Abstract
Purpose
Metabolic associated fatty liver disease is a novel concept defined as fatty liver associated with metabolic disorders. We investigated the effect of metabolic associated fatty liver disease on hepatocellular carcinoma patient mortality.
Patients and Methods
A total of 624 patients with hepatocellular carcinoma between 2012 and 2020 were enrolled in this retrospective study. Hepatic steatosis was diagnosed using computed tomography or magnetic resonance imaging. Metabolic associated fatty liver disease was defined based on the proposed criteria in 2020. Propensity score matching was performed for patients with metabolic associated fatty liver disease and those without the condition. A Cox proportional hazards regression model was used to evaluate the association between metabolic associated fatty liver disease and hepatocellular carcinoma patient outcomes.
Results
Patients with hepatocellular carcinoma and metabolic associated fatty liver disease tended to achieve better outcomes than did those without metabolic associated fatty liver disease after matching (p<0.001). Metabolic associated fatty liver disease was significantly associated with better prognosis in patients with concurrent hepatitis B infection (p<0.001). Moreover, high levels of hepatitis B viral DNA in serum samples was associated with a significantly increased risk of death in patients without non-metabolic associated fatty liver disease (p=0.045). Additionally, the association between metabolic associated fatty liver disease and survival in hepatitis B virus-related hepatocellular carcinoma was similar in all subgroups based on metabolic traits.
Conclusion
Metabolic associated fatty liver disease increases the survival rate of patients with hepatocellular carcinoma and hepatitis B virus infection. The potential interaction of steatosis and virus replication should be considered for future research and clinical treatment strategies.
Keywords: steatosis, diabetes, obesity, HBe Ag
Plain Language Summary
The current study explores the association between metabolic associated fatty liver disease (MAFLD) and chronic hepatitis B-related hepatocellular carcinoma (HBV-HCC) in terms of the prognosis. It shows that HBV-related HCC patients with MAFLD have a better outcome than those without MAFLD. Risk factors for HCC, such as positive HBeAg (Hepatitis B e-antigen), high load of HBV DNA, and metabolic traits, do not have a significant effect on the MAFLD group. A novel finding was that patients with MAFLD achieved better survival among HCC patients with hepatitis B infection, which may be due to the interaction between steatosis and virus replication. Drawing on discoveries in the interaction between MAFLD and HBV-HCC, we propose that further patient stratification is necessary for MAFLD group, considering the heterogeneous nature of MAFLD and the complex pathogenesis of HBV.
Introduction
Metabolic associated fatty liver disease (MAFLD) is a novel concept proposed by an international consensus in 2020, defined as hepatic steatosis with diabetes, obesity, or at least two metabolic abnormalities.1 Considering the increasing incidence of metabolic disorders, MAFLD has been associated with the development of various cancers.2,3 Hepatocellular carcinoma (HCC), the predominant primary malignancy of the liver, is one of the leading causes of mortality in patients with cancer.4,5 However, as MAFLD criteria have recently been developed based on consensus, there are limited data regarding the characteristics of patients with MAFLD-HCC, especially in Asians.
The higher incidence of HCC in Asia than in other regions of the world is still related to predominance of chronic hepatitis B (CHB).6,7 Unfortunately, the previous term NAFLD was defined as the exclusion of other chronic liver diseases, making it harder to focus on metabolic disorders in CHB patients.8 Thus, the new MAFLD definition makes its coexistence with CHB possible. Accompanied by the rapid increase in the prevalence of metabolic disorders, the coexistence of metabolic syndrome and CHB is commonly observed.9–11 In hepatitis B virus (HBV) carriers, metabolic factors such as obesity and diabetes are well-established risk factors for HCC; however, hepatitis steatosis is found to be associated with a lower incidence of HCC.12 Thus, owing to the combined effects of these metabolic factors, MAFLD represents a complex multisystem disorder, and the impact of MAFLD on the clinical outcomes of CHB-HCC patients remains unknown.
As such, we performed a retrospective analysis to investigate the effect of MAFLD on the long-term survival of patients with HCC. We also explore the association between MAFLD and CHB-related HCC in terms of the prognosis.
