Abstract
Background:
Cancer stem cells (CSC), as responsible issues to cancer development and progression, play a crucial role in tumorigenesis, recurrence, metastasis, and chemoresistance. Both hyperthermia and photodynamic therapy (PDT) may be effective for cancer treatment, particularly when combined with other therapeutic approaches. This study aimed to evaluate the effect of hyperthermia combined with PDT on colorectal CSC and the gene expression of the CSC markers, presenting a more effective approach for cancer therapy.
Methods:
The study was conducted in the Pasteur institute of Iran, Tehran, Iran in 2018. We evaluated the anticancer role of hyperthermia, Gold nanoparticles coated with curcumin (Cur-GNPs) in PDT and combination of the two approaches on cell viability and the expression of CSC markers, Nanog and Oct4 in colorectal cancer cell line HT-29. The cytotoxicity effect of Cur-GNPs against the cells was assessed in vitro. The cell viability was assessed using MTT assay, and the expression analysis of the CSC genes was evaluated using a q-real-time PCR.
Results:
Cell viability was decreased by PDT (P=0.015) and the combination therapy (P=0.006) but not by hyperthermia alone (P=0.4), compared to control. Also, the expression of CSC markers, Nanog and Oct4 was shown to significantly down-regulate in all hyperthermia, PDT and combination groups.
Conclusion:
Hyperthermia combined with PDT was indicated to be more efficient in eliminating tumors than hyperthermia or PDT alone.
Keywords: Colorectal cancer stem cell, Nanog, Hyperthermia, Photodynamic therapy
Introduction
Colorectal cancer (CRC) is the third most commonly detected cancer with 1.8 million cases worldwide in 2018 (1–3). Currently, conventional treatments for CRC include tumor surgery, radiation therapy, and chemotherapy (4). Despite advances in the therapeutics approaches, CRC has shown be resistant to these treatments alone (5). A subpopulation of CRC cells, named cancer stem cells (CSCs), contribute to resistance to radiation and chemotherapy and therefore, lead to failure of the cancer treatments (6–8). Colorectal CSCs (CCSCs) have the capacity of tumorigenesis, recurrence and metastasis (9). To overcome the drawbacks of conventional therapies, several studies have investigated the novel and combined strategies, including combination of ionizing radiation, hyperthermia and photodynamic therapy (PDT) (10–12).
Hyperthermia (also commonly known as thermal therapy or thermotherapy) occurs when an individual's body temperature (local, regional or whole body) increases (13). Hyperthermia has been found to be highly effective in CRC therapy specially when combined with chemotherapy, radiotherapy, or immunotherapy (14, 15). Mild hypothermia or fever-range hyperthermia had inhibitory effect on viability/proliferation in glioblastoma, prostate, lung and breast cancer cell lines (16). However, hyperthermia alone had no significant effect on breast cancer cells but its combination with radiotherapy might be useful through increasing apoptosis (17). Hence, we need to clear the possible discrepancy in our knowledge on anticancer effects of hyperthermia. Cancer therapy with light (i.e. PDT) is a noninvasive strategy that can also be effective as a supporting treatment for various types of cancer and malignant tumors (18). It use a photosensitizer which becomes activated by laser in a specific wavelength and reacts with oxygen to generate reactive oxidant species (ROS) in target tissues, leading to cell death (19, 20). ROS can unbalance redox system as well as damage DNA and protein. Moreover, PDT can dysregulate mitochondrial activity, autophagy, and CSCs dormancy (21). One of the most studied photosensitizer is curcumin (Cur) which has numerous therapeutic properties. An increasing body of evidence suggests that Cur might an appropriate photosensitizer for PDT (22). Curcumin-based PDT cannot effect on apoptosis in melanoma (23). To deliver the photosensitizer to the malignant tumor, gold nanoparticles (GNPs) have been widely used due to their biocompatibility and low toxicity (24).
We aimed to investigate whether hyperthermia and Cur-GNPs-mediated PDT alone and in combination could effect on the cell viability and the expression of cancer stem cell markers in CRC.
