Abstract
Background:
Local anesthetic lidocaine is one of the most common pain therapies, but high concentration of lidocaine induced neurotoxicity and its mechanism is unclear. Exosomal microRNAs (miRNAs) is implicated in neuronal diseases, but its role in lidocaine induced neurotoxicity remains to be elucidated.
Methods:
All the experiments were performed at Huzhou Key Laboratory of Molecular Medicine, Huzhou City, Jiangsu Province, China in 2022. Lidocaine was used to induce apoptosis of SH-SY5Y cells. Exosomes isolated from bone marrow mesenchymal stem cells (BMSC-exos) were used to co-treat SH-SY5Y cells with lidocaine. Cell apoptosis was measured using a flow cytometer. PKH-67 Dye was used for exosome uptake assay. miR-21-5p mimics/inhibitors, or negative controls were transfected with Lipo2000 to study its effect on lid-induced injury. Interactions between miR-21-5p and PDCD4 was analyzed by luciferase reporter assay.
Results:
Administration of BMSC-exo protected SH-SY5Y cells against lidocaine induced apoptosis. Suppressing miR-21-5p dramatically enhanced PDCD4, but miR-21-5p overexpression sharply down-regulated PDCD4. Mechanism study showed that miR-21-5p bound to 3′-UTR of PDCD4 to inhibit it. Suppressing miR-21-5p reversed the effect of BMSC-exo on Lid-induced injury. Results also indicate that miR-21-5p regulated lidocaine-induced injury through targeting PDCD4.
Conclusion:
BMSC-exos protected SH-SY5Y cells against lidocaine induced apoptosis through miR-21-5p by targeting PDCD4, which may develop new strategy in the management of lidocaine-induced neurotoxicity.
Keywords: Microrna, Exosomes, Lidocaine, Programmed cell death protein 4 (PDCD4), Apoptosis, Neuronal toxicity
Introduction
Pain is one of the most common miseries in humans and one of the most unbearable clinical symptoms in patients (1). It is now well accepted that pain management is a worldwide problem (2). For example, more than 40% of the population suffers from chronic pain in USA (3). The use of local anesthetics, which disrupt neural conduction, is one of the most common pain therapies (4). As a classic local anesthetic, lidocaine was prepared in early 1943 and was used in clinical trials in 1948 (5). Lidocaine became widely employed after its discovery because of its excellent efficacy (6). However, high concentration of lidocaine has been shown to induce neurotoxicity (7). The process of local anesthesia-induced neurotoxicity is complicated, and its mechanism is not fully understood.
Mesenchymal stem cells (MSCs) can be found in connective tissues of most organs (8), which contribute to tissue repair and regeneration (9). Bone marrow-derived MSCs (BMSCs) have emerged as the primary research focus and have been used to treat various diseases (10, 11). BMSCs have benefits in various neuro-diseases, including spinal cord injury (12). Studies indicate that MSCs’ benefits result from secreted factors such as extracellular vesicles (EVs) (13).
Exosomes are EVs (40–160 nm) surrounded by a lipid bilayer membrane (14). Studies demonstrate that exosome-enclosed microRNAs important roles in cell–cell interaction (15–17). miR-21-5p is abundant in cells and exosomes-derived from BMSCs (BMSC-exos) (13). Furthermore, miR-21 is involved in apoptosis and chemo-resistance (18, 19), and exosomal miR-21-5p from MSCs protected neurons and alleviated cognitive dysfunction (20). Data also support that miR-21 mediates apoptosis via programmed cell death protein 4 (PDCD4) (21).
However, the function of miR-21-5p/PDCD4 in neuronal toxicity remains unclear. This research aims to elucidate the protective role of miR-21-5p in lidocaine-induced neuronal toxicity, and to find the possible mechanisms.
Material and Methods
Cell culture
BMSCs and SH-SY5Y were maintained in DMEM (Sigma, Shanghai), 10% FBS (GIBCO, 16000e044; Carlsbad, CA) and 1% pen-strep (Solarbio, P1400, Beijing) and kept at 37°C with 5% CO2. All the experiments were performed at Huzhou Key Laboratory of Molecular Medicine, Huzhou City, Jiangsu Province, China in 2022.
