Abstract
Objective:
Endoplasmic reticulum-metallopeptidase 1 (ERMP1) is involved in cellular response to oxidative stress. However, its functional role in proliferation and progression of cancer cells remains unknown. The focus of this study was to investigate the molecular-mechanisms in which ERMP1 modulates the proliferation and progression of colorectal cancer (CRC) cells under normal and environment stress conditions.
Materials and Methods:
In this experimental study, ERMP1 expression was evaluated using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) in CRC cells. ERMP1 was knocked down using lentiviral transduction of ERMP1-specific shRNA into HCT116 cells. ERMP1 was also upregulated using lipofectamine transfection of ERMP1-overexpressing vector into SW48 cells. To evaluate the role of ERMP1 in the cellular and environmental stress conditions, ERMP1-downregulated cells were exposed to stressful conditions including starvation, serum free medium, and treatment with redox or chemotherapy agents for 72 hours. The expression of AKT, p-AKT, phospho-mammalian target of rapamycin (p-mTOR), β-catenin, p-β-catenin, E-cadherin, and Glucose-regulating protein 78 (GRP78) proteins was evaluated by western blotting. The expression of ERMP1, CYCLIN D, and c-MYC was evaluated by RT-qPCR. The cell surface localization of GRP78, cell cycle distribution, and apoptosis were determined by Flow cytometry.
Results:
ERMP1 knock-down reduced the cellular proliferation, inactivated the PI3K/AKT pathway, prompted the G1 arrest, and attenuated the free β-catenin and CYCLIN D expression. Opposite results were obtained in ERMP1- overexpressed cells. Knock-down of ERMP1 also reduced the GRP78 localization at the cell surface. Various environmental stress conditions differently affected the ERMP1-downregulated cells.
Conclusion:
ERMP1 functioned as an oncogene in CRC cells by promoting malignant characteristics. The phosphoinositide 3-kinases (PI3K)/AKT/β-catenin pathway and localization of GRP78 were closely related to the effects of ERMP1. Consequently, ERMP1 might be regarded as a promising target in therapeutic strategies related to CRC.
Keywords: β-catenin, Colorectal Cancer, ERMP1, GRP78, PI3K/AKT
Introduction
Endoplasmic reticulum-metallopeptidase 1 ( ERMP1) is a member of the M28 Zn-peptidase family with nine transmembrane-domains typically incorporated in the endoplasmic reticulum (ER) (1). ERMP1 gene is located at chromosome 9p24 and encodes an approximately 100 kDa protein. It was first recognized as a new, hypothetical gene named Felix-ina, expressed in the granulosa cells of ovarian follicles in rats. Its role in follicular organization was attributed to its proteolytic function on precursor proteins which is essential for intra-ovarian cell to cell communication (2). Primarily, ERMP1 was proposed as a candidate oncogene due to its localization on amplified chromosome in cancers (3). In 2016, it was identified as a chief player in the unfolded protein responses (UPR) and defense against oxidative stress in cancer cells. This identification was based on the findings, showing that ERMP1 expression is extremely affected by thapsigargin-induced ER stress and other oxidative stresses. The overexpression of ERMP1 is verified in multiple cancers including breast, ovary, colon and lung; meanwhile the maximum expression level is attributed to CRC (1).
Colorectal cancer (CRC) is regarded as one of the most common causes of morbidity and mortality from cancer and public health problem worldwide. Although CRC can occur at any age, it is mostly categorized as an old-age disease with the most occurrence in the fifth decade of human life. Unfortunately, it is highly metastatic and resistant to anticancer therapies (4). Despite progression in cancer therapy, survival is less than 5 years ,in most of the patients, which necessitates the exploration of molecular mechanisms and cancer-specific-cellular targets in CRC for novel therapeutic strategies (5). ERMP1 is suggested as a new target for anti-cancer therapies because of its effect on GRP78. Accordingly, ERMP1 mediates the ER stress-promoted activation of GRP78 and loss of ERMP1 suppresses the GRP78 expression (1). GRP78 was initially recognized by glucose deprivation led to the overexpression of this 78 kDa protein in chick embryo fibroblasts; therefore,, it is called glucose-regulated protein 78 (GRP78) (6). It was routinely regarded as an ER luminal protein because of a retention KDEL motif in carboxyl domain supposed to exert its anti-apoptotic effects in cancer just by regulating the activation of UPR. Later, a sub-fraction of this protein was detected at the cell surface of cancer cells as a multifunctional receptor that mediates the signaling pathways, a finding that unwrapped novel mechanisms wherein GRP78 exerts its anti-apoptotic and pro-proliferative effects in cancer cells. GRP78 upregulation is attributed to various stressful parameters such as nutrient deprivation, hypoxia, low pH condition, chemotherapy agents, and radiation (7).
In 2018, miR-148b was defined as a tumor suppressor because of its negative impact on ERMP1 expression in human endometrial cancer. Following the ERMP1 suppression, the proliferation of endometrial cells was suppressed (8). Recently, the correlation between ERMP1 and phosphatidylinositol-3-kinase (PI3K)/AKT pathway was distinguished. This association was ascribed to miR-328-3p suppressing the PI3K/AKT pathway via targeting ERMP1 in hepatocellular carcinoma and reduces the cancer cells proliferation and invasion (9).
The PI3K/AKT signaling pathway is implicated in the cellular proliferation and survival at both physiological and pathological conditions. Because cancer cells are under cellular and environment stresses, the PI3K/AKT pathway plays a critical role in cancer progression. Multiple targets of AKT included in glucose metabolism, protein synthesis, and cell cycle control comprise glycogen synthase kinase-3β (GSK-3β), insulin receptor substrate-1 (IRS-1), mammalian target of rapamycin (mTOR), cyclin-dependent kinase (CDK) inhibitors like p21 and p27, and so on. As to β-catenin, AKT/ GSK-3β/β-catenin transduction pathway activates an axis that positively mediates the cell cycle progression from G1 to S phase via inactivating GSK-3β, upregulating CYCLIN D, and suppressing some transcription factors which ultimately cause p27 depletion (10).
AKT is overactivated in most human cancers that causes the uncontrollable cell growth, metastasis, and angiogenesis of cancer cells by activating the PI3K/ AKT/mTOR pathway (11). The overactivation of proliferative pathways in cancer cells and application of chemotherapeutic agents induce stressful condition in tumor microenvironment (12) known as cellular and environment stresses and considered as a barrier for effective treatments (13).
Considering the important role of the AKT/GSK-3β/β-catenin pathways and GRP78 in cancers, we made an attempt to determine the functional role of ERMP1 in proliferation and progression of CRC cells and related pathways under normal or environment stress conditions.
Materials and Methods
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS. REC.1400.279).
Materials
In this experimental study, RPMI-1640, fetal bovine serum (FBS), and DMEM (Dulbecco’s Modified Eagle Medium) were acquired from Pan biotech (Germany). The pLKO.1, pCMV6-AN-GFP, and pCDNA3.1 (+)-ERMP1 vectors were prepared from Addgene (USA), OriGene (USA), and Biomatik (Canada) companies, respectively. Lipofectamine 2000 were obtained from Thermo Fisher (USA). The ERMP1 and scramble oligo sequences were purchased from metabion (Germany). Super ECL reagent, and BCA assay kit were purchased from Abcam (UK), and Thermo Scientific (USA), respectively. Annexin-V apoptosis detection kit, puromycin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), were obtained from MabTag (Germany), Santa Cruz (USA), and Sigma-Aldrich (USA), respectively. Antibodies for AKT (Cat. #4691), p-AKT. S473 (Cat. #9271), and p-β-Catenin (Cat. #9561) were purchased from cell signaling (USA). Also, antibodies for GAPDH (Cat. #sc-47724), E-Cadherin (Cat. #sc-71009), and β-Catenin (Cat. #sc-7963) were obtained from Santa Cruz Biotechnology (USA). p-mTOR S2448 (Cat. # 610301) antibody from BioLegend (USA), anti-rabbit IgG, and anti-mouse IgG peroxidase-conjugated secondary antibodies were acquired from Sigma-Aldrich (USA), and Alexa-conjugated secondary antibody was also purchased from Razi BioTech (IRAN). The designed shRNA against ERMP1 and scramble shRNA were prepared through Metabion Company (Germany)
Cell culture
Seven human CRC cell lines including HT29, HCT116, LS180, SW1116, SW742, SW480, SW48, and HEK293T cell line, as a human-embryonic-kidney cell line, were obtained from National cell bank of Iran for this experimental study. All cells were cultured in RPMI-1640 medium except LS180 and HEK293T cell lines cultured in DMEM, containing 10% FBS and 5% CO2 at 37˚C humidified incubator. Whenever the cells reached 70- 80% confluency, they were trypsinized and seeded in an appropriate plate for each experiment. Each experiment was performed 3 times independently, and at least the mean of 3 independent assays was represented.