Materials and methods
In vitro cytotoxicity evaluation of Cur-GNPs
The study was conducted in the Pasteur institute of Iran, Tehran, Iran in 2018. Synthesized and characterized gold nanoparticles (GNP) coated with Cur (Cur-GNPs) (25) were generously gifted by Seyed Mohammad Amini (Radiation Biology Research Center, Iran University of Medical Sciences, Tehran, Iran). The colon carcinoma cell line (HT-29) was purchased from National Bank of Pasteur Institute (Iran) and cultured in RPMI-1640 cell growth medium supplemented with penicillin (100U/ml), streptomycin (100 μg/ml), and 10% FBS. A standard humidified atmosphere (95% air & 5% CO2 at 37 °C) was provided for continuous cell culture.
In order to evaluate Cur-GNPs cytotoxicity, 104 cells/well were seeded into each well of a 96-well plate. After 24h, culture medium containing a definite concentration of Cur-GNPs was added and the cells were incubated for an additional 24h. Using the same procedure, Cur dark cytotoxicity was examined by 1h incubation. For photodynamic studies, we added fresh culture medium containing Cur-GNPs (128mg/ml) and Cur (6.4μg/ml) to cells, and they were incubated for 1h. Then, the culture medium was replaced and exposed to the 150mW (15.7mW/cm2) laser (Thor International Ltd, Amersham, Bucks, UK) for 2 min. The quantitative real-time PCR (qPCR) and MTT tests were completed after 24 h incubation.
In vitro treatment with hyperthermia, PDT and combination treatment
The cell line was cultured in RPMI-1640 supplemented with 10% of fetal bovine serum (FBS) (Gibco, USA) and incubated at 37 °C in 5% CO2. The medium was replaced every 2 d until the cells reached 80%–90% confluence. The cells were incubated in culture medium supplemented with 10% FBS in an incubator preheated to 42 and 43 ºC for 2 h. Control groups were incubated at 37 ºC for 2 h, as well. After hyperthermia treatment, the cells were incubated at 37 ºC for 2 h prior to analysis.
For PDT treatment, first the cells were seeded in 96-well plates and incubated with 5 μM solution of Cur-GNPs for 24 h. Cur-GNPs culture medium was then removed and the plate was washed with 350 μL PBS per well. Irradiation was accomplished at room temperature with a LED-based illumination device (PDT EDL-1; Hamamatsu Photonics K.K., Hamamatsu, Japan). The expriments were adjusted by low-power laser (32 mW 630 nm diode laser, 0.5 J/cm2, continuous mode for 360 sec). Following irradiation, fresh culture medium lacking phenol red was added, cells were incubated at 37 °C for 24 h. After the irradiation, the cells were enriched by fresh medium and incubated for 24 h. Then, cell viability was evaluated by MTT assay.
For combination therapy with both hyperthermia and PDT, a cell group was simultaneously exposed to hyperthermia and PDT with the aforementioned conditions. In order to minimize interference with the optical sensitizer and maximize the effect of the laser, the cell culture medium was enriched with low FBS (% 1). The preheated cells at 42 and 43 ºC for 2 h, were cultured in the presence of Cur-GNPs and treated with low-power laser. The cells with no treatment were considered as control in all experiments. Then, MTT test was performed to evaluate cell viability.
Cell morphology
To evaluate the effect of the hyperthermia and PDT on the cellular phenotype, the cells were evaluated using an inverted microscope.
Cell viability assay
After treatments, the cell viability was determined by MTT [3-(4, 5-dimethylthiazol-2-Yl)-2, 5-diphenyltetrazolium bromide] (BioIDEA, Iran) assay. For MTT assay, HT-29 cells were seeded in 96-well plates a day prior to the experiment. The cell viability was evaluated after hyperthermia exposure. Then, the culture medium was aspirated and 10µl MTT solution with a final concentration of 0.5 mg/mL was added. Afterward, 3 h of incubation at 37 °C, MTT solution was aspirated and 100µl well DMSO was added to each well. After 30 min incubation at 37 °C, the absorbance (570/630 nm) was measured using a microplate reader (BioTek, USA).
