Abstract
Objectives
To systematically review and evaluate metformin’s potential impact on vestibular schwannoma (VS) growth.
Data sources
PubMed, Cochrane Library, and Embase
Review Methods
Retrospective cohort study was performed on sporadic VS patients undergoing initial observation who had at least two magnetic resonance imaging (MRI) studies. Patients were stratified by metformin use during the observation period. Primary endpoint was VS growth, defined as at least 2mm increase in diameter. Survival free of tumor growth was evaluated between groups. Systematic review and meta-analysis were performed to produce a pooled odds ratio [OR]. Study heterogeneity was assessed and post-hoc power analysis performed.
Results
Total of 123 patients were included, of which 17% were taking metformin. Median patient age was 56.6 years (range, 25.1-84.5). There were no statistically significant differences between groups. Survival analysis did not demonstrate a statistically significant difference in time to VS growth between groups (hazard ratio=0.61, 95% confidence interval [CI]=0.29-1.29). Furthermore, logistic regression analysis did not demonstrate a statistically significant difference between groups in the odds of VS growth (OR=0.46, 95% CI=0.17-1.27). Systematic review identified 3 studies. Meta-analysis suggested that metformin reduces the odds of developing VS growth (pooled OR=0.45, 95% CI=0.29-0.71). Studies demonstrated low between-study heterogeneity. Power analysis demonstrated a sample size of 220 patients with equal randomization would be required to prospectively identify a true difference with 80% power.
Conclusions
Metformin use may reduce the odds of VS growth. A randomized trial would be ideal to identify an unbiased estimate of metformin’s effect on VS growth.
Lay summary
This article looks at whether taking metformin has an effect on slowing vestibular schwannoma growth by evaluating our own data and reviewing prior articles. In summary, taking metformin may decrease the odds of vestibular schwannoma growth.
Keywords: vestibular schwannoma, acoustic neuroma, metformin, systemic therapy, tumor growth
Introduction
Vestibular schwannomas (VS) are benign tumors associated with unregulated Schwann cell growth surrounding the vestibulocochlear nerve. They are the most common tumor of the cerebellopontine angle and have been found to have an increasing incidence rate primarily conferred by increased access and utilization of magnetic resonance imaging (MRI)1,2. As an increasing proportion of tumors are incidentally detected at a small size, upfront active observation for initial management has concomitantly increased, though decision making takes multiple factors into account3,4. As such, identification of factors which either predict or impede VS growth during this observation period has become a significant interest.
Numerous studies attempting to qualitatively and quantitatively evaluate risk factors for VS growth on a population basis have provided some insight as to which may play a role, namely initial tumor size, non-incidental diagnosis, and disequilibrium at presentation5–7. However, identification of factors which may prevent or impede VS growth remains even more elusive. Over the last two decades, multiple pharmacotherapies have been trialed, mostly in the neurofibromatosis type 2 (NF2) population, but none have demonstrated clear benefit8,9. Bevacizumab and aspirin have been amongst the most promising, but ultimately have failed to demonstrate consistent effects in more thorough reviews10–13. Similarly, studies evaluating the effects of metformin on sporadic VS growth have been variable14–16. As such, the objective of this study was to contribute another single institution’s experience of the effects of metformin on VS growth, and then to systematically review and meta-analyze the literature to perform a pooled analysis to provide additional insight into metformin’s true effect on sporadic VS growth.
Methods
Retrospective cohort data acquisition
The University of Texas MD Anderson Cancer Center Institutional Review Board approval was obtained prior to initiation of the study (#PA19-0106). All patients were identified through an ongoing maintained database of VS patients between February 2000 to May 2022. Subjects were included if they presented with radiographic diagnosis of a suspected sporadic VS, underwent initial observation, and had at least two serial brain MRI scans with a minimum of 3 months apart. Exclusion criteria included those who underwent prior microsurgery or radiosurgery, had less than two MRI scans, and those VS associated with suspected NF1 or NF2. Subjects were then stratified by use of metformin during the observation period, which was defined as patients documented of taking metformin at the time of initial clinic presentation. The initial daily dose of metformin was recorded for these individuals.