BMSCs characterization
BMSCs were purchased from Chinese Academy of Sciences and kept in NutriStem® XF Medium. Surface antigens of MSCs were characterized using a CytoFLEX Flow Cytometer (Beckman, Beijing) and the Human MSC Analysis Kit (BD Biosciences).
Isolation and characterization of exosomes
BMSCs were kept in exosome-free medium and medium was used for exosome isolation (22). The morphology was observed using a transmission electron microscopy. The expression of CD63, CD81 and TSG101 were detected by Western blot.
Exosomes uptake assay
PKH-67 (UR52303, Umibio) was provided to track exosomes being endocytosed by SH-SY5Y cells. In brief, preparation of PKH67 dyeing working solution: at room temperature, the “PKH67 linker” is mixed with “Diluent C” at a ratio of 1:9 in the dark. Exosome staining was performed at room temperature with PKH67 staining solution.
miRNA transfection
miR-21-5p mimic (5′-UAGCUAUAUCAGCACUGAUGAUUGA-3′), inhibitor (5′-UCAACUCAGUCUGAUAGCUA-3′), and NC (5′-CAGUAUUUUGUGUGUACA-3′) were purchased from Beyotime and transfected into cells using Lipo2000.
Cell transfection
PDCD4 (NM_014456.5) was amplified with the following primers and cloned into pCDNA3.1(+) plasmids (Clontech, USA), which containing the restriction enzyme cutting sites of Hind III and EcoR I, to overexpress PDCD4 (oePDCD4). Blank pCDNA3.1(+) was used as negative control transduction.
PDCD4-F: 5′-CCCAAGCTTATGGATGTAGAAAATGAGCAGATAC-3′ (Hind III)
PDCD4-R: 5′-CGGAATTCTCAGTAGCTCTCTGGTTTAAGACG-3′ (EcoR I)
Luciferase reporter assay
PDCD4 3′-UTR region was introduced to pGL3 plasmids to construct pGL3-PDCD4-WT or pGL3-PDCD4-MUT plasmids for transfection. Luminescence was detected 2 days after transfection.
Cell apoptosis
Cells after 24h-treatment by lidocaine (Lid), BMSC-exo, or oePDCD4 plasmids were stained by Annexin V Apoptosis Kit (Beyotime) and a Beckman CytoFLEX Flow Cytometer was used to analyze apoptosis.
Quantitative PCR (Q-PCR)
RNA was isolated and cDNA was made with a cDNA Synthesis Kit (Fisher, USA). Q-PCR was done on an ABI7300 system. The relative abundance of genes was quantified using 2−ΔΔCt with GAPDH or U6 as an internal control, and the primers used were shown in Table 1.
Table 1:
Primers used
| Gene | Primers (5′-3′) |
|---|---|
| miR-21-5p | RT: TCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA F: GCGCGTAGCTTATCAGACTG R: AGTGCAGGGTCCGAGGTATT |
| miR-125b-5p | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCACAA F: CGCGTCCCTGAGACCCTAAC R: AGTGCAGGGTCCGAGGTATT |
| miR-4454 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGGTGC F: GCGGGATCCGAGTCACG R: AGTGCAGGGTCCGAGGTATT |
| RNU6-1 | F: CTCGCTACGCCAGCTCA R: AATGCTTCACCAATTCGCGT |
| PDCD4 | F: ACTCCTAGAGCACCACAG R: TTCAGCAGCATATCAATC |
| GAPDH | F: GGATTGTCTGGCAGTAGCC R: ATTGTGAAAGGCAGGGAG |
Western blot
Proteins were extracted, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (23), and transferred to nitrocellulose membranes, blocked, and incubated with antibodies against CD63, CD81, TSG101, PDCD4, and GAPDH. Membranes were then immersed into HRP-conjugated secondary antibody solution (Beyotime). Signals were captured by a chemiluminescence system.
Statistical analysis
Data analysis was performed by Prism8.0.2 (GraphPad, San Diego, US). Data was shown as mean ± SD. One-way ANOVA was performed to calculate the significance among multiple groups. P values < 0.05 were considered as significant.