RNA extraction and cDNA synthesis
Total RNA was extracted from cells using high pure RNA isolation kit (Roche, Germany) according to the manufacturer’s instructions. The concentration of RNA was quantified using Nano Drop™ Lite Spectrophotometer (Thermo Fisher Scientific, USA). A 2% agarose gel electrophoresis was used to assess quality of RNA. Subsequently, cDNA was synthesized from the purified total RNA using a PrimeScript RT reagent Kit (Takara, Japan) according to the manufacturer's protocol.
Primer design for qPCR
The GeneRunner software was used to design the primers for amplification of interested genes. Additionally, the primer specificity was confirmed by Primer-BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast). The sequences of primers used in the current study are listed in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control for normalization (Table 1).
Table 1:
Primers designed for amplification of interested gene
| DNA fragment name | Primer name | Primer sequence | Product length |
|---|---|---|---|
| OCT4 | F | 5′ CTTGAATCCCGAATGGAAAGGG 3′ | 164 |
| R | 5′ GTGTATATCCCAGGGTGATCCTC 3′ | ||
| NANOG | F | 5′ TTTGTGGGCCTGAAGAAAACT 3′ | 116 |
| R | 5′ AGGGCTGTCCTGAATAAGCAG 3′ | ||
| GAPDH | F | 5′ CACCAGGGCTGCTTTTAAC 3′ | 190 |
| R | 5′ ATCTCGCTCCTGGAAGAT 3′ |
Quantitative Real-Time PCR
To analyse mRNA levels, qPCR was performed using SYBR® Premix Ex Taq™ II (Takara, Japan) and the ABI7500 system (Applied Biosystems; Thermo Fisher Scientific). The reaction system included 10 μLSYBR_ Premix Ex Taq_II (2×), 0.4 μL ROX dye, 2 μL template cDNA, 0.4 μL each primer, and 6.8 μL RNase-free water. The qPCR reactions were performed in the following cycling conditions: The initial denaturation step 95 ºC for 30 sec, then 40 cycles of 95 ºC for 5 sec and 60 ºC for 30 sec. All experiments were repeated at least three times.
Statistical analysis
The SPSS 19.0 (IBM Corp., Armonk, NY, USA) software was used for statistical analysis. Kruskal Wallis test was used to compare the experiments. Gene expression data derived from qPCR were analysed using GraphPad Prism 7.0 software. Data were presented as mean ± SEM. The P-values <0.05 were considered statistically significant.
Results
Cell morphology assay
The inverted microscope results showed that the hyperthermia and PDT had no effect on cell morphology.
Hyperthermia decreased expression of CSC marker but did not affect cell viability
Hyperthermia did not decrease cell viability of HT-29 cells compared to the control (37 °C) (Fig. 1a). However, expression of Nanog gene was significantly decreased in the HT-29 cells treated with hyperthermia at 42 °C (P=0.038), but not at 43 °C (P>0.05). Additionally, there was no significant difference in Oct4 gene expression between hyperthermia group and the control (Fig. 1b).
Fig. 1:
a) Cell viability after treatment with hyperthermia. b) Effect of hyperthermia on expression of Nanog and Oct4 measured by qPCR in HT-29 cell line. *P<0.05
PDT decreased cancer cell viability and downregulated expression of CSC markers
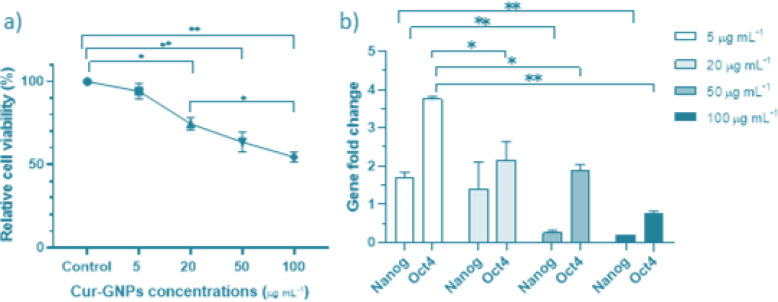
The cell viability of HT-29 was significantly decreased by Cur-GNPs-mediated PDT in a dose-dependent manner (Fig. 2a). Nanog and Oct4 gene expression also decreased (P<0.05) (Fig. 2b).