Materials and Methods
Patients
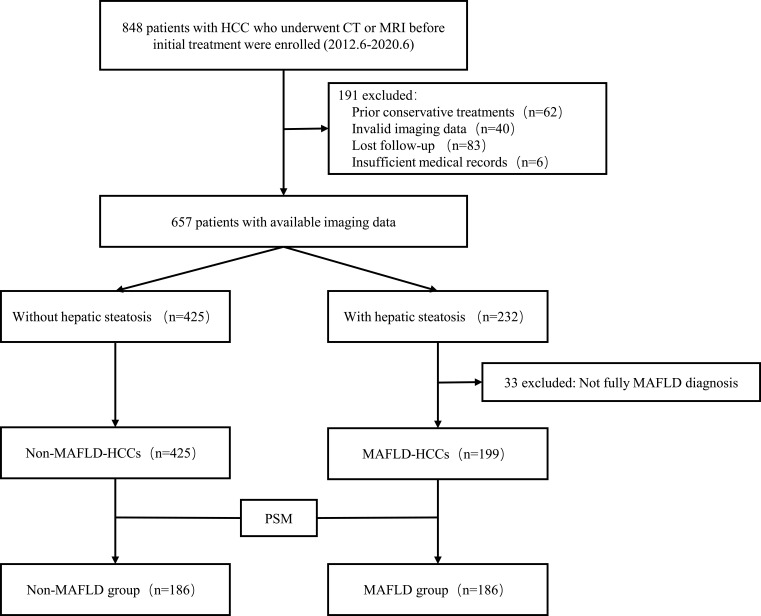
The study included a total of 848 patients with HCC who were admitted to Shandong Provincial Hospital Affiliated with Shandong First Medical University, Shandong, China, between June 2012 and June 2020. Patients underwent computed tomography (CT) or magnetic resonance imaging (MRI) before initial treatment. Patients were identified according to the following inclusion criteria: (1) Age ≥18 years; (2) Initial diagnosis of HCC confirmed by histopathology and radiographic evaluation; (3) Available imaging data for the assessment of hepatic steatosis; and (4) No other malignant neoplasms within the last 5 years. The exclusion criteria were as follows: (1) Patients with HCC who underwent transcatheter arterial chemoembolization or other conservative treatments; (2) Patients with hepatic steatosis with lacking body mass index (BMI) information; or (3) Patients without any MAFLD inclusion criteria. A total of 624 patients were enrolled, including 199 with MAFLD and 425 without MAFLD (Figure 1). This study was approved by the institutional review board of Shandong Provincial hospital, and the requirement for informed patient consent was waived due to the retrospective nature of the study.
Figure 1.
Patient flow diagram.
Abbreviations: CT, computed tomography; HCC, hepatocellular carcinoma; MAFLD, metabolic dysfunction-associated fatty liver disease; MRI, magnetic resonance imaging; PSM, propensity score matching.
Diagnosis of Liver Disease
MAFLD cases were defined as radiologically diagnosed hepatic steatosis in combination with at least one of the following characteristics: (1) Overweight (BMI ≥ 23 kg/m2 for Asians); (2) Type 2 diabetes mellitus; and (3) Evidence of metabolic dysregulation. Metabolic dysregulation was defined by the presence of two or more of the following metabolic abnormalities: (1) Blood pressure ≥130/85 mmHg or specific drug treatment; 2) Plasma triglycerides ≥1.70 mmol/L or drug treatment; 3) Plasma HDL-C < 1.0 mmol/L for men and <1.3 mmol/L for women; and 4) Prediabetes (ie, fasting glucose levels 5.6 to 6.9 mmol/L, or 2-hour post-load glucose levels 7.8 to 11.0 mmol or HbA1c 5.7% to 6.4%). Three additional proposed criteria (waist circumference, high-sensitivity C-reactive protein level, and homeostasis model assessment index) were not available for this cohort.
To evaluate steatosis, a clinical CT-MRI linear conversion formula was used.13 Quantification of liver fat content using MRI was based on the two-point Dixon method.14 Other etiologies of HCC, including HBV infection, hepatitis C virus (HCV) infection, and alcohol use, were defined according to generally agreed criteria. HCC staging was assessed using the Barcelona Clinic Liver Cancer (BCLC) and China Liver Cancer (CNLC) staging systems.15,16 The severity of liver cirrhosis was assessed using the Child-Pugh score and model for end-stage liver disease (MELD) score.17,18
Follow-Up and Outcomes
The prognostic information for patients was obtained from regular follow-ups every 3-months during the 1st year and 3–6 months thereafter. Disease recurrence was identified using radiological detection. The outcome of this study was overall survival (OS), calculated from the date of first treatment to the date of death related to any cause or the date of the last follow-up.
Statistical Analysis
R (version 4.1.3) and SPSS 26.0 statistical packages were used for data analyses in this study. Continuous data were presented as median with interquartile range. Categorical variables were presented as counts and percentages. Differences in categorical variables were compared using the Pearson’s chi-squared test or Fisher’s exact test, and continuous data were analyzed using the Mann–Whitney U-test.
Propensity score matching (PSM) was used to reduce the influence of confounding factors and selection bias. A total of 372 patients were enrolled after 1:1 matching with caliper distance (0.02), including age, sex, cirrhosis, etiology (HBV, HCV, and cryptogenic), tumor characteristics (histopathology, metastasis, and tumor size), initial therapy, and MELD score.
Survival curves were generated using the Kaplan-Meier method and estimated using a Log rank test. Univariate and multivariate Cox proportional hazard models were used to analyze the prognostic factors and were constructed using a forward stepwise approach. All reported p values were two-sided, and p values < 0.05 were considered significant.
Results
Baseline Characteristics
Among the 624 participants, the median age was 57.0 (49.3–64.0) years, and 83.3% of patients were male. The most common cause of HCC was chronic HBV infection (83.8%). Cirrhosis was present in 85.1% of patients. The BMI (p < 0.001) and proportion of hypertension (p = 0.003) were higher in the MAFLD group than in the non-MAFLD group. Patients with non-MAFLD-HCC were more frequently treated with liver resection (p = 0.008), had a higher incidence of HBV infection (p = 0.006), and had higher MELD scores (p = 0.040). After PSM, all matching variables were balanced, and 186 patients in each group were included in the analysis (Table 1).