Results
BMSC-exo protected against lidocaine induced apoptosis in a dose-dependent manner
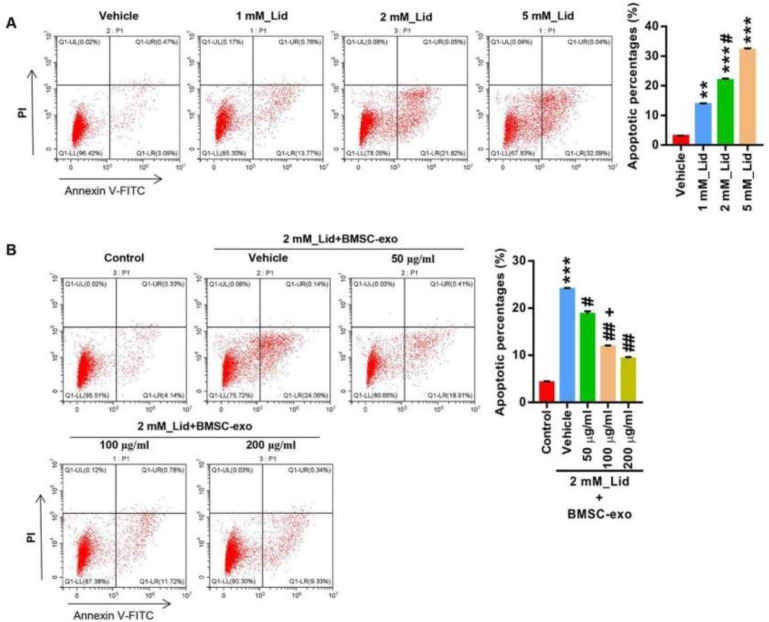
To study the neural toxicity of lidocaine, we first established a neuronal cell toxicity model using SH-SY5Y. Cells were provided with lidocaine (1, 2 and 5 mM). Lidocaine treatment dose-dependently increased the apoptosis of SH-SY5Y cells (Fig. 1A), indicated by an increase of Annexin V staining positive cells. The data suggested that the model of neural toxicity was successfully set which enables us to explore the toxic effects of lidocaine in neuronal cells. Next, we explored the effect of BMSC-exo on lidocaine induced neural toxicity. We first measured the cell surface markers of BMSCs using a flow cytometer and results confirmed that the cells were stained CD90+, CD105+, CD34-, and CD45- (Fig. 2A).
Fig. 1:
BMSC-exo protected against lidocaine-mediated apoptosis in a dose-dependent manner
SH-SY5Y cells were provided with lidocaine (0, 1, 2, and 5mM) for 24 hours. (A) Apoptosis analysis. **P < 0.01, ***P < 0.001 vs. Vehicle; #P < 0.05 vs. 1 mM_Lid. (B) SH-SY5Y cells were treated with idocaine (2mM) and BMSC-exo (0, 50, 100, and 200 μg/ml) for 24 hours, and apoptosis was analyzed. *** P < 0.001 vs. Ctrl; # P < 0.05, ## P < 0.01 vs. 2 mM_Lid+Vehicle; +P < 0.05 vs. 2 mM_Lid+50 μg/ml BMSC_exo
Fig. 2:
Isolation and identification of exosomes from human bone mesenchymal stem cells (BMSC-exo).
(A) Flow cytometry analysis of BMSC cell surface markers. (B-C) BMSC-exos were isolated using ultra-high-speed centrifugation and characterized by TEM (B) and immunoblotting analysis of exosomal markers (C). (D) Co-cultured of PKH-67-labeled BMSC-exos with SH-SY5Y cells showed that BMSC-exos could be endocytosed by SH-SY5Y cells. (E) miR-21-5p inhibitor or mimic was transfected into BMSCs and BMSC-exos were isolated using ultra-high-speed centrifugation. miR-21-5p expression was measured by Q-PCR. *P < 0.05, **P < 0.01 vs. NC
Then, BMSC-exos were isolated using ultra-high-speed centrifugation and characterized by TEM (Fig. 2B) and immunoblotting analysis of exosomal markers CD81, CD63, and TSG101 (Fig. 2C). We next proved that exosomes could be taken up by SH-SY5Y cells (Fig. 2D). The results indicated that BMSC-exos were successfully isolated and were ready for the study. Next, different concentrations of BMSC-exos (50, 100 and 200 μg/μL) was used to treat SH-SY5Y cells together with lidocaine (2 mM). The concentration of lidocaine (2 mM) was chosen based on the fact that it induced a decent amount of apoptosis and to avoidance of any possible ceiling effects. Administration of BMSC-exo dose-dependently alleviated lidocaine induced apoptosis of SH-SY5Y cells (Fig. 1B). The results demonstrate that BMSC-exo is able to protect neuronal cells from lidocaine induced injury.