RNA extraction and reverse transcription-quantitative polymerase chain reaction
Total RNA was elicited from the cells using the RNA extraction Kit (SinaClon, Iran), according to the manufacturer’s guidelines. Subsequently, cDNA was synthetized employing cDNA synthesis kit (SinaClon, Iran), according to the kit procedure. The mRNA expression analysis was performed using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) on QuanStudioTM 5 Real-Time PCR system. First, the mRNA expression level of ERMP1 was determined in 7 CRC cell lines (SW48, SW480, SW742, SW1116, LS180, HT29, and HCT116). Then, the cell line with the highest (HCT116) and lowest (SW48) expression of ERMP1 were selected for ERMP1 knock-down and overexpression, respectively. The primers that were used to detect each gene product are listed in Table S1 (See supplementary Online Information at www.celljournal.org). GAPDH was employed as a control gene to normalize all expression data. The relative expression levels (fold changes) were quantified by utilizing the 2-∆∆CT method.
Transduction with shRNA-encoding lentiviruses
Lentiviral-based transduction of short hairpin (sh) RNA was used in stable knock-down of the ERMP1 gene. Initially, ERMP1-specific shRNA oligos (shERMP1) or shRNA against scramble sequence (shscramble) (negative control) were cloned into pLKO.1 vector at Agel and EcoRI restriction sites and, then, amplified by transformation to the competent DH5α E.coli. Lentiviral particles expressing shERMP1 or shscramble were produced in 6-well plate containing HEK293T cells by co-transfecting 2 µg pLKO.1 plasmid (pLKO.1-shERMP1, or PLKO.1- shscramble) plus 0.5 µg enveloping-plasmid (pMD2.G) and 1.5 µg packaging-plasmid (pSPAX2) (which encode the viral capsid and transcriptional machinery).
HCT116 cells were seeded in the 6-well plate (1.0×105 cells/ well). After 24 hours, lentiviruses expressing shERMP1 or shscramble (negative control) were added with 8 μg/ml polybrene. Following transfection, the medium was replaced with fresh and complete medium. Then, puromycin selection with optimal dose (1 μg/ml) was carried out 72 hours post-transduction for two weeks. Finally, the efficacy of ERMP1 knock-down was assessed by RT-qPCR.
The upregulation of ERMP1 gene using overexpression vector
SW48 cells were seeded in 24-well plates to reach 70- 80% confluency on the day of transfection. After replacing the medium, transfection mixture was added to the cells (2.5 µl of Lipofectamine-2000 for each µg of pCDNA3.1 (+)-ERMP1, pCDNA3.1 or pCMV6-AN-GFP) and incubated for 4 hours at 37°C with 5% CO2 . To reduce cytotoxicity, we replaced the transfection medium with fresh one, and the cells were incubated for 48 hours. The efficiency of transfection was assessed by fluorescence microscopy using the pCMV6-AN-GFP vector as a control. Finally, the cells were collected for next steps.
MTT proliferation assay
Cells were plated in 96-well in a medium containing 10% FBS with about 5,000 and 7,000 cell/well for HCT116 and SW48, respectively. Seventy two hours after treatment or 48 hours post-transfection, 20 μl of MTT solution (5 mg/ ml) was added to each well and incubated for 4 hours at 37°C to quantify the cell viability. After 4 hours, the medium was removed and the resulting MTT formazans solubilized in 100 μl of dimethyl sulfoxide (DMSO) to check each solution spectrophotometrically at 570 nm.
Cell treatment
The ERMP1 stably knocked down HCT116 cell line or HCT116 scramble cell line were seeded in appropriate plates in 5 or 6 groups. The next day, the medium was changed in all groups. In the first group, the cells were exposed to the normal condition (medium was changed whenever it was necessary). The second group was exposed to the starvation condition (medium was not changed for 72 hours to induce starvation). The remaining groups were also exposed to the serum free medium (0.25% FBS) and medium containing Dithiothreitol [(DTT) 10 µM)], 5-Fluorouracil [(5-FU) 8 µM], and cisplatin (6 µM) for 72 hours, respectively.
Colony forming assay
The cells (200 cell/well) were seeded in 6-well plate and cultured for 2-3 weeks at 37°C in a 5% CO2 incubator. The medium was replaced every 7 days. After the colonies became visible (50 cell/colony), 95% methanol was used to fix them. Then, the colonies were stained with crystal violet followed by PBS washing to be viewed and counted by microscope (×40 magnification). Afterwards, 10% acetic acid was used to dissolve the crystal violet for quantification of the stained colonies. The solutions were added to a 96-well plate to determine the absorbance at 590 nm using the spectrophotometer (14).
Immunoblotting analysis
Western Blot assay was performed for protein analysis. Briefly, the cells were harvested and lysed to be centrifuged for 8 minutes with 10.000 g at 4˚C. The supernatant was ready to be used for loading on dodecyl sulfate (SDS)- polyacrylamide gel. Therefore, proteins were separated based on their molecular weight by electrophoresis in an SDS-polyacrylamide gel under denaturing conditions; then, they transferred to a nitrocellulose membrane using the wet transfer apparatus at 100 V or 400 mA. After blockage of nitrocellulose membrane with PBS-T + 5% skim milk at RT, proteins were ready for incubation with primary antibodies (anti-AKT, p-AKT, GAPDH, E-Cadherin, β-catenin, p-β-catenin and p-mTOR) for overnight with agitation. The membrane was then incubated with relative secondary antibody at RT. Finally, proteins were identified through a chemiluminescence imaging system (Bio-Rad Chemidoc XRS, USA).
Evaluation of the cell cycle by Flow Cytometry
The respective cells were cultured in 6-well plates, and after being detached by 0.25% Trypsin-EDTA solution on the day of experiment, the cells centrifuged at 1500 g for 5 minutes at 4°C to be washed in PBS and re-suspended in a hypotonic propidium iodide (PI) lysis buffer (0.1% Triton X-100, 1% sodium citrate, 0.5 µg/ml RNase A, 40 analyzed by flow cytometry (BD FACS Calibrator, Biosiences Company, USA).
Evaluation of the apoptosis by Flow Cytometry
Cells were cultured in 6-well plates and on the day of experiment, they were detached, washed with PBS, and re-suspended in 500 μl of annexin V binding buffer. Then, 5 μl of annexin V conjugate and 5 μl of PI solution were added to the samples to be incubated in the dark for 20 minutes. After centrifuging at 400 g for 5 minutes and re-suspending in 200 μl annexin V binding buffer, we analyzed each sample by flow cytometry.
Detection of the cell surface GRP78
The cells were harvested and re-suspended in ice cold PBS. Primary antibody (10 μg/ml) was added to 100 μl of the cell suspension and incubated for at least 30 minutes at 4°C. Then, the cells were washed 3 times with PBS and centrifuged for 5 minutes at 400 g. Alexa-conjugated secondary antibody dilution was added, and the cells were re-suspended in this solution to be incubated in dark for 20-30 minutes at 4°C. After being washed 3 times with PBS and centrifugation, the cells were ready to be analyzed with flow cytometry.
Statistical analysis
All data are reported as means ± SD from at least 3 independent assays. Statistical data analysis was conducted by using the GraphPad Prism 7.0 for Windows (GraphPad Software, Inc, USA). T test and the 1- or 2-way ANOVA tests were used for comparison of two and multiple groups, respectively. * P<0.05, ** P<0.01, *** P<0.001 and **** P<0.0001 were considered as statistically significant.
Results
ERMP1 is variously expressed in colorectal cancer cells
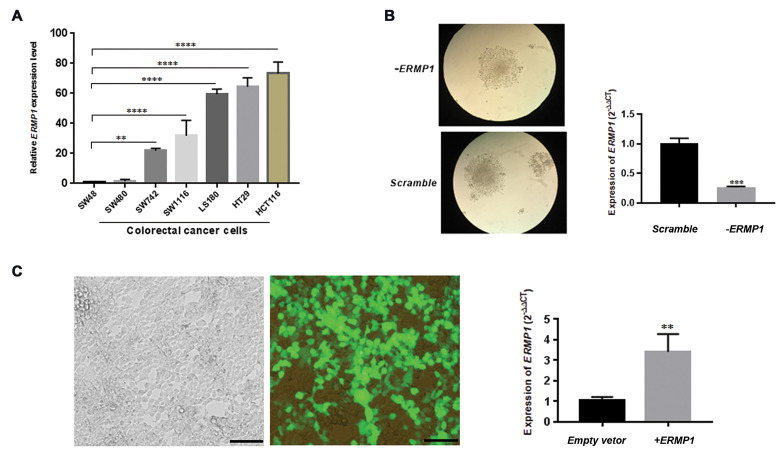
The total RNA was elicited from 7 CRC cell lines to assess the expression level of ERMP1 by RT- qPCR (Fig .1A). We found a relatively high expression level of ERMP1 in HCT116 cells P<0.0001 and a low expression in SW48 cells. Accordingly, to explore the biological function of ERMP1 in CRC, we selected HCT116 and SW48 cell lines to down- and up-regulate ERMP1, respectively (Fig .1B, C).