Table 1.
Baseline Characteristics of Total Cohort and PSM Cohort
| Total Cohort | PSM Cohort | |||||
|---|---|---|---|---|---|---|
| MAFLD (n=199) | Non-MAFLD (n=425) | p value | MAFLD (n=186) | Non-MAFLD (n=186) | p value | |
| Age (years)+ | 57.0 (48.0–63.5) | 56.0 (49.0–63.0) | 0.431 | 56.5 (48.0–63.8) | 57.0 (50.0–64.0) | 0.876 |
| Gender (male) | 163 (81.9) | 357 (84.0) | 0.514 | 153 (82.3) | 147 (79.0) | 0.431 |
| BMI (kg/m2)+ | 25.7 (24.1–28.0) | 24.8 (23.1–26.9) | 0.000 | 25.7 (24.0–28.0) | 24.9 (23.2–27.1) | 0.000 |
| Type 2 diabetes | 36 (18.1) | 70 (16.5) | 0.616 | 32 (17.2) | 39 (21.0) | 0.356 |
| Hypertension | 73 (36.7) | 107 (25.2) | 0.003 | 68 (36.6) | 42 (22.6) | 0.003 |
| Liver cirrhosis | 165 (82.9) | 366 (86.1) | 0.295 | 158 (84.9) | 158 (84.9) | 1.000 |
| HBV | 155 (77.9) | 368 (86.6) | 0.006 | 150 (80.6) | 149 (80.1) | 0.896 |
| Alcohol | 53 (26.6) | 106 (24.9) | 0.651 | 52 (28.0) | 47 (25.3) | 0.557 |
| ECOG PS (>0) | 45 (22.6) | 94 (22.1) | 0.890 | 41 (22.0) | 45 (24.2) | 0.623 |
| AFP (>1000 ng/mL) | 37 (19.1) | 81 (19.6) | 0.886 | 37 (20.3) | 42 (23.0) | 0.543 |
| Histopathology | 0.426 | 0.310 | ||||
| Well | 17 (9.9) | 28 (7.0) | 17 (10.3) | 10 (5.7) | ||
| Moderate | 120 (70.2) | 297 (74.4) | 115 (69.7) | 128 (73.1) | ||
| Poor | 34 (19.9) | 74 (18.6) | 33 (20.0) | 37 (21.1) | ||
| Tumor number (>1) | 32 (16.2) | 69 (16.2) | 0.981 | 32 (17.2) | 29 (15.6) | 0.674 |
| Tumor size (cm)+ | 3.8 (2.6–6.3) | 4.0 (2.7–6.9) | 0.184 | 3.9 (2.6–6.4) | 4.5 (2.7–7.0) | 0.066 |
| Microvascular invasion | 42 (21.2) | 94 (22.2) | 0.788 | 42 (22.6) | 52 (28.0) | 0.233 |
| Metastases | 9 (4.5) | 36 (8.5) | 0.077 | 9 (4.8) | 16 (8.6) | 0.213 |
| Satellite nodules | 21 (10.6) | 50 (11.8) | 0.665 | 21 (11.3) | 22 (11.8) | 0.871 |
| Child B and C | 18 (9.1) | 28 (6.6) | 0.273 | 17 (9.1) | 18 (9.7) | 0.859 |
| MELD+ | 7.0 (7.0–8.0) | 8.0 (7.0–9.0) | 0.040 | 7.0 (7.0–8.0) | 8.0 (7.0–9.0) | 0.089 |
| BCLC | 0.875 | 0.772 | ||||
| 0 | 14 (7.2) | 23 (5.4) | 10 (5.4) | 12 (6.5) | ||
| A | 38 (19.6) | 77 (18.2) | 37 (19.9) | 32 (17.2) | ||
| B | 89 (45.9) | 197 (46.6) | 87 (46.8) | 80 (43.0) | ||
| C | 50 (25.8) | 119 (28.1) | 49 (26.3) | 59 (31.7) | ||
| D | 3 (1.5) | 7 (1.7) | 3 (1.6) | 3 (1.6) | ||
| CNLC | 0.983 | 0.762 | ||||
| I | 130 (67.0) | 278 (65.9) | 122 (65.6) | 113 (60.8) | ||
| II | 13 (6.7) | 27 (6.4) | 13 (7.0) | 13 (7.0) | ||
| III | 48 (24.7) | 110 (26.0) | 48 (25.8) | 57 (30.6) | ||
| IV | 3 (1.6) | 7 (1.6) | 3 (1.6) | 3 (1.6) | ||
| Initial therapy | 0.008 | 0.587 | ||||
| Liver resection | 112 (56.3) | 281 (66.1) | 111 (59.7) | 103 (55.4) | ||
| Laparoscopic resection | 61 (30.7) | 120 (28.2) | 56 (30.1) | 69 (37.1) | ||
| Others | 26(13.0) | 24(5.7) | 19 (10.2) | 14 (7.5) | ||
| HBeAg positive | 43 (21.6) | 95 (22.6) | 0.789 | 40 (21.5) | 35 (18.8) | 0.518 |
| HBV DNA (log10 IU/mL)+ | 2.8 (1.8–4.9) | 3.0 (1.6–4.8) | 0.777 | 2.8 (1.9–4.9) | 2.3 (1.4–3.9) | 0.078 |
Notes: +Data are medians, with interquartile ranges in parentheses. p value of < 0.05 was considered statistically significant and shown in bold values. Except where indicated, Data are numbers of patients, with percentages in parentheses.
