389/fimmu.2022.864923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Vormann M. K. et al. , “ Implementation of a human renal proximal tubule on a chip for nephrotoxicity and drug interaction studies,” J. Pharm. Sci. 110, 1601–1614 (2021). 10.1016/j.xphs.2021.01.028 [DOI] [PubMed] [Google Scholar]
- 188. Kutys M. L. et al. , “ Uncovering mutation-specific morphogenic phenotypes and paracrine-mediated vessel dysfunction in a biomimetic vascularized mammary duct platform,” Nat. Commun. 11, 3377 (2020). 10.1038/s41467-020-17102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Beaurivage C. et al. , “ Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery,” Int. J. Mol. Sci. 20, 5661 (2019). 10.3390/ijms20225661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kramer B. et al. , “ High-throughput 3D microvessel-on-a-chip model to study defective angiogenesis in systemic sclerosis,” Sci. Rep. 12, 16930 (2022). 10.1038/s41598-022-21468-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Jung O. et al. , “ Development of human-derived, three-dimensional respiratory epithelial tissue constructs with perfusable microvasculature on a high-throughput microfluidics screening platform,” Biofabrication 14, 025012 (2022). 10.1088/1758-5090/ac32a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chhabra A. et al. , “ A vascularized model of the human liver mimics regenerative responses,” Proc. Natl. Acad. Sci. U. S. A. 119, e2115867119 (2022). 10.1073/pnas.2115867119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Lai B. F. L. et al. , “ A well plate-based multiplexed platform for incorporation of organoids into an organ-on-a-chip system with a perfusable vasculature,” Nat. Protoc. 16, 2158–2189 (2021). 10.1038/s41596-020-00490-1 [DOI] [PubMed] [Google Scholar]
- 194. Lee D. W., Choi N., and Sung J. H., “ A microfluidic chip with gravity-induced unidirectional flow for perfusion cell culture,” Biotechnol. Prog. 35, e2701 (2019). 10.1002/btpr.2701 [DOI] [PubMed] [Google Scholar]
- 195. Vera R. H. et al. , “ Interstitial fluid flow intensity modulates endothelial sprouting in restricted Src-activated cell clusters during capillary morphogenesis,” Tissue Eng. Part A 15, 175 (2009). 10.1089/ten.tea.2007.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kolesky D. B., Homan K. A., Skylar-Scott M. A., and Lewis J. A., “ Three-dimensional bioprinting of thick vascularized tissues,” Proc. Natl. Acad. Sci. U. S. A. 113, 3179–3184 (2016). 10.1073/pnas.1521342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Grebenyuk S., et al. , “ Large-scale perfused tissues via synthetic 3D soft microfluidics,” Nat. Commun. 14, 193 (2023). 10.1038/s41467-022-35619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hofer M. and Lutolf M. P., “ Engineering organoids,” Nat. Rev. Mater. 6, 402–420 (2021). 10.1038/s41578-021-00279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zhao Z. et al. , “ Organoids,” Nat. Rev. Methods Primers 2, 94 (2022). 10.1038/s43586-022-00174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang Y. S. et al. , “ Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors,” Proc. Natl. Acad. Sci. U. S. A. 114, E2293–E2302 (2017). 10.1073/pnas.1612906114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Jalili-Firoozinezhad S., Miranda C. C., and Cabral J. M. S., “ Modeling the human body on microfluidic chips,” Trends Biotechnol. 39, 838–852 (2021). 10.1016/j.tibtech.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 202. Gao G. et al. , “ Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing,” Appl. Phys. Rev. 6, 041402 (2019). 10.1063/1.5099306 [DOI] [Google Scholar]
- 203. Berg J. M. and Dallas T., “ Peristaltic pumps,” in Encyclopedia of Microfluidics and Nanofluidics (Springer, 2008), pp. 1626–1633. [Google Scholar]