Fig.1.
ERMP1 relative expressions are normalized to GAPDH and determined by RT-qPCR. A. ERMP1 expression level in 7 CRC cell lines (SW48, SW480, SW742, SW1116, LS180, HT29 and HCT116), SW48 is applied as a reference set to 1.0, which exhibited the lowest mRNA expression level. B. The RT-qPCR results of the ERMP1 gene in puromycin-resistant colonies of HCT116 transduced cells with ERMP1 and scramble shRNAs, The ERMP1 expression was significantly inhibited in HCT116 cells following transduction with lentivirus expressing ERMP1 shRNA, compared with HCT116 cells transduced with scramble lentivirus (P=0.0002). C. Transfection efficiency in SW48 cells is determined using pCMV6-AN-GFP vector as a control and RT-qPCR, which showed that pCDNA3.1 (+)-ERMP1 vector transfection obviously enhanced ERMP1 expression in SW48 cells (P=0.0095) (scale bar: 200 µm). Bars represent the mean ± SD. **; P<0.01, ***; P<0.001, ****; P<0.0001 vs. scramble control or empty vector. One-way ANOVA A. Multiple comparison and B, C. Unpaired t tests were utilized. RT-qPCR; Reverse transcription-quantitative polymerase chain reaction and CRC; Colorectal cancer.
Transduction of cells through lentiviral-based shRNA approach provided a quick and stable method to successfully diminish the ERMP1 expression (Fig .1B).
To overexpress ERMP1 gene, we designed and ordered pCDNA3.1 (+)- ERMP1 vector. Later, pCDNA3.1 (+)-ERMP1 vector was transformed into the E.coli and amplified. Then, it was extracted and confirmed via digestion with PmeI restriction enzyme. SW48 cells were transiently transfected with pCDNA3.1 (+)-ERMP1 and empty vector (pCDNA3.1). The transfection efficiency was checked 48 hours later as illustrated in Figure 1C.
ERMP1 mediates the proliferation of colorectal cancer cells
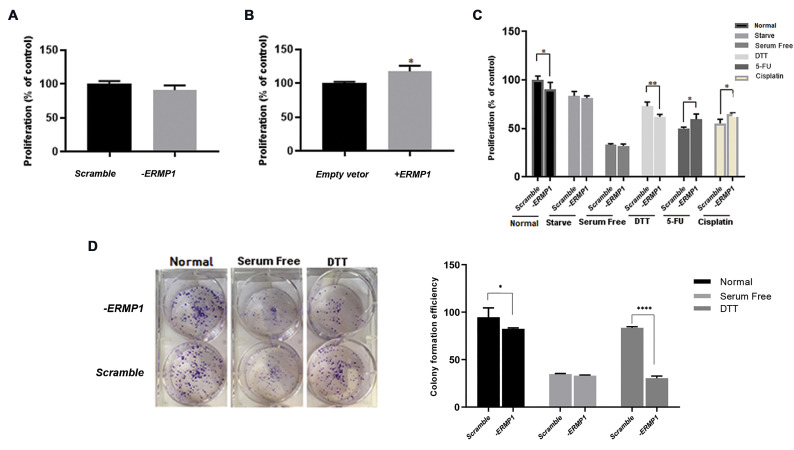
ERMP1 has a remarkable expression in various cancer types including CRC cancer prompted us to verify the cancer-related function of ERMP1. As shown in Figure 2A, MTT assay was conducted to evaluate the proliferation of ERMP1 shRNA-mediated knock-down HCT116 cells (-ERMP1 cells) compared to HCT116 control cells (transduced with scramble shRNA). The results of the proliferation assay in the SW48 cells transfected with pCDNA3.1 (+)-ERMP1 vector (+ERMP1 cells) compared to the SW48 control cells (transfected with empty vector) are also shown in Figure 2B. The current results indicated that ERMP1 overexpression promoted the proliferation rate in SW48 representing the probable role of the ERMP1 in proliferation pathways of CRC cells.
Fig.2.
The MTT and colony formation assays for proliferation analysis. A. Knock-down of ERMP1 non-significantly reduced the proliferation rate in HCT116 cells compared with scramble ones. B. Overexpression of ERMP1 significantly increased the proliferation rate in SW48 cells compared to the control empty vector ones (P=0.02). C. Exposure to various stressful conditions as a model of environmental stress, such as Dithiothreitol (DTT), 5-FU and cisplatin, differently affected the proliferation rate of -ERMP1 cells. D. Colony formation ability was reduced considerably by ERMP1 knock-down (P=0.03) and DTT treatment (P<0.0001). Data are reported as means ± SD. *; P<0.05, **; P<0.01, and ****; P<0.0001 compared with each scramble control or empty vector. A, B. Unpaired t test and C, D. two-way ANOVA multiple comparison tests were used.
As shown in Figure 2C, down-regulation of ERMP1 along with the DTT treatment, obviously (P=0.009) reduced the proliferation rate in -ERMP1 cells compared to the scramble control. Interestingly, 5-FU and cisplatin treatment elevated the proliferation rate in -ERMP1 cells compared to the scramble ones (P=0.03).
Additionally, as shown in Figure 2D, the colony formation assay was performed in the -ERMP1 cells to further determine the ERMP1 effects in CRC cells.
ERMP1 facilitates the AKT expression and subsequently promotes the PI3K/AKT pathway in colorectal cancer cells
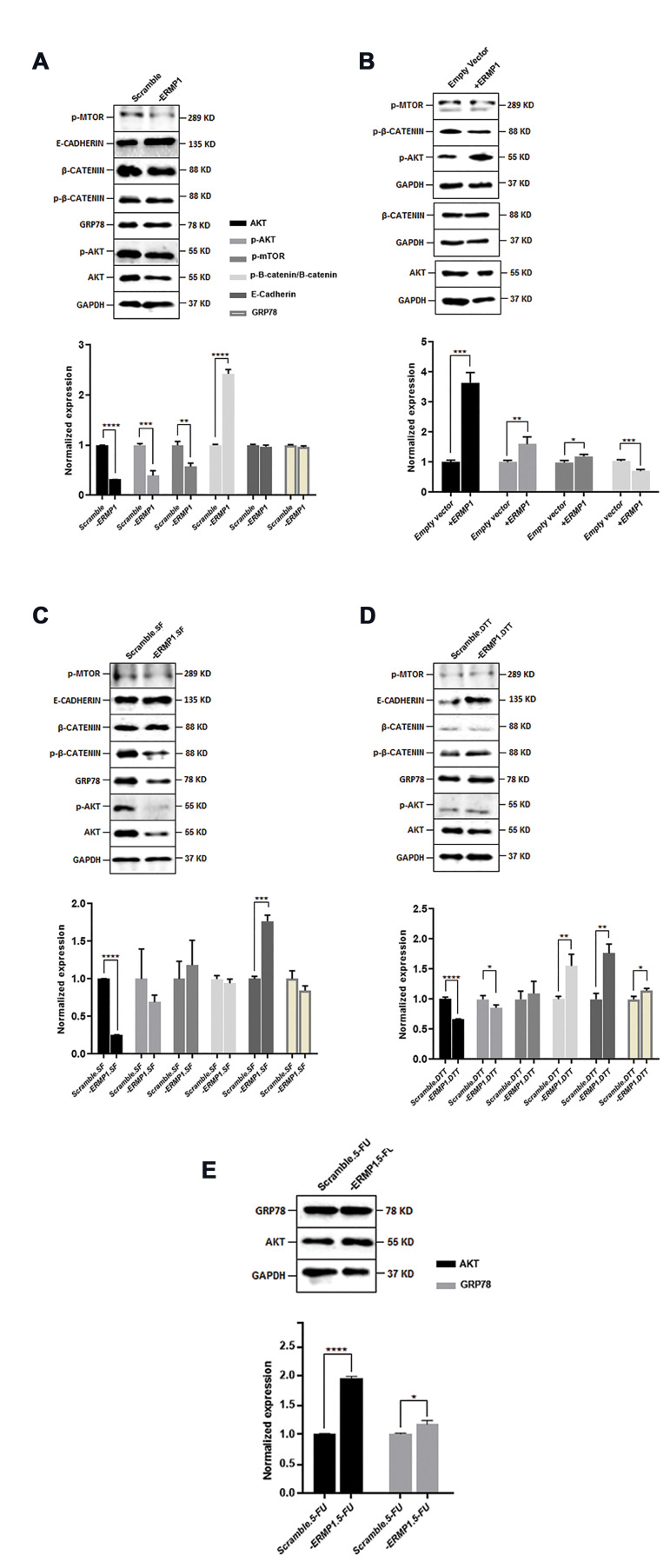
The PI3K/AKT pathway, as a crucial intracellular signal transduction pathway, contributes to survival, proliferation, and invasion of cancer cells in response to extracellular stimuli via mediating phosphorylation cascade. Therefore, the PI3K/AKT pathway was evaluated in -ERMP1 and +ERMP1 CRC cells. As illustrated in Figure S1A (See supplementary Online Information at www.celljournal.org) and Figure 3A, ERMP1 knock-down in HCT116 cells considerably reduced the expression ofAKTat both mRNA(P=0.007) and protein (P<0.0001) levels compared to scramble ones under normal condition. Moreover, the p-AKT protein level was also checked to distinguish; whether ERMP1 down-regulation affected the phosphorylation of AKT. Related results showed that the p-AKT level in -ERMP1 cells was decreased (P=0.0005).