Fig. 2:
a) Cell viability after treatment with PDT. b) Relative gene expression of Nanog and Oct4 induced by PDT with various concentrations of Cur-GNPs. *P<0.05, **P<0.01, ***P<0.001
Combination of PDT and hyperthermia was more effective in downregulating gene expression of CSC markers
Combination of hyperthemia and PDT was more effective in reducing cell viability than hyperthermia or PDT alone (compared to hyperthermia; P<0.01, compared to PDT; P<0.05), (Fig. 3a). Moreover, the combination of PDT and hyperthermia downregulated the gene expression of Nanog and Oct4 (P<0.05) (Fig. 3b).
Fig. 3:
a) Cell viability after treatment with a combination of PDT and hyperthermia b) Relative effect of PDT and hyperthermia combination therapy on gene expression of Nanog and Oct4. *P<0.05, **P<0.01, ***P<0.001
Discussion
Given the anticancer effects of hyperthermia and PDT, the present study aimed to investigate whether hyperthermia and Cur-GNPs-mediated PDT alone and in combination could effect on the cell viability and the expression of CCSC markers. Based on our findings, PDT but not hyperthermia, was effective in reducing cell viability. Both treatments significantly reduced gene expression of CCSCs markers. However, combination of hyperthermia and PDT was more effective than hyperthermia and PDT alone.
Hyperthermia exposure downregulated the stemness-related genes such as Abcg2 and Nanog in colon cancer cell lines (26). Hyperthermia might contribute to chemosensitization of CRC. In this regard, hyperthermia has been shown to enhance the effectiveness of chemotherapy and radiation treatment (27). The hyperthermia reduced the number of CSCs cells and downregulated expression of CSC markers such as Nanog (6, 26). We found similar results in our study where hyperthermia significantly reduced expression of Nanog, a CCSC marker, but not change cell viability. Although evidence has suggested the effectiveness of hyperthermia in cancer treatment, temperature elevation alone cannot remove cancer cells (28). On the other hand, hyperthermia can inhibit repair of chemotherapy- or radiotherapy-induced DNA damage (29) and activate extracellular heat shock proteins (HSPs) leading to the antitumor immune system from the heat-treated necrotic tumor cells (30). Thereby, a series of events have been revealed to cause cell death. After mild hyperthermia, some cellular functions can be recovered and some subpopulations of cells may be resistant to hyperthermic-induced cell death (15). Thus, inability to reduce cell viability may be due to the rate of temperature or short time hyperthermia exposure.
Furthermore, we used PDT to treat colorectal cancer cell HT-29. We found that the expression of CCSC markers as well as the cancer cell viability were significantly decreased by PDT. This finding was consistent with previous studies who reported a beneficial role for PDT in treating several types of cancer, including glioblastomas, lung, esophageal, bladder, colorectal and nasopharyngeal carcinomas (31). In this regard, PDT-generated ROS could change the activity of CSC markers Oct4 and Sox2 (21). Furthermore, ROS might attack DNA causing point mutations in key genes such as Ras (32) and p53 (33) which regulate several cellular processes, including regulate proliferation, differentiation and apoptosis (34). In addition, ROS binds to lipids and produces free radicals and peroxides that can damage cell membranes and change mitochondrial permeability leading to apoptosis (35).