Patient and tumor data were retrospectively collected, which included age at initial presentation, sex, race, initial tumor size, and length of observation. Tumor size was evaluated by width and length, which were defined as the largest tumor diameter using two-dimensional measurements in the anteroposterior and mediolateral planes, respectively, incorporating both intracanalicular and extracanalicular tumor components. The primary endpoint was VS growth, which was defined as at least 2mm increase in either measurement between the first and last MRI studies, in accordance with previous studies and what is readily used in generalized clinical practice17,18. Length of observation was defined as time between the date of initial MRI and date of first MRI denoting tumor growth or date of most recent MRI. If the patient underwent subsequent microsurgery or radiosurgery, the most recent MRI scan was defined as the last MRI immediately preceding intervention.
Systematic review
Systematic review of the current literature was performed in order to identify all reviews evaluating metformin and VS growth and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines19. To identify studies for inclusion, a keyword search of PubMed, Cochrane Library, and Embase was performed using the following: “(‘metformin’/exp OR metformin) AND (‘vestibular schwannoma’/exp OR ‘vestibular schwannoma’ OR (vestibular AND (‘schwannoma’/exp OR schwannoma)))” and “(‘metformin’/exp OR metformin) AND (‘acoustic neuroma’/exp OR ‘acoustic neuroma’ OR (acoustic AND (‘neuroma’/exp OR neuroma))).” The databases were searched from inception through September 25, 2022. To identify additional articles for inclusion, the reference lists of relevant articles were hand-searched. References were exported to EndNote (Clarivate Analytics, Philadelphia, PA, USA) and screened for relevance.
After duplicates were removed, 8 articles were identified. Titles and abstracts were first used to screen for relevance. Five full-text articles were then reviewed for eligibility. Inclusion criteria included a retrospective or prospective cohort study design which evaluated VS tumor growth in participants with sporadic VS who underwent initial observation and had at least 2 MRI scans. Intervention inclusion criteria was the use of metformin during the observation period. These participants were compared to similar participants from the same studies who did not use metformin. The outcome of interest was the impact of metformin use on tumor growth. Exclusion criteria included non-cohort study, abstract only, and included patients who underwent prior microsurgery or radiosurgery, had less than two MRI scans, or had VS associated with suspected NF1 or NF2. All manuscripts were assessed by 2 authors (B.D.L. and P.W.G.) to identify all studies meeting inclusion and exclusion criteria. Any discrepancy that arose was solved with discussion until consensus was obtained. Data regarding number of patients with and without metformin use, demographics, follow up, tumor characteristics, approach to tumor measurement, definition of tumor growth, statistical analysis, and tumor growth outcomes were obtained. Articles were then critically appraised to assess the level of evidence using the Oxford Center for Evidence-Based Medicine criteria20. The risk of bias was also critically assessed using the Joanna Briggs Institute Critical Appraisal Checklist for Cohort Studies21. An appraisal was performed for each study according to the checklist and an overall appraisal was determined. Eligible publications were reviewed to extract information regarding patient information, measurement of growth, statistical analysis, and outcomes. This search strategy is outlined in Figure 1.
Figure 1.

Detailed preferred reporting items for systematic reviews and meta-analyses flow-diagram.
Statistical analysis: retrospective cohort
Results were expressed as means for continuous variables and percentages for categorical variables. Fisher’s exact was used to compare groups for all categorical variables, while the Wilcoxon rank-sum test was used to compare groups for all continuous variables. A multivariable logistic regression model was fitted to assess the association between metformin use and tumor growth while including other covariates of interest. Backward selection was used to identify a final model, which was consistent with metformin use as the only covariate. Survival free of tumor growth was then evaluated using the Kaplan-Meier method, with time to event defined as time from date of initial MRI to date of first MRI denoting tumor growth or death, whichever occurred first. Patients alive without tumor growth were censored at last follow-up MRI. The Cox proportional hazards regression was used to compare metformin and non-metformin groups. P values <0.05 were considered statistically significant.