miR-21-5 negatively regulates PDCD4
Given the protective roles of BMSC-exo on neural toxicity, we explored the function of miR-21-5p in lidocaine induced neural toxicity. miR-21-5p was successfully silenced or up-regulated in SH-SY5Y cells as shown by Q-PCR (Fig. 3A, Fig. 2E). Silencing miR-21-5p sharply increased PDCD4, overexpressing miR-21-5p sharply suppressed PDCD4 (Fig. 3B-3C). The results of luciferase reporter gene assay showed that in WT PDCD4 3′-UTR transfected cells, luciferase activity was sharply upregulated with mi-21-5p inhibitor and was down-regulated with mi-21-5p mimic. Neither of these effects were observed in mut PDCD4 3′-UTR transfected cells (Fig. 3D). The findings show that mi-21-5p bound to the 3′-UTR to suppress PDCD4 in neuronal cells.
Fig. 3:
miR-21-5p negatively regulated PDCD4.
WT or mutant 3′-UTR of PDCD4 and miR-21-5p inhibitor/mimic were transfected into SH-SY5Y cells. (A) Q-PCR measurement of miR-21-5p expression. (B-C) Q-PCR and immunoblot analysis of PDCD4. ** P < 0.01, *** P < 0.001 vs. NC. (D) Luciferase reporter assay of PDCD4 and miR-21-5p. **P < 0.01, ***P < 0.001 vs. WT+NC
Inhibiting miR-21-5p reversed the effect of BMSC-exo on Lid-induced injury of SH-SY5Y cells
We then tested whether mi-21-5p is required for the protective effects of BMSC-exo. BMSCs were transfected by NC, inhibitor and mimic of mi-21-5p and exosomes were used to co-treat the SH-SY5Y cells together with lidocaine. As expected, lidocaine treatment significantly increased the apoptosis (P<0.001). Co-treatment of NC-exo decreased the cell apoptosis, compared to lidocaine treatment. Compared to NC-exo group, administration of inhibitor-exo increased the apoptosis, while administration of mimic-exo decreased the apoptosis of SH-SY5Y cells (Fig. 4A). The effects of mi-21-5p inhibitor and mimic transfection were validated with qPCR analysis of miR-21-5p. As expected, NC-exo treatment increased the level of mi-21-5p. Inhibitor-exo treatment decreased the amount of mi-21-5p, whereas mimic-exo treatment increased the amount of mi-21-5p (Fig. 4B). Correspondingly, our data showed that NC-exo treatment decreased PDCD4 expression. Inhibitor-exo treatment increased PDCD4, whereas mimic-exo treatment decreased PDCD4 (Figures 4C-4D).
Fig. 4:
Inhibition of miR-21-5p reversed the effect of BMSC-exo on lidocaine-induced injury.
SH-SY5Y cells were treated with lidocaine (2mM) and exosomes isolated from BMSC-transfected with inhibitor-exo) or mimic-exo (100 μg/ml) for 24 hours. (A) Apoptosis analysis. (B) miR-21-5p levels. (C-D) PDCD4 levels. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. Ctrl; # P < 0.05, ## P < 0.01 vs. 2 mM_Lid+Ctrl; +P < 0.05, ++ P < 0.01 vs. 2 mM_Lid+NC-exo
miR-21-5p regulated lidocaine-induced injury probably through targeting PDCD4
To further characterize the role of miR-21-5p/PDCD4 pathway in the protective effect of BMSC-exo, we successfully over-expressed PDCD4 (Figures 5A-5B). Then, SH-SY5Y cells were treated with both lidocaine and exosomes isolated from BMSCs transfected by either vector (vector-exo) control or PDCD4 (oePDCD4-exo). Administration of oePDCD4-exo diminished oePDCD4 increased lidocaine-induced PDCD4 expression (Fig. 5C). Consistent with these observation, over-expression of PDCD4 increased lidocaine induced apoptosis was significantly alleviated by administration of oePDCD4-exo (Fig. 5D).