In SW48 cells, it was demonstrated that ERMP1 overexpression led to an obvious increase in AKT expression at both mRNA (P=0.002) and protein (P=0.0002) level, compared to empty vector (Fig .S1B, See supplementary Online Information at www.celljournal.org, Fig .3B). In addition, p-AKT was also enhanced by ERMP1 overexpression (P=0.004). These results demonstrated that ERMP1 regulated the PI3K/AKT pathway by affecting the expression and phosphorylation of AKT in CRC cells.
Fig.3.
Evaluation of the ERMP1 effect on multiple proteins using Western blotting experiment. A. ERMP1 knock-down effects on PI3K/AKT/β-catenin pathway proteins as well as E-cadherin and GRP78. B. ERMP1 overexpression effects on PI3K/AKT/β-catenin pathway proteins. C. The impact of serum free stress on PI3K/AKT/β-catenin pathway proteins as well as E-cadherin and GRP78 in -ERMP1 cells. D. The effect of Dithiothreitol (DTT) exposure on PI3K/AKT/β-catenin pathway proteins as well as E-cadherin and GRP78 in -ERMP1 cells. E. AKT and GRP78 protein levels in 5-FU treated -ERMP1 cells. All details are explained in results. Data are presented as the mean ± SD. *; P<0.05, **; P<0.01, ***; P<0.001, and ****; P<0.0001 vs. each scramble control or empty vector, and unpaired t test is applied.
Interestingly, a reduction in AKT expression was also observed in -ERMP1 cells, which were exposed to serum free, and DTT treatment circumstances compared to their control scramble cells (Fig .S1C, D, See supplementary Online Information at www.celljournal.org, Fig .3C, D). Since the results of starvation with serum free conditions were in the same line, we continued our experiments with one of them (serum free).
The p-AKT protein level was also checked, and the results were different in each group. As displayed in Figure 3C, D, p-AKT was reduced in DTT-exposed -ERMP1 cells (P=0.03). These data illustrate that ERMP1 knock-down and DTT exert synergistic effects on AKT and p-AKT expression in CRC cells.
The AKT mRNA expression was measured in 5-FU and cisplatin exposed -ERMP cells, which showed an obvious increase in AKT expression at both mRNA (P=0.004) and protein (P<0.0001) levels compared to scramble treated cells for 5-FU-treated cells (Fig .S1E, See supplementary Online Information at www.celljournal.org, and Fig.3E) and at mRNA level for cisplatin-treated cells (P=0.003) (Fig .S1D, See supplementary Online Information at www.celljournal.org). Consequently, our results showed that ERMP1 promoted the expression of AKT and its phosphorylation, however this effect can alter subject to different circumstances in tumor microenvironment.
Additionally, we assessed the p-mTOR expression to determine whether the accelerating effects of ERMP1 on PI3K/AKT pathway can influence p-mTOR expression as downstream target of AKT.
Additionally, we assessed the p-mTOR expression to determine whether the accelerating effects of ERMP1 on PI3K/AKT pathway can influence p-mTOR expression as downstream target of AKT.
As displayed in Figure 3A, the p-mTOR protein expression level was reduced remarkably by ERMP1 knock-down compared with the scramble control (P=0.002); however, by serum free and DTT exposure, p-mTOR expression did not modify in -ERMP1 cells (Fig .3C, D).
In SW48 cells, overexpression of ERMP1 obviously increased (P=0.01) the p-mTOR expression compared with empty vector control (Fig .3B). These results illustrate that ERMP1 facilitates the expression of p-mTOR. Moreover, in the field of p-mTOR, DTT did not exhibit synergistic effect with ERMP1 knock-down; conversely, DTT inhibited the effects of ERMP1 knock-down.
ERMP1 induces the stability of β-catenin by facilitating AKT expression in colorectal cancer cells
Since phosphorylated AKT has the potency to regulate the function of multiple substrates involved in cellular survival such as GSK-3β becoming inactivated by p-AKT to trigger the stabilization of β-catenin for nucleus translocation, free β-catenin was measured in this study by western blotting to further investigate the ERMP1 effects on CRC cell lines. The results revealed that compared with the scramble control, the p-β-catenin/β-catenin relative ratio was significantly increased (P<0.0001) in -ERMP1 cells (Fig .3A) indicating a reduction in free β-catenin level by downregulation of ERMP1 in normal condition. In ERMP1 overexpressed cells, the p-β-catenin/β-catenin relative ratio was reduced (P=0.0008) in +ERMP1 cells (Fig .3B). Therefore, it appears that ERMP1 accounts for AKT-mediated GSK-3β/β-catenin pathway through stabilizing free β-catenin.
Thereafter, we examined the p-β-catenin/β-catenin relative ratio in -ERMP1 cells under serum free, and DTT treatment and a significant enhancement (P=0.008) was found in DTT-exposed cells (Fig .3C, D).
Stabilized β-catenin can translocate to the nucleus and incorporate with lymphoid enhancer factor/T cell factor (Lef/ Tcf) co-transcription factors to trigger transcription of target genes such as C-MYC and the CYCLIN D, which compromise in survival, proliferation and invasion of cancer cells. Hence, we evaluated C-MYC and the CYCLIN D expression at mRNA level to verify the possible role of ERMP1 in regulation of the transcriptional activity of β-catenin. However, the C-MYC expression did not show any change in -ERMP1 cells with an exception for DTT-exposed cells, in which C-MYC expression was reduced (P=0.0004) compared to the related control (Fig .S1A-D, See supplementary Online Information at www.celljournal.org). In addition, we assessed the C-MYC expression in 5-FU and cisplatin-treated -ERMP1 cells show an enhancement in the proliferation rate compared with the related controls. Relative results indicated that C-MYC expression was upregulated by 5-FU (P<0.0001) and cisplatin (P=0.002) treatment in -ERMP1 cells compared to scramble cells (Fig .S1E, F, See supplementary Online Information at www.celljournal.org).
As revealed in Figure S1A, B (See supplementary Online Information at www.celljournal.org) a lower expression level of CYCLIN D (P=0.002) was detected in -ERMP1 cells; however, in SW48 cells, CYCLIN D expression exhibited an obvious upregulation by ERMP1 overexpression. It was revealed that CYCLIN D expression was affected by downstream impact of ERMP1 on free β-catenin, unlike C-MYC. Because the outcomes in -ERMP1 an +ERMP1 cells were opposite, the next procedures were just carried out with -ERMP1 cells to overcome the limitations.
ERMP1 knock-down under serum free and DTT conditions enhances the E-cadherin expression in HCT116 cells
Phosphorylated AKT is implicated in downregulation of E-cadherin expression at mRNA level; thus, the E-cadherin expression was evaluated with western blotting to examine whether E-cadherin contributes to ERMP1 mediated β-catenin stabilization. As displayed in Figure 3A, E-cadherin protein expression was not changed through ERMP1 downregulation in HCT116 cells.
To further verify the ERMP1 correlation with E-cadherin, we also measured the expression of E-cadherin in -ERMP1 cells which were exposed to serum free and DTT (Fig.3C, D). The results revealed that serum free condition (P=0.0001) or DTT treatment (P=0.001), upregulated the E-cadherin expression in -ERMP1 cells compared to their scramble controls. These data may reflect the possibility that ERMP1 knock-down is not the solitary agent in downregulation of E-cadherin expression and ERMP1 silencing under stressful condition such as serum free and DTT treatment that can compact the membrane-bounded E-cadherin/β-catenin complex and inhibit a relocation of β-catenin into the nucleus, where β-catenin activates the transcription of the genes associated with enhancing cellular proliferation and progression in CRC cells.
ERMP1 knock-down reduces the GRP78 localization on the HCT116 cell surface
It is believed that ERMP1 has a role in upstream or within UPR and GRP78 overexpression. Therefore, we measured the GRP78 protein level in -ERMP1 cells. Based on our results, the expression of GRP78 at protein level was not changed by ERMP1 downregulation (Fig .3A). However, DTT treatment (P=0.04) and 5-FU exposition (P=0.01) led to the GRP78 overexpression in -ERMP1 cells compared to each scramble controls (Fig .3D, E).