Statistical analysis: systematic review
Systematic review of the literature identified 3 studies evaluating the effect of metformin on VS growth. Only one study16 reported a hazard ratio (HR) from a time-to-growth model while 2 studies14,15 reported odds ratios (OR) from evaluating VS growth as a binary outcome. A pooled OR was then obtained by meta-analyzing the results from two previous studies14,15 in conjunction with the results from the study herein with a fixed-effects model due to the lack of heterogeneity. The meta-analysis summarized data using the OR and its corresponding 95% confidence interval (CI).
Heterogeneity across studies was evaluated using Cochran’s Q-test. Higgins I2 statistic was then used to calculate the percentage of variation in the effect sizes across studies due to heterogeneity, where 0% indicates no observed heterogeneity and larger values indicate increasing heterogeneity. Finally, using the current study data, a two-sided Chi-squared test of equal proportions was used to calculate a post-hoc power analysis based on the same growth criteria used in this retrospective cohort study to direct future studies. All analyses were conducted using R version 4.1.2 and Stata v16 (StataCorp LLC, College Station, TX).
Results
Retrospective cohort
A total of 123 patients were included, of which 17% (n=21) were taking metformin. The mean patient age was 57.9 years and mean length of observation was 3.55 years. Slightly more patients were female (55.3%), and most patients were white (82.1%). There was no statistically significant difference between metformin and non-metformin groups regarding patient age, gender, race, length of observation, and initial tumor measurements. Demographic data and tumor characteristics are presented in Table 1.
Table 1.
Demographic and tumor characteristics between metformin and non-metformin users in the retrospective cohort study.
| No metformin | Metformin | Total | p value | |
|---|---|---|---|---|
| Total patients | 102 | 21 | 123 | NA |
| Mean age (years) | 57.2 | 60.9 | 57.9 | 0.256 |
| Male (%) | 43 (42) | 12 (57) | 55 (45) | 0.235 |
| Caucasian (%) | 85 (83) | 16 (76) | 101 (82) | 0.378 |
| Mean length of observation (years) | 3.47 | 3.92 | 3.55 | 0.861 |
| Mean initial length (mm) | 13.0 | 11.6 | 12.8 | 0.396 |
| Mean initial width (mm) | 8.5 | 8.0 | 8.4 | 0.749 |
| Tumor growth (%) | 53 (52) | 7 (33) | 60 (49) | 0.130 |
NA: not applicable, mm: millimeters
Overall, 60 patients (49%) experienced tumor growth throughout follow up. Survival free of tumor growth at 1, 3, 5, and 10 years was 87.3, 59.8, 39.0, and 30.2%. Survival analysis did not demonstrate a statistically significant difference in time to VS growth between metformin and non-metformin groups (HR=0.61, 95% CI=0.29-1.29, p=0.19). Figure 2 demonstrates a Kaplan-Meier plot of survival free of tumor growth for both groups.
Figure 2.
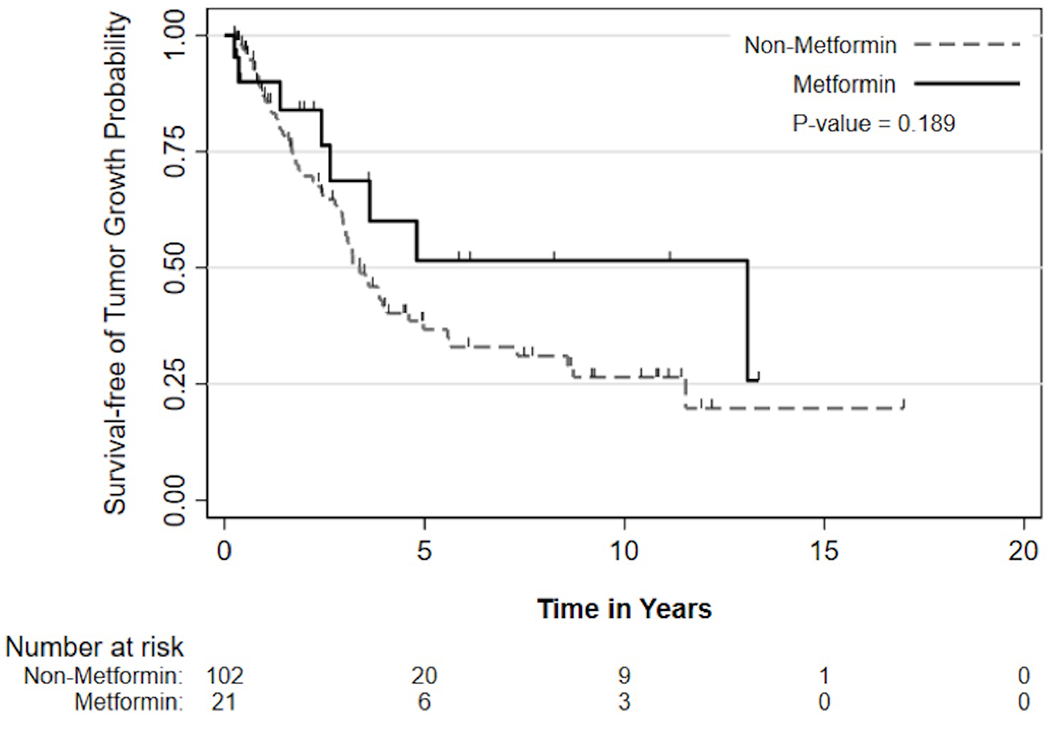
Kaplan-Meier curve of survival free of vestibular schwannoma growth for metformin and non-metformin groups. Number at risk denotes the remaining patients in each group who had not demonstrated tumor growth by the according timepoint.