According to other investigations, PDT combined by other methods could improve some cancer treatments. The effects of PBMT (Photobiomodulation therapy) combined with PDT have investigated in head and neck cancer (HNC) cell lines. Combination therapy of Photobiomodulation (PBM) and PDT functioned in some HNC cell lines such as SCC-25 cells by increasing PS uptake and production of ROS. However, the potential anticancer effect was not seen in SCC-4 cells compared to the PDT only group. PBM also improved cell metabolism by increasing uptake and oxygenation the reaction site, promoting PDT efficiency (36). A natural anthraquinone called Emodin, has cytotoxicity effects and anti-tumor activity in PDT. Moreover, emodin combined with PDT in cervical carcinoma cell lines was effective. The significant decrease in cell viability induced by the combination therapy (emodin-PDT) was due to apoptosis and autophagy mediated by ROS production in tumor cells (37).
Here, we used a combination of hyperthermia, and PDT. Cell viability and CCSC marker expression were significantly lower compared to hyperthermia, PDT or the control. Previous studies have also reported a decrease in cell viability and increased DNA fragmentation and ROS production (38, 39). The combination therapy induced the mitochondrial activation and increased the Caspases-9, −3 and poly ADP-ribose polymerase (PARP) expression leading to apoptosis (40). Hyperthermia increased efficacy of PDT on gastric cancer cells through elevated ROS production leading to enhanced PDT-induced cytotoxicity (38). The increased treatment efficiency in the combination therapy might be due to down-regulation of proliferation-related genes, increased ROS production and mitochondrial-dependent apoptosis (41). Our study showed the anti-cancer effects of combined hyperthermia and PDT in vitro system. Although, in vitro studies primarily support clinical applications, for prospective studies, it is suggested more investigation on several CRC cell lines, using various irradiation dose and animal models. In brief, we provided an important evidence of the effectiveness of hyperthermia combined with PDT in decreasing cell viability via regulating stemness-related pathways in CRC. However, the underlying molecular mechanisms of the combination of hyperthermia and PDT is still unknown. Future studies should focus on understanding the mechanism of cancer treatment via possible combination therapies.
Conclusion
Our results suggest that PDT in combination with hyperthermia may be more effective in treating and eliminate primary and aggressive colorectal tumors by affecting on cancer stem cells.
Journalism Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This work was supported by Grant No. 97-03-49-13165 from Iran University of Medical Sciences.
Footnotes
Conflict of interest
Authors declare no conflict of interests.
References
- 1. Akbari A, Mobini GR, Maghsoudi R, et al. ( 2016). Modulation of transforming growth factor-β signaling transducers in colon adenocarcinoma cells induced by staphylococcal enterotoxin B. Mol Med Rep, 13( 1): 909–914. [DOI] [PubMed] [Google Scholar]
- 2. Emami SS, Akbari A, Zare A-A, et al. ( 2019). MicroRNA expression levels and histopathological features of colorectal cancer. J Gastrointest Cancer, 50( 2): 276–284. [DOI] [PubMed] [Google Scholar]
- 3. Rawla P, Sunkara T, Barsouk A. ( 2019). Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol, 14( 2): 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takimoto R, Kamigaki T, Okada S, et al. ( 2019). Prognostic factors for colorectal cancer patients treated with combination of immune-cell therapy and first-line chemotherapy: A retrospective study. Anticancer Res, 39( 8): 4525–4532. [DOI] [PubMed] [Google Scholar]
- 5. Kim J-E, Shin J-Y, Cho M-H. ( 2012). Magnetic nanoparticles: an update of application for drug delivery and possible toxic effects. Arch Toxicol, 86( 5): 685–700. [DOI] [PubMed] [Google Scholar]