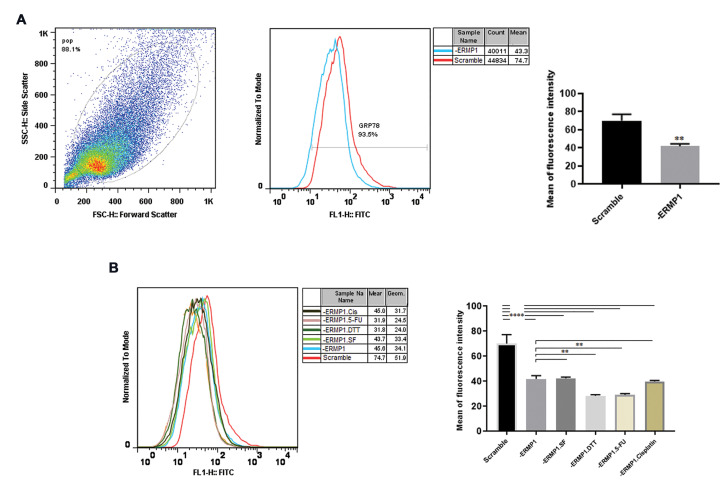
Because the biological effect of ERMP1 on GRP78 and UPRs pathways was not exactly determined, we aimed to elucidate the possible role of ERMP1 in localization of GRP78. The result showed that ERMP1 knock-down diminished (P=0.002) the localization of GRP78 at the cell surface (Fig .4A). GRP78 aggregates at the cell surface, initiates the multiple signals and, thereby, regulates the cell malignancy. Subsequently, to investigate; whether enhancement in the GRP78 protein level under DTT and 5-FU treatment increases the localization of GRP78 at the cell surface, we assessed csGRP78 under stressful circumstances in -ERMP1 cells. As revealed in Figure 4B, exposure to DTT (P=0.002) and 5-FU (P=0.003), not only substantially they did not enhance csGRP78, but also reduced its cell surface content compared with normal condition, which may suggest GRP78 has been trapped in ER lumen to alleviate ER stress. However, cisplatin treatment did not change csGRP78 compared with -ERMP1 cells in normal condition.
Fig.4.
Effect of ERMP1 knock-down on cell surface localization of GRP78 using Flowcytometry assay. A. Knock-down of ERMP1 significantly reduced the transportation of GRP78 on the cell surface (P<0.01). B. Following exposure to starvation, Dithiothreitol (DTT) and 5-FU, csGRP78 was decreased in -ERMP1 cells. Values are stated as the mean ± SD. **; P<0.01, and ****; P<0.0001 vs. each scramble control. A. Unpaired t test and B. One-way ANOVA multiple comparison tests were employed.
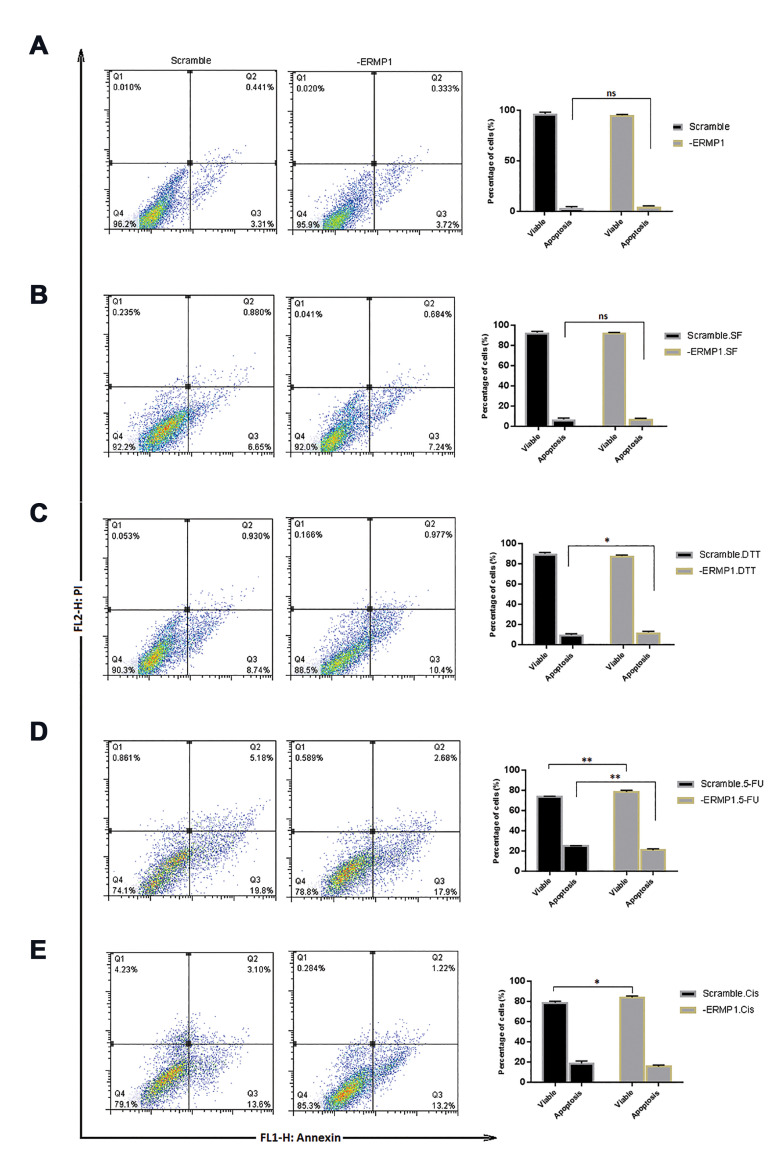
Knock-down of ERMP1 promotes apoptosis with Dithiothreitol treatment
MTT results showed that ERMP1 overexpression induced CRC cells proliferation, and its knock-down reduced the proliferation. In addition, we exposed -ERMP1 cells under stressful and DTT, 5-FU, and cisplatin conditions to identify the role of ERMP1 on proliferation under these circumstances. We found that, in response to tumor microenvironment, ERMP1 differently manipulated the cellular proliferation, suggesting that ERMP1 can differentially act in tumor microenvironment (its function in some conditions may be compensated with other proteins). Meanwhile, we examined cellular apoptosis assay to evaluate the effect of ERMP1 downregulation on apoptosis of -ERMP1 cells in normal condition and presence of environment stresses. The Figure 5 displays live, early/ late apoptotic and necrotic cells in the quadrants of flow cytometry (left part) and the comparison of total apoptotic and live cells in the curves (right part). According to the results, there was not any difference in apoptosis of -ERMP1 cells with its scramble control cells (Fig .5A). In the presence of stressful circumstances, just DTT exposure (P=0.04) triggered apoptosis in -ERMP1 cells, suggesting that ERMP1 is not the only factor in promotion of apoptosis in CRC cells. These results show that, in the absence of ERMP1, exposure to DTT induce apoptosis in HCT116 cells.
Fig.5.
Cellular apoptosis assay using flow cytometry analysis in -ERMP1 cells. A. Knock down of ERMP1 didn’t induced apoptosis in HCT116 cells. B. Serum free condition didn’t affect the apoptosis in -ERMP1 cells. C. DTT treatment induced apoptosis in -ERMP1 cells. D-E. 5-FU and cisplatin treatment reduced apoptosis in -ERMP1 cells. Quadrants represents four principal population: Q1; Necrotic, Q2; Late apoptotic, Q3; Early apoptotic, and Q4; Live cells. Each value is stated as the mean ± SD. *; P<0.05, **; P<0.01 vs. each scramble control and two-way ANOVA multiple comparison were applied, SF; Serum free, DTT; Dithiothreitol, CIS; Cisplatin, and Ns; Non-significant.
Analysis of cell apoptosis result of 5-FU treated -ERMP1 cells, revealed a reduction (P=0.001) in the percentage of apoptotic cells compared with that of scramble control, which suggests that ERMP1 downregulation alleviates the desired effects of 5-FU in CRC cells.