- 204. Lake J. R., Heyde K. C., and Ruder W. C., “ Low-cost feedback-controlled syringe pressure pumps for microfluidics applications,” PLoS One 12, e0175089 (2017). 10.1371/journal.pone.0175089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Mohammed B. S., Fields D. A., Mittendorfer B., Coggan A. R., and Klein S., “ Are peristaltic pumps as reliable as syringe pumps for metabolic research? assessment of accuracy, precision, and metabolic kinetics,” Metabolism 53, 875–878 (2004). 10.1016/j.metabol.2004.02.008 [DOI] [PubMed] [Google Scholar]
- 206. Snijder R. A., Konings M. K., Lucas P., Egberts T. C., and Timmerman A. D., “ Flow variability and its physical causes in infusion technology: A systematic review of in vitro measurement and modeling studies,” Biomed. Tech. 60, 277–300 (2015). 10.1515/bmt-2014-0148 [DOI] [PubMed] [Google Scholar]
- 207. Kurth F. et al. , “ Organs-on-a-chip engineering,” in Organ-on-a-chip: Engineered Microenvironments for Safety and Efficacy Testing (Academic Press, 2020), pp. 47–130. [Google Scholar]
- 208. Pisano M., Triacca V., Barbee K. A., and Swartz M. A., “ An in vitro model of the tumor–lymphatic microenvironment with simultaneous transendothelial and luminal flows reveals mechanisms of flow enhanced invasion,” Integr. Biol. 7, 525–533 (2015). 10.1039/C5IB00085H [DOI] [PubMed] [Google Scholar]
- 209. Chong A. et al. , “ A novel roller pump for physiological flow,” Artif. Organs 44, 818–826 (2020). 10.1111/aor.13670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Abello J., Raghavan S., Yien Y. Y., and Stratman A. N., “ Peristaltic pumps adapted for laminar flow experiments enhance in vitro modeling of vascular cell behavior,” J. Biol. Chem. 298, 102404 (2022). 10.1016/j.jbc.2022.102404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Batliner M. et al. , “ Evaluation of a novel flow-controlled syringe infusion pump for precise and continuous drug delivery at low flow rates: A laboratory study,” Anaesthesia 74, 1425–1431 (2019). 10.1111/anae.14784 [DOI] [PubMed] [Google Scholar]
- 212. Kalantarifard A., Alizadeh Haghighi E., and Elbuken C., “ Damping hydrodynamic fluctuations in microfluidic systems,” Chem. Eng. Sci. 178, 238–247 (2018). 10.1016/j.ces.2017.12.045 [DOI] [Google Scholar]
- 213. Mina S. G. et al. , “ Shear stress magnitude and transforming growth factor-βeta 1 regulate endothelial to mesenchymal transformation in a three-dimensional culture microfluidic device,” RSC Adv. 6, 85457–85467 (2016). 10.1039/C6RA16607E [DOI] [Google Scholar]
- 214. Li M., Tan Y., Stenmark K. R., and Tan W., “ High pulsatility flow induces acute endothelial inflammation through overpolarizing cells to activate NF-κB,” Cardiovasc. Eng. Technol. 4, 26 (2013). 10.1007/s13239-012-0115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. McIntyre M. P., van Schoor G., Uren K. R., and Kloppers C. P., “ Modelling the pulsatile flow rate and pressure response of a roller-type peristaltic pump,” Sens. Actuators, A 325, 112708 (2021). 10.1016/j.sna.2021.112708 [DOI] [Google Scholar]
- 216. Dincau, B. , Dressaire, E. , and Sauret, A. , “ Pulsatile flow in microfluidic systems,” Small 16, 1904032 (2020). 10.1002/smll.201904032 [DOI] [PubMed] [Google Scholar]
- 217. de Graaf M. N. S., Vivas A., van der Meer A. D., Mummery C. L., and Orlova V. V., “ Pressure-driven perfusion system to control, multiplex and recirculate cell culture medium for organs-on-chips,” Micromachines (Basel) 13, 1359 (2022). 10.3390/mi13081359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Huang S. B. et al. , “ An integrated microfluidic cell culture system for high-throughput perfusion three-dimensional cell culture-based assays: Effect of cell culture model on the results of chemosensitivity assays,” Lab Chip 13, 1133–1143 (2013). 10.1039/c2lc41264k [DOI] [PubMed] [Google Scholar]
- 219. Del Valle J. S. et al. , “ Dynamic in vitro culture of cryopreserved-thawed human ovarian cortical tissue using a microfluidics platform does not improve early folliculogenesis,” Front Endocrinol. 13, 936765 (2022). 10.3389/fendo.2022.936765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hajal C., Ibrahim L., Serrano J. C., Offeddu G. S., and Kamm R. D., “ The effects of luminal and trans-endothelial fluid flows on the extravasation and tissue invasion of tumor cells in a 3D in vitro microvascular platform,” Biomaterials 265, 120470 (2021). 10.1016/j.biomaterials.2020.120470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Qu D. et al. , “ Focal TLR4 activation mediates disturbed flow-induced endothelial inflammation,” Cardiovasc. Res. 116, 226–236 (2020). 10.1093/cvr/cvz046 [DOI] [PubMed] [Google Scholar]