Abbreviations: AFP, alpha fetoprotein; ALBI, albumin-bilirubin grade; BCLC, Barcelona Clinic Liver Cancer staging system; BMI, Body mass index; Child B and C, Child-Pugh class B and C; CNLC, China liver cancer staging system; ECOG PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; HBeAg, hepatitis B e antigen; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; PSM, propensity score matching; SMD, standardized mean difference.
Outcomes in HCC Patients
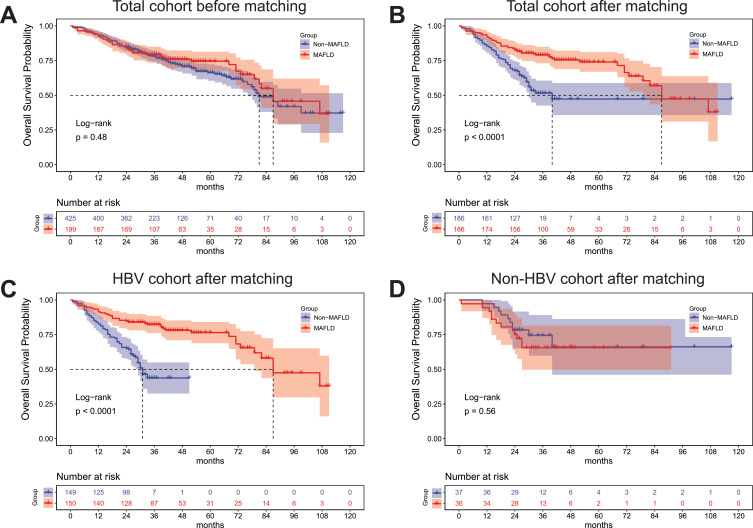
In the total cohort, the median follow-up was 87 months (95% CI 80.2–93.8), and the median OS between patients with and without MAFLD was not significantly different (p = 0.48) (Figure 2A). After PSM, the median OS were 87 and 40 months in the MAFLD and non-MAFLD groups, respectively (p < 0.001) (Figure 2B). For patients with CHB, the median OS was significantly lower in the non-MAFLD (31 months) than in the MAFLD group (87 months) (p < 0.001) (Figure 2C). For patients without HBV infection, the median OS was not significantly different between the MAFLD and non-MAFLD groups (p = 0.56) (Figure 2D).
Figure 2.
(A) OS before matching. (B) OS after matching. (C) OS in patients with hepatitis B after matching. (D) OS in patients without hepatitis B after matching.
Abbreviations: HCC, hepatocellular carcinoma; MAFLD, metabolic dysfunction-associated fatty liver disease; OS, overall survival.
Outcomes in MAFLD and Non-MAFLD Patients
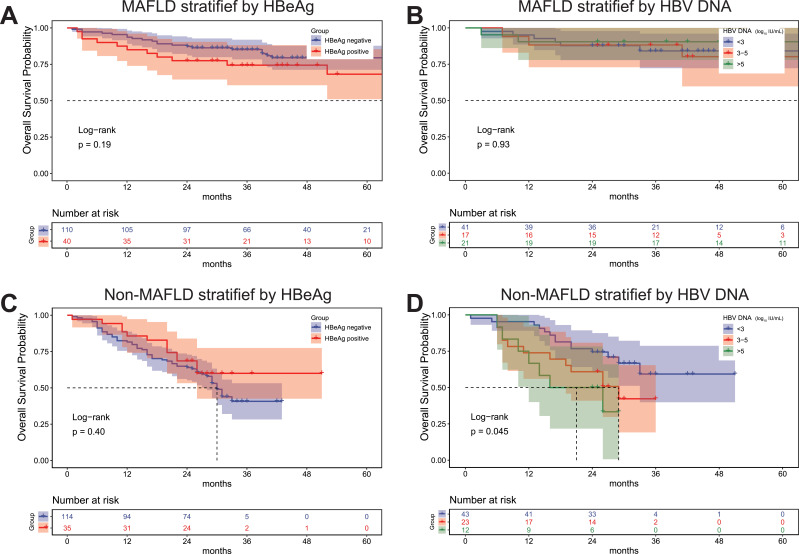
For CHB-HCC patients in the PSM cohort, the median OS of the MAFLD group was not significantly different between the hepatitis B e-antigen (HBeAg)-positive and HBeAg-negative groups (p = 0.19) (Figure 3A). A similar result was found among non-MAFLD individuals (p = 0.40) (Figure 3B). Moreover, the outcome of patients with MAFLD was not influenced by the serum HBV DNA level (p = 0.93), which was shown to be an important risk factor for non-MAFLD participants (p = 0.045) (Figure 3C and D).