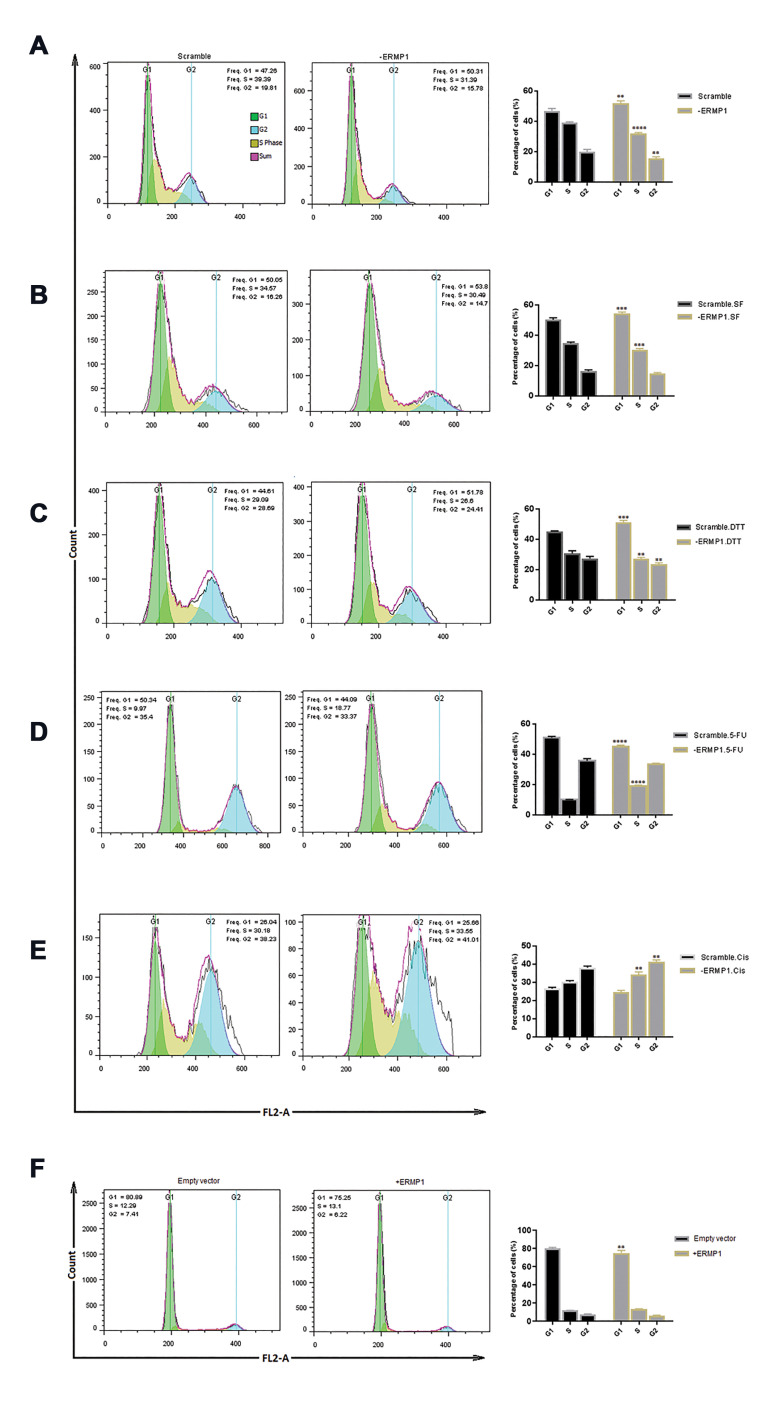
ERMP1 knock-down manipulates the cell cycle by promoting the G1 arrest
As cellular apoptotic results revealed that reduction in the proliferation rate of -ERMP1 cells was not the consequence of apoptosis, we assumed that ERMP1 exerts its effects on cell cycle progression. Thus, -ERMP1 cells were evaluated by flow cytometry to verify the cells distribution in various phases of the cell cycle. As illustrated in Figure 6A, -ERMP1 cells showed an obvious reduction in S phase (P<0.0001) of cell distribution as well as G2 (P=0.002) and a remarkable increase in G1 distribution (P=0.001) was also identified in starved -ERMP1 cells with lower/miner intense (Fig .6B). When -ERMP1 cells were under serum free circumstance, cells distribution in S phase decreased (P=0.004), which in turn accumulated the cells in G1 (P=0.0007) (Fig. 6C). Following DTT treatment for 72 hours, S phase of cell cycle reduced (0.008) which led to a drop in G2 (P=0.004), and then blocked the cells in the G1 phase (P=0.0001) compared with its scramble control (Fig .6D). These results suggested that ERMP1 downregulation induced cell cycle arrest in G1. Accumulation of cells in G1 phase could probably be correlated to CYCLIN D expression downregulated with ERMP1 knock-down, and all of these modifications ultimately; led to a decline in cellular proliferation.
Fig.6.
Cell cycle progression assay of -ERMP1 CRC cells using flow cytometry analysis. A-C. Results showed that the distribution of cells in S phase significantly decreased by knock-down of ERMP1 under normal condition or serum free and DTT exposure as compared with each scramble controls. D, E. However, following 5-FU and cisplatin treatment, the number of cells in S phase expanded compared with their scramble cells. F. Via ERMP1 overexpression, cell distribution in G1 has remarkably reduced without an obvious rise in S phase. Values are defined as the mean ± SD. **; P<0.01, ***; P<0.001, ****; P<0.0001 vs. each scramble control. Two-way ANOVA multiple comparison was used, SF; Serum free, DTT; Dithiothreitol, and CIS; Cisplatin.
In order to explore the cause of enhancement in the proliferation rate of 5-FU and cisplatin-treated -ERMP1 cells, we measured modifications in the cell cycle of these -ERMP1- treated cells. The results revealed that exposure to 5-FU and cisplatin for 72 hours elevated S phase distribution by reducing the cell numbers in G1 following 5-FU treatment (P<0.0001), compared with scramble control (Fig .6E, F).
In SW48 cells, overexpression of ERMP1 noticeably reduced the cell number in the G1 phase (P=0.0013). Therefore, it seems that ERMP1 contributes to cell cycle progression.
Taken together, these data demonstrate that -ERMP1- mediated proliferation suppression is due to cell cycle intervention.
Discussion
Knock-down and overexpression of ERMP1 in CRC cells revealed that ERMP1 could possess a role in proliferation of CRC cells under normal, stressful, and therapeutic condition detectable in tumor microenvironment because of high proliferation rate of cancer cells and therapy goals. In the same line with our study, a considerable loss (2- fold reduction) in cellular proliferation of MCF7 and SK-BR-3 cells has been observed subsequent to transfection with ERMP1 siRNAs (1). This loss in proliferation of breast cancer cells has been attributed to the ERMP1 function in UPR and oxidative stress. In another study, Qu et al. (8) indicated that miR-148b repressed the cellular proliferation and oxidative stress responses in human endometrial cancer RL95-2 cells by targeting ERMP1. In a recent observation, it has also been found that miR-328- 3p suppresses the proliferation and colony formation rate of Huh-7 hepatocellular carcinoma cells through binding to the 3ˊ-UTR end of the ERMP1 gene (9).
Cancer cells in tumor microenvironment are exposed to stressful conditions due to inner (high proliferation rate and nutrient deficiency) (15) or environment (therapy goals) factors (16). Accordingly, ERMP1 differently manipulated the cellular proliferation in response to stressful conditions and chemotherapeutic agents, suggesting that ERMP1 can possess dual effects in response to tumor microenvironment.
DTT, as a redox agent, exacerbated the ERMP1 knock-down effects, suggesting DTT as an efficient therapy in ERMP1 knock-down CRC cells. Our finding is in the same line with studies reported DTT as an inducer of cell death in multiple cancer cells (17-19).
It was also found that the effect of ERMP1 knock-down in HCT116 cells became reversed with 5-FU/cisplatin treatment, which suggests that more attention should be paid in deciding which drug or therapeutic manipulation is likely to function in ERMP1 knock-down CRCs. Besides, knock-down of ERMP1 can be considered as a stress accelerating resistance to anti-cancer drugs, as it has previously been declared that tumor microenvironment stress alleviates drug efficiency via modulating molecular-and biochemical-mechanisms (13). However, more studies are needed to explore the underlying molecular mechanisms. In agreement with our outcomes, it has been observed that applying drug treatment, with the aim of enhancing tumor microenvironment stress, increases the intratumor heterogeneity and contributes to chemotherapy resistance (20). Additionally, the consequence of exposition to harsh tumor microenvironment is proposed to develop invasive and aggressive features of cancer cells in an in-silico study (21). In accordance with our study, some preclinical evidence has also revealed that some anti-angiogenic drugs stimulate cancer metastasis and aggressiveness (22, 23).
The molecular mechanisms of ERMP1 function, as an extremely expressed protein in CRC was elucidated. Although studies documented that ERMP1 was implicated in UPR and oxidative stress responses of cancer cells, whether ERMP1 exerts its functional effects via PI3K/ AKT/mTOR pathway that has not yet been studied. Our results indicated that one of the unidentified mechanisms through which ERMP1 elicits its proliferative and progressive effects on CRC cells, is PI3K/AKT pathway. ERMP1 facilitated the AKT expression at both mRNA and protein level that subsequently promoted the PI3K/AKT pathway in CRC cells by enhancing the phosphorylation of AKT, which in turn augmented the phosphorylation of mTOR as well. PI3K/AKT/mTOR pathway has a significant role in modulating the survival, proliferation, progression, and invasion of CRC cells (24). AKT, as a key regulator of this pathway controls the function of multiple implicated in mediating the cell growth or proliferation, and cell cycle progression or apoptosis (11). Therefore, our results for the first time revealed that ERMP1 exerted its proliferative and colony forming impacts on CRC cells through PI3K/AKT pathway. In agreement with our study, Lu et al. (9) reported that the miR-328-3p overexpression suppressed the AKT phosphorylation by targeting ERMP1 and reduced the p-mTOR/mTOR ratio. Consequently, it decreases the malignant proliferation and invasion in liver cancer.