- 222. Hampel U., Garreis F., Burgemeister F., Eßel N., and Paulsen F., “ Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model,” Ocul. Surf. 16, 341–351 (2018). 10.1016/j.jtos.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 223. Kotsis F., Nitschke R., Doerken M., Walz G., and Kuehn E. W., “ Flow modulates centriole movements in tubular epithelial cells,” Pflugers Arch. 456, 1025–1035 (2008). 10.1007/s00424-008-0475-8 [DOI] [PubMed] [Google Scholar]
- 224. Pereiro I., Fomitcheva Khartchenko A., Petrini L., and Kaigala G. v., “ Nip the bubble in the bud: A guide to avoid gas nucleation in microfluidics,” Lab Chip 19, 2296–2314 (2019). 10.1039/C9LC00211A [DOI] [PubMed] [Google Scholar]
- 225. Cruz-Moreira D. et al. , “ Assessing the influence of perfusion on cardiac microtissue maturation: A heart-on-chip platform embedding peristaltic pump capabilities,” Biotechnol. Bioeng. 118, 3128–3137 (2021). 10.1002/bit.27836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Sung J. H. and Shuler M. L., “ Prevention of air bubble formation in a microfluidic perfusion cell culture system using a microscale bubble trap,” Biomed. Microdevices 11, 731–738 (2009). 10.1007/s10544-009-9286-8 [DOI] [PubMed] [Google Scholar]
- 227. Lochovsky C., Yasotharan S., and Günther A., “ Bubbles no more: In-plane trapping and removal of bubbles in microfluidic devices,” Lab Chip 12, 595–601 (2012). 10.1039/C1LC20817A [DOI] [PubMed] [Google Scholar]
- 228. Cheng H. B. and Lu Y. W., “ Applications of textured surfaces on bubble trapping and degassing for microfluidic devices,” Microfluid. Nanofluid. 17, 855–862 (2014). 10.1007/s10404-014-1368-0 [DOI] [Google Scholar]
- 229. Vivas A., van den Berg A., Passier R., Odijk M., and van der Meer A. D., “ Fluidic circuit board with modular sensor and valves enables stand-alone, tubeless microfluidic flow control in organs-on-chips,” Lab Chip 22, 1231–1243 (2022). 10.1039/D1LC00999K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. de Graaf M. N. S. et al. , “ Scalable microphysiological system to model three-dimensional blood vessels,” APL Bioeng. 3, 26105 (2019). 10.1063/1.5090986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. de Graaf M. N. S. et al. , “ Multiplexed fluidic circuit board for controlled perfusion of 3D blood vessels-on-a-chip,” Lab Chip 23, 168–181 (2023). 10.1039/D2LC00686C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Novak R. et al. , “ Robotic fluidic coupling and interrogation of multiple vascularized organ chips,” Nat. Biomed. Eng. 4, 407–420 (2020). 10.1038/s41551-019-0497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Herland A. et al. , “ Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips,” Nat. Biomed. Eng. 4, 421–436 (2020). 10.1038/s41551-019-0498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sung J. H. and Shuler M. L., “ A micro cell culture analog (μCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs,” Lab Chip 9, 1385–1394 (2009). 10.1039/b901377f [DOI] [PubMed] [Google Scholar]
- 235. Saateh A. et al. , “ Real-time impedimetric droplet measurement (iDM),” Lab Chip 19, 3815–3824 (2019). 10.1039/C9LC00641A [DOI] [PubMed] [Google Scholar]
- 236. Phelan M. A., Lelkes P. I., and Swaroop A., “ Mini and customized low-cost bioreactors for optimized high-throughput generation of tissue organoids,” Stem Cell Investig. 5, 565 (2018). 10.21037/sci.2018.09.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Qian X. et al. , “ Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure,” Cell 165, 1238–1254 (2016). 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Hoyle H. W., Stenger C. M. L., and Przyborski S. A., “ Design considerations of benchtop fluid flow bioreactors for bio-engineered tissue equivalents in vitro,” Biomater. Biosyst. 8, 100063 (2022). 10.1016/j.bbiosy.2022.100063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Rossi G., Manfrin A., and Lutolf M. P., “ Progress and potential in organoid research,” Nat. Rev. Genet. 19, 671–687 (2018). 10.1038/s41576-018-0051-9 [DOI] [PubMed] [Google Scholar]