Figure 3.
(A) Patients with MAFLD stratified by HBeAg status. (B) Patients with MAFLD stratified by HBV DNA levels. (C) Patients with non-MAFLD stratified by HBeAg status. (D) Patients with non-MAFLD stratified by HBV DNA levels.
Abbreviations: CHB, chronic hepatitis B; HCC, hepatocellular carcinoma; MAFLD, metabolic dysfunction-associated fatty liver disease; HBeAg, hepatitis B e-antigen; HBV, hepatitis B virus.
Risk Factors for Outcomes of Patients with Hepatitis B
For patients with CHB after matching, the univariate analysis in Table 2 showed that Eastern Cooperative Oncology Group (ECOG), alpha fetoprotein (AFP), liver function reserve (Child–Pugh), and tumor characteristics (histopathology, tumor size, microvascular invasion, metastasis, and satellite nodules) were significant factors for poor OS, as expected. MAFLD (p < 0.001) and higher BMI (p = 0.006) were associated with a significantly lower risk of mortality, which was also confirmed in the multivariate analysis. Three risk factors identified in the univariate analysis (ECOG performance status, tumor size, and microvascular invasion) remained significant in the multivariate analysis.
Table 2.
Prognostic Factor Analysis for Overall Survival in CHB-HCC Patients
| Variables | Overall Survival | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (years) | 1.00 (0.98–1.02) | 0.903 | ||
| Gender (male) | 0.75 (0.48–1.19) | 0.219 | ||
| MAFLD | 0.29 (0.19–0.46) | 0.000 | 0.36 (0.22–0.57) | 0.000 |
| BMI | 0.89 (0.83–0.94) | 0.000 | 0.91 (0.86–0.97) | 0.006 |
| Type 2 diabetes | 0.80 (0.45–1.44) | 0.464 | ||
| Hypertension | 0.69 (0.43–1.10) | 0.118 | ||
| Liver cirrhosis | 0.79 (0.48–1.31) | 0.358 | ||
| ECOG PS (>0) | 2.01 (1.35–2.99) | 0.001 | 2.13 (1.40–3.24) | 0.000 |
| Alcohol | 1.32 (0.88–1.97) | 0.186 | ||
| AFP (>1000 ng/mL) | 1.77 (1.17–2.68) | 0.007 | 0.81 (0.50–1.32) | 0.398 |
| Child B and C | 2.60 (1.54–4.38) | 0.000 | 1.58 (0.87–2.88) | 0.133 |
| Histological grade | 0.041 | 0.193 | ||
| Well | (ref) | (ref) | ||
| Moderate | 2.43 (0.76–7.76) | 0.133 | 1.91 (0.46–7.94) | 0.372 |
| Poor | 3.77 (1.13–12.61) | 0.031 | 2.76 (0.63–12.00) | 0.177 |
| Tumor number (>1) | 1.42 (0.90–2.26) | 0.136 | ||
| Tumor size | 1.15 (1.09–1.21) | 0.000 | 1.06 (1.00–1.13) | 0.048 |
| Microvascular invasion | 3.95 (2.67–5.83) | 0.000 | 3.01 (1.96–4.64) | 0.000 |
| Metastases | 2.33 (1.27–4.25) | 0.006 | 0.96 (0.48–1.93) | 0.911 |
| Satellite nodules | 2.05 (1.26–3.35) | 0.004 | 1.19 (0.69–2.03) | 0.538 |
Note: p value of < 0.05 was considered statistically significant and shown in bold values.
Abbreviations: AFP, alpha fetoprotein; BMI, Body mass index; Child B and C, Child-Pugh class B and C; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; HBsAg, Hepatitis B surface antigen; MAFLD, metabolic dysfunction-associated fatty liver disease.
Subgroup Analyses
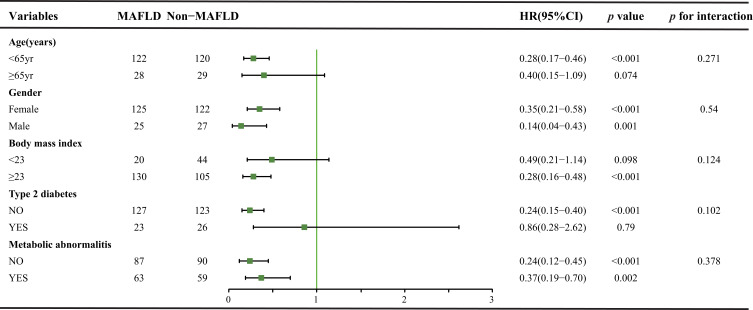
The study was stratified according to the types of metabolic traits (BMI, type 2 diabetes mellitus, and metabolic abnormalities proposed in the MAFLD criteria), and the outcome of patients with CHB-HCC was observed. As shown in Figure 4, clinical outcomes were significantly better in the MAFLD group, whereas the interaction effects between survival and metabolic traits were not statistically significant.