Phosphorylated AKT can in turn drive epithelial-mesenchymal transition (EMT) and reduce the transcription of E-cadherin that would impair cell adhesion and alleviate cellular motility and invasion in cancer cells (25). It has been revealed that activation of AKT with Contactin-1 in lung cancer diminishes E-cadherin expression (26). Consequently, we assessed the expression of E-cadherin and free β-catenin at protein level and identified that knock-down of ERMP1 remarkably attenuated the free β-catenin level without affecting the level of E-cadherin; however, exposition of these cells to serum free and DTT obviously increased the E-cadherin level and reduced free β-catenin. Moreover, ERMP1 overexpression obviously stabilized β-catenin to be free for activating the AKT-mediated GSK-3β/β-catenin pathway, and then prompted the transcription of CYCLIN D, which is demonstrated to cooperate in survival, proliferation, and invasion of cancer cells (27). Our results verified that the CYCLIN D expression was enhanced in +ERMP1 cells; however, it was reduced in -ERMP1 cells. The CYCLIN D down-regulation led to the cell cycle arrest of -ERMP1 cells at G1 phase. One mechanism in which ERMP1 can enhance the free β-catenin is through AKT phosphorylation. The p-AKT phosphorylates and inactivates GSK-3β, which causes a reduction in β-catenin degradation and eventually leads to the activation of Wnt/β-catenin signaling that ultimately trigger the CYCLIN D promoter activity (28). The role of CYCLIN D in G1 arrest has been illustrated in an observation that revealed adiponectin reduced the transcription of CYCLIN D via restraining AKT-induced phosphorylation of GSK-3β and attenuating free β-catenin in breast cancer cells (29). In CRC, it has been verified that peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), as a regulator of mitochondrial function, modulates SW620 and SW480 cells proliferation and invasion through AKT/GSK-3β/β-catenin pathway (30). In addition, another study has reported that CYCLIN D, as a downstream target of activated AKT/GSK-3β/β-catenin signaling, is induced by β-catenin as a consequence of PIK3CD overexpression in CRC cells in a way that, via blockage of AKT signaling or depletion of β-catenin, PIK3CD-regulated cell growth, and invasion becomes reversed (31).
As p-AKT has the potency to regulate the function of multiple substrates involved in cellular survival such as GSK-3β becomes inactivated by p-AKT to trigger the stabilization of β-catenin for nucleus translocation (32) and it was revealed for the first time in this study that downregulation of ERMP1 could reduce intracellular accumulation and nuclear activity of β-catenin, which then decreased the expression of CYCLIN D and elevated the G1 arrest. It appears that AKT is a determining marker in mediating ERMP1 effects, and G1 is the dominant phase in CRC cell cycle affected by ERMP1.
In response to ERMP1 knock-down, the protein level of E-cadherin did not change in normal condition and this may reflect the possibility that ERMP1 is not the solitary agent implicated in regulating E-cadherin expression; this is in accordance with the concept that various factors can be involved in inhibition of E-cadherin (26, 33) as applying starvation or exposure to serum free and DTT circumstances in our study reduced the expression of E-cadherin. Since E-cadherin and β-catenin cohesion, regularly found in the adherent junction, suppresses the release of β-catenin to nucleus and stabilizes the complex formation for cellular adhesion with restoring β-catenin membrane bounded, these results indicate that ERMP1 reduces the complex formation between E-cadherin and β-catenin that further facilitates the release of β-catenin to act as a transcription factor. Therefore, co-exerting serum free or DTT conditions following ERMP1 knock-down could inhibit the AKT-mediated EMT through restoring E-cadherin expression in CRC cell. Research on oral squamous carcinoma cells has revealed that AKT inhibition could bring back the E-cadherin expression, and thereby the mesenchymal-to-epithelial reverting transition (34).
According to our data, knock-down of ERMP1 did not promote apoptosis, possibly due to inability in affecting C-MYC expression with an exception to DTT-treated cells which further caused cell apoptosis and reduced C-MYC expression in ERMP1 downregulated cells. C-MYC promotes the progression through cell cycle phases not only by inducing cell-cycle regulators such as cyclins and cyclin-dependent kinases, but also via repressing the activity of a set of cell cycle inhibitors (35). It has been demonstrated that C-MYC expression is regulated through not only Wnt/β-catenin and PI3K/AKT/GSK-3 pathways, but also many other important signaling pathways (36); possibly because of that result, ERMP1 could not influence C-MYC expression, and ERMP1 was not the only factor to induce apoptosis in CRC cells.
All together, we provided evidence that ERMP1 through activating its downstream signaling could possibly modulate the G1 phase progression. G1 arrest found by ERMP1 downregulation may be attributed to the reduction of CYCLIN D expression, attained in -ERMP1 cells. Since G1 phase and G1 to S phase transition in cell cycle specifically regulates the cellular proliferation and differentiation, the proliferation of CRC cells was affected by ERMP1.
Our data also showed that GRP78 expression was not affected by ERMP1 knock-down, whereas reduction in csGRP78 was considerably explored in -ERMP1 cells. Since ERMP1 is an ER membrane protein (1), GRP78 has probably become trapped in ER membrane to initiate ER stress through ERMP1 downregulation. Nonetheless, in -ERMP1 cells co-existing with starvation, DTT and 5-FU circumstances, both expression and cell surface localization of GRP78 were decreased. The results of DTT are consistent with evidence documented that DTT by reducing GRP78 expression prompted ER stress-promoted HeLa cells apoptosis (17). Although it is proved that ERMP1 contributes to ER stress, whether it acts in upstream or within ER-related UPRs remains unclear, these data suggest that ERMP1 plays an important role in localization of GRP78 and exert its several biological effects via GRP78. GRP78 is upregulated in numerous cancers, and following translocation to the cell surface it acts as a receptor for various ligands that induce cell malignancy by promoting multiple responses such as cell proliferation, migration, invasion, stemness, and chemo-resistance (7, 37). In the case of CRC, csGRP78 also aggregate in the cell membrane, act as a biomarker for prognosis, and promote signaling pathways involved in survival and proliferation of CRCs such as PI3K/AKT pathway and Wnt/β-catenin through binding to α2-macroglobulin, complexing with PI3K, and promoting PI [3, 4, 5] P3 production and co-localization (38, 39). Besides, it has been suggested that GRP78 can indirectly suppress the interaction between β-catenin and E-cadherin through prevention of the E-cadherin expression in CRC (40), and co-existence of starvation or DTT in -ERMP1 cells seems to downregulate the expression of GRP78 and subsequently accelerate its preventive effects on E-cadherin. As a consequence, it seems that ERMP1exerts its effects on proliferation of CRC cells through not only AKT expression, but also translocation of GRP78 to the cell surface.
In summary, in an attempt to explore the mechanisms whereby ERMP1 enhances the proliferation of CRC cells, we found that ERMP1 played a role in accelerating AKT expression. As a consequence, the augmented p-AKT significantly increased free β-catenin, an affect that elevated the expression of CYCLIN D but not C-MYC; this suggests that activation of p-AKT plays a role in ERMP1-mediated stabilization of β-catenin and depletion of complex formation between E-cadherin and β-catenin. Thus, through enhancing the expression of AKT and facilitating cell surface translocation of GRP78, ERMP1 could promote the AKT/mTOR phosphorylation, free β-catenin stabilization, and subsequently prompt the cell cycle progression of CRC cells into the S phase
The limitation of the present study was lacking animal experiments. Our next plan is to assess the frequency of the ERMP1 overexpression in CRC patients and explore the clinical and pathological impact of ERMP1 overexpression in affected patients.
Conclusion
Our in vitro research suggests that ERMP1 acts as an oncogene possibly through accelerating the malignant characteristics of CRC cells via affecting the PI3K/AKT/mTOR/β-catenin signaling pathways and localizing GRP78; therefore, ERMP1 may be regarded as a promising point in therapeutic strategies related to CRC. Additionally, this evidence helps better explore the ERMP1 employed molecular mechanisms underlying CRC proliferation which may further suggest the effectiveness of ERMP1 suppression in combination with DTT for CRC treatment. Further in vivo studies are required to confirm these results.
Supplementary PDF
Acknowledgments
The authors would like to acknowledge Shiraz University of Medical Sciences for financially supporting this study (Grant No. 22208). The authors would also like to express their sincere gratitude to the National Institute for Medical Research Development (NIMAD) for financially supporting this project (Grant No. 943267). The authors would like to acknowledge the office of the president and vice-president for science and technology, Iran National Science Foundation (INSF) for financially supporting this project (Grant No. 99024306). The authors would also like to appreciate Dr. Nasrin Shokrpour at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for her valuable assistance in English editing of this manuscript. The authors declare that there is no conflict of interest.
Author’s Contributions
P.M., M.Z.; Conceptualization, Resources, Project administration, Writing, Reviewing, and Editing. N.R.-K., M.Z.; Methodology. N.R.-K.; Investigation, Software, Formal analysis, Visualization, and Writing. P.M., N.R., M.Z.; Data curation. P.M.; Supervision, Funding acquisition. All authors read and approved the final manuscript.