As there were no statistically significant covariates to include in the multivariable model, the final logistic regression model consisted of metformin group as the only covariate. Logistic regression analysis with this single covariate did not demonstrate a statistically significant difference between groups with regards to risk of tumor growth (OR=0.46, 95% CI=0.17-1.27, p=0.13). Moreover, a dose-effect was not observed as there was no significant association between metformin daily dosage and tumor growth (p=0.307).
Systematic review and meta-analysis
Study characteristics and data regarding demographics and tumor characteristics for the studies in the systematic review and meta-analysis are summarized in Table 2 and Table 3, respectively. All studies were judged to have a low risk of bias (Table 4). The meta-analysis demonstrated that metformin use is associated with decreased odds of VS growth when assessed as a binary outcome (pooled OR=0.45, 95% CI=0.29-0.71). These are graphically summarized in Figure 3. Studies demonstrated low heterogeneity and variance (I2=0%). A power analysis demonstrated that a total sample size of 220 patients (110 per study group) would be required to prospectively identify a true difference with approximately 80% power with an OR of 0.46.
Table 2.
Characteristics of included studies in the systematic review and meta-analysis.
| Authors | Publication year | Study design | Measurement of growth | Statistical analysis | Result of interest | Level of Evidence (1a-5) † |
|---|---|---|---|---|---|---|
| Feng et al. | 2020 | Retrospective cohort | Linear; >25% increase in sum of perpendicular diameter products | Multivariable logistic regression model for growth as binary outcome | Metformin use is associated with less growth (OR=0.39, p=0.02) | 3 |
| Tran et al. | 2021 | Retrospective cohort | Volumetric; >20% increase in volume | Multivariable logistic regression model for growth as binary outcome | Metformin use is associated with less growth (OR=0.50, p=0.036) | 3 |
| Armstrong et al. | 2021 | Retrospective cohort | Volumetric; >20% increase in volume | Survival free of growth using Cox proportional hazards regression model | Metformin use is not associated with time to growth (HR=0.75, p=0.37) | 3 |
According to Oxford Center for Evidence-Based Medicine criteria
OR: odds ratio, HR: hazard ratio
Table 3.
Demographics and tumor characteristics of included studies in the systematic review and meta-analysis.
| Authors | Feng et al. | Tran et al. | Armstrong et al. |
|---|---|---|---|
| Total patients | 149 | 387 | 361 |
| Patients taking metformin (%) | 42 (28) | 46 (12) | 19 (5) |
| Mean age (years) | 69.6 | 60.6 | ND |
| Male (%) | 80 (54) | 180 (47) | ND |
| Caucasian (%) | 81 (54) | ND | ND |
| Mean length of observation (months) | 30.6 | 30.8 | ND |
| Mean initial tumor size (mm) | 8 | 11.9 | ND |
| Tumor growth (%) | 65 (44) | 216 (56) | 319 (88) |
Table 4.