Fig. 5:
miR-21-5p regulated lidocaine-induced injury probably through targeting PDCD4.
PDCD4 plasmids were used to transfect SH-SY5Y to overexpress PDCD4. (A) Q-PCR and (B) immunoblot analysis of PDCD4. ***P < 0.001 vs. Vector. PDCD4-overexpression SH-SY5Y cells were treated with idocaine (2mM) and BMSC-exo (100μg/ml) for 24 hours. (C) Immunoblot analysis of PDCD4. (D) Flow cytometry analysis of apoptosis. **P < 0.01 vs. Control; ##P < 0.01 vs. 2 mM_Lid+Vehicle+Vector; +++P < 0.001 vs. 2 mM_Lid+exo+Vector
Discussion
This study, for the first time, showed that BMSC-derived exosomal miRNA-21-5p inhibits lid-induced apoptosis of SH-SY5Y via directly targeting PDCD4. miRNAs play a pivotal role in neurotoxicity and neurodegenerative diseases (24). For instance, studies showed that miR-497 increased neuronal death (25). Pietrao et al. reported that miR-425-5p was sharply decreased in traumatic brain injury (TBI) and can be used as a prognostic indicator (26). Low level of miR-153 has been demonstrated to cause Aβ accumulation (27). All these studies support that miRNAs play a very important role in neurotoxicity. Apoptosis is responsible for the maintenance of organismal homeostasis (28). Apoptosis also plays a critical role in various diseases including neurodegenerative diseases (29). Here, we showed that miR-21-5p protects SH-SY5Y cells against lidocaine induced apoptosis. These results improve our understanding of the lidocaine induced neurotoxicity.
miR-21-5p is involved in different pathological processes. For example, miR-21-5p was increased in tumors to promote invasiveness (30). miR-21-5p also involves in neuronal toxicity. Li et al. have showed that exosomal miR-21-5p protected TBI through suppressing autophagy (31). Another study showed that miR-21 protects against ischemic neuronal death (32). In consistent with published data, this study indicated that miR-21-5p from BMSC-exo protected SH-SY5Y cells against lidocaine induced apoptosis. Our data revealed a new role of miR-21-5p in the lidocaine induced neuronal toxicity, showing that exosomal miR-21-5p exerts its protective effect on SH-SY5Y cell apoptosis.
miRNAs work as small guide molecules in RNA silencing (34). One miRNA may regulate multiple downstream target genes (35). Different target genes of miR-21-5p have been discovered. For example, miR-21-5p promoted tumor peritoneal metastasis via targeting SMAD7 (36). Liu et al. have showed that miR-21-5p enhanced glycolysis via targeting PDHA1 (37). PDCD4 is a key protein involved in apoptosis (38). Overexpressing PDCD4 in breast cancer cells induced apoptosis (39). In another study, researcher showed that overexpressing PDCD4 led to hepatoma cell apoptosis (40). Wan et al. demonstrated that inhibiting miR-150 enhanced PDCD4-dependent caspase-8 activation to promote apoptosis of melanoma (38). miR-21-5p has been shown to target PDCD4 to regulate apoptosis and inflammation (41). Our results confirmed that overexpressing miR-21-5p sharply down-regulated PDCD4, suggesting that miR-21-5p targets PDCD4. This was further proved by luciferase reporter assay result that miR-21-5p suppressed PDCD4. These results broaden our understanding of miR-21-5p/PDCD4 in apoptosis, also help to expand our understanding of lidocaine-induced neurotoxicity. It could be inferred that exosomal miR-21-5p exerts its protective effect on lidocaine induced neuronal toxicity in SH-SY5Y cells probably through inhibiting PDCD4. It is worth to mention that only one cell line (SH-SY5Y) is used in this study, future studies using more cell lines will help to prove the findings of this study. To further elucidate the role of miR-21-5p/PDCD4 in lidocaine induced neurotoxicity, patient samples would provide more relevant data.