Figure 4 .
Subgroup analyses by two groups of non-MAFLD and MAFLD after matching.
Abbreviations: HR, hazard ratio; MAFLD, metabolic associated fatty liver disease.
Discussion
Our analyses showed that among patients with CHB-HCC, those with MAFLD experienced better long-term outcomes than did those without MAFLD. In addition, multivariate analysis indicated that MAFLD was an independent favorable prognostic factor for patients with CHB-HCC; moreover, the subgroup analyses defined by metabolic characteristics further confirmed these findings. Whilst the association between MAFLD and HCC has been explored in a few studies,19,20 the potential effect of MAFLD in patients with HCC with coexisting other etiologies remains largely unknown. The new definition of MAFLD has been endorsed and supported by the Chinese Society of Hepatology (CSH), and a large proportion of HCC patients in China has CHB as an etiology. With the increasing prevalence of MAFLD, it is relevant to explore the underlying interactions between MAFLD and CHB in HCC patients.21 To our knowledge, this is the first study wherein liver steatosis has been evaluated by quantitative imaging methods for the diagnosis of MAFLD to investigate the potential synergism between MAFLD and CHB in HCC progression.
Unlike the previous definition of NAFLD, exclusion of other etiologies, such as alcohol and viral infections, is not a prerequisite for the diagnosis of MAFLD.22 The epidemiological features of the HCC population in our study indicate that the incidence of MAFLD is increasing with concomitant HBV infection (Supplementary Figure 1). Furthermore, while the subclassification of MAFLD has not been completed,1 our evidence highlights the importance of this new criterion in individuals with HBV-related HCC.
Substantial epidemiological evidence indicates that being overweight is associated with an increased risk of HCC.23 Moreover, a recent meta-analysis demonstrated that overweight or obese is associated with higher all-cause mortality than is normal body weight.24 Conversely, we found a significantly lower risk of death in patients with HCC with a higher BMI (≥ 23 kg/m2). Controversial data have also been generated from NAFLD studies. Patients with lean NAFLD (BMI < 23 kg/m2) are at a higher risk of developing severe liver disease and worse outcomes, despite having a better histological and metabolic profile.19,25 However, our stratified analyses determined that both lean and overweight patients with MAFLD achieved a significant improvement in the clinical outcome of HCC. The main reason could be the heterogeneity of MAFLD influenced by multiple factors including age, sex, surveillance, alcohol intake, viral infection, and metabolic status.26–31 The clinical course of HCC is based on the balance of these diverse inputs. To reduce selection bias and the effect of baseline differences, strict PSM and multivariate analyses were performed. The results showed that the protective effect of MAFLD on HCC patient survival is independent of tumor characteristics and obesity. Nevertheless, the expected prognostic advantage of MAFLD might be counterbalanced by other causes in the total cohort, such as different treatment options, more aggressive tumor phenotypes, and worse health status of patients.20
Hepatic steatosis, defined as the abnormal accumulation of triglycerides within hepatocytes, is correlated with the progression of inflammation, fibrosis, and carcinogenesis.32,33 Although liver biopsy is a reference standard, non-invasive quantification of liver fat is now possible due to advances in imaging modalities conducted to evaluate the fat content in MAFLD participants.34–36 However, our results suggest that hepatic steatosis may not be an important factor for MAFLD-HCC patient outcomes (Supplementary Figure 2A and B). One potential reason is that 84.9% of the patients were classified as having mild steatosis, while the proportion of those with moderate to severe fatty liver was lower. Another possibility is that viral infections and cirrhosis status might confer a protective effect on steatosis, which remains controversial in clinical practice.37–39
HBV infection remains a global public health problem and plays a pivotal role in hepatocarcinogenesis.40 According to the updated Clinical Practice Guideline,41 HBeAg is essential for immune tolerance in patients with CHB, and positive serum HBeAg is associated with poor outcomes in HCC.42 However, in the MAFLD and non-MAFLD groups, no differences in survival were detected between HBeAg-positive and HBeAg-negative patients in our study. A previous study has verified that an increasing risk of HCC seen in HBeAg seropositive cases might be due to higher HBV DNA viral loads rather than the effect of HBeAg status.43 Interestingly, higher HBV DNA levels were significantly correlated with unfavorable outcomes in non-MAFLD participants, whereas no differences were detected in MAFLD patients. One prospective study found that hepatic steatosis was associated with a three-fold increase in hepatitis B surface antigen (HBsAg) seroclearance rate in HBV carriers.44 Moreover, the presence of hepatic steatosis was associated with a lower HBV DNA load in HCC patients,39 which might account for our results. Also, recent studies showed that steatosis in CHB patients was significant associated with lower risk of HCC incidence.39,45 Contrastingly, previous studies have shown that MAFLD is associated with the risk of liver fibrosis and inflammatory activity in CHB patients.46 Thus, additional well-designed studies are needed to confirm these data, which will be important to clarify the effect of MAFLD on liver disease progression in patients with CHB.