Risk of bias assessment for the studies in the systematic review and meta-analysis.
| Feng et al. | Tran et al. | Armstrong et al. | |
|---|---|---|---|
| Were the two groups similar and recruited from the same population? | Yes | Yes | Yes |
| Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Yes | Yes | Yes |
| Was the exposure measured in a valid and reliable way? | Yes | Yes | Yes |
| Were confounding factors identified? | Yes | Yes | No |
| Were strategies to deal with confounding factors stated? | Yes | No | NA |
| Were the participants free of the outcome at the start of the study (or at the moment of exposure)? | Unclear | Unclear | Yes |
| Were the outcomes measured in a valid and reliable way? | Yes | Yes | Yes |
| Was the follow up time reported and sufficient to be long enough for outcomes to occur? | Yes | Yes | Yes |
| Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | Yes | Yes | Yes |
| Were strategies to address incomplete follow up utilized? | NA | NA | NA |
| Was appropriate statistical analysis used? | Yes | Yes | Yes |
| Overall assessment | Low | Low | Low |
NA: not applicable
Figure 3.

Forest plot of available cohort studies evaluating the effect of metformin on vestibular schwannoma growth utilized in the meta-analysis. Individual and pooled odds ratios and confidence intervals are displayed.
Discussion
The study herein sought to better understand the relationship between metformin and VS growth by reporting another institution’s experience and meta-analyzing the relevant literature. Our institution’s experience did not identify a statistically significant association between metformin on VS growth or time to VS growth in survival analysis. However, the meta-analysis did demonstrate a statistically significant reduction in the odds of VS growth with metformin use.
The initial investigation of metformin and VS likely stemmed from studies evaluating metformin’s effect on cancer incidence in the diabetic population22. A recent systematic review found that patients taking metformin had a statistically significant lower risk of being diagnosed with liver, colorectal, stomach, pancreas, and esophageal cancer, as well as cancer-related mortality23. Specifically, exposure to metformin was associated with a 31% reduction in the risk of any cancer, and a 35% reduction in the risk of cancer mortality23. The proposed antitumor mechanism of action is multifactorial. Directly, metformin activates adenosine monophosphate-activated protein kinase (AMPK) which inhibits mammalian target of rapamycin (mTOR), thereby promoting autophagy and apoptosis. It also downregulates c-myc proto-oncogene, which reduces tumor growth through anti-inflammatory and antiangiogenic effects24–26. Metformin also inhibits oxidative phosphorylation in mitochondria, which creates reactive oxygen species and contributes to apoptosis27. Indirectly, maintenance of a more euglycemic state with metformin may also contribute to its antitumor properties as hyperglycemia and hyperinsulinemia have been linked to tumor growth and proliferation28–30. While these theories are promising, the true effects of each are unknown.
This study identified that 49% of all patients exhibited VS growth during follow up with a mean of 3.5 years of observation. This finding is in agreement with both prior natural history studies17,31, as well as the three studies evaluating VS growth with metformin14–16. Feng et al. was the first study to investigate metformin14. They evaluated 149 patients, 28% of which were taking metformin, and evaluated growth using linear measurements. They concluded that metformin users are significantly less likely to present with tumor growth at final follow-up on multivariable analysis (OR=0.39, p=0.02). Tran et al. was the largest study with 387 patients, 12% of which were metformin users15. They utilized volumetric measurements to assess for growth and found again that metformin use is significantly associated with reduced VS growth on multivariable analysis (OR=0.5, p=0.036). Finally, Armstrong et al. evaluated volumetric growth in 361 patients, 5% of which were metformin users16. In contrast to the prior two studies, they evaluated metformin’s effects in a time to event model using hazard regression models. They did not find a statistically significant association between metformin use and time to growth on multivariable analysis (HR=0.75, p=0.37). Finally, while the current study has the smallest cohort of all studies, it has the second largest proportion of metformin users at 17%. By evaluating survival free of tumor growth, we were able to evaluate metformin’s effects more analytically beyond assessing growth as a binary outcome, similar to Armstrong et al. However, by also employing logistic regression we were able to perform a meta-analysis in conjunction with Feng et al. and Tran et al. to provide statistical power.