- 240. Lancaster M. A. et al. , “ Cerebral organoids model human brain development and microcephaly,” Nature 501, 373–379 (2013). 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lancaster M. A. and Knoblich J. A., “ Generation of cerebral organoids from human pluripotent stem cells,” Nat. Protoc. 9, 2329–2340 (2014). 10.1038/nprot.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Sutcliffe M. and Lancaster M. A., “ A simple method of generating 3D brain organoids using standard laboratory equipment,” in Methods Molecular Biology ( Humana Press Inc., 2019), Vol. 1576, pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 243. Qian X. et al. , “ Generation of human brain region–specific organoids using a miniaturized spinning bioreactor,” Nat. Protoc. 13, 565–580 (2018). 10.1038/nprot.2017.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Mansour A. A. et al. , “ An in vivo model of functional and vascularized human brain organoids,” Nat. Biotechnol. 36, 432–441 (2018). 10.1038/nbt.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Schneeberger K. et al. , “ Large-scale production of LGR5-positive bipotential human liver stem cells,” Hepatology 72, 257–270 (2020). 10.1002/hep.31037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kumar S. V. et al. , “ Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells,” Development 146, dev172361 (2019). 10.1242/dev.172361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. McCauley H. A. and Wells J. M., “ Pluripotent stem cell-derived organoids: Using principles of developmental biology to grow human tissues in a dish,” Development 144, 958–962 (2017). 10.1242/dev.140731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. DiStefano T. et al. , “ Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors,” Stem Cell Rep. 10, 300–313 (2018). 10.1016/j.stemcr.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Nakazato T. et al. , “ Engineered three-dimensional cardiac tissues maturing in a rotating wall vessel bioreactor remodel diseased hearts in rats with myocardial infarction,” Stem Cell Rep. 17, 1170–1182 (2022). 10.1016/j.stemcr.2022.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Mansouri M. and Leipzig N. D., “ Advances in removing mass transport limitations for more physiologically relevant in vitro 3D cell constructs,” Biophys Rev 2, 021305 (2021). 10.1063/5.0048837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Huh D. et al. , “ Reconstituting organ-level lung functions on a chip,” Science 328, 1662–1668 (2010). 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kim H. J., Huh D., Hamilton G., and Ingber D. E., “ Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow,” Lab Chip 12, 2165–2174 (2012). 10.1039/c2lc40074j [DOI] [PubMed] [Google Scholar]
- 253. Musah S. et al. , “ Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip,” Nat. Biomed. Eng. 1, 0069 (2017). 10.1038/s41551-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Ferrari E., Nebuloni F., Rasponi M., and Occhetta P., “ Photo and soft lithography for organ-on-chip applications,” Methods Mol. Biol. 2373, 1–19 (2022). 10.1007/978-1-0716-1693-2 [DOI] [PubMed] [Google Scholar]
- 255. Ko J., Lee Y., Lee S., Lee S. R., and Jeon N. L., “ Human ocular angiogenesis-inspired vascular models on an injection-molded microfluidic chip,” Adv. Healthc. Mater. 8, 1900328 (2019). 10.1002/adhm.201900328 [DOI] [PubMed] [Google Scholar]
- 256. Guckenberger D. J., de Groot T. E., Wan A. M. D., Beebe D. J., and Young E. W. K., “ Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices,” Lab Chip 15, 2364–2378 (2015). 10.1039/C5LC00234F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Enrico A. et al. , “ 3D microvascularized tissue models by laser-based cavitation molding of collagen,” Adv. Mater. 34, 2109823 (2022). 10.1002/adma.202109823 [DOI] [PubMed] [Google Scholar]