This study has several limitations. First, although we performed PSM and multivariate analysis to balance the variables and enhance intergroup comparisons, there was still unidentified bias due to the retrospective nature of the study. Second, data on 3 metabolic parameters (waist circumference, insulin resistance, and C-reactive protein level) used to confirm the diagnosis of MAFLD were not available; thus, a few patients with lean MAFLDs were excluded. Third, because of incomplete information on antiviral treatments, treated patients may be biased to have more favorable outcomes than those of untreated patients. Additionally, the study by Goh et al showed that statin use is associated with a reduced risk of HCC development in patients with CHB;47 however, fewer patients in this study had dyslipidemia and required statins.
Conclusion
In conclusion, our study shows an increasing prevalence of MAFLD-HCC and indicates that MAFLD is associated with lower mortality in CHB-HCC patients, independent of metabolic risk factors. In addition, a novel finding of our study was that patients with MAFLD achieved better survival among HCC patients with hepatitis B infection. This may be due to the interaction between steatosis and virus replication. Further work is needed for patient stratification, considering the heterogeneous nature of MAFLD and the complex pathogenesis of HBV.
Acknowledgments
We would like to thank Editage for English language editing.
Funding Statement
This work was supported in part by grants from the National Natural Science Foundation of China (No. 82160124) and Regional Collaborative Innovation Project of Xinjiang Uygur Autonomous Region (No. 2022E02044).
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval and Informed Consent
Institutional Review Board approval was obtained. This study was approved by our institutional review board and the need for written informed consent was waived because of the retrospective nature of the study.
Informed Consent Statement
Patients were not required to give informed consent to the study because the analysis used anonymous clinical data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 2.Tarantino G, Crocetto F, Di Vito C, et al. Association of NAFLD and insulin resistance with non metastatic bladder cancer patients: a Cross-Sectional Retrospective Study. J Clin Med. 2021;10(2):346. doi: 10.3390/jcm10020346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh W-P, Dan YY, Goh G-B-B, Jin A, Wang R, Yuan J-M. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int. 2016;36(6):893–901. doi: 10.1111/liv.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidelines ECP. Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 6.Chan SL, Wong VWS, Qin S, Chan HLY. Infection and cancer: the case of hepatitis B. J Clin Oncol. 2016;34(1):83–90. doi: 10.1200/JCO.2015.61.5724 [DOI] [PubMed] [Google Scholar]
- 7.Vallet-Pichard A, Pol S. Review article: immunisation against hepatitis B virus infection and the prevention of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;53(11):1166–1182. doi: 10.1111/apt.16356 [DOI] [PubMed] [Google Scholar]
- 8.Brunt EM, Wong VWS, Nobili V, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80 [DOI] [PubMed] [Google Scholar]
- 9.Janicko M, Senajová G, Drazilová S, et al. Association between metabolic syndrome and hepatitis B virus infection in the Roma population in eastern Slovakia: a population-based study. Cent Eur J Public Health. 2014;22(Suppl):S37–S42. doi: 10.21101/cejph.a3900 [DOI] [PubMed] [Google Scholar]
- 10.Jiang D, Chen C, Liu X, et al. Concurrence and impact of hepatic steatosis on chronic hepatitis B patients: a systematic review and meta-analysis. Ann Transl Med. 2021;9(23):1718. doi: 10.21037/atm-21-3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng KI, Zheng M-H. Letter: hepatitis B and MAFLD - a consilience of risk factors for hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54(5):736–737. doi: 10.1111/apt.16532 [DOI] [PubMed] [Google Scholar]
- 12.Chen C-L, Yang H-I, Yang W-S, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135(1):111–121. doi: 10.1053/j.gastro.2008.03.073 [DOI] [PubMed] [Google Scholar]
- 13.Pickhardt PJ, Graffy PM, Reeder SB, Hernando D, Li K. Quantification of liver fat content with unenhanced MDCT: phantom and clinical correlation with MRI proton density fat fraction. AJR Am J Roentgenol. 2018;211(3):W151–W157. doi: 10.2214/AJR.17.19391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi T, Saitoh S, Takahashi J, et al. Hepatic fat quantification using the two-point Dixon method and fat color maps based on non-alcoholic fatty liver disease activity score. Hepatol Res. 2017;47(5):455–464. doi: 10.1111/hepr.12767 [DOI] [PubMed] [Google Scholar]
- 15.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 16.Xie D-Y, Ren Z-G, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. [DOI] [PubMed] [Google Scholar]
- 18.Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: the model for end-stage liver disease updated for the modern era. Gastroenterology. 2021;161(6):1887–1895.e4. doi: 10.1053/j.gastro.2021.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conci S, Cipriani F, Donadon M, et al. Hepatectomy for Metabolic Associated Fatty Liver Disease (MAFLD) related HCC: propensity case-matched analysis with viral- and alcohol-related HCC. Eur J Surg Oncol. 2022;48(1):103–112. doi: 10.1016/j.ejso.2021.07.015 [DOI] [PubMed] [Google Scholar]