Given the continued discrepancies between study outcomes, and even the outcomes presented herein, we are unable to argue causality between metformin use and VS growth. A true difference between metformin users and non-users would best be identified in a prospective trial, which would require 110 patients in each group based on the pooled OR found herein. While at this time we would not recommend prescribing metformin to non-diabetic patients with VS under active observation given unclear efficacy and potential metabolic side effects, it seems reasonable, however, to refer diabetic patients with VS under observation, particularly those who are not optimally controlled, to their Endocrinologist for consideration of metformin initiation.
While this study enhanced our understanding and has the highest level of evidence, to date, on the potential impact of metformin on VS growth, it has its limitations. First, as it is retrospective in nature it is subject to selection bias as some patients in either group may have been missed upon chart review. Secondly, this design precluded guaranteed medication compliance during observation, despite necessitating documented metformin use at first visit. Third, the use of linear measurements has been shown to lack sensitivity in measuring tumor growth when compared to volumetric measurements, which may have underestimated the rate of growth in both groups5. Fourth, reporting bias may exist in the systematic review as those studies which demonstrate a beneficial effect of metformin are more likely to be reported. Finally, the number of studies available for meta-analysis was limited based on available literature. With additional studies, a more precise result could be obtained. Ultimately, this information suggests that metformin remains a promising systemic therapy for VS patients and a randomized, placebo-controlled, prospective study is needed to identify a true effect.
Conclusion
There is conflicting evidence regarding the benefit metformin provides in inhibiting VS growth. Survival analysis does not demonstrate an association between the two, but a meta-analysis evaluating growth as a binary outcome suggests that metformin use is associated with a reduced odds of VS growth. An appropriately powered, randomized, prospective study is needed to identify a true difference between groups.
Funding:
This work is supported by the NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group.
Footnotes
Conflicts of interest:
MEN reports stock ownership in 3M, Amgen, Cardinal Health, Johnson & Johnson, Medtronic, and Pfizer.
PWG reports stock ownership in Amgen, Eli Lily, Merck, Medtronic, Novartis, Pfizer, and Roche.
There are no conflicts of interest to declare for the remaining authors.
Meeting information:
Abstract was accepted and will be presented as a podium presentation at the North American Skull Base Society Annual Meeting in Tampa, FL, USA 02/2023
References
- 1.Marinelli JP, Lohse CM, Grossardt BR, Lane JI, Carlson ML. Rising Incidence of Sporadic Vestibular Schwannoma: True Biological Shift Versus Simply Greater Detection. Otol Neurotol. 2020;41(6):813–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reznitsky M, Petersen M, West N, Stangerup SE, Caye-Thomasen P. Epidemiology Of Vestibular Schwannomas - Prospective 40-Year Data From An Unselected National Cohort. Clin Epidemiol. 2019;11:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gadot R, Anand A, Lovin BD, Sweeney AD, Patel AJ. Predicting surgical decision-making in vestibular schwannoma using tree-based machine learning. Neurosurg Focus. 2022;52(4):E8. [DOI] [PubMed] [Google Scholar]
- 4.Chan SA, Marinelli JP, Hahs-Vaughn DL, Nye C, Link MJ, Carlson ML. Evolution in Management Trends of Sporadic Vestibular Schwannoma in the United States Over the Last Half-century. Otol Neurotol. 2021;42(2):300–305. [DOI] [PubMed] [Google Scholar]
- 5.Lees KA, Tombers NM, Link MJ, et al. Natural History of Sporadic Vestibular Schwannoma: A Volumetric Study of Tumor Growth. Otolaryngol Head Neck Surg. 2018;159(3):535–542. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal Y, Clark JH, Limb CJ, Niparko JK, Francis HW. Predictors of Vestibular Schwannoma Growth and Clinical Implications. Otol Neurotol. 2010;31:807–812. [DOI] [PubMed] [Google Scholar]