- 6. Medema JP. ( 2013). Cancer stem cells: the challenges ahead. Nat Cell Biol, 15( 4): 338–44. [DOI] [PubMed] [Google Scholar]
- 7. Akbari A, Farahnejad Z, Akhtari J, et al. ( 2016). Staphylococcus aureus enterotoxin B downregulates the expression of transforming growth factor-beta (TGF-β) signaling transducers in human glioblastoma. Jundishapur J Microbiol, 9( 5): e27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cojoc M, Mäbert K, Muders MH, et al. ( 2015). A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol, 31: 16–27. [DOI] [PubMed] [Google Scholar]
- 9. Zhu P, Fan Z. ( 2018). Cancer stem cells and tumorigenesis. Biophys Rep, 4( 4): 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolosnjaj-Tabi J, Wilhelm C. ( 2017). Magnetic nanoparticles in cancer therapy: how can thermal approaches help? Nanomedicine (Lond), 12( 6): 573–575. [DOI] [PubMed] [Google Scholar]
- 11. Mobini GR., Ghahremani MH, Amanpour S, et al. ( 2016). Transforming growth factor beta-induced factor 2-linked X (TGIF2LX) regulates two morphogenesis genes, Nir1 and Nir2 in human colorectal. Acta Med Iran, 54( 5): 302–7. [PubMed] [Google Scholar]
- 12. Rentsch M, Schiergens T, Khandoga A, et al. ( 2016). Surgery for Colorectal Cancer-Trends, Developments, and Future Perspectives. Visc Med, 32( 3): 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song X, Kim HC, Kim SY, et al. ( 2012). Hyperthermia-enhanced TRAIL- and mapatumumab-induced apoptotic death is mediated through mitochondria in human colon cancer cells. J Cell Biochem, 113( 5): 1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lutgens L, van der Zee J, Pijls-Johannesma M, et al. ( 2010). Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev, 2010( 3): CD006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadhukha T, Niu L, Wiedmann TS, et al. ( 2013). Effective elimination of cancer stem cells by magnetic hyperthermia. Mol Pharm, 10( 4): 1432–1441. [DOI] [PubMed] [Google Scholar]
- 16. Kalamida D, Karagounis IV, Mitrakas A, et al. ( 2015). Fever-range hyperthermia vs. hypothermia effect on cancer cell viability, proliferation and HSP90 expression. PLoS One, 10( 1): e0116021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hadi F, Tavakkol S, Laurent S, et al. ( 2019). Combinatorial effects of radiofrequency hyperthermia and radiotherapy in the presence of magneto-plasmonic nanoparticles on MCF-7 breast cancer cells. J Cell Physiol, 234( 11): 20028–20035. [DOI] [PubMed] [Google Scholar]
- 18. Agostinis P, Berg K, Cengel KA, et al. ( 2011). Photodynamic therapy of cancer: an update. CA Cancer J Clin, 61( 4): 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. dos Santos AlF, de Almeida DRQ, Terra LF, et al. ( 2019). Photodynamic therapy in cancer treatment-an update review. J Cancer Metastasis Treat, 5: 25. [Google Scholar]
- 20. Kwiatkowski S, Knap B, Przystupski D, et al. ( 2018). Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed Pharmacother, 106: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Z-J, Wang K-P, Mo J-G, et al. ( 2020). Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species. World J Stem Cells, 12( 7): 562–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kazantzis K, Koutsonikoli K, Mavroidi B, et al. ( 2020). Curcumin derivatives as photosensitizers in photodynamic therapy: photophysical properties and in vitro studies with prostate cancer cells. Photochem Photobiol Sci, 19( 2): 193–206. [DOI] [PubMed] [Google Scholar]
- 23. Szlasa W, Supplitt S, Drąg-Zalesińska M, et al. ( 2020). Effects of curcumin based PDT on the viability and the organization of actin in melanotic (A375) and amelanotic melanoma (C32)–in vitro studies. Biomed Pharmacother, 132: 110883. [DOI] [PubMed] [Google Scholar]