- 258. Zhang B. et al. , “ 3D bioprinting: A novel avenue for manufacturing tissues and organs,” Engineering 5, 777–794 (2019). 10.1016/j.eng.2019.03.009 [DOI] [Google Scholar]
- 259. Mammoto T. and Ingber D. E., “ Mechanical control of tissue and organ development,” Development 137, 1407–1420 (2010). 10.1242/dev.024166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Bhatia S. N. and Ingber D. E., “ Microfluidic organs-on-chips,” Nat. Biotechnol. 32, 760–772 (2014). 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 261. Marsano A. et al. , “ Beating heart on a chip: A novel microfluidic platform to generate functional 3D cardiac microtissues,” Lab Chip 16, 599–610 (2016). 10.1039/C5LC01356A [DOI] [PubMed] [Google Scholar]
- 262. Visone R. et al. , “ A microscale biomimetic platform for generation and electro-mechanical stimulation of 3D cardiac microtissues,” APL Bioeng. 2, 046102 (2018). 10.1063/1.5037968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Basil M. C. and Levy B. D., “ Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation,” Nat. Rev. Immunol. 16, 51–67 (2016). 10.1038/nri.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Weisberg S. P., Ural B. B., and Farber D. L., “ Tissue-specific immunity for a changing world,” Cell 184, 1517–1529 (2021). 10.1016/j.cell.2021.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Goyal G. et al. , “ Ectopic lymphoid follicle formation and human seasonal influenza vaccination responses recapitulated in an organ-on-a-chip,” Adv. Sci. 9, 2103241 (2022). 10.1002/advs.202103241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Rowe R. G. and Daley G. Q., “ Induced pluripotent stem cells in disease modelling and drug discovery,” Nat. Rev. Genet. 20, 377–388 (2019). 10.1038/s41576-019-0100-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Colace T. V., Tormoen G. W., McCarty O. J. T., and Diamond S. L., “ Microfluidics and coagulation biology,” Annu. Rev. Biomed. Eng. 15, 283–303 (2013). 10.1146/annurev-bioeng-071812-152406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Neeves K. B., Onasoga A. A., and Wufsus A. R., “ The use of microfluidics in hemostasis: Clinical diagnostics and biomimetic models of vascular injury,” Curr. Opin. Hematol. 20, 417–423 (2013). 10.1097/MOH.0b013e3283642186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Tsai M. et al. , “ In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology,” J. Clin. Invest. 122, 408–418 (2012). 10.1172/JCI58753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Clevers H., “ Modeling development and disease with organoids,” Cell 165, 1586–1597 (2016). 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- 271. Beckwitt C. H. et al. , “ Liver ‘organ on a chip’,” Exp. Cell Res. 363, 15–25 (2018). 10.1016/j.yexcr.2017.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Kamm R. D. et al. , “ Perspective: The promise of multi-cellular engineered living systems,” APL Bioeng. 2, 40901 (2018). 10.1063/1.5038337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Aydin O. et al. , “ Principles for the design of multicellular engineered living systems,” APL Bioeng. 6, 010903 (2022). 10.1063/5.0076635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Garreta E. et al. , “ Rethinking organoid technology through bioengineering,” Nat. Mater. 20, 145–155 (2021). 10.1038/s41563-020-00804-4 [DOI] [PubMed] [Google Scholar]