Conclusions
This study indicated a novel role of miR-21-5p/PDCD4 axis, suggesting that BMSC-exos protected SH-SY5Y cells against lidocaine induced apoptosis through miR-21-5p by targeting PDCD4. These results highlighted the important role of miR-21-5p/PDCD4 signaling in lidocaine induced apoptosis, and may help to develop new strategy in the management of lidocaine-induced neurotoxicity.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
No finical support for this study was received.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1. Wang B, Wang S, Zhang Q, et al. ( 2019). Recent advances in polymer-based drug delivery systems for local anesthetics. Acta Biomater, 96: 55–67. [DOI] [PubMed] [Google Scholar]
- 2. Anderson T. ( 2016). Doctors lobby for better chronic pain management. Lancet, 388( 10062): 2856– 2858. [DOI] [PubMed] [Google Scholar]
- 3. Kuehn B. ( 2018). Chronic Pain Prevalence. JAMA, 320( 16): 1632. [DOI] [PubMed] [Google Scholar]
- 4. Tikhonov DB, Zhorov BS. ( 2017). Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants. J Gen Physiol, 149( 4): 465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donaldson M, Goodchild JH. ( 2018). Lidocaine turns 70: the evolution of dental local anesthesia. Gen Dent, 66( 3): 6–9. [PubMed] [Google Scholar]
- 6. Karm MH, Park FD, Kang M, et al. ( 2017). Comparison of the efficacy and safety of 2% lidocaine HCl with different epinephrine concentration for local anesthesia in participants undergoing surgical extraction of impacted mandibular third molars: A multicenter, randomized, double-blind, crossover, phase IV trial. Medicine (Baltimore), 96( 21): e6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim DD, Asif A, Kataria S. ( 2016). Presentation of Neurolytic Effect of 10% Lidocaine after Perineural Ultrasound Guided Injection of a Canine Sciatic Nerve: A Pilot Study. Korean J Pain, 29( 3): 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bianco P. ( 2014). “Mesenchymal” stem cells. Annu Rev Cell Dev Biol, 30: 677–704. [DOI] [PubMed] [Google Scholar]
- 9. Futrega K, Mosaad E, Chambers K, et al. ( 2018). Bone marrow-derived stem/stromal cells (BMSC) 3D microtissues cultured in BMP-2 supplemented osteogenic induction medium are prone to adipogenesis. Cell Tissue Res, 374( 3): 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J, Zhang W, Ran Q, et al. ( 2018). The Differentiation Balance of Bone Marrow Mesenchymal Stem Cells Is Crucial to Hematopoiesis. Stem Cells Int, 2018: 1540148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wislet-Gendebien S, Laudet E, Neirinckx V, et al. ( 2012). Mesenchymal stem cells and neural crest stem cells from adult bone marrow: characterization of their surprising similarities and differences. Cell Mol Life Sci, 69( 15): 2593–608. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Hao J, Li S, Shi X, et al. ( 2018). Bone marrow mesenchymal stem cells protect against nhexane-induced neuropathy through beclin 1-independent inhibition of autophagy. Sci Rep, 8( 1): 4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baglio SR, Rooijers K, Koppers-Lalic D, et al. ( 2015). Human bone marrow- and adiposemesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther, 6( 1): 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruivo CF, Adem B, Silva M, et al. ( 2017). The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res, 77( 23): 6480–6488. [DOI] [PubMed] [Google Scholar]
- 15. Beyer C, Zampetaki A, Lin NY, et al. ( 2015). Signature of circulating microRNAs in osteoarthritis. Ann Rheum Dis, 74( 3): e18. [DOI] [PubMed] [Google Scholar]
- 16. Zhou W, Fong MY, Min Y, et al. ( 2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 25( 4): 501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhuang G, Wu X, Jiang Z, et al. ( 2012). Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J, 31( 17): 3513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaudelot K, Gibier JB, Pottier N, et al. ( 2017). Targeting miR-21 decreases expression of multi-drug resistant genes and promotes chemosensitivity of renal carcinoma. Tumour Biol, 39( 7): 1010428317707372. [DOI] [PubMed] [Google Scholar]