References
- 1.Grandi A, Santi A, Campagnoli S, Parri M, De Camilli E, Song C, et al. ERMP1, a novel potential oncogene involved in UPR and oxidative stress defense, is highly expressed in human cancer. On-cotarget. 2016;7(39):63596–63610. doi: 10.18632/oncotarget.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Rudaz C, Luna F, Tapia V, Kerr B, Colgin L, Galimi F, et al. Fxna, a novel gene differentially expressed in the rat ovary at the time of folliculogenesis, is required for normal ovarian histogenesis. Development (Cambridge, England) 2007;134(5):945–957. doi: 10.1242/dev.02795. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Liu S, Liu G, Dombkowski A, Abrams J, Martin-Trevino R, et al. Identification and functional analysis of 9p24 amplified genes in human breast cancer. Oncogene. 2012;31(3):333–341. doi: 10.1038/onc.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 5.Sakata S, Larson DW. Targeted therapy for colorectal cancer. Surg Oncol Clin N Am. 2022;31(2):255–264. doi: 10.1016/j.soc.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci USA. 1977;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farshbaf M, Khosroushahi AY, Mojarad-Jabali S, Zarebkohan A, Valizadeh H, Walker PR. Cell surface GRP78: An emerging imaging marker and therapeutic target for cancer. J Control Release. 2020;328:932–941. doi: 10.1016/j.jconrel.2020.10.055. [DOI] [PubMed] [Google Scholar]
- 8.Qu J, Zhang L, Li L, Su Y. miR-148b functions as a tumor suppressor by targeting endoplasmic reticulum metallo protease 1 in human endometrial cancer cells. Oncol Res. 2018;27(1):81–88. doi: 10.3727/096504018X15202988139874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Lu H, Hu J, Li J, Lu W, Deng X, Wang X. miR-328-3p overexpression attenuates the malignant proliferation and invasion of liver cancer via targeting endoplasmic reticulum metallo protease 1 to inhibit AKT phosphorylation. Ann Transl Med. 2020;8(12):754–754. doi: 10.21037/atm-20-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64–64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. Recent Results Cancer Res. 2016;207:39–72. doi: 10.1007/978-3-319-42118-6_3. [DOI] [PubMed] [Google Scholar]
- 12.Saavedra-García P, Roman-Trufero M, Al-Sadah HA, Blighe K, López-Jiménez E, Christoforou M, et al. Systems level profiling of chemotherapy-induced stress resolution in cancer cells reveals druggable trade-offs. Proc Natl Acad Sci USA. 2021;118(17):e2018229118–e2018229118. doi: 10.1073/pnas.2018229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Akra L, Bae DH, Leck LYW, Richardson DR, Jansson PJ. The biochemical and molecular mechanisms involved in the role of tumor micro-environment stress in development of drug resistance. Biochim Biophys Acta Gen Subj. 2019;1863(9):1390–1397. doi: 10.1016/j.bbagen.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Z, Osipyan A, Song S, Chen D, Schut RA, van Merkerk R, et al. Thieno [2, 3-d] pyrimidine-2, 4 (1 H, 3 H)-dione derivative inhibits d-dopachrome tautomerase activity and suppresses the proliferation of non-small cell lung cancer cells. J Med Chem. 2022;65(3):2059–2077. doi: 10.1021/acs.jmedchem.1c01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18(9):1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiligada E. Chemotherapy: induction of stress responses. Endocr Relat Cancer. 2006;13(Suppl 1):S115–S124. doi: 10.1677/erc.1.01272. [DOI] [PubMed] [Google Scholar]
- 17.Xiang XY, Yang XC, Su J, Kang JS, Wu Y, Xue YN, et al. Inhibition of autophagic flux by ROS promotes apoptosis during DTT-induced ER/oxidative stress in HeLa cells. Oncol Rep. 2016;35(6):3471–3479. doi: 10.3892/or.2016.4725. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CW, Yang MD, Hsia TC, Chang WS, Hsu CM, Hsieh YH, et al. Dithiothreitol enhanced arsenic-trioxide-induced cell apoptosis in cultured oral cancer cells via mitochondrial dysfunction and endoplasmic reticulum stress. Environ Toxicol. 2017;32(1):17–27. doi: 10.1002/tox.22208. [DOI] [PubMed] [Google Scholar]
- 19.Mal’tseva VN, Goltyaev MV, Novoselov SV, Varlamova EG. Effects of sodium selenite and dithiothreitol on expression of endoplasmic reticulum selenoproteins and apoptosis markers in MSF7 breast adenocarcinoma cells. Mol Biol (Mosk) 2022;56(1):135–146. doi: 10.31857/S0026898422010062. [DOI] [PubMed] [Google Scholar]
- 20.Tan Z, Chan YJA, Chua YJK, Rutledge SD, Pavelka N, Cimini D, et al. Environmental stresses induce karyotypic instability in colorectal cancer cells. Mol Biol Cell. 2019;30(1):42–55. doi: 10.1091/mbc.E18-10-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127(5):905–915. doi: 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayanankutty A. PI3K/ Akt/ mTOR pathway as a therapeutic target for colorectal cancer: a review of preclinical and clinical evidence. Curr Drug Targets. 2019;20(12):1217–1226. doi: 10.2174/1389450120666190618123846. [DOI] [PubMed] [Google Scholar]
- 25.Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–234. doi: 10.1016/j.biochi.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Yan J, Wong N, Hung C, Chen WX, Tang D. Contactin-1 reduces Ecadherin expression via activating AKT in lung cancer. PLoS One. 2013;8(5):e65463–e65463. doi: 10.1371/journal.pone.0065463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He R, Du S, Lei T, Xie X, Wang Y. Glycogen synthase kinase 3β in tumorigenesis and oncotherapy (Review) Oncol Rep. 2020;44(6):2373–2385. doi: 10.3892/or.2020.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves M, Borges DP, Kimberly A, Martins Neto F, Oliveira AC, de Sousa JC, et al. Glycogen synthase kinase-3 beta expression correlates with worse overall survival in non-small cell lung cancer-A clinicopathological series. Front Oncol. 2021;11:621050–621050. doi: 10.3389/fonc.2021.621050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Lam JB, Chow KH, Xu A, Lam KS, Moon RT, et al. Adiponectin stimulates Wnt inhibitory factor-1 expression through epigenetic regulations involving the transcription factor specificity protein 1. Carcinogenesis. 2008;29(11):2195–2202. doi: 10.1093/carcin/bgn194. [DOI] [PubMed] [Google Scholar]
- 30.Yun SH, Park JI. PGC-1α regulates cell proliferation and invasion via AKT/GSK-3β/β-catenin pathway in human colorectal cancer SW620 and SW480 cells. Anticancer Res. 2020;40(2):653–664. doi: 10.21873/anticanres.13995. [DOI] [PubMed] [Google Scholar]
- 31.Chen JS, Huang JQ, Luo B, Dong SH, Wang RC, Jiang ZK, et al. PIK3CD induces cell growth and invasion by activating AKT/ GSK-3β/β-catenin signaling in colorectal cancer. Cancer Sci. 2019;110(3):997–1011. doi: 10.1111/cas.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prossomariti A, Piazzi G, Alquati C, Ricciardiello L. Are Wnt/βcatenin and PI3K/AKT/mTORC1 distinct pathways in colorectal cancer? Cell Mol Gastroenterol Hepatol. 2020;10(3):491–506. doi: 10.1016/j.jcmgh.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57–83. doi: 10.1016/j.critrevonc.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Hong KO, Kim JH, Hong JS, Yoon HJ, Lee JI, Hong SP, et al. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J Exp Clin Cancer Res. 2009;28(1):28–28. doi: 10.1186/1756-9966-28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Gutiérrez L, Delgado MD, León J. MYC oncogene contributions to release of cell cycle brakes. Genes (Basel) 2019;10(3):244–244. doi: 10.3390/genes10030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H, Weng H, Zhou H, Qu L. Attacking C-MYC: targeted and combined therapies for cancer. Curr Pharm Des. 2014;20(42):6543–6554. doi: 10.2174/1381612820666140826153203. [DOI] [PubMed] [Google Scholar]
- 37.Xi J, Chen Y, Huang S, Cui F, Wang X. Suppression of GRP78 sensitizes human colorectal cancer cells to oxaliplatin by downregulation of CD24. Oncol Lett. 2018;15(6):9861–9867. doi: 10.3892/ol.2018.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK, et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin Cancer Res. 2013;19(24):6802–6811. doi: 10.1158/1078-0432.CCR-13-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu R, Yang P, Wu HL, Li ZW, Li ZY. GRP78 secreted by colon cancer cells facilitates cell proliferation via PI3K/Akt signaling. Asian Pac J Cancer Prev. 2014;15(17):7245–7249. doi: 10.7314/apjcp.2014.15.17.7245. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Wang Y, Wu H, Zhang L, Yang P, Li Z. GRP78 enhances the glutamine metabolism to support cell survival from glucose deficiency by modulating the β-catenin signaling. Oncotarget. 2014;5(14):53–53. doi: 10.18632/oncotarget.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.