- 24. Tiwari PM, Vig K, Dennis VA, et al. ( 2011). Functionalized gold nanoparticles and their biomedical applications. Nanomaterials (Basel), 1( 1): 31–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaabani E, Amini SM, Kharrazi S, et al. ( 2017). Curcumin coated gold nanoparticles: synthesis, characterization, cytotoxicity, antioxidant activity and its comparison with citrate coated gold nanoparticles. Nanomed J, 4( 2): 115–125. [Google Scholar]
- 26. Gao F, Ye Y, Zhang Y, et al. ( 2013). Water bath hyperthermia reduces stemness of colon cancer cells. Clin Biochem, 46( 16–17): 1747–50. [DOI] [PubMed] [Google Scholar]
- 27. Moyer HR, Delman KA. ( 2008). The role of hyperthermia in optimizing tumor response to regional therapy. Int J Hyperthermia, 24( 3): 251–261. [DOI] [PubMed] [Google Scholar]
- 28. Chung H-J, Lee H-K, Kwon KB, et al. ( 2018). Transferrin as a thermosensitizer in radiofrequency hyperthermia for cancer treatment. Sci Rep, 8( 1): 13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pelicci PG, Dalton P, Orecchia R. ( 2011). Heating cancer stem cells to reduce tumor relapse. Breast Cancer Res, 13( 3): 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin F-C, Hsu C-H, Lin Y-Y. ( 2018). Nano-therapeutic cancer immunotherapy using hyperthermia-induced heat shock proteins: insights from mathematical modeling. Int J Nanomedicine, 13: 3529–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Daly SM, Abba ML, Gamal-Eldeen AM. ( 2017). The role of microRNAs in photodynamic therapy of cancer. Eur J Med Chem, 142: 550–555. [DOI] [PubMed] [Google Scholar]
- 32. Ozsvari B, Sotgia F, Lisanti MP. ( 2017). A new mutation-independent approach to cancer therapy: Inhibiting oncogenic RAS and MYC, by targeting mitochondrial biogenesis. Aging (Albany NY), 9( 10): 2098–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen Y-A, Lin C-H, Chi W-H, et al. ( 2013). Resveratrol impedes the stemness, epithelialmesenchymal transition, and metabolic reprogramming of cancer stem cells in nasopharyngeal carcinoma through p53 activation. Evid Based Complement Alternat Med, 2013: 590393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li H, Zhang J, Tong JHM, et al. ( 2019). Targeting the oncogenic p53 mutants in colorectal cancer and other solid tumors. Int J Mol Sci, 20( 23): 5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang B, Liu H, Yang H, et al. ( 2019). Combinatorial photochemotherapy on liver cancer stem cells with organoplatinum (ii) metallacage-based nanoparticles. J Mater Chem B, 7( 42): 6476–6487. [DOI] [PubMed] [Google Scholar]
- 36. de Faria CM, Costa CS, Bagnato VS, et al. ( 2021). Photobiomodulation effects on photodynamic therapy in HNSCC cell lines. J Photochem Photobiol B, 217: 112170. [DOI] [PubMed] [Google Scholar]
- 37. Galiardi-Campoy AEB, Machado FC, Carvalho T, et al. ( 2021). Effects of photodynamic therapy mediated by emodin in cervical carcinoma cells. Photodiagnosis Photodyn Ther, 35: 102394. [DOI] [PubMed] [Google Scholar]
- 38. Kurokawa H, Ito H, Terasaki M, et al. ( 2019). Hyperthermia enhances photodynamic therapy by regulation of HCP1 and ABCG2 expressions via high level ROS generation. Sci Rep, 9( 1): 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu J, Gao J, Wei QJ. ( 2016). Combination of photodynamic therapy with radiotherapy for cancer treatment. J Nanomater, 2016: 8507924. [Google Scholar]
- 40. Lu C-H, Kuo Y-Y, Lin G-B, et al. ( 2020). Application of non-invasive low-intensity pulsed electric field with thermal cycling-hyperthermia for synergistically enhanced anticancer effect of chlorogenic acid on PANC-1 cells. PLoS One, 15( 1): e0222126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noghreiyan AV, Imanparast A, Ara ES, et al. ( 2020). In-vitro investigation of cold atmospheric plasma induced photodynamic effect by Indocyanine green and Protoporphyrin IX. Photodiagnosis Photodyn Ther, 31: 101822. [DOI] [PubMed] [Google Scholar]