- 275. Takebe T. et al. , “ Massive and reproducible production of liver buds entirely from human pluripotent stem cells,” Cell Rep. 21, 2661–2670 (2017). 10.1016/j.celrep.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 276. Demers C. J. et al. , “ Development-on-chip: In vitro neural tube patterning with a microfluidic device,” Development 143, 1884–1892 (2016). 10.1242/dev.126847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Wang Y. et al. , “ A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium,” Biomaterials 128, 44–55 (2017). 10.1016/j.biomaterials.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Akhmanova M., Osidak E., Domogatsky S., Rodin S., and Domogatskaya A., “ Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research,” Stem Cells Int. 2015, 167025. 10.1155/2015/167025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Yin X. et al. , “ Engineering stem cell organoids,” Cell Stem Cell 18, 25–38 (2016). 10.1016/j.stem.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Fang Y. and Eglen R. M., “ Three-dimensional cell cultures in drug discovery and development,” SLAS Discov. 22, 456–472 (2017). 10.1177/1087057117696795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Gattazzo F., Urciuolo A., and Bonaldo P., “ Extracellular matrix: A dynamic microenvironment for stem cell niche,” Biochim. Biophys. Acta 1840, 2506–2519 (2014). 10.1016/j.bbagen.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Chan C. J., Heisenberg C. P., and Hiiragi T., “ Coordination of morphogenesis and cell-fate specification in development,” Curr. Biol. 27, R1024–R1035 (2017). 10.1016/j.cub.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 283. Wang Y. et al. , “ In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system,” Lab Chip 18, 3606–3616 (2018). 10.1039/C8LC00869H [DOI] [PubMed] [Google Scholar]
- 284. Takahashi Y., Sekine K., Kin T., Takebe T., and Taniguchi H., “ Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation,” Cell Rep. 23, 1620–1629 (2018). 10.1016/j.celrep.2018.03.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Miller P. G. and Shuler, M. L. , “ Design and demonstration of a pumpless 14 compartment microphysiological system,” Biotechnol. Bioeng. 113, 2213–2227 (2016). 10.1002/bit.25989 [DOI] [PubMed] [Google Scholar]
- 286. Ramadan Q., Nair Gourikutty S. B., and Zhang Q., “ OOCHIP: Compartmentalized microfluidic perfusion system with porous barriers for enhanced cell-cell crosstalk in organ-on-a-chip,” Micromachines (Basel) 11, 565 (2020). 10.3390/mi11060565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Kim J. Y. et al. , “ 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis,” J. Biotechnol. 205, 24–35 (2015). 10.1016/j.jbiotec.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 288. Ingber D. E., “ Human organs-on-chips for disease modelling, drug development and personalized medicine,” Nat. Rev. Genet. 23, 467–491 (2022). 10.1038/s41576-022-00466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Ma C., Peng Y., Li H., and Chen W., “ Organ-on-a-chip: A new paradigm for drug development,” Trends Pharmacol. Sci. 42, 119–133 (2021). 10.1016/j.tips.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. van den Berg A., Mummery C. L., Passier R., and van der Meer A. D., “ Personalised organs-on-chips: Functional testing for precision medicine,” Lab Chip 19, 198–205 (2019). 10.1039/C8LC00827B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Abbott R. D. et al. , “ Long term perfusion system supporting adipogenesis,” Methods 84, 84–89 (2015). 10.1016/j.ymeth.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. McCarthy M. et al. , “ Fat-on-a-chip models for research and discovery in obesity and its metabolic comorbidities,” Tissue Eng. Part B Rev. 26, 586–595 (2020). 10.1089/ten.teb.2019.0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Loskill P. et al. , “ WAT-on-a-chip: A physiologically relevant microfluidic system incorporating white adipose tissue,” Lab Chip 17, 1645–1654 (2017). 10.1039/C6LC01590E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Bernabeu M. et al. , “ Binding heterogeneity of plasmodium falciparum to engineered 3D brain microvessels is mediated by EPCR and ICAM-1,” mBio 10, e00420–19 (2019). 10.1128/mBio.00420-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Shirure V. S. et al. , “ Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids,” Lab Chip 18, 3687–3702 (2018). 10.1039/C8LC00596F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Shang M., Soon R. H., Lim C. T., Khoo B. L., and Han J., “ Microfluidic modelling of the tumor microenvironment for anti-cancer drug development,” Lab Chip 19, 369–386 (2019). 10.1039/C8LC00970H [DOI] [PubMed] [Google Scholar]