- 20.Vitale A, Svegliati-Baroni G, Ortolani A, et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: the ITA.LI.CA database. Gut. 2023;72(1):141–152. doi: 10.1136/gutjnl-2021-324915 [DOI] [PubMed] [Google Scholar]
- 21.Nan Y, An J, Bao J, et al. The Chinese society of hepatology position statement on the redefinition of fatty liver disease. J Hepatol. 2021;75(2):454–461. doi: 10.1016/j.jhep.2021.05.003 [DOI] [PubMed] [Google Scholar]
- 22.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 23.Hassan MM, Abdel-Wahab R, Kaseb A, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149(1):119–129. doi: 10.1053/j.gastro.2015.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. doi: 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagström H, Nasr P, Ekstedt M, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol Commun. 2018;2(1):48–57. doi: 10.1002/hep4.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aron-Wisnewsky J, Vigliotti C, Witjes J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279–297. doi: 10.1038/s41575-020-0269-9 [DOI] [PubMed] [Google Scholar]
- 27.Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17(4):e1003100. doi: 10.1371/journal.pmed.1003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063–1075. doi: 10.1016/j.jhep.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobili V, Mantovani A, Cianfarani S, et al. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. J Hepatol. 2019;71(4):802–810. doi: 10.1016/j.jhep.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 30.Seto W-K, Hui RWH, Mak L-Y, et al. Association between hepatic steatosis, measured by controlled attenuation parameter, and fibrosis burden in chronic hepatitis B. Clin Gastroenterol Hepatol. 2018;16(4):575–583.e2. doi: 10.1016/j.cgh.2017.09.044 [DOI] [PubMed] [Google Scholar]
- 31.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 32.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3(4):445–451. doi: 10.1586/egh.09.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickhardt PJ, Hahn L, Muñoz Del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202(4):752–758. doi: 10.2214/AJR.13.11367 [DOI] [PubMed] [Google Scholar]
- 34.Graffy PM, Pickhardt PJ. Quantification of hepatic and visceral fat by CT and MR imaging: relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br J Radiol. 2016;89(1062):20151024. doi: 10.1259/bjr.20151024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn L, Reeder SB, Muñoz Del Rio A, Pickhardt PJ. Longitudinal changes in liver fat content in asymptomatic adults: hepatic attenuation on unenhanced CT as an imaging biomarker for steatosis. AJR Am J Roentgenol. 2015;205(6):1167–1172. doi: 10.2214/AJR.15.14724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartoris R, Gregory J, Dioguardi Burgio M, Ronot M, Vilgrain V. HCC advances in diagnosis and prognosis: digital and imaging. Liver Int. 2021;Suppl 41(S1):73–77. doi: 10.1111/liv.14865 [DOI] [PubMed] [Google Scholar]
- 37.Chan AWH, Wong GLH, Chan H-Y, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32(3):667–676. doi: 10.1111/jgh.13536 [DOI] [PubMed] [Google Scholar]
- 38.Suliman I, Abdelgelil N, Kassamali F, Hassanein TI. The effects of hepatic steatosis on the natural history of HBV infection. Clin Liver Dis. 2019;23(3):433–450. doi: 10.1016/j.cld.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 39.Mak L-Y, Hui RW-H, Fung J, et al. Reduced hepatic steatosis is associated with higher risk of hepatocellular carcinoma in chronic hepatitis B infection. Hepatol Int. 2021;15(4):901–911. doi: 10.1007/s12072-021-10218-2 [DOI] [PubMed] [Google Scholar]
- 40.Yuen M-F, Chen D-S, Dusheiko GM, et al. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. doi: 10.1038/nrdp.2018.35 [DOI] [PubMed] [Google Scholar]
- 41.Lampertico P, Agarwal K, Berg T. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 42.Sun H-C, Zhang W, Qin L-X, et al. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol. 2007;47(5):684–690. doi: 10.1016/j.jhep.2007.06.019 [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Yang H-I, Lee M-H, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut. 2014;63(10):1648–1657. doi: 10.1136/gutjnl-2013-305785 [DOI] [PubMed] [Google Scholar]
- 44.Mak L-Y, Hui RW-H, Fung J, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73(4):800–806. doi: 10.1016/j.jhep.2020.05.040 [DOI] [PubMed] [Google Scholar]
- 45.Wong YJ, Nguyen VH, Yang H-I, et al. Impact of fatty liver on long-term outcomes in chronic hepatitis B: a systematic review and matched analysis of individual patient data meta-analysis. Clin Mol Hepatol. 2023;29(3):705–720. doi: 10.3350/cmh.2023.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Zhou J, Wu L, Zhu X, Deng H. MAFLD is associated with the risk of liver fibrosis and inflammatory activity in HBeAg-negative CHB patients. Diabetes Metab Syndr Obes. 2022;15:673–683. doi: 10.2147/DMSO.S351492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goh MJ, Sinn DH, Kim S, et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology. 2020;71(6):2023–2032. doi: 10.1002/hep.30973 [DOI] [PubMed] [Google Scholar]