- 7.Jethanamest D, Rivera AM, Ji H, Chokkalingam V, Telischi FF, Angeli SI. Conservative management of vestibular schwannoma: Predictors of growth and hearing. Laryngoscope. 2015;125(9):2163–2168. [DOI] [PubMed] [Google Scholar]
- 8.Tamura R, Toda M. A Critical Overview of Targeted Therapies for Vestibular Schwannoma. Int J Mol Sci. 2022;23(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long J, Zhang Y, Huang X, Ren J, Zhong P, Wang B. A Review of Drug Therapy in Vestibular Schwannoma. Drug Des Devel Ther. 2021;15:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu VM, Ravindran K, Graffeo CS, et al. Efficacy and safety of bevacizumab for vestibular schwannoma in neurofibromatosis type 2: a systematic review and meta-analysis of treatment outcomes. J Neurooncol. 2019;144(2):239–248. [DOI] [PubMed] [Google Scholar]
- 11.Killeen DE, Klesse L, Tolisano AM, Hunter JB, Kutz JW Jr. Long-Term Effects of Bevacizumab on Vestibular Schwannoma Volume in Neurofibromatosis Type 2 Patients. J Neurol Surg B Skull Base. 2019;80(5):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKeith S, Wasson J, Baker C, et al. Aspirin does not prevent growth of vestibular schwannomas: A case-control study. Laryngoscope. 2018;128(9):2139–2144. [DOI] [PubMed] [Google Scholar]
- 13.Marinelli JP, Lees KA, Tombers NM, Lohse CM, Carlson ML. Impact of Aspirin and Other NSAID Use on Volumetric and Linear Growth in Vestibular Schwannoma. Otolaryngol Head Neck Surg. 2019;160(6):1081–1086. [DOI] [PubMed] [Google Scholar]
- 14.Feng AY, Enriquez-Marulanda A, Kouhi A, Ali NE, Moore JM, Vaisbuch Y. Metformin Potential Impact on the Growth of Vestibular Schwannomas. Otol Neurotol. 2020;41(3):403–410. [DOI] [PubMed] [Google Scholar]
- 15.Tran S, Killeen DE, Qazi S, Balachandra S, Hunter JB. Association of Metformin With the Growth of Vestibular Schwannomas. Otolaryngol Head Neck Surg. 2021;164(1):182–187. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong MF, Lohse CM, Lees KA, Carlson ML. Association of Metformin With Volumetric Tumor Growth of Sporadic Vestibular Schwannomas. Otol Neurotol. 2021;42(7):1081–1085. [DOI] [PubMed] [Google Scholar]
- 17.Hunter JB, Francis DO, O’Connell BP, et al. Single Institutional Experience With Observing 564 Vestibular Schwannomas: Factors Associated With Tumor Growth. Otol Neurotol. 2016;37(10):1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanzaki J, Tos M, Sanna M, Moffat DA. New and Modified Reporting Systems from the Consensus Meeting on Systems for Reporting Results in Vestibular Schwannoma. Otol Neurotol. 2003;24:642–649. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman D, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The University of Oxford. OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. [Google Scholar]
- 21.The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Joanna Briggs Institute Critical Appraisal Checklist for Cohort Studies. The Joanna Briggs Institute, 2017. [Google Scholar]
- 22.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8(8):e71583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–170. [DOI] [PubMed] [Google Scholar]
- 25.Blandino G, Valerio M, Cioce M, et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun. 2012;3:865. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Xu W, Yan Z, et al. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res. 2018;37(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462(3):475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masur K, Vetter C, Hinz A, et al. Diabetogenic glucose and insulin concentrations modulate transcriptome and protein levels involved in tumour cell migration, adhesion and proliferation. Br J Cancer. 2011;104(2):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan W, Shen X, Lei J, et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int. 2014;2014:461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99(23):1793–1800. [DOI] [PubMed] [Google Scholar]
- 31.Marinelli JP, Carlson ML, Hunter JB, et al. Natural History of Growing Sporadic Vestibular Schwannomas During Observation: An International Multi-Institutional Study. Otol Neurotol. 2021;42(8):e1118–e1124. [DOI] [PubMed] [Google Scholar]