- 297. Monteran L. and Erez N., “ The dark side of fibroblasts: Cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment,” Front Immunol. 10, 1835 (2019). 10.3389/fimmu.2019.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Garnier L., Gkountidi A. O., and Hugues S., “ Tumor-associated lymphatic vessel features and immunomodulatory functions,” Front Immunol. 10, 00720 (2019). 10.3389/fimmu.2019.00720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Gabrilovich D. I., Ostrand-Rosenberg, S. , and Bronte, V. , “ Coordinated regulation of myeloid cells by tumours,” Nat. Rev Immunol. 12, 253–268 (2012). 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Gilkes D. M., Semenza G. L., and Wirtz D., “ Hypoxia and the extracellular matrix: Drivers of tumour metastasis,” Nat. Rev Cancer 14, 430–439 (2014). 10.1038/nrc3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. de Palma M., Biziato D., and T. v., Petrova , “ Microenvironmental regulation of tumour angiogenesis,” Nat. Rev Cancer 17, 457–474 (2017). 10.1038/nrc.2017.51 [DOI] [PubMed] [Google Scholar]
- 302. Han B., Qu C., Park K., Konieczny S. F., and Korc M., “ Recapitulation of complex transport and action of drugs at the tumor microenvironment using tumor-microenvironment-on-chip,” Cancer Lett. 380, 319–329 (2016). 10.1016/j.canlet.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Kumar V. and Varghese S., “ Ex vivo tumor-on-a-chip platforms to study intercellular interactions within the tumor microenvironment,” Adv. Healthc. Mater. 8, e1801198 (2019). 10.1002/adhm.201801198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Rajasekar S. et al. , “ IFlowPlate—A customized 384-well plate for the culture of perfusable vascularized colon organoids,” Adv. Mater. 32, 2002974 (2020). 10.1002/adma.202002974 [DOI] [PubMed] [Google Scholar]
- 305. Lai B. F. L. et al. , “ Recapitulating pancreatic tumor microenvironment through synergistic use of patient organoids and organ-on-a-chip vasculature,” Adv. Funct. Mater. 30, 2000545 (2020). 10.1002/adfm.202000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Hachey S. J. et al. , “ An in vitro vascularized micro-tumor model of human colorectal cancer recapitulates in vivo responses to standard-of-care therapy,” Lab Chip 21, 1333–1351 (2021). 10.1039/D0LC01216E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Hachey S. J. et al. , “ A human vascularized microtumor model of patient-derived colorectal cancer recapitulates clinical disease,” Transl. Res. 255, 97–108 (2022). 10.1016/j.trsl.2022.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Kim S., Park J., Kim J., and Jeon J. S., “ Microfluidic tumor vasculature model to recapitulate an endothelial immune barrier expressing FasL,” ACS Biomater. Sci. Eng. 7, 1230–1241 (2021). 10.1021/acsbiomaterials.0c01542 [DOI] [PubMed] [Google Scholar]
- 309. Zhang S., Wan Z., and Kamm R. D., “ Vascularized organoids on a chip: Strategies for engineering organoids with functional vasculature,” Lab Chip 21, 473–488 (2021). 10.1039/D0LC01186J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Rubin M. A., “ Health: Make precision medicine work for cancer care,” Nature 520, 290–291 (2015). 10.1038/520290a [DOI] [PubMed] [Google Scholar]
- 311. Pauli C. et al. , “ Personalized in vitro and in vivo cancer models to guide precision medicine,” Cancer Discov. 7, 462–477 (2017). 10.1158/2159-8290.CD-16-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Boussommier-Calleja A. et al. , “ The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model,” Biomaterials 198, 180–193 (2019). 10.1016/j.biomaterials.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Jeon J. S. et al. , “ Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation,” Proc. Natl. Acad. Sci. U. S. A. 112, 214–219 (2015). 10.1073/pnas.1417115112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Bahmaee H. et al. , “ Design and evaluation of an osteogenesis-on-a-chip microfluidic device incorporating 3D cell culture,” Front. Bioeng. Biotechnol. 8, 557111 (2020). 10.3389/fbioe.2020.557111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Bayir E., Sahinler M., Celtikoglu M. M., and Sendemir A., “ Bioreactors in tissue engineering: Mimicking the microenvironment,” in Biomaterials for Organ and Tissue Regeneration ( Elsevier, 2020), pp. 709–752. [Google Scholar]