- 19. Zhao Y, Zhao L, Ischenko I, et al. ( 2015). Anti-sense inhibition of microRNA-21 and microRNA-221 in tumor-initiating stem-like cells modulates tumorigenesis, metastasis, and chemotherapy resistance in pancreatic cancer. Target Oncol, 10( 4): 535– 48. [DOI] [PubMed] [Google Scholar]
- 20. Gao X, Xiong Y, Li Q, et al. ( 2020). Extracellular vesicle-mediated transfer of miR-21-5p from mesenchymal stromal cells to neurons alleviates early brain injury to improve cognitive function via the PTEN/Akt pathway after subarachnoid hemorrhage. Cell Death Dis, 11( 5): 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu X, He Y, Wang X, et al. ( 2017). Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther, 8( 1): 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Théry C, Amigorena S, Raposo G, et al. ( 2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol, 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 23. Jensen EC. ( 2012). The basics of western blotting. Anat Rec (Hoboken), 295: 369–371. [DOI] [PubMed] [Google Scholar]
- 24. Bas-Orth C, Koch M, Lau D, et al. ( 2020). A microRNA signature of toxic extrasynaptic N-methyl-D-aspartate (NMDA) receptor signaling. Molecular Brain, 13( 1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin KJ, Deng Z, Huang H, et al. ( 2010). miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis, 38( 1): 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Pietro V, Ragusa M, Davies D, et al. ( 2017). MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. J Neurotrauma, 34( 11): 1948–1956. [DOI] [PubMed] [Google Scholar]
- 27. Wang N, Qiu P, Cui W, et al. ( 2019). Recent Advances in Multi-target Anti-Alzheimer Disease Compounds (2013 Up to the Present). Curr Med Chem, 26( 30): 5684–5710. [DOI] [PubMed] [Google Scholar]
- 28. Singh R, Letai A, Sarosiek K. ( 2019). Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol, 20( 3): 175–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu X, Lai Y, Hua ZC. ( 2019). Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep, 39( 1): BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan JK, Blansit K, Kiet T, et al. ( 2014). The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol Oncol, 132( 3): 739–44. [DOI] [PubMed] [Google Scholar]
- 31. Li D, Huang S, Zhu J, et al. ( 2019). Exosomes from MiR-21-5p-Increased Neurons Play a Role in Neuroprotection by Suppressing Rab11a-Mediated Neuronal Autophagy In Vitro After Traumatic Brain Injury. Med Sci Monit, 25: 1871–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buller B, Liu X, Wang X, et al. ( 2010). MicroRNA-21 protects neurons from ischemic death. FEBS J, 277( 20): 4299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yelamanchili SV, Lamberty BG, Rennard DA, et al. ( 2015). MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PLoS Pathog, 11( 7): e1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oliveto S, Mancino M, Manfrini N, et al. ( 2017). Role of microRNAs in translation regulation and cancer. World J Biol Chem, 8( 1): 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu P, Wu Q, Yu J, et al. ( 2020). A Systematic Way to Infer the Regulation Relations of miRNAs on Target Genes and Critical miRNAs in Cancers. Front Genet, 11: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Q, Li B, Li Q, et al. ( 2018). Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis, 9( 9): 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu Z, Yu M, Fei B, et al. ( 2018). miR-21-5p targets PDHA1 to regulate glycolysis and cancer progression in gastric cancer. Oncol Rep, 40( 5): 2955–2963. [DOI] [PubMed] [Google Scholar]
- 38. Wan J, Yang J, Huang Y, et al. ( 2018). MicroRNA-150 inhibitors enhance cell apoptosis of melanoma by targeting PDCD4. Oncol Lett, 15( 2): 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y, Liu Z, Shen J. ( 2019). MicroRNA-421-targeted PDCD4 regulates breast cancer cell proliferation. Int J Mol Med, 43( 1): 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo J, Ozaki I, Xia J, et al. ( 2019). PDCD4 Knockdown Induces Senescence in Hepatoma Cells by Up-Regulating the p21 Expression. Front Oncol, 8: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao X, Huang X, Yang Q, et al. ( 2021). MicroRNA-21-5p targets PDCD4 to modulate apoptosis and inflammatory response to Clostridium perfringens beta2 toxin infection in IPEC-J2 cells. Dev Comp Immunol, 114: 103849. [DOI] [PubMed] [Google Scholar]