- 316. Zhao J. et al. , “ Bioreactors for tissue engineering: An update,” Biochem. Eng. J. 109, 268–281 (2016). 10.1016/j.bej.2016.01.018 [DOI] [Google Scholar]
- 317. Takebe T. et al. , “ Human elastic cartilage engineering from cartilage progenitor cells using rotating wall vessel bioreactor,” Transplant. Proc. 44, 1158–1161 (2012). 10.1016/j.transproceed.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 318. Correia C. et al. , Dynamic culturing of cartilage tissue: The significance of hydrostatic pressure. Tissue Eng. Part A 18, 1979–1991 (2012). 10.1089/ten.tea.2012.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Ren L. et al. , “ Biomechanical and biophysical environment of bone from the macroscopic to the pericellular and molecular level,” J. Mech. Behav. Biomed. Mater. 50, 104–122 (2015). 10.1016/j.jmbbm.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 320. Rayner S. G. et al. , “ Multiphoton-guided creation of complex organ-specific microvasculature,” Adv. Healthc. Mater. 10, e2100031 (2021). 10.1002/adhm.202100031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Daly A. C., Davidson M. D., and Burdick J. A., “ 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels,” Nat. Commun. 12, 753 (2021). 10.1038/s41467-021-21029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Ayan B. et al. , “ Aspiration-assisted bioprinting for precise positioning of biologics,” Sci. Adv. 6, eaaw5111 (2020). 10.1126/sciadv.aaw5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Kim T. Y. et al. , “ Directed fusion of cardiac spheroids into larger heterocellular microtissues enables investigation of cardiac action potential propagation via cardiac fibroblasts,” PLoS One 13, e0196714 (2018). 10.1371/journal.pone.0196714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Bantounas I. et al. , “ Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors,” Stem Cell Rep. 10, 766–779 (2018). 10.1016/j.stemcr.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Low J. H. et al. , “ Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network,” Cell Stem Cell 25, 373–387 (2019). 10.1016/j.stem.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Subramanian A. et al. , “ Single cell census of human kidney organoids shows reproducibility and diminished off-target cells after transplantation,” Nat. Commun. 10, 5462 (2019). 10.1038/s41467-019-13382-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Redd M. A. et al. , “ Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts,” Nat. Commun. 10, 584 (2019). 10.1038/s41467-019-08388-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Takahashi Y., Takebe T., and Taniguchi H., “ Methods for generating vascularized islet-like organoids via self-condensation,” Curr. Protoc. Stem Cell Biol. 45, e49 (2018). 10.1002/cpsc.49 [DOI] [PubMed] [Google Scholar]
- 329. Bertassoni L. E.et al. , “ Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs,” Lab Chip 14, 2202–2211 (2014). 10.1039/C4LC00030G [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See the supplementary material for Fig. 1, which shows the evolution of 3D cell culture and perfused 3D cell culture tendencies in publications for the last 30 years. Specifically, the percentage of cell culture publications including 3D ECM and perfused 3D ECM terms is analyzed, as well as the fold increase of publications including 3D ECM and perfused 3D ECM terms (compared with 1993–2002 decade), and the percentage of 3D ECM cell culture publications that include fluid flow term.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.